Abstract
Haemophilus ducreyi expresses a soluble cytolethal distending toxin (CDT) that kills HeLa, HEp-2, and other human epithelial cells in vitro. H. ducreyi CDT activity is encoded by a three-gene cluster (cdtABC), and antibody to the cdtC gene product can neutralize CDT activity in vitro (L. D. Cope, S. R. Lumbley, J. L. Latimer, J. Klesney-Tait, M. K. Stevens, L. S. Johnson, M. Purven, R. S. Munson, Jr., T. Lagergard, J. D. Radolf, and E. J. Hansen, Proc. Natl. Acad. Sci. USA 94:4056–4061, 1997). Culture supernatant fluid from a recombinant Escherichia coli strain containing the H. ducreyi cdtABC gene cluster readily killed both HeLa cells and HaCaT keratinocytes and had a modest inhibitory effect on the growth of human foreskin fibroblasts. Insertional inactivation of the cdtC gene in this recombinant E. coli strain eliminated the ability of this strain to kill HeLa cells and HaCaT keratinocytes. This mutated H. ducreyi cdtABC gene cluster was used to construct an isogenic H. ducreyi cdtC mutant. Monoclonal antibodies against the H. ducreyi CdtA, CdtB, and CdtC proteins were used to characterize protein expression by this cdtC mutant. Culture supernatant fluid from this H. ducreyi cdtC mutant did not detectably affect any of the human cells used in this study. The presence of the wild-type H. ducreyi cdtC gene in trans in this H. ducreyi mutant restored its ability to express a CDT that killed both HeLa cells and HaCaT keratinocytes. The isogenic H. ducreyi cdtC mutant was shown to be as virulent as its wild-type parent strain in the temperature-dependent rabbit model for experimental chancroid. Lack of expression of the H. ducreyi CdtC protein also did not affect the ability of this H. ducreyi mutant to survive in the skin of rabbits.
Haemophilus ducreyi is an extremely fastidious, gram-negative coccobacillus which causes chancroid, a sexually transmitted ulcerogenital disease that has a high degree of prevalence in some parts of Africa and Asia. In the United States, chancroid is uncommon (61), and outbreaks are often associated with prostitution, crack cocaine usage, and multiple sex partners (15, 39). Although chancroidal ulcers are usually relatively superficial, they can facilitate transmission of the human immunodeficiency virus (72).
There is a paucity of information concerning the gene products which allow H. ducreyi to cause genital ulcers. The organism is apparently not able to invade intact skin (64), and it is assumed that microabrasions sustained during sexual activity permit entry of the organism beneath the skin surface. The introduction of a number of new model systems for studying the interaction of H. ducreyi with host cells both in vitro and in vivo (8, 23, 24, 33, 68, 69, 71) has facilitated studies intended to identify virulence factors of this pathogen. In the past few years, a number of H. ducreyi gene products have been postulated to be involved directly or indirectly in virulence expression (7, 9–11, 17–19, 34, 37, 38, 48, 49, 59, 60, 66, 73), including at least two proteins which have cytotoxic activity.
The first of these two cytotoxins was originally described by Lagergard and colleagues (15, 31, 32, 54, 56) as being present in H. ducreyi culture supernatant fluid and active against several different types of human epithelial cell lines (e.g., HeLa cells) in vitro. The second cytotoxin, first described by Palmer and Munson (45), proved to be a hemolysin that was similar to the hemolysins expressed by Proteus mirabilis and Serratia marcescens, appeared to be bacterial cell-associated, and was found to kill human foreskin fibroblasts but not HeLa cells in culture (2, 43–45, 47, 70). It was recently shown that an isogenic H. ducreyi hemolysin-deficient mutant caused pustule formation in the human model for experimental chancroid (47).
The soluble cytotoxic activity in H. ducreyi culture supernatant fluid (15, 31, 32, 54, 56) was recently shown to be the result of the activity of a homolog of the cytolethal distending toxin (CDT) (13) expressed by a number of enteric pathogens, including Escherichia coli (26, 51, 62), Shigella species (27, 42), and Campylobacter species (28, 52). CDT activity is characterized by relatively slow morphological changes in cultured epithelial cells, including progressive cellular distention and death within 96 to 120 h (26).
The H. ducreyi CDT is chromosomally encoded by three genes, cdtA, cdtB, and cdtC (13), whose predicted protein products possess 24 to 51% identity with the CdtABC proteins from E. coli (51, 62). A monoclonal antibody (MAb) to the H. ducreyi CdtC protein neutralized CDT activity in vitro (13) and implicated at least the H. ducreyi cdtC gene product as being involved, directly or indirectly, in the expression of cytotoxic activity. As part of our continuing efforts to elucidate virulence mechanisms of H. ducreyi, we constructed an isogenic H. ducreyi cdtC mutant and tested this mutant in relevant in vitro and in vivo systems. Elimination of the ability to elaborate the CdtC protein caused this H. ducreyi mutant to be unable to kill HeLa cells and HaCaT keratinocytes in culture. In contrast, this mutation did not detectably affect the ability of H. ducreyi to cause skin lesions in the temperature-dependent rabbit model.
(Part of this research was presented by S. R. Lumbley at the 98th General Meeting of the American Society for Microbiology, Atlanta, Ga., 17 to 21 May 1998 [35].)
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains used for the majority of the experiments in this study are listed in Table 1. All H. ducreyi strains were cultivated on chocolate agar (CA) plates containing 1% (vol/vol) IsoVitaleX (BBL Microbiological Systems, Cockeysville, Md.) in a humidified atmosphere of 95% air–5% CO2 at 33°C as described previously (53). Stock cultures of all H. ducreyi strains were stored at −70°C in fetal bovine serum. H. ducreyi cultures used for cytotoxicity assays were grown in modified Columbia broth (74). H. ducreyi transformants were isolated on either CA containing chloramphenicol (2 μg/ml) or GC-heme agar (66) containing kanamycin (30 μg/ml). E. coli strains HB101 and DH5α were grown in Luria-Bertani (LB) broth or on LB agar medium at 37°C. For selection of E. coli recombinants, chloramphenicol, kanamycin, and tetracycline were used at final concentrations of 30, 30, and 15 μg/ml, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | Host strain for cloning experiments | 58 |
| HB101 | Host strain essential for propagating plasmids carrying mutated H. ducreyi DNA inserts used in electroporation | 5, 58 |
| DH5α(pJL300) | Contains H. ducreyi 35000 cdtABC gene cluster; expresses H. ducreyi CDT activity | This study |
| DH5α(pJL303) | Contains H. ducreyi cdtABC gene cluster with mutated cdtC gene; expresses no CDT activity | This study |
| DH5α(pJL303)(pJL300-C) | DH5α(pJL303) also containing pJL300-C with the H. ducreyi cdtC gene; expresses CDT activity | This study |
| DH5α(pJL303)(pLS88) | DH5α(pJL303) also containing pLS88; expresses no CDT activity | This study |
| H. ducreyi | ||
| 35000 | Wild-type strain isolated in Winnipeg, Manitoba, Canada; produces CDT | 21 |
| 35000.303 | Isogenic cdtC mutant with a cat cartridge inserted into the cdtC gene; does not express CDT activity | This study |
| 35000.303(pJL300-C) | 35000.303 containing pJL300-C with the cdtC gene; expresses CDT activity | This study |
| 35000.303(pLS88) | 35000.303 containing pLS88; expresses no CDT activity | This study |
| Plasmids | ||
| pBR322 | Cloning vector; Ampr Tetr | 58 |
| pJL300 | pBR322 with a 3-kb H. ducreyi 35000 DNA insert containing the cdtABC gene cluster | This study |
| pJL303 | pJL300 with an cat cartridge inserted into the BseRI site within the cdtC gene | This study |
| pLS88 | Cloning vector capable of replication in H. ducreyi; Kanr Smr Sulr | 16 |
| pJL300-C | pLS88 with a 813-bp PCR-derived insert containing the H. ducreyi cdtC gene | This study |
MAbs.
Female BALB/c mice were immunized with purified fusion proteins consisting of six histidine residues (His) coupled to most or all of the putative mature forms of the H. ducreyi CdtA, CdtB, and CdtC proteins as described previously (13). A synthetic peptide (EPTHRSGNILDYAILHDAHLPRREQARER) derived from the amino acid sequence of the H. ducreyi CdtB protein was covalently coupled to keyhole limpet hemocyanin; this peptide-protein conjugate was used to immunize mice as described previously (74). Splenocytes from the immunized mice were used in a hybridoma fusion protocol (57), and culture supernatant fluids from these fusions were screened for the presence of MAbs by means of an enzyme-linked immunosorbent assay (ELISA). The antigens used in this ELISA to identify the CdtA-reactive MAb 1G8 and the CdtC-reactive MAbs 8C9 and 9E9 were the purified, homologous His-tagged fusion proteins. The CdtC-reactive MAb 9E9 was used for colony blot radioimmunassays (RIAs); the CdtC-reactive MAb 8C9 was used in Western blot analysis. Cell envelopes from a recombinant E. coli strain expressing a glutathione S-transferase–CdtB fusion protein were used in the ELISA to identify the CdtB-reactive MAb 20B2. The oligonucleotide primers used for PCR-based amplification of the portion of the cdtB gene used in this fusion construct were 5′-GCGGATCCAACTCATCATCATCCCCACC-3′ and 5′-TCCCCCGGGGCGATCACGAACAAAACTAACAG-3′; the underlined sequences denote BamHI and SmaI restriction sites, respectively, used to insert this gene fragment into the pGEX-4T-2 vector (Pharmacia Biotech, Piscataway, N.J.).
Preparation of bacterial culture supernatant fluid for cytotoxicity testing.
After overnight growth (16 h), each E. coli or H. ducreyi culture was subjected to centrifugation at 7,600 × g for 15 min to remove bacterial cells and debris, followed by centrifugation at 140,000 × g for 2 h to remove membrane fragments. The resultant supernatant fluid was sterilized by filtration through a cellulose acetate filter (0.2-μm pore size) and either used immediately for cytotoxicity testing or stored at −70°C until used. H. ducreyi CDT activity in bacterial culture supernatant fluid was stable for 1 month at −70°C.
Cytotoxicity assays.
Three different human cell lines were used in cytotoxicity testing of bacterial culture supernatant fluids. HeLa cells (ATCC CRL7923) were grown in Dulbecco’s minimal essential medium (GIBCO-BRL, Gaithersburg, Md.) with 10% (vol/vol) fetal bovine serum, 1% (vol/vol) GlutaMAX I (GIBCO), and both penicillin and streptomycin. A spontaneously immortalized human keratinocyte line (HaCaT) that shows normal keratinization and differentiation in vitro (6) was grown in the same medium as the HeLa cells with the addition of 25 mM HEPES buffer. A human foreskin fibroblast line described previously (3) was grown in RPMI 1640 (Mediatech, Herndon, Va.) with 10% (vol/vol) fetal bovine serum, 1 mM sodium pyruvate, 1% (vol/vol) GlutaMAX I, and both penicillin and streptomycin. All three cell lines were incubated in an atmosphere of 95% air–5% CO2 at 37°C.
HeLa cells were seeded at 2 × 104 cells per well in a 24-well tissue culture plate; the HaCaT keratinocytes and human foreskin fibroblasts were seeded at 6 × 104 cells per well. After overnight incubation of the cells in 1 ml of the appropriate tissue culture medium, a 1-ml portion of filter-sterilized bacterial culture supernatant fluid was added to each well and the plates were incubated for 3 h as described above. Then, the fluid in each well was aspirated and replaced with fresh tissue culture medium. The plates were incubated for 72 to 96 h, at which time each well was photographed with an inverted phase-contrast microscope.
SDS-PAGE, Western blot, and colony blot analyses.
Whole-cell lysates were prepared from bacterial cells grown overnight on solidified medium as described previously (22). The bacterial cell suspension used to prepare the whole-cell lysate was adjusted to a density of 300 Klett units with a Klett-Summerson photoelectric colorimeter (Klett Manufacturing, New York, N.Y.). A 10-μl portion of each whole-cell lysate was used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins resolved by this method were transferred to nitrocellulose for Western blot analysis (30). Hybridoma culture supernatant fluids were used as the source of MAbs in all experiments. Affinity-purified and radioiodinated goat anti-mouse immunoglobulin was used as the secondary antibody in both Western blot analysis and the colony blot RIA (20).
Nucleotide sequence analysis.
A model 373A automated DNA sequencer (Applied Biosystems Inc., Foster City, Calif.) was used to determine the nucleotide sequences of DNAs contained in recombinant plasmids and PCR products. DNA sequences were assembled into larger contiguous sequences and analyzed by using AssemblyLign and MacVector DNA analysis software (version 6.0; Oxford Molecular Group, Campbell, Calif.).
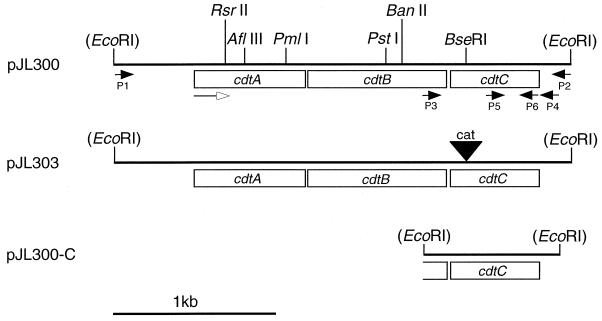
Cloning and mutagenesis of the H. ducreyi cdtABC gene cluster.
The Pfu DNA polymerase system (Stratagene, La Jolla, Calif.) was employed to amplify DNA for use in construction of a plasmid containing the wild-type H. ducreyi cdtABC gene cluster. The oligonucleotide primers P1 (5′-GGAATTCCTTGTAGATTATTCACCGTC-3′) and P2 (5′-CGGAATTCCAATTCCAGTTGATTCACC-3′) (Fig. 1) were used in a PCR to generate a 3-kb DNA product from the H. ducreyi 35000 chromosome. This PCR product contained approximately 500 bp of contiguous H. ducreyi chromosomal DNA both upstream and downstream of the cdtABC gene cluster, together with the restriction site for EcoRI at each end (underlined in the oligonucleotide primer sequences). After digestion with EcoRI, this PCR-derived product was ligated into the EcoRI site in pBR322, and the ligation reaction mixture was used to transform E. coli DH5α. After selection with tetracycline, the transformants were screened for reactivity with the H. ducreyi CdtC-reactive MAb 9E9. One of the tetracycline-resistant, MAb 9E9-reactive transformants was selected at random, and its recombinant plasmid was designated pJL300.
FIG. 1.
Partial restriction maps of the H. ducreyi 35000 cdtABC gene cluster in pJL300 and related constructs. Plasmid pJL300 was constructed by inserting the PCR-derived 3-kb cdtABC gene cluster into the EcoRI site in pBR322. Plasmid pJL303 was constructed by inserting a cat cartridge into the BseRI site in the cdtC gene within pJL300. Plasmid pJL300-C was constructed by inserting the 813-bp PCR product containing the cdtC open reading frame into the EcoRI site within pLS88. Oligonucleotide primers P1 to P6 are indicated and are described in detail in Materials and Methods. The open arrow indicates the direction of transcription. Restriction sites in parentheses are vector-based sites and are not present in H. ducreyi chromosomal DNA. A 1-kb size marker is indicated on the lower left.
Plasmid pJL300 was digested with BseRI, which cuts once within the H. ducreyi cdtC gene (Fig. 1), and the 1.4-kb chloramphenicol acetyltransferase gene (cat) cartridge was excised from the plasmid pUCΔECAT with the restriction enzyme BamHI. Both of these linear DNA molecules were treated with the Klenow fragment of DNA polymerase I (New England Biolabs, Beverly, Mass.) to create blunt ends, and then the cat cartridge was blunt-end ligated into the cdtC gene. The ligation mixture was used to transform E. coli DH5α, and the desired recombinants were selected by growth on LB medium supplemented with chloramphenicol and then screened for lack of reactivity with the H. ducreyi CdtC-reactive MAb 9E9 in the colony blot RIA. One of these chloramphenicol-resistant transformants that was unreactive with MAb 9E9 was chosen at random, and its recombinant plasmid was designated pJL303 (Fig. 1).
Construction of the isogenic H. ducreyi cdtC mutant.
Plasmid pJL303, containing the mutated cdtC gene, was used to transform E. coli HB101. This plasmid was purified from this recombinant strain by means of the Wizard Plus Minipreps DNA Purification System (Promega, Madison, Wis.). Approximately 10 μg of plasmid DNA was digested with NruI. After a phenol-chloroform extraction followed by a chloroform extraction, the linearized plasmid was precipitated by the addition of 1/10 volume of 3.0 M sodium acetate and 2 volumes of 100% ethanol, followed by two washes in 70% ethanol. The washed DNA was dissolved in 10 μl of distilled water and used to electroporate H. ducreyi 35000 as described previously (22). Chloramphenicol-resistant transformants were screened in a colony blot RIA for the absence of reactivity with the H. ducreyi CdtC-reactive MAb 9E9; one such transformant, designated 35000.303, was selected for further characterization.
Complementation analysis of E. coli and H. ducreyi strains.
DNA containing the H. ducreyi cdtC gene was amplified from the H. ducreyi 35000 chromosome by PCR with the Pfu DNA polymerase system (Stratagene) together with the oligonucleotide primers P3 (5′-GGAATTCTGCACATTTACCACGTAG-3′) and P4 (5′-GGAATTCATCTTACTGCGTCTGCCTGG-3′) (Fig. 1). Both primers contained EcoRI sites (underlined). This 813-bp fragment was ligated into the EcoRI site in pLS88 (16), and the ligation reaction mixture was used to transform E. coli DH5α. Kanamycin-resistant E. coli recombinants expressing the H. ducreyi CdtC protein were identified by their reactivity with the H. ducreyi CdtC-reactive MAb 9E9 in the colony blot RIA. The recombinant plasmid from one of these MAb 9E9-reactive transformants was designated pJL300-C and was used to electroporate both E. coli DH5α(pJL303) and the H. ducreyi cdtC mutant 35000.303; in both instances, the desired transformants were selected with kanamycin.
Southern blot analysis.
Purified chromosomal DNAs from H. ducreyi strains were digested to completion with PstI and used in Southern blot analysis as described previously (74). The probe for the cdtC gene was obtained by PCR with the oligonucleotide primers P5 (5′-ATGTTTTGCTTTCCTGGG-3′) and P6 (5′-ACCCTGATTTCTTCGCAC-3′) (Fig. 1) to amplify a 269-bp fragment from H. ducreyi 35000 chromosomal DNA. The probe for the cat cartridge was obtained by using the oligonucleotide primers 5′-CCAGGTTTTCACCGTAACACGC-3′ and 5′-TCCCAATGGCATCGTAAAGAAC-3′ to amplify a 331-bp fragment from this antibiotic resistance gene. Both probes were radiolabeled with [α-32P]dCTP by use of the Random-Primed DNA Labeling Kit (Boehringer-Mannheim Biochemicals, Indianapolis, Ind.) and purified on Quick Spin columns (Boehringer).
Virulence testing.
The temperature-dependent rabbit model for experimental chancroid was used to determine the relative virulences of the H. ducreyi wild-type and mutant strains used in this study (53). These studies were approved by the Institutional Animal Care and Research Advisory Committee. Briefly, eight New Zealand White adult male rabbits were used in this experiment. These animals were housed at a temperature of between 15 and 17°C. All other housing procedures and animal care procedures were performed in accordance with the standards of the U.S. Department of Agriculture and the Association for the Assessment and Accreditation of Laboratory Animal Care International. Ten-fold serial dilutions of the H. ducreyi strains were injected intradermally. Each animal was injected with both the wild-type strain and the cdtC mutant in this experiment, with one injection of each dilution of the inoculum. Inocula were coded prior to injection to prevent bias in scoring of the resultant lesions. Lesion characteristics were scored as described previously (53), using the following numeric values: 0, no change; 1, erythema; 2, induration; 3, nodule; and 4, pustule or necrosis. Lesion scores were recorded on days 2, 4, and 7 postinfection. On day 7 postinfection, material excised from lesions caused by injection of 105 CFU was cultured on CA plates. Statistical analyses were performed as described previously (65, 66).
RESULTS
Production of MAbs reactive with the protein products of the H. ducreyi cdtABC gene cluster.
To provide reagents necessary to confirm the isogenic nature of the desired H. ducreyi cdtC mutant, MAbs to the H. ducreyi CdtA, CdtB, and CdtC proteins were produced as described in Materials and Methods. It should be noted that the predicted sizes of the putative mature forms of the H. ducreyi CdtA, CdtB, and CdtC proteins are 23, 29, and 19 kDa, respectively (13).
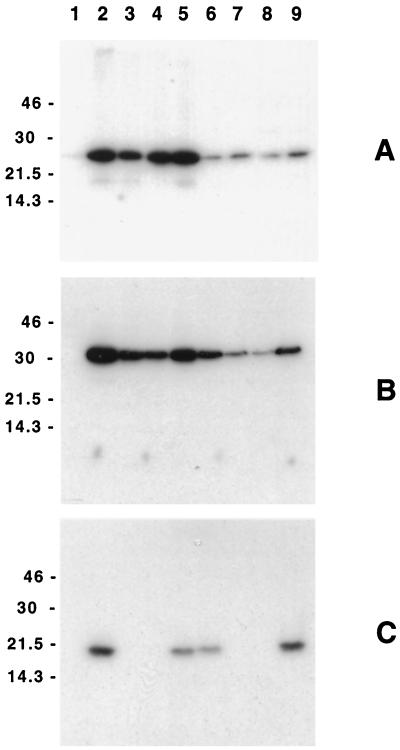
In Western blot analysis, MAb 1G8, raised against the His-CdtA fusion protein, bound a 24-kDa antigen in whole-cell lysates of the recombinant E. coli strain DH5α(pJL300) (Fig. 2A, lane 2), which contained the entire H. ducreyi 35000 cdtABC gene cluster. Repeated attempts to generate an H. ducreyi CdtB-reactive MAb by immunizing with a His-CdtB fusion protein (13) were unsuccessful. The use of an immunogen consisting of a CdtB-derived peptide coupled to keyhole limpet hemocyanin eventually yielded MAb 20B2, which bound a 30-kDa antigen expressed by the recombinant E. coli strain DH5α(pJL300) (Fig. 2B, lane 2). MAb 8C9, raised against the His-CdtC fusion protein, bound to a 20-kDa antigen expressed by E. coli DH5α(pJL300) (Fig. 2C, lane 2). None of these MAbs bound any antigens in the E. coli strain containing only the vector pBR322 (Fig. 2, lanes 1).
FIG. 2.
Western blot analysis of the H. ducreyi CdtABC proteins expressed by selected E. coli and H. ducreyi strains. Whole-cell lysates of these strains were probed with the H. ducreyi CdtA-reactive MAb 1G8 (A), the H. ducreyi CdtB-reactive MAb 20B2 (B), and the H. ducreyi CdtC-reactive MAb 8C9 (C). Lanes: 1, E. coli DH5α(pBR322); 2, E. coli DH5α(pJL300); 3, E. coli DH5α(pJL303); 4, E. coli DH5α(pJL303)(pLS88); 5, E. coli DH5α(pJL303)(pJL300-C); 6, wild-type H. ducreyi 35000; 7, H. ducreyi cdtC mutant 35000.303; 8, H. ducreyi 35000.303(pLS88); 9, H. ducreyi 35000.303(pJL300-C). Molecular size markers (in kilodaltons) are listed on the left of each panel.
The CdtA-directed MAb 1G8, the CdtB-directed MAb 20B2, and the CdtC-directed MAb 8C9 were shown to be reactive with the homologous proteins produced by seven different H. ducreyi strains (35000, Hd12, Cha-1, 541, 041, 135, and Hd13) (66) isolated in six different geographic locations (data not shown). This finding reinforced the likelihood that the CdtABC proteins are fairly well conserved among strains of H. ducreyi (13). These three MAbs did not bind to any proteins expressed by H. ducreyi 512, which has previously been shown to lack the cdtABC gene cluster (13).
H. ducreyi CDT readily kills HeLa cells and HaCaT keratinocytes.
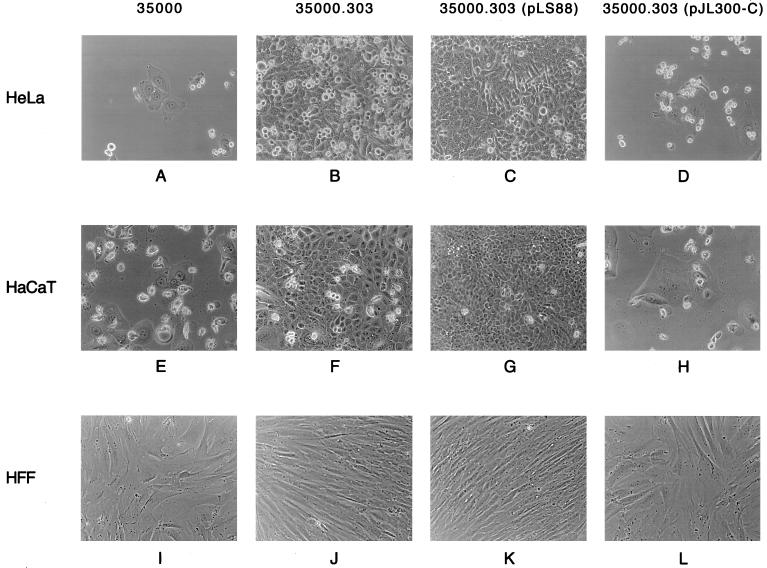
Recombinant H. ducreyi CDT was previously shown to kill HeLa cells in culture (13), and therefore these epithelial cells were included in these experiments as a positive control for cytotoxic activity. Culture supernatant fluid from E. coli DH5α(pJL300) was used as the source of H. ducreyi CDT for cytotoxicity testing that involved two different types of human cells potentially relevant to the infectious process in chancroid: keratinocytes (i.e., the HaCaT cell line) and human foreskin fibroblasts. The recombinant form of H. ducreyi CDT readily killed both HeLa cells (Fig. 3B) and HaCaT keratinocytes (Fig. 3G). Recombinant H. ducreyi CDT had only a modest effect on human foreskin fibroblasts as evidenced by an inhibition of growth (Fig. 3L). Culture supernatant fluid from a recombinant E. coli strain containing only the plasmid vector pBR322 had no effect on any of these three cell lines (Fig. 3A, F, and K).
FIG. 3.
CDT activity in culture supernatant fluids from recombinant strains of E. coli. HeLa cells (A to E), HaCaT keratinocytes (F to J), and human foreskin fibroblasts (HFF) (K to O) were exposed to filter-sterilized E. coli culture supernatant fluids as described in Materials and Methods and were photographed 72 h later (HeLa cells) or 96 h later (HaCaT cells and human foreskin fibroblasts). (A, F, and K) E. coli DH5α(pBR322); (B, G, and L) E. coli DH5α(pJL300); (C, H, and M) E. coli DH5α(pJL303); (D, I, and N) E. coli DH5α(pJL303)(pLS88); (E, J, and O) E. coli DH5α(pJL303)(pJL300-C). Magnification, ×37.
Inactivation of the H. ducreyi cdtC gene in E. coli.
Antibody against the H. ducreyi CdtC protein was previously shown to neutralize the activity of H. ducreyi CDT (13). To confirm the involvement of the H. ducreyi CdtC protein in CDT activity, the cdtC gene within the cloned H. ducreyi cdtABC gene cluster was inactivated by insertional mutagenesis. Specifically, the cdtC gene in pJL300 was disrupted by insertion of a cat cartridge into the BseRI site within this open reading frame, yielding the mutated plasmid pJL303 (Fig. 1). Western blot analysis of whole-cell lysates of the recombinant E. coli strain DH5α(pJL303) indicated that the mutated H. ducreyi cdtABC gene cluster did not express any CdtC protein detectable by Western blot analysis (Fig. 2C, lane 3). E. coli DH5α(pJL303) still expressed both the cdtA gene product (Fig. 2A, lane 3) and the cdtB gene product (Fig. 2B, lane 3), although the level of CdtB appeared to be somewhat reduced.
This insertion of the cat cartridge into the H. ducreyi cdtC gene eliminated the ability of culture supernatant fluid from the recombinant E. coli strain to kill HeLa cells (Fig. 3C) and HaCaT keratinocytes (Fig. 3H). This mutation also eliminated the modest inhibitory effect of the recombinant H. ducreyi CDT on human foreskin fibroblasts (Fig. 3M).
Construction of an isogenic H. ducreyi cdtC mutant.
Plasmid pJL303, containing the mutated cdtC gene, was linearized and used to electroporate the wild-type H. ducreyi strain 35000. A chloramphenicol-resistant transformant, 35000.303, was shown to be unable to express the CdtC protein as determined by Western blot analysis (Fig. 2C, lane 7). In contrast, the CdtC protein expressed by the wild-type parent strain (Fig. 2C, lane 6) was readily detected in this system. This mutant strain also expressed CdtA (Fig. 2A, lane 7) and CdtB (Fig. 2B, lane 7); again, the level of CdtB appeared to be somewhat less than that expressed by the wild-type parent strain (Fig. 2B, lane 6). To eliminate the possibility that an undetected secondary mutation had occurred in or near the cdtABC gene cluster during insertion of the cat cartridge or during allelic exchange, the entire cdtABC gene cluster containing the cat cartridge and immediately flanking DNA was amplified from this mutant strain by PCR and subjected to nucleotide sequence analysis to confirm that there were no other mutations in this DNA region.
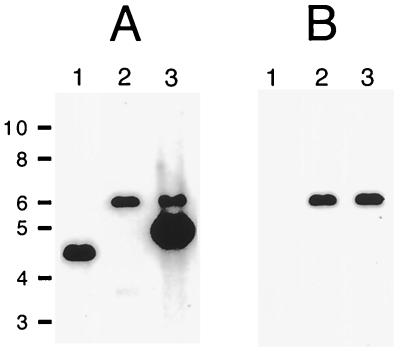
Southern blot analysis was used to confirm that H. ducreyi 35000.303 had only a single cat cartridge insertion in its chromosome. When chromosomal DNA from the wild-type parent strain 35000 was probed with a 269-bp fragment derived by PCR from the H. ducreyi cdtC gene (Fig. 1), a 4.5-kb PstI fragment hybridized to this probe (Fig. 4A, lane 1). This same probe hybridized to a 6-kb PstI fragment of chromosomal DNA from 35000.303 (Fig. 4A, lane 2). This result is consistent with the replacement, in strain 35000.303, of the wild-type cdtC gene with the mutated allele containing the 1.4-kb cat cartridge. When a 331-bp fragment derived from the cat cartridge was used as the probe, a 6-kb PstI fragment from 35000.303 also hybridized to this cat probe (Fig. 4B, lane 2). Chromosomal DNA from the wild-type parent strain 35000 did not hybridize to the cat probe (Fig. 4B, lane 1).
FIG. 4.
Southern blot analysis of chromosomal DNA preparations from H. ducreyi wild-type, mutant, and recombinant strains. Chromosomal DNAs were digested with PstI, resolved by agarose gel electrophoresis, and probed with either a 269-bp PCR product derived from the H. ducreyi cdtC gene (A) or a 331-bp PCR product derived from the cat cartridge (B). Lanes 1, strain 35000; lanes 2, cdtC mutant 35000.303; lanes 3, 35000.303(pJL300-C). Size markers (in kilobases) are on the left.
Culture supernatant fluid from the wild-type H. ducreyi strain 35000 readily killed both HeLa cells (Fig. 5A) and HaCaT keratinocytes (Fig. 5E) and had a modest inhibitory effect on human foreskin fibroblasts (Fig. 5I). In contrast, culture supernatant fluid from the H. ducreyi cdtC mutant 35000.303 did not kill either HeLa cells (Fig. 5B) or HaCaT keratinocytes (Fig. 5F) and also did not inhibit growth of human foreskin fibroblasts (Fig. 5J).
FIG. 5.
CDT activity in culture supernatant fluids from wild-type, mutant, and recombinant strains of H. ducreyi. HeLa cells (A to D), HaCaT keratinocytes (E to H), and human foreskin fibroblasts (HFF) (I to L) were exposed to filter-sterilized H. ducreyi culture supernatant fluids as described in Materials and Methods and were photographed 72 h later (HeLa cells) or 96 h later (HaCaT cells and human foreskin fibroblasts). (A, E, and I) wild-type H. ducreyi 35000; (B, F, and J) H. ducreyi cdtC mutant 35000.303; (C, G, and K) H. ducreyi 35000.303(pLS88); (D, H, and L) H. ducreyi 35000.303(pJL300-C). Magnification, ×42.
Complementation of the cdtC mutant.
Complementation analysis was used to eliminate the possibility that an unlinked secondary mutation was responsible for the lack of cytotoxicity observed with the H. ducreyi cdtC mutant 35000.303. The cdtC open reading frame together with a small amount of flanking DNA was amplified by PCR from the chromosome of H. ducreyi 35000 and cloned into the shuttle vector pLS88 to obtain pJL300-C (Fig. 1). When introduced into the recombinant E. coli strain DH5α(pJL303) containing the mutated cdtC gene, pJL300-C restored both expression of the CdtC protein (Fig. 2C, lane 5) and cytotoxicity of this strain for HeLa cells (Fig. 3E) and HaCaT keratinocytes (Fig. 3J). Culture supernatant fluid from E. coli DH5α(pJL303)(pJL300-C) also inhibited the growth of human foreskin fibroblasts (Fig. 3O). The presence of only the pLS88 vector in this same recombinant E. coli strain [DH5α(pJL303)] (Fig. 2, lanes 4, and Fig. 3D, I, and N) did not affect the phenotype of this strain in the relevant test systems.
When pJL300-C was introduced into the isogenic H. ducreyi cdtC mutant 35000.303 (Fig. 2C, lane 9), the presence of the wild-type H. ducreyi cdtC gene in trans restored expression of the CdtC protein. The presence of the pLS88 vector alone in this mutant had no detectable effect on its inability to express the CdtC protein (Fig. 2C, lane 8). Complementation with pJL300-C restored the ability of this H. ducreyi cdtC mutant to kill both HeLa cells (Fig. 5D) and HaCaT keratinocytes (Fig. 5H) and to inhibit the growth of human foreskin fibroblasts (Fig. 5L). The presence of only the pLS88 vector in this H. ducreyi mutant did not restore cytotoxicity for HeLa cells (Fig. 5C) and HaCaT keratinocytes (Fig. 5G).
Southern blot analysis confirmed that the wild-type cdtC gene in the complemented cdtC mutant was still present in the plasmid (Fig. 4). A chromosomal DNA preparation from 35000.303(pJL300-C) was digested with PstI, which cuts pJL300-C twice, yielding a 4.8-kb fragment containing the cloned H. ducreyi cdtC gene and a 0.8-kb fragment (data not shown). This 4.8-kb plasmid fragment bound the cdtC probe, as did the 6-kb PstI chromosomal fragment containing the mutated cdtC gene (Fig. 4A, lane 3). The cat probe bound only to the 6-kb PstI chromosomal fragment from this complemented mutant strain (Fig. 4B, lane 3).
Effect of the cdtC mutation on virulence of H. ducreyi.
The isogenic cdtC mutant strain 35000.303 was compared to the wild-type parent strain for its ability to produce lesions in the temperature-dependent rabbit model (53). In an experiment involving eight animals, the cdtC mutant proved to be as virulent as the wild-type parent strain with regard to lesion production (Table 2). In addition, in seven of eight animals, viable H. ducreyi cells were isolated from the lesions resulting from the injection of 105 CFU for both of the strains (data not shown). The numbers of CFU of the wild-type strain and the cdtC mutant recovered from the infected animals were equivalent (data not shown). Expression of H. ducreyi CDT in vivo was confirmed by Western blot-based analysis of serum obtained from rabbits that had been infected several times with viable H. ducreyi 35000; this serum contained antibodies directed against the H. ducreyi CdtC protein (data not shown).
TABLE 2.
Lesion formation by the wild-type H. ducreyi strain 35000 and the cdtC mutant strain 35000.303 in the temperature-dependent rabbit modela
| Strain | Inoculum size (CFU) | Mean lesion score (SD) on day:
|
P valueb | ||
|---|---|---|---|---|---|
| 2 | 4 | 7 | |||
| 35000 | 105 | 3.75 (0.46) | 3.88 (0.35) | 3.88 (0.35) | |
| 35000.303 | 105 | 4.00 (0) | 3.88 (0.35) | 3.88 (0.35) | |
| 0.104 | |||||
| 35000 | 104 | 3.00 (0) | 3.63 (0.52) | 3.75 (0.46) | |
| 35000.303 | 104 | 3.13 (0.35) | 3.63 (0.52) | 3.88 (0.35) | |
Eight rabbits were used in this experiment.
P value calculated for the difference between wild-type and test strain lesion scores. P values were calculated by using the lesion scores from both inoculum sizes and from all 3 days.
DISCUSSION
CDT was originally identified by Johnson and Lior (26, 28, 29), in studies of enteric pathogens, as the soluble factor in bacterial culture supernatant fluids which caused the appearance of giant elongated Chinese hamster ovary (CHO) cells after prolonged incubation. Subsequent studies from their and other laboratories revealed that CDT is a novel toxic activity released by some E. coli strains (26, 51, 62) and Shigella isolates (27, 42) and by many Campylobacter species (28, 40, 52). It has now been shown that CDT causes elongation followed by progressive cellular distention and cytotoxicity with certain mammalian cell lines (i.e., CHO, HeLa, HEp-2, and Vero) in vitro (29). Moreover, recent studies using HeLa cells have shown that E. coli CDT blocks the cell cycle at the G2/M transition, apparently by preventing Cdc2 protein kinase dephosphorylation and activation (12, 50, 75). In addition, E. coli CDT affects F-actin assembly by CHO cells in culture (4).
To date, the cdtABC gene clusters from a number of enteric pathogens have been sequenced. These include three E. coli strains (50, 51, 62), a Shigella dysenteriae strain (42), and one strain of Campylobacter jejuni (52, 75). The exact in vivo function of the enteric CDT proteins has not been determined, and a primary role for CDT in the pathogenesis of disease caused by these enteric pathogens has not been established (1). However, in one recent study using a suckling mouse model, recombinant CDT from S. dysenteriae was reported to be diarrheagenic (41).
Despite the recent identification of CDT homologs in numerous different organisms, including, most recently, Actinobacillus actinomycetemcomitans (36, 67), information on the exact nature of the CDT cytotoxic moiety remains limited. It has been reported that both A. actinomycetemcomitans CDT and H. ducreyi CDT, similar to that of E. coli, induce cell cycle arrest in the G2 phase (14, 67). At least in H. ducreyi, CDT activity in culture supernatant fluid was shown to involve a soluble protein with an apparent molecular mass in SDS-PAGE of approximately 20 kDa and an N-terminal amino acid sequence identical to that contained in the proposed mature form of the H. ducreyi CdtC protein (13, 55). Antibody to the H. ducreyi CdtC protein was shown to neutralize CDT activity in H. ducreyi culture supernatant fluid (13), and a similar result was reported recently for polyclonal antibody reactive with the CdtC protein expressed by A. actinomycetemcomitans (67). The last study also indicated that polyclonal antibody to the CdtA protein had a modest blocking effect on CDT activity (67).
It can be inferred from the accumulated data that at least the CdtC protein is involved, directly or indirectly, in the ability of H. ducreyi CDT to kill certain types of human cells. The functions of the CdtA and CdtB proteins remain to be determined, although it appears that all three gene products (i.e., CdtA, CdtB, and CdtC) must be expressed by the pathogen for CDT to be active in culture supernatant fluid (51, 52, 62, 67). All three proteins encoded by the H. ducreyi cdtABC gene cluster appear to have signal peptides, although the existence of a signal peptide has been confirmed only for the CdtC protein of H. ducreyi (13, 55).
Construction of an isogenic H. ducreyi cdtC mutant in the present study allowed us to confirm that CDT was responsible for the observed killing of HeLa cells by cell-free H. ducreyi culture supernatant fluids (13, 56). It had been previously shown that rapid killing of human foreskin fibroblasts by H. ducreyi required contact between the human cells and the bacterium (25). This contact-dependent killing of human foreskin fibroblasts was later shown to be the result of the activity of the HhdA hemolysin of H. ducreyi. Isogenic hemolysin-deficient mutants were unable to kill human foreskin fibroblasts in vitro when cocultivated with these human cells (2, 43) and had a reduced ability to kill human keratinocytes (i.e., HaCaT cells) (43). However, the isogenic hemolysin-deficient mutant still killed HeLa cells (43), a finding which indicated that this hemolysin was not the cytotoxic factor responsible for the killing of HeLa cells by H. ducreyi.
Culture supernatant fluid from the recombinant E. coli strain DH5α(pJL300) containing the intact H. ducreyi cdtABC gene cluster readily killed HaCaT keratinocytes (Fig. 3G). More importantly, culture supernatant fluid from the wild-type H. ducreyi strain killed these keratinocytes (Fig. 5E), whereas culture supernatant fluid from the isogenic H. ducreyi cdtC mutant had no apparent effect on these human cells (Fig. 5F). These results indicate that the soluble H. ducreyi CDT can kill keratinocytes, or at least the HaCaT keratinocyte cell line. It should be noted that the HhdA hemolysin of H. ducreyi also can affect keratinocytes as evidenced by the fact that a mutation which abolished hemolysin expression reduced but did not eliminate the ability of H. ducreyi cells to kill HaCaT cells during cocultivation (43). Moreover, the results of the present study indicate that the reduced killing of keratinocytes obtained with the hemolysin-deficient H. ducreyi mutant (43) was likely the result of CDT activity.
The cumulative results of cytotoxicity testing with culture supernatant fluids from the recombinant E. coli strain containing the H. ducreyi cdtABC gene cluster (Fig. 3L), the recombinant E. coli strain containing the cat cartridge insertion (Fig. 3M), and the wild-type H. ducreyi strain (Fig. 5I) and its isogenic cdtC mutant (Fig. 5J) indicated that H. ducreyi CDT can exert a negative albeit modest effect on human foreskin fibroblasts in culture. This effect was evidenced by an apparent inhibition of growth caused by CDT (Fig. 3L and 5I). Additional experiments in which decreasing numbers of human foreskin fibroblasts were exposed to culture supernatant fluid containing recombinant H. ducreyi CDT reinforced the impression that CDT exerted an inhibitory rather than a cytotoxic effect on these cells (35). These data are not in conflict with the reported inability of hemolysin-deficient H. ducreyi mutants to kill human foreskin fibroblasts in culture (2, 43). The cytotoxicity assays in the latter studies were terminated after only 24 to 30 h of incubation; this period of time is much too short to allow the CDT activity expressed by the hemolysin-deficient H. ducreyi mutant to be detected with human foreskin fibroblasts as the target cell line.
Interestingly, when the H. ducreyi cdtC mutant was tested in the temperature-dependent rabbit model for its ability to produce dermal lesions, this mutant proved to have a level of virulence expression that was not distinguishable from that of its wild-type parent strain. Other isogenic H. ducreyi mutants tested previously in this model, including both a hupA (hgbA) mutant unable to utilize hemoglobin (66) and a gmhA mutant expressing a drastically truncated lipooligosaccharide molecule (5), exhibited a reduced ability to form lesions. In these two instances, a reduction in virulence was correlated with a reduced ability to survive in vivo, as evidenced by the fact that viable mutant H. ducreyi could not be recovered from the lesions of these animals (5, 66). Attempts to recover viable H. ducreyi from lesions resulting from inoculation with the cdtC mutant revealed that this mutant apparently was able to survive in vivo as well as the wild-type parent strain (data not shown). These results suggest that, at least in this rabbit model, the ability of an H. ducreyi mutant to form dermal lesions may be an indication of its ability to survive and replicate in vivo. If this is the case, then elaboration of CDT, which could be involved in the development of ulcers, is not essential to survival of H. ducreyi in this rabbit model.
One limitation of the rabbit model, in contrast to naturally acquired H. ducreyi infection, is that the intradermal method of inoculation bypasses the keratinocytes (against which H. ducreyi CDT is relatively active) and introduces the bacteria into the dermis, where they initially interact with fibroblasts (against which H. ducreyi CDT is not very active). This artificial method of inoculation may protect keratinocytes from the action of CDT. However, H. ducreyi CDT is apparently soluble in vitro, which should allow this diffusible toxin access to the nearby epidermal keratinocytes. This exposure of keratinocytes to H. ducreyi CDT could also be facilitated by the breakdown in the basement membrane that occurs with lesion formation.
It is also possible that lesion development caused by H. ducreyi in rabbits simply may not involve CDT. Efforts to determine the sensitivities of various rabbit cells to H. ducreyi CDT have been initiated in this laboratory. In view of the demonstrated cytotoxic effect of H. ducreyi CDT on several different human cell lines, the possible involvement of this cytotoxin in the development of lesions in the experimental human challenge model will have to be addressed in the future (46, 47, 63, 64).
ACKNOWLEDGMENTS
This study was supported by Public Health Service grant AI32011 to E.J.H. C.K.W. was the recipient of a National Research Service Award (AI09845).
The HaCaT keratinocyte cell line was generously provided by N. E. Fusenig, German Cancer Research Center, Heidelberg, Germany. We thank Bruce A. Green for providing pUCΔECAT. We also thank Michelle Alfa for providing the human foreskin fibroblast cell line and many of the H. ducreyi strains used in this study.
REFERENCES
- 1.Albert M J, Faruque S M, Faruque A S G, Bettelheim K A, Neogi P K B, Bhuiyan N A, Kaper J B. Controlled study of cytolethal distending toxin-producing Escherichia coli infections in Bangladeshi children. J Clin Microbiol. 1996;34:717–719. doi: 10.1128/jcm.34.3.717-719.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfa M J, Degagne P, Totten P A. Haemophilus ducreyi hemolysin acts as a contact cytotoxin and damages human foreskin fibroblasts in cell culture. Infect Immun. 1996;64:2349–2352. doi: 10.1128/iai.64.6.2349-2352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfa M J, Stevens M K, Degagne P, Klesney-Tait J, Radolf J D, Hansen E J. Use of tissue culture and animal models to identify virulence-associated traits of Haemophilus ducreyi. Infect Immun. 1995;63:1754–1761. doi: 10.1128/iai.63.5.1754-1761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aragon V, Chao K, Dreyfus L A. Effect of cytolethal distending toxin on F-actin assembly and cell division in Chinese hamster ovary cells. Infect Immun. 1997;65:3774–3780. doi: 10.1128/iai.65.9.3774-3780.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer B A, Stevens M K, Hansen E J. Involvement of the Haemophilus ducreyi gmhA gene product in lipooligosaccharide expression and virulence. Infect Immun. 1998;66:4290–4298. doi: 10.1128/iai.66.9.4290-4298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boukamp P, Petrussevska R T, Breitkreutz D, Hornung J, Markham A, Fusenig N E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brentjens R J, Ketterer M, Apicella M A, Spinola S M. Fine tangled pili expressed by Haemophilus ducreyi are a novel class of pili. J Bacteriol. 1996;178:808–816. doi: 10.1128/jb.178.3.808-816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brentjens R J, Spinola S M, Campagnari A A. Haemophilus ducreyi adheres to human keratinocytes. Microb Pathog. 1994;16:243–247. doi: 10.1006/mpat.1994.1025. [DOI] [PubMed] [Google Scholar]
- 9.Campagnari A A, Karalus R, Apicella M A, Melaugh W, Lesse A J, Gibson B W. Use of pyocin to select a Haemophilus ducreyi variant defective in lipooligosaccharide biosynthesis. Infect Immun. 1994;62:2379–2386. doi: 10.1128/iai.62.6.2379-2386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campagnari A A, Wild L M, Griffiths G E, Karalus R J, Wirth M A, Spinola S M. Role of lipopolysaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect Immun. 1991;59:2601–2608. doi: 10.1128/iai.59.8.2601-2608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carson S D B, Thomas C E, Elkins C. Cloning and sequencing of a Haemophilus ducreyi fur homolog. Gene. 1996;176:125–129. doi: 10.1016/0378-1119(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 12.Comayras C, Tasca C, Peres S Y, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks HeLa cell cycle at the G2/M transition by preventing Cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson R S, Jr, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes-Bratti X, Chaves-Olarte E, Lagergard T, Thelestam M. The cytolethal distending toxin from chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. J Clin Invest. 1999;103:107–115. doi: 10.1172/JCI3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiCarlo R P, Armentor B S, Martin D H. Chancroid epidemiology in New Orleans men. J Infect Dis. 1995;172:446–452. doi: 10.1093/infdis/172.2.446. [DOI] [PubMed] [Google Scholar]
- 16.Dixon L G, Albritton W L, Willson P J. An analysis of the complete nucleotide sequence of the Haemophilus ducreyi broad-host-range plasmid pLS88. Plasmid. 1994;32:228–232. doi: 10.1006/plas.1994.1060. [DOI] [PubMed] [Google Scholar]
- 17.Elkins C, Chen C-J, Thomas C E. Characterization of the hgbA locus encoding a hemoglobin receptor from Haemophilus ducreyi. Infect Immun. 1995;63:2194–2200. doi: 10.1128/iai.63.6.2194-2200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisk A, Ison C A, Lagergard T. GroEL heat shock protein of Haemophilus ducreyi: association with cell surface and capacity to bind to eukaryotic cells. Infect Immun. 1998;66:1252–1257. doi: 10.1128/iai.66.3.1252-1257.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson B W, Campagnari A A, Melaugh W, Phillips N J, Apicella M A, Grass S, Wang J, Palmer K L, Munson R S., Jr Characterization of a transposon Tn916-generated mutant of Haemophilus ducreyi 35000 defective in lipooligosaccharide biosynthesis. J Bacteriol. 1997;179:5062–5071. doi: 10.1128/jb.179.16.5062-5071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulig P A, Patrick C C, Hermanstorfer L, McCracken G H, Jr, Hansen E J. Conservation of epitopes in the oligosaccharide portion of the lipooligosaccharide of Haemophilus influenzae type b. Infect Immun. 1987;55:513–520. doi: 10.1128/iai.55.3.513-520.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond G W, Lian C J, Wilt J C, Ronald A R. Antimicrobial susceptibility of Haemophilus ducreyi. Antimicrob Agents Chemother. 1978;13:608–612. doi: 10.1128/aac.13.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen E J, Latimer J L, Thomas S E, Helminen M E, Albritton W L, Radolf J D. Use of electroporation to construct isogenic mutants of Haemophilus ducreyi. J Bacteriol. 1992;174:5442–5449. doi: 10.1128/jb.174.16.5442-5449.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobbs M M, Paul T R, Wyrick P B, Kawula T H. Haemophilus ducreyi infection causes basal keratinocyte cytotoxicity and elicits a unique cytokine induction pattern in an in vitro human skin model. Infect Immun. 1998;66:2914–2921. doi: 10.1128/iai.66.6.2914-2921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobbs M M, San Mateo L R, Orndorff P E, Almond G, Kawula T H. Swine model of Haemophilus ducreyi infection. Infect Immun. 1995;63:3094–3100. doi: 10.1128/iai.63.8.3094-3100.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollyer T T, DeGagne P A, Alfa M J. Characterization of the cytopathic effect of Haemophilus ducreyi. Sex Transm Infect. 1994;21:247–257. doi: 10.1097/00007435-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Johnson W M, Lior H. Response of Chinese hamster ovary cells to a cytolethal toxin (CDT) of Escherichia coli and possible misinterpretation as heat-labile (LT) enterotoxin. FEMS Microbiol Lett. 1987;43:19–23. [Google Scholar]
- 27.Johnson W M, Lior H. Production of Shiga toxin and a cytolethal distending toxin (CLDT) by serogroups of Shigella ssp. FEMS Microbiol Lett. 1987;48:235–238. [Google Scholar]
- 28.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb Pathog. 1988;4:115–126. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 29.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microb Pathog. 1988;4:103–113. doi: 10.1016/0882-4010(88)90052-6. [DOI] [PubMed] [Google Scholar]
- 30.Kimura A, Gulig P A, McCracken G H, Jr, Loftus T A, Hansen E J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985;47:253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lagergard T. The role of Haemophilus ducreyi bacteria, cytotoxin, endotoxin and antibodies in animal models for study of chancroid. Microb Pathog. 1992;13:203–217. doi: 10.1016/0882-4010(92)90021-f. [DOI] [PubMed] [Google Scholar]
- 32.Lagergard T, Purven M. Neutralizing antibodies to Haemophilus ducreyi cytotoxin. Infect Immun. 1993;61:1589–1592. doi: 10.1128/iai.61.4.1589-1592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lammel C J, Dekker N P, Palefsky J, Brooks G F. In vitro model of Haemophilus ducreyi adherence to and entry into eukaryotic cells of genital origin. J Infect Dis. 1993;167:642–650. doi: 10.1093/infdis/167.3.642. [DOI] [PubMed] [Google Scholar]
- 34.Langford P R, Kroll J S. Distribution, cloning, characterization and mutagenesis of sodC, the gene encoding copper/zinc superoxide dismutase, a potential determinant of virulence in Haemophilus ducreyi. FEMS Immunol Med Microbiol. 1997;17:235–242. doi: 10.1111/j.1574-695X.1997.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 35.Lumbley S R, Latimer J L, Hansen E J. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Haemophilus ducreyi cytolethal distending toxin kills human keratinocytes but not human foreskin fibroblasts, abstr. B-288; p. 104. [Google Scholar]
- 36.Mayer M P A, Bueno L C, Hansen E J, DiRienzo J M. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:1227–1237. doi: 10.1128/iai.67.3.1227-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melaugh W, Campagnari A A, Gibson B W. The lipooligosaccharides of Haemophilus ducreyi are highly sialylated. J Bacteriol. 1996;178:564–570. doi: 10.1128/jb.178.2.564-570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melaugh W, Phillips N J, Campagnari A A, Tullius M V, Gibson B W. Structure of the major oligosaccharide from the lipooligosaccharide of Haemophilus ducreyi strain 35000 and evidence of additional glycoforms. Biochemistry. 1994;33:13070–13078. doi: 10.1021/bi00248a016. [DOI] [PubMed] [Google Scholar]
- 39.Mertz K J, Weiss J B, Webb R M, Levine W C, Lewis J S, Orle K A, Totten P A, Overbaugh J, Morse S A, Currier M M, Fishbein M, St. Louis M E. An investigation of genital ulcers in Jackson, Mississippi, with use of a multiplex polymerase chain reaction assay: high prevalence of chancroid and human immunodeficiency virus infection. J Infect Dis. 1998;178:1060–1066. doi: 10.1086/515664. [DOI] [PubMed] [Google Scholar]
- 40.Ohya T, Tominaga K, Nakazawa M. Production of cytolethal distending toxin (CLDT) by Campylobacter fetus subsp. fetus isolated from calves. J Vet Med Sci. 1993;55:507–509. doi: 10.1292/jvms.55.507. [DOI] [PubMed] [Google Scholar]
- 41.Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infect Immun. 1997;65:428–433. doi: 10.1128/iai.65.2.428-433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 43.Palmer K L, Goldman W E, Munson R S., Jr An isogenic haemolysin-deficient mutant of Haemophilus ducreyi lacks the ability to produce cytopathic effects on human foreskin fibroblasts. Mol Microbiol. 1996;21:13–19. doi: 10.1046/j.1365-2958.1996.00615.x. [DOI] [PubMed] [Google Scholar]
- 44.Palmer K L, Grass S, Munson R S., Jr Identification of a hemolytic activity elaborated by Haemophilus ducreyi. Infect Immun. 1994;62:3041–3043. doi: 10.1128/iai.62.7.3041-3043.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmer K L, Munson R S., Jr Cloning and characterization of the genes encoding the haemolysin of Haemophilus ducreyi. Mol Microbiol. 1995;18:821–830. doi: 10.1111/j.1365-2958.1995.18050821.x. [DOI] [PubMed] [Google Scholar]
- 46.Palmer K L, Schnizlein-Bick C T, Orazi A, John K, Chen C Y, Hood A F, Spinola S M. The immune response to Haemophilus ducreyi resembles a delayed-type hypersensitivity reaction throughout experimental infection of human subjects. J Infect Dis. 1998;178:1688–1697. doi: 10.1086/314489. [DOI] [PubMed] [Google Scholar]
- 47.Palmer K L, Thornton A C, Fortney K R, Munson R S, Jr, Spinola S M. Evaluation of an isogenic hemolysin-deficient mutant in the human model of Haemophilus ducreyi infection. J Infect Dis. 1998;178:191–199. doi: 10.1086/515617. [DOI] [PubMed] [Google Scholar]
- 48.Parsons L M, Limberger R J, Shayegani M. Alterations in levels of DnaK and GroEL result in diminished survival and adherence of stressed Haemophilus ducreyi. Infect Immun. 1997;65:2413–2419. doi: 10.1128/iai.65.6.2413-2419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parsons L M, Waring A L, Shayegani M. Molecular analysis of the Haemophilus ducreyi groE heat shock operon. Infect Immun. 1992;60:4111–4118. doi: 10.1128/iai.60.10.4111-4118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peres S Y, Marches O, Daigle F, Nougayrede J-P, Herault F, Tasca C, De Rycke J, Oswald E. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 51.Pickett C L, Cottle D L, Pesci E C, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pickett C L, Pesci E C, Cottle D L, Russell G, Erdem A N, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB genes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purcell B K, Richardson J A, Radolf J D, Hansen E J. A temperature-dependent rabbit model for production of dermal lesions by Haemophilus ducreyi. J Infect Dis. 1991;164:359–367. doi: 10.1093/infdis/164.2.359. [DOI] [PubMed] [Google Scholar]
- 54.Purven M, Falsen E, Lagergard T. Cytotoxin production in 100 strains of Haemophilus ducreyi from different geographic locations. FEMS Microbiol Lett. 1995;129:221–224. doi: 10.1111/j.1574-6968.1995.tb07583.x. [DOI] [PubMed] [Google Scholar]
- 55.Purven M, Frisk A, Lonnroth I, Lagergard T. Purification and identification of Haemophilus ducreyi cytotoxin by use of a neutralizing monoclonal antibody. Infect Immun. 1997;65:3496–3499. doi: 10.1128/iai.65.8.3496-3499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Purven M, Lagergard T. Haemophilus ducreyi, a cytotoxin-producing bacterium. Infect Immun. 1992;60:1156–1162. doi: 10.1128/iai.60.3.1156-1162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robertson S M, Frisch C F, Gulig P A, Kettman J R, Johnston K H, Hansen E J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982;36:80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 59.San Mateo L R, Hobbs M M, Kawula T H. Periplasmic copper-zinc superoxide dismutase protects Haemophilus ducreyi from exogenous superoxide. Mol Microbiol. 1998;27:391–404. doi: 10.1046/j.1365-2958.1998.00687.x. [DOI] [PubMed] [Google Scholar]
- 60.San Mateo L R, Toffer K L, Kawula T H. The sodA gene of Haemophilus ducreyi expresses a hydrogen peroxide-inhibitable superoxide dismutase. Gene. 1998;207:251–257. doi: 10.1016/s0378-1119(97)00642-2. [DOI] [PubMed] [Google Scholar]
- 61.Schmid G P, Sanders L L, Blount J H, Alexander E R. Chancroid in the United States: reestablishment of an old disease. JAMA. 1987;258:3265–3268. [PubMed] [Google Scholar]
- 62.Scott D A, Kaper J B. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spinola S M, Orazi A, Arno J N, Fortney K R, Kotylo P, Chen C-Y, Campagnari A A, Hood A F. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J Infect Dis. 1996;173:394–402. doi: 10.1093/infdis/173.2.394. [DOI] [PubMed] [Google Scholar]
- 64.Spinola S M, Wild L M, Apicella M A, Gaspari A A, Campagnari A A. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 65.Stevens M K, Klesney-Tait J, Lumbley S R, Walters K A, Joffe A M, Radolf J D, Hansen E J. Identification of tandem genes involved in lipooligosaccharide expression by Haemophilus ducreyi. Infect Immun. 1997;65:651–660. doi: 10.1128/iai.65.2.651-660.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stevens M K, Porcella S, Klesney-Tait J, Lumbley S, Thomas S E, Norgard M V, Radolf J D, Hansen E J. A hemoglobin-binding outer membrane protein is involved in virulence expression by Haemophilus ducreyi in an animal model. Infect Immun. 1996;64:1724–1735. doi: 10.1128/iai.64.5.1724-1735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sugai M, Kawamoto T, Peres S, Ueno Y, Komatsuzawa H, Fujiwara T, Kurihara H, Suginaka H, Oswald E. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Totten P A, Lara J C, Norn D V, Stamm W E. Haemophilus ducreyi attaches to and invades human epithelial cells in vitro. Infect Immun. 1994;62:5632–5640. doi: 10.1128/iai.62.12.5632-5640.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Totten P A, Morton W R, Knitter G H, Clark A M, Kiviat N B, Stamm W E. A primate model for chancroid. J Infect Dis. 1994;169:1284–1290. doi: 10.1093/infdis/169.6.1284. [DOI] [PubMed] [Google Scholar]
- 70.Totten P A, Norn D V, Stamm W E. Characterization of the hemolytic activity of Haemophilus ducreyi. Infect Immun. 1995;63:4409–4416. doi: 10.1128/iai.63.11.4409-4416.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trees D L, Arko R J, Morse S A. Mouse subcutaneous chamber model for in vivo growth of Haemophilus ducreyi. Microb Pathog. 1991;11:387–390. doi: 10.1016/0882-4010(91)90025-6. [DOI] [PubMed] [Google Scholar]
- 72.Trees D L, Morse S A. Chancroid and Haemophilus ducreyi: an update. Clin Microbiol Rev. 1995;8:357–375. doi: 10.1128/cmr.8.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tullius M V, Munson R S, Jr, Wang J, Gibson B W. Purification, cloning, and expression of a cytidine-5′-monophosphate N-acetylneuraminic acid synthetase from Haemophilus ducreyi. J Biol Chem. 1996;271:15373–15380. doi: 10.1074/jbc.271.26.15373. [DOI] [PubMed] [Google Scholar]
- 74.Ward C K, Lumbley S R, Latimer J L, Cope L D, Hansen E J. Haemophilus ducreyi secretes a filamentous hemagglutinin-like protein. J Bacteriol. 1998;180:6013–6022. doi: 10.1128/jb.180.22.6013-6022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitehouse C A, Balbo P B, Pesci E C, Cottle D L, Mirabito P M, Pickett C L. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]