Abstract
Background
Ventilator-associated pneumonia caused by Pseudomonas aeruginosa (PA) in hospitalised patients is associated with high mortality. The effectiveness of the bivalent, bispecific mAb MEDI3902 (gremubamab) in preventing PA nosocomial pneumonia was assessed in PA-colonised mechanically ventilated subjects.
Methods
EVADE (NCT02696902) was a phase 2, randomised, parallel-group, double-blind, placebo-controlled study in Europe, Turkey, Israel, and the USA. Subjects ≥ 18 years old, mechanically ventilated, tracheally colonised with PA, and without new-onset pneumonia, were randomised (1:1:1) to MEDI3902 500, 1500 mg (single intravenous dose), or placebo. The primary efficacy endpoint was the incidence of nosocomial PA pneumonia through 21 days post-dose in MEDI3902 1500 mg versus placebo, determined by an independent adjudication committee.
Results
Even if the initial sample size was not reached because of low recruitment, 188 subjects were randomised (MEDI3902 500/1500 mg: n = 16/87; placebo: n = 85) between 13 April 2016 and 17 October 2019. Out of these, 184 were dosed (MEDI3902 500/1500 mg: n = 16/85; placebo: n = 83), comprising the modified intent-to-treat set. Enrolment in the 500 mg arm was discontinued due to pharmacokinetic data demonstrating low MEDI3902 serum concentrations. Subsequently, enrolled subjects were randomised (1:1) to MEDI3902 1500 mg or placebo. PA pneumonia was confirmed in 22.4% (n = 19/85) of MEDI3902 1500 mg recipients and in 18.1% (n = 15/83) of placebo recipients (relative risk reduction [RRR]: − 23.7%; 80% confidence interval [CI] − 83.8%, 16.8%; p = 0.49). At 21 days post-1500 mg dose, the mean (standard deviation) serum MEDI3902 concentration was 9.46 (7.91) μg/mL, with 80.6% (n = 58/72) subjects achieving concentrations > 1.7 μg/mL, a level associated with improved outcome in animal models. Treatment-emergent adverse event incidence was similar between groups.
Conclusions
The bivalent, bispecific monoclonal antibody MEDI3902 (gremubamab) did not reduce PA nosocomial pneumonia incidence in PA-colonised mechanically ventilated subjects.
Trial registration Registered on Clinicaltrials.gov (NCT02696902) on 11th February 2016 and on EudraCT (2015-001706-34) on 7th March 2016.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04204-9.
Keywords: Monoclonal antibody, Prevention, Pharmacokinetics, Pseudomonas aeruginosa ventilator-associated pneumonia, Safety
Introduction
Pseudomonas aeruginosa (PA) is a common cause of ventilator-associated pneumonia (VAP) in hospitalised patients [1–3], associated with mortality rates > 30% in patients with antibiotic-susceptible and > 44% with multidrug-resistant (MDR) strains [4, 5]. Tracheobronchial colonisation with PA increases the odds of developing VAP by around eightfold [2, 6], but no specific guidelines exist for managing these patients before pneumonia is diagnosed. Current strategies involve antibiotic treatment post-diagnosis [1, 7, 8]. Although evidence suggests the earlier treatment may be beneficial [9], routine prophylactic antibiotics to prevent VAP can contribute to PA resistance [8, 10, 11]. Consequently, Infectious Diseases Society of America and the American Thoracic Society guidelines recommend withholding antibiotic treatment in patients with suspected VAP and endotracheal culture results below the diagnostic threshold for VAP (protected specimen brush [PSB] with < 103 colony-forming units [CFU]/mL, bronchoalveolar lavage [BAL] with < 104 CFU/mL) [12]. No systemic agents are currently approved for the pre-emptive treatment of ventilated patients with Pseudomonas airway colonisation to prevent PA pneumonia [7, 13], highlighting an unmet need for effective, targeted prevention.
Monoclonal antibodies are an attractive alternative to systemic antibiotics for the pre-emption of PA pneumonias. Their benefits include enhanced specificity, longer half-life, and complementary mechanism of action to antibiotics and do not induce antibiotic resistance [7, 14]. A placebo-controlled phase 2a study highlighted the potential for reducing PA pneumonia incidence in colonised, mechanically ventilated intensive care unit (ICU) patients dosed with a monovalent, monoclonal antibody against the PA PcrV protein [6].
MEDI3902 (gremubamab) is a first-in-class bivalent, bispecific human immunoglobulin G1 kappa monoclonal antibody that selectively binds to the PA PcrV protein and Psl exopolysaccharide involved in host cell cytotoxicity and PA colonisation and tissue adherence, respectively [15–17]. Prophylactic MEDI3902 administration protected against lethal PA in animal models [15, 18], with a significant reduction in the expression of genes encoding key inflammatory cytokines in animals who received MEDI3902 versus control immunoglobulin G [18].
The use of rapid diagnostic techniques, such as real-time PCR, in ICU settings can enable prompt identification of patients with bacterial colonisation of the lower respiratory tract before the onset of nosocomial pneumonia, bypassing the time required to obtain the results of conventional microbiological cultures which can take 48–72 h. Accordingly, rapid identification of patients with respiratory PA colonisation could assist in the timely initiation of pre-emptive or curative therapies. A phase 1, placebo-controlled, dose escalation study (NCT02255760) assessed an intravenous (IV) infusion of MEDI3902 in 56 healthy adults [16]. MEDI3902 serum concentrations through day 29 remained above the target therapeutic concentration of 5.3 μg/mL (based on a murine model of PA pneumonia where mice were inoculated with PA 5 × LD100, data on file) in subjects who received the highest doses (750, 1500, and 3000 mg), and dose-dependent increases in serum anticytotoxic and opsonophagocytic killing activities were observed [16]. MEDI3902 was well tolerated, supporting further assessment in PA-colonised subjects at risk for developing PA pneumonia. However, previous studies done in ICU patients for assessing the potential usefulness of monoclonal antibodies in preventing bacterial infections, while showing some non-statistically significant trends in favour of antibodies in post hoc analyses, were based on a limited number of patients and mostly negative, rendering difficult any conclusions [6, 14, 19]. Many factors that are not influenced by monoclonal antibodies can also contribute to the development of pneumonia, including disease severity, underlying immune function and concomitant therapies, warranting further studies before concluding that MEDI3902 represents a valuable complement to conventional measures for preventing lower respiratory tract infections caused by PA. Here, we present the results of a single-dose, proof-of-concept study of MEDI3902 for the pre-emptive treatment of PA nosocomial pneumonia in PA-colonised, mechanically ventilated subjects in the ICU.
Methods
Study design
EVADE (Clinicaltrials.gov NCT02696902; EudraCT 2015-001706-34) was a phase 2, randomised, parallel-group, double-blind, placebo-controlled study of MEDI3902 in mechanically ventilated patients with PA lower respiratory tract colonisation, confirmed by a polymerase chain reaction (PCR)-based test on tracheal aspirates collected no more than 36 h before randomisation. All randomised subjects were positive for PA by PCR, while 154/184 (83.7%) had positive cultures, highlighting the greater sensitivity of PCR for detecting PA airway colonisation in ventilated patients. EVADE was performed across 48 sites in 13 countries (Europe, Turkey, the USA, and Israel; Additional file 1: Tables S1 and S2).
The study was conducted within the European public–private partnership Combatting Bacterial Resistance In Europe—Molecules Against Gram-Negative Infections (COMBACTE-MAGNET) consortium [7] in accordance with the ethical principles of the Declaration of Helsinki and the International Council for Harmonization Guidance for Good Clinical Practice. The Antibiotic Resistance Leadership group also participated. Study-related documents were reviewed and approved by the local independent ethics committees or institutional review boards. All subjects/legally acceptable representatives provided written informed consent.
Subjects
Adults ≥ 18 years of age were eligible if they met the following key inclusion criteria: currently intubated and mechanically ventilated and expected to remain so for at least 72 h; tracheal PA colonisation as assessed by PCR (GeneXpert System with PA Xpert test cartridge [research use only], Cepheid, Sunnyvale, CA, USA) no more than 36 h and no diagnosis of new-onset pneumonia within 72 h before randomisation (patients with evidence of resolved pneumonia were eligible for inclusion); expected to survive for > 2 weeks and participate in the study through 49 days post-dose.
Key exclusion criteria were: acute confirmed or suspected pseudomonal disease or active pulmonary disease; a Clinical Pulmonary Infection Score (CPIS) of at least 6 measured no more than 24 h before treatment; an Acute Physiology and Chronic Health Evaluation-II (APACHE-II) score of at least 25 or a Sequential Organ Failure Assessment (SOFA) score of at least 12; and systemic or aerosolised colistin received for > 72 h within 96 h before randomisation. The Additional file 1: Methods detail full eligibility criteria.
The modalities of the screening process were left to the discretion of the investigators. In a minority of centres, mechanically ventilated patients were routinely screened for PA colonisation using conventional microbiological cultures of endotracheal aspirates (ETA) once or twice a week until death or weaning from mechanical ventilation, according to standard practice. When cultures grew positive for PA, informed consent was obtained and tracheal colonisation was then confirmed using PCR. The other centres did not use routine serial microbiological cultures for screening. Patients’ eligibility for study enrolment was regularly checked as long as they were expected to remain on mechanical ventilation for at least 72 h and did not meet any exclusion criteria (see above), and informed consent was obtained for using PCR on ETA.
Routine use of VAP prevention bundles was highly recommended. To check whether these prevention bundles had been implemented correctly, sites were asked to report the VAP bundle application in the electronic case report form for each enrolled patient (Table 1 and Additional file 1: Table S3).
Table 1.
Demographics and baseline characteristics (mITT)
| MEDI3902 500 mg (N = 16) | MEDI3902 1500 mg (N = 85) | Placebo (N = 83) | Total (N = 184) | |
|---|---|---|---|---|
| Age, years; mean (SD) | 62.7 (9.3) | 60.3 (15.2) | 64.1 (12.9) | 62.2 (13.8) |
| Age < 65 years; n (%) | 7 (43.8) | 42 (49.4) | 39 (47.0) | 88 (47.8) |
| Sex, male; n (%) | 10 (62.5) | 54 (63.5) | 62 (74.7) | 126 (68.5) |
| Race, n (%) | ||||
| Asian | 0 (0.0) | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Black or African American | 0 (0.0) | 2 (2.4) | 4 (4.8) | 6 (3.3) |
| Native Hawaiian or Other Pacific Islander | 0 (0.0) | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| White | 16 (100.0) | 81 (95.3) | 75 (90.4) | 172 (93.5) |
| Other | 0 (0.0) | 2 (2.4) | 2 (2.4) | 4 (2.2) |
| Weight, kg; mean (SD) | 82.5 (25.2) | 78.8 (19.5) | 84.4 (21.0) | 81.6 (20.7) |
| Height, cm; mean (SD) | 167.9 (10.0) | 169.1 (9.6) | 171.1 (10.0) | 169.9 (9.8) |
| BMI, kg/m2; mean (SD) | 29.5 (9.4) | 27.5 (6.4) | 29.0 (7.7) | 28.4 (7.3) |
| BMI ≤ 30 kg/m2; n (%) | 11 (68.8) | 60 (70.6) | 54 (65.1) | 125 (67.9) |
| Clinical severity scores at baseline; mean (SD) | ||||
| APACHE-II | 16.9 (2.9)* | 15.3 (5.4)† | 15.5 (5.2)‡ | 15.5 (5.1)§ |
| SOFA | 4.5 (2.4)* | 4.4 (2.7)† | 4.0 (2.1) | 4.2 (2.4)¶ |
| CPIS | 3.5 (1.5)* | 3.0 (1.5)# | 3.2 (1.5)# | 3.1 (1.5)** |
| Duration of mechanical ventilation; days, mean (SD) | 19.5 (15.5) | 25.2 (27.6) | 31.1 (28.4)†† | – |
| Previous PA infections ≤ 3 months before randomisation; n (%) | ||||
| Yes | 7 (43.8) | 25 (29.4) | 31 (37.8) | 63 (34.4) |
| No | 9 (56.3) | 60 (70.6) | 51 (62.2) | 120 (65.6) |
| Missing | 0 | 0 | 1 | 1 |
| Use of antibiotics in the 3 months before randomisation; n (%) | ||||
| Yes | 14 (93.3) | 59 (74.7) | 63 (81.8) | 136 (79.5) |
| No | 1 (6.7) | 20 (25.3) | 14 (18.2) | 35 (20.5) |
| Missing | 1 | 6 | 6 | 13 |
| CLSI susceptibility at baseline;‡‡ n (%) | ||||
| Any culture result, n | 16 | 83 | 82 | 181 |
| P. aeruginosa positive | 15 (93.8) | 72 (86.7) | 67 (81.7) | 154 (85.1) |
| Non-MDR | 6 (37.5) | 37 (44.6) | 25 (30.5) | 68 (37.6) |
| MDR§§ | 4 (25.0) | 12 (14.5) | 16 (19.5) | 32 (17.7) |
| XDR§§ | 4 (25.0) | 14 (16.9) | 21 (25.6) | 39 (21.5) |
| PDR§§ | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unknown¶¶ | 1 (6.3) | 9 (10.8) | 5 (6.1) | 15 (8.3) |
| P. aeruginosa negative## | 1 (6.3) | 11 (13.3) | 15 (18.3) | 27 (14.9) |
| MEDI3902 500 mg (N = 16) | MEDI3902 1500 mg (N = 85) | Placebo (N = 83) | MEDI3902 total (N = 101) | |
|---|---|---|---|---|
| P. aeruginosa PCR CT value; mean (SD) | 28.3 (3.7) | 28.5 (5.7) | 29.2 (6.2) | 28.5 (5.4) |
| White blood cell count, 103/μL; mean (SD) | 11.6 (4.4) | 13.2 (6.3) | 11.4 (6.1) | 12.9 (6.1) |
| Absolute Neutrophil count, 103/μL; mean (SD) | 9.1 (4.1) | 10.6 (6.0)*** | 8.4 (4.3)††† | 10.4 (5.7)‡‡‡ |
| Procalcitonin, μg/L; mean (SD) | 4.8 (15.5)§§§ | 1.0 (2.4)¶¶¶ | 0.61 (1.3)### | 1.5 (6.1)**** |
| CRP, mg/dL; mean (SD) | 7.9 (6.3) | 14.7 (37.9)†††† | 15.1 (34.3)¶¶¶ | 13.5 (34.6)‡‡‡‡ |
| Ventilator-associated pneumonia prevention† | ||||
| All 5 measures used, n (%) | 8 (50) | 36 (42.4) | 31 (37.3) | 44 (43.6) |
APACHE-II Acute Physiology and Chronic Health Evaluation-II, BMI body mass index, CLSI Clinical and Laboratory Standards Institute, CPIS Clinical Pulmonary Infection Score, CRP C-reactive protein, MDR multidrug-resistant, mITT modified intent-to-treat population, PCR CT polymerase chain reaction cycle threshold, PDR pan-drug-resistant, SD standard deviation, SOFA Sequential Organ Failure Assessment, XDR extensively drug resistant. †Preventive ventilator-associated pneumonia measures (bundles) were elevation of the head of the bed, daily sedation vacations and extubation readiness assessment, peptic ulcer disease prophylaxis, deep vein thrombosis prophylaxis, and daily oral care with chlorhexidine
*n = 15; †n = 82; ‡n = 82; §n = 179; ¶n = 180 #n = 81; **n = 177; ††n = 82; ‡‡minimum inhibitory concentrations were determined by CLSI broth microdilution at a centralised laboratory; §§MDR, PDR, and XDR as defined by;[29] ¶¶subjects with PA-positive culture results but missing minimum inhibitory concentration records; ##all randomised subjects were positive for PA by PCR within 36 h before randomisation, but not all had positive cultures; ***n = 83; †††n = 81; ‡‡‡n = 99; §§§n = 12; ¶¶¶n = 77; ###n = 74; ****n = 89; ††††n = 76; ‡‡‡‡n = 92
Randomisation and masking
Per protocol, subjects were randomised (1:1:1) to a single intravenous (IV) dose of MEDI3902 500 mg, 1500 mg, or placebo. Based on previous studies [19, 20] and pharmacokinetic (PK) data [21] received after the start of the study, a single MEDI3902 500 mg dose was not expected to maintain a target level of 1.7 μg/mL (derived from a murine model of PA pneumonia where mice were inoculated with PA 1 × LD100, data on file) for 21 days, and enrolment in this arm was discontinued after 16 subjects were dosed. Interim PK confirmed MEDI3902 1500 mg maintained the target level in 80% of patients through day 21 [21]. Subsequently, following protocol and statistical analysis plan (SAP) amendment, subjects were randomised (1:1) to MEDI3902 1500 mg or placebo (Additional file 1: Fig. S1), stratified by geographical region and duration of anti-PA antibiotic treatment within 96 h before randomisation (no antibiotic use, duration of no more than 72 h, duration > 72 h [except for systemic or aerosolised colistin; see exclusion criteria]). Subjects were followed until the end of the study period (day 50). An interactive web response system was used for randomisation to the treatment group and assignment of blinded investigational product kit numbers. To complete screening and ensure uniformity of inclusion criteria across sites, the eligibility of all potential patients was confirmed by the Clinical Coordinating Centre (Saint-Luc University Hospital, Brussels, Belgium). MEDI3902 and placebo were administered in a blinded fashion, and neither the subjects, their legal representatives, nor the investigators and sponsor staff involved in the treatment or clinical assessment of subjects were aware of the treatment received. The investigational products were handled by an unblinded investigational product manager at each site.
Endpoints and assessments
The primary efficacy endpoint was the incidence of nosocomial PA pneumonia through 21 days post-dose in MEDI3902 1500 mg recipients versus placebo as determined by a blinded independent Endpoint Adjudication Committee (EAC). The EAC included three experts in intensive care medicine and two radiologists that used prespecified, stringent and non-subjective criteria agreed upon by both the US Food and Drug Administration and the European Medicines Agency. Subjects must have met radiological (new or worsening infiltrate consistent with pneumonia on chest X-rays), clinical, and microbiological criteria concurrently to be diagnosed with PA pneumonia (Additional file 1: Methods). In subjects with suspected or confirmed pneumonia, tracheobronchitis, or bacteraemia, blood and respiratory specimens were collected, and chest X-rays were performed as clinically indicated, until clinical resolution. Primary safety endpoints included the incidence of treatment-emergent adverse events (TEAEs), serious AEs (SAEs), and AEs of special interest assessed through 49 days post-dose.
Secondary endpoints were MEDI3902 serum/ETA PK parameters and serum anti-drug antibody responses through 49 days post-dose. Blood samples were collected immediately before MEDI3902 dosing on day 1, at the end of infusion, and 8- and 24-h later and on days 4, 8, 15, 22, 29, and 50 of follow-up.
The Additional file 1: Methods include exploratory endpoints and sample collection details.
Statistical analysis
Following protocol/SAP amendment, planned enrolment was approximately 286 subjects randomised (1:1) to MEDI3902 1500 mg or placebo. Given the exploratory nature of the study, power was calculated based on Poisson regression with robust variance comparing MEDI3902 versus placebo groups (two-sided, α = 0.2), assuming a placebo group PA pneumonia incidence of 20%, a relative reduction of 50%, at least 80% power, and 20% adjustment for attrition. A relative reduction of 50% was considered clinically meaningful based on expert advice and published data [6].
Recruitment was stopped early after 168 patients were included (MEDI3902 1500 mg: n = 85; placebo: n = 83) due to slow enrolment.
Efficacy and PK were assessed in the modified intent-to-treat (mITT) population (all subjects randomised and dosed, analysed by randomised treatment). Following discontinued enrolment in the MEDI3902 500 mg arm and protocol/SAP amendment, efficacy was assessed in MEDI3902 1500 mg versus placebo recipients. The primary endpoint of nosocomial PA pneumonia was assessed by relative risk reduction (RRR), defined as 1—relative risk, and its corresponding two-sided 80% confidence interval (CI), as estimated from a Poisson regression model with robust variance and treatment group as a covariate (two-sided, α = 0.2). A positive RRR denoted less PA pneumonia in the MEDI3902 1500 mg group compared to placebo, and a negative RRR had more PA pneumonia in the MEDI3902 group. Patients with mixed culture results that included PA were counted towards the primary endpoint. Early discontinuation due to death from underlying disease was expected as the main cause of missing data. If no PA pneumonia occurred before discontinuation, the subject was considered as having no PA pneumonia infection in the primary efficacy analysis. No other imputation was applied to this analysis. Time to diagnosis of PA pneumonia, as judged by the EAC, was estimated by use of the Kaplan–Meier method. Safety was assessed in the as-treated population (all subjects randomised and dosed, analysed by treatment received). Safety and PK analyses included the 16 subjects who received MEDI3902 500 mg. Data were summarised descriptively, with no multiplicity adjustments.
Post hoc analyses
An adjusted post hoc analysis of the primary efficacy endpoint was done to address possible selection bias due to an imbalance in baseline covariates including ECMO, PA-positive cultures, absolute neutrophil counts (ANC) and procalcitonin (PCT) levels. The impact of key baseline covariates on the RRR of PA pneumonia and all-cause mortality in MEDI3902 1500 mg recipients versus placebo, and on MEDI3902 serum PK, was also assessed (Additional file 1: Methods). Efficacy data were calculated using PCT and ANC quartiles. The groups with high and statistically significant RRR with baseline levels of ≤ 0.55 μg/L for procalcitonin and ≤ 8.17 × 103 cells/μL for ANC correspond to combined quartiles 1–3 for procalcitonin and combined quartiles 1–2 for ANC.
Role of the funding source
The study sponsor was involved in study design, data collection, data analysis, and data interpretation, with input from the authors, and in the writing of the report.
Results
Subjects
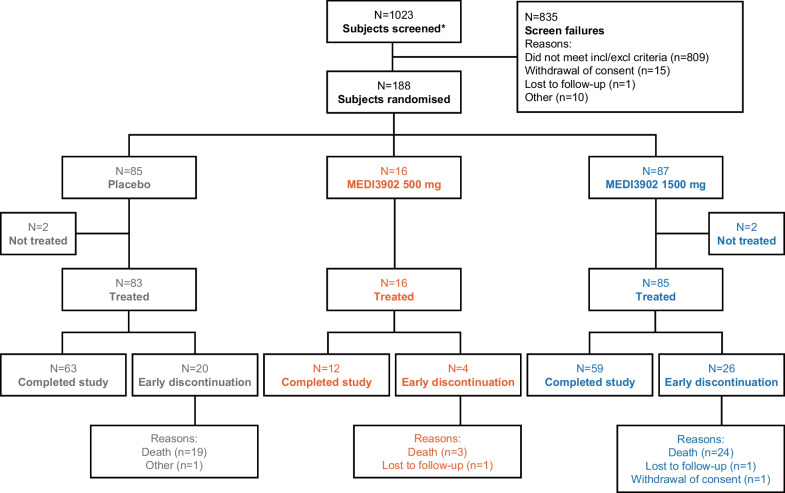
Subjects were randomised between 13 April 2016 and 17 October 2019; the study was completed on 4 December 2019. Of the 1023 subjects screened, 835 (81.6%) could not be included in the study (Fig. 1). Overall, 184 randomised subjects received MEDI3902 1500 mg (n = 85), 500 mg (n = 16), or placebo (n = 83) (Fig. 1); 134/184 (72.8%) subjects completed the study, including 59 (69.4%) MEDI3902 1500 mg, 12 (75.0%) 500 mg, and 63 (75.9%) placebo recipients. The most frequent reason for discontinuation was death in three (18.8%), 24 (28.2%), and 19 (22.9%) subjects in the 500 mg, 1500 mg and placebo groups, respectively.
Fig. 1.
Subject disposition. *Subjects signed informed consent
Baseline demographics, clinical severity scores, and Clinical and Laboratory Standards Institute (CLSI) PA antibiotic susceptibility results were generally similar between groups (Table 1). However, there was a numerically higher percentage of non-MDR PA-positive cultures in the MEDI3902 1500 (44.6%) mg group versus placebo (30.5%). Subjects in the MEDI3902 arms also had higher ANC (13.2 vs. 11.4 × 103 cells per microliter and PCT levels (1.0 vs. 0.61 μg/L) at baseline versus placebo (Table 1). All five components of the VAP prevention bundles were implemented in 42.4% and 37.3% of MEDI3902 and placebo recipients, respectively (Table 1 and Additional file 1: Table S3).
Efficacy
The PA pneumonia incidence was 19/85 (22.4%) in MEDI3902 1500 mg recipients and 15/83 (18.1%) in placebo recipients; RRR − 23.7% (80% CI − 83.8%, 16.8%; p = 0.49) (Table 2). The primary endpoint was not met. Similar results were observed regarding exploratory efficacy endpoints, including all-cause pneumonia, all-cause pneumonia or death, antibiotic usage and duration of mechanical ventilation (Table 2 and Additional file 1: Results and Table S4). Specifically, when patients who died before study completion without PA pneumonia were classified as failure (and not as success as done in the primary analysis), the incidence of all-cause pneumonia or death was 30/85 (35.3%) in MEDI3902 1500 mg recipients and 22/83 (26.5%) in placebo recipients; RRR − 33.3% (80% CI − 79.8%, 1.4%). The results of the time-to-event analysis are shown in the Additional file (Additional file 1: Fig. S2), with most PA pneumonia occurring 15 days after randomisation in both groups. No significant difference in the incidence of other serious PA infections (tracheobronchitis, bacteraemia, intraabdominal or deep skin and soft tissue infections) was observed between the placebo group and the MEDI3902 1500 mg group (Additional file 1: Table S5). To make sure that decisions made by the EAC were in agreement with those made by the physicians at the bedside, the number of subjects with an adverse event of PA pneumonia, not confirmed as PA pneumonia by the EAC, but who received anti-PA antibiotics, was tabulated by the treatment group (Additional file 1: Table S6). Clinicians detected and treated with antibiotics only three and 0 additional events of potential PA pneumonia in placebo recipients and MEDI3902 1500 mg recipients, respectively.
Table 2.
MEDI3902 phase II EVADE: efficacy through study day 22 (mITT)
| MEDI3902 500 mg (N = 16) | MEDI3902 1500 mg (N = 85) | Placebo (N = 83) | RRRa | 80% CIa | P valuea | |
|---|---|---|---|---|---|---|
| Primary endpoint | ||||||
| P. aeruginosa Pneumonia | 2 (12.5%) | 19 (22.4%) | 15 (18.1%) | − 23.7% | − 83.8 to 16.8% | 0.491 |
| Exploratory endpoints of interest (FDA) | ||||||
| All-cause pneumoniab | 3 (18.8%) | 25 (29.4%) | 17 (20.5%) | − 43.6% | − 104 to -1.1% | 0.186 |
| All-cause pneumonia or deathc | 4 (25.0%) | 30 (35.3%) | 22 (26.5%) | − 33.2% | − 79.8 to 1.4% | 0.222 |
RRR: relative risk reduction (a negative RRR denotes more PA pneumonia in the MEDI3902 1500 mg group compared to placebo); mITT = modified intent-to-treat population
All pneumonias were determined by an adjudication committee
aRelative risk reduction (MEDI3902 1500 mg vs. placebo), 80% confidence interval (CI), and p value based on Poisson regression with robust variance
bAll-cause pneumonia: P. aeruginosa pneumonia or non-P. aeruginosa pneumonia occurring through study day 22
cAll-cause pneumonia or death: P. aeruginosa pneumonia, non-P. aeruginosa pneumonia, or death occurring through study day 22
Additional post hoc analyses were done on baseline covariates (Additional file 1: Fig. S3). PCT and ANC had the greatest effect on RRR in PA pneumonia. At baseline, 115 patients (68% of the study population) had PCT levels below 0.55 μg/L. In that group of patients with a low PCT level, the incidence of PA pneumonia was 23.7% in the placebo and 12.5% in MEDI3902 1500 mg groups, corresponding to an 47.3% RRR (80% CI: 6.1% to 69.9%; p = 0.135). Similar results were observed in the subgroup of 83 patients (49% of the study population) with an ANC less than 8.17 × 103 cells/µL: PA pneumonia incidence was 17% and 2.8% in the placebo and MEDI3902 1500 mg group, respectively, corresponding to a 83.6% RRR (80% CI 39.5%, 95.5%; P = 0.038) (Additional file 1: Tables S7A and S7B). In subjects with ANC ≤ 8.17 × 103/µL, MEDI3902 treatment was associated with 56.5% RRR in all-cause mortality (80% CI 11.9%, 79.4%; P = 0.110). Conversely, RRRs were negative in patients with PCT levels above 0.55 μg/L and those with ANC above 8.17 × 103 cells/µL, denoting more PA pneumonia in the MEDI3902 group than in the placebo group (Additional file 1: Tables S7A and S7B).
Healthcare resource utilisation through 21 days post-dose for MEDI3902 1500 mg and placebo subjects with PA pneumonia were similar, including duration of mechanical ventilation (17.5 vs. 19.7 days, respectively), systemic antibiotic use (12.3 vs. 14.4 days) and duration of ICU stay (20.6 vs. 22.0 days) (Additional file 1: Table S4). Severity scores on day 1 of pneumonia onset and day of resolution, as well as changes in cellular and protein markers of inflammation, also did not demonstrate any significant differences between MEDI3902 patients and placebo patients with PA pneumonia (Additional file 1: Tables S8 and S10). The results of other exploratory analyses are described in the Additional file 1: Results, Tables S4 and S8-11 and Fig. S4.
Pharmacokinetics
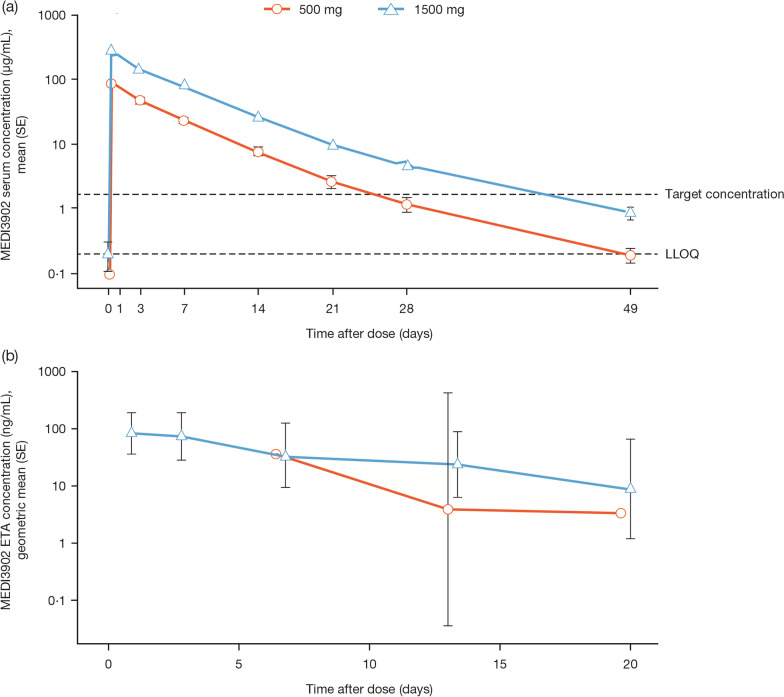
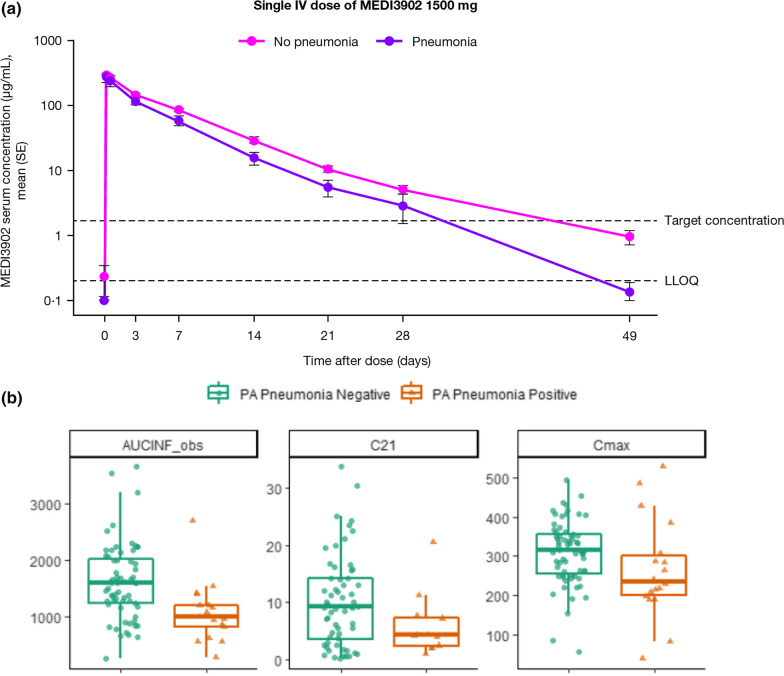
Mean serum concentration–time profiles of MEDI3902 are shown in Figs. 2a and 3a, b. At 21 days following a 1500 mg infusion, the mean (standard deviation [SD]) serum MEDI3902 concentration was 9.46 (7.91) μg/mL, with 80.6% (n = 58/72) subjects achieving concentrations > 1.7 μg/mL, the target level (Table 3). In ETA, the geometric mean (SD) MEDI3902 concentration was 8.73 (± 11) ng/mL at day 21 (Fig. 2b and Additional file 1: Fig. S5); however, significant correlations were not observed between MEDI3902 concentrations in serum and ETA (R2 = 0.000081; data not shown). MEDI3902 PK parameters in serum and ETA are summarised in Table 3. Mean clearance from serum was 1.27 L/day, with a mean half-life of 5.65 days following a 1500 mg infusion. On day 2, the median MEDI3902 ETA/serum PK ratio was 0.06%, increasing to 0.1% on day 4. A positive exposure–response relationship was observed after a 1500 mg IV infusion, with numerically higher serum MEDI3902 concentrations observed in subjects who did not develop PA pneumonia versus those who did (Fig. 3a, b and Additional file 1: Fig. S6).
Fig. 2.
Concentration–time profile following a single IV dose of MEDI3902 500 mg and 1500 mg in serum (a) and endotracheal aspirate (b) PK geometric mean profile. ETA = endotracheal aspirate. IV = intravenous. LLOQ = lower limit of quantification. PK = pharmacokinetic. SE = standard error
Fig. 3.
a Mean (SE) serum MEDI3902 concentrations in subjects with or without PA pneumonia. LLOQ = lower limit of quantification. SE = standard error. b MEDI3902 area under the curve from 0 to 21 days and concentrations obtained after a single IV dose of 1500 mg in patients with and without PA pneumonia. Cmax = maximal observed concentration; C21 = concentration 21 days post-dosing; AUC0to21 = Area under the curve from 0 to 21 days post-dose
Table 3.
MEDI3902 PK parameters
| PK parameter | MEDI3902 500 mg (N = 16) | MEDI3902 1500 mg (N = 85) | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| Serum | ||||
| Cmax, µg/mL | 16 | 87.6 (23.9) | 84 | 299 (94.1) |
| C21, µg/mL | 14 | 2.56 (2.23) | 72 | 9.46 (7.91) |
| AUC0–21, day*µg/mL | 16 | 418 (126) | 81 | 1410 (599) |
| AUC∞, day*µg/mL | 16 | 440 (135) | 81 | 1510 (675) |
| CL, L/day | 16 | 1.31 (0.64) | 81 | 1.27 (0.86) |
| t½, day | 16 | 6.56 (4.03) | 81 | 5.65 (2.69) |
| n/N | % | n/N | % | |
|---|---|---|---|---|
| Subjects with serum MEDI3902 levels > target level of 1.7 µg/mL on day 21 | 7/14 | 50.0% | 58/72 | 80.6% |
| Endotracheal aspirate† | ||||
| Cmax, µg/mL | – | – | n/a | 0.083 |
| AUC0–21, day*µg/mL | – | – | n/a | 0.704 |
| AUC∞, day*µg/mL | – | – | n/a | 0.784 |
| t½, day | – | – | n/a | 6.3 |
AUC0–21 = the area under the concentration–time curve from time zero to 21 days post-dose. AUC∞ = the area under the concentration–time curve from zero to infinity. CL = clearance. Cmax = maximum observed concentration. C21 = concentration 21 days post-dose. IV = intravenous. n/a = not applicable. SD = standard deviation. t½ = half-life
†Due to the large variability in measured endotracheal aspirate concentrations and limited samples per subject, it was not plausible to calculate endotracheal aspirate PK parameter at an individual level. Therefore, endotracheal aspirate PK parameters were calculated based on geometric mean concentration–time profile
Serum anti-drug antibodies
Serum anti-drug antibodies were detected at baseline in 2/85 (2.4%) subjects in the MEDI3902 1500 mg group, 2/16 (12.5%) in the 500 mg group, and 3/83 (3.6%) in the placebo group. The number of subjects with persistent serum anti-drug antibodies (defined as a positive test of at least 2 or the last post-baseline assessment) was similar between the MEDI3902 1500 mg (n = 4/85; 4.9%) and placebo (n = 4/83; 5.1%) groups. No differences were observed in MEDI3902 PK or safety profiles in subjects with and without post-baseline serum anti-drug antibodies responses.
Safety
The incidence of TEAEs, SAEs, and TEAEs of > grade 3 severity reported through 49 days post-dose was similar between MEDI3902 1500 mg and placebo groups (Table 4). TEAEs related to treatment were reported in three subjects receiving MEDI3902 1500 mg (sinus tachycardia [n = 1, grade 1, resolved the same day] and infusion-related reactions [n = 2, grade 1 and 3, resolved during the study]) and in one placebo recipient (acute kidney injury, grade 4). No treatment-related SAEs were reported.
Table 4.
Overall summary of TEAEs through 49 days post-dose
| Subjects,* n (%) with ≥ 1 event | MEDI3902 500 mg (N = 16) | MEDI3902 1500 mg (N = 85) | Placebo (N = 83) |
|---|---|---|---|
| TEAE | 15 (93.8) | 84 (98.8) | 81 (97.6) |
| Treatment-related TEAE | 0 (0.0) | 3 (3.5) | 1 (1.2) |
| TEAE of ≥ grade 3 severity† | 12 (75.0) | 60 (70.6) | 54 (65.1) |
| Death (grade 5 severity†) | 3 (18.8) | 24 (28.2) | 19 (22.9) |
| Serious‡ TEAE | 4 (25.0) | 38 (44.7) | 35 (42.2) |
| Serious‡ and/or ≥ grade 3 severity† TEAE | 12 (75.0) | 60 (70.6) | 55 (66.3) |
| Treatment-related serious‡ TEAE | 0 (0.0) | 0 (0.0) | 1 (1.2) |
| TEAE leading to discontinuation of treatment | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| AESI§ | 0 (0.0) | 2 (2.4) | 1 (1.2) |
| Treatment-related AESI§ | 0 (0.0) | 2 (2.4) | 0 (0.0) |
| AESI§ of ≥ grade 3 severity† | 0 (0.0) | 1 (1.2) | 1 (1.2) |
AE adverse event, AESI adverse event of special interest, TEAE treatment-emergent adverse event
*Subjects are counted once for each category regardless of the number of events; †grade 3, severe; grade 4, life-threatening; grade 5, fatal; ‡serious adverse event criteria: death, life-threatening, required inpatient hospitalisation, prolongation of existing hospitalisation, persistent or significant disability/incapacity, important medical event, congenital anomaly/disorder (in the offspring of the subject); §AESI defined as targeted AEs of hepatic function abnormalities, hypersensitivity reactions (including anaphylaxis), infusion-related reactions, and immune complex disease (including vasculitis, endocarditis, neuritis, and glomerulonephritis)
Overall, 46 deaths were reported until 49 days post-dose. A slightly higher number of deaths in the MEDI3902 1500 mg group (n = 24/85; 28.2%) versus placebo (n = 19/83; 22.9%; Table 4) was not statistically significant (RRR [80% CI] − 23.3% [− 73.3, 12.2%]; p = 0.43). No deaths were considered related to MEDI3902, as adjudicated by the blinded investigators and independent unblinded DSMB.
Discussion
The primary efficacy endpoint of reduction in PA pneumonia incidence, with a relative reduction of 50% considered clinically meaningful based on expert advice and published data [6], was not achieved. However, the certainty of our overall study findings may be limited given that the study was interrupted prematurely before the target number of patients was reached due to slow enrolment, and thus, the analyses may be underpowered. A single IV dose of MEDI3902 1500 mg provided PK serum exposure above the target level of 1.7 µg/mL for at least 21 days in most subjects, with MEDI3902 concentrations detectable in the ETA. The incidence of persistent serum anti-drug antibodies was low, occurring in < 5% of MEDI3902 1500 mg recipients. A positive exposure–response relationship was observed for the MEDI3902 1500 mg group, with a greater MEDI3902 area under the concentration–time curve from time zero to 21 days post-dose associated with a lower probability of PA pneumonia.
For mechanically ventilated critically ill subjects, high baseline inflammatory status or cachexia increases protein catabolism and volume distribution of many drugs [22], potentially lowering MEDI3902 exposure and increasing PA pneumonia susceptibility simultaneously. A higher MEDI3902 dose and/or direct administration into the tracheobronchial tree by aerosolisation may be considered for the most seriously ill subjects to achieve protection, especially since MEDI3902 ETA/serum PK ratio was low (0.1% on day 4).
An imbalance in baseline inflammation was reflected in PCT and ANC levels. Subjects with lower baseline PCT or ANC had slightly higher mean PK levels and may exhibit a greater MEDI3902 treatment response versus placebo against PA pneumonia. These results provide important lessons on pathogen-specific PA pneumonia complexity in ICU trials with potential implications for future study design. Whereas APACHE-II scores have been used as eligibility criteria to avoid enrolling the sickest patients [23], clinicians might also consider baseline levels of certain biomarkers as exclusion criteria and/or for stratifying patients by severity at randomisation, particularly in pre-emptive treatment studies of colonised patients. While PCT and ANC levels may not correlate directly with PA pneumonia, these markers may identify patients with higher bacterial load and higher inflammatory status, and therefore a higher risk of pneumonia. [24–26]. Conversely, patients with higher levels of these biomarkers (regardless of apparent pathogen levels) may be too sick to benefit from treatment, or may have progressed too far in the development of symptomatic pneumonia [27]. Whether MEDI3902 could have increased the rate of PA pneumonia in patients with high inflammatory status in the present study remains highly speculative. However, some potential deleterious effects of monoclonal antibodies have already been reported in other studies, including a large randomised trial having assessed the benefit of a combination of casirivimab and imdevimab given together in patients admitted to the hospital with COVID-19. Although the monoclonal combination improved survival and other clinical outcomes in patients who did not have detectable anti-SARS-CoV-2 antibodies (i.e. had not yet mounted their own humoral immune response), no clinical benefit was observed in patients who were seropositive at baseline, suggesting the possibility of a conflict between the subjects' own immune defences and the monoclonal antibodies [28]. Further research is required to understand the role of these surrogate biomarkers for the inflammatory response in risk stratification and treatment decisions.
The safety profile of MEDI3902 was acceptable, with a similar incidence of TEAEs and SAEs in the MEDI3902 1500 mg and placebo groups. TEAEs were generally reflective of the critically ill patient population and the numerical imbalance in deaths across treatment groups was expected due to the observed imbalance in baseline disease characteristics.
Limitations
This study has several limitations. First, the trial did not achieve its planned sample size, with the recruitment being stopped early due to low enrolment and thus was underpowered to detect small but clinically important treatment effects in the entire study population, as well as in specific subgroups of patients. The main contributing factor of low recruitment was that the proportion of patients meeting all the eligibility criteria was lower than expected, which resulted in an average of 4 patients randomised per site. Other major contributing factors included the unusually complex screening process and the difficulty for the attending clinicians to distinguish patients who were actually infected with PA from those only colonised, often resulting in the immediate administration of new antibiotics and making patients ineligible for randomisation. Second, the investigators may not have screened some eligible patients either because of the absence of consent or difficulties in identifying patients potentially colonised by PA. Since this number was not recorded, the extent of this bias cannot be ascertained. Third, the patient population was primarily Western European, thus reflecting the MDR profile from one region and the absence of strict guidelines for antibiotic treatment resulted in heterogeneity in antibiotic use. Fourth, colonisation, as determined by PCR, may not coincide with a positive PA culture and, since colonisation assays were not quantitative, active infection (rather than just colonisation) could not be ruled out at the time of treatment. Fifth, misclassification of pneumonia is also possible due to difficulty in diagnosis, particularly once new antibiotics were prescribed. However, the trial was double-blinded and a strict protocol was applied to diagnose PA pneumonia. Sixth, MEDI3902 endotracheal aspirate concentrations were rather low questioning whether adequate epithelial lining fluid levels of the monoclonal antibody were present in these patients to prevent infection. Efficacy may have increased with higher antibody doses, which were not assessed in this study. Furthermore, results derived from post hoc analyses should be interpreted with caution.
Conclusions
Among ICU patients requiring prolonged mechanical ventilation and colonised by PA as detected by PCR, a single intravenous dose of 1500 mg of the bivalent, bispecific monoclonal antibody MEDI3902 (gremubamab) was safe, with a number of AEs similar to placebo, but it did not reduce PA nosocomial pneumonia incidence. Whether MEDI3902 may help prevent PA pneumonia, with higher doses or in more specific patient populations, would require additional studies.
Supplementary Information
Additional file 1: Supplementary Methods, Results, Tables and Figures.
Acknowledgements
This research project receives support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no 115737, with resources comprising financial contributions from the European Union Seventh Framework Programme (FP7/2007–2013) and EFPIA companies in-kind contribution. Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under award number UM1AI104681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Medical writing support, under the direction of the authors, was provided by Claire Cairney, Ph.D. and Jennifer Stewart, PhD, MBA, CMC Connect, McCann Health Medical Communications, and was funded by AstraZeneca, Gaithersburg, USA. The additional writing support was provided by Sarah Legrand Demai, MA, Inserm CIC 1435, CHU Limoges, France.
The COMBACTE-MAGNET EVADE Study Group: Michael Joannidis: Medical University of Innsbruck, Austria; Walter Klimscha: SMZOST Donauspital Wien, Austria; Elisabeth De Waele: UZ Brussel, Belgium; Nicolas De Schryver: Clinique Saint-Pierre, Belgium; Jacques Devriendt: CHU Brugmann, Belgium; Vincent Huberlant: Centre Hospitalier Jolimont-Lobbes, Belgium; Pieter Depuydt: University Hospital Gent, Belgium; Marc Bourgeois: AZ Sint-Jan AV, Belgium; Sam Van Boxstael: Ziekenhuis Oost-Limburg, Belgium; Mladen Peric: Klinichki Bolnicki Centar Zagreb, Croatia; Jasminka Kopic: General Hospital Dr Josip Bencevic, Croatia; Michal Hanauer: Krajska zdravotni, a.s. – Nemocnice Decin, o.z., Czech Republic; Tomas Hruby: Krajska zdravotni, a.s. – Nemocnice Teplice, o.z., Czech Republic; Vladimir Sramek: Fakultni nemocnice u sv. Anny v Brne, Czech Republic; Petr Svoboda: Nemocnice Kyjov, prispevkova organizace, Czech Republic; Tomas Vymazal: Fakultni nemocnice v Motole, Czech Republic; Martin Novacek: Oblastni nemocnice Kolin, a.s., Czech Republic; Bruno François: Centre Hospitalier et Universitaire de Limoges, France; Djillali Annane: APHP Raymond-Poincaré de Garches, France; Jean Chastre: Groupe Hospitalier Pitié Salpétrière, France; Jean-Paul Mira: APHP Cochin, France; Bertrand Souweine: Centre Hospitalier Universitaire de Clermont Ferrand, France; Pierre-François Dequin: CHRU de Tours, France; Ferhat Meziani: Nouvel Hôpital Civil Strasbourg, France; François Stephan: Centre Chirurgical Marie Lannelongue, France; Saadalla Nseir: CHRU Lille, France; Sebastien Gibot: CHRU Nancy, France; Carole Schwebel: Hôpital Albert Michallon La Tronche, France; Alain Lepape: Centre Hospitalier Lyon Sud, France; Gaetan Plantefeve: Centre Hospitalier Victor Dupouy, France; Jean-Luc Diehl: APHP Hôpital Européen Georges-Pompidou, France; Christian Richard: APHP Hôpital de Bicêtre, France; Christian Lamer: Institut Mutualiste Montsouris, France; Kada Klouche: Centre Hospitalier Universitaire de Montpellier/Lapeyronie hospital, France; Samir Jaber: Centre Hospitalier Universitaire de Montpellier/Hôpital St Eloi, France; Epaminondas Zakynthinos: University Hospital of Larissa, Greece; Georgios Filntisis: Agioi Anargyroi Cancer Hospital, Greece; Apostolos Komnos: General Hospital of Larissa, Greece; Spyros Zakynthinos: Evangelismos General Hospital of Athens, Greece; Antonia Koutsoukou: Sotiria Chest Hospital of Athens, Greece; Georgios Saroglou: Metropolitan Hospital, Greece; Charikleia Nikolaou: Konstantopouleion General Hospital of Athens, Greece; Glykeria Vlachogianni: Agios Dimitrios General Hospital of Thessaloniki, Greece; Ioannis Pnevmatikos: University Hospital of Alexandroupolis, Greece; Konstantinos Mandragos: General Hospital of Athens Korgialenio Benakio Greek Red Cross, Greece; Ildiko Kremer: Pest Megyei Flór Ferenc Kórház, Hungary; Zsolt Dezso Rozgonyi: Orszagos Koranyi TBC es Pulmonologiai Intezet, Hungary; Zsuzsa Marjanek: Jávorszky Ödön Kórház, Hungary; Ignacio Martin-Loeches: St James University Hospital, Ireland; Pierre Singer: Rabin Medical Center, Israel; Vernon Van Heerden: Hadassah Medical Center, Israel; Yehuda Carmeli: Tel Aviv Sourasky Medical Center, Israel; Galia Rahav: Chaim Sheba Medical Center, Israel; Pedro Povoa: Centro Hospital de Lisboa Ocidental – Hospital São Francisco Xavier, Portugal; Antonio Alvarez Seoane: Centro Hospitalar Lisboa Norte, E.P.E. – Hospital de Santa Maria, Portugal; Pedro Moura: Unidade Local de Saúde do Alto Minho, EPE, Portugal; Filipe Gonzalez: Hospital Garcia de Orta, Portugal; Paula Ramirez: Hospital Universitari i Politecnic La Fe de Valencia, Spain; Antonio Torres Marti: Hospital Clinic de Barcelona, Spain; Miguel Sánchez-García: Hospital Clínico San Carlos Madrid, Spain; Ricard Ferrer Roca: Hospital Universitario Vall d'Hebron Barcelona, Spain; Lorena Oteiza: Hospital Universitario de Getafe Madrid, Spain; Dolores Escudero: Hospital Universitario Central de Asturias Oviedo, Spain; Enrique Piacentini: Hospital Mutua de Terrassa Barcelona, Spain; Paula Vera: Hospital de La Santa Creu i Sant Pau, Spain; Luis Tamayo: Hospital Universitario del Rio Hortega, Spain; Miguel Angel Gonzalez Gallego: Hospital Universitario Infanta Sofia, Spain; Borja Suberviola Canas: Hospital Universitario Marqués de Valdecilla, Spain; Iglesias Figueira: Hospital Universitario La Paz – PPDS, Spain; Rafael Leon: Hospital General Universitario Reina Sofia, Spain; Volkan Korten: Marmara University Research and Training Hospital, Turkey; Iftihar Koksal: Karadeniz Technical University Faculty of Medicine, Turkey; Murat Akova: Hacettepe Universitesi Tip Fakultesi Hastanesi, Turkey; Duncan Wyncoll: St Thomas’ Hospital, UK; Tony Whitehouse: Queen Elizabeth Hospital, UK; Phil Hopkins: King’s College Hospital, UK; Malcolm Sim: Southern General Hospital, UK; Yoav Golan: Tufts University Medical Center, UK; Marcus Zervos: Henry Ford Health Sys. Detroit, USA; Jose Vazquez: Georgia Regents Medical Center-Augusta, USA; Kartikeya Cherabuddi: University of Florida, USA; George Smulian: Univ. of Cincinnati, USA; Nadine Rouphael: Emory University Atlanta, USA; James Welker: Anne Arundel Health, USA; Mathew Sims: Beaumont Hospital Royal Oaks, USA; David Van Duin: UNC Chapel Hill, USA; Todd McCarthy: Univ. of Alabama, Birmingham, USA; Christopher Polk: Carolina Medical Center/Atrium Health, USA.
Abbreviations
- AE
Adverse event
- ANC
Absolute neutrophil count
- APACHE-II
Acute Physiology and Chronic Health Evaluation-II
- BAL
Bronchoalveolar lavage
- CI
Confidence interval
- CPIS
Clinical pulmonary infection score
- EAC
Endpoint Adjudication Committee
- ETA
Endotracheal aspirate
- ICU
Intensive care unit
- IV
Intravenous
- MDR
Multidrug-resistant
- mITT
Modified intent-to-treat
- PA
Pseudomonas aeruginosa
- PCR
Polymerase chain reaction
- PCT
Procalcitonin
- PK
Pharmacokinetic
- RRR
Relative risk reduction
- SAE
Serious adverse event
- SAP
Statistical analysis plan
- SOFA
Sequential Organ Failure Assessment
- TEAE
Treatment-emergent adverse event
- VAP
Ventilator-associated pneumonia
Author contributions
JC, BF, MS-G, AT, PE, DK, TLH, OA, KS, PR, AR, YW, AD, SC, DV, TB, MTE, and HSJ, conceptualised and designed the study. JC, BF, MBou, AK, RF, GR, NDS, AL, IK, C-EL, MS-G, AT, PE, OA, DET, AA, YW, AD, DV, FC, SM-K, LT, AO, and OB, collected the data. JC, BF, MS-G, AT, PE, DK, TLH, OA, KS, PR, JS, AR, DET, YJ, AD, MBon, HG, CR, MTE, and HSJ, analysed and interpreted the data. JC, BF, KS, PR, AR, and HSJ, verified the data. JC, BF, AR, MTE, and HSJ, drafted the manuscript. All authors had full access to study data, contributed to the development of the manuscript with medical writing support funded by the sponsor, critically reviewed and revised the manuscript. AR and MTE accessed and verified the data. JC and HSJ had final responsibility for the decision to submit for publication. All authors read and approved the final manuscript.
Funding
This research project receives support from the Innovative Medicines Initiative Joint Undertaking under grant agreement n° 115737 resources of which are composed of financial contribution from the European Union Seventh Framework Programme (FP7/2007‐2013) and EFPIA companies in-kind contribution. Other support from the National Institute of Allergy And Infectious Diseases of the National Institutes of Health, AstraZeneca.
Availability of data and materials
Data underlying the findings described in this manuscript can be obtained in accordance with AstraZeneca’s data sharing policy.
Declarations
Ethics approval and consent to participate
The study was conducted within the European public–private partnership Combatting Bacterial Resistance In Europe—Molecules Against Gram-Negative Infections (COMBACTE-MAGNET) consortium in accordance with the ethical principles of the Declaration of Helsinki and the International Council for Harmonization Guidance for Good Clinical Practice. Study-related documents were reviewed and approved by the local independent ethics committees or institutional review boards. All subjects/legally acceptable representatives provided written informed consent.
Consent for publication
Not applicable.
Competing interests
Jean Chastre received personal fees during the conduct of the study from COMBACTE-MAGNET, personal fees from outside of the submitted work from Aridis, Bayer, Inotrem, Shionogi, and Tigenix/Takeda, and grants from AstraZeneca/Medimmune. Bruno François consulted for AM-Pharma, Aridis, Enlivex, GSK, and Inotrem and was part of an adjudication committee for Takeda. Ricard Ferrer received fees for conferences from BD, Grifols, MSD, and Pfizer. Alain Lepape received personal fees outside of the study from Fresenius and Tigenix/Takeda (DSMD committee). Iftihar Koksal has served on advisory boards for Abbvie, MSD, Pfizer, Gilead, GSK, and Roche; lectures: Abbvie, Gilead, Pfizer, and MSD. Charles-Edouard Luyt received fees from Bayer Healthcare, ThermoFisher Brahms, Biomérieux, Faron, Carmat, Aerogen, and Merck Sharp & Dohme outside of the submitted work. Miguel Sánchez-García received funding for speaker fees from Biotest AG, Pfizer, Merck, Sharp & Dohme, AstraZeneca, Orion, and Cepheid; for consulting fees from Bayer, GlaxoSmithKline, Pfizer, and Masimo; for research grants from the European Union, 7th Framework Programme IMI, H2020; was part of an adjudication committee for Takeda. Antoni Torres has served on advisory boards for Pfizer, MSD, Biomérieux, Menarini, Chiesi, and Jansen; lectures: Pfizer and MSD; grants: Bayer, AstraZeneca, and Cardeas. Despoina Koulenti was part of the adjudication committee of EVADE. Thomas L. Holland consulted for Basilea Pharmaceutica, Genentech, and Motif Bio and took part in a Scientific Advisory Board for Motif Bio. Antonio Oliver received grants from and participated as a speaker and in advisory boards for Pfizer, MSD, and Shionogi. Olivier Barraud received speaker fees and/or travel grants from MSD, Pfizer, Roche, and Sanofi and has been a consultant to bioMérieux and Mylan. Herman Goossens received grants from European Union IMI Grant (in collaboration with Novartis). Omar Ali, Ahmad Akhgar, Pin Ren, Terramika Bellamy, Colin Reisner, Alexey Ruzin and Hasan S. Jafri were employees of AstraZeneca during the conduct of the study and hold shares in the company. Kathryn Shoemaker, David E. Tabor, Yuling Wu, Yu Jiang, Antonio DiGiandomenico, Susan Colbert and Mark Esser are employees of AstraZeneca and hold shares in the company. Marc Bourgeois, Apostolos Komnos, Galia Rahav, Nicolas De Schryver, Drieke Vandamme, Surbhi Malhotra-Kumar, Philippe Eggimann, Julien Sauser Frank Coenjaerts, Leen Timbermont, and Marc Bonten have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jean Chastre and Hasan S. Jafri contributed equally
Contributor Information
Jean Chastre, Email: jean.chastre@gmail.com.
Hasan S. Jafri, Email: hasan.jafri.md@gmail.com
The COMBACTE-MAGNET EVADE Study Group:
Michael Joannidis, Walter Klimscha, Elisabeth De Waele, Jacques Devriendt, Vincent Huberlant, Pieter Depuydt, Sam Van Boxstael, Mladen Peric, Jasminka Kopic, Michal Hanauer, Tomas Hruby, Vladimir Sramek, Petr Svoboda, Tomas Vymazal, Martin Novacek, Djillali Annane, Jean-Paul Mira, Bertrand Souweine, Pierre-François Dequin, Ferhat Meziani, François Stephan, Saadalla Nseir, Sebastien Gibot, Carole Schwebel, Gaetan Plantefeve, Jean-Luc Diehl, Christian Richard, Christian Lamer, Kada Klouche, Samir Jaber, Epaminondas Zakynthinos, Georgios Filntisis, Spyros Zakynthinos, Antonia Koutsoukou, Georgios Saroglou, Charikleia Nikolaou, Glykeria Vlachogianni, Ioannis Pnevmatikos, Konstantinos Mandragos, Ildiko Kremer, Zsolt Dezso Rozgonyi, Zsuzsa Marjanek, Ignacio Martin-Loeches, Pierre Singer, Vernon Van Heerden, Yehuda Carmeli, Pedro Povoa, Antonio Alvarez Seoane, Pedro Moura, Filipe Gonzalez, Paula Ramirez, Antonio Torres Marti, Ricard Ferrer Roca, Lorena Oteiza, Dolores Escudero, Enrique Piacentini, Paula Vera, Luis Tamayo, Miguel Angel Gonzalez Gallego, Borja Suberviola Canas, Iglesias Figueira, Rafael Leon, Volkan Korten, Murat Akova, Duncan Wyncoll, Tony Whitehouse, Phil Hopkins, Malcolm Sim, Yoav Golan, Marcus Zervos, Jose Vazquez, Kartikeya Cherabuddi, George Smulian, Nadine Rouphael, James Welker, Mathew Sims, David Van Duin, Todd McCarthy, and Christopher Polk
References
- 1.Ramírez-Estrada S, Borgatta B, Rello J. Pseudomonas aeruginosa ventilator-associated pneumonia management. Infect Drug Resist. 2016;9:7–18. doi: 10.2147/IDR.S50669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kollef MH, Chastre J, Fagon JY, Francois B, Niederman MS, Rello J, et al. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit Care Med. 2014;42(10):2178–2187. doi: 10.1097/CCM.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 3.Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51(Suppl. 1):S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 4.Micek ST, Wunderink RG, Kollef MH, Chen C, Rello J, Chastre J, et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Crit Care. 2015;19(1):219. doi: 10.1186/s13054-015-0926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tumbarello M, De Pascale G, Trecarichi EM, Spanu T, Antonicelli F, Maviglia R, et al. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med. 2013;39(4):682–692. doi: 10.1007/s00134-013-2828-9. [DOI] [PubMed] [Google Scholar]
- 6.François B, Luyt CE, Dugard A, Wolff M, Diehl JL, Jaber S, et al. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized, double-blind, placebo-controlled trial. Crit Care Med. 2012;40(8):2320–2326. doi: 10.1097/CCM.0b013e31825334f6. [DOI] [PubMed] [Google Scholar]
- 7.François B, Chastre J, Eggiman P, Laterre PF, Torres A, Sanchez M, et al. The SAATELLITE and EVADE clinical studies within the COMBACTE consortium: a public-private collaborative effort in designing and performing clinical trials for novel antibacterial drugs to prevent nosocomial pneumonia. Clin Infect Dis. 2016;63(Suppl. 2):S46–51. doi: 10.1093/cid/ciw245. [DOI] [PubMed] [Google Scholar]
- 8.Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT) Eur Respir J. 2017;50(3):1700582. doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 9.Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 10.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. [DOI] [PubMed]
- 11.Pang Z, Raudonis R, Glick BR, Lin TJ, Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37(1):177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tümmler B. Emerging therapies against infections with Pseudomonas aeruginosa. F1000Res. 2019;8(F1000 Faculty Rev-1371):1371.
- 14.Que YA, Lazar H, Wolff M, François B, Laterre PF, Mercier E, et al. Assessment of panobacumab as adjunctive immunotherapy for the treatment of nosocomial Pseudomonas aeruginosa pneumonia. Eur J Clin Microbiol Infect Dis. 2014;33(10):1861–1867. doi: 10.1007/s10096-014-2156-1. [DOI] [PubMed] [Google Scholar]
- 15.DiGiandomenico A, Keller AE, Gao C, Rainey GJ, Warrener P, Camara MM, et al. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci Transl Med. 2014;6(262):262ra155. doi: 10.1126/scitranslmed.3009655. [DOI] [PubMed] [Google Scholar]
- 16.Ali SO, Yu XQ, Robbie GJ, Wu Y, Shoemaker K, Yu L, et al. Phase 1 study of MEDI3902, an investigational anti-Pseudomonas aeruginosa PcrV and Psl bispecific human monoclonal antibody, in healthy adults. Clin Microbiol Infect. 2019;25(5):629.e1–.e6. [DOI] [PubMed]
- 17.Le HN, Tran VG, Vu TTT, Gras E, Le VTM, Pinheiro MG, et al. Treatment efficacy of MEDI3902 in Pseudomonas aeruginosa bloodstream infection and acute pneumonia rabbit models. Antimicrob Agents Chemother. 2019;63(8):e00710–e719. doi: 10.1128/AAC.00710-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le HN, Quetz JS, Tran VG, Le VTM, Aguiar-Alves F, Pinheiro MG, et al. MEDI3902 correlates of protection against severe Pseudomonas aeruginosa pneumonia in a rabbit acute pneumonia model. Antimicrob Agents Chemother. 2018;62(5):e02565–e2617. doi: 10.1128/AAC.02565-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francois B, Jafri HS, Chastre J, Sanchez-Garcia M, Eggimann P, Dequin PF, et al. Efficacy and safety of suvratoxumab for prevention of Staphylococcus aureus ventilator-associated pneumonia (SAATELLITE): a multicentre, randomised, double-blind, placebo-controlled, parallel-group, phase 2 pilot trial. Lancet Infect Dis. 2021;21(9):1313–1323. doi: 10.1016/S1473-3099(20)30995-6. [DOI] [PubMed] [Google Scholar]
- 20.Yu XQ, Robbie GJ, Wu Y, Esser MT, Jensen K, Schwartz HI, et al. Safety, tolerability, and pharmacokinetics of MEDI4893, an investigational, extended-half-life, anti-staphylococcus aureus alpha-toxin human monoclonal antibody. Healthy Adults Antimicrob Agents Chemother. 2017;61(1):e01020–e1116. doi: 10.1128/AAC.01020-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo X, François B, Bourgeois M, Komnos A, Rozgonyi ZD, Ali SA, et al. Interim pharmacokinetic analysis from the EVADE phase-2 clinical trial of MEDI3902, a bispecific monoclonal antibody against PcrV and Psl of Pseudomonas aeruginosa. In: 2018 E, editor. 28th European congress of clinical microbiology and infectious disease; 21–24 April 2018; Madrid, Spain: ECCMID; 2018. p. P2213.
- 22.O'Leary-Kelley C, Bawel-Brinkley K. Nutrition support protocols: enhancing delivery of enteral nutrition. Crit Care Nurse. 2017;37(2):e15–e23. doi: 10.4037/ccn2017650. [DOI] [PubMed] [Google Scholar]
- 23.Zeng J, Wang CT, Zhang FS, Qi F, Wang SF, Ma S, et al. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Int Care Med. 2016;42(6):1018–1028. doi: 10.1007/s00134-016-4303-x. [DOI] [PubMed] [Google Scholar]
- 24.Marik PE, Stephenson E. The ability of procalcitonin, lactate, white blood cell count and neutrophil-lymphocyte count ratio to predict blood stream infection. Analysis of a large database. J Crit Care. 2020;60:135–139. doi: 10.1016/j.jcrc.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Gregoriano C, Heilmann E, Molitor A, Schuetz P. Role of procalcitonin use in the management of sepsis. J Thorac Dis. 2020;12(Suppl 1):S5–S15. doi: 10.21037/jtd.2019.11.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med. 2020;38(3):641–647. doi: 10.1016/j.ajem.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Melsen WG, Rovers MM, Groenwold RHH, Bergmans DC, Camus C, Bauer TT, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13(8):665–671. doi: 10.1016/S1473-3099(13)70081-1. [DOI] [PubMed] [Google Scholar]
- 28.Group RC Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet (Lond Engl) 2022;399(10325):665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Methods, Results, Tables and Figures.
Data Availability Statement
Data underlying the findings described in this manuscript can be obtained in accordance with AstraZeneca’s data sharing policy.