Abstract
Peatlands are a major carbon (C) sink globally. Organic matter quality influence greenhouse gases production. However, little is known about how organic matter from different vegetation types, influences C composition and resultant greenhouse gases production in subtropical peatland. Anoxic incubation experiments were conducted using two types of peats with different botanical origin to assess C composition, CO2 and CH4 production. First peat had cypress dominance and the second knotted spikerush and water lily (spike + lily). Solid-state CPMAS 13C NMR determined C chemical stability, MESTA determined C thermal stability, stable isotopes for C source and gas chromatograph for carbon dioxide (CO2) and methane (CH4). The results indicated dominance of autochthonous C as indicated by δ13C signatures. Low thermal stable C (LTSC) dominated in litter, FL (fermentation layer) and spike + lily sediment, high thermal stable C was dominant in cypress peat. O-alkyl C strongly correlated with LTSC whereas aromatic C correlated negatively with R400 (LTSC:total C ratio). Generally, O-alkyl decreased and alkyl increased along litter-FL-peat continuum. Spike + lily peat exhibited initial stage of decomposition. Indicated by increased alkyl C, aromatic C and aromatic:O-alkyl ratio with increasing peat depth. Also, exhibited 3 times more CH4 and CO2 production compared to cypress peat that dominantly exhibited second stage of decomposition. O-alkyl C exhibited positive relationship with CH4 (P = 0.012, r2 = 0.57) and CO2 (P = 0.047, r2 = 0.41) production whereas R400 related positively with CH4 (P = 0.05, r2 = 0.40). Organic matter thermal and chemical composition varied between the peat types and thermally and chemically labile C influenced CO2 and CH4 production.
Keywords: Carbon composition, Carbon dioxide, Fermentation layer, Litter, Methane, Peat, Thermal stability, Vegetation types
Carbon composition; Carbon dioxide; Fermentation layer; Litter; Methane; Peat; Thermal stability; Vegetation types.
1. Introduction
Global peatlands cover approximately 3% of the earth's terrestrial area, of which 10% are located within the tropics (Chimner and Ewel, 2005; Upton et al., 2018). Peatlands act simultaneously as carbon (C) sink and sources, where they exchange large amounts of greenhouse gases with the atmosphere (Sjogersten et al., 2014). Generally, peat accumulation occurs when net primary productivity exceeds the rate of C loss through fires and decomposition. In the temperate zones, the loss of C is limited by anoxic conditions and cold temperatures (Hodgkins et al., 2018; Loisel et al., 2017). However, in (sub) tropical peatlands the year-round high temperatures would be expected to enhance microbial decomposition rates (Knorr et al., 2005), but the existence of large peat deposits remains enigmatic (Belyea and Baird, 2006; Hodgkins et al., 2018). Several factors have been attributed to accumulation of peat in the tropics, such as higher primary productivity (Gillman et al., 2015) that may allow faster litter deposition. Wright et al., 2013a, Wright et al., 2013b demonstrated that chemical composition of litter influences peat accumulation, consequentially influencing physical and chemical peat characteristics that may slow decomposition rates in the tropical peatland (Wieder and Vitt, 2006). Decomposition may also be slowed by high C recalcitrance as a result of initial rapid decay of plant litter (Chimner, 2004).
Vegetation types determines the quantity and quality of litter that contributes to the peat organic matter (Laiho 2006; Ward et al., 2015). Different litter species and tissue types degrade at different rates (Wright et al., 2013a, Wright et al., 2013b; Hoyos-Santillan et al., 2015) which is a major control on peat properties (Laiho 2006; Ward et al., 2015). Decomposition of organic matter is dependent on substrate quality, environmental conditions, decomposers presence and nutrient availability (Bragazza et al., 2012). Substrate quality is typically described as the concentration of C fractions and/or nutrients (Laiho, 2006). Carbon input is derived from vegetation through inputs of aboveground biomass as litter to the peat surface and also within the peat profile resulting from root exudates or senescence and microbial turnover (Chimner and Ewel 2005; Jauhiainen et al., 2005; Hirano et al., 2009). Consequently, differences in organic matter properties between contrasting vegetation types have been reported (Hoyos-Santillan et al., 2015; Upton et al., 2018). For example, Girkin et al. (2020) reported significant differences in both total C and C:N ratios for various peat types and argued that the differences could be attributed to contrasting vegetation and management practices. There remains a gap in the understanding and less attention has been given to the contribution of various peat types to larger scale peat C stock estimates (Loisel et al., 2017) as well as the C chemical composition and thermal stability in various sub-tropical peatlands.
Vegetation plays a key role in regulating greenhouse gases production, as specific litter inputs define initial peat properties (Cooper et al., 2019; Upton et al., 2018). Vegetation communities in tropical peatlands can regulate CH4 and CO2 production through labile C inputs (Girkin et al., 2020; Wright et al., 2011). Differences in fluxes between peat types are likely driven by contrasts in organic matter properties (Cooper et al., 2019) and/or nutrient availability (Hoyos Santillan et al., 2016; Sjögersten et al., 2011) and microbial community structure and function. Overall, surface peat C is more labile than deeper peat (Cooper et al., 2019). However, it has been indicated that in the (sub) tropics the surface peat has lower carbohydrates and greater aromatic content compared to the same depth in a temperate peat (Hodgkins et al., 2018), creating a reduced oxidation state and resulting to recalcitrance. The recalcitrance allows peat to persist in the (sub) tropical despite warm temperatures (Hodgkins et al., 2018) and is expected to have implication for CH4 and CO2 production throughout out the peat profile since labile C supply has been shown to limit heterotrophic microbial activity in a subtropical peatland (Wright et al., 2009). Approximately 50% of the subtropical Apalachicola National Forest (ANP) in Florida is covered by wetlands (Tschinkel and Hess, 1999). The dominant vegetation types in ANP wetlands include cypress (Taxodium distichum), Knotted Spikerush (Eleocharis equisetoides) and Waterlily species (Nuphar sp.) (Akinbi, 2018). But, limited information is available on the relationship between organic matter chemistry at molecular level and thermal stability with CH4 and CO2 production in the subtropical peatland under contrasting vegetation types.
Solid-state CPMAS 13C NMR and multi-element scanning thermal analysis (MESTA) provide advanced understanding of C chemical and thermal stability, respectively (Hsieh, 2007; Normand et al., 2017a, Normand et al., 2017b). Thermal stability analysis is fast and inexpensive, and involves oxidation or volatilization of organic C, which is conducted under certain temperature and pressure (Hsieh and Bugna, 2008; Schmidt and Noack, 2000). Thermal analysis allows different C compounds to decompose at different temperatures during heating cycle (Manning et al., 2005). Carbon recovered at temperature <400 °C is attributed to recovery of low thermal stable C while C recovered between 400 and 650 °C is attributed to more thermal stable C. The proportion of C compounds recovered at temperature <400 °C compared to the total C is considered the R400 index (Disnar et al., 2003). High R400 correspond to well preserved or fresh organic matter, while low R400 correspond to more degraded organic matter (Chawla et al., 2010; Disnar et al., 2008). Solid-state CPMAS 13C NMR identifies the organic functional groups in organic matter that vary in molecular composition and microbial utilization which includes alkyl, methoxyl, O-alkyl, aromatic and carboxyl based on chemical peak shifts (Knicker, 2011). Previously, a relationship has been reported between C thermal stability and NMR organic functional groups. The low thermal stable C correlates well with O-alkyl C, while high thermal stable C mainly correlates with aromatic compounds for example lignin or other polyphenols (Dell'Abate et al., 2002; Lopez-Capel et al., 2005; Manning et al., 2005; Plante et al., 2011; Strezov et al., 2004). However, there is limited information on the relationship between C thermal stability and NMR organic functional groups in the subtropical peatland and their influence on greenhouse gases production. The overall objective of the study was to evaluate variation in peat organic chemistry and thermal stability through the depth profile and their impact on CH4 and CO2 production rates under anoxic conditions and contrasting vegetation types in a subtropical peatland using an incubation experiment.
Four research hypotheses were addressed: (1) The source of organic matter in the study sites will be dominantly autochthonous (whereby the organic matter is dominantly generated from the vegetation growing in the respective wetlands), (2) Carbon thermal and chemical lability will be peat type dependent and will decrease with depth and R400 index will decrease with increasing peat depth, (3) Carbon thermal lability will be a potential predictor of chemical lability, and (4) Methane and CO2 production will increase with increasing chemical lability and low thermal stability of organic matter.
2. Materials and methods
2.1. Study site
Apalachicola National Forest (ANF) is located in the panhandle region of Florida between approximately 30°0′ and 30°30′ North latitude and 84°15′ and 85°0′ West longitude near Tallahassee, Florida. The ANF is the largest public forest in Florida which encompasses 2.28 × 109 m2 (564,000 acres) with an abundance of streams, lakes, fresh water and natural springs (Shrestha et al., 2007). The ANF consists of approximately 50% upland and 50% wetlands, with most of the uplands within the forest originally consisting of longleaf pine (Pinus palustris) in association with wiregrass (Aristida stricta). Slash pine (Pinus elliotti) mostly occupies the margins of wetlands and was associated to varying degrees with wetland understory species such as Titi (Cliftonia sp., Cyrilla sp.) and Gall berry (Ilex glabra, I. coriacea) (Tschinkel and Hess, 1999). The climate is humid subtropical, with an annual rainfall of 1670 mm (Gagnon et al., 2004). The study sites were peatlands classified as histosols that contained >40 cm accumulation of surface organic matter within 60 cm of the soil surface (Trettin and Jurgensen, 2003). The organic C ranged between 27% and 49%. As a result of organic matter input exceeding decomposition output the peat is more decomposed and recalcitrant compared to fresh litter (Craft, 2001). The study peatlands were dominated by different vegetation types which include cypress (Taxodium distichum), water lily (Nuphar advena), and Knotted spikerush (Eleocharis equisetoides). The study was conducted in the full-time inundated peatlands supporting the distinct vegetation types of interest. The sampling sites are located at 30°36′N latitude, 84°32′W longitude and 30°32′N latitude, 84°35′W longitude (Figure 1). Study site 1 was dominated by Taxodium distichum (Cypress) plant species and study site 2 was dominated by the Eleocharis equisetoides (Knotted Spikerush) and Nuphar advena (Waterlily) species.
Figure 1.
Global, regional, and local perspectives of the study area location in the panhandle of Florida near Tallahassee, Florida, USA. The location of the area in the southeast of the United States in provided for global reference (top right). The aerial perspective of the local area surrounding carbon site 1 (cypress site) and carbon site 2 (spike + lily) is provided in the bottom right image.
2.2. Experimental design
The litter, fermentation layer and peat samples were collected from the site dominated by Taxodium distichum (study site 1) hereafter referred to as cypress site and the site dominated by Eleocharis equisetoides (Knotted Spikerush) and Nuphar advena (Waterlily) (study site 2) hereafter referred as spike + lily site. Three composite replicates of litter, fermentation layer and peat were collected along three transects separated by approximately 50 m in each wetland type during dry season (spring 2017). Four samples were collected from each of the three transects and composited to make a composite sample. The peat and fermentation layer were collected using a soil core sampler. Fermentation layer was considered as the partially decomposed litter, whereby origin of material was still recognizable and was settled on top of the peat and under water. After separation of the fermentation layer from the peat, the peat was sectioned. Each of the four samples making a composite sample were sub-divided along the depth of the 30 cm core into four sub-samples. The peat sub-samples were composed of 0–5, 5–10, 10–20, and 20–30 cm depth sections. The sectioning was conducted in the laboratory, after sectioning the peat, the four samples collected along the same transect were composited according to their depth to make a composite sample. The litter and fermentation layer were composited along each transect with each of the three transects representing replications. The litter collected was specifically detached from the plant and floating on water. Samples were stored on ice for less than 3 h while in transport to the Florida A&M University laboratory. In the lab, after sectioning of the wet peat samples into 0–5 cm, 5–10 cm, 10–20 cm and 20–30 cm they were thoroughly mixed, composited, and transferred to a sterile mason jar for storage in the fridge until subsequent analyses.
2.3. Peat Chemical analysis
Peat pH was determined using wet soil at a 1:1 ratio (soil: deionized water) (McNeal, 1982) using Fisher Scientific pH meter (Fisher Scientific accumet AAE150 pH benchtop meter). Peat sub samples were oven dried at 70 °C for three days before ball milling using a Mixer Mill MM 400 (Retsch, Newton, PA, USA) at 25 Hz for 10 min. Total N, C, δ13C and δ15 N were analyzed with a Thermo Electron DeltaV Advantage isotope ratio mass spectrometer coupled with a ConFlo II interface linked to a Carlo Erba NA 1500 CNHS Elemental Analyzer. First, samples were manually loaded into tin capsules. Then the samples were placed in a 50-position automated Zero Blank sample carousel on a Carlo Erba NA1500 CNS elemental analyzer. The samples were combusted under high oxygen content in a quartz column at 1020 °C, then sample gas was transported in a He carrier stream and passed through a hot reduction column (650 °C) which consisted of elemental copper, the purpose of elemental copper was to remove oxygen. The effluent stream then passed through a magnesium perchlorate trap which is a chemical trap that removed water and was followed by a 0.7-meter GC column at 120 °C to separate N2 from CO2. The sample gas next passed into a ConFlo II preparation system and into the inlet of a Thermo Electron Delta V Advantage isotope ratio mass spectrometer which ran in a continuous flow mode and the sample gas was measured relative to laboratory reference N2 and CO2 gases. The C isotopic results were expressed in standard delta notation relative to VPDB and the nitrogen isotopic results are expressed in standard delta notation relative to AIR.
2.4. 13C Solid state nuclear magnetic resonance (ssNMR)
Samples were oven dried at 30 °C until constant weight was attained and then grounded into fine particles using Mixer Mill MM 400 (Retsch, Newton, PA, USA) at 25 Hz for 10 min. Ground samples were analyzed as indicated by (Ngatia et al., 2017) by magic angle spinning (MAS) Solid-state CPMAS 13C NMR. Bruker 300 MHz DRX NMR spectrometer was used for the samples analysis and it was equipped with a Bruker 4.0 mm double resonance MAS NMR probe. First, 4.0 mm zirconia rotors were used for samples packaging and capped using kel-F drive caps. Then the samples were spun to 9.5 kHz at RT using a Bruker pneumatic MAS unit. The 13C signals were enhanced by Cross Polarization: A 4.0 μs 1H π/2 pulse followed by a 1H spin-lock field of 45 kHz for 1.0 ms contact time, during this process the 13C RF field was ramped from 35 to 50 kHz. The 13C signals from the samples were recorded under the irradiation of the SPINAL64 decoupling sequence (Fung et al., 2000) with a 1H radiofrequency amplitude of 62.5 kHz. Accumulation of signals was achieved through accumulation of scans, the number of scans varied from 10,000 and 50,000, depending on the samples, with a recycle delay of 3 s. The spectral regions of MAS solid-state CPMAS 13C NMR were integrated and used to determine the contribution of each C functional group in the sample based on assignments from (Knicker, 2011): alkyl (0–45 ppm), methoxyl (45–60 ppm), O-alkyl (60–110 ppm), aromatic (110–140 ppm), phenolic (140–160 ppm), and carboxyl (160–220 ppm). Finally, total C concentration was used to estimate the concentration of each C functional groups (Ngatia et al., 2017). TopSpin 4.0.4 software was used for spectra integration.
2.5. Multi-element scanning analysis (MESTA)
Multi-element scanning thermal analysis (MESTA) technique (Hsieh, 2007) was used to generate C and N thermograms of the litter, fermentation layer and peat. Samples were oven dried at 30 °C and were ball milled using the Mixer Mill MM 400 (Retsch, Newton, PA, USA) at 25 Hz for 10 min. Ground samples were subjected to heating in a quartz combustion chamber at a rate of 50 °C min−1 starting from ambient temperature to 750 °C Throughout the analysis 33% oxygen in helium carrier gas was flushed through the sample chamber. As the samples were subjected to an oxidation process respective gases namely carbon dioxide, nitrogen dioxide and water were quantified and recorded over the combustion range. To calibrate the temperature range during each run cysteine standard was used. The samples contained high concentration of C, therefore, in order to obtain a uniform thermogram for thermo chemical analysis, sample dilution was done in a baked talk at a dilution ratio of 1:5 (sample: baked talk). Talk was baked at 900ᵒC for 30min. Different C compounds decompose during heating cycle at different temperatures, C thermogram acquired using MESTA reveals the temperature at which the C volatilizes (Hsieh, 2007). Carbon and N recovered at <400 °C and >400 °C temperature were considered as low thermal stable and high thermal stable C or N, respectively. This is consistent with Manning et al. (2005) who demonstrated that relatively labile cellulosic material normally decomposes at <400 °C and more refractory lignin and related materials usually decompose between 400 and 650 °C. The thermograms produced by MESTA technology in this study were used as a chemical signature for characterization and identifying specific chemical compounds in terms of thermal stability (Hsieh, 2007). Further the total C determined by C and N analyzer was used to calculate the low thermal stable C and high thermal stable C. Unlike thermogravimetric analysis method that uses weight balance to estimate the C loss (Schnitzer and Hoffman, 1966), MESTA method involves heating the sample, whereby the volatile components are oxidized to their respective oxides providing a more precise means of estimating C loss (Hsieh, 2007). The R400 index for C was calculated as the proportion of low thermal stable C recovered below 400 °C compared to total C.
2.6. Methane and carbon dioxide production
Serum bottles (120 mL) with butyl rubber lids and attached seals on the lid were prepared for gas sampling. Approximately 30 g of peat at field moisture content was transferred to a serum bottle for a given sample and 30 mL of deionized water was added in each bottle to completely submerge the peat sample; the mixture was kept in the dark at 25 °C for anaerobic peat incubation. Before sample incubation, oxygen free N2 gas was used to purge the sample for 5 min to ensure an absence of oxygen. The serum bottles remained tightly closed throughout the experiment to ensure anaerobic conditions. This experiment set up followed the procedure by Schipper and Reddy (1994). Both fermentation layer and peat were incubated under these conditions for 62 days. Headspace gas was sampled at 3 mL and 2 mL to determine CO2 and CH4 concentration, respectively, from the headspace of the anaerobic bottles using air-tight syringe. The CO2 concentration was determined using gas chromatography (Shimadzu® GC-8A, Shimadzu Scientific Instruments, Columbia, MD, USA), calibrated with CO2 standards with concentration 10,000 ppm. Methane concentration was determined using shimadzu® mini 2 gas chromatograph and calibrated using 1.83 ppm, 99.8 ppm and 1006 ppm as standards. Methane and CO2 concentration were calculated using Henry's Law as outlined by (Bridgham and Ye, 2013).
Carbon dioxide and CH4 concentration determined in this study is considered potential CO2 and CH4 production and not field emissions. This is because field CO2 and CH4 would factor in methane oxidation as a result of aeration and rhizosphere oxidation, in addition root decay and root exudation promote methane production (Segers, 1998). All these factors are not considered in this ex-situ experiment.
2.7. Statistical analysis
Statistical analyses for ANOVA and correlation were conducted using JMP (version 13.2.1). The analysis considered the mean and standard error of mean (SEM) for the response. Significant differences among the treatments for the variables were determined by analysis of variance using Tukey HSD test at α = 0.05.
3. Results
3.1. Biogeochemical parameters
The C data indicated flow of total C from the litter, to fermentation layer to peat (Table 1). Total C was significantly different between the three litter types and was in the order of cypress > spikerush > water lily (P < 0.001; Table 1). This translated to significantly higher C in the fermentation layer of the cypress site compared to spike + lily site (P = 0.01043; Table 1). The peat C decreased with increasing depth in both cypress site and spike + lily site. In the cypress site, the peat C was significantly higher in the 0–5 cm depth compared to other depths (P = 0.0248; Table 1), whereas in the Spike + lily site peat C was significantly higher in 0–10 depth compared to 10–30 depth (P = 0.001; Table 1).
Table 1.
Means of soil biogeochemical parameters at different depth (0–5, 5–10, 10–20, 20–30 cm) and at different sites (Cypress site and spike + lily). The means were determined using JMP (version 13.2.1; SAS Institute, 2007). The means were from three composite replicates, the data indicate mean ± SEM. The different superscript letters after SEM within a column indicate significant difference between sites means at P < 0.05.
| Depth | TC |
TN |
LOI |
13C |
15N |
pH | C: N |
|---|---|---|---|---|---|---|---|
| g kg−1 | g kg−1 | % | δ‰ | δ‰ | ratio | ||
| Litter | |||||||
| Cypress | 486 ± 1.2a | 6.5 ± 0.1c | −27.6 ± 0.1c | −2.0 ± 0.2c | 75.1 ± 1.0a | ||
| Spikerush | 450 ± 2.6b | 7.4 ± 0.1b | −25.5 ± 0.03b | 2.6 ± 0.02a | 60.6 ± 1.5b | ||
| Water Lily | 439 ± 0.3c | 18.1 ± 0.1a | −23.8 ± 0.03a | 1.1 ± 0.1b | 24.3 ± 0.1c | ||
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Fermentation layer | |||||||
| Cypress | 490 ± 6.9a | 17.3 ± 0.7a | 94.9 ± 1.0a | −28.1 ± 0.1a | −0.7 ± 0.1a | 4.2 ± 4.2a | 28.5 ± 1.1a |
| Spike + lily | 430 ± 23.1b | 26.0 ± 1.6b | 82.3 ± 3.9b | −26.6 ± 0.7b | 0.4 ± 0.04b | 4.9 ± 4.9b | 17 ± 0.9b |
| P value | 0.01043 | 0.0074 | 0.0337 | 0.0168 | 0.0014 | 0.0002 | 0.0012 |
| Sediment | |||||||
| Cypress | |||||||
| 0–5 cm | 445 ± 14.8a | 21.5 ± 1.2a | 85.4 ± 4.4a | −27.5 ± 0.1b | −0.1 ± 0.2a | 4.0 ± 0.1a | 20.8 ± 0.6ab |
| 5–10 cm | 431 ± 24.5ab | 21.8 ± 1.6a | 77.9 ± 3.7a | −26.9 ± 0.03a | 1.5 ± 0.2a | 3.8 ± 0.1b | 19.9 ± 0.9b |
| 10–20 cm | 376 ± 31.5ab | 16.1 ± 2.5ab | 64.7 ± 6.2ab | −27 ± 0.2a | 1.8 ± 0.2b | 3.8 ± 0.03ab | 24 ± 2.2ab |
| 20–30 cm | 333 ± 12.7b | 12.4 ± 0.8b | 44.6 ± 8.0b | −27 ± 0.1a | 0.7 ± 0.2c | 3.8 ± 0.02b | 26.7 ± 0.92a |
| P | 0.0248 | 0.0091 | 0.0051 | 0.0081 | 0.0002 | 0.0205 | 0.0246 |
| Spike + lily | |||||||
| 0–5 cm | 421 ± 0.56a | 27.2 ± 1.5ab | 83.6 ± 0.6a | −26.4 ± 0.2b | 0.2 ± 0.1b | 4.8 ± 0.2a | 15.5 ± 0.8ab |
| 5–10 cm | 440 ± 0.7a | 29.4 ± 0.3ab | 86.8 ± 2.8a | −25.7 ± 0.1a | 0.4 ± 0.1b | 4.6 ± 0.1a | 15 ± 0.3b |
| 10–20 cm | 350 ± 1.6b | 21.8 ± 1.5bc | 63.9 ± 3.5a | −26.5 ± 0.1b | 1.7 ± 0.2a | 4.8 ± 0.04a | 16.1 ± 0.4ab |
| 20–30 cm | 278 ± 2.1b | 15.3 ± 2.1c | 65.4 ± 17a | −26.8 ± 0.1b | 1.9 ± 0.1a | 4.8 ± 0.1a | 18.5 ± 1.1a |
| P value | 0.001 | 0.007 | 0.2223 | 0.0034 | <0.001 | 0.604 | 0.0364 |
Acronym: LOI – loss in ignition; TC – total carbon; TN – total nitrogen; C: N – carbon nitrogen ratio.
Total N was significantly different between the three litter types, but in contrast to total C it was in the order of water lily > spikerush > cypress (P < 0.0001; Table 1), whereby; water lily litter had almost three times more N compared to the other two types of litter. This translated to lower total N in the cypress fermentation layer compared to spike + lily fermentation layer (P = 0.0074; Table 1). Peat N decreased with increasing depth in both cypress (P = 0.0091) and spike + lily (P = 0.007) sites (Table 1).
The C:N ratio decreased from litter to fermentation layer to peat layer (Table 1). Cypress litter had significantly higher C:N ratio which was more than three times that of water lily, while spikerush C:N ratio was more than two times that of water lily (P<0.0001; Table 1). Cypress site fermentation layer had significantly higher C:N ratio compared to spike + lily site (P = 0.0012; Table 1). Peat C:N ratio increased with increasing depth in both cypress (P = 0.0246) and spike + lily (P = 0.0364) sites and was greatest in the 20–30 cm depth in both sites (Table 1).
The litter 13C stable isotope data indicated that water lily was significantly more enriched, followed by spikerush litter while cypress litter was the least enriched (P < 0.001; Table 1). As a result, the spike + lily site fermentation layer was more enriched compared to cypress site fermentation layer (P = 0.0168; Table 1). The peat 13C stable isotope signatures were closely similar to both litter and fermentation layer in the corresponding sites. However, significantly more enriched signatures for peat in cypress site were observed in 5–30 cm depth while in spike + lily site it was observed in only 5–10 cm depth. The litter 15N signatures were significantly different, spikerush signatures were more enriched followed by water lily while cypress litter was least enriched and negative (P < 0.0001; Table 1). In the fermentation layer, the spike + lily site 15N signatures were significantly enriched compared to cypress site (P = 0.0014; Table 1), whereas in the peat layer in both cypress and spike + lily sites the 15N signatures were significantly different (Table 1). In the spike + lily site the 15N signatures were more enriched with increasing depth (P < 0.001) but in the cypress site same signature were inconsistent with increasing depth (P = 0.0002).
The cypress site fermentation layer was significantly more acidic than the spike + lily site fermentation layer (P = 0.0002; Table 1). In cypress site peat, acidity increased with increasing depth; the 0–5 cm depth was significantly less acidic compared to 5–30 cm depth (P = 0.0205; Table 1). In the spike + lily site the peat acidity was not significantly different between the depth (P = 0.604; Table 1). The loss in ignition (LOI) was significantly higher in the cypress fermentation layer than in spike + lily fermentation layer. In cypress site the peat LOI decreased with increasing depth and was significantly higher in 0–10 cm than in the 20–30 cm (P = 0.0051). However, in the spike + lily site LOI had no significant differences between depths (Table 1).
3.2. Carbon and nitrogen thermograms
It was noted that in spike + lily site low thermal stable C was dominant in litter, fermentation layer as well as in the peat, however in the cypress site, there was a dominance of low thermal stable carbon in the litter and fermentation, on the other hand there was a dominance of high thermal stable C in the peat (Table 2; Figure 2). The three litter types exhibited dominance of low thermal stable C (Table 2; Figure 2). The spikerush litter contained relatively greater low thermal stable C but, lesser high thermal stable C compared to cypress and water lily litters (Table 2; Figure 2). The litter low thermal stable C was in the order of cypress > spikerush > water lily, while the high thermal stable C was in the order of water lily > cypress > spikerush (Table 2; Figure 2). The cypress, spikerush, and water lily litter low thermal stable C was 5, 8, and 3 times higher than high thermal stable C, respectively (Table 2; Figure 2). Cypress site fermentation layer contained relatively greater high thermal stable C compared to spike + lily site (Table 2; Figure 2). The low thermal stable C in the cypress site and spike + lily site fermentation layers were 4 and 7 times greater than high thermal stable C, respectively (Table 2; Figure 2). In cypress site peat, high thermal stable C was 3.9, 2.4, and 1.5 times higher than low thermal stable C in the 0–5, 5–10 and 10–20 cm depths, respectively. In contrast, spike + lily site low thermal stable C was 8.7, 6.6, and 3.0 times greater than high thermal stable C in 0–5, 5–10, and 10–20 cm depths, respectively. The R400 index was above 0.5 in all litter, fermentation layer, spike + lily peat but not in the cypress peat (Table 2). In cypress site R400 increased with peat depth while in spike + lily site it decreased with increasing peat depth (Table 2).
Table 2.
Thermal stability of carbon and R400 index. Low thermal stable C is attributed to C recovered at temperature <400 °C and high thermal stable C recovered at temperature >400 °C. R400 index if the proportion of C recovered at temperature <400 °C compared to total C.
| SITE | LTSC |
HTSC |
LTSN |
HTSN |
R400 |
|---|---|---|---|---|---|
| <400 °C |
>400 °C |
<400 °C |
>400 °C |
C |
|
| g kg−1 | INDEX | ||||
| Litter | |||||
| Cypress | 406 | 79 | 3 | 3.5 | 0.84 |
| Spike | 400 | 50 | 4.3 | 3.1 | 0.89 |
| Water lily | 338 | 100 | 8.7 | 9.4 | 0.77 |
| Cypress | |||||
| FL | 399 | 90 | 7.3 | 10 | 0.82 |
| 0–5 cm | 89 | 354 | 0.5 | 21 | 0.2 |
| 5–10cm | 127 | 303 | 1.6 | 20.2 | 0.3 |
| 10–20cm | 147 | 227 | 2.3 | 13.8 | 0.39 |
| 20–30 cm | 173 | 158 | 2.3 | 10.1 | 0.52 |
| Spike + Lily | |||||
| FL | 384 | 54 | 13.3 | 12.7 | 0.88 |
| 0–5 cm | 376 | 43 | 15.8 | 11.4 | 0.9 |
| 5–10 cm | 381 | 57 | 13.8 | 15.6 | 0.87 |
| 10–20 cm | 262 | 86 | 7.3 | 14.5 | 0.75 |
| 20–30 cm | 152 | 124 | 3.6 | 11.7 | 0.55 |
∗ LTSC – Low thermal stable C; HTSC – High thermal stable C; LTSN – Low thermal stable N; HTSN – High thermal stable N.
Figure 2.
Carbon thermograms for litter, fermentation layer and sediments at different sites and depths as determined by multielement scanning thermal analysis (MESTA). Carbon recovered at <400 °C is considered low thermal stable carbon (LTSC) and carbon recovered at >400 °C is considered high thermal stable carbon (HTSC) ∗FL-fermentation layer.
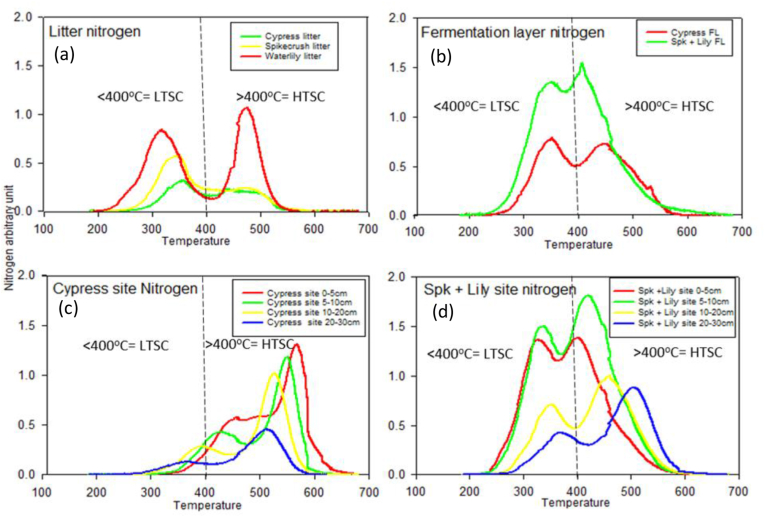
Both low and high thermal stable N were greater in water lily spikerush litter compared to the cypress and spikerush litters (Table 2; Figure 3). Low and high thermal stable N were in the order of water lily > spikerush > cypress and water lily > cypress > spikerush, respectively for litter (Table 2; Figure 3). For the fermentation layer, spike + lily site had more low thermal stable N and high thermal stable N compared to cypress site (Table 2; Figure 3). In the cypress peat low thermal stable N increased with depth, while high thermal stable N decreased. In spike + lily peat Low thermal stable N decreased with depth (Table 2; Figure 3).
Figure 3.
Nitrogen thermograms for litter, fermentation layer and sediments at different sites and depths as determined by multielement scanning thermal analysis (MESTA). Nitrogen recovered at <400 °C is considered low thermal stable nitrogen (LTSC) and nitrogen recovered at >400 °C is considered high thermal stable nitrogen (HTSC) ∗FL-fermentation layer.
3.3. Solid-state CPMAS 13C NMR spectra
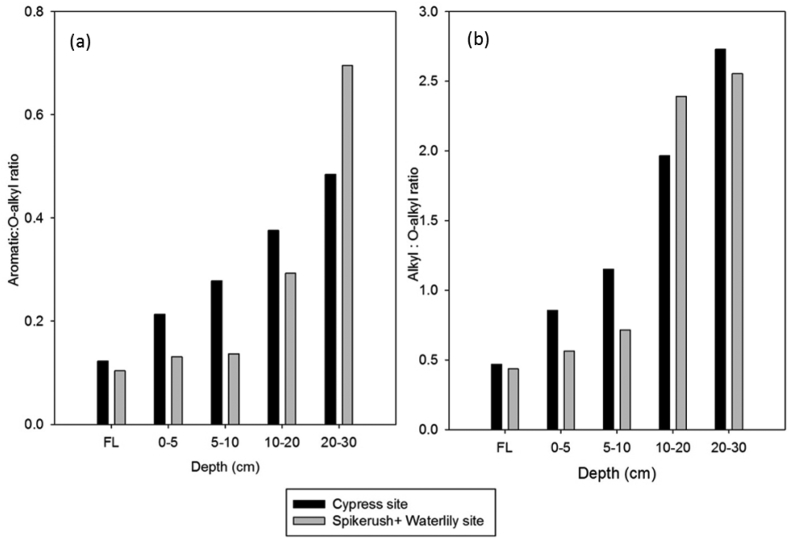
The results exhibited decreasing O-alkyl C and increasing alkyl C and aromatic C along litter – fermentation layer – peat continuum (Figure 4). O-alkyl C was dominant in all the litter types and the fermentation layer of both sites. The litter O-alkyl C content was in the order of spikerush > cypress > water lily; while fermentation layer O-alkyl C content was in the order of cypress > spike + lily site (Figure 4). In contrast, the cypress peat layer exhibited dominance of the alkyl C from 5-30 cm depths, while O-alkyl C was dominant in 0–5 cm peat depth. For spike + lily site, there was dominance of O-alkyl C in the 0–10 cm depth and dominance of alkyl C in 10–30 cm depth (Figure 4). From 0-5 cm to 20–30 cm depth, alkyl C increased by 21% and 13% and O-alkyl decreased by 62% and 75% in the cypress and spike + lily sites, respectively. The alkyl: O- alkyl ratio (humification index) increased from litter to fermentation layer to peat profile for both the cypress and spike + lily site (Figure 5). The humification indexes were 0.18–2.73 and 0.13–2.56 in cypress and spike + lily sites, respectively, as a result of decreasing O-alkyl and increasing alkyl along decomposition gradient. The alkyl:O-alkyl ratio was >1 for cypress and spike + lily peat at 5–30 cm and 10–30 cm depths, respectively. Aromatic:O-alkyl ratio increased with increasing depth in both sites. However, in the cypress site, aromatic C increased up to 10 cm depth and the trend was reversed to decreasing aromatic C in 10–30 cm depth (Figure 4).
Figure 4.
Soil Carbon composition in g kg −1 of litter, fermentation layer, different sites and depths as determined by Solid state 13 C NMR. ∗Cyp – cypress, spk-spikerush, Lily-water lily, FL-fermentation layer.
Figure 5.
Graph illustrating changes in aromatic:O-alkyl ratio and alkyl: O-alkyl ratio in fermentation layer and sediment at different depths and sites. ∗FL-fermentation layer. Acronym: LTC - low thermal stable carbon and HTC - high thermal stable carbon.
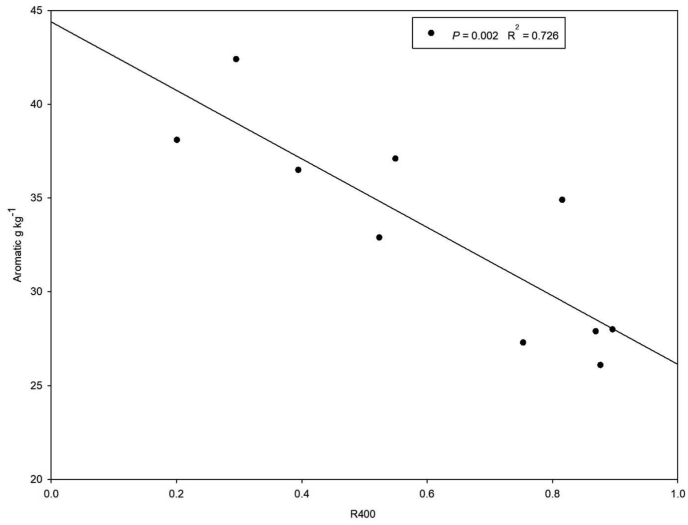
Low thermal stable C exihibited a positive relationship with O-alkyl C (P = 0.019; r2 = 0.52), high thermal stable C related significantly positively with aromatic C (Figure 6) but, R400 (P = 0.003; r2 = 0.69) related significantly negatively with aromatic C (P = 0.002; r2 = 0.73) (Figure 7).
Figure 6.
Relationship between low thermal stable carbon vs O-alkyl carbon and high thermal stable carbon vs aromatic.
Figure 7.
Relationship between R400 index vs aromatic carbon.
3.4. Methane and carbon dioxide production
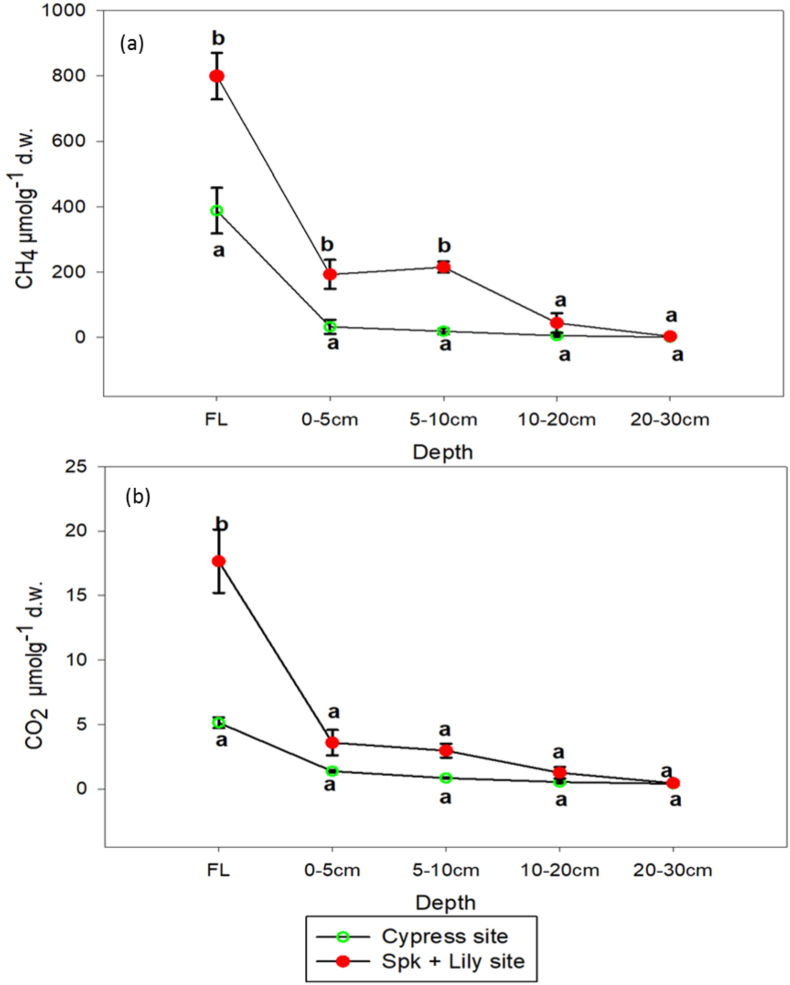
After 62 days of anaerobic incubation, CH4 production in the Spike + lily site fermentation layer was significantly greater than in Spike + lily site fermentation layer (Figure 8; P = 0.0146). In general, methane production was more than three-folds greater in the Spike + lily site fermentation layer and peat compared to cypress site fermentation layer. In Spike + lily site, CH4 production was significantly greater in the fermentation layer and 0–10 cm depth compared to Cypress site, but not significantly so for 20–30 cm depth. In Cypress site, 87% of CH4 was produced in the fermentation layer and 13% in the peat layer (0–30 cm), whereas in Spike + lily site, 64% was produced in fermentation layer and 36% in the peat layer (0–30 cm). Methane production decreased from fermentation layer to peat, and the production decreased with increasing depth in the peat (Figure 8).
Figure 8.
Comparison between cypress and spk + lily site on total CO2 and CH4 production in the fermentation layer and sediment. ∗FL-fermentation layer.
Carbon dioxide production was greater in the Spike + lily site compared to Cypress site (Figure 8). In the Spike + lily site, CO2 was significantly greater in the fermentation layer compared to Cypress site, but not significantly so for peat (Figure 8). The CO2 was 3.4, 2.6, 3, 1.3 times higher in the fermentation layer, 0–5, 5–10 and 10–20 depths of the spike + lily site compared to cypress site, respectively. In general, cypress fermentation layer and peat produced approximately three times more CO2 compared to spike + lily site. In the cypress site, 62% and 38% of the CO2 was produced from the fermentation layer and peat, respectively. In the spike + lily site, 68% and 32% CO2 was produced from the fermentation layer and peat respectively.
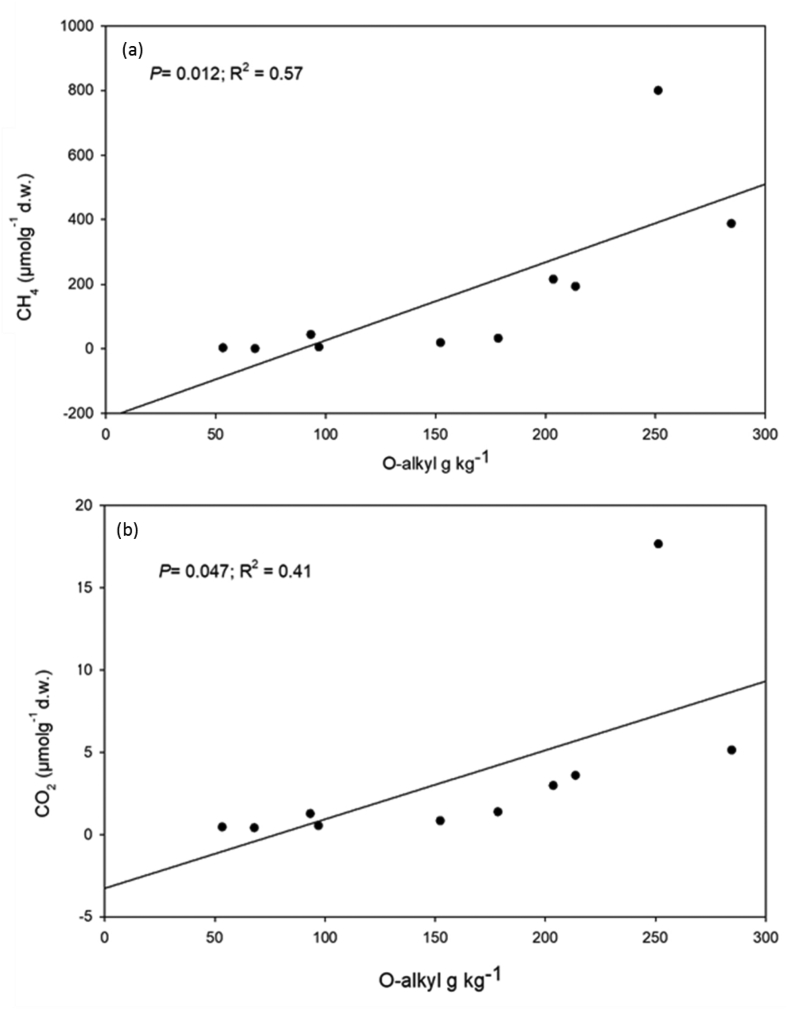
The R400 index exhibited significantly positive relationship with CH4 (P = 0.05; r2 = 0.4) but not significantly so for CO2 (P = 0.116; r2 = 0.28) production (Figure 9). The O-alkyl C exhibited a positive significantly relationship with both CH4 (P = 0.012; r2 = 0.58) and CO2 (P = 0.047; r2 = 0.41) production (Figure 10).
Figure 9.
Relationship between R400 index and CO2 and CH4 production.
Figure 10.
Relationship between O-alkyl carbon and CO2 and CH4 production.
4. Discussion
4.1. Characterization of organic matter
The study site peat exhibited high C content and acidity, typical of a (sub)tropical peat swamp forest (Kanokratana et al., 2010). The peat C chemical composition exhibited the dominance of O-alkyl and alkyl C and contrasted the expectation that the (sub)tropical peat would be dominated by aromatic C (Hodgkins et al., 2018) because of high recalcitrance resulting from initial rapid decay of plant litter (Chimner, 2004). Suggesting that higher primary productivity (Gillman et al., 2015) may have enhanced faster litter deposition or decomposers and nutrient limitations (Laiho 2006) could have supported organic matter accumulation. The low thermal stable C was the dominant form of thermal stable C apart from in the cypress peat, consequently the R400 was above 0.5 apart from at the cypress peat. The high R400 values are indicative of presence of well-preserved organic matter whereas low R400 values correspond to more degraded organic matter (Disnar et al., 2008).
This study showcased the relationship between the C thermal stability and C chemical stability. The low thermal stable C exhibited a positive relationship with O-alkyl C. O-alkyl C contains high content of polysaccharides found in fresh plant and microbial biomass (Baldock and Skjemstad, 2000). Furthermore, the high thermal stable C exhibited positive relationship with aromatic C, aromatic C is accumulated as a result of decomposition process and is considered to be stable, refractory and humified soil organic C (Hamdan et al., 2012). In addition, R400 index exhibited a negative relationship with aromatic C. The decreasing R400 is indicative of decrease in preserved or fresh organic matter (Chawla et al., 2010; Disnar et al., 2008). Therefore, high O-alkyl C, high low thermal stable C and high R400 were indicative of well-preserved or fresh organic matter. Suggesting that the fast and inexpensive thermal stability analysis could be used as a potential predictor of organic matter quality in similar peat. Further, increasing alkyl:O-alkyl and aromatic:O-alkyl ratios were coupled with increasing alkyl C and decreasing O-alkyl C. Both alkyl:O-alkyl and aromatic:O-alkyl ratios are indexes of the extent of organic matter decomposition (Baldock and Preston, 1995). As the alkyl:O-alkyl and aromatic:O-alkyl ratios increase, there is increasing accumulation of more stable C in expense of easily degradable C.
4.2. Source and peat decomposition of organic matter
The study indicated dominance of autochthonous source of C in the study sites and was consistent with (Saintilan et al., 2013), the 13C signatures were consistent along the decomposition continuum from the litter to fermentation layer to peat and indicated that organic matter was dominantly from C3 plants (Kohn, 2010). The 13C isotopic signature for C3 plants normally ranges from (−20 to −35) which is the range exhibited in this study (Kohn, 2010). In the peat the 13C signatures ranged between (−25 to −27) in all sites and depths, indicating low decomposition rates of organic matter with increasing peat depth. This is because wetlands are highly productive and can generate organic matter in enormous amounts and store the organic matter in a semi-decomposed state as a result of the anaerobic conditions (Mitsch and Gosselink, 2007).
We hypothesized that high thermal stable C would be dominant in the cypress site as a result of cypress woody nature (Moniruzzaman and Ono, 2013) and low thermal stable C would be dominant in the spike + lily site as a result of spikerush and water lily herbaceous nature (Still et al., 2003). The study hypothesis was not fully supported by the findings whereby, both cypress and spike + lily site exhibited dominance of low thermal stable C in the litter and fermentation layer that also corresponded with R400 > 0.5, this pattern persisted in the spike + lily site peat, however, cypress peat exhibited dominance of high thermal stable C and R400 < 0.5. Indicating that although the litter and fermentation layer composition were similar in terms of C thermal stability, the decomposition of the peat in the two different sites followed a different decomposition patterns. The discrepancy could potentially have been caused by different factors, which include levels of recalcitrance of decomposing detritus/substrate quality, decomposers present and nutrient availability (Laiho 2006).
The results from NMR analyses illustrated decreasing O-alkyl C, increasing alkyl and aromatic C along the litter-fermentation layer-peat continuum, the results were consistent with previous reported findings (Preston et al., 1989; Guggenberger et al., 1995; Quideau et al., 2001). Alkyl and aromatic C are considered to be stable, refractory and humified soil organic C (Hamdan et al., 2012). The observed increase in alkyl and aromatic C as well as increase of aromatic: O-alkyl ratio along peat depth signified the initial stage of decomposition and could be explained in the basis of selective utilization of the plant derived O-alkyl C and selective preservation of the more stable organic structures (Baldock et al., 1992; Quideau et al., 2000). However, it was evident in the cypress site that aromatic C increased up to 10 cm depth and the trend was reversed to decreasing aromatic C in 10–30 cm depth. The decreasing trend of aromatic C in 10–30 cm depth illustrated second stage of decomposition where the lignin molecules are exposed to microbial decomposition resulting in reduction in aromatic content (Baldock et al., 1997). Degradation of aromatic C is dominated by aerobic and anaerobic bacteria and aerobic fungi, however only a few archea are known to degrade aromatic C, and the archea mainly use strategies that require presence of molecular oxygen (Fuchs et al., 2011), suggesting that in the cypress site at 10–30 cm depth there was presence of oxygen enhancing aromatic C decomposition, the oxygen could have originated from the cypress rhizosphere (Moorberg et al., 2014). In addition (Davidson et al., 2006), research in Oxbow wetland in Mississippi dominated by cypress illustrated that rapid downward flow of surface water into the root zone is initiated after precipitation and has the potential to introduce oxygen to peats that would otherwise be anoxic.
Low thermal stable C related positively to O-alkyl C, and high thermal stable C related positively with aromatic C consequently R400 related negatively with aromatic C this was consistent with (Lopez-Capel et al., 2005; Ngatia et al., 2017), indicating that spike + lily peat dominated by low thermal stable C and O-alkyl C contained more labile C that is easily degradable while the cypress peat contained more stable C. The cypress which is a woody plant species contain high lignin content, aromatic C, lipids, waxes and cutin compared to herbaceous plants leading to slow decomposition and accumulation of lignin (Mackensen et al., 2003; Silver and Miya, 2001; Kögel-Knabner, 2002).
Organic matter decomposition was progressive along the decomposition continuum as evidence by increasing humification index as exhibited by alkyl:O-alkyl ratio and aromatic:O-alkyl ratio (Baldock and Preston, 1995; Zech et al., 1997). However, the presence of water in the permanently flooded wetlands slows down organic matter decomposition as a result of anaerobic conditions (Segnini et al., 2010). In addition, high and continuous production, high rates of organic matter input and slow decomposition in the anaerobic wetlands lead to large organic matter content, making soils an important C sink (Bot and Benites, 2005; Reddy and DeLaune, 2008).
4.3. Quality and stability of the organic matter and their relationship with greenhouse gases production
Decreasing CO2 and CH4 production were observed with increasing peat depth in both sites and the observation was consistent with Cooper et al. (2019), work which indicated that greenhouse gases production was greater in surface peats compared to deeper peats and was consistent with declining labile C. Overall the spike + lily site produced three times more CH4 and CO2 compared to cypress site. The high CH4 and CO2 production in the spike + lily site was consistent with dominance of low thermal stable C and R400 > 0.5 throughout the litter-fermentation layer-peat continuum. It was illustrated that O-alkyl that related positively with low thermal stable C favored both CH4 and CO2 production and was consistent with (Normand et al., 2017a, Normand et al., 2017b) findings. In contrast, the cypress site exhibited greater high thermal stable C, relatively higher accumulation of alkyl C, high C:N ratio, three times lower CO2 and CH4 production and consequentially high C storage in the peat compared to spike + lily site. Indicating that the amount of CH4 and CO2 produced as well as C stored in the two sites with different vegetation types is related to peat less thermally stable and chemically labile C which enhance microbial mineralization (Inglett et al., 2012; Silveria et al., 2008).
It was notable that in the spike + lily site the fermentation layer and 0–10 cm peat produced significantly high CH4 compared to cypress site. This was consistent with lower alkyl:O-alkyl and aromatic:O-alkyl ratios in spike + lily site compared to cypress site. Indicating that lower alkyl:O-alkyl and aromatic:O-alkyl ratios do favor both CH4 and CO2 production. As the alkyl:O-alkyl and aromatic:O-alkyl ratios increase, there is increasing accumulation of more stable C in expense of easily degradable C, the easily degradable organic materials are reported to increase CH4 production in soils (Yagi and Minami, 1990).
The alkyl:O-alkyl ratio was >1 for cypress and spike + lily peat at 5–30 cm and 10–30 cm depths, respectively, indicating a preferential loss of carbohydrates and humification of organic matter (Baldock et al., 1997). The >1 alkyl:O-alkyl ratio corresponded to the lowest CH4 production in both sites (<100 μmol g−1) indicating that organic matter humification negatively affected CH4 production. However, for fermentation layer in both sites, peat 0–5 cm depth in cypress site and 0–10 cm depth in spike + lily site, the alkyl:O-alkyl ratio was <1 reflecting weakly decomposed and less stable organic matter (Hamdan et al., 2012). The less <1 alkyl:O-alkyl ratio corresponded to highest CH4 production (200–800 μmol g−1).
5. Conclusion
The study indicated that the C stored in both peatland sites was autochthonous C, therefore C was mainly generated from the vegetation growing in the respective wetlands. The dominance of O-alkyl and alkyl C contrasted the expectation that aromatic C would be dominant in a tropical peatland as a result of rapid initial litter decomposition. Suggesting slow litter decomposition and accumulation of less decomposed organic matter. The strong positive relationship between O-alkyl C vs low thermal stable C, aromatic C vs high thermal stable C and negative relationship R400 vs aromatic C suggest that the fast and inexpensive thermal stability analysis could be useful as an organic matter quality predictor in this ecosystem. There was a decomposition gradient along litter-fermentation layer continuum. However, the spike + lily site entirely exhibited initial stage of decomposition whereas the cypress site exhibited initial stage of decomposition up to 10 cm depth and 10–30 cm depth exhibited the second stage of decomposition. Indicating different levels of organic matter decomposition in the two sites. The differences could have been as a result of substrate quality, nutrient availability and/or decomposer present. The CH4 and CO2 production decreased with peat depth and was positively related to O-alkyl, and R400 indicating that both thermal lability and chemical lability favored greenhouse gases production and were dependent on peat type.
Declarations
Author contribution statement
Lucy Ngatia, Johnny Grace: Conceived and designed the experiments; Performed the experiments.
Gbemisola Akinbi, Lucy Ngatia: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Robert Taylor: Analyzed and interpreted the data; Wrote the paper.
Lucy Ngatia; Tarek Abichou; Riqiang Fu; Chunhua Tan: Contributed reagents, materials, analysis tools or data.
Riqiang Fu, Chunhua Tan: Analyzed and interpreted the data.
Funding statement
Dr. Lucy Ngatia was supported by U.S. Forest Service [17-CA-11330140-027].
Riqiang Fu was supported by National Science Foundation, United States. [DMR-1644779].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Mr. Olumide Arigbede is highly appreciated for his assistance in data analysis. Mr. Pranavkumar D. Gajjar and Mr. Jwalit Nayak are highly appreciated for their assistance with fieldwork.
References
- Akinbi G.O. Florida Agricultural and Mechanical University; ProQuest: 2018. Effects of Vegetation Types on Carbon Dynamics, Microbial Response and Methanogenesis in Apalachicola National Forest Wetlands.http://famuproxy.fcla.edu/login?URL=?url=https://search.proquest.com/docview/2183395294?accountid=10913 [Google Scholar]
- Baldock J.A., Skjemstad J.O. Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Org. Geochem. 2000;31:697–710. [Google Scholar]
- Baldock J.A., Oades J.M., Waters A.G., Peng X., Vassallo A.M., Wilson M.A. Aspects of the chemical structure of soil organic materials as revealed by solid-state 13C NMR spectroscopy. Biogeochemistry. 1992;16:1–42. [Google Scholar]
- Baldock J.A., Oades J.M., Nelson P.N., Skene T.M., Golchin A., Clarke P. Assessing the extent of decomposition of natural organic materials using solid-state 13C NMR spectroscopy. Aust. J. Soil Res. 1997;35(5):1061–1083. [Google Scholar]
- Baldock J.A., Preston C.M. In: Carbon Forms and Functions in Forest Soils. McFee W.W., Kelly J.M., editors. Soil Sci.Soc. Am. Inc.; Madison, WI: 1995. Chemistry of carbon decomposition processes in forests as revealed by solid-state carbon-13 nuclear magnetic resonance; p. 89±117. [Google Scholar]
- Belyea L.R., Baird A.J. Beyond “the limits to peat bog growth”: Cross-scale feedback in peatland development. Ecol. Monogr. 2006;76(3):299–322. [Google Scholar]
- Bot A., Benites J. Food and Agriculture Organization of the United Nations. FAO Soils Bulletin 80, FAO; Rome: 2005. The Importance of Soil Organic Matter: Key to Drought-resistant Soil and Sustained Food Production. [Google Scholar]
- Bragazza L., Buttler A., Habermacher J., Brancaleoni L., Gerdol R., Fritze H., Hanajík P., Laiho R., Johnson D. High nitrogen deposition alters the decomposition of bog plant litter and reduces carbon accumulation. Global Change Biol. 2012;18:1163–1172. [Google Scholar]
- Bridgham S.D., Ye R. Methods in Biogeochemistry of Wetlands. 2013. Organic matter mineralization and decomposition; pp. 385–406. [Google Scholar]
- Chawla F., Steinmann P., Pfeifer H.R., Froidevaux P. Atmospheric deposition and migration of artificial radionuclides in alpine soils (val piora, Switzerland) compared to the distribution of selected major and trace elements. Sci. Total Environ. 2010;408(16):3292–3302. doi: 10.1016/j.scitotenv.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Chimner R. A Soil respiration rates of tropical peatlands in Micronesia and Hawaii. Wetlands. 2004;24:51–56. [Google Scholar]
- Chimner R.A., Ewel K.C. A tropical freshwater wetland: II. Production, decomposition, and peat formation. Wetl. Ecol. Manag. 2005;13(6):671–684. [Google Scholar]
- Cooper H.V., Vane C.H., Evers S., Aplin P., Girkin N.T., Sjögersten S. From peat swamp forest to oil palm plantations: the stability of tropical peatland carbon. Geoderma. 2019;342:109–111. [Google Scholar]
- Craft C.B. In: Wetland Soils. Richardson J.L., Vepraskas M.J., editors. Lewis Publishers; Boca Raton, FL: 2001. Biology of wetland soils; pp. 107–135. [Google Scholar]
- Davidson G.R., Laine B.C., Galicki S.J., Threlkeld S.T. Root-zone hydrology: why bald-cypress in flooded wetlands grow more when it rains. Tree-Ring Res. 2006;62(1):3–12. [Google Scholar]
- Dell'Abate M.T., Benedetti A., Trinchera A., Dazzi C. Humic substances along the profile of two Typic Haploxerert. Geoderma. 2002;107(3–4):281–296. [Google Scholar]
- Disnar J.-R., Guillet B., Keravis D., Di-Giovanni C., Sebag D. Soil organic matter (SOM) characterization by rock-Eval pyrolysis: scope and limitations. Org. Geochem. 2003;34(3):327–343. [Google Scholar]
- Disnar J.R., Jacob J., Morched-Issa M., Lottier N., Arnaud F. Assessment of peat quality by molecular and bulk geochemical analysis: application to the Holocene record of the Chautagne Marsh (Haute Savoie, France) Chem. Geol. 2008;254(1–2):101–112. [Google Scholar]
- Fuchs G., Boll M., Heider J. Microbial degradation of aromatic compounds — from one strategy to four. Nat. Rev. 2011;9:803 −816. doi: 10.1038/nrmicro2652. [DOI] [PubMed] [Google Scholar]
- Fung B., Khitrin A., Ermolaev K. An improved broadband decoupling sequence for liquid crystals and solids. J. Magn. Reson. 2000;142:97–101. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- Gagnon J.L., Jokela E.J., Moser W., Huber D.A. Characteristics of gaps and natural regeneration in mature longleaf pine flatwoods ecosystems. For. Ecol. Manage. 2004;187:373–380. [Google Scholar]
- Gillman L.N., Wright S.D., Cusens J., McBride P.D., Malhi Y., Whittaker R.J. Latitude, productivity and species richness. Global Ecol. Biogeogr. 2015;24:107–117. [Google Scholar]
- Girkin N.T., Dhandapani S., Evers S., Ostle N., Turner B.L., Sjögersten S. Interactions between labile carbon, temperature and land use regulate carbon dioxide and methane production in tropical peat. Biogeochemistry. 2020;147:87–97. [Google Scholar]
- Guggenberger G., Zech W., Haumaier L., Christensen B.T. Land-use effects on the composition of organic matter inparticle-size separates of soils: II. CPMAS and solution13C NMR analysis. Eur. J. Soil Sci. 1995;46:147–158. [Google Scholar]
- Hamdan R., El-Rifai H.M., Cheeseman A.W., Turner B.L., Reddy K.R., Cooper W.T. Linking phosphorus sequestration to carbon humification in wetlands soils by 31P and 13C NMR spectroscopy. Environ. Sci. Technol. 2012;46:4775–4782. doi: 10.1021/es204072k. [DOI] [PubMed] [Google Scholar]
- Hirano T., Jauhiainen J., Inoue T., Takahashi H. Controls on the carbon balance of tropical peatlands. Ecosystems. 2009;12:873–887. [Google Scholar]
- Hodgkins S.B., Richardson C.J., Dommain R., Wang H., Glaser P.H., Verbeke B., Winkler B.R., Cobb A.R., Rich V.I., Missilmani M., Flanagan N., Ho M., Hoyt A.,M.,, Harvey C.F., Vining S.R., Hough M.A., Moore T.R., Richard P.J.H., De La Cruz F.B., Toufaily J., Hamdan R., Cooper W.T., Chanton J.P. Tropical peatland carbon storage linked to global latitudinal trends in peat recalcitrance. Nat. Commun. 2018 doi: 10.1038/s41467-018-06050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos-Santillan J., Lomax B.H., Large D., Turner B.L., Boom A., Lopez O.R., Sjogersten S. Quality not quantity: organic matter composition controls of CO2 and CH4 fluxes in neotropical peat profiles. Soil Biol. Biochem. 2016;103:86–96. [Google Scholar]
- Hoyos-Santillan J., Lomax B.H., Large D., Turner B.L., Boom A., Lopez O.R., Sjögersten S. Getting to the root of the problem: litter decomposition and peat formation in lowland Neotropical peatlands. Biogeochemistry. 2015;126(1–2):115–129. [Google Scholar]
- Hsieh Y.P., Bugna G.C. Analysis of black carbon in sediments and soils using multielement scanning thermal analysis (MESTA) Org. Geochem. 2008;39(11):1562–1571. [Google Scholar]
- Hsieh Y.P. A novel multielemental scanning thermal analysis (MESTA) method for the identification and characterization of solid substances. J. AOAC Int. 2007;90:54–59. [PubMed] [Google Scholar]
- Inglett K.S., Inglett P.W., Reddy K.R., Osborne T.Z. Temperature sensitivity of greenhouse gas production in wetland soils of different vegetation. Biogeochemistry. 2012;108:77–90. [Google Scholar]
- Jauhiainen J., Takahashi H., Heikkinen J.E.P., Martikainen P.J., Vasander H. Carbon fluxes from a tropical peat swamp forest floor. Global Change Biol. 2005;11:1788–1797. [Google Scholar]
- Kanokratana P., Uengwetwanit T., Rattanachomsri U., Bunterngsook B., Nimchua T., Tangphatsornruang S., Plengvidhya V., Champreda V., Eurwilaichitr L. Insights into the phylogeny and metabolic potential of a primary tropical peat swamp forest microbial community by metagenomic analysis. Microb. Ecol. 2010;61:518–528. doi: 10.1007/s00248-010-9766-7. [DOI] [PubMed] [Google Scholar]
- Knicker H. Solid state CPMAS 13C and 15N NMR spectroscopy in organic geochemistry and how spin dynamics can either aggravate or improve spectra interpretation. Org. Geochem. 2011;42:867–890. [Google Scholar]
- Knorr W., Prentice I.C., House J.I., Holland E.A. Long-term sensitivity of soil carbon turnover to warming. Nature. 2005;433:298–301. doi: 10.1038/nature03226. [DOI] [PubMed] [Google Scholar]
- Kögel-Knabner I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002;34:139–162. [Google Scholar]
- Kohn M.J. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo) ecology and (paleo) climate. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19691–19695. doi: 10.1073/pnas.1004933107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho R. Decomposition in peatlands: reconciling seemingly contrasting results on the impacts of lowered water levels. Soil Biol. Biochem. 2006;38(8):2011–2024. [Google Scholar]
- Loisel J., van Bellen S., Pelletier L., Talbot J., Hugelius G., Karran D., Yu Z., Nichols J., Holmquist J. Insights and issues with estimating northern peatland carbon stocks and fluxes since the Last Glacial Maximum. Earth Sci. Rev. 2017;165:59–80. [Google Scholar]
- Lopez-Capel E., Sohi S.P., Gaunt J.L., Manning D.A.C. Use of thermogravimetry differential scanning calorimetry to characterize modelable soil organic matter fractions. Soil Sci. Soc. Am. J. 2005;69:136–140. [Google Scholar]
- Mackensen J., Bauhus J., Webber E. Decomposition rates of coarse woody debris—a review with particular emphasis on Australian tree species. Aust. J. Bot. 2003;51:27–37. [Google Scholar]
- Manning D.A.C., Lopez-Capel E., Bakker S. Seeing soil carbon: use of thermal analysis in the characterization of soil C reservoirs of differing stability. Mineral. Mag. 2005;69(4):425–435. [Google Scholar]
- McNeal E.O. In: Methods of Soil AnalysisPart 2. Chemical and Microbiological Properties. Page A.L., Miller R.H., Keeney D.R., editors. ASA Inc. SSSA Inc. Publishers; NY, USA: 1982. Soil pH and lime requirement; pp. 199–224. [Google Scholar]
- Mitsch W., Gosselink J. fourth ed. John Wiley & Sons; New York: 2007. Wetlands. [Google Scholar]
- Moniruzzaman M., Ono T. Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment. Bioresour. Technol. 2013;127:132–137. doi: 10.1016/j.biortech.2012.09.113. [DOI] [PubMed] [Google Scholar]
- Moorberg C.J., Vepraskas M.J., Niewoehner C.P. Phosphorus dissolution in the rhizosphere of bald cypress trees in restored wetland soils. Soil Sci. Soc. Am. J. 2014;79:343–355. [Google Scholar]
- Ngatia L.W., Hsieh Y.P., Nemours D., Fu R., Taylor R.W. Potential phosphorus eutrophication mitigation strategy: biochar carbon composition, thermal stability and pH influence phosphorus sorption. Chemosphere. 2017;180:201–211. doi: 10.1016/j.chemosphere.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Normand A.E., Smith A.N., Clark M.W., Long J.R., Reddy K.R. Chemical composition of soil organic matter in a subarctic peatland: influence of shifting vegetation communities. Soil Sci. Soc. Am. J. 2017;81:41–49. [Google Scholar]
- Normand A.E., Turner B.L., Lamit L.J., Smith A.N., Baiser B., Clark M.W., Hazlett C., Lilleskov E., Long J., Grover S., Reddy K.R. American Geophysical Union, Fall Meeting 2017; 2017. Soil Carbon Chemistry and Greenhouse Gas Production in Global Peatlands. abstract #B41M-06. [Google Scholar]
- Plante A.F., Fernández J.M., Haddix M.L., Steinweg J.M., Conant R.T. Biological, chemical and thermal indices of soil organic matter stability in four grassland soils. Soil Biol. Biochem. 2011;43:1051–1058. [Google Scholar]
- Preston C.M., Axelson D.E., Levesque M., Mathur S.P., Dinel H., Dudley R.L. Carbon-13 NMR and chemicalcharacterization of particle-size separates of peats differing in degree of decomposition. Org. Geochem. 1989;14:393–403. [Google Scholar]
- Quideau S.A., Anderson M.A., Graham R.C., Chadwick O.A., Trumbore S.E. Soil organic matter processes: characterization by 13C NMR and 14C measurements. For. Ecol. Manag. 2000;138:19–27. [Google Scholar]
- Quideau S., Chadwick O., Benesi A., Graham R., Anderson M. A direct link between forest vegetation type and soil organic matter composition. Geoderma. 2001;104:41–60. [Google Scholar]
- Reddy K.R., DeLaune R.D. CRC Press; Boca Raton, FL, USA: 2008. Biogeochemistry of Wetlands: Science and Applications. [Google Scholar]
- Saintilan N., Rogers K., Mazumder D., Woodroffe C. Allochthonous and autochthonous contributions to carbon accumulation and carbon store in southeastern Australian coastal wetlands. Estuar. Coast Shelf Sci. 2013;128:84–92. [Google Scholar]
- Schipper L.A., Reddy K.R. Methane production and emissions from four reclaimed and pristine wetlands of Southeastern United States. Soil Sci. Soc. Am. J. 1994;58:1270–1275. [Google Scholar]
- Schmidt M.W.I., Noack A.G. Black carbon in soils and sediments: analysis, distribution, implications and current challenges. Global Biogeochem. Cycles. 2000;14(3):777–793. [Google Scholar]
- Schnitzer M., Hoffman I. A thermogravimetric approach to the classification of organic soils. Soil Sci. Soc. Am. J. 1966;30:63–66. [Google Scholar]
- Segers R. Methane production and methane consumption:A review of processes underlying wetland methane fluxes. Biochemistry. 1998;41:23–51. [Google Scholar]
- Segnini A., Posadas A., Quiroz R., Milori D.M.B.P., Saab S.C., Neto L.M., Vaz C.M.P. Spectroscopic assessment of soil organic matter in wetlands from the High Andes. SSSAJ. 2010;74(6):2246–2253. [Google Scholar]
- Shrestha R.K., Stein T.V., Clark J. Valuing nature-based recreation in public natural areas of the Apalachicola River region, Florida. J. Environ. Manag. 2007;85:977–985. doi: 10.1016/j.jenvman.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Silveria M.L., Comerford N.M., Reddy K.R., Cooper W.T., El-Rifai H. Characterization of soil organic carbon pools by acid hydrolysis. Geoderma. 2008;144:405–414. [Google Scholar]
- Silver W.L., Miya R.K. Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia. 2001;129:407–419. doi: 10.1007/s004420100740. [DOI] [PubMed] [Google Scholar]
- Sjogersten S., Black C.R., Evers S., Hoyos-Santillan J., Wright E.L., Turner B.L. Tropical wetlands: a missing link in the global carbon cycle? Global Biogeochem. Cycles. 2014;28:1371–1386. doi: 10.1002/2014GB004844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögersten S., Cheesman A.W., Lopez O., Turner B.L. Biogeochemical processes along a nutrient gradient in a tropical ombrotrophic peatland. Biogeochemistry. 2011;104(1–3):147–163. [Google Scholar]
- Still C.J., Berry J.A., Collatz G.J., DeFries R.S. Global distribution of C3 and C4 vegetation: carbon cycle implications. Global Biogeochem. Cycles. 2003;17(1):1006. [Google Scholar]
- Strezov V., Moghtaderi B., Lucas J.A. Computational calorimetric investigation of the reactions during thermal conversion of wood biomass. Biomass Bioenergy. 2004;27(5):459–465. [Google Scholar]
- Trettin C.C., Jurgensen M.F. In: The Potential of US Forest Soils to Sequester Carbon and Mitigate the Greenhouse Effect. Kimble J.M., Heath L.S., Birdsey R.A., Lai R., editors. CRC Press; Boca Raton, FL: 2003. Carbon cycling in wetland forest soils; pp. 311–331. [Google Scholar]
- Tschinkel W.R., Hess C.A. Arboreal ant community of a pine forest in northern Florida. Ann. Entomol. Soc. Am. 1999;92:63–70. [Google Scholar]
- Wieder R.K., Vitt D.H., editors. Boreal Peatland Ecosystems. Springer; Heidelberg: 2006. [Google Scholar]
- Ward S.E., Orwin K.H., Ostle N.J., Briones M.J., Thomson B.C., Griffiths R.I., Oakley S., Quirk H., Bardgett R.D. Vegetation exerts a greater control on litter decomposition than climate warming in peatlands. Ecology. 2015;96(1):113–123. doi: 10.1890/14-0292.1. [DOI] [PubMed] [Google Scholar]
- Wright E., Black C.R., Cheesman A.W., Drage T., Large D., Turner B.L., Sjögersten S. Contribution of subsurface peat to CO2 and CH4 fluxes in a neotropical peatland. Global Change Biol. 2011;17:2867–2881. [Google Scholar]
- Wright E.L., Black C.R., Cheesman A.W., Turner B.L., Sjögersten S. Vol. 33. 2013. Wetlands. (217) [Google Scholar]
- Wright E.L., Black C.R., Turner B.L., Sjogersten S. Environmental controls of temporal and spatial variability in CO2 and CH4 fluxes in a neotropical peatland. Global Change Biol. 2013;19:3775–3789. doi: 10.1111/gcb.12330. [DOI] [PubMed] [Google Scholar]
- Wright A.L., Reddy K.R., Corstanje R. Patterns of heterotrophic microbial activity in eutrophic and oligotrophic peatlands. Eur. J. Soil Biol. 2009;45:131–137. [Google Scholar]
- Yagi K., Minami K. Effect of organic matter application on methane emission from some Japanese paddy fields, Soil Science and Plant Nutrition, 36:4,599-610 nitrogen and land use in New England, USA. Environ. Monit. Assess. 1990;131:71–81. [Google Scholar]
- Zech W., Senesi N., Guggenberger G., Kaiser K., Lechmann J., Miano T.M., Miltner A., Schroth G. Factors controlling humifi cation and mineralization of soil organic matter in the tropics. Geoderma. 1997;79:117–161. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.