Abstract
Ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) deficiency leads to cardiovascular calcification in infancy, fibroblast growth factor 23 (FGF23)-mediated hypophosphatemic rickets in childhood, and osteomalacia in adulthood. Excessive enthesis mineralization and cervical spine fusion have been previously reported in patients with biallelic ENPP1 deficiency, but their effect on quality of life is unknown. We describe additional musculoskeletal complications in patients with ENPP1 deficiency, namely osteoarthritis and interosseous membrane ossification, and for the first time evaluate health-related quality of life (HRQoL) in patients with this disease, both subjectively via narrative report, and objectively via the Brief Pain Inventory–Short Form, and a Patient Reported Outcome Measurement Information System Physical Function (PROMIS PF) short form. Residual pain, similar in magnitude to that identified in adult patients with X-linked hypophosphatemia, was experienced by the majority of patients despite use of analgesic medications. Impairment in physical function varied from mild to severe. To assess murine ENPP1 deficiency for the presence of enthesopathy, and for the potential response to enzyme replacement therapy, we maintained Enpp1asj/asj mice on regular chow for 23 weeks and treated cohorts with either vehicle or a long-acting form of recombinant ENPP1. Enpp1asj/asj mice treated with vehicle exhibited robust calcification throughout their Achilles tendons, whereas two-thirds of those treated with ENPP1 enzyme replacement exhibited complete or partial suppression of the Achilles tendon calcification. Our combined results document that musculoskeletal complications are a significant source of morbidity in biallelic ENPP1 deficiency, a phenotype which is closely recapitulated in Enpp1asj/asj mice. Finally, we show that a long-acting form of recombinant ENPP1 prevents the development of enthesis calcification at the relatively modest dose of 0.3 mg/kg per week, suggesting that suppression of enthesopathy may be attainable upon dose escalation. © 2021 American Society for Bone and Mineral Research (ASBMR). This article has been contributed to by US Government employees and their work is in the public domain in the USA.
Keywords: ENPP1 DEFICIENCY, ENTHESOPATHY, HEALTH-RELATED QUALITY OF LIFE, ENZYME REPLACEMENT THERAPY
Introduction
Ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) is a type-2 transmembrane protein whose extracellular activity hydrolyzes phosphodiester bonds of extracellular nucleotides such as adenosine triphosphate (ATP) to generate adenosine monophosphate (AMP) and inorganic pyrophosphate (PPi). Because PPi is the main physiologic inhibitor of hydroxyapatite deposition, biallelic ENPP1 deficiency leads to ectopic mineralization in early life, with high mortality in infancy resulting from arterial calcification and luminal narrowing.(1) Patients who survive, as well as ENPP1-deficient individuals who do not exhibit neonatal calcifications, can develop fibroblast growth factor 23 (FGF23)-mediated hypophosphatemic rickets in later life.(2) Other skeletal complications briefly mentioned in prior series include cervical spine fusion and enthesis calcification.(2)
The enthesis is the site of attachment of tendons or ligaments to bone, and is structured in four zones: the dense fibrous connective tissue zone, populated by fibroblast-type cells (tenocytes) and composed of collagen types I and III and decorin; the unmineralized fibrocartilage, populated by fibrochondrocytes and composed of collagen types I and II and aggrecan; the mineralized fibrocartilage, populated by hypertrophic chondrocytes and composed of collagen types II and X and aggrecan; and the bone, populated by osteoblasts, osteocytes, and osteoclasts, and composed of collagen type I.(3) The entheses thus represent a musculoskeletal structure that allows a smooth transition between two widely different tissues, the tendons or ligaments (compliant soft tissues) and bone (a stiff hard tissue).(3) An abrupt transition at this interface would lead to stress concentration between zones and increased risk of failure; conversely, a gradual transition in composition and structure over the enthesis alleviates stress concentrations.(4) Indeed, a gradual decrease in collagen fiber alignment and increase in mineral content is seen from the tendon toward the bone, creating a gradient of tissue stiffness.(4) A decrease in enthesis mineralization leads to decreased strength of this structure,(5) whereas an animal model with expansion of the mineralized fibrocartilage also manifests decreased enthesis strength.(6) Thus, mineralization must be appropriately regulated to achieve optimal mechanical properties of the entheses. Excessive enthesis mineralization has been described not just in patients with ENPP1 deficiency, but also with other etiologies of FGF23-dependent hypophosphatemic rickets, such as X-linked hypophosphatemia (XLH),(7,8) dentin matrix protein 1 (DMP1) deficiency,(9) and tumor-induced osteomalacia.(10) Another skeletal abnormality whose severity has recently been linked to elevated FGF23 is “ossification of the posterior longitudinal ligament” (OPLL),(11) and enthesopathy also appears intimately associated with the disease pathogenesis.(12) However, enthesopathy is not described in FGF23-independent forms of hypophosphatemic rickets, such as sodium-phosphate cotransporter IIc (SLC34A3) deficiency.(13) Indeed, transgenic mice overexpressing the secreted form of human FGF23 manifest enthesopathy,(9) and entheseal fibrochondrocytes are known to express the FGFR3/Klotho receptor and coreceptor, respectively, for FGF23.(8) FGF23 thus appears to be intimately related to the development of enthesopathy, although whether it exerts this effect directly or indirectly is not fully understood. For example, it appears that the FGF23-mediated suppression of 1,25-dihydroxyvitamin D synthesis is responsible for enhanced bone morphogenic protein (BMP) and Indian hedgehog (IHH) signaling, with consequent enthesopathy.(14)
Excessive enthesis mineralization does not represent the only musculoskeletal comorbidity seen in patients with FGF23-mediated hypophosphatemic rickets; osteoarthritis and interosseous membrane ossification have been described in patients with XLH.(15) Patients with ENPP1 deficiency, on the other hand, can additionally develop fusion of the posterior elements of the cervical spine,(2) a complication not seen in other etiologies of FGF23-mediated rickets.
Enthesopathy becomes prevalent with age in patients with XLH,(7) and with age and duration of disease in patients with tumor-induced osteomalacia.(10) Osteoarthritis also worsens with age in XLH patients.(16) Prior work has assessed the impact of skeletal complications on the quality of life (QoL) of adult patients with XLH(17); however, the disease burden from musculoskeletal complications of ENPP1 deficiency has not been previously assessed.
Conventional treatment of rickets worsens the enthesopathy in a mouse model of XLH,(9,18) although it has not been shown to prevent nor promote enthesopathy in patients.(19) It is unknown whether burosumab can prevent the development of enthesis calcification in patients, but an anti-FGF23 blocking antibody has been shown to attenuate the enthesopathy in a mouse model of XLH.(14) Although ENPP1 enzyme replacement therapy has been previously shown to improve survival,(20) cardiovascular function,(21) and complications from rickets(22) in mouse models of ENPP1 deficiency, no prior work has evaluated the effect of treatment on musculoskeletal comorbidities.
In this work, we characterize for the first time the musculoskeletal comorbidities in adults with ENPP1 deficiency, evaluate their effect on QoL, and assess the treatment response to administration of a novel, long-acting form of recombinant enzyme in a preclinical model of the disease.
Patients and Methods
Patients
Five adult individuals with biallelic ENPP1 pathogenic variants were evaluated at the National Institutes of Health (NIH) Clinical Center and enrolled in research protocol 76-HG-0238 (“Diagnosis and Treatment of Patients with Inborn Errors of Metabolism and Other Genetic Disorders”, identifier: NCT00369421) and 18-HG-0064 (“Study of People with Generalized Arterial Calcification of Infancy (GACI) or Autosomal Recessive Hypophosphatemic Rickets Type 2 (ARHR2)”, identifier: NCT03478839). The aforementioned protocols were approved by the National Human Genome Research Institute (NHGRI) Institutional Review Board (IRB). For consistency, naming convention was retained with prior papers characterizing other aspects of the disease (P8 and P11 previously reported in Ferreira et al.(2) in 2020; P16, P17, and P18 previously reported in Ferreira et al.(22) in 2021).
Seven adult individuals with ENPP1 deficiency were enrolled in the study “Understanding the Spectrum of ENPP1 Deficiency and Acute ABCC6 Deficiency Through the Eyes of Patients and Parents; Burden of Illness Perspectives from Patients and Parents who Speak English, French or German”, approved by the Western Institutional Review Board, Inc. (WIRB® protocol #20200966). One of these seven patients was also enrolled in the aforementioned NIH protocols (AdultENPP1-01 is the same as P8).
Standard imaging studies such as radiographs and computed tomography (CT) were obtained for the patients enrolled in the NIH studies. For patients enrolled in the WIRB study, qualitative data was obtained via narrative statement of symptoms, a questionnaire to assess the severity of pain and its impact on daily functions (the Brief Pain Inventory–Short Form [BPI-SF]),(23) and the Patient Reported Outcome Measurement Information System Physical Function (PROMIS PF) 12-item custom short form from the Item Bank v2.0.(24)
The BPI-SF was provided by The University of Texas MD Anderson Cancer Center, which granted permission for use of the questionnaire. Two separate domains are measured by the BPI: pain intensity (severity) and the impact of pain on functioning (interference). Pain severity is measured by scoring pain at its “worst,” “least,” “average,” and “now” (current pain), plus a composite of the four pain items (a mean severity score); each item is scored from 0 (no pain) to 10 (pain as bad as you can imagine). The BPI also measures how much pain has interfered with seven activities of daily living; BPI pain interference was scored as the mean of the seven interference items, with each item scored from 0 (does not interfere) to 10 (completely interferes).
Each item of the PROMIS PF form was scored as follows: 5, without any difficulty; 4, with a little difficulty; 3, with some difficulty; 2, with much difficulty; and 1, unable to do. The total score was then normalized to a T-score, corresponding to a mean in the US general population of 50, with a standard deviation of 10. The degree of impairment of physical function was measured based on patient classification of clinical severity in adults with rheumatic diseases,(25) as follows: >65.0, no impairment in physical function; 45.1–65.0, mild impairment in physical function; 35.1–45.0, moderate impairment in physical function; ≤35.0, severe impairment in physical function.
Mice
Enpp1asj/asj mouse model
Animal care and maintenance were provided through Yale University Animal Resource Center (YARC) at Yale University (New Haven). All procedures were approved by the Animal Care and Use Committee of Yale University and complied with the US National Institutes of Health guide for the care and use of laboratory animals. Enpp1asj/+ mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA; stock number 012810) and homozygous Enpp1asj/asj mice were generated in vitro by the Yale Genome Editing Center using eggs obtained from 3-week-old to 6-week-old female Enpp1asj/asj mice fertilized with sperm obtained from male Enpp1asj/asj mice. The fertilized oocytes were transferred into five pseudopregnant Balb/c mice, yielding 13 male and 17 female Enpp1asj/asj mice. In addition, six wild-type (WT) mice, three males and three females, were generated from heterozygous Enpp1asj/WT breeding pairs to use as controls. All animals were housed in pathogen-free conditions, pups were weaned on day 21, and were maintained on regular chow for the entire 23-week experiment.
The Enpp1asj/asj mice were randomly assigned to cohorts such that eight male and 11 female mice were treated with weekly subcutaneous injections of 0.3 mg/kg of clone BL-1118 (a long-acting variant of ENPP1-Fc described previously(26)) between days 14 and 161, and five male and six female mice were treated with weekly injections of vehicle. The six WT sibling pairs were similarly dosed with weekly injections of vehicle. All animals also received weekly ip injections of anti-murine CD4 (75 μL on week 2 and 50 μL on weeks 3–23) (clone GK 1.5; Life Technologies Corporation, Grand Island, NY, USA; cat #16–0041-95) to suppress immune rejection of the recombinant human biologic used in this study. All animals were terminally bled on day 161 and their Achilles tendons were isolated as described.(8)
Vehicle formulation
Enpp1-Fc was formulated in vehicle as to deliver 16 μL vehicle/gram of body weight. Vehicle consisted of AmericanBio 10× PBS (Canton, MA, USA; Stock# AB11072) diluted to 1× with endotoxin-free water and supplemented with 14μM CaCl2 and 14μM ZnCl2.
BL-1118 expression, purification, and characterization
The sequence of ENPP1 clone BL-1118 is listed in Fig. S1. The expression, purification, and characterization of the biologic for in vivo use was performed as described.(22,26)
Measurement of plasma analytes
Blood plasma was prepared and PPi assayed as described.(27,28) Parathyroid hormone (PTH) was measured with mouse PTH (1–84) ELISA kits purchased from Quidel Corporation (San Diego, CA, USA; catalog number as 60–2305). Intact FGF23 was quantified using Mouse/Rat FGF-23 (intact) ELISA kit (Quidel Corporation; catalog number 60–6800). For all aforementioned ELISA experiments, 45-μL plasma samples were used. Plasma phosphate and calcium was determined by the George M. O’Brien Kidney Center at Yale.
Quantification of enthesis calcification
Tendon samples were fixed in 70% ethanol, embedded in paraffin, and sectioned in 5-μm increments. Tendon sections were stained with Alizarin Red (ALR) to quantify calcified tissue (red) and Picro-Sirius Red (PSR) to capture tendon collagen fibers (green). The slides were imaged under normal light using an Olympus BX43 (Olympus Life Science, Waltham, MA, USA) and a SPOT Idea 5 MP camera (SPOT Imaging, Sterling Heights, MI, USA).
To capture the full tendon, each sample was imaged multiple times and then stitched together using a plug-in for ImageJ software (NIH, Bethesda, MD, USA; https://imagej.nih.gov/ij/).(29) To analyze the captured images, they were processed through a MATLAB script (MathWorks, Natick, MA, USA) that was repurposed from a previous code.(30) The program converted the red, green, blue (RGB) images to the hue, saturation, lightness (HSL) color-space. Conversion to the HSL color-space reduces the variables defining each pixel and aids in analysis. After converting to HSL, the program iterated through each pixel in an image and selected pixels within a certain threshold to isolate the colors of interest.
In order to isolate the red pixels associated with calcified tissue, the HSL parameters were as follows: H = 340–20 degrees, S = 0.1–1.0, L = 0.1–0.93. The area fraction of calcified tissue (Ac) was calculated by dividing the pixels that were identified as red by thresholding, divided by the total number of pixels in the sample (see Fig. S2). Comparison of different area fractions of calcified tissue allowed for a quantitative analysis of the severity of enthesis calcification between different samples.
Statistics
GraphPad Prism 9 (GraphPad Software, Inc., La Jolla, CA, USA) was used to statistically analyze enthesis calcification using ANOVA comparison of means with Bonferroni post hoc testing with p < 0.05 considered significant. Statistical significance was explicitly stated when 0.05 < p < 0.001 and denoted by *** when p < 0.001, and **** when p < 0.0001.
Results
Clinical and radiographic findings
We summarized pertinent clinical findings with special emphasis on musculoskeletal signs and symptoms in adult patients with ENPP1 deficiency from the NIH cohort.
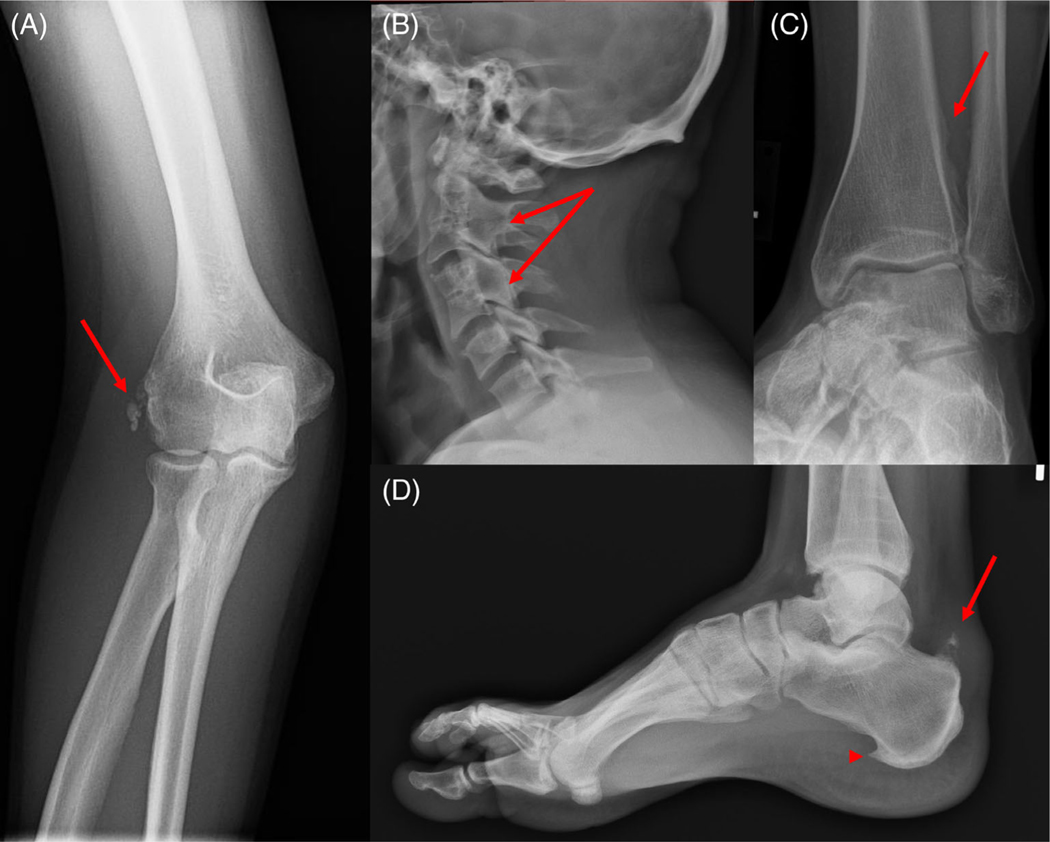
P8 was diagnosed with GACI during the first month of life, and was subsequently diagnosed with hypophosphatemic rickets at 8 years of age. She was treated with calcitriol and phosphate, which was discontinued when she achieved skeletal maturity at 17 years of age, only to be resumed 18 months later given the appearance of musculoskeletal pain. Ankle tendinitis was diagnosed at 18 years old. Around the same time, she found it more difficult to get up and down the stairs early in the morning. Imaging had revealed partial fusion through the cervical spine at 15 years of age, as well as evidence of early disc degeneration of C6/C7 and endplate abnormalities with degenerative changes at L2/L3 with an associated annular bulge. At age 25 years, radiographs revealed calcific enthesopathy affecting the common extensor origin of the right elbow (Fig. 1A) and fusion of the posterior vertebral bodies, articular processes, and laminae (Fig. 1B). At age 26 years she complained of left knee, right elbow, and cervical and lumbar pain that had started 2 years earlier with mild severity, but with progressive worsening, elicited by movement. Physical examination revealed residual genu valgum (intermalleolar distance 11.6 cm).
Fig. 1.
(A) Calcification of the common extensor tendon enthesis (arrow) (P8, 25 years 6 months). (B) Fusion of the C2-C3 and C4-C5 posterior vertebral bodies, articular processes, and laminae (arrows) (P8, 25 years 6 months). (C) Mild calcification of the interosseous membrane of the leg (arrow) (P11, 25 years 3 months). (D) Calcification of the Achilles tendon enthesis (arrow) and enthesophyte in the plantar fascia (arrowhead) (P11, 25 years 3 months).
P11 presented with GACI at 29 days of age and was subsequently diagnosed with hypophosphatemic rickets at 14 years old; his medical history was previously summarized.(31) Since the time of that report, he spontaneously ruptured his left Achilles tendon at age 23 years. After initial conservative management, he required surgical repair 7 months later. Radiographic examination at age 26 years revealed vertebral body fusion of the upper cervical spine, and ossification of the interosseous membrane of the leg (Fig. 1C). Ultrasound assessment of the foot and ankle revealed mildly thickened Achilles tendon bilaterally, whereas radiographs showed bilateral calcaneal enthesophytes both anteriorly and posteriorly, more pronounced on the left side (Fig. 1D).
P16 presented with GACI in infancy, subsequently developed genu valgum, and was diagnosed with hypophosphatemic rickets at age 6 years. She underwent hemiepiphyseal stapling and multiple foot/arch correction surgeries. At 35 years of age, she complained of bilateral lower extremity pain, with bilateral leg bowing. Knee radiographs showed degenerative changes bilaterally with mild joint space narrowing involving the right medial knee joint compartment, and osteophytes involving both the medial and lateral knee joint compartments of the left knee. Right hand radiographs revealed prominent sclerosis of the cortical surfaces, particularly within the carpus, as well as polyarticular degenerative changes with a distal predominance. Left hand radiographs similarly showed polyarticular degenerative changes and cortical sclerosis, with prominent subchondral cysts within the carpal bones (see Fig. S3A). CT of the lower extremities showed degenerative features in the knees and ankles.
P17 (P16’s older sister) was noted to have bowed legs at 2 years of age, in the absence of any prior cardiovascular signs or symptoms. She later developed bilateral genu valgum and was diagnosed with hypophosphatemic rickets at age 6 years, when she initiated therapy with phosphate and calcitriol. The patient self-discontinued medications in her teens. She reports having experienced musculoskeletal pain for as long as she can remember, with worsening during adolescence to the point of near-daily symptoms, precipitating treatment with acetaminophen and ibuprofen in her early 30s. At age 38 years, she reported daily mild bilateral leg pain that was exacerbated by activity and was worse in winter. She had bowed legs; knee radiographs revealed bilateral degenerative changes with mild joint space narrowing (see Fig. S3B). Hand radiographs showed mild degenerative changes and mild periarticular calcifications at the fifth metacarpophalangeal joints bilaterally. CT imaging revealed moderate degenerative changes in the knees and ankles. More recently, she required two intraarticular knee steroid injections, at ages 39 and 40 years. At the time of this writing, at age 40 years, she experiences knee pain, and occasional ankle pain with activity, as well as infrequent hip pain, and lower-to-middle back pain after waking in the morning. She experiences stiffness and decreased mobility of wrists (“can’t do pushups”), and fatigue.
P18 was diagnosed with mitral valve stenosis in infancy and genu valgum in early childhood. He was diagnosed with pseudoxanthoma elasticum at age 43 years following sudden unilateral left-sided loss of vision. Around age 50 years there was onset of severe joint and bone pain, primarily in the knees, with radiation to calves and feet, exacerbated by physical activity. Physical examination at age 56 years revealed “cobblestone” skin appearance of the neck and around the umbilicus, and genu valgum with an intermalleolar distance of 8.0 cm. CT imaging revealed moderate to severe degenerative changes of the hip (see Fig. S3C), knees, ankles, and feet, as well as calcification of the posterior longitudinal ligament in the cervical spine.
Health-related QoL
The burden of disease due to musculoskeletal symptoms was assessed in a cohort of seven adult patients (average age: 31.6 years). A narrative overview of the patients’ symptoms regarding pain, stiffness, mobility, and/or fatigue, and their impact on daily life is presented in Table 1. Stiffness was noted in four of seven patients, fatigue in six of seven patients, and mobility issues in four in seven (in one patient leading to wheelchair use in his 40s).
Table 1.
Narrative Report Pertaining to Pain, Stiffness, Mobility/Fatigue, and Impact of Disease
| Patient | Age (years) | Pain | Stiffness | Mobility/fatigue | Life impact |
|---|---|---|---|---|---|
|
| |||||
| AdultENPP1-01 | 30 | Pain all the time, worse in winter (cold weather) and mornings, starting at age 25 years. Getting worse. | In neck and spine; “bit of arthritis,” “definitely more in the mornings.” Started at age 25 years, getting worse. | Limited range of movement; can’t walk as fast as other people, “get quite tired,” “fatigued all the time.” Started at age 25 years, getting worse. | “Have to limit what I do based on how I feel, which is frustrating.” “Used to enjoy running, cannot run much anymore.” “Might need to reduce hours at work.” “Makes me a bit unhappy.” “Sometimes it is difficult to concentrate because of the pain.” “When and if I have children, will I struggle to play with them.” |
| AdultENPP1-02 | 50 | Hip and shoulder pain, always there, started “in my 30s.” Bad back pain starting in mid-20s until 40s, then had lower back fused. Gotten worse. | In hips and shoulder; trying physical therapy and massage. Gotten worse. | “Takes a bit to get going” from rest. Started “in my late 30s.” “Fatigued all the time.” Gotten worse. | “When it hurts it is difficult to get things done and take care of things.” “Some days definitely worse than others,” “I will sometimes just be flat on my back all day and can’t do anything.” “If I don’t take phosphorus I can’t function,” but “phosphorus can upset my stomach, cause diarrhea,” “frequency of needing to take medication” is burdensome. |
| AdultENPP1-03 | 31 | In feet, ankles, wrists, and back. Worse when sitting. Started around age 5 years. Symptom stayed the same. | In spine, started at 27 years old. Symptom stayed the same. | “Gets tired with any activity,” “when walking in particular.” Going up or down stairs takes much longer. Started at age 19 or 20 years. | “Would like to work without worry, help in the garden and just work normally.” No socialization issues, but “would like to meet more friends,” regrets that “will probably never be married.” |
| AdultENPP1-04 | 46 | In back, shoulders, arm. Started at age 30–32 years. Symptom worsening. | None. | “Can’t walk at all,” “can’t walk up and down stairs,” starting at age 40 years. Uses wheelchair for mobility. Fatigued all the time. Symptoms worsening. | “Stuck at home.” |
| AdultENPP1-05 | 21 | Especially in knees. “Cannot walk without pain.” Since childhood, getting worse. | Upper and lower body stiffness. Symptom stayed the same. | “Exhaustion” getting worse. | “Can’t do sports of any kind.” |
| AdultENPP1-06 | 20 | Knee (worst), foot, and hip pain daily. | None. | “Fatigued from an activity such as walking or running.” | “Try to avoid exerting myself.” |
| AdultENPP1-07 | 23 | Muscle pain, “feels like growing pains in my legs,” once every 3 months, randomly in the evenings, lasts a couple of hours. | None. | None. | “Sometimes I wish I could do some of the more rigorous sports but honestly it doesn’t prevent me much” from doing what I wish. |
Pain was objectively assessed using the BPI-SF questionnaire (Table 2). Six of seven patients had musculoskeletal pain, five of seven required treatment (4/5 used NSAIDs, 1/5 used muscle relaxants), but none achieved complete pain relief despite treatment (relief percentage varied from 20% to 70%, average 44%). In order of decreasing frequency, the location of pain involved hips (5/7), knees (5/7), neck (4/7), shoulders (3/7), ankle (3/7), upper back (2/7), and lower back (2/7). The mean worst pain score was 4.9, the mean least pain score was 3.1, the mean average pain score was 4.3, and the mean current pain score was 4.9. The composite mean severity score was 4.3, and the mean interference score 4.2.
Table 2.
Results of the Brief Pain Inventory–Short Form
| Patient | Age (years) | Worst pain | Least pain | Average pain | Pain right now | Mean pain severity score | Mean pain interference score | Pain location | Pain treatment | Relief with treatment |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| AdultENPP1-01 | 30 | 5 | 2 | 4 | 4 | 3.75 | 3.1 | Neck, left hip, left knee, lower back | Paracetamol, naproxen, amitriptyline, codeine, physiotherapy | 50% |
| AdultENPP1-02 | 50 | 8 | 6 | 7 | 7 | 7 | 6.57 | Right shoulder, arm, hand, left hip, foot, upper back, head | Methocarbamol, L-dopa, massage | 20% |
| AdultENPP1-03 | 31 | 6 | 3 | 6 | 6 | 5.25 | 5.71 | Knees, ankles, neck, upper back, lower back | Ibuprofen | 70% |
| AdultENPP1-04 | 46 | 4 | 2 | 3 | 6 | 3.75 | 4 | Back, right hip, right knee, right ankle, neck, both shoulders | Topical diclofenac | 30% |
| AdultENPP1-05 | 21 | 7 | 7 | 7 | 8 | 7.25 | 6.86 | Neck, shoulders, elbows, knees wrists, ankles, hips | Diclofenac, ibuprofen | 50% |
| AdultENPP1-06 | 20 | 4 | 2 | 3 | 3 | 3 | 3.14 | Knee, hip, feet, and legs | - | - |
| AdultENPP1-07 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | - |
Table 3 summarizes the results of the PROMIS questionnaire. Regarding physical function as assessed by T-score, two patients were mildly impaired, four were moderately impaired, and one was severely impaired.
Table 3.
Results of PROMIS Physical Function Form
| Patient | Walk a block on flat ground (PFC6r1) | Walk at normal speed (PFC38) | Walk up and down 2 steps (PFC29) | Walk up and down steps at normal pace (PFA21) | Sit on and get up from toilet (PFC45r1) | Bend and pick up clothing from floor (PFA9) | Cut your food using utensils (PFA20) | Dress self, tie shoe laces, button clothes (PFA16r1) | Reach into high cupboard (PFA17) | Does health limit care of personal needs (PFB43) | Does health limit any sports (PFB45) | Carry out every day physical activities (Global06) | T-score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| AdultENPP1-01 | 5 | 4 | 5 | 4 | 5 | 5 | 5 | 5 | 4 | 5 | 3 | 3 | 43.7 |
| AdultENPP1-02 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | 5 | 57.2 |
| AdultENPP1-03 | 3 | 3 | 4 | 2 | 5 | 3 | 5 | 5 | 4 | 4 | 3 | 3 | 36.3 |
| AdultENPP1-04 | 5 | 5 | 5 | 3 | 5 | 4 | 5 | 4 | 4 | 5 | 3 | 4 | 41.6 |
| AdultENPP1-05 | 1 | 1 | 2 | 1 | 2 | 2 | 3 | 1 | 1 | 2 | 2 | 1 | 22.3 |
| AdultENPP1-06 | 5 | 3 | 5 | 3 | 5 | 5 | 5 | 5 | 4 | 5 | 2 | 3 | 40.9 |
| AdultENPP1-07 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 58.6 |
Entheses in Enpp1asj/asj mice and response to ENPP1 enzyme replacement.
To evaluate the possibility that enthesis calcification may respond to enzyme replacement therapy, we examined the Achilles tendons of 23-week-old Enpp1asj/asj mice maintained on a regular chow diet and dosed with either vehicle or a recently-described long-acting form of human ENPP1-Fc which had been shown to normalize plasma [PPi] for up to 10 days following a single subcutaneous dose of 0.3 mg/kg.(26) The biologic—hereafter referred to as BL-1118—was administered to Enpp1asj/asj mice at the weekly subcutaneous dose of 0.3 mg/kg BL-1118 between weeks 2 and 23, and raised plasma PPi above vehicle-treated Enpp1asj/asj mice, but did not normalize plasma PPi relative to their WT sibling pairs (2235 ± 621 vs. 1358 ± 268, Table 4). In addition, intact FGF-23 was elevated in both dosed and undosed Enpp1asj/asj mice at 23 weeks, but PTH was elevated only in the dosed Enpp1asj/asj mice. The PTH findings in undosed Enpp1asj/asj mice are consistent with the development of a low-bone turnover previously observed in 23-week Enpp1asj/asj mice on regular chow.(27)
Table 4.
Plasma Analytes in 23-Week-Old Enpp1WT and Dosed and Undosed Enpp1asj/asj Mice
| Parameter | Unit | Enpp1wt + PBS | ENPP1asj/asj + PBS | Enpp1asj/asj + BL-1118 |
|---|---|---|---|---|
|
| ||||
| Calcium | mg/dL | 6.6 ± 0.5 | 7.0 ± 0.8 | 6.9 ± 0.4 |
| Phosphate | mg/dL | 6.4 ± 0.9 | 5.7 ± 0.5 | 5.5 ± 0.8 |
| PTH | pg/mL | 137 ± 80 | 229 ± 89 | 524 ± 248*(↑) |
| FGF23 | pg/mL | 172 ± 28 | 439 ± 215*(↑) | 614 ± 167***(↑) |
| PPi | nM | 2235 ± 621 | 51 ± 22**** (↓) | 1358 ± 268**(↓) |
FGF3 =fibroblastic growth factor 23; PPi = pyrophosphate; PTH = parathyroid hormone.
Text in bold represents abnormal values.
p < 0.05,
p < 0.01,
p < 0.001,
p < 0.0001.
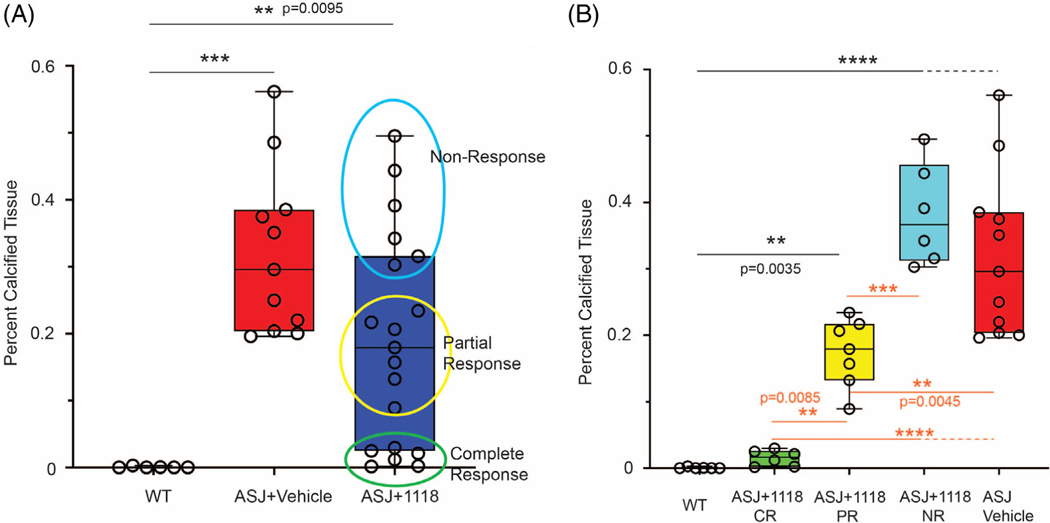
Enthesopathy in the murine Achilles tendons was evaluated both histologically and using custom MATLAB software to quantitate the red pixels in photomicrographs of Alizarin-Red–stained sections. The Achilles tendons of vehicle-treated Enpp1asj/asj mice revealed substantial calcifications throughout the length of the tendon, whereas tendon calcification was entirely suppressed in approximately one-third of the Enpp1asj/asj mice treated with BL-1118 (6/19 mice), whereas an additional one-third of the treated mice exhibited significant reductions in tendon calcification (7/19 mice, Fig. 2A,B). The response of the enthesopathy to enzyme replacement was evident in some dosed animals upon quantification of tendon calcification assessed by Alizarin Red staining in the Achilles tendons of dosed Enpp1asj/asj and vehicle-treated WT sibling pairs, but the large variation in calcifications in the dosed Enpp1asj/asj mice suggested a nonuniform response, inviting the clinical groupings of complete responders, partial responders, and nonresponders (Fig. 3A,B). Complete responders exhibited tendon histology comparable to WT animals, partial responders exhibited some tendon calcifications, but less than those present in vehicle-treated Enpp1asj/asj mice, which exhibited calcifications comparable to the nonresponders (Fig. 3B).
Fig. 2.
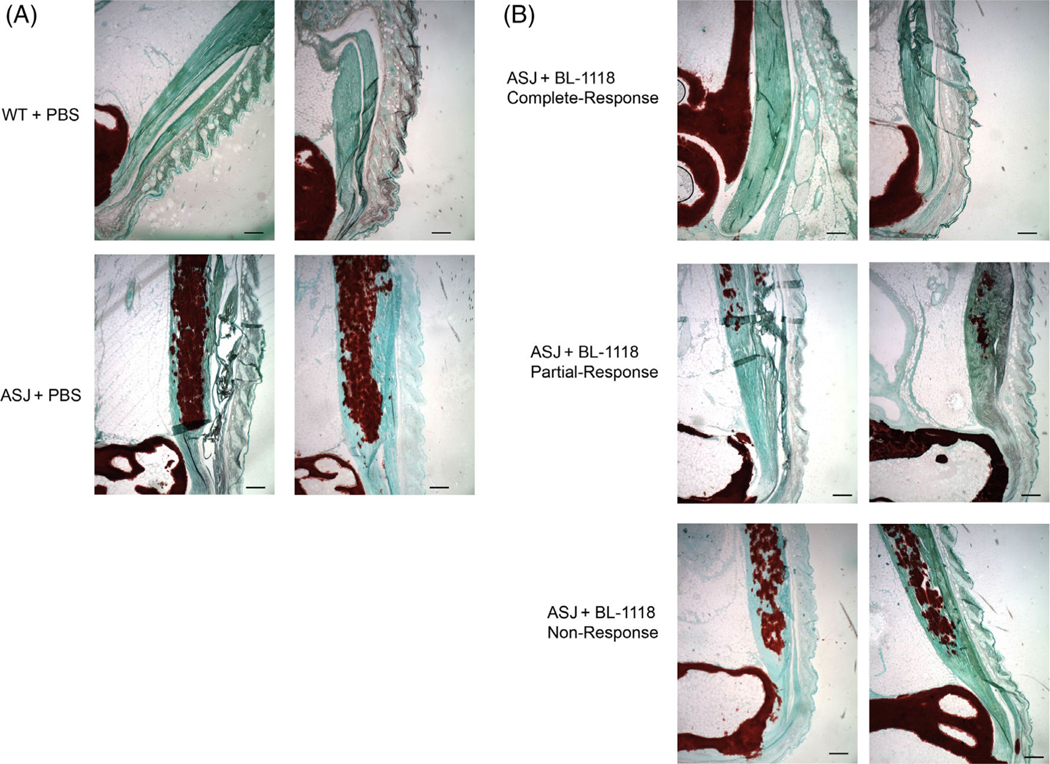
(A) Histopathology of representative Achilles entheses in 23-week-old WT (top) and Enpp1asj/asj mice (bottom) treated with vehicle. Calcific deposits are highlighted by Alizarin Red deposits embedded in the Achilles tendon, which appears green. (B) Histopathology of representative Achilles entheses in 23-week-old Enpp1asj/asj mice treated with weekly subcutaneous injections of 0.3 mg/kg BL-1118. Top panels illustrate representative histologic response in complete responders, middle panels illustrate the histologic response in partial responders, and bottom panels illustrate the histologic response in nonresponders. Scale bar = 10 μm.
Fig. 3.
(A) Quantification of calcified tissue in Alizarin Red–stained sections in WT and Enpp1asj/asj mice treated with vehicle, and Enpp1asj/asj mice treated with BL-1118. Although there was not significant difference in the WT and treated Enpp1asj/asj mice, the data spread suggested that the response could be grouped into complete, partial, and nonresponders (circled data points). (B) Calcified tissue in Alizarin Red–stained sections where treated Enpp1asj/asj mice are analyzed according to treatment response (CR, 6 animals; PR, 7 animals; NR, 6 animals). Black type denotes statistical significance between WT and treatment groups. Orange type denotes statistical significance between treatment groups. Statistical significance is explicitly stated between 0.05 > p > 0.001; ***p < 0.0001, ****p < 0.0001 (ANOVA comparison of means). CR, complete response; NR, nonresponse; PR, partial response.
Discussion
Our study is the first that has systematicaly characterized late-onset musculoskeletal complications in patients with ENPP1 deficiency. Just as in XLH, patients with ENPP1 deficiency not only develop enthesis calcification, but also osteoarthritis and interosseous membrane ossification. We show that these musculoskeletal complications are an important cause of morbidity in adulthood, leading to decreased QoL. Both BPI-SF and PROMIS PF conform to US Food and Drug Administration (FDA) requirements for use of patient-reported outcomes (PRO) in clinical trials.(32) In addition, prior papers support the use of the BPI-SF as an endpoint in XLH clinical trials,(33) and PROMIS annual assessment is recommended for adults with XLH.(34) In one study evaluating adult patients with XLH (average age 49.8 years), the mean worst pain in the BPI-SF was 6, the mean pain severity 4, and the mean pain interference also 4(35); in another study (average age 45.6 years), the mean worst pain was 5.1, the mean pain severity was 3.7, and the mean pain interference was 4.2.(36) Despite the fact that our ENPP1-deficient patient population was younger (average age 31.6 years), and that the musculoskeletal complications appear to develop over time, the mean worst pain was 4.9, the mean severity score was 4.3, and the mean interference score 4.2, similar to the BPI-SF scores reported in patients with XLH. These scores reflect the use of pain medication, which clearly did not provide full alleviation of symptoms.
ENPP1 deficiency can be associated with elevation of PTH in both humans(28,37) and mice.(22,27) This is also true of other models of elevated FGF23, such as in Hyp mice(38) and humans affected with XLH.(39) Our murine model is consistent with these observations. Doses used in this experiment did not result in a substantial correction of either circulating FGF23 or PTH. Dose escalation with the BL-1118 is underway to understand the dose-response of FGF23, PTH, phosphate, and ALP to more potent forms of the biologic.
Conventional therapy with calcitriol and phosphorus supplementation does not improve the enthesopathy in patients with XLH, and leads to nephrocalcinosis in patients with ENPP1 deficiency.(22) Although inhibition of FGF23 via antibody therapy has been shown to attenuate the enthesopathy in a mouse model of XLH,(14) it is unknown whether this approach can prevent this complication in humans. It has been shown, however, that burosumab leads to pain reduction and improvement in stiffness and mobility in adult patients with XLH.(40) Despite these encouraging findings in XLH patients, burosumab appears to lead to worsening cardiovascular calcification in patients with ENPP1 deficiency,(41,42) so better therapeutic options are needed.
Our study documents that ENPP1 enzyme replacement therapy attenuates the development of enthesis calcification in a mouse model of ENPP1 deficiency. We employed a recently described long-acting isoform of ENPP1-Fc, which we developed combining protein engineering to increase biologic half-life and stability, with upstream and downstream bioprocessing methods to enhance favorable glycosylations in the biologic.(26) In prior studies, we dosed Enpp1asj/asj mice with 10 mg/kg once a day to obtain either suppression of vascular calcification(20) or normalization of bone mineralization,(22) whereas here we dosed mice with BL-1118 at 0.3 mg/kg once a week, representing a reduction in the mass dose per week of over 200-fold. We chose this dose because our optimization studies demonstrated that Enpp1asj/asj mice dosed with 0.3 mg/kg of BL-1118 elevated plasma PPi into the normal range, defined as 1.5–2.5μM, for up to 8 days.(26) Here, we found that this weekly dose elevated [PPi] in Enpp1asj/asj mice to nearly the desired minimum target level of 1.5μM (i.e., 1.36μM), but this level was not sufficient to suppress enthesis calcification in all the animals, suggesting that higher concentrations of plasma PPi may be required to fully inhibit enthesis calcifications in some animals. The variation in clinical response may be due to individual variations in metabolic factors governing extracellular PPi, such as tissue-nonspecific alkaline phosphatase (TNAP) concentrations or other upstream and downstream factors responsible for the metabolism of PPi. Supporting this notion are the large variations in plasma PPi noted in Enpp1asj/asj mice, which exhibit similar ENPP1-Fc plasma concentrations (Stabach and colleagues(26); Fig. S8), and by the large variation of clinical symptoms exhibited in GACI patients, in which siblings with identical ENPP1 genetic variants cause mortality in one child but are relatively well tolerated in another.(2)
Although the once-a-week 0.3-mg/kg dose fell short of normalizing plasma PPi to WT levels and did not obtain a uniform response in all animals, the dose was sufficient to completely suppress enthesis calcification in about one-third (6/19) of the mice. Although some increase in potency was likely due to the use of the human ENPP1 isoform (which exhibits a half-life of approximately 36 hours) instead of the mouse isoform used in previous studies (which exhibits a half-life of approximately 8 hours), the findings validate the rational optimization approach we employed to increase ENPP1 potency.(26) Moreover, the incomplete biomarker response obtained in the dosed mice, in which plasma PPi was not normalized compared to WT levels, suggests that complete suppression of enthesis calcification may occur upon dose escalation. Finally, the suppression of enthesis mineralization occurred in the absence of correction of PTH or FGF23 levels, suggesting the dominance of ENPP1 effects in the pathogenesis of these lesions, and less dependence on PTH or FGF23. Further studies are planned to quantitate the dose response of BL-1118 on the phenotypes present in ENPP1 deficiency discussed herein, including enthesis calcification, spinal fusion, and hearing loss.
Our study has some limitations. First, radiographs only detect damage associated with chronic enthesis inflammation, not early disease. Thus, the usefulness of radiography is limited, and the use of ultrasonography or magnetic resonance imaging (MRI) of individual joints would allow a more accurate evaluation earlier in the disease course.(43) Second, enthesis-specific scoring systems—such as the Leeds Enthesitis Index (LEI)—are available, and have been shown to assess treatment response in other disorders associated with enthesopathy.(44) Similarly, other surveys to assess QoL and burden of disease are available, and have been used in cohorts of patients with XLH.(17,33,36,45) Last, the number of patients included was relatively small, which precluded the correlation of burden of disease with specific musculoskeletal complications such as enthesopathy. Despite this last limitation, ours represents the largest case series of adult patients to date. Future prospective studies should be undertaken to assess each joint individually, both through physical exam for tenderness (using validated indices) and via imaging (ultrasonography and/or MRI) of the most commonly affected joints; if possible, the presence of each musculoskeletal comorbidity should be correlated with QoL surveys.
In conclusion, we show that musculoskeletal complications represent an important source of morbidity in ENPP1 deficiency, that the mouse model faithfully recapitulates these complications, and that low doses of a long-acting form of ENPP1 enzyme replacement therapy (BL-1118) can prevent enthesis calcification in a significant proportion of mice. Further work will evaluate whether dose escalation of BL-1118 and/or the addition of a bone-targeting moiety to the biologic are capable of uniformly eliminating the musculoskeletal complications of ENPP1 deficiency.
Supplementary Material
Acknowledgments
D.T.B. acknowledges helpful discussions with Carolyn M. Macica (Quinnipiac University). This work was supported in part by the Intramural Research Program of the National Human Genome Research Institute (ZIA HG200407 to C.R.F.), and by Inozyme Pharma and the National Institutes of Health (R01 DK121326) to D.T.B. Funding to the George M O’Brien Kidney Center at Yale, NIH grant P30DK079310, supported the evaluation of plasma analytes.
Footnotes
Authors’ roles: Carlos R. Ferreira: conceptualization; formal analysis; investigation; resources; writing - original draft; writing - review & editing. Anenya Jai Ansh: investigation; writing - review & editing.Catherine Nester: conceptualization; writing - review & editing.Christine O’Brien: methodology; writing - review & editing. Paul R. Stabach: formal analysis; investigation; writing - review & editing. Sae-Il Murtada: investigation; writing - review & editing. Ethan R. Lester: investigation; writing - review & editing. Gus Khursigara: conceptualization; writing - review & editing. Liz Molloy: methodology; writing - review & editing. Thomas O. Carpenter: conceptualization; writing – review & editing. Demetrios T. Braddock: conceptualization; formal analysis; investigation; resources; writing - original draft; writing - review & editing.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4487.
Conflict of Interests
C.R.F. reports a collaboration with Inozyme Pharma, Inc. as part of a Cooperative Research and Development Agreement. P.R.S. and D.T.B. are inventors of patents owned by Yale University for therapeutics treating ENPP1 deficiency. D.T.B is an equity holder and receives research and consulting support from Inozyme Pharma, Inc. G.K. and C.N. are full-time employees of Inozyme Pharma, Inc. G.K., C.N. and T.O.C own stock in Inozyme Pharma, Inc. T.O.C. has received consulting fees from Ultragenyx, Alexion, Inozyme, Regeneron and Clementia, and honoraria from Kyowa Kirin. A.J. A, C.O., S.M., E.R.L., and L.M. report no conflict of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Ziegler SG, Gahl WA & Ferreira CR Generalized arterial calcification of infancy. In: Adam MP, Ardinger HH, Pagon RA, et al. , (eds). GeneReviews®. 2014. Available from: https://www.ncbi.nlm.nih.gov/books/NBK253403/ [Google Scholar]
- 2.Ferreira CR, Hackbarth ME, Ziegler SG, et al. Prospective phenotyping of long-term survivors of generalized arterial calcification of infancy (GACI). Genet Med. 2020;23:396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calejo I, Costa-Almeida R, Reis RL, Gomes ME. Enthesis tissue engineering: biological requirements meet at the interface. Tissue Eng Part B Rev. 2019;25(4):330–356. [DOI] [PubMed] [Google Scholar]
- 4.Genin GM, Kent A, Birman V, et al. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys J. 2009;97(4): 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deymier AC, An Y, Boyle JJ, et al. Micro-mechanical properties of the tendon-to-bone attachment. Acta Biomater. 2017;1(56):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marinovich R, Soenjaya Y, Wallace GQ, et al. The role of bone sialoprotein in the tendon-bone insertion. Matrix Biol. 2016;52–54:325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polisson RP, Martinez S, Khoury M, et al. Calcification of entheses associated with X-linked hypophosphatemic osteomalacia. N Engl J Med. 1985;313(1):1–6. [DOI] [PubMed] [Google Scholar]
- 8.Liang G, Katz LD, Insogna KL, Carpenter TO, Macica CM. Survey of the enthesopathy of X-linked hypophosphatemia and its characterization in Hyp mice. Calcif Tissue Int. 2009;85(3):235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macica CM, Karaplis AC, Bai X, Desai S, Falet J-P. Mineralizing enthesopathy is a common feature of renal phosphate-wasting disorders attributed to FGF23 and is exacerbated by standard therapy in Hyp mice. Semin Arthritis Rheum. 2013;42(5):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gafni RI, Spector EJ, Hartley IR, Redd B, Mitnik GL, Collins MT. SUN-364 enthesophytes are a common feature of FGF23-mediated hypophosphatemia due to tumor-induced osteomalacia. J Endocr Soc. 2020;4-(Supplement 1):SUN-364. 10.1210/jendso/bvaa046.960. [DOI] [Google Scholar]
- 11.Kawaguchi Y, Kitajima I, Nakano M, et al. Increase of the serum FGF-23 in ossification of the posterior longitudinal ligament. Global Spine J. 2019;9(5):492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Song D, Wang X, Shen X, Li Y, Yuan W. Is ossification of posterior longitudinal ligament an enthesopathy? Int Orthop. 2011; 35(10):1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen A, Ro H, Mundra VRR, et al. Description of 5 novel SLC34A3/NPT2c mutations causing hereditary hypophosphatemic rickets with hypercalciuria. Kidney Int Rep. 2019;4(8):1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu ES, Martins JS, Zhang W, Demay MB. Molecular analysis of enthesopathy in a mouse model of hypophosphatemic rickets. Development. 2018;145(15):dev163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steele A, Gonzalez R, Garbalosa JC, et al. Osteoarthritis, osteophytes, and enthesophytes affect biomechanical function in adults with X-linked hypophosphatemia. J Clin Endocrinol Metab. 2020;105(4):e1798–e1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck-Nielsen SS, Brusgaard K, Rasmussen LM, et al. Phenotype presentation of hypophosphatemic rickets in adults. Calcif Tissue Int. 2010;87(2):108–119. [DOI] [PubMed] [Google Scholar]
- 17.Che H, Roux C, Etcheto A, et al. Impaired quality of life in adults with X-linked hypophosphatemia and skeletal symptoms. Eur J Endocrinol. 2016;174(3):325–333. [DOI] [PubMed] [Google Scholar]
- 18.Karaplis AC, Bai X, Falet J-P, Macica CM. Mineralizing enthesopathy is a common feature of renal phosphate-wasting disorders attributed to FGF23 and is exacerbated by standard therapy in hyp mice. Endocrinology. 2012;153(12):5906–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor J, Olear EA, Insogna KL, et al. Conventional therapy in adults with X-linked hypophosphatemia: effects on enthesopathy and dental disease. J Clin Endocrinol Metab. 2015;100(10):3625–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albright RA, Stabach P, Cao W, et al. ENPP1-Fc prevents mortality and vascular calcifications in rodent model of generalized arterial calcification of infancy. Nat Commun. 2015;1(6):10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan T, Sinkevicius KW, Vong S, et al. ENPP1 enzyme replacement therapy improves blood pressure and cardiovascular function in a mouse model of generalized arterial calcification of infancy. Dis Model Mech. 2018;11(10):dmm035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira CR, Kavanagh D, Oheim R, et al. Response of the ENPP1-deficient skeletal phenotype to oral phosphate supplementation and/or enzyme replacement therapy: comparative studies in humans and mice. J Bone Miner Res. 2021;36(5):942–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 24.Fidai MS, Saltzman BM, Meta F, et al. Patient-reported outcomes measurement information system and legacy patient-reported outcome measures in the field of orthopaedics: a systematic review. Art Ther. 2018;34(2):605–614. [DOI] [PubMed] [Google Scholar]
- 25.Nagaraja V, Mara C, Khanna PP, et al. Establishing clinical severity for PROMIS® measures in adult patients with rheumatic diseases. Qual Life Res. 2018;27(3):755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stabach PR, Zimmerman K, Adame A, et al. Improving the pharmacodynamics and in vivo activity of ENPP1-fc through protein and glycosylation engineering. Clin Transl Sci. 2021;14(1):362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oheim R, Zimmerman K, Maulding ND, et al. Human heterozygous ENPP1 deficiency is associated with early onset osteoporosis, a phenotype recapitulated in a mouse model of Enpp1 deficiency. J Bone Miner Res. 2020;35(3):528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotwal A, Ferrer A, Kumar R, et al. Clinical and biochemical phenotypes in a family with ENPP1 mutations. J Bone Miner Res. 2020;35(4):662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preibisch S, Saalfeld S, Tomancak P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics. 2009;25(11):1463–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bersi MR, Bellini C, Wu J, Montaniel KRC, Harrison DG, Humphrey JD. Excessive adventitial remodeling leads to early aortic maladaptation in angiotensin-induced hypertension. Hypertension. 2016;67(5):890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira CR, Ziegler SG, Gupta A, Groden C, Hsu KS, Gahl WA. Treatment of hypophosphatemic rickets in generalized arterial calcification of infancy (GACI) without worsening of vascular calcification. Am J Med Genet A. 2016;170A(5):1308–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Center for Drug Evaluation and. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims [Internet]. U.S. Food and Drug Administration; 2020. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims [Google Scholar]
- 33.Theodore-Oklota C, Bonner N, Spencer H, Arbuckle R, Chen C-Y, Skrinar A. Qualitative research to explore the patient experience of X-linked hypophosphatemia and evaluate the suitability of the BPI-SF and WOMAC® as clinical trial end points. Value Health. 2018;21(8):973–983. [DOI] [PubMed] [Google Scholar]
- 34.Dahir K, Roberts MS, Krolczyk S, Simmons JH. X-linked hypophosphatemia: a new era in management. J Endocr Soc. 2020;4(12):bvaa151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpenter TO, Peacock M, Weber T, et al. Efficacy and safety of KRN23 in adults with X-linked hypophosphatemia (XLH): data from a phase 2 extension study. Osteoporos Int. 2017;28(1):S336. [Google Scholar]
- 36.Skrinar A, Dvorak-Ewell M, Evins A, et al. The lifelong impact of X-linked hypophosphatemia: results from a burden of disease survey. J Endocr Soc. 2019;3(7):1321–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capelli S, Donghi V, Maruca K, et al. Clinical and molecular heterogeneity in a large series of patients with hypophosphatemic rickets. Bone. 2015;79:143–149. [DOI] [PubMed] [Google Scholar]
- 38.Kiebzak GM, Roos BA, Meyer RA. Secondary hyperparathyroidism in X-linked hypophosphatemic mice. Endocrinology. 1982;111(2):650–652. [DOI] [PubMed] [Google Scholar]
- 39.Schmitt CP, Mehls O. The enigma of hyperparathyroidism in hypophosphatemic rickets. Pediatr Nephrol. 2004;19(5):473–477. [DOI] [PubMed] [Google Scholar]
- 40.Portale AA, Carpenter TO, Brandi ML, et al. Continued beneficial effects of burosumab in adults with X-linked hypophosphatemia: results from a 24-week treatment continuation period after a 24-week double-blind placebo-controlled period. Calcif Tissue Int. 2019;105(3):271–284. [DOI] [PubMed] [Google Scholar]
- 41.Stern R, Levi DS, Gales B, Rutsch F, Salusky IB. Correspondence on “Prospective phenotyping of long-term survivors of generalized arterial calcification of infancy (GACI)” by Ferreira et al. Genet Med. 2021; 23:2006–2007. 10.1038/s41436-021-01228-4. [DOI] [PubMed] [Google Scholar]
- 42.Ziegler SG, Ferreira CR. Response to Stern. Genet Med. 2021;23:2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakewell C, Aydin SZ, Ranganath VK, Eder L, Kaeley GS. Imaging techniques: options for the diagnosis and monitoring of treatment of enthesitis in psoriatic arthritis. J Rheumatol. 2020;47(7):973. [DOI] [PubMed] [Google Scholar]
- 44.Mease PJ, Van den Bosch F, Sieper J, Xia Y, Pangan AL, Song I-H. Performance of 3 Enthesitis indices in patients with peripheral spondyloarthritis during treatment with adalimumab. J Rheumatol. 2017; 44(5):599–608. [DOI] [PubMed] [Google Scholar]
- 45.Hughes M, Macica C, Meriano C, Doyle M. Giving credence to the experience of X-linked hypophosphatemia in adulthood: an interprofessional mixed-methods study. J Patient Cent Res Rev. 2020;7(2): 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.