Abstract
Understanding the pharmacodynamics of cannabinoids is an essential subject due to the recent increasing global acceptance of cannabis and its derivation for recreational and therapeutic purposes. Elucidating the interaction between cannabinoids and the vascular system is critical to exploring cannabinoids as a prospective therapeutic agent for treating vascular-associated clinical conditions. This review aims to examine the effect of cannabinoids on the vascular system and further discuss the fundamental pharmacological properties and mechanisms of action of cannabinoids in the vascular system. Data from literature revealed a substantial interaction between endocannabinoids, phytocannabinoids, and synthetic cannabinoids within the vasculature of both humans and animal models. However, the mechanisms and the ensuing functional response is blood vessels and species-dependent. The current understanding of classical cannabinoid receptor subtypes and the recently discovered atypical cannabinoid receptors and the development of new synthetic analogs have further enhanced the pharmacological characterization of the vascular cannabinoid receptors. Compelling evidence also suggest that cannabinoids represent a formidable therapeutic candidate for vascular-associated conditions. Nonetheless, explanations of the mechanisms underlining these processes are complex and paradoxical based on the heterogeneity of receptors and signaling pathways. Further insight from studies that uncover the mechanisms underlining the therapeutic effect of cannabinoids in the treatment of vascular-associated conditions is required to determine whether the known benefits of cannabinoids thus currently outweigh the known/unknown risks.
Keywords: cannabinoids, signaling, vascular pharmacodynamics, pharmacognosy
The endocannabinoid system (ECS) consists of novel signaling pathways of lipid mediators (endocannabinoids) that can be produced in essentially all cell types of mammalian species. ECS has been implicated in the synthesis, release, transport, and degradation of many important biological events. This unique system in the human body has been linked with several physiological functions of the nervous system and various peripheral tissues and organs. Its modulation holds therapeutic promise in a wide range of different diseases and pathological conditions.
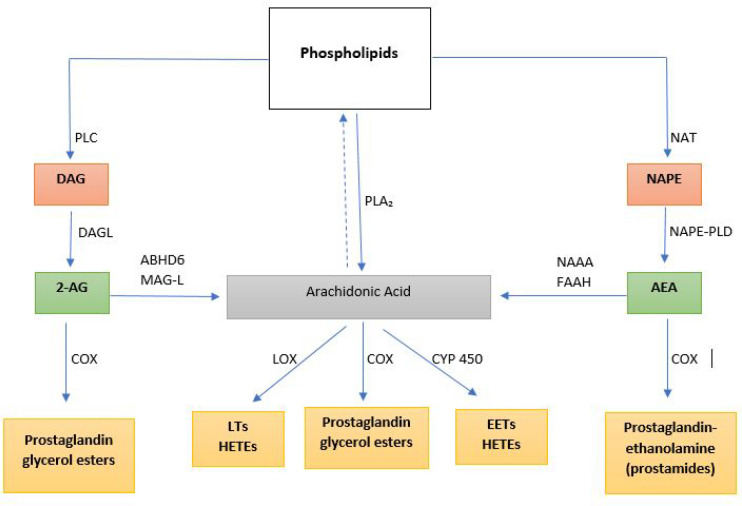
Cannabinoids encompass all-natural and synthetic compounds that elicit a cannabinoidergic effect via the activation of cannabinoid-specific receptors and other atypical receptors.1−3 Currently, cannabinoids are categorized into three broad groups based on their source, which include: (i) phytocannabinoids, (ii) endocannabinoids, and (iii) synthetic cannabinoids.4 Phytocannabinoids are the sole derivatives of Cannabis spp, although other plant sources (Radula and Helichrysum genera) have been reported,5 whereas endocannabinoids are naturally synthesized in mammals.1,6−11 By the end of the 20th century, two endocannabinoids, anandamide (N-arachidonoyl-ethanolamine, AEA) and 2-arachidonoyl-glycerol (2-AG), were fully characterized and remains the most studied endocannabinoid compounds (Figure 1).12−14 Three other pharmacologically distinct endocannabinoids, 2-arachidonyl-glyceryl ether (noladin, 2-AGE), O-arachidonoyl-ethanolamine (virhodamine), and N-arachidonoyl-dopamine (NADA), have also been recently described with their characteristics yet to be established.15−18 In the past decade, many synthetic cannabinoids have also been developed to either mimic or antagonize the effects of the naturally derived cannabinoids.19
Figure 1.
Endocannabinoid anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are formed from arachidonic acid-containing phospholipids. AEA is formed from a two-step catalysis of phospholipids to form N-arachidonoylphosphatidylethanolamine (NAPE). NAPE is cleaved by phospholipase D (PLD) to form AEA. AEA is metabolized by the enzyme fatty acid amide hydrolase (FAAH) and by N-acylethanolamine-hydrolyzing acid amidase (NAAA) to form arachidonic acid or by COX to form prostaglandin ethanolamide (prostamides). 2-AG is synthesized from diacylglycerol (formed from phosphoinositides by the action of phospholipase C) by the action of diacylglycerol lipase (DAGL). 2-AG is metabolized either via COX to form prostaglandin glycerol esters or by both monoacylglycerol lipase (MAG-L) and α,β-hydrolase domain-containing 6 (ABHD6) to form arachidonic acid. Additionally, arachidonic acid can be synthesized directly from phospholipids by phospholipase A2 (PLA2) which is further metabolized by lipoxygenase (LOX) to produce leukotrienes (LTs), cyclooxygenase (COX) to form prostaglandin glycerol esters, and cytochrome P450 (CYP) enzymes to form eicosanoids. EETs: epoxyeicosatrienoic acids; HETEs: hydroxyeicosatetraenoic acids.
Many studies have recently implicated the use of cannabis and its derivatives in certain cardiovascular disorders suggesting a positive correlation between cannabinoids and vascular events.20−22 However, no unified mechanism(s) have been put forward to explain the development of these vascular-related complications, and a direct link for ECS with cardiovascular morbidity and mortality remains to be determined.22,23 The vascular effects of the cannabinoids are complex, vary with species, and at different regions of the vascular bed.24−26 Moreover, several mechanisms are believed to be responsible for the vascular effects of the cannabinoids which include, but are not limited to, interference with the sympathetic nerve function and direct actions on vascular smooth muscle and endothelial cells.27
Despite the substantial progress being made in narrowing the research in the pharmacology of cannabinoids, further exploration of mechanistic effects in the vascular system remains. Here in this review, we will highlight the distribution, interactions, and stimulatory effect of different cannabinoid ligands on various cannabinoid receptors in the vasculature from existing experimental and human studies, and provide insight into the therapeutic targets for this system in the management of vascular-related complications.
Cannabinoid Receptor Ligands and Signaling
Literature until the mid 1980s indicated no direct evidence for the existence of cannabinoid receptors and seem to suggest that cannabinoids induced their effects in a nonreceptor-dependent manner.23 The observations of the existence of cannabinoid receptors have been reviewed in detail elsewhere.23,28 Cannabinoid receptors belong to the G protein-coupled receptor (GPCR) superfamily, and like several other GPCRs, the cannabinoid receptors activate multiple intracellular signal transduction pathways.29,30
The first cannabinoid (CB1) receptor was identified from several previously cloned “orphan” GPCR of the human cerebral cortex and testis, which was followed by the description of the second cannabinoid (CB2) receptor three years later.31−33 However, the very low-affinity binding by some of the naturally occurring cannabinoids, oleoylethanolamide (OEA) and palmitoylethanolamide (PEA), and phytocannabinoids cannabinol (CBN) and cannabidiol (CBD) to CB1 and CB2 receptors seem to suggest the possible existence of a non-CB1/CB2 cannabinoid receptor. This insight led to the description of a potential third cannabinoid receptor, G protein-coupled receptor 55 (GPR55).34 GPR55 is a member of the rhodopsin-like (class A) GCPRs. It is structurally associated with some of the orphan and lysophospholipid-sensitive receptors in the purinoreceptor family as GPR23, GPR92, GPR35, and P2Y5. These receptors are distinct from CB1 and CB2 receptors at the phylogenetic level of a family of lipid receptors.35,36 Other “orphan” G-protein-coupled receptors, including GPR119, GPR18, and atypical receptors including peroxisome proliferator activated receptor (PPAR), transient receptor protential cation channel (TRPV), and 5-hydroxytryptamine (5-HT) are also believed to mediate cannabinoidergic responses.34,36−38
CB1 and CB2 Receptor Ligands and Signaling Pathways
The CB1 receptor, although highly expressed in the brain, is not limited to this organ but also present in a variety of other tissues and organs such as skeletal muscle, liver, gastrointestinal tract, pancreas, and adipose tissue,39−42 although no relationship has been observed between the levels of expression and activity of the receptor in the brain.43 The CB2 receptor is primarily expressed in immune cells, including B cells, monocytes, and T cells as well the hematopoietic systems.39,44 However, recent studies suggest a low level of expression for CB2 receptors in the brain as well.45−48
The efficiency of the cannabinoid receptor signaling at different sites is dependent on the activating cannabinoid ligand and its affinity to the different CB receptor subtypes.49,50 With CB1 and CB2 receptors being part of the GPCR family, the cannabinoids have the ability to activate different intrinsic signaling pathways downstream that are generated via the activation of different Gα-subunits, dependent on the type of ligand and activated cannabinoid receptor.23,51−53
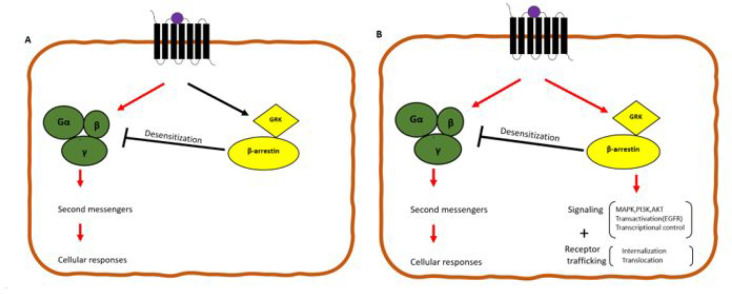
In line with these findings, GPCRs also exhibit “bias signalling”.51,54−58 Bias signalling posits that different ligands acting on the same receptor could induce different responses through different signalling pathways (Figure 2). The interaction of other cannabinoids eliciting distinct cannabinoidergic effects via bias signaling of cannabinoid receptors makes the precise characterization of the individual signalling contribution challenging. It also limits the potential to harness precise therapeutically relevant GPCR signalling elicited by cannabinoids. Furthermore, the resulting signalling pathways are also dependent on the cell/tissue types, the molecular structure of the ligands, conformational change of the receptor, microenvironment, and the type of stimulus that necessitated the signalling.51,59
Figure 2.
(A) Binary switch model of GPCRs, where upon stimulation, the GPCR activates heterotrimeric G-proteins causing its dissociation and subsequent formation of effector second messengers and cellular responses with the GRK-β-arrestin pathway serving as a negative feedback loop maintaining homeostasis by desensitization of GPCR interactions with G-proteins. (B) Balanced signaling system: β-arrestin is coupled to numerous signaling mediators aside acting as a negative feedback regulator of GPCR-G-protein signaling, and these two signaling pathways are independent of each other. Biased signaling is when a ligand-receptor-effector complex results in conformations using distinct pathways relative to other pathways. CB receptor signaling can be mediated through β-arrestins and G-proteins suggesting biased signaling. Abbreviations: GRK, G protein-coupled receptor kinase; MAPK, mitogen-activated protein kinase; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; and EGFR, epidermal growth factor receptor.
Both CB1 and CB2 receptors also appear to activate the arrestin pathway, independent of G-protein signaling.60,61 Indeed, the activation of either the G-protein and/or the arrestin pathways appears to depend on the type of cannabinoid ligand and the receptor, leading to the difference in specific receptor conformations that affect the downstream signaling pathway.62−64 The potential of these cannabinoid ligands to selectively engage specific pathways provides the opportunity to develop therapeutically specific treatments to use as the fundamental basis for the subclassification of various group of ligands.
“Orphan” Receptors GPR55, GPR18, and GPR35 Ligands and Signaling Pathways
Beyond the classical CB1 and CB2 cannabinoid receptors, orphan receptors GPR55, GPR18, and GPR35 represent putative cannabinoid receptor sites65−67 for vascular effects of cannabinoids. Up to now, many of these claims remain provisional because of limited success in the lack of direct evidence from specific ligands. However, several cannabinoid ligands have been described to possess an affinity for these so-called cannabinoid “orphan” G-couple protein-coupled receptors. Among phytocannabinoids, CBD, tetrahydrocannabinol (THC), and cannabigerol (CBG) can elicit mild to strong activation of GPR55 receptors.68,69 However, only a modest number of studies indicate interactions between synthetic cannabinoids and GPR55.69 Synthetic radioisomer CBDs, termed abnormal CBDs (abn-CBD), have also recently been determined to elicit effects on the orphan receptors, with vasorelaxant properties without psychoactive effects.70
It is also recognized that cannabinergic activation of GPR18 involves variable signal transduction pathways characteristic of a bias agonism.71 Two active ligands, kynurenic acid and 2-arachidonyl lysophosphatidic acid, are confirmed to activate GPR35 receptors.72−74 The characterization of the signaling pathway with the activation of GPR35 receptors by kynurenic acid has been linked to the GTPγS-mediated transduction pathway.73 Further studies are warranted to determine the precise signaling of some of these orphan receptors in the vascular system and their potential for therapeutic use.
Other Atypical Cannabinoid Receptors and Ligand Signaling Pathways
Recent studies have suggested the presence of other atypical cannabinoid receptors that elicit cannabinoid effects independent of both CB1 and CB2 receptors in the vascular system. Cannabinoid effects can be mediated by the direct or indirect activation of receptors, including PPARγ, members of the TRPV family and serotonin 5HT1A receptors.75−79 However, the claim that cannabinoids activate 5-HT receptors remains tentative, with conflicting reports from different studies.80−82 Unlike other cannabinoid receptors, the cannabinoid-activated signal transduction pathway of these atypical cannabinoid receptors remains less defined in the vasculature.
Distribution of Cannabinoid Receptors in the Vascular System
Recently, there has been a significant interest in the expression and pharmacological actions of cannabinoids on the vascular system. The endocannabinoid system is involved in modulating the vasoactivity of both central and peripheral blood vessels. However, the vascular effects of cannabinoids is complex, with the underlying mechanisms for eliciting these effects appearing nascent. Indeed, cannabinoid receptors are variably expressed within different parts of the circulatory system.83−115
In addition to the traditional CB1/CB2 receptors in the various vascular beds, there are non-CB1/CB2 receptors in the vascular endothelial cells in various vascular beds.116−122 However, the pharmacology and molecular signaling mechanisms are yet to be fully established.123,124 The delayed characterization of the molecular identity of these receptors stem from the inconsistencies of the results obtained from various abn-CBD-receptor vascular studies.125−128
The vasoactive effects of cannabinoids involve both the well-established CB1/CB2 receptors as well as the yet-to-be-identified non-CB1, non-CB2 Gi/o protein-coupled receptors and appear to be based on the particular vascular bed being investigated.123,129−131 Differentiating the pathway(s) utilized by various cannabimimetic ligands in eliciting their effects on the vascular system present an exciting field for further rigorous research to offer targeted treatment options.
Role of Cannabinoids in Vasorelaxation
The primary vascular effect of cannabinoids are believed to be vasorelaxation, although vasoconstrictor effects are reported due to the modulation of specific vasoactive compounds. As well, the varying effect of cannabinoids in different vascular beds is due to multiple mechanisms to elicit their effect,132−134 as well as on experimental conditions used.135−138
In vivo experiments suggest the vascular effect of cannabinoids to follow three distinct phases characterized by a vagal-mediated fall in blood pressure (phase I), transient sympathetic pressor response (phase II), and a prolonged hypotensive effect (phase III).133,134,139−141
In general, vasorelaxant effects of cannabinoids involve the stimulation of both classical and nonclassical cannabinoid receptors and the intracellular downstream activation of NO and arachidonic acid metabolites. There is no general concord about the implication of these vascular targets on the effects of cannabinoids, as the precise mechanism(s) of vasorelaxation remains yet unclear and controversial. However, several mechanisms of cannabinoid-mediated vasorelaxation have been proposed. They involve endothelial-mediated vasorelaxation, activation of K channels in the vascular smooth muscle cells, inhibition of the voltage-gated calcium channels of the vascular smooth muscle cell, activation of CB receptors in vascular smooth muscle cells, release of the calcitonin gene-related peptide (CGRP) from sensory neurons coupled to the vanilloid receptor (TRPV1), and inhibition of transmitter release from sympathetic nerve endings at the presynaptic level. We will discuss these in turn.
Endothelium-Mediated Vasorelaxation
Vascular endothelial cells have many endocrine functions, regulating vascular tone under physiological conditions by the production and release of vasoactive substances, including NO, with profound effects on the overall function of the vascular system.142,143 Crosstalk between NO and the endocannabinoid signaling pathway in normal and pathological conditions play a critical role in affecting vascular health, with emerging evidence suggesting that endocannabinoid mediators regulate NO bioavailability and signaling. The stimulatory or an inhibitory effect of cannabinoids on NO bioavailability depend on the species, vascular bed, and/or activation of specific receptors.144,145 Deutsch et al.146 demonstrated the role of the endogenous cannabinoid agonist, AEA, in stimulating a CB1 receptor-mediated release of NO from perfused rat renal arterial segments. Mukhopadhay and his colleagues147 also reported that AEA and methanandamide evoke vasodilation in juxtamedullary afferent arterioles and aortic rings of rabbits via activation of NO, independent of the cyclooxygenase (COX) pathway (i.e., through the production prostacyclin). Other investigators have further highlighted NO’s role in cannabinoid-induced relaxations in the rat mesenteric vasculature.130,148,149 Interestingly, NO-induced vasodilation is not universal with all cannabinoids. THC is reported to induce endothelial dysfunction caused by oxidative stress and reduced nitric oxide production in the isolated rat superior mesenteric artery and aortic rings in myography studies.83,150
There is also evidence to suggest a NO-independent role in cannabinoid-mediated vasorelaxation via the endothelium. In a study conducted by Vanessa and Hiley,151 an abn-CBD-induced NO-independent relaxation of rat mesenteric artery was found, as the relaxation was unaffected by the NO synthase inhibitor, L-NG-nitroarginine methyl ester (L-NAME). However, in the same study, an endothelium-dependent activation of the K channels was also found through a novel highly selective cannabinoid receptor agonist SR141716A-sensitive pathway.151 A similar observation was made in a study by White and Hiley,152 who reported a NO-independent vasorelaxant response in mesenteric arterial bed elicited by AEA. AEA has also been shown to dilate coronary arteries independent of NO production.153,154 The heterogeneous signaling of different cannabinoids in endothelium-dependent relaxation can be attributed to the predominant endothelium factor, such as endothelium-derived hyperpolarizing factor (EDHF), of a given vascular bed and represents a possible target in the therapeutic use of cannabinoids in vascular disorders.
Cannabinoid-Mediated Ion Channels in Vasorelaxation
In addition to the other mechanisms by which cannabinoids mediate vasorelaxation, cannabinoids act on multiple GPCR-independent targets, modulating voltage-gated channels, ligand-gated ion channel receptors, and ion-transporting membrane proteins. This includes the transient potential receptor class (TRP) channels to elicit vasoactive responses.155−157 These channels are crucial in shaping action potentials and controlling the membrane potential and cell excitability. Therefore, they regulate a wide array of physiological processes and serve as potential therapeutic targets for the treatment of cardiovascular disorders.
Randall et al.154 suggested that the endogenous cannabinoid, AEA, activates the endothelium-derived hyperpolarizing factor (EDHF) to induce relaxation of smooth muscle cells directly in the isolated perfused rat mesenteric arterial bed which was blocked by the cannabinoid receptor antagonist, SR141716A. However, subsequent studies suggested that vasorelaxant responses of AEA are independent of K channel blockers (which contribute to hyperpolarization); blocking of the K channels with apamin and charybdotoxin did not affect the vasorelaxant responses to anandamide directly in the precontracted rat small mesenteric artery, suggesting a differential sensitivity of AEA to K channel blockers.152 Further evidence was provided by Zygmunt et al.,158 who demonstrated that AEA caused smooth muscle hyperpolarization only in the presence of a functional endothelium in rat hepatic arteries.
In rat retinal arterioles, abn-CBD-induced vasorelaxation in response to endothelin-1 (ET-1) mediated vasoconstriction has been shown to be due to a novel endothelial non-CB1/CB2-dependent mechanism (0-1918; a selective, silent antagonist of a putative, sensitive, endothelial anandamide receptor target). It has been shown that it is a consequence of the activation of a small-conductance Ca -activated K channel in rat retinal arterioles.159,160
Vasodilation produced by the synthetic cannabinoid arachidonylcyclopropylamide (ACPA) have been reported to be, in part, dependent on an intact endothelium and are believed to involve the large-conductance, Ca-activated K channels.161,162 The role of EDHF-mediated vasodilation thus appear to differ in various vascular beds. However, they are very crucial as a backup mechanism during NO pathway impairment. Markedly different mechanisms utilized by cannabinoids to relax blood vessels, independent of NO or EDHF, also present promising therapeutics targets for endothelial dysfunction and vascular aging.
TRPV1-Mediated Vasorelaxation
TRPV belongs to a diverse superfamily of the transient receptor potential (TRP), with proteins associated with TRP forming cation channels that trigger multiple stimuli. As such, they can act as sensory domains for TRPV response to endogenous ligands, heat, chemicals, mechanical, and osmotic stress.163−165 TRPV channels are expressed (localized) in the vascular smooth muscle, endothelial cells, and in the perivascular nerves.166,167
The role of TPRV receptors in the noncannabinoid receptor-associated effects of cannabinoids has been described in several studies within the context of dysregulation in the expression and signaling of TRPs, leading to the development and progression of multiple vascular disorders.168,169 The vasorelaxant properties of the anandamide-like monounsaturated fatty acid oleoylethanolamide (OEA) are mediated via TRPV1 channels.148,170
There is a discrepancy as to whether TRPV1-mediated vasorelaxation is endothelium-dependent or endothelium-independent. In a study by Járai and co-workers,123 AEA was reported to induce vasorelaxation in both endothelium-intact and endothelium-denuded rat mesenteric arteries. Only the endothelium-denuded vessels were sensitive to the effects of TRPV1 receptors inhibition. However, Hoi and Hiley reported a capsaicin-sensitive relaxation in the small mesenteric arteries of rats, which were only observed in endothelium-intact blood vessels but not in denuded vessels.130 This suggests possible multiple signaling pathways for AEA-induced TRPVI-mediated vasorelaxation.
The activation of TPRV1 by cannabinoids results in the subsequent production of NO and prostacyclin (PGI2) by endothelial cells, opening the intermediate and small conductance K channels (IKca/SKca) and leading to vasodilation of the vasculature.164 In perivascular sensory nerves, the activation of the TRPV1 channels releases calcitonin gene-related peptide (CGRP), which causes relaxation of vascular smooth muscle cells.170 Furthermore, AEA has also been reported to induce relaxation in rat mesenteric arteries by stimulating the release of CGRP from capsaicin-sensitive sensory nerves through the activation of TPVR1.76,171,172 Determining the detailed signaling mechanism involved in cannabinoid TRPV1-mediated vasorelaxation is warranted and could aid in developing therapeutic strategies when the predominant NO and EDHF mechanism is dysfunctional in the vascular system.
Presynaptic Sympathetic Nerve Endings in Cannabinoid-Mediated Vasorelaxation
Activation of presynaptic endocannabinoid receptors has also appeared to mediate the vasoactive response produced by cannabinoids. This presents new insights into how cannabinoids modulate presynaptic cannabinoid receptors and neurotransmitter release and their downstream effect on vascular tone in conditions of sympathetic overstimulation. Their central and peripheral nervous system activity appears to inhibit the excitatory transmitter release from synaptic vesicles.173 This effect has been described as occurring via the inhibition of the voltage-dependent Ca channel, the activation of the K channel, and the direct inhibition of the vesicle release of the excitatory transmitters.174 The CB1 receptor is known to be the primary site that mediates sympathetic nervous system-induced vasorelaxation of cannabinoids as it is highly expressed at the presynaptic nerve endings.175
Presynaptically located CB1 receptors have been found on the adrenal medulla, suggesting their possible involvement in releasing adrenaline.176 The stimulation of the presynaptic CB1 receptors in the rat periventricular neurone have been demonstrated to be either dependent and/or independent of the type of catecholamine released from the adrenal medulla.176−181 Furthermore, the neurogenic vasopressor response of catecholamines appeared to be abolished by administering CB1 receptor agonist WIN 55,212-2 and CP-55,940 in a dose-dependent manner. The effect is thought to be mediated via the activation of CB1 receptors that are located presynaptically on the postganglionic sympathetic nerve fibers innervating resistance vessels.182 Endocannabinoids have also been suggested to play an important role in the initial phase of lipopolysaccharide-induced septic shock. Activation of the presynaptic inhibitory cannabinoid CB1 receptor, which consequently inhibits the neurogenic vasopressor effect183,184 is implicated, suggesting a critical neural-cannabinoid role in modulating the vascular system. The sympathoinhibitory effects of cannabinoids play a crucial role in sympathetic and vagal neuroeffector transmission in regulating heart rate and arterial blood pressure in normal and diseased conditions.
Vasoconstriction/Vasopressor Effects of Cannabinoids on the Vascular System
On the basis of reports in the literature, the vascular effects of cannabinoids that are mediated via the activation of CB receptors appear to be primarily associated with vasorelaxation in both animals and humans. Vasoconstriction resulting from the activation of classical cannabinoid receptors is rarely reported, and there is little information on the vascular contractile responses mediated by the activation of CB receptors.185 Although there is a consensus on the vasoconstrictor effect of cannabinoids, there is no unifying mechanism underlining this effect. For instance, although some cannabinoids elicit their vasopressor effect in the second phase of the so-called “triphase cardiovascular cannabinoid effect” in whole animals, the mechanism underlining this phase is poorly understood as diverse pathways are suggested to be involved. Also, a different mechanism involving activation of atypical cannabinoid receptors by prostanoids is implicated in direct vasoconstrictor response to endocannabinoids.
Wagner et al.186 first demonstrated the role of CB1 receptors in causing vasoconstriction. AEA was shown to induce vasoconstriction in isolated rat coronary arteries. AEA also produces vasoconstriction via CB1 receptors in spontaneously hypertensive rats but not in normotensive rats in vivo.187 To corroborate the role of CB1 receptors in producing vasoconstriction, Tamaki et al.188 further demonstrated that AEA could constrict isolated rat mesenteric arteries via the activation of CB1 receptors at high concentrations followed by long-lasting vasodilatation in a concentration-dependent manner.
Many cannabinoids, including 2-AG, anandamide, and metanandamide have been found to exhibit biphasic and triphasic effects on blood pressure in vivo, of which vasoconstriction/vasopressor response is a critical component.189−191 As described earlier, the triphasic effect elicited by cannabinoids is characterized by initial bradycardia and hypotensive effect (phase 1), which is followed by a transient pressor effect, vasoconstriction (phase II), and finally, a prolonged decrease in blood pressure (phase III).133,192 Interestingly, many CB1 receptor agonists, including WIN55212-2 or CP55940, cannot induce the transient vasopressor effect observed as described for phase II.175,193 It has been suggested that the vasopressor effect of anandamide is mediated via TPRV1 as was reported by Pacher et al. in anesthetized TRPV1 knockout mice.194 Similarly, methanandamide and capsaicin have been shown to induce a similar pressor effect in anesthetized rats; however, no mechanism was described to be responsible for such effect.195
Although the mechanism(s) underlining the transient pressor effect is poorly understood, Kwolek et al. provided a possible explanation of the potential mechanism(s) underlining these pressor effects in urethane-anesthetized rats.196 The first mechanism is thought to be mediated via the central nervous system, as this effect was reversed using a β-adrenoceptor antagonist, propanolol, and an N-methyl-d-aspartate receptor (NMDA) receptor antagonist, MK-801. The second mechanism is suggested to be originating from the periphery, possibly involving blood vessels, and noted to be sensitive to the actions of nifedipine, ruthenium, and pentobarbital, perhaps pointing to the possible involvement of the L-type sensitive Ca and other ion channels.
The indirect target site of action involving the prostanoid receptors TP and EP has also been suggested to be involved in vasoconstriction by means of endocannabinoid metabolites. Lefebvre et al.189 reported 2-AG induced contractions of the rat aortic rings via the conversion of 2-AG to thromboxane A2 (TXA2) (a potent vasoconstrictor), an effect that was blocked entirely by the TXA2 receptor (TP receptor) antagonist, GR32191.
The definitive pathway utilized in eliciting cannabinoid-induced vasoconstriction is yet to be wholly defined, and this is particularly important as vascular responses to cannabinoids seem to be enhanced in certain pathological conditions such as inflammation and hypertension. Further work is required to establish the extent of vascular actions of cannabinoids and their therapeutic application in physiological and pathophysiological situations
Medicinal Prospect of Cannabinoids in Vascular-Related Pathologies
More recently, cannabinoids have emerged as therapeutic targets for the management of a host of pathologies197−200 and a subject of investigation in varying experimental protocols. The main avenue of interest in the vascular system has been the preclinical investigations into the therapeutic potential of cannabinoids in the management of vascular-associated complications.
Role of Cannabinoids in Hypertension
The consensus about the potential therapeutic effects of cannabinoids in the vascular system depends on the various cannabinoids used and the stage/model of hypertension. Other factors, such as age and sex, may also be an extra layer to accessing the therapeutic effects of cannabinoids in hypertension. In humans and whole animals, these therapeutic effects can be categorized into short-term and long-term.
Away from the psychoactive effects, cannabinoids have been reported to elicit complex effects on blood pressure involving either a triphasic, which is elicited by endogenous cannabinoids and a biphasic or monophasic (which is reported in phytocannabinoids and synthetic cannabinoids) blood pressure responses in vivo.201−205
This effect, as described earlier, is characterized by bradycardia, a drop in BP, a brief pressure response resulting from increased cardiac contractility and blood flow in the mesenteric and renal vascular beds and, finally, a prolonged marked decrease in BP in the third phase.202 The biphasic phase involves only phases II and III, while the monophasic phase involves only phase III. The difference in short-term effects is generally reported to depend on the cannabinoids used.
The cardioprotective effect mediated by cannabinoids in hypertension involves the reduction in inotropy and peripheral vascular resistance, which are the critical components of blood pressure control. Presynaptic CB receptors have also been suggested to modulate sympathetic effects on BP.184,206 This effect results from the activation of both cardiac and vascular CB receptors by endogenous, Phyto, and synthetic cannabinoids.94,207−214 In a study by Bátkai et al.94 using three different rat models of hypertension, the therapeutic effect of cannabinoids was unmasked only in the hypertensive groups involving a decrease in cardiac contractility and reduction in vascular resistance. On the other hand, this suggests that the inhibition of cannabinoid receptors can be a promising therapeutic approach in heart failures by inhibiting the negative inotropy effects of cannabinoids. Furthermore, the difference in cannabinoid effects in the heart and various vascular beds could be as a result of the relative concentration of endogenous ligands and receptors, as well as the type of cannabinoids used.94,130,145,185
Hypertension is a multifactorial disease due to the complex interaction among intrinsic and extrinsic factors, broadly grouped into environmental, genetics, and sex steriods.215−217 A healthy blood vessel ensures a healthy cardiovascular function and better blood pressure regulation. Arterial remodelling as a cause or consequence of arterial diseases is known to exacerbate hypertension, and the long-term effects of cannabinoids on hypertension involve a yet-to-be-explored role of cannabinoid in modulating/inhibiting the various factors that lead to hypertension beyond this paper.218,219
In the vascular system, the long-term effects of cannabinoids in regulating blood pressure involve the maintenance of vascular endothelial function. The loss of the endothelial function as a modulator of vascular tone and maintenance of vessel integrity by actively suppressing thrombosis, vascular inflammation, and arterial remodelling alters vascular hemodynamics.220−222 Cross-talk between NO and cannabinoid signaling pathways (nitrergic signaling) as well as with other vasorelaxant mediators such as prostaglandin, ion channels which are involved in the modulation of vascular tone, suggest a vasoprotective endothelial effect of chronic use of cannabinoids in hypertension.223−227
The role of cannabinoids in hypertension seems very promising. However, further investigations are needed to assess the therapeutic effects of cannabinoids for short-term and long-term hypertension treatments.
Role of Cannabinoids in Inflammation
For over 2000 years, Chinese healers have claimed the anti-inflammatory properties of cannabinoids, suggesting that it heals rheumatism,228 and so has Indian folklore medicine, where it has been described as a remedy against inflammation, chronic pain, and asthma.229 Over the past decade, the potential of cannabinoid pharmacotherapy in inflammation has received much attention. Cannabinoids have been demonstrated to interact with various inflammatory processes, including endothelial inflammatory response,230−232 chemotaxis,233−238 adhesion of inflammatory cells to the endothelium,239−245 as well as its involvement in the release of a variety of proinflammatory mediators.246−256
Cannabinoids in Atherosclerosis
Atherosclerosis is an inflammatory disease that is characterized by arterial wall lesions containing cholesterol, immune infiltrates, and connective tissue elements.257 Multiple risk factors have been identified, including hypertension, smoking, diabetes, obesity, and genetic predisposition. Atherosclerosis is implicated as a major cause of mortality due to potentiating myocardial ischemia, myocardial infarction, coronary artery diseases, and cerebrovascular diseases.258−262 Endothelial dysfunction has been implicated as an early indicator for atherosclerosis, as it plays a pivotal role in the degeneration of vascular structure, initiating the pathogenesis and progression of atherosclerosis.263,264 There is evidence from a number of studies suggesting a role for cannabinoids in the pathogenesis of atherosclerosis, but the exact function of the endocannabinoid system during atherosclerosis is yet to be fully understood. Emerging evidence suggests that CB1 and CB2 receptors play a significant role in the pathogenesis of atherosclerosis.265 It appears that activation of CB2 and inhibition of CB1 receptors reduces the development of atherosclerosis, whereas the activation of the latter receptors may enhance the progression of atherosclerosis.266,267 A growing body of evidence has established a link between cannabinoids use and endothelial cell functioning. A low dose of THC was shown to reduce the progression of atherosclerosis in both human and mouse atherosclerotic plaque.266 This observation was associated with lowering levels of the T-helper cell 1-derived interferon γ and inhibition of macrophage chemotaxis.266 In a longitudinal epidemiological study, cannabinoids were shown alter not only the function of T-helper cells but also B-lymphocytes.268 However, unlike T-helper cells, the role of B-lymphocytes in the pathogenesis of atherosclerosis in the presence of cannabinoids is less explored.244,269−272
The elucidation of the pharmacodynamics of cannabinoids and their relationship to atherosclerosis could open an alternative therapeutic prospect for the management of the disease.
Role of Cannabinoids in Thrombosis
A fully functional endothelium and vasculature enable the efficient flow of blood to tissues and organs. Thrombotic-induced pathologies are a leading global cause of mortality, accounting for 50% of vascular-related deaths in the western world.273,274 Cannabinoids play a crucial role in regulating platelet function in thrombosis, and the expression of CB1 receptors in human platelets.275 In general, evidence for the thrombotic effect of cannabinoids appear paradoxical.
Many clinical reports have associated cannabis use with thrombogenic development culminating into acute coronary artery disease.276−281 However, over the past decade, there have been many discrepancies concerning the influence of endocannabinoids on the formation of thrombus and platelet aggregation. While some studies indicate a cannabinoid-mediated procoagulating effect on human platelet cells,282,283 additional research has shown that cannabinoids efficiently inhibit platelet aggregation.274,284−287
The prothrombic effect of endogenous cannabinoids reported may be due to different mechanisms of disease development. Some evidence suggests that the development of atherosclerosis may lead to the formation of thrombus.288,289 However, it appears not to always be the case. In an ex-vivo observation, young individuals produced thrombotic coronary artery occlusion without underlying atherosclerosis290,291 via a different mechanism not associated with the development of atherosclerosis. Indeed, the in vitro treatment of blood with THC induces direct expression of glycoprotein IIb-IIIa and P-selectin, which are requisites for platelet coagulation282 but not atherosclerosis. These findings are supported by a case report in two patients with coronary artery thrombotic lesions being successfully treated with glycoprotein IIb-IIIa inhibitors.292 The platelet activation by cannabinoids has been suggested not to be mediated via the activation of CB1/2 receptors.293 Almaghrabi et al.294 showed that vanilloid-like agents (capsaicin, N-arachidonoyldopamine, and N-oleoyldopamine) inhibit platelet aggregation but not via the activation of cannabinoid receptors or TRPV1 channels. Other findings suggest that THC prolongs lipopolysaccharide-activated tissue factor protein expression in activated monocytes, leading to a pro-coagulation effect295 and not via the activation of cannabinoid receptors.
Whatever the mechanism, thrombotic occlusions do play a significant role in atherosclerotic complications, and the pathophysiology of this process is regulated by the key factors responsible for maintaining the integrity of a blood vessel. The role of cannabinoids in modulating these factors, on the other hand, may represent a potentially promising target for the pharmacotherapy of atherosclerotic complications but requires much further investigation.
Conclusion
The pharmacology of cannabinoids in the vascular system is a promising field that should provide further insights into the therapeutic uses of cannabinoids in the vascular system. A literature survey suggests that cannabinoids elicit their vascular response via both classical and atypical receptors. The type of activated receptor(s) is highly dependent on the type of agonist, dose/concentration, type of tissue, and experimental setup (in vivo or in vitro). In addition, the literature at times contains paradoxical findings related to the actions of cannabinoids in relation to the vascular system. Currently, there is no unified evidence for the vasoactive effects of cannabinoids in the vascular system. However, most of the studies demonstrate a vasodilatory role rather than vasoconstrictive effects. This is primarily due to several factors such as the concentration, intrinsic vessel characteristics, baseline vascular tone, experimental conditions, and species. This is also partly due to, but not limited to, the variety of receptors in the vascular system utilized by cannabinoids in eliciting their diverse effects. Further studies to elucidate the various interactions between cannabinoids and the vascular system would provide additional insights into how these interactions facilitate the regulation of normal vascular functions and their modifications in pathophysiological conditions.
In a coda, exploration of the vascular effect of cannabinoids presents an alternative therapeutic option for the management of vascular-associated pathologies and some cardiovascular diseases.
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Pharmacology & Translational Science virtual special issue “New Drug Modalities in Medicinal Chemistry, Pharmacology, and Translational Science”.
References
- Pertwee R. G.Handbook of cannabis; Oxford University Press, 2014. [Google Scholar]
- Mackie K. Cannabinoid receptors: where they are and what they do. Journal of neuroendocrinology 20 Suppl 2008, 20, 10–14. 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Mechoulam R.; Gaoni Y.. Recent advances in the chemistry of hashish. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products/Progrès dans la Chimie des Substances Organiques Naturelles; Springer, 1967; pp 175–213. [DOI] [PubMed] [Google Scholar]
- Śledziński P.; Zeyland J.; Słomski R.; Nowak A. The current state and future perspectives of cannabinoids in cancer biology. Cancer medicine 2018, 7, 765–775. 10.1002/cam4.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appendino G.; Gibbons S.; Giana A.; Pagani A.; Grassi G.; Stavri M.; Smith E.; Rahman M. M. Antibacterial cannabinoids from Cannabis sativa: a structure– activity study. J. Nat. Prod. 2008, 71, 1427–1430. 10.1021/np8002673. [DOI] [PubMed] [Google Scholar]
- Turner C. E.; Elsohly M. A.; Boeren E. G. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J. Nat. Prod. 1980, 43, 169–234. 10.1021/np50008a001. [DOI] [PubMed] [Google Scholar]
- ElSohly M. A.; Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life sciences 2005, 78, 539–548. 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Ross S.; EISohly M. Constituents of Cannabis sativa L. XXVIII—A review of the natural constituents: 1980–1994. Zagazig J. Pharm. Sci. 1995, 4, 1–10. 10.21608/zjps.1995.169714. [DOI] [Google Scholar]
- ElSohly M.; Gul W. Constituents of cannabis sativa. Handbook of cannabis 2014, 3, 3. 10.1093/acprof:oso/9780199662685.003.0001. [DOI] [Google Scholar]
- Denisenko Y. K.; Lobanova E. G.; Novgorodtseva T. P.; Gvozdenko T. A.; Nazarenko A. V. The role of arachidonic acid metabolites (endocannabinoids and eicosanoids) in the immune processes: a review. International Journal of Chemical and Biomedical Science 2015, 1, 70–78. [Google Scholar]
- Burstein S. H.; Young J. K.; Wright G. E. Relationships between eicosanoids and cannabinoids. Are eicosanoids cannabimimetic agents?. Biochemical pharmacology 1995, 50, 1735–1742. 10.1016/0006-2952(95)00242-1. [DOI] [PubMed] [Google Scholar]
- Devane W. A.; Hanus L.; Breuer A.; Pertwee R. G.; Stevenson L. A.; Griffin G.; Gibson D.; Mandelbaum A.; Etinger A.; Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Mechoulam R.; Ben-Shabat S.; Hanus L.; Ligumsky M.; Kaminski N. E.; Schatz A. R.; Gopher A.; Almog S.; Martin B. R.; Compton D. R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochemical pharmacology 1995, 50, 83–90. 10.1016/0006-2952(95)00109-D. [DOI] [PubMed] [Google Scholar]
- Sugiura T.; Kondo S.; Sukagawa A.; Nakane S.; Shinoda A.; Itoh K.; Yamashita A.; Waku K. 2-Arachidonoylgylcerol: a possible endogenous cannabinoid receptor ligand in brain. Biochemical and biophysical research communications 1995, 215, 89–97. 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- BISOGNO T.; MELCK D.; BOBROV M. Y.; GRETSKAYA N. M.; BEZUGLOV V. V.; DE PETROCELLIS L.; DI MARZO V. N-acyl-dopamines: novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem. J. 2000, 351, 817–824. 10.1042/bj3510817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. M.; Bisogno T.; Trevisani M.; Al-Hayani A.; De Petrocellis L.; Fezza F.; Tognetto M.; Petros T. J.; Krey J. F.; Chu C. J. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 8400–8405. 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A. C.; Sauer J.-M.; Knierman M. D.; Becker G. W.; Berna M. J.; Bao J.; Nomikos G. G.; Carter P.; Bymaster F. P.; Leese A. B.; et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. Journal of Pharmacology and Experimental Therapeutics 2002, 301, 1020–1024. 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- Petrocellis L. D.; Cascio M. G.; Marzo V. D. The endocannabinoid system: a general view and latest additions. British journal of pharmacology 2004, 141, 765–774. 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makriyannis A.; Deng H.. Cannabimimetic indole derivatives. U.S. Patent US6900236B1, 2007.
- Wolff V.; Jouanjus E. Strokes are possible complications of cannabinoids use. Epilepsy & Behavior 2017, 70, 355–363. 10.1016/j.yebeh.2017.01.031. [DOI] [PubMed] [Google Scholar]
- Richter J. S.; Quenardelle V.; Rouyer O.; Raul J. S.; Beaujeux R.; Gény B.; Wolff V.. A Systematic Review of the Complex Effects of Cannabinoids on Cerebral and Peripheral Circulation in Animal Models. Frontiers in Physiology 2018, 9, 10.3389/fphys.2018.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Saluja S.; Kumar A.; Agrawal S.; Thind M.; Nanda S.; Shirani J. Cardiovascular complications of marijuana and related substances: A review. Cardiology and therapy 2018, 7, 45–59. 10.1007/s40119-017-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R. G. Cannabinoid pharmacology: the first 66 years. British journal of pharmacology 2006, 147, S163–S171. 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton D. R.; Harris L.; Lichtman A.; Martin B.. Marihuana. In Pharmacological aspects of drug dependence, Schuster C. R., Kuhar M. J., Eds.; Springer, 1996; pp 83–158, 10.1007/978-3-642-60963-3_3. [DOI] [Google Scholar]
- Wagner J. A.; Varga K.; Kunos G. Cardiovascular actions of cannabinoids and their generation during shock. Journal of Molecular Medicine 1998, 76, 824–836. 10.1007/s001090050287. [DOI] [PubMed] [Google Scholar]
- Kunos G.; Járai Z.; Varga K.; Liu J.; Wang L.; Wagner J. A. Cardiovascular effects of endocannabinoids—the plot thickens. Prostaglandins & other lipid mediators 2000, 61, 71–84. 10.1016/S0090-6980(00)00056-3. [DOI] [PubMed] [Google Scholar]
- Hillard C. J. Endocannabinoids and Vascular Function. J. Pharmacol. Exp. Ther. 2000, 294, 27–32. [PubMed] [Google Scholar]
- Pertwee R. G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacology & therapeutics 1997, 74, 129–180. 10.1016/S0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Rosenbaum D. M.; Rasmussen S. G.; Kobilka B. K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader C. D.; Fong T. M.; Tota M. R.; Underwood D.; Dixon R. A. Structure and function of G protein-coupled receptors. Annual review of biochemistry 1994, 63, 101–132. 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. A brief history of cannabinoid and endocannabinoid pharmacology as inspired by the work of British scientists. Trends in pharmacological sciences 2006, 27, 134–140. 10.1016/j.tips.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Howlett A.; Barth F.; Bonner T.; Cabral G.; Casellas P.; Devane W.; Felder C.; Herkenham M.; Mackie K.; Martin B. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Devane W. A.; Dysarz F. r.; Johnson M. R.; Melvin L. S.; Howlett A. C. Determination and characterization of a cannabinoid receptor in rat brain. Molecular pharmacology 1988, 34, 605–613. [PubMed] [Google Scholar]
- Begg M.; Pacher P.; Batkai S.; Osei-Hyiaman D.; Offertaler L.; Mo F. M.; Liu J.; Kunos G. Evidence for novel cannabinoid receptors. Pharmacol Ther 2005, 106, 133–145. 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Balenga N. A.; Henstridge C. M.; Kargl J.; Waldhoer M.. Pharmacology, signaling and physiological relevance of the G protein-coupled receptor 55. In Advances in pharmacology; Elsevier, 2011; pp 251–277. [DOI] [PubMed] [Google Scholar]
- Brown A. J. Novel cannabinoid receptors. British journal of pharmacology 2007, 152, 567–575. 10.1038/sj.bjp.0707481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D.; Page J.; Dunn E.; Bradshaw H. B. Delta(9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 2012, 165, 2414–2424. 10.1111/j.1476-5381.2011.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligresti A.; De Petrocellis L.; Di Marzo V. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles Through Complex Pharmacology. Physiol. Rev. 2016, 96, 1593–1659. 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- Demuth D. G.; Molleman A. Cannabinoid signalling. Life sciences 2006, 78, 549–563. 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Pagotto U.; Marsicano G.; Cota D.; Lutz B.; Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocrine reviews 2006, 27, 73–100. 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- Glass M.; Faull R.; Dragunow M. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 1997, 77, 299–318. 10.1016/S0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Herkenham M.; Lynn A. B.; Johnson M. R.; Melvin L. S.; de Costa B. R.; Rice K. C. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 1991, 11, 563–583. 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett A. C.; Breivogel C. S.; Childers S. R.; Deadwyler S. A.; Hampson R. E.; Porrino L. J. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology 2004, 47, 345–358. 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Munro S.; Thomas K. L.; Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61. 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Van Sickle M. D.; Duncan M.; Kingsley P. J.; Mouihate A.; Urbani P.; Mackie K.; Stella N.; Makriyannis A.; Piomelli D.; Davison J. S.; Marnett L. J.; Di Marzo V.; Pittman Q. J.; Patel K. D.; Sharkey K. A. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332. 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Atwood B. K.; Mackie K. CB2: a cannabinoid receptor with an identity crisis. British journal of pharmacology 2010, 160, 467–479. 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral G.; Raborn E.; Griffin L.; Dennis J.; Marciano-Cabral F. CB2 receptors in the brain: role in central immune function. British journal of pharmacology 2008, 153, 240–251. 10.1038/sj.bjp.0707584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempel A. V.; Stumpf A.; Zhang H.-Y.; Özdoǧan T.; Pannasch U.; Theis A.-K.; Otte D.-M.; Wojtalla A.; Rácz I.; Ponomarenko A.; Xi Z.-X.; Zimmer A.; Schmitz D. Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron 2016, 90, 795–809. 10.1016/j.neuron.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggio P. H. Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown. Curr. Med. Chem. 2010, 17, 1468–1486. 10.2174/092986710790980005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V.; De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand?. Philosophical Transactions of the Royal Society B: Biological Sciences 2012, 367, 3216–3228. 10.1098/rstb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibsen M. S.; Connor M.; Glass M. Cannabinoid CB(1) and CB(2) Receptor Signaling and Bias. Cannabis and cannabinoid research 2017, 2, 48–60. 10.1089/can.2016.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets-Garcia A.; Bains J.; Marsicano G. CB1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity. Neuropsychopharmacology 2018, 43, 4. 10.1038/npp.2017.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V. CB1 receptor antagonism: biological basis for metabolic effects. Drug discovery today 2008, 13, 1026–1041. 10.1016/j.drudis.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Bologna Z.; Teoh J.-P.; Bayoumi A. S.; Tang Y.; Kim I.-M. Biased G Protein-Coupled Receptor Signaling: New Player in Modulating Physiology and Pathology. Biomolecules & therapeutics 2017, 25, 12–25. 10.4062/biomolther.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. E.; Melcher K.; Xu H. E. Understanding the GPCR biased signaling through G protein and arrestin complex structures. Curr. Opin. Struct. Biol. 2017, 45, 150–159. 10.1016/j.sbi.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Shukla A. K. Biasing GPCR Signaling from Inside. Science Signaling 2014, 7, pe3–pe3. 10.1126/scisignal.2005021. [DOI] [PubMed] [Google Scholar]
- Grundmann M.; Kostenis E. Temporal bias: time-encoded dynamic GPCR signaling. Trends in pharmacological sciences 2017, 38, 1110–1124. 10.1016/j.tips.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Bologna Z.; Teoh J.-p.; Bayoumi A. S.; Tang Y.; Kim I.-m. Biased G protein-coupled receptor signaling: new player in modulating physiology and pathology. Biomolecules & therapeutics 2017, 25, 12. 10.4062/biomolther.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou S.; Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. International journal of molecular sciences 2018, 19, 833. 10.3390/ijms19030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G.; Reyes-Resina I.; Rivas-Santisteban R.; Sanchez de Medina V.; Morales P.; Casano S.; Ferreiro-Vera C.; Lillo A.; Aguinaga D.; Jagerovic N.; Nadal X.; Franco R. Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochemical pharmacology 2018, 157, 148–158. 10.1016/j.bcp.2018.08.046. [DOI] [PubMed] [Google Scholar]
- Ibsen M. S.; Finlay D. B.; Patel M.; Javitch J. A.; Glass M.; Grimsey N. L. Cannabinoid CB1 and CB2 receptor-mediated arrestin translocation: species, subtype, and agonist-dependence. Frontiers in pharmacology 2019, 10, 350. 10.3389/fphar.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueras-Ortiz C.; Yudowski G. A. The multiple waves of cannabinoid 1 receptor signaling. Molecular pharmacology 2016, 90, 620–626. 10.1124/mol.116.104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soethoudt M.; van Gils N.; van der Stelt M.; Heitman L. H. Protocol to Study beta-Arrestin Recruitment by CB1 and CB2 Cannabinoid Receptors. Methods in molecular biology (Clifton, N.J.) 2016, 1412, 103–111. 10.1007/978-1-4939-3539-0_11. [DOI] [PubMed] [Google Scholar]
- Dhopeshwarkar A.; Mackie K. Functional Selectivity of CB2 Cannabinoid Receptor Ligands at a Canonical and Noncanonical Pathway. Journal of pharmacology and experimental therapeutics 2016, 358, 342–351. 10.1124/jpet.116.232561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales P.; Hurst D. P.; Reggio P. H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Progress in the chemistry of organic natural products 2017, 103, 103–131. 10.1007/978-3-319-45541-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving A.; Abdulrazzaq G.; Chan S. L.; Penman J.; Harvey J.; Alexander S. P. Cannabinoid receptor-related orphan G protein-coupled receptors. Advances in pharmacology 2017, 80, 223–247. 10.1016/bs.apha.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Shore D. M.; Reggio P. H. The therapeutic potential of orphan GPCRs, GPR35 and GPR55. Frontiers in pharmacology 2015, 6, 69. 10.3389/fphar.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner J. E.; Jensen J. B.; Chen H.-Y.; Lu H.-C.; Hille B.; Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 2699–2704. 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E.; Larsson N.; Sjögren S.; Hjorth S.; Hermansson N. O.; Leonova J.; Elebring T.; Nilsson K.; Drmota T.; Greasley P. J. The orphan receptor GPR55 is a novel cannabinoid receptor. British journal of pharmacology 2007, 152, 1092–1101. 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X.; Sun C.; Abame M. A.; Shi W.; Xie Y.; Xu W.; Zhu F.; Zhang Y.; Shen J.; Aisa H. A. Synthesis of CBD and its derivatives bearing various C4′-side chains with a late-stage diversification method. Journal of organic chemistry 2020, 85, 2704–2715. 10.1021/acs.joc.9b02880. [DOI] [PubMed] [Google Scholar]
- Console-Bram L.; Brailoiu E.; Brailoiu G. C.; Sharir H.; Abood M. E. Activation of GPR18 by cannabinoid compounds: a tale of biased agonism. Br. J. Pharmacol. 2014, 171, 3908–3917. 10.1111/bph.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S.; Ota R.; Shima M.; Yamashita A.; Sugiura T. GPR35 is a novel lysophosphatidic acid receptor. Biochemical and biophysical research communications 2010, 395, 232–237. 10.1016/j.bbrc.2010.03.169. [DOI] [PubMed] [Google Scholar]
- Wang J.; Simonavicius N.; Wu X.; Swaminath G.; Reagan J.; Tian H.; Ling L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- Zhao P.; Abood M. E. GPR55 and GPR35 and their relationship to cannabinoid and lysophospholipid receptors. Life Sci. 2013, 92, 453–457. 10.1016/j.lfs.2012.06.039. [DOI] [PubMed] [Google Scholar]
- Begg M.; Pacher P.; Bátkai S.; Osei-Hyiaman D.; Offertáler L.; Mo F. M.; Liu J.; Kunos G. Evidence for novel cannabinoid receptors. Pharmacology & therapeutics 2005, 106, 133–145. 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Zygmunt P. M.; Petersson J.; Andersson D. A.; Chuang H.-h.; Sørgård M.; Di Marzo V.; Julius D.; Högestätt E. D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452. 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- Campos A. C.; Ortega Z.; Palazuelos J.; Fogaca M. V.; Aguiar D. C.; Diaz-Alonso J.; Ortega-Gutierrez S.; Vazquez-Villa H.; Moreira F. A.; Guzman M.; Galve-Roperh I.; Guimaraes F. S. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. International Journal of Neuropsychopharmacology 2013, 16, 1407–1419. 10.1017/S1461145712001502. [DOI] [PubMed] [Google Scholar]
- Ligresti A.; De Petrocellis L.; Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016, 96, 1593. 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- Marzo V.; Petrocellis L. D. Endocannabinoids as regulators of transient receptor potential (TRP) channels: a further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr. Med. Chem. 2010, 17, 1430–1449. 10.2174/092986710790980078. [DOI] [PubMed] [Google Scholar]
- Resstel L. B. M.; Tavares R. F.; Lisboa S. F. S.; Joca S. R. L.; Corrêa F. M. A.; Guimarães F. S. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. British journal of pharmacology 2009, 156, 181–188. 10.1111/j.1476-5381.2008.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barann M.; Molderings G.; Brüss M.; Bönisch H.; Urban B. W.; Göthert M. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. British journal of pharmacology 2002, 137, 589–596. 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopinho A. A.; Guimarães F. S.; Corrêa F. M. A.; Resstel L. B. M. Cannabidiol inhibits the hyperphagia induced by cannabinoid-1 or serotonin-1A receptor agonists. Pharmacol., Biochem. Behav. 2011, 98, 268–272. 10.1016/j.pbb.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Rajesh M.; Mukhopadhyay P.; Haskó G.; Liaudet L.; Mackie K.; Pacher P. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and-independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. British journal of pharmacology 2010, 160, 688–700. 10.1111/j.1476-5381.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M.; Mukhopadhyay P.; Haskó G.; Pacher P. Cannabinoid CB1 receptor inhibition decreases vascular smooth muscle migration and proliferation. Biochemical and biophysical research communications 2008, 377, 1248–1252. 10.1016/j.bbrc.2008.10.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch D G; Goligorsky M S; Schmid P C; Krebsbach R J; Schmid H H; Das S K; Dey S K; Arreaza G; Thorup C; Stefano G; Moore L C Production and physiological actions of anandamide in the vasculature of the rat kidney. J. Clin. Invest. 1997, 100, 1538–1546. 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T.; Kodaka T.; Nakane S.; Kishimoto S.; Kondo S.; Waku K. Detection of an endogenous cannabimimetic molecule, 2-arachidonoylglycerol, and cannabinoid CB1 receptor mRNA in human vascular cells: is 2-arachidonoylglycerol a possible vasomodulator?. Biochemical and biophysical research communications 1998, 243, 838–843. 10.1006/bbrc.1998.8187. [DOI] [PubMed] [Google Scholar]
- LIU J.; GAO B.; MIRSHAHI F.; SANYAL A. J.; KHANOLKAR A. D.; MAKRIYANNIS A.; KUNOS G. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem. J. 2000, 346, 835–840. PMCID: PMC1220920 10.1042/bj3460835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan N. M.; Blazquez C.; Alvarez L.; Gallily R.; Schlesinger M.; Guzman M.; Mechoulam R. A cannabinoid quinone inhibits angiogenesis by targeting vascular endothelial cells. Molecular pharmacology 2006, 70, 51–59. 10.1124/mol.105.021089. [DOI] [PubMed] [Google Scholar]
- Kunos G.; Bátkai S.; Offertáler L.; Mo F.; Liu J.; Karcher J.; Harvey-White J. The quest for a vascular endothelial cannabinoid receptor. Chemistry and physics of lipids 2002, 121, 45–56. 10.1016/S0009-3084(02)00145-7. [DOI] [PubMed] [Google Scholar]
- Bonz A.; Laser M.; Küllmer S.; Kniesch S.; Babin-Ebell J.; Popp V.; Ertl G.; Wagner J. A. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. Journal of cardiovascular pharmacology 2003, 41, 657–664. 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- Mach F.; Montecucco F.; Steffens S. Cannabinoid receptors in acute and chronic complications of atherosclerosis. British journal of pharmacology 2008, 153, 290–298. 10.1038/sj.bjp.0707517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis F.; Beiras-Fernandez A.; Sodian R.; Kaczmarek I.; Reichart B.; Beiras A.; Schelling G.; Kreth S. Substantially altered expression pattern of cannabinoid receptor 2 and activated endocannabinoid system in patients with severe heart failure. Journal of molecular and cellular cardiology 2010, 48, 1187–1193. 10.1016/j.yjmcc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Piotrowska Ż.; Niezgoda M.; Łebkowski W.; Filipek A.; Domian N.; Kasacka I. Sex differences in distribution of cannabinoid receptors (CB1 and CB2), S100A6 and CacyBP/SIP in human ageing hearts. Biology of sex differences 2018, 9, 1–12. 10.1186/s13293-018-0209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bátkai S.; Pacher P.; Osei-Hyiaman D.; Radaeva S.; Liu J.; Harvey-White J.; Offertáler L.; Mackie K.; Rudd M. A.; Bukoski R. D.; Kunos G. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation 2004, 110, 1996–2002. 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne D.; Lepicier P.; Lagneux C.; Bouchard J. The endogenous cardiac cannabinoid system: a new protective mechanism against myocardial ischemia. Archives des Maladies du Coeur et des Vaisseaux 2006, 99, 242–246. [PubMed] [Google Scholar]
- Hajrasouliha A. R.; Tavakoli S.; Ghasemi M.; Jabehdar-Maralani P.; Sadeghipour H.; Ebrahimi F.; Dehpour A. R. Endogenous cannabinoids contribute to remote ischemic preconditioning via cannabinoid CB2 receptors in the rat heart. European journal of pharmacology 2008, 579, 246–252. 10.1016/j.ejphar.2007.09.034. [DOI] [PubMed] [Google Scholar]
- Krylatov A.; Maslov L.; Ermakov S. Y.; Lasukova O.; Barzakh E.; Crawford D.; Pertwee R. Significance of cardiac cannabinoid receptors in regulation of cardiac rhythm, myocardial contractility, and electrophysiologic processes in heart. Biology Bulletin 2007, 34, 28–35. 10.1134/S1062359007010049. [DOI] [PubMed] [Google Scholar]
- Pacher P.; Bátkai S.; Osei-Hyiaman D.; Offertáler L.; Liu J.; Harvey-White J.; Brassai A.; Járai Z.; Cravatt B. F.; Kunos G. Hemodynamic profile, responsiveness to anandamide, and baroreflex sensitivity of mice lacking fatty acid amide hydrolase. American Journal of Physiology-Heart and Circulatory Physiology 2005, 289, H533–H541. 10.1152/ajpheart.00107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Ma S.; Wang Q.; Hu W.; Wang D.; Li X.; Su T.; Qin X.; Zhang X.; Ma K.; et al. Effects of cannabinoid receptor type 2 on endogenous myocardial regeneration by activating cardiac progenitor cells in mouse infarcted heart. Science China Life Sciences 2014, 57, 201–208. 10.1007/s11427-013-4604-z. [DOI] [PubMed] [Google Scholar]
- Di Filippo C.; Rossi F.; Rossi S.; D’Amico M. Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: involvement of cytokine/chemokines and PMN. Journal of leukocyte biology 2004, 75, 453–459. 10.1189/jlb.0703303. [DOI] [PubMed] [Google Scholar]
- Montecucco F.; Lenglet S.; Braunersreuther V.; Burger F.; Pelli G.; Bertolotto M.; Mach F.; Steffens S. CB2 cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. Journal of molecular and cellular cardiology 2009, 46, 612–620. 10.1016/j.yjmcc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Chen Y.; McCarron R. M.; Ohara Y.; Bembry J.; Azzam N.; Lenz F. A.; Shohami E.; Mechoulam R.; Spatz M. Human brain capillary endothelium: 2-arachidonoglycerol (endocannabinoid) interacts with endothelin-1. Circulation research 2000, 87, 323–327. 10.1161/01.RES.87.4.323. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D.; Lange A. R.; Campbell W. B.; Hillard C. J.; Harder D. R. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. American Journal of Physiology-Heart and Circulatory Physiology 1999, 276, H2085–H2093. 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- Golech S. A.; McCarron R. M.; Chen Y.; Bembry J.; Lenz F.; Mechoulam R.; Shohami E.; Spatz M. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Mol. Brain Res. 2004, 132, 87–92. 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Lu T.-S.; Avraham H. K.; Seng S.; Tachado S. D.; Koziel H.; Makriyannis A.; Avraham S. Cannabinoids inhibit HIV-1 Gp120-mediated insults in brain microvascular endothelial cells. J. Immunol. 2008, 181, 6406–6416. 10.4049/jimmunol.181.9.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Hilton D. A.; Hanemann C. O.; Zajicek J. Cannabinoid Receptor and N-acyl Phosphatidylethanolamine Phospholipase D—Evidence for Altered Expression in Multiple Sclerosis. Brain Pathology 2011, 21, 544–557. 10.1111/j.1750-3639.2011.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryciw D. H.; McAinch A. J. Cannabinoid receptors in the kidney. Current opinion in nephrology and hypertension 2016, 25, 459–464. 10.1097/MNH.0000000000000249. [DOI] [PubMed] [Google Scholar]
- Francois H.; Lecru L. The role of cannabinoid receptors in renal diseases. Curr. Med. Chem. 2018, 25, 793–801. 10.2174/0929867324666170911170020. [DOI] [PubMed] [Google Scholar]
- Barutta F.; Mastrocola R.; Bellini S.; Bruno G.; Gruden G. Cannabinoid receptors in diabetic kidney disease. Current diabetes reports 2018, 18, 1–7. 10.1007/s11892-018-0975-7. [DOI] [PubMed] [Google Scholar]
- Barutta F.; Bruno G.; Mastrocola R.; Bellini S.; Gruden G. The role of cannabinoid signaling in acute and chronic kidney diseases. Kidney International 2018, 94, 252–258. 10.1016/j.kint.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Jenkin K. A.; McAinch A. J.; Grinfeld E.; Hryciw D. H. Role for cannabinoid receptors in human proximal tubular hypertrophy. Cellular Physiology and Biochemistry 2010, 26, 879–886. 10.1159/000323997. [DOI] [PubMed] [Google Scholar]
- Lecru L.; Desterke C.; Grassin-Delyle S.; Chatziantoniou C.; Vandermeersch S.; Devocelle A.; Vernochet A.; Ivanovski N.; Ledent C.; Ferlicot S.; Dalia M.; Said M.; Beaudreuil S.; Charpentier B.; Vazquez A.; GIron-Michel J.; Azzarone B.; Durrbach A.; Francois H. Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney International 2015, 88, 72–84. 10.1038/ki.2015.63. [DOI] [PubMed] [Google Scholar]
- Bátkai S.; Osei-Hyiaman D.; Pan H.; El-Assal O.; Rajesh M.; Mukhopadhyay P.; Hong F.; Harvey-White J.; Jafri A.; Hasko G.; Huffman J. W.; Gao B.; Kunos G.; Pacher P. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007, 21, 1788–1800. 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat A.; Teixeira-Clerc F.; Lotersztajn S. Cannabinoid signaling and liver therapeutics. Journal of hepatology 2013, 59, 891–896. 10.1016/j.jhep.2013.03.032. [DOI] [PubMed] [Google Scholar]
- Parfieniuk A.; Flisiak R. Role of cannabinoids in chronic liver diseases. World journal of gastroenterology: WJG 2008, 14, 6109. 10.3748/wjg.14.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman G.; Maor Y.; Abu-Lafi S.; Horowitz M.; Gallily R.; Batkai S.; Mo F.-M.; Offertaler L.; Pacher P.; Kunos G.; Mechoulam R. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 2428–2433. 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowska H.; Baranowska M.; Schlicker E.; Kozlowski M.; Laudanski J.; Malinowska B. Identification of the vasodilatory endothelial cannabinoid receptor in the human pulmonary artery. Journal of hypertension 2007, 25, 2240–2248. 10.1097/HJH.0b013e3282ef7a0a. [DOI] [PubMed] [Google Scholar]
- Herradón E.; Martín M.; López-Miranda V. Characterization of the vasorelaxant mechanisms of the endocannabinoid anandamide in rat aorta. British journal of pharmacology 2007, 152, 699–708. 10.1038/sj.bjp.0707404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Miranda V.; Dannert M. T.; Herradón E.; Alsasua A.; Martín M. I. Cytochrome P450 pathway contributes to methanandamide-induced vasorelaxation in rat aorta. Cardiovascular drugs and therapy 2010, 24, 379–389. 10.1007/s10557-010-6261-9. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S.; Chapnick B. M.; Howlett A. C. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. American Journal of Physiology-Heart and Circulatory Physiology 2002, 282, H2046–H2054. 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- McCollum L.; Howlett A. C.; Mukhopadhyay S. Anandamide-mediated CB1/CB2 cannabinoid receptor-independent nitric oxide production in rabbit aortic endothelial cells. Journal of Pharmacology and Experimental Therapeutics 2007, 321, 930–937. 10.1124/jpet.106.117549. [DOI] [PubMed] [Google Scholar]
- Baranowska-Kuczko M.; MacLean M. R.; Kozłowska H.; Malinowska B. Endothelium-dependent mechanisms of the vasodilatory effect of the endocannabinoid, anandamide, in the rat pulmonary artery. Pharmacological research 2012, 66, 251–259. 10.1016/j.phrs.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Járai Z.; Wagner J. A.; Varga K.; Lake K. D.; Compton D. R.; Martin B. R.; Zimmer A. M.; Bonner T. I.; Buckley N. E.; Mezey E.; et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 14136–14141. 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko A. I. Endothelial atypical cannabinoid receptor: do we have enough evidence?. Br. J. Pharmacol. 2014, 171, 5573–5588. 10.1111/bph.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D.; Hu S. S.; Rimmerman N.; Juknat A.; Vogel Z.; Walker J. M.; Bradshaw H. B. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC neuroscience 2010, 11, 1–14. 10.1186/1471-2202-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J. Y.; Vo A. C. 2-Arachidonylglyceryl ether and abnormal cannabidiol-induced vascular smooth muscle relaxation in rabbit pulmonary arteries via receptor-pertussis toxin sensitive G proteins-ERK1/2 signaling. European journal of pharmacology 2007, 559, 189–195. 10.1016/j.ejphar.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Penumarti A.; Abdel-Rahman A. A. The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. Journal of Pharmacology and Experimental Therapeutics 2014, 349, 29–38. 10.1124/jpet.113.209213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matouk A. I.; Taye A.; El-Moselhy M. A.; Heeba G. H.; Abdel-Rahman A. A. The effect of chronic activation of the novel endocannabinoid receptor GPR18 on myocardial function and blood pressure in conscious rats. Journal of cardiovascular pharmacology 2017, 69, 23. 10.1097/FJC.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. A.; Varga K.; Járai Z.; Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension 1999, 33, 429–434. 10.1161/01.HYP.33.1.429. [DOI] [PubMed] [Google Scholar]
- Hoi P. M.; Hiley C. R. Vasorelaxant effects of oleamide in rat small mesenteric artery indicate action at a novel cannabinoid receptor. British journal of pharmacology 2006, 147, 560–568. 10.1038/sj.bjp.0706643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidaut-Russell M.; Devane W. A.; Howlett A. C. Cannabinoid receptors and modulation of cyclic AMP accumulation in the rat brain. Journal of neurochemistry 1990, 55, 21–26. 10.1111/j.1471-4159.1990.tb08815.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Miranda V.; Herradon E.; Martin M Vasorelaxation caused by cannabinoids: mechanisms in different vascular beds. Current vascular pharmacology 2008, 6, 335–346. 10.2174/157016108785909706. [DOI] [PubMed] [Google Scholar]
- Malinowska B.; Baranowska-Kuczko M.; Schlicker E. Triphasic blood pressure responses to cannabinoids: do we understand the mechanism?. Br. J. Pharmacol. 2012, 165, 2073–2088. 10.1111/j.1476-5381.2011.01747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendizabal V.; Adler-Graschinsky E. Cannabinoids as therapeutic agents in cardiovascular disease: a tale of passions and illusions. British journal of pharmacology 2007, 151, 427–440. 10.1038/sj.bjp.0707261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall M. D.; Kendall D. A.; O’Sullivan S. The complexities of the cardiovascular actions of cannabinoids. British journal of pharmacology 2004, 142, 20–26. 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake K. D.; Compton D. R.; Varga K.; Martin B. R.; Kunos G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J. Pharmacol Exp Ther 1997, 281, 1030–1037. [PubMed] [Google Scholar]
- Stein E. A.; Fuller S. A.; Edgemond W. S.; Campbell W. B. Physiological and behavioural effects of the endogenous cannabinoid, arachidonylethanolamide (anandamide), in the rat. Br. J. Pharmacol. 1996, 119, 107–114. 10.1111/j.1476-5381.1996.tb15683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosersky D. S. Antihypertensive effects of delta9-tetrahydrocannabinol. Arch. Int. Pharmacodyn. Ther. 1978, 233, 76–81. [PubMed] [Google Scholar]
- Pacher P.; Bátkai S.; Kunos G. Blood pressure regulation by endocannabinoids and their receptors. Neuropharmacology 2005, 48, 1130–1138. 10.1016/j.neuropharm.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P.; Bátkai S.; Kunos G. Cardiovascular pharmacology of cannabinoids. Handbook of experimental pharmacology 2005, 168, 599–625. 10.1007/3-540-26573-2_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iring A.; Ruisanchez É.; Leszl-Ishiguro M.; Horváth B.; Benko R.; Lacza Z.; Járai Z.; Sándor P.; Di Marzo V.; Pacher P.; Benyó Z. Role of Endocannabinoids and Cannabinoid-1 Receptors in Cerebrocortical Blood Flow Regulation. PLoS One 2013, 8, e53390 10.1371/journal.pone.0053390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H.; Fukuta H.; Dickens E. J.; Suzuki H. Cellular mechanisms of nitric oxide-induced relaxation of corporeal smooth muscle in the guinea-pig. Journal of physiology 2002, 538, 573–581. 10.1113/jphysiol.2001.013049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. A.; Weisbrod R. M.; Gericke M.; Yaghoubi M.; Bierl C.; Bolotina V. M. Mechanism of nitric oxide–induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circulation research 1999, 84, 210–219. 10.1161/01.RES.84.2.210. [DOI] [PubMed] [Google Scholar]
- Stanley C. P.; Hind W. H.; Tufarelli C.; O’Sullivan S. E. Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovascular research 2015, 107, 568–578. 10.1093/cvr/cvv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan S. E.; Kendall D. A.; Randall M. D. Vascular effects of Δ9-tetrahydrocannabinol (THC), anandamide and N-arachidonoyldopamine (NADA) in the rat isolated aorta. European journal of pharmacology 2005, 507, 211–221. 10.1016/j.ejphar.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G.; Goligorsky M. S.; Schmid P. C.; Krebsbach R. J.; Schmid H. H.; Das S.; Dey S.; Arreaza G.; Thorup C.; Stefano G.; et al. Production and physiological actions of anandamide in the vasculature of the rat kidney. J. Clin. Invest. 1997, 100, 1538–1546. 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S.; Chapnick B. M.; Howlett A. C. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. American Journal of Physiology-Heart and Circulatory Physiology 2002, 282, H2046. 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- AlSuleimani Y. M.; Hiley C. R. Mechanisms of vasorelaxation induced by oleoylethanolamide in the rat small mesenteric artery. European journal of pharmacology 2013, 702, 1–11. 10.1016/j.ejphar.2013.01.006. [DOI] [PubMed] [Google Scholar]
- O’Sullivan S. E.; Tarling E. J.; Bennett A. J.; Kendall D. A.; Randall M. D. Novel time-dependent vascular actions of Δ 9-tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor gamma. Biochemical and biophysical research communications 2005, 337, 824–831. 10.1016/j.bbrc.2005.09.121. [DOI] [PubMed] [Google Scholar]
- O’Sullivan S. E.; Kendall D. A.; Randall M. D. Further characterization of the time-dependent vascular effects of Δ9-tetrahydrocannabinol. Journal of Pharmacology and Experimental Therapeutics 2006, 317, 428–438. 10.1124/jpet.105.095828. [DOI] [PubMed] [Google Scholar]
- Vanessa Ho W. S.; Hiley C. R. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. British journal of pharmacology 2003, 138, 1320–1332. 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.; Hiley C. R. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. British journal of pharmacology 1997, 122, 1573–1584. 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall M. D.; Kendall D. A. Involvement of a cannabinoid in endothelium-derived hyperpolarizing factor-mediated coronary vasorelaxation. European journal of pharmacology 1997, 335, 205–209. 10.1016/S0014-2999(97)01237-5. [DOI] [PubMed] [Google Scholar]
- Randall M. D.; Alexander S. P.; Bennett T.; Boyd E. A.; Fry J. R.; Gardiner S. M.; Kemp P. A.; Mcculloch A. I.; Kendall D. A. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochemical and biophysical research communications 1996, 229, 114–120. 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- Console-Bram L.; Marcu J.; Abood M. E. Cannabinoid receptors: nomenclature and pharmacological principles. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2012, 38, 4–15. 10.1016/j.pnpbp.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L.; Nabissi M.; Santoni G.; Ligresti A. Actions and regulation of ionotropic cannabinoid receptors. Adv. Pharmacol. 2017, 80, 249–289. 10.1016/bs.apha.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Lin Y.-F. Potassium channels as molecular targets of endocannabinoids. Channels 2021, 15, 408–423. 10.1080/19336950.2021.1910461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt P. M.; Högestätt E. D.; Waldeck K.; Edwards G.; Kirkup A. J.; Weston A. H. Studies on the effects of anandamide in rat hepatic artery. British journal of pharmacology 1997, 122, 1679–1686. 10.1038/sj.bjp.0701601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre J.; Dong A.; Straiker A.; Zhu J.; Howlett S. E.; Bagher A.; Denovan-Wright E.; Yu D.-Y.; Kelly M. E. Cannabinoid and lipid-mediated vasorelaxation in retinal microvasculature. Eur. J. Pharmacol. 2014, 735, 105–114. 10.1016/j.ejphar.2014.03.055. [DOI] [PubMed] [Google Scholar]
- Parmar N.; Ho W. S. V. N-arachidonoyl glycine, an endogenous lipid that acts as a vasorelaxant via nitric oxide and large conductance calcium-activated potassium channels. British journal of pharmacology 2010, 160, 594–603. 10.1111/j.1476-5381.2009.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Dyck E.; Andrade-Urzúa F.; Elizalde A.; Ferrer-Villada T.; Dagnino-Acosta A.; Huerta M.; Osuna-Calleros Z.; Rangel-Sandoval C.; Sánchez-Pastor E. ACPA and JWH-133 modulate the vascular tone of superior mesenteric arteries through cannabinoid receptors, BKCa channels, and nitric oxide dependent mechanisms. Pharmacological Reports 2017, 69, 1131–1139. 10.1016/j.pharep.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pastor E.; Andrade F.; Sánchez-Pastor J.; Elizalde A.; Huerta M.; Virgen-Ortiz A.; Trujillo X.; Rodríguez-Hernández A. Cannabinoid receptor type 1 activation by arachidonylcyclopropylamide in rat aortic rings causes vasorelaxation involving calcium-activated potassium channel subunit alpha-1 and calcium channel, voltage-dependent, L type, alpha 1C subunit. European journal of pharmacology 2014, 729, 100–106. 10.1016/j.ejphar.2014.02.016. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G.; Heller S. The mechanosensitive nature of TRPV channels. Pflügers Archiv 2005, 451, 193–203. 10.1007/s00424-005-1424-4. [DOI] [PubMed] [Google Scholar]
- Baylie R.; Brayden J. TRPV channels and vascular function. Acta Physiologica 2011, 203, 99–116. 10.1111/j.1748-1716.2010.02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W. TRPV channels’ role in osmotransduction and mechanotransduction. Transient Receptor Potential (TRP) Channels 2007, 179, 473–487. 10.1007/978-3-540-34891-7_28. [DOI] [PubMed] [Google Scholar]
- Yin J.; Kuebler W. M. Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem. Biophys. 2010, 56, 1–18. 10.1007/s12013-009-9067-2. [DOI] [PubMed] [Google Scholar]
- Jardin I.; Dionisio N.; Lopez J.; Salido G.; Rosado J. Pharmacology of TRP channels in the vasculature. Current vascular pharmacology 2013, 11, 480–489. 10.2174/1570161111311040011. [DOI] [PubMed] [Google Scholar]
- Yao X.; Garland C. J. Recent developments in vascular endothelial cell transient receptor potential channels. Circulation research 2005, 97, 853–863. 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- Inoue R.; Jensen L. J.; Shi J.; Morita H.; Nishida M.; Honda A.; Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circulation research 2006, 99, 119–131. 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- Movahed P.; Jönsson B. A.; Birnir B.; Wingstrand J. A.; Jørgensen T. D.; Ermund A.; Sterner O.; Zygmunt P. M.; Högestätt E. D. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J. Biol. Chem. 2005, 280, 38496. 10.1074/jbc.M507429200. [DOI] [PubMed] [Google Scholar]
- Ralevic V.; Kendall D. A. Cannabinoid inhibition of capsaicin-sensitive sensory neurotransmission in the rat mesenteric arterial bed. European journal of pharmacology 2001, 418, 117–125. 10.1016/S0014-2999(01)00940-2. [DOI] [PubMed] [Google Scholar]
- White R.; Vanessa Ho W -S; Bottrill F. E; Ford W. R; Hiley C R. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. British journal of pharmacology 2001, 134, 921–929. 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M. A.; Marty A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). British journal of pharmacology 2004, 142, 9–19. 10.1038/sj.bjp.0705726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B.; Schlicker E.. Effects of cannabinoids on neurotransmission. In Cannabinoids; Springer, 2005; pp 327–365, 10.1007/3-540-26573-2_11. [DOI] [PubMed] [Google Scholar]
- Malinowska B.; Baranowska-Kuczko M.; Schlicker E. Triphasic blood pressure responses to cannabinoids: do we understand the mechanism?. British journal of pharmacology 2012, 165, 2073–2088. 10.1111/j.1476-5381.2011.01747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhoffer N.; Hansen H. H.; Fernandez-Ruiz J. J.; Szabo B. Effects of cannabinoids on adrenaline release from adrenal medullary cells. Br. J. Pharmacol. 2001, 134, 1319–1327. 10.1038/sj.bjp.0704359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzȩda E.; Schlicker E.; Toczek M.; Zalewska I.; Baranowska-Kuczko M.; Malinowska B. CB1 receptor activation in the rat paraventricular nucleus induces bi-directional cardiovascular effects via modification of glutamatergic and GABAergic neurotransmission. Naunyn-Schmiedeberg's Arch Pharmacol 2017, 390, 25–35. 10.1007/s00210-016-1302-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K.; Lake K. D.; Huangfu D.; Guyenet P. G.; Kunos G. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension 1996, 28, 682–686. 10.1161/01.HYP.28.4.682. [DOI] [PubMed] [Google Scholar]
- Vizi E. S.; Katona I.; Freund T. F. Evidence for presynaptic cannabinoid CB(1) receptor-mediated inhibition of noradrenaline release in the guinea pig lung. Eur. J. Pharmacol. 2001, 431, 237–244. 10.1016/S0014-2999(01)01413-3. [DOI] [PubMed] [Google Scholar]
- Ishac E. J.; Jiang L.; Lake K. D.; Varga K.; Abood M. E.; Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br. J. Pharmacol. 1996, 118, 2023–2028. 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska B.; Godlewski G.; Bucher B.; Schlicker E. Cannabinoid CB1 receptor-mediated inhibition of the neurogenic vasopressor response in the pithed rat. Naunyn Schmiedebergs Arch Pharmacol 1997, 356, 197–202. 10.1007/PL00005041. [DOI] [PubMed] [Google Scholar]
- Malinowska B.; Godlewski G.; Bucher B.; Schlicker E. Cannabinoid CB1 receptor-mediated inhibition of the neurogenic vasopressor response in the pithed rat. Naunyn-Schmiedeberg's Arch Pharmacol 1997, 356, 197–202. 10.1007/pl00005041. [DOI] [PubMed] [Google Scholar]
- Godlewski G.; Malinowska B.; Schlicker E. Presynaptic cannabinoid CB1 receptors are involved in the inhibition of the neurogenic vasopressor response during septic shock in pithed rats. British journal of pharmacology 2004, 142, 701–708. 10.1038/sj.bjp.0705839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E.; Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends in pharmacological sciences 2001, 22, 565–572. 10.1016/S0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Stanley C.; O’Sullivan S. E. Vascular targets for cannabinoids: animal and human studies. British journal of pharmacology 2014, 171, 1361–1378. 10.1111/bph.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. A.; Abesser M.; Karcher J.; Laser M.; Kunos G. Coronary vasodilator effects of endogenous cannabinoids in vasopressin-preconstricted unpaced rat isolated hearts. Journal of cardiovascular pharmacology 2005, 46, 348–355. 10.1097/01.fjc.0000175437.87283.f2. [DOI] [PubMed] [Google Scholar]
- Wheal A.; Bennett T.; Randall M.; Gardiner S. Cardiovascular effects of cannabinoids in conscious spontaneously hypertensive rats. British journal of pharmacology 2007, 152, 717–724. 10.1038/sj.bjp.0707410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki C.; Nawa H.; Takatori S.; Oda S.; Sendo T.; Zamami Y.; Kawasaki H. Anandamide Induces Endothelium-Dependent Vasoconstriction and CGRPergic Nerve–Mediated Vasodilatation in the Rat Mesenteric Vascular Bed. Journal of pharmacological sciences 2012, 118, 496–505. 10.1254/jphs.11236FP. [DOI] [PubMed] [Google Scholar]
- Lefebvre B.; Caron F.; Stanke-Labesque F.; Hardy G.; Bessard G.; Mallaret M. 2-Arachidonoyl glycerol induces contraction of isolated rat aorta: role of cyclooxygenase-derived products. Cardiovasc. Res. 2004, 63, 155–160. 10.1016/j.cardiores.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Zakrzeska A.; Schlicker E.; Baranowska M.; Kozlowska H.; Kwolek G.; Malinowska B. A cannabinoid receptor, sensitive to O-1918, is involved in the delayed hypotension induced by anandamide in anaesthetized rats. Br. J. Pharmacol. 2010, 160, 574–584. 10.1111/j.1476-5381.2009.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwolek G.; Zakrzeska A.; Schlicker E.; Gothert M.; Godlewski G.; Malinowska B. Central and peripheral components of the pressor effect of anandamide in urethane-anaesthetized rats. Br. J. Pharmacol. 2005, 145, 567–575. 10.1038/sj.bjp.0706195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P.; Bátkai S.; Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. Journal of physiology 2004, 558, 647–657. 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K.; Lake K.; Martin B. R.; Kunos G. Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur. J. Pharmacol. 1995, 278, 279–283. 10.1016/0014-2999(95)00181-J. [DOI] [PubMed] [Google Scholar]
- Pacher P.; Bátkai S.; Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. Journal of physiology 2004, 558, 647–657. 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska B.; Kwolek G.; Gothert M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedebergs Arch Pharmacol 2001, 364, 562–569. 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- Kwolek G.; Zakrzeska A.; Schlicker E.; Göthert M.; Godlewski G.; Malinowska B. Central and peripheral components of the pressor effect of anandamide in urethane-anaesthetized rats. British journal of pharmacology 2005, 145, 567–575. 10.1038/sj.bjp.0706195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E. Immunoactive cannabinoids: therapeutic prospects for marijuana constituents. Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 9363–9364. 10.1073/pnas.180314297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson P. J.; Guy G. W.; Di Marzo V. Cannabinoids and schizophrenia: therapeutic prospects. Current pharmaceutical design 2014, 20, 2194–2204. 10.2174/13816128113199990427. [DOI] [PubMed] [Google Scholar]
- Whittle B. A.; Guy G. W.; Robson P. Prospects for new cannabis-based prescription medicines. Journal of Cannabis Therapeutics 2001, 1, 183–205. 10.1300/J175v01n03_12. [DOI] [Google Scholar]
- Eagleston L. R. M.; Kalani N. K.; Patel R. R.; Flaten H. K.; Dunnick C. A.; Dellavalle R. P.. Cannabinoids in dermatology: a scoping review. Dermatology online journal 2018, 24, 10.5070/D3246040706. [DOI] [PubMed] [Google Scholar]
- Laragh J. H.; Case D. B.; Atlas S. A.; Sealey J. E. Captopril compared with other antirenin system agents in hypertensive patients: its triphasic effects on blood pressure and its use to identify and treat the renin factor. Hypertension 1980, 2, 586–593. 10.1161/01.HYP.2.4.586. [DOI] [PubMed] [Google Scholar]
- Malinowska B.; Baranowska-Kuczko M.; Schlicker E. Triphasic blood pressure responses to cannabinoids: do we understand the mechanism?. British journal of pharmacology 2012, 165, 2073–2088. 10.1111/j.1476-5381.2011.01747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake K. D.; Martin B. R.; Kunos G.; Varga K. r. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension 1997, 29, 1204–1210. 10.1161/01.HYP.29.5.1204. [DOI] [PubMed] [Google Scholar]
- King K. M.; Myers A. M.; Soroka-Monzo A. J.; Tuma R. F.; Tallarida R. J.; Walker E. A.; Ward S. J. Single and combined effects of Δ9-tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. British journal of pharmacology 2017, 174, 2832–2841. 10.1111/bph.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid B. G. Cannabinoids for treating cardiovascular disorders: putting together a complex puzzle. Journal of microscopy and ultrastructure 2018, 6, 171. 10.4103/JMAU.JMAU_42_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishac E. J.; Jiang L.; Lake K. D.; Varga K.; Abood M. E.; Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB 1 receptors on peripheral sympathetic nerves. British journal of pharmacology 1996, 118, 2023–2028. 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimian Azari E.; Kerrigan A.; O’Connor A. Naturally occurring cannabinoids and their role in modulation of cardiovascular health. Journal of Dietary Supplements 2020, 17, 625–650. 10.1080/19390211.2020.1790708. [DOI] [PubMed] [Google Scholar]
- Kaschina E. Cannabinoid CB1/CB2 receptors in the heart: expression, regulation, and function. Cannabinoids in Health and Diseas. INTECH 2016, 169–185. 10.5772/62822. [DOI] [Google Scholar]
- Alfulaij N.; Meiners F.; Michalek J.; Small-Howard A. L.; Turner H. C.; Stokes A. J. Cannabinoids, the Heart of the Matter. Journal of the American Heart Association 2018, 7, e009099 10.1161/JAHA.118.009099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Haar J.; Talebi S.; Ghobadi F.; Singh S.; Chirurgi R.; Rajeswari P.; Kalantari H.; Hassen G. W. Synthetic cannabinoids and their effects on the cardiovascular system. Journal of emergency medicine 2016, 50, 258–262. 10.1016/j.jemermed.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Bátkai S.; Pacher P. Endocannabinoids and cardiac contractile function: pathophysiological implications. Pharmacol. Res. 2009, 60, 99–106. 10.1016/j.phrs.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. A.; Járai Z.; Bátkai S.; Kunos G. Hemodynamic effects of cannabinoids: coronary and cerebral vasodilation mediated by cannabinoid CB1 receptors. European journal of pharmacology 2001, 423, 203–210. 10.1016/S0014-2999(01)01112-8. [DOI] [PubMed] [Google Scholar]
- Jones R. T. Cardiovascular system effects of marijuana. Journal of Clinical Pharmacology 2002, 42, 58S–63S. 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- Baranowska-Kuczko M.; Kozłowska H.; Kloza M.; Karpińska O.; Toczek M.; Harasim E.; Kasacka I.; Malinowska B. Protective role of cannabinoid CB1 receptors and vascular effects of chronic administration of FAAH inhibitor URB597 in DOCA-salt hypertensive rats. Life sciences 2016, 151, 288–299. 10.1016/j.lfs.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Bell E.; Pedersen A. The causes of hypertension. Ann. Int. Med. 1930, 4, 227–237. [Google Scholar]
- Hall J. E.; Granger J. P.; Carmo J. M.; Silva A. A.; Dubinion J.; George E.; Hamza S.; Speed J.; Hall M. E. Hypertension: physiology and pathophysiology. Comprehensive Physiology 2012, 2, 2393–2442. 10.1002/cphy.c110058. [DOI] [PubMed] [Google Scholar]
- Staessen J. A.; Wang J.; Bianchi G.; Birkenhäger W. H. Essential hypertension. Lancet 2003, 361, 1629–1641. 10.1016/S0140-6736(03)13302-8. [DOI] [PubMed] [Google Scholar]
- Kopustinskiene D. M.; Masteikova R.; Lazauskas R.; Bernatoniene J. Cannabis sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. 10.3390/antiox11040660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booz G. W. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radical Biol. Med. 2011, 51, 1054–1061. 10.1016/j.freeradbiomed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T. J. Arterial stiffness or endothelial dysfunction as a surrogate marker of vascular risk. Canadian Journal of Cardiology 2006, 22, 72B–80B. 10.1016/S0828-282X(06)70990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudic R. D.; Sessa W. C. Nitric oxide in endothelial dysfunction and vascular remodeling: clinical correlates and experimental links. American journal of human genetics 1999, 64, 673. 10.1086/302304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras F.; Rivera M.; Vasquez J.; De la Parte M.; Velasco M. Endothelial dysfunction in arterial hypertension. Journal of Human Hypertension 2000, 14, S20–S25. 10.1038/sj.jhh.1000982. [DOI] [PubMed] [Google Scholar]
- Lipina C.; Hundal H. S. The endocannabinoid system:‘NO’longer anonymous in the control of nitrergic signalling?. Journal of Molecular Cell Biology 2017, 9, 91–103. 10.1093/jmcb/mjx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A.; Marnett L. J. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem. Rev. 2011, 111, 5899–5921. 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowska-Kuczko M.; Kozłowska H.; Kloza M.; Kusaczuk M.; Harasim-Symbor E.; Biernacki M.; Kasacka I.; Malinowska B. Vasoprotective Endothelial Effects of Chronic Cannabidiol Treatment and Its Influence on the Endocannabinoid System in Rats with Primary and Secondary Hypertension. Pharmaceuticals 2021, 14, 1120. 10.3390/ph14111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih H.-C.; Lin C.-L.; Wu S.-C.; Kwan A.-L.; Hong Y.-R.; Howng S.-L. Upregulation of estrogen receptor α and mediation of 17β-estradiol vasoprotective effects via estrogen receptor α in basilar arteries in rats after experimental subarachnoid hemorrhage. Journal of neurosurgery 2008, 109, 92–99. 10.3171/JNS/2008/109/7/0092. [DOI] [PubMed] [Google Scholar]
- Chambliss K. L.; Shaul P. W. Estrogen modulation of endothelial nitric oxide synthase. Endocrine reviews 2002, 23, 665–686. 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- Barrie N.; Manolios N. The endocannabinoid system in pain and inflammation: its relevance to rheumatic disease. European journal of rheumatology 2017, 4, 210. 10.5152/eurjrheum.2017.17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R.Cannabinoids as therapeutic agents; CRC Press: Boca Raton, FL, 1986; 10.1201/9780429260667. [DOI] [Google Scholar]
- Alhouayek M.; Muccioli G. G. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends in pharmacological sciences 2014, 35, 284–292. 10.1016/j.tips.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Alhouayek M.; Masquelier J.; Cani P. D.; Lambert D. M.; Muccioli G. G. Implication of the anti-inflammatory bioactive lipid prostaglandin D2-glycerol ester in the control of macrophage activation and inflammation by ABHD6. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 17558–17563. 10.1073/pnas.1314017110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M.; Abais J.; Boini K.; Li P.-L.; Ritter J. Protective Action of Prostamide E2 from Homocysteine-induced NLRP3 Inflammasome Activation and Podocyte Injury. FASEB J. 2015, 29, 808.12. 10.1096/fasebj.29.1_supplement.808.12. [DOI] [Google Scholar]
- Bleul C. C.; Fuhlbrigge R. C.; Casasnovas J. M.; Aiuti A.; Springer T. A. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). Journal of Experimental Medicine 1996, 184, 1101–1109. 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.; Stella N. CB2 receptor-mediated migration of immune cells: it can go either way. British journal of pharmacology 2008, 153, 299–308. 10.1038/sj.bjp.0707523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZFARB L.; NEEDLE M.; CHAVEZ-CHASE M. Dose-Related Inhibition of Leukocyte Migration by Marihuana and Delta-9-Tetrahydrocannabinol (THC) in Vitro. Journal of Clinical Pharmacology 1974, 14, 35–41. 10.1002/j.1552-4604.1974.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Gaul C. C.; Mellors A. Delta-9-tetrahydrocannabinol and decreased macrophage migration inhibition activity. Res. Commun. Chem. Pathol. Pharmacol. 1975, 10, 559–564. [PubMed] [Google Scholar]
- Ghosh S.; Preet A.; Groopman J. E.; Ganju R. K. Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Molecular immunology 2006, 43, 2169–2179. 10.1016/j.molimm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Alberich Jorda M.; Verbakel S. E.; Valk P. J. M.; Vankan-Berkhoudt Y. V.; Maccarrone M.; Finazzi-Agro A.; Lowenberg B.; Delwel R. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood 2002, 99, 2786–2793. 10.1182/blood.V99.8.2786. [DOI] [PubMed] [Google Scholar]
- Benyó Z.; Ruisanchez É.; Leszl-Ishiguro M.; Sándor P.; Pacher P. Endocannabinoids in cerebrovascular regulation. American Journal of Physiology-Heart and Circulatory Physiology 2016, 310, H785–H801. 10.1152/ajpheart.00571.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton J. C.; Glass M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Current neuropharmacology 2007, 5, 73–80. 10.2174/157015907780866884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S. H.; Haskó J.; Skuba A.; Fan S.; Dykstra H.; McCormick R.; Reichenbach N.; Krizbai I.; Mahadevan A.; Zhang M.; Tuma R.; Son Y.-J.; Persidky Y.; et al. Activation of cannabinoid receptor 2 attenuates leukocyte–endothelial cell interactions and blood–brain barrier dysfunction under inflammatory conditions. J. Neurosci. 2012, 32, 4004–4016. 10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Valdepeñas L.; Martínez-Orgado J. A.; Benito C.; Millán Á.; Tolón R. M.; Romero J. Cannabidiol reduces lipopolysaccharide-induced vascular changes and inflammation in the mouse brain: an intravital microscopy study. Journal of neuroinflammation 2011, 8, 5. 10.1186/1742-2094-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H.; Mukhopadhyay P.; Rajesh M.; Patel V.; Mukhopadhyay B.; Gao B.; Haskó G.; Pacher P. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J. Pharmacol. Exp. Ther. 2009, 328, 708–714. 10.1124/jpet.108.147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Liu Y.; Zhang W.; Xue J.; Wu Y. Z.; Xu W.; Liang X.; Chen T.; Kishimoto C.; Yuan Z. WIN55212–2 ameliorates atherosclerosis associated with suppression of pro-inflammatory responses in ApoE-knockout mice. Eur. J. Pharmacol. 2010, 649, 285–292. 10.1016/j.ejphar.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Yuan Z.; Liu Y.; Xue J.; Tian Y.; Liu W.; Zhang W.; Shen Y.; Xu W.; Liang X.; et al. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. Journal of cardiovascular pharmacology 2010, 55, 292–298. 10.1097/FJC.0b013e3181d2644d. [DOI] [PubMed] [Google Scholar]
- Rajesh M.; Mukhopadhyay P.; Bátkai S.; Haskó G.; Liaudet L.; Huffman J. W.; Csiszar A.; Ungvari Z.; Mackie K.; Chatterjee S.; Pacher P. CB2-receptor stimulation attenuates TNF-α-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. American journal of physiology. Heart and circulatory physiology 2007, 293, H2210. 10.1152/ajpheart.00688.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bátkai S.; Osei-Hyiaman D.; Pan H.; El-Assal O.; Rajesh M.; Mukhopadhyay P.; Hong F.; Harvey-White J.; Jafri A.; Hasko G.; Huffman J. W.; Gao B.; Kunos G.; Pacher P. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007, 21, 1788–1800. 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M.; Pan H.; Mukhopadhyay P.; Batkai S.; Osei-Hyiaman D.; Haskó G.; Liaudet L.; Gao B.; Pacher P. Pivotal Advance: Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis. Journal of leukocyte biology 2007, 82, 1382–1389. 10.1189/jlb.0307180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco F.; Burger F.; Mach F.; Steffens S. The CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. American Journal of Physiology-Heart and Circulatory Physiology 2008, 294, H1145. 10.1152/ajpheart.01328.2007. [DOI] [PubMed] [Google Scholar]
- Watzl B.; Scuderi P.; Watson R. R. Marijuana components stimulate human peripheral blood mononuclear cell secretion of interferon-gamma and suppress interleukin-1 alpha in vitro. International journal of immunopharmacology 1991, 13, 1091–1097. 10.1016/0192-0561(91)90160-9. [DOI] [PubMed] [Google Scholar]
- Weigang Zhu; Toshihisa Igarashi; Zhong Tian Qi; Newton C.; Widen R. E.; Friedman H.; Klein T. W. Delta-9-Tetrahydrocannabiol (THC) decreases the number of high and intermediate affinity IL-2 receptors of the IL-2 dependent cell line NKB61A2. International journal of immunopharmacology 1993, 15, 401–408. 10.1016/0192-0561(93)90051-Y. [DOI] [PubMed] [Google Scholar]
- Srivastava M. D.; Srivastava B.; Brouhard B. Δ9 tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology 1998, 40, 179–185. 10.1016/S0162-3109(98)00041-1. [DOI] [PubMed] [Google Scholar]
- Onaivi E.; Chakrabarti A.; Gwebu E.; Chaudhuri G. Neurobehavioral effects of Δ9-THC and cannabinoid (CB1) receptor gene expression in mice. Behavioural brain research 1995, 72, 115–125. 10.1016/0166-4328(96)00139-8. [DOI] [PubMed] [Google Scholar]
- Smith S. R.; Terminelli C.; Denhardt G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J Pharmacol Exp Ther. 2000, 293, 136–150. [PubMed] [Google Scholar]
- Berdyshev E. V.; Boichot E.; Germain N.; Allain N.; Anger J.-P.; Lagente V. Influence of fatty acid ethanolamides and Δ9-tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. European journal of pharmacology 1997, 330, 231–240. 10.1016/S0014-2999(97)01007-8. [DOI] [PubMed] [Google Scholar]
- Selvi E.; Lorenzini S.; Garcia-Gonzalez E.; Maggio R.; Lazzerini P. E.; Capecchi P. L.; Balistreri E.; Spreafico A.; Niccolini S.; Pompella G.; Natale M. R.; Guideri F.; Laghi Pasini F.; Galeazzi M.; Marcolongo R. Inhibitory effect of synthetic cannabinoids on cytokine production in rheumatoid fibroblast-like synoviocytes. Clinical and experimental rheumatology 2008, 26, 574–581. [PubMed] [Google Scholar]
- Mach F.; Steffens S. The role of the endocannabinoid system in atherosclerosis. Journal of neuroendocrinology 2008, 20, 53–57. 10.1111/j.1365-2826.2008.01685.x. [DOI] [PubMed] [Google Scholar]
- Tousoulis D.; Charakida M.; Stefanadis C. Endothelial function and inflammation in coronary artery disease. Heart (British Cardiac Society) 2006, 92, 441–444. 10.1136/hrt.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzilli M.; Merz C. N.; Boden W. E.; Bonow R. O.; Capozza P. G.; Chilian W. M.; DeMaria A. N.; Guarini G.; Huqi A.; Morrone D.; Patel M. R.; Weintraub W. S. Obstructive coronary atherosclerosis and ischemic heart disease: an elusive link!. Journal of the American College of Cardiology 2012, 60, 951–956. 10.1016/j.jacc.2012.02.082. [DOI] [PubMed] [Google Scholar]
- Caselli C.; Prontera C.; Liga R.; De Graaf M. A.; Gaemperli O.; Lorenzoni V.; Ragusa R.; Marinelli M.; Del Ry S.; Rovai D.; Giannessi D.; Aguade-Bruix S.; Clemente A.; Bax J. J.; Lombardi M.; Sicari R.; Zamorano J.; Scholte A. J.; Kaufmann P. A.; Knuuti J.; Underwood S. R.; Clerico A.; Neglia D. Effect of Coronary Atherosclerosis and Myocardial Ischemia on Plasma Levels of High-Sensitivity Troponin T and NT-proBNP in Patients With Stable Angina. Arterioscler., Thromb., Vasc. Biol. 2016, 36, 757–764. 10.1161/ATVBAHA.115.306818. [DOI] [PubMed] [Google Scholar]
- Heyden S.; Gerber C. J. Atherosclerotic cerebrovascular disease —Its nature and management. American Journal of Medicine 1969, 46, 763–773. 10.1016/0002-9343(69)90027-8. [DOI] [PubMed] [Google Scholar]
- Tugcu A.; Jin Z.; Homma S.; Elkind M. S. V.; Rundek T.; Yoshita M.; DeCarli C.; Nakanishi K.; Shames S.; Wright C. B.; Sacco R. L.; Di Tullio M. R. Atherosclerotic Plaques in the Aortic Arch and Subclinical Cerebrovascular Disease. Stroke 2016, 47, 2813–2819. 10.1161/STROKEAHA.116.015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaperonis E. A.; Liapis C. D.; Kakisis J. D.; Dimitroulis D.; Papavassiliou V. G. Inflammation and Atherosclerosis. European Journal of Vascular and Endovascular Surgery 2006, 31, 386–393. 10.1016/j.ejvs.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A.; García-Cardeña G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens S.; Pacher P. Targeting cannabinoid receptor CB2 in cardiovascular disorders: promises and controversies. British journal of pharmacology 2012, 167, 313–323. 10.1111/j.1476-5381.2012.02042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens S.; Veillard N. R.; Arnaud C.; Pelli G.; Burger F.; Staub C.; Zimmer A.; Frossard J.-L.; Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 2005, 434, 782. 10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- Sugamura K.; Sugiyama S.; Nozaki T.; Matsuzawa Y.; Izumiya Y.; Miyata K.; Nakayama M.; Kaikita K.; Obata T.; Takeya M.; Ogawa H. Activated Endocannabinoid System in Coronary Artery Disease and Anti-Inflammatory Effects of Cannabinoid 1 Receptor Blockade on Macrophages. Circulation 2009, 119 (1), 28–36. 10.1161/CIRCULATIONAHA.108.811992. [DOI] [PubMed] [Google Scholar]
- Cabral G. A.; Staab A.. Effects on the immune system. In Cannabinoids, Springer, 2005; pp 385–423, 10.1007/3-540-26573-2_13. [DOI] [PubMed] [Google Scholar]
- Malfait A.; Gallily R.; Sumariwalla P.; Malik A.; Andreakos E.; Mechoulam R.; Feldmann M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 9561–9566. 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Yuan Z.; Liu Y.; Xue J.; Tian Y.; Liu W.; Zhang W.; Shen Y.; Xu W.; Liang X.; Chen T. Activation of cannabinoid CB2 receptor ameliorates atherosclerosis associated with suppression of adhesion molecules. J. Cardiovasc Pharmacol 2010, 55, 292–298. 10.1097/FJC.0b013e3181d2644d. [DOI] [PubMed] [Google Scholar]
- Hoyer F. F.; Steinmetz M.; Zimmer S.; Becker A.; Lütjohann D.; Buchalla R.; Zimmer A.; Nickenig G. Atheroprotection via cannabinoid receptor-2 is mediated by circulating and vascular cells in vivo. Journal of molecular and cellular cardiology 2011, 51, 1007–1014. 10.1016/j.yjmcc.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Montecucco F.; Di Marzo V.; da Silva R. F.; Vuilleumier N.; Capettini L.; Lenglet S.; Pagano S.; Piscitelli F.; Quintao S.; Bertolotto M.; Pelli G.; Galan K.; Pilet L.; Kuzmanovic K.; Burger F.; Pane B.; Spinella G.; Braunersreuther V.; Gayet-Ageron A.; Pende A.; Viviani G. L.; Palombo D.; Dallegri F.; Roux-Lombard P.; Santos R. A. S.; Stergiopulos N.; Steffens S.; Mach F. The activation of the cannabinoid receptor type 2 reduces neutrophilic protease-mediated vulnerability in atherosclerotic plaques. European heart journal 2012, 33, 846–856. 10.1093/eurheartj/ehr449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B. Blood coagulation. Lancet 2000, 355, 1627–1632. 10.1016/S0140-6736(00)02225-X. [DOI] [PubMed] [Google Scholar]
- De Angelis V.; Koekman A. C.; Weeterings C.; Roest M.; de Groot P. G.; Herczenik E.; Maas C. Endocannabinoids control platelet activation and limit aggregate formation under flow. PloS one 2014, 9, e108282 10.1371/journal.pone.0108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deusch E.; Kress H. G.; Kraft B.; Kozek-Langenecker S. A. The procoagulatory effects of delta-9-tetrahydrocannabinol in human platelets. Anesthesia and analgesia 2004, 99, 1127–1130. 10.1213/01.ANE.0000131505.03006.74. [DOI] [PubMed] [Google Scholar]
- Casier I.; Vanduynhoven P.; Haine S.; Vrints C.; Jorens P. G. Is recent cannabis use associated with acute coronary syndromes? An illustrative case series. Acta cardiologica 2014, 69, 131–136. 10.1080/AC.69.2.3017293. [DOI] [PubMed] [Google Scholar]
- Salhan D.; Abdulfattah O.; Roy S.; Kandel S.; Agu C.; Basunia M.; Enriquez D.; Quist J.; Schmidt F. M. Cannabis-Induced VTE: Is It a Safe Recreational Drug?. Chest 2016, 150, 909A. 10.1016/j.chest.2016.08.1009. [DOI] [Google Scholar]
- Dean S. A.; Jufer-Phipps R.; Fowler D. R.; Kutys R.; Ladich E.; Alexander R. Acute Coronary Artery Thrombosis Associated with Synthetic Cannabinoid Intoxication. Academic Forensic Pathology 2015, 5, 127–132. 10.23907/2015.014. [DOI] [Google Scholar]
- Yurtdaş M.; Aydın M. K. Acute myocardial infarction in a young man; fatal blow of the marijuana: a case report. Korean circulation journal 2012, 42, 641–645. 10.4070/kcj.2012.42.9.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti D.; Spagnolo A.; De Matteis V.; Filograna L.; De Giovanni N. Coronary thrombosis and marijuana smoking: a case report and narrative review of the literature. Drug testing and analysis 2016, 8, 56–62. 10.1002/dta.1898. [DOI] [PubMed] [Google Scholar]
- Tatli E.; Yilmaztepe M.; Altun G.; Altun A. Cannabis-induced coronary artery thrombosis and acute anterior myocardial infarction in a young man. International journal of cardiology 2007, 120, 420–422. 10.1016/j.ijcard.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Deusch E.; Kress H. G.; Kraft B.; Kozek-Langenecker S. A. The procoagulatory effects of delta-9-tetrahydrocannabinol in human platelets. Anesthesia & Analgesia 2004, 99, 1127–1130. 10.1213/01.ANE.0000131505.03006.74. [DOI] [PubMed] [Google Scholar]
- Maccarrone M.; Bari M.; Menichelli A.; Giuliani E.; Del Principe D.; Finazzi-Agrò A. Human platelets bind and degrade 2-arachidonoylglycerol, which activates these cells through a cannabinoid receptor. European journal of biochemistry 2001, 268, 819–825. 10.1046/j.1432-1327.2001.01942.x. [DOI] [PubMed] [Google Scholar]
- Formukong E.; Evans A.; Evans F. The inhibitory effects of cannabinoids, the active constituents of Cannabis sativa L. on human and rabbit platelet aggregation. J. Pharm. Pharmacol. 2011, 41, 705–709. 10.1111/j.2042-7158.1989.tb06345.x. [DOI] [PubMed] [Google Scholar]
- Levy R.; Schurr A.; Nathan I.; Dvilanski A.; Livne A. Impairment of ADP-induced platelet aggregation by hashish components. Thrombosis and haemostasis 1976, 36, 634–640. 10.1055/s-0038-1648084. [DOI] [PubMed] [Google Scholar]
- Schäfer A.; Pfrang J.; Neumüller J.; Fiedler S.; Ertl G.; Bauersachs J. The cannabinoid receptor-1 antagonist rimonabant inhibits platelet activation and reduces pro-inflammatory chemokines and leukocytes in Zucker rats. British journal of pharmacology 2008, 154, 1047–1054. 10.1038/bjp.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis V.; Koekman A. C.; Weeterings C.; Roest M.; de Groot P. G.; Herczenik E.; Maas C. Endocannabinoids control platelet activation and limit aggregate formation under flow. PloS one 2014, 9, e108282 10.1371/journal.pone.0108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meens M. J. T.; Pfenniger A.; Kwak B. R.. Risky communication in atherosclerosis and thrombus formation. Swiss medical weekly 2012, 142, 10.4414/smw.2012.13553. [DOI] [PubMed] [Google Scholar]
- Libby P.; Ridker P. M.; Maseri A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Dahdouh Z.; Roule V.; Lognoné T.; Sabatier R.; Grollier G. Cannabis and coronary thrombosis: what is the role of platelets?. Platelets 2012, 23, 243–245. 10.3109/09537104.2011.601824. [DOI] [PubMed] [Google Scholar]
- Velibey Y.; Sahin S.; Tanık O.; Keskin M.; Bolca O.; Eren M. Acute myocardial infarction due to marijuana smoking in a young man: guilty should not be underestimated. American journal of emergency medicine 2015, 33, 1114.e1. 10.1016/j.ajem.2015.01.032. [DOI] [PubMed] [Google Scholar]
- Noamen A.; Hajlaoui N.; Mehdi G.; Haouala H. Cannabis induced myocardial infarction underlying mechanism and the place of glycoprotein IIb/IIIa inhibitors in its management: Two illustrative cases of acute anterior myocardial infarction related to cannabis. J. Integr. Cardiol. 2016, 2, 292. 10.15761/JIC.1000162. [DOI] [Google Scholar]
- Grambow E.; Strüder D.; Klar E.; Hinz B.; Vollmar B. Differential effects of endogenous, phyto and synthetic cannabinoids on thrombogenesis and platelet activity. BioFactors 2016, 42, 581–590. 10.1002/biof.1294. [DOI] [PubMed] [Google Scholar]
- Almaghrabi S.; Geraghty D.; Ahuja K.; Adams M. Inhibition of platelet aggregation by vanilloid-like agents is not mediated by transient receptor potential vanilloid-1 channels or cannabinoid receptors. Clin. Exp. Pharmacol. Physiol. 2016, 43, 606–611. 10.1111/1440-1681.12569. [DOI] [PubMed] [Google Scholar]
- Williams J. C.; Klein T. W.; Goldberger B. A.; Sleasman J. W.; Mackman N.; Goodenow M. M. Δ 9-Tetrahydrocannabinol (THC) enhances lipopolysaccharide-stimulated tissue factor in human monocytes and monocyte-derived microvesicles. Journal of Inflammation 2015, 12, 39. 10.1186/s12950-015-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]