Abstract
Population size changes and gene flow are processes that can have significant impacts on evolution. The aim of this study was to investigate the relationship of geography to patterns of gene flow and population size changes in a pair of closely related Sphagnum (peatmoss) species: S. recurvum and S. flexuosum. Both species occur in eastern North America, and S. flexuosum also occurs in Europe. Genetic data from restriction‐site‐associated DNA sequencing (RAD‐seq) were used in this study. Analyses of gene flow were accomplished using coalescent simulations of site frequency spectra (SFSs). Signatures of gene flow were confirmed by f 4 statistics. For S. flexuosum, genetic diversity of plants in glaciated areas appeared to be lower than that in unglaciated areas, suggesting that glaciation can have an impact on effective population sizes. There is asymmetric gene flow from eastern North America to Europe, suggesting that Europe might have been colonized by plants from eastern North America after the last glacial maximum. The rate of gene flow between S. flexuosum and S. recurvum is lower than that between geographically disjunct S. flexuosum populations. The rate of gene flow between species is higher among sympatric plants of the two species than between currently allopatric S. flexuosum populations. There was also gene flow from S. recurvum to the ancestor S. flexuosum on both continents which occurred through secondary contact. These results illustrate a complex history of interspecific gene flow between S. flexuosum and S. recurvum, which occurred in at least two phases: between ancestral populations after secondary contact and between currently sympatric plants.
Keywords: demographic history, effective population size, gene flow, genetic diversity, glaciation, sphagnum
This study has shown that there are multiple phases of gene flow between closely related peatmosses. Most of the gene flow occured in the past via secondary contact, the lesser extent of gene flow is currently ongoing between only sympatric populations.

1. INTRODUCTION
Inferring patterns of demography and gene flow among diverging populations is crucial to understanding speciation processes (Edelman & Mallet, 2021; Ellstrand, 2014; Nielsen et al., 2009). Gene flow, the movement of genetic material between individuals from differentiated populations, can act as a homogenizing force among partially divergent species. In some instances, however, gene flow can augment genetic diversity within populations and provide potentially adaptive alleles or even promote the speciation process (Abbott et al., 2013; Morjan & Rieseberg, 2004; Richards & Martin, 2017; Slatkin, 1987; Suarez‐Gonzalez et al., 2018). Rates of gene flow can obviously be affected by physical distances; proximate individuals are more likely to exchange genetic material than more distant ones. Gene flow can occur between populations within the same species (intraspecific gene flow) and between populations of different species (interspecific gene flow). Since individuals from different species usually have some degree of reproductive isolation, interspecific gene flow in general should occur at lower levels than intraspecific gene flow (Edelman & Mallet, 2021; Ellstrand, 2014). Changes in population size through time can also influence the genetic makeup of populations. For example, a population expansion can create an excess of rare alleles that can mimic signatures of selection. Population sizes can be influenced by changes in environmental conditions that cause populations to contract or expand (Nielsen et al., 2009). Major environmental changes such as glaciation can have profound effects on population sizes (Abbott & Brochmann, 2003). Founder events following dispersal or range expansions can also significantly impact the genetic makeup of populations (Hewitt, 1996).
Developments in sequencing technologies have made genome‐scale data in non‐model organisms much easier to acquire. Such large datasets allow for the analyses of complex evolutionary models (Ekblom & Galindo, 2011). Statistical methods have been developed to compare demographic models that include different effective population sizes through time and variable gene flow histories (Beichman et al., 2018; Excoffier et al., 2013). This can be instrumental in understanding the speciation process of closely related species, which involves a complex interaction of population divergence, population size changes, and gene flow.
Peatmosses (Sphagnum spp.) are semiaquatic to terrestrial plants that grow in bogs, fens, forests, and seepages (Rydin et al., 2013). Sphagnum is of unparalleled ecological importance because some 25%–30% of the entire terrestrial pool of carbon is estimated to be bound up in partially decomposed peat within Sphagnum‐dominated peatlands (Gorham, 1991; Yu, 2011). Thus, understanding evolutionary and ecological processes in Sphagnum has profound implications for biogeochemistry and the control of global climate (Weston et al., 2018).
There are around 300–500 species of Sphagnum worldwide, and although the Sphagnum clade is hundreds of millions of years old, most extant species seem to have emerged through relatively recent diversification during the last 10–15 million years (Shaw et al., 2010). Sphagnum is capable of long‐distance dispersal via either spores or vegetative fragments (Sundberg, 2013). Many species have intercontinental ranges, with some degree of population structure across their geographic ranges (Kyrkjeeide et al., 2016). Long‐distance dispersal allows populations in different geographic regions, even between continents, to remain connected by gene flow (Shaw et al., 2015; Stenøien et al., 2011). Multiple species of Sphagnum often occupy the same habitat, usually by specializing in different microhabitats. In fact, many sites have ten or more sympatric species. Different Sphagnum species in the same habitat can hybridize, at least occasionally (Cronberg, 1998; Cronberg & Natcheva, 2002). In addition to hybridization occurring in current populations, recent analyses using genomic data showed signatures of ancient introgressions between Sphagnum species (Meleshko et al., 2021). Many Sphagnum species occur in northern areas that were covered by ice during the last glacial maximum (LGM) and have experienced significant shifts in geographical range during the recent past (Abbott & Brochmann, 2003; Gignac et al., 2000). These attributes, interspecific gene flow, recent range changes, and the potential for long distance dispersal can make the demographic history of Sphagnum species very complex. Moreover, the broad intercontinental geographic ranges of individual Sphagnum species add a layer of potential demographic and evolutionary complexity compared to most seed plant species that have much more restricted geographic ranges (Frahm & Vitt, 1993; Qian, 1999). Understanding patterns of gene flow and population size changes in closely related Sphagnum species is required to fully understand speciation processes and Sphagnum diversification.
This study focused on two closely related Sphagnum species: S. recurvum P. Beauv. and S. flexuosum Dozy & Molk (Figure 1). These species are members of the so‐called S. recurvum complex (Flatberg, 1992), which is part of the subgenus Cuspidata. Phylogenetic analyses (Duffy et al., 2020) have shown that S. flexuosum and S. recurvum are closely related. Sphagnum recurvum is restricted to eastern North America, with the exception of a single disjunct population in the Azores, while S. flexuosum occurs in both eastern North America and western Europe. Analyses of genetic structure have shown that European S. flexuosum are nested within a clade of eastern North American plants, suggesting that European plants were derived from eastern North America (Duffy et al., 2020).
FIGURE 1.

Field‐derived photographs of (a) Sphagnum flexuosum and (b) Sphagnum recurvum. Photos: Blanka Aguero (with permission).
The geographic distributions of S. recurvum and S. flexuosum provide a natural experiment for testing factors that impact patterns of interspecific gene flow, intraspecific gene flow between continents, and population size changes in these closely related species. Plant communities in Europe and eastern North America have been affected differently during the LGM. Europe suffered more diversity lost during the LGM (Adams & Woodward, 1989; Svenning, 2003). Fossil records have shown that there are many woody plant genera that existed in Europe during the Upper Tertiary (25–2 Mya) but now persist only in eastern North America and Asia (Adams & Woodward, 1989). One explanation for this pattern is that with the Appalachian Mountains oriented in a north–south direction, plants in eastern North America were able to freely migrate during cold periods of the Pleistocene, whereas plants in Europe were more likely blocked by the east–west orienting Alps (Hewitt, 1996; Soltis et al., 2006). Another explanation for greater diversity loss in Europe during the LGM is that southern refugia in Europe had dry climates that could not support many mesic temperate plants (Svenning, 2003). Most of the mesic temperate tree species in Europe that survived the LGM were restricted to only the Mediterranean and Black Sea regions (Svenning et al., 2008). Since S. flexuosum can occur only in moist habitats, the S. flexuosum population in Europe might have suffered a severe bottleneck or was possibly eliminated completely during the LGM, only to be reestablished by plants from eastern North America. This can result in S. flexuosum plants in Europe having lower genetic diversity and a smaller effective population size than plants in eastern North America. If within eastern North America, S. flexuosum survived glaciation south of the ice, we might predict lower genetic diversity among plants in glaciated versus unglaciated areas. On the other hand, if spore‐producing Sphagnum plants are highly proficient dispersers, any such genetic signal of migration and population bottlenecks could have been erased.
Opportunities for interspecific gene flow between S. recurvum and S. flexuosum were likely impacted by their intercontinental ranges. Hybridization between the species is obviously more likely between plants currently growing on the same continent, but intercontinental migration within these spore‐reproducing plants makes it possible that plants now disjunct across the Atlantic Ocean could bear signatures of gene flow as well (Shaw et al., 2014; Stenøien et al., 2011). There are several possibilities for interspecific gene flow between S. flexuosum and S. recurvum: between presently allopatric plants (i.e., eastern North American S. recurvum and European S. flexuosum), between presently sympatric plants (S. recurvum and eastern North American S. flexuosum), or between plants ancestral to current population systems (S. recurvum and the ancestor of both S. flexuosum populations).
The goals of this study were to answer the following questions. (1) are eastern North American versus European metapopulation systems within S. flexuosum connected by intraspecific gene flow? If so, is the rate of gene flow symmetrical between the two continents? (2) Is the rate of interspecific gene flow between S. flexuosum and S. recurvum higher between plants currently sympatric on the same continent than between plants currently separated on different continents? (3) Is there evidence of gene flow between S. recurvum and those ancestral to the currently disjunct populations within S. flexuosum? And if so, was that gene flow limited to the period during and after speciation, did it occur after secondary contact, or was it continuous? (4) Is genetic diversity in S. flexuosum lower in glaciated than unglaciated areas of eastern North America and Europe?
2. METHODS
2.1. Taxon sampling
Restriction‐site‐associated DNA sequencing (RAD‐seq) raw reads from Duffy et al. (2020) were used in this study. For DNA‐extraction, library preparation, sequencing protocols, and data availability, see Duffy et al. (2020). A total of 60 samples were divided into three groups for the present study: S. recurvum (16 samples), S. flexuosum from eastern North America (28 samples, hereafter “ENA S. flexuosum”), and S. flexuosum from Europe (16 samples, hereafter “EUR S. flexuosum”). All our European samples of S. flexusosum were collected from a relatively small area in Norway, which limits some generalities about the species in “Europe.” Recently collected samples from other areas were not available. Nevertheless, the questions we address should be relatively robust to this sampling limitation (see discussion). Figure 2 shows the geographical locations of samples used in this study. In addition to S. flexuosum and S. recurvum samples, one sample of S. cuspidatulum Müll. Hal. and two samples of S. fallax H. Klinggr. were also included for the introgression analysis. RAD‐seq reads for S. fallax samples were obtained from Duffy et al. (2020), while the S. cuspidatulum sample was acquired from in silico digestion of the genomic resequencing sample (see data availability for more information). Specimen voucher information is provided in the appendix (Table A1).
FIGURE 2.

Geographic locations of S. recurvum and S. flexuosum samples used in this study.
2.2. RAD‐seq data assembly
The RAD‐seq raw reads were assembled using ipyrad version 0.7.29 (Eaton, 2014) with default parameters except noted here. The reads were aligned to the S. divinum (v1.1) reference genome (https://phytozome‐next.jgi.doe.gov/), which is an outgroup species relative to S. recurvum and S. flexuosum (Shaw et al., 2016) in order to infer derived versus ancestral alleles. The samples were treated as haploid. Based on a previous study (Duffy et al., 2020), a read clustering threshold of 0.9 was used to maximize the number of variable sites. Loci presented in less than 80% of the samples were discarded.
2.3. Genetic diversity and introgression analysis
Within population nucleotide diversity (π), and pairwise Fst, genetic distance (Dxy) and number of fixed, shared, and monomorphic sites among ENA S. flexuosum, EUR S. flexuosum, and S. recurvum were calculated by the R package popgenome (Pfeifer et al., 2014). Nucleotide diversity and genetic distance are defined as the average pairwise nucleotide differences between samples within and between populations, respectively (Nei & Li, 1979). For the analysis comparing genetic diversity among geographic regions within S. flexuosum, two subsets of ENA S. flexuosum samples were created: Maryland (ten samples) and central New York (nine samples). These subsets have similar distributional ranges to the Norwegian (EUR) collections. The samples from Europe and central New York represent glaciated regions, and samples from Maryland come from an unglaciated region. Our sampling is insufficient to confirm any relationship between glacial history and genetic diversity but can yield a preliminary assessment. Nucleotide diversity was calculated for each group of S. flexuosum samples using the same method as above. Jackknife resampling was used to calculate the variance of nucleotide diversity estimates; n subsamples of each group was made by excluding one sample from the dataset, where n is the number samples in the group. Statistical differences of nucleotide diversity estimates were analyzed by ANOVA and post‐hoc Student's t‐test using Bonferroni correction for multiple comparisons. Additional samples of ENA S. flexuosum samples were excluded from these geographic comparisons so we could use samples from comparable areas, but these were included in other analyses.
Two ABBA/BABA site pattern statistics were calculated using the program Dsuite (Malinsky et al., 2021) to detect signatures of introgression: Patterson's D statistics (Green et al., 2010) and f 4 ratios (Patterson et al., 2012). In the introgression analyses, outgroup samples of S. cuspidatulum and S. fallax were also included. Sphagnum cuspidatulum is a tropical species from Southeast Asia (Eddy, 1977). Phylogenetic analyses have shown that S. cuspidatulum is strongly supported as sister to S. recurvum (unpublished data). Including this species in the analyses allows for an inference about introgression between S. recurvum and the ancestor of S. flexuosum Europe and eastern North America. Sphagnum fallax was also included as an outgroup because it is one of the closest relatives to the “S. flexuosum + S. recurvum” clade (Duffy et al., 2020).
2.4. Demographic modeling
Multiple demographic models were compared using the approximate‐likelihood method in fastsimcoal2 (fsc26, Excoffier et al., 2013). This method uses site frequency spectra (SFS) as input. Unfolded SFS were calculated using easySFS pipeline (https://github.com/isaacovercast/easySFS). It is possible to calculate unfolded SFS in this case because the RAD‐seq reads were aligned to an outgroup reference genome, thus retaining information about derived and ancestral alleles. Since SFS requires each site to have no missing data, the easySFS pipeline allows SNPs to be subsampled from the dataset. This reduces the number of samples but increases the number of SNPs with no missing data. The populations were subsampled as follows: S. recurvum: 8 of 16 samples, ENA S. flexuosum: 16 of 28 samples, EUR S. flexuosum: 11 of 26 samples. Unfolded SFS were generated both using all SNPs and one SNP per RAD‐seq locus.
The demographic models tested all utilize the same bifurcating history: S. recurvum diverged from S. flexuosum and then S. flexuosum diverged into two allopatric populations; that is, Europe (EUR) and eastern North America (ENA). The differences among the models are the presence/absence of gene flow between populations. There are eight possible gene flow events: six between the three current populations and two between ancestral populations. Of the eight possible gene flow events, two are between populations of the same species (intraspecific gene flow) and six are between populations of different species (interspecific gene flow) (Figure B1). A total of 29 demographic models with several combinations of gene flow events were included in the analysis, out of 128 possible combinations (Table B1). This includes models with no gene flow, with all possible patterns of gene flow, and with only gene flow between current populations. We also tested models in which one of the eight gene flow events was excluded. Further model testing was designed by excluding multiple gene flow events that might impact the likelihood of the model when absent. All gene flows were treated as temporally continuous. After identifying the best demographic model, further comparisons were conducted by modifying the gene flow between S. recurvum and the ancestor of the two allopatric S. flexuosum populations as continuous gene flow, early gene flow shortly after divergence of the population systems, and secondary contact.
Approximate likelihoods for each demographic model were calculated by fastsimcoal2 in two steps, following Bagley et al. (2017). First, demographic parameters were inferred from the SFS containing all SNPs. According to Excoffier et al. (2013), the use of linked SNPs should not bias demographic parameter estimation and can help increase the amount of information for parameter inference. At least 100 independent runs were performed for each model. In each run, the expected SFSs were generated from 50,000 simulations, and the demographic parameters were optimized in 40 ECM cycles. In the second set of analyses, the best demographic parameters for each model were used to compute the approximate likelihood based on SFS containing only one SNP per RAD‐seq locus. In this case, the expected SFS was generated from 10 million simulations to increase the accuracy of the approximate likelihood. This approximate likelihood was then used to calculate an Akaike information criterion (AIC) for the model.
Confidence intervals of demographic parameters in the best model were obtained from parametric bootstrap. Demographic parameters in the best model were used to simulate 100 independent SFSs. For each of the simulated SFS, ten independent runs were performed using 50,000 simulations and 40 ECM cycles. Demographic parameters from the best run of each simulated SFS were then combined to calculate confidence intervals.
3. RESULTS
3.1. RAD‐seq reads assembly
The total number of raw reads from 60 samples was 90,196,383, ranging from 375,249 to 2,594,985 reads per sample (median ± SD = 1,565,588.5 ± 572,048.9). The assembly pipeline yielded 14,874 loci that are present in more than 80% of the samples, and 13,756 of those contained one or more SNPs. The mean SNP coverage was 75.12%.
3.2. Genetic diversity analysis
The DNA sequence matrix used in these calculations contained 282,865 sites, of which 12,307 were biallelic. S. recurvum has higher nucleotide diversity (π = 0.00626) than S. flexuosum (π = 0.00462). Both F st and genetic distance (D xy) values between S. recurvum and S. flexuosum (F st = 0.594, D xy = 0.0134) are higher than the value between allopatric ENA and EUR populations of S. flexuosum (F st = 0.104, D xy = 0.00482). EUR S. flexuosum and S. recurvum share 1549 polymorphic sites and have 1403 fixed differences. ENA S. flexuosum and S. recurvum share 2061 polymorphic sites and have 1323 fixed differences. These measurements suggest that differentiation between S. recurvum and S. flexuosum is higher than that of the allopatric populations of S. flexuosum (Table 1).
TABLE 1.
Nucleotide diversity within populations (π) and pairwise comparisons of the fixation index (Fst), genetic distance (Dxy), shared polymorphic sites, and fixed differences between S. recurvum and populations within S. flexuosum
| Within population | ||
|---|---|---|
| Population | N | Nucleotide diversity |
| S. recurvum | 16 | 0.00627 |
| ALL S. flexuosum | 44 | 0.00463 |
| EUR S. flexuosum | 16 | 0.00403 |
| ENA S. flexuosum | 28 | 0.00460 |
| Between population | ||||
|---|---|---|---|---|
| Population pair | Fst | Dxy | Shared polymorphic sites | Fixed Differences |
| ALL S. flexuosum and S. recurvum | 0.594 | 0.0134 | 2287 | 1274 |
| EUR S. flexuosum and ENA S. flexuosum | 0.104 | 0.00482 | 2904 | 0 |
| EUR S. flexuosum and S. recurvum | 0.615 | 0.0134 | 1549 | 1403 |
| ENA S. flexuosum and S. recurvum | 0.597 | 0.0135 | 2061 | 1323 |
Abbreviations: ALL, including both ENA and EUR samples; ENA, eastern North America; EUR, Europe.
Within S. flexuosum, nucleotide diversity in ENA S. flexuosum (π = 0.00460) is higher than that of EUR S. flexuosum (π = 0.00403) (Table 1). However, these estimates were incomparable since EUR S. flexuosum samples were collected from much smaller range than ENA S. flexuosum. Nevertheless, when ENA S. flexuosum was reduced into two subsets with comparable sampling range as EUR S. flexuosum, estimates for the three regions are significantly different (p < .05). Plants from Maryland (ENA, unglaciated) have the highest nucleotide diversity (π = 0.00436), followed by central New York (ENA, glaciated) (π = 0.00418), and central Norway (EUR, glaciated) has the lowest nucleotide diversity (π = 0.00403) (Table 2).
TABLE 2.
Nucleotide diversity (π) for populations within S. flexuosum and statistical comparison using ANOVA and Student's t‐test
| Summary | ||||
|---|---|---|---|---|
| Population | Continent | Glaciation during LGM | N | Nucleotide diversity (SD) |
| Maryland | ENA | No | 10 | 0.00436 (1.2 × 10−4) |
| Upstate New York | ENA | Yes | 9 | 0.00418 (9.7 × 10−5) |
| Central Norway | EUR | Yes | 16 | 0.004031 (1.2 × 10−5) |
| ANOVA | |||||
|---|---|---|---|---|---|
| Source of variation | SS | df | MS | F | p‐value (α = 0.05) |
| Between groups | 7.27 × 10−7 | 2 | 3.63 × 10−7 | 57.1 | 2.75 × 10−11 |
| Within groups | 2.03 × 10−7 | 32 | 6.36 × 10−9 | ||
| Total | 9.29 × 10−7 | 34 | |||
| Post‐hoc Student's t‐test | |
|---|---|
| Population comparisons | p‐value (α = 0.0167) |
| Maryland – Upstate New York | 0.000987 |
| Maryland – Central Norway | 3.91 × 10−13 |
| Upstate New York – Central Norway | 6.89 × 10−5 |
Abbreviations: ENA, eastern North America; EUR, Europe.
3.3. Introgession estimates: ABBA/BABA site patterns analyses
In all four species trios (Table 3), P1 and P2 share more derived alleles (BBAA sites, pattern concordant with species tree) than either P1 or P2 with P3 (ABBA/BABA sites, patterns discordant with species tree), confirming the topology of phylogenetic relationships used in this analysis (Figure 3). For the discordant site patterns, under a hypothetical scenario with only incomplete lineage sorting and the phylogeny being (([P1, P2], P3), outgroup), it is expected that P1 and P2 share equal numbers of derived alleles with P3. That is, the number of ABBA and BABA sites should be roughly equal (D‐statistics not significantly different from zero). In all four species trios, D‐statistics were significantly different from zero (p < .05). Table 3 and Figure 3 summarize ABBA/BABA site patterns statistics for each species trio.
TABLE 3.
ABBA/BABA site pattern statistics. Z‐score and p‐value of D statistics were computed by jackknife. Significant p‐value (<.0125) suggests the presence of introgression.
| no. of species trio | P1 | P2 | P3 | D statistics | Z‐score | p‐value | f4‐ratio | BBAA sites | ABBA sites | BABA sites | Putative introgression pair |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ENA S. flexuosum | EUR S. flexuosum | S. cuspidatulum | 0.0534438 | 2.50067 | .00620 | 0.00475 | 5441.56 | 392.791 | 352.936 | EUR S. flexuosum and S. cuspidatulum |
| 2 | S. cuspidatulum | S. recurvum | ENA S. flexuosum | 0.13912 | 5.08219 | 1.87 E‐07 | 0.0695 | 1900.53 | 1486.8 | 1123.63 | S. recurvum and ENA S. flexuosum |
| 3 | S. cuspidatulum | S. recurvum | EUR S. flexuosum | 0.112862 | 4.49452 | 3.49 E‐06 | 0.0557 | 1921.5 | 1473.49 | 1174.62 | S. recurvum and EUR S. flexuosum |
| 4 |
EUR S. flexuosum |
ENA S. flexuosum | S. recurvum | 0.0362019 | 2.67152 | .00378 | 0.00949 | 8913.22 | 1203.59 | 1119.49 | ENA S. flexuosum and S. recurvum |
Note: f 4‐ratio indicates proportion of the genome involved in introgression. In all of species trios, S. fallax was used as an outgroup population.
FIGURE 3.

ABBA/BABA statistics analysis. The double‐headed arrows represent the putative introgression events, numbers of species trio are based on Table 3, and f 4 values represent the proportion of genome involved in introgression. Thick arrow is used for introgression events with f 4 higher than 0.01.
These non‐zero D statistics value suggest a signature of introgression, and f 4‐ratio values show the proportions of genomes that were introgressed (Table 3). In the first species trio, EUR S. flexuosum (P1) shares more derived alleles with S. cuspidatulum (P3) relative to ENA S. flexuosum (P2), suggesting introgression between EUR S. flexuosum and S. cuspidatulum. In the second and third species trios, S. recurvum (P2) shares more derived alleles with both ENA and EUR S. flexuosum (P3), relative to S. cuspidatulum (P1), suggesting introgression between S. flexuosum and S. recurvum. In the fourth species trio, ENA S. flexuosum (P2) shares more derived alleles with S. recurvum (P3), relative to EUR S. flexuosum (P1), suggesting introgression between ENA S. flexuosum and S. recurvum.
3.4. Demographic history
Of 33 demographic models tested, the best model was “model 10 with secondary contact” (Figure 4). This model consists of three gene flow events: from ENA S. flexuosum to EUR S. flexuosum, from ENA S. flexuosum to S. recurvum, and from S. recurvum to the lineage ancestral to ENA and EUR S. flexuosum. In this model, there is also an isolation period after S. recurvum diverged from the ancestral population of S. flexuosum. For effective population sizes, EUR S. flexuosum has the smallest effective population size, followed by ENA S. flexuosum, and then S. recurvum with the largest. Figure 5 shows variation in demographic parameter estimates from parametric bootstrap. Tables B1 and B2 show demographic parameters, approximate likelihoods, and AIC values for all 33 demographic models tested. Table B2 provides 95% confidence intervals for demographic parameters in “full migration”, “full migration with secondary contact”, “model 10”, and “model 10 with secondary contact” models based on parametric bootstrap. Figures B2, B3, B4, B5 show boxplots of demographic parameters estimates for the models included in Table B2.
FIGURE 4.

Best demographic model (“model 10 with secondary contact”). The width of the boxes is roughly proportional to the effective population sizes (Ne); the height of the boxes is roughly proportional to divergence time; arrows represent the presence and direction of gene flow; thick arrows is used when gene flow rate exceeds 0.01.
FIGURE 5.

Variations in gene flow rates and effective population sizes of the best demographic model as inferred from parametric bootstrap.
4. DISCUSSION
4.1. Gene flow between S. flexuosum and S. recurvum
There is much evidence that hybridization is widespread in plants and can have significant evolutionary impacts (Rieseberg, 1995; Rieseberg & Carney, 1998; Suarez‐Gonzalez et al., 2018). One possible outcome of hybridization is introgression, where hybrids backcross to one or both parental species. After generations of backcrossing, a small genomic fraction can be transferred from one species to another (Abbott et al., 2013; Edelman & Mallet, 2021). In mosses, the initial F1 hybrid is the short‐lived sporophyte generation, but meiosis in the sporangia (capsules) of such hybrids yields recombinant haploid gametophytes with allelic representation from the two parental species across loci. There is no (or little) heterozygosity to shield hybridity from natural selection. In Sphagnum, it is common for many species to grow intimately mixed, and demonstrably recombinant individuals have been detected in Sphagnum (Cronberg, 1989; Cronberg, 1998; Cronberg & Natcheva, 2002). Allopolyploid species with diploid gametophytes and tetraploid sporophytes have also been documented in Sphagnum from all over the world (for example, Karlin et al., 2010; Ricca & Shaw, 2010; Såstad et al., 2001), and these provide further evidence that hybridization can and does occur in the genus.
The results from both ABBA/BABA statistics and demographic modeling strongly suggest that there has been gene flow between S. flexuosum and S. recurvum. Furthermore, interspecific gene flow occurred in at least two phases: before and after the divergence between European and eastern North American plants within S. flexuosum. Demographic modeling indicates that the first hybridization event(s) occurred between S. recurvum and the ancestor of divergent North American and European S. flexuosum and the second event(s) between S. recurvum and regionally sympatric North American plants of S. flexuosum after the divergence of the European clade. Two phases of gene flow can also be indirectly inferred from ABBA/BABA statistics. When S. cuspidatulum is treated as P1 and S. recurvum is P2, the results indicate that S. recurvum shares more derived alleles with both European and eastern North American S. flexuosum. This pattern suggests hybridization between S. recurvum and the ancestor of European and eastern North American S. flexuosum. When European S. flexuosum is treated as P1, North American S. flexuosum as P2, and S. recurvum as P3 in the analyses, it is eastern North American S. flexuosum that shares more derived alleles with S. recurvum. This suggests that there was another introgression event that occurred between S. recurvum and S. flexuosum in eastern North America, but not with S. flexuosum in Europe. Consistent with that interpretation, S. recurvum shares more polymorphic sites, fewer fixed differences, and a lower estimated F st with eastern North American S. flexuosum than with European S. flexuosum.
The best demographic model suggests that gene flow between S. recurvum and the ancestor of European and eastern North American S. flexuosum occurred during secondary contact after a period of isolation. From this, it can be inferred that speciation of S. recurvum and S. flexuosum may have occurred in allopatry. While their ranges are currently sympatric in eastern North America today, S. flexuosum and S. recurvum might have had allopatric distributions in the past. Duffy et al. (2022) showed that some continuously distributed eastern North American species of Sphagnum exhibit population structure that suggests regional divergence that presumably developed during previous periods of allopatry.
Based on f 4 values, at least 5.5% of the genome has been transferred between S. flexuosum and S. recurvum in the first phase of gene flow. The best demographic models suggest that the direction of transfer has been from S. recurvum to S. flexuosum. This direction of gene flow is consistent across all of the demographic models tested (Table B1). The best model indicates that the population size of S. flexuosum after divergence from S. recurvum was very small (Ne = 21), relative to the ancestral population size (Ne = 5769). With this small population size, genetic drift can have an enormous influence on the gene pool; beneficial alleles might be eliminated, or slightly deleterious mutations could be fixed. Gene flow with S. recurvum during secondary contact could have reintroduced beneficial alleles in the ancestral population that were lost or could have introduced new alleles that originated within S. recurvum.
Since S. recurvum is restricted to eastern North America (except for one site in the Azores), this first phase of interspecific gene flow also suggests that the ancestral population of the current S. flexuosum populations occurred in eastern North America. This is consistent with the phylogenetic inference that S. flexuosum in Europe was derived from plants in eastern North America (Duffy et al., 2020).
The second phase of gene flow occurred between S. recurvum and eastern North American S. flexuosum. Both the rate of gene flow estimated by demographic modeling and the f 4 value from ABBA/BABA statistics clearly indicate that the magnitude of interspecific gene flow before the divergence of European and North American S. flexuosum was higher than in the second phase after they diverged. This result suggests that reproductive isolation between sympatric S. flexuosum and S. recurvum in eastern North America is strong, even if not absolute. The relatively high estimate for interspecific gene flow before the divergence of S. flexuosum compared to gene flow after divergence of continental populations is consistent across most demographic models tested.
In contrast to the earlier phase of introgression between S. recurvum and the ancestor of North American and European S. flexuosum, the direction of the second phase of interspecific gene flow appears from the best demographic model to have occurred from eastern North American S. flexuosum into S. recurvum. The inferred direction of gene flow is not consistent across the models tested, but the occurrence of gene flow is strongly and consistently supported. Since this gene flow occurs only with North American S. flexuosum, it could contribute to the differentiation between intercontinentally disjunct populations of S. flexuosum.
Gene flow could potentially result in merger of two differentiated clades. However, even if hybridization is still occurring between S. recurvum and S. flexuosum in eastern North America, this appears unlikely because the rate of gene flow is low. If the value of parameters in the best demographic model are assumed to be accurate, the value of N e m (number of individuals migrating per generation) between S. flexuosum and S. recurvum is 452*(1.13*10−4) = 0.051, or around 1 individual per 20 generation. Under Wright's Island model for haploid organisms, , in order to have an equilibrium F st of 0.1, the value of N e m has to be 4.5 individuals per generation (Cutter, 2019). The current rate of gene flow between S. flexuosum and S. recurvum is too low to homogenize the two species in the long run.
4.2. Relative genetic diversity and intercontinental gene flow in S. flexuosum
The last glacial maximum caused huge changes in species distributions and genetic structure of organisms in the Northern Hemisphere (Abbott & Brochmann, 2003; Hewitt, 2000). Plants in areas previously covered by ice sheets could have survived in local refugia or could have been extirpated and recolonized from another continent after the ice sheet receded. It has been shown that for angiosperms, Europe suffered more diversity losses during the last glacial maximum than did those in North America (Adams & Woodward, 1989; Svenning, 2003). With its intercontinental amphi‐Atlantic distribution, and with most of its current distribution located in areas previously glaciated, S. flexuosum provides an opportunity to compare genetic diversity between glaciated and unglaciated regions in Europe and eastern North America.
Due to the limited sampling range of EUR S. flexuosum samples, a generalized comparison of genetic diversity between S. flexuosum in eastern North America and Europe cannot be made with the sample we have. In order to compare samples from the two continents, we selected two subsets of ENA S. flexuosum samples from smaller regions: Maryland and central New York. Our estimates indicate that European plants have lower nucleotide diversity than both of the eastern North American regions. Moreover, within eastern North America, S. flexuosum plants from the unglaciated region (Maryland) had higher nucleotide diversity than plants from glaciated regions (central New York) (Table 2). Since all of EUR S. flexuosum samples were collected from central Norway, which is also glaciated, this suggests that plants in unglaciated areas have higher genetic diversity than plants in glaciated areas. Nevertheless, this observation needs to be tested with additional sampling from more glaciated and unglaciated areas.
Previous analyses of phylogenetic structure have shown that European S. flexuosum forms a monophyletic group that is nested within eastern North American plants (Duffy et al., 2020), suggesting that extant plants originated in North America and subsequently expanded to Europe. This inference coincides with our best demographic model, which indicates that gene flow has occurred in one direction from eastern North America to Europe. European and North American plants of S. flexuosum are clearly genetically similar, with F st values much lower than interspecific F st of either S. flexuosum group (European, North American) with S. recurvum. Moreover, there are no fixed differences between S. flexuosum in eastern North America versus Europe in contrast to thousands of fixed differences between S. recurvum and S. flexuosum at the species level (Table 1).
However, when considering demographic models with all possible migration events, migration rates and directions between S. flexuosum in Europe and eastern North America are not consistent (Tables B2 and B3). In the “full migration” model, the migration rate from eastern North America to Europe is 20 times higher than the rate from Europe to eastern North America. This pattern corresponds to the best demographic model. However, in the “full migration with secondary contact” model, the pattern is reversed; the migration rate from eastern North America to Europe is approximately half the migration rate from Europe to eastern North America. Nevertheless, the inference that S. flexuosum plants in Europe and eastern North America are connected by gene flow is consistent across most of the demographic models tested.
Integrating inferences about contrasting levels of genetic diversity between plants of S. flexuosum in North America and Europe, phylogenetic relationships among plants on the two continents, and evidence for intercontinental gene flow, we suggest the following historical scenario. Sphagnum flexuosum was extirpated in Europe during the LGM and was recolonized by North American plants during the Holocene. A founder effect associated with that recolonization gave rise to the lower level of genetic diversity in Europe relative to North America. An alternative scenario is that S. flexuosum originated in eastern North America and expanded its range to Europe, subsequent to speciation. Then, S. flexuosum plants in Europe have persisted through the glaciation periods in some refugia within the continent. A weak signal of introgression between European S. flexuosum and S. cuspidatulum, currently restricted to Asia, could suggest that during glaciation, S. flexuosum persisted in some part of Europe that is close to Asia. This is possible, although if that were the case, we might expect a stronger introgression signal between European S. flexuosum and S. capidatulum than we detected. The weak introgression signal detected here could have come from long‐distance dispersal between current populations of S. flexuosum in Europe and S. cuspidatulum in Asia. Although we cannot date the origin of S. flexuosum confidently because of the absence of fossils for calibration, recent estimates for the diversification of extant Sphagnum species suggest dates on the order of at least 10 million years ago (e.g., Shaw et al., 2010, 2019). Nevertheless, it seems unlikely that a genetic signature of any bottleneck associated with that ancient speciation process and range expansion persists today. Another important signature of recent population divergence is that there is no fixed difference between S. flexuosum in eastern North America and Europe. Thus, the difference in genetic diversity detected here is more likely to be caused by recent events and possibly associated with glaciation. This scenario corresponds to an earlier work by Ledent et al. (2019) which showed that post‐glacial assembly of European bryophytes involves high contribution of migrants from other continents.
Stenøien et al. (2011) inferred from microsatellite data that European plants of the the amphi‐Atlantic species Sphagnum angermanicum were established relatively recently from eastern North America plants via long‐distance dispersal. European plants of S. angermanicum, like those of S. flexuosum, are less genetically diverse than are those in eastern North America. In contrast, demographic analyses of other amphi‐Atlantic bryophytes have shown that levels of genetic diversity in European and North American populations are similar; bottleneck events of similar magnitudes have also been inferred on both continents (Désamoré et al., 2016). This is also the case in some circumarctic angiosperms (Brochmann & Brysting, 2008). Such demographic patterns could reflect the occurrence of northern refugia in both Europe and North America where both bryophytes and Arctic angiosperms could survive the glaciation. This discrepancy in the effects of the LGM can be explained by the difference in plant response to climate during the LGM. Paleoclimactic data have shown that ice‐free areas in Europe were drier than in eastern North America, which can produce severe effects on plants that cannot tolerate drought (Svenning, 2003). A study using species distribution modeling on European trees during the LGM has shown that boreal species have existed in northern refugia across the plains of Central and Eastern Europe, while nemoral species were restricted to southern refugia such as the Mediterranean and Black Sea regions (Svenning et al., 2008). Furthermore, a comparison of niche requirements of the relictual and extinct plant taxa in Europe has shown that relictual taxa are more cold and drought‐tolerant than the extinct taxa (Svenning, 2003). Studies of genetic diversity of bryophytes within glaciated and unglaciated areas of Europe also yielded similar patterns. Plants of the epiphytic bryophyte Leucodon sciuroides, which relies on host trees, have lower genetic diversity in glaciated areas than unglaciated areas (Cronberg, 2000). On the other hand, the cold‐tolerant Hylocomium splendens appears to have a center of genetic diversity in Northern Scandinavia, which was glaciated (Cronberg et al., 1997). Thus, since Sphagnum requires mesic habitats, it is reasonable to expect that Sphagnum in Europe would have been affected by the LGM in ways similar to temperate angiosperms that were less able to tolerate drought.
4.3. Limitations associated with the inference of demographic models
Our results provide some clear inferences about genetic diversity and gene flow (both intraspecific and interspecific) in S. flexuosum and S. recurvum. There are, nevertheless, important limitations and uncertainty associated with the data and approaches used in this study. With regard to sampling, all our collections of S. flexuosum in Europe came from central Norway (Figure 2; Table A1), even though S. flexuosum is widespread in Europe. Furthermore, central Norway is the northern edge of S. flexuosum in Europe (Laine et al., 2018) and might not represent the actual genetic diversity of S. flexuosum in Europe. Thus, the effective population size of EUR S. flexuosum as inferred from the demographic model might be lower than the actual value. Instead of using effective population size estimates from the demographic model, we reduce the scope of the question to only comparing the genetic diversity of S. flexuosum in glaciated areas and unglaciated areas within Europe and eastern North America. In this case, genetic diversity was used as a proxy for effective population size since the two values are correlated according to the neutral model (Ellegren & Galtier, 2016; Kimura, 1983). Variation in mutation rates can alter the relationship between genetic diversity and effective population size, but since this study focuses on plants from the same species, it can be assumed that the mutation rates are similar in all the groups being compared. There are empirical evidence showing positive correlation between genetic diversity and effective population sizes (Hague & Routman, 2016; Leimu et al., 2006).
There are also caveats regarding the interpretation of demographic models. Sphagnum life history does not strictly correspond to the Wright–Fisher model used in SFS simulations. The mutation rate of Sphagnum is unknown, and the default value of 2.8 × 10−8 mutations per site per generation was used in this study. Estimated values for demographic parameters should be considered relative values, not absolute values.
Moreover, in complex demographic models, different combinations of parameters can give similar approximate likelihoods. The most complex model in this study contained 19 parameters, and it can be difficult to reach the global optimum in parameter space. For some models, there can be a set of parameters that explain the data even better than the best model, but those set of parameters were not evaluated. It is also possible that 100 independent runs per model are not enough to adequately cover the parameter space. This can be problematic if there are multiple demographic models with similar approximate likelihood but have substantially different values of demographic parameters. In this case, it will be difficult to determine the best demographic model. Thus, in addition to the best demographic model reported here, it is prudent to compare parameter estimates of the best model with other models that have similar approximate likelihoods, especially the “full migration model” which contains all demographic parameters.
5. CONCLUSIONS
This study supports the interpretation that S. flexuosum in glaciated areas has lower genetic diversity than unglaciated areas, that plants in Europe are derived from eastern North America, and that the population systems disjunct across the Atlantic Ocean are still connected by gene flow. Interspecific gene flow between S. flexuosum and S. recurvum occurred in at least two phases: before and after population divergence of S. flexuosum. Gene flow before population divergence of S. flexuosum has much higher magnitude than gene flow after population divergence, and it occurred through secondary contact. Gene flow after population divergence of S. flexuosum occurred only between sympatric plants in eastern North America.
AUTHOR CONTRIBUTIONS
Karn Imwattana: Conceptualization (equal); formal analysis (lead); investigation (lead); writing – original draft (lead); writing – review and editing (equal). Aaron Duffy: Data curation (supporting). Blanka Aguero: Data curation (supporting). Jon Shaw: Conceptualization (supporting); supervision (lead); writing – review and editing (equal).
ACKNOWLEDGMENTS
We would like to thank George Tiley for useful discussions about the inference of demographic models. We would like to thank Rossarin Pollawath for helping with S. cuspidatulum sample collection. The two anonymous reviewers have provided constructive suggestions. The corresponding author has been supported by the Department of Biology, Duke University, and the Queen Sirikit Scholarship, the Crown Property Bureau, Thailand. Genome assembly and annotation for the S. divinum reference (v1.1) is available at Phytozome (https://phytozome‐next.jgi.doe.gov/) This research was supported by NSF grant DEB‐1928514.
APPENDIX A.
TABLE A1.
Voucher information of the specimens used in this study, for more information see Duffy et al. (2020).
| DNA isolate | Species | Geographic region | Collectors | Col. nr. | Country | State/province | County/district | Collection date | Latitude | Longtitude |
|---|---|---|---|---|---|---|---|---|---|---|
| SB4974 | S. flexuosum | Eastern North America | A. Garrett | A030 | USA | West Virginia | Grant Co. | 20‐Jun‐13 | 39.45417 | −79.38663 |
| SB4975 | S. flexuosum | Eastern North America | A. Garrett | A031 | USA | West Virginia | Grant Co. | 20‐Jun‐13 | 39.45417 | −79.38663 |
| SB4976 | S. flexuosum | Eastern North America | A. Garrett | A033 | USA | Maryland | Garrett Co. | 20‐Jun‐13 | 39.56775 | −79.29842 |
| SB4980 | S. flexuosum | Eastern North America | A. Garrett | A043 | USA | Maryland | Garrett Co. | 21‐Jun‐13 | 39.56448 | −79.23805 |
| SB4985 | S. flexuosum | Eastern North America | B. Shaw | 18,906 | USA | Maryland | Garrett Co. | 21‐Jun‐13 | 39.5645 | −79.2382 |
| SB4987 | S. flexuosum | Eastern North America | B. Shaw | 1107a/1 | USA | Maryland | Garrett Co. | 21‐Jun‐13 | 39.66966 | −79.08571 |
| SB4996 | S. flexuosum | Eastern North America | A. Garrett | A065 | USA | Maryland | Garrett Co. | 21‐Jun‐13 | 39.66565 | −79.08935 |
| SB4997 | S. flexuosum | Eastern North America | A. Garrett | A066 | USA | Maryland | Garrett Co. | 21‐Jun‐13 | 39.66565 | −79.08935 |
| SB5002 | S. flexuosum | Eastern North America | B. Shaw | 18927 | USA | Pennsylvania | Elk Co. | 22‐Jun‐13 | 41.6802 | −78.5248 |
| SB5003 | S. flexuosum | Eastern North America | B. Shaw | 18930 | USA | Pennsylvania | Elk Co. | 22‐Jun‐13 | 41.6802 | −78.5248 |
| SB5007 | S. flexuosum | Eastern North America | B. Shaw | 18937 | USA | Pennsylvania | Elk Co. | 22‐Jun‐13 | 41.6808 | −78.5253 |
| SB5009 | S. flexuosum | Eastern North America | A. Garrett | A075 | USA | Pennsylvania | McKean Co | 22‐Jun‐13 | 41.68032 | −78.52475 |
| SB5011 | S. flexuosum | Eastern North America | A. Garrett | A083 | USA | Pennsylvania | McKean Co | 22‐Jun‐13 | 41.68055 | −78.525 |
| SB5019 | S. flexuosum | Eastern North America | A. Garrett | A100 | USA | New York | St. Lawrence Co. | 23‐Jun‐13 | 44.15123 | −74.9561 |
| SB5022 | S. flexuosum | Eastern North America | B. Shaw | 18953 | USA | New York | St. Lawrence Co. | 23‐Jun‐13 | 44.1511 | −74.9562 |
| SB5023 | S. flexuosum | Eastern North America | B. Shaw | 18957 | USA | New York | St. Lawrence Co. | 23‐Jun‐13 | 44.1511 | −74.9562 |
| SB5024 | S. flexuosum | Eastern North America | B. Shaw | 18958 | USA | New York | St. Lawrence Co. | 23‐Jun‐13 | 44.1511 | −74.9562 |
| SB5025 | S. flexuosum | Eastern North America | B. Shaw | 18959 | USA | New York | St. Lawrence Co. | 23‐Jun‐13 | 44.1511 | −74.9562 |
| SB5039 | S. flexuosum | Eastern North America | B. Shaw | 18974 | USA | New York | Franklin Co | 23‐Jun‐13 | 44.4262 | −74.245 |
| SB5042 | S. flexuosum | Eastern North America | M. Reeve | 660 | USA | ME | Waldo Co. | 10‐Nov‐10 | 44.41333 | −69.01833 |
| SB5043 | S. flexuosum | Eastern North America | J. Shaw | 16491 | USA | Maryland | Garrett Co. | 20‐Jun‐13 | 39.5975 | −79.27472 |
| SB5091 | S. flexuosum | Eastern North America | B. Shaw | 18992 | USA | New York | Rensselaer Co. | 24‐Jun‐13 | 42.6449 | −73.4094 |
| SB5106 | S. flexuosum | Eastern North America | B. Shaw | 18,935 | USA | Pennsylvania | McKean Co | 22‐Jun‐13 | 41.6808 | −78.5253 |
| SB5108 | S. flexuosum | Eastern North America | A. Garrett | A074 | USA | Pennsylvania | McKean Co | 22‐Jun‐13 | 41.68032 | −78.52475 |
| SB5120 | S. flexuosum | Eastern North America | A. Garrett | A126 | USA | New York | Warren Co. | 24‐Jun‐13 | 43.67917 | −73.78852 |
| SB5121 | S. flexuosum | Eastern North America | A. Garrett | A127 | USA | New York | Warren Co. | 24‐Jun‐13 | 43.67892 | −73.78842 |
| AG198 | S. flexuosum | Europe | A. Garrett | A161 | Norway | Sør‐Trøndelag | Trondheim | 12‐Aug‐13 | 63.4224 | 10.26592 |
| AG201 | S. flexuosum | Europe | A. Garrett | A166 | Norway | Sør‐Trøndelag | Trondheim | 12‐Aug‐13 | 63.42235 | 10.26598 |
| AG209 | S. flexuosum | Europe | A. Garrett | A178 | Norway | Sør‐Trøndelag | Orkdal | 13‐Aug‐13 | 63.36198 | 9.85645 |
| AG231 | S. flexuosum | Europe | A. Garrett | A214 | Norway | Sør‐Trøndelag | Melhus | 14‐Aug‐13 | 63.30583 | 10.38778 |
| AG234 | S. flexuosum | Europe | A. Garrett | A219 | Norway | Sør‐Trøndelag | Melhus | 14‐Aug‐13 | 63.3043 | 10.38412 |
| AG239 | S. flexuosum | Europe | A. Garrett | A227 | Norway | Sør‐Trøndelag | Klæbū | 14‐Aug‐13 | 63.30897 | 10.39018 |
| AG247 | S. flexuosum | Europe | A. Garrett | A241 | Norway | Nord‐Trøndelag | Steinkjer | 15‐Aug‐13 | 63.93202 | 11.692 |
| AG257 | S. flexuosum | Europe | A. Garrett | A258 | Norway | Nord‐Trøndelag | Grong | 15‐Aug‐13 | 64.3565 | 12.33225 |
| AG259 | S. flexuosum | Europe | A. Garrett | A260 | Norway | Nord‐Trøndelag | Grong | 15‐Aug‐13 | 64.3565 | 12.33225 |
| AG263 | S. flexuosum | Europe | A. Garrett | A264 | Norway | Nord‐Trøndelag | Grong | 15‐Aug‐13 | 64.3567 | 12.33262 |
| AG268 | S. flexuosum | Europe | A. Garrett | A274 | Norway | Nord‐Trøndelag | Høylandet | 16‐Aug‐13 | 64.66405 | 12.17537 |
| AG271 | S. flexuosum | Europe | A. Garrett | A280 | Norway | Nord‐Trøndelag | Høylandet | 16‐Aug‐13 | 64.65497 | 12.17522 |
| AG273 | S. flexuosum | Europe | A. Garrett | A282 | Norway | Nord‐Trøndelag | Høylandet | 16‐Aug‐13 | 64.65493 | 12.17517 |
| AG281 | S. flexuosum | Europe | A. Garrett | A291 | Norway | Sor‐Trøndelag | Klæbū | 17‐Aug‐13 | 63.2495 | 10.45597 |
| AG395 | S. flexuosum | Europe | A. Garrett | A278 | Norway | Nord‐Trøndelag | Høylandet | 16‐Aug‐13 | 64.6577 | 12.18405 |
| SB5361 | S. flexuosum | Europe | A. Garrett | A282 | Norway | Nord‐Trøndelag | Høylandet | 16‐Aug‐13 | 63.30897 | 10.39018 |
| SB4977 | S. flexuosum | Eastern North America | A. Garrett | A037 | USA | Maryland | Garrett Co. | 21‐Jun‐13 | 39.56513 | −79.23867 |
| SB5241 | S. flexuosum | Eastern North America | A. Garrett | A146 | USA | Pennsylvania | Pike Co. | 25‐Jun‐13 | 41.3794 | −75.0792 |
| SB4982 | S. recurvum | Eastern North America | A. Garrett | A045 | USA | Maryland | Garrett Co. | 21‐Jun‐13 | 39.56362 | −79.23695 |
| SB4983 | S. recurvum | Eastern North America | B. Shaw | 18904 | USA | Maryland | Garrett Co. | 21‐Jun‐13 | 39.5645 | −79.2382 |
| SB4986 | S. recurvum | Eastern North America | B. Shaw | 18907 | USA | Maryland | Garrett Co. | 21‐Jun‐13 | 39.5645 | −79.2382 |
| SB4989 | S. recurvum | Eastern North America | B. Shaw | 18916 | USA | Maryland | Garrett Co. | 21‐Jun‐13 | 39.6669 | −79.0873 |
| SB4995 | S. recurvum | Eastern North America | A. Garrett | A064 | USA | Maryland | Garrett Co. | 21‐Jun‐13 | 39.66565 | −79.08935 |
| SB5016 | S. recurvum | Eastern North America | B. Shaw | 18,945 | USA | New York | Chenango Co. | 22‐Jun‐13 | 42.496 | −75.8268 |
| SB5045 | S. recurvum | Eastern North America | J. Atwood | 8 | USA | Connecticut | New London Co. | 28‐Jul‐02 | 41.59333 | −71.87 |
| SB5048 | S. recurvum | Eastern North America | T. Neily | 766 | Canada | Nova Scotia | Yarmouth Co. | 22‐Aug‐12 | 43.91326 | −65.88168 |
| SB5109 | S. recurvum | Eastern North America | A. Garrett | A142 | USA | Pennsylvania | Pike Co. | 25‐Jun‐13 | 41.3794 | −75.0792 |
| SB5113 | S. recurvum | Eastern North America | B. Shaw | 18,941 | USA | New York | Chenango Co. | 22‐Jun‐13 | 42.4966 | −75.8275 |
| SB5136 | S. recurvum | Eastern North America | B. Shaw | 17,793 | USA | North Carolina | Dare Co. | 9‐Dec‐13 | 35.80115 | −75.88329 |
| SB5140 | S. recurvum | Eastern North America | Ferreira M. | 1 | Portugal | Azores | Terceira | 4‐Jul‐08 | 38.73 | −27.27 |
| SB5196 | S. recurvum | Eastern North America | B. Aguero | 19,470 | USA | North Carolina | Dare Co. | 17‐Apr‐18 | 35.80102 | −75.88355 |
| SB5231 | S. recurvum | Eastern North America | B. Piatkowski | 2018‐40 | USA | Florida | Franklin | 17‐May‐18 | 30.00172 | −85.00005 |
| SB5233 | S. recurvum | Eastern North America | B. Aguero | M02A/2 | USA | North Carolina | Transylvania Co. | 01‐May‐18 | 35.35137 | −82.77266 |
| SB5234 | S. recurvum | Eastern North America | B. Aguero | 19,605 | USA | North Carolina | Brunswick Co. | 04‐Jun‐18 | 34.09144 | −75.88355 |
| AG255 | S. fallax | Europe | A. Garrett | A254 | Norway | Nord‐Trøndelag | Sltaeginkjer | 15‐Aug‐13 | 63.93458 | 11.69667 |
| SB5032 | S. fallax | Eastern North America | B. Aguero | 18,965 | USA | New York | Franklin Co. | 23‐Jun‐13 | 44.4258 | −74.2436 |
| IUSS | S. cuspidatulum | Asia | K. Imwattana | KI‐149 | Thailand | Chiang Mai | Chiang Mai | 4‐Jul‐18 | 18.58885 | 98.48516 |
APPENDIX B.
Demographic models tested in this study.
FIGURE B1.

Diagram showing variable names for the demographic parameters in Figures B2, B3, B4, B5 and Tables B1
TABLE B1.
List of all tested demographic models and the inferred parameters, see Figure B1 for the definition of parameter names.
| Model name | NPOP1 | NPOP2 | NPOP3 | NANC1 | NANC2 | TDIV1 | TDIV2 | TSIO | migr12 | migr13 | migr21 | migr23 | migr31 | migr32 | manc13 | manc31 | k | Lhood | AIC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full_migration | 141 | 478 | 893 | 240 | 6554 | 208 | 3139 | 7.55 E‐04 | 2.85 E‐07 | 0.0151132 | 8.56 E‐07 | 7.17 E‐06 | 9.57 E‐05 | 6.72 E‐05 | 0.0010497 | 17 | −14700.7 | 67733.2 | |
| no_migration | 1562 | 3894 | 3842 | 3095 | 19,950 | 239 | 3666 | 9 | −14831.0 | 68317.4 | |||||||||
| recent_migration | 259 | 295 | 681 | 2884 | 2625 | 545 | 743 | 0.012888 | 1.72 E‐07 | 0.0021118 | 3.97 E‐05 | 1.91 E‐04 | 1.03 E‐06 | 15 | −14782.2 | 68104.5 | |||
| nomig12 | 106 | 400 | 763 | 255 | 3749 | 223 | 2254 | 1.51 E‐08 | 0.0238204 | 4.15 E‐06 | 4.97 E‐05 | 1.48 E‐04 | 8.72 E‐05 | 0.0010908 | 16 | −14694.1 | 67700.7 | ||
| nomig13 | 2947 | 6594 | 13,519 | 2825 | 98,886 | 2228 | 37,768 | 2.04 E‐04 | 5.99 E‐04 | 1.33 E‐08 | 4.31 E‐08 | 1.61 E‐07 | 1.88 E‐08 | 8.73 E‐05 | 16 | −14691.5 | 67688.9 | ||
| nomig21 | 925 | 499 | 1871 | 403 | 10,640 | 461 | 4719 | 0.0077547 | 3.42 E‐09 | 4.07 E‐07 | 4.46 E‐05 | 1.27 E‐05 | 3.58 E‐09 | 6.83 E‐04 | 16 | −14721.8 | 67828.2 | ||
| nomig23 | 1736 | 1256 | 4526 | 1124 | 30,897 | 853 | 12,209 | 0.0030612 | 1.54 E‐07 | 2.59 E‐05 | 8.00 E‐06 | 1.32 E‐06 | 1.48 E‐08 | 2.64 E‐04 | 16 | −14700.1 | 67728.5 | ||
| nomig31 | 23 | 84 | 176 | 44 | 795 | 38 | 400 | 1.38 E‐05 | 2.55 E‐05 | 0.1347102 | 9.47 E‐05 | 5.61 E‐04 | 4.93 E‐06 | 0.0062277 | 16 | −14708.8 | 67768.6 | ||
| nomig32 | 154 | 110 | 346 | 76 | 5213 | 54 | 1348 | 0.0430574 | 1.11 E‐07 | 4.41 E‐05 | 5.68 E‐05 | 2.30 E‐07 | 4.41 E‐06 | 0.0034757 | 16 | −14710.9 | 67778.2 | ||
| nomig_a13 | 36 | 203 | 275 | 53 | 6054 | 65 | 1053 | 2.12 E‐05 | 1.85 E‐07 | 0.0762748 | 1.33 E‐05 | 1.84 E‐07 | 4.33 E‐05 | 0.0051892 | 16 | −14696.4 | 67711.4 | ||
| nomig_a31 | 598 | 791 | 1431 | 3070 | 20,615 | 1445 | 8005 | 0.0057039 | 9.35 E‐09 | 0.0013189 | 4.12 E‐08 | 7.70 E‐05 | 2.33 E‐05 | 0.0032208 | 16 | −14752.0 | 67967.4 | ||
| model1 | 41 | 163 | 241 | 46 | 2799 | 41 | 782 | 0.0664278 | 1.26 E‐05 | 2.78 E‐05 | 1.40 E‐07 | 0.0054293 | 14 | −14713.7 | 67786.9 | ||||
| model2 | 34 | 151 | 261 | 67 | 1587 | 83 | 598 | 0.0857 | 5.35 E‐05 | 4.53 E‐07 | 3.13 E‐04 | 7.70 E‐08 | 0.0046777 | 15 | −14687.0 | 67666.0 | |||
| model3 | 45 | 183 | 367 | 122 | 4215 | 53 | 2093 | 2.43 E‐05 | 0.057202 | 8.75 E‐08 | 1.44 E‐04 | 2.27 E‐04 | 0.0017993 | 15 | −14703.8 | 67743.5 | |||
| model4 | 112 | 453 | 782 | 157 | 46,458 | 200 | 3726 | 5.16 E‐04 | 0.0303858 | 5.39 E‐07 | 3.96 E‐05 | 7.78 E‐08 | 0.0020168 | 15 | −14712.0 | 67781.4 | |||
| model5 | 36 | 137 | 225 | 51 | 1369 | 54 | 528 | 0.0684546 | 1.97 E‐08 | 2.48 E‐04 | 0.0053256 | 13 | −14692.0 | 67685.4 | |||||
| model6 | 51 | 213 | 312 | 73 | 1468 | 54 | 652 | 0.0569785 | 2.89 E‐05 | 1.30 E‐04 | 1.18 E‐07 | 0.003058 | 14 | −14691.7 | 67685.7 | ||||
| model7 | 211 | 1060 | 1598 | 331 | 11,533 | 208 | 4697 | 0.0134329 | 2.36 E‐08 | 7.47 E‐08 | 6.24 E‐07 | 6.92 E‐04 | 14 | −14697.0 | 67710.3 | ||||
| model8 | 919 | 3266 | 5489 | 1240 | 36,042 | 2177 | 14,000 | 1.46 E‐06 | 0.0029642 | 7.92 E‐09 | 1.83 E‐05 | 2.61 E‐04 | 14 | −14696.4 | 67707.4 | ||||
| model9 | 96 | 289 | 585 | 122 | 3830 | 91 | 1653 | 2.69 E‐07 | 0.0340505 | 1.90 E‐06 | 6.94 E‐05 | 0.0019851 | 14 | −14697.4 | 67712.2 | ||||
| model10 | 49 | 170 | 307 | 58 | 9279 | 42 | 1416 | 0.0595581 | 1.52 E‐05 | 0.0040193 | 12 | −14681.4 | 67634.4 | ||||||
| model11 | 53 | 171 | 278 | 63 | 1215 | 29 | 705 | 0.0447198 | 2.17 E‐06 | 2.44 E‐07 | 0.0032825 | 13 | −14699.5 | 67719.8 | |||||
| model12 | 701 | 3312 | 5080 | 979 | 38,440 | 1542 | 12,062 | 0.0040156 | 1.22 E‐09 | 1.37 E‐05 | 2.80 E‐04 | 13 | −14694.4 | 67696.0 | |||||
| model13 | 105 | 370 | 634 | 140 | 3520 | 88 | 1516 | 0.021999 | 3.67 E‐06 | 4.07 E‐09 | 0.0016853 | 13 | −14701.0 | 67726.4 | |||||
| model14 | 3645 | 16,267 | 26,557 | 6074 | 145,983 | 4308 | 61,643 | 2.50 E‐06 | 7.27 E‐04 | 7.05 E‐08 | 3.85 E‐05 | 13 | −14698.0 | 67712.7 | |||||
| model15 | 60 | 40 | 109 | 20 | 1481 | 24 | 360 | 0.0857143 | 1.83 E‐08 | 3.66 E‐08 | 0.0129705 | 13 | −14714.4 | 67788.1 | |||||
| model16 | 16 | 82 | 134 | 28 | 600 | 17 | 269 | 0.1444892 | 0.0078581 | 11 | −14714.5 | 67784.8 | |||||||
| model17 | 1611 | 7187 | 8715 | 114,779 | 43,420 | 8624 | 9977 | 0.0014391 | 8.21 E‐06 | 12 | −14836.5 | 68348.7 | |||||||
| model18 | 15,596 | 29,750 | 46,581 | 31,654 | 174,203 | 2047 | 53,352 | 1.39 E‐06 | 3.03 E‐06 | 11 | −14764.5 | 68015.2 | |||||||
| early_gene_flow | 196 | 634 | 871 | 290 | 3095 | 58 | 1766 | 143 | 3.16 E‐06 | 4.40 E‐08 | 0.0126008 | 8.56 E‐06 | 8.29 E‐05 | 9.51 E‐05 | 2.09 E‐08 | 9.65 E‐04 | 18 | −14694.7 | 67707.5 |
| secondary_contact | 629 | 802 | 2306 | 68 | 177 | 441 | 27,447 | 871 | 0.003833 | 4.67 E‐06 | 0.002031 | 7.05 E‐08 | 3.70 E‐05 | 1.01 E‐06 | 9.66 E‐05 | 0.0042645 | 18 | −14681.2 | 67645.6 |
| model10_with_early_gene_flow | 2948 | 12,332 | 16,923 | 4239 | 109,882 | 2090 | 42,452 | 2730 | 8.53 E‐04 | 9.64 E‐07 | 6.89 E‐05 | 13 | −14715.0 | 67791.2 | |||||
| model10_with_secondary_contact | 45 | 245 | 452 | 21 | 5769 | 80 | 3098 | 274 | 0.052726 | 1.13 E‐04 | 0.013594 | 13 | −14659.2 | 67533.9 |
TABLE B2.
Demographic parameters and confidence intervals of the full migration model and model 10 (the best demographic model) with continuous ancestral migration and secondary contact, see Figure B1 for the definition of parameter names.
| RUN | NPOP1 | NPOP2 | NPOP3 | NANC1 | NANC2 | TDIV1 | TDIV2 | TISO | migr12 | migr13 | migr21 | migr23 | migr31 | migr32 | manc13 | manc31 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full migration | ||||||||||||||||
| Original_run | 141 | 478 | 893 | 240 | 6554 | 208 | 3139 | 7.55 E‐04 | 2.85 E‐07 | 0.015113 | 8.56 E‐07 | 7.17 E‐06 | 9.57 E‐05 | 6.72 E‐05 | 0.00105 | |
| mean | 301.79 | 979.99 | 1793.61 | 1962.77 | 9785.92 | 733.94 | 3615.44 | 0.005058 | 4.57 E‐05 | 0.086108 | 3.82 E‐05 | 3.01 E‐05 | 0.000527 | 0.00014 | 0.006146 | |
| median | 29 | 107 | 178 | 54 | 1042.5 | 37 | 555 | 5.11 E‐05 | 1.05 E‐06 | 0.074566 | 6.11 E‐07 | 1.24 E‐06 | 0.000293 | 1.06 E‐05 | 0.004333 | |
| SD | 1054.163 | 3362.891 | 6434.168 | 13664.72 | 42747.65 | 4557.508 | 11570.83 | 0.012711 | 9.89 E‐05 | 0.071233 | 9.02 E‐05 | 0.000101 | 0.000596 | 0.000308 | 0.006414 | |
| upper 95% CI | 508.406 | 1639.117 | 3054.707 | 4641.056 | 18164.46 | 1627.212 | 5883.322 | 0.007549 | 6.51 E‐05 | 0.100069 | 5.59 E‐05 | 4.99 E‐05 | 0.000644 | 0.0002 | 0.007403 | |
| lower 95% CI | 202.1424 | 658.7231 | 1194.887 | 1053.123 | 6225.686 | 415.0065 | 2462.309 | 0.003578 | 3.29 E‐05 | 0.066494 | 2.72 E‐05 | 2.04 E‐05 | 0.000401 | 0.000101 | 0.004695 | |
| Model 10 | ||||||||||||||||
| Original_run | 49 | 170 | 307 | 58 | 9279 | 42 | 1416 | 0.059558 | 1.52 E‐05 | 0.004019 | ||||||
| mean | 345.88 | 1029.2 | 1674.24 | 467.51 | 15536.13 | 197.48 | 4892.03 | 0.085014 | 5.35 E‐06 | 0.00432 | ||||||
| median | 51 | 172 | 272.5 | 61.5 | 2129.5 | 34 | 915.5 | 0.068852 | 1.74 E‐07 | 0.003497 | ||||||
| SD | 784.8463 | 2060.191 | 3304.299 | 1016.397 | 42606.33 | 410.0477 | 10308.92 | 0.077057 | 1.2 E‐05 | 0.004 | ||||||
| upper 95% CI | 499.7099 | 1432.997 | 2321.883 | 666.7237 | 23886.97 | 277.8493 | 6912.579 | 0.100117 | 7.71 E‐06 | 0.005104 | ||||||
| lower 95% CI | 247.9369 | 748.3325 | 1219.151 | 336.8322 | 10854.28 | 143.0215 | 3537.165 | 0.065391 | 3.84 E‐06 | 0.00332 | ||||||
| Full migration with secondary contact | ||||||||||||||||
| Original_run | 629 | 802 | 2306 | 68 | 177 | 441 | 27,447 | 871 | 0.003833 | 4.67 E‐06 | 0.002031 | 7.05 E‐08 | 3.7 E‐05 | 1.01 E‐06 | 9.66 E‐05 | 0.004265 |
| mean | 859.46 | 806.22 | 2526.23 | 356.77 | 13940.39 | 378.04 | 15306.57 | 3087.61 | 0.022695 | 2.52 E‐05 | 0.010984 | 1.46 E‐05 | 6.03 E‐05 | 8.09 E‐06 | 0.00022 | 0.007995 |
| median | 221.5 | 226 | 658 | 77 | 1315.5 | 87.5 | 6668 | 717 | 0.01289 | 1.56 E‐06 | 0.001609 | 4.29 E‐07 | 8.47 E‐06 | 1.94 E‐07 | 7.41 E‐05 | 0.003757 |
| SD | 2513.873 | 2047.719 | 6397.056 | 1285.493 | 42266.01 | 843.9441 | 21051.35 | 9919.357 | 0.029148 | 5.24 E‐05 | 0.021975 | 4.12 E‐05 | 0.000137 | 2.62 E‐05 | 0.000577 | 0.010577 |
| upper 95% CI | 1352.179 | 1207.573 | 3780.053 | 608.7265 | 22224.53 | 543.453 | 19432.63 | 5031.804 | 0.028408 | 3.54 E‐05 | 0.015291 | 2.26 E‐05 | 8.72 E‐05 | 1.32 E‐05 | 0.000333 | 0.010068 |
| lower 95% CI | 594.4329 | 569.5357 | 1785.34 | 237.4596 | 9584.383 | 271.5232 | 11497.77 | 2101.376 | 0.017127 | 1.82 E‐05 | 0.007987 | 1.01 E‐05 | 4.32 E‐05 | 5.5 E‐06 | 0.000155 | 0.006022 |
| Model 10 with secondary contact | ||||||||||||||||
| Original_run | 45 | 245 | 452 | 21 | 5769 | 80 | 3098 | 274 | 0.052726 | 0.000113 | 0.013594 | |||||
| mean | 405.74 | 1920.28 | 3083.07 | 978.77 | 17966.4 | 554.84 | 25922.16 | 3334.15 | 0.045681 | 2.08 E‐05 | 0.00962 | |||||
| median | 110.5 | 628 | 1076 | 65 | 1511.5 | 172 | 12653.5 | 755 | 0.024446 | 4.49 E‐06 | 0.004538 | |||||
| SD | 1338.282 | 4759.928 | 6134.585 | 4075.481 | 39986.49 | 946.531 | 32264.66 | 7749.457 | 0.047288 | 4.59 E‐05 | 0.011854 | |||||
| upper 95% CI | 668.0434 | 2853.226 | 4285.449 | 1777.564 | 25803.75 | 740.3601 | 32246.03 | 4853.044 | 0.05495 | 2.98 E‐05 | 0.011943 | |||||
| lower 95% CI | 274.8035 | 1361.048 | 2243.122 | 630.3674 | 12908.86 | 409.7294 | 19601.94 | 2382.953 | 0.034911 | 1.49 E‐05 | 0.007279 | |||||
FIGURE B2.

Variations in effective population sizes (N) inferred from parametric bootstrap of the “full migration model” and “model 10”.
FIGURE B3.
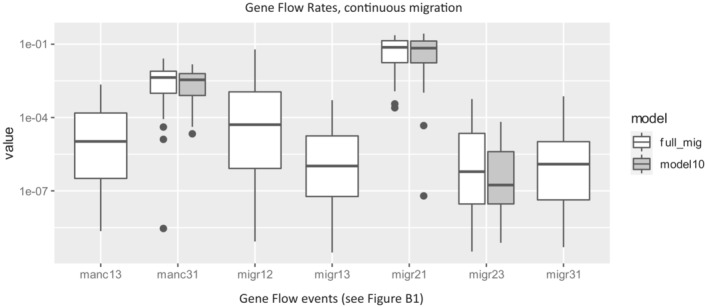
Variations in gene flow rates inferred from parametric bootstrap of the “full migration model” and “model 10”.
FIGURE B4.

Variations in effective population sizes inferred from parametric bootstrap of the “full migration model with secondary contact” and “model 10 with secondary contact”.
FIGURE B5.

Variations in gene flow rates inferred from parametric bootstrap of the “full migration model with secondary contact” and “model 10 with secondary contact”.
Imwattana, K. , Aguero, B. , Duffy, A. , & Shaw, A. J. (2022). Demographic history and gene flow in the peatmosses Sphagnum recurvum and Sphagnum flexuosum (Bryophyta: Sphagnaceae). Ecology and Evolution, 12, e9489. 10.1002/ece3.9489
DATA AVAILABILITY STATEMENT
In silico digested reads from a genomic resequencing sample of S. cuspidatulum (Library IUSS) are available in Dryad (https://doi.org/10.5061/dryad.1c59zw3xc). Demultiplexed Illumina reads from RAD‐seq samples of S. flexuosum, S. recurvum, and S. fallax are available in Dryad https://doi.org/10.5061/dryad.1g1jwsts7 (Duffy et al., 2020). For the list of samples used in this study, see Appendix A.
REFERENCES
- Abbott, R. , Albach, D. , Ansell, S. , Arntzen, J. W. , Baird, S. J. E. , Bierne, N. , Boughman, J. , Brelsford, A. , Buerkle, C. A. , Buggs, R. , Butlin, R. K. , Dieckmann, U. , Eroukhmanoff, F. , Grill, A. , Cahan, S. H. , Hermansen, J. S. , Hewitt, G. , Hudson, A. G. , Jiggins, C. , … Zinner, D. (2013). Hybridization and speciation. Journal of Evolutionary Biology, 26(2), 229–246. 10.1111/j.1420-9101.2012.02599.x [DOI] [PubMed] [Google Scholar]
- Abbott, R. J. , & Brochmann, C. (2003). History and evolution of the arctic flora: in the footsteps of Eric Hultén. Molecular Ecology, 12(2), 299–313. 10.1046/j.1365-294x.2003.01731.x [DOI] [PubMed] [Google Scholar]
- Adams, J. M. , & Woodward, F. I. (1989). Patterns in tree species richness as a test of the glacial extinction hypothesis. Nature, 339(6227), 699–701. 10.1038/339699a0 [DOI] [Google Scholar]
- Bagley, R. K. , Sousa, V. C. , Niemiller, M. L. , & Linnen, C. R. (2017). History, geography and host use shape genomewide patterns of genetic variation in the redheaded pine sawfly (Neodiprion lecontei). Molecular Ecology, 26(4), 1022–1044. 10.1111/mec.13972 [DOI] [PubMed] [Google Scholar]
- Beichman, A. C. , Huerta‐Sanchez, E. , & Lohmueller, K. E. (2018). Using genomic data to infer historic population dynamics of nonmodel organisms. Annual Review of Ecology, Evolution, and Systematics, 49(1), 433–456. 10.1146/annurev-ecolsys-110617-062431 [DOI] [Google Scholar]
- Brochmann, C. , & Brysting, A. K. (2008). The Arctic – An evolutionary freezer? Plant Ecology & Diversity, 1(2), 181–195. 10.1080/17550870802331904 [DOI] [Google Scholar]
- Cronberg, N. (1989). Patterns of variation in morphological characters and isoenzymes in populations of Sphagnum capillifolium (Ehrh.) Hedw. And S. rubellum Wils. From two bogs in southern Sweden. Journal of Bryology, 15(4), 683–696. 10.1179/jbr.1989.15.4.683 [DOI] [Google Scholar]
- Cronberg, N. (1998). Population structure and interspecific differentiation of the peat moss sister species Sphagnum rubellum and S. capillifolium (Sphagnaceae) in northern Europe. Plant Systematics and Evolution, 209, 139–158. [Google Scholar]
- Cronberg, N. (2000). Genetic diversity of the epiphytic bryophyte Leucodon sciuroides in formerly glaciated versus nonglaciated parts of Europe. Heredity, 84(6), 710–720. 10.1046/j.1365-2540.2000.00719.x [DOI] [PubMed] [Google Scholar]
- Cronberg, N. , Molau, U. , & Sonesson, M. (1997). Genetic variation in the clonal bryophyte Hylocomium splendens at hierarchical geographical scales in Scandinavia. Heredity, 78(3), 293–301. 10.1038/hdy.1997.44 [DOI] [Google Scholar]
- Cronberg, N. , & Natcheva, R. (2002). Hybridization between the peat moss, Sphagnum capillifolium, and S. Quinquefarium (Sphagnaceae, Bryophyta) as inferred by morphological characters and isozyme markers. Plant Systematics and Evolution, 234, 53‐70. [Google Scholar]
- Cutter, A. D. (2019). A primer of molecular population genetics. Oxford University Press. [Google Scholar]
- Désamoré, A. , Patiño, J. , Mardulyn, P. , Mcdaniel, S. F. , Zanatta, F. , Laenen, B. , & Vanderpoorten, A. (2016). High migration rates shape the postglacial history of amphi‐Atlantic bryophytes. Molecular Ecology, 25(21), 5568–5584. 10.1111/mec.13839 [DOI] [PubMed] [Google Scholar]
- Duffy, A. M. , Aguero, B. , Stenøien, H. K. , Flatberg, K. I. , Ignatov, M. S. , Hassel, K. , & Shaw, A. J. (2020). Phylogenetic structure in the Sphagnum recurvum complex (Bryophyta) in relation to taxonomy and geography. American Journal of Botany, 107(9), 1283–1295. 10.1002/ajb2.1525 [DOI] [PubMed] [Google Scholar]
- Duffy, A. M. , Ricca, M. , Robinson, S. , Aguero, B. , Johnson, M. G. , Stenøien, H. K. , Flatberg, K. I. , Hassel, K. , & Shaw, A. J. (2022). Heterogeneous genetic structure in eastern north American peat mosses (sphagnum). Biological Journal of the Linnean Society, 135(4), 692–707. 10.1093/biolinnean/blab175 [DOI] [Google Scholar]
- Eaton, D. A. R. (2014). PyRAD: Assembly of de novo RADseq loci for phylogenetic analyses. Bioinformatics, 30(13), 1844–1849. 10.1093/bioinformatics/btu121 [DOI] [PubMed] [Google Scholar]
- Eddy, A. (1977). Sphagnales of tropical Asia. Bulletin of the British Museum (Nat. Hist.) Botany, 5, 359–445. [Google Scholar]
- Edelman, N. B. , & Mallet, J. (2021). Prevalence and adaptive impact of introgression. Annual Review of Genetics, 55(1), 1–19. 10.1146/annurev-genet-021821-020805 [DOI] [PubMed] [Google Scholar]
- Ekblom, R. , & Galindo, J. (2011). Applications of next generation sequencing in molecular ecology of non‐model organisms. Heredity, 107(1), 1–15. 10.1038/hdy.2010.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren, H. , & Galtier, N. (2016). Determinants of genetic diversity. Nature Reviews Genetics, 17(7), 422–433. 10.1038/nrg.2016.58 [DOI] [PubMed] [Google Scholar]
- Ellstrand, N. C. (2014). Is gene flow the most important evolutionary force in plants? American Journal of Botany, 101(5), 737–753. 10.3732/ajb.1400024 [DOI] [PubMed] [Google Scholar]
- Excoffier, L. , Dupanloup, I. , Huerta‐Sánchez, E. , Sousa, V. C. , & Foll, M. (2013). Robust demographic inference from genomic and SNP data. PLoS Genetics, 9(10), e1003905. 10.1371/journal.pgen.1003905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatberg, K. I. (1992). European taxa in the Sphagnum recurvum complex. 2. Amended descriptions of Sphagnum brevifolium and S. fallax . Lindbergia: A Journal of Bryology, 17, 96‐110. [Google Scholar]
- Frahm, J. P. , & Vitt, D. H. (1993). Comparisons between the moss floras of North America and Europe. Nova Hedwigia, 56(3–4), 307–333. [Google Scholar]
- Gignac, L. D. , Halsey, L. A. , & Vitt, D. H. (2000). A bioclimatic model for the distribution of sphagnum‐dominated peatlands in North America under present climatic conditions. Journal of Biogeography, 27(5), 1139–1151. 10.1046/j.1365-2699.2000.00458.x [DOI] [Google Scholar]
- Gorham, E. (1991). Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecological Applications, 1(2), 182–195. 10.2307/1941811 [DOI] [PubMed] [Google Scholar]
- Green, R. E. , Krause, J. , Briggs, A. W. , Maricic, T. , Stenzel, U. , Kircher, M. , Patterson, N. , Li, H. , Zhai, W. , & Fritz, M. H.‐Y. (2010). A draft sequence of the Neandertal genome. Science, 328, 710–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague, M. T. J. , & Routman, E. J. (2016). Does population size affect genetic diversity? A test with sympatric lizard species. Heredity, 116(1), 92–98. 10.1038/hdy.2015.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt, G. M. (1996). Some genetic consequences of ice ages, and their role in divergence and speciation. Biological Journal of the Linnean Society, 58(3), 247–276. 10.1006/bijl.1996.0035 [DOI] [Google Scholar]
- Karlin, E. F. , Gardner, G. P. , Lukshis, K. , Boles, S. , & Shaw, A. J. (2010). Allopolyploidy in Sphagnum mendocinum and S. papillosum (Sphagnaceae). The Bryologist, 113(1), 114–119. 10.1639/0007-2745-113.1.114 [DOI] [Google Scholar]
- Kimura, M. (1983). The neutral theory of molecular evolution. Cambridge University Press. [Google Scholar]
- Kyrkjeeide, M. O. , Hassel, K. , Flatberg, K. I. , Shaw, A. J. , Brochmann, C. , & Stenøien, H. K. (2016). Long‐distance dispersal and barriers shape genetic structure of peatmosses (sphagnum) across the northern hemisphere. Journal of Biogeography, 43(6), 1215–1226. 10.1111/jbi.12716 [DOI] [Google Scholar]
- Laine, J. , Flatberg, K. I. , Harju, P. , Timonen, T. , Minkkinen, K. J. , Laine, A. , Tuittila, E. S. , & Vasander, H. T. (2018). Sphagnum mosses: The stars of European mires. Sphagna Ky. [Google Scholar]
- Ledent, A. , Désamoré, A. , Laenen, B. , Mardulyn, P. , McDaniel, S. F. , Zanatta, F. , Patiño, J. , & Vanderpoorten, A. (2019). No borders during the post‐glacial assembly of European bryophytes. Ecology Letters, 22(6), 973–986. 10.1111/ele.13254 [DOI] [PubMed] [Google Scholar]
- Leimu, R. , Mutikainen, P. , Koricheva, J. , & Fischer, M. (2006). How general are positive relationships between plant population size, fitness and genetic variation? Journal of Ecology, 94(5), 942–952. 10.1111/j.1365-2745.2006.01150.x [DOI] [Google Scholar]
- Malinsky, M. , Matschiner, M. , & Svardal, H. (2021). Dsuite ‐ fast D‐statistics and related admixture evidence from VCF files. Molecular Ecology Resources, 21(2), 584–595. 10.1111/1755-0998.13265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meleshko, O. , Martin, M. D. , Korneliussen, T. S. , Schröck, C. , Lamkowski, P. , Schmutz, J. , Healey, A. , Piatkowski, B. T. , Shaw, A. J. , Weston, D. J. , Flatberg, K. I. , Szövényi, P. , Hassel, K. , & Stenøien, H. K. (2021). Extensive genome‐wide phylogenetic discordance is due to incomplete lineage sorting and not ongoing introgression in a rapidly radiated bryophyte genus. Molecular Biology and Evolution, 38(7), msab063–msab2766. 10.1093/molbev/msab063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morjan, C. L. , & Rieseberg, L. H. (2004). How species evolve collectively: Implications of gene flow and selection for the spread of advantageous alleles. Molecular Ecology, 13(6), 1341–1356. 10.1111/j.1365-294x.2004.02164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei, M. , & Li, W. H. (1979). Mathematical model for studying genetic variation in terms of restriction endonucleases. Proceedings of the National Academy of Sciences, 76(10), 5269–5273. 10.1073/pnas.76.10.5269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, R. , Hubisz, M. J. , Hellmann, I. , Torgerson, D. , Andrés, A. M. , Albrechtsen, A. , Gutenkunst, R. , Adams, M. D. , Cargill, M. , Boyko, A. , Indap, A. , Bustamante, C. D. , & Clark, A. G. (2009). Darwinian and demographic forces affecting human protein coding genes. Genome Research, 19(5), 838–849. 10.1101/gr.088336.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, N. , Moorjani, P. , Luo, Y. , Mallick, S. , Rohland, N. , Zhan, Y. , Genschoreck, T. , Webster, T. , & Reich, D. (2012). Ancient admixture in human history. Genetics, 192(3), 1065–1093. 10.1534/genetics.112.145037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer, B. , Wittelsbürger, U. , Ramos‐Onsins, S. E. , & Lercher, M. J. (2014). PopGenome: An efficient swiss Army knife for population genomic analyses in R. Molecular Biology and Evolution, 31(7), 1929–1936. 10.1093/molbev/msu136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, H. (1999). Spatial pattern of vascular plant diversity in North America north of Mexico and its floristic relationship with Eurasia. Annals of Botany, 83(3), 271–283. 10.1006/anbo.1998.0816 [DOI] [Google Scholar]
- Ricca, M. , & Shaw, A. J. (2010). Allopolyploidy and homoploid hybridization in the Sphagnum subsecundum complex (Sphagnaceae: Bryophyta). Biological Journal of the Linnean Society, 99(1), 135–151. 10.1111/j.1095-8312.2009.01340.x [DOI] [Google Scholar]
- Richards, E. J. , & Martin, C. H. (2017). Adaptive introgression from distant Caribbean islands contributed to the diversification of a microendemic adaptive radiation of trophic specialist pupfishes. PLoS Genetics, 13(8), e1006919. 10.1371/journal.pgen.1006919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieseberg, L. H. (1995). The role of hybridization in evolution: Old wine in new skins. American Journal of Botany, 82(7), 944–953. 10.1002/j.1537-2197.1995.tb15711.x [DOI] [Google Scholar]
- Rieseberg, L. H. , & Carney, S. E. (1998). Plant hybridization. New Phytologist, 140(4), 599–624. 10.1046/j.1469-8137.1998.00315.x [DOI] [PubMed] [Google Scholar]
- Rydin, H. , Jeglum, J. K. , Rydin, H. , & Jeglum, J. K. (2013). The biology of peatlands. Oxford University Press. [Google Scholar]
- Såstad, S. M. , Sten Øien, H. K. , Flatberg, K. I. , & Bakken, S. (2001). The narrow endemic sphagnum troendelagicum is an allopolyploid derivative of the widespread S. balticum and S. tenellum. Systematic Botany, 26(1), 66–74. [Google Scholar]
- Shaw, A. J. , Carter, B. E. , Aguero, B. , Costa, D. P. , & Crowl, A. A. (2019). Range change evolution of peat mosses (sphagnum) within and between climate zones. Global Change Biology, 25(1), 108–120. 10.1111/gcb.14485 [DOI] [PubMed] [Google Scholar]
- Shaw, A. J. , Devos, N. , Cox, C. J. , Boles, S. B. , Shaw, B. , Buchanan, A. M. , Cave, L. , & Seppelt, R. (2010). Peatmoss (sphagnum) diversification associated with Miocene northern hemisphere climatic cooling? Molecular Phylogenetics and Evolution, 55(3), 1139–1145. 10.1016/j.ympev.2010.01.020 [DOI] [PubMed] [Google Scholar]
- Shaw, A. J. , Devos, N. , Liu, Y. , Cox, C. J. , Goffinet, B. , Flatberg, K. I. , & Shaw, B. (2016). Organellar phylogenomics of an emerging model system: Sphagnum (peatmoss). Annals of Botany, 118(2), 185–196. 10.1093/aob/mcw086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, A. J. , Golinski, G. K. , Clark, E. G. , Shaw, B. , Stenøien, H. K. , & Flatberg, K. I. (2014). Intercontinental genetic structure in the amphi‐Pacific peatmoss Sphagnum miyabeanum (Bryophyta: Sphagnaceae). Biological Journal of the Linnean Society, 111(1), 17–37. 10.1111/bij.12200 [DOI] [Google Scholar]
- Shaw, A. J. , Shaw, B. , Stenøien, H. K. , Golinski, G. K. , Hassel, K. , & Flatberg, K. I. (2015). Pleistocene survival, regional genetic structure and interspecific gene flow among three northern peat‐mosses: Sphagnum inexspectatum, S. orientale and S. miyabeanum . Journal of Biogeography, 42(2), 364–376. 10.1111/jbi.12399 [DOI] [Google Scholar]
- Slatkin, M. (1987). Gene flow and the geographic structure of natural populations. Science, 236(4803), 787–792. 10.1126/science.3576198 [DOI] [PubMed] [Google Scholar]
- Soltis, D. E. , Morris, A. B. , McLachlan, J. S. , Manos, P. S. , & Soltis, P. S. (2006). Comparative phylogeography of unglaciated eastern North America. Molecular Ecology, 15(14), 4261–4293. 10.1111/j.1365-294x.2006.03061.x [DOI] [PubMed] [Google Scholar]
- Stenøien, H. K. , Shaw, A. J. , Shaw, B. , Hassel, K. , & Gunnarsson, U. (2011). North American origin and recent European establishments of the amphi‐Atlantic peat moss Sphagnum angermanicum . Evolution, 65(4), 1181–1194. 10.1111/j.1558-5646.2010.01191.x [DOI] [PubMed] [Google Scholar]
- Suarez‐Gonzalez, A. , Lexer, C. , & Cronk, Q. C. B. (2018). Adaptive introgression: A plant perspective. Biology Letters, 14(3), 20170688. 10.1098/rsbl.2017.0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg, S. (2013). Spore rain in relation to regional sources and beyond. Ecography, 36(3), 364–373. 10.1111/j.1600-0587.2012.07664.x [DOI] [Google Scholar]
- Svenning, J. (2003). Deterministic Plio‐Pleistocene extinctions in the European cool‐temperate tree flora. Ecology Letters, 6(7), 646–653. 10.1046/j.1461-0248.2003.00477.x [DOI] [Google Scholar]
- Svenning, J. , Normand, S. , & Kageyama, M. (2008). Glacial refugia of temperate trees in Europe: Insights from species distribution modelling. Journal of Ecology, 96(6), 1117–1127. 10.1111/j.1365-2745.2008.01422.x [DOI] [Google Scholar]
- Weston, D. J. , Turetsky, M. R. , Johnson, M. G. , Granath, G. , Lindo, Z. , Belyea, L. R. , Rice, S. K. , Hanson, D. T. , Engelhardt, K. A. M. , Schmutz, J. , Dorrepaal, E. , Euskirchen, E. S. , Stenøien, H. K. , Szövényi, P. , Jackson, M. , Piatkowski, B. T. , Muchero, W. , Norby, R. J. , Kostka, J. E. , … Shaw, A. J. (2018). The Sphagnome project: Enabling ecological and evolutionary insights through a genus‐level sequencing project. New Phytologist, 217(1), 16–25. 10.1111/nph.14860 [DOI] [PubMed] [Google Scholar]
- Yu, Z. (2011). Holocene carbon flux histories of the world's peatlands. The Holocene, 21(5), 761–774. 10.1177/0959683610386982 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
In silico digested reads from a genomic resequencing sample of S. cuspidatulum (Library IUSS) are available in Dryad (https://doi.org/10.5061/dryad.1c59zw3xc). Demultiplexed Illumina reads from RAD‐seq samples of S. flexuosum, S. recurvum, and S. fallax are available in Dryad https://doi.org/10.5061/dryad.1g1jwsts7 (Duffy et al., 2020). For the list of samples used in this study, see Appendix A.


