Abstract
Evolutionary theory predicts a late-life decline in the force of natural selection, possibly leading to late-life deregulations of the immune system. A potential outcome of such deregulations is the inability to produce specific immunity against target pathogens. We tested this possibility by infecting multiple Drosophila melanogaster lines (with bacterial pathogens) across age groups, where either individual or different combinations of Imd- and Toll-inducible antimicrobial peptides (AMPs) were deleted using CRISPR gene editing. We show a high degree of non-redundancy and pathogen-specificity of AMPs in young flies: in some cases, even a single AMP could confer complete resistance. However, ageing led to drastic reductions in such specificity to target pathogens, warranting the action of multiple AMPs across Imd and Toll pathways. Moreover, use of diverse AMPs either lacked survival benefits or even accompanied survival costs post-infection. These features were also sexually dimorphic: females required a larger repertoire of AMPs than males but extracted equivalent survival benefits. Finally, age-specific expansion of the AMP-repertoire was accompanied with ageing-induced downregulation of negative-regulators of the Imd pathway and damage to renal function post-infection, as features of poorly regulated immunity. Overall, we could highlight the potentially non-adaptive role of ageing in producing less-specific AMP responses, across sexes and pathogens.
Keywords: ageing, antimicrobial peptides, pathogen resistance, sexual dimorphism, immune specificity, immune senescence
1. Introduction
Ageing often leads to physiological senescence, including immune senescence, characterized by exaggerated and over-reactive pro-inflammatory responses [1,2]. In several insects (e.g. fruit flies and flour beetles), older individuals show increased expression of antimicrobial peptides (AMPs) [3]; higher haemolymph antibacterial activity or phenoloxidase response (PO) after infection, without any significant survival benefits [4]. Instead, increased immunity often induces lethal immunopathological damage in older individuals, increasing their mortality rate [2,5]. Similar effects are also reported in vertebrates, where an increase in chronic inflammatory state with age leads to maladaptive impacts of the innate immune system [6]. For example, older mice die faster owing to an elevated level of interleukin-17 and neutrophil activation, causing hepatocyte necrosis [1]. By and large, older individuals are thus more likely to experience the detrimental effects of overactive immunity in both invertebrate and vertebrate species.
Age-specific hyper-activation of immunity is consistent with the evolutionary theory of ageing which predicts a progressive decline in the force of natural selection with age [7,8]—natural selection that optimizes organismal physiology for development and reproduction early in life, can become too weak to effectively regulate the late-life performance in older individuals [9]. For example, poor regulatory mechanisms in several evolutionarily conserved signalling pathways such as insulin/insulin-like growth factor signalling can result in suboptimal levels of gene expression in late life, with myriad negative health effects [10–12]. Such changes in conserved signalling pathways might also interfere with the optimal induction and regulation of costly immune pathways in aged individuals. Several experiments on age-specific changes in the expression of negative regulators of immunity support this hypothesis [13]: e.g. reduced expression of anti-inflammatory cytokine interleukin-10 not only causes over-activation of cytotoxic inflammatory pathways in older mice but also promotes their muscular, cardiovascular and metabolic dysfunction [14]. In older mice and humans, a rapid age-specific decline of another immunomodulatory molecule, MANF, increases the levels of pro-inflammatory cytokines and activated macrophages [14,15]. These changes in immunity and fitness effects are thus an outcome of age-related malfunctioning of regulatory units of immune pathways.
A further potential manifestation of such a deregulated ageing immune system is the progressive loss of specificity to pathogens. Perhaps, younger individuals can optimize their immune responses by acting selectively on pathogens with a limited set of immune effectors (e.g. AMPs, [16,17] By contrast, older individuals, owing to their poorly regulated immunity, might show nonspecific activation of a higher number of immune effectors against an equivalent dose of antigenic exposure. Deployment of more immune effectors can also collectively increase the cytotoxicity of immune responses, elevating the risk of morbidity and mortality with ageing [2,5]. Indeed, prior experiments with older mice showed that pathways leading to increased production of antigen non-specific antibodies can enhance the risk of autoimmune responses with no improvement in pathogen clearance ability or survival [18]. However, experiments measuring the functional expansion of the available immune repertoire with ageing and their role in overall infection outcome is currently missing.
In the present work, we tested the impact of ageing on specific interactions between immune effectors and bacterial infections, using multiple D. melanogaster lines where different combinations of AMPs (host-encoded short, cationic peptides involved in innate immunity) from the Imd and Toll pathways were knocked out by CRISPR/Cas9 gene editing [16]. Note that AMPs specific to Imd or Toll pathways are primarily induced upon recognition of either Gram-negative or Gram-positive bacteria (including fungal pathogens) respectively [19,20]. We targeted AMPs as they have been recently shown to possess a high degree of non-redundancy, non-interchangeability and specificity against a range of pathogens in young flies [16]. Only a small subset of the total AMP repertoire provides the most effective protection against specific pathogens so that in some cases, even a single AMP is sufficient to control the growth of specific pathogens (e.g. Imd- pathway-responsive AMPs Diptericins or Drosocin can alone contribute to defence against Providencia rettgeri and Enterobacter cloacae infection respectively). Such specificity of AMP responses might also indicate potentially higher adaptive benefits associated with using fewer immune effectors in young individuals, thereby, avoiding the net fitness costs of general immune activation [21]. Indeed, earlier experiments suggest that toxic levels of AMP expression, due to suppression of negative immune regulators of Imd pathway (or increased transcriptional activation of its positive regulators) in young flies, can lead to reduced lifespan or extensive neurodegeneration causing faster ageing [22]. We hypothesize that the general loss of regulation in an ageing immune system might also accompany loss of such controlled specific AMP actions, deploying more AMPs to counter equivalent infection levels, but without any added survival benefits.
In addition, the age-specific role of AMPs can be sex-specific, with a strong sex-by-age interaction [23]. For instance, overexpression of relish or Toll-responsive defensin can reduce male lifespan more than that of females in D. melanogaster [5]. Also, previous studies with flies infected with P. rettgeri indicated D. melanogaster males had higher Diptericin expression [24]. A relatively higher expression of Diptericin transcripts in males is perhaps needed to support its exclusive role against P. rettgeri infection, whereas low expression in females opens up possibilities where Diptericin is either dispensable or requires compensatory actions of other AMPs. However, there are no direct experiments to test these possibilities of sex-specific expansion of AMP use.
2. Material and methods
(a) . Fly strains and maintenance
To test the role of ageing on AMP-driven specific immunity, we used multiple Drosophila melanogaster lines where different combinations of multiple and individual AMPs were knocked out mostly using the CRISPR/Cas9 gene editing or homologous recombination (details described in [16,17]; table 1). We used null mutants for 10 known Drosophila AMPs that are expressed upon systemic infection. These include mutations from six single gene families including Defensin (DefSK3), Attacin C (AttCMi), Attacin D (AttDSK3), Drosocin (DroSK4) Metchnikowin (MtkR1) and Drosomycin (DrsR1) loci and two small deletion removing Diptericins DptA and DptB (DptSK1), or the gene cluster containing Drosocin and Attacins AttA, AttB (Dro-AttABSK2). The iso-w1118 (DrosDel isogenic) wild-type was used as the genetic background for mutant isogenization (see [16,17,25]). We also used ‘ΔAMPs’ flies where independent mutations were recombined into a background lacking 10 inducible AMPs. However, we note that the impact of ΔAMPs could be due to AMPs having specific effects or combinatorial action of multiple co-expressed AMPs. To tease apart these effects, we also included various combined mutants where different groups of AMPs were deleted based on the pathways that they are controlled by: (1) Group B—flies lacking AMPs such as Attacins, Drosocin and Diptericins (AttCMi; AttDSK1; DroSK4; DptSK1; Dro-AttABSK2) (exclusively regulated by Imd pathway); and (2) Group C—flies lacking the two Toll-regulated antifungal peptide genes Metchnikowin and Drosomycin (MtkR1; DrsR1) (mostly regulated by Toll pathway). We also referred to flies with single mutations lacking Defensin (DefSk3) (co-regulated by Imd and Toll pathways) as group A. Finally, we also included fly line where group-A, B and C mutants were combined to generate flies lacking AMPs either from groups A and B (AB), or A and C (AC), or B and C (BC).
Table 1.
Brief description of fly lines used in the study, where either individual or different combinations of Imd- and Toll-inducible AMPs were deleted using CRISPR gene editing (see [16,17]. ‘+’ and ‘−’ denote the presence and absence of individual AMPs in each of the fly lines.
| fly lines | Attacin-A | Attacin-B | Attacin-C | Attacin-D | Diptericin-A | Diptericin-B | Drosocin | Defensin | Drosomycin | Metchnikowin |
|---|---|---|---|---|---|---|---|---|---|---|
| iso-w1118 | + | + | + | + | + | + | + | + | + | + |
| ΔAMPs | − | − | − | − | − | − | − | − | − | − |
| group-AB | − | − | − | − | − | − | − | − | + | + |
| group-BC | − | − | − | − | − | − | − | + | − | − |
| group-AC | + | + | + | + | + | + | + | − | − | − |
| group-A (DefSK3) | + | + | + | + | + | + | + | − | + | + |
| group-B | − | − | − | − | − | − | − | + | + | + |
| group-C | + | + | + | + | + | + | + | + | − | − |
| AttDSK3 | + | + | + | − | + | + | + | + | + | + |
| AttCMi | + | + | − | + | + | + | + | + | + | + |
| DroSK4 | + | + | + | + | + | + | − | + | + | + |
| Dro-AttABSK2 | − | − | + | + | + | + | − | + | + | + |
| DptSK1 | + | + | + | + | − | − | + | + | + | + |
| ΔAMPs+Dpt | − | − | − | − | + | + | − | − | − | − |
| MtkR1 | + | + | + | + | + | + | + | + | + | − |
| DrsR1 | + | + | + | + | + | + | + | + | − | + |
We maintained all fly stocks and experimental individuals on a standard cornmeal diet also known as Lewis medium (6.1 g agar, 93.6 g brown sugar, 68 g maize, 18.7 g instant yeast, 15 ml Tegosept Drosophila antifungal agent in 1 l distilled water; see [26]) at a constant temperature of 25°C on a 12 : 12 h light: dark cycle at 60% humidity. To generate the experimental flies, we reared flies at a larval density of approximately 70 eggs per 6 ml food. We collected adult males and females as virgins and held at a density of 25 flies/sex/food vial for the experiment described below. Also, we used virgin flies for all the experiments to exclude the confounding effects of mating, thereby gaining a clearer understanding of age-specific changes in AMP use alone in subsequent experiments. Female iso-w1118 flies undergo reproductive senescence within 25 days post-eclosion (Reproductive output measured for 18 h; mean ± SE: 3-day-old = 6.75 ± 0.77 versus 24-day-old = 3.17 ± 0.53, p < 0.001). Hence, in our experiments, we used 3- and 25-day-old individuals (post-eclosion) as ‘young’ and ‘old’ adults, respectively. We transferred the adults to fresh food vials every 3 days, during the entire experimental window. By screening the single mutants, along with combined genotypes, we were able to compare the changes in specific immunity as a function of possible interactions between AMPs of different groups' versus function of individual AMPs with ageing.
(b) . Infection protocol and the assay for post-infection survival
For all the infections, we either used Gram-negative bacteria Providencia rettgeri (strain Dmel; see [16]) or Pseudomonas entomophila (strain originally isolated from wild-caught D. melanogaster in Guadeloupe; see [27]). Both are natural pathogens of Drosophila that activate the IMD pathway [20] and could impose significant mortality [28,29]. To quantify post-infection survivorship, we infected flies (septic injury method) in the thorax region with a 0.1 mm minutien pin (Fine Science Tools) dipped into a bacterial suspension made from 5 ml overnight culture (optical density of 0.95, measured at 600 nm) of either Providencia rettgeri or Pseudomonas entomophila, adjusted to OD of 0.1 and 0.05 respectively, using sterile phosphate buffer solution (1× PBS) (See electronic supplementary material, methods for details). This procedure can deliver approximately 34 (±2) or 40 (±3) P. rettgeri versus P. entomophila cells respectively to each fly. In total, we infected 160–280 flies/sex/infection treatment/bacterial pathogen/age group/fly genotypes and then held them in food vials in a group of 20 individuals (For each treatment, sex, age group, pathogen type, we thus had 8–14 food vials as independent replicate batches of flies). We carried out sham infection with a pin dipped in sterile 1× PBS.
We then recorded their survival every 4 h (±2) for 5 days. Due to logistical challenges of handling a large number of flies, we infected each sex and age groups with P. rettgeri (or P. entomophila) separately in multiple batches, where they were handled as: (i) Groups AB, BC and AC; (ii) Groups A, B and C; (iii) Imd-responsive; and (iv) Toll-responsive single mutants for P. rettgeri; or (i) Groups AB, BC, AC, A, B and C; (ii) Imd-responsive; and (iii) Toll-responsive single mutants for P. entomophila. Every time, we also assayed iso-w1118 flies as a control to facilitate a meaningful comparison across different batches. Therefore, although sexes and age groups for each mutant were not directly comparable, their relative effects with respect to control iso-w1118 were estimated across sexes, age groups and pathogen types. Note that we compared each mutant separately with iso-w1118 flies since we only wanted to capture their changes in infection susceptibility relative to control flies. For each batch of flies, across pathogen types, sexes and age groups, we analysed the survival data with a mixed-effects Cox model, using the R package ‘coxme’ [30]. We specified the model as: survival approximately fly lines (individual AMP mutant lines versus iso-w1118) + (1|food vials), with fly lines as a fixed effect and replicate food vials as a random effect. Since none of the fly lines had any mortality after sham infection, we were able to quantify the susceptibility of each infected mutant lines (AMP knockouts) with respect to control flies (iso-w1118 group) as the estimated hazard ratio of infected AMP mutants versus control flies (hazard ratio = rate of deaths occurring in infected AMP mutants /rate of deaths occurring in iso-w1118 group). A hazard ratio significantly greater than one indicated a higher risk of mortality in the AMP mutant individuals.
Note that the above experimental design allowed us to repeat the assay for post-infection survival of young and old iso-w1118 flies infected with P. rettgeri (or P. entomophila) in 4 (or 3) independently replicated experiments. We thus estimated the effects of ageing on their post-infection survival, using a mixed-effects Cox model specified as: survival∼age + (1|food vials + 1|replicate experiment), with age as a fixed effect, and food vials and replicate experiments as random effects.
(c) . Assay for the bacterial load
Mortality of control flies (iso-w1118) injected with the experimental infection dose began around 24 h and 20 h after infection with P. rettgeri and P. entomophila, respectively (electronic supplementary material, figure S1A). We therefore used these time-points to estimate the bacterial load across the age groups as a measure of the pathogen clearance ability across AMPs (see electronic supplementary material, methods for detailed protocol). We homogenized flies in a group of 6 in the sterile 1X PBS (n = 8–15 replicate groups/sex/treatment/age group/fly lines), followed by plating them on Luria agar [31]. Due to logistical challenges with large number of experimental flies, we handled each sex, age group and pathogen type separately and in multiple batches as described above.
Also, similar to post-infection survival data, we were only interested in comparing the changes in bacterial load for each mutant line relative to control iso-w1118 flies across experimental groups. We thus analysed the bacterial load data of each mutant genotype with iso-w1118 flies separately across age groups, sexes and pathogen types. Since residuals of bacterial load data were non-normally distributed (confirmed using Shapiro-Wilks's test), we log-transformed the data, but residuals were still non-normally distributed. Subsequently, we analysed the log-transformed data, using a generalized linear model best fitted to gamma distribution, with fly lines (i.e. control iso-w1118 line versus individual AMP knockout line) as a fixed effect.
(d) . Assay for the Malpighian tubule activity, as a proxy for immunopathological damage
Malpighian tubules (MTs), the fluid-transporting excretory epithelium in all insects, are prone to increased immunopathology following an immune activation due to their position in the body and the fact that they cannot be protected with an impermeable membrane due to their functional requirement [2,32]. Previous experiments have shown that the risk of such immunopathological damage can increase further with ageing in mealworm beetle Tenebrio molitor [2]. It is possible that loss of specific AMP responses with ageing in Drosophila were also associated with increased immunopathological damage to MTs. We thus estimated the fluid transporting capacity of functional MTs dissected from experimental iso-w1118 females across age groups, after immune challenge with 0.1 OD P. rettgeri (n = 12–20 females/infection treatment/age group), using a modified ‘oil drop’ technique as outlined in previous studies [32,33] (also see electronic supplementary material, methods). Our preliminary data suggest that the most consistent impacts of P. rettgeri infection on MT activity appear only after 3.5 h post-infection. In the present experiments, we thus decided to assay MT activity at 4 h post-infection. Also, we only assayed females as they had significantly higher mortality after P. rettgeri infection with ageing (electronic supplementary material, figure S1A, B; table S4A), whereas males did not show any effects of ageing. Females were thus more meaningful to assay for immunopathology to explain the post-infection survival costs despite the expansion of AMP pool. Overall, this method provides a functional estimate of their physiological capacity by assaying the ability to transport saline across the active cell wall into the tubule lumen. The volume of the secreted saline droplet is negatively correlated with the level of immunopathological damage to MTs. Since we collected the flies across the age groups on different days, we analysed the MT activity data as a function of infection status for each age group separately, using a generalized linear mixed model best fitted to a quasi-binomial distribution.
(e) . Gene expression assay
Finally, we note that transcription of negative regulators of Imd pathway such as pirk and caudal are important to ensure an appropriate level of immune response following infection with gram-negative bacterial pathogens, thereby avoiding the immunopathological effects [34,35]. While pirk interferes with the interaction of PGRP-LC (peptidoglycan recognition protein) and PGRP-LE with the molecule Imd to limit the activation of the Imd pathway, caudal downregulates the expression of AMPs [34]. To examine whether the non-specific expansion of AMP repertoire was associated with the lower expression of these negative regulators, we estimated their relative expression level in both young and old iso-w1118 individuals infected with P. rettgeri at 24 h post-infection (i.e. beginning of the post-infection mortality; see electronic supplementary material, figure S1A), by using qPCR (as outlined in [36]) (n = total 15–21 flies in a group of 3 homogenized in Trizol reagent/infection treatment/age group and sex combination).
In addition, we also estimated the expression of the Imd pathway NF-κB transcription factor Relish and peptidoglycan recognition protein PGRP-LC, both act as positive regulators of Imd pathway [20,37] and hence, can serve as a proxy for overactivated Imd pathway and higher AMP expression in older flies [5] (also see electronic supplementary material, methods). We analysed the gene expression data using ANOVA (see electronic supplementary material, methods, section iv for details).
3. Results
We began our observation with high mortality and increased bacterial load in AMP-deficient flies (ΔAMPs) infected with P. rettgeri, regardless of their sex and age (figure 1a,b,e,f; electronic supplementary material, table S3), suggesting that AMPs are critically important to prevent pathogen growth, thereby increasing the post-infection survival costs (figure 1a,b,e,f; electronic supplementary material, figure S2–S3, table S2–S3). Older iso-w1118 control females infected with P. rettgeri also showed higher mortality (electronic supplementary material, figure S1A,B, table S4A) and increased bacterial load (electronic supplementary material, figure S1C, table S4B) than their younger counterparts, suggesting negative effects of ageing on fitness and pathogen clearance ability. By contrast, both young and old males had similar post-infection mortality rate (electronic supplementary material, figure S1A-B; table S4A) with comparable bacterial load (electronic supplementary material, figure S1C, table S4B), indicating that ageing did not impact male's ability to survive post-infection and clear pathogens, at least at the infection dose and the experimental window of 5-days post-infection. Nevertheless, in the subsequent assays, these results from male flies infected with P. rettgeri enabled us to directly compare the relative effects of deleting different Imd- versus Toll-responsive AMPs across age groups (against a common baseline).
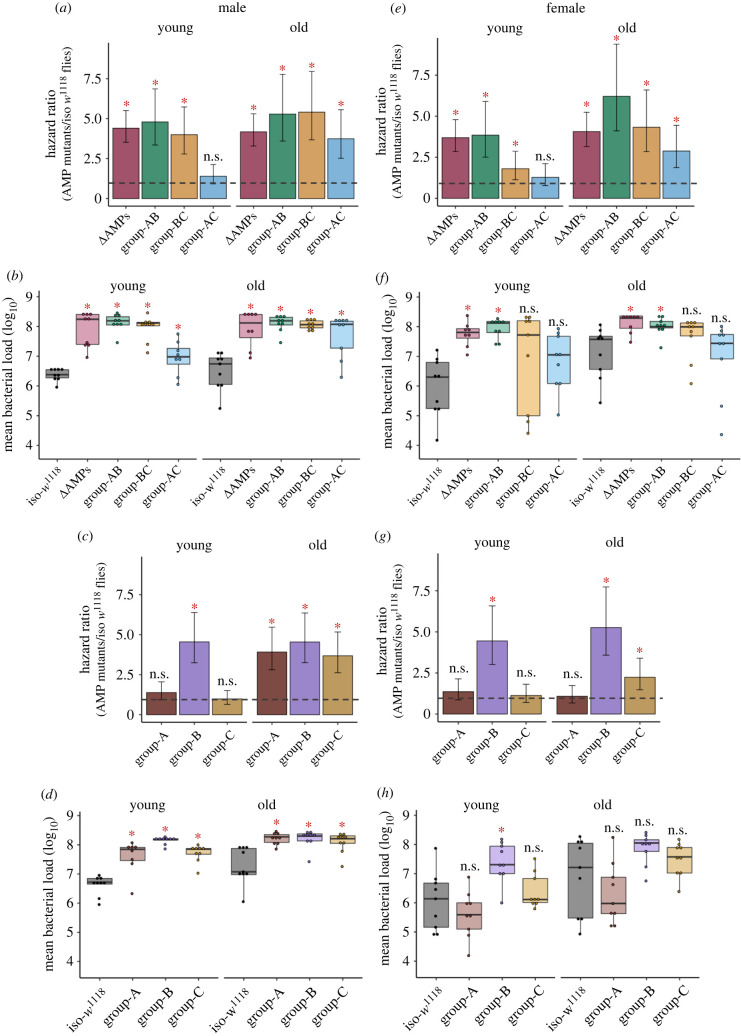
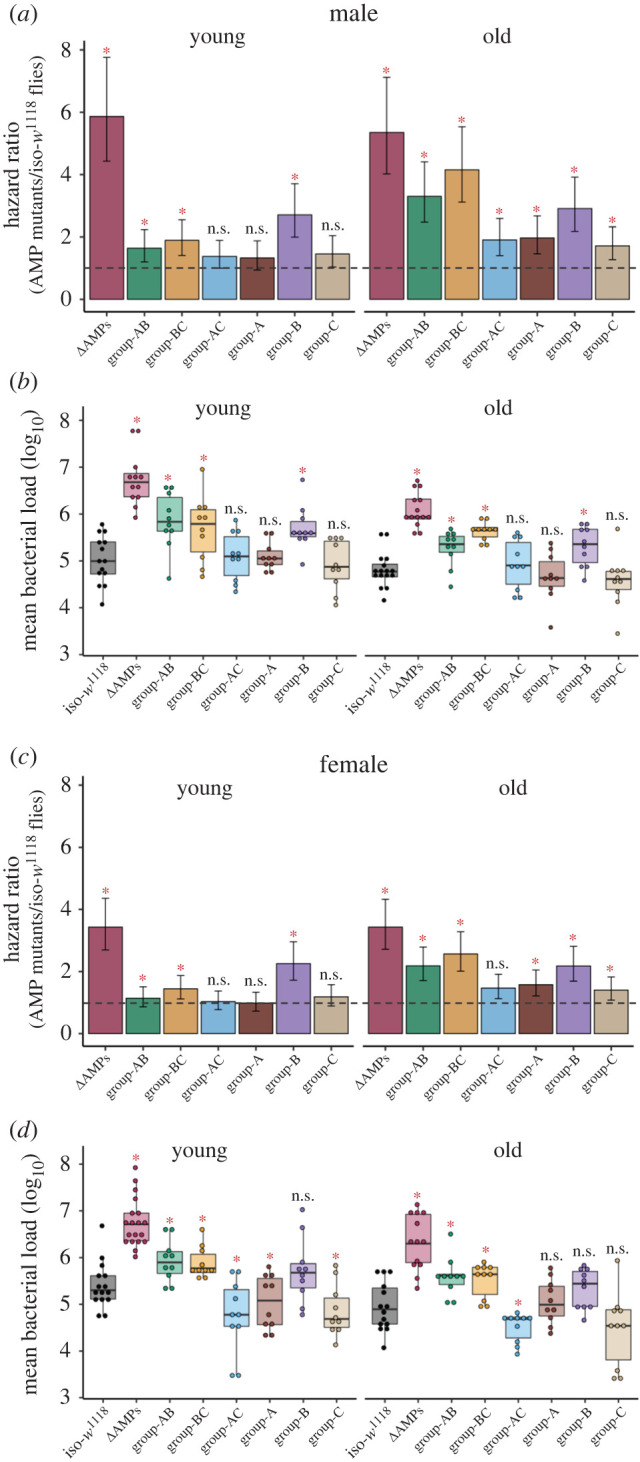
Figure 1.
Infection with Providencia rettgeri in multiple AMP-knockouts. The estimated hazard ratios, including their lower and upper limits of the 95% confidence interval, calculated from survival curves (160–180 flies/sex/infection treatment/age group/fly line; see electronic supplementary material, figure S2, S3), and bacterial load (n = 8–9 replicate groups/sex/treatment/age group/fly line) measured at 24 h after P. rettgeri infection across sexes and age groups. Hazard ratios for double combination of AMP-knockouts (i.e. group-AB, BC & AC; table 1 for details about the fly lines) in males (a) and females (e). Bacterial loads for double combination of AMP-knockouts in males (b) and females (f). Hazard ratios for single combination of AMP-knockouts (e.g. group-A, B & C) in males (c) and females (g). Bacterial load for single combination of compound of AMP-knockouts in males (d) and females (h). In panels (a,c,e,g), hazard ratios significantly greater than 1 (hazard ratio = 1; shown as horizontal dashed grey lines), indicated by asterisk (*), suggests higher infection susceptibility of mutant flies than the iso-w1118 control flies. In panels (b,d,f,h), each data point represents the bacterial load of flies pooled in a group of 6. Mutant fly lines that had significantly different bacterial load from wild-type iso-w1118 are indicated by asterisks. ns = not significant. Group A: flies lacking Defensin; Group B: flies lacking AMPs such as Drosocin, Diptericins and Attacins; Group C: flies lacking Metchnikowin and Drosomycin. Group A, B and C mutants were combined to generate flies lacking AMPs either from groups A and B (AB), or A and C (AC), or B and C (BC). (Online version in colour.)
(a) . Ageing leads to an expansion of the required AMP repertoire against P. rettgeri infection
To gain a broad understanding of how AMP specificity changes with age, we first tested mutants lacking different groups of AMPs either from Imd- (e.g. group B) or Toll pathways (e.g. group C) (pathway-specific), or combined mutants lacking pathway-specific mutants in different combinations (e.g. group AB, BC or AC) (table 1 for description of mutants). As reported in a previous study by Hanson and co-workers [16], young males lacking group-AB and -BC AMPs were highly susceptible to P. rettgeri infection (figure 1a; electronic supplementary material, table S2), and this was generally associated with 10–100-fold increased bacterial loads in these mutants relative to the iso-w1118 control (figure 1b; electronic supplementary material, table S3). Subsequent assays with pathway-specific (i.e. Imd- or Toll pathway) AMP combinations (group A, B or C) confirmed that such effects were primarily driven by the deletion of Imd-regulated group-B AMPs that were shared between both AB and BC combinations (figure 1c; electronic supplementary material, figure S2; table S2), and were important to control the bacterial growth (figure 1d; electronic supplementary material, table S3). We found a comparable pattern in young females as well, except that flies lacking group BC combinations of AMPs showed increased susceptibility to infection, but were not associated with increased bacterial loads (figure 1e–h; electronic supplementary material, figure S3; table S2–3).
By contrast to young flies, most of the pathway-specific or combined mutants became highly susceptible to P. rettgeri infection with age, except females of group-A mutants flies lacking Def. This would suggest a possible sexually dimorphic effect of Defensin in P. rettgeri infection, which appear to be important for males, but not females (figure 1c,g; electronic supplementary material, figure S2–S3; table S2). Regardless of this slight variation across sexes, our results clearly demonstrated that only having functional Imd-regulated group-B AMPs was not sufficient to protect older flies against P. rettgeri infection. Also, these results indicated that a single AMP Dpt-driven protection against P. rettgeri infection, as suggested by [16,17], may not be applicable to older males and females (also see [38]). High susceptibility of older mutants lacking group A or C AMPs (figure 1c,g; electronic supplementary material, table S2) and increased bacterial growth (figure 1d,h; electronic supplementary material, table S3) therein clearly indicated that other AMPs responsive to Gram-positive bacteria (e.g. Def) or fungal pathogens (e.g. Mtk, Drs) might be needed as well (figure 2c,d; electronic supplementary material, table S5–6).
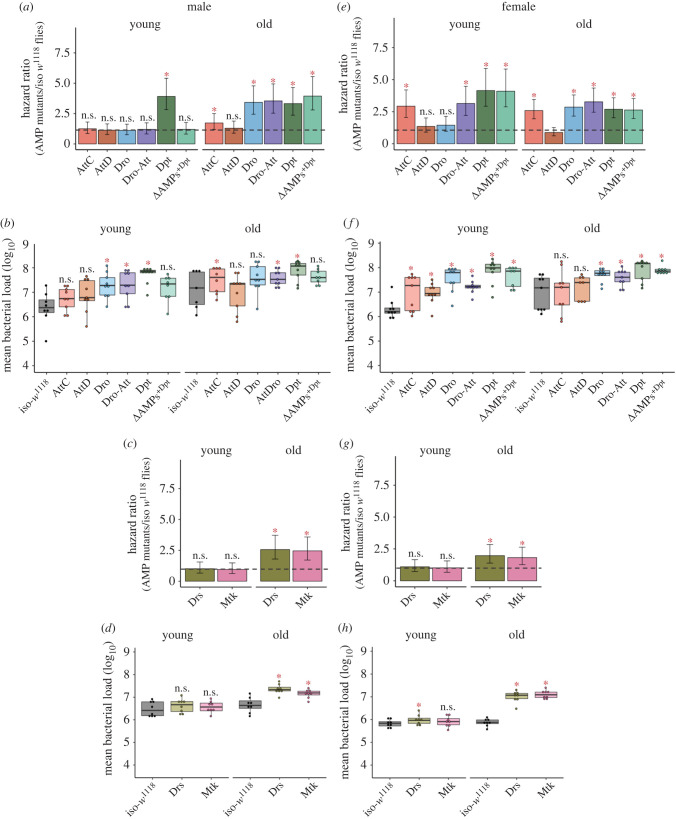
Figure 2.
Infection with Providencia rettgeri in individual AMP-knockouts. The estimated hazard ratios, including their lower and upper limits of the 95% confidence interval, calculated from survival curves (160–180 flies/sex/infection treatment/ age group/fly line; see electronic supplementary material, figure S2, S3) and bacterial load (n = 8–9 replicate groups/sex/treatment/age group/fly line) measured at 24 h after P. rettgeri infection across sexes and age groups. Hazard ratios for Imd-responsive single AMP (e.g. Dpt, AttC, AttD, Dro; table 1 for details about the fly lines) and Dro-Att knockouts in males (a) and females (e). Bacterial load of Imd-responsive single AMP and Dro-Att knockouts in males (b) and females (f). Hazard ratios for Toll-responsive single AMP knockouts (e.g. Drs & Mtk) in males (c) and females (g). Bacterial loads of Toll-responsive single AMP knockouts in males (d) and females (h) respectively. In panels (a,c,e,g), hazard ratios significantly greater than 1 (hazard ratio = 1; shown as horizontal dashed grey lines), indicated by asterisk (*), suggests higher infection susceptibility of mutant flies than the iso-w1118 control flies. In panels (b,d,f,h), each data point represents the bacterial load of flies pooled in a group of 6. Mutant fly lines that had significantly different bacterial load from wild-type iso-w1118 are indicated by asterisks (*). ns = not significant. (Online version in colour.)
(b) . Dpt-specificity against P. rettgeri infection is sex-specific and disappears with age
Next, we decided to test the role of individual AMPs deleted in the pathway-specific or compound mutants across age groups and sexes. Interestingly, Dpt provided complete protection against P. rettgeri only in young males, but not in females or older males (figure 2a,e; electronic supplementary material, table S5). This was verified by using fly lines where DptA and DptB are introduced on an AMP-deficient background (ΔAMPs+Dpt). Dpt reintroduction could fully restore survival as that of wild-type flies only in young males, and this was associated with a decrease in CFUs compared to the Dpt deletion mutant (figure 2b; electronic supplementary material, table S6). However, reintroduction of functional DptA and DptB (ΔAMPs+Dpt) in young or old females flies did not result in lower CFUs (figure 2f; electronic supplementary material, table S6) and these flies remained highly susceptible to P. rettgeri (figure 2e; electronic supplementary material, table S2). Young (or old) females also showed increased bacterial loads and associated higher susceptibility when other Imd-regulated group-B AMPs such as AttC and Dro-Att (or Dro and Dro-Att in old females) were deleted (figure 2e,f; electronic supplementary material, table S5–6). Older females lacking AttC showed increased infection susceptibility as well, but did not have increased bacterial load (figure 2e,f; electronic supplementary material, table S5–6). Older ΔAMPs+Dpt males, on the other hand, could limit the bacterial burden as low as that of the control iso-w1118 flies (figure 2b; electronic supplementary material, table S6), but still showed very high post-infection mortality (figure 2a; electronic supplementary material, table S5). These results from older males thus suggested that the ability to clear pathogens might not always translate into an improved ability to survive after infection (figure 2a,b; electronic supplementary material, table S5–6).
Why did females always require AMPs other than Dpt after P. rettgeri infection? Although the mechanisms behind sex-specific expansion of AMP repertoire are unknown, a possible explanation is that females show inherently lower expression level of Dpt relative to males. Consequently, they may require the joint expression of other AMPs to complement the lower Dpt expression, thereby enhancing the protection against P. rettgeri infection. Indeed, a previous study has already demonstrated lower Dpt expression in iso-w1118 females than males after P. rettgeri infection (see [24]), although the causal link between reduced Dpt expression and proportional increase in the compensatory action of other AMPs is not yet experimentally validated.
Older males and females also relied on Toll-responsive AMPs against P. rettgeri infection. In addition to the role of Def (included in group-A) as described above in older males (but not in older females; compare figure 1d,h; electronic supplementary material, table S3), older flies of both sexes showed increased microbe loads and increased mortality when Toll-regulated AMPs from such as Drs and Mtk were deleted (figure 2c,d,g,h; electronic supplementary material, table S5–6), raising a possibility of crosstalk between Toll and Imd immune-signalling pathways [24,39]. Taken together, these results describe ageing as a major driver behind the loss of specificity of AMP responses.
Additionally, we also note that a few other mutations such as deletion of Dro and Dro-Att, which otherwise had no effects on the survival of P. rettgeri-infected young males, caused a significant increase in the bacterial load (figure 2a,b; electronic supplementary material, table S5–6). Together, these results not only underscored the multifaceted role of AMPs, but also provided a functional resolution at the level of single AMPs such as Dpt which in addition to playing the canonical role in resisting the infection, also aided in withstanding the effects of increased pathogen growth, caused by the dysfunction of other AMPs (figure 2a,b; electronic supplementary material, table S5–6).
(c) . Expansion of the required AMP repertoire does not improve (and even reduces) survival in both older males and females infected with P. entomophila
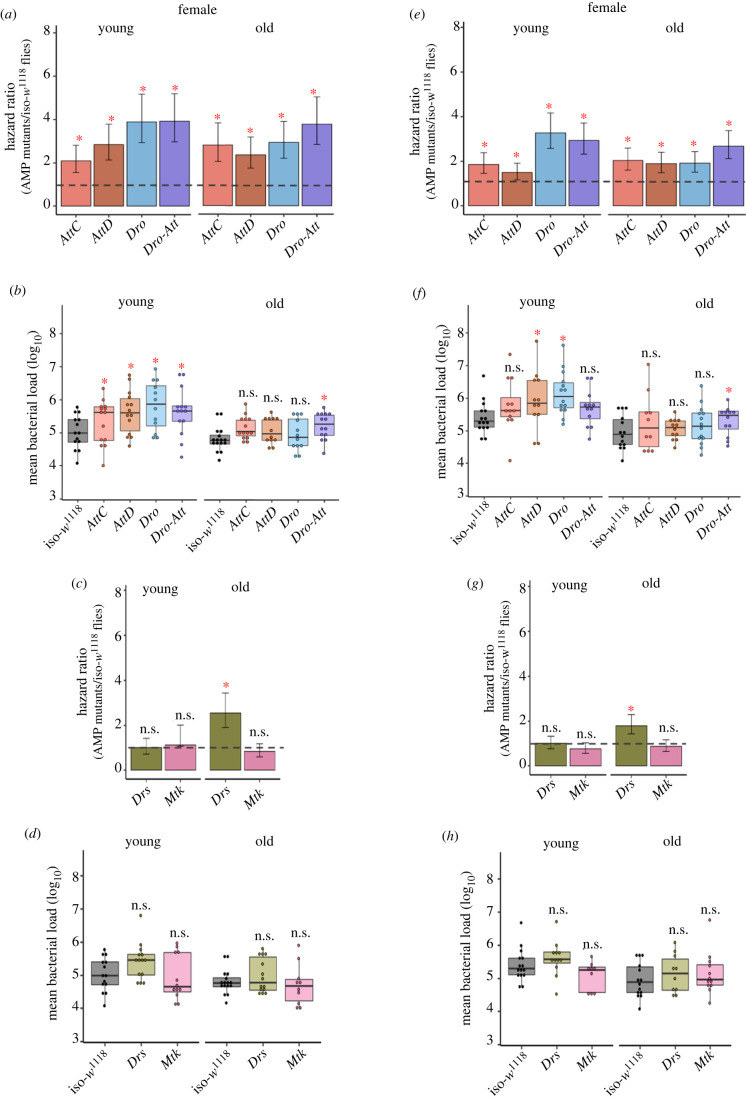
To test if age-related loss of AMP specificity was specific to P. rettgeri, or also occurred with other infections, we investigated the AMP repertoire in young and old flies infected with the Gram-negative bacteria P. entomophila. Similarly, older flies required a larger repertoire of AMPs (figure 3a,c; electronic supplementary material, table S7) and yet, died faster than young flies (old versus young: 4-fold versus 2-fold; figure 3a,c; S4–5). By contrast to younger flies, where only group-B, -AB and -BC mutants were susceptible to P. entomophila infection, all the other pathway-specific or combined mutants of older males and females were also highly sensitive to infection (figure 3a,c; electronic supplementary material, table S7). However, further experiments with single AMP mutants revealed that the antibacterial protection in both young males and females was still limited only to the exclusively Imd-regulated group-B AMPs (i.e. Diptericins, Attacins and Drosocin) (figure 3b,d; electronic supplementary material, table S8), where several of them individually caused a significant increase in microbe loads and reduction in post-infection survival (figure 4a,b,e,f; electronic supplementary material, table S9–10). By contrast, older flies also needed additional action of Toll-regulated AMP Drs (figure 4c,g; electronic supplementary material, table S9), though it is striking that increased mortality was not associated with increased microbe loads relative to iso-w1118 in this case (figure 4d,h; electronic supplementary material, table S10). Overall, this is comparable to P. rettgeri infection where potential crosstalk between Toll & Imd immune-signalling pathways has already been implicated with ageing (figure 2; electronic supplementary material, table S5–6). Also, the broad similarity between age-specific expansion and cross-reactivity of AMP repertoire against two different pathogens indicated the possibility where non-specificity can indeed be a generalized feature of ageing immunity. Moreover, the increased mortality in older flies infected with P. entomophila, despite involving a higher number of AMPs, was perhaps an indication of their exacerbated cytotoxic effects with age [5].
Figure 3.
Infection with Pseudomonas entomophila in multiple AMP-knockouts. The estimated hazard ratios, including their lower and upper limits of the 95% confidence interval, calculated from survival curves (180–280 flies/treatment/age groups/sex/fly line; see electronic supplementary material figure S4, S5) and bacterial load (n = 9–15 replicate groups/sex/treatment/age group/fly line) measured at 20 h after P. entomophila infection across sexes and age groups. Hazard ratios for double (i.e. group-AB, BC & AC) and single combination (i.e. group-A, B, C) of AMP-knockouts in males (a) and females (c). Bacterial loads for double and single combination of AMP-knockouts in males (b) and females (d). In panels (a,c) hazard ratios significantly greater than 1 (hazard ratio = 1; shown as horizontal dashed grey lines), indicated by asterisk (*), suggests higher infection susceptibility of mutant flies than the iso-w1118 control flies. In panels (b,d) each data point represents the bacterial load of flies pooled in a group of 6. Mutant fly lines that had significantly different bacterial load from wild-type iso-w1118 are indicated by asterisks. ns = not significant. table 1 or the main text for the description of different fly groups. (Online version in colour.)
Figure 4.
Infection with Pseudomonas entomophila in individual AMP-knockouts. The estimated hazard ratios, including their lower and upper limits of the 95% confidence interval, calculated from survival curves (180–280 flies/sex/infection treatment/ age group/fly line; see electronic supplementary material, figures S4 and S5) and bacterial load (n = 9–15 replicate groups/sex/treatment/age group/fly line) measured at 20 h after P. entomophila infection across sexes and age groups. Hazard ratios for Imd-responsive single AMP (e.g. AttC, AttD, Dro; table 1 for details about the fly lines) and Dro-Att knockouts in males (a) and females (e). Bacterial load of Imd-responsive single AMPs and Dro-Att knockouts in males (b) and females (f). Hazard ratios for Toll-responsive single AMP knockouts (e.g. Drs & Mtk) in males (c) and females (g). Bacterial loads of Toll-responsive single AMP knockouts in males (d) and females (h) respectively. In panels (a,c,e,g), hazard ratios significantly greater than 1 (hazard ratio = 1; shown as horizontal dashed grey lines), indicated by asterisk (*), suggests higher infection susceptibility of mutant flies than the iso-w1118 control flies. In panels (b,d,f,h), each data point represents the bacterial load of flies pooled in a group of 6. Mutant fly lines that had significantly different bacterial load from wild-type iso-w1118 are indicated by asterisks (*). n.s. = not significant. (Online version in colour.)
(d) . Ageing-induced expansion of the required AMP repertoire was associated with downregulation of negative immune regulators and a trend of reduced renal purging post-infection
The expansion of the AMP repertoire in older flies could reflect a compensatory action to balance the lower per capita efficiency of their individual AMPs. This would enable flies to maintain an equivalent post-infection survival as that of younger flies against similar infection dose (e.g. old versus young males infected with P. rettgeri; figure 1a; electronic supplementary material, table S2). However, any benefits of recruiting multiple AMPs, may have been outweighed by the costs of expressing them (suggested in [5]) as higher immune activity, in general, accelerates the ageing process by imposing immunopathological damage to vital organs such as Malpighian tubules (MTs) [2]. We also expected that the expansion of the AMP repertoire might have similar consequences in our experimental older flies. This is closely reflected by our results where P. rettgeri infection significantly reduced renal function in older females, measured as MT secretion (figure 5a; electronic supplementary material, table S11). Since functional MTs are needed to purge excessive ROS produced during immune responses as a physiological adaptation to prevent tissue damage in Drosophila [33], reduced MT activity might not only exacerbates the effects of pathogenic infection, but can also causes late-life costs [2].
Figure 5.
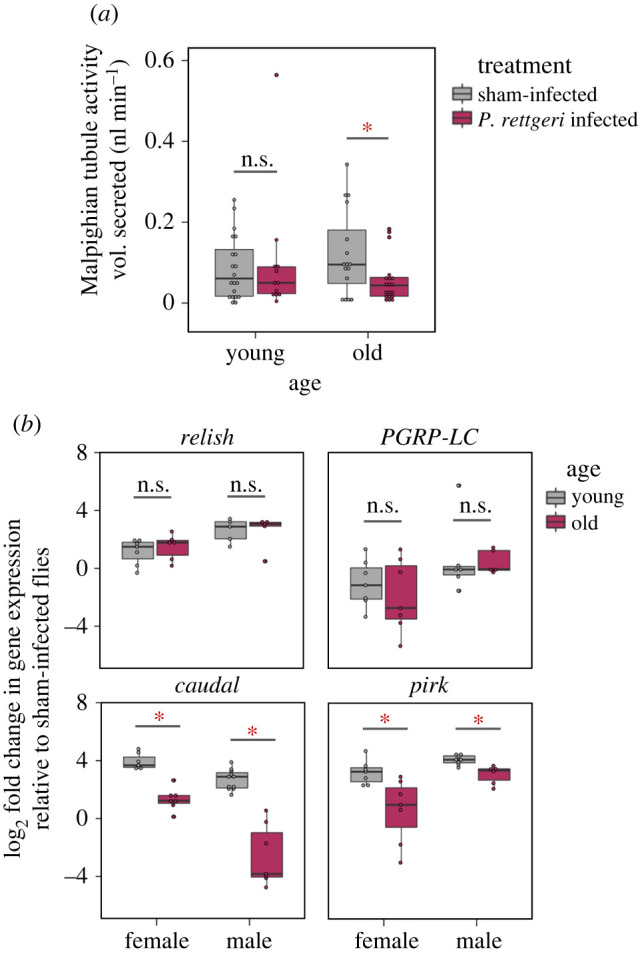
Ageing-associated immune dysregulation and immunopathology. (a) Malpighian tubule (MT) activity (n = 12–20 females/infection treatment/age group), as a proxy for immunopathological damage, measured at 4 h after infection with 0.1 OD of P. rettgeri. Statistically significant difference between groups are indicated by asterisk (*). (b) Expression of positive (Relish, PGRP-LC) and negative (Caudal, Pirk) regulators of Imd pathway across sexes and age groups after P. rettgeri infection, relative to an internal control rp49 (n = total 15–21 flies homogenized in Trizol in a group of 3/Infection treatment/age group/sex-combination). n.s. = not significant. (Online version in colour.)
Finally, we also found ageing-associated downregulation of the major negative regulators of Imd-signalling such as Caudal & Pirk in older flies (figure 5b; electronic supplementary material, table S12), which has been previously linked to the production of toxic levels of AMP production, causing reduced lifespan, locomotor defects and extensive neurodegeneration [5,22,36]. However, the expression of PGRP-LC and Relish did not change with ageing, suggesting that the expansion of the AMP pool was neither associated with increased detection of bacterial antigens nor an increase in the overall transcription of AMPs respectively. Instead, it might possibly be associated with leaky molecular signals associated with the downregulation of negative regulators (e.g. Caudal and Pirk) that fail to limit the responses to fewer AMPs upon infection. Overall, based on these results, we speculate that the observed expansion of AMP repertoire with age is therefore most likely to represent a suboptimal body condition, characterized by a poorly regulated immune system and increased physiological costs.
4. Discussion
Recent studies performed functional validation of Drosophila AMPs, revealing remarkable specificity and non-redundant interactions with subsets of pathogens that they target [16]. In the present work, we analysed these specific AMPs responses primarily as a function of ageing that alters the regulation and relative investment in immune responses [2,4]. We used two bacterial entomopathogens P. rettgeri and P. entomophila to induce various levels of AMP responses inside a fly host, ranging from a single AMP to pathway-specific expression (e.g. Imd versus Toll; [16,17]). Further, although sex profoundly impacts the relative use of AMPs [24], previous studies addressing AMP specificity have almost entirely focused on males [16,38]. We thus also included both males and females in our experiments to test the sex-specific effect of ageing on AMP functions (estimated as post-infection survival followed for 5 days). In fact, we showed that the efficiency of these AMP responses is strictly age-driven with a high degree of sexual dimorphism. For example, the classic Dpt-driven protection against P. rettgeri, as shown by previous studies [16], is only limited to young males, whereas females also needed other Imd-regulated AMPs. Although the reason is unclear, we speculate that multiple AMPs were needed possibly to compensate for the inherently lower expression level of Dpt transcript in females than in males ([24]; also shown by [40]). Alternatively, the observed pattern of AMP use can be attributed to the inherent divergence in fitness optima across sexes, where males, typically subjected to stronger intrasexual selection and greater variation in reproductive success, invest lesser in immunity [41,42]. Females, on the other hand, can invest more in immunity which is possibly reflected by their ability to employ more diverse immune effectors such as AMPs. Finally, the observed sexual dimorphism can also be due to the differences in the body size between males versus females. Males, due to their smaller body size, might receive higher density of pathogens per mg of their body tissue, which might cause them more severe infections. However, this is unlikely to explain our results because males still needed fewer AMPs to clear bacterial cells and survive after infection. However, regardless of sex, ageing led to a more drastic expansion of AMP repertoire across pathogens used in our experiments. Instead of deploying only canonical expression of Imd-responsive AMPs, older males and females also used AMPs from Toll pathways, suggesting the possibility of a novel ageing-induced functional cross-talk to create generalized and less specific protection against a wider variety of Gram-negative bacterial infections [39].
Surprisingly, despite using more diverse AMPs, older flies either lacked any survival benefits or were associated with survival costs after P. rettgeri and P. entomophila infection respectively. We thus speculate that the less specific use of AMPs with ageing was unnecessary, perhaps indicating an immune system failing to control over-reactive immune responses with potentially immunopathological effects [1,2,5,43]. This notion was further supported by reduced expression levels of negative regulators of immune responses such as caudal and pirk in older flies, which have been previously implicated in over-activating Imd-signalling and AMP expression. In addition, reduced renal function or Malpighian tubule activity in infected older flies suggested that expanded AMP repertoire might not be able to prevent the plausible physiological costs of bacterial infection. Although not verified experimentally, we suspect a causal role of overactivated AMPs here. This is because (a) overactive and simultaneously expressed multiple AMPs can impose cytotoxic effects [5], and (b) reduced Malpighian tubule activity is already a known manifestation of immunopathological costs caused by overactive insect immune components [2,44], reducing fitness by accumulating toxic metabolites [33].
We also note an alternative possibility where the age-specific increase in AMP expression could have been beneficial. For example, since ageing can lead to the accumulation of diverse microbes in the body cavity [45,46], this might warrant the overexpression of multiple AMPs to tackle the antigenic diversity of many microbial species to maintain the health [5,45]. Indeed, previous experiments have found that highly expressed Imd-responsive AMPs such as CecA1 and Dro were needed to maintain health while extending the lifespan in Drosophila [47]. However, the benefits of non-specific, highly expressed immune responses may still not be able to outweigh the net costs of overreactive immune responses. In fact, the detrimental effects of overreactive immunity with ageing have been supported by recent analyses linking weaker strength of purifying selection in older individuals and high frequency of non-synonymous and disease-causing mutations [48]. This in turn can lead to poorly regulated gene expression networks in older animals with increased cancer risk in a range of species, including humans. Taken together, non-specific AMP responses with ageing are thus a more likely feature of a deregulated immune system of older individuals [22].
Finally, the use of a diverse array of AMP deletion mutants allowed us to capture the enormous functional diversity of AMPs, revealing dynamic age- and sex-specific changes in their pathogen clearance ability. For instance, the role of AMPs in improving survival after infection can either be mediated via increased bacterial clearance or even without any changes in bacterial load relative to control iso-w1118 flies (e.g. role of AttC in young versus old females against P. rettgeri infection), which might suggest their divergent roles in both pathogen resistance versus infection tolerance respectively [49]. Also, the same AMPs that enable the host to prevent bacterial growth across different life-history contexts (e.g. compare the role of Dpt against P. rettgeri infection in old versus young males), might not always translate into better survival, suggesting the possibility of either relatively increased (immunopathological) costs of bacterial clearance or the additional roles of other AMPs. Also, older individuals showed an increased divergence between individual AMPs versus their combined action (e.g. Dpt versus group-B mutants in older females), possibly indicating greater complexity associated with a higher number of AMPs in use versus their various interactions. Although we did not find much evidence of synergism or additive effects between individual AMPs (but see the older males infected with P. entomophila), the indispensability of each AMP to maintain the fitness post-infection in older flies suggested the mutually non-exclusive and intertwined nature of their activity with ageing. We hope that these results will motivate future studies to investigate the deeper mechanistic details underlying the loss of specific AMP function and its broader role in ageing. Also, with the growing importance of AMPs in developing novel antibiotics and autoimmune disease research, identifying age or sex as major sources of variability in AMP functions and fitness impacts might have significant importance for therapeutic and gerontological research.
Acknowledgements
We thank Basabi Bagchi, Joy Bose and Srijan Seal for feedback on the manuscript. We are grateful to Bruno Lemaitre and Mark Hanson for generously providing us the fly lines and bacterial strains. We thank Srijan Seal, Katy M. Monteith, Raghav Pavan Thunga and Devshuvam Banerji for laboratory assistance.
Contributor Information
Arun Prakash, Email: arunpadmaprakash@gmail.com.
Imroze Khan, Email: imroze.khan@ashoka.edu.in.
Data accessibility
Data and code are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.12jm63z1s [50].
Supplementary material is available online [51].
Authors' contributions
B.S.: data curation, formal analysis, investigation, methodology, writing—review and editing; A.P.: data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing; S.S.: investigation, methodology, writing—review and editing; P.F.V.: funding acquisition, resources, writing—review and editing; I.K.: conceptualization, data curation, formal analysis, funding acquisition, project administration, resources, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We thank the grant supplement from SERB-DST India (no. ECR/2017/003370) to I.K. and Ashoka University for supporting this research. The research was also funded by a Society in Science Branco Weiss fellowship and a Chancellor's Fellowship (University of Edinburgh) both awarded to P.F.V. A.P. was funded by a Darwin Trust PhD Studentship (University of Edinburgh).
References
- 1.Stout-Delgado HW, Du W, Shirali AC, Booth CJ, Goldstein DR. 2009. Aging promotes neutrophil-induced mortality by augmenting IL-17 production during viral infection. Cell Host Microbe 6, 446-456. ( 10.1016/j.chom.2009.09.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan I, Agashe D, Rolff J. 2017. Early-life inflammation, immune response and ageing. Proc. R. Soc. B 284, 20170125. ( 10.1098/rspb.2017.0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zerofsky M, Harel E, Silverman N, Tatar M. 2005. Aging of the innate immune response in Drosophila melanogaster. Aging Cell 4, 103-108. ( 10.1111/j.1474-9728.2005.00147.x) [DOI] [PubMed] [Google Scholar]
- 4.Khan I, Prakash A, Agashe D. 2016. Immunosenescence and the ability to survive bacterial infection in the red flour beetle Tribolium castaneum. J. Anim. Ecol. 85, 291-301. ( 10.1111/1365-2656.12433) [DOI] [PubMed] [Google Scholar]
- 5.Badinloo M, Nguyen E, Suh W, Alzahrani F, Castellanos J, Klichko VI, Orr WC, Radyuk SN. 2018. Overexpression of antimicrobial peptides contributes to aging through cytotoxic effects in Drosophila tissues. Arch. Insect Biochem. Physiol. 98, e21464. ( 10.1002/arch.21464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw AC, Goldstein DR, Montgomery RR. 2013. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 13, 875-887. ( 10.1038/nri3547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams GC. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398-411. ( 10.1111/j.1558-5646.1957.tb02911.x) [DOI] [Google Scholar]
- 8.Hamilton WD. 1966. The moulding of senescence by natural selection. J. Theor. Biol. 12, 12-45. ( 10.1016/0022-5193(66)90184-6) [DOI] [PubMed] [Google Scholar]
- 9.Maklakov AA, Chapman T. 2019. Evolution of ageing as a tangle of trade-offs: energy versus function. Proc. R. Soc. B 286, 20191604. ( 10.1098/rspb.2019.1604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenyon CJ. 2010. The genetics of ageing. Nature 464, 504-512. ( 10.1038/nature08980) [DOI] [PubMed] [Google Scholar]
- 11.Flatt T, Partridge L. 2018. Horizons in the evolution of aging. BMC Biol. 16, 93. ( 10.1186/s12915-018-0562-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlsson H, Ivimey-Cook E, Duxbury EML, Edden N, Sales K, Maklakov AA. 2021. Ageing as ‘early-life inertia’: disentangling life-history trade-offs along a lifetime of an individual. Evol. Lett. 5, 551-564. ( 10.1002/evl3.254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neves J, Sousa-Victor P. 2020. Regulation of inflammation as an anti-aging intervention. FEBS J. 287, 43-52. ( 10.1111/febs.15061) [DOI] [PubMed] [Google Scholar]
- 14.Mohanty S, et al. 2015. Prolonged proinflammatory cytokine production in monocytes modulated by interleukin 10 after influenza vaccination in older adults. J. Infect. Dis. 211, 1174-1184. ( 10.1093/infdis/jiu573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neves J, Zhu J, Sousa-Victor P, Konjikusic M, Riley R, Chew S, Qi Y, Jasper H, Lamba DA. 2016. Immune modulation by MANF promotes tissue repair and regenerative success in the retina. Science 353, aaf3646. ( 10.1126/science.aaf3646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson MA, Dostálová A, Ceroni C, Poidevin M, Kondo S, Lemaitre B. 2019. Synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach. Elife 8, e44341. ( 10.7554/eLife.44341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson MA, Dostálová A, Ceroni C, Poidevin M, Kondo S, Lemaître B. 2019. Correction: synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach. Elife 8, e48778. ( 10.7554/eLife.48778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruce D, Whitcomb JP, August A, McDowell MA, Cantorna MT. 2009. Elevated non-specific immunity and normal Listeria clearance in young and old vitamin D receptor knockout mice. Int. Immunol. 21, 113-122. ( 10.1093/intimm/dxn129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valanne S, Wang J-H, Rämet M. 2011. The Drosophila Toll signaling pathway. J. Immunol. 186, 649-656. ( 10.4049/jimmunol.1002302) [DOI] [PubMed] [Google Scholar]
- 20.Myllymäki H, Valanne S, Rämet M. 2014. The Drosophila Imd signaling pathway. J. Immunol. 192, 3455-3462. ( 10.4049/jimmunol.1303309) [DOI] [PubMed] [Google Scholar]
- 21.Moret Y. 2003. Explaining variable costs of the immune response: selection for specific versus non-specific immunity and facultative life history change. Oikos 102, 213–216. ( 10.1034/j.1600-0706.2003.12496.x) [DOI] [Google Scholar]
- 22.Kounatidis I, Chtarbanova S, Cao Y, Hayne M, Jayanth D, Ganetzky B, Ligoxygakis P. 2017. NF-κB immunity in the brain determines fly lifespan in healthy aging and age-related neurodegeneration. Cell Rep. 19, 836-848. ( 10.1016/j.celrep.2017.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belmonte RL, Corbally M-K, Duneau DF, Regan JC. 2020. Sexual dimorphisms in innate immunity and responses to infection in Drosophila melanogaster. Front. Immunol. 10, 3075. ( 10.3389/fimmu.2019.03075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duneau DF, et al. 2017. The Toll pathway underlies host sexual dimorphism in resistance to both Gram-negative and Gram-positive bacteria in mated Drosophila. BMC Biol. 15, 124. ( 10.1186/s12915-017-0466-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira ÁG, Naylor H, Esteves SS, Pais IS, Martins NE, Teixeira L. 2014. The Toll-dorsal pathway is required for resistance to viral oral infection in Drosophila. PLoS Pathog. 10, e1004507. ( 10.1371/journal.ppat.1004507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siva-Jothy JA, Prakash A, Vasanthakrishnan RB, Monteith KM, Vale PF. 2018. Oral bacterial infection and shedding in Drosophila melanogaster. JoVE 135, e57676. ( 10.3791/57676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vodovar N, Vinals M, Liehl P, Basset A, Degrouard J, Spellman P, Boccard F, Lemaitre B. 2005. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc. Natl Acad. Sci. USA 102, 11 414-11 419. ( 10.1073/pnas.0502240102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galac MR, Lazzaro BP. 2011. Comparative pathology of bacteria in the genus Providencia to a natural host, Drosophila melanogaster. Microbes Infect. 13, 673-683. ( 10.1016/j.micinf.2011.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dieppois G, Opota O, Lalucat J, Lemaitre B. 2015. Pseudomonas entomophila: a versatile bacterium with entomopathogenic properties. In Pseudomonas (eds Ramos J-L, Goldberg JB, Filloux A), pp. 25-49. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 30.Therneau T. 2015. Mixed Effects Cox Models, in: Mixed Effects Cox Models. CRAN repository.
- 31.Prakash A, Monteith KM, Vale PF. 2022. Mechanisms of damage prevention, signalling and repair impact disease tolerance. Proc. R. Soc. B 289, 20220837. ( 10.1098/rspb.2022.0837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dow JAT, Maddrell SHP, Görtz A, Skaer NJV, Brogan S, Kaiser K. 1994. The malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J. Exp. Biol. 197, 421-428. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Rommelaere S, Kondo S, Lemaitre B. 2020. Renal purge of hemolymphatic lipids prevents the accumulation of ROS-induced inflammatory oxidized lipids and protects drosophila from tissue damage. Immunity 52, 374-387.e6. ( 10.1016/j.immuni.2020.01.008) [DOI] [PubMed] [Google Scholar]
- 34.Lee K-Z, Ferrandon D. 2011. Negative regulation of immune responses on the fly: immune responses on the fly. EMBO J. 30, 988-990. ( 10.1038/emboj.2011.47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleino A, Silverman N. 2014. The Drosophila IMD pathway in the activation of the humoral immune response. Dev. Comp. Immunol. 42, 25-35. ( 10.1016/j.dci.2013.05.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prakash A, Monteith KM, Vale PF. 2021. Negative regulation of IMD contributes to disease tolerance during systemic bacterial infection in Drosophila (preprint). bioRxiv. ( 10.1101/2021.09.23.461574) [DOI] [Google Scholar]
- 37.Myllymäki H, Valanne S, Rämet M. 2014 The Drosophila Imd Signaling Pathway. J. Immunol 192, 3455–62. ( 10.4049/jimmunol.1303309) [DOI] [PubMed]
- 38.Unckless RL, Howick VM, Lazzaro BP. 2016. Convergent balancing selection on an antimicrobial peptide in Drosophila. Curr. Biol. 26, 257-262. ( 10.1016/j.cub.2015.11.063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishide Y, Kageyama D, Yokoi K, Jouraku A, Tanaka H, Futahashi R, Fukatsu T. 2019. Functional crosstalk across IMD and Toll pathways: insight into the evolution of incomplete immune cascades. Proc. R. Soc. B 286, 20182207. ( 10.1098/rspb.2018.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prakash A. 2022. Immune regulation of disease tolerance and immune priming in Drosophila. ( 10.7488/era/2279) [DOI]
- 41.Zuk M. 1990. Reproductive strategies and disease susceptibility: an evolutionary viewpoint. Parasitol. Today 6, 231-233. ( 10.1016/0169-4758(90)90202-F) [DOI] [PubMed] [Google Scholar]
- 42.Rolff J. 2002. Bateman's principle and immunity. Proc. R. Soc. Lond. B 269, 867-872. ( 10.1098/rspb.2002.1959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldstein DR. 2010. Aging, imbalanced inflammation and viral infection. Virulence 1, 295-298. ( 10.4161/viru.1.4.12009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadd BM, Siva-Jothy MT. 2006. Self-harm caused by an insect's innate immunity. Proc. R. Soc. B. 273, 2571-2574. ( 10.1098/rspb.2006.3574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren C, Webster P, Finkel SE, Tower J. 2007. Increased internal and external bacterial load during Drosophila aging without life-span trade-off. Cell Metab. 6, 144-152. ( 10.1016/j.cmet.2007.06.006) [DOI] [PubMed] [Google Scholar]
- 46.Arias-Rojas A, Iatsenko I. 2022. The role of microbiota in Drosophila melanogaster aging. Front. Aging 3, 909509. ( 10.3389/fragi.2022.909509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loch G, Zinke I, Mori T, Carrera P, Schroer J, Takeyama H, Hoch M. 2017. Antimicrobial peptides extend lifespan in Drosophila. PLoS ONE 12, e0176689. ( 10.1371/journal.pone.0176689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng C, Kirkpatrick M. 2021. Molecular evolution and the decline of purifying selection with age. Nat. Commun. 12, 2657. ( 10.1038/s41467-021-22981-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider DS, Ayres JS. 2008. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol. 8, 889-895. ( 10.1038/nri2432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shit B, Prakash A, Sarkar S, Vale PF, Khan I. 2022. Data and code from: Ageing leads to reduced specificity of antimicrobial peptide responses in Drosophila melanogaster. Dryad Digital Repository. ( 10.5061/dryad.12jm63z1s) [DOI] [PMC free article] [PubMed]
- 51.Shit B, Prakash A, Sarkar S, Vale PF, Khan I. 2022. Data from: Ageing leads to reduced specificity of antimicrobial peptide responses in Drosophila melanogaster. Figshare. ( 10.6084/m9.figshare.c.6277190) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Shit B, Prakash A, Sarkar S, Vale PF, Khan I. 2022. Data and code from: Ageing leads to reduced specificity of antimicrobial peptide responses in Drosophila melanogaster. Dryad Digital Repository. ( 10.5061/dryad.12jm63z1s) [DOI] [PMC free article] [PubMed]
- Shit B, Prakash A, Sarkar S, Vale PF, Khan I. 2022. Data from: Ageing leads to reduced specificity of antimicrobial peptide responses in Drosophila melanogaster. Figshare. ( 10.6084/m9.figshare.c.6277190) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data and code are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.12jm63z1s [50].
Supplementary material is available online [51].