Corresponding Author

Key Words: cardiac magnetic resonance, echocardiography, mitral valve
Mitral Regurgitation: Frequent but Often Misunderstood and Undertreated
Mitral regurgitation (MR) is the most frequent valve disease in the developed world,1,2 where degenerative (DMR) and functional (FMR) causes predominate, and in low-income countries, where rheumatic disease remains prevalent.3 MR heterogeneity affects presentation, severity, consequences, and treatment, often creating diagnostic confusion. Indeed, we were previously taught that symptoms and left ventricle (LV) function were the diagnostic foci in MR.4 Now we also evaluate the left atrium (LA),5 hemodynamics, tricuspid regurgitation,6 right ventricle function,7 and even hormonal responses.8 All of these variables are profoundly heterogeneous and affect outcome in all forms of MR. MR physiologic response heterogeneity contributes to difficult clinical decision-making and to MR undertreatment. Indeed, despite valve repair availability/excellence, MR remains deeply undertreated,9,10 particularly FMR but also DMR, with excess mortality and heart failure rates10,11 warranting decisive modification of clinical processes, particularly with expansion of transcatheter therapies that may improve these outcomes.12 But, at the root of MR evaluation is regurgitation severity, measured in cm2 of effective regurgitant orifice (ERO) and in mL of regurgitant volume (RVol).13 These measurements ultimately predict mortality and complication rates.14 Progress in applying quantitative methods has led to strong, independent, and incremental prognostic power in expert hands14 and also in routine practice.15,16 This progress has allowed advancing the practice to orderly grading and rigorous quantification.17
This crucial approach is amplified in the case series reported in this issue of JACC: Case Reports by Faza et al.18 They present a compelling series of patients with various MR etiologies, analyzing obstacles to MR severity assessment, and describing individualized pathways to diagnosis and treatment. The first case involves a patient with a flail posterior leaflet where the flow convergence is difficult to detect due to P3 location. MR quantitation by Echo-Doppler and LV volumetric methods is complemented by magnetic resonance imaging. Remarkably, average RVol by 2 echo methods was almost identical to that obtained by magnetic resonance imaging, confirming MR severity and need for transcatheter edge-to-edge repair. The second case involves a patient with mid-late systolic MR whereby large jet extension overestimated MR severity and truncated continuous-wave Doppler was key to diagnosis and quantitation. The third case is a patient with MitraClip detachment and MR recurrence. Clip interposition makes MR assessment tenuous and was addressed by transesophageal echo. The fourth case is a patient with multiple jets making cursory MR assessment by color-flow imaging difficult. The fifth case is a patient with FMR whereby low RVol may complicate evaluation. All valve disease and cardiac imaging specialists should carefully review these cases to understand the pitfalls and apply the lessons to their clinical practice.
Evidently, cases can be disputed and lessons adjusted. For example, in case 1, assessing MR should involve scanning the entire mitral valve, locating flail segment and flow convergence and allowing PISA calculation. Also, incongruous measurements (eg, pulsed mitral time-velocity-integral) should be weeded out to improve RVol calculations. It is also important to underscore limitations of calculating RVol by subtraction of large volumes (with magnetic resonance imaging or echo) that causes errors-propagation. In case 2, full quantitation showing “discordance” of ERO and RVol provides a full physiological picture of partial (Mid-Late) MR. In case 3, a persistently flail valve is generally easily diagnosed, but nondetached clips with multiple jets represent true challenges to MR assessment. Similarly, case 4, with multiple jets, can be quantified by summation of regurgitant flows of all jets (by measuring each flow convergence) to calculate summated ERO/RVol. Finally, case 5 of FMR demonstrates how FMR and DMR are different diseases with different scales of severity. Despite “low” RVol, the authors considered it severe and worthy of treatment. It is not a measurement error due to regurgitant orifice shape, it is an obligatory low RVol due to low total stroke volume with reduced LV function.
However, potential drawbacks in this case series should not distract from essential facts. In most cases, the authors, after diagnosing MR mechanism, strove to measure ERO and RVol to determine appropriate therapy. Although guidelines propose MR quantitation for difficult cases, we concur with striving to quantify MR as often as possible, integrating results with observed cardiac remodeling, possibly remeasuring in discordant cases, reporting final ERO and RVol values, and ultimately reporting conclusions based on ERO and RVol interpretation in the larger context.
The 3 Rules of Comprehensive Echocardiography: Quantify, Quantify, Quantify
Although the PISA (flow convergence) method is most often used because of its direct nature and visual confirmation of the calculations,13 criticisms are not infrequent. Yet, MR quantitation is a major difference between screening echocardiography that only detects MR and comprehensive echocardiography, detailing MR etiologies and mechanisms, fully quantifying MR, and yielding therapeutic decisions in conjunction with quantification of MR physiologic responses (LV, LA, etc). Criticisms are often based on misconceptions. The regurgitant orifice may appear larger by color-vena-contracta than by PISA19 but this difference is mostly due to blooming color pitfall. Also, noncircular regurgitant orifices are cited as affecting flow convergence radius. Indeed, with high aliasing velocity, elliptic orifices would appear with smaller radii, possibly underestimating MR, but with lower aliasing velocity, flow convergence is larger and regurgitant orifice shape has little effect on measurements.20 Practically, what are the appropriate steps to effective measurements and interpretation of MR quantitation?
-
1.
Locate the flow convergence center by scanning thoroughly the mitral valve, which provides also mechanistic information (eg, P3 flail or anterior leaflet perforation).
-
2.
With flow convergence located, zoom tightly (no need to see the entire LA) for precise measurements then move the color baseline in the direction of the jet, down in MR imaged transthoracically.
-
3.
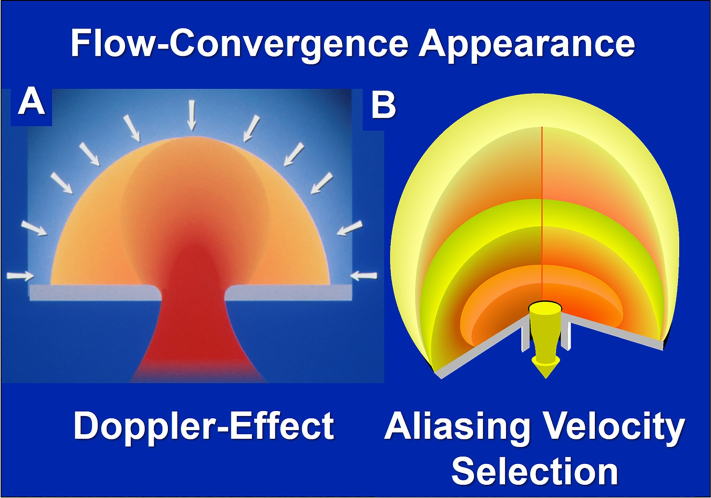
Adjust aliasing velocity to obtain the desired flow convergence shape. The desirable shape is a “ball” sitting on the jet, reflecting a true flow-hemisphere (Figure 1A), the sides of which are progressively lost to visualization toward the 90-degree angle, due to the Doppler effect. With too-high aliasing velocity, the flow convergence appears pancake-like, and with too-low aliasing velocity, it appears like a football or rugby ball (Figure 1B). If the flow convergence shape is irredeemably deformed, reject it (Figure 2). Record long segments of multiple beats to average and reduce measurement variability.
-
4.
Measure flow-convergence radius: The regurgitant orifice position is marked by the black line of horizontal flow (do not remove color-flow) and the junction blue-yellow marks the radius upper limit in the direction of the ultrasound beam. Time the measurement, not to largest flow convergence but to peak MR velocity, most often concomitant to electrocardiogram T-wave (Figure 2A). Integrate continuous Doppler MR velocity tracing aligned with regurgitant flow convergence (not jet).
-
5.
Measure 2 flow convergences with double jets. The common radius is calculated as: R = √(R12 + R22) (or sum Flows, ERO, and RFlow)
-
6.
Calculate ERO and RVol. Confront to flow-volume/hemodynamics/remodeling. If incoherent, then remeasure. If possible, consider Quantitative Doppler LV volume measurements (requires notable training), examine physiological coherence, and reconcile differences.
-
7.
Understand physiological discordances: For any given ERO, the RVol will be lower with a stiff LA and that translates into a lower regurgitant time-velocity integral due to equalization trend of LV and LA pressures. This explains usually stronger outcome prediction by ERO.
-
8.
Interpret ERO and RVol in context: Similar biological values yield different interpretations in different contexts (eg, creatinine values interpreted by sex/body-size):
-
•
Holosystolic organic MR: severe MR starts at ERO 0.40 cm2, and at 0.60 cm2 may be considered very severe. But also, ERO 0.30-0.40 cm2 is associated with notable excess mortality and may require treatment.15
-
•
Mid-late-systolic MR: disregard ERO (or adjust to % systole with MR) and consider MR severe for RVol ≥60 mL/beat.21
-
•
Functional MR: ERO and RVol are generally smaller but excess mortality starts at lower orifices and increases exponentially. Recent data suggest ERO ≥0.30 cm2 as an appropriate threshold for severe FMR and 0.20-0.29 cm2 as moderately severe FMR.16
-
•
Atrial FMR: A poorly know entity with lower ERO and RVol but generally normal LV function. Thresholds for severe MR are undefined.11
Figure 1.
Imaging Characteristics Affecting the Flow Convergence Assessment
(A) The appearance of the flow convergence in the case of a hemispheric flow convergence. Because color-flow imaging is based on Doppler effect, with an increasing angle vs the beam of ultrasound, the flow display decreases and disappears at 90 degrees. Thus, a hemispheric flow convergence will appear as a “ball sitting on the jet.” Furthermore, this Doppler effect underscores the importance of measuring the radius in the direction of the ultrasound beam. (B) Schematic representation of the effect of the aliasing velocity on the shape of the flow convergence. With too-low aliasing velocity, the flow convergence appears football-like (pale yellow shape) and with too-high aliasing velocity the flow convergence appears pancake-like. Both are inappropriate for measurement, whereas the intense-yellow medium shape is appropriate for measurement.
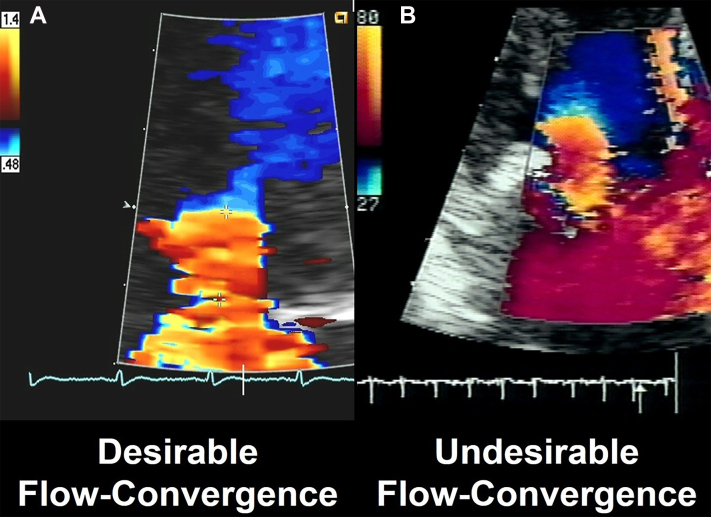
Figure 2.
Imaging of the Flow Convergence
(A) A desirable flow convergence appropriate for measurement in shape and timing. (B) An undesirable, deformed flow convergence inappropriate for measurement.
In summary, for MR, learn the lessons of the cases presented that underscore versatility, quantification, integration, and interpretation of ERO and RVol in the greater context. This approach is crucial for centers aiming to be Valvular Heart Disease Centers and is the path forward to address MR undertreatment.
Funding Support and Author Disclosures
Dr Enriquez-Sarano consults for Edwards Lifescience, ChemImage, HighLife, and Artivion (not related to this paper). Dr Messika-Zeitoun consults for Edwards Lifescience. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Nkomo V.T., Gardin J.M., Skelton T.N., Gottdiener J.S., Scott C.G., Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.d'Arcy J.L., Coffey S., Loudon M.A., et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515–3522. doi: 10.1093/eurheartj/ehw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuhlke L., Engel M.E., Karthikeyan G., et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study) Eur Heart J. 2015;36:1115–1122a. doi: 10.1093/eurheartj/ehu449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford M., Souchek J., Oprian C., et al. Determinants of survival and left ventricular performance after mitral valve replacement. Circulation. 1990;81:1173–1181. doi: 10.1161/01.cir.81.4.1173. [DOI] [PubMed] [Google Scholar]
- 5.Essayagh B., Antoine C., Benfari G., et al. Prognostic implications of left atrial enlargement in degenerative mitral regurgitation. J Am Coll Cardiol. 2019;74:858–870. doi: 10.1016/j.jacc.2019.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Essayagh B., Antoine C., Benfari G., et al. Functional tricuspid regurgitation of degenerative mitral valve disease: a crucial determinant of survival. Eur Heart J. 2020;41:1918–1929. doi: 10.1093/eurheartj/ehaa192. [DOI] [PubMed] [Google Scholar]
- 7.van Wijngaarden A.L., Mantegazza V., Hiemstra Y.L., et al. Prognostic impact of extra-mitral valve cardiac involvement in patients with primary mitral regurgitation. J Am Coll Cardiol Img. 2022;15:961–970. doi: 10.1016/j.jcmg.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Clavel M.A., Tribouilloy C., Vanoverschelde J.L., et al. Association of B-Type Natriuretic Peptide with Survival of Patients with Degenerative Mitral Regurgitation. J Am Coll Cardiol. 2016;68:1297–1307. doi: 10.1016/j.jacc.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 9.Dziadzko V., Clavel M.A., Dziadzko M., et al. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet. 2018;391:960–969. doi: 10.1016/S0140-6736(18)30473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messika-Zeitoun D., Candolfi P., Vahanian A., et al. Dismal outcomes and high societal burden of mitral valve regurgitation in France in the recent era: a nationwide perspective. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dziadzko V., Dziadzko M., Medina-Inojosa J.R., et al. Causes and mechanisms of isolated mitral regurgitation in the community: clinical context and outcome. Eur Heart J. 2019;40:2194–2202. doi: 10.1093/eurheartj/ehz314. [DOI] [PubMed] [Google Scholar]
- 12.Benfari G., Sorajja P., Pedrazzini G., et al. Association of transcatheter edge-to-edge repair with improved survival in older patients with severe, symptomatic degenerative mitral regurgitation. Eur Heart J. 2022;43:1626–1635. doi: 10.1093/eurheartj/ehab910. [DOI] [PubMed] [Google Scholar]
- 13.Enriquez-Sarano M., Miller F.A.J., Hayes S.N., Bailey K.R., Tajik A.J., Seward J.B. Effective mitral regurgitant orifice area: clinical use and pitfalls of the proximal isovelocity surface area method. J Am Coll Cardiol. 1995;25:703–709. doi: 10.1016/0735-1097(94)00434-R. [DOI] [PubMed] [Google Scholar]
- 14.Enriquez-Sarano M., Avierinos J.F., Messika-Zeitoun D., et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–883. doi: 10.1056/NEJMoa041451. [DOI] [PubMed] [Google Scholar]
- 15.Antoine C., Benfari G., Michelena H.I., et al. Clinical outcome of degenerative mitral regurgitation: Critical importance of echocardiographic quantitative assessment in routine practice. Circulation. 2018;138:1317–1326. doi: 10.1161/CIRCULATIONAHA.117.033173. [DOI] [PubMed] [Google Scholar]
- 16.Benfari G., Antoine C., Essayagh B., et al. Functional mitral regurgitation outcome and grading in heart failure with reduced ejection fraction. J Am Coll Cardiol Img. 2021;14:2303–2315. doi: 10.1016/j.jcmg.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Zoghbi W.A., Adams D., Bonow R.O., et al. Recommendations for noninvasive evaluation of Native valvular regurgitation: A report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Faza N.N., Chebrolu L.B., El-Tallawi K.C., Zoghbi W.A. An integrative, multiparametric approach to mitral regurgitation evaluation: a case-based illustration. J Am Coll Cardiol Case Rep. 2022;4:1231–1241. doi: 10.1016/j.jaccas.2022.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng X., Levine R.A., Hua L., et al. Diagnostic value of vena contracta area in the quantification of mitral regurgitation severity by color Doppler 3D echocardiography. Circ Cardiovasc Imaging. 2011;4:506–513. doi: 10.1161/CIRCIMAGING.110.961649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rifkin R.D., Sharma S. An alternative isovelocity surface model for quantitation of effective regurgitant orifice area in mitral regurgitation with an elongated orifice application to functional mitral regurgitation. J Am Coll Cardiol Img. 2010;3:1091–1103. doi: 10.1016/j.jcmg.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Topilsky Y., Michelena H., Bichara V., Maalouf J., Mahoney D.W., Enriquez-Sarano M. Mitral valve prolapse with mid-late systolic mitral regurgitation: pitfalls of evaluation and clinical outcome compared with holosystolic regurgitation. Circulation. 2012;125:1643–1651. doi: 10.1161/CIRCULATIONAHA.111.055111. [DOI] [PubMed] [Google Scholar]