Abstract
Introduction
The aim of this study was to evaluate the efficacy of pemetrexed and platinum plus pembrolizumab by baseline tumor burden.
Methods
A total of 616 patients in the intention-to-treat population of the KEYNOTE-189 study were included in this analysis. Baseline tumor burden subgroups were identified on the basis of extent of distant metastasis (M1a versus M1b), median number (≤3 versus >3) of organ systems with lesions, or symptom severity score of patient-reported lung cancer-associated symptoms (≤median versus >median). Overall survival (OS), progression-free survival (PFS), and PFS-2 were evaluated by Kaplan-Meier and univariate Cox methods. Objective response rate was analyzed using logistic regression models, and duration of response was analyzed descriptively. Efficacy outcomes were also analyzed according to the programmed death-ligand 1 expression levels.
Results
OS and PFS were significantly improved with pemetrexed and platinum plus pembrolizumab in all baseline tumor burden subgroups (M1a stage: OS hazard ratio [HR] = 0.54, p = 0.0037; PFS HR = 0.48, p = 0.0001; M1b stage: OS HR = 0.58, p ≤ 0.0001; PFS HR = 0.51, p ≤ 0.0001; number of organ systems with lesion ≤ 3: OS HR = 0.49, p ≤ 0.0001 PFS HR = 0.41, p ≤ 0.0001; >3: OS HR = 0.67, p = 0.0068; PFS HR = 0.59, p = 0.0001; symptom severity score ≤ median: HR = 0.51, p ≤ 0.0001; PFS HR 0.49, p ≤ 0.0001; > median: OS HR = 0.60, p = 0.0003; PFS HR = 0.48, p ≤ 0.0001). PFS2 and objective response rate were also improved with pemetrexed and platinum plus pembrolizumab in all baseline tumor burden subgroups. Efficacy outcomes were generally consistent regardless of programmed death-ligand 1 expression levels.
Conclusions
Pemetrexed and platinum plus pembrolizumab were found to have relevant efficacy regardless of the extent of baseline tumor burden and the variables used to define it. These results further support pemetrexed and platinum plus pembrolizumab as the standard of care in the first-line treatment of metastatic nonsquamous NSCLC.
Keywords: Metastatic nonsquamous non–small cell lung cancer, Pemetrexed, Pembrolizumab, Tumor burden, Immune checkpoint inhibitors, Chemotherapy
Introduction
The recent advent of immune checkpoint inhibitors (ICIs), alone or in combination with chemotherapy,1 has greatly reduced the risk of disease progression and improved survival in treatment-naive patients with metastatic nonsquamous NSCLC (NSQ-NSCLC).2, 3, 4, 5, 6
Pemetrexed has been the standard of care in combination with platinum-based chemotherapy for the treatment of patients with advanced NSQ-NSCLC for more than a decade.7, 8, 9 In 2017, the U.S. Food and Drug Administration approved pemetrexed and platinum in combination with pembrolizumab as a first-line treatment of metastatic NSQ-NSCLC, on the basis of the objective response rate (ORR) of the cohort G of the phase 2 KEYNOTE-021 study.10,11 The efficacy and the manageable safety profile of pemetrexed and platinum in combination with pembrolizumab were subsequently confirmed in the phase 3 KEYNOTE-189 study.12 After a median follow-up of 10.5 months, pemetrexed and platinum plus pembrolizumab significantly improved overall survival (OS) and progression-free survival (PFS), compared with pemetrexed and platinum plus placebo.12 The improvement in OS, PFS, and ORR with pemetrexed and platinum plus pembrolizumab was observed irrespectively of tumor programmed death-ligand 1 (PD-L1) expression levels.13,14 Importantly, efficacy benefits were consistently maintained at a longer follow-up of approximately 4 years.2 The effectiveness of pemetrexed and platinum plus pembrolizumab in the first-line treatment of patients with NSQ-NSCLC has also been revealed in the real-world clinical setting, with results comparable with those of the KEYNOTE-189 trial.15
Baseline tumor burden generally defined by tumor volume, tumor diameter, location, or number of metastatic lesions16 is being increasingly recognized as an important determinant of clinical outcomes and treatment decisions.16, 17, 18, 19, 20 The presence of high tumor burden at baseline is considered a negative prognostic factor in various cancer types, including metastatic NSCLC.16,21, 22, 23 Preclinical studies24 and emerging clinical evidence also indicate that the extent of tumor burden at baseline influences treatment responses and outcomes to ICI monotherapy in advanced NSCLC.18,24
Results from the KEYNOTE-189 study revealed a similar improvement in PFS and OS by pemetrexed and platinum plus pembrolizumab in patients with poor prognostic factors, including presence of brain metastases, liver metastases, and M1b stage.13,14 Data on the impact of baseline tumor burden to treatment with pemetrexed and platinum plus pembrolizumab are lacking. The aim of this post hoc analysis was to evaluate the efficacy benefit of pemetrexed and platinum plus pembrolizumab by baseline tumor burden.
Materials and Methods
Study Design
KEYNOTE-189 (NCT02578680) study design, eligibility criteria, and patient baseline and demographic characteristics have been previously reported12 and are briefly summarized in the Supplementary Materials. The trial protocol and all amendments were approved by the appropriate ethics panel at each study center. The trial was conducted in accordance with the Declaration of Helsinki and was overseen by an external monitoring committee. All patients provided informed written consent before enrollment.
Patients
The intention-to-treat population included 616 patients, randomized to pemetrexed and platinum plus pembrolizumab (n = 410) or pemetrexed and platinum plus placebo (n = 206).13 All patients in the intention-to-treat population were included in this analysis. All patients provided written informed consent before enrollment. The results reported herein are from the data cutoff date of May 20, 2019. Median time from randomization to data cutoff was 31 months (26.5–38.8 mo).14
Outcomes
Efficacy end points included OS, PFS, PFS-2, ORR, and duration of response (DoR).
Tumor Burden at Baseline
We used three distinct variables to define tumor burden at baseline.
Extent of Distant Metastases
The KEYNOTE-189 study enrolled patients with histologically or cytologically confirmed diagnosis of stage IV M1a or M1b NSQ-NSCLC,12 according to the TNM classification system seventh edition.25 Stage M1a defines patients with intrathoracic metastases only (lesions in the contralateral lobe or pleura or pleural/pericardial effusion), whereas stage M1b indicates the presence of extrathoracic metastases.26 Thus, in this post hoc analysis, we used the degree of metastatic involvement, defined by the M1a and M1b staging, as a measure of baseline tumor burden. We categorized patients into two distinct baseline tumor burden subgroups: patients with low tumor burden (stage M1a) and patients with high tumor burden (stage M1b).
Number of Organ Systems With Lesions
Evaluation of the number of organ systems with lesions was based on the imaging assessment at the time of randomization and was used as a second variable. Organ systems included in the baseline tumor burden assessment are listed in Supplementary Table 1. Lesions were identified at baseline, and each lesion was mapped onto organ systems. Each organ system was counted regardless of the number of lesions present. The median number of organ systems with lesions (n = 3) was used as a cutoff value to categorize patients in two subgroups: patients with median number of organ system with lesions less than or equal to 3 (low tumor burden) and patients with median number of organ systems with lesions of greater than 3 (high tumor burden).
Patient-Reported Lung Cancer-Associated Symptoms (Symptom Severity Score)
Quality of life and patient-reported outcomes from the KEYNOTE-189 study have been previously reported27 using the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30,28 Quality of Life Questionnaire Lung Cancer 13 (QLQ-LC13),29 and EuroQoL 5D.28 In this study, the patient population with available baseline QLQ-LC13 assessment included 357 patients in the pemetrexed and platinum plus pembrolizumab arm and 179 in the pemetrexed and platinum plus placebo arm. The symptom severity score was derived from six questions assessing lung cancer-associated symptoms from the patient-reported QLQ-LC13 instrument (coughing, dyspnea, hemoptysis, pain in arm or shoulder, pain in the chest, pain in other parts of body) administered at baseline (cycle 1). The patient scoring of symptoms was translated into a numeric rating for each of the six symptoms and added up to a total score, which was standardized to a scale ranging from 0 (absence of symptom) to 100 (maximum symptom) by linear transformation. The median score (n = 122.2) was used as a cutoff value to categorize patients in two subgroups: patients with symptom severity score less than or equal to median (low tumor burden) and patient with symptom severity score greater than median (high tumor burden).
Statistical Analysis
Cohorts of interest for statistical analysis were defined by the three variables used to define tumor burden. Descriptive, within-treatment arm, and within-cohort summary statistics for OS, PFS, and PFS-2 were derived using the Kaplan-Meier method. Within each cohort, between-treatment arm comparative hazard ratio (HR) estimates for OS, PFS, and PFS-2 were obtained using univariate stratified Cox models; the stratification variables were the original stratification variables of the KEYNOTE-189 study (PD-L1 expression, choice of platinum-based drug, and smoking history). ORR was analyzed using logistic regression models. DoR was analyzed using the Kaplan–Meier method for within-treatment arm, within-cohort descriptive summaries. Analyses by PD-L1 expression levels (tumor proportion score [TPS] <1% and ≥1%) within each baseline tumor burden subgroup were also performed.
Results
Patients
Baseline Tumor Burden Subgroups
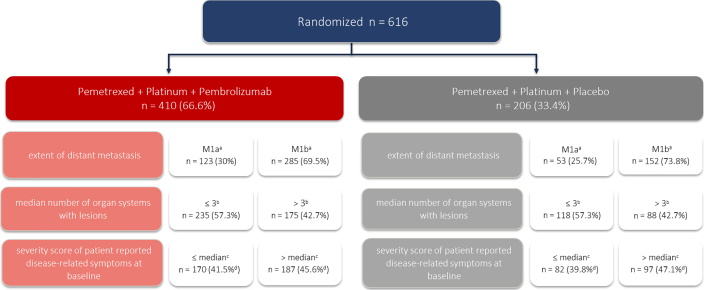
Figure 1 reveals the population analysis and the categorization of baseline tumor burden subgroups. The proportion of patients with M1a or M1b stage, number of organ systems with lesions less than or equal to 3 or greater than 3, or a symptom severity score less than or equal to median or greater than median was balanced across the two treatment arms. Nevertheless, both treatment arms had a higher number of patients with M1b stage (69.5% versus 73.8%) than with M1a stage (30% versus 25.7%), with less than or equal to 3 organ system with lesions (57.3% versus 57.3%) than with greater than 3 organ systems with lesions (42.7% versus 42.7%), and with symptom severity score greater than median (45.6% versus 47.1%) than with symptom severity score less than or equal to median (41.5% versus 39.8%) (Fig. 1).
Figure 1.
Identification of the analysis population and characterization of baseline tumor burden subgroups. aThere were two patients with missing data in the treatment arm and one patient in the control arm. bMedian number of organ systems with lesions was 3. cMedian score = 122.2, on the basis of six lung-specific symptoms from the patient-reported QLQ-LC-13 instrument (coughing, dyspnea, hemoptysis, pain in arm or shoulder, pain in the chest, pain in other parts of body); the number of patients with available lung cancer-associated symptoms at baseline from the QLQ-LC13 instrument was 357 in the pemetrexed + platinum + pembrolizumab arm and 179 in the pemetrexed + platinum + placebo arm. dPercentage calculations are provided on the basis of total patient population (n = 410 and N = 206) and not total number of patients with available lung cancer-associated symptoms at baseline from the QLQ-LC13 instrument. QLC-LC13, Quality of Life Questionnaire Lung Cancer 13.
Baseline Demographic and Disease Characteristics
Baseline demographic and disease characteristics were generally balanced across subgroups (Supplementary Tables 2–4). An increased proportion of older patients (aged ≥65 y) was present in the pemetrexed and platinum plus pembrolizumab arm compared with the pemetrexed and platinum plus placebo arm of the following tumor burden subgroups: M1b stage (50.2% versus 38.2%), greater than 3 organ systems with lesions (49.1% versus 34.1%), and symptom severity score less than or equal to median (57.6% versus 46.3%). In addition, the proportion of patients with Eastern Cooperative Oncology Group performance status (ECOG PS) 1 was higher in both treatment arms of the subgroups with M1b stage, greater than 3 organ systems with lesions, and symptom severity score > median versus those with M1a stage, less than or equal to 3 organ system with lesions, and symptom severity score less than or equal to median (Supplementary Tables 2–4).
OS and PFS by Baseline Tumor Burden
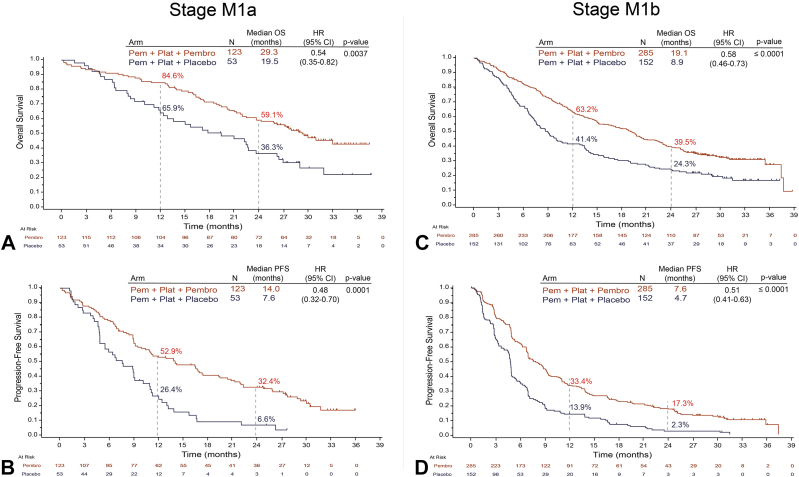
In the subgroup with M1a stage, median OS was 29.3 months with pemetrexed and platinum plus pembrolizumab and 19.5 months with pemetrexed and platinum plus placebo (HR = 0.54, 95% confidence interval [CI]: 0.35–0.82, p = 0.0037) (Fig. 2A). In the subgroup with M1b stage, median OS was 19.1 months with pemetrexed and platinum plus pembrolizumab and 8.9 months with pemetrexed and platinum plus placebo (HR = 0.58, 95% CI: 0.4–0.73, p ≤ 0.0001) (Fig. 2C). PFS was also significantly improved with pemetrexed and platinum plus pembrolizumab in both M1a and M1b stages. In the subgroup with M1a stage, median PFS was 14.0 month with pemetrexed and platinum plus pembrolizumab and 7.6 months with pemetrexed and platinum plus placebo (HR = 0.48, 95% CI: 0.32–0.70, p = 0.0001) (Fig. 2B). In the subgroup with M1b stage, median PFS was 7.6 months with pemetrexed and platinum plus pembrolizumab and 4.7 months with pemetrexed and platinum plus placebo (HR = 0.51, 95% CI: 0.41–0.63, p ≤ 0.0001) (Fig. 2D). Estimated OS and PFS rates at 12 months and 24 months were higher with pemetrexed and platinum plus pembrolizumab than with pemetrexed and platinum plus placebo in both M1a and M1b subgroups (Fig. 2).
Figure 2.
OS and PFS by M1a and M1b staging. Illustrated are Kaplan-Meier estimates of OS and PFS in patients with M1a stage NSQ-NSCLC (A and B) or M1b stage NSQ-NSCLC (C and D). Tick marks indicate censoring of data at the last time the patient was known to be alive. CI, confidence interval; HR, hazard ratio; NSQ-NSCLC, nonsquamous NSCLC; OS, overall survival; Pem, pemetrexed; Pembro, pembrolizumab; PFS, progression-free survival; Plat, platinum.
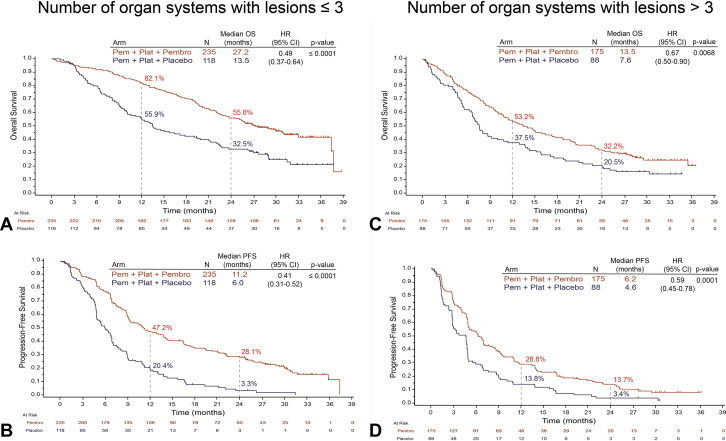
A significant improvement in OS with pemetrexed and platinum plus pembrolizumab compared with the pemetrexed and platinum plus placebo arm was also observed when tumor burden was assessed by the number of organ systems with lesions. In the subgroup with less than or equal to 3 organ systems with lesions, median OS was 27.2 months with pemetrexed and platinum plus pembrolizumab combination and 13.5 months with pemetrexed and platinum plus placebo (HR = 0.49, 95% CI: 0.37–0.64, p ≤ 0.0001) (Fig. 3A). In the subgroup with greater than 3 organ systems with lesions, median OS was 13.5 months with pemetrexed and platinum plus pembrolizumab and 7.6 months with pemetrexed and platinum plus placebo (HR = 0.67, 95% CI: 0.50–0.90, p = 0.0068) (Fig. 3C). Median PFS was 11.2 months with pemetrexed and platinum plus pembrolizumab and 6.0 months with pemetrexed and platinum plus placebo (HR = 0.41, 95% CI: 0.31–0.52, p ≤ 0.0001) in the subgroup of patients with less than or equal to 3 organ systems with lesions (Fig. 3B). In patients with greater than 3 organ systems with lesions, median PFS was 6.2 months in the pemetrexed and platinum plus pembrolizumab arm and 4.6 months in the pemetrexed and platinum plus placebo arm (HR = 0.59, 95% CI: 0.45–0.78, p = 0.0001) (Fig. 3D). Estimated OS and PFS rates at 12 months and 24 months were improved with pemetrexed and platinum plus pembrolizumab compared with pemetrexed and platinum plus placebo across both subgroups (Fig. 3).
Figure 3.
OS and PFS by number of organ systems with lesion. Illustrated are Kaplan-Meier estimates of OS and PFS in patients with less than or equal to 3 organ systems with lesions (A and B) or greater than 3 organ systems with lesions (C and D). Tick marks indicate censoring of data at the last time the patient was known to be alive. CI, confidence interval; HR, hazard ratio; NSQ-NSCLC, nonsquamous NSCLC; OS, overall survival; Pem, pemetrexed; Pembro, pembrolizumab; PFS, progression-free survival; Plat, platinum.
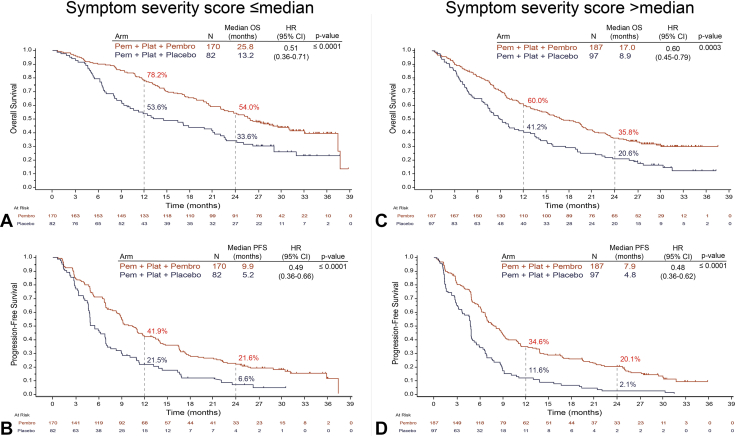
Treatment with pemetrexed and platinum plus pembrolizumab significantly improved median OS in the subgroup with symptom severity score less than or equal to median compared with pemetrexed and platinum plus placebo (25.8 mo versus 13.2 mo; HR = 0.51, 95% CI: 0.36–0.71; p ≤ 0.0001) (Fig. 4A). A similar improvement was observed in the subgroup with symptom severity score greater than median with pemetrexed and platinum plus pembrolizumab compared with pemetrexed and platinum plus placebo (17.0 mo versus 8.9 mo; HR = 0.60, 95% CI: 0.45–0.79, p = 0.0003) (Fig. 4C). In addition to the improvement in OS, median PFS was significantly longer in the subgroup with symptom severity score less than or equal to median treated with pemetrexed and platinum plus pembrolizumab compared with pemetrexed and platinum plus placebo (9.9 mo versus 5.2 mo; HR = 0.49, 95% CI: 0.36–0.66, p ≤ 0.0001) (Fig. 4B). Similarly, in patients with symptom severity score > median, median PFS was 7.9 months in the pemetrexed and platinum plus pembrolizumab arm and 4.8 months in the pemetrexed and platinum plus placebo arm (HR = 0.48, 95% CI: 0.36–0.62, p ≤ 0.0001) (Fig. 4D). The estimated OS and PFS rates at 12 and 24 months were higher in the pemetrexed and platinum plus pembrolizumab arm compared with the pemetrexed and platinum plus placebo arm across both subgroups (Fig. 4).
Figure 4.
OS and PFS by severity score of patient-reported lung cancer-associated symptoms at baseline. Illustrated are Kaplan-Meier estimates of OS and PFS in patients with symptom severity score less than or equal to the median (A and B) or greater than the median (C and D). Tick marks indicate censoring of data at the last time the patient was known to be alive. CI, confidence interval; HR, hazard ratio; NSQ-NSCLC, nonsquamous NSCLC; OS, overall survival; Pem, pemetrexed; Pembro, pembrolizumab; PFS, progression-free survival; Plat, platinum.
In the primary analysis of the KEYNOTE-189 study, the superior efficacy of pemetrexed and platinum plus pembrolizumab was maintained regardless of tumor PD-L1 expression levels.12 Similarly, in this post hoc analysis, OS and PFS were improved with pemetrexed and platinum plus pembrolizumab in PD-L1–negative (TPS < 1%) and in PD-L1–positive (TPS ≥ 1%) patients across all baseline tumor burden subgroups (Supplementary Tables 5–6).
ORR and Median DoR by Baseline Tumor Burden
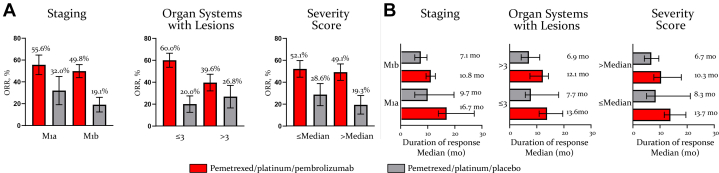
ORR with pemetrexed and platinum plus pembrolizumab was 55.6% in the subgroup with M1a stage and 49.8% in the M1b stage subgroup, compared with 32% and 19.1% with pemetrexed and platinum plus placebo, respectively (Fig. 5A). DoR also favored pemetrexed and platinum plus pembrolizumab compared with pemetrexed and platinum plus placebo in M1a stage (16.7 mo versus 9.7 mo) and in M1b stage (10.8 mo versus 7.1 mo) subgroups (Fig. 5B). A similar improvement in ORR and DoR was observed with pemetrexed and platinum plus pembrolizumab compared with pemetrexed and platinum plus placebo in the tumor burden subgroups with less than or equal to 3 organ systems with lesions (60.0% versus 20.0%; 13.6 mo versus 7.7 mo) and greater than 3 organ systems with lesions (39.6% versus 26.8%; 12.1 mo versus 6.9 mo) (Fig. 5A and B). Similarly, the proportion of patients who experienced an ORR and longer DoR was higher with pemetrexed and platinum plus pembrolizumab than with pemetrexed and platinum plus placebo in the subgroup with symptom severity score less than or equal to median (52.1% versus 28.6%; 13.7 mo versus 8.3 mo) and greater than median (49.1% versus 19.3%; 10.3 mo versus 6.7 mo) (Fig. 5A and B). The ORR and DoR benefits with pemetrexed and platinum plus pembrolizumab were also maintained across all baseline tumor burden subgroups irrespectively of PD-L1 expression levels (Supplementary Table 7), except for a shorter DoR with pemetrexed and platinum plus pembrolizumab in the subgroup with greater than 3 organ systems with lesion and PD-L1 TPS less than 1% (Supplementary Table 7). The lack of difference in DoR benefit between the treatment arms in this subgroup of patients is likely due to the small sample size (Supplementary Table 7).
Figure 5.
ORR and DoR by baseline tumor burden. Illustrated are the ORR (A) and median DoR (B) by tumor burden subgroups. Responses are based on BICR assessment per RECIST version 1.1. In the M1a compared with M1b stage subgroups, 39.7% versus 23.3% of patients receiving pemetrexed and platinum plus pembrolizumab and 15.6% versus 6.5% of patients receiving pemetrexed and platinum plus placebo had a DoR that lasted for 24 months. In the subgroup with number of organ systems with lesions less than or equal to 3 compared with greater than 3, 33.7% versus 17.2% of patients receiving pemetrexed and platinum plus pembrolizumab and 0.0% versus 11.6% of patients receiving pemetrexed and platinum plus placebo had a DoR that lasted for 24 months. In the subgroup with symptom severity score less than or equal to the median compared with greater than the median, 27.7% versus 29.2% of patients receiving pemetrexed and platinum plus pembrolizumab and 20.2% versus 0.0% of patients receiving pemetrexed and platinum plus placebo had a DoR that lasted for 24 months. BICR, blinded independent central review; DoR, duration of response; ORR, objective response rate; RECIST, Response Evaluation Criteria in Solid Tumors.
PFS-2 by Baseline Tumor Burden
In the primary analysis of the KEYNOTE-189 study, treatment with pemetrexed and platinum plus pembrolizumab resulted in an improvement of median PFS-2 compared with pemetrexed and platinum plus placebo (17.0 mo versus 9.0 mo).13 In the present analysis, the PFS-2 benefit of pemetrexed and platinum plus pembrolizumab was maintained across all baseline tumor burden subgroups. In the subgroup with M1a stage, median PFS-2 was 23.0 months with pemetrexed and platinum plus pembrolizumab and 15.0 months with pemetrexed and platinum plus placebo (HR = 0.57, 95% CI: 0.38–0.86, p = 0.0059) (Table 1). Similar improvement was observed with pemetrexed and platinum plus pembrolizumab compared with pemetrexed and platinum plus placebo in patients with M1b stage (median 15.1 mo versus 7.6 mo [HR = 0.50, 95% CI: 0.40–0.63, p ≤ 0.0001]) (Table 1). Estimated PFS-2 rates at 12 and 24 months were higher in the pemetrexed and platinum plus pembrolizumab across both subgroups (Table 1).
Table 1.
PFS-2 by Baseline Tumor Burden
| Staging |
Organ Systems With Lesions |
Symptom Severity Score |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1a |
M1b |
≤3 |
>3 |
≤Median |
>Median |
|||||||
| Pem/plat/pembro (n = 123) | Pem/plat/placebo (n = 53) | Pem/plat/pembro (n = 285) | Pem/plat/placebo (n = 152) | Pem/plat/pembro (n = 235) | Pem/plat/placebo (n = 118) | Pem/plat/pembro (n = 175) | Pem/plat/placebo (n = 88) | Pem/plat/pembro (n = 170) | Pem/plat/placebo (n = 82) | Pem/plat/pembro (n = 187) | Pem/plat/placebo (n = 97) | |
| Events, % | 58.5 | 81.1 | 71.6 | 88.8 | 58.3 | 83.1 | 80.0 | 92.0 | 64.1 | 84.1 | 71.1 | 89.7 |
| Median, mo | 23.0 | 15.0 | 15.1 | 7.6 | 22.5 | 10.5 | 10.3 | 6.6 | 20.4 | 10.1 | 13.8 | 7.6 |
| Stratified HR (CI 95%) | 0.57 | 0.50 | 0.44 | 0.60 | 0.43 | 0.54 | ||||||
| (0.38–0.86) | (0.40–0.62) | (0.33–0.57) | (0.45–0.80) | (0.31–0.59) | (0.41–0.72) | |||||||
| Estimated rates, % | ||||||||||||
| 12 mo | 76.3 | 60.2 | 57.8 | 33.6 | 77.2 | 49.1 | 45.1 | 29.5 | 71.7 | 47.5 | 55.1 | 33.0 |
| 24 mo | 49.0 | 23.3 | 33.4 | 13.8 | 48.3 | 21.5 | 24.5 | 9.1 | 45.6 | 22.5 | 29.1 | 10.3 |
Note: PFS-2 was defined as the time from randomization to second/subsequent tumor progression on next-line treatment or death from any cause.
CI, confidence interval; HR, hazard ratio; Pem, pemetrexed; Pembro, pembrolizumab; PFS, progression-free survival; Plat, platinum.
In the subgroup with less than or equal to 3 organ systems with lesions, median PFS-2 was 22.5 months with pemetrexed and platinum plus pembrolizumab and 10.5 months with pemetrexed and platinum plus placebo (HR = 0.43, 95% CI: 0.33–0.57, p ≤ 0.0001) (Table 1). In the subgroup with greater than 3 organ systems with lesions, median PFS-2 was 10.3 months with pemetrexed and platinum plus pembrolizumab and 6.6 months with pemetrexed and platinum plus placebo (HR = 0.60, 95% CI: 0.45–0.80, p = 0.0005) (Table 1). Estimated PFS-2 rates at 12 and 24 months were higher in the pemetrexed and platinum plus pembrolizumab arm across both subgroups (Table 1).
In the subgroup with symptom severity score less than or equal to median, median PFS-2 was 20.4 months with pemetrexed and platinum plus pembrolizumab and 10.1 months with pemetrexed and platinum plus placebo (HR = 0.43, 95% CI: 0.31–0.59, p ≤ 0.0001) (Table 1). In patients with symptom severity score greater than median, median PFS-2 was 13.8 months with pemetrexed and platinum plus pembrolizumab and 7.6 months with pemetrexed and platinum plus placebo (HR = 0.55, 95% CI: 0.41–0.72, p ≤ 0.0001) (Table 1). Estimated PFS-2 rates at 12 and 24 months were higher in the pemetrexed and platinum plus pembrolizumab across both subgroups (Table 1).
The PFS-2 benefit with pemetrexed and platinum plus pembrolizumab was maintained across all baseline tumor burden subgroups regardless of PD-L1 expression levels (Supplementary Table 8).
Discussion
To the best of our knowledge, this is the first manuscript reporting on the efficacy of pemetrexed and platinum plus pembrolizumab combination by baseline tumor burden defined using various criteria. The results of this post hoc efficacy analysis reveal the superior clinical benefit of pemetrexed and platinum plus pembrolizumab compared with pemetrexed and platinum plus placebo across all baseline tumor burden subgroups. Notably, the treatment effect was maintained in the low or high baseline tumor burden subgroups irrespectively of the criteria used for their characterization. Importantly, the clinical benefit with pemetrexed and platinum plus pembrolizumab across baseline tumor burden subgroups is comparable with the benefit in the overall study population from the updated analysis of the KEYNOTE-189 study.13 Taken together, these results underscore the efficacy of pemetrexed and platinum plus pembrolizumab combination across clinically distinct patient populations.
We used three distinct variables to evaluate the extent of tumor burden at baseline, and according to these variables, we categorized the analysis population in the following two main groups: patients with low tumor burden (M1a stage, or ≤3 organ systems with lesions, or symptom severity score ≤ median) and patients with high tumor burden (M1b stage, or >3 organ systems with lesions, or symptom severity score > median). The extent of metastatic involvement assessed by M1a and M1b staging was used as a surrogate marker for tumor burden given the difference in OS outcomes between M1a and M1b stages.30 Previous results from the KEYNOTE-189 study revealed consistent OS and PFS benefits with pemetrexed and platinum plus pembrolizumab in patients with M1a stage or M1b stage disease.14 Here, we further reveal that in addition to OS and PFS, pemetrexed and platinum plus pembrolizumab significantly prolonged PFS-2 in both M1a (p = 0.0059) and M1b (p ≤ 0.0001) subgroups with the same degree of magnitude compared with pemetrexed and platinum plus placebo. Higher response rates and prolonged DoR were also observed in both subgroups.
The extent of baseline tumor burden was also evaluated by the number of organ systems with metastatic lesions. Notably, organ systems were included in the assessment irrespectively of the number of lesions or the size of the lesions present. Moreover, we did not exclude organ systems on the basis of the size of the lesions because small metastatic lesions can potentially have biological and clinical significance. The improvement in OS, PFS, and PFS-2, including the proportion of patients achieving an objective response, was greater with pemetrexed and platinum plus pembrolizumab than with pemetrexed and platinum plus placebo across both organ systems with lesions subgroups. Nevertheless, the treatment effect was slightly less pronounced in the subgroup of greater than 3 organ systems with lesions compared with the subgroup of less than or equal to 3 organ systems with lesions as illustrated by the higher HRs for OS, PFS, and PFS-2.
Liver and brain metastases are poor prognostic factors in NSCLC26,30 and could have a negative impact on treatment outcomes irrespectively of the extent of baseline tumor burden. Consistent with this possibility, in the study by Nagasaka et al.,31 although baseline tumor volume did not predict response to ICI therapy, the presence of liver metastases was associated with worse OS outcome. It is important to note that in our analysis, the assessment of the number of organ systems with lesions also included the liver and brain. Moreover, in a previous analysis from the KEYNOTE-189 study, although patients with liver or brain metastases had a worse outcome compared with patients without liver or brain metastases, the treatment effect of pemetrexed and platinum plus pembrolizumab was similar among patients with or without liver or brain metastases.13
A novel aspect of this study is the inclusion of burden of symptoms as an additional variable to assess baseline tumor burden. We quantified burden of symptoms by generating a symptom severity score of patient-reported disease-related symptoms from the EORTC QLQ-LC13 questionnaire.29 The rationale for using burden of symptoms as a surrogate marker of tumor burden is supported by several lines of evidence. Patients with metastatic lung cancer have a higher burden of symptoms than patients with other tumor types.32 Moreover, high baseline burden of symptoms was reported to be an independent negative predictor of OS in patients with advanced NSCLC.32, 33, 34 In their study, Wang et al.34 reported that severity of baseline cough, a symptom included in our assessment of symptom severity score, was an independent risk for decreased OS. Consistent with the findings obtained when using M staging or number of organ systems with lesions to characterize baseline tumor burden, pemetrexed and platinum plus pembrolizumab conferred a greater clinical benefit than pemetrexed and platinum plus placebo irrespectively of the degree of the baseline symptom severity score.
Notably, the present analysis was not intended to include any formal statistical comparison between high and low tumor burden subgroups. Nevertheless, we observed numerically higher OS in the low tumor burden subgroups than in the high tumor burden subgroups particularly when tumor burden was measured by organ systems with lesions and symptom severity score. In patients with low tumor burden, pemetrexed and platinum plus pembrolizumab lowered the risk of death more than in patients with high tumor burden (M1a stage: 46% versus M1b stage: 42%; ≤3 organ systems with lesions: 51% versus >3: 33%; severity symptoms score ≤ median: 49% versus > median: 40%). The PFS benefit favoring pemetrexed and platinum plus pembrolizumab over pemetrexed and platinum plus placebo also tended to be numerically higher in patients with low tumor burden. Nevertheless, the observed OS and PFS HRs stand out more in terms of numerical similarities between high and low tumor burden subgroups; overall, we therefore interpreted these results as revealing a consistency of efficacy between high and low tumor burden subgroups.
Several studies have investigated the role of baseline tumor burden as a prognostic factor in NSCLC. In a pooled analysis from 1461 patients with NSCLC treated with atezolizumab in the context of the OAK, BIRCH, POPLAR, BIRCH, and FIR trials, a large baseline tumor burden, measured as the sum of the longest diameters of target lesions, was found to be an independent prognostic factor of worse OS and PFS.35 Similarly, in a retrospective analysis of 83 patients with NSCLC receiving ICI therapy, high tumor burden was a negative prognostic factor for OS.20
The role of baseline tumor burden as a predictive factor to response to treatment with ICIs has also been investigated. In a recent exploratory analysis from the IMpower 150 study, the clinical benefit of atezolizumab (Tecentriq) in combination with bevacizumab (Avastin) and carboplatin plus paclitaxel (ABCP) was observed across patients with high and low baseline tumor burden, which was defined as the sum of the longest diameter of target lesions or the number of metastatic sites at baseline versus bevacizumab (Avastin), carboplatin, and paclitaxel (BCP).36 Mixed results however have been reported from several retrospective studies using tumor size, tumor volume, or number of metastatic lesions as surrogate markers for tumor burden. In a recent retrospective real-world analysis of patients with metastatic NSCLC and high PD-L1 expression, baseline tumor burden did not predict response to pembrolizumab.37 Similarly, Nagasaka et al.31 reported a lack of association between baseline tumor volume and efficacy outcomes in patients with NSCLC treated with nivolumab or pembrolizumab. Conversely, in other retrospective single-institution studies, high baseline tumor burden was associated with decreased PFS and OS benefits to ICI monotherapy.18
The lack of consistency in the predicting value of tumor burden to ICI therapy might be explained by the different cutoff values used to define high versus low tumor burden. Nevertheless, a favorable clinical response to ICI therapy in small tumors compared with large tumors was also reported in preclinical studies.38 Although the underlying biological basis is not well understood, differences in tumor immunity and immunosuppressive tumor microenvironment signals between small tumors and large tumors may contribute to the favorable response to treatment with ICIs found in small tumors.39
When interpreting the results presented in this manuscript, the following points should be taken into consideration. The subgroup analyses across PD-L1 expression levels comprised relatively small sample sizes. Thus, although both PD-L1–negative and PD-L1–positive patients across all baseline tumor burden subgroups benefited from treatment with pemetrexed and platinum plus pembrolizumab, outcomes by PD-L1 expression revealed greater variations consistent with the small sample sizes. In addition, our study was not designed to investigate the prognostic significance of baseline tumor burden; instead, the study investigated whether the benefit of pemetrexed and platinum plus pembrolizumab was maintained across patient populations with varying degrees of disease involvement at the time of presentation. Last, to overcome potential biases owing to the lack of standardization for definition of tumor burden, we used three distinct variables to characterize baseline tumor burden thus allowing for a more comprehensive assessment of total tumor burden at baseline.
In conclusion, this is the first systematic analysis reporting on the efficacy of pemetrexed and platinum plus pembrolizumab across baseline tumor burden. The results presented in here reveal the superior benefit of pemetrexed and platinum plus pembrolizumab irrespectively of the extent of baseline tumor burden and the variables used to define it. These findings further substantiate the use of pemetrexed and platinum plus pembrolizumab as the standard of care for the first-line treatment of patients with NSQ-NSCLC.
CRediT Authorship Contribution Statement
Shirish Gadgeel: Acquisition of data for the work, Analysis of data for the work, Interpretation of data for the work, Critical revision of the work for important intellectual content.
Jhanelle E. Gray: Interpretation of data for the work, Critical revision of the work for important intellectual content.
Maria Teresa Rizzo: Design of the work, Interpretation of data for the work, Drafting of the work, Critical revision of the work for important intellectual content.
Patrick Peterson: Design of the work, Interpretation of data for the work, Drafting of the work, Critical revision of the work for important intellectual content.
Jong Seok Kim: Design of the work, Interpretation of data for the work, Critical revision of the work for important intellectual content.
Delvys Rodríguez-Abreu: Acquisition of data for the work, Analysis of data for the work, Interpretation of data for the work, Critical revision of the work for important intellectual content.
Data Sharing
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data-sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank, or annotated case report forms, will be provided in a secure data-sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org/.
Acknowledgments
This study was supported by Eli Lilly and Company and Merck Sharp & Dohme Corp. The authors thank the patients, their caregivers, and the investigators and their support staff for participating in the KEYNOTE-189 trial. Medical writing and editorial assistance were provided by Eglantine Julle-Daniere.
Footnotes
Disclosure: Dr. Gadgeel reports receiving personal fees for advisory board from Eli Lilly and Company, Merck, AstraZeneca, Genentech/Roche, Mirati, Daichii, Janssen, and Blueprint; and other personal fees from AstraZeneca (IMDC). Dr. Gray reports receiving grants and personal fees from AstraZeneca, Bristol-Myers Squibb, Genentech, Merck & Co., Inc., and Novartis; personal fees from AbbVie, Axiom HC Strategies, Blueprint Medicines, Celgene, Daiichi Sankyo, Inc., EMD Serono—Merck KGaA, Inivata, Janssen Scientific, Jazz Pharmaceuticals, Loxo Oncology Inc., OncoCyte Biotechnology, Sanofi Pharmaceuticals, and Takeda Pharmaceuticals; and grants from Boehringer Ingelheim, G1 Therapeutics, Ludwig Institute of Cancer Research, and Pfizer. Drs. Rizzo and Peterson are employees and shareholders of Eli Lilly and Company. Dr. Kim was an employee of Eli Lilly and Company. Dr. Rodríguez-Abreu reports receiving personal fees and other from AstraZeneca, Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Eli Lilly and Company, Novartis, and Pfizer.
Cite this article as: Gadgeel S, Gray JE, Rizzo MT, Peterson P, Kim JS, Rodríguez-Abreu D. Pemetrexed and Platinum Plus Pembrolizumab in Patients With Metastatic Nonsquamous NSCLC by Tumor Burden at Baseline: a Post Hoc Efficacy Analysis of KEYNOTE-189. JTO Clin Res Rep. 2022;3:100389.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org/ and at 10.1016/j.jtocrr.2022.100389.
Supplementary Data
References
- 1.Remon J., Passiglia F., Ahn M.J., et al. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol. 2020;15:914–947. doi: 10.1016/j.jtho.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Gray J., Rodríguez-Abreu D., Powell S.F., et al. FP13. 02 Pembrolizumab + pemetrexed-platinum vs pemetrexed-platinum for metastatic NSCLC: 4-year follow-up from KEYNOTE-189. J Thorac Oncol. 2021;16:S224. [Google Scholar]
- 3.Paz-Ares L., Ciuleanu T.E., Cobo M., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 4.Reck M., Rodríguez-Abreu D., Robinson A.G., et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non–small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 5.Reck M., Schenker M., Lee K.H., et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non–small-cell lung cancer with high tumour mutational burden: patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur J Cancer. 2019;116:137–147. doi: 10.1016/j.ejca.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Socinski M.A., Nishio M., Jotte R.M., et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol. 2021;16:1909–1924. doi: 10.1016/j.jtho.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Hanauske A.R., Chen V., Paoletti P., Niyikiza C. Pemetrexed disodium: a novel antifolate clinically active against multiple solid tumors. Oncologist. 2001;6:363–373. doi: 10.1634/theoncologist.6-4-363. [DOI] [PubMed] [Google Scholar]
- 8.Schaer D.A., Geeganage S., Amaladas N., et al. The folate pathway inhibitor pemetrexed pleiotropically enhances effects of cancer immunotherapy. Clin Cancer Res. 2019;25:7175–7188. doi: 10.1158/1078-0432.CCR-19-0433. [DOI] [PubMed] [Google Scholar]
- 9.Scagliotti G.V., Parikh P., von Pawel J., et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 10.Borghaei H., Langer C.J., Gadgeel S., et al. 24-Month overall survival from KEYNOTE-021 cohort G: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous non–small cell lung cancer. J Thorac Oncol. 2019;14:124–129. doi: 10.1016/j.jtho.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Langer C.J., Gadgeel S.M., Borghaei H., et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi L., Rodríguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 13.Gadgeel S., Rodríguez-Abreu D., Speranza G., et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non–small-cell lung cancer. J Clin Oncol. 2020;38:1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Abreu D., Powell S.F., Hochmair M.J., et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32:881–895. doi: 10.1016/j.annonc.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Velcheti V., Hu X., Piperdi B., Burke T. Real-world outcomes of first-line pembrolizumab plus pemetrexed-carboplatin for metastatic nonsquamous NSCLC at US oncology practices. Sci Rep. 2021;11:9222. doi: 10.1038/s41598-021-88453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higuera Gómez O., Moreno Paul A., Ortega Granados A.L., et al. “High tumor burden” in metastatic nonsmall cell lung cancer: defining the concept. Cancer Manag Res. 2021;13:4665–4670. doi: 10.2147/CMAR.S302928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph R.W., Elassaiss-Schaap J., Kefford R., et al. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin Cancer Res. 2018;24:4960–4967. doi: 10.1158/1078-0432.CCR-17-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsurada M., Nagano T., Tachihara M., et al. Baseline tumor size as a predictive and prognostic factor of immune checkpoint inhibitor therapy for non-small cell lung cancer. Anticancer Res. 2019;39:815–825. doi: 10.21873/anticanres.13180. [DOI] [PubMed] [Google Scholar]
- 19.Miyawaki T., Kenmotsu H., Mori K., et al. Association between clinical tumor burden and efficacy of immune checkpoint inhibitor monotherapy for advanced non–small-cell lung cancer. Clin Lung Cancer. 2020;21:e405–e414. doi: 10.1016/j.cllc.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Sakata Y., Kawamura K., Ichikado K., et al. Comparisons between tumor burden and other prognostic factors that influence survival of patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer. 2019;10:2259–2266. doi: 10.1111/1759-7714.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iacovelli R., Lanoy E., Albiges L., Escudier B. Tumour burden is an independent prognostic factor in metastatic renal cell carcinoma. BJU Int. 2012;110:1747–1754. doi: 10.1111/j.1464-410X.2012.11518.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Sung C., Dartois C., et al. Elucidation of relationship between tumor size and survival in non-small-cell lung cancer patients can aid early decision making in clinical drug development. Clin Pharmacol Ther. 2009;86:167–174. doi: 10.1038/clpt.2009.64. [DOI] [PubMed] [Google Scholar]
- 23.Warner A.B., Postow M.A. Bigger is not always better: tumor size and prognosis in advanced melanoma. Clin Cancer Res. 2018;24:4915–4917. doi: 10.1158/1078-0432.CCR-18-1311. [DOI] [PubMed] [Google Scholar]
- 24.Kim S.I., Cassella C.R., Byrne K.T. Tumor burden and immunotherapy: impact on immune infiltration and therapeutic outcomes. Front Immunol. 2021;11:629722. doi: 10.3389/fimmu.2020.629722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edge S.B., Compton C.C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 26.Gibson A.J.W., Li H., D’Silva A., et al. Impact of number versus location of metastases on survival in stage IV M1b non-small cell lung cancer. Med Oncol. 2018;35:117. doi: 10.1007/s12032-018-1182-8. [DOI] [PubMed] [Google Scholar]
- 27.Garassino M.C., Gadgeel S., Esteban E., et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:387–397. doi: 10.1016/S1470-2045(19)30801-0. [DOI] [PubMed] [Google Scholar]
- 28.Aaronson N.K., Ahmedzai S., Bergman B., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 29.Bergman B., Aaronson N.K., Ahmedzai S., Kaasa S., Sullivan M. The EORTC QLQ-LC13: a modular supplement to the EORTC core quality of life questionnaire (QLQ-C30) for use in lung cancer clinical trials. Eur J Cancer. 1994;30:635–642. doi: 10.1016/0959-8049(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 30.Dias M., Coutinho D., Linhas R., et al. Non-small cell lung cancer: are M1a and M1b the same stage? Eur Respir J. 2015;46(suppl 59):4288. [Google Scholar]
- 31.Nagasaka M., Abdallah N., Crosby M., et al. A retrospective study evaluating the pretreatment tumor volume (PTV) in non-small cell lung cancer (NSCLC) as a predictor of response to program death-1 (PD-1) inhibitors. Lung Cancer Targets Ther. 2019;10:95–105. doi: 10.2147/LCTT.S219886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batra A., Yang L., Boyne D.J., Harper A., Cheung W.Y., Cuthbert C.A. Associations between baseline symptom burden as assessed by patient-reported outcomes and overall survival of patients with metastatic cancer. Support Care Cancer. 2021;29:1423–1431. doi: 10.1007/s00520-020-05623-6. [DOI] [PubMed] [Google Scholar]
- 33.McGee S.F., Zhang T., Jonker H., et al. The impact of baseline Edmonton Symptom Assessment Scale scores on treatment and survival in patients with advanced non–small-cell lung cancer. Clin Lung Cancer. 2018;19:e91–e99. doi: 10.1016/j.cllc.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 34.Wang X.S., Shi Q., Lu C., et al. Prognostic value of symptom burden for overall survival in patients receiving chemotherapy for advanced nonsmall cell lung cancer. Cancer Interdiscip Int J Am Cancer Soc. 2010;116:137–145. doi: 10.1002/cncr.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hopkins A.M., Kichenadasse G., McKinnon R.A., Rowland A., Sorich M.J. Baseline tumor size and survival outcomes in lung cancer patients treated with immune checkpoint inhibitors. Semin Oncol. 2019;46:380–384. doi: 10.1053/j.seminoncol.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Jotte R.M., Batus M., Bernicker E., et al. IMpower150: exploratory efficacy analysis in patients (pts) with bulky disease. J Clin Oncol. 2020;38(suppl 15):e21637. e21637. [Google Scholar]
- 37.Schakenraad A., Hashemi S., Twisk J., et al. The effect of tumor size and metastatic extent on the efficacy of first line pembrolizumab monotherapy in patients with high PD-L1 expressing advanced NSCLC tumors. Lung Cancer. 2021;162:36–41. doi: 10.1016/j.lungcan.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Guisier F., Cousse S., Jeanvoine M., Thiberville L., Salaun M. A rationale for surgical debulking to improve anti-PD1 therapy outcome in non small cell lung cancer. Sci Rep. 2019;9:16902. doi: 10.1038/s41598-019-52913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitney R.B., Levy J.G., Smith A.G. Influence of tumor size and surgical resection on cell-mediated immunity in mice. J Natl Cancer Inst. 1974;53:111–116. doi: 10.1093/jnci/53.1.111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.