Introduction
Intake of sufficient concentrations of vitamins is directly related to good health status. The diverse physiological roles of vitamins are generally grouped into water-soluble (or B-vitamins) and fat-soluble vitamins. All B vitamins are essential for cell functions such as glycolysis, gluconeogenesis, fatty acid metabolisms, and amino acids (1). Most vitamins also act as signaling molecules through phosphatases, kinases, or transcription factors (2). Some of the vitamins (e.g., vitamins C, E, and A) have received wide attention in part because of their antioxidant activities via radical scavenging, regulation, and antioxidant enzymes or antioxidant reactive elements mechanisms (3–5). Many effects of vitamins on health are still widely studied, such as the role of vitamins in acute respiratory distress syndrome and their microbiota regulation properties (6). One of the vitamins with newly discovered functions (e.g., a link with fatty liver) is vitamin D, which is one of the four fat-soluble vitamins. It is well characterized by its role in controlling the metabolism of calcium and phosphate. The consequence is healthy mineralization of the bones and regulation of the immune system. The active form of vitamin D (1,25-dihydroxy vitamin D) has been demonstrated in experimental experiments to exhibit immunologic effects on several constituents of the immune system and the integrity of the endothelial membrane. Low blood 25-hydroxyvitamin D levels were associated with an elevated risk of developing some immune-related conditions and diseases, including diabetes, multiple sclerosis, rheumatoid arthritis, respiratory infection, and nonalcoholic fatty liver disease (NAFLD) (7).
The insufficiency of vitamin D frequently coexists with NAFLD, and they share many cardiometabolic risk factors (8). This fact is supported by Liu et al. (9), who showed that a lower amount of vitamin D is an independent risk factor for NAFLD. In addition, the most prevalent long-term liver disease in children is pediatric NAFLD, whose incidence is growing along with increasing rates of obesity and overweight (10). These conditions call for nutritional intervention, as evidenced by a significant improvement in clinical markers of NAFLD in people who received vitamin D supplementation (11, 12). Therefore, the main objective of this article is to analyze the most recent research on the connection between NAFLD and vitamin D in obese children to emphasize the need for early screening for NAFLD in this population.
Vitamin D deficiency and diseases
Vitamin D is a fat-soluble micronutrient that has two equal forms, namely vitamin D2 and vitamin D3, which are biologically inert. Vitamin D2 is obtained from the diet as ergosterol, primarily from mushrooms and fungi, and is then transformed to ergocalciferol by ultraviolet light, while vitamin D3 (cholecalciferol) is endogenously produced in the skin through the effect of UV-B on 7-dehydrocholesterol (13). Once absorbed from the intestine, vitamins D2 and D3 are metabolized in the liver and then form 25-hydroxyvitamin D [25(OH)D], consisting of 25(OH)D2 and 25(OH)D3. Vitamin 25(OH)D (also called calcidiol) is further converted so that a 1,25-dihydroxy vitamin D 1,25(OH)2D is formed. Vitamin D has both skeletal (calcemic) and extra-skeletal (non-calcemic) functions. The skeletal function is the function of vitamin D in terms of maintaining bone mineral homeostasis and bone growth, while the non-calcemic function is its function in modulating the innate and adaptive immune systems (14).
Vitamin D is often associated with several diseases, from infectious diseases to malignancies. Higher serum vitamin D levels are suggested to regulate calcium homeostasis and reduce the risk of obesity, insulin resistance, metabolic syndrome, and malignancy (15). Vitamin D deficiency may be caused by decreased dietary intake or impaired absorption, decreased sun exposure, decreased endogenous synthesis, and end-organ vitamin D resistance due to hereditary issues (16). It has also been demonstrated that personal traits and the environment have an impact on how vitamin D is synthesized in the skin (17). While obesity has been defined as a risk factor for vitamin D deficiency due to metabolic impairment and adiposity status (18), obese children also spend less time playing outside and more time indoors (19), reducing sun exposure related to vitamin D synthesis. Surprisingly, 10.8% of children in South China have vitamin D deficiency, while vitamin D insufficiency reaches 39% (20). The prevalence of hypovitaminosis D in America is 60.4%, with an insufficiency of 44.6% and a deficiency of 15.8% (21). In Indonesia, vitamin D insufficiency (25–49 nmol/L) reaches 45.1%, while the prevalence of insufficiency (50–74 nmol/L) and sufficient (≥75 nmol/L) reach 49.3% and 5.6% (22). In Jakarta, vitamin D insufficiency in elementary school children was 75.9% and vitamin D deficiency was 15%. Vitamin D insufficiency in Manado, North Sulawesi, is 34%, while vitamin D deficiency is 64% in adolescents aged 10–18 years (23).
Non-alcoholic fatty liver disease in obese children
Non-alcoholic fatty liver disease is a long-term liver disease that occurs due to the accumulation of excess fat in the liver organs. Non-alcoholic fatty liver (NAFL), a more benign disease, and non-alcoholic steatohepatitis (NASH), a more severe illness, are both subsets of NAFLD (24). NAFLD in the pediatric population is defined as long-standing liver steatosis in children (≤ 18 years) thatis not caused by metabolic or genetic disorders, malnutrition, infections, ethanol consumption, or the use of steatogenic drugs (25, 26). Steatosis, as a sign of NAFLD, is formed due to the rate of taking fatty acids from the liver from plasma and de novo lipogenesis, which is much higher than the rate of oxidation of fatty acids. Excessive levels of intrahepatic triglycerides mark the presence of an imbalance between metabolic interactions. Steatosis is related to a combination of metabolic disorders of glucose, fatty acids, and lipoproteins. On the other hand, clinical conditions such as obesity, insulin resistance, and type 2 diabetes have all been linked to NAFLD (27). The unusual metabolism of fatty acids combined with elevated adipose tissue and liver-systemic inflammation is the key to the formation of risk factors for the occurrence of NAFLD (28). However, significant pathophysiological interactions between circulating lipids, adiponectin, muscle and liver tissues, pancreas, and gastrointestinal hormones in connection to nutrition, exercise, and inflammation also need to be considered when discussing the pathophysiology of NAFLD (29).
The prevalence of NAFLD in children reaches 7.6% of the general population and 34% in pediatric patients with obesity (30). The prevalence of children diagnosed with NAFLD has increased annually in the last 10 years (31, 32). The incidence of NAFLD incidence among children increased by 62%, that is, from 0.036% in 2009 to 0.0582% in 2018 (31). Obesity itself is closely related and is the major precursor of the appearance of NAFLD. The prevalence of NAFLD prevalence also has a positive relationship with body mass index (BMI). Analysis of liver histology showed that the picture of steatosis in obese patients reached 65−85%. Several other studies that have been conducted have found that a decrease in 25 (OH)D levels in the body increases the occurrence of NAFLD (33–36). Those studies found a negative association between vitamin D status with NAFLD, fibrosis, and NASH in adolescents and children. Furthermore, vitamin D insufficiency was also found to be more common in obese patients than in those of normal weight. Many findings on the prevalence of NAFLD are summarized in Table 1.
Table 1.
Table of the proportion of cases of vitamin D insufficiency or deficiency in obese children with NAFLD and obese children without NAFLD.
| Studies | Total sample |
Number of insufficiency/deficiency cases vitamin D |
||
|---|---|---|---|---|
| NAFLD | Non-NAFLD | NAFLD | Non-NAFLD | |
| Black et al. (37) | 156 | 838 | Insufficient: 79 (50.6%) Deficient: 27 (17.3%) |
Insufficient: 316 (37.7%) Deficient: 93 (11%) |
| Malespin et al. (38) | 12 | 134 | Deficient: 10 (83.3%) | Deficient: 66 (49.2%) |
| Sezer et al. (39) | 58 | 53 | Insufficient: 17 (29.3%) Deficient: 39 (67.2%) |
Insufficient: 13 (24.5%) Deficient: 40 (75.4%) |
| Cho et al. (40) | 215 | 3,663 | Deficient: 183 (85.1%) | Deficient: 2,835 (77.4%) |
Discussion
Lower serum levels of 25(OH)D in obese children are caused by the uptake of 25(OH)D in the body's adipose tissue, causing its bioavailability to decrease. Vitamin D plays an important role in inhibiting the adipogenesis process so that a decrease in its levels in the blood will trigger adipogenesis so that the synthesis process of adipocytes continues. Research shows that NAFLD is more prevalent in obese children. Elizabeth et al. (41) found that the incidence of NAFLD is more common in children with obesity. Anderson et al. (30) showed that the prevalence of NAFLD in healthy children was 7.6% (95%CI; 5.5−10.3%), while in obese children it was 34% (95%CI; 27.8−41.2%) (30). Obesity is a significant factor in the development of hepatosteatosis. Several theories were developed to understand the mechanism of the course of obesity that causes hepatosteatosis. One of the theories stated that an injury to hepatocytes may be caused by oxidative stress and insulin resistance (40). Kitade et al. (27) found that a high accumulation of lipids in the liver activates macrophages and Kupfer cells, causing an increase in insulin resistance, as well as liver inflammation and fibrogenesis.
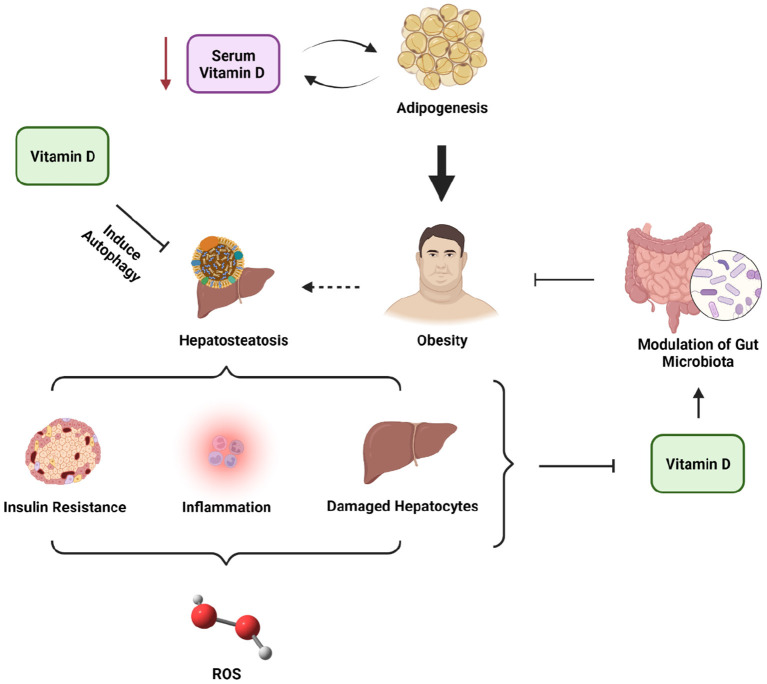
Such a broad role of vitamin D has shown that, in addition to maintaining bone mineral homeostasis, vitamin D also plays an important role in cell proliferation, namely as an anti-inflammatory agent and immunomodulatory. However, the function of vitamin D is not restricted to those functions alone. Vitamin D can also protect insulin sensitivity and secretion. Insufficient vitamin D levels were evidenced to contribute to the development of insulin resistance and NAFLD and worsen NAFLD conditions through activation of TLR (toll-like receptor) due to endotoxin exposure, thus causing liver inflammation and oxidative stress (35, 42, 43) (Figure 1). Vitamin D has been shown to protect against liver steatosis caused by a high-fat diet (HFD) or free fatty acid (FFA) by activating autophagy in mice cells (44). Additionally, vitamin D was also known to modulate the gut microbiota, which may be beneficial in the pathophysiology of NAFLD (45, 46).
Figure 1.
The roles of vitamin D in NAFLD and obesity.
Another mechanism that also causes inflammation in the liver is due to a decrease in 25 (OH)D levels, namely by disrupting tight junctions and damaging the intestinal barrier so that there is a translocation of bacteria and their endotoxins to the portal circulation, which increases inflammation in the liver and triggers hepatocyte apoptosis (47) (Figure 1). Some exposure to the above mechanisms suggests that a reduction in 25(OH)D levels along with obesity increases the risk of NAFLD in children. Due to the “pleiotropic” effects of vitamin D, which include roles in immunomodulation and control of inflammation, vitamin D insufficiency has recently been associated with the etiology and complexity of NAFLD (48). The roles of vitamin D in ameliorating NAFLD and obesity conditions are presented in Figure 1. Interestingly, many studies could not establish a direct relationship between vitamin D levels and the incidence of NAFLD in children and adolescents (37–40), which may be explained due to the observational study designs in each study, especially cohort, cross-sectional, and case-control. Therefore, more solid evidence is needed to determine the direct contributors or mechanisms of NAFLD in children and adolescents.
Future implications
The level of 25(OH)D in obese children with NAFLD is lower than in healthy children and obese children who do not suffer from NAFLD. For clinical purposes, we suggest that it is necessary to detect 25 (OH)D levels in obese children so that early treatment can be performed to prevent NAFLD. More cohort research and clinical trials are needed on vitamin D supplementation in obese children with NAFLD who experience vitamin D insufficiency or deficiency to improve NAFLD while also establishing the direct cause of NAFLD in children and adolescents.
Author contributions
JM, CP, VP, FL, and FN contributed to the conceptualization with the design of the opinion study and drafted the manuscript. AT and FN edited-revised and approved the final version of the submitted manuscript. All authors and contributors contributed to the opinion article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We offer a sincere thank you to the Chairman of the Indonesian Association of Clinical Nutrition Physicians, as well as Professor Nurpudji Astuti Taslim, MD., MPH., PhD., Sp.GK(K) and Professor Hardinsyah, Ph.D. [President of Federation of Asian Nutrition Societies (FANS)] who have reviewed and provided suggestions, as well as input on the draft of this opinion article. We also thank Dr. Nelly Mayulu, MD., who has given her views on the nutrition programs that are important now and in the future. Cheers to William Ben Gunawan for his contribution to the technical assistance and creation of the figure. We also express our gratitude to two institutions, namely Sam Ratulangi University and the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia.
References
- 1.Godoy-Parejo C, Deng C, Zhang Y, Liu W, Chen G. Roles of vitamins in stem cells. Cell Mol Life Sci. (2020) 77:1771–91. 10.1007/s00018-019-03352-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zingg JM. Vitamin E: a role in signal transduction. Annu Rev Nutr. (2015) 17:135–73. 10.1146/annurev-nutr-071714-034347 [DOI] [PubMed] [Google Scholar]
- 3.Farbstein D, Kozak-Blickstein A, Levy AP. Antioxidant vitamins and their use in preventing cardiovascular disease. Molecules. (2010) 15:8098–110. 10.3390/molecules15118098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins MR, Izadi A, Kaviani M. Antioxidants and exercise performance: with a focus on vitamin E and C supplementation. Int J Environ Res Public Health. (2020) 17:8452. 10.3390/ijerph17228452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaner WS, Shmarakov IO, Traber MG. Vitamin A and vitamin E: will the real antioxidant please stand up? Annu Rev Nutr. (2021) 41:105–31. 10.1146/annurev-nutr-082018-124228 [DOI] [PubMed] [Google Scholar]
- 6.Erol N, Saglam L, Saglam YS, Erol HS, Altun S, Aktas MS, et al. The protection potential of antioxidant vitamins against acute respiratory distress syndrome: a rat trial. Inflammation. (2019) 42:1585–94. 10.1007/s10753-019-01020-2 [DOI] [PubMed] [Google Scholar]
- 7.Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. (2020) 12:2097. 10.3390/nu12072097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliades M, Spyrou E. Vitamin D: a new player in non-alcoholic fatty liver disease? World J Gastroenterol. (2015) 21:1718–27. 10.3748/wjg.v21.i6.1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Liu Y, Wan B, Zhang H, Wu S, Zhu Z, et al. Association between vitamin D status and non-alcoholic fatty liver disease: a population-based study. J Nutr Sci Vitaminol. (2019) 65:303–8. 10.3177/jnsv.65.303 [DOI] [PubMed] [Google Scholar]
- 10.Brecelj J, Orel R. Non-alcoholic fatty liver disease in children. Medicina. (2021) 57:719. 10.3390/medicina57070719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakpal M, Satsangi S, Mehta M, Duseja A, Bhadada S, Das A, et al. Vitamin D supplementation in patients with nonalcoholic fatty liver disease: a randomized controlled trial. JGH Open. (2017) 1:62–7. 10.1002/jgh3.12010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabrizi R, Moosazadeh M, Lankarani KB, Akbari M, Heydari ST, Kolahdooz F, et al. The effects of vitamin D supplementation on metabolic profiles and liver function in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr. (2017) 11(Suppl 2):S975–82. 10.1016/j.dsx.2017.07.025 [DOI] [PubMed] [Google Scholar]
- 13.Ahmed LHM, Butler AE, Dargham SR, Latif A, Robay A, Chidiac OM, et al. Association of vitamin D2 and D3 with type 2 diabetes complications. BMC Endocr Disord. (2020) 20:65. 10.1186/s12902-020-00549-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zmijewski MA. Vitamin D and human health. Int J Mol Sci. (2019) 20:145. 10.3390/ijms20010145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodwell VW, Bender DA, Botham KM, Kennelly PJ, Weil PA. Harper's Illustrated Biochemistry, 13th ed. New York, NY: The McGraw-Hill Education; (2015), p. 470–1. [Google Scholar]
- 16.Sizar O, Khare S, Goyal A, Givler A. Vitamin D Deficiency. [Updated 2022 Jul 18]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; (2022). [Google Scholar]
- 17.Cashman KD. Vitamin D deficiency: defining, prevalence, causes, and strategies of addressing. Calcif Tissue Int. (2020) 106:14–29. 10.1007/s00223-019-00559-4 [DOI] [PubMed] [Google Scholar]
- 18.Peterson CA, Belenchia AM. Vitamin D deficiency & childhood obesity: a tale of two epidemics. Mo Med. (2014) 111:49–53. [PMC free article] [PubMed] [Google Scholar]
- 19.Malden S, Gillespie J, Hughes A, Gibson AM, Farooq A, Martin A, et al. Obesity in young children and its relationship with diagnosis of asthma, vitamin D deficiency, iron deficiency, specific allergies and flat-footedness: a systematic review and meta-analysis. Obes Rev. (2021) 22:e13129. 10.1111/obr.13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Y, Ke HJ, Liu Y, Fu M, Ning J Yu L, et al. Prevalence of vitamin D insufficiency among children in southern China: a cross-sectional survey. Medicine. (2018) 97:e11030. 10.1097/MD.0000000000011030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dura-Trave T, Gallinas-Victoriano F, Urretavizcaya-Martinez M, Ahmed-Mohamed L, Malumbres-Chacon M, Moreno-Gonzalez P. Vitamin D deficiency in children. An Pediatr. (2019) 77:1–17. 10.5772/intechopen.89208 [DOI] [Google Scholar]
- 22.Ernawati F, Budiman B. Status vitamin D terkini anak Indonesia usia 2 – 12,9 tahun. Gizi Indon. (2015) 38:73–80. 10.36457/gizindo.v38i1.169 [DOI] [Google Scholar]
- 23.Pangestu YM, Warouw SM, Tatura SNN. Hubungan kadar 25-hydroksivitamin D dan high molecular weight adiponectin pada remaja obes. Sari Pediatri. (2015) 7:64–70. 10.14238/sp17.1.2015.64-70 [DOI] [Google Scholar]
- 24.Pouwels S, Sakran N, Graham Y, Leal A, Pintar T, Yang W, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. (2022) 22:63. 10.1186/s12902-022-00980-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Temple JL, Cordero P, Li J, Nguyen V, Oben JA. A guide to non-alcoholic fatty liver disease in childhood and adolescence. Int J Mol Sci. (2016) 17:947. 10.3390/ijms17060947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vos M, Abrams S, Barlow S, Caprio S, Daniels S, Kohli R, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. (2017) 64:319–34. 10.1097/MPG.0000000000001482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitade H, Chen G, Ni Y, Ota T. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients. (2017) 9:387. 10.3390/nu9040387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrescu M, Vlaicu SI, Ciumărnean L, Milaciu MV, Mărginean C, Florea M, et al. Chronic inflammation – a link between nonalcoholic fatty liver disease (NAFLD) and dysfunctional adipose tissue. Medicina. (2022) 58:641. 10.3390/medicina58050641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petta S, Gastaldelli A, Rebelos E, Bugianesi E, Messa P, Miele L, et al. Pathophysiology of non alcoholic fatty liver disease. Int J Mol Sci. (2016) 17:2082. 10.3390/ijms17122082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and metaanalysis. PLoS ONE. (2015) 10:e0140908. 10.1371/journal.pone.0140908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahota AK, Shapiro WL, Newton KP, Kim ST, Chung J, Schwimmer JB. Incidence of nonalcoholic fatty liver disease in children: 2009–2018. Pediatrics. (2020) 146:e20200771. 10.1542/peds.2020-0771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandes DM, Pantangi V, Azam M, Salomao M, Iuga AC, Lefkowitch JH, et al. Pediatric nonalcoholic fatty liver disease in New York City: an autopsy study. J Pediatr. (2018) 200:174–80. 10.1016/j.jpeds.2018.04.047 [DOI] [PubMed] [Google Scholar]
- 33.Shanshan Z, Yuhui W, Fei L, Jie L, Liangchang X, Jiheng Q, et al. The level of vitamin d in children and adolescents with nonalcoholic fatty liver disease: a meta-analysis. Biomed Res Int. (2019) 2019:7643542. 10.1155/2019/7643542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yodoshi T, Orkin S, Arce-Clachar AC, Bramlage K, Liu C, Fei L, et al. Vitamin D deficiency: prevalence and association with liver disease severity in pediatric nonalcoholic fatty liver disease. Eur J Clin Nutr. (2020) 74:427–35. 10.1038/s41430-019-0493-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed M, Abdel Ghany M, Abdel Hakeem GL, Kamal A, Khattab R, Abdalla A, et al. Assessment of vitamin D status in a group of Egyptian children with non-alcoholic fatty liver disease (multicenter study). Nutr Metab. (2016) 13:53. 10.1186/s12986-016-0112-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang YL, Chen H, Wang CL, Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: from “two hit theory” to “multiple hit model”. World J Gastroenterol. (2018) 24:2974–83. 10.3748/wjg.v24.i27.2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black LJ, Jacoby P, She Ping-Delfos WC, Mori TA, Beilin LJ, Olynyk JK, et al. Low serum 25-hydroxyvitamin D concentrations associate with non-alcoholic fatty liver disease in adolescents independent of adiposity. J Gastroenterol Hepatol. (2014) 29:1215–22. 10.1111/jgh.12541 [DOI] [PubMed] [Google Scholar]
- 38.Malespin M, Sleesman B, Lau A, Wong SS, Cotler SJ. Prevalence and correlates of suspected nonalcoholic fatty liver disease in Chinese American children. J Clin Gastroenterol. (2015) 49:345–9. 10.1097/MCG.0000000000000121 [DOI] [PubMed] [Google Scholar]
- 39.Sezer OB, Bulus S. Hizli NA, Yllmaz D, Ramadan SU. Low 25-hidroksivitaminD level is not an independent risk factor for hepatosteatosis in obese children. J Pediatr Endocrinol Metab. (2016) 29:783–8. 10.1515/jpem-2015-0426 [DOI] [PubMed] [Google Scholar]
- 40.Cho YH, Kim JW, Shim JO, Yang HR, Chang JY, Moon JS, et al. Association between vitamin d deficiency and suspected nonalcoholic fatty liver disease in an adolescent population. Pediatr Gastroenterol Hepatol Nutr. (2019) 22:233–41. 10.5223/pghn.2019.22.3.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elizabeth LY, Shahrokh G, Kathryn EH, Jorge EA, Janis D, Nidhi PG, et al. Prevalence of nonalcoholic fatty liver disease in children with obesity. J Pediatr. (2019) 207:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth CL, Elfers CT, Figlewicz DP, Melhorn SJ, Morton GJ, Hoofnagle A, et al. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and Toll-like receptor activation. Hepatology. (2012) 55:1103–11. 10.1002/hep.24737 [DOI] [PubMed] [Google Scholar]
- 43.Dongiovanni P, Lanti C, Riso P, Valenti L. Nutritional therapy for nonalcoholic fatty liver disease. J Nutr Biochem. (2016) 29:1–11. 10.1016/j.jnutbio.2015.08.024 [DOI] [PubMed] [Google Scholar]
- 44.Li R, Guo E, Yang J, Li A, Yang Y, Liu S, et al. 1,25(OH)2 D3 attenuates hepatic steatosis by inducing autophagy in mice. Obesity. (2017) 25:561–71. 10.1002/oby.21757 [DOI] [PubMed] [Google Scholar]
- 45.Waterhouse M, Hope B, Krause L, Morrison M, Protani MM, Zakrzewski M, et al. Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur J Nutr. (2019) 58:2895–910. 10.1007/s00394-018-1842-7 [DOI] [PubMed] [Google Scholar]
- 46.Singh P, Rawat A, Alwakeel M, Sharif E, Al Khodor S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci Rep. (2020) 10:21641. 10.1038/s41598-020-77806-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mangin M, Sinha R, Fincher K. Inflammation and vitamin D: the infection connection. Inflamm Res. (2014) 63:803–19. 10.1007/s00011-014-0755-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pacifico L, Osborn JF, Bonci E, Pierimarchi P, Chiesa C. Association between vitamin D levels and nonalcoholic fatty liver disease: potential confounding variables. Mini Rev Med Chem. (2019) 19:310–32. 10.2174/1389557518666181025153712 [DOI] [PubMed] [Google Scholar]