Abstract
Ferroptosis is a type of regulated cell death (RCD), and it plays an important role in the occurrence of diseases, especially the development of tumors. Ferroptosis of tumor cells affects the antitumor immunity and the immune response to treatment to varying degrees. Ferroptosis also plays a key role in immune cells. This review outlines the mechanism of the immune‐related effects of ferroptosis pathways in tumor progression and treatment, and it discusses potential methods for improving antitumor immunity and enhancing the efficacy of current cancer treatments by targeting ferroptosis.
Keywords: ferroptosis, immune, lipid peroxidation, therapy, tumor microenvironment
1. INTRODUCTION
Ferroptosis is a newly discovered oxidative cell death triggered by excessive iron‐mediated lipid peroxidation. 1 , 2 It was first reported in 2012 when it was found that some small molecules (e.g., erastin and RSL3) could specifically induce a non‐apoptotic form of RCD in cancer cells; this RCD could be blocked by lipophilic antioxidants or iron chelators (e.g., vitamin E and ferrostatin‐1). 3 The morphological characteristics of ferroptotic cells are different from those of apoptotic cells. Apoptotic cells are characterized by cellular shrinkage, membrane blebbing, chromatin condensation, and nuclear fragmentation, whereas ferroptotic cells show typical necrotic features, such as an incomplete plasma membrane and the release of intracellular contents, especially damage‐associated molecular patterns (DAMPs). 4 Ultrastructural analysis has also shown that the mitochondria of apoptotic cells become larger, 5 whereas the mitochondria of ferroptotic cells lose their structural integrity and become smaller in size, the mitochondrial bilayer membrane density increases, cristae decrease or disappear, and the outer membrane ruptures. In addition, ferroptotic cells had normal‐sized nuclei without chromatin aggregation. 3
Here, we outline the role of the ferroptosis pathway in the tumor microenvironment (TME). Ferroptosis of tumor cells and immune cells in the TME is regulated by various factors, thereby inhibiting or promoting tumor progression. The impact of ferroptosis on tumors may be environmentally dependent. Changes in tumor genetics and staging, as well as changes in host factors, will alter the role of ferroptosis. The immune system not only plays a vital role in preventing the occurrence, development, and metastasis of tumors but also determines the response of tumors to treatment. Tumor cells can evade the surveillance of the immune system in a variety of ways, such as reducing immunogenicity, directly suppressing the immune response, and promoting the formation of an immunosuppressive network. 6 Recently, immunotherapy has promoted a successful antitumor immune response that combats tumors by activating the immune system. One of the most striking examples is immune checkpoint blockade therapy, which can reactivate the ability of T cells to kill tumor cells. 7 , 8 In addition, other immunotherapies, including cytokine therapy, dendritic cell vaccines, and chimeric antigen receptor T cells, can promote antitumor immunity.
Recent studies have found that the ferroptosis pathway is involved in the survival, apoptosis, differentiation, activation, and effector functions of various immune cells. 9 , 10 , 11 Moreover, tumor ferroptosis can regulate tumor growth by modulating the immune response. By combining immune checkpoint therapy with ferroptosis inducers, tumor resistance can be eliminated. As understanding about the multi‐layered relationship between ferroptosis pathways and tumor immune responses has progressed, and as the potential to target ferroptosis pathways in cancer treatment has developed, it has become appropriate to summarize and discuss the latest findings about ferroptosis pathways in tumor immunity and immunotherapy.
2. MECHANISM OF FERROPTOSIS
2.1. Inhibition of system Xc‐
System Xc‐, a heterodimeric cell surface antiporter for amino acids, contains two integral proteins: one is a twelve‐pass transmembrane transporter protein named SLC7A11, and the other is a single‐pass transmembrane regulatory protein named SLC3A2 that is linked with SLC7A11 by a disulfide bridge. 12 By controlling the process of bidirectional transmembrane transfer of extracellular cystine and intracellular glutamic acid, System Xc‐ improves the concentration of intracellular cystine. 13 Cystine acts as a necessary substrate for biosynthesis of glutathione (GSH), which is an essential antioxidant that prevents damage to important cellular components. 14 Likewise, intracellular cystine depletion occurs when system Xc‐ is inhibited. Several chemical molecules and their derivatives can inhibit system Xc‐. For instance, the classical oncogenic molecule, erastin—also known as the first compound discovered to induce ferroptosis—achieves its function by inhibiting system Xc‐. 3
2.2. The role of GPX4 in ferroptosis
As a GSH‐dependent enzyme, GPX4 can decrease lipid hydroperoxide (LOOH) to harmless alcohol (LOH). Specifically, GPX4 restricts the biosynthesis of highly reactive lipid alkoxy radicals. 15 Appropriate functioning of GPX4 seems essential for cell survival because GPX4 can efficiently remove phospholipid hydroperoxides. If GPX4 does not effectively clear excessive phospholipid hydroperoxides in cells, an iron‐involved catalytic reaction will be eventually triggered, thus causing ferroptosis. 16 When GPX4 activity is suppressed, reactive oxygen species (ROS) quickly accumulate in cells and mediate a continual cell damage, exposing cells to a high risk of death (Figure 1). The loss of GPX4 also appears lethal to murine embryos. 15 , 17 Inactivation of GPX4 is a key process to cause ferroptosis, and this process can be mediated by RSL3, a small molecule; overexpression of GPX4 can block its function. Ferroptosis can be induced in human oncogenic HRAS cells by knocking down GPX4. 17 In addition, lipophilic antioxidants and iron chelators can suppress ferroptotic cell death induced by GPX4 deletion in murine cells, which indicates that GPX4 activity is necessary to prevent ferroptosis. 15 , 18 One study showed that GPX4 activity plays an important role in several cancers driven by oncogenic mutations and dedifferentiated states. 19 However, GPX4 is not limited to one mode of cell death. Some studies have reported that the link between GPX4 activity and sensitivity regulates cell death pathways, such as apoptosis, 20 necrosis, 21 and pyrolysis. 22 However, whether these cell death pathways really have similar metabolic characteristics remains to be explored. 23
FIGURE 1.
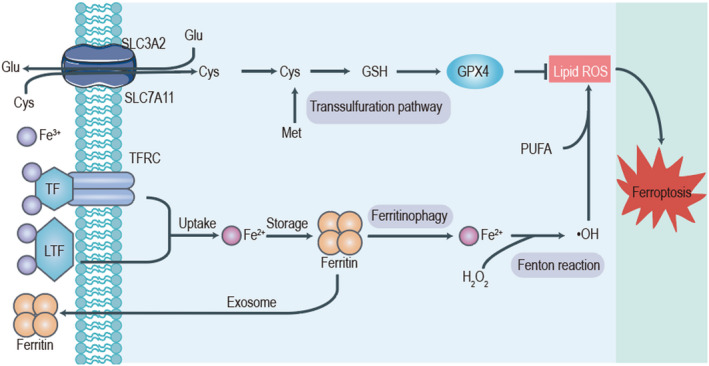
Mechanism of ferroptosis. The mechanism of ferroptosis involves two core parts: one is the xc‐GSH‐GPX4 system; the other is iron metabolism. System Xc‐ is composed of SLC7A11 and SLC3A2; it is responsible for transporting extracellular cystine into the cell and transporting intracellular glutamate out of the cell. Cystine is subsequently involved in the synthesis of GSH, an important antioxidant substance in cells that can inhibit ferroptosis. In addition, transferrin (TF) and lactotransferrin (LTF) import iron into cells. Excessive free Fe2+ induces the production of lipid reactive oxygen species (ROS) through the Fenton reaction and ultimately promotes the occurrence of ferroptosis in the cell.
2.3. The effect of iron in ferroptosis
Iron is a necessary participant in ferroptosis. Cell ferroptosis induced by erastin, RSL3, or a physiological stimulus (e.g., high concentrations of extracellular glutamate) is less likely to occur when iron chelators are added into the growth medium. In addition, both membrane‐permeable (e.g., ciclopirox, and 2,2‐bipyridyl) and membrane‐impermeable (e.g., deferoxamine) iron chelators have been effective in preventing ferroptosis. 3 , 17 Moreover, when TFRC (a gene that encodes the transferrin receptor, which is needed to absorb transferrin‐iron complexes) was silenced, erastin and cystine deprivation failed to cause ferroptosis. 24 Conversely, when the growth medium was supplemented with divalent iron (e.g., ferric ammonium citrate) or iron‐bound transferrin, erastin‐induced ferroptosis was augmented. 3 , 24 These results strongly support the essential role of iron in ferroptosis (Figure 1).
Iron chelators may block ferroptosis by keeping iron from offering electrons to oxygen—an offering that is necessary for ROS—but several puzzles remain before a redox‐independent role of iron in ferroptosis is clear. 25 A study of the properties of iron chelators showed that lipophilic iron chelators pass through the cell membrane, thus reducing the level of free intracellular iron from a “redox‐active” iron pool. 26 This iron pool provides the ability to catalyze the synthesis of soluble radicals, which can start or accelerate oxidative polyunsaturated fatty acid (PUFA) fragmentation; after ROS synthesis is inhibited, cell death may be blocked. 27 If iron metabolism is out of control, ROS can be generated, and this process is the most obvious mechanism for ferroptosis. 1
With the help of transferrin (TF) and transferrin receptor 1 (TFR1), circulated iron (Fe3+) can be absorbed into cells; then, an iron oxide reductase named six‐transmembrane epithelial antigen of the prostate 3 deoxidizes Fe3+ to Fe2+. Iron will eventually release into the labile iron pool (LIP). If the LIP continues to be formed, the process of ROS generation mediated by the interaction between Fe2+ and hydrogen peroxide (H2O2), also known as the Fenton reaction, may develop as a result of the high solubility of Fe2+ and its ability to transfer electrons. 28 Unlike RAS‐unmutated ferroptosis‐insensitive cells, RAS‐mutated ferroptosis‐sensitive cells express a higher level of TFR1 and a lower level of ferritin light chain and ferritin heavy chain 1, which are iron‐storage proteins. When cells continually absorb iron as the intracellular iron storage capacity decreases, iron overload develops and eventually leads to ferroptosis. 29 Therefore, maintaining homeostasis of iron metabolism in cells is vital for occurrence and progression of ferroptosis; compared with normal cells, cancer cells are more likely to have iron toxicosis and ROS accumulation because of a strong iron dependency. This distinguishing feature may be applied in cancer treatments by inducing ferroptosis in cancer cells. 30 , 31
Some additional mechanistic pathways are worthy of discussion. Nuclear factor (erythroid‐derived 2) like 2 (Nrf2) can regulate cellular iron metabolism, and Nrf2 activity has inhibited ferroptosis in hepatocellular carcinoma cells. 32 The classical tumor suppressor P53 is an important regulator of the ferroptosis process as well. Furthermore, analyses of ferroptosis‐related molecules have identified various ways to regulate the process of ferroptosis in vitro and in vivo.
2.4. Lipid oxidation: Result of ferroptosis
When system Xc‐ is inhibited or GPX4 is inactivated, cellular iron‐dependent ROS accumulate and PUFAs are exhausted, ultimately leading to ferroptosis. 17 , 18 , 33 ROS are usually synthesized using the PUFA chains from membrane lipids. PUFAs are easily oxidized either through an enzymatic (e.g., lipoxygenase‐catalyzed) or a non‐enzymatic (e.g., ROS‐catalyzed) method, and this oxidation contributes to the occurrence of LOOH. 27 LOOH can be transformed into several harmful lipid radicals, such as the alkoxy radical LOH. These lipid radicals can steal protons from nearby PUFAs, thus starting a new wave of lipid oxidation, causing damage to the cellular lipid membrane. 27
Ultimately, PUFAs become fragmented after oxidation and damage by free toxic radicals. 27 Ferritin 1 (a kind of small‐molecule antioxidant) can prevent murine cancer cell ferroptosis induced by erastin or GPX4 inactivation from an L‐ROS (lipid ROS) stockpile, increasing PUFA exhaustion and, eventually, ferroptotic cell death; this finding indicates that lipid ROS‐ mediated damage is necessary for ferroptosis 17 , 18 , 33 (Figure 1). The complicated interplay of iron, cysteine, and lipid metabolism maintains an important role in ferroptosis.
3. FERROPTOTIC CANCER CELLS IN THE IMMUNE RESPONSE
3.1. Immune cell activation
Previous studies have confirmed that ferroptosis belongs to a type of immunogenic cell death (ICD) process 4 , 34 , 35 and therefore has some characteristics of immunogenic cell death. Cells undergoing typical ICD can release DAMPs, such as HMGB1(high‐mobility group protein box 1), adenosine triphosphate (ATP), and calreticulin (CRT). These DAMPs may interact with pattern recognition receptors, phagocytosis receptors, (receptor for advanced glycation endproducts) RAGE, toll‐like receptor 2 (TLR2), toll‐like receptor 4 (TLR4), and purinergic receptors on immune cells to promote their immune response and adaptive immunity driven by cytotoxic T lymphocytes. 36 , 37 , 38 Therefore, research on the immunogenicity of ferroptotic tumor cells and the exploration of its influence on the effect of antitumor treatments have important scientific and clinical significance.
Wen et al. 4 found that HMGB1 released by ferroptotic cells could lead to upregulation of tumor necrosis factor (TNF) expression in macrophages. An increase in HMGB1 and the infiltration of leukocytes were also observed in the ferroptotic tissue of a murine model of experimental pancreatitis. 39 Interleukin‐33 is a DAMP with high pro‐inflammatory activity. 40 One study has shown not only that interleukin‐33 in the kidney and plasma increased in a murine model of acute kidney injury with ferroptosis but also that a ferroptosis inhibitor could inhibit this increase. 41 Another study confirmed that 1‐steaoryl‐2‐15‐HpETE‐sn‐glycero‐3‐phosphatidylethanolamine (SAPE‐OOH) is an “eat me” signal on the surface of ferroptotic cell membranes that increased the phagocytic function of macrophages by targeting TLR2 42 (Figure 2).
FIGURE 2.
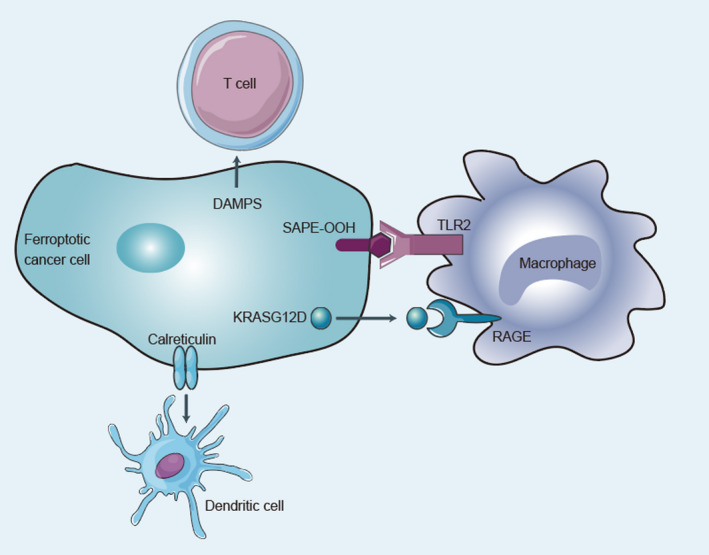
Effect of ferroptotic cancer cells in the tumor microenvironment. Ferroptotic cancer cells can affect immune cells in the tumor microenvironment in many ways to promote or inhibit the antitumor immune response. Various damage‐associated molecular patterns (DAMPs) produced by ferroptotic cancer cells can promote the differentiation and maturation of T cells and dendritic cells to enhance their immune functions. In addition, 1‐steaoryl‐2‐15‐HpETE‐sn‐glycero‐3‐phosphatidylethanolamine (SAPE‐OOH) on the surface can promote the phagocytosis of macrophages through toll‐like receptor 2 (TLR2). Conversely, ferroptotic cancer cells can secrete a large amount of KRASG12D, which promotes the polarization of M1 macrophages into M2 macrophages and damages their ability to kill tumor cells.
Calreticulin is a soluble endoplasmic reticulum–related chaperone protein on the plasma membrane that is released when cells are stressed or die. It can eliminate cancer cells by promoting the phagocytosis of phagocytes. 43 , 44 , 45 , 46 , 47 In ROS‐mediated ferroptotic cancer cells, calreticulin is transferred to the cell surface. 48 , 49 Together, these results underscore the idea that ferroptotic cells are able to release signals that let immune cells locate them (Figure 2).
Efimova et al. 34 established a preclinical model to prove that RSL3, a GPX4 inhibitor, induced the death of murine fibrosarcoma MCA205 cells through ferroptosis rather than via apoptosis or necrosis. The authors compared the effects of early and late ferroptotic cancer cells (MCA205 cells treated with RSL3 for 1 h and 24 h, respectively) on the maturation of murine bone marrow–derived dendritic cells (BMDCs) and concluded that the former released HMGB1 and that ATP contributed to the maturation of BMDCs, whereas the latter did not. This finding is the first confirmation that ferroptosis is indeed a kind of ICD. 34 In addition, ferroptosis induced by photodynamic therapy (PDT) promoted the release of HMGB1/ATP from cancer cells and the subsequent phagocytosis by macrophages and activation and maturation of BMDCs. 50 These studies have expanded our understanding of the relationship between ferroptosis and ICD and have consolidated the effect of ICD induction to promote immunity after ferroptosis is induced.
In addition to relying on the secretion of annexin A1, the release of type‐1 interferons, and the surface exposure of calreticulin—methods that also apply to apoptotic ICD—ferroptotic 36 ICD may depend on other DAMPs. The possibility that certain combinations of DAMPs that have not been defined so far may play a role in different types of ICDs has been proposed. Therefore, additional research is urgently needed to support or disprove the hypothesis that different cell death patterns, such as apoptosis, ferroptosis, and necrosis, may cause ICD via different approaches despite sharing only a small portion of DAMPs. 51
Previous research has revealed that apoptotic cells can produce a series of signals during their death, including “find me” and “eat me” signals, which help communicate with immune cells. 52 Recent studies have demonstrated that ferroptotic cells also produce a corresponding “find me” signal that involves lipid mediators and is able to help immune cells find dead or dying ferroptotic cells. Ferroptotic cancer cells in vitro can be located and engulfed by macrophages efficiently with the help of such signals. 53 Additionally, ferroptotic cells can secrete several oxidative products of AA (arachidonic acid) as the potential signals to regulate antitumor immunity and of lipid mediators. Accordingly, as a ferroptotic signal, LOX (lipoxygenase) not only plays a key role in the oxidation of esterified PUFAs but also promotes the release of such signals from ferroptotic cells, thereby regulating antitumor immunity. Specifically, ferroptotic cells can secrete eicosanoids, including 5‐HETE, 11‐HETE, and 15‐HETE, when GPX4 is depleted. 18 In cells stimulated by TNF or IL‐1, increased GPX4 activity suppresses the synthesis of pro‐inflammatory lipid mediators, including LTB4, that plays an important role in tumorigenesis and reduces pro‐inflammatory activity activated by the nuclear factor‐kappa B pathway. 54 , 55
Esterified eicosanoids are a family of molecules formed under the direct catalyzation of lipoxygenase on SN‐2 fatty acids or by re‐esterification of free eicosanoids. There is a growing interest in their biological role and speculation that they may have the same role as that of immunoregulatory signaling molecules as free eicosanoids. 56 Analyses of lipid components in the ferroptotic cells have confirmed the presence of a large number of doubly and triply oxidized arachidonic acids, including albumin phosphatidylethanolamine (PE), which may be formed under catalyzation by arachidonic acid 15‐lipoxygenase (ALOX15). 57 Previous research has described the function of lyso‐phospholipids (a kind of hydrolysis product of PE) in APC attraction to apoptotic cells. 58 Furthermore, evidence has confirmed that by providing more AA, cancer cells undergoing ferroptosis have accelerated the biosynthesis of eicosanoids, which eventually promoted antitumor immunity. 59
In conclusion, ferroptosis of tumor cells appears to not only lead to tumor cell death but also promote tumor antigen presentation, immune cell localization, and activation of effector immune cell function in the TME.
3.2. Immune response inhibition
Several molecules secreted from ferroptotic cells may promote tumor progression by suppressing immune cells. Cells undergoing ferroptosis can release molecules, including the mutated KRASG12D protein, that promote tumor development. 60 , 61 When the KRASG12D protein is released and absorbed by macrophages via a specific cell surface receptor called RAGE (an advanced glycation end‐product), it induces fatty acid oxidation and promotes the conversion of macrophages into M2‐like tumor‐associated macrophages (TAMs) and activates the tumor‐promoting effect of macrophages. In fact, the expression level of KRASG12D in TAMs has been positively related to poor prognosis of pancreatic cancer, suggesting that KRASG12D may be a target for tumor immunotherapy 60 (Figure 2).
A recent study demonstrated that stearoyl‐CoA desaturase‐1 (SCD1) and fatty acid binding protein (FABP4) play roles in tumor recurrence by reducing the sensitivity of cancer cells to ferroptosis. Specifically, SCD1 secreted by cancer cells increased the production of monounsaturated fatty acids (MUFAs). FABP4 increased the synthesis of lipid droplets under hypoxic circumstances, which both promoted tumor migration and protected cancer cells from ferroptosis. 62
Ferroptotic cancer cells can generate oxidized lipids, such as 5‐hydroxyeicosatetraenoic acid (5‐HETE), that suppress immune cells to modulate antitumor immunity. 18 The level of GPX4 activity has been negatively related to the production of pro‐inflammatory lipids, such as leukotriene B4 (LTB4), that are essential for tumorigenesis. 9 , 54 Free eicosanoids and esterified eicosane also regulate the immune response. Specifically, oxidized phosphatidylcholine suppressed the maturation of dendritic cells by activating Nrf2 and the differentiation of T‐helper 17 cells. 63 Moreover, oxidized lipids and lipid droplets also modulate antitumor immune reactions, which can be decomposed to oxygenated neutral lipids, free PUFAs, or PUFA triacylglycerols, that could trigger inaccurate antigen cross presentation and incomplete antitumor immunity. 64 , 65 All these lipids suppress antitumor immunity through various mechanisms.
Recent studies have found several links between ferroptotic tumor cells and increased production of prostaglandin E2 (PGE2), which is an important immune modulator that inhibits antitumor immunity. 17 , 66 , 67 PGE2 suppresses the accumulation of classical type 1 dendritic cells and the secretion of CC chemokine ligand 5 (CCL5) and chemokine lymphotactin by natural killer (NK) cells. 67 , 68 , 69 Moreover, PGE2 can also affect an acquired immune response by directly suppressing the function of cytotoxic T cells. 70 A study by Kurtova et al. 71 found that the release of PGE2 during chemotherapy was associated with the resistance of a tumor to treatment, and its mechanism was promotion of tumor cell regeneration, which could be blocked by co‐targeting PGE2. Therefore, researchers assumed that tumor cells undergoing ferroptosis are likely to secrete more PGE2 because of the inactivation of GPX4, whereas tumor cells treated with chemotherapy would produce more PGE2, that in turn would promote the growth of ferroptosis‐sensitive tumor cells. Such assumptions lead to the question of how to keep the balance between antitumor immune reactions induced by ferroptosis and the immunosuppressive effect of PGE2 which was promoted by ferroptosis‐sensitive cells. 56 , 72 , 73
One bold hypothesis states that ferroptotic cells in a tumor may inhibit the immune response and promote tumor growth. However, this hypothesis requires more in‐depth research to validate the concept and determine the immunoregulatory role of ferroptosis‐sensitive cells in antitumor immunity. This research may include the analysis of eicosanoids (as immunosuppressive factors) during different levels of GPX4 activity. 18
4. FERROPTOSIS IN TUMOR‐INFILTRATING IMMUNE CELLS
4.1. Effector T‐cell subsets
Studies have shown that the increased expression of CD36 in tumor‐infiltrating CD8+ T cells in the TME led to tumor progression and poor survival rates in human and mouse cancers. However, genetic downregulation of CD36 in effector CD8+ T cells promoted the production of cytotoxic cytokines and eradication of tumors. 74 In addition, lipid peroxidation and ferroptosis induced by CD36 inhibited the production of cytotoxic cytokines in tumor‐infiltrating CD8+ T cells. Therefore, blocking CD36 or inhibiting ferroptosis in CD8+ T cells can effectively restore their antitumor functions; more importantly, binding to anti–PD‐1 antibodies had a stronger antitumor effect. 75
Overexpression of GPX4 or the ferroptotic inhibitor 1 (FSP1) has protected CD8+ T cells from ferroptosis without impairing their function. The genetic deletion of ACSL4 could also inhibit the ferroptosis of CD8+ T cells but impairs its antitumor function. 76 A study has shown that conditional deletion of GPX4 induced T‐cell ferroptosis in mice and led to a lack of immune response to infection. 9 This finding suggests that GPX4 and ferroptosis play important roles in the immune response mediated by T cells.
4.2. Myeloid‐derived suppressor cells
Myeloid‐derived suppressor cells (MDSCs) are a type of immunosuppressive cells that accumulate in large amounts under pathological conditions to suppress T‐cell immune responses. 77 Neutral ceramidase (N‐ceramide hydrolase) is overexpressed in tumor‐infiltrating bone marrow mesenchymal stem cells of colon cancer as an MDSC survival factor. ASAH2 inhibits the p53 pathway of bone marrow mesenchymal stem cells in the TME by destroying the stability of the p53 protein, thereby protecting bone marrow mesenchymal stem cells from ferroptosis. As an inhibitor of ASAH2, NC06 can reduce GSH synthesis and increase lipid ROS to activate ferroptosis in bone marrow mesenchymal stem cells and promote the activation of tumor‐infiltrating cytotoxic T lymphocyte (CTL) to inhibit tumor growth in vivo. 78 Therefore, targeting ASAH2 with NC06 to induce MDSC ferroptosis may be a potentially effective approach to tumor immunotherapy.
4.3. Macrophages
In the process of cancer therapy, eliminating dead cells via macrophages is very important in maintaining immune homeostasis. Studies have found that macrophages engulf asbestos and cause its ferroptosis, which increases catalytic iron content inside and outside the cell. Furthermore, this ferroptosis promotes the development of mesothelial carcinoma by activating the Wnt/β‐catenin pathway. 10 In addition, studies have shown that macrophages can induce the ferroptotic cascade through erythrophagocytosis. A similar pro‐ferroptosis effect can also occur during treatment with ferric citrate or radiation‐induced hemorrhage. 79 , 80 , 81
The expression level of inducible nitric oxide synthase in M1 macrophages is higher than that of M2 macrophages, so more nitric oxide is produced (thus inhibiting lipid peroxidation), and the ferroptosis of M1 macrophages is inhibited. 82 This study showed that nitric oxide plays an important role in regulating the ferroptosis of macrophages, and it supports a potential therapeutic opportunity for inhibiting M2 macrophages through ferroptosis to enhance the antitumor immune response.
5. FERROPTOSIS IN CANCER THERAPY
5.1. Targeting ferroptosis
Killing tumor cells by activating regulatory cell death is an effective anticancer treatment. Although some results have been achieved in clinical cancer treatment, drug resistance of tumor cells remains a troublesome problem. 83 , 84 , 85 The discovery of ferroptosis may improve the clinical treatment of tumors, and exploring the activation of ferroptosis may provide new therapeutic targets 3 (Figure 3).
FIGURE 3.
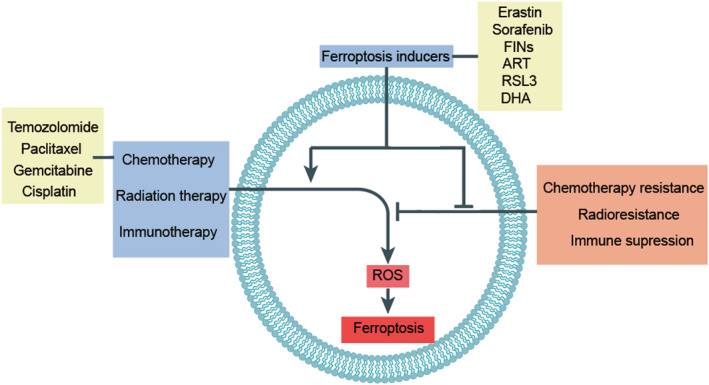
Roles of ferroptosis in antitumor therapy. Three common antitumor treatment methods—chemotherapy, radiotherapy, and immunotherapy—have played important roles in antitumor treatment. However, when they face tumor resistance, radiotherapy resistance, and immunosuppression, they are often ineffective. Ferroptosis has introduced some new methods. These three common treatment methods can promote the ferroptosis of tumor cells together with ferroptosis inducers. Conversely, ferroptosis inducers can enhance these three treatments by reversing tumor resistance, radiotherapy resistance, and immunosuppression.
Studies have shown that certain refractory tumor cells are abnormally sensitive to ferroptosis. 86 , 87 For example, traditional therapies induce apoptosis in cancer cells but are generally ineffective in cancer cells in a mesenchymal state. The enhanced synthesis of PUFA‐PL in these cancer cells makes them strongly dependent on GPX4 to detoxify lipid peroxides for survival. Therefore, these cells are highly susceptible to ferroptosis. 87 For example, certain recurrent breast cancer cells with mesenchymal features exhibit a high sensitivity to ferroptosis. 88 Likewise, mesenchymal gastric cancer cells overexpressing very long‐chain fatty acid protein 5 (ELOVL5), a key enzyme involved in PUFA synthesis, are particularly susceptible to ferroptosis. 89 In addition, subpopulations of dedifferentiated melanoma cells may also be susceptible to ferroptosis due to low levels of GSH. 87 CD44‐overexpressing mesenchymal cancer cells may increase sensitivity to ferroptosis by increasing intracellular iron load. 90 In conclusion, cancer cells prone to ferroptosis tend to have the following metabolic characteristics, including high levels of PUFAs, overloaded iron pools, and vulnerable GPX4–GSH defenses. These types of patients may gain more benefits from targeting ferroptosis.
Convincing evidence has shown that activation of the ferroptosis of tumor cells is a promising new method for cancer treatment. In theory, small molecules that can induce ferroptosis can be found through systematic screening or by inhibiting key molecules related to ferroptosis resistance, such as GPX4 and cystine/glutamate reverse transporter SLC7A11, to achieve ferroptosis activation. We review here some ways to make progress in cancer treatment by activating ferroptosis.
Systematic screening identified some small molecules, such as erastin, 3 sorafenib, 91 artemisinin (ART), 92 dihydroartemisinin (DHA), 93 RSL3, and ferritin‐inducing compounds (FINs), that induced cell death through ferroptosis. For example, in tumor cells that are resistant to sorafenib, the combination of erastin and sorafenib has exerted a powerful antitumor effect. 94 For example, ART can induce ROS and ferroptosis in pancreatic ductal adenocarcinoma (PDAC) cell lines. Ferroptosis inhibitors can block lipid peroxidation and cell death induced by ART, thereby increasing cell survival. 92 Studies have shown that DHA specifically promoted cell death in head and neck cancer (HNC) cells by inducing ferroptosis and apoptosis. 93 In addition, 10 different ART derivatives not only killed tumor cells by inducing apoptosis, autophagy, or necroptosis (as previously established) but also killed tumor cells by ferroptosis. 95 A larger study discovered that a group of small molecules named FINs can induce ferroptosis. 96 FIN56 has not only promoted the reduction of GPX4 protein levels but also prevented the production of lipophilic antioxidants, which ultimately triggered the ferroptosis of cells. 96 However, this method still has great potential to screen more novel ferroptosis inducers.
GPX4 is the cause of ferroptosis induced by lipid peroxidation signals; therefore, knocking down the expression of GPX4 is enough to induce ferroptosis, 17 and GPX4 inhibitors may be potential candidates for tumor treatment. Because RSL3 effectively inhibits GPX4, identifying GPX4 inhibitors using the structure of RSL3 is a current research interest. For example, a recent study found that covalently targeting a binding site on GPX4 can induce cell ferroptosis. 97 Ideally, the co‐crystal structure of GPX4 and RSL3 will prompt research on GPX4 inhibitors for therapeutic use. By inhibiting the key active site of GPX4 selenocysteine and systematically screening ferroptosis inducers, it may be possible to combat tumor chemotherapy resistance. However, we should take into account that GPX4 is an essential protein for life, and knocking out GPX4 greatly increased lethality in mouse embryos. 98 Compared with normal cells, though, cancer cells are more sensitive to GPX4 inhibition. Therefore, treating tumors by inhibiting GPX4 may be a potential treatment method, although the side effects caused by the lack of this key enzyme should be considered in future studies.
To date, study on the safety and effectiveness of ferroptosis‐related drugs is limited in vivo and mainly involves PE, (1S, 3R)‐RSL3, and sorafenib. The structure of PE is similar to that of erastin, which is more soluble in water and more stable and suitable for experiments in vivo. 17 In a xenograft murine tumor model, PE has induced ferroptosis to inhibit tumor growth. In addition, when retinoblastoma protein is reduced, sorafenib can effectively eliminate tumors in murine xenograft models of HCC. 99 2‐nitroimidazoles can induce ferroptosis of glioma stem cells under hypoxic conditions. 100 Solanine can induce ferroptosis of liver cancer cells by inhibiting GPX4 and GSH to increase the level of intracellular ROS. 101 However, evaluation of drug safety has not been involved in these studies and must be considered in the future.
Because the state of Nrf2 greatly affects the therapeutic response of HCC cells to ferroptosis‐targeted therapy, 91 the expression of Nrf2 must be suppressed to enhance antitumor efficacy during ferroptosis‐targeted therapy. Furthermore, the iron‐rich tumor environment and the clinical application of sorafenib in the treatment of RCCs indicate that targeting ferroptosis in RCC may be a treatment option. 102 , 103 In addition, recent studies have found that autophagy can cause ferroptosis by reducing the level of ferritin in tumor cells. 104 Overexpression of NCOA4 has always increased the degradation of ferritin and promoted ferroptosis. 104
Previous studies have shown that inhibition of the cystine/glutamate antiporter SLC7A11 can significantly reverse the resistance of HNC cells to cisplatin, whereas the deficiency of SLC7A11 will not affect the development and survival of mice 103 ; compared with chemotherapy, this inhibition may have better efficacy and fewer side effects in antitumor treatments that target ferroptosis. However, inducing ferroptosis by targeting system Xc‐ may not be an effective treatment for certain tumors that do not rely on system Xc‐. Recent studies have found that tumor cells that are sensitive to erastin‐induced ferroptosis are resistant because they lack cysteinyl‐tRNA synthetase. 105 The lack of cysteinyl‐tRNA synthetase increases the accumulation of cystathionine, which activates the trans‐sulfurization pathway and ultimately makes tumor cells resistant to ferroptosis. 105 However, the sensitivity of tumor cells to erastin can be restored by inhibiting the trans‐sulfide pathway. In addition, some tumors, such as diffuse large B‐cell lymphoma, not only rely on system Xc‐ but also have defective trans‐sulfurization pathways. Inhibiting system Xc‐ to induce ferroptotic cancer cells may be an effective therapeutic target. 17 Therefore, the genetic composition of tumor cells determines to a large extent whether antitumor therapy can be carried out by targeting system Xc‐. In general, targeting important molecules in the ferroptosis pathway to treat tumors remains an important direction of exploration for tumor‐targeted therapy.
However, cancer cells can acquire resistance to ferroptosis through different escape mechanisms. Therefore, avoiding cancer cell resistance to ferroptosis is critical to targeting ferroptosis. For example, reducing DJ1 inhibits the activity of S‐adenosyl homocysteine hydrolase (SAHH) and thus the transsulfuration pathway, which resensitizes xenograft tumors to targeting ferroptosis. 106 Similarly, targeting other enzymes involved in the trans‐sulfuration pathway, such as cystathionine β‐synthase (CBS) and glycine N‐methyltransferase (GNMT), can also enhance tumor sensitivity to ferroptosis 107 , 108 . Furthermore, inhibition of pyruvate dehydrogenase kinase 4 (PDK4) by accelerating pyruvate oxidation can resensitize xenograft tumors to ferroptosis‐inducing agents. 109 Tumor cell resistance to ferroptosis can also be reversed by targeting oncogene‐induced downstream effectors. For example, PI3K can promote tumor cell resistance to ferroptosis through mTOR complex 1 (mTORC1). Thus, the combination of mTORC1 inhibitors and ferroptosis inducers exerted a powerful tumor‐suppressor effect. 110 Overall, blocking the escape pathways of cancer cells to avoid cancer cell resistance to ferroptosis is an important strategy for cancer therapy (Figure 3).
Because different cancers have different metabolisms, their susceptibility to ferroptosis is different. But, it has not been possible to determine which cancer is more suitable for ferroptosis‐related treatment in the future. However, after reviewing the extensive literature, we found that diffuse large B‐cell lymphoma and renal cell carcinoma are particularly sensitive to GPX4‐mediated ferroptosis 17 and adrenocortical carcinoma is very sensitive to DDT‐mediated ferroptosis. 111 Among all cancers, alterations in iron metabolism genes were most abundant in clear cell renal cell carcinoma, and the mRNA levels of 38 iron metabolism genes were most significantly associated with prognosis in clear cell renal cell carcinoma. 112 In addition, a large number of studies on the mechanism of ferroptosis sensitivity of tumor cells have provided many new targets for targeted ferroptosis therapy, such as lung cancer 113 , 114 , 115 , 116 and colorectal cancer. 117 , 118 , 119 , 120 This may indicate that these tumors are more suitable for ferroptosis‐related therapy.
5.2. Chemotherapy
Studies have shown that erastin and cisplatin synergistically inhibit the growth of ovarian cancer cells, and inhibition may be manipulated by a mechanism mediated by ROS, which enhances cisplatin therapy and provides a new strategy for overcoming cisplatin resistance. 121 A recent study showed that classic chemotherapeutic drugs can also induce ferroptosis in cancer cells, which is a new mechanism by which chemotherapeutic drugs exert their effects. For example, cisplatin can reduce the level of GSH in cancer cells to inhibit the effect of GPX4 and ultimately induce ferroptosis of cancer cells 122 (Table 1). Furthermore, when free iron levels increase as a result of ferritinophagy, cisplatin can trigger ferroptosis. 123 More evidence has shown that ferroptosis inducers, such as erastin, can synergistically promote the anticancer effect of cisplatin by antagonizing system Xc‐ or GPX4 in a variety of cancers. 122 , 123 , 124 , 125 In addition, octanoylanilide hydroxamic acid (SAHA), as a histone deacetylase inhibitor, can induce tumor cells to produce ROS to make them sensitive to the effects of cisplatin. 126
TABLE 1.
Antitumor therapy together with ferroptosis inducers
| Tumor type | Ferroptosis inducer | Combination | Target | Mechanism | Ref. |
|---|---|---|---|---|---|
| Acute myeloid leukemia | Erastin | Cytarabine and doxorubicin /adriamycin | GPX4 | JNK and p38 synergistically promote erastin‐induced ferroptosis | [140] |
| Breast cancer | Albiziabioside A | Dichloroacetate | GPX4 | AlbA‐DCA can inhibit GPX4 and eliminate M2 macrophages to promote antitumor immunity | [156] |
| Breast cancer | Ferroptosis inducers | Oxygen‐boosted PDT | – | PDT promotes the accumulation of lymphocytes at the tumor site and stimulates the secretion of IFN‐γ | [158] |
| Glioblastomas | Sulfasalazine | Gamma knife radiosurgery | System Xc‐ | SSZ inhibits the uptake of cystine and therefore reduces the level of GSH, thus increasing the intracellular ROS | [149] |
| Head and neck cancer | Trigonelline | Artesunate | NRF2 | NRF2 inhibitor trigonelline can induce lipid peroxide accumulation | [134] |
| Lung cancer | RSL3 | Cisplatin | GPX4 | RSL3 enhances the therapeutic effect of cisplatin | [123] |
| Melanoma | TGF‐β inhibitor | PD‐1 antibody | ROS | PD‐1 antibody and TGF‐β inhibitor cooperatively polarize M2‐TAMs into M1‐TAMs and promote the Fenton reaction with Fe ions discharged from magnetic nanoclusters | [155] |
| NSCLC | Erastin | Cisplatin | GSH‐GPX4 | Cisplatin can deplete the GSH and inactivate the GPX4 together | [122] |
| NSCLC | Erastin | Cisplatin | ROS | SAHA and erastin, the inducers of ROS‐mediated cell death, strongly enhanced the effect of cisplatin in WT EGFR cells | [126] |
| Ovarian cancer | Erastin | Cisplatin | System Xc‐ | Erastin can inhibit system Xc‐ and potentiate the cytotoxic effects of cisplatin to eradicate tumor cells | [125] |
| PDAC | Erastin | Gemcitabine | System Xc‐ | Both SLC7A11‐KO cell lines exhibit amino acid stress with induction of ATF4 and cell death | [124] |
| PDAC | Erastin | Cisplatin | System Xc‐ | Both SLC7A11‐KO cell lines exhibit amino acid stress with induction of ATF4 and cell death | [124] |
| Sarcoma | Anti–PD‐L1/Anti‐ CTLA‐4 mAb | Ionizing radiation | System Xc‐ | Radiation therapy and interferon synergistically reduce the expression level of SLC7A11 | [151] |
The ferroptosis caused by ferritinophagy is related to the antitumor mechanism of ART and its derivatives. 93 , 127 , 128 Ferritin binds to NCOA4 and is transported to the lysosome. However, ART and its derivative dihydroartemisinin (DAT) increase the degradation of ferritin in the lysosome, which ultimately increases intracellular iron and triggers ferroptosis. 129 , 130 In addition, recent literature has shown that DAT can regulate iron homeostasis by changing the ratio of iron regulatory proteins/iron response elements and so increase the level of free iron in tumor cells. 127 In this manner, the synergistic treatment of ART and transferrin induced ferroptosis in pancreatic ductal adenocarcinoma by promoting iron transport into cells and accelerating the release of free iron from lysosomes. 92
Traditional chemotherapeutics can upregulate GPX4 and system Xc‐ to inhibit cancer cell death and ferroptosis. For example, gemcitabine can increase the expression level of heat shock protein 70 family protein 5 (HSPA5), thereby preventing the degradation of the GPX4 protein and ferroptosis in cancer cells. 131 However, it is possible to prevent the protective effect of gemcitabine on tumors and promote the eradication of pancreatic cancer by combining treatment with erastin or inhibiting the HSPA5‐GPX4 pathway 124 (Table 1). Temozolomide (TMZ) promoted the expression of SLC7A11 at the gene and protein levels through the Nrf2 and ATF4 pathways. In addition, TMZ can enhance the activity of cystathionine γ‐lyase (CTH); when SLC7A11 was blocked, CTH was an important enzyme that ensured the supply of cysteine and the synthesis of GSH in the trans‐sulfide pathway. This finding may explain the mechanism by which gliomas with high system Xc‐ expression levels are more likely to be treated by the combination of erastin and TMZ. 132 , 133 Artesunate can be used for chemotherapy of HNC cells, but it can activate the Nrf2 antioxidant pathway. Therefore, inhibition of Nrf2 can induce lipid oxidation of HNC and reverse its ferroptosis resistance. 134
Other classic chemotherapy drugs enhance their anticancer effects by combining with ferroptosis inducers. Paclitaxel (PTX), a classic chemotherapy drug widely used to treat a variety of cancers, can not only upregulate the expression of p53 and p21 genes but also downregulate the expression of SLC7A11 and SLC1A5. 135 , 136 A recent study has shown that the combined use of PTX and RSL3 can cause the ferroptosis of cancer cells. 137 Natália et al. 138 found that metformin can induce ferroptosis in breast cancer cells by reducing the level of intracellular GSH. In addition, MAP30, a biologically active protein isolated from balsam pear seeds, can increase the therapeutic effect of cisplatin on ovarian cancer by inducing cell ferroptosis. 139 Similarly, erastin can significantly enhance the inhibitory effects of cytarabine and adriamycin on leukemia cells. Its mode of action has nothing to do with RAS and partly depends on the induction of ferroptosis. The c‐Jun N‐terminal kinases (JNKs)/p38‐MAPK pathway is an important cause of erastin‐induced leukemia cell death 140 (Table 1). In addition, the ERK‐MAPK pathway plays a major role in RAS‐dependent ferroptotic cells. 141 Fingolimod, a new type of immunosuppressant, also induces ferroptosis and autophagy through the PP2A/AMPK pathway. 142 These findings suggest that promotion of erastin as an anticancer treatment via activation of the MAPK pathway may be a promising approach.
5.3. Radiation therapy
The ionizing radiation of radiotherapy always damages cells by inducing DNA double‐strand breaks. 143 In addition, the activation of oxidases and the radiolysis of water will indirectly lead to GSH depletion and increased ROS. 144 , 145 , 146 Studies have shown that depletion of glutathione promotes the antitumor effect of radiotherapy. 147 , 148 The expression of SLC7A11 in glioblastoma or glioma cells is higher than that in normal brain tissue. Therefore, inhibition of system Xc‐ by ferroptosis inducers can enhance the radiotherapy effect of glioblastoma. 149 Khorsandi et al. 150 proved that synergistic treatment with pre‐irradiation and gallic acid could greatly inhibit the survival of tumor cells, mainly by inhibiting GPX4.
Recently, Lei et al. 150 demonstrated that in most cancer cell lines, ionizing radiation (IR) promotes the expression of ferroptosis genes, but it also induces ROS through the ACSL4/LPCAT3/ALOX and SLC7A11/GPX4 pathways. In addition, inhibition of SLC7A11 or GPX4 can increase the radiosensitivity of tumor cells to IR. 150 Ferroptosis may play an important role in combined radiotherapy and CD8+ T cell therapy. Studies have shown that the ataxia telangiectasia mutant, which is upregulated by radiotherapy, can synergize with interferon (IFN) to downregulate the expression of SLC7A11. 151 A recent study found that itraconazole can induce the ferroptosis of tumor cells by increasing the level of iron in the lysosome and thus can reverse the resistance of nasopharyngeal carcinoma stem cells to radiotherapy. 152 These findings provide new directions for improving the therapeutic effect of radiotherapy or regulating ferroptosis to treat radiotherapy‐resistant tumors (Figure 3).
5.4. Immunotherapy
Recent studies have shown that cytotoxic CD8+ T cells can suppress the expression level of system Xc‐ by secreting IFN‐γ and thus promote the ferroptosis of cancer cells. 153 This finding suggests that the combination therapy of immunotherapy and a ferroptosis inducer may be a promising direction for research. PD‐1 antibody treatment can transform M2 macrophages into M1 macrophages, enhancing the antitumor function of macrophages. 154 Nanoparticles are composed of PD‐1 antibodies and transforming growth factor beta inhibitors, which synergistically enhance the antitumor immune response, increasing H2O2 levels in M1 macrophages and inducing a Fenton reaction. The subsequent generation of hydroxyl radicals induces ferroptosis in tumor cells. 155 A new drug, AlbA‐DCA, inhibits the progression of breast cancer by downregulating the expression of GPX4 and eliminating precancerous M2 TAMs 156 (Table 1).
M1 phagocytes have a higher resistance to ferroptosis than M2 phagocytes do. 82 Studies have shown that radiated tumor cell–released microparticles (RT‐MPs) can transform M2 macrophages into M1 macrophages. 157 In addition, RT‐MPs can also promote the ferroptosis of tumor cells and trigger ICD, which improves the clearance of tumor cells by macrophages. 154 A recent study found that PDT can effectively induce ferroptosis and phenotypic maturation of bone marrow mesenchymal stem cells in cancer cells. 50 The combination of PDT and ferroptosis inducers can reduce the level of GSH in tumor tissues and increase the level of the lipid peroxidation product malondialdehyde. In addition, PDT promoted not only the infiltration of lymphocytes at the tumor site but also the secretion of IFN‐γ. 158 Therefore, the combination of PDT and immunotherapy to promote the ferroptosis of tumor cells and exert anti‐tumor effects requires more exploration.
6. PERSPECTIVE
Since the discovery of ferroptosis, continual evidence has suggested inextricable links between ferroptosis and tumor immunity. Many studies have suggested that the immunogenicity of ferroptotic cells will increase. For example, two immunogenic markers, HMGB1 and CRT, were increased in ferroptotic cells. 4 , 46 In addition, ferroptosis can directly affect the functions of various immune cells, suggesting that the function of immune cells can be regulated by targeting ferroptosis to treat cancer. Therefore, exploration of the interaction mechanism between ferroptosis and immunity can provide ideas for new antitumor treatments.
Targeting metabolic signatures of ferroptosis, such as enrichment of PUFA‐PLs, iron overload, and unbalanced ferroptosis defense system, have uncovered a series of novel cancer therapeutic targets. The drugs developed with these targets can not only be used for antitumor therapy alone but also can be combined with conventional therapy that can induce apoptosis, which can further improve the therapeutic effect. For example, the resistance of tumor cells to chemotherapy and radiotherapy has been a major problem in antitumor therapy. However, the emergence of ferroptosis offers hope for a solution to this problem. The combination of cisplatin and ferroptosis inducers restored sensitivity to cells that were originally cisplatin‐resistant. In addition, some other refractory tumors, such as radio resistant and immunosuppressive types, can also be greatly improved by ferroptosis inducers.
Finally, in order to realize the full potential of ferroptosis in tumor treatment strategies, there are some additional issues that need to be addressed urgently in future studies. Thorough histological and pharmacological analysis must be performed to confirm whether ferroptosis inducers have potentially toxic effects on normal tissues. In addition, it is critical to discover predictive biomarkers that can predict tumor response to ferroptosis‐inducing therapy, especially those that can be detected directly in patient fluids and biopsy specimens. These biomarkers help stratify tumor patients for further antitumor treatment with ferroptosis‐inducing therapy. In conclusion, both further exploration of the mechanism of ferroptosis and the development of new drugs are expected to provide more help for antitumor therapy.
AUTHOR CONTRIBUTIONS
Chuandong Gong: Writing – original draft (lead). Qiankun Ji: Writing – original draft (equal). Miaojing Wu: Writing – original draft (equal). Zewei Tu: Writing – review and editing (supporting). Kunjian Lei: Writing – review and editing (supporting). Min Luo: Writing – review and editing (supporting). Junzhe Liu: Writing – review and editing (supporting). Li Lin: Writing – review and editing (supporting). Kuangxun Li: Writing – review and editing (supporting). Jingying Li: Project administration (equal). Kai Huang: Project administration (equal). Xingen Zhu: Project administration (lead).
FUNDING INFORMATION
This research was funded by the National Natural Science Foundation (grant nos. 82002660, 81960456, 81760445, and 81760446), the Key Research and Development projects in Jiangxi (grant nos. 20212BBG73021), Jiangxi Training Program for academic and technical leaders of major disciplines – Young talents program (grant no. 20212BCJ23023), and Jiangxi Province Department of Education Science and technology research project (grant no. GJJ210177).
CONFLICT OF INTEREST
No competing interests exist.
Gong C, Ji Q, Wu M, et al. Ferroptosis in tumor immunity and therapy. J Cell Mol Med. 2022;26:5565‐5579. doi: 10.1111/jcmm.17529
Chuandong Gong, Qiankun Ji, and Miaojing Wu contribute equally to this work.
Contributor Information
Jingying Li, Email: jingyingli@ncu.edu.cn.
Kai Huang, Email: kaihuang@ncu.edu.cn.
Xingen Zhu, Email: ndefy89006@ncu.edu.cn.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Xie Y, Hou W, Song X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369‐379. doi: 10.1038/cdd.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273‐285. doi: 10.1016/j.cell.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron‐dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060‐1072. doi: 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510(2):278‐283. doi: 10.1016/j.bbrc.2019.01.090 [DOI] [PubMed] [Google Scholar]
- 5. Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347‐364. doi: 10.1038/s41422-019-0164-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263‐274. doi: 10.1038/nrc1586 [DOI] [PubMed] [Google Scholar]
- 7. Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175(2):313‐326. doi: 10.1016/j.cell.2018.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zou W, Wolchok JD, Chen L. PD‐L1 (B7‐H1) and PD‐1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. doi: 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med. 2015;212(4):555‐568. doi: 10.1084/jem.20140857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ito F, Yanatori I, Maeda Y, et al. Asbestos conceives Fe(II)‐dependent mutagenic stromal milieu through ceaseless macrophage ferroptosis and beta‐catenin induction in mesothelium. Redox Biol. 2020;36:101616. doi: 10.1016/j.redox.2020.101616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muri J, Thut H, Bornkamm GW, Kopf M. B1 and marginal zone B cells but not follicular B2 cells require Gpx4 to prevent lipid peroxidation and ferroptosis. Cell Rep. 2019;29(9):2731‐2744.e4. doi: 10.1016/j.celrep.2019.10.070 [DOI] [PubMed] [Google Scholar]
- 12. Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274(17):11455‐11458. doi: 10.1074/jbc.274.17.11455 [DOI] [PubMed] [Google Scholar]
- 13. Liang C, Zhang X, Yang M, Dong X. Recent progress in ferroptosis inducers for cancer therapy. Adv Mater. 2019;31(51):e1904197. doi: 10.1002/adma.201904197 [DOI] [PubMed] [Google Scholar]
- 14. Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122(3168):501‐514. doi: 10.1126/science.122.3168.501 [DOI] [PubMed] [Google Scholar]
- 15. Seiler A, Schneider M, Forster H, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15‐lipoxygenase dependent‐ and AIF‐mediated cell death. Cell Metab. 2008;8(3):237‐248. doi: 10.1016/j.cmet.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 16. Shah R, Shchepinov MS, Pratt DA. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent Sci. 2018;4(3):387‐396. doi: 10.1021/acscentsci.7b00589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1‐2):317‐331. doi: 10.1016/j.cell.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180‐1191. doi: 10.1038/ncb3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu H, Schreiber SL, Stockwell BR. Targeting dependency on the GPX4 lipid peroxide repair pathway for cancer therapy. Biochemistry. 2018;57(14):2059‐2060. doi: 10.1021/acs.biochem.8b00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nomura K, Imai H, Koumura T, Kobayashi T, Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia‐induced apoptosis. Biochem J. 2000;351(Pt 1):183‐193. doi: 10.1042/0264-6021:3510183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Canli O, Alankus YB, Grootjans S, et al. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood. 2016;127(1):139‐148. doi: 10.1182/blood-2015-06-654194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang R, Zeng L, Zhu S, et al. Lipid peroxidation drives gasdermin D‐mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe. 2018;24(1):97‐108.e4. doi: 10.1016/j.chom.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muller T, Dewitz C, Schmitz J, et al. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci. 2017;74(19):3631‐3645. doi: 10.1007/s00018-017-2547-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59(2):298‐308. doi: 10.1016/j.molcel.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10(1):9‐17. doi: 10.1038/nchembio.1416 [DOI] [PubMed] [Google Scholar]
- 26. Petrat F, de Groot H, Rauen U. Subcellular distribution of chelatable iron: a laser scanning microscopic study in isolated hepatocytes and liver endothelial cells. Biochem J. 2001;356(Pt 1):61‐69. doi: 10.1042/0264-6021:3560061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng Z, Li Y. What is responsible for the initiating chemistry of iron‐mediated lipid peroxidation: an update. Chem Rev. 2007;107(3):748‐766. doi: 10.1021/cr040077w [DOI] [PubMed] [Google Scholar]
- 28. Lachaier E, Louandre C, Ezzoukhry Z, et al. Ferroptosis, a new form of cell death relevant to the medical treatment of cancer. Med Sci. 2014;30(8‐9):779‐783. doi: 10.1051/medsci/20143008016 [DOI] [PubMed] [Google Scholar]
- 29. Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron‐dependent, nonapoptotic cell death in oncogenic‐RAS‐harboring cancer cells. Chem Biol. 2008;15(3):234‐245. doi: 10.1016/j.chembiol.2008.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35(6):830‐849. doi: 10.1016/j.ccell.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 31. Lei P, Bai T, Sun Y. Mechanisms of ferroptosis and relations with regulated cell death: a review. Front Physiol. 2019;10:139. doi: 10.3389/fphys.2019.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shin D, Kim EH, Lee J, Roh JL. Nrf2 inhibition reverses resistance to GPX4 inhibitor‐induced ferroptosis in head and neck cancer. Free Radic Biol Med. 2018;129:454‐462. doi: 10.1016/j.freeradbiomed.2018.10.426 [DOI] [PubMed] [Google Scholar]
- 33. Skouta R, Dixon SJ, Wang J, et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136(12):4551‐4556. doi: 10.1021/ja411006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Efimova I, Catanzaro E, Van der Meeren L, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020;8(2):e001369. doi: 10.1136/jitc-2020-001369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang R, Xu J, Zhang B, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13(1):110. doi: 10.1186/s13045-020-00946-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Galluzzi L, Vitale I, Warren S, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8(1):e000337. doi: 10.1136/jitc-2019-000337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Medina CB, Ravichandran KS. Do not let death do us part: ‘find‐me’ signals in communication between dying cells and the phagocytes. Cell Death Differ. 2016;23(6):979‐989. doi: 10.1038/cdd.2016.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kepp O, Zitvogel L, Kroemer G. Clinical evidence that immunogenic cell death sensitizes to PD‐1/PD‐L1 blockade. Oncoimmunology. 2019;8(10):e1637188. doi: 10.1080/2162402X.2019.1637188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Y, Wang Y, Liu J, Kang R, Tang D. The circadian clock protects against ferroptosis‐induced sterile inflammation. Biochem Biophys Res Commun. 2020;525(3):620‐625. doi: 10.1016/j.bbrc.2020.02.142 [DOI] [PubMed] [Google Scholar]
- 40. Rickard JA, O'Donnell JA, Evans JM, et al. RIPK1 regulates RIPK3‐MLKL‐driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157(5):1175‐1188. doi: 10.1016/j.cell.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 41. Martin‐Sanchez D, Ruiz‐Andres O, Poveda J, et al. Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid‐Induced AKI. J Am Soc Nephrol. 2017;28(1):218‐229. doi: 10.1681/ASN.2015121376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Luo X, Gong HB, Gao HY, et al. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ. 2021;28(6):1971‐1989. doi: 10.1038/s41418-020-00719-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gardai SJ, McPhillips KA, Frasch SC, et al. Cell‐surface calreticulin initiates clearance of viable or apoptotic cells through trans‐activation of LRP on the phagocyte. Cell. 2005;123(2):321‐334. doi: 10.1016/j.cell.2005.08.032 [DOI] [PubMed] [Google Scholar]
- 44. Zhou F, Feng B, Yu H, et al. Tumor microenvironment‐activatable prodrug vesicles for nanoenabled cancer chemoimmunotherapy combining immunogenic cell death induction and CD47 blockade. Adv Mater. 2019;31(14):e1805888. doi: 10.1002/adma.201805888 [DOI] [PubMed] [Google Scholar]
- 45. Yue W, Chen L, Yu L, et al. Checkpoint blockade and nanosonosensitizer‐augmented noninvasive sonodynamic therapy combination reduces tumour growth and metastases in mice. Nat Commun. 2019;10(1):2025. doi: 10.1038/s41467-019-09760-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Y, Liu L, Jin L, et al. Oxidative stress‐induced calreticulin expression and translocation: new insights into the destruction of melanocytes. J Invest Dermatol. 2014;134(1):183‐191. doi: 10.1038/jid.2013.268 [DOI] [PubMed] [Google Scholar]
- 47. Yu B, Choi B, Li W, Kim DH. Magnetic field boosted ferroptosis‐like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat Commun. 2020;11(1):3637. doi: 10.1038/s41467-020-17380-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johnson S, Michalak M, Opas M, Eggleton P. The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol. 2001;11(3):122‐129. doi: 10.1016/s0962-8924(01)01926-2 [DOI] [PubMed] [Google Scholar]
- 49. Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi‐process calcium‐buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417(3):651‐666. doi: 10.1042/BJ20081847 [DOI] [PubMed] [Google Scholar]
- 50. Turubanova VD, Balalaeva IV, Mishchenko TA, et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J Immunother Cancer. 2019;7(1):350. doi: 10.1186/s40425-019-0826-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tang D, Kepp O, Kroemer G. Ferroptosis becomes immunogenic: implications for anticancer treatments. Oncoimmunology. 2020;10(1):1862949. doi: 10.1080/2162402X.2020.1862949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Elliott MR, Ravichandran KS. The dynamics of apoptotic cell clearance. Dev Cell. 2016;38(2):147‐160. doi: 10.1016/j.devcel.2016.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kloditz K, Fadeel B. Three cell deaths and a funeral: macrophage clearance of cells undergoing distinct modes of cell death. Cell Death Discov. 2019;5:65. doi: 10.1038/s41420-019-0146-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li C, Deng X, Zhang W, et al. Novel allosteric activators for ferroptosis regulator glutathione peroxidase 4. J Med Chem. 2019;62(1):266‐275. doi: 10.1021/acs.jmedchem.8b00315 [DOI] [PubMed] [Google Scholar]
- 55. Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181‐193. doi: 10.1038/nrc2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morgan AH, Dioszeghy V, Maskrey BH, et al. Phosphatidylethanolamine‐esterified eicosanoids in the mouse: tissue localization and inflammation‐dependent formation in Th‐2 disease. J Biol Chem. 2009;284(32):21185‐21191. doi: 10.1074/jbc.M109.021634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. D'Herde K, Krysko DV. Ferroptosis: oxidized PEs trigger death. Nat Chem Biol. 2017;13(1):4‐5. doi: 10.1038/nchembio.2261 [DOI] [PubMed] [Google Scholar]
- 58. Lauber K, Bohn E, Krober SM, et al. Apoptotic cells induce migration of phagocytes via caspase‐3‐mediated release of a lipid attraction signal. Cell. 2003;113(6):717‐730. doi: 10.1016/s0092-8674(03)00422-7 [DOI] [PubMed] [Google Scholar]
- 59. Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer‐acquired drug resistance and immune evasion. Nat Rev Cancer. 2019;19(7):405‐414. doi: 10.1038/s41568-019-0149-1 [DOI] [PubMed] [Google Scholar]
- 60. Dai E, Han L, Liu J, et al. Autophagy‐dependent ferroptosis drives tumor‐associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16(11):2069‐2083. doi: 10.1080/15548627.2020.1714209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dai E, Han L, Liu J, et al. Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING‐dependent DNA sensor pathway. Nat Commun. 2020;11(1):6339. doi: 10.1038/s41467-020-20154-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Casemore DP. Tuberculosis in south Cleveland 1968‐‐74. A laboratory based survey. Public Health. 1978;92(6):264‐271. doi: 10.1016/s0033-3506(78)80078-x [DOI] [PubMed] [Google Scholar]
- 63. Rothe T, Gruber F, Uderhardt S, et al. 12/15‐Lipoxygenase‐mediated enzymatic lipid oxidation regulates DC maturation and function. J Clin Invest. 2015;125(5):1944‐1954. doi: 10.1172/JCI78490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Veglia F, Tyurin VA, Mohammadyani D, et al. Lipid bodies containing oxidatively truncated lipids block antigen cross‐presentation by dendritic cells in cancer. Nat Commun. 2017;8(1):2122. doi: 10.1038/s41467-017-02186-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Polonelli L, Dettori G, Morace G, Rosa R, Castagnola M, Schipper MA. Antigenic studies on Rhizopus microsporus, Rh. rhizopodiformis, progeny and intermediates (Rh. chinensis). Antonie Van Leeuwenhoek. 1988;54(1):5‐17. doi: 10.1007/BF00393954 [DOI] [PubMed] [Google Scholar]
- 66. Veglia F, Tyurin VA, Blasi M, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569(7754):73‐78. doi: 10.1038/s41586-019-1118-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188(1):21‐28. doi: 10.4049/jimmunol.1101029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zelenay S, van der Veen AG, Bottcher JP, et al. Cyclooxygenase‐dependent tumor growth through evasion of immunity. Cell. 2015;162(6):1257‐1270. doi: 10.1016/j.cell.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bottcher JP, Bonavita E, Chakravarty P, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172(5):1022‐1037.e14. doi: 10.1016/j.cell.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang D, DuBois RN. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis. 2015;36(10):1085‐1093. doi: 10.1093/carcin/bgv123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kurtova AV, Xiao J, Mo Q, et al. Blocking PGE2‐induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517(7533):209‐213. doi: 10.1038/nature14034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Uderhardt S, Herrmann M, Oskolkova OV, et al. 12/15‐lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36(5):834‐846. doi: 10.1016/j.immuni.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 73. Bluml S, Kirchberger S, Bochkov VN, et al. Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40. J Immunol. 2005;175(1):501‐508. doi: 10.4049/jimmunol.175.1.501 [DOI] [PubMed] [Google Scholar]
- 74. CD36 activity causes ferroptosis in tumor‐infiltrating CD8(+) T cells. Cancer Discov. 2021;11(5):OF24. doi: 10.1158/2159-8290.CD-RW2021-039 [DOI] [PubMed] [Google Scholar]
- 75. Ma X, Xiao L, Liu L, et al. CD36‐mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33(5):1001‐1012.e5. doi: 10.1016/j.cmet.2021.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Drijvers JM, Gillis JE, Muijlwijk T, et al. Pharmacologic screening identifies metabolic vulnerabilities of CD8(+) T cells. Cancer Immunol Res. 2021;9(2):184‐199. doi: 10.1158/2326-6066.CIR-20-0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Veglia F, Sanseviero E, Gabrilovich DI. Myeloid‐derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21(8):485‐498. doi: 10.1038/s41577-020-00490-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhu H, Klement JD, Lu C, et al. Asah2 represses the p53‐Hmox1 axis to protect myeloid‐derived suppressor cells from ferroptosis. J Immunol. 2021;206(6):1395‐1404. doi: 10.4049/jimmunol.2000500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Youssef LA, Rebbaa A, Pampou S, et al. Increased erythrophagocytosis induces ferroptosis in red pulp macrophages in a mouse model of transfusion. Blood. 2018;131(23):2581‐2593. doi: 10.1182/blood-2017-12-822619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang X, Xing X, Liu H, et al. Ionizing radiation induces ferroptosis in granulocyte‐macrophage hematopoietic progenitor cells of murine bone marrow. Int J Radiat Biol. 2020;96(5):584‐595. doi: 10.1080/09553002.2020.1708993 [DOI] [PubMed] [Google Scholar]
- 81. Wang H, An P, Xie E, et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017;66(2):449‐465. doi: 10.1002/hep.29117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kapralov AA, Yang Q, Dar HH, et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol. 2020;16(3):278‐290. doi: 10.1038/s41589-019-0462-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pommier Y, Sordet O, Antony S, Hayward RL, Kohn KW. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene. 2004;23(16):2934‐2949. doi: 10.1038/sj.onc.1207515 [DOI] [PubMed] [Google Scholar]
- 84. Liu L, Yang M, Kang R, et al. HMGB1‐induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia. 2011;25(1):23‐31. doi: 10.1038/leu.2010.225 [DOI] [PubMed] [Google Scholar]
- 85. Sui X, Chen R, Wang Z, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838. doi: 10.1038/cddis.2013.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hangauer MJ, Viswanathan VS, Ryan MJ, et al. Drug‐tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551(7679):247‐250. doi: 10.1038/nature24297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tsoi J, Robert L, Paraiso K, et al. Multi‐stage differentiation defines melanoma subtypes with differential vulnerability to drug‐induced iron‐dependent oxidative stress. Cancer Cell. 2018;33(5):890‐904.e5. doi: 10.1016/j.ccell.2018.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lin CC, Yang WH, Lin YT, et al. DDR2 upregulation confers ferroptosis susceptibility of recurrent breast tumors through the Hippo pathway. Oncogene. 2021;40(11):2018‐2034. doi: 10.1038/s41388-021-01676-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lee JY, Nam M, Son HY, et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci U S A. 2020;117(51):32433‐32442. doi: 10.1073/pnas.2006828117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Muller S, Sindikubwabo F, Caneque T, et al. CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nat Chem. 2020;12(10):929‐938. doi: 10.1038/s41557-020-0513-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sun X, Ou Z, Chen R, et al. Activation of the p62‐Keap1‐NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173‐184. doi: 10.1002/hep.28251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Eling N, Reuter L, Hazin J, Hamacher‐Brady A, Brady NR. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2(5):517‐532. doi: 10.18632/oncoscience.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lin R, Zhang Z, Chen L, et al. Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett. 2016;381(1):165‐175. doi: 10.1016/j.canlet.2016.07.033 [DOI] [PubMed] [Google Scholar]
- 94. Dixon SJ, Patel DN, Welsch M, et al. Pharmacological inhibition of cystine‐glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 2014;3:e02523. doi: 10.7554/eLife.02523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ooko E, Saeed ME, Kadioglu O, et al. Artemisinin derivatives induce iron‐dependent cell death (ferroptosis) in tumor cells. Phytomedicine. 2015;22(11):1045‐1054. doi: 10.1016/j.phymed.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 96. Shimada K, Skouta R, Kaplan A, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12(7):497‐503. doi: 10.1038/nchembio.2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113(34):E4966‐E4975. doi: 10.1073/pnas.1603244113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yant LJ, Ran Q, Rao L, et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34(4):496‐502. doi: 10.1016/s0891-5849(02)01360-6 [DOI] [PubMed] [Google Scholar]
- 99. Louandre C, Marcq I, Bouhlal H, et al. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett. 2015;356(2 Pt B):971‐977. doi: 10.1016/j.canlet.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 100. Koike N, Kota R, Naito Y, et al. 2‐Nitroimidazoles induce mitochondrial stress and ferroptosis in glioma stem cells residing in a hypoxic niche. Commun Biol. 2020;3(1):450. doi: 10.1038/s42003-020-01165-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jin M, Shi C, Li T, Wu Y, Hu C, Huang G. Solasonine promotes ferroptosis of hepatoma carcinoma cells via glutathione peroxidase 4‐induced destruction of the glutathione redox system. Biomed Pharmacother. 2020;129:110282. doi: 10.1016/j.biopha.2020.110282 [DOI] [PubMed] [Google Scholar]
- 102. Kroll MH, Jiji V, Jiji R. Microcytic hypochromic anemia associated with renal cell carcinoma. South Med J. 1984;77(5):635‐637. doi: 10.1097/00007611-198405000-00024 [DOI] [PubMed] [Google Scholar]
- 103. Sato H, Shiiya A, Kimata M, et al. Redox imbalance in cystine/glutamate transporter‐deficient mice. J Biol Chem. 2005;280(45):37423‐37429. doi: 10.1074/jbc.M506439200 [DOI] [PubMed] [Google Scholar]
- 104. Hou W, Xie Y, Song X, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425‐1428. doi: 10.1080/15548627.2016.1187366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hayano M, Yang WS, Corn CK, Pagano NC, Stockwell BR. Loss of cysteinyl‐tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23(2):270‐278. doi: 10.1038/cdd.2015.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cao J, Chen X, Jiang L, et al. DJ‐1 suppresses ferroptosis through preserving the activity of S‐adenosyl homocysteine hydrolase. Nat Commun. 2020;11(1):1251. doi: 10.1038/s41467-020-15109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhu J, Berisa M, Schworer S, Qin W, Cross JR, Thompson CB. Transsulfuration activity can support cell growth upon extracellular cysteine limitation. Cell Metab. 2019;30(5):865‐876.e5. doi: 10.1016/j.cmet.2019.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang L, Cai H, Hu Y, et al. A pharmacological probe identifies cystathionine beta‐synthase as a new negative regulator for ferroptosis. Cell Death Dis. 2018;9(10):1005. doi: 10.1038/s41419-018-1063-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Song X, Liu J, Kuang F, et al. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep. 2021;34(8):108767. doi: 10.1016/j.celrep.2021.108767 [DOI] [PubMed] [Google Scholar]
- 110. Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K‐AKT‐mTOR signaling suppresses ferroptosis via SREBP‐mediated lipogenesis. Proc Natl Acad Sci U S A. 2020;117(49):31189‐31197. doi: 10.1073/pnas.2017152117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Belavgeni A, Bornstein SR, von Massenhausen A, et al. Exquisite sensitivity of adrenocortical carcinomas to induction of ferroptosis. Proc Natl Acad Sci U S A. 2019;116(44):22269‐22274. doi: 10.1073/pnas.1912700116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang S, Chang W, Wu H, et al. Pan‐cancer analysis of iron metabolic landscape across the Cancer Genome Atlas. J Cell Physiol. 2020;235(2):1013‐1024. doi: 10.1002/jcp.29017 [DOI] [PubMed] [Google Scholar]
- 113. Vu NT, Kim M, Stephenson DJ, MacKnight HP, Chalfant CE. Ceramide kinase inhibition drives ferroptosis and sensitivity to cisplatin in mutant KRAS lung cancer by dysregulating VDAC‐mediated mitochondria function. Mol Cancer Res. 2022. doi: 10.1158/1541-7786.MCR-22-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang Y, Qiu S, Wang H, et al. Transcriptional Repression of Ferritin Light Chain Increases Ferroptosis Sensitivity in Lung Adenocarcinoma. Front Cell Dev Biol. 2021;9:719187. doi: 10.3389/fcell.2021.719187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhang X, Yu K, Ma L, et al. Endogenous glutamate determines ferroptosis sensitivity via ADCY10‐dependent YAP suppression in lung adenocarcinoma. Theranostics. 2021;11(12):5650‐5674. doi: 10.7150/thno.55482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wu H, Liu A. Long non‐coding RNA NEAT1 regulates ferroptosis sensitivity in non‐small‐cell lung cancer. J Int Med Res. 2021;49(3):300060521996183. doi: 10.1177/0300060521996183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yang C, Zhang Y, Lin S, Liu Y, Li W. Correction for: Suppressing the KIF20A/NUAK1/Nrf2/GPX4 signaling pathway induces ferroptosis and enhances the sensitivity of colorectal cancer to oxaliplatin. Aging. 2021;13(14):19077. doi: 10.18632/aging.203382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yang C, Zhang Y, Lin S, Liu Y, Li W. Suppressing the KIF20A/NUAK1/Nrf2/GPX4 signaling pathway induces ferroptosis and enhances the sensitivity of colorectal cancer to oxaliplatin. Aging. 2021;13(10):13515‐13534. doi: 10.18632/aging.202774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ye S, Xu M, Zhu T, et al. Cytoglobin promotes sensitivity to ferroptosis by regulating p53‐YAP1 axis in colon cancer cells. J Cell Mol Med. 2021;25(7):3300‐3311. doi: 10.1111/jcmm.16400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang R, Su Q, Yin H, Wu D, Lv C, Yan Z. Inhibition of SRSF9 enhances the sensitivity of colorectal cancer to erastin‐induced ferroptosis by reducing glutathione peroxidase 4 expression. Int J Biochem Cell Biol. 2021;134:105948. doi: 10.1016/j.biocel.2021.105948 [DOI] [PubMed] [Google Scholar]
- 121. Cheng Q, Bao L, Li M, Chang K, Yi X. Erastin synergizes with cisplatin via ferroptosis to inhibit ovarian cancer growth in vitro and in vivo. J Obstet Gynaecol Res. 2021;47(7):2481‐2491. doi: 10.1111/jog.14779 [DOI] [PubMed] [Google Scholar]
- 122. Guo J, Xu B, Han Q, et al. Ferroptosis: a novel anti‐tumor action for cisplatin. Cancer Res Treat. 2018;50(2):445‐460. doi: 10.4143/crt.2016.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhang X, Sui S, Wang L, et al. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. J Cell Physiol. 2020;235(4):3425‐3437. doi: 10.1002/jcp.29232 [DOI] [PubMed] [Google Scholar]
- 124. Daher B, Parks SK, Durivault J, et al. Genetic ablation of the cystine transporter xCT in PDAC cells inhibits mTORC1, growth, survival, and tumor formation via nutrient and oxidative stresses. Cancer Res. 2019;79(15):3877‐3890. doi: 10.1158/0008-5472.CAN-18-3855 [DOI] [PubMed] [Google Scholar]
- 125. Sato M, Kusumi R, Hamashima S, et al. The ferroptosis inducer erastin irreversibly inhibits system xc‐ and synergizes with cisplatin to increase cisplatin's cytotoxicity in cancer cells. Sci Rep. 2018;8(1):968. doi: 10.1038/s41598-018-19213-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yamaguchi H, Hsu JL, Chen CT, et al. Caspase‐independent cell death is involved in the negative effect of EGF receptor inhibitors on cisplatin in non‐small cell lung cancer cells. Clin Cancer Res. 2013;19(4):845‐854. doi: 10.1158/1078-0432.CCR-12-2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chen GQ, Benthani FA, Wu J, Liang D, Bian ZX, Jiang X. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 2020;27(1):242‐254. doi: 10.1038/s41418-019-0352-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Du J, Wang T, Li Y, et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic Biol Med. 2019;131:356‐369. doi: 10.1016/j.freeradbiomed.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 129. Yang ND, Tan SH, Ng S, et al. Artesunate induces cell death in human cancer cells via enhancing lysosomal function and lysosomal degradation of ferritin. J Biol Chem. 2014;289(48):33425‐33441. doi: 10.1074/jbc.M114.564567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Torii S, Shintoku R, Kubota C, et al. An essential role for functional lysosomes in ferroptosis of cancer cells. Biochem J. 2016;473(6):769‐777. doi: 10.1042/BJ20150658 [DOI] [PubMed] [Google Scholar]
- 131. Zhu S, Zhang Q, Sun X, et al. HSPA5 regulates ferroptotic cell death in cancer cells. Cancer Res. 2017;77(8):2064‐2077. doi: 10.1158/0008-5472.CAN-16-1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chen L, Li X, Liu L, Yu B, Xue Y, Liu Y. Erastin sensitizes glioblastoma cells to temozolomide by restraining xCT and cystathionine‐gamma‐lyase function. Oncol Rep. 2015;33(3):1465‐1474. doi: 10.3892/or.2015.3712 [DOI] [PubMed] [Google Scholar]
- 133. Sehm T, Rauh M, Wiendieck K, Buchfelder M, Eyupoglu IY, Savaskan NE. Temozolomide toxicity operates in a xCT/SLC7a11 dependent manner and is fostered by ferroptosis. Oncotarget. 2016;7(46):74630‐74647. doi: 10.18632/oncotarget.11858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin‐resistant head and neck cancer cells to artesunate‐induced ferroptosis. Redox Biol. 2017;11:254‐262. doi: 10.1016/j.redox.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lv C, Qu H, Zhu W, et al. Low‐dose paclitaxel inhibits tumor cell growth by regulating glutaminolysis in colorectal carcinoma cells. Front Pharmacol. 2017;8:244. doi: 10.3389/fphar.2017.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Giannakakou P, Robey R, Fojo T, Blagosklonny MV. Low concentrations of paclitaxel induce cell type‐dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: molecular determinants of paclitaxel‐induced cytotoxicity. Oncogene. 2001;20(29):3806‐3813. doi: 10.1038/sj.onc.1204487 [DOI] [PubMed] [Google Scholar]
- 137. Ye J, Jiang X, Dong Z, Hu S, Xiao M. Low‐concentration PTX and RSL3 inhibits tumor cell growth synergistically by inducing ferroptosis in mutant p53 hypopharyngeal squamous carcinoma. Cancer Manag Res. 2019;11:9783‐9792. doi: 10.2147/CMAR.S217944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Dias Lopes NM, Marinello PC, Sanches LJ, et al. Patterns of cell death induced by metformin in human MCF‐7 breast cancer cells. Pathol Res Pract. 2020;216(11):153199. doi: 10.1016/j.prp.2020.153199 [DOI] [PubMed] [Google Scholar]
- 139. Chan DW, Yung MM, Chan YS, et al. MAP30 protein from Momordica charantia is therapeutic and has synergic activity with cisplatin against ovarian cancer in vivo by altering metabolism and inducing ferroptosis. Pharmacol Res. 2020;161:105157. doi: 10.1016/j.phrs.2020.105157 [DOI] [PubMed] [Google Scholar]
- 140. Yu Y, Xie Y, Cao L, et al. The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol Cell Oncol. 2015;2(4):e1054549. doi: 10.1080/23723556.2015.1054549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yagoda N, von Rechenberg M, Zaganjor E, et al. RAS‐RAF‐MEK‐dependent oxidative cell death involving voltage‐dependent anion channels. Nature. 2007;447(7146):864‐868. doi: 10.1038/nature05859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhong Y, Tian F, Ma H, et al. FTY720 induces ferroptosis and autophagy via PP2A/AMPK pathway in multiple myeloma cells. Life Sci. 2020;260:118077. doi: 10.1016/j.lfs.2020.118077 [DOI] [PubMed] [Google Scholar]
- 143. Baidoo KE, Yong K, Brechbiel MW. Molecular pathways: targeted alpha‐particle radiation therapy. Clin Cancer Res. 2013;19(3):530‐537. doi: 10.1158/1078-0432.CCR-12-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ashwood‐Smith MJ. The effect of whole‐body x‐irradiation on the glutathione content of rat thymus. Int J Radiat Biol Relat Stud Phys Chem Med. 1961;3:125‐132. doi: 10.1080/09553006114550141 [DOI] [PubMed] [Google Scholar]
- 145. Azzam EI, Jay‐Gerin JP, Pain D. Ionizing radiation‐induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327(1‐2):48‐60. doi: 10.1016/j.canlet.2011.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Fierro S, Yoshikawa M, Nagano O, Yoshimi K, Saya H, Einaga Y. In vivo assessment of cancerous tumors using boron doped diamond microelectrode. Sci Rep. 2012;2:901. doi: 10.1038/srep00901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bump EA, Yu NY, Brown JM. Radiosensitization of hypoxic tumor cells by depletion of intracellular glutathione. Science. 1982;217(4559):544‐545. doi: 10.1126/science.7089580 [DOI] [PubMed] [Google Scholar]
- 148. Allalunis‐Turner MJ, Day RS 3rd, McKean JD, et al. Glutathione levels and chemosensitizing effects of buthionine sulfoximine in human malignant glioma cells. J Neurooncol. 1991;11(2):157‐164. doi: 10.1007/BF02390175 [DOI] [PubMed] [Google Scholar]
- 149. Sleire L, Skeie BS, Netland IA, et al. Drug repurposing: sulfasalazine sensitizes gliomas to gamma knife radiosurgery by blocking cystine uptake through system Xc‐, leading to glutathione depletion. Oncogene. 2015;34(49):5951‐5959. doi: 10.1038/onc.2015.60 [DOI] [PubMed] [Google Scholar]
- 150. Lei G, Zhang Y, Koppula P, et al. The role of ferroptosis in ionizing radiation‐induced cell death and tumor suppression. Cell Res. 2020;30(2):146‐162. doi: 10.1038/s41422-019-0263-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lang X, Green MD, Wang W, et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9(12):1673‐1685. doi: 10.1158/2159-8290.CD-19-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Xu Y, Wang Q, Li X, Chen Y, Xu G. Itraconazole attenuates the stemness of nasopharyngeal carcinoma cells via triggering ferroptosis. Environ Toxicol. 2021;36(2):257‐266. doi: 10.1002/tox.23031 [DOI] [PubMed] [Google Scholar]
- 153. Wang W, Green M, Choi JE, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270‐274. doi: 10.1038/s41586-019-1170-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wan C, Sun Y, Tian Y, et al. Irradiated tumor cell‐derived microparticles mediate tumor eradication via cell killing and immune reprogramming. Sci Adv. 2020;6(13):eaay9789. doi: 10.1126/sciadv.aay9789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zhang F, Li F, Lu GH, et al. Engineering magnetosomes for ferroptosis/immunomodulation synergism in cancer. ACS Nano. 2019;13(5):5662‐5673. doi: 10.1021/acsnano.9b00892 [DOI] [PubMed] [Google Scholar]
- 156. Wei G, Sun J, Luan W, et al. Natural product albiziabioside A conjugated with pyruvate dehydrogenase kinase inhibitor dichloroacetate to induce apoptosis‐ferroptosis‐M2‐TAMs polarization for combined cancer therapy. J Med Chem. 2019;62(19):8760‐8772. doi: 10.1021/acs.jmedchem.9b00644 [DOI] [PubMed] [Google Scholar]
- 157. Sindrilaru A, Peters T, Wieschalka S, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121(3):985‐997. doi: 10.1172/JCI44490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Xu T, Ma Y, Yuan Q, et al. Enhanced ferroptosis by oxygen‐boosted phototherapy based on a 2‐in‐1 nanoplatform of ferrous hemoglobin for tumor synergistic therapy. ACS Nano. 2020;14(3):3414‐3425. doi: 10.1021/acsnano.9b09426 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


