Abstract
Purpose
To describe clinical manifestations and short-term prognosis of ocular motility disorders following coronavirus disease-2019 (COVID-19) vaccination.
Methods
Ocular motility disorders were diagnosed by clinical assessment, high-resolution magnetic resonance imaging, and laboratory testing. Clinical manifestations, short-term prognosis, and rate of complete recovery were analyzed.
Results
Sixty-three patients (37 males, 26 females) with a mean age of 61.6 ± 13.3 years (range, 22–81 years) were included in this study. Among 61 applicable patients with sufficient information regarding medical histories, 38 (62.3%) had one or more significant underlying past medical histories including vasculopathic risk factors. The interval between initial symptoms and vaccination was 8.6 ± 8.2 (range, 0–28) days. Forty-two (66.7%), 14 (22.2%), and 7 (11.1%) patients developed symptoms after the first, second, and third vaccinations, respectively. One case of internuclear ophthalmoplegia, 52 cases of cranial nerve palsy, two cases of myasthenia gravis, six cases of orbital diseases (such as myositis, thyroid eye disease, and IgG-related orbital myopathy), and two cases of comitant vertical strabismus with acute onset diplopia were found. Among 42 patients with follow-up data (duration: 62.1 ± 40.3 days), complete improvement, partial improvement, no improvement, and exacerbation were shown in 20, 15, 3, and 4 patients, respectively.
Conclusion
This study provided various clinical features of ocular motility disorders following COVID-19 vaccination. The majority of cases had a mild clinical course while some cases showed a progressive nature. Close follow-up and further studies are needed to elucidate the underlying mechanisms and long-term prognosis.
Keywords: Coronavirus disease 2019 (COVID-19), Ocular motility disorders, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Ophthalmoplegia
Introduction
Since December 2019, the pandemic of coronavirus disease-19 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus, has had a tremendous impact on global public health, causing great morbidity and mortality with drastic effects on the economy and social life around the globe [1, 2]. COVID-19 vaccines against SARS-CoV-2 have shown excellent and promising results in preventing symptomatic COVID-19 [3–6]. They have been developed at an unprecedented speed in an unprecedented disastrous situation. As clinical trials for vaccines involve limited numbers of people, inevitably, several complications undocumented through phase III studies were first reported after their commercial release [7–9]. Neuro-ophthalmic manifestations including ocular motility disorders after the administration of COVID-19 vaccines have been reported sporadically [10–19], although their incidence is very low. Due to their rare occurrence, these reports covered clinical information for only a small number of patients. A nationwide multicenter study was needed to investigate clinical spectrum and understand possible underlying pathomechanisms of ocular motility disorders occurring after COVID-19 vaccination. Thus, the objective of this nationwide multicenter study was to determine the clinical spectrum and short-term outcomes of ocular motility disorders that occurred after COVID-19 vaccination in Korea.
Materials and methods
This study was conducted by the Korean Neuro-Ophthalmology Society inviting participants via email with a multicenter retrospective design. The Institutional Review Board of the appropriate institution (Samsung Medical Center, Pusan National University hospital, Kangnam Sacred Heart Hospital, Seoul National University Hospital, Konkuk University Hospital, Asan Medical Center, Keimyung University Hospital, Busan Paik Hospital, Samsung Changwon Hospital, Kim’s Eye Hospital, Chungbuk National University Hospital, Yeungnam University Hospital, Chung-Ang University Gwangmyeong Hospital, Jeonbuk National University Hospital, Seoul National University Bundang Hospital, Kangwon National University Hospital, Korea university Hospital, Hallym University Sacred Heart Hospital, Ajou University hospital, Nowon Eulji medical center, Kangdong Sacred Heart Hospital) approved this study and waived the informed consent.
This study was conducted according to the tenets of the Declaration of Helsinki. It included patients of 21 Korean hospitals who had been diagnosed as ocular motility disorders which caused binocular diplopia, abnormal head postures, or gaze palsy after vaccination against COVID-19 within four weeks from February 2021 to January 2022.
Patients with a history of neurological or ophthalmic disorders that might affect ocular motility, extraocular muscle surgery, or head/ocular trauma were excluded from this study. Patients with other identifiable causes of ocular motility disorders such as tumorous lesions or infection involving ocular motor pathways were also excluded. In addition, cases without a sufficient evaluation of diplopia due to lack of cooperation or refusal to test were excluded.
Data regarding demographics including age, sex, smoking history, baseline comorbidities including diabetes mellitus (DM), hypertension, hypercholesterolemia, underlying neurological condition, and ophthalmic disease, surgical history, trauma history, and current medication were collected. Type and frequency of vaccine before initial symptoms and the time interval between vaccination and initiation of symptoms were investigated. Clinical features included systemic and ocular manifestations. The diagnosis was based on clinical features including ocular deviation, duction, and version. Additionally, pupil exam, slit lamp exam, fundus exam, laboratory test, and magnetic resonance imaging (MRI) were used as auxiliary tests for diagnosis.
Results
Baseline characteristics
Sixty-three patients (37 males and 26 females) with a mean age of 61.6 ± 13.3 years (range, 22–81 years) were included in this study. Among 61 applicable patients with sufficient information regarding social, medical, and surgical histories, 38 (62.3%) had one or more significant underlying diseases or vasculopathic risk factors. Demographics and comorbidities of patients are summarized in Table 1. Among two patients with a history of malignancy, one had a postoperative status of colon cancer and the other had a postoperative status of kidney cancer. None of them was diagnosed with metastasis as the cause of cranial nerve palsy. Four patients had histories of autoimmune diseases. However, the ocular motor pathway was not involved before the current events. Of the four patients with autoimmune diseases, two patients had rheumatic arthritis, one patient had Behcet’s disease, and the other one patient had granulomatosis with polyangiitis.
Table 1.
Demographics and comorbidities of patients
| Total | Patients ≥ 50 years old | Patients < 50 years old | |
|---|---|---|---|
| Number of patients | 63 | 52 | 11 |
| Age, years (mean ± SD (range)) | 61.6 ± 13.3 (22–81) | 66.5 ± 8.0 (51–81) | 38.7 ± 8.0 (22–45) |
| Sex, n (M:F) | 37:26 | 29:23 | 8:3 |
| Vasculopathic comorbidities, n (%) | 38/61* (62.3) | 36/51* (70.6) | 2/10* (20.0) |
| Diabetes mellitus | 18/61* (29.5) | 17/51* (33.3) | 1/10* (10.0) |
| Hypertension | 19/61* (31.1) | 19/51* (37.3) | 0/10* (0.0) |
| Hypercholesterolemia | 11/61* (18.0) | 11/51* (21.6) | 0/10* (0.0) |
| Other cardiovascular disease | 6/61* (9.8) | 5/51* (9.8) | 1/10* (10.0) |
| Previous malignancies, n (%) | 2/61* (3.3) | 2/51* (3.9) | 0/10* (0.0) |
| Autoimmune diseases, n (%) | 4/61* (6.6) | 3/51* (5.9) | 1/10* (10.0) |
| Current smokers, n (%) | 6/45* (13.3) | 5/38* (13.2) | 1/7* (14.3) |
n numbers, SD standard deviation, M male, F female
*Number of applicable patients with sufficient information of comorbidities and social history
Vaccine information
The interval between vaccination and symptom onset of ocular motility disorders was 8.6 ± 8.2 (range, 0–28) days. Forty-two (66.7%), 14 (22.2%), and 7 (11.1%) patients developed symptoms after the first, second, and third vaccinations, respectively. Types of vaccines are presented in Table 2.
Table 2.
Types of vaccines before the onset of symptoms
| Number and type of vaccines preceding onset | Number of patients (%) |
|---|---|
| First | 42 (66.7) |
| BNT162b2, Pfizer-BioNTech | 10 |
| ChAdOx1 nCoV-19/ AZD1222, Oxford-AstraZeneca | 23 |
| mRNA-1273, Moderna | 4 |
| Ad26.COV2, Janssen Johnson & Johnson | 1 |
| Unspecified | 4 |
| Second | 14 (22.2) |
| BNT162b2, Pfizer-BioNTech | 4 |
| ChAdOx1 nCoV-19/ AZD1222, Oxford-AstraZeneca | 8 |
| mRNA-1273, Moderna | 1 |
| Unspecified | 1 |
| Third | 7 (11.1) |
| BNT162b2, Pfizer-BioNTech | 5 |
| Unspecified | 2 |
Clinical features of each ocular motility disorder
In this study, one case of supranuclear disorder, 52 cases of cranial nerve palsy, two cases of neuromuscular junction abnormality, six cases of the orbital lesions, and two cases of acute comitant vertical strabismus with diplopia were observed (Table 3).
Table 3.
Classification of ocular motility disorders
| Number of patients (%) (n = 63) | Number of patients ≥ 50 years old (%) (n = 52) | Number of patients < 50 years old (%) (n = 11) | |
|---|---|---|---|
| Supranuclear palsy | 1 (1.6) | 1 (1.9) | 0 (0) |
| Skew deviation | 1 (1.6) | 1 (1.9) | 0 (0) |
| Monocranial nerve palsy | 46 (73.0) | 39 (75.1) | 7 (63.7) |
| Oculomotor palsy | 9 (14.3) | 7 (13.5) | 2 (18.2) |
| Trochlear palsy | 12 (19.0) | 11 (21.2) | 1 (9.1) |
| Abducence palsy | 25 (39.7) | 21 (40.4) | 4 (36.4) |
| Multicranial nerve palsy | 6 (9.5) | 4 (7.7) | 2 (18.2) |
| Tolosa-Hunt syndrome | 1 (1.6) | 1 (1.9) | 0 (0) |
| Orbital apex syndrome | 1 (1.6) | 1 (1.9) | 0 (0) |
| Miller Fisher syndrome | 4 (6.3) | 2 (3.9) | 2 (18.2) |
| Myasthenia gravis | 2 (3.2) | 1 (1.9) | 1 (9.1) |
| Orbital disease | 6 (9.5) | 6 (11.5) | 0 (0) |
| Myositis | 2 (3.2) | 2 (3.9) | 0 (0) |
| Thyroid eye disease | 3 (4.8) | 3 (5.7) | 0 (0) |
| IgG-related orbital myopathy | 1 (1.6) | 1 (1.9) | 0 (0) |
| Others | 2 (3.2) | 1 (1.9) | 1 (9.1) |
| Comitant hyptertropia | 2 (3.2) | 1 (1.9) | 1 (9.1) |
n numbers
Supranuclear disorders
A 60-year-old man with a history of hypertension was diagnosed with skew deviation at 16 days following the first dose of AstraZeneca COVID-19 vaccination. He showed an acute infarction with diffusion restriction observed at the midline and anterior pons and the pontomedullary junction in MRI. He had normal platelet count, fibrinogen, prothrombin time, and D-dimer.
Cranial nerve palsy
Isolated cranial nerve palsy was diagnosed in 46 patients following COVID-19 vaccination. Symptoms of patients occurred in 28 cases after the first vaccination (AstraZeneca in 16, Pfizer-BioNTech in 9, Moderna in 1, and unspecified in 2), 12 cases after the second vaccination (AstraZeneca in 7, Pfizer-BioNTech in 4, Moderna in 1), and six cases after the third vaccination (Pfizer-BioNTech in 4 and unspecified in 2). Twenty-eight (62.2%) patients had predisposing or concurrent vasculopathic comorbidities among 45 applicable patients with sufficient information (Table 4). Seven (15.2%) patients complained of combined headaches. Oculomotor palsy in 9, trochlear nerve palsy in 12, and abducens nerve palsy in 25 were presented. Among the 9 patients with oculomotor nerve palsy, only one patient had a complete palsy while the other 8 patients had an incomplete palsy (Fig. 1). Five patients showed ptosis. One patient showed pupillary involvement. Among 46 patients with isolated cranial nerve palsy, neuroimaging workup revealed intracerebral hemorrhage in two patients, ischemic stroke in two patients, acute subtle ischemic change in the brain stem in one patient, a hyperintense lesion in the brainstem suggesting demyelinating lesion in one patient, diffuse leptomeningeal enhancement in one patient, and enhancing lesion in the cavernous sinus suggesting an inflammatory process in one patient. Other patients did not show any significant abnormality in neuroimaging.
Table 4.
Demographics and comorbidities of patients who developed isolated ocular motor cranial nerve palsy
| Total | Patients ≥ 50 years old (%) | Patients < 50 years old (%) | |
|---|---|---|---|
| Number of patients | 46 | 39 | 7 |
| Age, years (mean ± SD) | 63.3 ± 12.5 | 67.4 ± 8.2 | 40.7 ± 6.9 |
| Sex, n (M:F) | 26:20 | 22:17 | 4:3 |
| Vasculopathic comorbidities, n (%) | 28/45* (62.2) | 27/39* (69.2) | 1/6* (16.7) |
| Diabetes mellitus | 13/45* (28.9) | 12/39* (30.8) | 1/6* (16.7) |
| Hypertension | 13/45* (28.9) | 13/39* (33.3) | 0/6* (0.0) |
| Hypercholesterolemia | 9/45* (20.0) | 9/39* (23.1) | 0/6* (0.0) |
| Other cardiovascular disease | 3/45* (6.7) | 3/39* (7.7) | 0/6* (0.0) |
| Previous malignancies, n (%) | 0/45* (0.0) | 0/39* (0.0) | 0/6* (0.0) |
| Autoimmune diseases, n (%) | 2/45* (4.4) | 1/39* (2.6) | 1/6* (16.7) |
| Current smokers, n (%) | 4/33* (12.1) | 4/29* (13.8) | 0/4* (0.0) |
SD standard deviation, n numbers, M male, F female
*Number of applicable patients with sufficient information on comorbidities and social history
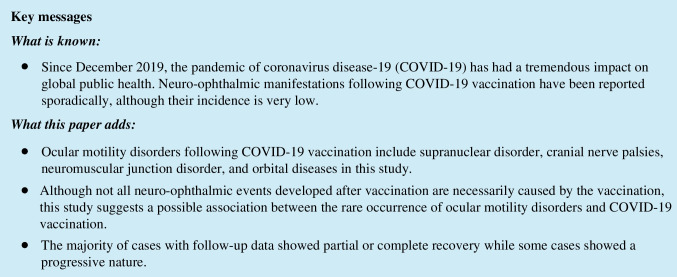
Fig. 1.
A 76-year-old male patient with hypertension and chronic renal failure who developed a right oculomotor nerve palsy at 2 weeks after the second dose of the AstraZeneca COVID-19 vaccine. A The patient showed ptosis, limited adduction, and depression in the right eye. B The right pupil (upper figure) was mildly dilated and fixed. C Axial FLAIR image demonstrated an increased signal intensity (white arrow) of the right cavernous sinus and oculomotor nerve, suggesting an inflammatory process. The patient received high-dose intravenous methylprednisolone pulse therapy (1 g/day for 3 days) followed by oral prednisone in tapering dosage. The patient showed partial recovery at 1 month of follow-up
There were six cases with multiple cranial nerve palsy, and four of them had Miller Fisher syndrome. Among these four patients with Miller Fisher syndrome, symptom occurred in three patients after the first injection (Moderna in 1 and unspecified in 2) and one patient after the second AstraZeneca injection. The anti-GQ1b antibody was positive in two patients (Fig. 2). Antibody-negative patients were diagnosed with a combination of typical clinical features and electromyographic features. Tolosa-Hunt syndrome was diagnosed in one patient after the first AstraZeneca COVID-19 vaccination. In this patient, MRI showed asymmetric soft tissue lesion involving the left cavernous sinus with subtle optic nerve sheath enhancement. He showed total ophthalmoplegia except for a small degree of abduction. There were no other accompanying symptoms. One patient with previously diagnosed granulomatosis and polyangiitis who was on cyclophosphamide and rituximab medications developed inflammatory lesions involving bilateral orbital apex after the first unspecified vaccination.
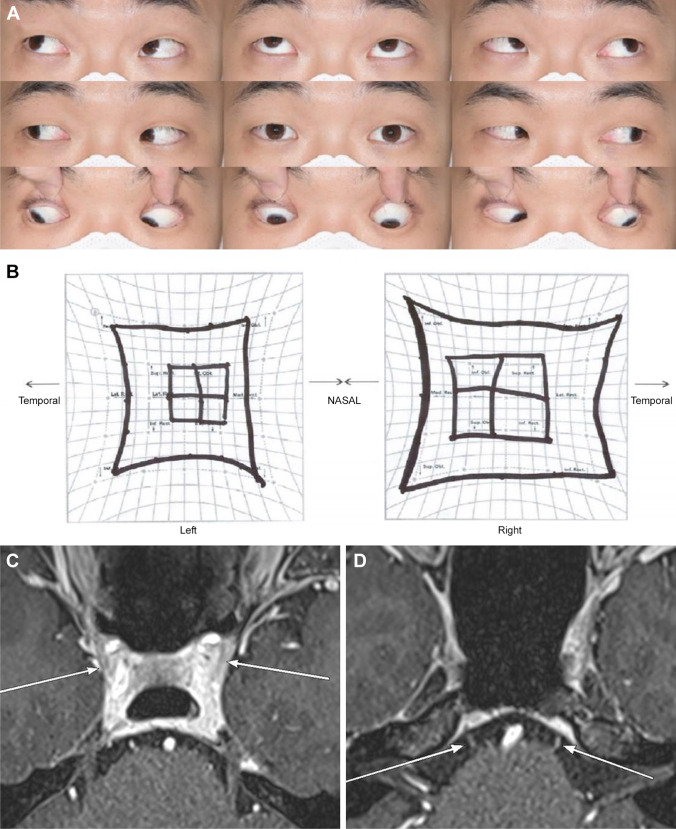
Fig. 2.
A 31-year-old healthy male patient who developed diplopia and ataxia at four weeks after the first dose of the Janssen Johnson & Johnson COVID-19 vaccine injection. A, B The patient showed limited elevation in both eyes, more in the left eye with mildly limited adduction and abduction. Axial magnetic resonance imaging demonstrated multifocal enhancement of 3rd (cavernous sinus segment, white arrows) (C), 6th (cisternal segment, white arrows) (D), and 7th cranial nerves. Blood work was positive for anti-GQ1b IgG antibody. The patient was diagnosed with Miller Fisher syndrome. Because the patient’s symptoms were already recovering at the presentation, the patient is on a close follow-up without treatment
Neuromuscular junction abnormality
Ocular myasthenia gravis was diagnosed in two patients. One developed it at 7 days after the first AstraZeneca COVID-19 vaccine injection, and the other one developed it at two days after the first Moderna COVID-19 vaccination. Anti-acetylcholine antibody test and ice test were positive in one patient. In the other patient, the diagnosis was made based on clinical features including symptom fluctuation and good response to Mestinon.
Orbital lesion
Three patients developed a thyroid eye disease at 3, 5, and 18 days after the first AstraZeneca COVID-19 vaccination, respectively. Serum thyroid-stimulating hormone receptor antibody was detected in all three patients. There was no history of previous diagnosis of thyroid dysfunction or thyroid eye disease. One patient developed orbital IgG4 related disorder one day after the third Pfizer-BioNTech COVID-19 vaccination. Serum immunoglobulin G4 was elevated in this patient. Two patients developed the symptom of myositis after the first Pfizer-BioNTech vaccine and the second unspecified vaccine, respectively. Radiologic findings were consistent with the diagnosis of orbital lesions in all patients.
Other
Two patients who were not classified into other disease groups showed comitant vertical strabismus with new-onset acute diplopia following the first Pfizer-BioNTech vaccination and the first Moderna COVID-19 vaccination, respectively.
Treatment and prognosis
Steroid was administrated in 14 (22.2%) patients: high-dose intravenous methylprednisolone injections in eight patients and oral steroids in six patients. Pyridostigmine was tried in two patients with ocular myasthenia gravis. For the patient having granulomatosis with polyangiitis, rituximab was administered. Aceclofenac was taken without steroids by the patient with Tolosa-Hunt syndrome. Intravenous immunoglobulin was administrated to two patients with Miller Fisher syndrome.
The follow-up period of total subjects in this study was 62.1 ± 40.3 days (range, 2–141 days) in 42 patients with follow-up data. There were 20 (47.6%) patients with complete improvement, 15 (35.7%) with partial improvement, three (7.1%) with no improvement, and four (9.5%) with exacerbation. The recovery period was 70.9 ± 61.4 days in the case of complete improvement. In the subgroup of patients with isolated cranial nerve palsy, among 21 patients who were followed up for one month or more (average follow-up period: 2.6 ± 1.1 months [range, 1–5 months]), motor defects showed complete resolution in 11 (52.4%) patients, partial recovery in seven (33.3%), and no improvement in three (14.3%). Six of 21 patients received steroid treatment. Two of the 11 patients with complete recovery received steroid treatment.
Discussion
In this study, ocular motility disorders following COVID-19 vaccination included supranuclear disorder such as skew deviation, cranial nerve palsies including isolated third, fourth, and sixth cranial nerve palsies, and multiple cranial nerve palsies due to Tolosa-Hunt syndrome, orbital apex syndrome, and Miller Fisher syndrome, neuromuscular junction disorders such as myasthenia gravis, and orbital diseases such as myositis, thyroid eye disease, and IgG-related orbital myopathy. The mean interval between vaccination and symptom onset was 9 days (range, 0–28 days). After the first, second, and third vaccinations, 67%, 22%, and 11% of patients developed symptoms, respectively. This distribution might be related to the fact that this study was conducted before completion of the third vaccination in Korea.
Supranculear disorder
In this cohort, a 60-year-old patient with a history of hypertension had a skew deviation due to acute infarction of the brainstem that developed 16 days after the first injection of an AstraZeneca COVID-19 vaccine. The temporal relationship between vaccination and stroke did not necessarily mean that the stroke was caused by the vaccine. Stroke following other types of vaccination such as varicella or influenza vaccination has also been described in the literature [20–22]. Regarding COVID-19 vaccines, ischemic stroke in the context of vaccine-induced immune thrombotic thrombocytopenia has been described in multiple studies [23–28]. However, there were only a few case reports that described primary ischemic stroke without thrombocytopenia [29]. The time interval between vaccination and stroke onset was three days in a previous report [29], shorter than that in our case. The patient did not have significant comorbidities in the previous report [29]. The causal relationship in this study was unclear because the patient in this study had a history of hypertension that might have contributed to the natural development of stroke. Although the causal relationship and possible mechanisms are both unclear, vaccines may contribute to atherosclerotic plaque instability and rupture in a similar way to a viral infection [30]. However, from epidemiologic perspectives, vaccines can reduce the possibility of thromboembolic events caused by viral infections [31, 32]. In addition, previous data have shown a significant increase in stroke incidence during the early period of the COVID-19 pandemic [33]. Hence, while suspicion might be required to identify very rare thromboembolic events following vaccination, it is also important to understand that these possible adverse events are rare and far less common than thromboembolic events following COVID-19 infection itself.
Cranial nerve palsy
Among 46 patients with isolated ocular motor cranial nerve palsy in this study, seven patients were younger than 50 years. Of six young or middle-aged patients with sufficient information on medical and social histories, five patients did not have any vasculopathic risk factors such as DM, hypertension, hypercholesterolemia, other cardiovascular diseases, or smoking history. Among 39 patients aged 50 years or more, 12 (30.8%) patients did not have predisposing vasculopathic risk factors. In this study, the overall prevalence of hypertension, diabetes, dyslipidemia, and current smoker in patients with isolated cranial nerve palsy were 28.9%, 28.9%, 20.0%, and 12.1%, respectively, comparable to their prevalence in the general population of Korea. The prevalence of hypertension in th general population of Korea was reported to be 67% (in people ≥ 20 years of age) in 2018 [34]. The prevalence of diabetes was reported to be 13.8% in people ≥ 30 years of age and 27.6% in people aged ≥ 65 years [35]. The prevalence of dyslipidemia (in people ≥ 20 years of age) and current smoking (in people ≥ 19 years of age) were 45.6% [36] and 36.7% in men [37], respectively, and 31.3% [36] and 7.5% in women [37], respectively. The reported overall prevalence of clinical atherosclerotic cardiovascular disease was 10.1% among Korean people aged ≥ 18 years in 2015 [38]. One of the most common etiologies of ocular motor cranial nerve palsy has been thought to be microvascular ischemia, especially in the elderly [39–46], accounting for 83.5–99% of cases in previous studies [46–50]. Jung et al. have previously reported the prevalence of vasculopathic risk factors in Korean patients with ocular motor cranial nerve palsy (mean age of patients: 64.5 ± 10.2 years, 33 (61.1%) of patients aged ≤ 60 years) [51]. In their study, the overall prevalence of hypertension, diabetes, dyslipidemia, ischemic heart disease, and current smoker in those with isolated cranial nerve palsy were 63.0%, 53.7%, 24.1%, 7.4%, and 22.2% [51], respectively, much higher than those in our study.
Ocular motor nerve palsies following various vaccinations have been reported, with onset ranging from two days to three weeks after vaccination [52–56], although their occurrence is very rare. The pathophysiology behind postimmunization ocular nerve palsies remains unknown. It is hypothesized to be related to immune-mediated damage resulting in demyelination or localized vasculitis [52–56]. Several case reports of isolated ocular motor nerve palsies following various COVID-19 vaccines have been described [10–13]. Reyes-Capo et al. have reported the development of an acute abducens nerve palsy following a febrile illness in a healthy 59-year-old woman at 2 days after receiving a Pfizer-BioNTech COVID-19 vaccine [10]. MRI of the brain and orbits showed no abnormal finding [10]. The authors suggested a viral-like inflammatory reaction to the vaccine, inciting an immune-mediated indirect insult along the nerve [10].
Pereira and Haslett have also reported the development of an acute abducens nerve palsy in a healthy 65-year-old man at three days after receiving the second dose of the AstraZeneca COVID-19 vaccine [11]. Pawar et al. have reported the development of abducens nerve palsy in a 23-year-old young man following Covishield COVID-19 vaccine (Serum Institute of India) [12]. Results of neuroimaging and laboratory test were normal in both cases [11, 12]. Another unique case report was an 88-year-old man with a history of cardiovascular disease who developed oculomotor nerve palsy at three days after the first administration of Moderna COVID-19 vaccine in the right deltoid. He received a second Pfizer-BioNTech COVID-19 vaccine at a different site uneventfully [13]. While neuroimaging workup of patients with isolated cranial nerve palsy in the current study revealed various pathologies including intracerebral hemorrhage, acute ischemic changes, demyelinating lesion, diffuse leptomeningeal enhancement, and enhancing lesion in the cavernous sinus, the majority of patients did not show any significant abnormalities in neuroimaging.
Among 21 patients with isolated cranial nerve palsy who were followed up for 1 month or more in this study, 52% of patients showed complete recovery, 33% showed partial recovery, and 14% showed no improvement. Only two patients out of the 11 with complete recovery received steroid treatment. Among previously reported cases with ocular motor nerve palsies following COVID-19 vaccination, sensorimotor defect of the patient remained unchanged in the report of Reyes-Capo et al. [10], while the ocular motor dysfunctions had resolved within three months in the report of Pereira and Haslett [11] and within 5 days after steroid therapy in the report of Cicales et al. [13]. It has also been reported that postimmunization ocular motor nerve palsies following other types of vaccines tend to resolve spontaneously within 6 months [52, 53, 56].
In this study, there were six cases of multiple ocular motor cranial nerve palsy, including Miller Fisher syndrome in four patients, Tolosa-Hunt syndrome in one patient, and cavernous involvement of granulomatosis with polyangiitis in one patient. There have been several previous reports of Miller Fisher syndrome after receiving Pfizer-BioNTech COVID-19 vaccine [14, 15] or AstraZeneca COVID-19 vaccine [16, 17]. All of these cases showed complete or significant improvement after immunoglobulin treatment. In this study, one patient with Miller Fisher syndrome who received immunoglobulin treatment completely recovered at four weeks after the symptom onset. The other three patients with Miller Fisher syndrome were followed up for less than 3 weeks. Previous reports and our patient suggest an excellent prognosis of Miller Fisher syndrome developed after vaccination.
Neuromuscular junction disorder
In this study, ocular myasthenia gravis was diagnosed in two patients. Reports of myasthenia gravis after other types of vaccination are rare. One after human papillomavirus vaccination [57] and one after hepatitis B vaccination [58] have been reported. There were only a few case reports describing the occurrence of myasthenia gravis following COVID-19 vaccination [18, 19, 59–61]. Like other autoimmune diseases, antibodies produced by an inflammatory reaction to an external agent can trigger an immune response that cross-reacts with acetylcholine receptors due to molecular resemblance [62, 63]. Molecular mimicry could play a key role in the development of myasthenia gravis. However, the causal association between the development of myasthenia gravis and vaccination remains unclear. It necessitates further research.
Orbital diseases
In this study, three patients without a history of thyroid disease or related symptoms developed a thyroid eye disease and one patient developed orbital IgG4-related disorder. There have been multiple reports on the development of Graves’ disease following COVID-19 vaccination [64–72]. One case with a relapse of IgG4-related nephritis following Pfizer-BioNTech COVID-19 vaccination has been reported [73]. The pathogenesis of thyroid eye disease and IgG4-related diseases following vaccination could be induced via immune stimulation by the vaccine like other immune-mediated reactions related to vaccination [74], although those cases could also be coincidental.
Vaccines are administered to otherwise healthy people often in large numbers [75]. They could show side effects in healthy individuals, although the probability is very low [7]. This may alter the perception of risk compared to therapeutic drugs prescribed for patients with an already existing disorder [7], especially in countries where the case fatality rate and disease-related deaths are relatively low [75]. However, there is no doubt that effective and massive vaccination is essential to end this disastrous pandemic caused by COVID-19. Vaccines against COVID-19 showed excellent results in preventing symptomatic COVID-19 [3–6]. The most presumed mechanism of possible vaccine side effects is similar to that occurring after an infection. The probability of vaccine side effects is much less than that following an infection. In very few cases, reported neuro-ophthalmologic symptoms may occur following COVID-19 vaccination. We hope that this research will serve as a helpful reference for physicians who will manage these patients.
Although all neuro-ophthalmic events developed after vaccination are not necessarily caused by the vaccination, this study suggests a possible association between the rare occurrence of ocular motility disorders and COVID-19 vaccination. The majority of cases with follow-up data showed partial or complete recovery while some cases showed a progressive nature. Close follow-up and further studies are needed to elucidate the underlying mechanisms and long-term prognosis.
Acknowledgements
The authors would like to thank Dong Hyun Kim, Yung-Ju Yoo, Young-Woo Suh, Joo Yeon Lee, Seung Ah Chung, Eun Hye Jung, Youn Joo Choi MD, Jeong-Min Hwang, Ga-In Lee, Min Chae Kang, and Jiho Kim for supporting to proceed this study.
Author contribution
Kyung-Ah Park and Hyeshin Jeon wrote the main manuscript text and prepared the tables and figures. Kyung-Ah Park, Hyeshin Jeon, Dong Gyu Choi, Jae Ho Jung, Hyun-Jin Shin, Byung Joo Lee, Yeji Moon, Se-Youp Lee, Dong Cheol Lee, Soon Young Cho, Seong-Joon Kim, Sei Yeul Oh, Sunghyuk Moon, Shin Yeop Oh, Daye Diana Choi, Mi Young Choi, Won Jae Kim, Ungsoo Samuel Kim, Haeng-Jin Lee, and Yikyung Kim prepared and analyzed the dataset. Dong Gyu Choi, Kyung-Ah Park, and Hyeshin Jeon conceived of the idea and supervised the findings of this work.
Declarations
Ethical approval
This study was approved by the local ethics committee.
Conflict of interest
The authors declare no competing interests.
Footnotes
Meeting Presentation: This study was presented in the 127th annual meeting of the Korean Ophthalmological Society.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kyung-Ah Park and Hyeshin Jeon contributed equally to this study as co-first authors.
References
- 1.Bonaccorsi G, Pierri F, Cinelli M, Flori A, Galeazzi A, Porcelli F, Schmidt AL, Valensise CM, Scala A, Quattrociocchi W, Pammolli F. Economic and social consequences of human mobility restrictions under COVID-19. Proc Natl Acad Sci U S A. 2020;117:15530–15535. doi: 10.1073/pnas.2007658117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonotti M, Zech ST (2021) The human, economic, social, and political costs of COVID-19. RecovCiv during COVID-19 1–36. 10.1007/978-981-33-6706-7_1
- 3.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O'Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/s0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr, Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC, Group CCT Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng JY, Margo CE. Ocular adverse events following vaccination: overview and update. Surv Ophthalmol. 2022;67:293–306. doi: 10.1016/j.survophthal.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Eleiwa TK, Gaier ED, Haseeb A, ElSheikh RH, Sallam AB, Elhusseiny AM. Adverse ocular events following COVID-19 vaccination. Inflamm Res. 2021;70:1005–1009. doi: 10.1007/s00011-021-01506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng XL, Betzler BK, Testi I, Ho SL, Tien M, Ngo WK, Zierhut M, Chee SP, Gupta V, Pavesio CE, de Smet MD, Agrawal R. Ocular adverse events after COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29:1216–1224. doi: 10.1080/09273948.2021.1976221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes-Capo DP, Stevens SM, Cavuoto KM. Acute abducens nerve palsy following COVID-19 vaccination. J aapos. 2021;25:302–303. doi: 10.1016/j.jaapos.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira A, Haslett RS. Acute abducens nerve palsy following the second dose of the AstraZeneca COVID-19 vaccine. J Pediatr Ophthalmol Strabismus. 2021;58:e49–e50. doi: 10.3928/01913913-20210920-01. [DOI] [PubMed] [Google Scholar]
- 12.Pawar N, Ravindran M, Padmavathy S, Chakrabarty S. Acute abducens nerve palsy after COVID-19 vaccination in a young adult. Indian J Ophthalmol. 2021;69:3764–3766. doi: 10.4103/ijo.IJO_1968_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicalese MP, Ferrua F, Barzaghi F, Cerri F, Moro M, Aiuti A, Silvani P. Third cranial nerve palsy in an 88-year-old man after SARS-CoV-2 mRNA vaccination: change of injection site and type of vaccine resulted in an uneventful second dose with humoral immune response. BMJ Case Rep. 2022;15:e246485. doi: 10.1136/bcr-2021-246485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abičić A, Adamec I, Habek M. Miller Fisher syndrome following Pfizer COVID-19 vaccine. Neurol Sci. 2021;43:1495. doi: 10.1007/s10072-021-05776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michaelson NM, Lam T, Malhotra A, Schiff ND, MacGowan DJL. Miller Fisher syndrome presenting after a second dose of Pfizer-BioNTech vaccination in a patient with resolved COVID-19: a case report. J Clin Neuromuscul Dis. 2021;23:113–115. doi: 10.1097/cnd.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 16.Dang YL, Bryson A. Miller-Fisher syndrome and Guillain-Barre syndrome overlap syndrome in a patient post Oxford-AstraZeneca SARS-CoV-2 vaccination. BMJ Case Rep. 2021;14:e246701. doi: 10.1136/bcr-2021-246701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishiguchi Y, Matsuyama H, Maeda K, Shindo A, Tomimoto H. Miller Fisher syndrome following BNT162b2 mRNA coronavirus 2019 vaccination. BMC Neurol. 2021;21:452. doi: 10.1186/s12883-021-02489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, Haddad A, Elias M, Zisman D, Naffaa ME, Brodavka M, Cohen Y, Abu-Much A, Abu Elhija M, Bridgewood C, Langevitz P, McLorinan J, Bragazzi NL, Marzo-Ortega H, Lidar M, Calabrese C, Calabrese L, Vital E, Shoenfeld Y, Amital H, McGonagle D. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel) 2021;9:435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galassi G, Rispoli V, Iori E, Ariatti A, Marchioni A. Coincidental onset of ocular myasthenia gravis following ChAdOx1 n-CoV-19 vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Isr Med Assoc J. 2022;24:9–10. [PubMed] [Google Scholar]
- 20.Famularo G, Minisola G, Gasbarrone L. Ischemic stroke after influenza vaccination. Ann Pharmacother. 2015;49:747. doi: 10.1177/1060028015578452. [DOI] [PubMed] [Google Scholar]
- 21.Lin YP, Li TH, Chen WH. Ischaemic stroke and influenza A H1N1 vaccination: a case report. Arch Med Sci. 2011;7:345–348. doi: 10.5114/aoms.2011.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wirrell E, Hill MD, Jadavji T, Kirton A, Barlow K. Stroke after varicella vaccination. J Pediatr. 2004;145:845–847. doi: 10.1016/j.jpeds.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Al-Mayhani T, Saber S, Stubbs MJ, Losseff NA, Perry RJ, Simister RJ, Gull D, Jäger HR, Scully MA, Werring DJ. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. J Neurol Neurosurg Psychiatry. 2021;92:1247–1248. doi: 10.1136/jnnp-2021-326984. [DOI] [PubMed] [Google Scholar]
- 24.Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet. 2021;397:e11. doi: 10.1016/s0140-6736(21)00872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost. 2021;19:1771–1775. doi: 10.1111/jth.15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hidayat R, Diafiri D, Zairinal RA, Arifin GR, Azzahroh F, Widjaya N, Fani DN, Mesiano T, Kurniawan M, Al R, Giantini A, Haris S. Acute ischaemic stroke incidence after coronavirus vaccine in indonesia: case series. Curr Neurovasc Res. 2021;18:360–363. doi: 10.2174/1567202618666210927095613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenda J, Lovrič D, Škerget M, Milivojević N. Treatment of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia related acute ischemic stroke. J Stroke Cerebrovasc Dis. 2021;30:106072. doi: 10.1016/j.jstrokecerebrovasdis.2021.106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancuso M, Lauretti DL, Cecconi N, Santini M, Lami V, Orlandi G, Casagli S, Ghetta A, Liberti G, Elena BM, Siciliano G, Cosottini M. Arterial intracranial thrombosis as the first manifestation of vaccine-induced immune thrombotic thrombocytopenia (VITT): a case report. Neurol Sci. 2022;43:1–5. doi: 10.1007/s10072-021-05800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alammar MA. Ischemic stroke after AstraZeneca (Covid-19) vaccination. Saudi Med J. 2021;42:1136–1139. doi: 10.15537/smj.2021.42.10.20210326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keller TT, van der Meer JJ, Teeling P, van der Sluijs K, Idu MM, Rimmelzwaan GF, Levi M, van der Wal AC, de Boer OJ. Selective expansion of influenza A virus-specific T cells in symptomatic human carotid artery atherosclerotic plaques. Stroke. 2008;39:174–179. doi: 10.1161/strokeaha.107.491282. [DOI] [PubMed] [Google Scholar]
- 31.Lam F, Chen TL, Shih CC, Lin CS, Yeh CC, Lee YJ, Hu CJ, Chiou HY, Liao CC. Protective effect of influenza vaccination on outcomes in geriatric stroke patients: a nationwide matched cohort study. Atherosclerosis. 2019;282:85–90. doi: 10.1016/j.atherosclerosis.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Modin D, Claggett B, Køber L, Schou M, Jensen JUS, Solomon SD, Vardeny O, Knop FK, Nielsen SD, Fralick M, Torp-Pedersen C, Gislason G, Biering-Sørensen T. Influenza vaccination is associated with reduced cardiovascular mortality in adults with diabetes: a nationwide cohort study. Diabetes Care. 2020;43:2226–2233. doi: 10.2337/dc20-0229. [DOI] [PubMed] [Google Scholar]
- 33.Wu J, Mamas MA, Mohamed MO, Kwok CS, Roebuck C, Humberstone B, Denwood T, Luescher T, de Belder MA, Deanfield JE, Gale CP. Place and causes of acute cardiovascular mortality during the COVID-19 pandemic. Heart. 2021;107:113–119. doi: 10.1136/heartjnl-2020-317912. [DOI] [PubMed] [Google Scholar]
- 34.Kim HC, Cho SMJ, Lee H, Lee HH, Baek J, Heo JE. Korea hypertension fact sheet 2020: analysis of nationwide population-based data. Clin Hypertens. 2021;27:8. doi: 10.1186/s40885-021-00166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung CH, Son JW, Kang S, Kim WJ, Kim HS, Kim HS, Seo M, Shin HJ, Lee SS, Jeong SJ, Cho Y, Han SJ, Jang HM, Rho M, Lee S, Koo M, Yoo B, Moon JW, Lee HY, Yun JS, Kim SY, Kim SR, Jeong IK, Mok JO, Yoon KH. Diabetes fact sheets in Korea, 2020: an appraisal of current status. Diabetes Metab J. 2021;45:1–10. doi: 10.4093/dmj.2020.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho SMJ, Lee H, Lee HH, Baek J, Heo JE, Joo HJ, Hong SJ, Kim HC. Dyslipidemia fact sheets in Korea 2020: an analysis of nationwide population-based data. J Lipid Atheroscler. 2021;10:202–209. doi: 10.12997/jla.2021.10.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S, Choi S, Kim J, Park S, Kim YT, Park O, Oh K. Trends in health behaviors over 20 years: findings from the 1998–2018 Korea National Health and Nutrition Examination Survey. Epidemiol Health. 2021;43:e2021026. doi: 10.4178/epih.e2021026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Kim S, Han S, Rane PP, Fox KM, Qian Y, Suh HS. Prevalence and incidence of atherosclerotic cardiovascular disease and its risk factors in Korea: a nationwide population-based study. BMC Public Health. 2019;19:1112. doi: 10.1186/s12889-019-7439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobson DM, McCanna TD, Layde PM. Risk factors for ischemic ocular motor nerve palsies. Arch Ophthalmol. 1994;112:961–966. doi: 10.1001/archopht.1994.01090190109029. [DOI] [PubMed] [Google Scholar]
- 40.Patel SV, Mutyala S, Leske DA, Hodge DO, Holmes JM. Incidence, associations, and evaluation of sixth nerve palsy using a population-based method. Ophthalmology. 2004;111:369–375. doi: 10.1016/j.ophtha.2003.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Sanders SK, Kawasaki A, Purvin VA. Long-term prognosis in patients with vasculopathic sixth nerve palsy. Am J Ophthalmol. 2002;134:81–84. doi: 10.1016/s0002-9394(02)01439-3. [DOI] [PubMed] [Google Scholar]
- 42.Dosunmu EO, Hatt SR, Leske DA, Hodge DO, Holmes JM. Incidence and etiology of presumed fourth cranial nerve palsy: a population-based study. Am J Ophthalmol. 2018;185:110–114. doi: 10.1016/j.ajo.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang C, Leavitt JA, Hodge DO, Holmes JM, Mohney BG, Chen JJ. Incidence and Etiologies of Acquired Third Nerve Palsy Using a Population-Based Method. JAMA Ophthalmol. 2017;135:23–28. doi: 10.1001/jamaophthalmol.2016.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung EH, Kim SJ, Lee JY, Cho BJ. The incidence and etiology of sixth cranial nerve palsy in Koreans: a 10-year nationwide cohort study. Sci Rep. 2019;9:18419. doi: 10.1038/s41598-019-54975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung EH, Kim SJ, Lee JY, Cho BJ. The incidence and etiologies of third cranial nerve palsy in Koreans: a 10-year nationwide cohort study. Ophthalmic Epidemiol. 2020;27:460–467. doi: 10.1080/09286586.2020.1773870. [DOI] [PubMed] [Google Scholar]
- 46.Tamhankar MA, Biousse V, Ying GS, Prasad S, Subramanian PS, Lee MS, Eggenberger E, Moss HE, Pineles S, Bennett J, Osborne B, Volpe NJ, Liu GT, Bruce BB, Newman NJ, Galetta SL, Balcer LJ. Isolated third, fourth, and sixth cranial nerve palsies from presumed microvascular versus other causes: a prospective study. Ophthalmology. 2013;120:2264–2269. doi: 10.1016/j.ophtha.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bendszus M, Beck A, Koltzenburg M, Vince GH, Brechtelsbauer D, Littan T, Urbach H, Solymosi L. MRI in isolated sixth nerve palsies. Neuroradiology. 2001;43:742–745. doi: 10.1007/s002340100572. [DOI] [PubMed] [Google Scholar]
- 48.Akagi T, Miyamoto K, Kashii S, Yoshimura N. Cause and prognosis of neurologically isolated third, fourth, or sixth cranial nerve dysfunction in cases of oculomotor palsy. Jpn J Ophthalmol. 2008;52:32–35. doi: 10.1007/s10384-007-0489-3. [DOI] [PubMed] [Google Scholar]
- 49.Chou KL, Galetta SL, Liu GT, Volpe NJ, Bennett JL, Asbury AK, Balcer LJ. Acute ocular motor mononeuropathies: prospective study of the roles of neuroimaging and clinical assessment. J Neurol Sci. 2004;219:35–39. doi: 10.1016/j.jns.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Murchison AP, Gilbert ME, Savino PJ. Neuroimaging and acute ocular motor mononeuropathies: a prospective study. Arch Ophthalmol. 2011;129:301–305. doi: 10.1001/archophthalmol.2011.25. [DOI] [PubMed] [Google Scholar]
- 51.Jung JS, Kim DH. Risk factors and prognosis of isolated ischemic third, fourth, or sixth cranial nerve palsies in the Korean population. J Neuroophthalmol. 2015;35:37–40. doi: 10.1097/wno.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 52.Bourtoulamaiou A, Yadav S, Nayak H. Benign recurrent sixth (abducens) nerve palsy following measles-mumps-rubella vaccination. Case Rep Pediatr. 2015;2015:734516. doi: 10.1155/2015/734516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Werner DB, Savino PJ, Schatz NJ. Benign recurrent sixth nerve palsies in childhood. Secondary to immunization or viral illness. Arch Ophthalmol. 1983;101:607–608. doi: 10.1001/archopht.1983.01040010607016. [DOI] [PubMed] [Google Scholar]
- 54.Woo EJ, Winiecki SK, Ou AC. Motor palsies of cranial nerves (excluding VII) after vaccination: reports to the US Vaccine Adverse Event Reporting System. Hum Vaccin Immunother. 2014;10:301–305. doi: 10.4161/hv.27032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grewal DS, Zeid JL. Isolated abducens nerve palsy following neonatal hepatitis B vaccination. J aapos. 2014;18:75–76. doi: 10.1016/j.jaapos.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 56.Leiderman YI, Lessell S, Cestari DM. Recurrent isolated sixth nerve palsy after consecutive annual influenza vaccinations in a child. J aapos. 2009;13:317–318. doi: 10.1016/j.jaapos.2008.12.137. [DOI] [PubMed] [Google Scholar]
- 57.Chung JY, Lee SJ, Shin BS, Kang HG. Myasthenia gravis following human papillomavirus vaccination: a case report. BMC Neurol. 2018;18:222. doi: 10.1186/s12883-018-1233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biron P, Montpetit P, Infante-Rivard C, Léry L. Myasthenia gravis after general anesthesia and hepatitis B vaccine. Arch Intern Med. 1988;148:2685. doi: 10.1001/archinte.1988.00380120123025. [DOI] [PubMed] [Google Scholar]
- 59.Chavez A, Pougnier C. A case of COVID-19 vaccine associated new diagnosis myasthenia gravis. J Prim Care Community Health. 2021;12:21501327211051933. doi: 10.1177/21501327211051933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee MA, Lee C, Park JH, Lee JH. Early-onset myasthenia gravis following COVID-19 vaccination. J Korean Med Sci. 2022;37:e50. doi: 10.3346/jkms.2022.37.e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tagliaferri AR, Narvaneni S, Azzam MH, Grist W. A case of COVID-19 vaccine causing a myasthenia gravis crisis. Cureus. 2021;13:e15581. doi: 10.7759/cureus.15581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sriwastava S, Tandon M, Kataria S, Daimee M, Sultan S. New onset of ocular myasthenia gravis in a patient with COVID-19: a novel case report and literature review. J Neurol. 2021;268:2690–2696. doi: 10.1007/s00415-020-10263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koneczny I, Herbst R. Myasthenia gravis: pathogenic effects of autoantibodies on neuromuscular architecture. Cells. 2019;8:671. doi: 10.3390/cells8070671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.di Filippo L, Castellino L, Giustina A. Occurrence and response to treatment of Graves' disease after COVID vaccination in two male patients. Endocrine. 2022;75:19–21. doi: 10.1007/s12020-021-02919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goblirsch TJ, Paulson AE, Tashko G, Mekonnen AJ. Graves' disease following administration of second dose of SARS-CoV-2 vaccine. BMJ Case Rep. 2021;14:e246432. doi: 10.1136/bcr-2021-246432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lui DTW, Lee KK, Lee CH, Lee ACH, Hung IFN, Tan KCB. Development of Graves' disease after SARS-CoV-2 mRNA vaccination: a case report and literature review. Front Public Health. 2021;9:778964. doi: 10.3389/fpubh.2021.778964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patrizio A, Ferrari SM, Antonelli A, Fallahi P. A case of Graves' disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J Autoimmun. 2021;125:102738. doi: 10.1016/j.jaut.2021.102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pujol A, Gómez LA, Gallegos C, Nicolau J, Sanchís P, González-Freire M, López-González ÁA, Dotres K, Masmiquel L. Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA-based SARS-CoV2 vaccination: from Graves' disease to silent thyroiditis. J Endocrinol Invest. 2021;45:1–8. doi: 10.1007/s40618-021-01707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sriphrapradang C, Shantavasinkul PC. Graves' disease following SARS-CoV-2 vaccination. Endocrine. 2021;74:473–474. doi: 10.1007/s12020-021-02902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vera-Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sánchez Valadez TI, Jara LJ. Two cases of graves' disease following SARS-CoV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021;31:1436–1439. doi: 10.1089/thy.2021.0142. [DOI] [PubMed] [Google Scholar]
- 71.Weintraub MA, Ameer B, Sinha Gregory N. Graves disease following the SARS-CoV-2 vaccine: case series. J Investig Med High Impact Case Rep. 2021;9:23247096211063356. doi: 10.1177/23247096211063356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zettinig G, Krebs M. Two further cases of Graves' disease following SARS-Cov-2 vaccination. J Endocrinol Invest. 2022;45:227–228. doi: 10.1007/s40618-021-01650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masset C, Kervella D, Kandel-Aznar C, Fantou A, Blancho G, Hamidou M. Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine. Kidney Int. 2021;100:465–466. doi: 10.1016/j.kint.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rubinstein TJ. Thyroid Eye Disease Following COVID-19 Vaccine in a Patient With a History Graves' Disease: A Case Report. Ophthalmic Plast Reconstr Surg. 2021;37:e221–e223. doi: 10.1097/iop.0000000000002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duclos P, Bentsi-Enchill A. Current thoughts on the risks and benefits of immunisation. Drug Saf. 1993;8:404–413. doi: 10.2165/00002018-199308060-00002. [DOI] [PubMed] [Google Scholar]