Abstract
School closures were enforced as measures to restrain the COVID-19 pandemic, based on the assumption that young children may play a key role in SARS-CoV-2 spread. This study aims to determine the prevalence of SARS-CoV-2 IgG antibodies in children and corresponding parents, in order to improve surveillance and estimate the prevalence of asymptomatic or subclinical COVID-19 cases. A prospective multicenter study was conducted between March and June 2021 in Greece. Children admitted to the hospital or examined in outpatient clinics for reasons other than COVID-19 and their parents were tested for anti-Spike SARS-CoV-2 IgG in serum. A questionnaire about clinical and demographic data was completed. The study included 823 participants: 427 children and 396 corresponding parents. The overall seroprevalence was 16.4% in parents and 13.8% in children. Among families with ≥ 1 seropositive child or parent, the combination of a seropositive parent and a corresponding seronegative child was 29.6%, a seronegative parent and a corresponding seropositive child was 24.7%, and a seropositive child with a corresponding seropositive parent was 45.7%. Age, level of education, and school or work attendance were not significantly associated with increased seropositivity. On the contrary, ethnic minority of Roma, close contact with known COVID-19 case, previous symptoms consistent with COVID-19, and mass gatherings were risk factors for seropositivity.
Conclusion: The spread of SARS-CoV-2 during a period of lockdown in Greece was low in children and comparable to adults most likely due to intrafamilial transmission. Accordingly, it is unlikely that children have boosted virus transmission.
|
What is Known: • In the earliest months of the pandemic, it was demonstrated that children had significantly lower seroprevalence rates than the older age groups, due to the fact that children had decreased exposure to the virus, because of early public health interventions, such as school and day care closure. • Later, further studies reported that children have similar incidence rate of SARS-CoV-2 infection compared to adults in households and community settings. | |
|
What is New: • In this seroprevalence study, the spread of SARS-CoV-2 infection during a period of lockdown in Greece with the predominance of the Alpha-variant was particularly low in children and comparable to adults, most likely due to intrafamilial transmission. • These study findings will be useful for decisions regarding non-pharmaceutical interventions during the pandemic, and especially, to guide in designing and implementing appropriate containment measures for schools and social gatherings. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-022-04681-8.
Keywords: Seroprevalence, COVID-19, Children, Parents, Seroepidemiology, Household transmission
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of the novel disease 2019 (COVID-19), has spread rapidly around the world since first identified in Wuhan, China, in December 2019 [1]. COVID-19 pandemic is characterized by sustained human-to-human transmission, threatening the global public health and resulting in worldwide severe morbidity and mortality [2]. The global range and high fatality rate of this pandemic are causing economic damage, related to public panic and aggressive policy measures to contain the disease worldwide, and it has made SARS-CoV-2 the focus of the scientific world. The first COVID-19 case was reported in Greece on 26 February 2020 and on 10 March 2020, with a total of 89 active COVID-19 cases and 0 death; the Greek government decided to suspend the operation of all educational institutions throughout the country [3]. Gradually, restrictive measures were extended and resulted in a general lockdown on the 23rd of March, when 649 active cases and two deaths had been recorded [4]. In November 2020, the Greek government decided a new school closure due to the second pandemic wave with gradual opening of the educational institutions in April 2021.
Seroprevalence studies are important tools for determining the true extent of an outbreak, mapping its distribution, identifying hotspots and at-risk groups needing special attention, and designing public health strategies to reduce community transmission [5, 6]. Also, serosurveys can reveal the proportion of asymptomatic or subclinical infections in the general population, resulting in proper public health measures and control strategies [5, 6]. For this purpose, the immunoassays must have high sensitivity and specificity, in order to avoid the misleading public health interventions by overestimating the level of immunity and prematurely easing restrictions [7]. It is worth noting that seroprevalence of antibodies to SARS-CoV-2 varies with country, study population, time of sampling, and laboratory testing method [7].
The risk of transmission from symptomatic and pre-symptomatic patients is considered to be higher. However, asymptomatic infection is common, especially in the pediatric population, and the potential of viral transmission from asymptomatic patients, who may have similar viral loads with symptomatic cases, has been reported [8–10]. Furthermore, studies have shown that as much as 44% of SARS-CoV-2 transmission can take place when individuals are pre-symptomatic or asymptomatic [11]. Generally, mildly affected or asymptomatic individuals are not screened. As a result, the number of confirmed SARS-CoV-2 infections is largely underestimated [12]. In this context, seroprevalence surveys are of utmost importance to assess the proportion of the population that has already developed antibodies against the virus and might potentially be protected against subsequent infection [13]. As recommended by the WHO, monitoring changes of seroprevalence over time is also crucial at the beginning of an epidemic to anticipate its dynamics and plan an adequate public health response [14]. Also, studying the seroprevalence in both children and parents may be a useful tool to evaluate intrafamilial transmission and the possibility of children to be the drivers of the COVID-19 transmission. In the earliest months of the pandemic, it was demonstrated that children (5–9 years) had significantly lower seroprevalence rates than the older age groups, suggesting that infection was less prevalent in children than in adolescents and adults [15]. These results may have been due to the fact that children had decreased exposure to the virus, because of early public health interventions, such as school and day care closure. Later, further studies reported that children have similar incidence rate of SARS-CoV-2 infection compared to adults in households and community settings [16–18].
The purpose of this study was to estimate the prevalence of SARS-CoV-2 IgG antibodies in children and their parents in order to examine the extent of potential underdiagnosis of asymptomatic or subclinical infections. This information may also help elucidate the role of children in the transmission of infection within the family.
Material and methods
Study design and participants
A period prevalence, multicenter, prospective study was conducted from March 1, 2021 to June 30, 2021 with the participation of seven university departments of pediatrics that were distributed throughout the country, representing all 7 Regional Health Directorates of Greece. Children and adolescents 0–16 years of age and their parents presenting in the emergency department or in the outpatient clinics or admitted to the hospital for any reason were candidates for enrollment in the study.
It is worth noting that on 10 March 2020, the operation of all educational institutions throughout the country was suspended. In May 2020, the schools were open for 1 month, and in September 2020, the schools re-opened, but in November 2020, a new school closure was performed due to the second pandemic wave with gradual opening of the educational in April 2021. Therefore, the study period included a period after the school opening and the general lockdown implemented in Greece. The Alpha-variant was the predominant variant during the study period, while in July 2021, the Delta variant arose representing the most prevalent variant.
The study participation was random and voluntary. Informed consent was obtained from all the participants at the time of enrollment. Children or parents, who had symptoms consistent with acute COVID-19, according to the Centers for Disease Control and Prevention (CDC) definition [19] and/or a positive SARS-CoV-2 PCR test at the time of enrollment were excluded, in order to avoid the overestimation of the SARS-CoV-2 infection prevalence. In addition, parents who had received COVID-19 vaccination before enrollment and participants with congenital or acquired immunodeficiency were also excluded from the study. On the other hand, we included the participants with previous symptoms consistent with COVID-19 or previous SARS-CoV-2 infection, in order to estimate the true prevalence of SARS-CoV-2 IgG antibodies, representing the prevalence in the community and to examine the extent of potential underdiagnosis of asymptomatic or subclinical infections. Siblings or more than one parent per household consisting of more than one pair of children-parents residing in the same household were eligible. Parents were asked to complete a questionnaire on age, sex, ethnic origin, medical history, level of education, close contact with a confirmed COVID-19 case, occupation, school, previous travel, or symptoms compatible with COVID-19. Clinical, demographic, and epidemiological data of the parents and their children were recorded.
Close contact with a confirmed COVID-19 case is defined as the person who is within 6 feet of a case infected with SARS-CoV-2 for a cumulative total of 15 min or more over a 24-h period, starting from 2 days before illness onset (or, for asymptomatic patients, 2 days prior to test specimen collection) until the time the patient is isolated [20]. Previous travel is defined as any travel history outside the person’s community by different means of public transportation, such as airplane, ship, train, or bus during the period from the pandemic’s start to the time of enrollment. School attendance is defined as the duration that children attended school, during the interval from the beginning of the outbreak until the study recruitment. In addition, the implementation of preventive measures in schools included mask wearing, while regular mass testing was implemented in April 2021, after the re-opening of schools. Data on mask wearing at school was reported by the participants, in order to evaluate if this is a risk factor for the increased seroprevalence. Finally, the participants were questioned if they attend frequently social gatherings and about the number of persons in these gatherings. If there were different group sizes in the gatherings, the highest number was recorded.
Serological testing
Venous blood samples were collected for the purpose of the study, and immunoglobulin G (IgG) against SARS-CoV-2 was detected in serum samples by using emergency use authorization (EUA)-approved and CE-marked SARS-CoV-2 IgG II Quant assay kit (Abbott Laboratories, Chicago, USA), according to the manufacturer’s instructions. The SARS-CoV-2 IgG II Quant assay is an automated, two-step chemiluminescent microparticle immunoassay (CMIA) used for the qualitative and quantitative determination of IgG antibodies to the receptor-binding domain (RBD) of the S1 subunit of the spike protein of SARS-CoV-2 in human serum on the ARCHITECT i Systems. The analytical measurement interval is stated as 21 to 40,000 AU/ml, and positivity cutoff is ≥ 50 AU/ml.
Statistical analysis
Seroprevalence with 95% confidence interval (CI) was estimated for each prefecture [21]. Continuous variables are expressed as medians and interquartile range (IQR) and categorical variables as frequencies and relative frequencies.
The relationship between the main outcome measure (SARS-VoV-2 infection) and participants’ characteristics was assessed using either chi-square analysis or Fisher’s exact test for categorical variables and Mann–Whitney U test for continuous variables. Mann–Whitney U test was performed for continuous data since there was deviation from normal distribution (Shapiro–Wilk normality test). In univariate analysis, the percentage of infected and the proportional ratio (PR) with 95% CIs are presented. Multivariate analysis was performed using a logistic regression for binary outcomes (positive vs negative), and odds ratios (OR) with 95% CIs were calculated. Variables with P < 0.05 on univariate analyses were included in the final model. All tests were 2-sided, and a p value < 0.05 was considered to indicate statistical significance. Statistical analysis was performed using Excel and IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 823 participants were enrolled, 427 children (males: 224 (52.5%) and females: 203 (47.5%)) and 396 corresponding parents (males: 97 (24.5%) and females: 299 (75.5%)). A total of 363 pairs of one parent and one child, 21 families with 3 members (either 1 child and 2 parents or 2 children and 1 parent) and only 1 family with 2 parents and 2 children, as well as 30 children without parents were included in the study. The children without parents who participated in the study were immigrants or parents who had refused to give blood. The median age of children was 8.1 years (IQR 5.4), and the median age of the parents was 39.4 years (IQR 9.7). A rate of 5.9% of the children and 6.3% of the parents belonged to the Roma minority, and 14.1% of the children and 15.4% of the parents were immigrants. Previous COVID-19 was reported in 24 (5.6%) children and 29 parents (7.3%), previous symptoms consistent with COVID-19 in 107 (25.1%) children and 79 (19.9%) parents, while close contact with confirmed COVID-19 case in 62 (14.5%) children and 62 (15.7%) parents. Tables 1 and 2 summarize the demographic and clinical characteristics of all the individuals included in the study.
Table 1.
Demographics and clinical characteristics of the children and parents participating in the study
| Characteristics | Children (n = 427) | Parents (n = 396) | |
|---|---|---|---|
| Sex | Male | 224 (52.5%) | 97 (24.5%) |
| Female | 203 (47.5%) | 299 (75.5%) | |
| Age (years) (median (IQR)) | 8.1 (5.4) | (9.7) | |
| Age groups | |||
| 0–1 years | 58 (13.5%) | ||
| 2–5 years | 91 (21.3%) | ||
| 6–12 years | 166 (38.9%) | ||
| 13–16 years | 108 (25.3%) | ||
| Ethnic minority | 99 (23.2%) | 88 (22.2%) | |
| Roma | 25 (5.9%) | 25 (6.3%) | |
| Immigrants | 60 (14.1%) | 61 (15.4%) | |
| No | 342 (80.1%) | 310 (78.3%) | |
| Residency in a camp | 13 (3.1%) | 9 (2.3%) | |
| Level of education | No education | 26 (7.9%) | 0 (0.0%) |
| Kindergarten | 37 (11.2%) | 0 (0.0%) | |
| Pre-school | 38 (11.5%) | 0 (0.0%) | |
| Elementary school | 121 (36.7%) | 37 (10.7%) | |
| High school | 106 (25.8%) | 166 (8.1%) | |
| University | 2 (0.6%) | 127 (36.6%) | |
| Master degree | - | 15 (4.3%) | |
| PhD | - | 2 (0.6%) | |
| Underlying disease* | 103 (24.1%) | 84 (21.2%) | |
| Previous known SARS-CoV-2 infection | 24 (5.6%) | 29 (7.3%) | |
*Underlying disease: any medical condition or disease that interferes with daily life or activities and requires continuous medical care or treatment, except for the congenital and acquired immunodeficiency
Table 2.
Demographics and clinical characteristics of the children and parents participating in the study
| Characteristics | Children (n = 427) | Parents (n = 396) | |
|---|---|---|---|
| History of symptoms consistent with COVID-19 | 107 (25.1%) | 79 (19.9%) | |
| Close contact with COVID-19 cases | 62 (14.5%) | 62 (15.7%) | |
| Travel | 52 (12.2%) | 59 (14.9%) | |
| School attendance | 304 (71.2%) | 2 (0.5%) | |
| Number of children in the class (median (IQR)) | 19.4 (4.6) | - | |
| Duration of school attendance (months) (median (IQR)) | 4.6 (1.6) | ||
| Wearing mask at school | Always | 137 (52.3%) | |
| Occasionally | 87 (33.2%) | ||
| Rarely | 38 (14.5%) | ||
| COVID-19 case in the class | 36 (8.4%) | ||
| Number of cases at school (median (IQR)) | 1.4 (2.5) | ||
| Closure of the class due to COVID-19 case | 32 (7.5%) | ||
| Work attendance | 213 (53.8%) | ||
| Teleworking | 49 (22.4%) | ||
| Duration of teleworking (months) (median (IQR)) | 10 (26.1) | ||
| Wearing mask at work | Always | 162 (77.9%) | |
| Occasionally | 31 (14.9%) | ||
| Rarely | 15 (7.3%) | ||
| Use of public transport | 40 (9.4%) | 83 (21.0%) | |
| Participation in social gatherings | Yes | 110 (29.8%) | 92 (27.1%) |
| Rarely | 115 (31.2%) | 122 (35.9%) | |
| Never | 144 (39.0%) | 126 (37.1%) | |
| Number of persons at gatherings (median (IQR)) | 4.3 (3.2) | 4.5 (3.4) | |
| Number of families at gatherings (median (IQR)) | 2.2 (1.5) | 2.0 (1.4) | |
| Wearing mask at gatherings | 11 (5.7%) | 14 (7.4%) | |
| Frequent visits by relatives | 274 (64.1%) | 254 (64.1%) | |
| Number of family members (median (IQR)) | 4.4 (1.4) | 4.3 (1.4) | |
A total of 59 children and 65 parents were seropositive with a seroprevalence of 13.8% and 16.4%, respectively. Moreover, 58 children and 62 parents were seropositive from the 363 pairs of parent/child, and 1 child and 3 parents were seropositive from the group that included 21 families with 3 members. In total, there were 81 families with at least one seropositive individual, either child or parent. In detail, there were only seropositive children in 20 families, and only seropositive parents in 24 families, while there was seropositivity in both children and parents in 37 families. So, the combination of a seropositive parent and a corresponding seronegative child was 29.6%, the combination of a seronegative parent and a corresponding seropositive child was 24.7%, and the combination of a seropositive child and a corresponding seropositive parent was 45.7%. In order to clarify whether seroprevalence increased more in children than in adults over the course of the study, we tried to compare the seroprevalence in parents and children between the 2 time periods, March/April and May/June. During the period May/June, the seroprevalence in both children and parents increased. In parents, there was a statistically significant increase in seroprevalence from March/April (10.3%) to May/June (22.8%) (PR (95% CI): 2.58 (1.47–4.53), p = 0.001) (Suppl. Table 1). On the other hand, in children seroprevalence increased from March/April (11.1%) to May/June (16.4%) (PR (95% CI): 1.59 (0.91–2.79), p = 0.103) (Suppl. Table 1), but the difference was not statistically significant.
Table 3 describes the seroprevalence differences in children related to their demographic and clinical characteristics. Previous COVID-19 symptoms were present in 21/59 (35.6%) seropositive children, while previous known SARS-CoV-2 infection was reported in 18/59 (30.5%) of them. There was no correlation between age and seroprevalence (p = 0.841), as well as between the level of education and seropositivity (p > 0.05). School attendance was not a risk factor for seropositivity (p = 0.22), but known close contact with a confirmed COVID-19 case in the class, resulting in closure of the class, was associated with seropositivity (PR (95% CI): 2.35 (1.25–4.42), p = 0.01). Frequent gatherings with other people, close contact with a confirmed COVID-19 case, and previous symptoms consistent with COVID-19 were related to seropositivity (PR (95% CI): 2.16 (1.18–3.95), p = 0.008; PR (95% CI): 4.03 (2.59–6.27), p < 0.001; and PR (95% CI): 1.66 (1.03–2.70), p = 0.041, respectively] (p = 0.008, p < 0.001, and p = 0.041, respectively). Children belonging to the ethnic minority of Roma were statistically associated with increased seroprevalence (PR (95% CI): 2.50 (1.33–4.69), p = 0.008).
Table 3.
Seroprevalence differences in children related to their demographic and clinical characteristics
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Children (Ν = 414) |
Seropositive N (%) or median (IQR) |
PR (95% CI) | P-value | OR (95% CI) | P-value | |
| Sex | Male | 219 | 29 (13.2) | 0.86 (0.54–1.38) | 0.534 (C) | ||
| Female | 195 | 30 (15.4) | Ref | ||||
| Age (years) | Seropositivea | 59 | 2 (1) | 0.841 (M-W) | |||
| Seronegativeb | 355 | 1 (1) | |||||
| Ethnic minority | Yes | 97 | 15 (15.5) | 1.11 (0.65–1.91) | 0.696 (C) | ||
| No | 317 | 44 (13.9) | Ref | ||||
| Roma | 24 | 8 (33.3) | 2.50 (1.33–4.69) | 0.008 (C) | 0.999 | ||
| Immigrants | 60 | 7 (11.7) | 0.88 (0.41–1.85) | 0.725 (C) | 0.70 (0.14–3.62) | 0.673 | |
| No | 330 | 44 (13.3) | Ref | ||||
| Residence in a campc | Yes | 12 | 2 (16.7) | 1.17 (0.32–4.25) | 0.811 (F) | ||
| No | 401 | 57 (14.2) | Ref | ||||
| Level of education | No education | 25 | 6 (24.0) | 1.68 (0.48–5.92) | 0.408 (C) | ||
| Kindergarten | 35 | 7 (20.0) | 1.40 (0.41–4.84) | 0.437 (C) | |||
| Preschool | 38 | 3 (7.9) | 0.55 (0.12–2.50) | 0.361 (F) | |||
| Elementary school | 119 | 11 (9.2) | 0.65 (0.20–2.13) | 0.442 (C) | |||
| High school | 84 | 14 (16.7) | 1.17 (0.37–3.69) | 0.791 (C) | |||
| Symptoms consistent with COVID-19 | Yes | 103 | 21 (20.4) | 1.66 (1.03–2.70) | 0.041 (C) | 1.43 (0.41–5.01) | 0.573 |
| No | 310 | 38 (12.3) | Ref | ||||
| Close contact with COVID-19c | Yes | 60 | 24 (40.0) | 4.03 (2.59–6.27) | < 0.001 (C) | 4.82 (1.22–19.00) | 0.025 |
| No | 353 | 35 (9.9) | Ref | ||||
| Travel | Yes | 49 | 6 (12.2) | 0.84 (0.38–1.86) | 0.669 (C) | ||
| No | 365 | 53 (14.5) | Ref | ||||
| School Attendancec | Yes | 298 | 39 (13.1) | 0.73 (0.45–1.20) | 0.220 (C) | ||
| No | 112 | 20 (17.9) | Ref | ||||
| Number of children in the classc | Positive | 36 | 20 (5) | 0.919 (M-W) | |||
| Negative | 220 | 20 (7) | |||||
| Wearing mask at schoolc | Occasionally | 219 | 31 (14.2) | 0.87 (0.39–1.95) | 0.742 (C) | ||
| Rarely | 37 | 6 (16.2) | Ref | ||||
| COVID-19 case in the classc | Yes | 35 | 10 (28.6) | 2.35 (1.25–4.42) | 0.010 (C) | ||
| No | 222 | 27 (12.2) | Ref | ||||
| Number of COVID-19 cases at schoolc | Seropositive | 16 | 2 (2) | 0.006 (M-W) | 1.24 (0.85–1.79) | 0.260 | |
| Seronegative | 124 | 0 (2) | |||||
| Closure of the class due to COVID-19c | Yes | 32 | 9 (28.1) | 2.48 (1.22–5.06) | 0.014 (C) | 1.59 (0.35–7.33) | 0.550 |
| No | 150 | 17 (11.3) | Ref | ||||
| Use of means of public transportc | Yes | 39 | 2 (5.1) | 0.48 (0.12–2.00) | 0.294 (F) | ||
| No | 150 | 16 (10.7) | Ref | ||||
| Frequent social gatheringsc | Yes | 222 | 43 (19.4) | 2.16 (1.18–3.95) | 0.008 (C) | 0.89 (0.24–3.20) | 0.853 |
| No | 134 | 12 (9.0) | Ref | ||||
| Number of persons at gatheringsc | Seropositive | 30 | 4 (4) | 0.304 (M-W) | |||
| Seronegative | 159 | 4 (3) | |||||
| Number of families at gatheringsc | Seropositive | 30 | 2 (2) | 0.808 (M-W) | |||
| Seronegative | 156 | 2 (2) | |||||
| Wearing mask at gatheringsc | Yes | 8 | 3 (37.5) | 2.42 (0.93–6.31) | 0.126 (F) | ||
| No | 181 | 28 (15.5) | Ref | ||||
| Number of family membersc | Seropositive | 55 | 4 (1) | 0.133 (M-W) | |||
| Seronegative | 303 | 4 (1) | |||||
| Underlying diseasec | Yes | 101 | 14 (13.9) | 0.96 (0.55–1.68) | 0.897 (C) | ||
| No | 313 | 45 (14.4) | Ref | ||||
13 children were excluded from the analysis because their questionnaires were not filled out sufficiently. "Bold" is about the statistical significant p-values
C chi-square test, F Fisher’s exact test, M-W Mann–Whitney U test, CI confidence interval, OR odds ratios, PR proportional ratio
aAge- > seropositive: the median age of the seropositive children
bAge- > seronegative: the median age of the seronegative children
cPlease, note that there is missing data
Table 4 shows the seroprevalence differences in parents related to their demographic and clinical characteristics. Previous COVID-19 symptoms were present in 48/65 (73.8%) seropositive parents, while previous known SARS-CoV-2 infection was reported in 25/65 (38.5%) of them. Age, level of education, occupation, and teleworking were not correlated with seropositivity (p = 0.084, p = 0.519, p = 0.450, and p = 0.414). History of previous symptoms compatible with COVID-19 and known close contact with SARS-CoV-2-infected individual were associated with seropositivity (PR (95% CI): 11.37 (6.93–18.64), p < 0.001 and PR (95% CI): 3.61 (2.38–5.46), p < 0.001, respectively). Finally, individuals who belonged to the Roma minority were at higher risk to be seropositive (PR (95% CI): 2.18 (1.17–4.08), p = 0.022).
Table 4.
Seroprevalence differences in parents related to their demographic and clinical characteristics
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Parents (Ν = 392) |
Seropositive N (%) or median (IQR) |
PR (95% CI) | P-value | OR (95% CI) | P-value | |
| Sex | Male | 96 | 12 (12.5) | 0.70 (0.39–1.25) | 0.216 (C) | ||
| Female | 296 | 53 (17.9) | Ref | ||||
| Age (years) | Seropositivea | 65 | 39 (12.0) | 0.084 (M-W) | 0.99 (0.95–1.04) | 0.780 | |
| Seronegativeb | 327 | 41 (11.0) | |||||
| Ethnic minority | Yes | 86 | 18 (20.9) | 1.36 (0.84–2.22) | 0.220 (C) | ||
| No | 306 | 47 (15.4) | Ref | ||||
| Roma | 24 | 8 (33.3) | 2.18 (1.17–4.08) | 0.022 (C) | 6.15 (1.28–29.46) | 0.023 | |
| Immigrants | 60 | 10 (16.7) | 1.09 (0.59–2.04) | 0.783 (C) | 2.17 (0.56–8.40) | 0.262 | |
| No | 308 | 47 (15.3) | Ref | ||||
| Residency in a campc | Yes | 8 | 3 (37.5) | 2.32 (0.92–5.83) | 0.132 (F) | ||
| No | 383 | 62 (16.2) | |||||
| Level of educationc | < High school | 202 | 33 (16.3) | 0.86 (0.54–1.36) | 0.519 (C) | ||
| BSc, MSc, PhD | 142 | 27 (19.0) | |||||
| History of symptoms consistent with COVID-19c | Yes | 78 | 48 (61.5) |
11.37 (6.93–18.64) |
< 0.001 (C) | 11.70 (3.77–36.32) | < 0.001 |
| No | 314 | 17 (5.4) | Ref | ||||
| History of close contact with COVID-19c | Yes | 61 | 26 (42.6) | 3.61 (2.38–5.46) | < 0.001 (C) | 2.54 (0.78–8.27) | 0.122 |
| No | 330 | 39 (11.8) | Ref | ||||
| Travelc | Yes | 58 | 7 (12.1) | 0.69 (0.33–1.44) | 0.313 (C) | ||
| No | 336 | 58 (17.3) | |||||
| Work attendancec | Yes | 211 | 35 (16.6) | 0.84 (0.53–1.33) | 0.450 (C) | ||
| No | 126 | 25 (19.8) | Ref | ||||
| Teleworkingc | Yes | 49 | 10 (20.4) | 1.32 (0.68–2.54) | 0.414 (C) | ||
| No | 168 | 26 (15.5) | |||||
| Duration of teleworking (months) | Seropositive | 8 | 5.5 (2) | 0.805 (M-W) | |||
| Seronegative | 37 | 6 (3) | |||||
| Wearing mask at workc | Yes | 160 | 31 (16.2) | 0.70 (0.25–2.00) | 0.522 (F) | ||
| Occasionally | 13 | 3 (23.1) | Ref | ||||
| Use of means of public transportation | Yes | 82 | 11 (13.4) | 0.84 (0.45–1.54) | 0.558 (C) | ||
| No | 280 | 45 (16.1) | Ref | ||||
| Parent with a child attending school | Yes | 256 | 44 (17.2) | 1.14 (0.64–2.00) | 0.658 (C) | ||
| No | 136 | 21 (15.4) | Ref | ||||
| Frequent mass gatheringsc | Yes | 213 | 42 (19.7) | 1.36 (0.82–2.25) | 0.229 (C) | ||
| No | 124 | 18 (14.5) | Ref | ||||
| Number of persons at gatheringsc | Seropositive | 27 | 5 (4) | 0.002 (M-W) | 1.11 (0.96–1.28) | 0.163 | |
| Seronegative | 156 | 4 (3) | |||||
| Number of families at gatheringsc | Seropositive | 27 | 2 (1) | 0.942 (M-W) | |||
| Seronegative | 153 | 2 (1) | |||||
| Wearing mask at gatheringsc | Yes | 13 | 1 (7.7) | 0.51 (0.08–3.46) | 0.696 (C) | ||
| No | 172 | 26 (15.1) | Ref | ||||
| Number of family membersc | Seropositive | 60 | 4 (1) | 0.616 (M-W) | |||
| Seronegative | 277 | 4 (1) | |||||
| Underlying disease | Yes | 83 | 11 (13.3) | 0.76 (0.42–1.38) | 0.358 (F) | ||
| No | 309 | 54 (17.5) | Ref | ||||
Four participants’ parents were excluded from the analysis because their questionnaires were not filled out sufficiently. "Bold" is about the statistical significant p-values
C chi-square test, F Fisher’s exact test, M-W Mann–Whitney U test, CI confidence interval, OR odds ratios, PR proportional ratio
aAge- > seropositive: the median age of the seropositive children
bAge- > seronegative: the median age of the seronegative children
cPlease, note that there is missing data
Multivariate analysis revealed that known close contact with COVID-19 was associated with increased seropositivity in children (OR (95% CI): 4.82 (1.22–19.00), p = 0.025). In addition, parents who belonged to the Roma minority as well as those with a history of previous symptoms compatible with SARS-CoV-2 infection were more likely to be positive (OR (95% CI): 6.15 (1.28–29.46), p < 0.023 and OR (95% CI): 11.70 (3.77–36.32), p < 0.001, respectively).
Discussion
Inadequate knowledge about the prevalence of SARS-CoV-2 infection in a certain population challenges public health response. Changing testing strategies has made it very difficult for countries to estimate the proportion of the infected population. Well-designed serosurveys play an important role to determine the prevalence of SARS-CoV-2 infection and its burden in the general population or in selected groups, and they are useful for monitoring transmission trends [13]. In addition, such studies may provide some evidence about the effectiveness of different non-pharmaceutical interventions in a certain population. School closures were enforced as measures to restrain the COVID-19 pandemic, based on the assumption that young children may play a key role in SARS-CoV-2 spread. To date, data on the seroprevalence of children and the household transmission are sparse. This is the first multicenter seroprevalence study of SARS-CoV-2 IgG antibodies in children and corresponding parents in Greece, providing evidence for the potential underdiagnosis of asymptomatic or subclinical COVID-19 cases. The prevalence of infection was found to be comparable in children and adults arguing against the role of children as drivers of transmission.
Our study shows that the seroprevalence in children and parents at the time the study was conducted was 13.8% and 16.4%, respectively, indicating that there was no significant difference between these two groups. Also, there was no difference between the number of families with at least one seropositive child or parent or both of them. These results showed that seroprevalence in children was low and comparable to that of adults possibly implying that children are not the source of the infection neither the drivers of transmission. These findings are similar to those of one serosurvey in children and parents residing in Southwest Germany [22]. However, one seroprevalence study performed in children and family members in Brussels suggested that children may be the primary source of infection and transmission in households, as children usually have mild or no symptoms and they are underdiagnosed [23]. In the current study, young age was not a significant factor for higher seroprevalence, and children were not identified as the primary case of SARS-CoV-2 infection in households. These findings may contribute to plan for school-based measures, in order to reduce transmission risk within and between classrooms.
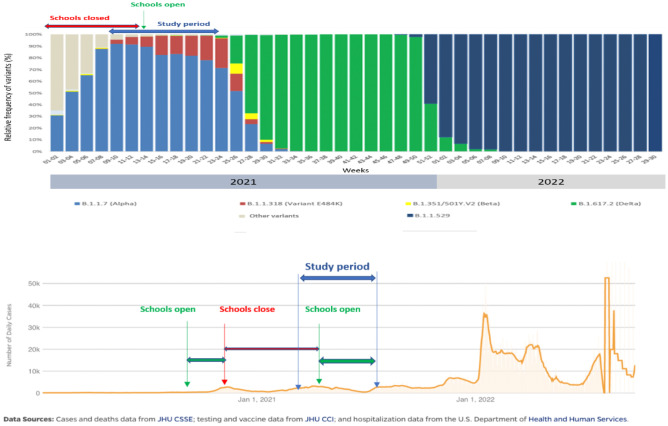
Current data about the potential transmission of COVID-19 from children to adults are controversial, as a few studies support a lower transmission rate from children to adults, while other studies show increased infectivity in young children compared to older individuals [24–27]. Our findings about similar infection rates between children and adults are consistent with other household transmission studies [21, 28]. However, some studies, especially those performed in the pre-Omicron period, suggest that children are less susceptible to SARS-CoV-2 infection than adults and particularly in the early phase of the pandemic, when children were largely protected from community exposures due to non-pharmaceutical interventions, including closure of schools and teleworking [29]. In addition, these controversies in the results may be due to the methods of detecting SARS-CoV-2 infection among household contacts, as individuals with asymptomatic or mildly symptomatic infection are rarely tested, resulting in the underestimation of SARS-CoV-2 infections. A recent meta-analysis about child transmission of SARS-CoV-2 showed that children may be less likely to transmit COVID-19 compared to adults, and household transmission remains the most prominent source of child-to-adult and child-to-child transmission [30]. Further studies are needed to be performed, in order to clarify this emergent issue (Fig. 1).
Fig. 1.
Graphical overview of the predominant virus variants (adapted from the National Public Health Organization (NPHO), available in https://eody.gov.gr/epidimiologika-statistika-dedomena/ektheseis-epidimiologikis-epitirisis-loimoxis-apo-ton-sars-cov-2/. A and the course of the pandemic in Greece (adapted from the Center for Systems Science and Engineering (CSSE) COVID-19 Data at Johns Hopkins University (JHU), available in https://coronavirus.jhu.edu/region/Greece) B, indicating the closure of schools and the study period
Another important finding of this serosurvey was that people belonging to the ethnic minority of Roma were associated with increased seropositivity. It has been shown that Roma communities are more vulnerable to infectious disease outbreaks, including hepatitis, measles, poliomyelitis, and tuberculosis [31], and Roma children present increased incidence of respiratory infections compared to non-Roma children [32–34]. This increased seropositivity may be due to the overcrowded households, poverty, and poor sanitation of this population. This is in line with one study indicating that large household membership and greater household crowding were associated with increased odds for SARS-CoV-2 infection [35].
School attendance for children or absence of teleworking was not associated with increased seropositivity. These data provide more information about how the schools contribute to the spread of the SARS-CoV-2 infection, as major policy decisions on temporary school or class closures have been implemented globally in order to prevent transmission. However, close contact with confirmed COVID-19 case either at school or in the household or at social gatherings are risk factors for higher infectivity. Therefore, the implementation of distancing and contact restriction measures may be the most effective methods to control the spread of the COVID-19. Surprisingly, our study showed that wearing mask is not associated with decreased seropositivity, and this may be related to the fact that these results were based on the information given by the participants, resulting in study bias.
One of the strengths of this study is the prospective design, and that is multicenter, representing all 7 Regional Health Directorates of the country. However, a potential limitation is the voluntary participation of the individuals participating in the study, leading to potential selection bias in our results. For example, the seroprevalence may be overestimated if the parents were more willing to participate, because they believed that their children and/or themselves had been exposed to SARS-CoV-2 infection. Vaccinated people were excluded from the study, as measurement of anti-N antibodies for SARS-CoV-2 was not performed, and this might have led to another selection bias. Additional limitation is a possible cluster/family effect on the results. Considering that the majority of the family clusters are very small (363 families with a pair of one parent and one child), the correction in the analysis could not be easy and with minimal results. Another limitation is that during the time period before the initiation of the serosurvey, the country was on lockdown, and the schools were closed, resulting in relatively low seroprevalence. In order to clarify this, we compared the seroprevalence between the period March/April and the period May/June, and it was found that there was an increase in seropositivity among both parents and children; however, it was higher and the difference statistically significant only in the parent group. This finding may indirectly indicate less efficient transmission of SARS-CoV-2 in schools. Moreover, the design of the study did not allow to trace the sequence of infections within a family and therefore to evaluate the role of children in intrafamilial transmission. Finally, these seroprevalence results of our study concern the pre-Omicron period and, in particular, the Alpha period, just before the Delta wave. Further seroepidemiological studies are currently underway, in order to evaluate the seroprevalence during the Omicron period. It is known that Omicron variant is more transmissible and can cause milder disease compared to other variants [36]. So, it is expected that seroprevalence will be higher during the Omicron period compared to previous ones.
In conclusion, in this seroprevalence study, the spread of SARS-CoV-2 infection during a period of lockdown in Greece with the predominance of the Alpha variant was particularly low in children and comparable to adults, most likely due to intrafamilial transmission. These study findings will be useful for decisions regarding non-pharmaceutical interventions during the pandemic, and especially, to guide in designing and implementing appropriate containment measures for schools and social gatherings.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- CI
Confidence interval
- CMIA
Chemiluminescent microparticle immunoassay
- COVID-19
Coronavirus disease 2019
- EUA
Emergency use authorization
- IgG
Immunoglobulin G
- IQR
Interquartile range
- OR
Odds ratios
- PR
Proportional ratio
- RBD
Receptor-binding domain
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
Authors’ contributions
All authors contributed to the study conception and design. Maria Tsolia, Vassiliki Spoulou, Athanasios Michos, Despoina Gkentzi, Ekaterini Siomou, Vassiliki Papaevangelou, George Syrogiannopoulos, and Emmanouil Galanakis contributed to the conception and design of the work. Material preparation and data collection were performed by Dimitra Dimopoulou, Eleni Vergadi, Ekaterini Tsiligianni, Eleni Papadimitriou, Artemis Mavridi, Spyridon Giannakopoulos, Georgia Tsiourvopoulou, Maria Palyvou, Evangelia Angeli, Nikitas Brikos, and Irini Eleftheriou. Data analysis was performed by Maria Kyritsi, Katerina Dadouli, Christos Hadjichristodoulou, Maria Tsolia, and Ioanna Grivea. The first draft of the manuscript was written by Dimitra Dimopoulou, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declarations
Ethics approval
The study was approved by the “P. & A. Kyriakou” Children’s Hospital’s Ethical and Research Committee (approval number: 507/20.10.2020). All procedures were conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
For all participants, written informed consent was obtained from the parents at the time of the enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19 – studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 2.WHO Coronavirus Disease (COVID-19) Dashboard (2020). https://covid19.who.int/. Accessed 20 Sept 2020
- 3.Greek Ministry of Education and Religious Affairs (2020) Temporary ban on the operation of all educational structures. Athens. Greek. Available from: https://www.minedu.gov.gr/news/44308-10-03-20-prosorini-apagorefsi-tis-ekpaideftikis-leitourgias-olon-tonekpaideftikon-domon. Accessed 26 Sept 2022
- 4.European Union Agency for Fundamental Rights (FRA) (2020) Coronavirus COVID-19 outbreak in the EU Fundamental Rights Implications. Vienna: FRA. Available from: https://fra.europa.eu/sites/default/files/fra_uploads/greece-report-covid-19-april-2020_en.pdf. Accessed 26 Sept 2022
- 5.Bryant JE, Azman AS, Ferrari MJ et al (2020) Serology for SARS-CoV-2: apprehensions, opportunities, and the path forward. Sci Immunol 5:eabc6347 [DOI] [PubMed]
- 6.Valdivia A, Torres I, Huntley D, Alcaraz MJ, Albert E, Solano de la Asunción C, González C, Ferrer J, Navarro D. Caveats in interpreting SARS-CoV-2 IgM(+)/IgG(-) antibody profile in asymptomatic health care workers. J Med Virol. 2020 doi: 10.1002/jmv.26400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peeling RW, Olliaro PL. The time to do serosurveys for COVID-19 is now. Lancet Respir Med. 2020;8:836–838. doi: 10.1016/S2213-2600(20)30313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byambasuren O, Cardona M, Bell K, Clark J, McLaws M.L, Glasziou P (2020) Estimating the extent of true asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. medRxiv [DOI] [PMC free article] [PubMed]
- 9.Pan X, Chen D, Xia Y, Wu X, Li T, Ou X, Zhou L, Liu J. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20:410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L, Zhang X, Zhang X, Wei Z, Zhang L, Xu J, et al. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16–23 years outside Wuhan and characteristics of young patients with COVID-19: a prospective contact-tracing study. J Infect. 2020;80:e1–e13. doi: 10.1016/j.jinf.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He X, Lau, EH, Wu P et al (2020) Temporal dynamics in viral shedding and transmissibility of COVID-19. medRxiv [DOI] [PubMed]
- 12.Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19 - studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 14.WHO (2020) Population-based age-stratified seroepidemiological investigation protocol for COVID-19 virus infection. https://apps.who.int/iris/handle/10665/331656. Accessed 26 Sept 2022
- 15.Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. doi: 10.1093/biomet/26.4.404. [DOI] [Google Scholar]
- 17.Chung E, Chow EJ, Wilcox NC, Burstein R, Brandstetter E, Han PD, et al. Comparison of symptoms and RNA levels in children and adults with SARS-CoV-2 infection in the community setting. JAMA Pediatr. 2021;175(10):e212025. doi: 10.1001/jamapediatrics.2021.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawood FS, Porucznik CA, Veguilla V, Stanford JB, Duque J, Rolfes MA, et al. Incidence rates, household infection risk, and clinical characteristics of SARS-CoV-2 infection among children and adults in Utah and New York City. New York JAMA Pediatr. 2022;176(1):59–67. doi: 10.1001/jamapediatrics.2021.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Centers for Disease Control and Prevention (CDC) (2022). Symptoms of COVID-19. Available in: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed 26 Sept 2022
- 20.The Centers for Disease Control and Prevention (CDC) (2022). Contact tracing. Available in: https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/appendix.html. Accessed 26 Sept 2022
- 21.McLean HQ, Grijalva CG, Hanson KE, Zhu YG, Deyoe JE, Meece JK et al (2022) Household transmission and clinical features of SARS-CoV-2 infections by age in 2 US Communities. Pediatrics [DOI] [PMC free article] [PubMed]
- 22.Tönshoff B, Müller B, Elling R, Renk H, Meissner P, Hengel H, et al. Prevalence of SARS-CoV-2 infection in children and their parents in Southwest Germany. JAMA Pediatr. 2021;175:586–593. doi: 10.1001/jamapediatrics.2021.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dethioux L, Dauby N, Montesinos I, Rebuffat E, Hainaut M. SARS-CoV-2 seroprevalence in children and their family members, July-October 2020, Brussels. Eur J Pediatr. 2022;181:1009–1016. doi: 10.1007/s00431-021-04284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee B, Raszka WV (2020) COVID-19 transmission and children: the child is not to blame. Pediatrics e2020004879 [DOI] [PubMed]
- 25.Merckx J, A. Labrecque J, S. Kaufman J, Transmission of SARS-CoV-2 by children. Dtsch Arztebl Int. 2020;117:553–560. doi: 10.3238/arztebl.2020.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li F, Li Y-Y, Liu M-J, et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect Dis. 2021;21:617–628. doi: 10.1016/S1473-3099(20)30981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun K, Wang W, Gao L et al (2021) Transmission heterogeneities, kinetics, and controllability of SARS-CoV-2. Science 371:eabe2424 [DOI] [PMC free article] [PubMed]
- 28.Laws RL, Chancey RJ, Rabold EM et al (2021) Symptoms and transmission of SARS-CoV-2 among children - Utah and Wisconsin, March-May 2020. Pediatrics 147 [DOI] [PubMed]
- 29.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175:143–156. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverberg SL, Zhang BY, Li SNJ, Burgert C, Shulha HP, Kitchin V, et al. Child transmission of SARS-CoV-2: a systematic review and meta-analysis. BMC Pediatr. 2022;22:172. doi: 10.1186/s12887-022-03175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook B, Wayne GF, Valentine A, Lessios A, Yeh E. Revisiting the evidence on health and health care disparities among the Roma: a systematic review 2003–2012. Int J Public Health. 2013;58:885–911. doi: 10.1007/s00038-013-0518-6. [DOI] [PubMed] [Google Scholar]
- 32.Dostal M, Topinka J, Sram RJ. Comparison of the health of Roma and non-Roma children living in the district of Teplice. Int J Public Health. 2010;55:435–441. doi: 10.1007/s00038-010-0133-8. [DOI] [PubMed] [Google Scholar]
- 33.Kaditis AG, Gourgoulianis K, Tsoutsou P, Papaioannou AI, Fotiadou A, Christina M, et al. Spirometric values in Gypsy (Roma) children. Respir Med. 2008;102:1321–1328. doi: 10.1016/j.rmed.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 34.Monasta L, Andersson N, Ledogar RJ, Cockcroft A. Minority health and small numbers epidemiology: a case study of living conditions and the health of children in 5 foreign Romá camps in Italy. Am J Public Health. 2008;98:2035–2041. doi: 10.2105/AJPH.2007.129734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emeruwa UN, Ona S, Shaman JL, Turitz A, Wright JD, Gyamfi-Bannerman C, Melamed A. Associations between built environment, neighborhood socioeconomic status, and SARS-CoV-2 infection among pregnant women in New York City. JAMA. 2020;324:390–392. doi: 10.1001/jama.2020.11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kannan S, Shaik Syed Ali P, Sheeza A (2021) Omicron (B.1.1.529) - variant of concern - molecular profile and epidemiology: a mini review. Eur Rev Med Pharmacol Sci 25:8019–8022 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.