Abstract
Objective
Integration of environmentally sustainable digital health interventions requires robust evaluation of their carbon emission life-cycle before implementation in healthcare. This scoping review surveys the evidence on available environmental assessment frameworks, methods, and tools to evaluate the carbon footprint of digital health interventions for environmentally sustainable healthcare.
Materials and Methods
Medline (Ovid), Embase (Ovid). PsycINFO (Ovid), CINAHL, Web of Science, Scopus (which indexes IEEE Xplore, Springer Lecture Notes in Computer Science and ACM databases), Compendex, and Inspec databases were searched with no time or language constraints. The Systematic Reviews and Meta-analyses Extension for Scoping Reviews (PRISMA_SCR), Joanna Briggs Scoping Review Framework, and template for intervention description and replication (TiDiER) checklist were used to structure and report the findings.
Results
From 3299 studies screened, data was extracted from 13 full-text studies. No standardised methods or validated tools were identified to systematically determine the environmental sustainability of a digital health intervention over its full life-cycle from conception to realisation. Most studies (n = 8) adapted publicly available carbon calculators to estimate telehealth travel-related emissions. Others adapted these tools to examine the environmental impact of electronic health records (n = 2), e-prescriptions and e-referrals (n = 1), and robotic surgery (n = 1). One study explored optimising the information system electricity consumption of telemedicine. No validated systems-based approach to evaluation and validation of digital health interventions could be identified.
Conclusion
There is a need to develop standardised, validated methods and tools for healthcare environments to assist stakeholders to make informed decisions about reduction of carbon emissions from digital health interventions.
Keywords: carbon emissions, environmental sustainability, digital health technologies, assessment methods and tools
INTRODUCTION
The highest authority on climate science, the Intergovernmental Panel on Climate Change’s most recent report, emphasises the worsening impacts on human and planetary health which are overwhelmingly impacting marginalized and disadvantaged communities globally, thus intensifying climate justice issues.1 The healthcare industry, as the 5th largest contributor to planetary pollution,2,3 is increasingly addressing its contribution to climate change.4 Health information technologies, often referred to as digital health technologies (DHT) and encompassing telehealth, artificial intelligence (AI), the Internet of Things (IoT), and electronic medical/health records (EMR/EHR), may hold solutions to reducing healthcare’s carbon footprint.5 For example, telehealth reduces transport-associated carbon emissions, fuel consumption, the consumption of paper, and personal protective equipment.6 However, DHT’s benefits must be balanced against significant carbon impacts generated by infrastructure such as computer networks and computer systems powering DHTs, the emissions generated by data storage and transfer, rare minerals mining and materials synthesized for technology production, and technology’s end-of-life waste management and disposal problems.7,8
To assess if DHT can be a means of reducing healthcare’s carbon emissions, there needs to be a transparent, standardised, and accessible way to evaluate environmental impacts over the full “life-cycle” before DHT are implemented in healthcare settings. Decision-makers need the knowledge, standardised methods, and validated tools to assist this evaluation process. However, it is not clear what frameworks and methods exist for this context that could prompt busy decision-makers to consider DHTs carbon emissions mitigation. Identifying standardised environmental assessment methods and tools for healthcare would permit cyclical standardised reassessment and identification of efficiencies in the system that may further support carbon emission reduction. Therefore, the availability, dissemination, and implementation of DHT carbon impact methods and validated tools is an important issue considering the worsening impacts of global climate change.1
Several different frameworks exist to help stakeholders consider environmental sustainability when evaluating technology in general. For example, Health Technology Assessment (HTA) involves multidisciplinary, systematic evaluation of properties, effects and/or impacts of healthcare technology that spans medical, socio-economic, and ethical issues related to direct and indirect consequences of health technology.9 HTA is inclusive of drugs, biologicals, devices, medical and surgical procedures, public health programs, settings of care, screening programs, support systems (eg, blood bank, clinical laboratory, EMR, telemedicine systems), and organisational and managerial systems (eg, medication adherence program and healthcare payments).10 To assess the above products in the context of environmental sustainability, the HTA adapts existing environmental frameworks, the Life Cycle Assessment (LCA) of the technology, Environmentally Extended Input-Output Analysis (EEIOA) and Comprehensive Environmental Assessment (CEA).11 Strengths, weaknesses, and examples of the application of these frameworks are described in Table 1.
Table 1.
Description, strengths, and weaknesses of LCA, EEIOA, and CEA frameworks
| Framework | Description | Methods | Strengths | Weaknesses | Examples of application |
|---|---|---|---|---|---|
| Life-Cycle Assessment (LCA) | Methodological framework for assessment and comparison of products’ potential environmental impacts considering the entire product life-cycle.12 |
|
Detailed consideration of the environmental impacts (including climate change, acidification, toxicological stress on human health and ecosystems, noise etc.) during the products’ entire life-cycle.12 |
|
|
| Environmentally Extended Input-Output Analysis (EEIOA) | Analytical framework to calculate hidden or indirect environmental impacts of products.15 | Mathematical input-output matrix using measurements of direct environmental impacts for each studied sector.15 | Simple and rapid method13 to efficiently estimate the carbon emissions generated by each individual unit of output such as upstream and downstream carbon emission of a single product.15 | The method rests on the assumption of homogeneity13 and a narrow focus on environmental impact, if exploring only single environmental impactor (eg, carbon emissions).16 | Method has been applied to analyse water, ecological, biodiversity and nitrogen footprints.15 |
| Comprehensive Environmental Assessment (CEA/CEASS) | Framework and a process, integrating various risk-assessment methods, used for research planning and risk management.17 |
|
Provides a holistic, systems-based and transparent analysis incorporating both qualitative and quantitative information.17 | Includes labor-intensive analytic methods. Limited data or newly emerging issues might result in premature structuring of the judgment process.17 | EPA case studies for selected nanomaterials, sustainability of biofuels.17 |
The LCA framework facilitates methodological estimation of the environmental impacts attributable to the life cycle of a product spanning the design/development, resource extraction, production of the product, production of materials and manufacturing provision, use/consumption, and end-of-life activities (collection, sorting, reuse, recycling, waste disposal).12 The EEIOA framework facilitates calculations between economic consumption activities and their environmental impact. For example, all direct and indirect greenhouse gas emission activities and associated costs caused by a nation can be calculated using this framework.15,18 The CEA consists of a framework and procedures that provide a systematic sequence of processes to organise information about environmental issues arising in the context of a product’s life-cycle. These include: the risk of release of the product’s primary material into the environment (air, water, sediment, and soil), the product’s alteration of environmental conditions (physical, chemical, biological, and social) impacting the release of substances from the product or changes arising from the product’s exposure (cumulative or aggregate) to specific environmental conditions.17
These frameworks have been linked to qualitative and quantitative evaluation methodologies, including Cost-Utility Analysis (CUA), Cost-Benefit Analysis (CBA), Multi-Criteria Decision Analysis (MCDA), and Weight of Evidence (WoE), that assess systems for environmental risk using many sources of data.11 However, it remains to be seen how these frameworks and methods can be applied in the context of DHT interventions. This scoping review examined the evidence on relevant environmental assessment frameworks, methods, instruments and tools, their validity, reliability, feasibility, and usefulness, to calculate a carbon footprint of DHT interventions. The scoping review was guided by two questions:
What methods and assessment tools exist to help health informaticians assess the carbon footprint of DHT in healthcare?
How valid, reliable, feasible, and useful are tools developed to assess carbon footprint of DHT in healthcare?
MATERIALS AND METHODS
Prior to undertaking the scoping review, a detailed scoping review protocol including definition of terms, such as population, concept, and context, was developed and registered with the Open Science Framework.19
Review frameworks
The scoping review employed the Joanna Briggs Scoping Review Methodological Framework using the 5-stage “Population, Concept, Context” (PCC) scoping review framework.20–23 The PRISMA-ScR (Preferred Reporting Items for Systematic Review and Meta-analyses Extension for Scoping Reviews)24 was used to build a search strategy and provide a framework for reporting findings.19
Ethics permission
Ethics permission to conduct the scoping review was not required as the information collected is publicly available through databases. Patients and/or the public were not involved in the design, conduct, reporting, or dissemination of this research. Due to the nature of the study, patient consent for publication was not required.
Inclusion and exclusion criteria
Studies were eligible if they:
reported results of primary research;
described DHT (including data management and storage) supporting digital health interventions as defined by the “Classification of Digital Health Interventions” published by the World Health Organization. This classification scheme organizes digital health interventions into 4 overarching groups based on the targeted primary user as: interventions for clients, interventions for healthcare providers, interventions for health systems and resource managers, and interventions for data services25;
defined, outlined, or mentioned a method and/or assessment instrument or tool that helps assess digital health intervention’s and DHT’s environmental impact;
were based on original data;
applied quantitative, qualitative, or mixed methods;
reported study design that was one or more of: randomized controlled trial; experimental (including quasi-experimental); case control; cohort; case series; or case report;
were systematic or scoping reviews that related to the research question (to ensure thorough acknowledgment of the literature).
To maximise the number of articles identified, no language or time limitations were imposed on the search. Reference lists of selected articles relevant to the research questions were also reviewed and relevant references were identified and added to the articles to screen. Articles were excluded from the scoping review if they did not meet the inclusion criteria or if the publication type was a commentary article; dissertation; conference abstract; or review other than a systematic or scoping review.
Search strategy
The search strategy was developed in consultation with an information specialist who tested the initial search and scanned literature to identify terminology related to carbon footprint calculators, frameworks, assessment methods, instruments, and tools. Subsequent searches were conducted to combine these concepts with informatics, digital health, e-health, medical or health technologies, and related terms. These databases were searched: Medline (Ovid), Embase (Ovid). PsycINFO (Ovid), CINAHL, Web of Science and Scopus, Compendex, and Inspec covering content from ACM, IEEE, and Springer Lecture Notes in Computer Science. The reference list of papers reporting studies selected for inclusion in the scoping review was also searched to identify additional relevant studies. Medline Ovid search strategy is shown in Supplementary Appendix S1.19
Screening, article selection, and data extraction
References identified through database searches were imported to EndNote 20 software, then to Covidence for automatic duplicate removal and screening. Further duplicate articles identified in Covidence were manually removed. The screening process of article and data extraction were performed as per the scoping review protocol with the template for intervention description and replication (TiDieR) Checklist and guideline used to describe an intervention in the context of environmental sustainability.19,26
Data analysis, synthesis, and presentation
Narrative synthesis was used to provide an overview of the findings focusing on specific frameworks, methods, and assessment tools. With respect to evaluations of interventions, the focus was on ease of implementation, strengths, weaknesses, validity, reliability, and the outcomes of interventions. Table 2 and Supplementary Appendix S2 include a rationale for each of the intervention studies listed and how these related to the objectives of the scoping review. The presented data focused on the framework, method, and the assessment tool used.
Table 2.
A summary of the studies that report on frameworks, methods, and tools to estimate carbon emissions of DHTs
| Study details/country | Study design/study settings | Aims/objectives | Framework | Assessment tools | Measured outcomes | Key findings |
|---|---|---|---|---|---|---|
| Bartlett et al. (2022)27/UK | Retrospective audit/Geriatric Medicine Outpatients Clinic. | To estimate the carbon footprint, including. the effect of virtual consultations and the use of personal protective equipment, to inform design of a service that meets the needs of both patients and the environment. | Greenhouse Gas protocol (https://ghgprotocol.org/). |
|
|
|
| Blenkinsop et al. (2021)28/UK | Retrospective audit/Outpatient epilepsy clinics. | To determine potential savings and risks over the short term from telemedicine through virtual clinics. | None reported. |
|
|
|
| Connor et al. (2019)29/UK | Prospective study/Urology outpatient clinic. | To evaluate the clinical, fiscal and environmental impact of a specialist-led acute ureteric colic virtual clinic pathway. | None reported. |
|
|
|
| Di Giacomo & Hakansson (2011)30/Croatia | Methods study/case study. | To propose a method for assessing potential reduction of future CO2 emissions. | Life-Cycle Assessment. | None provided. |
|
|
| Filfilan et al. (2021)31/France | Prospective study/Urology outpatient clinics. | To assess the reduction in carbon footprint of teleconsultation compared to in-person consultations. | Not reported. |
|
|
|
| Garcia-Berna et al. (2021)32/Spain | Case report/settings not defined. | To evaluate the energy efficiency of 5 personal health records and present recommendations to developers; including precise guidelines regarding sustainability and to create more energy-efficient systems. | The Framework for Energy Efficiency Testing to Improve eNvironmental Goals of the Software (FEETINGS). | Energy Efficient Tester (EET). |
|
|
| Habib & Marimuthu (2014)33/Kuwait | Simulation study/Hospital network | To develop a capacity planning tool to reduce greenhouse emissions within a hospital offering telemedicine services by optimizing the task flow and reducing the backbone communications. | Not reported. |
|
Direct proportionality relation between consumed energy and produced carbon associated with data flow and data generated. |
|
| Holmner et al. (2014)34/Sweden | Retrospective audit/2 rehabilitation units. | To evaluate the potential of telemedicine services (videoconferencing technology) to reduce traveling and thus carbon emissions in the healthcare sector. | Life-Cycle Assessment. | None reported. |
|
|
| Paquette & Lin (2019)35/USA | Retrospective audit/virtual outpatient vascular clinic. | To analyse the impact of outpatient telemedicine services on the travel burden of vascular surgery patients regarding distance, time, and cost, and the associated emission of environmental pollutants. | None reported. |
|
Emission of CO2, CO, N2O, volatile organic compounds per mile driven (grams). |
|
| Polisena et al. (2018)11/Canada | Scoping review. | To identify articles on frameworks, methods, or case studies on the environmental impact assessment of health technologies. | Not applicable. | Not applicable. | Not applicable. |
|
| Turley et al. (2011)36/USA | Case study | To develop a model to measure the environmental impact of a single healthcare system-wide use of electronic health records. | EcoHealth Footprint. |
|
|
|
| Vidal-Alaball et al. (2019)37/Spain | Retrospective audit. | To evaluate the effect of a telemedicine program in lowering a procedure’s environmental footprint by reducing the emission of atmospheric pollutants due to a reduction in the number of hospital visits involving journeys by road. | None reported. | Google maps. |
|
|
| Woods et al. (2015)38/USA | Retrospective audit/Obstetrics and Gynecology. | To quantify the carbon footprint of 3 surgical modalities: robotically assisted laparoscopy, laparoscopy and laparotomy based on their energy consumed and waste produced. | Greenhouse Gas Protocol published by the World Business Council for Sustainable Development and the World Resources Institute. |
|
Emissions calculated as kg CO2e per patient. |
|
GHG: greenhouse gases; CO2e: carbon dioxide equivalent.
RESULTS
Search and selection of relevant studies
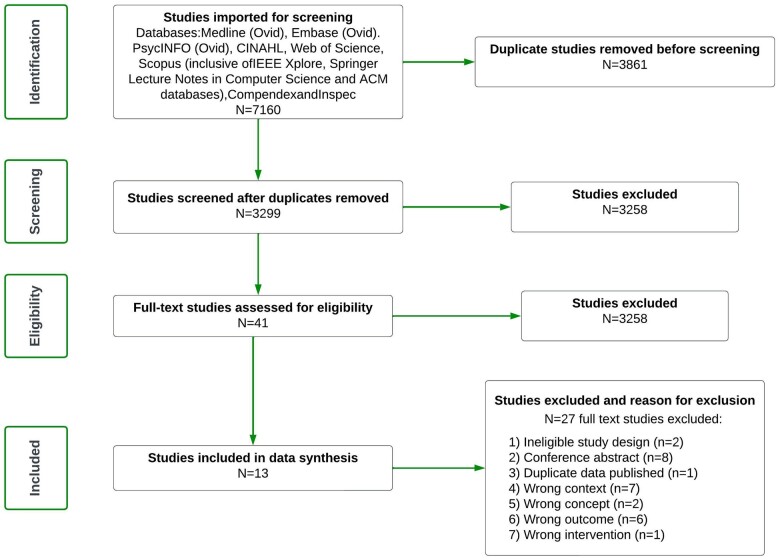
The scoping review was undertaken between March 2022 and April 2022. The search retrieved 7160 studies of which 3861 were duplicates. The remaining 3299 studies were screened for title and abstract with 3258 deemed irrelevant. Full text was requested for 41 articles with 40 full text studies assessed for eligibility. For one article, full text could not be obtained. Thereafter, 13 articles were included for full text data extraction (Figure 1). The level of agreement between 2 reviewers at full text screening was reflected by a Cohen’s kappa of 0.66. Data extracted from all studies are presented in Supplementary Appendix S2. A summary table of different frameworks, and DHTs to which they have been applied, referencing specific studies identified in this review, is presented in Table 2.
Figure 1.
PRISMA flow chart representing the study outcome following database mining and importing of all articles on March 17, 2022.
Study settings, design, and measured outcomes
Most studies (n = 8, 62%) focused on using tools to estimate travel-related carbon emissions in the context of telehealth in healthcare.27–29,31,33–35,37 The remaining studies adapted the tools to examine the impact of EHRs (n = 2, 15%),32,36 e-prescriptions and e-referrals (n = 1, 8%),30 and robotic surgery (n = 1, 8%).38 One study was a scoping review focused on health technology, inclusive of EMR.11 The studies examined came from the United Kingdom (n = 3, 23%),27–29 United States (n = 3, 23%),35,36,38 Spain (n = 2, 15%),32,37 Canada (n = 1, 8%),11 Croatia (n = 1, 8%),30 France (n = 1, 8%),31 Kuwait (n = 1, 8%),33 and Sweden (n = 1, 8%).34 Most of the studies examined an outpatient context (n = 5, 38%),27–29,31,35 followed by hospital settings (n = 3, 23%),30,38 a rehabilitation unit (n = 1, 8%),34 a single healthcare system (n = 1, 8%),36 and primary care (n = 1, 8%).37 Two studies (15%) did not specify their health care setting or context.11,32
The study designs included retrospective audits (n = 6, 46%),27,28,34,35,37,38 prospective studies (n = 2, 15%),29,31 a case report (n = 1, 8%),32 a case study (n = 1, 8%),36 a methods study (n = 1, 8%),30 a scoping review (n = 1, 8%),11 and a simulation study (n = 1, 8%).33 The sampling methods implemented were mostly purposive (n = 10, 77%),27–29,31,32,34–38 followed by simulation modeling (n = 1, 8%),33 theoretical sampling (n = 1, 8%),30 and one, a scoping review used database mining (n = 1, 8%).11 The outcomes measured for DHT environmental impact included carbon dioxide equivalent (n = 10, 77%),27–31,33–37 energy consumption (n = 6, 46%),27,28,31,32,34,36 other greenhouse gas emissions (n = 4, 31%),33,35–37 waste and toxic chemicals (n = 2, 15%),35,36 materials (eg, paper, X-ray-related consumables, fuel) (n = 2, 15%),35,36 water consumption (n = 2, 15%),27,36 and other environmental considerations (eg, trees to be planted to offset emissions) (n = 2, 15%).29,36 Note that some studies measured more than one outcome.
Identified frameworks, methods, and tools
There was no consistent application of any specific framework to estimate DHT carbon emissions. One study used LCA to retrospectively demonstrate the impact of telehealth that, in addition to travel emissions, encompassed equipment and infrastructure emissions (computers, monitors, local area network components, and video codecs), emissions generated during design, manufacturing and disposal and equipment recycling, and meeting time.34 This demonstrated that selecting telehealth equipment with greater screen resolution and more user-friendly interfaces generated greater carbon emissions.34 This study was the most thorough assessment of telehealth’s contribution to carbon emissions identified in this scoping review. A second study that used the LCA framework did so within the context of e-referrals and e-prescribing.30
The additional frameworks identified through the scoping review included the Eco-Health Footprint and the Framework for Energy Efficiency Testing to Improve eNvironmental Goals of the Software (FEETINGS). The Eco-Health Footprint, developed by the Global Safety and Health Initiatives and used by Turley and colleagues,36 could not be accessed publicly. The FEETINGS and associated guidelines, that target software engineers to prospectively consider energy efficiency of their software in the context of environmental sustainability, was publicly accessible.39 Habib and Marimuthu33 also focused on telehealth, took an approach called “the green synthesis” framework to develop a capacity planning tool to prospectively optimise energy consumption of an IT system through algorithmics. This provides an avenue for the prospective design of energy-efficient telehealth services inclusive of EMR use.33
Overall, the methodologies and approaches to the analysis of DHT’s contribution to carbon emissions varied widely amongst the literature surveyed. No consistency was found in how methods and tools, such as carbon footprint calculators, were employed. Studies did not describe sufficient details of their DHT intervention to facilitate transparent interpretation of the carbon emission data provided. With respect to tools, regardless of the DHT used, all studies referred to some form of their national carbon footprint calculator as summarised in Table 2.
DHT assessments
EMR/EHR were the subject of 2 (15%) studies.32,36 Garcia-Berna et al.32 focused on the energy consumption of 5 web-based EMR software applications using the Energy Efficient Tester and Framework for Energy Efficiency Testing to Improve eNvironmental Goals of the Software (FEETINGS). They demonstrated that the more complex the user interface, the more energy the software consumed. Specifically, progress bars, animations, icons, images, and scroll bars seemed to be environmentally damaging. Turley et al.36 focused on EHR-related downstream carbon savings made on travel-related emissions, paper consumption, plastic, and electronic waste. They also reported where EHR may emit carbon that then needed to be mitigated: an important factor to acknowledge.36
It was reported that up to 15 000 metric tonnes of carbon production per year could be eliminated by implementing e-prescribing and e-referral services.30 Robotically assisted laparoscopy’s carbon emissions were 38% higher than conventional laparoscopic surgery and 77% greater than laparotomy.38 No reports were identified on the environmental sustainability of mobile apps.
DISCUSSION
While global healthcare is the 5th largest polluter on the planet, accounting for approximately 4.4% of global greenhouse gas net emissions, it is less well known that information and communication technology emissions account for 3.7% of global carbon emissions.40 By choosing to implement DHTs with low carbon emission footprints, decision-makers will mitigate healthcare’s carbon emissions and the impact of DHT on climate injustice that disproportionately affect vulnerable populations. Often obsolete information technology components (e-waste) are transported, treated, recycled, and destroyed in developing and impoverished nations.7 E-waste recycling processes may locally increase pollutants and toxins, impacting the health of those who are already vulnerable.41 Child labor is common in impoverished countries, potentially exposing children to toxic e-waste chemicals, linked to negative health outcomes, including cancer.7 Hence, there is an incentive to identify the best frameworks, methods, and tools to correctly identify DHTs with low carbon and other pollutant emissions.
Implications for future advancement of DHT
The purpose of this research was: (1) to discover which methods and assessment tools exist to assess the carbon footprint of DHTs, and (2) to examine the validity, reliability, feasibility, and usefulness of these tools to assess the carbon footprint of DHTs in healthcare. Our findings have several implications for experts, researchers, healthcare leaders and the public. In summary:
Multiple carbon frameworks and methods have been applied to a variety of healthcare settings and contexts. This variability is likely required, given healthcare’s complexity through the continuum of care and areas of practice.
While researchers have applied frameworks and methods to their studies, the scoping review did not identify validated tools that would improve the transparency of the identified processes.
There is a need for researchers to rigorously evaluate these frameworks and methods for use in healthcare’s diverse contexts. More effort is needed to support appropriate implementation of validated and reliable tools as stakeholder’s knowledge, expertise, skills, and attitudes will vary across settings.
More transparent, standardised approaches to assessing DHT carbon emissions are needed. To address this gap, multiple disciplines are required to advance and apply these methods strategically, uniformly, and purposefully.
User friendliness and robust implementation guidelines may be the key for stakeholders to ensure organisation-wide ownership and commitment to sustainable, successful change.42
A publicly available, multilanguage repository should be developed permitting stakeholders to retrieve pertinent information on specific products that capture the entire life-cycle of a digital health intervention. For example, a repository could help healthcare organisations adopt and implement carbon evaluation frameworks by providing a useful, easy to use platform to gather information to operationalize carbon assessments and decisions.
The basic principles that these frameworks provide (ie, LCA questions on how materials are sourced, manufactured, and recycled) can catalyse process changes and be integrated into existing supply chain practices for healthcare. For example, organisations may integrate the Life-Cycle Assessment framework on vendor contract requirements (eg, Request for Purchase on a contract for electronic blood glucose monitors) which include disclosure on where raw materials were sourced, emissions produced during development, and the percentage of a product that is recyclable.
As demand from healthcare organisations for DHT increases, their collective market power to demand manufacturers publish transparent carbon emissions on DHT production must be used to support purchasing decisions.4
The shift by hospitals and private healthcare systems towards using DHTs to focus on disease prevention (rather than treatment) will reduce the need for carbon intensive treatment and facilities.
Due to the complexity of the healthcare systems, across which emissions ought to be assessed and reduced, simple analysis techniques may well be inadequate. Consequently, although it was not specifically identified in the literature we surveyed, we note that advances in machine learning models might hold a key to developing future, sustainable digital healthcare systems and interventions.43 Such tools may enable collection of data to support carbon emission calculations, and facilitate building models needed for responsible design, implementation, and decision-making about future DHT interventions. Overall, these implications drive at the need for stakeholders to advance acceptable carbon emission evaluation frameworks to evaluate DHTs, that can be integrated into the global health care environment.
Variation in environmental sustainability frameworks, methods, and tools
This scoping review has established that there was no single framework or method consistently used to assess the carbon footprint of DHT. Furthermore, no descriptions of the methods for adapting frameworks to the context of digital healthcare were identified. While this makes it challenging to reproduce the results of past studies, this review’s findings may reflect complexity of the healthcare. The life-cycle of DHT in healthcare has a multitude of contexts (eg, software, hardware, imaging, written text, printing reports, virtual health services). Consequently, different tools may be required to measure different aspects of environmental impact.
The integration of any DHT in the healthcare context is multifaceted. For example, an EMR in one organisation alone needs to be interoperable to allow electronic ordering of the laboratory tests, e-prescribing, barcode scanning, medication administration, integration with bedside monitoring devices, anaesthetic machines, workflow, productivity, and many mobile applications. Considering this complexity, which parameter should therefore be selected as a measured outcome for environmental sustainability? If, by way of example, integration of EMR leads to fewer request for laboratory tests, should this be taken as an environmentally sustainable outcome, given that we know that requesting fewer blood tests reduces carbon emissions?44 These issues are compounded by the need to conduct system updates, staff training, maintain sustainable clinical practice and manage interoperability with external organisations’ EMRs/EHRs. Similarly, not all DHT interventions are created equal. For example, during this scoping review it was noted that in many cases “telehealth” delivery consisted of a phone call to patient’s home. This cannot be considered in the same category as videoconferencing because its use of internet bandwidth is associated with greater carbon consumption.5,8,34 Specifically, Holmner et al.34 estimate that for a one hour telehealth meeting, the net carbon emissions would increase by more than 200% when increasing the bandwidth from 4 to 10 MB. In contrast, a decrease to 1 MB would result in 4-fold reduction in energy consumption for data transfer and a 3-fold reduction in total carbon emissions.
Assessment of the environmental impact of DHT interventions is multifaceted as it needs to consider (1) the DHT used, (2) how the DHT transforms clinical practice and patient outcomes, and (3) the DHTs impact on organisational function. Therefore, assessment needs a “Systems Thinking” approach to fully support achievement of sustainability goals.45,46 A Systems Thinking approach acknowledges that all systems are composed of interconnected parts and that changes to one part may affect another.47 Systems Thinking has been applied widely to analyse the behavior of complex interactions between components of a system, and has specifically been presented for advancing product life-cycle sustainability assessments.46 Hence, a holistic assessment of the environmental impact of DHTs needs to consider what impacts the adoption of the technology has on different elements of the systems to which it is connected (eg, societal, organisational, staffing, and patient). This is counter to a reductive approach, that breaks the system down into potentially independent components to explore them individually, eliminating any possibility of understanding how effects on components might be interconnected. For a systems-level understanding of DHT’s environmental impact to be reached, multiple variables must be simultaneously considered in the assessment.
Development and validation of frameworks, methods, and tools
Based on the findings of this scoping review, the development and validation of frameworks, methods, and tools for assessing the environmental impact of digital health interventions are in their infancy. For example, none of the studies reviewed reported on tool validity, reliability, feasibility, or how useful they were when assessing carbon emissions of DHT. That said, it is possible that these tools were validated and evaluated elsewhere and not reported in the format of publications that could be retrieved through databases our review explored.
Development of tools for assessment of different types of phenomena is a systematic and long process requiring specific methodological expertise, time and effort, and the involvement of all stakeholders.48 Health science research typically emphasises rigor in the development and implementation process, as well as the psychometric soundness of the aspects of the developed tool capturing its validity and reliability (for examples, see Refs 49,50). For example, the methods employed to evaluate the psychometric rigor of a tool, the reliability and validity of clinical impact, or the verification of an implemented prototype design, are approached differently when grounding the research in health informatics than in mathematics, engineering, computer software development, or environmental sciences. This is because each requires different knowledge transfer, translation of meaning, and transformation of thought.45,47 Future focus needs to be on developing and validating tools that accurately and appropriately measure the right outcomes to inform decision-making at different levels regarding healthcare sustainability.
Limitations of the review
The search strategy for this scoping review intended to capture all related search terms. However, it is recognised that unconsidered terms and definitions may exist. As the research team is international and multilingual, no language barriers were imposed in this search. Nevertheless, it is acknowledged that the databases searched may not capture reports in some languages. Grey literature was also not included in this review, meaning that some relevant, novel ideas may have been overlooked. As a scoping review does not require assessment of study quality, we did not make recommendations about the extent to which each framework could contribute to DHT’s reduction of carbon emissions. However, there is a clear paucity of original primary research in this area to answer the research questions in greater depth. Therefore, we highlighted substantial gaps in the existing research, directing us towards important new avenues of research for the environmental sustainability and carbon emission reduction of digital healthcare.
CONCLUSION
During this scoping review, no specific, easy to implement methods or tools to facilitate assessment of DHTs carbon emission in healthcare context could be identified. There is a genuine need to develop an interdisciplinary, standardised, sustainable approach to assessing DHT carbon emissions prospectively as part of addressing healthcare’s contribution to climate change. The formation of close interdisciplinary collaborations between clinicians in real-life clinical settings, consumers/patients, environmental scientists, engineers, and computer scientists is crucial to developing such resources, and to help healthcare implement environmentally conscious and environmentally sustainable digital health solutions. The latest IPCC (The Intergovernmental Panel on Climate Change) report issues a dire warning for the health of people and our planet. Interdisciplinary work has never been more important than right now, since every day we do something to minimise carbon emissions has the potential to divert us further from a dire future towards something better. By creating digital health interventions that are environmentally sustainable through responsible design, development, and implementation, we have a real opportunity to make a difference.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
AUTHOR CONTRIBUTIONS
The initial project idea was conceived, managed, and supervised by ZLT. All authors then contributed to the refinement of the idea, scoping review process, and to the formal analysis of the results. ZLT drafted the first version of the manuscript. All authors contributed to discussions on the direction of the scoping review, and subsequent manuscript revisions and all agreed to the final manuscript version.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
Supplementary Material
Acknowledgement
We thank our respective employers for supporting our work and for sharing our vision for environmentally sustainable healthcare. We also thank Ms Raluca Radu for her helpful comments in the early stages of this project’s development. This article was a collaboration within a nursing informatics network and was conducted in multiprofessional teams. All the authors have connection to health informatics, and backgrounds as registered nurses, public health nurses and/or computer scientists.
CONFLICT OF INTEREST STATEMENT
ZLT is a member of Climate and Health Alliance, Alliance of Nurses for Healthy Environments, World Health Organization Global Community of Practice for Nursing and Midwifery specific to Climate Change, Climate Reality Leadership Corps, and Australian College of Nursing Emission Reduction Policy Chapter. The remaining authors report no conflict of interest.
Contributor Information
Zerina Lokmic-Tomkins, School of Nursing and Midwifery, Monash University, Clayton, Melbourne, Victoria, Australia.
Shauna Davies, Faculty of Nursing, University of Regina, Regina, Saskatchewan, Canada.
Lorraine J Block, School of Nursing, University of British Columbia, Vancouver, British Columbia, Canada.
Lindy Cochrane, Brownless Biomedical Library, University of Melbourne, Parkville, Victoria, Australia.
Alan Dorin, Department of Data Science and Artificial Intelligence, Faculty of Information Technology, Monash University, Melbourne, Victoria, Australia.
Hanna von Gerich, Department of Nursing Science, University of Turku and Turku University Hospital, Turku, Finland.
Erika Lozada-Perezmitre, Nursing Faculty FE-BUAP, Benemerita Universidad Autonoma de Puebla, Puebla, México.
Lisa Reid, College of Nursing and Health Sciences, Flinders University, Bedford Park, South Australia, Australia.
Laura-Maria Peltonen, Department of Nursing Science, University of Turku, Turku, Finland.
Data Availability
The data available are presented in this article and in its Supplementary Material. Access to EndNote library available on request.
REFERENCES
- 1. The Intergovernmental Panel on Climate Change (IPCC). Sixth assessment cycle (AR6) climate change 2022: impacts, adaptation and vulnerability. 2022. https://www.ipcc.ch/report/ar6/wg2/. Accessed March 1, 2022.
- 2. Watts N, Amann M, Ayeb-Karlsson S, et al. The Lancet Countdown on health and climate change: from 25 years of inaction to a global transformation for public health. Lancet 2018; 391 (10120): 581–630. [DOI] [PubMed] [Google Scholar]
- 3. Lenzen M, Malik A, Li M, et al. The environmental footprint of health care: a global assessment. Lancet Planet Health 2020; 4 (7): e271–9. [DOI] [PubMed] [Google Scholar]
- 4. Health Care Without Harm. Health care’s climate footprint: how the health sector contributes to the global climate crisis and opportunities for action. 2019. https://noharm-uscanada.org/ClimateFootprintReport. Accessed March 2, 2022.
- 5. Holmner Å, Rocklöv J, Ng N, Nilsson M.. Climate change and eHealth: a promising strategy for health sector mitigation and adaptation. Glob Health Action 2012; 5 (1): 18428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Purohit A, Smith J, Hibble A.. Does telemedicine reduce the carbon footprint of healthcare? A systematic review. Future Healthc J 2021; 8 (1): e85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization. Soaring e-waste affects the health of millions of children, WHO warns. https://www.who.int/news/item/15-06-2021-soaring-e-waste-affects-the-health-of-millions-of-children-who-warns#:~:text=Other%20adverse%20child%20health%20impacts,as%20cancer%20and%20cardiovascular%20disease.&text=%E2%80%9CImproper%20e%2Dwaste%20management%20is%20the%20cause. Accessed March 22, 2022.
- 8. Thompson M. The environmental impacts of digital health. Digit Health 2021; 7: 205520762110334. [Google Scholar]
- 9. National Library of Medicine. HTA 101: glossary. Secondary HTA 101: glossary. 2017. https://www.nlm.nih.gov/nichsr/hta101/ta101013.html#HTA. Accessed February 28, 2022.
- 10. National Library of Medicine. HTA 101: II. Fundamental concepts. Secondary HTA 101: II. Fundamental concepts. 2019. https://www.nlm.nih.gov/nichsr/hta101/ta10104.html. Accessed March 1, 2022.
- 11. Polisena J, De Angelis G, Kaunelis D, Gutierrez-Ibarluzea I.. Environmental impact assessment of a health technology: a scoping review. Int J Technol Assess Health Care 2018; 34 (3): 317–26. [DOI] [PubMed] [Google Scholar]
- 12. Rebitzer G, Ekvall T, Frischknecht R, et al. Life cycle assessment part 1: framework, goal and scope definition, inventory analysis, and applications. Environ Int 2004; 30 (5): 701–20. [DOI] [PubMed] [Google Scholar]
- 13. Parvatker AG, Tunceroglu H, Sherman JD, et al. Cradle-to-gate greenhouse gas emissions for twenty anesthetic active pharmaceutical ingredients based on process scale-up and process design calculations. ACS Sustainable Chem Eng 2019; 7 (7): 6580–91. [Google Scholar]
- 14. Kløverpris NH. Establishing LCA in the healthcare sector. In: Benetto E, Gericke K, Guiton M, eds. Designing Sustainable Technologies, Products and Policies: From Science to Innovation. Cham: Springer International Publishing; 2018: 89–94. [Google Scholar]
- 15. Kitzes J. An introduction to environmentally-extended input-output analysis. Resources 2013; 2 (4): 489–503. [Google Scholar]
- 16. Marsh K, Ganz ML, Hsu J, Strandberg-Larsen M, Gonzalez RP, Lund N.. Expanding health technology assessments to include effects on the environment. Value Health 2016; 19 (2): 249–54. [DOI] [PubMed] [Google Scholar]
- 17. Powers CM, Dana G, Gillespie P, et al. Comprehensive environmental assessment: a meta-assessment approach. Environ Sci Technol 2012; 46 (17): 9202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Minx JC, Wiedmann T, Wood R, et al. Input–output analysis and carbon footprinting: an overview of applications. Econ Syst Res 2009; 21 (3): 187–216. [Google Scholar]
- 19. Lokmic-Tomkins Z, Davies S, Block LJ, et al. Assessing the carbon footprint of digital health interventions: a scoping review protocol. 2022. osf.io/hrb4w. [DOI] [PMC free article] [PubMed]
- 20. Arksey H, O’Malley L.. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005; 8 (1): 19–32. [Google Scholar]
- 21. Levac D, Colquhoun H, O’Brien KK.. Scoping studies: advancing the methodology. Implement Sci 2010; 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil H. Chapter 11: Scoping reviews (2020 version). In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. Adelaide, Australia: JBI; 2020. https://synthesismanual.jbi.global. 10.46658/JBIMES-20-12/. [DOI] [Google Scholar]
- 23. Peters MDJ, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth 2020; 18 (10): 2119–26. [DOI] [PubMed] [Google Scholar]
- 24. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169 (7): 467–73. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization. Classification of Digital health Interventions v1.0: a shared language to describe the uses of digital technology for health. 2018. https://www.who.int/reproductivehealth/publications/mhealth/classification-digital-health-interventions/en/. Accessed February 27, 2022.
- 26. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: g1687. [DOI] [PubMed] [Google Scholar]
- 27. Bartlett S, Keir S.. Calculating the carbon footprint of a Geriatric Medicine clinic before and after COVID-19. Age Ageing 2022; 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blenkinsop S, Foley A, Schneider N, Willis J, Fowler HJ, Sisodiya SM.. Carbon emission savings and short-term health care impacts from telemedicine: an evaluation in epilepsy. Epilepsia 2021; 62 (11): 2732–40. [DOI] [PubMed] [Google Scholar]
- 29. Connor MJ, Miah S, Edison MA, et al. Clinical, fiscal and environmental benefits of a specialist-led virtual ureteric colic clinic: a prospective study. BJU Int 2019; 124 (6): 1034–9. [DOI] [PubMed] [Google Scholar]
- 30. Di Giacomo P, Hakansson P.. A method to measure the reduction of CO2 emissions in e-health applications. Stud Health Technol Inform 2011; 169: 970–4. [PubMed] [Google Scholar]
- 31. Filfilan A, Anract J, Chartier-Kastler E, et al. Positive environmental impact of remote teleconsultation in urology during the COVID-19 pandemic in a highly populated area. Prog Urol 2021; 31 (16): 1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garcia-Berna JA, Fernandez-Aleman JL, Carrillo de Gea JM, et al. Energy efficiency in software: a case study on sustainability in personal health records. J Clean Prod 2021; 282: 124262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Habib S, Marimuthu PN.. Green synthesis of hospital enterprise network. Int J Med Eng Inform 2014; 6 (1): 26–42. [Google Scholar]
- 34. Holmner A, Ebi KL, Lazuardi L, Nilsson M.. Carbon footprint of telemedicine solutions–unexplored opportunity for reducing carbon emissions in the health sector. PLoS One 2014; 9 (9): e105040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paquette S, Lin JC.. Outpatient telemedicine program in vascular surgery reduces patient travel time, cost, and environmental pollutant emissions. Ann Vasc Surg 2019; 59: 167–72. [DOI] [PubMed] [Google Scholar]
- 36. Turley M, Porter C, Garrido T, et al. Use of electronic health records can improve the health care industry’s environmental footprint. Health Aff (Millwood) 2011; 30 (5): 938–46. [DOI] [PubMed] [Google Scholar]
- 37. Vidal-Alaball J, Franch-Parella J, Lopez SF, Garcia CF, Mendioroz PJ.. Impact of a telemedicine program on the reduction in the emission of atmospheric pollutants and journeys by road. Int J Environ Res Public Health 2019; 16 (22): 4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Woods DL, McAndrew T, Nevadunsky N, et al. Carbon footprint of robotically-assisted laparoscopy, laparoscopy and laparotomy: a comparison. Int J Med Robotics Comput Assist Surg 2015; 11 (4): 406–12. [DOI] [PubMed] [Google Scholar]
- 39. Mancebo J, Calero C, Garcia F, Moraga MA, Garcia-Rodriguez de Guzman I.. FEETINGS: framework for energy efficiency testing to improve environmental goal of the software. Sustain Comput Inform Syst 2021; 30:100558. [Google Scholar]
- 40. Ferreboeuf H. Project shift. Lean ICT: towards digital sobriety. https://theshiftproject.org/en/article/lean-ict-our-new-report/. Accessed May, 2022.
- 41. Abalansa S, El Mahrad B, Icely J, Newton A.. Electronic waste, an environmental problem exported to developing countries: the GOOD, the BAD and the UGLY. Sustainability 2021; 13 (9): 5302. [Google Scholar]
- 42. Carlile PR. Transferring, translating, and transforming: an integrative framework for managing knowledge across boundaries. Organ Sci 2004; 15 (5): 555–68. [Google Scholar]
- 43. Rolnick D, Donti PL, Kaack LH, et al. Tackling climate change with machine learning. ACM Comput Surv 2023; 55 (2): 1–96. [Google Scholar]
- 44. McAlister S, Barratt AL, McGain F.. The carbon footprint of pathology testing. Med J Aust 2020; 213 (10): 477–77.e1. [DOI] [PubMed] [Google Scholar]
- 45. Kutty AA, Abdella GM, Kucukvar M, Onat NC, Bulu M.. A system thinking approach for harmonizing smart and sustainable city initiatives with United Nations sustainable development goals. Sustain Dev 2020; 28 (5): 1347–65. [Google Scholar]
- 46. Onat NC, Kucukvar M, Halog A, Cloutier S.. Systems thinking for life cycle sustainability assessment: a review of recent developments, applications, and future perspectives. Sustainability 2017; 9 (5): 706. [Google Scholar]
- 47. Checkland P. Systems Thinking, Systems Practice: Includes a 30-Year Retrospective. Chichester, England: John Wiley & Sons, Ltd.; 2022. [Google Scholar]
- 48. DeVellis R. Scale Development: Theory and Applications. 3rd ed. London: Sage; 2012. [Google Scholar]
- 49. DeVon HA, Block ME, Moyle-Wright P, et al. A psychometric toolbox for testing validity and reliability. J Nurs Scholarsh 2007; 39 (2): 155–64. [DOI] [PubMed] [Google Scholar]
- 50. Dima AL. Scale validation in applied health research: tutorial for a 6-step R-based psychometrics protocol. Health Psychol Behav Med 2018; 6 (1): 136–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data available are presented in this article and in its Supplementary Material. Access to EndNote library available on request.