Abstract
The US Food and Drug Administration (FDA) created the Sentinel System in response to a requirement in the FDA Amendments Act of 2007 that the agency establish a system for monitoring risks associated with drug and biologic products using data from disparate sources. The Sentinel System has completed hundreds of analyses, including many that have directly informed regulatory decisions. The Sentinel System also was designed to support a national infrastructure for a learning health system. Sentinel governance and guiding principles were designed to facilitate Sentinel’s role as a national resource. The Sentinel System infrastructure now supports multiple non-FDA projects for stakeholders ranging from regulated industry to other federal agencies, international regulators, and academics. The Sentinel System is a working example of a learning health system that is expanding with the potential to create a global learning health system that can support medical product safety assessments and other research.
Keywords: Sentinel, learning health system, real-world data, real-world evidence
FDA SENTINEL SYSTEM AND A COMMON INFORMATICS INFRASTRUCTURE
The US Food and Drug Administration (FDA) created the Sentinel System in response to a requirement in the FDA Amendments Act of 2007 (FDAAA 2007) that FDA “link and analyze safety data from multiple sources” to monitor risks associated with drug and biologic products.1 The system’s initial instantiation, the Mini-Sentinel pilot (2009–2015), developed and tested a distributed data network and its associated analytic infrastructure as well as the governance needed to support FDA’s mission.2,3 Since 2016, the FDA has used the Sentinel System to meet its regulatory requirements under FDAAA, assess the characteristics of real-world data (RWD) sources, better use RWD in distributed data environments,4,5 and better understand how to generate real-world evidence (RWE) to support regulatory decision-making.6–8
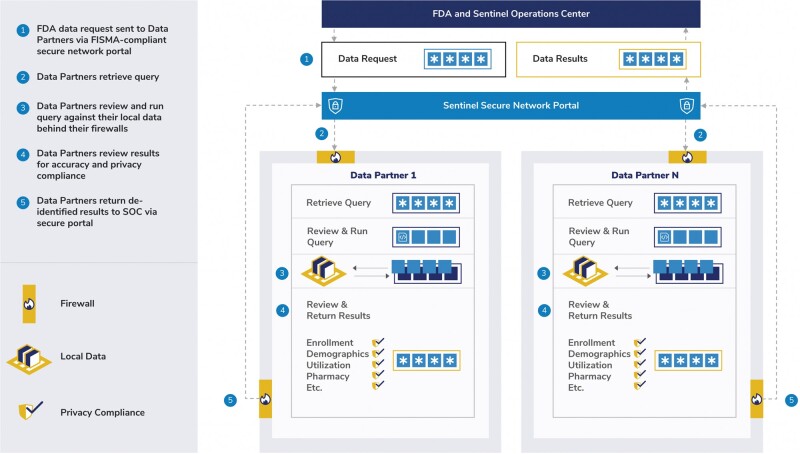
The Sentinel System uses a secure distributed data network, common data model (Sentinel Common Data Model),9 curated data, and distributed analytic tools to conduct analyses in more than a dozen data partners. Data partners maintain physical and operational control of their data, which are collected as part of routine clinical practice or operations. They regularly transform their source data into the version-controlled Sentinel Common Data Model and have to pass a robust data quality assurance process before the transformed data can be used for any queries. Health insurance claims are the primary data source, augmented by electronic health record (EHR) data for about 5% of available health plan members.10 Sentinel also has access to several EHR-only data sources.11,12 Work is directed by FDA and coordinated by the Sentinel Operations Center.13 The Sentinel Operations Center has created a suite of publicly available analytic tools compatible with the Sentinel Common Data Model to standardize and expedite the execution of queries within the distributed data network. Figure 1 shows the workflow of a typical Sentinel query. The Sentinel Common Data Model and analytic tools enable rapid query creation, execution, and reporting ranging from simple descriptive queries to complex comparative analyses. Over the years, the System’s capabilites have been expanded to include new data domains (eg, laboratory results, prescriptions, inpatient medication administrations, mother-infant linkage, patient-reported measures),9 updated data characterization and curation,14 enhanced analytic tools (including tools for assessing medication use in pregnancy and maternal and infant outcomes),15–23 and new data sources.
Figure 1.
Architecture of the US Food and Drug Administration Sentinel System. FDA: Food and Drug Administration; FISMA: Federal Information Security Management Act; SOC: Sentinel Operations Center.
Sentinel governing principles were jointly developed by the Sentinel Operations Center, FDA, and scientific and data partners to facilitate Sentinel’s role as a national resource. These principles include:
Contracting language that allows data partners to use their curated Sentinel Common Data Model-formatted database for any purpose while maintaining robust conflict of interest policies to ensure independence of evaluations24,25;
A data modeling approach focused on the most granular data elements possible to maximize analytic flexibility and extensibility13;
Public posting of Sentinel reports, tools, data curation and other processes, and lessons learned9,26–28;
Public training and resources to help others use the Sentinel Common Data Model and tools29,30; and
Support for improving the use of RWD, including approaches for extracting and using EHR data31 and collecting data directly from patients.7
USES OF SENTINEL’S COMMON INFORMATICS INFRASTRUCTURE TO SUPPORT A LEARNING HEALTH SYSTEM
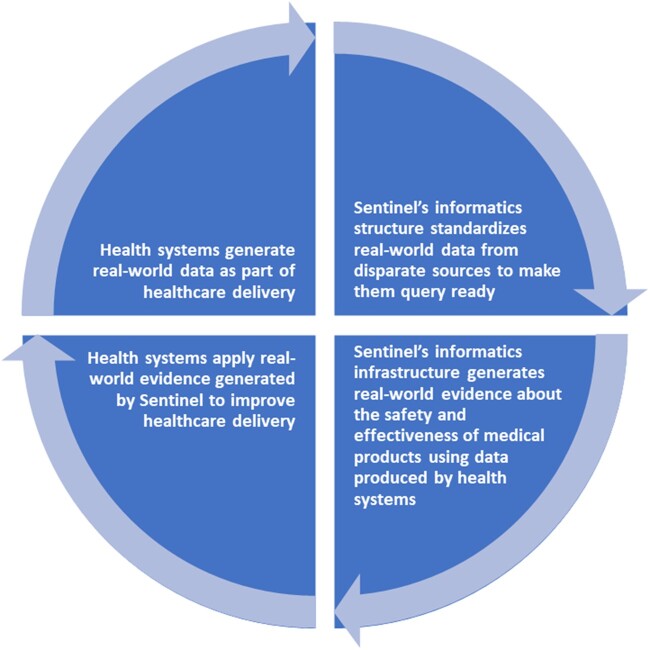
The use of RWD collected by a diverse group of health plans and delivery systems for evidence generation, along with the guiding principles described above, allows the Sentinel System to support FDA’s obligations under FDAAA and regulatory decision-making, and also more broadly serve as a national infrastructure for a learning health system, in which evidence is generated and applied as part of real-world clinical care (Figure 2).3,32,33 The Sentinel System has completed hundreds of analyses and published approximately 200 papers in the peer-reviewed literature,34 with results used to inform regulatory decisions35 and FDA Advisory Committee meetings.36 Going beyond medical product safety, FDA has used the Sentinel infrastructure to gather information about the performance of its regulated medical products, including interactions and interventions with patients. The FDA built a mobile application, the MyStudies app, to obtain patient reported data not available in the Sentinel Common Data Model. The FDA MyStudies app lets patients provide data that can be linked to traditional clinical trials, real-world pragmatic trials, observational studies, and registries and obtain electronic consent.7,37 FDA also sponsored the IMPACT Afib (IMplementation of a randomized controlled trial to imProve treatment with oral AntiCoagulanTs in patients with Atrial Fibrillation) trial, a proof-of-concept randomized trial conducted using the Sentinel infrastructure to inform future interventional studies that are designed to utilize existing healthcare data.6,38 These projects both inform FDA’s understanding of RWE generation and provide examples and tools for the larger research community. FDA also used Sentinel to describe the natural history and epidemiology of COVID-19.11
Figure 2.
Uses of Sentinel’s common informatics infrastructure to support a learning health system.
Further, approximately 40 publicly reported large-scale research projects are known to have used or are using the Sentinel informatics infrastructure; many other projects and systems use Sentinel informatics infrastructure for internal decision-making. These “leveraged projects” use Sentinel curated data, Sentinel Common Data Model, analytic tools, and secure distributed querying architecture on behalf of investigators at, or funded by, the US National Institutes of Health, the US Centers for Disease Control and Prevention (CDC), the Patient-Centered Outcomes Research Institute, the Reagan-Udall Foundation’s Innovation in Medical Evidence Development and Surveillance, the Academy of Managed Care Pharmacy Biologics and Biosimilars Collective Intelligence Consortium, and individual pharmaceutical companies.39,40 Details of these projects are described in Table 1. Like Sentinel, participation by data partners in these leveraged projects is completely voluntary. Many of these projects use Sentinel for activities similar to FDA’s use, namely medical product safety surveillance, including studies of medication safety, vaccine safety, and medication and vaccine use during pregnancy. In the coming years, many of the ongoing studies will be published and several will be submitted to FDA and European Medicines Agency as part of regulatory commitments. Importantly, several projects use the infrastructure and curated data for studies unrelated to medical product safety surveillance, such as patterns of cancer screening, diffusion of medical products, and natural history and epidemiology of disease.41
Table 1.
Leveraged projects that have used the US FDA Sentinel resources, by funding source
Treatment patterns among patients with epilepsy
| Funding source |
Leveraged project | Reference | |
|---|---|---|---|
| Organization | Brief information | ||
| Biologics and Biosimilars Collective Intelligence Consortium (BBCIC) |
|
Completed | |
|
Assessment of the utilization and patient characteristics related to the use of biologics and biosimilars
| |||
| Characterization of the use of originator and follow-on insulin products and users of these products | 74 , 75 | ||
| Utilization patterns and patient characteristics for anti-inflammatory originator biologics and their biosimilars | 76 | ||
| Utilization patterns and patient characteristics for short-acting granulocyte-colony stimulating factor (G-CSF) products | 77 | ||
| Utilization patterns and patient characteristics for the originator and follow-on insulin glargine drugs | 78 | ||
| Utilization patterns and patient characteristics for trastuzumab originator and biosimilar products | 79 | ||
| Utilization patterns and patient characteristics for erythropoietin stimulating agents in hemodialysis patients | White paper in progress to be published on the BBCIC website (www.bbcic.org) | ||
|
Examination of clinical outcomes associated with biologics use
| |||
| Medical-attended severe hypoglycemia, modified major adverse cardiac events, and hemoglobin A1c level related to long- and intermediate-acting insulin | 80 | ||
| Rates of serious infections among autoimmune disease patients using anti-inflammatory biologics | 81 | ||
| G-CSF use in patients with breast or lung cancer receiving and risk of chemotherapy-induced febrile neutropenia | 82 | ||
| Methodologic studies/thought leadership | |||
| Approaches for comparative analyses for product switching | 83 | ||
| Use of National Drug Codes of biologics and biosimilars in physician-office claims | 84 | ||
| International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) to ICD-10-CM codes mapping for conditions of interest | 85 | ||
| Barriers and facilitators to conduct high-quality, large-scale safety and comparative effectiveness research: the Biologics and Biosimilars Collective Intelligence Consortium experience | 86 | ||
| Ongoing | |||
| Inferential study to examine the comparative effectiveness and safety study of G-CSFs | No publications yet | ||
| Utilization patterns and patient characteristics for bevacizumab originator and biosimilar products | No publications yet | ||
| Innovation in Medical Evidence Development and Surveillance (IMEDS) |
|
Completed | |
| Evaluation of venous thromboembolism (VTE) rates in new users of second and fourth generation oral contraceptives | 87 | ||
| Assessment of the impact of the FDA’s 2010 proton pump inhibitor (PPI) class label change on the PPI dispensing patterns, incident fractures, and osteoporosis screening or interventions | 88 | ||
| Evaluation of the risks of VTE among rheumatoid arthritis patients treated with biologic and nonbiologic disease-modifying antirheumatic drugs (DMARDs) | 89 | ||
| Assessment of pain medication treatment patterns and incidence of total join replacement in persons with osteoarthritis | 90 | ||
| Ongoing | |||
| Multiple studies for FDA and European Medicines Agency (EMA) commitments, including a study examining the risk of angioedema with a drug for heart failure, a study of diabetic ketoacidosis risk associated with a diabetes medication, and an investigation of pregnancy exposures and outcomes among women treated with a biologic product for psoriasis | 91 , 92 | ||
| National Institutes of Health (NIH) Collaboratory Distributed Research Network (DRN) |
|
Completed | |
| Cancer screening rates and follow-up screenings for breast, colorectal, and cervical cancers. Used Sentinel analytic tools and data to assess screening rates among health plan members, the rate of abnormal findings, and the rate and timing of follow-up screenings after abnormal findings | 41 | ||
| Assessment of the prevalence of Alzheimer’s disease and dementia in Medicare Advantage populations | 93 | ||
| A multisite study of chemotherapy-induced peripheral neuropathy among over 400 000 patients receiving neurotoxic and nonneurotoxic chemotherapies; results were used to support an NIH grant application | 94 | ||
| Statin use in older patients with and without cardiovascular disease and type 2 diabetes mellitus; feasibility assessment for a pragmatic clinical trial | 95 | ||
| Antibiotic dispensing following pediatric ambulatory and emergency department encounters | 46 | ||
| Considerations for using distributed research networks to conduct aspects of randomized trials | 96 | ||
| Antidopaminergic-Antiparkinsonian medication prescribing cascade in persons with Alzheimer’s disease | 97 | ||
| Practical challenges in the conduct of pragmatic trials embedded in health plans: lessons of IMPACT-AFib, an FDA-Catalyst trial | 98 | ||
| Prescribing cascades in persons with Alzheimer’s disease: engaging patients, caregivers, and providers in a qualitative evaluation of print educational materials | 99 | ||
| Regulated industry and others |
|
Completed | |
| Feasibility assessment for an observational study on the effect of long-acting beta agonists with inhaled corticosteroid therapy on asthma mortality | 100 | ||
| Treatment patterns among patients with epilepsy | No publications yet | ||
| Vaccine use during pregnancy | 101 | ||
| Comparative effectiveness of novel asthma therapies | 102 | ||
| Replication of an FDA Sentinel System report103 on the risks of acute myocardial infarction and stroke among mirabegron users | 60 | ||
| Comparison of the Observational Medical Outcomes Partnership (OMOP) and Mini-Sentinel Common Data Models, focusing on data model applicability to signal detection and early signal refinement | 104 | ||
| Ongoing | |||
| Safety and effectiveness of an adult vaccine (multiple studies in different patient populations); 2 of these studies are supporting FDA regulatory commitments, and one is supporting an EMA requirement | 105 | ||
| Evaluation of the safety and effectiveness of Covid-19 vaccines in the real-world environment; in collaboration with multiple industry sponsors | No publications yet | ||
| Descriptive analysis of exposure to monoclonal antibodies (asthma, systemic lupus erythematous) during pregnancy to support ongoing registries for these products | No publications yet | ||
SELECT EXAMPLES OF HOW SENTINEL CONTRIBUTES TO A LEARNING HEALTH SYSTEM
Use of the Sentinel System by FDA has led to multiple labeling changes or decisions that no labeling changes were needed. These regulatory decisions, which were informed by data generated from clinical care, help guide clinical practice. For example, a Sentinel analysis found an elevated risk of nonmelanoma skin cancer associated with long-term use of hydrochlorothiazide, a commonly used antihypertensive medication.42 The results were consistent with findings from studies conducted in Europe. FDA used the Sentinel results to make a label change to all hydrochlorothiazide-containing products to include information about the risk of nonmelanoma skin cancer.43 FDA has also used the Sentinel System to examine systemic corticosteroid use for COVID-19 in outpatient settings.44 The study found increasing use of systemic corticosteroids to treat outpatient COVID-19 cases. Findings from the analysis were used in a health advisory by the CDC to advise against using systemic corticosteroid in patients with mild to moderate COVID-19.45
Beyond FDA, the Sentinel informatics infrastructure has been used by the National Institutes of Health to inform clinical trial designs to evaluate patterns in cancer screening and care, and to assess antibiotic prescribing patterns. A study found that potentially inappropriate antibiotic prescribing patterns decreased unevenly and that antibiotic stewardship initiatives in the outpatient setting were warranted.46 The Sentinel informatics infrastructure is also being used to evaluate the safety and effectiveness of COVID-19 vaccines in collaboration with multiple industry sponsors. These studies are examples of how Sentinel is being used as a national resource to support a learning health system.
SENTINEL HELPING EXPAND COLLABORATIONS IN THE UNITED STATES AND ACROSS THE GLOBE
Sentinel was built on the experiences of other research networks47 (eg, the CDC-funded Vaccine Safety Datalink,48 the Health Care Systems Research Network,49 the National Institutes of Health-funded Cancer Research Network50) and now other networks have adapted the Sentinel Common Data Model and tools. The Patient-Centered Clinical Research Network (PCORnet)51 common data model and analytic toolkit were based on the Sentinel Common Data Model and tools, and PCORnet adopted the same secure distributed networking software (PopMedNet) as Sentinel.28 This alignment has facilitated collaboration between PCORnet and FDA, with several PCORnet partners joining Sentinel as collaborating institutions and several Sentinel partners joining PCORnet. FDA collaboration with PCORnet includes COVID-19 projects,12,52 cofunding of the PCORnet RELIANCE (RofLumilast or Azithromycin to preveNt COPD Exacerbations) trial that will link trial data with the Medicare fee-for-service data to provide additional information on the primary and select secondary outcomes; the project will also test distributed regression methods with vertically partitioned data.53
FDA is leveraging the Sentinel infrastructure and lessons learned to help establish international collaborations with other regulatory authorities and data networks. Recent examples include an investigation of the impact of the recall of angiotensin receptor blockers due to nitrosamine contamination on the use of these medications54 and COVID-19 related activities.55 Health Canada and researchers at the Canadian Network for Observational Drug Effect Studies (CNODES) have adopted the Sentinel Common Data Model and analytic approaches,56,57 enabling the exact same analysis to be run in CNODES as among Sentinel data partners.58 Through a partnership with the University of Southern Denmark and the Danish Medicines Agency, cohorts extracted from the Danish National Healthcare Databases have been formatted in the Sentinel Common Data Model for joint analyses. The Taiwan Ministry of Science and Technology has funded transformation of the National Health Insurance Research Database, which includes longitudinal claims data with information on over 23 million individuals, into the Sentinel Common Data Model.59 The use of the Sentinel Common Data Model by numerous countries is efficient and convenient for conducting studies but more importantly, it ensures that studies are conducted in the exact same way, resulting in true study replication in different data sources and patient populations. In response to frequent requests for information from regulatory authorities around the world, FDA and the Sentinel Operations Center have provided technical advice on how to operate a secure distributed data network, Sentinel Common Data Model, data quality review, and analytic tools for RWE generation to regulators and researchers around the world, including Singapore, Canada, South Korea, Japan, Taiwan, European Union, and China, and to promote worldwide regulatory collaborations.
Other uses of the Sentinel System infrastructure include analyses conducted by pharmaceutical companies and contract research organizations60 that have formatted their internal data resources into the Sentinel Common Data Model to enable use of the Sentinel tools and to help the companies better understand how FDA uses the Sentinel System. These companies often have data in multiple data models (eg, Observational Medical Outcomes Partnership [OMOP], Sentinel Common Data Model) to make use of the analytic tools best suited to their needs. In addition, health data and analytics companies are using Sentinel analytic tools such as its implementation of TreeScan™61—a data mining method to identify unexpected potential adverse events follow medical product use—to support commercial offerings.
AREAS FOR FURTHER INVESTMENT
FDA continues to invest in promoting the expanded use of the Sentinel infrastructure to a broader set of stakeholders. Over the past few years, FDA has conducted multiple public trainings targeting industry, academic, and regulatory stakeholders.29 The trainings regularly reach capacity and are attended by a range of stakeholders. The publicly available Sentinel reports are also becoming a source to guide research. A recent review article described Sentinel analyses focused on nephrology and outlined pathways for researchers to leverage Sentinel infrastructure for generating RWE in renal care.62 Another study used Sentinel reports describing the results of mapping International Classification of Diseases, ninth revision, Clinical Modification (ICD-9-CM) to ICD-10-CM codes, including variation in incidence and prevalence.63
The recently created Sentinel Innovation Center64 is working to improve Sentinel’s capabilities through the development of new approaches for extracting structured and unstructured EHR data for incorporation into Sentinel Common Data Model and use in distributed querying, new ways to identify health outcomes of interest in RWD,65 and investigation of new causal inference methods.66 In addition, the Sentinel Community Building and Outreach Center (CBOC) is focused on expanding the community of users through projects designed to increase awareness of the Sentinel Initiative to a more diverse scientific community, improve usability of the Sentinel tools and infrastructure, and enable stakeholders to more effectively contribute to advancing the scientific foundation of the Sentinel System.67
SUMMARY
The Sentinel System has grown from a pilot program into an important part of FDA’s postmarketing drug safety system. Consistent with FDA’s initial vision, the Sentinel System infrastructure is now supporting multiple non-FDA projects for stakeholders ranging from regulated industry, to other federal agencies, international regulators, and academics.40 Demand for information on how to make use of Sentinel System data resources and tools is growing, and resources to support this growth are expanding. The Sentinel System is a working example of a learning health system that is expanding with the potential to create a global learning health system that can support medical product safety assessments and other research.
Experiences from the Sentinel System’s creation, growth, and use beyond FDA can help inform the creation and expansion of other learning health systems across the globe. Establishing a strong set of governing principles for the collaboration was critical in fostering Sentinel’s initial success by helping all partners focus on meeting FDA’s needs while keeping an eye towards future uses within and beyond FDA. The core principles that fostered collaboration within Sentinel and the expansion of capabilities for FDA while providing governance and technical resources to the community enabled FDA to meet the dual goals of having a robust postmarketing active safety surveillance system and creating a national resource to support evidence generation well-beyond FDA. The Sentinel governance and culture encouraged the data partners to use their Sentinel Common Data Model-formatted data and the Sentinel analytic tools for other purposes and encouraged the Sentinel Operations Center to support leveraged projects; those other uses of Sentinel infrastructure were celebrated as successes and not viewed as competing interests or a distraction. Beyond the core Sentinel governing principles, consistent engagement with stakeholders through public meetings, seminars, trainings, and scientific dissemination were critical to the acceptance of the Sentinel informatics infrastructure as a viable option as a national data resource. It also was critical that the public communications and engagements were scientifically rigorous and transparent. Although this paper focuses on Sentinel, we note many other large-scale collaborations using RWD to generate RWE around the world.68–72 Our hope is that these efforts, taken together and through a spirit of collaboration, can help expand the depth and breadth of research and public health surveillance capabilities across the world.
FUNDING
This work was supported in part by the U.S. Food and Drug Administration (FDA) through the Department of Health and Human Services (HHS) contract number 75F40119F19001.
AUTHOR CONTRIBUTIONS
JSB conceived of the idea for this work. JSB, ABM, YHN, and ST prepared the first draft of the manuscript. All authors revised the manuscript for important intellectual content and approved the final manuscript to be submitted for publication. All authors agree to be accountable for all aspects of the work.
ETHICS APPROVAL
The authors state that no ethical approval was needed.
ACKNOWLEDGMENTS
The authors thank Dayna Sylvester, Sarah Malek, and Juliane Reynolds for their administrative and project management support.
CONFLICT OF INTEREST STATEMENT
None declared.
Contributor Information
Jeffrey S Brown, Department of Population Medicine, Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, Massachusetts, USA.
Aaron B Mendelsohn, Department of Population Medicine, Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, Massachusetts, USA.
Young Hee Nam, Department of Population Medicine, Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, Massachusetts, USA.
Judith C Maro, Department of Population Medicine, Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, Massachusetts, USA.
Noelle M Cocoros, Department of Population Medicine, Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, Massachusetts, USA.
Carla Rodriguez-Watson, Reagan-Udall Foundation for the Food and Drug Administration, Washington, District of Columbia, USA.
Catherine M Lockhart, Biologics and Biosimilars Collective Intelligence Consortium, Alexandria, Virginia, USA.
Richard Platt, Department of Population Medicine, Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, Massachusetts, USA.
Robert Ball, Office of Surveillance and Epidemiology, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland, USA.
Gerald J Dal Pan, Office of Surveillance and Epidemiology, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland, USA.
Sengwee Toh, Department of Population Medicine, Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, Massachusetts, USA.
Data Availability
No new data were generated or analyzed in support of this article.
REFERENCES
- 1. Food and Drug Administration Amendments Act of 2007. Pub. L. No. 110-85. 21 U.S.C. Stat 823. https://www.govinfo.gov/content/pkg/PLAW-110publ85/pdf/PLAW-110publ85.pdf. Accessed February, 2022.
- 2. Platt R, Carnahan RM, Brown JS, et al. The U.S. Food and Drug Administration’s Mini-Sentinel program: status and direction. Pharmacoepidemiol Drug Saf 2012; 21 (Suppl 1): 1–8. [DOI] [PubMed] [Google Scholar]
- 3. Behrman RE, Benner JS, Brown JS, et al. Developing the Sentinel System–a national resource for evidence development. N Engl J Med 2011; 364 (6): 498–9. [DOI] [PubMed] [Google Scholar]
- 4. Ball R, Robb M, Anderson SA, et al. The FDA’s Sentinel Initiative—a comprehensive approach to medical product surveillance. Clin Pharmacol Ther 2016; 99 (3): 265–8. [DOI] [PubMed] [Google Scholar]
- 5. Platt R, Brown JS, Robb M, et al. The FDA Sentinel Initiative—an evolving national resource. N Engl J Med 2018; 379 (22): 2091–3. [DOI] [PubMed] [Google Scholar]
- 6. Cocoros NM, Pokorney SD, Haynes K, et al. FDA-Catalyst—using FDA’s Sentinel Initiative for large-scale pragmatic randomized trials: approach and lessons learned during the planning phase of the first trial. Clin Trials 2019; 16 (1): 90–7. [DOI] [PubMed] [Google Scholar]
- 7. Wyner Z, Dublin S, Chambers C, et al. The FDA MyStudies app: a reusable platform for distributed clinical trials and real-world evidence studies. JAMIA Open 2020; 3 (4): 500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sansing-Foster V, Haug N, Mosholder A, et al. Risk of psychiatric adverse events among Montelukast users. J Allergy Clin Immunol Pract 2021; 9 (1): 385–93.e12. [DOI] [PubMed] [Google Scholar]
- 9. Sentinel Initiative. Sentinel Common Data Model. https://www.sentinelinitiative.org/methods-data-tools/sentinel-common-data-model. Accessed July 14, 2022.
- 10.Sentinel Initiative. How Sentinel Gets Its Data. https://www.sentinelinitiative.org/about/how-sentinel-gets-its-data. Accessed July 14, 2022.
- 11. Cocoros NM, Fuller CC, Adimadhyam S, et al. ; FDA-Sentinel COVID-19 Working Group. A COVID-19-ready public health surveillance system: the Food and Drug Administration’s Sentinel System. Pharmacoepidemiol Drug Saf 2021; 30 (7): 827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sentinel Initiative. Drug Assessments. https://www.sentinelinitiative.org/assessments/drugs. Accessed July 14, 2022.
- 13. Curtis LH, Weiner MG, Boudreau DM, et al. Design considerations, architecture, and use of the Mini-Sentinel distributed data system. Pharmacoepidemiol Drug Saf 2012; 21(Suppl 1): 23–31. [DOI] [PubMed] [Google Scholar]
- 14. Sentinel Initiative. Data Quality Review and Characterization Programs. https://www.sentinelinitiative.org/methods-data-tools/sentinel-common-data-model/data-quality-review-and-characterization-programs. Accessed July 14, 2022.
- 15. Sentinel Initiative. Query Builder. https://www.sentinelinitiative.org/methods-data-tools/routine-querying-tools/query-builder. Accessed July 14, 2022.
- 16. Liu W, Menzin TJ, Woods CM, et al. Phosphodiesterase type 5 inhibitor use among pregnant and reproductive-age women in the United States. Pharmacoepidemiol Drug Saf 2021; 30 (2): 126–34. [DOI] [PubMed] [Google Scholar]
- 17. Bird ST, Gelperin K, Sahin L, et al. First-trimester exposure to gadolinium-based contrast agents: a utilization study of 4.6 million U.S. pregnancies. Radiology 2019; 293 (1): 193–200. [DOI] [PubMed] [Google Scholar]
- 18. Eworuke E, Panucci G, Goulding M, et al. Use of tumor necrosis factor-alpha inhibitors during pregnancy among women who delivered live born infants. Pharmacoepidemiol Drug Saf 2019; 28 (3): 296–304. [DOI] [PubMed] [Google Scholar]
- 19. Taylor LG, Bird ST, Sahin L, et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf 2017; 26 (5): 592–6. [DOI] [PubMed] [Google Scholar]
- 20. Mott K, Reichman ME, Toh S, et al. Use of antidiabetic drugs during pregnancy among U.S. women with livebirth deliveries in the Mini-Sentinel System. BMC Pregnancy Childbirth 2019; 19 (1): 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Illoh OA, Toh S, Andrade SE, et al. Utilization of drugs with pregnancy exposure registries during pregnancy. Pharmacoepidemiol Drug Saf 2018; 27 (6): 604–11. [DOI] [PubMed] [Google Scholar]
- 22. Kawai A. Developing a mother-infant cohort in Sentinel’s PRISM Program as a resource to monitor the safety of vaccine use during pregnancy. In: 33rd International Conference on Pharmacoepidemiology & Therapeutic Risk Management; August 25, 2017; Montréal, Canada.
- 23. Suarez E. Sentinel mother-infant linkage and pregnancy analyses. In: Canadian Mother-Child Cohort (CAMCCO) Active Surveillance 1st Team and Stakeholders Symposium; February 20, 2020; Montréal, Canada.
- 24. Forrow S, Campion DM, Herrinton LJ, et al. The organizational structure and governing principles of the Food and Drug Administration’s Mini-Sentinel pilot program. Pharmacoepidemiol Drug Saf 2012; 21(Suppl 1): 12–7. [DOI] [PubMed] [Google Scholar]
- 25. Sentinel Initiative. Principles and Policies: Data. https://www.sentinelinitiative.org/principles-and-policies-data. Accessed July 14, 2022.
- 26. Sentinel Initiative. Routine Querying Tools. https://www.sentinelinitiative.org/methods-data-tools/routine-querying-tools. Accessed July 14, 2022.
- 27. PopMedNet. PopMedNet. 2012. https://www.popmednet.org/. Accessed July 14, 2022.
- 28. Davies M, Erickson K, Wyner Z, et al. Software-enabled distributed network governance: the PopMedNet experience. EGEMS (Wash DC) 2016; 4 (2): 1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sentinel Initiative. Sentinel Training Center. https://www.sentinelinitiative.org/engage-sentinel/sentinel-training-center. Accessed July 14, 2022.
- 30. Sentinel Initiative. Medicare Claims Synthetic Public Use Files in Sentinel Common Data Model Format. https://www.sentinelinitiative.org/methods-data-tools/software-packages-toolkits/medicare-claims-synthetic-public-use-files-sentinel. Accessed July 14, 2022.
- 31. Gibson TB, Nguyen MD, Burrell T, et al. Electronic phenotyping of health outcomes of interest using a linked claims-electronic health record database: findings from a machine learning pilot project. J Am Med Inform Assoc 2021; 28 (7): 1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedman C, Rubin J, Brown J, et al. Toward a science of learning systems: a research agenda for the high-functioning learning health system. J Am Med Inform Assoc 2015; 22 (1): 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Institute of Medicine. The National Academies Collection: reports funded by National Institutes of Health. In: Grossmann C, Powers B, McGinnis JM, eds. Digital Infrastructure for the Learning Health System: The Foundation for Continuous Improvement in Health and Health Care: Workshop Series Summary. Washington, DC: National Academies Press (US; ); 2011. [PubMed] [Google Scholar]
- 34. Sentinel Initiative. Publications & Presentations. https://www.sentinelinitiative.org/news-events/publications-presentations. Accessed July 14, 2022.
- 35. Sentinel Initiative. FDA Safety Communications & Labeling Changes. https://www.sentinelinitiative.org/news-events/fda-safety-communications-labeling-changes. Accessed July 14, 2022.
- 36. Sentinel Initiative. FDA Advisory Committee Meetings. https://www.sentinelinitiative.org/communications/fda-advisory-committee-meetings. Accessed July 14, 2022.
- 37. Rothschild CW, Dublin S, Brown JS, et al. Use of a mobile app to capture supplemental health information during pregnancy: implications for clinical research. Pharmacoepidemiol Drug Saf 2022; 31 (1): 37–45. [DOI] [PubMed] [Google Scholar]
- 38. Pokorney SD, Cocoros N, Al-Khalidi HR, et al. Effect of mailing educational material to patients with atrial fibrillation and their clinicians on use of oral anticoagulants: a randomized clinical trial. JAMA Netw Open 2022; 5 (5): e2214321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Curtis LH, Brown J, Platt R.. Four health data networks illustrate the potential for a shared national multipurpose big-data network. Health Aff (Millwood) 2014; 33 (7): 1178–86. [DOI] [PubMed] [Google Scholar]
- 40.Sentinel Initiative. Sentinel as a National Resource. https://www.sentinelinitiative.org/methods-data-tools/sentinel-national-resource. Accessed July 14, 2022.
- 41. Raman SR, Brown JS, Curtis LH, et al. Cancer screening results and follow-up using routinely collected electronic health data: estimates for breast, colon, and cervical cancer screenings. J Gen Intern Med 2019; 34 (3): 341–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eworuke E, Haug N, Bradley M, et al. Risk of nonmelanoma skin cancer in association with use of hydrochlorothiazide-containing products in the United States. JNCI Cancer Spectrum 2021; 5 (2): pkab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. U.S. Food & Drug Administration. FDA Approves Label Changes to Hydrochlorothiazide to Describe Small Risk of Non-Melanoma Skin Cancer. https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-label-changes-hydrochlorothiazide-describe-small-risk-non-melanoma-skin-cancer#:~:text=%5B8%2F20%2F2020%5D,HCTZ%20use%20and%20to%20encourage. Accessed July 14, 2022.
- 44. Bradley MC, Perez-Vilar S, Chillarige Y, et al. Systemic corticosteroid use for COVID-19 in US outpatient settings from April 2020 to August 2021. JAMA 2022; 327 (20): 2015–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Centers for Disease Control and Prevention Health Alert Network. Updated Information on Availability and Use of Treatments for Outpatients with Mild to Moderate COVID-19 Who Are at Increased Risk for Severe Outcomes of COVID-19. https://emergency.cdc.gov/han/2022/han00463.asp?ACSTrackingID=USCDC_511-DM80614&ACSTrackingLabel=HAN%20463%20-%20General%20Public&deliveryName=USCDC_511-DM80614. Accessed July 14, 2022.
- 46. Agiro A, Sridhar G, Gordon A, et al. Antibiotic dispensing following pediatric visits in the US emergency departments and outpatient settings from 2006 to 2016. Pharmacol Res Perspect 2019; 7 (5): e00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weeks J, Pardee R.. Learning to share health care data: a brief timeline of influential common data models and distributed health data networks in U.S. Health Care Research. EGEMS (Wash DC) 2019; 7 (1): 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen RT, Glasser JW, Rhodes PH, et al. Vaccine Safety Datalink project: a new tool for improving vaccine safety monitoring in the United States. The Vaccine Safety Datalink Team. Pediatrics 1997; 99 (6): 765–73. [DOI] [PubMed] [Google Scholar]
- 49. Ross TR, Ng D, Brown JS, et al. The HMO research network virtual data warehouse: a public data model to support collaboration. EGEMS (Wash DC) 2014; 2 (1): 1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Doria-Rose VP, Greenlee RT, Buist DSM, et al. Collaborating on data, science, and infrastructure: the 20-year journey of the cancer research network. EGEMS (Wash DC) 2019; 7 (1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Collins FS, Hudson KL, Briggs JP, et al. PCORnet: turning a dream into reality. J Am Med Inform Assoc 2014; 21 (4): 576–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sentinel Initiative. Characteristics of Patients Using Anti-Diabetic Agents in the Patient-Centered Clinical Research Network (PCORnet): An Exploratory Query of a Network of Electronic Health Records. https://www.sentinelinitiative.org/methods-data-tools/methods/characteristics-patients-using-anti-diabetic-agents-patient-centered. Accessed July 14, 2022.
- 53.Sentinel Initiative. FDA-Catalyst Alignment with the CMS Linkage to the PCORI RELIANCE Trial. https://www.sentinelinitiative.org/methods-data-tools/fda-catalyst-projects/fda-catalyst-alignment-cms-linkage-pcori-reliance-trial. Accessed July 14, 2022.
- 54. Sentinel Initiative. Quantitative Assessment of the Impact of Nitrosamine Contamination and Angiotensin Receptor Blockers (ARB) Recall on ARB Utilization: A Multinational Study. https://www.sentinelinitiative.org/methods-data-tools/methods/quantitative-assessment-impact-nitrosamine-contamination-and-angiotensin. Accessed July 14, 2022.
- 55. Sentinel Initiative. COVID-19 Pregnancy Study Implementation. https://www.sentinelinitiative.org/methods-data-tools/methods/covid-19-pregnancy-study-implementation. Accessed July 14, 2022.
- 56. Suissa S, Henry D, Caetano P, et al. CNODES: the Canadian network for observational drug effect studies. Open Med 2012; 6 (4): e134. [PMC free article] [PubMed] [Google Scholar]
- 57. Platt RW, Platt R, Brown JS, et al. How pharmacoepidemiology networks can manage distributed analyses to improve replicability and transparency and minimize bias. Pharmacoepidemiol Drug Saf 2020; 29 (S1): 3–7. [DOI] [PubMed] [Google Scholar]
- 58. Platt R. Medical product and performance evaluation programs using distributed data sources. In: 33rd Annual ICPE Conference; August, 2017; Montreal, CN.
- 59. Huang K, Lin F-J, Ou H-T, et al. Building an active medical product safety surveillance system in Taiwan: adaptation of the U.S. Sentinel System common data model structure to the National Health Insurance Research Database in Taiwan. Pharmacoepidemiol Drug Saf 2021; 30 (1): 97–101. [DOI] [PubMed] [Google Scholar]
- 60. Simeone JC, Nordstrom BL, Appenteng K, et al. Replication of Mini-Sentinel study assessing Mirabegron and cardiovascular risk in non-Mini-Sentinel databases. Drugs Real World Outcomes 2018; 5 (1): 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang SV, Maro JC, Baro E, et al. Data mining for adverse drug events with a propensity score-matched tree-based scan statistic. Epidemiology 2018; 29 (6): 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Adimadhyam S, Barreto EF, Cocoros NM, et al. Leveraging the capabilities of the FDA’s Sentinel System to improve kidney care. J Am Soc Nephrol 2020; 31 (11): 2506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nam YH, Mendelsohn AB, Panozzo CA, et al. Health outcomes coding trends in the US Food and Drug Administration’s Sentinel System during transition to International Classification of Diseases-10 coding system: a brief review. Pharmacoepidemiol Drug Saf 2021; 30 (7): 838–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sentinel Innovation Center. Sentinel Innovation Center Master Plan. 2021. https://www.sentinelinitiative.org/sites/default/files/communications/publications-presentations/IC-Master-Plan.pdf. Accessed July 14, 2022.
- 65. Brown JS, Maro JC, Nguyen M, et al. Using and improving distributed data networks to generate actionable evidence: the case of real-world outcomes in the Food and Drug Administration’s Sentinel System. J Am Med Inform Assoc 2020; 27 (5): 793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Desai RJ, Matheny ME, Johnson K, et al. Broadening the reach of the FDA Sentinel System: a roadmap for integrating electronic health record data in a causal analysis framework. NPJ Digit Med 2021; 4 (1): 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sentinel Community Building and Outreach Center. Sentinel Community Building and Outreach Center Master Plan. 2021. https://www.sentinelinitiative.org/sites/default/files/communications/publications-presentations/CBOC-Master-Plan.pdf. Accessed July 14, 2022.
- 68. Haendel MA, Chute CG, Bennett TD, et al. ; N3C Consortium. The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment. J Am Med Inform Assoc 2021; 28 (3): 427–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Brat GA, Weber GM, Gehlenborg N, et al. International electronic health record-derived COVID-19 clinical course profiles: the 4CE Consortium. NPJ Digit Med 2020; 3 (1): 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Recalde M, Roel E, Pistillo A, et al. Characteristics and outcomes of 627 044 COVID-19 patients living with and without obesity in the United States, Spain, and the United Kingdom. Int J Obes (Lond) 2021; 45 (11): 2347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. European Health Data & Evidence Network. https://www.ehden.eu/. Accessed July 14, 2022.
- 72. Andersen M, Bergman U, Choi N-K, et al. ; AsPEN Collaborators. The Asian Pharmacoepidemiology Network (AsPEN): promoting multi-national collaboration for pharmacoepidemiologic research in Asia. Pharmacoepidemiol Drug Saf 2013; 22 (7): 700–4. [DOI] [PubMed] [Google Scholar]
- 73. AMCP Task Force on Biosimilar Collective Intelligence Systems. Utilizing data consortia to monitor safety and effectiveness of biosimilars and their innovator products. J Manag Care Spec Pharm 2015; 21 (1): 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McMahill-Walraven CN, Kent DJ, Panozzo CA, et al. Harnessing the biologics and biosimilars collective intelligence consortium to evaluate patterns of care. J Manag Care Spec Pharm 2019; 25 (11): 1156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McMahill-Walraven C, Kent D, Marshall J, et al. Differentiation of adults with type 1 versus type 2 diabetes in administrative claims analysis: experience from the Biologics and Biosimilars Collective Intelligence Consortium. AMCP Nexus; 2018; BBCIC.
- 76. Mendelsohn AB, Nam YH, Marshall J, et al. Utilization patterns and characteristics of users of biologic anti-inflammatory agents in a large, US commercially insured population. Pharmacol Res Perspect 2021; 9 (1): e00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mendelsohn AB, Marshall J, McDermott CL, et al. Patient characteristics and utilization patterns of short-acting recombinant granulocyte colony-stimulating factor (G-CSF) biosimilars compared to their reference product. Drugs Real World Outcomes 2021; 8 (2): 125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nam YH, Marshall J, McDermott C, et al. Utilization patterns and characteristics of patients treated with the originator and the follow-on insulin glargine in the United States. AMCP; 2021; Virtual.
- 79. Nam YH, Mendelsohn AB, Marshall J, et al. Utilization and patient characteristics for the trastuzumab originator, biosimilars, and other HER2 inhibitors in the United States. AMCP Nexus; 2021; Denver, CO.
- 80. Kent DJ, McMahill-Walraven CN, Panozzo CA, et al. Descriptive analysis of long- and intermediate-acting insulin and key safety outcomes in adults with type 2 diabetes mellitus. J Manag Care Spec Pharm 2019; 25 (11): 1162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang J, Sridhar G, Barr CE, et al. Incidence of serious infections and design of utilization and safety studies for biologic and biosimilar surveillance. J Manag Care Spec Pharm 2020; 26 (4): 417–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pawloski PA, McDermott CL, Marshall JH, et al. BBCIC research network analysis of first-cycle prophylactic G-CSF use in patients treated with high-neutropenia risk chemotherapy [published online ahead of print Aug 16, 2021]. J Natl Compr Canc Netw 2021; doi: 10.6004/jnccn.2021.7027. [DOI] [PubMed] [Google Scholar]
- 83. Desai RJ, Kim SC, Curtis JR, et al. ; Biologics and Biosimilars Collective Intelligence Consortium (BBCIC) Switching Workgroup. Methodologic considerations for noninterventional studies of switching from reference biologic to biosimilars. Pharmacoepidemiol Drug Saf 2020; 29 (7): 757–69. [DOI] [PubMed] [Google Scholar]
- 84. Zhang J, Haynes K, Mendelsohn AB, et al. Capture of biologic and biosimilar dispensings in a consortium of U.S.-based claims databases: utilization of national drug codes and Healthcare Common Procedure Coding System modifiers in medical claims. Pharmacoepidemiol Drug Saf 2020; 29 (7): 778–85. [DOI] [PubMed] [Google Scholar]
- 85. He M, Santiago Ortiz AJ, Marshall J, et al. Mapping from the International Classification of Diseases (ICD) 9th to 10th revision for research in biologics and biosimilars using administrative healthcare data. Pharmacoepidemiol Drug Saf 2020; 29 (7): 770–7. [DOI] [PubMed] [Google Scholar]
- 86. Lockhart CM, McDermott CL, Felix T, et al. Barriers and facilitators to conduct high-quality, large-scale safety and comparative effectiveness research: the Biologics and Biosimilars Collective Intelligence Consortium experience. Pharmacoepidemiol Drug Saf 2020; 29 (7): 811–3. [DOI] [PubMed] [Google Scholar]
- 87. Bate A, Sobel RE, Marshall J, et al. Oral contraceptives and VTE across the Sentinel Data Network—an IMEDS evaluation pilot assessment. In: 32nd International Conference on Pharmacoepidemiology & Therapeutic Risk Management; 2016; Dublin, Ireland.
- 88. Sobel RE, Bate A, Marshall J, et al. Do FDA label changes work? Assessment of the 2010 class label change for proton pump inhibitors using the Sentinel System’s analytic tools. Pharmacoepidemiol Drug Saf 2018; 27 (3): 332–9. [DOI] [PubMed] [Google Scholar]
- 89. Maro JC, Menzin T, Hornbuckle K, et al. Risk of venous thromboembolism in rheumatoid arthritis patients treated with biologic and non-biologic DMARDS. Ann Rheum Dis 2018; 77(Suppl 2): 932. [Google Scholar]
- 90. Shinde M, Rodriguez-Watson C, Zhang T, et al. Patient characteristics, pain treatment patterns, and incidence of total joint replacement in a population with osteoarthritis. In: 37th International Conference on Pharmacoepidemiology & Therapeutic Risk Management; 2021; Virtual.
- 91. Connolly J, Mendelsohn A, Rodriguez-Watson C, et al. Cohort study assessing angioedema risk in black heart failure patients using angiotensin-converting enzyme inhibitors in the United States—interim results. In: 37th International Conference on Pharmacoepidemiology & Therapeutic Risk Management; 2021; Virtual.
- 92. Huang T-Y, Rodriguez-Watson C, Wang T, et al. A new United States database for pharmacoepidemiology studies in type 2 diabetes mellitus: the IMEDS distributed database. In: 37th International Conference on Pharmacoepidemiology & Therapeutic Risk Management; 2021; Virtual.
- 93. Jutkowitz E, Bynum JPW, Mitchell SL, et al. Diagnosed prevalence of Alzheimer’s disease and related dementias in Medicare Advantage plans. Alzheimers Dement (Amst) 2020; 12 (1): e12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gewandter JS, Kleckner AS, Marshall JH, et al. Chemotherapy-induced peripheral neuropathy (CIPN) and its treatment: an NIH Collaboratory study of claims data. Support Care Cancer 2020; 28 (6): 2553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Panozzo CA, Curtis LH, Marshall J, et al. Incidence of statin use in older adults with and without cardiovascular disease and diabetes mellitus, January 2008–March 2018. PLoS One 2019; 14 (12): e0223515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Marsolo KA, Brown JS, Hernandez AF, et al. Considerations for using distributed research networks to conduct aspects of randomized trials. Contemp Clin Trials Commun 2020; 17: 100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Singh S, Cocoros NM, Haynes K, et al. Antidopaminergic-Antiparkinsonian medication prescribing cascade in persons with Alzheimer’s disease. J Am Geriatr Soc 2021; 69 (5): 1328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Garcia CJ, Haynes K, Pokorney SD, et al. Practical challenges in the conduct of pragmatic trials embedded in health plans: lessons of IMPACT-AFib, an FDA-catalyst trial. Clin Trials 2020; 17 (4): 360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bloomstone S, Anzuoni K, Cocoros N, et al. Prescribing cascades in persons with Alzheimer’s disease: engaging patients, caregivers, and providers in a qualitative evaluation of print educational materials. Ther Adv Drug Saf 2020; 11: 2042098620968310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Johannes CB, McQuay LJ, Midkiff KD, et al. The feasibility of using multiple databases to study rare outcomes: the potential effect of long-acting beta agonists with inhaled corticosteroid therapy on asthma mortality. Pharmacoepidemiol Drug Saf 2017; 26 (4): 446–58. [DOI] [PubMed] [Google Scholar]
- 101. Panozzo CA, Purcell B, Andrade S, et al. Safety of Trumenba vaccine among pregnant women in the United States: planning and design of a large-scale multi-site observational study. Pharmacoepidemiol Drug Saf 2017; 26 (S2): 410. [Google Scholar]
- 102. Wu AC, McMahon PM, Welch E, et al. Characteristics of new adult users of mepolizumab with asthma in the USA. BMJ Open Resp Res 2021; 8 (1): e001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sentinel Initiative. PROMPT: Mirabegron Surveillance. https://www.sentinelinitiative.org/drugs/assessments/prompt-mirabegron-surveillance. Accessed July 14, 2022.
- 104. Xu Y, Zhou X, Suehs BT, et al. A comparative assessment of observational medical outcomes partnership and Mini-Sentinel common data models and analytics: implications for active drug safety surveillance. Drug Saf 2015; 38 (8): 749–65. [DOI] [PubMed] [Google Scholar]
- 105. GSK. Targeted safety study to assess the real-world safety of Shingrix vaccine in adults aged 50 and older in the United States (US). 2020. https://www.gsk-studyregister.com/en/trial-details/?id=209452. Accessed July 14, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this article.