Summary
Here, we provide a step-by-step protocol for generating human induced pluripotent stem cell (hiPSC)-based microglial mouse brain chimeras. In addition, we detail steps for intracerebral injection of pathological tau and magnetic cell isolation of human microglia from chimeric mouse brains for single-cell RNA sequencing. Human microglia developed in chimeric mouse brains recapitulate the pathophysiology of microglia in human brain tissue, offering unprecedented opportunities to study human microglial senescence in vivo.
For complete details on the use and execution of this protocol, please refer to (Jin et al., 2022b).
Subject areas: Cell isolation, Single Cell, Model Organisms, Neuroscience, Stem Cells
Graphical abstract
Highlights
-
•
Transplant hiPSC-derived PMPs to create human-mouse microglial brain chimeras
-
•
Examine the responses of human microglia to pathological tau in chimeric brains
-
•
Isolate human microglia from chimeric brains for single-cell RNA-seq analysis
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Herewe provide a step-by-step protocol for generating human-induced-pluripotent-stem-cell (hiPSC)-based microglial mouse brain chimeras. In addition, we detail steps for intracerebral injection of pathological tau and magnetic cell isolation of human microglia from chimeric mouse brains for single-cell RNA sequencing. Human microglia developed in chimeric mouse brains recapitulate the pathophysiology of microglia in human brain tissue, offering unprecedented opportunities to study human microglial senescence in vivo.
Before you begin
Prepare the needed materials before starting the cell differentiation and transplantation. Refer to the key resources table for a complete list of materials. All procedures are performed in a Class II biological safety cabinet with standard aseptic techniques. Cells are cultured in a humidified 37°C incubator with 5% CO2.
Institutional permissions
The control (Cont) and Down syndrome (DS) human induced pluripotent stem cell (hiPSC) lines used in this study were approved by the Rutgers University committee on stem cell research. All animal work was performed with the approval of the Rutgers University Institutional Animal Care and Use Committee. The Rag2−/−hCSF1 immunodeficient mice (C;129S4-Rag2tm1.1Flv Csf1tm1(CSF1)Flv Il2rgtm1.1Flv/J, The Jackson Laboratory) were used in this study. Others who wish to replicate this protocol will need approval from their respective funding agencies and/or institutions.
Cell culture: Thawing and maintaining hiPSC
Timing: 2 h
The protocol below describes the steps for hiPSC recovery and maintenance. The cell culture is performed in a Class II biological safety cabinet.
Note: In this protocol, we used Cont and DS hiPSC lines generated and fully characterized as described in our previous studies (Chen et al., 2014; Xu et al., 2019).
Medium change: Every two days
-
1.
Thaw Matrigel on ice.
Note: Thawing Matrigel too quickly or not on ice will cause it to polymerize.
-
2.
Dilute the Matrigel to DMEM/F12 (1:100) and coat 6-well plates with 2 mL Matrigel, then incubate in 37°C incubator with 5% CO2 for at least 1 h.
-
3.
Warm mTeSR plus medium at 25°C (∼ 20 min) beforehand.
-
4.
Move Matrigel-coated 6-well plates from incubator and place them in tissue culture hood.
-
5.
Take cryovials of hiPSCs (Chen et al., 2014; Xu et al., 2019) from the liquid nitrogen storage container, immediately transfer them to a 37°C water bath, and thaw for 1–2 min.
-
6.
Transfer contents of one cryovial to a 15 mL tube, and then add 5 mL of DMEM/F12 medium in a dropwise manner to the cells.
-
7.
Spin at 300 g for 5 min, aspirate supernatant, and resuspend the cell pellet in 2 mL mTeSR plus medium. Add Y-27632 (1: 1000) or the combination of chroman 1 (50 nM), emricasan (5 μM), polyamines (1×), and trans-ISRIB (0.7 μM) (CEPT) compound to each well, as described in a previous study (Chen et al., 2021).
-
8.
Aspirate Matrigel from each well of a 6-well plate and add the 2 mL mTeSR plus suspension with hiPSCs to each well.
-
9.
Change the mTeSR plus on the next day to remove dead cells and Y-27632 or CEPT compound.
-
10.
Change media every two days till the hiPSCs reach a confluence of 70%–80%.
Differentiation of hiPSCs to primitive macrophage progenitors (PMPs)
Timing: 7 days for steps 11 to 16
Timing: 4–6 weeks for steps 17 to 22
For the PMP differentiation, we follow a published protocol (Haenseler et al., 2017) with some modifications (Jin et al., 2022a, 2022b; Xu et al., 2020).
Note: In the previous study (Haenseler et al., 2017), the embryoid body (EB) differentiation was four days. In our studies, we use six days for both DS and Cont hiPSC cell lines to obtain efficient PMP differentiation.
Prepare the EB medium before starting this step (store up to 2 weeks at 4°C).
Medium change: daily.
This step will produce EBs that are ready for differentiation into PMPs.
-
11.
Aspirate mTeSR plus medium, wash the well with 2 mL of 25°C PBS, and then aspirate the PBS.
-
12.
Add 2 mL ReleSR and aspirate, then place the plate in a humidified 37°C incubator with 5% CO2 for 4–6 min.
-
13.
Add 2 mL mTeSR 1 and tap the plate to see if the majority of the hiPSC colonies (> 80%) are detached from the plate.
-
14.
Transfer these detached hiPSC colonies to one well of a low attachment 6-well plate, then add Y-27632 or CEPT compound to the well (1: 1000) on the first day.
CRITICAL: The Y-27632 or CEPT compound is added to improve the viability of hiPSCs and the formation of EBs.
-
15.On the second day:
-
a.Collect all EBs with medium into an Eppendorf tube and wait till the EBs sink to the bottom of the tube.
-
b.Remove the supernatant medium with a P1000 pipette without agitating the EBs.
-
c.Add 2 mL fresh EB medium.
-
d.Transfer the EBs with medium back to the wells.
-
a.
-
16.
Repeat step 15 until day 7.
Before starting this step, prepare the factory medium (store up to 2 weeks at 4°C).
Medium change: once a week.
-
17.
Warm factory medium at 25°C (∼ 20 min) beforehand.
-
18.
Collect the EBs after 7 days of differentiation, transfer them into an Eppendorf tube, and aspirate the EB medium.
-
19.
Plate the EBs in a 10-cm dish with the factory medium.
-
20.
After seven days, gently aspirate the media and add 10 mL of fresh factory medium.
-
21.
Repeat step 20 once a week.
CRITICAL: The attachment of EBs during the first three weeks of plating is not stable. It is crucial to be gentle when changing the medium.
-
22.
After 3–4 weeks, the PMPs start to be released into the supernatant.
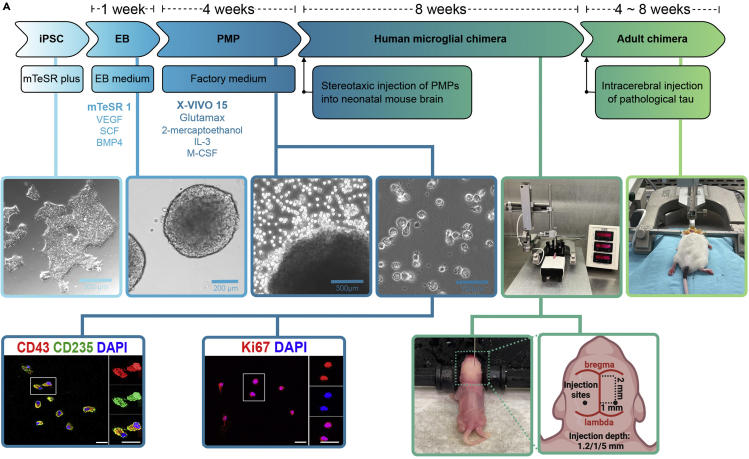
Note: For cell transplantation experiments, we recommend collecting PMPs after EBs are plated for 6–8 weeks. The identity of these hiPSC-derived PMPs can be confirmed by immunostaining with CD235, a YS primitive hematopoietic progenitor marker, and CD43, a hematopoietic progenitor-like cell marker. These PMPs are also highly proliferative, as indicated by expressing Ki67, a cell proliferation marker (Figure 1).
Figure 1.
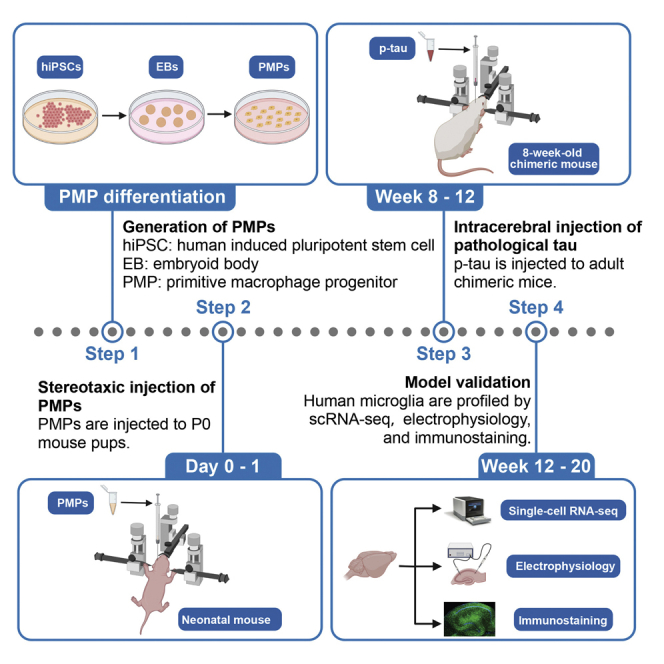
Timeline for generating of iPSC-based Human Microglial Chimeras to Study Senescence of Human Microglia
(A) Diagram showing the procedures of the hiPSC-based chimeras to study the senescence of human microglia. Stage-specific medium changes are listed below the cell culture stages. Mice are injected with human PMPs at postnatal day 0, and p-Tau at 2 months old post-injection of PMPs. Representative images of CD235+, CD43+, and Ki67+ cells in PMPs. Scale bars, 20 mm and 10 mm.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat anti-PSD95 (1:400 dilution) | Abcam | Catalog# AB12093 |
| Mouse anti-AT8 (1:250 dilution) | Invitrogen | Catalog# MN1020 |
| Mouse anti-CD43 (1:25 dilution) | Invitrogen | Catalog# 14-0439-82 |
| Mouse anti-CD45 (1:40 dilution) | Invitrogen | Catalog# 14-9457-82 |
| Mouse anti-CD68 (1:100 dilution) | Invitrogen | Catalog# MA5-13324 |
| Rabbit anti-CD235 (1:100 dilution) | Invitrogen | Catalog# PA5-27154 |
| Rabbit anti-Ferritin (1:250 dilution) | MilliporeSigma | Catalog# F6136 |
| Rabbit anti-Iba1 (1:100 dilution) | Wako | Catalog# 019-19741 |
| Mouse anti-Ki67(1:200 dilution) | Thermo Fisher Scientific | Catalog# RM-9106-S |
| Rabbit anti-TMEM119 (1:200 dilution) | Invitrogen | Catalog# PA5-62505 |
| Donkey anti-goat 488 (1:500 dilution) | Invitrogen | Catalog# A11055 |
| Donkey anti-goat 594 (1:500 dilution) | Invitrogen | Catalog# A11058 |
| Donkey anti-goat 647 (1:500 dilution) | Invitrogen | Catalog# A21447 |
| Goat anti-mouse 488 (1:500 dilution) | Invitrogen | Catalog# A11029 |
| Goat anti-mouse 594 (1:500 dilution) | Invitrogen | Catalog# A11032 |
| Goat anti-mouse 647 (1:500 dilution) | Invitrogen | Catalog# A21235 |
| Goat anti-rabbit 488 (1:500 dilution) | Invitrogen | Catalog# A27034 |
| Goat anti-rabbit 594 (1:500 dilution) | Invitrogen | Catalog# A11037 |
| Goat anti-rabbit 647 (1:500 dilution) | Invitrogen | Catalog# A21245 |
| Biological samples | ||
| Human brain tissue samples | UCI-ADRC | See Table 1 for detailed information |
| Chemicals, peptides, and recombinant proteins | ||
| 2-Mercaptoethanol | Gibco | Catalog# 21985-023 |
| Adult brain dissociation kit | Miltenyi Biotec | Catalog# 130-107-677 |
| BMP4 | Peprotech | Catalog# 120-05ET |
| Chroman 1 | MedChem Express | Catalog# HY-15392 |
| DPBS | Cytiva HyClone | Catalog# SH30028.02 |
| DMEM/F12 | Cytiva HyClone | Catalog# SH3002201 |
| DNase I recombinant | Roche | Catalog# 4536282001 |
| Emricasan | SelleckChem | Catalog# S7775 |
| GlutaMAX | Gibco | Catalog# 35050-061 |
| IL-3 | Peprotech | Catalog# 200-03 |
| Corning® Matrigel® hESC-Qualified Matrix | MilliporeSigma | Catalog# CLS354277 |
| M-CSF | Peprotech | Catalog# 300-25 |
| Mouse cell depletion cocktail | Miltenyi Biotec | Catalog# 130-104-694 |
| mTeSR 1 | Stemcell Technologies | Catalog# 85850 |
| mTeSR plus | Stemcell Technologies | Catalog# 100-0276 |
| Polyamine supplement (1000×) | MilliporeSigma | Catalog# P8483 |
| ReLeSR | Stemcell Technologies | Catalog# 100-0484 |
| SCF | Peprotech | Catalog# 300-07 |
| Trans-ISRIB | R&D Systems | Catalog# 5284 |
| VEGF | Peprotech | Catalog# 100-20 |
| X-VIVOTM 15 | Lonza | Catalog# 04-418Q |
| Y-27632 | Tocris | Catalog# 1254 |
| Ketamine | Covetrus | Catalog# 11695-0703-1 |
| Xylazine | Covetrus | Catalog# 11695-4024-1 |
| Bupivacaine | MP Biomedicals | Catalog# 154904 |
| Penicillin-Streptomycin | Cytiva HyClone | Catalog# SV30010 |
| Critical commercial assays | ||
| Chromium Next GEM Single Cell 3′ GEM, Library & Gel Bead Kit v3.1 | 10x Genomics | Catalog# 1000128 |
| Chromium Next GEM Chip G Single Cell Kit | 10x Genomics | Catalog#1000127 |
| Single Index Kit T Set A | 10x Genomics | Catalog#1000213 |
| Deposited data | ||
| scRNA-seq data | NCBI GEO: GSE189227 | |
| Experimental models: Cell lines | ||
| Cell lines | Coriell Institute for Medical Research | See Table 2 for detailed information |
| Experimental models: Organisms/strains | ||
| Mice: Rag2−/−hCSF1 | The Jackson Laboratory | IMSR_JAX:017708 Genotype: C;129S4-Rag2tm1.1FlvCsf1tm1(CSF1)FlvIl2rgtm1.1Flv/J Age: P0 Sex: both |
| Software and algorithms | ||
| Adobe Illustrator | Adobe | https://www.adobe.com/products/illustrator.html |
| Adobe Photoshop | Adobe | https://www.adobe.com/products/photoshop.html |
| BioRender | BioRender.Inc | https://biorender.com/ |
| Cell Ranger v6.1.1 | 10x Genomics | https://github.com/10XGenomics/cellranger |
| Cluster Profiler 4.0.5 | https://guangchuangyu.github.io/software/clusterProfiler/ | |
| Clampfit 10.5 | Molecular Devices | https://support.moleculardevices.com/s/ |
| GraphPad Prism 9.2.0 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| ImageJ | NIH | https://imagej.net/software/fiji/ |
| Imaris 9.5.1 | Bitplane | https://imaris.oxinst.com |
| Zen 2.3 | Carl Zeiss | N/A |
| Other | ||
| Digital stereotaxic device | David KOPF | Model 940 |
| Mouse and Neonates Adaptor | Stoeling | Catalog# 51625 |
| Syringe 1701RN | Hamilton | Catalog# 7653-01 |
| Small Hub RN Needle | Hamilton | Catalog# nc1767272 |
| GentleMACS Octo Diss. with Heating Units | Miltenyi Biotec | Catalog# 130-096-427 |
| QuadroMACS Separator | Miltenyi Biotec | Catalog# 130-091-051 |
| Chromium X | 10×Genomics | Catalog# PN-1000323 |
| High Speed Rotary Micromotor Kit | Foredom | Catalog# K.1070 |
| Micro Drill Bit, Uncoated | Kyocera | Catalog# 07289, #77 (Bit Size) |
| LS Columns | Miltenyi Biotec | Catalog# 130-042-401 |
| MidiMACS Separator | Miltenyi Biotec | Catalog# 130-042-302 |
| 70 μm cell strainer | Bio TC | Catalog# C4070BTC |
Note: After diluting the growth factors at stock concentrations and preparing aliquots for all growth factors, they were all stored at −20°C. All the cell culture medium will be stored at 4°C, and the X-VIVOTM 15 should be kept from light.
.
Table 1.
Basic information of the human brain tissues obtained from the University of California Alzheimer’s Disease Research Center (UCI-ADRC) and the Institute for Memory Impairments and Neurological Disorders
| Case no. | Disorder | Sex | Age (years) | PMI (hours) | Tangle stage | Plaque stage |
|---|---|---|---|---|---|---|
| 38 | Trisomy 21 (AD Present) | Female | 63 | 3 | Stage 6 | Stage C |
| 8 | Trisomy 21 (AD Present) | Male | 70 | 4.75 | Stage 6 | Stage C |
| 34 | Normal | Female | 91 | 3.33 | Stage 3 | Stage A |
| 6 | Normal | Male | 84 | 4.3 | Stage 3 | None |
Table 2.
Basic information of Down syndrome patient fibroblasts from Coriell Institute for Medical Research
| Coriell’s cat. no. | Sex | Age | Race | Cell type | Karyotype | Generated iPSC lines |
|---|---|---|---|---|---|---|
| GM04616 | female | 3 days | Caucasian | Fibroblast | 47,XX,+21 | DS1 iPSC |
| AG08942 | male | 21 years | Caucasian | Fibroblast | 47,XY,+21 | DS2 iPSC |
| AG06872 | female | 1 year | Caucasian | Fibroblast | 47,XX,+21 | Tri-DS iPSCs |
Materials and equipment
EB medium
| Reagent | Stock concentration | Final concentration |
|---|---|---|
| mTeSR1 | 1× | 1× |
| VEGF | 50 μg/mL | 50 ng/mL |
| SCF | 20 μg/mL | 20 ng/mL |
| BMP4 | 10 μg/mL | 50 ng/mL |
| Penicillin-Streptomycin | 100× | 1× |
Factory medium
| Reagent | Stock concentration | Final concentration |
|---|---|---|
| X-VIVOTM 15 | 1× | 1× |
| GlutaMAX | 100× | 1× |
| 2-mercaptoethanol | 1000× | 1× |
| IL-3 | 25 μg/mL | 25 ng/mL |
| M-CSF | 100 μg/mL | 100 ng/mL |
| Penicillin-Streptomycin | 100× | 1× |
Step-by-step method details
This protocol describes all steps of cell transplantation, intracerebral brain injection of pathological tau, and magnetic isolation of human microglia for single-cell RNA sequencing (scRNA-seq) (Figure 1).
Stereotaxic injection of PMPs into neonatal mouse brain
Timing: 4–6 h
All instruments are sterilized by autoclaving or using a hot bead sterilizer. The surgery is performed in a Class II biological safety cabinet.
-
1.Prepare the PMPs.
-
a.Collect the supernatant from a 10 cm dish and flow through a 70 μm cell strainer.
-
b.Collect the flow-through and centrifuge at 400 g for 5 min.
-
c.Discard the supernatant and resuspend the cell pellets in 5 mL PBS.
-
d.Count the PMPs and add DNase (100 U/mL).
-
e.Centrifuge at 400 g for 5 min.
-
f.After centrifugation, aspirate supernatant and resuspend the PMP cell pellet to 1 × 105 cells/μL with PBS. Keep the cells on ice till transplantation.
-
a.
CRITICAL: The typical yield of PMPs is around 40-fold higher than the number of inputs hiPSCs. Before resuspending the PMPs cell pellet, it is recommended to perform additional quick centrifugation to remove all supernatant in order to get a more accurate dilution.
-
2.Anesthetize P0, sex unspecified neonatal pups via hypothermia:
-
a.Place pups (within 24 h of birth) in protective latex sleeves.
-
b.Immerse up to the neck in crushed ice (4°C).
-
a.
Note: A 4–5 min induction time is required.
Note: The pups should never be placed in direct contact with ice in order to avoid frostbite of the skin.
-
3.
After anesthetizing (pinch the tail and no movement), fix the heads of the pups on a stereotaxic device equipped with a neonatal mouse adaptor.
-
4.
Load the cell suspension into the Hamilton syringe (5–6 μL for each pup).
CRITICAL: It is recommended that load cells into the Hamilton syringe right before cell injection. Keeping cells in a Hamilton syringe for too long may cause significant cell death.
-
5.
The surgical site will be treated first with an antiseptic scrub and then with an antiseptic solution (povidone-iodine scrub and solution).
Note: This step will be repeated 3 times, and the antiseptic solution will be applied last before surgery.
-
6.
Insert the needle directly through the skull to the hippocampus injection sites (coordinates: 1.0 mm bilaterally from the midline, 2.0 mm posterior bregma, and 1.2/1.5 mm depth) using a digital stereotaxic device.
-
7.Start the cell injection:
-
a.The pups are injected with 0.5 μL (50,000 in 0.5 μL PBS) of cells into each site (total of four sites),
-
a.
Note: Keep the needle at each depth (1.5 mm and 1.2 mm) for at least one minute following injection.
-
8.
After the cell transplantation (approximately 30 min per pup), the pups are kept on a heating pad (37°C) for about 5–10 min of recovery. The pups are returned to dams after recovery.
CRITICAL: Before returning the pups to the dam, it is recommended to make sure that they are warm, pink, breathing, and capable of spontaneous movements.
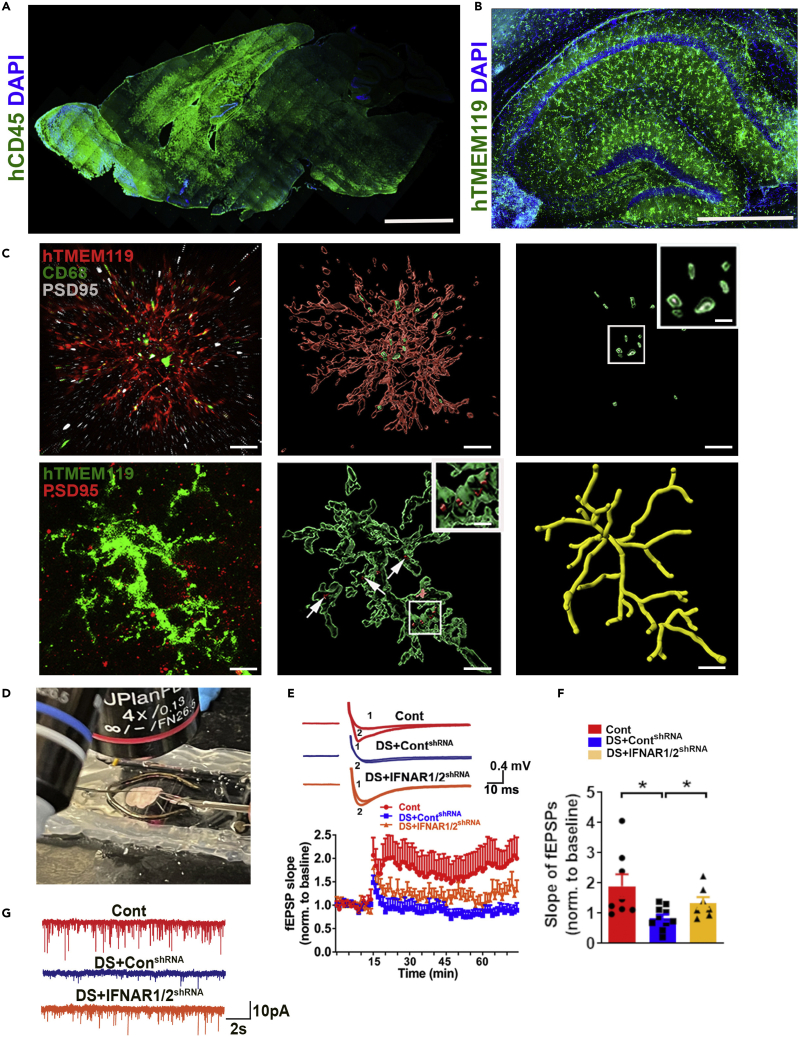
Note: At 4–5 months post-transplantation, we recommend immunostaining with a human-specific marker expressed by microglia (hCD45) to examine the distribution of microglia (Figure 2A). At 3 months post-transplantation, our slices showed a wide distribution of xenografted, hTMEM119+ human microglia in the hippocampal region (Figure 2B). Normally, at six months post-transplantation, about 7% of total cells are donor-derived human microglia on our previous scRNA-seq analysis of chimeric brains (Xu et al., 2020). The synaptic pruning and phagocytic function of microglia can be examined by staining hTMEM119/PSD95 or hTMEM119/CD68/PSD95 (Figure 2C). These chimeras can be used to study functional synaptic neurotransmission by patch-clamp recording of miniature excitatory postsynaptic currents (mEPSCs) and study long-term synaptic plasticity by inducing long-term potentiation (LTP) in hippocampal slices from microglial chimeric mice at 3 months post-transplantation (Figures 2D–2G).
Figure 2.
Modeling microglial phenotypes in human-mouse microglial chimeras
(A) Representative images from sagittal brain sections showing the distribution of transplanted hiPSC-derived microglia at 4–5 months. Scale bar: 1 mm.
(B) Representative images from sagittal brain sections showing the distribution of transplanted hiPSC-derived microglia at 3 months in the hippocampus. Scale bar: 500 μm.
(C) Representative images showing colocalization of hTMEM119+ and PSD95+ staining (arrows indicate PSD95+ puncta), and colocalization of hTMEM119+, CD68+, and PSD95+ staining. Scale bars, 5 and 1 mm.
(D) Representative image showing LTP recording from CA1 region on a chimeric mouse brain slice.
(E) Representative traces of baseline (1) and last 10 min (2) fEPSP after 4 × 100 Hz LTP induction. Quantification of LTP after LTP induction in 3- to 4-month-old chimeras (n = 7–10 slices from 3 to 4 mice/group).
(F) Quantification of the last 10 min of fEPSP slope after LTP induction (n = 7–10 slices from 3 to 4 mice/group). One-way ANOVA with Bonferroni post-hoc test, ∗p < 0.05.
(G) Representative traces of mEPSCs in CA1 hippocampal pyramidal neurons.
Intracerebral injection of pathological tau into adult chimeric mouse brain
Timing: 4–6 h
All instruments are sterilized by autoclaving or using a hot bead sterilizer. The surgery is conducted in a Class II biological safety cabinet, and the whole procedure is performed on a heating pad.
-
9.Soluble tau preparation:
-
a.DSAD tau: soluble S1 fractions containing hyperphosphorylated tau (p-tau) from human brain tissues collected from DS individuals with Alzheimer’s disease (DSAD), following the methods as described before (Sanchez-Mejias et al., 2016),
-
b.Control (Cont) tau: cont tau was collected from age- and sex-matched healthy control brain tissues.
-
a.
-
10.
Weigh the mice and calculate the appropriate dose for anesthesia. Administrate intraperitoneally a mixture of ketamine (80 mg/kg) and xylazine (8 mg/kg).
CRITICAL: The ketamine and xylazine are diluted with 0.9% saline and mixed at the concentration of 80 mg/mL and 8 mg/mL. Then the ketamine/xylazine mixture will be injected at 0.01 mL/g intraperitoneally.
-
11.
Immediately place the animal on a heating pad to maintain body temperature after the injection of anesthetics.
Note: The animal should reach deep anesthesia within 10 min.
CRITICAL: Confirm the effective anesthesia by pinching the tail.
-
12.
Shave the fur on the skull and clean the skin with the iodine-based wash. Apply lubricant to their eyes.
-
13.
Before cutting the skin, inject the bupivacaine (2 mg/kg) subcutaneously along the incision line.
-
14.
Make a straight midline incision through the skin with surgical scissors from the back of the neck to the interpupillary line and gently push aside the connective tissue on top of the skull.
-
15.
Place the animal on the platform of the stereotaxic apparatus and fix the head with ear bars.
-
16.
Use the position of bregma as the reference (stereotaxic zero) of the X and Y coordinates and the defined stereotaxic coordinates (2.0 mm bilaterally from the midline and 2.5 mm posterior bregma) to mark the intended sites of injection on the skull.
-
17.
Adjust the drill to 20,000 rpm and make a single burr-hole in the skull at the injection sites.
-
18.Injection:
-
a.Load the Cont or DSAD tau into the Hamilton syringe (7–8 μL at a concentration of 0.8 μg protein/μL for each 2-month-old chimeric mouse),
-
b.Position the syringe over the burr-hole and inject pathological tau proteins into two depths in the hippocampus (1.8- and 2.4-mm depth in both sides of the mice brain).
-
a.
CRITICAL: The injection of the fluid should be very slow to avoid an acute increase of intracranial pressure and facilitate diffusion of the fluid (1 μL each depth, total 4 μL per mouse).
-
19.
When the injection is completed, allow a minimum of 1 additional minute of rest time before starting to withdraw the syringe from the brain.
-
20.
Pull the edges of the skin together and suture the skin at 3 separate points. Place the animal in the temperature-controlled cage until complete recovery.
-
21.
Once the animal recovers, the analgesic treatment may be administered.
-
22.
After the surgery, the injected mice will be monitored every day for the first week.
CRITICAL: Before tau injection, we recommend sampling chimeric mice from each batch of cell transplantation experiments to confirm the wide microglia distribution.
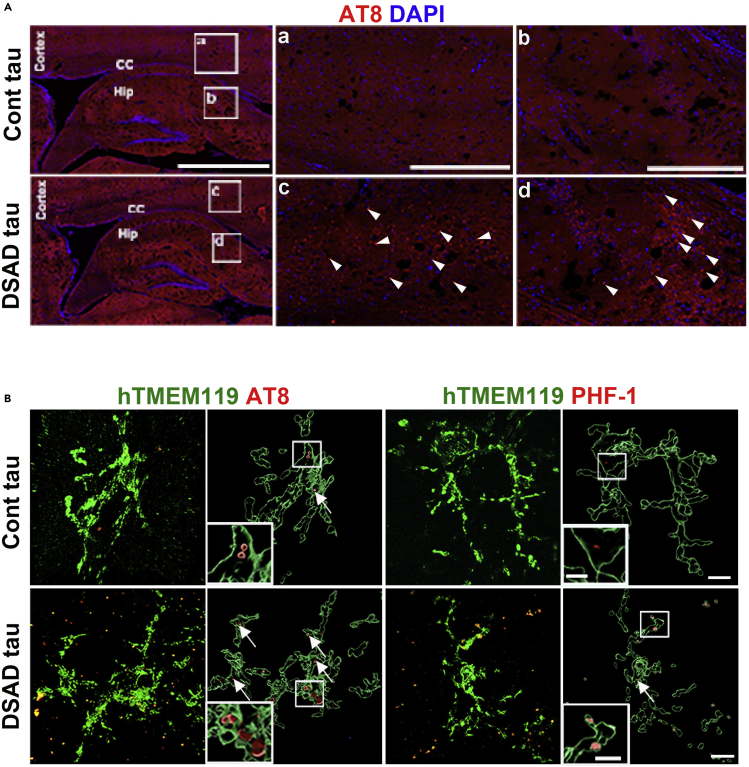
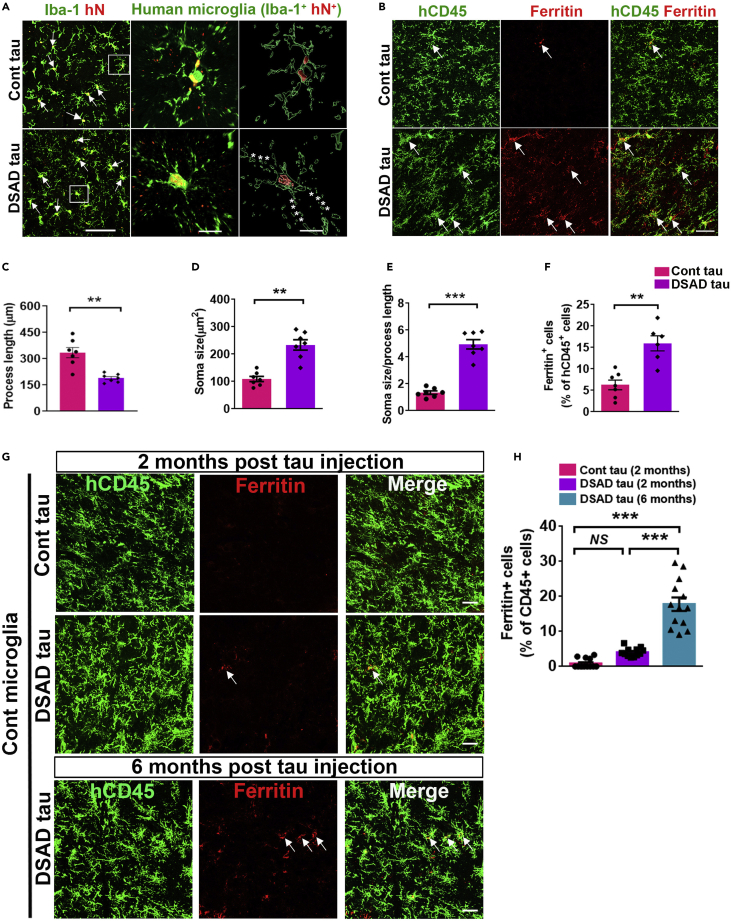
Note: At two weeks post-injection, we observed that AT8+ p-tau was distributed widely in the mouse brains that received DSAD tau (Figure 3A). To characterize the responses of human microglia to p-tau, we examined phagocytosis of p-tau by double-staining p-tau with human microglia at 1–2 months post tau injection. hTMEM119+ DS microglia engulfed a large number of AT8+ or PHF-1+ p-tau in the DSAD Tau injection group (Figure 3B). In the Cont tau injection group, few p-tau was seen in DS microglia. Both control and DS microglial chimeras can be used to study the senescence of human microglia in vivo. DS exhibits premature and accelerated aging (Gensous et al., 2020; Zigman, 2013). In line with this, in the DS chimeric brains injected with soluble tau, we usually observe senescent phenotypes such as beading with shortened processes and fragmentation morphological changes and an increased number of ferritin+/hCD45+ DS microglia at 8 weeks post-transplantation (Figures 4A–4F). In control microglial chimeras, a small number of control microglia displayed the senescent phenotypes at 2 months post-injection of pathological tau proteins, and significantly more were seen at 6 months post-injection of tau proteins (Figures 4G and 4H).
Figure 3.
Quality control of human brain tissue-derived soluble tau
(A) Representative images of sagittal brain sections showing the distribution of AT8 at two weeks post-injection. Arrowheads indicate AT8+ p-tau. Scale bar: 500 μm and 50 μm in the original and enlarged images, respectively.
(B) Representative images of hTMEM119, AT8, and PHF-1 staining in 4- to 5-month-old chimeras receiving an injection of Cont or DSAD tau at 8 weeks. Arrows indicate AT8+ or PHF-1+ p-tau. Scale bars, 7 and 3 μm.
Figure 4.
Pathological tau induces senescence in human microglial chimeras
(A) Representative images of Iba+/hN+ human microglia in Cont and DSAD tau groups. Arrows indicate Iba+/hN+ human microglia. Asterisks indicate fragmented processes. Scale bars, 50 and 10 μm.
(B) Representative images showing colocalization of hCD45+ and ferritin+ staining in Cont and DSAD tau groups. Arrows indicate ferritin+ and/or hCD45+ staining. Scale bars, 50 μm.
(C) Quantification of the process length in cells (n = 7 mice/group). Two-tailed unpaired t test with Welch’s correction, ∗∗p < 0.01.
(D) Quantification of the soma size in cells (n = 7 mice/group). Two-tailed unpaired t test with Welch’s correction, ∗∗p < 0.01.
(E) Quantification of the soma size/process length in cells (n = 7 mice/group). Two-tailed unpaired t test with Welch’s correction, ∗∗∗p < 0.001.
(F) Quantification of the percentage of ferritin in hCD45+ cells (n = 6–7 mice/group). Two-tailed unpaired t test with Welch’s correction, ∗∗p < 0.01.
(G) Representative raw fluorescent super-resolution, 3D surface rendered, and 3D skeletonization images of mouse microglia (Iba+hN-) in Cont tau and DSAD tau chimeric mice. Scale bar: 5 μm.
(H) Quantification of the process length, branch numbers, and endpoints in mouse microglia (Iba+hN-) (n=7 mice per group). One-way ANOVA with Bonferroni post-hoc test, ∗∗∗p < 0.001, NS, not significant. Data are presented as mean ± SEM.
Magnetic isolation of human microglia from chimeric mouse brain tissue for scRNA-seq
Timing: 4–6 h
To focus on hiPSC-derived human microglia for scRNA-seq, we isolate and enrich human microglia from chimeric brains by removing mouse cells using a magnetic cell sorting-based mouse cell depletion kit. This is performed by following procedures described previously (Hasselmann et al., 2019) with modifications (Jin et al., 2022b).
-
23.
After two months of tau injection, the mice are perfused with ice-cold PBS. Then, the whole brain is dissected, and the cerebellum is removed.
-
24.
Tissue dissociation is then performed utilizing the Adult Brain Dissociation Kit and the gentleMACS Octo Dissociator with Heaters according to manufacturer instructions.
-
25.
The cell pellets from mouse brains are resuspended in 160 μL MACS buffer (0.5% BSA in 1× PBS) + 40 μL Mouse cell removal beads and incubated at 4°C for 15 min.
-
26.
The resulting samples are then isolated using LS columns and the MidiMACS™ separator, and the human cells are collected in the flow-through.
-
27.
Using centrifugation (10 min, 400g, 4°C), the cells are pelleted and resuspended to 1,000 cells per microliter in MACS buffer for scRNA-seq library preparation.
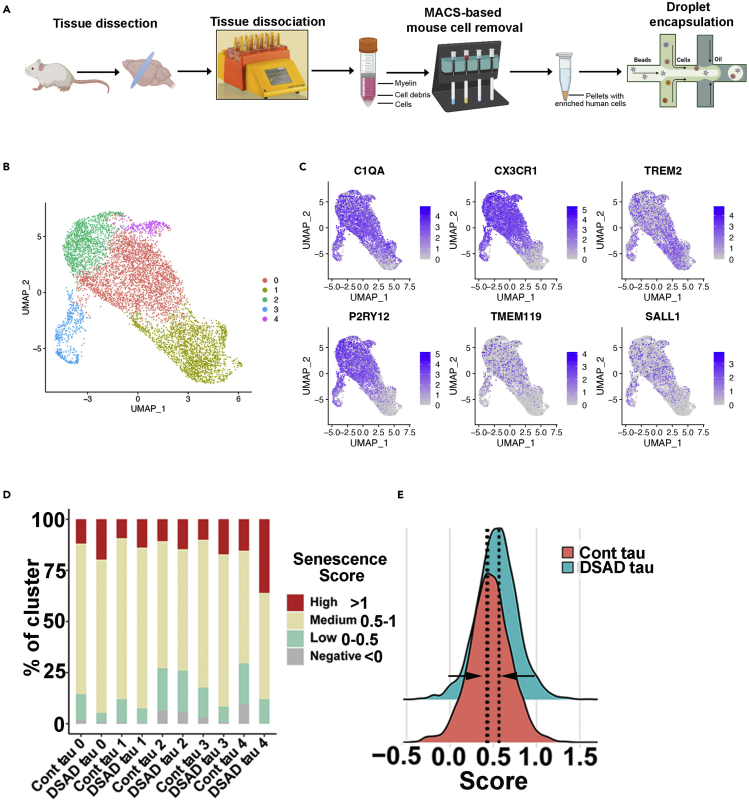
Note: As shown in Figure 5A, using this method, we isolated human microglia and performed scRNA-seq. We identified 5 clusters and all clusters highly expressed microglial markers, including C1QA, CX3CR1, TREM2, TMEM119, P2RY12, and SALL1 (Figures 5B and 5C). Compared to Cont tau group, overall DSAD tau group as well as each cluster showed a shift towards a greater senescence signature (Figures 5C and 5D). In our microglia chimeric mouse brains, there are usually a large number of human microglia (about 8% of total brain cells) (Xu et al., 2020). Even without any purification steps, ample numbers of human microglia can also be obtained for scRNA-seq. In this case, gene expression profile of the host mouse brain cells can be analyzed (Xu et al., 2020).
Figure 5.
scRNA-seq analysis of microglial chimeras receiving an injection of soluble tau
(A) A schematic diagram showing the design of the scRNA-seq experiment.
(B) A UMAP plot showing independent microglia subclusters (clusters 0–4).
(C) Dot plots showing the representative conserved microglial markers from each subcluster.
(D) Bar plot of the percentage of cells with a negative (<0), low (0–0.5), medium (0.5–1), or high (>1) senescence score in the custom senescence signature in each cluster.
(E) Ridge plot showing the senescence score in DSAD and Cont tau groups.
Expected outcomes
Current knowledge on human microglial pathology in aging-related neurodegenerative diseases is largely gained from postmortem human brain tissue (Shahidehpour et al., 2021; Xue and Streit, 2011). In particular, for DSAD, none of the mouse models of DS reliably reproduces pathologies, even in aged mice (Choong et al., 2015). Recent advances in stem cell technology have led to the efficient generation of microglia from hiPSCs (Haenseler et al., 2017; Jiang et al., 2020), providing an unlimited source of human microglia to study their pathophysiology. Recently, we have developed new hiPSC-based microglial chimeric mouse brain models, in which hiPSC-derived microglia undergo maturation and develop appropriate functions (Jin et al., 2022a, 2022b; Xu et al., 2020). This new model provides unprecedented opportunities to investigate human microglial changes during aging and neurodegenerative diseases.
This protocol provides instructions on generation of human-mouse microglial chimeras with a wide microglia distribution at two months post-transplantation (Figure 3A). These microglia exhibit not only synaptic pruning but also phagocytic functions (Figure 3B). Particularly, in the hippocampal CA1 region, the pyramidal neurons are surrounded by donor-derived human microglia. As such, our chimeric mouse model facilitates the investigation on how microglia alter synaptic neurotransmission and plasticity. Electrophysiological recordings can be performed in hippocampal slices from these microglial chimeric to examine the impact of engrafted human microglia on synaptic functions (Figures 3C and 3D).
These human microglial chimeras can readily be used for immunostaining of senescent markers, such as ferritin, listed in the key resources table. In our experience, ferritin+ dystrophic human microglia are detectable as early as two months after the p-tau injection (Figure 4B). In line with this, the cellular senescence can also be assessed by scRNA-seq. We found that the DSAD tau injection group displayed a shift towards a greater senescence signature (Figures 5D and 5E).
Quantification and statistical analysis
All data are represented as mean ± SEM. When only two independent groups were compared, significance was determined by using two-tailed unpaired t test with Welch’s correction (Figures 4C–4F). When three groups were compared, one-way ANOVA with Bonferroni post-hoc test was used (Figures 2F and 4H). A p-value of < 0.05 was considered significant. All the analyses were done in GraphPad Prism v.9. All experiments were independently performed at least three times with similar results.
Limitations
Using hiPSC lines, this protocol efficiently generates PMPs and delivers them into mice brains, which will later give rise to human microglial cells. Successful generation of chimeras with mature microglia depends on the generation of high purity PMPs that are proliferative and skilled stereotaxic cell transplantation technique in neonatal brains.
Troubleshooting
Problem 1
The efficiency of PMP generation is low.
Potential solution
The efficiency can be impacted by the quality of starting cells. The quality of starting hiPSCs should be carefully controlled before differentiation. The low efficiency of EB differentiation also could arise from the inappropriate cultural conditions. Therefore, it is recommended to change EB medium daily before plating them into the factory medium (steps 15 and 16 of differentiation of hiPSCs to PMPs).
Problem 2
PMPs are lost during collection.
Potential solution
When collecting supernatant with PMPs from plates for cell transplantation (step 1 of stereotaxic injection of PMPs into neonatal mouse brain), PMPs may aggregate to form cell clusters. Thus, pipetting the supernatant up and down for a few times to dissociate them into single cells in a 15 mL tube is recommended. Then, place the PMPs through a 70 μm cell strainer, collect the flow-through, and centrifuge at 400 g for 5 min to spin down PMPs.
Problem 3
There is significant cell death during the surgery.
Potential solution
During the cell preparation in step 1 of stereotaxic injection of PMPs into neonatal mouse brain, it’s recommended to add DNase to remove DNA released by dead cells. The PMPs should be put on the ice immediately after centrifugation and kept on ice for the duration of the surgery.
Problem 4
The number of human microglia is low after magnetic isolation.
Potential solution
In step 26 of magnetic isolation of human microglia from chimeric mouse brain tissue for scRNA-seq: if debris removal solution from the adult brain dissociation kit is used, strictly follow the manufacturer's protocol. Particularly, in the centrifugation step – the key step for debris removal, the samples should be spun down with full acceleration and deceleration in 4°C. The debris should be removed completely. Otherwise, this could significantly compromise the quality of the cell preparations and subsequent magnetic isolation.
Problem 5
Adult animals have limited cell distribution or low number of human cells after transplantation.
Potential solution
In step 6 of stereotaxic injection of PMPs into neonatal mouse brain, delivering cells to the injection sites accurately is critical for the migration and distribution of donor-derived human microglia. And it is recommended that 0.6 μL PBS with cells are injected per injection site. Injecting large volumes may cause damage to the brain tissue, resulting in a limited distribution of donor-derived human microglia.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, peng.jiang@rutgers.edu.
Materials availability
This study did not generate new unique materials or reagents.
Acknowledgments
This work was partly supported by grants from the NIH (R01NS102382, R01NS122108, R01DA056906, and R01AG073779 to P.J.). We thank the UCI-ADRC, which is funded by NIH/NIA Grant P30AG066519, and the Bright focus Foundation (BFF17-0008), for providing us with DSAD and healthy control human brain tissues.
Author contributions
M.J. and P.J. conceived the project and wrote the protocol. M.J. designed, performed, and analyzed the experimental protocol. Z.M. assisted with figure preparation. P.J. supervised the study.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Mengmeng Jin, Email: mengmeng.jin@rutgers.edu.
Peng Jiang, Email: peng.jiang@rutgers.edu.
Data and code availability
The scRNA-seq datasets generated in the original study (Jin et al., 2022b) have been deposited to NCBI GEO (GSE189227) and are publicly available.
This paper does not report original code.
References
- Chen C., Jiang P., Xue H., Peterson S.E., Tran H.T., McCann A.E., Parast M.M., Li S., Pleasure D.E., Laurent L.C., et al. Role of astroglia in Down's syndrome revealed by patient-derived human-induced pluripotent stem cells. Nat. Commun. 2014;5:4430. doi: 10.1038/ncomms5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Tristan C.A., Chen L., Jovanovic V.M., Malley C., Chu P.H., Ryu S., Deng T., Ormanoglu P., Tao D., et al. A versatile polypharmacology platform promotes cytoprotection and viability of human pluripotent and differentiated cells. Nat. Methods. 2021;18:528–541. doi: 10.1038/s41592-021-01126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong X.Y., Tosh J.L., Pulford L.J., Fisher E.M.C. Dissecting Alzheimer disease in Down syndrome using mouse models. Front. Behav. Neurosci. 2015;9:268. doi: 10.3389/fnbeh.2015.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensous N., Bacalini M.G., Franceschi C., Garagnani P. Down syndrome, accelerated aging and immunosenescence. Semin. Immunopathol. 2020;42:635–645. doi: 10.1007/s00281-020-00804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenseler W., Sansom S.N., Buchrieser J., Newey S.E., Moore C.S., Nicholls F.J., Chintawar S., Schnell C., Antel J.P., Allen N.D., et al. A highly efficient human pluripotent stem cell microglia model displays a neuronal-Co-culture-Specific expression profile and inflammatory response. Stem Cell Rep. 2017;8:1727–1742. doi: 10.1016/j.stemcr.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmann J., Coburn M.A., England W., Figueroa Velez D.X., Kiani Shabestari S., Tu C.H., McQuade A., Kolahdouzan M., Echeverria K., Claes C., et al. Development of a chimeric model to study and manipulate human microglia in vivo. Neuron. 2019;103:1016–1033.e10. doi: 10.1016/j.neuron.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P., Turkalj L., Xu R. High-fidelity modeling of human microglia with pluripotent stem cells. Cell Stem Cell. 2020;26:629–631. doi: 10.1016/j.stem.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M., Alam M.M., Alice L., Jiang P. Rag2-/- accelerates lipofuscin accumulation in the brain: implications for human stem cell brain transplantation studies. Stem Cell Rep. 2022 doi: 10.1016/j.stemcr.2022.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M., Xu R., Wang L., Alam M.M., Ma Z., Zhu S., Martini A.C., Jadali A., Bernabucci M., Xie P., et al. Type-I-interferon signaling drives microglial dysfunction and senescence in human iPSC models of down syndrome and Alzheimer's disease. Cell Stem Cell. 2022;29:1135–1153.e8. doi: 10.1016/j.stem.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mejias E., Navarro V., Jimenez S., Sanchez-Mico M., Sanchez-Varo R., Nuñez-Diaz C., Trujillo-Estrada L., Davila J.C., Vizuete M., Gutierrez A., Vitorica J. Soluble phospho-tau from Alzheimer's disease hippocampus drives microglial degeneration. Acta Neuropathol. 2016;132:897–916. doi: 10.1007/s00401-016-1630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidehpour R.K., Higdon R.E., Crawford N.G., Neltner J.H., Ighodaro E.T., Patel E., Price D., Nelson P.T., Bachstetter A.D. Dystrophic microglia are associated with neurodegenerative disease and not healthy aging in the human brain. Neurobiol. Aging. 2021;99:19–27. doi: 10.1016/j.neurobiolaging.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Brawner A.T., Li S., Liu J.J., Kim H., Xue H., Pang Z.P., Kim W.Y., Hart R.P., Liu Y., Jiang P. OLIG2 drives abnormal neurodevelopmental phenotypes in human iPSC-based organoid and chimeric mouse models of down syndrome. Cell Stem Cell. 2019;24:908–926.e8. doi: 10.1016/j.stem.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Li X., Boreland A.J., Posyton A., Kwan K., Hart R.P., Jiang P. Human iPSC-derived mature microglia retain their identity and functionally integrate in the chimeric mouse brain. Nat. Commun. 2020;11:1577. doi: 10.1038/s41467-020-15411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q.S., Streit W.J. Microglial pathology in Down syndrome. Acta Neuropathol. 2011;122:455–466. doi: 10.1007/s00401-011-0864-5. [DOI] [PubMed] [Google Scholar]
- Zigman W.B. Atypical aging in Down syndrome. Dev. Disabil. Res. Rev. 2013;18:51–67. doi: 10.1002/ddrr.1128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The scRNA-seq datasets generated in the original study (Jin et al., 2022b) have been deposited to NCBI GEO (GSE189227) and are publicly available.
This paper does not report original code.