Abstract
Phosphorus, often in the form of inorganic phosphate (Pi), is critical to cellular function on many levels; it is required as an integral component of kinase signaling, in the formation and function of DNA and lipids, and energy metabolism in the form of ATP. Accordingly, crucial aspects of cell mitosis – such as DNA synthesis and ATP energy generation – elevate the cellular requirement for Pi, with rapidly dividing cells consuming increased levels. Mechanisms to sense, respond, acquire, accumulate, and potentially seek Pi have evolved to support highly proliferative cellular states such as injury and malignant transformation. As such, manipulating Pi availability to target rapidly dividing cells presents a novel strategy to reduce or prevent unrestrained cell growth. Currently, limited knowledge exists regarding how modulating Pi consumption by pre-cancerous cells might influence the initiation of aberrant growth during malignant transformation, and if reducing the bioavailability or suppressing Pi consumption by malignant cells could alter tumorigenesis. The concept of targeting Pi-regulated pathways and/or consumption by pre-cancerous or tumor cells represents a novel approach to cancer prevention and control, although current data remains insufficient as to rigorously assess the therapeutic value and physiological relevance of this strategy. With this review, we present a critical evaluation of the paradox of how an element critical to essential cellular functions can, when available in excess, influence and promote a cancer phenotype. Further, we conjecture how Pi manipulation could be utilized as a therapeutic intervention, either systemically or at the cell level, to ultimately suppress or treat cancer initiation and/or progression.
Keywords: dietary phosphate, phosphate transport, cell proliferation, tumor progression, osteopontin, phosphate addiction
Introduction
Phosphorus/inorganic phosphate (Pi) is rarely lacking in the human diet and has therefore been largely neglected in terms of health-related research in the context of normal kidney function. Relatively little is understood about long-term consequences of altered serum Pi levels on health and disease with the underlying mechanism(s) associated with cellular Pi consumption just beginning to be explored. However, emerging data suggests the need for a paradigm shift from viewing Pi as only a passive element of systemic and cellular function to that of an active modulator of tissue and cellular behavior. Although increased serum Pi levels as a result of either hereditary syndromes or kidney disease are known to result in significant health consequences [1], there is a growing appreciation that increased serum Pi as a result of increased dietary consumption in adults [2], and the ensuing changes in serum Pi levels, influence age-associated disease progression as observed with bone metabolism [3–8] and cardiovascular function [9–12]. The importance of regulating systemic Pi levels is further highlighted by knockout or mutation of the phosphaturic hormone fibroblast growth factor 23 (FGF23) or its co-receptor Klotho in mice; loss or reduction of these hormones results in hyperphosphatemia, inflammation and a premature-aging syndrome that can be corrected with a low Pi diet [13, 14]. Yet, the cellular requirement for Pi is complex. In early life, a period of rapid growth, dietary Pi is critical for bone and mineral formation, as well as energy metabolism. Severely restricted young rats (0.02% Pi diet) die within eight weeks [15] and weanling rats fed a low Pi diet (0.2%) have reduced bone mineralization rates [16]. Taken together, the results suggest a stage- and tissue-specific relationship between the cell and tissue requirements for Pi related to cellular processes ranging from growth to senescence. It has been theorized that the mechanisms underlying these observations involve changes in extra and intracellular Pi concentration which, in turn, alter glucose metabolism, increase oxidative stress, and inflammation [17, 18]. These biological processes are being increasingly appreciated for their roles in tumorigenesis and tumor progression. Whereas Pi levels and cellular consequences related to bone, kidney, and cardiovascular disease are currently an active area of investigation, the role of Pi consumption and metabolism in cancer has only begun to be investigated. Here we discuss data supporting the concept that both pre- and fully malignant cells require additional Pi, causing a Pi “addiction”, and how Pi consumption can be exploited for health benefits and disease treatment.
1. Regulation of systemic, cellular, and microenvironment Pi
To better understand the role of excess Pi in influencing complex diseases such as cancer, it is important to first understand how Pi is regulated in a healthy adult. Although Pi homeostasis is typically described as being tightly regulated comparable to calcium, in fact, Pi lacks the tight control at the systemic and cellular levels [19]. Whereas calcium levels are maintained in a very narrow range in the serum and the intracellular levels are much lower than the extracellular levels, Pi levels have more fluctuation in the serum and are substantially higher intracellularly than extracellularly [20]. This increased range, provides multiple points for therapeutic intervention and suggests the possibility of a therapeutic window of opportunity to modulate Pi levels without detrimental systemic or cellular effects.
1.1. Endocrine homeostasis of systemic Pi
Serum Pi levels in health humans are generally reported in the clinical laboratory reference range of 2.5 to 4.5 mg/dL (0.81 to 1.45 mmol/L) although this can vary slightly with fasting, dietary consumption, age, gender, hormone status, and diurnal fluctuations [19–29]. Sustained low (<0.81 mmol/L (<2.5 mg/dL for hypophosphatemia) and high (>1.46 mmol/L (generally 7–9mg/dL for severe hyperphosphatemia)) serum Pi levels are known to result in significant health consequences and are generally the result of either hereditary or disease acquired syndromes [20, 30–34]. For healthy adults, dietary intake provides the main source of serum Pi with the intestine being the main site of absorption, the kidney being the main site of excretion, and the skeleton being the main site of storage [35]. Total body Pi is approximately 500 to 800 grams of which 85–90% is in the skeleton and 10–15% resides in other tissues [36, 37]. Due in part to the increased consumption of convenience foods, such as highly processed “enhanced/restructured” meats and fast foods [38], the amount of Pi in the American diet continues to rise well above levels considered high by the FDA [3, 39]. Changes in serum Pi levels, in the absence of disease, are generally proportional to dietary intake [19, 40] and a high Pi meal can significantly increase serum Pi levels for up to 8 hours [41–43]. With the goal of keeping serum Pi levels in balance, a number of tissues sense changes in Pi levels and produce endocrine factors such as parathyroid hormone (PTH) and fibroblast growth factor-23 (FGF23) (reviewed [44–48]) (Fig.1). Increased intake of Pi leads to increased circulating levels of PTH and FGF23 which, in turn, act on the kidneys to decrease Pi reabsorption through the downregulation of Pi transporters Slc34a1 and Slc34a3 via internalization. These events also trigger a decrease in active vitamin D (D3) produced in the kidney as a result of a decrease in the synthesizing enzyme 1α hydroxylase (CYP27B1) and increase in the degrading enzyme 24-hydroxylase (CYP24A1). The decrease in serum vitamin D is presumed to reduce Pi absorption from the intestines as well as generate a negative feedback loop to reduce PTH and FGF23 (Fig.1). The net result is increased Pi excretion in the urine and decreased absorption in the gut which brings serum Pi levels back into balance. While PTH acts by binding to the PTH receptor, FGF23 requires both the FGF receptor as well as the co-factor Klotho. Mice with mutations in the Klotho gene, which decreases FGF23 efficacy, are characterized by hyperphosphatemia, osteoporosis, vascular calcification, and thinning skin among other premature aging related pathologies [49–51]. Remarkably, most of this robust aging phenotype can be corrected with a low Pi diet [52, 53], emphasizing the potential contribution of dietary Pi and serum Pi to health, disease, and the aging process and its modulation as a potential therapeutic intervention.
Figure 1: Regulation of serum Pi levels.
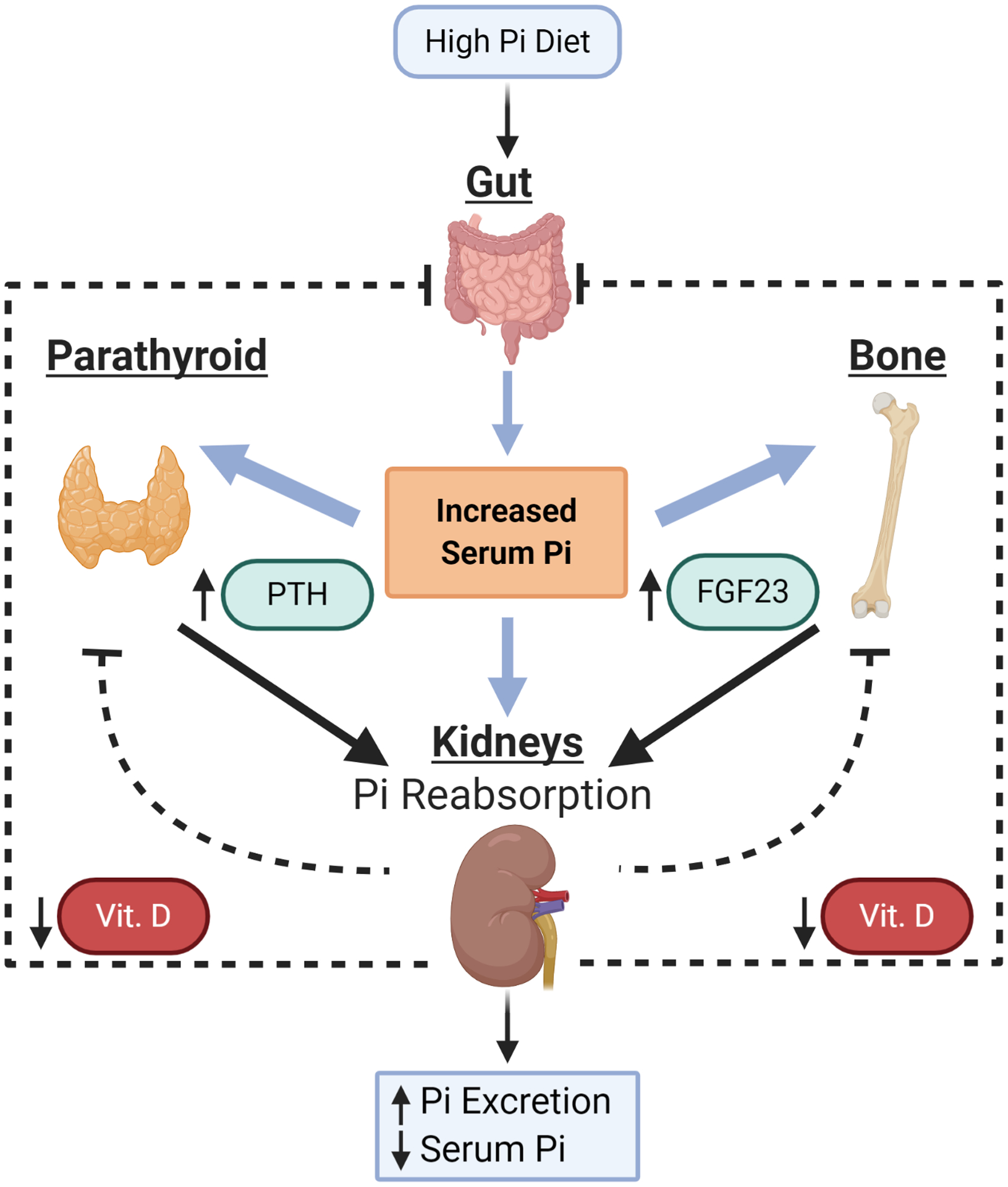
In response to a high Pi diet serum Pi levels increase along with FGF23, expressed in osteoblasts and osteocytes, while PTH is increased in the parathyroid gland. Both proteins act on receptors in the kidney to reduce the number of Pi-transporters thereby increasing Pi excretion and lowering serum Pi levels. Simultaneously, the synthesis of vitamin D3 is decreased in the kidney which is thought to decrease Pi absorption in the gut and to generate a negative feedback loop to decrease synthesis and release of PTH and FGF23.
1.2. Cell autonomous regulation of intracellular Pi
Intracellular Pi levels are difficult to precisely quantify because much of the Pi is bound and may vary substantially between cell types; however, levels are generally estimated to be in the range of 50–100mM with approximately 5mM existing as free inorganic phosphate [54]. The primary mechanism by which cells regulate and possibly sense the extracellular distribution and levels of Pi is through a family of sodium dependent Pi-transporters (reviewed in [55]). Type II transporters (current nomenclature Slc34a1-3) are thought to be responsible mainly for absorption in the intestine and resorption in the kidney (reviewed in [56]), although recent data suggests the possibility of more diverse functions [57]. Type III transporters (current nomenclature Slc20a1-2) are expressed more ubiquitously with evidence highlighting important roles in calcifying tissues (reviewed in [58]). Although the potential role(s) of Pi-transporters as active regulators of cell function - as opposed to “housekeeping” ion transport functions - are not fully understood, cell culture studies have linked changes in extracellular Pi concentrations to changes in cell behavior in numerous cell types of varying function [59–70]. Most cells express more than one of the Pi-transporters and therefore sorting out individual roles for specific transporters has been challenging. To date, two Pi-transporters Slc20a1 and Slc34a2 have been linked to modulation of cell behavior related to the cancer phenotype (discussed below). While active Pi transport is a critical function of transporters in the kidney and intestine, in other cell types, it is possible that these membrane proteins also act as Pi-sensors of extracellular concentrations in addition to ion channels [71].
1.3. Regulation of Pi in the cellular microenvironment; paracrine/autocrine and ectoenzymes
Although systemic and cellular regulation of Pi are at least partially understood, the control of Pi levels in the microenvironment is less so. Similar to serum, interstitial or extracellular fluid is broadly thought to contain 0.5–2mM Pi, but data are limited and levels likely vary by tissue environment [40, 72–74]. Supporting the increased Pi demand of tumors a recent study identified an almost two-fold increase in tumor microenvironment interstitial Pi relative to corresponding normal tissue [75]. There are a number of factors in the cellular microenvironment that are known to alter the cellular handling of Pi. Cell surface enzymes (ectoenzymes) are known to increase extracellular Pi through cleavage of various available forms of organic phosphates including ATP. Under physiological conditions, the generation of free extracellular Pi is normally associated with mineralizing cells and the activity of the established ectoenzyme Alkaline phosphatase. Alkaline phosphatase (ALP) is actually four unique genes/enzymes (Tissue non-specific ALP (TNALP; or also referred to as liver/bone/kidney), Placental, Intestinal, and Germ Cell) which are membrane bound but can also be measured in circulation and have been found elevated in many different cancers [76–79]. The extracellular environment can also influence the amount of cellular Pi uptake by autocrine and paracrine signaling. A number of factors have been identified that will directly influence the kinetics of transport of Pi into cells including parathyroid hormone (PTH), insulin like growth factor-1 (IGF-1), platelet derived growth factor (PDGF) [80], calcium [81], IL-8 [82], insulin [83] and the phorbol ester, 12-O-tetradecanocylphorbol-13-acetate (TPA) [84, 85]. Interestingly, two recent studies have identified the coordinated requirement of FGF receptor signaling for both extracellular Pi-induced cell signaling and gene expression [86, 87]. The coordination of Pi transport with growth factor stimulation would be advantageous so as to allow cells to not enter a proliferative state unless a key building block is present and begs the question as to whether this coordination can be decoupled to inhibit a cell from proliferating.
2. Functional consequences of Pi availability on cell proliferation and transformation
Traditionally, Pi has been viewed as a required but passive player in cellular behavior. Growing evidence suggests a more active role for this common nutritional element in influencing various cellular functions. While diet has become increasingly appreciated for its profound effects on functional genomics and gene expression, the molecular and cellular response to long-term changes in serum Pi levels have only begun to be investigated. Specifically, based on the essential requirement of Pi for numerous critical cellular functions, it follows that rapidly proliferating cells would have an increased need to Pi. Evidence supporting this concept comes from in vivo studies in mice and humans that have demonstrated increased Pi uptake and retention in tumors relative to the corresponding non-malignant tissue controls [88–92]. More recently, cell-based studies have revealed that exposure of a variety of cell types to elevated Pi will alter growth properties, signal transduction pathways and gene expression, through a myriad of cell autonomous, autocrine, and paracrine effectors. In vitro investigations have begun to uncover diverse downstream functional implications of elevated Pi on cell behavior as a result of the cells ability to sense and respond to changes in extracellular Pi.
2.1. Functional consequences of Pi on transformation and energy metabolism
Cancerous transformation is the process by which a healthy cell acquires the traits of a malignant cell such as uninhibited growth, the ability of normally adherent cells to grow in suspension and altered energy metabolism. Early studies using the NIH3T3 focus formation assay, cell proliferation in limited growth medium, established Pi as a required nutrient in the regulation and stimulation of transformation [93, 94]. A recent study utilizing the transformation-sensitive epidermal keratinocyte JB6 cell line, which respond to various tumor promoters, such as TPA (12-O-tetradecanoylphorbol-13-acetate), with anchorage-independent growth in soft agar and tumorigenicity found that addition of Pi, 1–2 mM in addition to the 1 mM in the medium, resulted in a significant increase in cell proliferation and significantly increased anchorage-independent growth over TPA alone [67]. Further, the same study demonstrated that Pi alone (in the absence of TPA) augmented soft agar growth, suggesting that Pi is not only a required nutrient in the transformation process but excess Pi itself can actively promote it. Elevated Pi availability has also been demonstrated to alter cellular energy metabolism, another aspect of malignant transformation. Pi was originally determined as a required element for mitochondrial respiration but more recently increased Pi was shown to actively increase oxidative phosphorylation and glycolysis pathways and ATP production [67, 95–97]. The Pi-induced changes in cellular energy metabolism appear to be driven by changes in protein abundance and not linked to changes in gene expression, as might be predicted [98]. Further, oxidative phosphorylation was demonstrated as necessary for certain Pi-induced changes in gene expression such as OPN [86]. Taken together, these results identified that increased Pi availability is sufficient to promote altered cellular energy metabolism as well as anchorage-independent growth in cell models, indicative of cell transformation towards a malignant phenotype.
2.2. Functional consequences of Pi on cell proliferation
Fundamentally, cancer is a disease driven, in large part, by excessive and unrestrained cell growth, and a number of cell-based studies have demonstrated that the availability of Pi influences cell proliferation acting similar to a mitogen. Over four decades ago Pi was identified as a limiting nutrient in the proliferation of Swiss 3T3 fibroblast-like cells [99–101]. Further, contact inhibited 3T3 cells respond to serum stimulation with a rapid increase in Pi transport [102–104]. Whereas these studies identified Pi as a required nutrient for cell growth, other studies suggest that excess Pi, added to culture medium already sufficient in Pi, can actively promote cell proliferation [67–69, 98, 102, 105]. This tight association of Pi with cell functions associated with energy metabolism and proliferation has inspired the growth rate hypothesis, proposing that tumors, or any fast growing tissue, have an increased demand for Pi [88]. However, it should be noted that a number of studies have found an increase in apoptosis in certain cell types, including cancer cells, and under certain culture conditions, highlighting the complexity of the cellular response to excess Pi [106–111]. Whether these complexities and inconsistencies result from specific culture conditions or differences in cell types still remains to be determined. Collectively, these results identify Pi as a nutrient that when in excess can promote proliferation and transformation but also highlight the possibility that Pi consumption can be manipulated to control cell growth, particularly in rapidly dividing cells.
2.3. Effects of Pi on cell signaling in cell culture and in vivo
The biochemical mechanisms by which elevated extracellular Pi might alter cell functions are also being investigated. Although not fully elucidated, data suggest that increased extracellular Pi generates a complex, temporally controlled series of specific signaling events likely as specific as those elicited via many traditional signaling molecules. Two specific signaling pathways identified as responsive to elevated Pi are the growth related ras-ERK1/2 pathway [67, 68, 112, 113] and protein translation Akt- eIF4E-BP1 [68] cascades (Fig.2). Further, a cell culture study found Pi-induced post-transcriptional regulation of the AP-1 factor Fra-1, resulted in increased protein levels [98] demonstrating one mechanisms by which Pi might influence protein abundance. Both ERK1/2 and Akt become phosphorylated in response to increasing levels of Pi in multiple cell types, and inhibition of these proteins by either pharmacological or siRNA knockdown results in elimination of downstream Pi-induced effects on gene expression [68, 112]. Specifically, the response of the MAPK (ERK) pathway to altered Pi levels has also been identified in drosophila, suggesting an evolutionarily conserved mechanism linking ERK1/2 signaling with elevated Pi levels [114]. Additional signaling proteins identified as Pi responsive include PKC [112] and nitric oxide [115]. These results are also supported by in vivo studies from mice fed a high Pi diet for 4 weeks [116], emphasizing the relevance of the cell culture studies to mammalian physiology. Taken together, these studies identify an increase in extracellular Pi as an initiator of surprisingly specific and conserved signal transduction pathways.
Figure 2: Effects of increased Pi availability on cellular signaling.
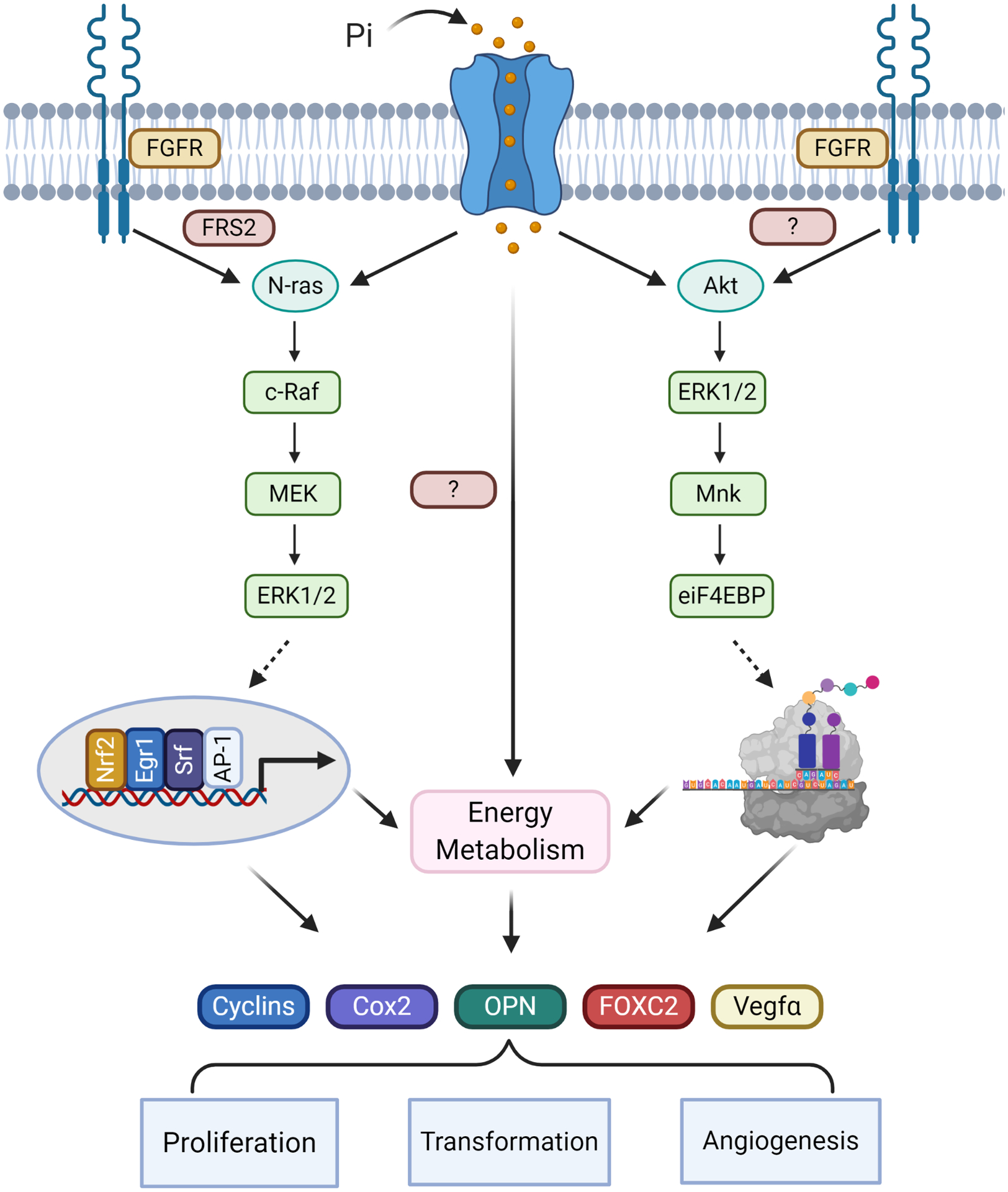
Cell-based studies have identified specific membrane and signaling events associated with increased extracellular Pi availability including the coordinated requirement of sodium dependent Pi-transporters/sensors as well as FGF receptor signaling. Downstream signaling proteins include the FGF receptor associated factor FRS2, as well as N-ras, c-raf, Mek and ERK1/2, leading to activation of transcription factors such as AP-1, Srf, Nrf2, and Egr-1 and expression of genes associated with cell growth and metabolism. A second pathway identified as Pi-responsive includes Akt, ERK1/2, Mnk ultimately resulting in the regulation of eIF4E-BP1, a key component of the protein translation machinery. A third Pi-stimulated and required cellular event is changes in energy metabolism including an increase in oxidative phosphorylation. Stimulation of these pathways have been identified in varying cell types in response to elevated extracellular Pi. Inhibitor studies suggest that the three pathways are required for different aspects of the Pi-induced alterations in cell functions related to altered gene expression, increased proliferation and metabolism, and angiogenesis.
2.4. Pi-induced gene expression correlates with changes in cell behavior
The molecular mechanisms underlying Pi-induced increased proliferation and transformation are also currently being investigated. Studies identifying specific genes responsive to elevated extracellular Pi support the idea that changes in extracellular Pi could directly and actively alter cell behavior. Many of the original studies on Pi-induced changes in cell function focused on calcifying cells such as osteoblasts and vascular smooth muscle cells and found the secreted factor osteopontin (OPN) as the first identified Pi-responsive gene [70, 112, 117]. Subsequent studies, confirmed and identified elevated extracellular Pi as altering gene expression in various cell types, including: osteoblasts and cementoblasts [63, 86, 118], keratinocytes [67], vascular smooth muscle [70, 119] and bronchial epithelial cells [68] among many others. To date, hundreds of genes have been identified that are temporally upregulated or downregulated by varying extracellular Pi levels. Consistent across multiple diverse cell types, many of the same genes have been identified as Pi-responsive, suggesting conserved regulatory networks. The functions of these Pi-responsive genes/proteins generally fall into two categories, regulation of calcification or cell proliferation/cancer [98]. Examples of Pi-responsive genes/proteins tightly linked to cell proliferation and cancer progression include c-fos (Fos), Egr1 (Ngfi-A, Krox24), Cyclin D1 (Ccnd1), Nrf2 (Nfe2l2) and Osteopontin (Spp1), Cox2 (Ptgs2), Fra1 (Fosl1), among many others [86, 98, 120, 121] (Fig.2). Based on the identification of these specific genes a number of transcriptional regulators of the Pi-response have been identified, including the activator protein 1 (AP-1) dimers and Egr-1 which have been demonstrated to respond to changes in extracellular Pi with rapid post-translational modifications, DNA binding, and transcriptional activation [67, 86, 122] (Fig.2). AP-1 is known to regulate genes associated with proliferation, differentiation, and apoptosis, and inhibiting activation of AP-1 is considered a therapeutic target for cancer prevention [123]. Although the accompanying changes in endocrine factors complicates the understanding of the impact of serum Pi levels on gene expression in vivo, studies have found increased Pi-responsive genes in response to high Pi diets in various tissues [8, 124–126] supporting direct Pi-responsive gene expression at least in mice.
2.5. Elevated Pi stimulates OPN in vitro and in vivo
One of the most robust and commonly identified Pi-responsive genes is osteopontin (OPN; Spp1, 2ar, eta-1) [117], a circulating cytokine-like factor. Cell culture studies have determined that elevated Pi strongly stimulated OPN expression from multiple cell types through specific signaling pathways [59, 63, 70, 86, 98, 112, 113, 117, 118, 121, 127–130] and a high Pi diet has been demonstrated to increase circulating OPN levels in both mice and humans [8, 12, 67]. OPN is a particularly interesting Pi-responsive gene as it has been linked to neoplastic transformation [131] and overexpression of OPN has been intimately associated with cancer progression and metastasis arising from many tissue types (reviewed in [132, 133]). OPN can influence changes in cell behavior such as proliferation, cell survival, promotion of angiogenesis and can acts as an immune-modulatory factor [134–137]. OPN modulates cell function by acting as an endocrine, paracrine, or autocrine cytokine (reviewed in [136, 138]) through its ability to bind multiple cell surface receptors, such as CD44 and multiple β-integrins pairs [136, 139]. At present, a clear mechanistic role for elevated OPN in the context of elevated Pi has not been established, although protection against calcification has been suggested as at least one function [140]. Due to the diverse functions of OPN, elucidating how Pi-induced OPN influences disease progression will aid in determining if OPN represents a therapeutic target to impinge on Pi-induced changes in cell and tissue behavior.
3: Role of Pi consumption in detecting and modulating cancer initiation and/or progression
Malignancy is a multistage, complex process often involving both genetic and environmental factors [141, 142]. The cell-based studies described above suggest that Pi availability might represent an environmental factor that acts to promote tumorigenesis. Nutritional intervention represents a cancer prevention opportunity that can be easily manipulated; however, insufficient information currently prevents investigators from capitalizing on this intriguing opportunity [143]. The possibility that altering the level of a common dietary element might alter tumor formation and/or progression would represent a significant opportunity to intervene in potentially numerous cancers [144]. Although limited, some evidence from both mice and humans supports the notion that Pi represents a dietary and serum factor that might influence malignancy [67, 145–147] and therefore a factor that might be modulated for therapeutic benefit.
3.1. Dietary and serum Pi and human cancer
To date, information on the possible relationship between consumption and serum Pi availability and cancer in humans is limited. A recent twenty-four year follow-up of the Health Professionals Study used Cox proportional hazards modeling to assess the association between dietary Pi and calcium intake and prostate cancer [146]. The study identified a positive correlation between Pi intake and increased risk of poorly differentiated and clinically advanced prostate cancer, independent of calcium intake [146]. Additionally, the association between serum Pi and risk of cancer was analyzed in a population-based observational assessment of serum collected under mostly fasting conditions from the Swedish Apolipoprotein Mortality Risk (AMORIS) study [147]. Multivariate Cox proportional hazard regression analyses found no overall correlation between serum Pi with cancer risk, however, a statistical correlation was identified with cancer from specific tissues [147]. Increasing serum Pi quartiles in men correlated with increased risk of pancreatic, lung, thyroid, bone, and “other” cancers with an association with liver and gallbladder cancers only in the highest quartile. In women, high serum Pi correlated with increased risk of esophageal, lung, and non-melanoma skin cancers with an association with stomach and bone cancers only in the highest quartile [147]. While only associative these studies are some of the first to provide a link between dietary Pi intake and serum Pi levels with cancer in humans. Future studies focusing specifically on Pi consumption are needed to further understand the role of Pi in human cancer initiation and progression, and to design potential interventional strategies aimed at limiting Pi intake in at-risk populations.
3.2. Cellular and serum Pi levels as a diagnostic and prognostic marker
Almost a century ago phosphorus, in the form of the isotope 32P, was investigated as a diagnostic marker for cancer as tumors exhibited increased 32P uptake relative to healthy tissue [148–150]. In addition to cellular uptake, increased serum Pi levels have been investigated for the potential to be a negative prognostic cancer marker. One study found that serum Pi and calcium levels were significantly elevated in 50 patients with disseminated breast cancer, before endocrine treatment, and without change in renal clearance of tubular reabsorption of calcium or of Pi [151]. More recently, an investigation of whether baseline Pi serum levels were prognostic in terms of stage and overall patient survival in newly diagnosed non-small cell and small cell lung cancer patients (130 patients) found that pre-treatment Pi serum levels were elevated outside of the normal range, additionally, serum Pi levels were predictive of disease stage, with a significant impact on patient survival [152]. A cohort of 110 patients with multiple myeloma, presented with serum Pi levels that were evaluated and a multivariate analysis (Cox’s proportional hazards regression model) showed that the serum Pi level was a significant negative prognostic factor in this patient population [153]. Finally, a retrospective analysis of 1241 colorectal cancer patients was assessed for an association of postoperative hyperphosphatemia with overall patient survival and suggested that serum Pi levels represent a negative prognostic marker of colorectal cancer patients after surgery [154]. Although correlative, these results support a link between the cancerous state and increased cellular Pi consumption and serum availability in vivo.
3.3. Influence of dietary Pi consumption on cancer initiation and progression in preclinical mouse models
A direct association between dietary Pi consumption and cancer has been provided by two different mouse studies. The two-stage skin carcinogenesis model induces papilloma (tumor) formation through chemical carcinogen treatment of the skin of mice [155]. This cancer model was used in combination with isocaloric diets that contained high Pi (1.2%) or low Pi (0.2%) levels, with calcium kept constant at 0.6%. While mouse weight remained stable, the mice on the high Pi diet exhibited a corresponding significant increase in serum Pi and decrease in serum calcium [67]. Mice on the high Pi diet developed twice as many papillomas as mice fed the low Pi diet, and these papillomas developed at an earlier time point (initiation) and were initially larger (progression) [67]. High Pi diet mice also had significantly elevated serum PTH and OPN levels suggesting an additional mechanism by which Pi consumption might influence tumorigenesis. A second pre-clinical cancer model that represents an example of oncogene driven tumorigenesis is the KrasLA1 model of spontaneous lung cancer [156]. KrasLA1 mice were fed a diet with normal Pi levels (0.5%) or high Pi diet (1.0%) for 4 weeks [145] resulting in a corresponding increase in serum Pi in the high Pi diet fed mice. Pathohistological analyses of the lungs revealed a significant increase in the total number of tumors including both small (<1.5mm diameter) and large tumors (>1.5mm) as well as proliferation index (as indicated by PCNA staining). These data suggest that the high Pi diet influenced both tumor initiation and tumor growth and progression [145]. Interestingly, the same KrasLA1 mouse model was used with a very low Pi diet (0.1%) and identified an increase in lung tumor number relative to the control Pi diet (0.5%) [157]. The results suggest the possibility of a U-shaped safety curve regarding Pi intake versus risk of tumorigenesis in the presence of an activating oncogenic mutation.
4: How tumor cells might increase systemic Pi availability: novel prevention strategies?
Throughout this review, we present the hypothesis that due to the importance and necessity of Pi for many critical cell functions, a rapidly growing tumor mass will require additional nutrients, with Pi being one of the most important. This hypothesis is supported by a recent study in which electron paramagnetic resonance was used to non-invasively assess the chemical nature of the tumor microenvironment in vivo [75]. The most dramatic differences in tumors vs. non-malignant tissues, were observed with interstitial Pi levels, more so than hypoxic or acidic regions. Interstitial Pi represented the only parameter in this study that also allowed for discrimination between non-metastatic and highly metastatic tumors [75]. Another recent study found Pi levels three times greater in the cyst fluid from malignant brain tumors than in cerebrospinal fluid [158]. So, if cancerous cells require additional Pi how can a rapidly expanding tumor cell population obtain additional Pi and what are the potential avenues of therapeutic intervention? We propose four distinct, although not mutually exclusive, means by which a cancerous cell and a growing tumor mass could obtain additional Pi (Fig.3): 1) indirectly, through increased systemic levels, most likely from diet, 2) actively, by the generation of free Pi in the microenvironment and/or autocrine/paracrine stimulated transport, 3) directly, by cell autonomous transport mediated by increased transporter membrane abundance, or 4) actively, by the generation of increased blood flow to supply nutrients such as Pi.
Figure 3: Possible mechanism by which a growing tumor might acquire Pi.
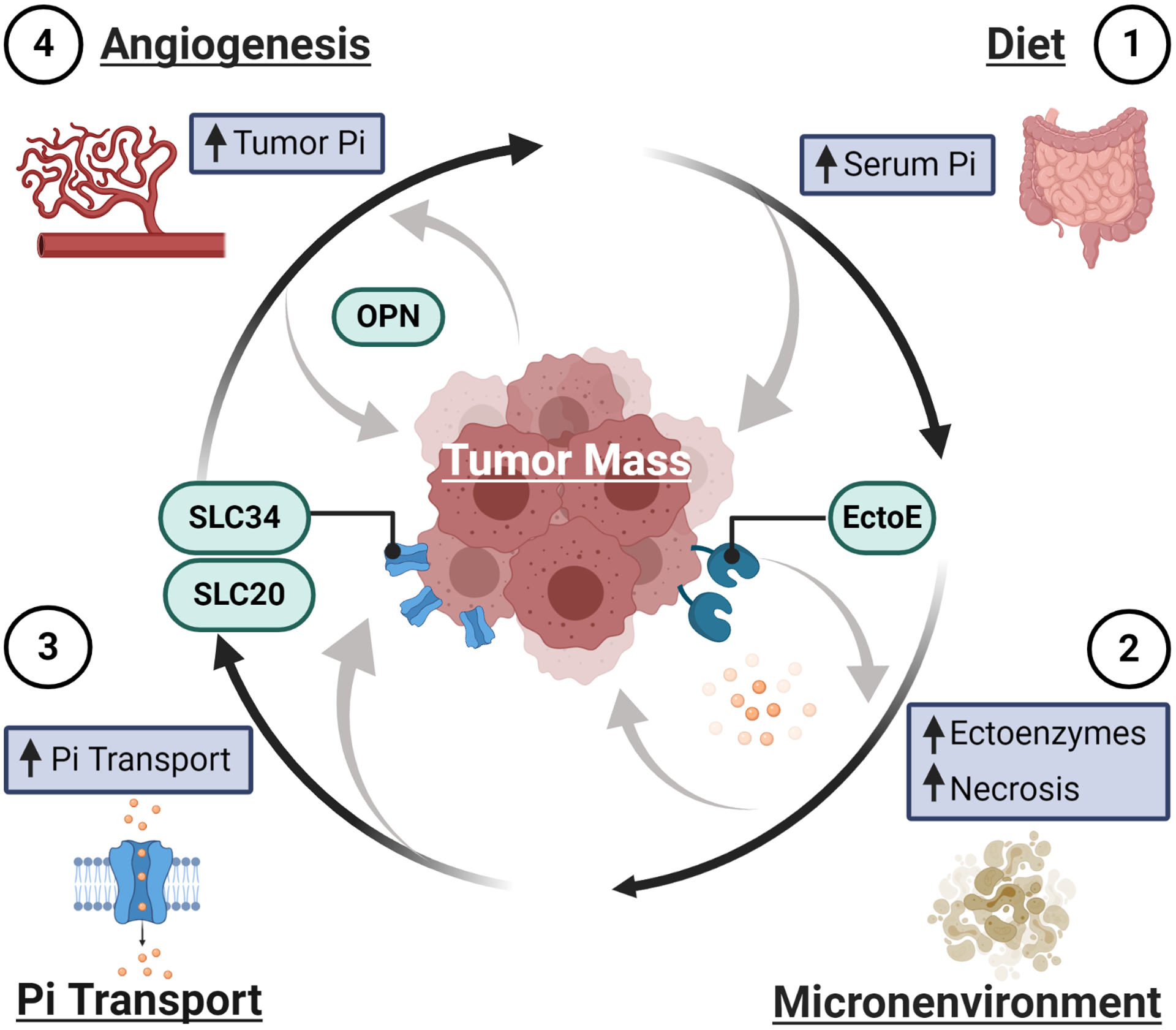
1) Pi might be acquired through increased serum Pi levels subsequent to a high Pi meal or sustained high Pi diet. 2) Pi from the tumor microenvironment could be obtained through the actions of ectoenzymes such as alkaline phosphatase, to generate an increase in local Pi, and through the necrosis of surrounding tissue and intratumoral cells which would release cellular contents including substantial Pi. 3) Increased transport of Pi into the cell could be accomplished by an increase in Pi-transporter abundance or increased transport kinetics stimulated by autocrine/paracrine factors. 4) The growing tumor might acquire additional sources of Pi through increased blood flow by neovascularization/angiogenesis.
4.1. Dietary Pi consumption and absorption: a prevention or therapeutic opportunity
The results from preclinical mouse studies suggest the intriguing possibility that manipulating Pi intake might represent a novel strategy to either reduce the likelihood of cancer initiation or slowing tumor progression as a growing tumor might acquire needed Pi through elevated serum levels (Fig.3). As noted above, many Western-style diets are high in Pi which could lead to a transient increase in serum Pi or a long-term sustained increase that could be utilized by an initiated cell or growing tumor. The current dietary recommended allowance for Pi is 700mg/d; the typical American adult consumes 1200–1500 mg/d with the 90th percentile at ~2500 mg/d [3, 39, 159]. These calculations are likely underestimated [160] as nutrient composition databases do not fully account for Pi as a food additive [3, 161]. In fact, a diet high in processed foods, such as convenience and fast foods, has been estimated to more than double Pi daily intake [162]. Compounding the high overall daily intake, Pi from highly processed foods is often in the form of inorganic salts, which are more efficiently absorbed than naturally occurring phosphates that can require enzymatic digestion (80–100% vs. 40–60%, respectively). Importantly, these diets often do not have the corresponding increase in calcium (Ca) which is observed with a diet high in dairy for example. In addition to managing Pi intake through dietary considerations, Pi absorption might also be therapeutically targeted. Pi transport occurs in the small intestine through transcellular transport, which is mainly handled by the Slc34a2 transporter [163]. Importantly, paracellular transport also contributes significantly to absorption [164]; therefore, attempts to specifically target individual Pi-transporters in the digestive tract may prove difficult. However, Pi-binders, such as sevelamer and lanthanum carbonate are currently being clinically used to reduce absorption and lower serum Pi levels in chronic kidney disease patients [165] and two investigational drugs, Tenapanor, which reduces Pi absorption by inhibiting the intestinal sodium/hydrogen exchanger 3 [166], and EOS789, a pan-Pi transport inhibitor [167], could represent a novel approach to lower Pi serum levels in the context of cancer prevention or treatment.
4.2. Generation of free Pi in the microenvironment
In addition to acquiring Pi from the circulation, we speculate that tumor cells might also evolve the means to actively generate free Pi in the microenvironment. (Fig.3). Two possibilities include: i) an increase in levels of a Pi generating ectoenzyme, and ii) the necrosis of intratumoral or surrounding cells and subsequent release of intracellular Pi. Circulating levels of the ectoenzyme ALP, both TNAP and placental, have been studied and utilized as prognostic markers that correlate with various cancers and possibly relate to bone metastasis [76, 77]. In addition to serum levels, ALP has also been found expressed by tumor cells of various origins [78, 79] suggestive of an active generation, however, functional evidence for these enzymes in cancer initiation or progression has not been reported. A recent review of the literature has also linked the increased expression of ecto-nucleotidases and ecto-phosphatases to the possible generation of increased free Pi in the tumor microenvironment [168]. Another possible source of Pi for a growing tumor mass could be the adjacent non-malignant tissue or cells within the growing tumor mass. Cells contain a substantial amount of Pi and during necrosis or tumor lysis [169–171], this Pi would be released into the local microenvironment producing a substantial pool to be utilized by a growing tumor. Currently, these mechanisms for tumor Pi acquisition are mostly speculative and therefore the therapeutic value of targeting Pi in the tumor microenvironment remains essentially unknown.
4.3. Increased expression/abundance of Pi transporters; Slc34a2 and Slc20a1
To date, two particular Pi-transporters have been linked to proliferation and cancerous cells: Slc34a2 and Slc20a1. The type-II co-transporter, Slc34a2 (NAPI-IIb, NaPi-3b, NPTIIb) has a more limited tissue expression profile than other Pi-transporters, and RNA/protein is expressed at high levels mainly in lung, seminal vesicles, and female genital tract. Overexpression of Slc34a2 has been linked to cancer in humans in ovarian cancer [172, 173], papillary thyroid cancers [174] and breast cancer samples [175]. Slc34a2 has also recently been demonstrated as necessary for tumor cell growth from various cancers [176–181]. Knockdown of Slc34a2 in the lungs of KrasLA1 mice resulted in suppressed lung cancer growth, and decreased cancer cell proliferation and angiogenesis while increasing apoptosis [182]. Increased expression of RNA and protein levels of Slc34a2 have been identified in the lungs of mice consuming a high Pi diet [116, 145], as well as increased expression in human lung cancer cells exposed to elevated Pi in vitro [68]. Likewise, functional studies have identified the requirement specifically of Slc20a1 (Pit-1, Glvr-1) for Pi-induced changes in cell behavior including proliferation, transformation and tumor growth both in cell culture and a xenograft mouse model [113, 183–187]. Interestingly, some studies suggest that the effect on cell proliferation is independent from its transport function. Gene expression profiling studies in human cancer cells have identified Slc20a1 as more highly expressed in a number of tumor types [188–190] and high levels were associated with poor 10-year survival rate in estrogen receptor-positive breast cancer [191]. Taken together, growing evidence does support an association between increased abundance of Pi-transporters and tumorigenesis (Fig.3) which generates the question; can Pi transport at the cell membrane level be therapeutically targeted. At least one broad Pi-transport inhibitor has been identified that is used clinically as an antiviral, the pyrophosphate analog Foscavir (Foscarnet, phosphonoformic acid). However, it is a relatively weak inhibitor, at least for intestinal Pi absorption and requires levels that could cause nephrotoxicity. A Slc34a2 inhibitor, ASP3325 was recently reported to provide no reduction in serum Pi when given orally with the goal of reduced Pi absorption [192] although targeting Pi transport in a tumor might represent a different scenario. Collectively, these results identify at least two specific Pi-transporters as possible cancer therapeutic targets although challenges of off-target effects particularly on the intestine and kidney, if given systemically would need to be addressed and overcome.
4.4. Effects of Pi on angiogenesis
A primary mechanism by which rapidly expanding tumors acquire additional nutrients and oxygen is through a new or expanded blood supply which can be achieved by neovascularization and/or angiogenesis. This might represent an additional means whereby a tumor could obtain additional Pi. To better understand the mechanism(s) by which a high Pi environment might alter cancer progression, a recent study utilized lung and breast cancer cell lines in combination with the human umbilical vascular endothelial cell (HUVEC) vessel formation model. Exposure of cancer cells to elevated Pi stimulated expression of the transcription factor FOXC2 and OPN, and conditioned medium from the Pi-stimulated cancer cells stimulated migration and tube formation in the HUVEC model. Mechanistically, the requirement of FOXC2 for Pi-induced OPN expression and secretion from cancer cells was identified as necessary for the angiogenic response [121]. These results identify an additional mechanism by which Pi availability and Pi-responsive factors might influence the process of cancer progression and increase the Pi supply to a growing tumor (Fig.3). In regard to therapeutically inhibiting this process, OPN is being investigated for targeting with antibody neutralization strategies with limited success [193] possibly because the high circulating levels and whether OPN could be targeted specifically in the tumor microenvironment remains to be determined.
5: Conclusions and Future Directions
Here we have presented evidence supporting Pi consumption, both at the systemic and cellular level, as a possible driver of various stages of malignancy and as such a novel therapeutic target for the possible prevention, control and/or treatment of cancer. Although speculative at times, we have also described various mechanisms by which tumors might acquire Pi but also how these processes might be therapeutically targeted including; 1) reduced dietary Pi intake, 2) inhibition of Pi absorption in the intestine, 3) inhibition of Pi generation in the tumor microenvironment, 4) targeting Pi transporters at the tumor cell surface, and 5) neutralization of Pi-induced tumor secreted paracrine factors that generate new sources of Pi, such as OPN (Fig.3). Although these strategies present therapeutic challenges, the novel idea that Pi acts as an important systemic signaling molecule capable of altering cell behavior through the regulation of signaling pathways and gene expression provides a unique and potentially powerful target for the development of cancer prevention and therapeutic interventional strategies. Therefore, even with the challenges described above, defining how Pi availability through either dietary consumption or generation within the microenvironment influences cell metabolism, systemic energy metabolism, and cell behavior will provide valuable future information about long-term health and disease initiation.
Many key issues and challenges still need to be addressed regarding a possible role of Pi in tumorigenesis and cancer progression, and in defining its value as a therapeutic target. 1) There is substantial and growing evidence from cell-based studies suggesting that increased Pi availability alters cell behavior towards increased proliferation and a more tumorigenic phenotype; however, whether this translates to the in vivo environment has not been fully established. This may be complicated by the complexities of nutrition in which the presence or absence of other nutrients might significantly alter the cellular or systemic response to Pi, a challenge associated with most diet and cancer studies [194]. Further, cancer often requires decades to develop. Therefore, maintaining a specific diet in humans for extended periods of time is both challenging and difficult to correlate with a lifestyle choice that might change with age or geographic location. 2) A challenge with targeting cellular Pi consumption is associated with defining an appropriate therapeutic window. Can cellular Pi metabolism be therapeutically targeted without disrupting the Pi metabolism of healthy cells? If cells require additional Pi, is that requirement at a level that is sufficiently different from baseline needs to generate a therapeutic window of opportunity? Pi is necessary for certain cell functions and therefore targeting Pi therapeutically produces a number of obstacles mainly surrounding possible off-target effects on healthy cells as well as Pi homeostasis regulated by Pi-transporters in the intestine and kidney. Although we have focused mostly on excess Pi in this review, it should be noted that a low Pi environment might also generate a more cancerous phenotype by putting selective survival pressure on cells to seek Pi, although this has yet to be investigated. 3) Finally, one challenge associated with correlating mouse and human data is that most serum from mice is collected from ad lib feeding; whereas, the vast majority of human serum is collected under fasting conditions. Do these different conditions affect serum Pi levels in the context of normal renal function, or levels of Pi-responsive circulating factors?
The effects of high Pi consumption on health and disease, in the context of normal renal function, have only begun to be investigated. A comprehensive understanding of the common dietary element Pi in regulating cell behavior both in the healthy state and in response to challenges represents multiple significant interventional opportunities to alter the initiation, promotion, and/or progression of various age-related diseases, such as osteoporosis, cardiovascular disease and cancer. If a low Pi diet is to be used, future studies will be required to determine the lower limits for adult human consumption before negative health consequences become a complication. In the coming age of personalized medicine, a more complete understanding of how individual dietary components influence cells and tissues will greatly improve our ability to manipulate diet for health benefits as well as enhance existing therapies. The idea that dietary Pi could be manipulated for health benefits represents an attractive future novel strategy for influencing disease initiation and progression.
Acknowledgments
This effort was supported in part by the VA Office of Research and Development Biomedical Laboratory Research & Development Service Award (I01BX001516), and grants from the Emory University Research Committee grant (00067461), Winship Cancer Institute Invest grant (98439) and the NIH (R21AR073593). The content of this manuscript is solely the responsibility of the authors and does not represent the views of the Department of Veterans Affairs, National Institutes of Health, or the United States Government. Figures were created with Biorender.com.
Footnotes
CRediT authorship contribution statement
Jamie Arnst: Investigation, Writing – Editing, Visualization. George R. Beck Jr.: Conceptualization, Investigation, Writing - original draft, Visualization.
Disclosure statement
The authors declare no competing financial interests.
References:
- [1].Lee R, Weber TJ, Disorders of phosphorus homeostasis, Curr Opin Endocrinol Diabetes Obes 17(6) (2010) 561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Calvo MS, Moshfegh AJ, Tucker KL, Assessing the health impact of phosphorus in the food supply: issues and considerations, Adv Nutr 5(1) (2014) 104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Calvo MS, Dietary phosphorus, calcium metabolism and bone, J Nutr 123(9) (1993) 1627–33. [DOI] [PubMed] [Google Scholar]
- [4].Kemi VE, Karkkainen MU, Karp HJ, Laitinen KA, Lamberg-Allardt CJ, Increased calcium intake does not completely counteract the effects of increased phosphorus intake on bone: an acute dose-response study in healthy females, Br J Nutr 99(4) (2008) 832–9. [DOI] [PubMed] [Google Scholar]
- [5].Huttunen MM, Pietila PE, Viljakainen HT, Lamberg-Allardt CJ, Prolonged increase in dietary phosphate intake alters bone mineralization in adult male rats, J Nutr Biochem 17(7) (2006) 479–84. [DOI] [PubMed] [Google Scholar]
- [6].Huttunen MM, Tillman I, Viljakainen HT, Tuukkanen J, Peng Z, Pekkinen M, Lamberg-Allardt CJ, High dietary phosphate intake reduces bone strength in the growing rat skeleton, J Bone Miner Res 22(1) (2007) 83–92. [DOI] [PubMed] [Google Scholar]
- [7].Draper HH, Sie TL, Bergan JG, Osteoporosis in aging rats induced by high phosphorus diets, J Nutr 102(9) (1972) 1133–41. [DOI] [PubMed] [Google Scholar]
- [8].Gutierrez OM, Luzuriaga-McPherson A, Lin Y, Gilbert LC, Ha SW, Beck GR Jr., Impact of Phosphorus-Based Food Additives on Bone and Mineral Metabolism, J Clin Endocrinol Metab 100(11) (2015) 4264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Giachelli CM, The emerging role of phosphate in vascular calcification, Kidney Int 75(9) (2009) 890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Onufrak SJ, Bellasi A, Shaw LJ, Herzog CA, Cardarelli F, Wilson PW, Vaccarino V, Raggi P, Phosphorus levels are associated with subclinical atherosclerosis in the general population, Atherosclerosis 199(2) (2008) 424–31. [DOI] [PubMed] [Google Scholar]
- [11].Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G, Relation between serum phosphate level and cardiovascular event rate in people with coronary disease, Circulation 112(17) (2005) 2627–33. [DOI] [PubMed] [Google Scholar]
- [12].Gutierrez OM, Porter AK, Viggeswarapu M, Roberts JL, Beck GR Jr., Effects of phosphorus and calcium to phosphorus consumption ratio on mineral metabolism and cardiometabolic health, J Nutr Biochem 80 (2020) 108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ohnishi M, Razzaque MS, Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging, Faseb Journal 24(9) (2010) 3562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T, Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism, J Clin Invest 113(4) (2004) 561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Day HG, McCollum EV, Mineral metabolism, growth, and symptomatology of rats on a diet extremely deficient in phosphorus, Journal of Biological Chemistry 130(1) (1939) 269–283. [Google Scholar]
- [16].Baylink D, Wergedal J, Stauffer M, Formation, mineralization, and resorption of bone in hypophosphatemic rats, J Clin Invest 50(12) (1971) 2519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kuro-o M, A potential link between phosphate and aging--lessons from Klotho-deficient mice, Mech Ageing Dev 131(4) (2010) 270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Izquierdo MC, Perez-Gomez MV, Sanchez-Nino MD, Sanz AB, Ruiz-Andres O, Poveda J, Moreno JA, Egido J, Ortiz A, Klotho, phosphate and inflammation/ageing in chronic kidney disease, Nephrol Dial Transplant 27 Suppl 4 (2012) iv6–10. [DOI] [PubMed] [Google Scholar]
- [19].Favus M, Bushinsky D, Lemann J, Chapter 13. Regulation of Calcium, Magnesium, and Phosphate Metabolism, Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism (2006). [Google Scholar]
- [20].Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, Sixth Edition ed., Elsevier, St. Louis, Missouri: 2018. [Google Scholar]
- [21].Huang CL, Moe OW, Clinical assessment of phosphorus status, balance and renal handling in normal individuals and in patients with chronic kidney disease, Curr Opin Nephrol Hypertens 22(4) (2013) 452–8. [DOI] [PubMed] [Google Scholar]
- [22].Markowitz M, Rotkin L, Rosen JF, Circadian rhythms of blood minerals in humans, Science 213(4508) (1981) 672–4. [DOI] [PubMed] [Google Scholar]
- [23].Gomez P, Coca C, Vargas C, Acebillo J, Martinez A, Normal reference-intervals for 20 biochemical variables in healthy infants, children, and adolescents, Clin Chem 30(3) (1984) 407–12. [PubMed] [Google Scholar]
- [24].Perry HM 3rd, Province MA, Droke DM, Kim GS, Shaheb S, Avioli LV, Diurnal variation of serum calcium and phosphorus in postmenopausal women, Calcif Tissue Int 38(2) (1986) 115–8. [DOI] [PubMed] [Google Scholar]
- [25].Greenberg BG, Winters RW, Graham JB, The normal range of serum inorganic phosphorus and its utility as a discriminant in the diagnosis of congenital hypophosphatemia, J Clin Endocrinol Metab 20 (1960) 364–79. [DOI] [PubMed] [Google Scholar]
- [26].Zhang D, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K, Effects of sex and postmenopausal estrogen use on serum phosphorus levels: a cross-sectional study of the National Health and Nutrition Examination Survey (NHANES) 2003–2006, Am J Kidney Dis 63(2) (2014) 198–205. [DOI] [PubMed] [Google Scholar]
- [27].Aitken JM, Gallagher MJ, Hart DM, Newton DA, Craig A, Plasma growth hormone and serum phosphorus concentrations in relation to the menopause and to oestrogen therapy, J Endocrinol 59(3) (1973) 593–8. [DOI] [PubMed] [Google Scholar]
- [28].Keating FR Jr., Jones JD, Elveback LR, Randall RV, The relation of age and sex to distribution of values in healthy adults of serum calcium, inorganic phosphorus, magnesium, alkaline phosphatase, total proteins, albumin, and blood urea, J Lab Clin Med 73(5) (1969) 825–34. [PubMed] [Google Scholar]
- [29].Body JJ, Cryer PE, Offord KP, Heath H 3rd, Epinephrine is a hypophosphatemic hormone in man. Physiological effects of circulating epinephrine on plasma calcium, magnesium, phosphorus, parathyroid hormone, and calcitonin, J Clin Invest 71(3) (1983) 572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vervloet MG, van Ballegooijen AJ, Prevention and treatment of hyperphosphatemia in chronic kidney disease, Kidney Int 93(5) (2018) 1060–1072. [DOI] [PubMed] [Google Scholar]
- [31].Koumakis E, Cormier C, Roux C, Briot K, The Causes of Hypo- and Hyperphosphatemia in Humans, Calcif Tissue Int (2020). [DOI] [PubMed] [Google Scholar]
- [32].Manghat P, Sodi R, Swaminathan R, Phosphate homeostasis and disorders, Ann Clin Biochem 51(Pt 6) (2014) 631–56. [DOI] [PubMed] [Google Scholar]
- [33].Clinkenbeard EL, White KE, Heritable and acquired disorders of phosphate metabolism: Etiologies involving FGF23 and current therapeutics, Bone 102 (2017) 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Root AW, Genetic disorders of calcium, phosphorus, and bone homeostasis, Translational Science of Rare Diseases 3 (2018) 1–36. [Google Scholar]
- [35].Berndt T, Kumar R, Phosphatonins and the regulation of phosphate homeostasis, Annu Rev Physiol 69 (2007) 341–59. [DOI] [PubMed] [Google Scholar]
- [36].Takeda E, Taketani Y, Morita K, Tatsumi S, Katai K, Nii T, Yamamoto H, Miyamoto K, Molecular mechanisms of mammalian inorganic phosphate homeostasis, Adv Enzyme Regul 40 (2000) 285–302. [DOI] [PubMed] [Google Scholar]
- [37].Tenenhouse HS, Regulation of phosphorus homeostasis by the type iia na/phosphate cotransporter, Annu Rev Nutr 25 (2005) 197–214. [DOI] [PubMed] [Google Scholar]
- [38].Sherman RA, Mehta O, Phosphorus and potassium content of enhanced meat and poultry products: implications for patients who receive dialysis, Clin J Am Soc Nephrol 4(8) (2009) 1370–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Calvo MS, Park YK, Changing phosphorus content of the U.S. diet: potential for adverse effects on bone, J Nutr 126(4 Suppl) (1996) 1168S–80S. [DOI] [PubMed] [Google Scholar]
- [40].I. Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference, The National Academies Collection: Reports funded by National Institutes of Health, Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride, National Academies Press (US) Copyright © 1997, National Academy of Sciences., Washington (DC), 1997. [Google Scholar]
- [41].Portale AA, Halloran BP, Morris RC Jr., Physiologic regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men, J Clin Invest 83(5) (1989) 1494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Portale AA, Halloran BP, Murphy MM, Morris RC Jr., Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in humans, J Clin Invest 77(1) (1986) 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nishida Y, Taketani Y, Yamanaka-Okumura H, Imamura F, Taniguchi A, Sato T, Shuto E, Nashiki K, Arai H, Yamamoto H, Takeda E, Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men, Kidney Int 70(12) (2006) 2141–7. [DOI] [PubMed] [Google Scholar]
- [44].Blumsohn A, What have we learnt about the regulation of phosphate metabolism?, Curr Opin Nephrol Hypertens 13(4) (2004) 397–401. [DOI] [PubMed] [Google Scholar]
- [45].White KE, Larsson TE, Econs MJ, The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis: frizzled related protein-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23, Endocr Rev 27(3) (2006) 221–41. [DOI] [PubMed] [Google Scholar]
- [46].Rowe PS, The wrickkened pathways of FGF23, MEPE and PHEX, Crit Rev Oral Biol Med 15(5) (2004) 264–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA, Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice, Endocrinology 146(12) (2005) 5358–64. [DOI] [PubMed] [Google Scholar]
- [48].Prie D, Beck L, Urena P, Friedlander G, Recent findings in phosphate homeostasis, Curr Opin Nephrol Hypertens 14(4) (2005) 318–24. [DOI] [PubMed] [Google Scholar]
- [49].Arking DE, Krebsova A, Macek M Sr., Macek M Jr., Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I, Dietz HC, Association of human aging with a functional variant of klotho, Proc Natl Acad Sci U S A 99(2) (2002) 856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI, Mutation of the mouse klotho gene leads to a syndrome resembling ageing, Nature 390(6655) (1997) 45–51. [DOI] [PubMed] [Google Scholar]
- [51].Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M, Suppression of aging in mice by the hormone Klotho, Science 309(5742) (2005) 1829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Morishita K, Shirai A, Kubota M, Katakura Y, Nabeshima Y, Takeshige K, Kamiya T, The progression of aging in klotho mutant mice can be modified by dietary phosphorus and zinc, J Nutr 131(12) (2001) 3182–8. [DOI] [PubMed] [Google Scholar]
- [53].Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y, Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system, Mol Endocrinol 17(12) (2003) 2393–403. [DOI] [PubMed] [Google Scholar]
- [54].Baker SB, Worthley LI, The essentials of calcium, magnesium and phosphate metabolism: part I. Physiology, Crit Care Resusc 4(4) (2002) 301–6. [PubMed] [Google Scholar]
- [55].Biber J, Hernando N, Forster I, Phosphate transporters and their function, Annu Rev Physiol 75 (2013) 535–50. [DOI] [PubMed] [Google Scholar]
- [56].Tenenhouse HS, Phosphate transport: molecular basis, regulation and pathophysiology, J Steroid Biochem Mol Biol 103(3–5) (2007) 572–7. [DOI] [PubMed] [Google Scholar]
- [57].Lundquist P, Murer H, Biber J, Type II Na+-Pi cotransporters in osteoblast mineral formation: regulation by inorganic phosphate, Cell Physiol Biochem 19(1–4) (2007) 43–56. [DOI] [PubMed] [Google Scholar]
- [58].Collins JF, Bai L, Ghishan FK, The SLC20 family of proteins: dual functions as sodium-phosphate cotransporters and viral receptors, Pflugers Arch 447(5) (2004) 647–52. [DOI] [PubMed] [Google Scholar]
- [59].Beck GR Jr., Inorganic phosphate as a signaling molecule in osteoblast differentiation, J Cell Biochem 90(2) (2003) 234–43. [DOI] [PubMed] [Google Scholar]
- [60].Mansfield K, Teixeira CC, Adams CS, Shapiro IM, Phosphate ions mediate chondrocyte apoptosis through a plasma membrane transporter mechanism, Bone 28(1) (2001) 1–8. [DOI] [PubMed] [Google Scholar]
- [61].Fujita T, Meguro T, Izumo N, Yasutomi C, Fukuyama R, Nakamuta H, Koida M, Phosphate stimulates differentiation and mineralization of the chondroprogenitor clone ATDC5, Jpn J Pharmacol 85(3) (2001) 278–81. [DOI] [PubMed] [Google Scholar]
- [62].Julien M, Magne D, Masson M, Rolli-Derkinderen M, Chassande O, Cario-Toumaniantz C, Cherel Y, Weiss P, Guicheux J, Phosphate stimulates matrix Gla protein expression in chondrocytes through the extracellular signal regulated kinase signaling pathway, Endocrinology 148(2) (2007) 530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Foster BL, Nociti FH Jr., Swanson EC, Matsa-Dunn D, Berry JE, Cupp CJ, Zhang P, Somerman MJ, Regulation of cementoblast gene expression by inorganic phosphate in vitro, Calcif Tissue Int 78(2) (2006) 103–12. [DOI] [PubMed] [Google Scholar]
- [64].Kanatani M, Sugimoto T, Kano J, Kanzawa M, Chihara K, Effect of high phosphate concentration on osteoclast differentiation as well as bone-resorbing activity, J Cell Physiol 196(1) (2003) 180–9. [DOI] [PubMed] [Google Scholar]
- [65].Yates AJ, Oreffo RO, Mayor K, Mundy GR, Inhibition of bone resorption by inorganic phosphate is mediated by both reduced osteoclast formation and decreased activity of mature osteoclasts, J Bone Miner Res 6(5) (1991) 473–8. [DOI] [PubMed] [Google Scholar]
- [66].Mozar A, Haren N, Chasseraud M, Louvet L, Maziere C, Wattel A, Mentaverri R, Morliere P, Kamel S, Brazier M, Maziere JC, Massy ZA, Phosphate inhibits RANKL induce NF-kappa B activation during the differentiation of monocytes-macrophages to osteoclast-like cells: Possible role in CKD-MBD, Nephrology Dialysis Transplantation 22 (2007) 139–140. [Google Scholar]
- [67].Camalier CE, Young MR, Bobe G, Perella CM, Colburn NH, Beck GR Jr., Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis, Cancer Prev Res (Phila) 3(3) (2010) 359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Chang SH, Yu KN, Lee YS, An GH, Beck GR Jr., Colburn NH, Lee KH, Cho MH, Elevated inorganic phosphate stimulates Akt-ERK1/2-Mnk1 signaling in human lung cells, Am J Respir Cell Mol Biol 35(5) (2006) 528–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Roussanne MC, Lieberherr M, Souberbielle JC, Sarfati E, Drueke T, Bourdeau A, Human parathyroid cell proliferation in response to calcium, NPS R-467, calcitriol and phosphate, Eur J Clin Invest 31(7) (2001) 610–6. [DOI] [PubMed] [Google Scholar]
- [70].Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM, Phosphate regulation of vascular smooth muscle cell calcification, Circ Res 87(7) (2000) E10–7. [DOI] [PubMed] [Google Scholar]
- [71].Bergwitz C, Juppner H, Phosphate sensing, Adv Chronic Kidney Dis 18(2) (2011) 132–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].MacLean DA, Imadojemu VA, Sinoway LI, Interstitial pH, K(+), lactate, and phosphate determined with MSNA during exercise in humans, Am J Physiol Regul Integr Comp Physiol 278(3) (2000) R563–71. [DOI] [PubMed] [Google Scholar]
- [73].Fogh-Andersen N, Altura BM, Altura BT, Siggaard-Andersen O, Composition of interstitial fluid, Clin Chem 41(10) (1995) 1522–5. [PubMed] [Google Scholar]
- [74].Sharpe RM, Gonadotrophin-induced accumulation of ‘interstitial fluid’ in the rat testis, J Reprod Fertil 55(2) (1979) 365–71. [DOI] [PubMed] [Google Scholar]
- [75].Bobko AA, Eubank TD, Driesschaert B, Dhimitruka I, Evans J, Mohammad R, Tchekneva EE, Dikov MM, Khramtsov VV, Interstitial Inorganic Phosphate as a Tumor Microenvironment Marker for Tumor Progression, Sci Rep 7 (2017) 41233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lim SM, Kim YN, Park KH, Kang B, Chon HJ, Kim C, Kim JH, Rha SY, Bone alkaline phosphatase as a surrogate marker of bone metastasis in gastric cancer patients, BMC Cancer 16 (2016) 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rao SR, Snaith AE, Marino D, Cheng X, Lwin ST, Orriss IR, Hamdy FC, Edwards CM, Tumour-derived alkaline phosphatase regulates tumour growth, epithelial plasticity and disease-free survival in metastatic prostate cancer, Br J Cancer 116(2) (2017) 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Benham FJ, Fogh J, Harris H, Alkaline phosphatase expression in human cell lines derived from various malignancies, Int J Cancer 27(5) (1981) 637–44. [DOI] [PubMed] [Google Scholar]
- [79].Takahara N, Herz F, Singer RM, Hirano A, Koss LG, Induction of alkaline phosphatase activity in cultured human intracranial tumor cells, Cancer Res 42(2) (1982) 563–8. [PubMed] [Google Scholar]
- [80].Caverzasio J, Bonjour JP, Characteristics and regulation of Pi transport in osteogenic cells for bone metabolism, Kidney Int 49(4) (1996) 975–80. [DOI] [PubMed] [Google Scholar]
- [81].Schmid C, Keller C, Schlapfer I, Veldman C, Zapf J, Calcium and insulin-like growth factor I stimulation of sodium-dependent phosphate transport and proliferation of cultured rat osteoblasts, Biochem Biophys Res Commun 245(1) (1998) 220–5. [DOI] [PubMed] [Google Scholar]
- [82].Cecil DL, Rose DM, Terkeltaub R, Liu-Bryan R, Role of interleukin-8 in PiT-1 expression and CXCR1-mediated inorganic phosphate uptake in chondrocytes, Arthritis Rheum 52(1) (2005) 144–54. [DOI] [PubMed] [Google Scholar]
- [83].Polgreen KE, Kemp GJ, Leighton B, Radda GK, Modulation of Pi transport in skeletal muscle by insulin and IGF-1, Biochim Biophys Acta 1223(2) (1994) 279–84. [DOI] [PubMed] [Google Scholar]
- [84].Mohrmann I, Mohrmann M, Biber J, Murer H, Stimulation of Na+/phosphate cotransport in LLC-PK1 cells by 12-O-tetradecanoylphorbol 13-acetate (TPA), Biochim Biophys Acta 860(1) (1986) 35–43. [DOI] [PubMed] [Google Scholar]
- [85].Moroney J, Smith A, Tomei LD, Wenner CE, Stimulation of 86Rb+ and 32Pi movements in 3T3 cells by prostaglandins and phorbol esters, J Cell Physiol 95(3) (1978) 287–94. [DOI] [PubMed] [Google Scholar]
- [86].Camalier CE, Yi M, Yu LR, Hood BL, Conrads KA, Lee YJ, Lin Y, Garneys LM, Bouloux GF, Young MR, Veenstra TD, Stephens RM, Colburn NH, Conrads TP, Beck GR Jr., An integrated understanding of the physiological response to elevated extracellular phosphate, J Cell Physiol 228(7) (2013) 1536–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Yamazaki M, Ozono K, Okada T, Tachikawa K, Kondou H, Ohata Y, Michigami T, Both FGF23 and extracellular phosphate activate Raf/MEK/ERK pathway via FGF receptors in HEK293 cells, J Cell Biochem 111(5) (2010) 1210–21. [DOI] [PubMed] [Google Scholar]
- [88].Elser JJ, Kyle MM, Smith MS, Nagy JD, Biological stoichiometry in human cancer, PLoS One 2(10) (2007) e1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Thomas CI, Harrington H, Bovington MS, Uptake of radioactive phosphorus in experimental tumors. III. The biochemical fate of P32 in normal and neoplastic ocular tissue, Cancer Res 18(9) (1958) 1008–11. [PubMed] [Google Scholar]
- [90].Jones HB, Chaikoff IL, Lawrence JH, Phosphorus metabolism of neoplastic tissues (mammary carcinoma, lymphoma, lymphosarcoma) as indicated by radioactive phosphorus, Cancer Research 40 (1940) 243–250. [Google Scholar]
- [91].Albaum H, Goldfeder A, Eisler L, Incorporation and turnover of radiophosphorus in mouse mammary tumors (dbrB and C3H), Cancer Res 12(3) (1952) 188–91. [PubMed] [Google Scholar]
- [92].Marshak A, Uptake of Radioactive Phosphorus by Nuclei of Liver and Tumors, Science 92(2394) (1940) 460–1. [DOI] [PubMed] [Google Scholar]
- [93].Rubin H, Chu BM, Solute concentration effects on the expression of cellular heterogeneity of anchorage-independent growth among spontaneously transformed BALB/c3T3 cells, In Vitro 20(7) (1984) 585–96. [DOI] [PubMed] [Google Scholar]
- [94].Rubin H, Sanui H, Complexes of inorganic pyrophosphate, orthophosphate, and calcium as stimulants of 3T3 cell multiplication, Proc Natl Acad Sci U S A 74(11) (1977) 5026–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Brazy PC, Gullans SR, Mandel LJ, Dennis VW, Metabolic requirement for inorganic phosphate by the rabbit proximal tubule, J Clin Invest 70(1) (1982) 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Brazy PC, Gullans SR, Mandel LJ, Dennis VW, Interactions between phosphate transport and oxidative metabolism in the rabbit proximal tubule, Adv Exp Med Biol 151 (1982) 65–9. [DOI] [PubMed] [Google Scholar]
- [97].Wilson DF, Owen CS, Holian A, Control of mitochondrial respiration: a quantitative evaluation of the roles of cytochrome c and oxygen, Arch Biochem Biophys 182(2) (1977) 749–62. [DOI] [PubMed] [Google Scholar]
- [98].Conrads KA, Yi M, Simpson KA, Lucas DA, Camalier CE, Yu LR, Veenstra TD, Stephens RM, Conrads TP, Beck GR Jr., A combined proteome and microarray investigation of inorganic phosphate-induced pre-osteoblast cells, Mol Cell Proteomics 4(9) (2005) 1284–96. [DOI] [PubMed] [Google Scholar]
- [99].Holley RW, Kiernan JA, Control of the initiation of DNA synthesis in 3T3 cells: low-molecular weight nutrients, Proc Natl Acad Sci U S A 71(8) (1974) 2942–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Weber MJ, Edlin G, Phosphate transport, nucleotide pools, and ribonucleic acid synthesis in growing and in density-inhibited 3T3 cells, J Biol Chem 246(6) (1971) 1828–33. [PubMed] [Google Scholar]
- [101].Hilborn DA, Serum stimulation of phosphate uptake into 3T3 cells, J Cell Physiol 87(1) (1976) 111–21. [DOI] [PubMed] [Google Scholar]
- [102].Cunningham DD, Pardee AB, Transport changes rapidly initiated by serum addition to “contact inhibited” 3T3 cells, Proc Natl Acad Sci U S A 64(3) (1969) 1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Barsh GS, Greenberg DB, Cunningham DD, Phosphate uptake and control of fibroblasts growth, J Cell Physiol 92(1) (1977) 115–28. [DOI] [PubMed] [Google Scholar]
- [104].de Asua LJ, Rozengurt E, Dulbecco R, Kinetics of early changes in phosphate and uridine transport and cyclic AMP levels stimulated by serum in density-inhibited 3T3 cells, Proc Natl Acad Sci U S A 71(1) (1974) 96–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Engstrom W, Zetterberg A, Phosphate and the regulation of DNA replication in normal and virus-transformed 3T3 cells, Biochem J 214(3) (1983) 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Meleti Z, Shapiro IM, Adams CS, Inorganic phosphate induces apoptosis of osteoblast-like cells in culture, Bone 27(3) (2000) 359–66. [DOI] [PubMed] [Google Scholar]
- [107].Rahabi-Layachi H, Ourouda R, Boullier A, Massy ZA, Amant C, Distinct Effects of Inorganic Phosphate on Cell Cycle and Apoptosis in Human Vascular Smooth Muscle Cells, J Cell Physiol 230(2) (2015) 347–55. [DOI] [PubMed] [Google Scholar]
- [108].Di Marco GS, Hausberg M, Hillebrand U, Rustemeyer P, Wittkowski W, Lang D, Pavenstadt H, Increased inorganic phosphate induces human endothelial cell apoptosis in vitro, Am J Physiol Renal Physiol 294(6) (2008) F1381–7. [DOI] [PubMed] [Google Scholar]
- [109].Zhong M, Carney DH, Jo H, Boyan BD, Schwartz Z, Inorganic phosphate induces mammalian growth plate chondrocyte apoptosis in a mitochondrial pathway involving nitric oxide and JNK MAP kinase, Calcif Tissue Int 88(2) (2011) 96–108. [DOI] [PubMed] [Google Scholar]
- [110].Sapio L, Sorvillo L, Illiano M, Chiosi E, Spina A, Naviglio S, Inorganic Phosphate Prevents Erk1/2 and Stat3 Activation and Improves Sensitivity to Doxorubicin of MDA-MB-231 Breast Cancer Cells, Molecules 20(9) (2015) 15910–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Spina A, Sorvillo L, Di Maiolo F, Esposito A, D’Auria R, Di Gesto D, Chiosi E, Naviglio S, Inorganic phosphate enhances sensitivity of human osteosarcoma U2OS cells to doxorubicin via a p53-dependent pathway, J Cell Physiol 228(1) (2013) 198–206. [DOI] [PubMed] [Google Scholar]
- [112].Beck GR Jr., Knecht N, Osteopontin regulation by inorganic phosphate is ERK1/2-, protein kinase C-, and proteasome-dependent, J Biol Chem 278(43) (2003) 41921–9. [DOI] [PubMed] [Google Scholar]
- [113].Kimata M, Michigami T, Tachikawa K, Okada T, Koshimizu T, Yamazaki M, Kogo M, Ozono K, Signaling of extracellular inorganic phosphate up-regulates cyclin D1 expression in proliferating chondrocytes via the Na+/Pi cotransporter Pit-1 and Raf/MEK/ERK pathway, Bone 47(5) (2010) 938–47. [DOI] [PubMed] [Google Scholar]
- [114].Bergwitz C, Rasmussen MD, DeRobertis C, Wee MJ, Sinha S, Chen HH, Huang J, Perrimon N, Roles of major facilitator superfamily transporters in phosphate response in Drosophila, PloS one 7(2) (2012) e31730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Teixeira CC, Mansfield K, Hertkorn C, Ischiropoulos H, Shapiro IM, Phosphate-induced chondrocyte apoptosis is linked to nitric oxide generation, Am J Physiol Cell Physiol 281(3) (2001) C833–9. [DOI] [PubMed] [Google Scholar]
- [116].Jin H, Chang SH, Xu CX, Shin JY, Chung YS, Park SJ, Lee YS, An GH, Lee KH, Cho MH, High dietary inorganic phosphate affects lung through altering protein translation, cell cycle, and angiogenesis in developing mice, Toxicol Sci 100(1) (2007) 215–23. [DOI] [PubMed] [Google Scholar]
- [117].Beck GR Jr., Zerler B, Moran E, Phosphate is a specific signal for induction of osteopontin gene expression, Proc Natl Acad Sci U S A 97(15) (2000) 8352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Beck GR Jr., Moran E, Knecht N, Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2, Exp Cell Res 288(2) (2003) 288–300. [DOI] [PubMed] [Google Scholar]
- [119].Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM, Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers, Circ Res 89(12) (2001) 1147–54. [DOI] [PubMed] [Google Scholar]
- [120].Conrads KA, Yu LR, Lucas DA, Zhou M, Chan KC, Simpson KA, Schaefer CF, Issaq HJ, Veenstra TD, Beck GR Jr., Conrads TP, Quantitative proteomic analysis of inorganic phosphate-induced murine MC3T3-E1 osteoblast cells, Electrophoresis 25(9) (2004) 1342–52. [DOI] [PubMed] [Google Scholar]
- [121].Lin Y, McKinnon KE, Ha SW, Beck GR Jr., Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression, Mol Carcinog 54(9) (2015) 926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Meng Z, Camalier CE, Lucas DA, Veenstra TD, Beck GR Jr., Conrads TP, Probing early growth response 1 interacting proteins at the active promoter in osteoblast cells using oligoprecipitation and mass spectrometry, J Proteome Res 5(8) (2006) 1931–9. [DOI] [PubMed] [Google Scholar]
- [123].Matthews CP, Colburn NH, Young MR, AP-1 a target for cancer prevention, Curr Cancer Drug Targets 7(4) (2007) 317–24. [DOI] [PubMed] [Google Scholar]
- [124].Suyama T, Okada S, Ishijima T, Iida K, Abe K, Nakai Y, High phosphorus diet-induced changes in NaPi-IIb phosphate transporter expression in the rat kidney: DNA microarray analysis, PLoS One 7(1) (2012) e29483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Peri-Okonny P, Baskin KK, Iwamoto G, Mitchell JH, Smith SA, Kim HK, Szweda LI, Bassel-Duby R, Fujikawa T, Castorena CM, Richardson J, Shelton JM, Ayers C, Berry JD, Malladi VS, Hu MC, Moe OW, Scherer PE, Vongpatanasin W, High-Phosphate Diet Induces Exercise Intolerance and Impairs Fatty Acid Metabolism in Mice, Circulation 139(11) (2019) 1422–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Katsumata S, Matsuzaki H, Katsumata-Tsuboi R, Uehara M, Suzuki K, Effects of high phosphorus diet on bone metabolism-related gene expression in young and aged mice, J Nutr Metab 2014 (2014) 575932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Beck GR Jr., Sullivan EC, Moran E, Zerler B, Relationship between alkaline phosphatase levels, osteopontin expression, and mineralization in differentiating MC3T3-E1 osteoblasts, J Cell Biochem 68(2) (1998) 269–80. [DOI] [PubMed] [Google Scholar]
- [128].Fatherazi S, Matsa-Dunn D, Foster BL, Rutherford RB, Somerman MJ, Presland RB, Phosphate regulates osteopontin gene transcription, J Dent Res 88(1) (2009) 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sage AP, Lu J, Tintut Y, Demer LL, Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro, Kidney Int 79(4) (2011) 414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Chen NX, O’Neill KD, Duan D, Moe SM, Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells, Kidney Int 62(5) (2002) 1724–31. [DOI] [PubMed] [Google Scholar]
- [131].Senger DR, Perruzzi CA, Papadopoulos A, Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation, Anticancer Res 9(5) (1989) 1291–9. [PubMed] [Google Scholar]
- [132].Oates AJ, Barraclough R, Rudland PS, The role of osteopontin in tumorigenesis and metastasis, Invasion Metastasis 17(1) (1997) 1–15. [PubMed] [Google Scholar]
- [133].Rittling SR, Chambers AF, Role of osteopontin in tumour progression, Br J Cancer 90(10) (2004) 1877–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Giachelli CM, Steitz S, Osteopontin: a versatile regulator of inflammation and biomineralization, Matrix Biol 19(7) (2000) 615–22. [DOI] [PubMed] [Google Scholar]
- [135].Cantor H, Shinohara ML, Regulation of T-helper-cell lineage development by osteopontin: the inside story, Nat Rev Immunol 9(2) (2009) 137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Denhardt DT, Giachelli CM, Rittling SR, Role of osteopontin in cellular signaling and toxicant injury, Annu Rev Pharmacol Toxicol 41 (2001) 723–49. [DOI] [PubMed] [Google Scholar]
- [137].O’Regan AW, Hayden JM, Berman JS, Osteopontin augments CD3-mediated interferon-gamma and CD40 ligand expression by T cells, which results in IL-12 production from peripheral blood mononuclear cells, J Leukoc Biol 68(4) (2000) 495–502. [PubMed] [Google Scholar]
- [138].El-Tanani MK, Campbell FC, Kurisetty V, Jin D, McCann M, Rudland PS, The regulation and role of osteopontin in malignant transformation and cancer, Cytokine Growth Factor Rev 17(6) (2006) 463–74. [DOI] [PubMed] [Google Scholar]
- [139].Bayless KJ, Meininger GA, Scholtz JM, Davis GE, Osteopontin is a ligand for the alpha4beta1 integrin, J Cell Sci 111 (Pt 9) (1998) 1165–74. [DOI] [PubMed] [Google Scholar]
- [140].Paloian NJ, Leaf EM, Giachelli CM, Osteopontin protects against high phosphate-induced nephrocalcinosis and vascular calcification, Kidney Int 89(5) (2016) 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Morris RJ, A perspective on keratinocyte stem cells as targets for skin carcinogenesis, Differentiation 72(8) (2004) 381–6. [DOI] [PubMed] [Google Scholar]
- [142].Kemp CJ, Multistep skin cancer in mice as a model to study the evolution of cancer cells, Semin Cancer Biol 15(6) (2005) 460–73. [DOI] [PubMed] [Google Scholar]
- [143].Mayne ST, Playdon MC, Rock CL, Diet, nutrition, and cancer: past, present and future, Nat Rev Clin Oncol 13(8) (2016) 504–15. [DOI] [PubMed] [Google Scholar]
- [144].Key TJ, Schatzkin A, Willett WC, Allen NE, Spencer EA, Travis RC, Diet, nutrition and the prevention of cancer, Public Health Nutr 7(1A) (2004) 187–200. [DOI] [PubMed] [Google Scholar]
- [145].Jin H, Xu CX, Lim HT, Park SJ, Shin JY, Chung YS, Park SC, Chang SH, Youn HJ, Lee KH, Lee YS, Ha YC, Chae CH, Beck GR Jr., Cho MH, High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling, Am J Respir Crit Care Med 179(1) (2009) 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wilson KM, Shui IM, Mucci LA, Giovannucci E, Calcium and phosphorus intake and prostate cancer risk: a 24-y follow-up study, Am J Clin Nutr 101(1) (2015) 173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Wulaningsih W, Michaelsson K, Garmo H, Hammar N, Jungner I, Walldius G, Holmberg L, Van Hemelrijck M, Inorganic phosphate and the risk of cancer in the Swedish AMORIS study, BMC Cancer 13 (2013) 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Ackerman NB, Shahon DB, Marvin JF, The Diagnosis of Thyroid Cancer with Radioactive Phosphorus, Surgery 47(4) (1960) 615–622. [Google Scholar]
- [149].Selverstone B, Sweet WH, Robinson CV, The Clinical Use of Radioactive Phosphorus in the Surgery of Brain Tumors, Ann Surg 130(4) (1949) 643–50. [PMC free article] [PubMed] [Google Scholar]
- [150].Ackerman NB, Shahon DB, Mc FA, Wangensteen OH, Recognition of gastric cancer by in vivo radioautography, Ann Surg 152 (1960) 602–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Gardner B, Graham WP 3rd, Gordan GS, Loken HF, Thomas AN, Teal JS, Calcium and Phosphate Metabolism in Patients with Disseminated Breast Cancer: Effect of Androgens and of Prednisone, J Clin Endocrinol Metab 23 (1963) 1115–24. [DOI] [PubMed] [Google Scholar]
- [152].Kouloulias V, Tolia M, Tsoukalas N, Papaloucas C, Pistevou-Gombaki K, Zygogianni A, Mystakidou K, Kouvaris J, Papaloucas M, Psyrri A, Kyrgias G, Gennimata V, Leventakos K, Panayiotides I, Liakouli Z, Kelekis N, Papaloucas A, Is there any potential clinical impact of serum phosphorus and magnesium in patients with lung cancer at first diagnosis? A multi-institutional study, Asian Pac J Cancer Prev 16(1) (2015) 77–81. [DOI] [PubMed] [Google Scholar]
- [153].Umeda M, Okuda S, Izumi H, Nagase D, Fujimoto Y, Sugasawa Y, Arai C, Natori K, Katoh M, Kuraishi Y, Prognostic significance of the serum phosphorus level and its relationship with other prognostic factors in multiple myeloma, Ann Hematol 85(7) (2006) 469–73. [DOI] [PubMed] [Google Scholar]
- [154].Ye Z, Palazzo JP, Lin L, Lai Y, Guiles F, Myers RE, Han J, Xing J, Yang H, Postoperative hyperphosphatemia significantly associates with adverse survival in colorectal cancer patients, J Gastroenterol Hepatol 28(9) (2013) 1469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Slaga TJ, Budunova IV, Gimenez-Conti IB, Aldaz CM, The mouse skin carcinogenesis model, J Investig Dermatol Symp Proc 1(2) (1996) 151–6. [PubMed] [Google Scholar]
- [156].Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T, Somatic activation of the K-ras oncogene causes early onset lung cancer in mice, Nature 410(6832) (2001) 1111–6. [DOI] [PubMed] [Google Scholar]
- [157].Xu CX, Jin H, Lim HT, Ha YC, Chae CH, An GH, Lee KH, Cho MH, Low dietary inorganic phosphate stimulates lung tumorigenesis through altering protein translation and cell cycle in K-ras(LA1) mice, Nutr Cancer 62(4) (2010) 525–32. [DOI] [PubMed] [Google Scholar]
- [158].Dahlberg D, Struys EA, Jansen EE, Morkrid L, Midttun O, Hassel B, Cyst Fluid From Cystic, Malignant Brain Tumors: A Reservoir of Nutrients, Including Growth Factor-Like Nutrients, for Tumor Cells, Neurosurgery 80(6) (2017) 917–924. [DOI] [PubMed] [Google Scholar]
- [159].Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M, Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis, N Engl J Med 359(6) (2008) 584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Uribarri J, Calvo MS, Hidden sources of phosphorus in the typical American diet: does it matter in nephrology?, Semin Dial 16(3) (2003) 186–8. [DOI] [PubMed] [Google Scholar]
- [161].Sullivan CM, Leon JB, Sehgal AR, Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients, J Ren Nutr 17(5) (2007) 350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Bell RR, Draper HH, Tzeng DY, Shin HK, Schmidt GR, Physiological responses of human adults to foods containing phosphate additives, J Nutr 107(1) (1977) 42–50. [DOI] [PubMed] [Google Scholar]
- [163].Levi M, Gratton E, Forster IC, Hernando N, Wagner CA, Biber J, Sorribas V, Murer H, Mechanisms of phosphate transport, Nat Rev Nephrol 15(8) (2019) 482–500. [DOI] [PubMed] [Google Scholar]
- [164].Saurette M, Alexander RT, Intestinal phosphate absorption: The paracellular pathway predominates?, Exp Biol Med (Maywood) 244(8) (2019) 646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Carfagna F, Del Vecchio L, Pontoriero G, Locatelli F, Current and potential treatment options for hyperphosphatemia, Expert Opin Drug Saf 17(6) (2018) 597–607. [DOI] [PubMed] [Google Scholar]
- [166].Block GA, Rosenbaum DP, Yan A, Chertow GM, Efficacy and Safety of Tenapanor in Patients with Hyperphosphatemia Receiving Maintenance Hemodialysis: A Randomized Phase 3 Trial, J Am Soc Nephrol 30(4) (2019) 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Tsuboi Y, Ohtomo S, Ichida Y, Hagita H, Ozawa K, Iida M, Nagao S, Ikegami H, Takahashi T, Horiba N, EOS789, a novel pan-phosphate transporter inhibitor, is effective for the treatment of chronic kidney disease-mineral bone disorder, Kidney Int 98(2) (2020) 343–354. [DOI] [PubMed] [Google Scholar]
- [168].Lacerda-Abreu MA, Russo-Abrahao T, Cosentino-Gomes D, Nascimento MTC, Carvalho-Kelly LF, Gomes T, Rodrigues MF, Konig S, Rumjanek FD, Monteiro RQ, Meyer-Fernandes JR, H(+)-dependent inorganic phosphate transporter in breast cancer cells: Possible functions in the tumor microenvironment, Biochim Biophys Acta Mol Basis Dis 1865(9) (2019) 2180–2188. [DOI] [PubMed] [Google Scholar]
- [169].Howard SC, Jones DP, Pui CH, The tumor lysis syndrome, N Engl J Med 364(19) (2011) 1844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Rampello E, Fricia T, Malaguarnera M, The management of tumor lysis syndrome, Nat Clin Pract Oncol 3(8) (2006) 438–47. [DOI] [PubMed] [Google Scholar]
- [171].Wilson FP, Berns JS, Tumor lysis syndrome: new challenges and recent advances, Adv Chronic Kidney Dis 21(1) (2014) 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Rangel LB, Sherman-Baust CA, Wernyj RP, Schwartz DR, Cho KR, Morin PJ, Characterization of novel human ovarian cancer-specific transcripts (HOSTs) identified by serial analysis of gene expression, Oncogene 22(46) (2003) 7225–32. [DOI] [PubMed] [Google Scholar]
- [173].Shyian M, Gryshkova V, Kostianets O, Gorshkov V, Gogolev Y, Goncharuk I, Nespryadko S, Vorobjova L, Filonenko V, Kiyamova R, Quantitative analysis of SLC34A2 expression in different types of ovarian tumors, Exp Oncol 33(2) (2011) 94–8. [PubMed] [Google Scholar]
- [174].Kim HS, Kim do H, Kim JY, Jeoung NH, Lee IK, Bong JG, Jung ED, Microarray analysis of papillary thyroid cancers in Korean, Korean J Intern Med 25(4) (2010) 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Chen DR, Chien SY, Kuo SJ, Teng YH, Tsai HT, Kuo JH, Chung JG, SLC34A2 as a novel marker for diagnosis and targeted therapy of breast cancer, Anticancer Res 30(10) (2010) 4135–40. [PubMed] [Google Scholar]
- [176].Jiang Z, Hao Y, Ding X, Zhang Z, Liu P, Wei X, Xi J, The effects and mechanisms of SLC34A2 on tumorigenicity in human non-small cell lung cancer stem cells, Tumour Biol 37(8) (2016) 10383–92. [DOI] [PubMed] [Google Scholar]
- [177].He J, Zhou M, Li X, Gu S, Cao Y, Xing T, Chen W, Chu C, Gu F, Zhou J, Jin Y, Ma J, Ma D, Zou Q, SLC34A2 simultaneously promotes papillary thyroid carcinoma growth and invasion through distinct mechanisms, Oncogene 39(13) (2020) 2658–2675. [DOI] [PubMed] [Google Scholar]
- [178].Zhang L, Guo X, Zhang L, Yang F, Qin L, Zhang D, Qin Y, SLC34A2 regulates miR-25-Gsk3beta signaling pathway to affect tumor progression in gastric cancer stem cell-like cells, Mol Carcinog 57(3) (2018) 440–450. [DOI] [PubMed] [Google Scholar]
- [179].Liu L, Yang Y, Zhou X, Yan X, Wu Z, Solute carrier family 34 member 2 overexpression contributes to tumor growth and poor patient survival in colorectal cancer, Biomed Pharmacother 99 (2018) 645–654. [DOI] [PubMed] [Google Scholar]
- [180].Russo-Abrahao T, Lacerda-Abreu MA, Gomes T, Cosentino-Gomes D, Carvalho-de-Araujo AD, Rodrigues MF, Oliveira ACL, Rumjanek FD, Monteiro RQ, Meyer-Fernandes JR, Characterization of inorganic phosphate transport in the triple-negative breast cancer cell line, MDA-MB-231, PLoS One 13(2) (2018) e0191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Ye W, Chen C, Gao Y, Zheng ZS, Xu Y, Yun M, Weng HW, Xie D, Ye S, Zhang JX, Overexpression of SLC34A2 is an independent prognostic indicator in bladder cancer and its depletion suppresses tumor growth via decreasing c-Myc expression and transcriptional activity, Cell Death Dis 8(2) (2017) e2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Hong SH, Minai-Tehrani A, Chang SH, Jiang HL, Lee S, Lee AY, Seo HW, Chae C, Beck GR Jr., Cho MH, Knockdown of the sodium-dependent phosphate co-transporter 2b (NPT2b) suppresses lung tumorigenesis, PloS one 8(10) (2013) e77121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Li X, Yang HY, Giachelli CM, Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification, Circ Res 98(7) (2006) 905–12. [DOI] [PubMed] [Google Scholar]
- [184].Suzuki A, Ghayor C, Guicheux J, Magne D, Quillard S, Kakita A, Ono Y, Miura Y, Oiso Y, Itoh M, Caverzasio J, Enhanced expression of the inorganic phosphate transporter Pit-1 is involved in BMP-2-induced matrix mineralization in osteoblast-like cells, J Bone Miner Res 21(5) (2006) 674–83. [DOI] [PubMed] [Google Scholar]
- [185].Yoshiko Y, Candeliere GA, Maeda N, Aubin JE, Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization, Mol Cell Biol 27(12) (2007) 4465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Beck L, Leroy C, Salaun C, Margall-Ducos G, Desdouets C, Friedlander G, Identification of a novel function of PiT1 critical for cell proliferation and independent of its phosphate transport activity, J Biol Chem 284(45) (2009) 31363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Byskov K, Jensen N, Kongsfelt IB, Wielsoe M, Pedersen LE, Haldrup C, Pedersen L, Regulation of cell proliferation and cell density by the inorganic phosphate transporter PiT1, Cell Div 7(1) (2012) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Harima Y, Togashi A, Horikoshi K, Imamura M, Sougawa M, Sawada S, Tsunoda T, Nakamura Y, Katagiri T, Prediction of outcome of advanced cervical cancer to thermoradiotherapy according to expression profiles of 35 genes selected by cDNA microarray analysis, Int J Radiat Oncol Biol Phys 60(1) (2004) 237–48. [DOI] [PubMed] [Google Scholar]
- [189].Cao D, Hustinx SR, Sui G, Bala P, Sato N, Martin S, Maitra A, Murphy KM, Cameron JL, Yeo CJ, Kern SE, Goggins M, Pandey A, Hruban RH, Identification of novel highly expressed genes in pancreatic ductal adenocarcinomas through a bioinformatics analysis of expressed sequence tags, Cancer Biol Ther 3(11) (2004) 1081–9; discussion 1090–1. [DOI] [PubMed] [Google Scholar]
- [190].Walker LC, Waddell N, Ten Haaf A, kConFab I, Grimmond S, Spurdle AB, Use of expression data and the CGEMS genome-wide breast cancer association study to identify genes that may modify risk in BRCA1/2 mutation carriers, Breast Cancer Res Treat 112(2) (2008) 229–36. [DOI] [PubMed] [Google Scholar]
- [191].Sato K, Akimoto K, Expression Levels of KMT2C and SLC20A1 Identified by Information-theoretical Analysis Are Powerful Prognostic Biomarkers in Estrogen Receptor-positive Breast Cancer, Clin Breast Cancer 17(3) (2017) e135–e142. [DOI] [PubMed] [Google Scholar]
- [192].Larsson TE, Kameoka C, Nakajo I, Taniuchi Y, Yoshida S, Akizawa T, Smulders RA, NPT-IIb Inhibition Does Not Improve Hyperphosphatemia in CKD, Kidney Int Rep 3(1) (2018) 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Boumans MJ, Houbiers JG, Verschueren P, Ishikura H, Westhovens R, Brouwer E, Rojkovich B, Kelly S, den Adel M, Isaacs J, Jacobs H, Gomez-Reino J, Holtkamp GM, Hastings A, Gerlag DM, Tak PP, Safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of the monoclonal antibody ASK8007 blocking osteopontin in patients with rheumatoid arthritis: a randomised, placebo controlled, proof-of-concept study, Ann Rheum Dis 71(2) (2012) 180–5. [DOI] [PubMed] [Google Scholar]
- [194].Taylor PR, Greenwald P, Nutritional interventions in cancer prevention, J Clin Oncol 23(2) (2005) 333–45. [DOI] [PubMed] [Google Scholar]


