Abstract
Inactivation of the gbpA gene of Streptococcus mutans increases virulence in a gnotobiotic rat model and also promotes in vivo accumulation of organisms in which gtfB and gtfC have recombined to reduce virulence (K. R. O. Hazlett, S. M. Michalek, and J. A. Banas, Infect. Immun. 66:2180–2185, 1998). These changes in virulence were hypothesized to result from changes in plaque structure. We have utilized an in vitro plaque model to test the hypothesis that the absence of GbpA alters S. mutans plaque structure and that the presence of gtfBC recombinant organisms within a gbpA background restores a wild-type (wt)-like plaque structure. When grown in the presence of sucrose within hydroxyapatite-coated wells, the wt S. mutans plaque consisted primarily of large aggregates which did not completely coat the hydroxyapatite surface, whereas the gbpA mutant plaque consisted of a uniform layer of smaller aggregates which almost entirely coated the hydroxyapatite. If 25% of the gbpA mutants used as inoculum were also gtfBC recombinants (gbpA/25%gtfBC), a wt-like plaque was formed. These changes in plaque structure correlated with differences in susceptibility to ampicillin; gbpA plaque organisms were more susceptible than organisms in either the wt or gbpA/25%gtfBC plaques. These data allow the conclusion that GbpA contributes to S. mutans plaque biofilm development. Since the changes in plaque structure detailed in this report correlate well with previously observed changes in virulence, it seems likely that S. mutans biofilm structure influences virulence. A potential model for this influence, which can account for the gtfBC recombination compensating gbpA inactivation, is that the ratio of glucan to glucan-binding protein is a critical factor in plaque development.
Streptococcus mutans is the primary etiologic agent of smooth-surface tooth decay in humans (14). The primary virulence traits of S. mutans are sucrose-dependent adherence, aciduricity, and acidogenicity (14). Sucrose-dependent adherence is mediated by glucans, the products of the extracellular glucosyltransferase (GTF) enzymes, which have glucan-binding properties (8). GTF-I, GTF-SI, and GTF-S, encoded by the gtfB, gtfC, and gtfD genes, respectively, polymerize the glucose moieties of sucrose into glucans. The importance of the GTFs, particularly the products of the gtfB and gtfC genes, in cariogenicity has been established by many labs (16, 26, 27). Ueda and Kuramitsu found that within S. mutans the tandemly arranged, highly homologous gtfB and gtfC genes could spontaneously recombine at a frequency of 10−3 to form a single hybrid gtfBC gene (24). This recombination resulted in a dramatic decrease in the synthesis of water-insoluble glucan (24) and a reduction in virulence (26). When the intact gtfB and gtfC genes were cloned in Escherichia coli, it was found that recombination of these genes was RecA dependent and resulted in recombinant gtfBC genes with markedly dissimilar sites of recombination (24). It was later reported that gtfBC recombination within S. mutans was not RecA dependent and that a variety of in vivo-generated gtfBC recombinants had similar sites of recombination (11).
S. mutans also synthesizes three glucan-binding proteins with no known enzymatic activities, GbpA, GbpB, and GbpC, whose contributions to virulence, or properties associated with virulence, have begun to be explored. GbpC appears to be anchored to the cell wall, is partially similar to members of the Spa family of oral streptococcal proteins, and is involved in rapid dextran-dependent aggregation under defined, stressful growth conditions (19). The contribution of GbpC to virulence has yet to be documented. Immunization with GbpB has been reported to be caries protective (23), although the actual function of GbpB is unknown and the gene encoding it has yet to be cloned.
Like the GTFs, GbpA is a secreted protein found in association with the cell surface and in the extracellular medium. The carboxyl-terminal three-quarters of GbpA, which has homology to the carboxyl-terminal repeat domains of the GTFs (1), mediates binding to α-1,6 glucosidic linkages (10, 18) present in water-soluble and, to a lesser extent, water-insoluble glucans and undergoes a conformational shift upon binding to dextran (9). Analysis of gbpA transcriptional regulation, as determined with a gbpA::cat reporter construct, indicated that gbpA was maximally expressed under anaerobic and neutral-pH conditions but that sucrose did not induce gbpA expression (3).
Previously we utilized wild-type (wt) and gbpA-isogenic strains to test the hypothesis that GbpA contributes to the virulence of S. mutans (11). Contrary to expectations, the gbpA strain was hypercariogenic in the gnotobiotic rat model. Since the colonization levels of gbpA mutant and wt S. mutans were not significantly different, the increased virulence of the gbpA strain was not due to an increase in the number of adherent organisms. In vitro, the gbpA mutant plaque was more resistant to mechanical stress than that of the wt, suggesting that they were structurally different (11). On the basis of these findings, we hypothesized that gbpA S. mutans was hypercariogenic due to an altered plaque structure which modified either acid production or diffusion. After 35 days in vivo, the gbpA strain had become enriched to various degrees with organisms with reduced GTF activity due to recombination involving the highly homologous, continguous gtfB and gtfC genes. The incidence of gtfBC recombinant organisms within the gbpA background was inversely correlated with caries development, such that gbpA S. mutans containing 22.33% gtfBC recombinant organisms was not significantly different from wt S. mutans in cariogenicity or colonization levels. These results suggested the possibility that the reduced GTF activity of gtfBC recombinant organisms restored a less cariogenic, more wt-like plaque structure.
The purpose of the present work was to test the hypothesis that the absence of GbpA alters S. mutans plaque structure and that the presence of gtfBC recombinant organisms within a gbpA background restores a wt-like plaque structure. We show that the loss of GbpA dramatically alters the structure of S. mutans plaque biofilm and that gtfBC recombination within a gbpA background partially restores a wt-like plaque structure. We also show that these changes in plaque structure both have functional consequences and correlate with changes in virulence. Our findings have implications both for further understanding S. mutans cariogenicity and for the study of biofilms. To our knowledge, this work, in conjunction with our previous findings, represents the first experimental evidence that changes in biofilm structure influence virulence.
(Part of this work was conducted by K. R. O. Hazlett in partial fulfillment of the requirements for a Ph.D. from Albany Medical College, Albany, N.Y.)
MATERIALS AND METHODS
Bacteria and their cultivation.
Construction of the gbpA strains of S. mutans UA130 (serotype c) has been described previously (2). When grown on mitis salivarius (MS) agar, nonrecombinant (GTF-wt) S. mutans produces rough colonies whereas gtfBC recombinant S. mutans produces smooth colonies. The laboratory gbpA gtfBC strain used in this work was a spontaneous mutant isolated by streaking S. mutans UA130 gbpA on MS agar (Difco Laboratories, Grand Island, N.Y.) plates and picking a smooth colony. This isolate was phenotypically indistinguishable from gtfBC recombinant organisms previously recovered from gbpA mutant-infected rats. PCR amplification of the gtfB-gtfC region (11) confirmed that this isolate was a gtfBC recombinant. The clinical gbpA gtfBC strains were recovered from gbpA S. mutans-infected gnotobiotic rats (11). Broth cultures of gbpA gtfBC strains were individually mixed with broth cultures of the nonrecombinant gbpA strain such that the resulting mixture (termed gbpA/25%gtfBC) contained 25% gtfBC recombinant organisms. These mixtures were immediately used to generate frozen glycerol stocks. By plating the glycerol stocks of the gbpA/25%gtfBC mixtures on MS agar and enumerating the smooth and rough colonies, we confirmed that inoculation broths generated from the glycerol stocks contained the appropriate ratio of recombinants and nonrecombinants. This ratio of nonrecombinants to recombinants was used to mimic the previously observed level of in vivo accumulation of gtfBC organisms by gbpA S. mutans (11). Glycerol stocks of S. mutans UA130 wt, gbpA, and the gbpA/25%gtfBC mixtures were stored at −70°C and used to generate overnight chemically defined medium (CDM) broth cultures. S. mutans strains were routinely grown anaerobically at 37°C in CDM (JRH Biosciences, Lenexa, Kansas). Growth on Todd-Hewitt (Difco Laboratories) and MS agar plates was used to confirm culture purity and colony morphology, respectively. The gbpA and gbpA/25%gtfBC strains were maintained in vitro with erythromycin at 25 μg/ml.
In vitro S. mutans plaques.
The method described by Schilling et al. (20) was used in the preparation of hydroxyapatite-coated plates. Briefly, 333 μl (96-well plates [product no. 25860; Corning, Corning, N.Y.] and 16-well Nunc Lab-Tek Chamber slides [Fisher Scientific, Pittsburgh, Pa.]) or 2.5 ml (24-well plates [product no. 3524; Costar, Cambridge, Mass.]) of a 2.5 mM CaCl2 · H2O–7.5 mM KH2PO4–250 mM triethanolamine solution (pH 7.3) was added to each of the wells of tissue culture plates. The plates were incubated at 75°C without lids for 90 min. Following the incubation, the supernatants were carefully aspirated, the plates were allowed to dry, and the process was repeated three times. Hydroxyapatite-coated plates and lids were sterilized prior to use by exposure to 2 kJ of UV (254-nm) radiation.
To generate S. mutans plaques on deposited hydroxyapatite, overnight CDM cultures were diluted with fresh CDM to an optical density at 540 nm (OD540) of 1.5. Either 280 μl (96-well plates) or 1.5 ml (240-well plates) of CDM containing 5% sucrose was added to each of the sterile, hydroxyapatite-coated wells. Either 20 μl (96-well plates) or 100 μl (24-well plates) of CDM broth culture (OD540 = 1.5) was added to each of the medium-containing wells. The plates were incubated overnight on a hematological rotator, within a 37°C CO2 incubator, at a rotation speed of 10 rpm and an angle of 60°C less than horizontal. Following overnight incubation, the supernatants were aspirated, fresh CDM containing 5% sucrose was added to the wells, and the plates were incubated overnight as described above. Unless stated otherwise, data presented here were derived from 4-day-old plaques.
Light microscopy.
S. mutans plaques grown within the wells of hydroxyapatite-coated 24-well plates were photographed unmagnified against a black background with a Polaroid MP-4 Land camera and at low magnification with a 35-mm camera coupled to an Olympus IM inverted microscope with a 4× objective.
Confocal microscopy.
S. mutans plaques for use in confocal microscopy were generated within the hydroxyapatite-coated wells of 16-well Nunc Lab-Tek Chamber slides (Fisher Scientific). Following 4 days of growth, plaques were rinsed twice with 300 μl of TKS buffer (10 mM Tris-HCl, 50 mM KCl, 5% sucrose; pH 7.0)/well, stained for 15 min in the dark with 200 μl of the LIVE Baclight Bacterial Gram Stain fluorescent dye mixture (5 μM SYTO 9, 7 μM hexidium iodide, and 0.3% dimethyl sulfoxide in TKS buffer) (Molecular Probes, Eugene, Oreg.)/well, and rinsed once with 300 μl of TKS buffer/well. The well walls were gently removed, 50 μl of TKS was deposited on each plaque, and the plaques were covered with a 22-mm by 50-mm coverslip which was secured with superglue. Because the silicone sealing gasket (which previously connected the well walls to the slide) was left intact on the slide, the coverslip did not disturb the S. mutans plaques.
Plaques were examined by confocal microscopy with a Noran OZ confocal laser imaging system (Noran Instruments, Madison, Wis.) on a Nikon Diaphot 200 inverted microscope equipped with a 20× 0.75 N.A. objective lens and a Kr/Ar laser. Optical sections were collected at 1-μm steps through a sample depth of ∼130 μm. Three-dimensional volumes were rendered by using the Noran InterVision 3D Analysis software. Maximum-intensity projection images were constructed, and TIFF images of y-z slices were made randomly through the rendered volumes. Peak-to-base heights of individual aggregates were measured on the TIFF images by using the Sigma ScanPro 4 program (Jandel Scientific, San Rafael, Calif.). Solid volumes were also rendered to reveal the surface morphology of the aggregates in each sample.
Determination of GTF activity.
To visualize water-insoluble GTF activity of S. mutans, cell-associated proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by incubation with Triton X-100 (Fisher Biotech) and sucrose (18). Specifically, overnight CDM broth cultures were diluted into 20 ml of Todd-Hewitt broth, grown to an OD540 of 1.3, and harvested by centrifugation. Cells were resuspended in 150 μl of 2× cracking buffer (0.038 M Tris-HCl [pH 6.8], 1% SDS, 2.5% 2-mercaptoethanol 15% glycerol) and incubated at 25°C for 2 h. A 25-μl volume of cell-free supernatant was resolved by SDS-PAGE. Following electrophoresis, the SDS was eluted from the gel by two 30-min washes with 50 mM Tris buffer, pH 7.5. In situ water-insoluble GTF activity was visualized by incubation of the gel for 16 h in phosphate-buffered saline (PBS; pH 6.5) containing 2% sucrose, 2% Triton X-100, and 0.05% 11,000-molecular-weight dextran (Sigma, St. Louis, Mo.). The gels were briefly rinsed with PBS and incubated in a 45% methanol–10% acetic acid solution for 20 min to enhance the glucan bands. Gels were photographed against a black background. Scanning densitometry was used to quantify water-insoluble GTF activity. The glucan signals were normalized to the fructan signals; the normalized glucan signals were divided by the normalized wt glucan signal and presented as a percentage of wt GTF activity.
Sequencing of gtfBC recombination junctions.
A combination of restriction digestions, Southern blotting analysis, and PCR analyses indicated that the recombination junctions of all gtfBC gene fusions were contained on 1.8-kb HindIII fragments of chromosomal DNA. Cloning of this region was achieved by PCR amplification of gtfBC gene fusions (11), digestion of the 5.3-kb PCR products with HindIII (Promega, Madison, Wis.), and ligation of the 1.8-kb fragments to HindIII-digested, alkaline phosphatase (Promega) treated pUC19. INVαF′ One Shot competent cells (Invitrogen, Carlsbad, Calif.) were transformed with ligation mixtures as per the manufacturer’s instructions and plated on 2× yeast extract-tryptone agar plates containing 100 μg of ampicillin (Sigma)/ml and 40 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside Gold Biotechnology, St. Louis, Mo.)/ml. Clones containing a 1.8-kb HindIII insert were sequenced in both directions with universal M13 primers and primers gtfB@1639 (5′-GCAACTATTCAAGCAAAAATTG) and gtfC@2107 (GAAAGTGCCGTATTATAGTG), using a Perkin-Elmer ABI Prism 310 Genetic Analyzer. The published sequences of the gtfB (21) and gtfC (25) genes and the DNA analysis program MacDNASIS Pro version 3.5 (Hitachi Software, San Bruno, Calif.) were used for sequence alignment and analysis.
Antibiotic sensitivity.
S. mutans plaques were generated within the wells of hydroxyapatite-coated 96-well plates as described above. On the 4th day of growth, plaques were incubated with fresh CDM containing 0 or 100 μg of ampicillin (Sigma)/ml for 2 h while rotating within a 37°C CO2 incubator. Following ampicillin challenge, the plaques were washed three times with 300 μl of CDM and incubated with 250 μl of CDM containing 5% sucrose and 1 μCi of [3H]thymidine/ml for 16 h while rotating at 37°C. Labeled plaques were subsequently rinsed twice with 300 μl of PBS, digested with 250 μl of 1 M NaOH–10 mM EDTA for 2 h at 37°C, and subjected to scintillation counting. The percentage of plaque organisms killed was calculated as follows: 1.0 − (mean counts per minute of the challenged plaque organisms/mean counts per minute of the unchallenged plaque organisms of the respective genotype).
Statistical analysis.
Data analysis and determination of significance were performed with the unpaired, two-tailed Student t test (data are presented as means ± standard deviations) or, if appropriate, the unpaired, two-tailed nonparametric Mann-Whitney test (data are presented as medians with upper and lower 95% confidence intervals). Differences were considered significant when a P value of ≤0.05 was obtained.
RESULTS
The contribution of GbpA and GtfBC to S. mutans plaque structure.
We previously hypothesized that the enhanced virulence of gbpA S. mutans in the gnotobiotic rat model resulted from a change in plaque structure and that the accumulation of recombinant gtfBC genes by gbpA S. mutans attenuated virulence by restoring a wt-like plaque structure (11). To test the hypothesis that inactivation of gbpA and recombination of the gtfB and gtfC genes within a gbpA background reciprocally alter plaque structure, we used an in vitro plaque model in which S. mutans wt, gbpA, and gbpA/25%gtfBC plaques were grown within hydroxyapatite-coated wells of microtiter dishes and examined by light and confocal microscopy. The gbpA mutant mixture containing 25% gbpA gtfBC recombinant organisms was used to mimic the level of in vivo accumulation of gtfBC organisms (22.33%) by gbpA S. mutans in the gnotobiotic rat model (11).
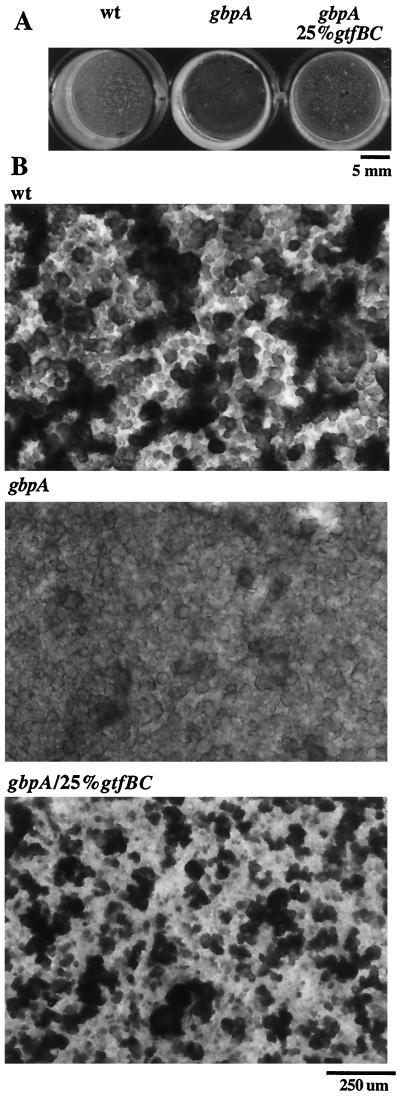
Following overnight plaque deposition and aspiration of supernatants, gross differences were noted among these plaques. Figure 1A shows S. mutans wt, gbpA, and gbpA/25%gtfBC plaques after 4 days of growth in hydroxyapatite-coated wells of a 24-well plate. While the plaque deposited by wt S. mutans appeared as an opaque, granular, thick layer, the plaque deposited by gbpA S. mutans appeared as a translucent, amorphous, thinner layer. As the percentage of gtfBC organisms within the gbpA inocula was increased from 0 to 25%, the opaque and granular patterns were partially restored. Restoration of these patterns was characteristic of spontaneous laboratory gtfBC recombinants as well as gtfBC recombinants recovered from gbpA mutant-infected gnotobiotic rats (clinical recombinants), suggesting that this is a general phenomenon of gbpA gtfBC recombinant organisms. It should be noted that for recombinants with higher-than-average recombinant levels of GTF activity (C5-2 and A6-1 [see Fig. 4]), a recombinant proportion of greater than 25% of the inoculum was required to restore plaque structure. The plaque deposited by nonrecombinant laboratory gbpA strains was indistinguishable from the plaque produced by nonrecombinant clinical gbpA strains (data not shown). Both laboratory and clinical gbpA strains produced wt-like plaques when gtfBC recombinant (either spontaneous or clinical) organisms were included in the inoculum. Plaques produced by gbpA/100%gtfBC S. mutans were as opaque and granular as wt plaques but appeared thinner than the other S. mutans plaques, perhaps due to the marked decrease in GTF activity. Quantification of plaque organisms in this model by the crystal violet release assay (11) revealed no differences in cell number among these plaques (data not shown).
FIG. 1.
S. mutans plaques deposited in hydroxyapatite-coated wells of 24-well plates. S. mutans strains were grown in hydroxyapatite-coated wells on a rotator for 4 days in CDM with 5% sucrose as described in the text. Supernatants were aspirated, and the plaques were photographed. (A) Unmagnified hydroxyapatite wells containing S. mutans plaques photographed against a black background. (B) S. mutans plaques at low magnification, viewed with an inverted microscope with a 4× objective. With the exception of the focal plane, light micrographs of S. mutans plaques were taken under identical photographic conditions (magnification, lighting, exposure time, etc.).
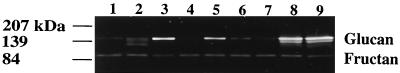
FIG. 4.
GTF activity of S. mutans UA130 gbpA gtfBC, gbpA, and wt. Equivalent levels of cell-associated proteins were resolved by SDS-PAGE and incubated at 37°C in PBS (pH 6.5) containing sucrose and Triton X-100. Gels were photographed against a black background. The positions of SDS-PAGE molecular mass standards are indicated in the left margin. The positions of water-insoluble glucan and fructan are indicated in the right margin. Lanes 1 to 6: contain gbpA gtfBC isolates recovered from six individual gbpA mutant-infected gnotobiotic rats from two separate determinations of cariogenicity. Lanes: 1, B3-B; 2, D2-A; 3, C5-2; 4, C3-2; 5, A6-1; 6, A2-1; 7 to 9, laboratory strains (7, gbpA spontaneous gtfBC; 8, gbpA; 9, wt).
Examination of these plaques by light microscopy offered insight into the possible mechanisms underlying the differences in plaque appearance. Figure 1B shows 4-day-old S. mutans wt, gbpA, and gbpA/25%gtfBC plaques deposited in hydroxyapatite-coated wells, as viewed with an inverted microscope. While the wt plaque consisted of many large aggregates adherent to smaller aggregates, which were themselves adherent to the hydroxyapatite, the S. mutans gbpA plaque consisted of primarily smaller aggregates adherent to the hydroxyapatite. Notably, the smaller aggregates of the gbpA plaque more completely covered the hydroxyapatite, whereas the mixture of smaller and larger aggregates of the wt plaque left larger areas of the hydroxyapatite uncovered. As the proportion of gtfBC organisms within the gbpA inocula was increased from 0 to 25%, the capacity of gbpA S. mutans to form large aggregates was restored.
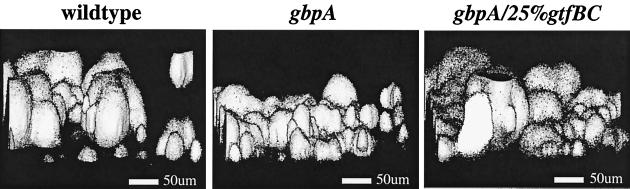
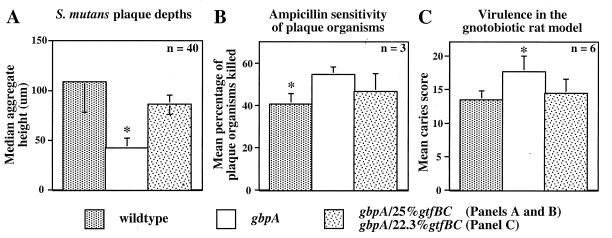
To extend the above-described observations and to perform quantitative analysis, confocal laser scanning microscopy (CLSM) was used to examine S. mutans plaques. Figure 2 shows solid renderings of data collected by CLSM of wt, gbpA, and gbpA/25%gtfBC S. mutans plaques. The renderings in Fig. 2 have been tilted (x = −75, y = −5, z = 0) to give the impression of viewing a terrain from above and slightly to one side. As was seen by light microscopy, wt and gbpA/25%gtfBC plaques were composed primarily of large aggregates, while the gbpA plaque consisted of uniformly smaller aggregates. To quantify the differences in plaque depth, the peak-to-base distance of individual aggregates was measured. As shown in Figure 3A, the median aggregate heights of the wt and gbpA/25%gtfBC plaques were both significantly greater than that of the gbpA plaque (P < 0.0001) but were not statistically different from each other.
FIG. 2.
Tilted saggital solid renderings of S. mutans plaques acquired by CLSM. S. mutans strains were grown in hydroxyapatite-coated wells of a Nunc Lab-Tek Chamber slide on a rotator for 4 days in CDM with 5% sucrose as described in the text. Fluorescently stained plaques were optically sectioned at 1-μm increments by CLSM; saggital views of solid renderings were tilted (x = −75, y = −5, z = 0) by using the InterVision 3-D Analysis program, converted to gray scale by using Adobe Photoshop 3.0.4 (Adobe Systems Inc., Mountain View, Calif.), and assembled by using Canvas 5.02 (Deneba Software, Miami, Fla.). All parameters of data collection, analysis, and presentation were applied identically to each of the S. mutans plaques.
FIG. 3.
Quantitative analysis of structural and functional aspects of S. mutans plaques. Asterisks indicate significant differences between adjacent columns. (A) Median heights (in micrometers) with upper and lower 95% confidence intervals of aggregates comprising S. mutans plaques (P < 0.0001). (B) Sensitivity of S. mutans plaque organisms to ampicillin challenge. Four-day-old S. mutans plaques were treated for 2 h with fresh CDM medium containing 0 or 100 μg of ampicillin/ml, washed three times, and incubated with CDM containing 5% sucrose and 1 μCi of [3H]thymidine/ml. The percentage of plaque organisms killed was calculated as follows: 1.0 − (mean counts per minute of the challenged plaque organisms/mean counts per minute unchallenged plaque organisms of the respective genotype) (P < 0.02). (C) Virulence of S. mutans in the gnotobiotic rat model, as previously reported in the form of tabular data (Table 3 in reference 11 [enamel involvement, buccal surfaces]), represented here in graphical form to facilitate comparison with results from the in vitro plaque model (P < 0.03).
Characterization of gtfBC recombinant organisms.
It thus appears that the presence of gtfBC recombinants within a gbpA population can restore a wt-like plaque structure whether the gbpA gtfBC recombinants were obtained as spontaneous laboratory isolates or recovered from gbpA mutant-infected gnotobiotic rats. Since recombinants could potentially vary based on the point of recombination and the active site retained in the hybrid enzyme, the genotypic and phenotypic characteristics of several gbpA gtfBC recombinants were examined for insoluble GTF activity and the site(s) of recombination. An early analysis (11) provided evidence that the recombinants recovered from infected rats all recombined near the same region. There existed the possibility that selection for certain recombinants occurred even if multiple sites of recombination were possible.
Cell-associated proteins from wt, gbpA, and representative gbpA gtfBC isolates were extracted, resolved by SDS-PAGE, and developed for determination of insoluble GTF activity. Since inactivation of gbpA does not influence Gtf-S or Gtf-I activity, as determined by the radiolabeled-sucrose assay (unpublished data), and since the genes affected by gtfBC recombination encode primarily Gtf-I activity, we focused on the Gtf-I activity of the strains used in this work. As with the radioactive-sucrose method, no differences in insoluble GTF activity were found between wt and gbpA S. mutans by the activity gel method (Fig. 4). All gbpA gtfBC isolates exhibited a marked reduction (50 to 90%) in insoluble glucan synthesis relative to that of nonrecombinant S. mutans, although differences in insoluble GTF activity among the isolates were apparent (Fig. 4). The degree of GTF activity reduction likely influenced the extent to which particular recombinants accumulated within individual animals. Among gbpA mutant-infected rats which experienced wt levels of caries, a general trend was noted: the greater the accumulation of a recombinant within a gbpA mutant-infected rat, the greater the GTF activity of the recombinant. Similarly, gbpA mutant-infected rats which experienced elevated levels of caries harbored low levels of recombinants which retained higher-than-average levels of recombinant GTF activity (26.7% of wt GTF activity). These observations make sense since it appears that a certain level of insoluble glucan reduction by gbpA S. mutans is necessary for restoration of plaque structure. When both the levels of recombinant GTF activity and the degree of accumulation of the recombinants were considered, it appeared that in order to restore plaque structure and cariogenicity, gbpA S. mutans accumulated gtfBC recombinant organisms to a degree such that the total insoluble glucan production of the mixed population was approximately 80% of wt levels.
The regions which contained the gtfB gtfC recombination junctions of 11 recombinant isolates were cloned and sequenced. Among these were seven UA130 gbpA gtfBC isolates recovered from various animals representing both animal experiments, the UA130 gbpA spontaneous gtfBC recombinant described here, the previously described strain GS-5 spontaneous recombinant SP2 (24), an independent GS-5 spontaneous recombinant, and a strain 3209 recA spontaneous recombinant. Using the Higgins-Sharp algorithm of the MacDNASIS program, the sequences of these isolates were multiply aligned along with the published sequences of the GS-5 gtfB and gtfC genes (21, 25). Ten of these isolates had recombination junctions within a region 270 bp downstream of the catalytic site of gtfB and spanning approximately 200 bp. Only the recombination junction of SP2 was in a markedly different region, approximately 580 bp upstream of the gtfB catalytic site. Nucleotide differences existed among several gtfBC genes, and multiple recombinations could not be ruled out. While an intact recA gene was not required for the recombination (11), our results suggested that gtfBC recombination within the mammalian host was mediated by the homology exhibited by these genes, as has been documented for in vitro-cultivated S. mutans (24, 26).
These observations, combined with our finding that gbpA S. mutans does not have a higher basal level of spontaneous gtfBC recombination in vitro (data not shown), favor a role for the selection of recombinants within a gbpA plaque, rather than an increased frequency of recombination, as the mechanism of recombinant accumulation within gnotobiotic rats.
The contribution of GbpA and GtfBC to S. mutans plaque function.
Dental plaque, being composed of adherent aggregates of cells imbedded in a matrix of extracellular polysaccharide surrounded by fluid-filled spaces, exhibits features common to biofilms (13). A characteristic of biofilm organisms is decreased susceptibility to biocide agents compared to that of their planktonic counterparts (17). It has recently been reported that Pseudomonas aeruginosa organisms within a thin, unstructured, mutant biofilm were more susceptible to a biocidal agent than were organisms within the wt biofilm (6). Since the plaque deposited by gbpA S. mutans was thinner and less structured than wt and gbpA/25%gtfBC plaques, we hypothesized that gbpA plaque organisms would be more susceptible to a biocidal agent. To test this hypothesis, 4-day-old S. mutans plaques were challenged for 2 h with a dose of ampicillin lethal to planktonic cells and incubated overnight in the presence of [3H]thymidine. As shown in Fig. 3B, gbpA plaque organisms were significantly (P < 0.02) more susceptible to ampicillin challenge than wt plaque organisms. As the percentage of gtfBC organisms within the gbpA inocula was increased, the sensitivity of plaque organisms to ampicillin decreased. No differences in ampicillin sensitivity were found among these strains when planktonic cultures were tested (data not shown). These results indicate that the observed changes in plaque structure have functional consequences.
DISCUSSION
Having observed that inactivation of the gbpA gene of S. mutans increased virulence and promoted accumulation of virulence-attenuating gtfBC recombinant organisms in vivo, we hypothesized that these findings might have resulted from changes in plaque structure (11). Here we report the results of experiments designed to test the hypothesis that the absence of GbpA alters plaque structure and that the presence of gtfBC recombinant organisms within a gbpA background compensates for this alteration. Our findings have implications for further understanding S. mutans cariogenicity as well as for the study of biofilms and the relationship between biofilm structure and virulence.
Several previous observations have suggested that the absence of GbpA may have altered the structure of S. mutans plaque. An analysis of virulence in the gnotobiotic rat model revealed that while levels of colonization of gbpA and wt S. mutans were not significantly different, gbpA S. mutans was hypercariogenic (11). In vitro, gbpA mutant plaque was more resistant to mechanical stress than wt plaque (11). Since levels of acid production in batch cultures of wt and gbpA S. mutans were not different (11), we hypothesized that the hypercariogenicity of gbpA S. mutans in vivo resulted from a change in plaque structure which either increased acid production or decreased the diffusion of acid away from the tooth enamel. We have subsequently found no differences in the cell numbers of (as was found in vivo) or in the rates of acid production by wt, gbpA, or gbpA/25%gtfBC S. mutans in our plaque model (unpublished data). In light of this and the finding that gbpA mutant plaque is composed of smaller aggregates which more completely coat the underlying substratum, we hypothesize that GbpA mediates aggregation and that gbpA S. mutans is hypercariogenic in vivo due to the altered plaque structure, which, we speculate, forms a tighter barrier between the tooth surface and the saliva. We propose that a tighter barrier would allow for sustained exposure of the tooth enamel to demineralizing conditions by decreasing both the influx of salivary buffering capacity and the efflux of bacterially derived acids. We are presently developing techniques to directly test this barrier hypothesis.
Several observations led us to believe that data derived from our in vitro plaque model are relevant to our previous findings concerning cariogenicity. First, in both the gnotobiotic rat model and our in vitro plaque model, wt and gbpA/25%gtfBC S. mutans strains gave results which were highly similar to each other yet distinct from those of gbpA S. mutans. In the rats, this pattern was observed in measurements of cariogenicity, and in our in vitro model, this pattern was repeated with respect to plaque structure, aggregate size, and ampicillin sensitivity (Fig. 3). Second, in our plaque model, clinical isolates (those recovered from gbpA mutant-infected rats) produced plaques which were grossly indistinguishable from their laboratory counterparts. This finding also supports the hypothesis that gtfBC recombination was the change (as opposed to some other in vivo-selected adaptation) which attenuated the hypercariogenicity of gbpA S. mutans in the gnotobiotic rat model.
The ability of gtfBC recombination to compensate for gbpA inactivation in terms of plaque structure, aggregate size, ampicillin sensitivity, and cariogenicity led us to consider the possibility that the ratio of glucan to glucan-binding protein may be an important parameter in S. mutans plaque development. This hypothesis predicts that inactivation of other GBP-encoding genes would impart both an altered plaque structure phenotype and a propensity toward in vivo accumulation of organisms with reduced GTF activity. Since GbpA is the quantitatively predominant S. mutans nonenzymatic glucan-binding protein (22), it is possible that these effects would be most marked in the gbpA background. Obviously, many questions would have to be addressed in order to validate the glucan–glucan-binding protein hypothesis, yet the fact that a 20% reduction in GTF activity within a gbpA background restored every parameter measured (cariogenicity, plaque structure, etc.) to near-wt levels suggests that this hypothesis warrants further investigation.
While the objective of this investigation was to elucidate the contribution of GbpA and gtfBC recombination to S. mutans plaque development and cariogenicity, we feel that our results have important implications to the study of biofilms. Biofilms are communities of bacteria which develop on solid surfaces as pillar-like structures separated by fluid-filled spaces (5). These structures are composed of bacteria embedded in a matrix of extracellular polysaccharide. While the universal characteristics of biofilm structure—copious extracellular polysaccharide production and decreased antibiotic susceptibility—have been well documented, the analysis of specific gene products in biofilm formation has just recently begun (12). Davies et al. have shown that P. aeruginosa mutants defective in acylated homoserine lactone production form a thin, flat, unstructured biofilm (6). By screening a Pseudomonas fluorescens transposon mutagenesis library, O’Toole and Kolter isolated mutants defective in biofilm initiation. Of 24 mutants isolated, 21 contained transposon insertions in genes of unknown function (17). Burne et al. have found that S. mutans polysaccharide synthesis genes are differentially regulated in biofilms (4); Mack et al. have reported that defects in production of a unique polysaccharide (PIA) by Staphylococcus epidermidis impair biofilm formation (15). Given the association of biofilms with extracellular polysaccharides, it might seem elementary that inactivation of a gene encoding a nonenzymatic polysaccharide-binding protein would alter biofilm structure, yet to our knowledge this communication represents the first report to this effect and, in conjunction with our previous findings, presents the first experimental evidence that changes in biofilm structure influence virulence. It has been suggested that interfering with the ability to form mature biofilms by disrupting cell-to-cell communication may be a novel method for attenuating the negative impact of biofilms in medicine and industry (6, 7). Our findings suggest that disruption of mature biofilm structure can have unanticipated, undesirable ramifications.
ACKNOWLEDGMENTS
We thank M. M. Vickerman for providing a recA mutant strain of S. mutans 3209 and H. K. Kuramitsu for providing the gtfBC recombinant strain SP2 of S. mutans GS-5. We are grateful to Justin D. Radolf and Melissa Caimano for critical review of the manuscript.
The research efforts of J.A.B. and K.R.O.H. were supported by grant DE10058 from the National Institute of Dental Research. The research efforts of J.E.M. were supported by grant S10 RR12894-01A1 from the National Institutes of Health.
REFERENCES
- 1.Banas J A, Russell R R B, Ferretti J J. Sequence analysis of the gene for the glucan-binding protein of Streptococcus mutans Ingbritt. Infect Immun. 1990;58:667–673. doi: 10.1128/iai.58.3.667-673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banas J A, Gilmore K S. Analysis of Streptococcus mutans and Streptococcus downei mutants insertionally inactivated in the gbp and gtfS genes. In: Dunny G M, Cleary P P, McKay L L, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, D.C: American Society for Microbiology; 1991. pp. 281–283. [Google Scholar]
- 3.Banas J A, Potvin H C, Singh R N. The regulation of Streptococcus mutans glucan-binding protein A expression. FEMS Microbiol. 1997;154:289–292. doi: 10.1111/j.1574-6968.1997.tb12658.x. [DOI] [PubMed] [Google Scholar]
- 4.Burne R A, Chen Y Y, Penders J E. Analysis of gene expression in Streptococcus mutans in biofilms in vitro. Adv Dent Res. 1997;11:100–109. doi: 10.1177/08959374970110010101. [DOI] [PubMed] [Google Scholar]
- 5.Costerton J R, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 6.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 7.Dixon B. Biofilms: cultural diversity in action. ASM News. 1998;64:484–485. [Google Scholar]
- 8.Gibbons R L, van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- 9.Haas W, MacColl R, Banas J A. Circular dichroism analysis of the glucan binding domain of Streptococcus mutans glucan binding protein-A. Biochim Biophys Acta. 1998;1384:112–120. doi: 10.1016/s0167-4838(98)00005-3. [DOI] [PubMed] [Google Scholar]
- 10.Haas W, Banas J A. The glucan-binding domain of the Streptococcus mutans glucan-binding protein. Adv Exp Med Biol. 1997;418:707–708. doi: 10.1007/978-1-4899-1825-3_165. [DOI] [PubMed] [Google Scholar]
- 11.Hazlett K R O, Michalek S M, Banas J A. Inactivation of the gbpA gene of Streptococcus mutans increases virulence and promotes in vivo accumulation of recombinations between the glucosyltransferase B and C genes. Infect Immun. 1998;66:2180–2185. doi: 10.1128/iai.66.5.2180-2185.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolter R, Losick R. One for all and all for one. Science. 1998;280:226–227. doi: 10.1126/science.280.5361.226. [DOI] [PubMed] [Google Scholar]
- 13.Larsen T, Fiehn N E. Development of a flow method for susceptibility testing of oral biofilms in vitro. APMIS. 1995;103:339–344. doi: 10.1111/j.1699-0463.1995.tb01117.x. [DOI] [PubMed] [Google Scholar]
- 14.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mack D, Nedelmann M, Krokotsch A, Schwarzkopf A, Heesemann J, Laufs R. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect Immun. 1994;62:3244–3253. doi: 10.1128/iai.62.8.3244-3253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munro C, Michalek S M, Macrina F L. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect Immun. 1991;59:2316–2323. doi: 10.1128/iai.59.7.2316-2323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Toole G A, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 18.Russell R R B. Glucan-binding proteins of Streptococcus mutans serotype c. J Gen Microbiol. 1979;112:197–201. doi: 10.1099/00221287-112-1-197. [DOI] [PubMed] [Google Scholar]
- 19.Sato Y, Yamamoto Y, Kizaki H. Cloning and sequence analysis of the gbpC gene encoding a novel glucan-binding protein of Streptococcus mutans. Infect Immun. 1997;65:668–675. doi: 10.1128/iai.65.2.668-675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schilling K M, Carson R G, Bosko C A, Golikeri G D, Bruinooge A, Hoyberg K, Waller A M, Hughes N P. A microassay for bacterial adherence to hydroxyapatite. Colloids Surf. 1994;3:31–38. [Google Scholar]
- 21.Shiroza T, Udea S, Kuramitsu H K. Sequence analysis of the gtfB gene from Streptococcus mutans. J Bacteriol. 1987;169:4263–4270. doi: 10.1128/jb.169.9.4263-4270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith D J, Akita H, King W F, Taubman M A. Purification and antigenicity of a novel glucan-binding protein of Streptococcus mutans. Infect Immun. 1994;62:2545–2552. doi: 10.1128/iai.62.6.2545-2552.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith D J, Taubman M A. Experimental immunization of rats with a Streptococcus mutans 59-kilodalton glucan-binding protein protects against dental caries. Infect Immun. 1996;64:3069–3073. doi: 10.1128/iai.64.8.3069-3073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueda S, Kuramitsu H K. Molecular basis for the spontaneous generation of colonization-defective mutants of Streptococcus mutans. Mol Microbiol. 1988;2:135–140. doi: 10.1111/j.1365-2958.1988.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 25.Ueda S, Shiroza T, Kuramitsu H K. Sequence analysis of the gtfC gene from Streptococcus mutans GS-5. Gene. 1988;69:101–109. doi: 10.1016/0378-1119(88)90382-4. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita Y, Bowen W H, Kuramitsu H K. Molecular analysis of a Streptococcus mutans strain exhibiting polymorphism in the tandem gtfB and gtfC genes. Infect Immun. 1992;60:1618–1624. doi: 10.1128/iai.60.4.1618-1624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita Y, Bowen W H, Burne R A, Kuramitsu H K. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]