Abstract

Luminescence-based techniques play an increasingly important role in all areas of biochemical research, including investigations on G protein-coupled receptors (GPCRs). One quite recent and popular addition has been made by introducing bioluminescence resonance energy transfer (BRET)-based binding assays for GPCRs, which are based on the fusion of nanoluciferase (Nluc) to the N-terminus of the receptor and the occurring energy transfer via BRET to a bound fluorescent ligand. However, being based on BRET, the technique is strongly dependent on the distance/orientation between the luciferase and the fluorescent ligand. Here we describe an alternative strategy to establish BRET-based binding assays for GPCRs, where the N-terminal fusion of Nluc did not result in functioning test systems with our fluorescent ligands (e.g., for the neuropeptide Y Y1 receptor (Y1R) and the neurotensin receptor type 1 (NTS1R)). Instead, we introduced Nluc into their second extracellular loop and we obtained binding data for the fluorescent ligands and reported standard ligands (in saturation and competition binding experiments, respectively) comparable to data from the literature. The strategy was transferred to the angiotensin II receptor type 1 (AT1R) and the M1 muscarinic acetylcholine receptor (M1R), which led to affinity estimates comparable to data from radioligand binding experiments. Additionally, an analysis of the binding kinetics of all fluorescent ligands at their respective target was performed using the newly described receptor/Nluc-constructs.
Keywords: G protein-coupled receptor, bioluminescence resonance energy transfer, ligand binding, assay development, binding kinetics
G protein-coupled receptors (GPCRs) represent one of the largest protein families with more than 800 members encoded in the human genome.1 All GPCRs share a common architecture, as they all comprise an extracellular N-terminus, an intracellular C-terminus, and seven transmembrane domains, which are connected by three extracellular (ECL1–3) and three intracellular loops (ICL1–3).2,3 Due to their abundant expression in humans and their involvement in various (patho)physiological processes, GPCRs represent a very attractive target family in therapy and drug discovery.4,5 A mandatory step in the development of novel drug candidates is the assessment of their binding properties to their putative target. In addition to the determination of affinities, investigations on the kinetics of ligand binding are of particular interest.6−8 In the last few decades, fluorescence-based techniques emerged as alternative or complementary methods to the widely used radioligand binding assays, as they offer distinct advantages, e.g., in terms of handling, safety, and costs of waste disposal.9−11 Especially, proximity-based methods exploiting bioluminescence resonance energy transfer (BRET) or (time-resolved) Förster resonance energy transfer ((TR-)FRET) have gained popularity because of their high-throughput capability, the lower impact of nonspecific binding, and the possibility of performing kinetic measurements in real time without any separation or washing steps.12−14 Stoddart et al. introduced a procedure to quantify binding of a fluorescent ligand based on BRET by fusing the very brightly blue light-emitting nanoluciferase (Nluc, λmax ≈ 460 nm)15 to the N-terminus of a GPCR to serve as a bioluminescent donor.16 Prerequisites for this technique to give robust results are an overlap of the excitation spectrum of the fluorescent ligand (acceptor) with the emission spectrum of the luciferase (donor), an appropriate distance (≈< 10 nm) between donor and acceptor and their correct orientation toward each other.13,17 However, although this technique has been used successfully for the determination of binding affinities and binding kinetics at several GPCRs across different classes,18−23 we noticed that it was not universally applicable for all ligand–receptor combinations we wanted to assess. Therefore, we aimed at the development of an alternative strategy for those receptor–ligand pairs, for which the N-terminal fusion of Nluc did not result in functioning BRET binding assays.16 The neuropeptide Y Y1 receptor (Y1R) was first taken as a model receptor due to the lack of a specific BRET signal using an N-terminally Nluc-tagged Y1R in combination with our fluorescent ligand UR-CM138 (1). We examined the insertion of Nluc into unstructured regions within the ECL2 and ECL3 of the Y1R, and the former ultimately enabled BRET binding experiments at this receptor. This approach, i.e., insertion of Nluc into the ECL2, was then transferred to the neurotensin receptor type 1 (NTS1R), and the applicability was also tested at two receptors with slightly shorter N-termini, the angiotensin II receptor type 1 (AT1R) and the M1 muscarinic acetylcholine receptor (M1R). Besides the determination of the affinities of fluorescently labeled and unlabeled ligands to their target, we performed a detailed analysis of the binding kinetics of the fluorescent ligands with a particular focus on the NTS1R and the AT1R.
Results and Discussion
Search for a Strategy To Establish a BRET Binding Assay at the NPY Y1R
The approach to establish a BRET binding assay described by Stoddart et al.16 was pursued for the Y1R, and Nluc was fused to its N-terminus (Nluc-Y1R(Nterm)). However, no specific signal was detectable in BRET saturation binding experiments (Figure 1B) with the high-affinity fluorescent Y1R ligand UR-CM13824 (1, see Figure 2). Membrane expression of the tagged receptor could be proven by radioligand saturation binding experiments with [3H]UR-MK29925 (see Supporting Figure S1A and Supporting Table S1). Furthermore, the retained ability of the radioligand to bind to the modified receptor with high affinity ruled out an abrogation of receptor binding upon fusion with the luciferase. At this point, we hypothesized that shortening the N-terminal domain of the Y1R might lead to a higher BRET signal and saturable binding of fluorescent ligand 1, because the efficiency of BRET is strongly dependent on the distance between donor and acceptor.13,17 As a model system to test this hypothesis, we utilized a Y1R deletion mutant described by Lindner et al.,26 lacking the first 31 amino acids. We removed these same amino acids from the N-terminus and fused Nluc to Asp32 of the Y1R via a short linker yielding the construct Nluc-Y1R(Δ1–31). Although the ability of this mutant to bind the endogenous agonist neuropeptide Y (NPY) was slightly impaired,26 it still represents the mutant that allows the greatest possible proximity between Nluc and the fluorophore. Interestingly, 1 was now able to elicit a specific and saturable signal in a BRET-based saturation binding experiment at the luciferase-tagged truncated Y1R (see Figure 1B). However, the determined equilibrium dissociation constant (pKd ± SEM (Nluc-Y1R(Δ1–31) = 8.54 ± 0.05, cf. Table 1) was found to be inconsistent with the previously obtained results from radioligand competition binding studies at the wild-type Y1R (pKi = 9.95),24 although the radioligand [3H]UR-MK299 still showed saturable binding and a high affinity to the truncated and modified receptor (see Supporting Figure S1B and Supporting Table S1).
Figure 1.
Impact of different attachment and insertion sites of Nluc at the Y1R on BRET-based binding. (A) Crystal structure of the Y1R in complex with UR-MK299 (PDB-ID: 5ZBQ).27 Receptor sites addressed by attachment or insertion of Nluc are indicated by different colors; the N-terminus was artificially extended for the illustration, as it was not completely resolved in the crystal structure. Structure visualization was performed with UCSF Chimera.28 (B) Binding isotherms from BRET saturation binding experiments with 1 at HEK293T cells stably expressing the respective Nluc-Y1R receptor constructs Nluc-Y1R(Nterm), Nluc-Y1R(Δ1–31), Nluc-Y1R(Y192), or Nluc-Y1R(Q291). Nonspecific binding was determined in the presence of BIBO3304 (500-fold excess over the respective concentration of 1). Data are shown as means ± errors of one representative experiment from a set of three to four independent experiments, each performed in triplicate. Error bars of total and nonspecific binding represent the SEM, the error bars for specific binding represent propagated errors. (C) Displacement curves from BRET competition binding experiments with 1 (c = 0.5 nM) and reported Y1R ligands at Nluc-Y1R(Y192) and Nluc-Y1R(Q291), stably expressed in HEK293T cells. Data are shown as means ± SEM of at least three independent experiments, each performed in triplicate. Abbreviation: solv.: solvent control.
Figure 2.
Structures of the investigated fluorescent ligands 1–4.
Table 1. Equilibrium Dissociation Constants (pKd values) of the Fluorescent Y1R Ligand 1 Obtained from BRET Saturation Binding Experiments.
| compound | receptor (construct) | pKd (BRET)a | N |
|---|---|---|---|
| 1 | Nluc-Y1R(Nterm) | n.a. | – |
| Nluc-Y1R(Δ1–31) | 8.54 ± 0.05 | 3 | |
| Nluc-Y1R(Y192) | 9.62 ± 0.05 | 4 | |
| Nluc-Y1R(Q291) | 9.54 ± 0.07 | 4 |
Determined by BRET saturation binding experiments at intact HEK293T cells stably expressing the respective receptor construct. Data are shown as means ± SEM of N independent experiments performed in triplicate. n.a.: not applicable.
In 2018, the structure of the human Y1R was published in complex with UR-MK299 (PDB-ID: 5ZBQ),27 which represents the parent compound of the used fluorescent ligand 1. The crystal structure showed that the diphenylacetic acid moiety, which served as the attachment point for the fluorophore in 1 (see Figure 2), is pointing toward the extracellular region of the receptor (see Figure 1A). However, the N-terminus of the Y1R is comparably long in size and, even though this region of the receptor was not fully resolved, seems to be pointing away from the ligand binding pocket. This might be an explanation why the N-terminal fusion of Nluc did not yield specific BRET (Figure 1B). The distance could apparently be reduced by truncation of the N-terminus, which resulted in a higher BRET signal, but compromised the binding of 1 to the receptor.
Making use of the structure of the Y1R, we pursued a different strategy: to position the luciferase in a more favorable orientation toward the fluorescent ligand, we inserted Nluc into unstructured regions within the second (ECL2) or third extracellular loop (ECL3) of the receptor right after Tyr192 (Nluc-Y1R(Y192)) or Gln291 (Nluc-Y1R(Q291)), respectively. In BRET saturation binding experiments, 1 showed saturable binding at both Nluc-Y1R(Y192) and Nluc-Y1R(Q291) (see Figure 1B). However, in contrast to the construct Nluc-Y1R(Δ1–31), the equilibrium dissociation constants for 1 (pKd ± SEM (Nluc-Y1R(Y192)) = 9.62 ± 0.05; pKd ± SEM (Nluc-Y1R(Q291)) = 9.54 ± 0.07) were both in very good agreement with the results from radioligand competition binding experiments (pKi = 9.95).24
Interestingly, the signal-to-background ratio obtained for Nluc-Y1R(Y192) was comparatively high considering the low number of receptors per cell estimated by radioligand saturation binding experiments (≈ 2500 receptors/cell, see Supporting Figure S1C). This confirms the sensitivity of the BRET-based approach in general, which has already been shown by establishing Nluc-based BRET binding assays for receptors under endogenous promotion (e.g., adenosine A2B receptors) achieved by means of genome editing.29
Then BRET competition binding experiments were performed with 1 and different structurally diverse Y1R ligands (for structures, see Supporting Figure S2) at Nluc-Y1R(Y192) and Nluc-Y1R(Q291). The obtained affinities (pKi values) of the investigated antagonists (UR-MK299, BIBO3304, BIBP3226, BMS193885, and PD160170) were in very good agreement with data reported in the literature (cf. Table 2 for Nluc-Y1R(Y192) and Supporting Table S2 for Nluc-Y1R(Q291)). Interestingly, the agonist pNPY (porcine NPY) was not able to displace the fluorescent tracer from Nluc-Y1R(Q291) (Figure 1C, right panel). Apparently, the insertion of the luciferase into the ECL3 of the Y1R had a negative impact and disrupted the binding of pNPY. In contrast, pNPY was able to displace 1 from Nluc-Y1R(Y192) with a pKi of 7.49 ± 0.08, which is lower than most reported affinity estimates found in the literature (cf. Table 2). However, it is in very good agreement with the pKi value for pNPY determined in flow cytometry-based competition binding experiments using 1 as the fluorescent probe (pKi = 7.58), suggesting that the observed discrepancy occurs due to properties of the probe and not the used test system.24
Table 2. Binding Data (pKi Values) of Standard Y1R Ligands from BRET Competition Binding Experiments with the Fluorescent Ligand 1 at Nluc-Y1R(Y192) Compared to Data from the Literature.
| compound | pKi (BRET)a | N | reference values (literature)b |
|---|---|---|---|
| pNPY | 7.49 ± 0.08 | 5 | 7.58–9.7524,25,27,30−32 |
| UR-MK299 | 10.20 ± 0.06 | 6 | 10.1125 |
| BIBO3304 | 9.31 ± 0.11 | 5 | 8.76–9.6027,30,31 |
| BIBP3226 | 8.38 ± 0.11 | 5 | 8.14–9.0025,27,30,33,34 |
| BMS193885 | 8.23 ± 0.01 | 4 | 7.66–8.4827,35,36 |
| PD160170 | 7.45 ± 0.05 | 5 | 7.30,37 7.3238 |
Determined by BRET competition binding experiments with 1 (c = 0.5 nM, Kd = 0.24 nM) at intact HEK293T cells stably expressing Nluc-Y1R(Y192). Data are shown as means ± SEM of N independent experiments, each performed in triplicate.
Reference binding data from the literature, which were obtained from radioactivity-based or fluorescence-based competition binding experiments in different expression systems. Data originate from experiments performed in sodium-containing or sodium-free buffers. For the agonist pNPY, a wide range of pKi values has been reported depending on the expression system, the used buffer, and the tracer molecule.
Transfer of the Novel Strategy to Other GPCRs (NTS1R, AT1R, M1R)
We wanted to investigate if the presented strategy, i.e., the insertion of Nluc into the second extracellular loop of a GPCR to establish BRET binding assays, might be more widely applicable. Therefore, the approach was transferred to the NTS1R, which comprises an even longer N-terminus (67 amino acids) than the Y1R. Furthermore, the AT1R and the M1R, which both comprise shorter N-terminal domains, were investigated. Structural information was available for all three receptors,39−41 which allowed educated guessing of potential insertion sites by detecting unstructured regions within the extracellular loops and estimating the distance between the luciferase and the binding pocket (for snake plots of all generated constructs, see Supporting Figure S3). The NT(8–13) (neurotensin (8–13))-based NTS1R ligand UR-AP178 (2),42 the angiotensin II-derived AT1R ligand UR-AP177 (3), and the MR ligand UR-AP175 (4)43 were used as fluorescent ligands (see Figure 2). All tested ligands carried the fluorescence label Py-5, as it was previously shown to be ideally suited for a combination with Nluc in BRET binding assays.18
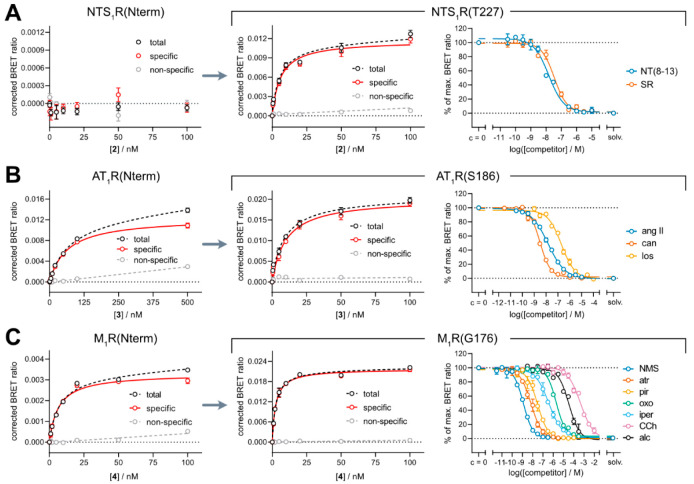
By analogy with the results for the Y1R, no specific binding of 2 was observed in BRET saturation binding experiments at the N-terminally Nluc-tagged NTS1 receptor (Nluc-NTS1R(Nterm), see Figure 3A). Again, this observation suggested that the length and orientation of the N-terminus of a given receptor with respect to the fluorescent ligand might play an important role in whether the N-terminal fusion of Nluc16 results in a functioning BRET binding assay. According to our novel strategy, Nluc was inserted into the ECL2 of the NTS1R downstream of Thr227 (Nluc-NTS1R(T227)). Membrane localization and retained binding properties of the receptor–luciferase fusion protein were confirmed by radioligand saturation binding. The radioligand [3H]UR-MK30044 bound to the modified NTS1 receptor in a saturable manner with an affinity not more than half a log unit below the pKd values obtained at unmodified receptors (see Supporting Figure S1D and Supporting Table S1).44 In contrast to Nluc-NTS1R(Nterm), a specific BRET signal could be observed in BRET saturation binding experiments with 2 at Nluc-NTS1R(T227) (see Figure 3A). The pKd value for 2 was found to be comparable to the literature-described value (pKd ± SEM (Nluc-NTS1R(T227)) = 8.32 ± 0.08).42 Similarly, BRET competition binding experiments with 2 yielded pKi values for the reference agonist NT(8–13) and the antagonist SR142948 (for structures, see Supporting Figure S2) with no more than half an order of magnitude difference from data described in the literature (cf. Table 4). Moreover, Nluc-NTS1R(T227) was still functional in a Fura-2 Ca2+ assay (see Supporting Figure S4A).
Figure 3.
BRET binding data at the NTS1R (A), the AT1R (B), and the M1R (C). Shown are binding isotherms from saturation binding experiments with the fluorescent ligands 2 (A), 3 (B), or 4 (C) at HEK293T cells stably expressing the N-terminally Nluc-tagged receptors (Nluc-NTS1R(Nterm) in A, Nluc-AT1R(Nterm) in B, or Nluc-M1R(Nterm) in C) or the respective receptor constructs with Nluc inserted into the second extracellular loop (Nluc-NTS1R(T227) in A, Nluc-AT1R(S186) in B, or Nluc-M1R(G176) in C). Nonspecific binding was assessed in the presence of an excess of SR142948 (A, 100-fold excess), candesartan (B, 100-fold excess) or atropine (C, 500-fold excess) (for all experiments: excess over the concentration of the fluorescent ligand). Data are shown as means ± errors of one representative experiment from a set of at least three independent experiments, each performed in triplicate. Error bars of total and nonspecific binding represent the SEM, whereas error bars of specific binding represent propagated errors. In the right section of the figure, displacement curves from BRET competition binding experiments with the fluorescent ligands 2 (A, c = 5 nM), 3 (B, c = 10 nM) or 4 (C, c = 5 nM) and corresponding standard ligands at the respective receptor construct, stably expressed in HEK293T cells, are shown. Data are shown as means ± SEM of at least four independent experiments, each performed in triplicate. Abbreviations: solv.: solvent control; NT(8–13): neurotensin (8–13); SR: SR142948; ang II: angiotensin II; can: candesartan; los: losartan; NMS: N-methylscopolamine; atr: atropine; pir: pirenzepine; oxo: oxotremorine; iper: iperoxo; CCh: carbachol; alc: alcuronium.
Table 4. Binding Data (pKi values) of Standard NTS1R, AT1R, and M1R Ligands from BRET Competition Binding Experiments at the Indicated Receptor Construct Using 2 (Nluc-NTS1R(T227)), 3 (Nluc-AT1R(S186)), or 4 (Nluc-M1R(G176)) as the Fluorescent Probe.
| receptor construct | compound | pKi (BRET)a | N | reference values (literature)b |
|---|---|---|---|---|
| Nluc-NTS1R(T227) | NT(8–13) | 8.24 ± 0.16 | 4 | 8.27–9.8542,44,50−52 |
| SR142948 | 7.93 ± 0.12 | 4 | 8.05–8.9942,44,50,53 | |
| Nluc-AT1R(S186) | angiotensin II | 8.15 ± 0.07 | 5 | 7.61–9.6244,46−49 |
| candesartan | 8.82 ± 0.04 | 5 | 8.46–10.2841,44,46−48 | |
| losartan | 7.06 ± 0.05 | 4 | 7.23–8.0041,44,46,48,49 | |
| Nluc-M1R(G176) | carbachol | 3.89 ± 0.09 | 6 | 3.46–4.5254−59 |
| oxotremorine | 6.00 ± 0.11 | 5 | 5.48–5.8655,57,59 | |
| iperoxo | 7.00 ± 0.07 | 6 | 6.4656 | |
| atropine | 8.60 ± 0.09 | 6 | 8.50–9.7055,60−65 | |
| NMS | 9.41 ± 0.03 | 5 | 9.49–10.2255,62,66 | |
| pirenzepine | 7.91 ± 0.10 | 5 | 6.85–8.2955,60,63,64,67 | |
| alcuronium | 4.99 ± 0.08 | 4 | 5.01,57 5.2555 |
Determined by BRET competition binding experiments with 2 (Nluc-NTS1R(T227), c = 5 nM, Kd = 4.82 nM), 3 (Nluc-AT1R(S186), c = 10 nM, Kd = 9.02 nM), or 4 (Nluc-M1R(G176), c = 5 nM, Kd = 2.26 nM) at intact HEK293T cells stably expressing the indicated receptor construct. Data are shown as means ± SEM of N independent experiments, each performed in triplicate.
Reference binding data from literature determined by radioactivity-based or fluorescence-based competition binding experiments in different expression systems.
Next, we extended our approach to the AT1R and the M1R, both comprising shorter N-termini. As a BRET binding assay for the N-terminally Nluc-tagged AT1R (Nluc-AT1R(Nterm)) had been reported,16 we expected that BRET saturation binding experiments with the angiotensin II-derived ligand 3 would also yield a concentration-dependent and saturable specific BRET signal (see Figure 3B). Surprisingly, the pKd value for 3 at Nluc-AT1R(Nterm) (pKd ± SEM = 7.24 ± 0.07) was not comparable to results from radioligand competition binding experiments (pKi ± SD = 8.69 ± 0.05, for displacement curve, see Supporting Figure S5) at untagged receptors. As the ECL2 was the most convincing insertion site for both the Y1R and the NTS1R, we followed this approach for the AT1R as well by introducing Nluc downstream of Ser186 (Nluc-AT1R(S186)). The generated receptor–luciferase fusion protein was still capable of binding the AT1R radioligand [3H]UR-MK29244 (see Supporting Figure S1E) and was functionally active in a Fura-2 Ca2+ assay (see Supporting Figure S4B). The fluorescent ligand 3 showed saturable binding to the modified receptor but, in comparison to the N-terminally tagged variant,16 bound with higher affinity (pKd ± SEM (Nluc-AT1R(S186)) = 8.04 ± 0.08). Although being more in line, the obtained pKd value was still slightly lower than the results from radioligand competition binding experiments. However, the CHO cells used for the radioligand competition binding experiments were stably cotransfected with the Gα16 subunit, which stabilizes the present AT1Rs in an active receptor conformation, thus favoring agonist binding.45 This presumably led to a higher affinity estimate for 3, a compound derived from the endogenous AT1R agonist angiotensin II. The same explanation also holds true for the discrepancy between the pKd value of the radiolabeled agonist [3H]UR-MK29244 from radioligand saturation binding experiments at Nluc-AT1R(S186) and the previously reported results from experiments at CHO-AT1R cells stably coexpressing Gα16 (see Supporting Figure S1E and Supporting Table S1).44 BRET competition binding experiments with 3 and the agonist angiotensin II or the antagonists candesartan and losartan (Figure 3B, right panel; structures see Supporting Figure S2) resulted in pKi values in very good agreement with previously reported radioligand competition binding data (cf. Table 4), especially when compared with affinities determined at cells devoid of the stably coexpressed Gα16 subunit.46−49
BRET saturation binding experiments with the fluorescent MR ligand 4 at the N-terminally Nluc-tagged M1 receptor (Nluc-M1R(Nterm)) resulted in a specific BRET signal (Figure 3C) and a pKd value of 8.22 ± 0.06 (cf. Table 3), which was comparable, although slightly lower than results from radioligand competition binding experiments.43 Applying our novel strategy, Nluc was inserted into the ECL2 of the receptor right after Gly176 (Nluc-M1R(G176)). Compared to the results at Nluc-M1R(Nterm), a clear increase in signal-to-background ratio (see Figure 3C) was observed. At the same time, the pKd value originating from BRET saturation binding experiments at Nluc-M1R(G176) (pKd ± SEM = 8.65 ± 0.04) matched well with the results at Nluc-M1R(Nterm) and even better with the pKi value from radioligand competition binding experiments at untagged M1 receptors.43 Both of these observations, i.e., the increase in signal-to-background ratio by inserting Nluc into the ECL2 and the maintained pKd/pKi values, could also be observed for the TAMRA-labeled MR ligand UR-CG07243 (6) and the Py-1-labeled MR ligand UR-CG07443 (7, see Supporting Figure S6 and Supporting Table S3), both based on the same precursor.
Table 3. Equilibrium Dissociation Constants (pKd values) of the Investigated Fluorescent Ligands 2, 3, and 4 Obtained from BRET Saturation Binding Experiments.
| compound | receptor construct | pKd (BRET)a | N |
|---|---|---|---|
| 2 | Nluc-NTS1R(Nterm) | n.a. | – |
| Nluc-NTS1R(T227) | 8.32 ± 0.08 | 4 | |
| 3 | Nluc-AT1R(Nterm) | 7.24 ± 0.07 | 3 |
| Nluc-AT1R(S186) | 8.04 ± 0.08 | 5 | |
| 4 | Nluc-M1R(Nterm) | 8.22 ± 0.06 | 5 |
| Nluc-M1R(G176) | 8.65 ± 0.04 | 4 |
Determined by BRET saturation binding experiments at intact HEK293T cells stably expressing the indicated receptor construct. Data are shown as means ± SEM of N independent experiments performed in triplicate.
BRET competition binding experiments at the Nluc-M1R(G176) with 4 and several reference ligands yielded pKi values in good agreement with reported data from the literature (cf. Table 4). Even the allosteric modulator alcuronium could still bind to the modified receptor and displace the dualsteric ligand 4 completely from Nluc-M1R(G176).
Investigations on the Binding Kinetics at the Generated Constructs
One of the most useful characteristics of the BRET binding assay is the possibility to record the association and dissociation of fluorescent ligands in real time. Therefore, kinetic BRET binding experiments were conducted to obtain more information about the binding behavior of the fluorescent ligands at their target receptor/Nluc fusion proteins (see Figure 4 and Table 5).
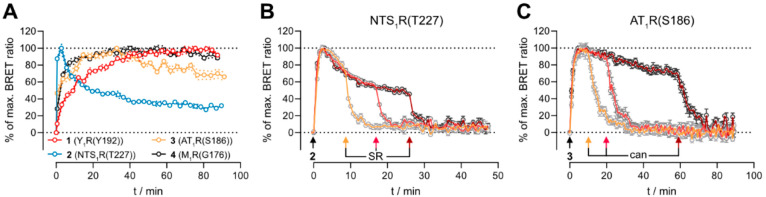
Figure 4.
Association and dissociation kinetics (specific binding) of the fluorescent ligands 1 (Nluc-Y1R(Y192), c = 0.5 nM), 2 (Nluc-NTS1R(T227)), c = 10 nM), 3 (Nluc-AT1R(S186), c = 10 nM), and 4 (Nluc-M1R(G176), c = 5 nM) from kinetic BRET binding experiments at intact HEK293T cells stably expressing the respective constructs (note the differing time scales). Association was initiated at t = 0 min by the addition of the respective fluorescent ligand. Dissociation was initiated at the indicated time points by the addition of BIBO3304 (Nluc-Y1R(Y192), c = 500 nM), SR142948 (Nluc-NTS1R(T227)), c = 2.5 μM), candesartan (Nluc-AT1R(S186), c = 2.5 μM), or atropine (Nluc-M1R(G176), c = 5 μM). Data are shown as means ± propagated errors and are representative of three independent experiments, each performed in triplicate. Abbreviations: SR: SR142948; can: candesartan; atr: atropine.
Table 5. Kinetic Constants of the Fluorescent Ligands 1–4 Determined in BRET-Based Binding Experiments.
| receptor construct | compound | koff [min–1]a | kon [nM–1 min–1]b | pKdkinetic,c | pKdequilibrium,d |
|---|---|---|---|---|---|
| Nluc-Y1R (Y192) | 1 | 0.004 ± 0.001 | 0.143 ± 0.005 | 10.62 ± 0.07 | 9.62 ± 0.05 |
| Nluc-NTS1R(T227) | 2 | 0.830 ± 0.051 | 0.120 ± 0.012 | 8.16 ± 0.07 | 8.32 ± 0.08 |
| Nluc-AT1R(S186) | 3 | 0.236 ± 0.008 | 0.077 ± 0.005 | 8.51 ± 0.04 | 8.04 ± 0.08 |
| Nluc-M1R(G176) | 4 | 0.178 ± 0.002 | 0.022 ± 0.007 | 8.04 ± 0.15 | 8.65 ± 0.04 |
Dissociation rate constant (koff). Determined by kinetic BRET binding experiments with 1 (c = 0.5 nM), 2 (c = 10 nM), 3 (c = 10 nM) and 4 (c = 5 nM) at intact HEK293T cells stably expressing the indicated receptor construct. Data represent the means ± SEM of three independent experiments performed in triplicate.
Association rate constant (kon). Determined by kinetic BRET binding experiments with 1 (c = 0.5 nM), 2 (c = 10 nM), 3 (c = 10 nM) and 4 (c = 5 nM) at intact HEK293T cells stably expressing the indicated receptor construct. Data represent the means ± SEM of three independent experiments performed in triplicate.
Kinetically derived dissociation constant (Kdkinetic), which was transformed into the pKd value for each independent experiment; indicated values represent means ± SEM of the pKdkinetic values. Determined by kinetic BRET binding experiments with 1 (c = 0.5 nM), 2 (c = 10 nM), 3 (c = 10 nM) and 4 (c = 5 nM) at intact HEK293T cells stably expressing the indicated receptor construct. Data represent the means ± SEM of three independent experiments performed in triplicate.
The fluorescent Y1R ligand 1 (c = 0.5 nM) showed an association to Nluc-Y1R(Y192) within 60 min and very slow dissociation kinetics (≈ 40% still bound after 4 h). The slow dissociation kinetics also contribute to the deviation of the kinetically derived dissociation constant (pKdkinetic) from the results from equilibrium experiments (cf. Table 5). However, as described above, the slow dissociation of the fluorescent tracer from the receptor did not preclude the complete displacement of the fluorescent ligand in competition binding experiments and pKi values in good agreement with data from the literature (cf. Table 2). Association of the fluorescent NTS1R ligand 2 (c = 10 nM) occurred rapidly within 2 min, and the ligand could be displaced completely from Nluc-NTS1R(T227) within 10 min (see Figure 4). This observation was consistent with previous results from confocal microscopy experiments with similar fluorescent ligands based on the same pharmacophore.42 The fluorescent ligands 3 (c = 10 nM) and 4 (c = 5 nM) showed a moderate association rate to their respective targets. After addition of a competitive ligand, both compounds could be displaced completely from their receptors within the observed time period (see Figure 4). The determined pKdkinetic values were matching the respective pKd values from equilibrium saturation binding experiments.
Interestingly, the association kinetics of ligands 2 and 3 to their respective target showed a peak followed by a decrease in signal without the addition of a competitive ligand when observing them for a longer time. In contrast, the kinetic traces of the antagonists 1 at Nluc-Y1R(Y192) and 4 at Nluc-M1R(G176) both reached a stable plateau (see Figure 5A). The qualitative curve shapes were independent of the ligand concentration used (see Supporting Figure S7 for exemplary kinetic traces of BRET saturation binding experiments). Similar observations have been previously reported for BRET binding assays at the receptor tyrosine kinase VEGFR2 and were explained by agonist-dependent internalization of the receptor and subsequent dissociation of the ligand–receptor complex.68,69 Furthermore, immunostaining studies at the NTS1R and the AT1R indicated that agonist binding can cause internalization and that the ligand and the receptor are then localized in different compartments within the cells.70,71 As the fluorescent ligands used are both putative agonists at their target, we assumed that this uncoupling of ligand and receptor after internalization was responsible for the observed signal decay in our BRET-based binding assay. To investigate this assumption, BRET saturation binding experiments were performed at cell homogenates of HEK293T cells stably expressing Nluc-NTS1R(T227) or Nluc-AT1R(S186), as receptor internalization cannot occur in cell homogenates. The fluorescent ligands 2 and 3 bound in a saturable manner to the homogenates, and after association, the BRET signal was stable over a longer period of time (see Supporting Figure S8). This corroborated our assumption that the signal decay shown in Figure 5A was indeed caused by internalization processes. It should be noted that the determined pKd values for 2 and 3 at the cell homogenates differed by around two log units from the results obtained using intact cells (cf. Supporting Table S4), presumably due to the uncoupling of the heterotrimeric G protein from the receptor in homogenates, which consequently results in a lower agonist affinity to the free receptor.72,73
Figure 5.
(A) Comparison of the association kinetics (specific binding) of the fluorescent ligands 1 (Nluc-Y1R(Y192), c = 0.5 nM), 2 (Nluc-NTS1R(T227), c = 5 nM), 3 (Nluc-AT1R(S186), c = 5 nM), and 4 (Nluc-M1R(G176), c = 5 nM) from BRET-based binding experiments at intact HEK293T cells stably expressing the respective construct. (B, C) Dissociation of 2 (c = 10 nM) from Nluc-NTS1R(T227) (B) or 3 (c = 10 nM) from Nluc-AT1R(S186) (C). The fluorescent ligands were added at the time point t = 0 min. Dissociation was initiated after different time points (indicated by differently colored arrows) by the addition of SR142948 (B, c = 2.5 μM) or candesartan (C, c = 2.5 μM). Data are shown as means ± propagated errors. Data shown are representative of at least three independent experiments, each performed in triplicate. Data in A were sampled from kinetic saturation binding experiments performed at the respective construct.
The aforementioned experiments suggested the dissociation of receptor and fluorescent ligand after internalization. We hypothesized that it should be possible to abolish the residual BRET signal at any given time point by addition of a competitor, because the residual BRET should only originate from fluorescent ligands bound to receptors at the cell surface. Therefore, further kinetic BRET binding experiments were performed by analogy with the experiments depicted in Figure 4, with the following modification: dissociation of the fluorescent ligands was initiated after different time points by addition of a competitive ligand, when the BRET signal had already started to decrease (Figure 5B and 5C). Independent of the time point of dissociation initiation, both fluorescent ligands could be displaced completely from Nluc-NTS1R(T227) or Nluc-AT1R(S186) with similar dissociation kinetics. Therefore, we conclude that the detected BRET signal originates from ligand-bound receptors at the membrane and not from internalized receptors.
As expected, following the time course of BRET competition binding experiments at Nluc-NTS1R(T227) and Nluc-AT1R(S186) with 2 and 3, respectively, resulted in similar kinetic traces (see Supporting Figure S9). However, despite the steady signal decrease over time, the pIC50 values of the investigated competitive ligands determined at different time points stabilized quickly (maximum: 45 min for losartan and angiotensin II) (see Supporting Figures S9F and S9G), suggesting equilibrium.
Conclusion
Here we present a complementary approach to establish BRET binding assays for GPCRs by inserting the bioluminescent donor Nluc into the ECL2 of the receptor instead of fusing it to the N-terminus. This strategy proved especially useful for class A GPCRs with slightly longer N-termini (exceeding 40 amino acids), e.g., the Y1R and the NTS1R, as BRET-based binding assays with a specific signal and retained affinity of our fluorescent ligands were only possible with the herein presented approach.
It should however be noted that it is not only the length of the N-terminal domain, which decides whether the N-terminal fusion of Nluc results in a functioning BRET binding assay. The used fluorescent ligand also plays a substantial role. For example, a BRET-based binding assay with an N-terminally Nluc-tagged Y1R has recently been described in combination with a TAMRA-labeled pNPY as the fluorescent ligand,74 even though this approach did not work robustly with our labeled ligand UR-CM138 (1). Moreover, BRET binding assays have been described for receptors with substantially larger N-terminal domains than the receptors tested in this study, especially for receptors of the class F.22,75 Therefore, the combination of fluorescent ligand and receptor seems to be the main determinant whether the N-terminal fusion of Nluc results in a functioning binding assay.
Our strategy, i.e., insertion of Nluc into the ECL2 of a GPCR was also applicable to receptors with shorter N-terminal domains. For those receptors, compared to the N-terminal fusion of Nluc, our approach resulted in an affinity estimate for the fluorescent ligand comparable or even more in line with literature. BRET competition binding experiments at all receptor constructs yielded affinity values comparable to literature-described data for several standard ligands. Kinetic binding experiments with the agonistic ligands 2 and 3 at Nluc-NTS1R(T227) and Nluc-AT1R(S186), respectively, resulted in association curves, which did not end in plateaus, but showed a decline after a peak was reached. This could be explained by receptor internalization, occurring in live cells, as plateau-reaching association curves could be obtained by BRET saturation binding experiments with 2 and 3 at cell homogenates.
Taken together, the herein presented strategy, i.e., insertion of Nluc into the ECL2 of a GPCR, will be of high value for the establishment of BRET binding assays and represents an alternative approach in particular but not exclusively for receptors, for which an N-terminal Nluc fusion delivers no or insufficient BRET upon addition of a fluorescent ligand.
Experimental Section
Materials
Dulbecco’s Modified Eagle’s Medium (DMEM), l-glutamine, fetal calf serum (FCS), HEPES, and Triton X-100 were from Sigma-Aldrich (Munich, Germany). Trypsin/EDTA (0.05%/0.02%) was from Biochrom (Berlin, Germany). Leibovitz’s L-15 medium (L-15) and geneticin (G418) were from Fisher Scientific (Nidderau, Germany). Bovine serum albumin (BSA) and bacitracin were from SERVA Electrophoresis (Heidelberg, Germany). Furimazine (Nano-Glo Live Cell Substrate) was purchased from Promega (Mannheim, Germany). The pcDNA3.1 vector was from Thermo Fisher (Nidderau, Germany).
Alcuronium chloride (alc), atropine sulfate (atr), carbachol (CCh), iperoxo iodide (iper), N-methyl scopolamine bromide (NMS), and pirenzepine dihydrochloride (pir) were from Sigma-Aldrich (Munich, Germany). BMS193885, oxotremorine sesquifumarate (oxo), PD160170, and SR142948 (SR) were from Tocris Bioscience (Bristol, UK). Candesartan (can) and losartan potassium salt (los) were kindly provided by Hexal AG (Holzkirchen, Germany). Neurotensin(8–13) (NT(8–13)) and porcine neuropeptide Y (pNPY) were from SynPeptide (Shanghai, China), whereas angiotensin II (ang II) was from Bachem (Bubendorf, Switzerland). The radioligand [3H]NMS (specific activity = 80 Ci/mmol) was purchased from American Radiolabeled Chemicals Inc. (St. Louis, MO). The syntheses of UR-MK299 and the radioligand [3H]UR-MK29925 as well as the syntheses of the radioligands [3H]UR-MK292 and [3H]UR-MK300 were reported elsewhere.44 The syntheses of the fluorescent ligands UR-CM138 (1,Y1R), UR-AP178 (2, NTS1R), and UR-AP175 (4, M1R) are described elsewhere.24,42,43 Syntheses of the fluorescent AT1R ligand UR-AP177 (3) and the Y1R ligand BIBP3226 can be found in the Supporting Information. Stock solutions of the fluorescent ligands were prepared in DMSO and stored in aliquots at −80 °C. Stock solutions of angiotensin II and NT(8–13) were prepared in a mixture of ethanol and 50 mM HCl (30:70). Stock solutions of the other standard ligands were prepared in H2O or in DMSO, whenever the compound was insoluble in H2O.
Generation of Plasmids
Plasmids containing the sequences of the investigated human GPCRs (neuropeptide Y Y1 receptor (Y1R), neurotensin receptor type 1 (NTS1R), angiotensin II receptor type 1 (AT1R), and M1 muscarinic acetylcholine receptor (M1R)) were purchased from the cDNA Resource Center (Rolla, MO). The vector encoding the nanoluciferase (Nluc) was obtained from Promega (Mannheim, Germany). All constructs in this study were generated using standard PCR and restriction techniques. The vectors encoding the N-terminally Nluc-tagged receptors (Nterm) were prepared by exchanging the receptor sequence in the previously described pcDNA3.1 NLuc-hH4R18 with the respective GPCR of interest. The pcDNA3.1 Nluc-Y1R(Δ1–31) encoding Nluc fused N-terminally to a truncated Y1 receptor (lacking amino acids 1–31) was obtained analogously. For the constructs with the luciferase being located within the ECLs, Nluc was integrated into the receptor sequence downstream of the indicated amino acids (e.g., pcDNA3.1 Nluc-Y1R(Y192): after the tyrosine in position 192). A short flexible linker sequence consisting of Gly and Ser was used to connect the luciferase to the receptor at both the 5′ and 3′ ends. The sequence of all generated plasmids was verified by sequencing (Eurofins Genomics, Ebersberg, Germany).
Cell Culture and Generation of Stable Transfectants
HEK293T cells (kind gift from Prof. Dr. Wulf Schneider, Institute for Medical Microbiology and Hygiene, University of Regensburg, Germany) were cultivated in DMEM supplemented with 2 mM l-glutamine and 10% FCS at 37 °C in a water-saturated atmosphere (containing 5% CO2). One day before transfection, the cells were seeded at a density of 3 × 105 cells/mL in six-well plates (Sarstedt, Nümbrecht, Germany). On the following day, cells were transfected with 2 μg of the respective cDNA using X-tremeGENE HP (Roche Diagnostics, Mannheim, Germany) as the transfection reagent (used according to manufacturer’s protocol). Stably transfected cells were selected with 1 mg/mL G418, while cultivation was continued with 600 μg/mL G418. All cells were regularly tested for mycoplasma infection using the Venor GeM Mycoplasma Detection Kit (Minerva Biolabs, Berlin, Germany) and were negative.
Radioligand Binding Experiments
Radioligand saturation binding experiments at intact, suspended HEK293T cells stably expressing Nluc-Y1R(Nterm), Nluc-Y1R(Δ1–31), Nluc-Y1R(Y192), Nluc-M1R(Nterm), or Nluc-M1R(G176) were performed according to a procedure described for CHO cells76 with the following minor modifications: After reaching ≈80% confluency, the cells were detached from the cell culture flask by trypsinization. After centrifugation (400g, 6 min), the cells were resuspended in L-15 medium with 1% BSA and the cell density was adjusted to 1.25 × 106 (for the Nluc-Y1R constructs and Nluc-M1R(G176)) or 1.25 × 105 (for Nluc-M1R(Nterm)) cells/mL. [3H]UR-MK29925 or [3H]NMS were used as radioligands for experiments at the Nluc-Y1R constructs or Nluc-M1R constructs, respectively. Nonspecific binding was assessed in the presence of either BIBO3304 (for the Nluc-Y1R constructs, 500-fold excess over the concentration of radioligand) or atropine (for the Nluc-M1R constructs, 1000-fold excess over the concentration of radioligand). The wells were prefilled with 20 μL of L-15 containing the respective radioligand (10-fold more concentrated than the final assay concentration) and 20 μL of L-15 (total binding) or 20 μL of L-15 containing the suitable competitive ligand (nonspecific binding). A 160 μL amount of the density-adjusted cell suspension was added to the wells, and the plate was incubated under gentle shaking at 23 °C for 90 min (for the Nluc-Y1R constructs) and 3 h (for the Nluc-M1R constructs), respectively.
Saturation binding experiments at Nluc-NTS1R(T227) and Nluc-AT1R(S186) were essentially conducted following a described protocol44 with modifications; in detail: saturation binding was investigated with [3H]UR-MK300 at intact HEK293T cells stably expressing Nluc-NTS1R(T227) and with [3H]UR-MK292 at intact HEK293T cells stably expressing Nluc-AT1R(S186). Experiments were performed at room temperature (rt) in white 96-well plates with clear bottoms (Corning Inc., Tewksbury, MA). Two days before the experiment, the plates were treated with poly-d-lysine hydrobromide (Sigma-Aldrich, Munich, Germany) for 10 min. Subsequently, the plates were washed once with PBS and left to dry overnight at rt. Dulbecco’s PBS (D-PBS) with Ca2+ and Mg2+ (1.8 mM CaCl2, 2.68 mM KCl, 1.47 mM KH2PO4, 3.98 mM MgSO4, 136.9 mM NaCl and 8.06 mM Na2HPO4), supplemented with 1% BSA and 100 μg/mL of the protease inhibitor bacitracin, served as binding buffer. Washing steps were performed using D-PBS at rt (prior to the incubation) or ice-cold (after incubation). Cells were seeded 1 day before the experiment in 100 μL of DMEM with 2 mM l-glutamine and 10% FCS at a density of 1 × 105 cells/well. On the day of the experiment, the culture medium was carefully removed using a multichannel pipet (Transferpette S-12, Brand, Wertheim, Germany), and the cells were washed once with D-PBS (200 μL) and covered with binding buffer (160 μL). For the assessment of total binding, 20 μL of binding buffer and the same volume of binding buffer containing the radioligand (10-fold more concentrated than the final concentration) were added. Nonspecific binding was determined in the presence of NT(8–13) (for Nluc-NTS1R(T227)) or angiotensin II (for Nluc-AT1R(S186)) in 500-fold excess over the respective concentration of the radioligand by adding binding buffer (20 μL) containing the competitor (10-fold more concentrated than the final concentration) and binding buffer (20 μL) containing the radioligand (10-fold more concentrated than the final concentration). The plates were gently shaken for 2 h at rt. After incubation, the liquid was carefully removed using a multichannel pipet, the cells were washed twice with ice-cold D-PBS (200 μL) and treated with lysis solution (urea (8 M), acetic acid (3 M) and Triton X-100 (1%) in water) (25 μL). The plates were shaken for 20 min, a liquid scintillator (Ultima Gold (PerkinElmer, Waltham, MA)) (200 μL) was added, and the plates were sealed with a transparent sealing tape (permanent seal for microplates, PerkinElmer, prod. no. 1450-461). Complete mixing of scintillator and lysis solution was achieved by turning the plates upside down multiple times. The plates were kept in the dark for at least 30 min prior to the measurement of the radioactivity (dpm) with a MicroBeta2 plate counter (PerkinElmer, Rodgau, Germany). Radioligand competition binding experiments with [3H]UR-MK292 at intact CHO-AT1-Gα16-mtAEQ cells were performed as described.44
BRET Binding Assay at Intact HEK293T Cells
BRET binding experiments were essentially performed as described18 with the following minor modifications: 1 day prior to the experiment, HEK293T cells stably expressing the respective Nluc-receptor fusion construct were detached from the cell culture dish by trypsinization, centrifuged (500g, 5 min), and resuspended in L-15 with 5% FCS and 10 mM HEPES (pH 7.4). After adjusting the cell density to 1.4 × 106 cells/mL, the cells were seeded in a volume of 70 μL per well into white 96-well plates (Brand, Wertheim, Germany) and incubated overnight in a water-saturated atmosphere (no additional CO2). On the day of the experiment, serial dilutions of the fluorescent ligands and the competitors (all 10-fold more concentrated than the final assay concentration) were prepared in assay buffer consisting of L-15 medium with 10 mM HEPES (pH 7.4) and 2% BSA. For saturation binding experiments, 10 μL of assay buffer (total binding) or 10 μL of assay buffer containing a competitive ligand (for the assessment of nonspecific binding, for the Nluc-Y1R constructs: BIBO3304 in a 500-fold excess; for the Nluc-NTS1R constructs: SR142948 in a 100-fold excess; for the Nluc-AT1R constructs: candesartan in a 100-fold excess; for the Nluc-M1R constructs: atropine in a 500-fold excess; the excess always refers to the respective concentration of fluorescent ligand used) were added to the wells. Subsequently, 10 μL of assay buffer containing the investigated fluorescent ligand was added to all wells. After an incubation period of 60 min at 27 °C, 10 μL of the Nluc substrate furimazine (prediluted 1:1000 before use) was added and after an equilibration time of 5 min inside the plate reader (prewarmed to 27 °C), the measurement was started.
For competition binding experiments at Nluc-Y1R(Y192), Nluc-Y1R(Q291), Nluc-NTS1R(T227), and Nluc-M1R(G176), 10 μL of a solution of the investigated competitive ligand (various concentrations) and 10 μL of a solution of the suitable fluorescent ligand (for Nluc-Y1R(Y192)/ Nluc-Y1R(Q291): cfinal(1) = 0.5 nM; for Nluc-NTS1R(T227): cfinal(2) = 5 nM; for Nluc-M1R(G176): cfinal(4) = 5 nM) were added. For competition binding experiments at Nluc-AT1R(S186), cells were preincubated with the competitor for 30 min before the addition of the fluorescent ligand 3 (cfinal = 10 nM). A positive control containing only fluorescent ligand and no competitor (100% value), as well as a negative control (buffer, 0% value), was included in every experiment. After an incubation period of 60 min at 27 °C, 10 μL of furimazine (prediluted 1:1000 before use) was added to the cells and after equilibration for 5 min, the measurement was started.
For kinetic saturation and competition binding experiments, 10 μL of the substrate furimazine (prediluted 1:1000 before use) and 10 μL of assay buffer (total binding) or 10 μL of a solution of the competitive ligand (nonspecific binding and competition binding experiments) were added at the same time. After a short equilibration (5 min), 10 μL of the solution of the investigated fluorescent ligand was added to the cells (final concentrations of the fluorescent ligands in competition binding experiments: see above) and the measurement was started immediately. For kinetic BRET competition binding experiments at Nluc-AT1R(S186), the cells were preincubated with the competitive ligand for 30 min as described above prior to the addition of the substrate and the fluorescent ligand 3.
For recording association and dissociation kinetics, 20 μL of assay buffer (total binding) or 20 μL of assay buffer containing the competitive ligand (nonspecific binding, for Nluc-Y1R(Y192): BIBO3304, cfinal = 500 nM; for Nluc-NTS1R(T227): SR142948, cfinal = 2.5 μM; for the AT1R(S186): candesartan, cfinal = 2.5 μM; for the M1R(G176): atropine, cfinal = 5 μM) were added to the cells. After the addition of 10 μL of furimazine (prediluted 1:1000 before use), cells were equilibrated inside the thermostated plate reader (27 °C) for 5 min and the measurement was started. After the first cycle (t = 0 min), 50 μL of assay buffer containing the fluorescent ligands 1 (Nluc-Y1R(Y192), cfinal = 0.5 nM), 2 (Nluc-NTS1R(T227), cfinal = 10 nM), 3 (Nluc-AT1R(S186), cfinal = 10 nM), or 4 (Nluc-M1R(G176), cfinal = 5 nM) were added via the injector module. Dissociation was initiated at the indicated time points by the addition of 50 μL of assay buffer containing the competitive ligand (for Nluc-Y1R(Y192): BIBO3304, cfinal = 500 nM; Nluc-NTS1R(T227): SR142948, cfinal = 2.5 μM; Nluc-AT1R(S186): candesartan, cfinal = 2.5 μM; Nluc-M1R(G176): atropine, cfinal = 5 μM).
All BRET measurements were performed at a temperature of 27 °C using a TECAN GENiosPro or a TECAN InfiniteLumi plate reader (Tecan Austria GmbH, Grödig, Austria). The bioluminescence of the luciferase was detected using a 460 ± 25 nm band-pass (460/25 BP, GENiosPro) filter or a 460 ± 35 nm band-pass (460/35 BP, InfiniteLumi) filter. The emission originating from the fluorescent ligand was detected through a 610 nm long-pass (610 LP) filter with both readers. Integration times for equilibrium experiments were set to 100 ms for both channels except for experiments at Nluc-Y1R(Y192), where a longer integration time (300 ms) was used for both channels. Kinetic experiments (except for on–off-kinetics) were monitored using an integration time of 1000 ms for the 610 LP filter to reduce noise. For the determination of on–off-kinetics, the following integration times (460 BP/610 LP) were used: Nluc-Y1R(Y192) 1000 ms/1000 ms, Nluc-NTS1R(T227) and Nluc-AT1R(S186): 100 ms/500 ms, Nluc-M1R(G176): 1000 ms/1000 ms
Preparation of HEK293T Cell Homogenates
Cell homogenates of HEK293T cells stably expressing Nluc-AT1R(S186) or Nluc-NTS1R(T227) were prepared as described77 with the following minor modifications: after cell lysis and the centrifugation of the lysate (23 000 rpm, 4 °C, 45 min), the pellet was resuspended in ice-cold binding buffer (50 mM Tris-HCl, 1 mM EDTA, pH 7.4) and homogenized using a 1 mL syringe (Injekt-F, B. Braun Melsungen AG, Melsungen, Germany) and a needle with 0.4 mm diameter (BD Microlance, Becton Dickinson, Heidelberg, Germany). The protein concentration was determined by the Bradford method, and aliquots of the homogenates were stored at −80 °C until further use.
BRET Binding Assay at HEK293T Cell Homogenates
To perform kinetic BRET saturation binding experiments at cell homogenates of HEK293T expressing Nluc-AT1R(S186) or Nluc-NTS1R(T227), the homogenate was thawed and centrifuged (16 000g, 4 °C, 10 min). The pellet was resuspended in ice-cold binding buffer, and the protein concentration was adjusted to 1.5 μg/μL (Nluc-AT1R(S186)) or 0.9 μg/μL (Nluc-NTS1R(T227)). Next, 10 μL of the concentration-adjusted cell homogenates was added to each well in a white 96-well plate (Brand, Wertheim, Germany) together with 60 μL of binding buffer. The assay was performed as described above following the protocol for BRET saturation binding experiments at intact cells using a Tecan GENiosPro plate reader. The integration times were set to 100 ms (460/25 BP filter) and 500 ms (610 LP filter).
Fura-2 Ca2+ Assay
The Fura-2 calcium assays were essentially performed as previously described.78 Therefore, HEK293T cells stably expressing Nluc-NTS1R(T227) or Nluc-AT1R(S186) were incubated with Fura-2 AM (Merck Biochrom, Berlin, Germany). The Ca2+ responses were measured in cuvettes using a LS50B luminescence spectrophotometer (PerkinElmer, Rodgau, Germany).
Data Analysis
Data analysis was performed using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA). Specific binding data (dpm) from radioligand saturation binding experiments were plotted against the free radioligand concentration and fitted by an equation for hyperbolic binding (“one site-specific binding”, GraphPad Prism 8.0) yielding Kd and Bmax values. Total and nonspecific binding were analyzed simultaneously using the “one site-total and non-specific binding” model in Prism 8.0. The free radioligand concentration was calculated by subtraction of the amount of specifically bound radioligand from the total radioligand concentration. The number of binding sites per cell was calculated from the Bmax values as described.44 Error propagation was calculated as described.79
Obtained data from radioligand competition binding experiments at the AT1 receptor were normalized to the radioactivity measured in the presence of the radioligand [3H]UR-MK292, but in the absence of competitor (100%) and analyzed by a four-parameter logistic equation (Prism 8.0) yielding pIC50 values. These were transformed into pKi values using the Cheng–Prusoff equation (logarithmic form),80 for which the mean and SD was calculated.
For BRET experiments, the “raw BRET ratio” was calculated by dividing the emission detected through the 610 LP filter (acceptor) by the emission detected through the 460 BP filter (donor). A baseline correction was performed for all values by subtracting the BRET ratio of a buffer control, yielding “corrected BRET ratios”. For saturation binding experiments, total and nonspecific binding were fitted simultaneously applying a one-site binding model (“one site-total and non-specific binding”; Prism 8.0), which fits total binding by a hyperbolic curve and nonspecific binding by linear regression. Specific binding was fitted by an equation describing hyperbolic binding (“one site-specific binding”, Prism 8.0). The obtained Kd values were transformed into pKd values, for which means and SEMs were calculated.
For competition binding experiments, the data were normalized to the BRET ratio obtained for the negative control (buffer, 0% value) and the BRET ratio obtained for wells containing fluorescent ligand, but no competitor (100% value). The normalized data were then fitted by a four-parameter logistic equation (Prism 8.0). The obtained pIC50 values were subsequently transformed into pKi values by means of the Cheng–Prusoff equation (logarithmic form),80 for which means and SEMs were calculated.
Kinetic data were normalized to the BRET ratio before the addition of a fluorescent ligand (0%) and the maximal BRET ratio obtained after association reached a plateau (100%). The data from combined association and dissociation experiments were then analyzed by an “association then dissociation” fit (Prism 8.0) yielding estimates for kon, koff, and Kdkinetic values for each independent experiment. The obtained Kdkinetic values were transformed into pKdkinetic values for every experiment, and means and SEMs were calculated for the pKdkinetic values.
Acknowledgments
The authors thank Maria Beer-Krön, Brigitte Wenzl and Lydia Schneider for expert technical assistance. The authors thank Dr. Max Keller for help with the analytical characterization of the fluorescent ligands and for fruitful discussions. Financial support by the Deutsche Forschungsgemeinschaft (Research Training Group GRK1910) is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.2c00162.
Binding isotherms from radioligand saturation binding experiments; structures of the competitive ligands used in the course of the study; snake plots of the investigated GPCRs with an indication of the insertion sites for Nluc; functional validation of Nluc-NTS1R(T227) and AT1R(S186) in a Fura-2 Ca2+ assay; displacement curve from radioligand competition binding experiments with 3 at the AT1R; BRET-based binding experiments with the muscarinic acetylcholine receptor ligands 6 and 7; kinetic traces (BRET binding) of the binding of fluorescent ligands 1–4 to their respective targets; saturation binding experiments with 2 and 3 at cell homogenates of HEK293T cells expressing Nluc-NTS1R(T227) or Nluc-AT1R(S186); kinetic traces from BRET competition binding experiments at Nluc-NTS1R(T227) or Nluc-AT1R(S186); Supporting Tables: pKd values of the used radioligands obtained from radioligand saturation binding experiments; pKi values of reported standard antagonists from BRET competition binding experiments at Nluc-Y1R(Q291); pKd values of the fluorescent muscarinic acetylcholine receptor ligands 6 and 7 from BRET saturation binding experiments; pKd values of 2 and 3 from BRET saturation binding experiments at cell homogenates; synthesis and analytical characterization of fluorescent ligand 3 and BIBP3226 (PDF)
Author Present Address
† Section of Receptor Biology & Signaling, Department of Physiology & Pharmacology, Karolinska Institutet, S-17165, Stockholm, Sweden
Author Present Address
‡ Ramboll Italy Srl, Viale Edoardo Jenner 53, 20159 Milano, Italy.
Author Present Address
§ AbbVie Deutschland GmbH & Co. KG, 67061 Ludwigshafen am Rhein, Germany.
Author Contributions
L.G. conceived the project with input from G.B. and T.L. L.G. established the BRET assay including the molecular cloning, performed all BRET experiments, and analyzed the data. C.M. and A.P. synthesized and characterized the used fluorescent ligands. L.S. performed radioligand binding experiments. G.B. supervised the research. L.G., G.B., and T.L. wrote the manuscript with input from all coauthors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Special Issue
Published as part of the ACS Pharmacology & Translational Science virtual special issue "GPCR Signaling".
Supplementary Material
References
- Fredriksson R.; Lagerström M. C.; Lundin L.-G.; Schiöth H. B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan A. J.; Deupi X.; Lebon G.; Tate C. G.; Schertler G. F.; Babu M. M. Molecular signatures of G-protein-coupled receptors. Nature 2013, 494, 185–194. 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- Peeters M. C.; van Westen G. J. P.; Li Q.; Ijzerman A. P. Importance of the extracellular loops in G protein-coupled receptors for ligand recognition and receptor activation. Trends Pharmacol. Sci. 2011, 32, 35–42. 10.1016/j.tips.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Santos R.; Ursu O.; Gaulton A.; Bento A. P.; Donadi R. S.; Bologa C. G.; Karlsson A.; Al-Lazikani B.; Hersey A.; Oprea T. I.; Overington J. P. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discovery 2017, 16, 19–34. 10.1038/nrd.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser A. S.; Attwood M. M.; Rask-Andersen M.; Schiöth H. B.; Gloriam D. E. Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discovery 2017, 16, 829–842. 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C.; Castro M.; Rinken A.; Leurs R.; Hill S. J.; Vischer H. F. Ligand residence time at G-protein–coupled receptors—why we should take our time to study it. Mol. Pharmacol. 2015, 88, 552–560. 10.1124/mol.115.099671. [DOI] [PubMed] [Google Scholar]
- Swinney D. C.; Haubrich B. A.; Van Liefde I.; Vauquelin G. The role of binding kinetics in GPCR drug discovery. Curr. Top. Med. Chem. 2015, 15, 2504–2522. 10.2174/1568026615666150701113054. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Monsma F. Binding kinetics and mechanism of action: toward the discovery and development of better and best in class drugs. Expert Opin. Drug Discovery 2010, 5, 1023–1029. 10.1517/17460441.2010.520700. [DOI] [PubMed] [Google Scholar]
- Sridharan R.; Zuber J.; Connelly S. M.; Mathew E.; Dumont M. E. Fluorescent approaches for understanding interactions of ligands with G protein coupled receptors. Biochim. Biophys. Acta Biomembr. 2014, 1838, 15–33. 10.1016/j.bbamem.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart L. A.; White C. W.; Nguyen K.; Hill S. J.; Pfleger K. D. Fluorescence- and bioluminescence-based approaches to study GPCR ligand binding. Br. J. Pharmacol. 2016, 173, 3028–3037. 10.1111/bph.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R.; Xie X. Tools for GPCR drug discovery. Acta Pharmacol. Sin. 2012, 33, 372–384. 10.1038/aps.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami-Nemini A.; Roux T.; Leblay M.; Bourrier E.; Lamarque L.; Trinquet E.; Lohse M. J. Time-resolved fluorescence ligand binding for G protein–coupled receptors. Nat. Protoc. 2013, 8, 1307–1320. 10.1038/nprot.2013.073. [DOI] [PubMed] [Google Scholar]
- Stoddart L. A.; Kilpatrick L. E.; Hill S. J. NanoBRET approaches to study ligand binding to GPCRs and RTKs. Trends Pharmacol. Sci. 2018, 39, 136–147. 10.1016/j.tips.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Tahtaoui C.; Parrot I.; Klotz P.; Guillier F.; Galzi J. L.; Hibert M.; Ilien B. Fluorescent pirenzepine derivatives as potential bitopic ligands of the human M1 muscarinic receptor. J. Med. Chem. 2004, 47, 4300–4315. 10.1021/jm040800a. [DOI] [PubMed] [Google Scholar]
- Hall M. P.; Unch J.; Binkowski B. F.; Valley M. P.; Butler B. L.; Wood M. G.; Otto P.; Zimmerman K.; Vidugiris G.; Machleidt T.; Robers M. B.; Benink H. A.; Eggers C. T.; Slater M. R.; Meisenheimer P. L.; Klaubert D. H.; Fan F.; Encell L. P.; Wood K. V. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012, 7, 1848–1857. 10.1021/cb3002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart L. A.; Johnstone E. K. M.; Wheal A. J.; Goulding J.; Robers M. B.; Machleidt T.; Wood K. V.; Hill S. J.; Pfleger K. D. G. Application of BRET to monitor ligand binding to GPCRs. Nat. Methods 2015, 12, 661–663. 10.1038/nmeth.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. Phys. 1948, 437, 55–75. 10.1002/andp.19484370105. [DOI] [Google Scholar]
- Bartole E.; Grätz L.; Littmann T.; Wifling D.; Seibel U.; Buschauer A.; Bernhardt G. UR-DEBa242: a Py-5-labeled fluorescent multipurpose probe for investigations on the histamine H3 and H4 receptors. J. Med. Chem. 2020, 63, 5297–5311. 10.1021/acs.jmedchem.0c00160. [DOI] [PubMed] [Google Scholar]
- Fernández-Dueñas V.; Qian M.; Argerich J.; Amaral C.; Risseeuw M. D. P.; Van Calenbergh S.; Ciruela F. Design, synthesis and characterization of a new series of fluorescent metabotropic glutamate receptor type 5 negative allosteric modulators. Molecules 2020, 25, 1532. 10.3390/molecules25071532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grätz L.; Tropmann K.; Bresinsky M.; Müller C.; Bernhardt G.; Pockes S. NanoBRET binding assay for histamine H2 receptor ligands using live recombinant HEK293T cells. Sci. Rep. 2020, 10, 13288. 10.1038/s41598-020-70332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare B. L.; Bruell S.; Sethi A.; Gooley P. R.; Lew M. J.; Hossain M. A.; Inoue A.; Scott D. J.; Bathgate R. A. D. Multi-component mechanism of H2 relaxin binding to RXFP1 through NanoBRET kinetic analysis. iScience 2019, 11, 93–113. 10.1016/j.isci.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozielewicz P.; Bowin C. F.; Turku A.; Schulte G. A NanoBRET-based binding assay for Smoothened allows real-time analysis of ligand binding and distinction of two binding sites for BODIPY-cyclopamine. Mol. Pharmacol. 2020, 97, 23–34. 10.1124/mol.119.118158. [DOI] [PubMed] [Google Scholar]
- Zhao P.; Liang Y.-L.; Belousoff M. J.; Deganutti G.; Fletcher M. M.; Willard F. S.; Bell M. G.; Christe M. E.; Sloop K. W.; Inoue A.; Truong T. T.; Clydesdale L.; Furness S. G. B.; Christopoulos A.; Wang M.-W.; Miller L. J.; Reynolds C. A.; Danev R.; Sexton P. M.; Wootten D. Activation of the GLP-1 receptor by a non-peptidic agonist. Nature 2020, 577, 432–436. 10.1038/s41586-019-1902-z. [DOI] [PubMed] [Google Scholar]
- Müller C.; Gleixner J.; Tahk M.-J.; Kopanchuk S.; Laasfeld T.; Weinhart M.; Schollmeyer D.; Betschart M. U.; Lüdeke S.; Koch P.; Rinken A.; Keller M. Structure-based design of high-affinity fluorescent probes for the neuropeptide Y Y1 receptor. J. Med. Chem. 2022, 65, 4832–4853. 10.1021/acs.jmedchem.1c02033. [DOI] [PubMed] [Google Scholar]
- Keller M.; Weiss S.; Hutzler C.; Kuhn K. K.; Mollereau C.; Dukorn S.; Schindler L.; Bernhardt G.; König B.; Buschauer A. Nω-Carbamoylation of the argininamide moiety: an avenue to insurmountable NPY Y1 receptor antagonists and a radiolabeled selective high-affinity molecular tool ([3H]UR-MK299) with extended residence time. J. Med. Chem. 2015, 58, 8834–8849. 10.1021/acs.jmedchem.5b00925. [DOI] [PubMed] [Google Scholar]
- Lindner D.; Walther C.; Tennemann A.; Beck-Sickinger A. G. Functional role of the extracellular N-terminal domain of neuropeptide Y subfamily receptors in membrane integration and agonist-stimulated internalization. Cell. Signal. 2009, 21, 61–68. 10.1016/j.cellsig.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Han S.; Keller M.; Kaiser A.; Bender B. J.; Bosse M.; Burkert K.; Kögler L. M.; Wifling D.; Bernhardt G.; Plank N.; Littmann T.; Schmidt P.; Yi C.; Li B.; Ye S.; Zhang R.; Xu B.; Larhammar D.; Stevens R. C.; Huster D.; Meiler J.; Zhao Q.; Beck-Sickinger A. G.; Buschauer A.; Wu B. Structural basis of ligand binding modes at the neuropeptide Y Y1 receptor. Nature 2018, 556, 520–524. 10.1038/s41586-018-0046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E. F.; Goddard T. D.; Huang C. C.; Couch G. S.; Greenblatt D. M.; Meng E. C.; Ferrin T. E. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- White C. W.; Johnstone E. K. M.; See H. B.; Pfleger K. D. G. NanoBRET ligand binding at a GPCR under endogenous promotion facilitated by CRISPR/Cas9 genome editing. Cell. Signal. 2019, 54, 27–34. 10.1016/j.cellsig.2018.11.018. [DOI] [PubMed] [Google Scholar]
- Keller M.; Erdmann D.; Pop N.; Pluym N.; Teng S.; Bernhardt G.; Buschauer A. Red-fluorescent argininamide-type NPY Y1 receptor antagonists as pharmacological tools. Bioorg. Med. Chem. 2011, 19, 2859–2878. 10.1016/j.bmc.2011.03.045. [DOI] [PubMed] [Google Scholar]
- Liu M.; Richardson R. R.; Mountford S. J.; Zhang L.; Tempone M. H.; Herzog H.; Holliday N. D.; Thompson P. E. Identification of a cyanine-dye labeled peptidic ligand for Y1R and Y4R, based upon the neuropeptide Y C-terminal analogue, BVD-15. Bioconjugate Chem. 2016, 27, 2166–2175. 10.1021/acs.bioconjchem.6b00376. [DOI] [PubMed] [Google Scholar]
- Richardson R. R.; Groenen M.; Liu M.; Mountford S. J.; Briddon S. J.; Holliday N. D.; Thompson P. E. Heterodimeric analogues of the potent Y1R antagonist 1229U91, lacking one of the pharmacophoric C-terminal structures, retain potent Y1R affinity and show improved selectivity over Y4R. J. Med. Chem. 2020, 63, 5274–5286. 10.1021/acs.jmedchem.0c00027. [DOI] [PubMed] [Google Scholar]
- Keller M.; Bernhardt G.; Buschauer A. [3H]UR-MK136: a highly potent and selective radioligand for neuropeptide Y Y1 receptors. ChemMedChem. 2011, 6, 1566–1571. 10.1002/cmdc.201100197. [DOI] [PubMed] [Google Scholar]
- Rudolf K.; Eberlein W.; Engel W.; Wieland H. A.; Willim K. D.; Entzeroth M.; Wienen W.; Beck-Sickinger A. G.; Doods H. N. The first highly potent and selective non-peptide neuropeptide Y Y1 receptor antagonist: BIBP3226. Eur. J. Pharmacol. 1994, 271, R11–R13. 10.1016/0014-2999(94)90822-2. [DOI] [PubMed] [Google Scholar]
- Antal-Zimanyi I.; Bruce M. A.; Leboulluec K. L.; Iben L. G.; Mattson G. K.; McGovern R. T.; Hogan J. B.; Leahy C. L.; Flowers S. C.; Stanley J. A.; Ortiz A. A.; Poindexter G. S. Pharmacological characterization and appetite suppressive properties of BMS-193885, a novel and selective neuropeptide Y1 receptor antagonist. Eur. J. Pharmacol. 2008, 590, 224–232. 10.1016/j.ejphar.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Poindexter G. S.; Bruce M. A.; LeBoulluec K. L.; Monkovic I.; Martin S. W.; Parker E. M.; Iben L. G.; McGovern R. T.; Ortiz A. A.; Stanley J. A.; Mattson G. K.; Kozlowski M.; Arcuri M.; Antal-Zimanyi I. Dihydropyridine neuropeptide Y Y1 receptor antagonists. Bioorg. Med. Chem. Lett. 2002, 12, 379–382. 10.1016/S0960-894X(01)00761-2. [DOI] [PubMed] [Google Scholar]
- Wielgosz-Collin G.; Duflos M.; Pinson P.; Le Baut G.; Renard P.; Bennejean C.; Boutin J.; Boulanger M. 8-Amino-5-nitro-6-phenoxyquinolines: potential non-peptidic neuropeptide Y receptor ligands. J. Enzyme Inhib. Med. Chem. 2002, 17, 449–453. 10.1080/1475636021000005758. [DOI] [PubMed] [Google Scholar]
- Wright J.; Bolton G.; Creswell M.; Downing D.; Georgic L.; Heffner T.; Hodges J.; MacKenzie R.; Wise L. 8-Amino-6-(arylsulphonyl)-5-nitroquinolines: novel nonpeptide neuropeptide Y1 receptor antagonists. Biorg. Med. Chem. Lett. 1996, 6, 1809–1814. 10.1016/0960-894X(96)00319-8. [DOI] [Google Scholar]
- Thal D. M.; Sun B.; Feng D.; Nawaratne V.; Leach K.; Felder C. C.; Bures M. G.; Evans D. A.; Weis W. I.; Bachhawat P.; Kobilka T. S.; Sexton P. M.; Kobilka B. K.; Christopoulos A. Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature 2016, 531, 335–340. 10.1038/nature17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. F.; Noinaj N.; Shibata Y.; Love J.; Kloss B.; Xu F.; Gvozdenovic-Jeremic J.; Shah P.; Shiloach J.; Tate C. G.; Grisshammer R. Structure of the agonist-bound neurotensin receptor. Nature 2012, 490, 508–513. 10.1038/nature11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Unal H.; Gati C.; Han G. W.; Liu W.; Zatsepin N. A.; James D.; Wang D.; Nelson G.; Weierstall U.; Sawaya M. R.; Xu Q.; Messerschmidt M.; Williams G. J.; Boutet S.; Yefanov O. M.; White T. A.; Wang C.; Ishchenko A.; Tirupula K. C.; Desnoyer R.; Coe J.; Conrad C. E.; Fromme P.; Stevens R. C.; Katritch V.; Karnik S. S.; Cherezov V. Structure of the angiotensin receptor revealed by serial femtosecond crystallography. Cell 2015, 161, 833–844. 10.1016/j.cell.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M.; Mahuroof S. A.; Hong Yee V.; Carpenter J.; Schindler L.; Littmann T.; Pegoli A.; Hübner H.; Bernhardt G.; Gmeiner P.; Holliday N. D. Fluorescence labeling of neurotensin(8–13) via arginine residues gives molecular tools with high receptor affinity. ACS Med. Chem. Lett. 2020, 11, 16–22. 10.1021/acsmedchemlett.9b00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber C. G.; Pegoli A.; Müller C.; Grätz L.; She X.; Keller M. Differently fluorescence-labelled dibenzodiazepinone-type muscarinic acetylcholine receptor ligands with high M2R affinity. RSC Med. Chem. 2020, 11, 823–832. 10.1039/D0MD00137F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M.; Kuhn K. K.; Einsiedel J.; Hübner H.; Biselli S.; Mollereau C.; Wifling D.; Svobodová J.; Bernhardt G.; Cabrele C.; Vanderheyden P. M. L.; Gmeiner P.; Buschauer A. Mimicking of arginine by functionalized Nω-carbamoylated arginine as a new broadly applicable approach to labeled bioactive peptides: high affinity angiotensin, neuropeptide Y, neuropeptide FF, and neurotensin receptor ligands as examples. J. Med. Chem. 2016, 59, 1925–1945. 10.1021/acs.jmedchem.5b01495. [DOI] [PubMed] [Google Scholar]
- De Lean A.; Stadel J. M.; Lefkowitz R. J. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled β-adrenergic receptor. J. Biol. Chem. 1980, 255, 7108–7117. 10.1016/S0021-9258(20)79672-9. [DOI] [PubMed] [Google Scholar]
- Bhuiyan M. A.; Ishiguro M.; Hossain M.; Nakamura T.; Ozaki M.; Miura S.; Nagatomo T. Binding sites of valsartan, candesartan and losartan with angiotensin II receptor 1 subtype by molecular modeling. Life Sci. 2009, 85, 136–140. 10.1016/j.lfs.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Le M. T.; Vanderheyden P. M.; Szaszák M.; Hunyady L.; Vauquelin G. Angiotensin IV is a potent agonist for constitutive active human AT1 receptors. Distinct roles of the N- and C-terminal residues of angiotensin II during AT1 receptor activation. J. Biol. Chem. 2002, 277, 23107–23110. 10.1074/jbc.C200201200. [DOI] [PubMed] [Google Scholar]
- Verheijen I.; Fierens F. L.; Debacker J. P.; Vauquelin G.; Vanderheyden P. M. Interaction between the partially insurmountable antagonist valsartan and human recombinant angiotensin II type 1 receptors. Fundam. Clin. Pharmacol. 2000, 14, 577–585. 10.1111/j.1472-8206.2000.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Wingler L. M.; McMahon C.; Staus D. P.; Lefkowitz R. J.; Kruse A. C. Distinctive activation mechanism for angiotensin receptor revealed by a synthetic nanobody. Cell 2019, 176, 479–490. 10.1016/j.cell.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C.; Maschauer S.; Hübner H.; Gmeiner P.; Prante O. Synthesis and evaluation of a 18F-labeled diarylpyrazole glycoconjugate for the imaging of NTS1-positive tumors. J. Med. Chem. 2013, 56, 9361–9365. 10.1021/jm401491e. [DOI] [PubMed] [Google Scholar]
- Lundquist; Dix T. A. Synthesis and human neurotensin receptor binding activities of neurotensin(8–13) analogues containing position 8 α-azido-N-alkylated derivatives of ornithine, lysine, and homolysine. J. Med. Chem. 1999, 42, 4914–4918. 10.1021/jm9903444. [DOI] [PubMed] [Google Scholar]
- Schindler L.; Bernhardt G.; Keller M. Modifications at Arg and Ile give neurotensin(8–13) derivatives with high stability and retained NTS1 receptor affinity. ACS Med. Chem. Lett. 2019, 10, 960–965. 10.1021/acsmedchemlett.9b00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gully D.; Labeeuw B.; Boigegrain R.; Oury-Donat F.; Bachy A.; Poncelet M.; Steinberg R.; Suaud-Chagny M. F.; Santucci V.; Vita N.; Pecceu F.; Labbé-Jullié C.; Kitabgi P.; Soubrié P.; Le Fur G.; Maffrand J. P. Biochemical and pharmacological activities of SR 142948A, a new potent neurotensin receptor antagonist. J. Pharmacol. Exp. Ther. 1997, 802–812. [PubMed] [Google Scholar]
- Abdul-Ridha A.; López L.; Keov P.; Thal D. M.; Mistry S. N.; Sexton P. M.; Lane J. R.; Canals M.; Christopoulos A. Molecular determinants of allosteric modulation at the M1 muscarinic acetylcholine receptor. J. Biol. Chem. 2014, 289, 6067–6079. 10.1074/jbc.M113.539080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G. Z.; Kameyama K.; Rinken A.; Haga T. Ligand binding properties of muscarinic acetylcholine receptor subtypes (m1-m5) expressed in baculovirus-infected insect cells. J. Pharmacol. Exp. Ther. 1995, 378–384. [PubMed] [Google Scholar]
- Fish I.; Stößel A.; Eitel K.; Valant C.; Albold S.; Huebner H.; Möller D.; Clark M. J.; Sunahara R. K.; Christopoulos A.; Shoichet B. K.; Gmeiner P. Structure-based design and discovery of new M2 receptor agonists. J. Med. Chem. 2017, 60, 9239–9250. 10.1021/acs.jmedchem.7b01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubík J.; Bacáková L.; El-Fakahany E. E.; Tucek S. Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetylcholine receptors. Mol. Pharmacol. 1997, 52, 172–179. 10.1124/mol.52.1.172. [DOI] [PubMed] [Google Scholar]
- Matucci R.; Bellucci C.; Martino M. V.; Nesi M.; Manetti D.; Welzel J.; Bartz U.; Holze J.; Tränkle C.; Mohr K.; Mazzolari A.; Vistoli G.; Dei S.; Teodori E.; Romanelli M. N. Carbachol dimers with primary carbamate groups as homobivalent modulators of muscarinic receptors. Eur. J. Pharmacol. 2020, 883, 173183. 10.1016/j.ejphar.2020.173183. [DOI] [PubMed] [Google Scholar]
- Richards M. H.; van Giersbergen P. L. Human muscarinic receptors expressed in A9L and CHO cells: activation by full and partial agonists. Br. J. Pharmacol. 1995, 114, 1241–1249. 10.1111/j.1476-5381.1995.tb13339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley N. J.; Bonner T. I.; Buckley C. M.; Brann M. R. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol. Pharmacol. 1989, 469–476. [PubMed] [Google Scholar]
- Christopoulos A.; Pierce T. L.; Sorman J. L.; El-Fakahany E. E. On the unique binding and activating properties of xanomeline at the M1 muscarinic acetylcholine receptor. Mol. Pharmacol. 1998, 1120–1130. [PubMed] [Google Scholar]
- Fruchart-Gaillard C.; Mourier G.; Marquer C.; Ménez A.; Servent D. Identification of various allosteric interaction sites on M1 muscarinic receptor using 125I-Met35-oxidized muscarinic toxin 7. Mol. Pharmacol. 2006, 69, 1641–1651. 10.1124/mol.105.020883. [DOI] [PubMed] [Google Scholar]
- Huang F.; Buchwald P.; Browne C. E.; Farag H. H.; Wu W. M.; Ji F.; Hochhaus G.; Bodor N. Receptor binding studies of soft anticholinergic agents. AAPS PharmSci. 2001, 3, 44–56. 10.1208/ps030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M.; Tränkle C.; She X.; Pegoli A.; Bernhardt G.; Buschauer A.; Read R. W. M2 Subtype preferring dibenzodiazepinone-type muscarinic receptor ligands: effect of chemical homo-dimerization on orthosteric (and allosteric?) binding. Bioorg. Med. Chem. 2015, 23, 3970–3990. 10.1016/j.bmc.2015.01.015. [DOI] [PubMed] [Google Scholar]
- Keov P.; López L.; Devine S. M.; Valant C.; Lane J. R.; Scammells P. J.; Sexton P. M.; Christopoulos A. Molecular mechanisms of bitopic ligand engagement with the M1 muscarinic acetylcholine receptor. J. Biol. Chem. 2014, 289, 23817–23837. 10.1074/jbc.M114.582874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bello F.; Barocelli E.; Bertoni S.; Bonifazi A.; Camalli M.; Campi G.; Giannella M.; Matucci R.; Nesi M.; Pigini M.; Quaglia W.; Piergentili A. 1,4-Dioxane, a suitable scaffold for the development of novel M3 muscarinic receptor antagonists. J. Med. Chem. 2012, 55, 1783–1787. 10.1021/jm2013216. [DOI] [PubMed] [Google Scholar]
- Esqueda E. E.; Gerstin E. H. Jr.; Griffin M. T.; Ehlert F. J. Stimulation of cyclic AMP accumulation and phosphoinositide hydrolysis by M3 muscarinic receptors in the rat peripheral lung. Biochem. Pharmacol. 1996, 52, 643–658. 10.1016/0006-2952(96)00339-5. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L. E.; Friedman-Ohana R.; Alcobia D. C.; Riching K.; Peach C. J.; Wheal A. J.; Briddon S. J.; Robers M. B.; Zimmerman K.; Machleidt T.; Wood K. V.; Woolard J.; Hill S. J. Real-time analysis of the binding of fluorescent VEGF165a to VEGFR2 in living cells: effect of receptor tyrosine kinase inhibitors and fate of internalized agonist-receptor complexes. Biochem. Pharmacol. 2017, 136, 62–75. 10.1016/j.bcp.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach C. J.; Kilpatrick L. E.; Woolard J.; Hill S. J. Comparison of the ligand-binding properties of fluorescent VEGF-A isoforms to VEGF receptor 2 in living cells and membrane preparations using NanoBRET. Br. J. Pharmacol. 2019, 176, 3220–3235. 10.1111/bph.14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein L.; Meinel L.; Pratt R. E.; Dzau V. J.; Kobilka B. K. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol. Endocrinol. 1997, 11, 1266–1277. 10.1210/mend.11.9.9975. [DOI] [PubMed] [Google Scholar]
- Vandenbulcke F.; Nouel D.; Vincent J. P.; Mazella J.; Beaudet A. Ligand-induced internalization of neurotensin in transfected COS-7 cells: differential intracellular trafficking of ligand and receptor. J. Cell Sci. 2000, 113, 2963–2975. 10.1242/jcs.113.17.2963. [DOI] [PubMed] [Google Scholar]
- Park P. S. H.; Lodowski D. T.; Palczewski K. Activation of G protein-coupled receptors: beyond two-state models and tertiary conformational changes. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 107–141. 10.1146/annurev.pharmtox.48.113006.094630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samama P.; Cotecchia S.; Costa T.; Lefkowitz R. J. A mutation-induced activated state of the β2-adrenergic receptor. Extending the ternary complex model. J. Biol. Chem. 1993, 268, 4625–4636. 10.1016/S0021-9258(18)53442-6. [DOI] [PubMed] [Google Scholar]
- Tang T.; Tan Q.; Han S.; Diemar A.; Löbner K.; Wang H.; Schüss C.; Behr V.; Mörl K.; Wang M.; Chu X.; Yi C.; Keller M.; Kofoed J.; Reedtz-Runge S.; Kaiser A.; Beck-Sickinger A. G.; Zhao Q.; Wu B. Receptor-specific recognition of NPY peptides revealed by structures of NPY receptors. Sci. Adv. 2022, 8, eabm1232. 10.1126/sciadv.abm1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozielewicz P.; Turku A.; Bowin C.-F.; Petersen J.; Valnohova J.; Cañizal M. C. A.; Ono Y.; Inoue A.; Hoffmann C.; Schulte G. Structural insight into small molecule action on Frizzleds. Nat. Commun. 2020, 11, 414. 10.1038/s41467-019-14149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoli A.; She X.; Wifling D.; Hubner H.; Bernhardt G.; Gmeiner P.; Keller M. Radiolabeled dibenzodiazepinone-type antagonists give evidence of dualsteric binding at the M2 muscarinic acetylcholine receptor. J. Med. Chem. 2017, 60, 3314–3334. 10.1021/acs.jmedchem.6b01892. [DOI] [PubMed] [Google Scholar]
- Bartole E.; Littmann T.; Tanaka M.; Ozawa T.; Buschauer A.; Bernhardt G. [3H]UR-DEBa176: a 2,4-diaminopyrimidine-type radioligand enabling binding studies at the human, mouse, and rat histamine H4 Receptors. J. Med. Chem. 2019, 62, 8338–8356. 10.1021/acs.jmedchem.9b01342. [DOI] [PubMed] [Google Scholar]
- Müller M.; Knieps S.; Geßele K.; Dove S.; Bernhardt G.; Buschauer A. Synthesis and neuropeptide Y Y1 receptor antagonistic activity of N,N-disubstituted ω-guanidino- and ω-aminoalkanoic acid amides. Arch. Pharm. 1997, 330, 333–342. 10.1002/ardp.19973301104. [DOI] [PubMed] [Google Scholar]
- Grätz L.; Laasfeld T.; Allikalt A.; Gruber C. G.; Pegoli A.; Tahk M.-J.; Tsernant M.-L.; Keller M.; Rinken A. BRET- and fluorescence anisotropy-based assays for real-time monitoring of ligand binding to M2 muscarinic acetylcholine receptors. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118930. 10.1016/j.bbamcr.2020.118930. [DOI] [PubMed] [Google Scholar]
- Cheng Y.-C.; Prusoff W. H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.