Abstract
Objective
To compare maternal mortality in eight countries with enhanced surveillance systems.
Design
Descriptive multicountry population based study.
Setting
Eight countries with permanent surveillance systems using enhanced methods to identify, document, and review maternal deaths. The most recent available aggregated maternal mortality data were collected for three year periods for France, Italy, and the UK and for five year periods for Denmark, Finland, the Netherlands, Norway, and Slovakia.
Population
297 835 live births in Denmark (2013-17), 301 169 in Finland (2008-12), 2 435 583 in France (2013-15), 1 281 986 in Italy (2013-15), 856 572 in the Netherlands (2014-18), 292 315 in Norway (2014-18), 283 930 in Slovakia (2014-18), and 2 261 090 in the UK (2016-18).
Outcome measures
Maternal mortality ratios from enhanced systems were calculated and compared with those obtained from each country’s office of vital statistics. Age specific maternal mortality ratios; maternal mortality ratios according to women’s origin, citizenship, or ethnicity; and cause specific maternal mortality ratios were also calculated.
Results
Methods for identifying and classifying maternal deaths up to 42 days were very similar across countries (except for the Netherlands). Maternal mortality ratios up to 42 days after end of pregnancy varied by a multiplicative factor of four from 2.7 and 3.4 per 100 000 live births in Norway and Denmark to 9.6 in the UK and 10.9 in Slovakia. Vital statistics offices underestimated maternal mortality by 36% or more everywhere but Denmark. Age specific maternal mortality ratios were higher for the youngest and oldest mothers (pooled relative risk 2.17 (95% confidence interval 1.38 to 3.34) for women aged <20 years, 2.10 (1.54 to 2.86) for those aged 35-39, and 3.95 (3.01 to 5.19) for those aged ≥40, compared with women aged 20-29 years). Except in Norway, maternal mortality ratios were ≥50% higher in women born abroad or of minoritised ethnicity, defined variously in different countries. Cardiovascular diseases and suicides were leading causes of maternal deaths in each country. Some other conditions were also major contributors to maternal mortality in only one or two countries: venous thromboembolism in the UK and the Netherlands, hypertensive disorders in the Netherlands, amniotic fluid embolism in France, haemorrhage in Italy, and stroke in Slovakia. Only two countries, France and the UK, had enhanced methods for studying late maternal deaths, those occurring between 43 and 365 days after the end of pregnancy.
Conclusions
Variations in maternal mortality ratios exist between high income European countries with enhanced surveillance systems. In-depth analyses of differences in the quality of care and health system performance at national levels are needed to reduce maternal mortality further by learning from best practices and each other. Cardiovascular diseases and mental health in women during and after pregnancy must be prioritised in all countries.
Introduction
Maternal mortality, a key indicator of the quality of maternal healthcare, is at historic lows in high income countries.1 Most of these countries seem to be in stage IV or V of the obstetric transition, in which maternal deaths are mostly due to extra-obstetrical conditions.2 International comparisons of maternal mortality are important as they can highlight national similarities or differences, both of which are valuable for determining priorities in maternal health. Cause specific profiles of maternal mortality provide warnings about the quality of maternal care in particular conditions. They can lead to the identification, analysis, and remediation of inadequate practices, as they have for postpartum haemorrhage in France and Italy and for venous thromboembolism in the UK.3 4 5
Historically, cross country comparisons of maternal mortality have been based on data from vital statistics offices, based on death certificates. Several studies, however, showed that vital statistics underestimate maternal mortality and provide inaccurate information about the cause of death, thus preventing meaningful international comparisons.6 7 8 9 10 Previous reports highlighted the resulting inability to use these data for informative comparisons between European countries, as many of the variations observed reflect differences in measurement methods rather than actual differences in maternal mortality.11 12 13 For example, because countries with the least advanced case ascertainment methods are likely to miss the most cases, they may paradoxically seem to have the lowest maternity mortality ratios.6 7
In response to these major limitations, several countries have implemented enhanced surveillance systems for maternal mortality to optimise and standardise the successive steps of identifying, documenting, classifying, and learning lessons from every maternal death.14 We hypothesised that these enhanced systems ensure that intercountry variability of maternal mortality is not due to differences in data collection methods and that they provide reliable and comparable data. The International Network of Obstetric Survey Systems (INOSS), an international collaboration of researchers and clinicians collecting population based data on severe maternal complications and rare diseases in pregnancy, offered the opportunity to gather and compare such data.15 Our aim was to compare maternal mortality patterns between countries with national data obtained through enhanced maternal mortality surveillance systems.
Methods
Study design and data sources
Eight countries collaborating in the INOSS and using enhanced surveillance systems for maternal mortality participated in this descriptive population based study: Denmark, Finland, France, Italy, the Netherlands, Norway, Slovakia, and the UK.15 We defined enhanced surveillance systems as those with specific prerequisites for the three essential steps of this process: the identification, documentation, and review of maternal deaths. For the first step, completeness of case identification must be ensured by at least one type of linkage between the death register and one of the following: birth register, pregnancy loss register, and hospital discharge database. We considered the Dutch system to be enhanced despite the lack of linkage for the identification of women who died (prevented by data privacy regulations). Because the Netherlands is a relatively small country where reporting of maternal deaths up to one year after the end of pregnancy is mandatory, we considered its maternity mortality surveillance system “possibly enhanced” and included its data in this analysis. The second step, documentation of each case, requires going beyond the content of the death certificate and including data from the birth certificate, birth register, medical records, hospital discharge database, or a confidential inquiry. Finally, the third step—case review—must be conducted by a committee auditing all maternal deaths. Table 1 describes the main features of the enhanced surveillance system in each participating country. Full protocols are published elsewhere.5 16 17 18 19 20 21
Table 1.
Methods used to identify, document, and review maternal deaths in enhanced surveillance system of each country
| Denmark | Finland | France | Italy | Netherlands | Norway | Slovakia | UK | |
|---|---|---|---|---|---|---|---|---|
| Identification of cases | ||||||||
| Death certificate content | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Pregnancy checkbox in death certificate | - | - | Yes | Yes | - | - | Yes | Scotland only |
| Linkage of deaths and birth registers | Yes | Yes | Yes | - | - | Yes | Yes | Yes |
| Linkage of deaths and pregnancy loss registers | Yes | Yes | - | - | - | Yes | Yes | - |
| Linkage of death register and hospital discharge database | Yes | Yes | Yes | Yes | - | Yes | - | Yes |
| Direct notification | Voluntary | Voluntary | Voluntary | Mandatory | Mandatory | Voluntary | Mandatory | Mandatory |
| Documentation of each case, information source | ||||||||
| Content of death certificate | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Content of birth certificate | - | - | - | - | - | Yes | Yes | - |
| ICD-10 codes/procedure codes from hospital discharge data | Yes | Yes | Yes | Yes | - | Yes | - | - |
| Copy of medical records | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Confidential enquiry | Yes | - | Yes | Yes | Yes | Yes | Yes | Yes |
| Review of cases | ||||||||
| National committee | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| ICD-MM definition of maternal death | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Enhanced surveillance for maternal deaths up to 1 year* | - | - | Yes | - | - | - | - | Yes |
| Funding | ||||||||
| Specific funding to run system | Partly by Danish Society of Obstetricians and Gynecologists | No | Yes—from National Public Health Agency | Yes—from National Health Institute | No | No | No | Yes—from Healthcare Quality Improvement Partnership† |
No case identification after 42 days in Denmark and Slovakia; no linkage for case identification in Netherlands; no case review by national committee of late pregnancy associated deaths in Finland, Italy, and Norway.
On behalf of NHS England, NHS Wales, the Health and Social Care Division of the Scottish Government, the Northern Ireland Department of Health, and the States of Jersey, Guernsey and the Isle of Man.
Collected data
We collected the most recent aggregated and complete dataset available at the time of the study design from a time period that varied according to the number of births in each country: a three year period for France, Italy, and the UK and a five year period for Denmark, Finland, the Netherlands, Norway, and Slovakia. The Finnish data cover deaths from 2008 to 2012, and the other countries provided data from 2013 and after (table 2).
Table 2.
Maternal deaths and maternal mortality ratios per 100 000 live births according to enhanced surveillance systems and to vital statistics
| Country and period | Live births | Enhanced surveillance system* | Vital statistics | ||||
|---|---|---|---|---|---|---|---|
| No | MMR (95%CI) | No | MMR (95%CI) | Underestimation (%)† | |||
| Denmark (2013-17) | 297 835 | 10 | 3.4 (1.6 to 6.2) | 9 | 3.0 (1.4 to 5.7) | 10 | |
| Finland (2008-12) | 301 169 | 22 | 7.3 (4.6 to 11.0) | 11‡ | 3.6 (1.8 to 6.5) | 50 | |
| France (2013-15) | 2 435 583 | 196 | 8.0 (7.0 to 9.3) | 125 | 5.1 (4.2 to 6.1) | 36 | |
| Italy (2013-15) | 1 281 986 | 112 | 8.7 (7.2 to 10.5) | 48 | 3.7 (2.8 to 5.0) | 57 | |
| Netherlands (2014-18) | 856 572 | 40 | 4.7 (3.3 to 6.4) | 25 | 2.9 (1.9 to 4.3) | 38 | |
| Norway (2014-18) | 292 315 | 8 | 2.7 (1.2 to 5.4) | 3 | 1.0 (0.2 to 3.0) | 62 | |
| Slovakia (2014-18) | 283 930 | 31 | 10.9 (7.4 to 15.5) | 12 | 4.2 (2.2 to 7.4) | 61 | |
| UK (2016-18) | 2 261 090 | 217 | 9.6 (8.4 to 11.0) | 95§ | 4.2 (3.4 to 5.1) | 56 | |
CI=confidence interval; MMR=maternal mortality ratio.
Maternal mortality up to 42 days after end of pregnancy.
Number of maternal deaths up to 42 days according to enhanced surveillance system (ESS)–number of maternal deaths according to vital statistics/number of maternal deaths up to 42 days according to ESS×100.
In Finland, vital statistics do not include suicides as maternal deaths.
UK number of maternal deaths from vital statistics from 2015 to 2017
We included deaths at any time during pregnancy and up to one year after the end of pregnancy, regardless of pregnancy duration and/or site.14 Late deaths were those occurring between 43 days and one year after the end of pregnancy. Pregnancy associated deaths were those occurring within one year of the end of pregnancy, regardless of their cause. Maternal deaths were those with a cause related to or aggravated by the pregnancy or its management, but not from an accidental or coincidental cause, as per the World Health Organization’s application of ICD-10 (international classification of diseases, 10th revision) to deaths during pregnancy, childbirth, and puerperium (ICD-MM), used by all countries.22
We requested the following aggregated numbers from enhanced systems in each country: pregnancy associated deaths, maternal deaths, and maternal deaths by age group (<20, 20-24, 25-29, 30-34, 35-39, ≥40 years) and by migrant status or ethnicity defined by the categorisations available in each country (current citizenship, country of birth, or ethnicity). We also collected the number of maternal deaths by underlying cause, as classified by the national committees and according to the following groups, also based on ICD-MM but with further developments for some groups relevant for high income settings: pregnancies with abortive outcomes (ectopic pregnancy, post-abortum haemorrhage, post-abortum infection or pulmonary embolism, other abortive outcomes), obstetric haemorrhage (uterine atony, morbidly adherent placenta, placenta previa, uterine rupture, placenta abruption and other obstetric haemorrhage), pregnancy related infection, hypertensive disorders of pregnancy (pre-eclampsia, eclampsia, HELLP syndrome), venous thrombosis and thromboembolism (cerebral pulmonary or unspecified), amniotic fluid embolism, anaesthesia complications, other direct causes, cardiovascular causes (peripartum cardiomyopathy, ischaemic cardiomyopathy, other cardiomyopathy, other cardiac, aortic dissection, arterial rupture), stroke (arterial, haemorrhagic, ischaemic), indirect sepsis (influenza, pneumonia, meningitis, urinary sepsis, other indirect sepsis), malignancy (trophoblastic or other), other indirect causes, suicide, illicit drug or alcohol misuse, and unascertained.22 Finally, we obtained the number of maternal deaths according to the country’s office of vital statistics during the same time periods (official numbers provided by death registers and not reviewed by a committee). We collected the numbers of live births and maternities (see each country’s definition of a live birth and maternity in appendix 1) over the same specified time period, overall, as well as by age and origin.
Analysis
We calculated maternal mortality ratios, as defined by WHO, as the number of maternal deaths during the study period per 100 000 live births during the same time period (number of maternities was unavailable for Italy).23 We calculated the maternal mortality ratios up to 42 days after the end of pregnancy, with their 95% confidence intervals, according to the enhanced surveillance systems and compared them with those obtained from vital statistics in each country. To quantify between country variation in maternal mortality ratios up to 42 days, we divided the highest maternal mortality ratio by the lowest one and also calculated the maximum absolute difference in maternal mortality ratios by subtracting the smallest maternal mortality ratio from the highest one, with its 95% confidence interval. We also calculated maternal mortality ratios up to one year for countries where enhanced surveillance of maternal mortality was conducted for late deaths. We calculated age specific maternal mortality ratios and those for women with migrant or minoritised ethnic backgrounds (based on current citizenship, country of birth, or ethnicity) for each country. For age, we also calculated the pooled relative risks and 95% confidence intervals of death by maternal age group, including data from all the countries that used linkage for case identification, with women aged 20-29 as the reference group, using a random effects meta-analysis model with inverse variance weighting and the DerSimonian and Laird method.24
Finally, in each country, we calculated the proportion of each cause among all maternal deaths, as well as the cause specific maternal mortality ratios per 100 000 live births. We did this for maternal mortality up to 42 days in all countries and for maternal mortality up to one year in countries where enhanced surveillance took place for late deaths. We used Stata v13 for analyses.
Data availability
Data on the citizenship of women who died and the number of live births according to citizenship were available for five countries: Finland, France, Italy, Norway, and Slovakia (appendix 2). The country of birth of women who died and the number of live births according to country of birth were available for six countries: Denmark, Finland, France, Norway, Slovakia, and the UK. Maternal ethnicity of women who died and the number of live births were available for two countries: the Netherlands (ethnicity reported by healthcare providers) and the UK (self-reported ethnicity).
Patient and public involvement
No patients were involved in setting the research question, as we thought that discussing the topic of maternal mortality with mothers may be difficult. Being involved in the maternal mortality surveillance systems rather than directly speaking to patients inspired this research. However, although there was no direct patient involvement in this paper, members of the public have read our manuscript. All agreed on the importance of the topic and of the specific findings of this study.
Results
The maternal mortality ratios up to 42 days varied by a multiplicative factor of four between countries (table 2; fig 1). The lowest maternal mortality ratios were in Norway (2.7, 95% confidence interval 1.2 to 5.4) and Denmark (3.4, 1.6 to 6.2) and the highest in the UK (9.6, 8.4 to 11.0) and Slovakia (10.9, 7.4 to 15.5), all per 100 000 live births, with a maximum absolute difference of 8.2 (3.9 to 12.5) per 100 000 live births. Compared with the enhanced systems, vital statistics offices underestimated by 36% or more the number of maternal deaths and the corresponding maternal mortality ratio in every country except Denmark (table 2; fig 1).
Fig 1.
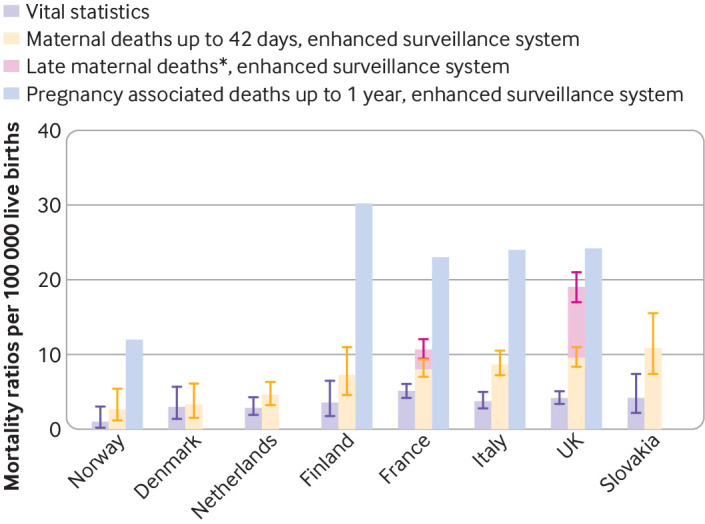
Maternal and pregnancy associated mortality ratios up to 1 year after end of pregnancy, in countries with enhanced surveillance systems (listed from lowest to highest maternal mortality ratio up to 42 days). Error bars represent 95% confidence intervals. Pregnancy associated deaths are those occurring within 1 year of end of pregnancy, regardless of their cause. Maternal deaths are those with cause related to or aggravated by pregnancy or its management but not from accidental or coincidental cause. In Norway, Finland, and Italy, pregnancy associated deaths are identified up to 1 year after end of pregnancy, but cases occurring between 43 days and 1 year are not reviewed, which is why late maternal deaths were unavailable. In Denmark and Slovakia, pregnancy associated deaths are identified only up to 42 days after end of pregnancy; accordingly, pregnancy associated deaths up to 1 year and late maternal deaths were unavailable for these two countries. In the Netherlands, results up to 1 year after pregnancy end were considered not reliable as linkage is not used to identify deaths, and they therefore not included. *Maternal deaths between 43 and 365 days after end of pregnancy
Enhanced methods for identification and/or review of late maternal deaths (between 43 days and one year after the end of pregnancy) were not available for Denmark, Finland, Italy, the Netherlands, Norway, or Slovakia. In the two countries where enhanced surveillance of maternal mortality extended up to one year, maternal mortality ratios up to one year varied from 10.8 (9.5 to 12.1) in France to 19.1 (17.0 to 21.0) in the UK, per 100 000 live births. The contribution of late deaths to total maternal mortality up to one year was 25% in France and 50% for the UK (fig 1).
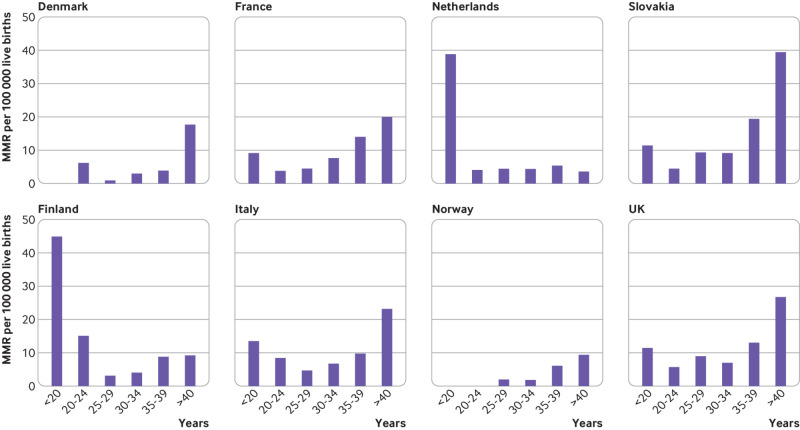
Age specific maternal mortality ratios were highest for the oldest mothers in all countries except the Netherlands (fig 2; appendix 3). In the pooled analysis, using data from all countries except the Netherlands, women aged under 20 years had a higher risk of maternal mortality than did those aged 20-29 (pooled relative risk 2.17, 95% confidence interval 1.38 to 3.43), as did women aged 35-39 (2.10, 1.54 to 2.86) and those aged over 40 (3.95, 3.01 to 5.19) (results not in table).
Fig 2.
Age specific maternal mortality ratios, in countries with enhanced surveillance systems. Maternal mortality up to 42 days after end of pregnancy. MMR=maternal mortality ratio
In Finland and Slovakia, all deaths occurred in women who were both citizens of and born in that country. For the other countries, we observed disparities in maternal mortality according to where the women were from regardless of the categorisation used, with maternal mortality ratios ≥50% higher in minoritised compared with reference subgroups, except in Norway (fig 3; appendix 4). Among the three countries with data available for citizenship, maternal mortality ratios were higher for women with a foreign nationality in both France and Italy but not in Norway. Women born abroad had a higher maternal mortality ratio than those born in Denmark and in France; this was not the case in either the UK or Norway. In both countries collecting ethnicity data, women who were not white had higher maternal mortality ratios.
Fig 3.
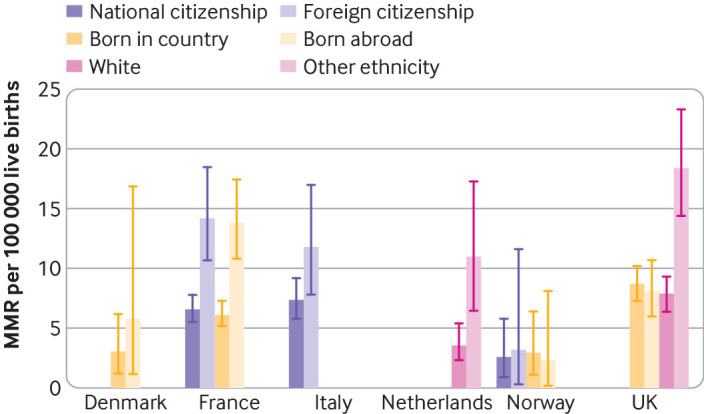
Maternal mortality ratios by women’s migrant or minoritised ethnic background according to various categorisations, in countries with enhanced surveillance systems. Maternal mortality up to 42 days after end of pregnancy; categories presented are those available in each country. Error bars represent 95% confidence intervals. Because all maternal deaths in Finland and Slovakia occurred in women who were citizens and natives of the country, these countries are not presented in this figure. MMR=maternal mortality ratio
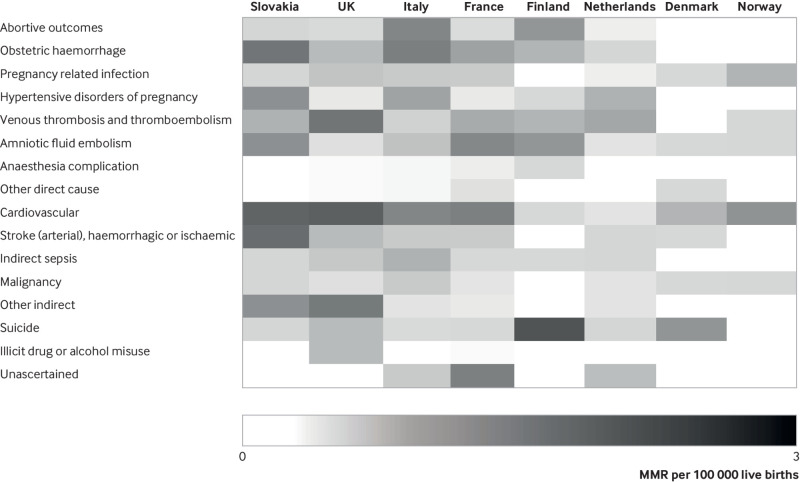
Major contributors to maternal deaths in all countries were cardiovascular diseases (for maternal mortality up to 42 days and up to one year) (table 3; table 4; fig 4) and suicide (for maternal mortality up to one year) (appendix 5 and 6). In addition, specific conditions contributed notably more to maternal mortality in some countries than in others. These include venous thromboembolism in the UK and the Netherlands, hypertensive disorders in the Netherlands, amniotic fluid embolism in France, haemorrhage in Italy, and stroke in Slovakia.
Table 3.
Cause specific maternal mortality* (numbers, proportion of all maternal deaths, and maternal mortality ratios) in countries with enhanced surveillance systems (Denmark, Finland, France, and Italy)
| Cause of death | Denmark (10/297 835)† | Finland (22/301 169)† | France (196/2 435 583)† | Italy (112/1 281 986)† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No (%) | MMR‡ | No (%) | MMR‡ | No (%) | MMR‡ | No (%) | MMR‡ | ||||
| Abortive outcomes§ | 0 | - (0.0 to1.2) | 3 (13.6) | 1.0 (0.24to3.2) | 7 (3.6) | 0.3 (0.1 to 0.5) | 15 (13.4) | 1.2 (0.6 to 1.9) | |||
| Obstetric haemorrhage¶ | 0 | - (0.0 to 1.2) | 2 (9.2) | 0.7 (0.1 to 2.4) | 21 (10.7) | 0.9 (0.5 to 1.3) | 16 (14.3) | 1.2 (0.7 to 2.0) | |||
| Pregnancy related infection | 1 (10.0) | 0.3 (0.0 to1.9) | 0 | - (0.0 to 1.2) | 11 (5.6) | 0.5 (0.2 to 0.8) | 6 (5.4) | 0.5 (0.1 to 1.0) | |||
| Hypertensive disorders of pregnancy** | 0 | - (0.0 to 1.2) | 1 (4.5) | 0.3 (0.01 to 1.9) | 4 (2.0) | 0.2 (0.0 to 0.4) | 11 (9.8) | 0.9 (0.4 to 1.5) | |||
| Venous thrombosis and thromboembolism | 0 | - (0.0 to 1.2) | 2 (9.2) | 0.7 (0.1 to 2.4) | 19 (9.7) | 0.8 (0.5 to 1.2) | 5 (4.5) | 0.4 (0.1 to 0.9) | |||
| Amniotic fluid embolism | 1 (10.0) | 0.3 (0.0 to 1.9) | 3 (13.6) | 1.0 (0.24 to 3.2) | 28 (14.3) | 1.1 (0.7 to 1.7) | 7 (6.3) | 0.5 (0.2 to 1.1) | |||
| Anaesthesia complication | 0 | - (0.0 to 1.2) | 1 (4.5) | 0.3 (0.01 to 1.9) | 3 (1.5) | 0.1 (0.0 to 0.3) | 1 (0.9) | 0.1 (0.0 to 0.4) | |||
| Other direct causes | 1 (10.0) | 0.3 (0.0 to 1.9) | 0 | - (0.0 to 1.2) | 6 (3.1) | 0.2 (0.0 to 0.5) | 1 (0.9) | 0.1 (0.0 to 0.4) | |||
| Cardiovascular†† | 2 (20.0) | 0.7 (0.1 to 2.4) | 1 (4.5) | 0.3 (0.01 to 1.9) | 30 (15.3) | 1.2 (0.8 to 1.7) | 15 (13.4) | 1.2 (0.6 to 1.9) | |||
| Stroke (arterial), haemorrhagic or ischaemic | 1 (10.0) | 0.3 (0.0 to 1.9) | 0 | - (0.0 to 1.2) | 11 (5.6) | 0.5 (0.2 to 0.8) | 6 (5.4) | 0.5 (0.2 to 1.0) | |||
| Indirect sepsis‡‡ | 0 | - (0.0 to 1.2) | 1 (4.5) | 0.3 (0.01 to 1.9) | 8 (4.1) | 0.3 (0.1 to 0.6) | 9 (8.0) | 0.7 (0.3 to 1.3) | |||
| Malignancy§§ | 1 (10.0) | 0.3 (0.0 to 1.9) | 0 | - (0.0 to 1.2) | 5 (2.6) | 0.2 (0.0 to 0.4) | 6 (5.4) | 0.5 (0.1 to 1.0) | |||
| Other indirect causes | 0 | - (0.0 to 1.2) | 0 | - (0.0 to 1.2) | 4 (2.0) | 0.2 (0.0 to 0.4) | 3 (2.7) | 0.2 (0.0 to 0.7) | |||
| Suicide | 3 (30.0) | 1.0 (0.2 to 2.9) | 8 (36.4) | 2.7 (1.2 to 5.2) | 8 (4.1) | 0.3 (0.1 to 0.6) | 4 (3.6) | 0.3 (0.0 to 0.8) | |||
| Illicit drug or alcohol misuse | 0 | - (0.0 to 1.2) | 0 | - (0.0 to 1.2) | 1 (0.5) | 0.0 (0.0 to 0.2) | 0 | - (0.0 to 0.3) | |||
| Unascertained | 0 | - (0.0 to 1.2) | 0 | - (0.0 to 1.2) | 30 (15.3) | 1.2 (0.8 to 1.7) | 7 (6.3) | 0.5 (0.2 to1.1) | |||
Maternal mortality up to 42 days after end of pregnancy.
Total number of maternal deaths/number of live births.
Maternal mortality ratios per 100 000 live births (95% confidence interval or one sided 97.5% confidence interval).
Ectopic pregnancy, post-abortion (spontaneous, elective, and medically indicated) haemorrhage, post-abortion infection, post-abortion pulmonary embolism, and other abortive outcomes.
Haemorrhage by uterine atony, morbidly adherent placenta, placenta praevia, uterine rupture, placenta abruption, and other obstetric haemorrhage.
Pre-eclampsia, eclampsia, and HELLP syndrome.
Peripartum cardiomyopathy, ischaemic cardiomyopathy, other cardiomyopathy, other cardiac, aortic dissection, and arterial rupture.
Influenza, pneumonia, meningitis, urinary sepsis, and other indirect sepsis.
In all countries, deaths after trophoblastic tumours were considered maternal. For other tumours, definitions of maternal death varied between countries. In Denmark, only pregnancy associated deaths (PADs) from breast cancers were considered maternal. In Finland, no formal rules were used to classify PAD as maternal, and cases were discussed individually. In France, maternal deaths were PADs from all hormone sensitive cancers or any other type of cancer for which pregnancy delayed diagnosis or treatment. In Italy, all PADs from cancers of breast, ovary, cervix, skin, blood, or brain were considered maternal.
Table 4.
Cause specific maternal mortality* (numbers, proportion of all maternal deaths, and maternal mortality ratios) in countries with enhanced surveillance systems (Netherlands, Norway, Slovakia, and UK)
| Cause of death | Netherlands (40/856 572)† | Norway (8/292 315)† | Slovakia (31/283 930)† | UK (217/2 261 090)† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No (%) | MMR‡ | No (%) | MMR‡ | No (%) | MMR‡ | No (%) | MMR‡ | ||||
| Abortive outcomes§ | 1 (2.5) | 0.1 (0.0 to 0.6) | 0 | - (0.0 to 1.3) | 1 (3.2) | 0.4 (0.0 to 2.0) | 7 (3.2) | 0.3 (0.1 to 0.6) | |||
| Obstetric haemorrhage¶ | 3 (7.5) | 0.4 (0.0 to 1.0) | 0 | - (0.0 to 1.3) | 4 (12.9) | 1.4 (0.3 to 3.6) | 14 (6.5) | 0.6 (0.3 to 10.4) | |||
| Pregnancy related infection | 1 (2.5) | 0.1 (0.0 to 0.6) | 2 (25.0) | 0.7 (0.0 to 2.5) | 1 (3.2) | 0.4 (0.0 to 2.0) | 12 (5.5) | 0.5 (0.2 to 0.9) | |||
| Hypertensive disorders of pregnancy** | 6 (15.0) | 0.7 (0.2 to 1.5) | 0 | - (0.0 to 1.3) | 3 (9.7) | 1.1 (0.2 to 3.1) | 4 (1.8) | 0.2 (0.0 to 0.4) | |||
| Venous thrombosis and thromboembolism | 7 (17.5) | 0.8 (0.3 to 1.7) | 1 (12.5) | 0.3 (0.0 to 1.9) | 2 (6.5) | 0.7 (0.0 to 2.5) | 33 (15.2) | 1.5 (1.0 to 2.0) | |||
| Amniotic fluid embolism | 2 (5.0) | 0.2 (0.0 to 0.8) | 1 (12.5) | 0.3 (0.0 to 1.9) | 3 (9.7) | 1.1 (0.2 to 3.1) | 6 (2.8) | 0.3 (0.1 to 0.6) | |||
| Anaesthesia complication | 0 | - (0.0 to 0.6) | 0 | - (0.0 to 1.3) | 0 | - (0.0 to 1.3) | 1 (0.5) | 0.0 (0.0 to 0.3) | |||
| Other direct causes | 0 | - (0.0 t0 .6) | 0 | - (0.0 t o1.3) | 0 | - (0.0 to 1.3) | 1 (0.5) | 0.0 (0.0 to 0.3) | |||
| Cardiovascular†† | 2 (5.0) | 0.2 (0.0 to 0.8) | 3 (37.5) | 1.0 (0.0 to 3.0) | 6 (19.4) | 2.1 (0.7 to 4.6) | 50 (23.0) | 2.2 (1.6 to 2.9) | |||
| Stroke (arterial), haemorrhagic or ischaemic | 3 (7.5) | 0.4 (0.0 to 1.0) | 0 | - (0.0 to 1.3) | 5 (16.1) | 1.8 (0.5 to 4.1) | 14 (6.5) | 0.6 (0.3 to 1.0) | |||
| Indirect sepsis‡‡ | 3 (7.5) | 0.4 (0.0 to 1.0) | 0 | - (0.0 to 1.3) | 1 (3.2) | 0.4 (0.0 to 2.0) | 11 (5.1) | 0.5 (0.2 to 0.9) | |||
| Malignancy§§ | 2 (5.0) | 0.2 (0.0 to 0.8) | 1 (12.5) | 0.3 (0.0 to 1.9) | 1 (3.2) | 0.4 (0.0 to 2.0) | 6 (2.8) | 0.3 (0.0 to 0.6) | |||
| Other indirect causes | 2 (5.0) | 0.2 (0.0 to 0.8) | 0 | - (0.0 to 1.3) | 3 (9.7) | 1.1 (0.2 to 3.1) | 30 (13.8) | 1.3 (0.9 to 1.9) | |||
| Suicide | 3 (7.5) | 0.4 (0.0 to 1.0) | 0 | - (0.0 to 1.3) | 1 (3.2) | 0.4 (0.0 to 2.0) | 14 (6.5) | 0.6 (0.3 to 1.0) | |||
| Illicit drug or alcohol misuse | 0 | - (0.0 to 0.6) | 0 | - (0.0 to 1.3) | 0 | - (0.0 to 1.3) | 14 (6.5) | 0.6 (0.3 to 1.0) | |||
| Unascertained | 5 (12.5) | 0.6 (0.2 to 1.4) | 0 | - (0.0 to 1.3) | 0 | - (0.0 to 1.3) | 0 | - (0.0 to 0.3) | |||
Maternal mortality up to 42 days after end of pregnancy.
Total number of maternal deaths/number of live births..
Maternal mortality ratios per 100 000 live births (95% confidence interval or one sided 97.5% confidence interval).
Ectopic pregnancy, post-abortion (spontaneous, elective, and medically indicated) haemorrhage, post-abortion infection, post-abortion pulmonary embolism, and other abortive outcomes.
Haemorrhage by uterine atony, morbidly adherent placenta, placenta praevia, uterine rupture, placenta abruption, and other obstetric haemorrhage.
Pre-eclampsia, eclampsia, and HELLP syndrome.
Peripartum cardiomyopathy, ischaemic cardiomyopathy, other cardiomyopathy, other cardiac, aortic dissection, and arterial rupture.
Influenza, pneumonia, meningitis, urinary sepsis, and other indirect sepsis.
In all countries, deaths after trophoblastic tumours were considered maternal. For other tumours, definitions of maternal death varied between countries. In Netherlands, maternal deaths were pregnancy associated deaths (PADs) from all hormone sensitive cancers or any other type of cancer for which pregnancy delayed diagnosis or treatment. In Norway, PADs from breast and cervical cancers were considered maternal deaths. In Slovakia, PADs from any type of cancer were considered maternal deaths. UK’s definition of maternal death due to cancer was PADs after cancer of breast, ovary, or uterus.
Fig 4.
Cause specific maternal mortality ratios, in countries with enhanced surveillance systems. Maternal mortality up to 42 days after end of pregnancy. Countries are listed from lowest to highest all cause maternal mortality ratio (MMR)
Discussion
This comparison of maternal mortality data from enhanced national surveillance systems in eight European countries shows that maternal mortality ratios up to 42 days after the end of pregnancy varied by a multiplicative factor of four—from 2.7 and 3.4 per 100 000 live births in Norway and Denmark to 9.6 in the UK and 10.9 in Slovakia. The analysis showed similarities between countries in the subgroups of women at high risk (older and with migrant or minoritised ethnic backgrounds) and with cardiovascular disease and suicide as leading causes of death, but also disparities between countries for other important causes of maternal mortality.
Importance of enhanced systems
Our analysis confirms that national vital statistics registers are not a valid source for surveillance of maternal mortality in high income countries, as previously reported with older data from some of these countries and others.6 8 9 10 The substantial underestimation of maternal mortality from vital statistics alone observed in our study underpins that previous reports on national maternal mortality levels worldwide using this source of data for high income countries are not informative for this category of countries.25 26 This strongly supports the current WHO initiative to integrate data from enhanced surveillance systems in countries where they exist when reporting international comparisons of these deaths.1 12 The extent of this underestimation may vary between countries, as vital statistics in some countries, such as Finland, did not include suicides as maternal deaths when these data were collected, whereas those in other countries, such as France and the UK, did. Our detailed description of enhanced systems also highlights the major importance of data linkage for maternal death case finding; it is a key component of an enhanced maternal mortality surveillance system. We hypothesise that the singular maternal mortality pattern found in the Netherlands (the only participating country not using linkage) might be explained by the absence of linkage to birth records, which might have resulted in missing some deaths, especially those in the late postpartum period or from non-obstetric causes. Keeping the Dutch data in our analysis illustrates our point on the importance of linkage and shows how some strict data privacy rules (barrier to the implementation of data linkage in this country) might prevent the ascertainment of maternal deaths and the calculation of valid maternal mortality ratios. Receiving official support from national health authorities might help enhanced systems to highlight the specificities of maternal mortality measurement and of the methods needed; as table 1 shows, the two countries with extended enhanced surveillance up to one year after the end of pregnancy are receiving specific funding from national health agencies to run the system. Government support, beyond the visibility it brings, is certainly a guarantee of the quality of the system’s operation.
Other interesting findings were the heterogeneity between countries in the methods used to study late maternal deaths (those occurring between 43 days and one year after the end of pregnancy), both in terms of case identification or review—not conducted for these late deaths in most countries—and of obviously heterogeneous classification rules, which make interpreting national variations in maternal mortality up to one year difficult. Differences of classification were particularly striking for late deaths from cancer. Deaths from or after trophoblastic tumours were considered maternal in all countries. In France, maternal deaths also included those from hormone sensitive cancers or any cancer for which the pregnancy delayed diagnosis or treatment, whereas the UK’s definition of maternal deaths due to cancer specified cancers of the breast, ovaries, or uterus. In Italy, all deaths from cancers of the breast, ovaries, cervix, skin, blood, or brain were considered maternal. This heterogeneity is likely due to the lack of precision in the ICD-MM guidelines regarding the classification of late maternal deaths. Further standardisation of classification is urgently needed to allow relevant international comparisons of those late deaths and of maternal mortality up to one year to be conducted.27 All lessons learnt about the importance of enhanced surveillance of maternal mortality in high income countries may also be valuable for low and middle income countries.
Differences in maternal mortality profiles between countries
We showed that maternal mortality up to 42 days after the end of pregnancy varied by a factor of four among the participating countries. As methods for identifying and classifying maternal deaths up to 42 days were very similar across countries (except for the Netherlands), we believe that the differences in the level and profile of causes of maternal mortality in this time frame are not at all or very little related to measurement variations. Several possible explanations for these variations exist. Firstly, differences could exist in baseline characteristics of pregnant women between countries. Wide variations exist in the proportions, among all births, of younger and older mothers and of mothers with a migrant or minoritised ethnic background (see appendix 7 and 8 for distribution in the different countries). These may partially explain the differences in maternal mortality ratios, as those characteristics were associated with higher risks of maternal death. A standardisation of the sociodemographic variables collected for each maternal death and each birth, not only on geographical origin but also other dimensions of the social status, would help to further explore this hypothesis. Other characteristics not reported in our study, such as body mass index, also vary between countries, and this may be matched with variations in cardiovascular maternal mortality in our study.28 Alternatively, variations in maternal mortality ratio up to 42 days may reflect differences in the quality of healthcare provided or performance of healthcare systems between countries. Differences in maternal mortality from causes that are not strongly related to individual characteristics, such as haemorrhage or amniotic fluid embolism, may suggest explanatory hypotheses related to care. Finally, organisation of care in large countries with high numbers of births and of maternity units is probably more challenging than for countries with a smaller perinatal community. Future research on individual data is needed to disentangle these non-exclusive explanatory hypotheses.
Between country variation of maternal mortality was the key focus of our study, but we acknowledge that national rates may mask within country variations, as previously highlighted in France and in the Netherlands.29 30 Although the characteristics of women may vary from one region to another, a meaningful national profile of pregnant women exists, and the organisation of care and recommendations for good practice are defined according to a national standard. Furthermore, the appropriate regional categorisation may vary from one country to another; thus, in the two studies cited above, France isolated the overseas territories and the Netherlands the urban regions, in relation to each national question of healthcare organisation. Sticking to the national scale in our analysis therefore seemed justified.
Similarities in causes of maternal mortality between countries
Although differences in the main causes of death were highlighted in some countries, we also noted similarities. In particular, cardiovascular diseases and suicides were leading causes of maternal death in most countries. Although the increasing importance of cardiovascular causes and suicides within the maternal deaths has already been highlighted in individual country reports, the major contribution of our analysis is to compare these country patterns with homogenised data. For example, cardiovascular mortality is the first cause of maternal mortality up to 42 days in France and in the UK, but the specific maternal mortality ratio due to this cause is twice as important in the UK (2.2 v 1.2 per 100 000 live births); the same observation can be made concerning maternal suicide mortality up to one year between these two countries (2.9 v 1.4 per 100 000 live births).
This shared pattern underlines the importance of women’s mental and cardiovascular health and the need to develop strategies before, during, and after pregnancy to prevent the morbidity and mortality these problems can cause.31 32 33 34 35 36 37 38 This is a substantial challenge, as the management of these conditions implies an extension of maternal care to coordinate various medical disciplines and levels of care, from preconception to postpartum. Most high income countries are in stage IV or even V of the obstetric transition.2 In stage IV, indirect causes of maternal mortality become more important, especially those related to non-communicable diseases. Improved quality of care and the elimination of health system delays are needed to reduce maternal mortality further. Stage V is the ultimate goal: the avoidance of all avoidable maternal deaths. At that point, the main concerns would be real progress against structural violence (for example, gender inequality), effective care of vulnerable populations (for example, displaced people, immigrants, and disadvantaged racial, ethnic, and sexual minorities), and finally successfully sustaining this quality of care over the long term.
Conclusion
We report variations in maternal mortality ratios up to 42 days between European countries with enhanced surveillance systems that minimise measurement variability. Although these variations may result from differences in the sociodemographic characteristics of pregnant women between countries, they also raise questions about differential quality of healthcare provided and the performance of health systems. To further reduce maternal mortality by learning from best practices and each other, in-depth analyses of differences in quality of care and health system performance at national levels are needed. Cardiovascular diseases and mental health in women during and after pregnancy need to be prioritised in all countries.
What is already known on this topic
Despite its rarity in high income countries, maternal mortality remains an important health indicator of the quality of the care provided and health system performance
International comparisons of maternal mortality rates and causes can highlight similarities and differences between countries, providing valuable information about priorities in maternal health
Some countries have set up enhanced systems for maternal mortality surveillance, expected to minimise the variability of measurement and provide comparable data between countries
What this study adds
Enhanced methods to identify, document, and review maternal deaths are needed to provide reliable maternal mortality data in high income countries
Maternal mortality ratios up to 42 days after the end of pregnancy varied by a factor of four, from 2.7 and 3.4 per 100 000 live births in Denmark and Norway to 9.6 in the UK and 10.9 in Slovakia
Cardiovascular diseases and suicides were leading causes of maternal deaths in all countries
Web extra.
Extra material supplied by authors
Supplementary information: Appendices 1-8
Contributors: CD, MS, AKallianidis, and CDT designed the study. CDT and MS were responsible for the French data. AKallianidis and KB were responsible for the data from the Netherlands. MB and SD were responsible for the Italian data. BB and MJ were responsible for the Danish data. MG was responsible for the Finnish data. LN was responsible for the Norwegian data. MKnight was responsible for the UK data. MKorbel and AKristufkova were responsible for the Slovak data. CD collected the data. CD, MS, AKallianidis, and CDT did the analysis and wrote the draft manuscript. All authors contributed significantly to writing the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. CDT is the guarantor.
Funding: This study received no specific funding.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Dissemination of the results to women, families, healthcare practitioners, and policy makers will be undertaken at the international level through the INOSS website and social media account. The results will be presented at the FIGO conference in Paris (2023). At the national level, the people in charge of the obstetric surveillance systems of each country involved in the study will disseminate the results to healthcare practitioners through professional bodies’ websites and conferences and to women through patients’ associations.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
As the study was conducted on aggregated data only, no ethics approval was needed.
Data availability statement
No additional data available.
References
- 1.World Health Organization, UNICEF, UNFPA, World Bank Group, United Nations. Maternal mortality: levels and trends, 2000 to 2017. 2019. https://www.who.int/publications/i/item/9789241516488.
- 2. Souza JP, Tunçalp Ö, Vogel JP, et al. Obstetric transition: the pathway towards ending preventable maternal deaths. BJOG 2014;121(Suppl 1):1-4. 10.1111/1471-0528.12735 [DOI] [PubMed] [Google Scholar]
- 3. Morau E, Ducloy JC, Le Roux S, Weber P, Dreyfus M, Comité National d’Experts sur la Mortalité Maternelle . [Maternal deaths due to haemorrhage in France 2013-2015]. Gynecol Obstet Fertil Senol 2021;49:60-6. 10.1016/j.gofs.2020.11.011 [DOI] [PubMed] [Google Scholar]
- 4. Donati S, Buoncristiano M, Lega I, D’Aloja P, Maraschini A, ItOSS working group . The Italian Obstetric Surveillance System: Implementation of a bundle of population-based initiatives to reduce haemorrhagic maternal deaths. PLoS One 2021;16:e0250373. 10.1371/journal.pone.0250373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knight M, Bunch K, Tuffnell D, et al., eds. MBRRACE-UK. Saving Lives, Improving Mothers’ Care - Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2016-18. National Perinatal Epidemiology Unit. University of Oxford, 2020. [Google Scholar]
- 6. Saucedo M, Bouvier-Colle MH, Chantry AA, Lamarche-Vadel A, Rey G, Deneux-Tharaux C. Pitfalls of national routine death statistics for maternal mortality study. Paediatr Perinat Epidemiol 2014;28:479-88. 10.1111/ppe.12153 [DOI] [PubMed] [Google Scholar]
- 7. Deneux-Tharaux C, Berg C, Bouvier-Colle MH, et al. Underreporting of pregnancy-related mortality in the United States and Europe. Obstet Gynecol 2005;106:684-92. 10.1097/01.AOG.0000174580.24281.e6 [DOI] [PubMed] [Google Scholar]
- 8. Schuitemaker N, Van Roosmalen J, Dekker G, Van Dongen P, Van Geijn H, Gravenhorst JB. Underreporting of maternal mortality in The Netherlands. Obstet Gynecol 1997;90:78-82. 10.1016/S0029-7844(97)00128-2 [DOI] [PubMed] [Google Scholar]
- 9. Berdzuli N, Lomia N, Staff AC, Kereselidze M, Lazdane G, Jacobsen AF. Maternal Mortality in Georgia: Incidence, Causes and Level of Underreporting: A National Reproductive Age Mortality Study 2014. Int J Womens Health 2020;12:277-86. 10.2147/IJWH.S227349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donati S, Maraschini A, Lega I, D’Aloja P, Buoncristiano M, Manno V, Regional Maternal Mortality Working Group . Maternal mortality in Italy: Results and perspectives of record-linkage analysis. Acta Obstet Gynecol Scand 2018;97:1317-24. 10.1111/aogs.13415 [DOI] [PubMed] [Google Scholar]
- 11.Euro-Peristat Project. European Perinatal Health Report. Core indicators of the health and care of pregnant women and babies in Europe in 2015. 2018. https://www.europeristat.com/index.php/reports/european-perinatal-health-report-2015.html.
- 12. Bouvier-Colle MH, Mohangoo AD, Gissler M, et al. Euro-Peristat Scientific Committee . What about the mothers? An analysis of maternal mortality and morbidity in perinatal health surveillance systems in Europe. BJOG 2012;119:880-9, discussion 890. 10.1111/j.1471-0528.2012.03330.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Creanga AA. Maternal mortality in the developed world: a review of surveillance methods, levels and causes of maternal deaths during 2006-2010. Minerva Ginecol 2017;69:608-17. [DOI] [PubMed] [Google Scholar]
- 14. Berg C, Danel I, Atrash H, Zane S, Bartlett L. Strategies to reduce pregnancy-related deaths: from identification and reveiw to action. Center for Disease Control and Prevention, 2001. [Google Scholar]
- 15. Knight M, INOSS . The International Network of Obstetric Survey Systems (INOSS): benefits of multi-country studies of severe and uncommon maternal morbidities. Acta Obstet Gynecol Scand 2014;93:127-31. 10.1111/aogs.12316 [DOI] [PubMed] [Google Scholar]
- 16. Deneux-Tharaux C, Saucedo M, Comité National d’Experts sur la Mortalité Maternelle . [Enhanced system for maternal mortality surveillance in France, context and Methods]. Gynecol Obstet Fertil Senol 2021;49:3-8. 10.1016/j.gofs.2020.11.005 [DOI] [PubMed] [Google Scholar]
- 17. Vangen S, Bødker B, Ellingsen L, et al. Maternal deaths in the Nordic countries. Acta Obstet Gynecol Scand 2017;96:1112-9. 10.1111/aogs.13172 [DOI] [PubMed] [Google Scholar]
- 18. Donati S, Maraschini A, Dell’Oro S, Lega I, D’Aloja P, Regional Maternal Mortality Working Group . The way to move beyond the numbers: the lesson learnt from the Italian Obstetric Surveillance System. Ann Ist Super Sanita 2019;55:363-70. [DOI] [PubMed] [Google Scholar]
- 19. Bødker B, Hvidman L, Weber T, et al. Reduction in maternal mortality in Denmark over three decades. Dan Med J 2021;68:A02210143. [PubMed] [Google Scholar]
- 20. Korbeľ M, Krištúfková A, Daniš J, Némethová B, Kaščák P, Nižňanská Z. Maternal morbidity and mortality in Slovak Republic in the years 2007-2015. Ceska Gynekol 2019;84:129-39. [PubMed] [Google Scholar]
- 21. Kallianidis AF, Schutte JM, Schuringa LEM, et al. Confidential enquiry into maternal deaths in the Netherlands, 2006-2018. Acta Obstet Gynecol Scand 2022;101:441-9. 10.1111/aogs.14312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. The WHO application of ICD-10 to deaths during pregnancy, childbirth and puerperium: ICD-MM. 2012. https://apps.who.int/iris/handle/10665/70929.
- 23.World Health Organization. The global heath observatory. https://www.who.int/data/gho/indicator-metadata-registry/imr-details/26
- 24. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 25. Alkema L, Chou D, Hogan D, et al. United Nations Maternal Mortality Estimation Inter-Agency Group collaborators and technical advisory group . Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet 2016;387:462-74. 10.1016/S0140-6736(15)00838-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. GBD 2015 Maternal Mortality Collaborators . Global, regional, and national levels of maternal mortality, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1775-812. 10.1016/S0140-6736(16)31470-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van den Akker T, Bloemenkamp KWM, van Roosmalen J, Knight M, Netherlands Audit Committee Maternal Mortality and Morbidity. UK Confidential Enquiry into Maternal Deaths . Classification of maternal deaths: where does the chain of events start? Lancet 2017;390:922-3. 10.1016/S0140-6736(17)31633-1 [DOI] [PubMed] [Google Scholar]
- 28. Devlieger R, Benhalima K, Damm P, et al. Maternal obesity in Europe: where do we stand and how to move forward?: A scientific paper commissioned by the European Board and College of Obstetrics and Gynaecology (EBCOG). Eur J Obstet Gynecol Reprod Biol 2016;201:203-8. 10.1016/j.ejogrb.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 29. Saucedo M, Deneux-Tharaux C, Bouvier-Colle M-H. Understanding regional differences in maternal mortality: a national case-control study in France. BJOG 2012;119:573-81. 10.1111/j.1471-0528.2011.03220.x [DOI] [PubMed] [Google Scholar]
- 30. de Graaf JP, Schutte JM, Poeran JJ, van Roosmalen J, Bonsel GJ, Steegers EA. Regional differences in Dutch maternal mortality. BJOG 2012;119:582-8. 10.1111/j.1471-0528.2012.03283.x [DOI] [PubMed] [Google Scholar]
- 31. Egidy Assenza G, Dimopoulos K, Budts W, et al. Management of acute cardiovascular complications in pregnancy. Eur Heart J 2021;42:4224-40. 10.1093/eurheartj/ehab546 [DOI] [PubMed] [Google Scholar]
- 32. Siegmund AS, Pieper PG, Bilardo CM, et al. Cardiovascular determinants of impaired placental function in women with cardiac dysfunction. Am Heart J 2022;245:126-35. 10.1016/j.ahj.2021.11.020 [DOI] [PubMed] [Google Scholar]
- 33. Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. European Society of Gynecology (ESG) Association for European Paediatric Cardiology (AEPC) German Society for Gender Medicine (DGesGM) ESC Committee for Practice Guidelines . ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011;32:3147-97. 10.1093/eurheartj/ehr218 [DOI] [PubMed] [Google Scholar]
- 34. Silversides CK, Grewal J, Mason J, et al. Pregnancy Outcomes in Women With Heart Disease: The CARPREG II Study. J Am Coll Cardiol 2018;71:2419-30. 10.1016/j.jacc.2018.02.076 [DOI] [PubMed] [Google Scholar]
- 35. Roos-Hesselink J, Baris L, Johnson M, et al. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry Of Pregnancy And Cardiac disease (ROPAC). Eur Heart J 2019;40:3848-55. 10.1093/eurheartj/ehz136 [DOI] [PubMed] [Google Scholar]
- 36. Cantwell R. Mental disorder in pregnancy and the early postpartum. Anaesthesia 2021;76(Suppl 4):76-83. 10.1111/anae.15424 [DOI] [PubMed] [Google Scholar]
- 37. National Institute for Health and Care Excellence . Surveillance report 2017 – Antenatal and postnatal mental health: clinical management and service guidance (2014). NICE guideline CG192. NICE, 2017. [PubMed] [Google Scholar]
- 38. Lega I, Maraschini A, D’Aloja P, et al. Regional maternal mortality working group . Maternal suicide in Italy. Arch Womens Ment Health 2020;23:199-206. 10.1007/s00737-019-00977-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Appendices 1-8
Data Availability Statement
Data on the citizenship of women who died and the number of live births according to citizenship were available for five countries: Finland, France, Italy, Norway, and Slovakia (appendix 2). The country of birth of women who died and the number of live births according to country of birth were available for six countries: Denmark, Finland, France, Norway, Slovakia, and the UK. Maternal ethnicity of women who died and the number of live births were available for two countries: the Netherlands (ethnicity reported by healthcare providers) and the UK (self-reported ethnicity).
No additional data available.