Abstract
There is policy impetus for provision of self-care support (SCS) for children with long-term conditions (LTCs). However, it is not clear what SCS should consist of and how it can be delivered in routine care. This review aimed to synthesise the literature, specifically on SCS of diet and the gut as these components are essential for optimal growth and development and enhanced quality of life. Using an integrative review methodology, studies conducted between January 1990 and July 2020 were systematically identified and methodological quality assessed using the Mixed Methods Appraisal Tool. Twenty-five studies were included. SCS of diet and the gut consisted of support in developing and applying specific knowledge and skills and practical help with incorporating the demands of self-care into everyday life. Key requisites for models of SCS in the context of delivery and uptake in routine care were starting early, keeping it going, being flexible and choosing appropriate outcomes. This review contributes new understanding on the provision of SCS of diet and the gut for school-age children with LTCs, including identification of gaps in the literature and further research needs.
Keywords: Child, diet, gastrointestinal, self-care, systematic review
Introduction
Enabling school-age children with long-term conditions (LTCs) to take an active role in their care is essential if the development of life-long self-care skills, attitudes and behaviours is to become a normal, expected aspect of routine care (Fung, 2020; Taylor et al., 2014).
Self-care may be defined as the broad range of activities carried out to live well with a LTC (Kirk et al., 2010). The term ‘self-care’ is often used interchangeably with ‘self-management’; however, self-management more narrowly relates to managing the LTC well (Pelicand et al., 2013). Self-care incorporates self-management and also health promotion (Bee et al., 2018). This broad perspective is particularly appropriate in the care of children with LTCs, for whom developmental, psychosocial and healthcare needs are closely intertwined (Aujoulat et al., 2006) and where anticipatory guidance may pre-empt some of the challenges faced.
Whilst the burden of performing daily self-care rests with patients and their families, healthcare professionals (HCPs) can help to enable self-care. There is policy impetus to support the self-care of children with LTCs in the United Kingdom (Department of Health and Department for Education and Skills, 2004; NHS England, 2014, 2019). However, it is not clear what self-care support (SCS) should consist of and how it can be delivered as part of routine care. This uncertainty is important to address as laying a solid foundation for self-care in childhood may better prepare children for living well over their life course, to navigate challenges during adolescence (Christian and D’Auria, 2006) and to improve outcomes in adulthood (Culhane et al., 2013).
In LTCs with a diet and/or gastrointestinal (GI)-related component of care, a specific focus on SCS of these components is warranted. This is because self-care of diet and/or the gut is essential for controlling symptoms and optimising children’s growth and development, which, in turn, may enhance quality of life (Shoff et al., 2013; Spinks and Guest, 2017; Rocha et al., 2013; Wagner et al., 2008). Although diet and the gut are central to care in cystic fibrosis (CF) (Turck et al., 2016), studies on SCS in CF are limited. This review therefore sought to learn from a broad range of other childhood-onset LTCs with a diet and/or GI component, such as type 1 diabetes (T1DM), as a first step in developing a model for SCS of diet and the gut in CF.
Two systematic reviews on SCS for children with LTCs have been conducted (Bee et al., 2018; Kirk et al., 2013). Both provide some evidence to inform the development of a model for SCS. For example, SCS interventions that directly targeted children were effective, particularly in improving psychosocial well-being (Kirk et al., 2013). However, in both systematic reviews, the included studies predominantly focused on children with asthma and therefore did not consider self-care of diet and/or the gut. In addition, the findings of both are limited with respect to informing how SCS can be delivered as part of routine care. Further evidence synthesis is therefore required, of both qualitative and quantitative studies, to move beyond examining effectiveness and obtain a comprehensive understanding of context (Pawson et al., 2005).
For the current review, an integrative review methodology was chosen to systematically capture breadth and depth through integrating findings from qualitative, quantitative and mixed methods studies in a single synthesis (Leeman et al., 2015). The aim of this review was to identify, critically appraise and synthesise evidence from primary studies on SCS of diet and the gut in school-age children with LTCs, to obtain a comprehensive understanding of what is already known, what the gaps are and what new knowledge is needed.
Two review questions were addressed:
• What is SCS of diet and the gut for school-age children with LTCs?
• What models of SCS have worked, when and how, in the routine care of school-age children with LTCs (including enablers for and barriers to, delivery and uptake)?
Methods
This review was conducted in accordance with the methodological guidelines of Whittemore and Knafl (2005). A protocol was developed and registered with PROSPERO (CRD42019144941) (Cave et al., 2019). The reporting of the review followed the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) guidance (Moher et al., 2009).
Inclusion criteria
This review considered studies (i) that included children of school-age (4–16 years) with any physical LTC with a diet and/or GI-related component of care or the following exemplar LTCs: CF, T1DM, coeliac disease, phenylketonuria or inflammatory bowel disease; (ii) in which children were actively involved in self-care of diet and/or the gut having received some type of SCS and/or (iii) investigated enablers for and barriers to, delivery and uptake of SCS. All types of studies were considered: qualitative, quantitative and mixed methods. The inclusion and exclusion criteria for the review are detailed in Supplementary Material 1.
Search strategy
The search strategy aimed to find both published literature and grey literature. A comprehensive search of electronic databases was conducted, including CINAHL, Medline, Embase, PsycINFO, Scopus, Web of Science and the Cochrane Library. Additional information sources for grey literature and unpublished studies included OpenGrey, the ISRCTN registry and ClinicalTrials.gov.
The search dates in each database were from January 1990 to July 2020. This date range reflects the development of policy and research in self-care/self-management of LTCs since the 1990s. The search strategy was modified for each database, included use of database-specific subject headings, free-text terms and variations relating to diet/gut self-care, children and LTCs. An example of the search strategy is included in Supplementary Material 2.
Additional search strategies included citation searching, targeted author searches and hand searching reference lists of included studies, review articles and key journals. Searches were limited to studies published in English.
Study selection
Following the search, all potentially eligible studies were collated and duplicates removed. A two-stage screening process was adopted. Stage one involved the screening of titles and abstracts. Studies that met the inclusion criteria were taken forward to stage two which involved full-text screening of the studies against the inclusion criteria. Where required, companion papers were sought or authors emailed to request missing or additional information (Hong et al., 2018). Both stages were led by LC, independently checked by LM and GM and consensus reached by discussion.
Data extraction
Data were extracted from the eligible studies using a pre-piloted data extraction table. The data extracted included research aim, study design and methods, participants, intervention/exposure to SCS, setting, key findings and supporting data for quality appraisal. Data were extracted by LC and 10% of the full extraction checked for accuracy and completeness by LM and GM. Any discrepancies that arose between the reviewers were resolved through discussion.
Quality appraisal
The methodological quality of included studies was critically appraised using the Mixed Methods Appraisal Tool (MMAT) (version 2018) (Hong et al., 2018). MMAT was chosen as it is a validated tool, has comprehensive guidelines and allowed concomitant appraisal of core methodological criteria for the included study designs. Quality appraisal was conducted by LC and 10% of the full assessment independently checked by LM and GM. Any discrepancies that arose between the reviewers were resolved through discussion.
Data analysis and synthesis
Due to the heterogeneity across included studies, studies could not be combined statistically, and a narrative analysis and synthesis were therefore conducted. Data extracted from quantitative studies were converted into textual descriptions to facilitate integration with data from qualitative studies. Assembled data were then systematically coded, organised into categories and iteratively compared across the studies to begin identifying patterns, variations and relationships (Whittemore and Knafl, 2005). LC conducted the analysis and developed a set of integrated findings in the form of themes. Themes were verified following discussion with all authors and presented as a narrative summary.
Results
Study inclusion
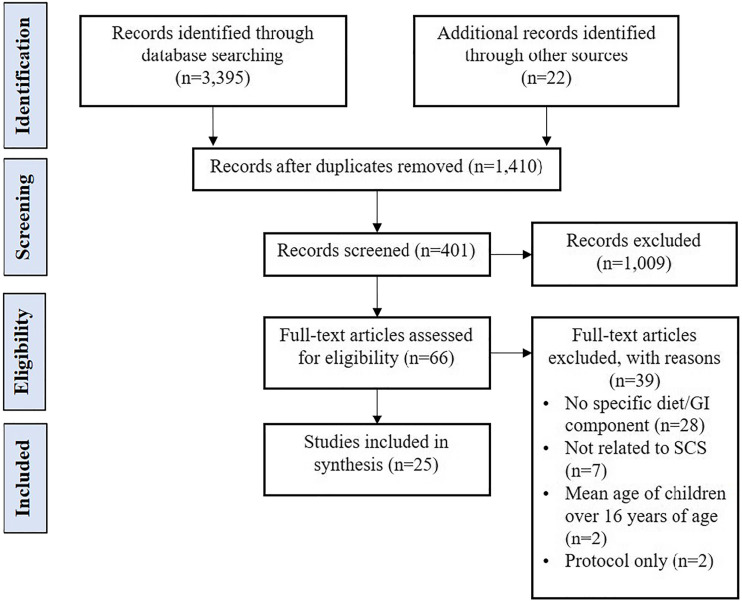
The search strategy identified 3417 records, summarised using a PRISMA flow diagram (Figure 1) (Moher et al., 2009). A total of 27 articles reporting on 25 studies met the review inclusion criteria.
Figure 1.
Selection process: PRISMA flow diagram (Moher et al., 2009).
Description of the included studies
Characteristics of the included studies are summarised in Supplementary Material 3. Overall, studies included children and adolescents aged 2–19 years, predominantly with T1DM (n = 12, 48%) and CF (n = 9, 36%), followed by phenylketonuria (n = 2, 8%), coeliac disease (n = 1, 4%) and concurrent coeliac disease and T1DM (n = 1, 4%). No studies that included children and adolescents with inflammatory bowel disease met the inclusion criteria. Of the 25 studies, 18 were quantitative, five mixed methods and two qualitative designs; 12 were conducted in Europe, 11 in North America, with the remainder conducted in Australia and Brazil.
The majority of studies (n = 19, 76%) provided models of SCS, whilst other studies (n = 6, 24%) informed the context of SCS. Several studies had limited detail about the diet/GI-related component of care (Cooper et al., 2018; Cottrell et al., 1996; Fiallo-Scharer et al., 2019; Nabors et al., 2014), the dietary self-care programme (Austin et al., 2011, 2013) or routine support (Rankin et al., 2018a). However, they were included, as they were potentially relevant in answering the review questions.
Quality appraisal
Only one study had insufficient information for appraisal using MMAT (Bell, 2004). Overall, whilst the majority of studies appraised had some limitations, they generally rated fair to good. No studies were excluded based on these findings though this was taken into consideration in the synthesis, in judging the strength of evidence for developing themes. MMAT findings are presented in Supplementary Material 4.
Findings of the review
Six themes were identified through synthesis of the included studies. Two themes related to the first review question: (i) support in developing and applying specific knowledge and skills and (ii) practical help with incorporating the demands of self-care into everyday life. Four themes related to the second review question: (i) starting early, (ii) keeping it going, (iii) being flexible and (iv) choosing appropriate outcomes. Themes are described in the following narrative.
First review question: What is SCS of diet and the gut for school-age children with LTCs?
Support in developing and applying specific knowledge and skills
There was consistency across the studies in supporting children and adolescents’ capability to self-care, beginning with development of essential, through to more advanced, knowledge and skills, with repeated opportunities to practice and develop confidence in their application.
A common starting point for school-age children in many of the studies was knowing how to identify which foods contained fat, carbohydrate (CHO), protein or gluten, as this set the foundation for selecting foods to eat, or restrict or avoid completely, as appropriate to the LTC (Boon et al., 2020; Christie et al., 2016; Fishman et al., 2018; Singh et al., 2000; Sparapani et al., 2017). Further knowledge was required to estimate how much fat, CHO, phenylalanine or protein was contained in foods (Coates et al., 2013; Witalis et al., 2017), possibly through developing the skill of reading food labels (Bell, 2004; Culhane, 2013; Rankin et al., 2018a). However, as this skill also relied on knowing how to estimate portion sizes, Spiegel et al. (2012) encouraged dietitians to support children and adolescents to repeatedly practice estimating portion sizes using real food and food models, alongside measuring actual portions. An alternative approach, in which adolescents with T1DM took photos of their own foods using an app (Frøisland and Årsand, 2015), had the advantage of facilitating estimation of both CHO content and portion sizes, and with further development, the app may be a useful tool for SCS.
More advanced knowledge was required to begin making sense of the complex relationships between CHOs, blood glucose and insulin (Coates et al., 2013; Christie et al., 2016; Frøisland and Årsand, 2015); fats, absorption and enzymes (pancreatic enzyme replacement therapy (PERT)) (Boon et al., 2020) and food containing gluten and absorption (Connan et al., 2019). In several studies, promoting a visual understanding of these relationships through use of an app (Frøisland and Årsand, 2015), an interactive e-learning module (Connan et al., 2019) or a video game (Sparapani et al., 2017), helped children and adolescents to make sense of what was happening inside their bodies as a result of coeliac disease and T1DM.
In many of the studies, the advanced skill of self-monitoring enabled recognition and management of GI symptoms (Bell, 2004; Cottrell et al., 1996; Culhane, 2013; Stapleton, 2001), hypo and hyperglycaemia (Nabors et al., 2014) and also tracking adherence to daily goals (Stapleton, 2001). Some studies combined building knowledge on how PERT or insulin works, with skills on administering and understanding what happens if too little or too much is taken (Bell, 2004; Davis et al., 2004). Further to this, studies focused on the advanced skill of titrating the dose of PERT to fat intake (Bell, 2004; Owen et al., 2013; Revert et al., 2018) and the dose of insulin to CHO (Christie et al., 2016; Coates et al., 2013; Price et al., 2016). Only one study (Rankin et al., 2018a), together with a companion study to Coates et al. (2013) (Chaney et al., 2010), highlighted poor mathematical comprehension as a barrier to performing this complex self-care task. Children adopted strategies to limit the need for complex maths skills such as choosing foods with CHO values they could remember or using mobile phones to contact their parents about CHO contents (thus remaining reliant on their parents) (Rankin et al., 2018a). In the trial of an app for CF, the enzyme dose calculation was the most used function by both children and their parents (Boon et al., 2020). This highlights numeracy as a vital core skill for self-care of diet and the gut in T1DM and CF.
Practical help with incorporating the demands of self-care into everyday life
Across many of the studies, SCS consisted of practical help for children and adolescents to have sustained opportunities and motivation to perform daily self-care.
In several studies, performing daily self-care relied on the creation of supportive physical environments, in which there was availability of planned foods for children with CF (Stapleton, 2001) and low protein foods for adolescents with phenylketonuria (Singh et al., 2000).
Children and adolescents’ ability to perform self-care also varied with the support received from HCPs, parents and friends (Austin et al., 2013; Boon et al., 2020; Frøisland and Årsand, 2015; Kyngäs et al., 1998; Rankin et al., 2018b; Revert et al., 2018; Singh et al., 2000; Spiegel et al., 2012; Stapleton, 2001; Witalis et al., 2017). Adolescents who felt their HCPs understood their dietary self-care challenges, accepted them as they were and provided them with choices were more motivated toward dietary self-care (Austin et al., 2013). Equally, children and adolescents valued HCPs who gave tailored advice and timely feedback (Boon et al., 2020; Frøisland and Årsand, 2015), particularly where this was simple practical advice relevant to their immediate situation and they could take action (Frøisland and Årsand, 2015). Kyngäs et al. (1998) highlighted the need for HCPs to ensure consultations are not dominated by disease-monitoring activities such as blood tests, to permit time for discussion of perceived barriers to self-care. In other studies, this extended to discussing other factors that may affect motivation to self-care, such as attitudes and beliefs in adolescents with phenylketonuria (Singh et al., 2000), goal setting in children with T1DM (Nabors et al., 2014) and emotions around food intake in children with T1DM (Sparapani et al., 2017).
Across several studies, HCPs provided practical support to parents in positively accepting their child’s growing independence (Witalis et al., 2017). They facilitated a balance of parents not exerting too much control (Austin et al., 2011; Kyngäs et al., 1998) or having too little involvement, as adolescents with T1DM who collaborated more with their parents had better metabolic control (Spiegel et al., 2012) and the value of an app to adolescents with T1DM was greater with parental support (Cooper et al., 2018). In one study, Rankin et al. (2018b) suggested HCPs could assist small friendship groups, to enable close friends of children with T1DM to provide support at school in the form of monitoring and prompting self-care tasks; furthermore, they suggested that raising awareness among school peers may help to normalise performance of self-care throughout the school day.
Second review question: What models of SCS have worked, when and how, in the routine care of school-age children with LTCs (including enablers for and barriers to, delivery and uptake)
Four themes related to the second review question: (i) starting early, (ii) keeping it going, (iii) being flexible and (iv) choosing appropriate outcomes.
Starting early
Across the included studies, models of SCS were more successful when started early in the disease course or in early childhood (as appropriate to the LTC).
Starting SCS early on in the disease course (Boon et al., 2020; Revert et al., 2018; Stapleton, 2001) negated having to change established behaviours and reverse poor metabolic control. Significant challenges were encountered where there was a wide variation in how long study participants had been diagnosed with their LTC or had been performing dietary self-care. For example, between one and 17 years since diagnosis for participants with T1DM (Coates et al., 2013), between one and 11.7 years since starting a gluten-free diet (Connan et al., 2019) and between 6 months and 9 years already counting CHOs (Spiegel et al., 2012). In the study by Christie et al. (2016), participants with the highest HbA1c (poorer metabolic control) were less likely to attend group education sessions; in addition, significantly more children (8–12 years) attended, compared with teenagers (13–16 years) (n = 62, 64% vs. n = 42, 44%). This finding was consistent across several studies, where children in the younger age groups were the more receptive and keener to learn (Bell, 2004; Culhane, 2013; Fishman et al., 2018; Owen et al., 2013).
Keeping it going
Across the included studies, models of SCS were more successful when the intervention or exposure to SCS was of longer duration. For example, between six and 12 months (Boon et al., 2020; Cooper et al., 2018; Fiallo-Scharer et al., 2019; Owen et al., 2013) to over 3 years (Revert et al., 2018). Provision of ongoing input, with regular reiteration of topics to reinforce knowledge and skills (Owen et al., 2013), correct misconceptions, misinformation or fill gaps (Culhane, 2013), together with further top-ups, enabled tailoring of SCS to meet specific and changing needs of children over time. This was not possible where SCS was delivered, for example, as an intensive 5-day block (Nabors et al., 2014; Price et al., 2016; Singh et al., 2000) or as a very brief intervention, for example, two six-hour group sessions (Cottrell et al., 1996) or viewing a CD-ROM for approximately 30 min (Davis et al., 2004).
Whilst provision of regular ongoing SCS may enable behaviours leading to the formation of self-care habits and routines, this was poorly addressed in the included studies.
Being flexible
Models of SCS across the included studies employed various modes of delivery to accommodate differing needs and preferences. Study participants engaged well when SCS utilised a range of interactive (rather than passive) learning (Bell, 2004; Boon et al., 2020; Connan et al., 2019; Owen et al., 2013; Price et al., 2016; Spiegel et al., 2012; Stapleton, 2001). For example, adolescents with T1DM practised CHO counting in practical cookery sessions (Price et al., 2016) and children with CF learnt the fat content of foods though doing hands-on labelling activities and matching pair games (Owen et al., 2013).
SCS interventions that were integrated into routine clinic visits (Bell, 2004; Culhane, 2013; Fiallo-Scharer et al., 2019; Revert et al., 2018), either in a group-based format or on an individual basis, were not without challenges, chiefly due to time constraints in busy clinics. However, further challenges were encountered when SCS was delivered as an optional extra. For example, in groups in the clinic setting but independent to regular outpatient clinic (Christie et al., 2016; Coates et al., 2013), HCPs were trying to organise and deliver sessions in addition to their usual workload, often following little or no training; children and families also had competing demands, for example, school and work commitments. In a 10-week home-based programme, though carers enjoyed helping their child learn and learning themselves, some carers reported being too busy to easily fit in daily recording and weekly paper-based exercises with their child (Stapleton, 2001). Further to this, given the choice of completing the ADNAT app at home or in clinic, the majority of adolescents chose clinic (Cooper et al., 2018) (though this relied on having access to Wi-Fi in clinics) and for individual sessions with a dietitian, incorporating these as part of outpatient clinic visits was preferred over separate home visits (Owen et al., 2013).
Across the included studies, it was clear that integrating SCS into routine care required organisational commitment, with prioritisation and active support of HCPs at a service level (Christie et al., 2016; Cooper et al., 2018; Revert et al., 2018).
Choosing appropriate outcomes
Evaluating success of the models of SCS relied on the choice of appropriate outcomes. Many of the included studies chose outcomes commonly used in clinical practice. For example, HbA1c as a measure of glycaemic control (Christie et al., 2016; Coates et al., 2013; Cooper et al., 2018; Fiallo-Scharer et al., 2019; Frøisland and Årsand, 2015; Price et al., 2016; Spiegel et al., 2012) and weight or BMI z-score as a measure of nutritional status (Boon et al., 2020; Coates et al., 2013; Cottrell et al., 1996; Owen et al., 2013; Revert et al., 2018; Stark et al., 2009). However, such outcomes may not be sensitive enough to detect clinically meaningful change or sustained behaviour change over the short duration of SCS interventions observed in the majority of included studies. Perhaps surprisingly (for studies related to SCS of diet and the gut), few included patient-reported outcomes, such as control of symptoms or quality of life (the exceptions being Christie et al., 2016; Cottrell et al., 1996; Fiallo-Scharer et al., 2019; Price et al., 2016), and only one study focused on GI-related quality of life (Boon et al., 2020).
The limited choice of outcomes in the included studies may, in part, reflect how the majority of SCS interventions lacked a theoretical basis to their development. Only four of the 19 intervention studies reported using an underlying theory or model of behaviour change (Cooper et al., 2018; Price et al., 2016; Singh et al., 2000; Stapleton, 2001). However, more encouragingly, eight of the 19 intervention studies (Boon et al., 2020; Christie et al., 2016; Coates et al., 2013; Connan et al., 2019; Cooper et al., 2018; Fiallo-Scharer et al., 2019; Price et al., 2016; Stapleton, 2001) reported involvement of patients and families in their development.
Discussion
The aim of this integrative review was to identify, critically appraise and synthesise evidence from primary studies on SCS of diet and the gut in school-age children with LTCs. Synthesis of the 25 eligible studies identified six themes that collectively contribute new understanding of what constitutes SCS of diet and the gut, together with key requisites for models of SCS in the context of delivery and uptake in routine care.
SCS of diet and the gut throughout the school-age years was found to be complex and dynamic, yet on a continuum as the child grows. It included supporting stepwise development and application of a specific knowledge and skill set. This may be facilitated by the use of age/developmental stage competency checklists (Bell, 2004; Culhane, 2013; Fishman et al., 2018), such as those currently used in UK practice with children and adolescents with T1DM (Thornton et al., 2016) and in the USA with children and adolescents with CF (CF R.I.S.E, 2016). Particular emphasis is needed on numeracy skills, with a means of assessment and tailored support as appropriate (Moosa and Segal, 2011; Mulvaney et al., 2013). Visual resources may also be required. However, as images can be interpreted in many ways, involving children in the design and selection of images is essential to ensure images are meaningful to them and evoke positive emotional responses (Houts et al., 2006). Further studies are also needed combining visual resources with hands-on practical experience, as this may have more impact than visualisation alone (Evans et al., 2009).
SCS of diet and the gut also included providing practical help with incorporating the demands of self-care into everyday life. This encompassed attention to the fine detail, to enable both proactive and responsive tailoring of support. Further work is needed to identify how this collaborative approach can be implemented, for example, regarding expectations and roles of children, families and HCPs (Smith and Kendal, 2018). The included studies did not address the development of routines and habits in sustaining daily self-care. However, when treatment burden is high, routine is key to motivation (Calthorpe et al., 2020). More research is needed on how best to support habit formation throughout childhood and whether self-care behaviours established during childhood can be maintained despite challenges (such as lack of time and competing demands) during adolescence and through to adulthood (Hoo et al., 2019; Lally and Gardner, 2013).
In the included studies, models of SCS were more successful when started early on in the disease course and were ongoing, to allow tailoring to changing needs and priorities over time. In addition, to remain relevant, models need to adapt to advances in treatments and technologies. For example, in the new era of modulator therapies in CF, increased fat absorption and decreased gut inflammation may contribute to weight gain (Stallings et al., 2018), prompting greater emphasis on diet quality (Sutherland et al., 2018) and a more individualised approach to diet and PERT (McDonald et al., 2020).
Implementation of SCS was more successful when SCS interventions were embedded within routine care, rather than being an optional extra. However, there was no one size fits all; flexibility is needed in terms of what SCS interventions and activities can be accessed (such as interactive e-learning, mobile apps (Day, 2020), hands-on activities and learning through fun play sessions (La Banca et al., 2020)) to meet individual preferences and target specific needs at any one time. This complex picture was further compounded by the need for a whole system approach, where there is strong leadership and organisational support for HCPs to implement SCS in routine care, as reported previously (Taylor et al., 2014). To enable this, further studies are needed to identify training and supervision needs of HCPs and how system constraints such as limited consultation times and workload pressures can be adjusted (Eaton et al., 2015).
Outcomes of the included studies reflected more of a focus on self-management than self-care, that is, taking care of one’s own condition rather than taking care of oneself (Pelicand et al., 2013). Further work is needed to develop models of SCS that more accurately reflect the broad range of activities involved in self-care, identify outcomes that capture that breadth (Feillet et al., 2010) as well as the shorter-, medium- and longer-term effects of SCS. To facilitate this, models of SCS need to be theoretically informed (Kirk et al., 2013) and co-developed with patients and their families, as highlighted previously (Bee et al., 2018; Kirk et al., 2013). Furthermore, outcomes of SCS of diet and the gut of most value to patients and their families need to be explored (De Wit et al., 2020; Kirk et al., 2013; Ye et al., 2017).
Implications for practice
The findings of this review suggest several implications for practice. Enabling children to gradually take an active role from early on in their care, requires a more child-centred focus (Coyne et al., 2016). HCPs need to support children to have time, space and repeated opportunities to apply their developing knowledge and skills, alongside helping to address factors that may affect their motivation to self-care. A greater emphasis on the health promotion aspect of self-care, adopting a more proactive, less reactive approach, will also require more time and potentially additional training and support for HCPs. Investment in SCS for the long term within organisations will therefore need the support of policy makers.
Strengths and limitations of this review
In this review, varied data sources were used to identify primary research of all study designs, in both published and grey literature. Whilst this allowed exploration of multiple aspects of SCS of diet and the gut, analysis and synthesis of data from such a diverse range of studies are complex and can introduce bias and inaccuracy (Whittemore and Knafl, 2005). To combat this, a systematic and rigorous approach was adopted, with involvement of multiple authors at each stage. Most studies were conducted in high-income countries; however, the results may also be relevant in low- and middle-income countries. The methodological quality of some of the included studies (as presented in Supplementary Material 4) is a limitation, as is the inclusion of only English language studies, as some relevant non-English studies may have been omitted.
Conclusion
Findings from this review suggest what SCS of diet and the gut for school-age children with LTCs consists of and indicate key requirements for models of SCS to work in the context of routine care. As such, the findings form a foundation for further work. In particular, more research is needed directly with children, their families and HCPs to advance understanding of their needs and preferences for SCS and inform development of a theory- and evidence-based model for SCS of diet and the gut.
Supplemental Material
Supplemental Material, sj-pdf-1-chc-10.1177_13674935211029124 for Self-care support of diet and the gut in the routine care of school-age children with long-term conditions: An integrative review by Laurie Cave, Linda J Milnes and Gretl A McHugh in Journal of Child Health Care
Supplemental Material, sj-pdf-2-chc-10.1177_13674935211029124 for Self-care support of diet and the gut in the routine care of school-age children with long-term conditions: An integrative review by Laurie Cave, Linda J Milnes and Gretl A McHugh in Journal of Child Health Care
Supplemental Material, sj-pdf-3-chc-10.1177_13674935211029124 for Self-care support of diet and the gut in the routine care of school-age children with long-term conditions: An integrative review by Laurie Cave, Linda J Milnes and Gretl A McHugh in Journal of Child Health Care
Supplemental Material, sj-pdf-4-chc-10.1177_13674935211029124 for Self-care support of diet and the gut in the routine care of school-age children with long-term conditions: An integrative review by Laurie Cave, Linda J Milnes and Gretl A McHugh in Journal of Child Health Care
Author contributions: LC, LM and GM were involved in the design of the review and developing the protocol. LC had a lead role in study selection, data extraction, quality appraisal, data analysis and synthesis, with contributions and supervision from LM and GM at every stage. All authors contributed to the drafting of the paper, critically reviewed the manuscript and approved the final version submitted for publication.
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Laurie Cave, Clinical Doctoral Research Fellow, ICA-CDRF-2018-04-ST2-017, is funded by Health Education England (HEE)/National Institute for Health Research (NIHR) for this research project. The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the UK Department of Health and Social Care.
Supplemental material: Supplemental material for this article is available online.
ORCID iD
Laurie Cave https://orcid.org/0000-0001-8184-0201
References
- Aujoulat I, Simonelli F, Deccache A. (2006) Health promotion needs of children and adolescents in hospitals: a review. Patient Education and Counseling 61(1): 23–32. [DOI] [PubMed] [Google Scholar]
- Austin S, Guay F, Senécal C, et al. (2013) Longitudinal testing of a dietary self-care motivational model in adolescents with diabetes. Journal of Psychosomatic Research 75(2): 153–159. [DOI] [PubMed] [Google Scholar]
- Austin S, Senécal C, Guay F, et al. (2011) Dietary self-care in adolescents with type 1 diabetes: report from the juvenile diabetes and dietary study. Canadian Journal of Diabetes 35(1): 39–45. [Google Scholar]
- Bee P, Pedley R, Rithalia A, et al. (2018) Self-care support for children and adolescents with long-term conditions: the REfOCUS evidence synthesis. Health Services and Delivery Research 6(3). [PubMed] [Google Scholar]
- Bell S. (2004) Workshop: a nutrition and enzyme education programme for children with cystic fibrosis. In: 27th European cystic fibrosis conference, Birmingham, UK, 12–17 June 2004. European Cystic Fibrosis Nutrition Group. [Google Scholar]
- Boon M, Calvo-Lerma J, Claes I, et al. (2020) Use of a mobile application for self-management of pancreatic enzyme replacement therapy is associated with improved gastro-intestinal related quality of life in children with cystic fibrosis. Journal of Cystic Fibrosis 19(4): 562–568. [DOI] [PubMed] [Google Scholar]
- Calthorpe RJ, Smith SJ, Rowbotham NJ, et al. (2020) What effective ways of motivation, support and technologies help people with cystic fibrosis improve and sustain adherence to treatment? BMJ Open Respiratory Research 7(1): e000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave L, Milnes L, McHugh G. (2019) Self-care support of diet and the gut in the routine care of school-age children with long-term conditions: a systematic integrative review. Available at: www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019144941 (accessed November 2020). [DOI] [PMC free article] [PubMed]
- CFRISE (2016) CF milestones. Available at: www.cfrise.com/cystic-fibrosis-milestones (accessed 04 August 2020).
- Chaney D, Coates V, Shevlin M. (2010) Running a complex educational intervention for adolescents with type 1 diabetes–Lessons learnt. Journal of Diabetes Nursing 14: 370–379. [Google Scholar]
- Christian BJ, D’Auria JP. (2006) Building life skills for children with cystic fibrosis: effectiveness of an intervention. Nursing Research 55(5): 300–307. [DOI] [PubMed] [Google Scholar]
- Christie D, Thompson R, Sawtell M, et al. (2016) Effectiveness of a structured educational intervention using psychological delivery methods in children and adolescents with poorly controlled type 1 diabetes: a cluster-randomized controlled trial of the CASCADE intervention. BMJ Open Diabetes Research & Care 4(1): e000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates V, Chaney D, Bunting B, et al. (2013) Evaluation of the effectiveness of a structured diabetes education programme (CHOICE) on clinical outcomes for adolescents with type 1 diabetes: a randomised controlled trial. Journal of Diabetes and Metabolism 4(6): 2155–6156. [Google Scholar]
- Connan V, Marcon MA, Mahmud FH, et al. (2019) Online education for gluten‐free diet teaching: development and usability testing of an e‐learning module for children with concurrent celiac disease and type 1 diabetes. Pediatric Diabetes 20(3): 293–303. [DOI] [PubMed] [Google Scholar]
- Cooper H, Lancaster GA, Gichuru P, et al. (2018) A mixed methods study to evaluate the feasibility of using the adolescent diabetes needs assessment tool app in paediatric diabetes care in preparation for a longitudinal cohort study. Pilot and Feasibility Studies 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell CK, Young GA, Creer TL, et al. (1996) The development and evaluation of a self-management program for cystic fibrosis. Pediatric Asthma, Allergy & Immunology 10(3): 109–118. [Google Scholar]
- Coyne I, Hallström I, Söderbäck M. (2016) Reframing the focus from a family-centred to a child-centred care approach for children’s healthcare. Journal of Child Health Care 20(4): 494–502. [DOI] [PubMed] [Google Scholar]
- Culhane S. (2013) Development of an in-clinic education tool for pediatric cystic fibrosis patients. Journal of the Academy of Nutrition and Dietetics 113(9): A32. [Google Scholar]
- Culhane S, George C, Pearo B, et al. (2013) Malnutrition in cystic fibrosis. Nutrition in Clinical Practice 28(6): 676–683. [DOI] [PubMed] [Google Scholar]
- Davis MA, Quittner AL, Stack CM, et al. (2004) Controlled evaluation of the STARBRIGHT CD-ROM program for children and adolescents with cystic fibrosis. Journal of Pediatric Psychology 29(4): 259–267. [DOI] [PubMed] [Google Scholar]
- Day H. (2020) The use of app-based technology to promote self-management within paediatric diabetes. Diabetes Care for Children & Young People 9(3): DCCYP054. [Google Scholar]
- de Wit M, Versloot J, Zenlea I, et al. (2020) Using person-reported outcomes (PROs) to motivate young people with diabetes. Current Diabetes Reports 20(7): 23–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Department for Education and Skills (2004) National Service Framework for Children, Young People and Maternity Services: Children and Young People Who Are Ill. London: Department of Health. [Google Scholar]
- Eaton S, Roberts S, Turner B. (2015) Delivering person centred care in long term conditions. BMJ 350: h181. [DOI] [PubMed] [Google Scholar]
- Evans S, Daly A, Hopkins V, et al. (2009) The impact of visual media to encourage low protein cooking in inherited metabolic disorders. Journal of Human Nutrition and Dietetics 22(5): 409–413. [DOI] [PubMed] [Google Scholar]
- Feillet F, MacDonald A, Hartung D, et al. (2010) Outcomes beyond phenylalanine: an international perspective. Molecular Genetics and Metabolism 99(Suppl 1): S79–S85. [DOI] [PubMed] [Google Scholar]
- Fiallo-Scharer R, Palta M, Chewning BA, et al. (2019) Impact of family-centered tailoring of pediatric diabetes self-management resources. Pediatric Diabetes 20(7): 1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman LN, Kearney J, DeGroote M, et al. (2018) Creation of experience-based Celiac Benchmarks. Journal of Pediatric Gastroenterology and Nutrition 67(1): e6–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøisland DH, Årsand E. (2015) Integrating visual dietary documentation in mobile-phone-based self-management application for adolescents with type 1 diabetes. Journal of Diabetes Science and Technology 9(3): 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung CY. (2020) Dr. Me project: teaching children self-care for self-limiting illnesses in primary schools. Future Healthcare Journal 7(2): 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong QN, Fàbregues S, Bartlett G, et al. (2018) The mixed methods appraisal tool (MMAT) version 2018 for information professionals and researchers. Education for Information 34: 1–7. [Google Scholar]
- Hoo ZH, Gardner B, Arden MA, et al. (2019) Role of habit in treatment adherence among adults with cystic fibrosis. Thorax 74: 197–199. [DOI] [PubMed] [Google Scholar]
- Houts PS, Doak CC, Doak LG, et al. (2006) The role of pictures in improving health communication: a review of research on attention, comprehension, recall, and adherence. Patient Education and Counseling 61(2): 173–190. [DOI] [PubMed] [Google Scholar]
- Kirk S, Beatty S, Callery P, et al. (2010) Evaluating self-care support for children and young people with long-term conditions. Southampton: National Institute for Health Research Service Delivery and Organisation Programme. [Google Scholar]
- Kirk S, Beatty S, Callery P, et al. (2013) The effectiveness of self-care support interventions for children and young people with long-term conditions: a systematic review. Child: Care, Health and Development 39(3): 305–324. [DOI] [PubMed] [Google Scholar]
- Kyngäs H, Hentinen M, Barlow JH. (1998) Adolescents’ perceptions of physicians, nurses, parents and friends: help or hindrance in compliance with diabetes self‐care? Journal of Advanced Nursing 27(4): 760–769. [DOI] [PubMed] [Google Scholar]
- La Banca RO, Brandão MCdM, Sparapani VdC, et al. (2020) A fun way to learn about diabetes: using therapeutic play in a Brazilian camp. Journal of Pediatric Nursing 53: e35–e40. [DOI] [PubMed] [Google Scholar]
- Lally P, Gardner B. (2013) Promoting habit formation. Health Psychology Review 7(sup1): S137–S158. [Google Scholar]
- Leeman J, Voils CI, Sandelowski M. (2015) Conducting mixed methods literature reviews: synthesising the evidence needed to develop and implement complex social and health interventions. In: Hesse-Biber S, Burke Johnson R. (eds) The Oxford Handbook of Multimethod and Mixed Methods Research Inquiry. New York: Oxford University Press. [Google Scholar]
- McDonald CM, Alvarez JA, Bailey J, et al. (2020) Academy of nutrition and dietetics: 2020 cystic fibrosis evidence analysis center evidence-based nutrition practice guideline. Journal of the Academy of Nutrition and Dietetics. Epub ahead of print 2020 June 23. DOI: 10.1016/j.jand.2020.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339: b2535–b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosa F, Segal D. (2011) Assessing maths literacy skills in type 1 diabetic children and their caregivers. Journal of Endocrinology, Metabolism and Diabetes of South Africa 16(3): 146–153. [Google Scholar]
- Mulvaney SA, Lilley JS, Cavanaugh KL, et al. (2013) Validation of the diabetes numeracy test with adolescents with type 1 diabetes. Journal of Health Communication 18(7): 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabors LA, Kichler JC, Burbage ML, et al. (2014) Children’s learning and goal-setting at a diabetes camp. Diabetes Spectrum 27(4): 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS England (2014) The NHS five year forward view. Available at: www.england.nhs.uk/publication/nhs-five-year-forward-view/ (accessed March 2020).
- NHS England (2019) The NHS long-term plan. Available at: www.longtermplan.nhs.uk/publication/nhs-long-term-plan/ (accessed 20 March 2020).
- Owen E, Bryon M, Prasad A, et al. (2013) 282 Evaluation of nutrition education in a group of children with moderate to severe cystic fibrosis (CF). Journal of Cystic Fibrosis 12: S120. [DOI] [PubMed] [Google Scholar]
- Pawson R, Greenhalgh T, Harvey G, et al. (2005) Realist review -a new method of systematic review designed for complex policy interventions. Journal of Health Services Research & Policy 10(Suppl 1): 21–34. [DOI] [PubMed] [Google Scholar]
- Pelicand J, Fournier C, Le Rhun A, et al. (2013) Self-care support in paediatric patients with type 1 diabetes: bridging the gap between patient education and health promotion? A review. Health Expectations 18(3): 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KJ, Knowles JA, Fox M, et al. (2016) Effectiveness of the kids in control of food (KICk-OFF) structured education course for 11-16 year olds with Type 1 diabetes. Diabetic Medicine 33(2): 192–203. [DOI] [PubMed] [Google Scholar]
- Rankin D, Harden J, Barnard K, et al. (2018. a) Barriers and facilitators to taking on diabetes self-management tasks in pre-adolescent children with type 1 diabetes: a qualitative study. BMC Endocrine Disorders 18(1): 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin D, Harden J, Barnard KD, et al. (2018. b) Pre-adolescent children’s experiences of receiving diabetes-related support from friends and peers: a qualitative study. Health Expectations 21(5): 870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revert K, Audran L, Pengam J, et al. (2018) A quality improvement program to improve nutritional status of children with cystic fibrosis aged 2-12 years old over a 3 year period at CF center Roscoff, Brittany. Orphanet Journal of Rare Diseases 13(Suppl 1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha JC, van Spronsen FJ, Almeida MF, et al. (2013) Early dietary treated patients with phenylketonuria can achieve normal growth and body composition. Molecular Genetics and Metabolism 110: S40–S43. [DOI] [PubMed] [Google Scholar]
- Shoff SM, Tluczek A, Laxova A, et al. (2013) Nutritional status is associated with health-related quality of life in children with cystic fibrosis aged 9-19years. Journal of Cystic Fibrosis 12(6): 746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RH, Kable JA, Guerrero NV, et al. (2000) Impact of a camp experience on phenylalanine levels, knowledge, attitudes, and health beliefs relevant to nutrition management of phenylketonuria in adolescent girls. Journal of the American Dietetic Association 100(7): 797–803. [DOI] [PubMed] [Google Scholar]
- Smith J, Kendal S. (2018) Parents’ and health professionals’ views of collaboration in the management of childhood long-term conditions. Journal of Pediatric Nursing 43: 36–44. [DOI] [PubMed] [Google Scholar]
- Sparapani VdC, Fels S, Nascimento LC. (2017) The value of children’s voices for a video game development in the context of type 1 diabetes: focus group study. JMIR Diabetes 2(2): e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel G, Bortsov A, Bishop FK, et al. (2012) Randomized nutrition education intervention to improve carbohydrate counting in adolescents with type 1 diabetes study: is more intensive education needed? Journal of the Academy of Nutrition and Dietetics 112(11): 1736–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinks J, Guest S. (2017) Dietary management of children with type 1 diabetes. Paediatrics and Child Health 27(4): 176–180. [Google Scholar]
- Stallings VA, Sainath N, Oberle M, et al. (2018) Energy balance and mechanisms of weight gain with ivacaftor treatment of cystic fibrosis gating mutations. The Journal of Pediatrics 201: 229–237.e4. [DOI] [PubMed] [Google Scholar]
- Stapleton DR. (2001) Development, implementation and evaluation of a nutrition education and behaviour program for children with ystic fibrosis. PhD Thesis, Curtin University, Australia. [Google Scholar]
- Stark LJ, Quittner AL, Powers SW, et al. (2009) Randomized clinical trial of behavioral intervention and nutrition education to improve caloric intake and weight in children with cystic fibrosis. Archives of Pediatrics & Adolescent Medicine 163(10): 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R, Katz T, Liu V, et al. (2018) Dietary intake of energy-dense, nutrient-poor and nutrient-dense food sources in children with cystic fibrosis. Journal of Cystic Fibrosis 17(6): 804–810. [DOI] [PubMed] [Google Scholar]
- Taylor SJC, Pinnock H, Epiphaniou E, et al. (2014) A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions: PRISMS – Practical systematic Review of Self-Management Support for long-term conditions. Health Services and Delivery Research 2(53). [PubMed] [Google Scholar]
- Thornton H, Campbell F, Seagrave V, et al. (2016) Goals of diabetes education. Available at: www.digibete.org/wp-content/uploads/2018/01/GoDe_Book_v7_INTERACTIVE.pdf (accessed 21 August 2020).
- Turck D, Braegger CP, Colombo C, et al. (2016) ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clinical Nutrition 35(3): 557–577. [DOI] [PubMed] [Google Scholar]
- Wagner G, Berger G, Sinnreich U, et al. (2008) Quality of life in adolescents with treated coeliac disease: influence of compliance and age at diagnosis. Journal of Pediatric Gastroenterology and Nutrition 47(5): 555–561. [DOI] [PubMed] [Google Scholar]
- Whittemore R, Knafl K. (2005) The integrative review: updated methodology. Journal of Advanced Nursing 52(5): 546–553. [DOI] [PubMed] [Google Scholar]
- Witalis E, Mikoluc B, Mikoluc B, et al. (2017) Phenylketonuria patients’ and their parents’ knowledge and attitudes to the daily diet - multi-centre study. Nutrition & Metabolism 14: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye CY, Jeppson TC, Kleinmaus EM, et al. (2017) Outcomes that matter to teens with type 1 diabetes. The Diabetes Educator 43(3): 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-chc-10.1177_13674935211029124 for Self-care support of diet and the gut in the routine care of school-age children with long-term conditions: An integrative review by Laurie Cave, Linda J Milnes and Gretl A McHugh in Journal of Child Health Care
Supplemental Material, sj-pdf-2-chc-10.1177_13674935211029124 for Self-care support of diet and the gut in the routine care of school-age children with long-term conditions: An integrative review by Laurie Cave, Linda J Milnes and Gretl A McHugh in Journal of Child Health Care
Supplemental Material, sj-pdf-3-chc-10.1177_13674935211029124 for Self-care support of diet and the gut in the routine care of school-age children with long-term conditions: An integrative review by Laurie Cave, Linda J Milnes and Gretl A McHugh in Journal of Child Health Care
Supplemental Material, sj-pdf-4-chc-10.1177_13674935211029124 for Self-care support of diet and the gut in the routine care of school-age children with long-term conditions: An integrative review by Laurie Cave, Linda J Milnes and Gretl A McHugh in Journal of Child Health Care