Abstract

Due to the lack of treatment options for the genetic disease primary hyperoxaluria (PH), including three subtypes PH1, PH2, and PH3, caused by accumulation of oxalate forming kidney stones, there is an urgent need for the development of a drug therapy aside from siRNA drug lumasiran for patients with PH1. After the recent success of drug therapies based on small interfering RNA (siRNA), nedosiran is currently being developed for the treatment of three types of PH as a siRNA-based modality. Through specific inhibition of lactate dehydrogenase enzyme, the key enzyme in biosynthesis of oxalate in liver, phase 1, 2, and 3 clinical trials of nedosiran have achieved the desired primary end point of reduction of urinary oxalate levels in patients with PH1. More PH2 and PH3 patients need to be tested for efficacy. It has also produced a favorable secondary end point on safety and toxicity in PH patients. In addition to common injection site reactions that resolved spontaneously, no severe nedosiran treatment-associated adverse events were reported. Based on the positive results in the clinical studies, nedosiran is a candidate siRNA drug to treat PH patients.
Keywords: nedosiran, siRNA drug, primary hyperoxaluria
Primary hyperoxaluria (PH) is a group of rare genetic disorders associated with chronic kidney diseases and renal failure caused by kidney stones from accumulation of calcium oxalate (CaOx). It is estimated that the prevalence of PH is approximately 1–3 cases per 100,000 individuals in Europe1−3 and also approximately 1–2 per 100,000 in the United States.4 There has been lack of treatment options for PH for a long time since the first case was diagnosed in 1925.5 In additional to symptomatic relief, dual liver–kidney transplant is only option for curing PH, but it is an invasive and costly option.
Recently, with the rapid growth of small interfering RNA (siRNA)-based therapeutics,6 treatment and management of PH by siRNA drugs have shown promise.7−9 This review discusses the drug design, development, clinical studies, and management of PH by a candidate siRNA drug, nedosiran, developed by Dicerna Pharmaceuticals.
Pathology and Genetics of PH
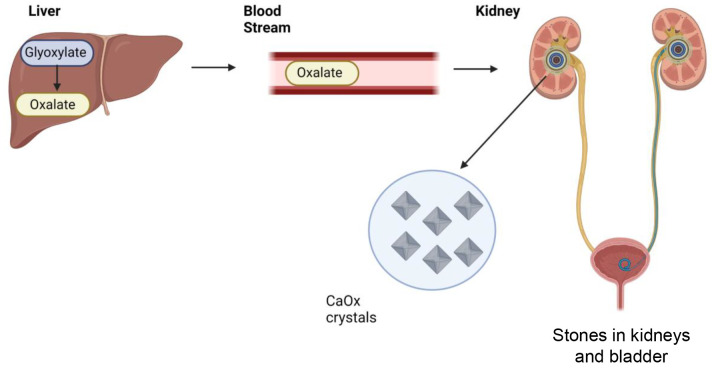
PH is the result of excessive synthesis of oxalate in liver.10,11 Since oxalate is excreted by the kidneys, excessive oxalate accumulates in the kidneys and can increase risk of the precipitation of insoluble calcium oxalate crystals, which may lead to kidney stone formation and nephrocalcinosis and may result in chronic kidney diseases and kidney failure (Figure 1).
Figure 1.
Progression of primary hyperoxaluria (PH). Accumulation of calcium oxalate (CaOx) crystals that form stones in the kidneys and bladder is due to the excessive production of oxalate in the liver.
PH is categorized into three distinct subtypes, PH type 1 (PH1), type 2 (PH2), and type 3 (PH3), based on the genetic mutations in the genes encoding key enzymes in biosynthesis of oxalate in the liver. PH1 is the most common and severe form accounting for 80% of the cases, whereas PH2 and PH3 account for 10% each.12 PH1 is caused by genetic mutations in the alanine–glyoxylate aminotransferase (AGXT) gene encoding the alanine–glyoxylate aminotransferase enzyme (AGT), which converts alanine and glyoxylate into glycine and pyruvate in peroxisomes of hepatocytes (Figure 2).13 Deficiency of the AGT enzyme can result in the accumulation of glyoxylate in peroxisomes and cytosol,14,15 which is the substrate of oxalate converted by lactate dehydrogenase enzyme (LDH) in the cytosol. Mutations in the AGXT gene cause the deficiency of AGT leading to failed transamination of glyoxylate to glycine.16 In addition to be converted to oxalate by LDH in both cytosol and mitochondria, glyoxylate can also be converted to glycolate by glyoxylate reductase (GR). PH2 is caused by the genetic mutations in the glyoxylate reductase gene (GRHPR) encoding the GR enzyme.17,18 Deficiency of GR enzyme can result in more conversion of oxalate from glyoxylate in cytosol (Figure 2). PH3 involves functional loss of the 4-hydroxy-2-oxoglutarate aldolase (HOGA1) enzyme in mitochondria encoded by the HOGA1 gene (Figure 2).19,20 Mutations in the HOGA1 gene (also called DHDPSL gene) are responsible for PH321 and have been associated with PH3 patients in different populations.22,23 The exact mechanism by which genetic mutations in the HOGA1 gene contribute to downstream oxalate accumulation is unclear. PH3 likely exists in the first months to years of life with early symptomatic nephrolithiasis.24 LDH is the critical enzyme in the last step of the biosynthesis pathways of oxalate formation in liver; therefore, reduction of LDH has potential for the treatment of all known types of PH.25
Figure 2.
Biosynthesis pathways of oxalate in hepatocytes and pathological formation of PH1, PH2, and PH3 due to genetic mutations in the alanine-glycine aminotransferase (AGXT), glyoxylate reductase (GRHPR), and 4-hydroxy-2-oxoglutarate aldolase (HOGA1) genes, respectively. The overaccumulation of oxalate in the cytosol of hepatocytes is a result of overconversion from glyoxylate by the enzyme lactate dehydrogenase (LDH) encoded by the LDH gene. Deficiency of the AGT enzyme in the peroxisomes caused by the genetic mutations in the AGXT gene results in less conversion of glyoxylate to pyruvate in peroxisomes and more accumulation of oxalate in the cytosol (PH1). Lumasiran is approved to treat PH1 patients by inhibiting the overproduction of glyoxylate from glycolate in peroxisomes through inhibition of the glycolate oxidase (GO) enzyme. Deficiency of glyoxylate reductase (GR) in mitochondria caused by genetic mutations in the GRHPR gene can result in overaccumulation of glyoxylate (PH2). Functional loss of the HOGA1 enzyme in mitochondria encoded by the HOGA1 gene can result in the overaccumulation of glyoxylate (PH3). Nedosiran targets the degradation of the mRNA of LDH and reduces the production of oxalate from all types of PH patients.
When a patient is diagnosed with PH and has large kidney stones, which cause pain or block urine flow, kidney stones need to be managed by removing them or breaking them up so they can pass in the urine. If PH has caused severe kidney function damage, a kidney transplant or kidney and liver transplant can cure certain types of PH, but these procedures have a high risk of rejection. Reduction of oxalate production in liver is the basis for the treatment of PH. In addition to dietary changes and high fluid intake, medications, such as vitamin B-6, oral phosphates and citrate, and thiazide diuretics, have certain effectiveness in reducing oxalate in the urine. Recently, the Food and Drug Administration (FDA) has approved a siRNA drug, lumasiran, to treat PH1 patients.
Development of siRNA-Based Drugs for PH
In the pathways of oxalate biosynthesis in liver, excess of some key enzymes can result in the accumulation of oxalate, and these enzymes can serve as targets for the development of siRNA-based therapeutics. In 2020, the FDA approved an siRNA drug, lumasiran, developed by Alnylam Pharmaceuticals, for the treatment of PH1 patients in all age groups.26 Lumasiran works by reducing the production of oxalate through the siRNA-mediated degradation of hydroxyacid oxidase 1 (HAO1) mRNA, also called glycolate oxidase (GO) enzyme mRNA, which further results in decreased HAO1/GO enzyme production (Figure 2).7,27 Reduction of HAO1/GO enzyme production in peroxisomes can result in less accumulation of glyoxylate in peroxisomes and cytosol, leading to less accumulation of oxalate in liver and kidneys. Lumasiran has shown promising clinical benefits in PH1 with sustained lowering of oxalate levels, acceptable safety, and encouraging results on clinical outcomes in clinical trials28−31 and also benefits for management of PH1 in small infants32 and twins33 in the postmarketing studies.
There is no therapeutic drug available yet for the management of PH2 and PH3. Thus, a new drug is needed that would help to treat all three types of PH. Encouragingly, Dicerna Pharmaceuticals is developing a siRNA-based drug, nedosiran, which has potential to treat all three types of PH.34
Chemistry of Nedosiran
Nedosiran is a synthetic, chemically modified, double-stranded siRNA, consisting of a 22-base antisense strand and a 36-base sense stand. Of these bases, 19 have a 2′-fluoro-ribonucleotide (2′-F) substitution and 35 have a 2-O-methyl-ribonucleotide (2′-OMe) substitution. In addition, a phosphorothioate group is added to the two nucleotides at the 5′-end of the sense and both the 5′ and 3′ ends of the antisense strands. Its sense strand is conjugated with a triantennary N-acetyl-d-galactosamine amino sugar residue (GalNAc). The GalNAc–siRNA conjugates are a solution to solve the problem of siRNA delivery to hepatocytes in liver.35
Principle of Drug Action of Nedosiran
Nedosiran is designed to inhibit production of hepatic LDH, resulting in reduction of oxalate accumulation (Figure 2).36 By inhibition of hepatic LDH, the key enzyme responsible for converting glyoxylate to oxalate, oxalate would not accumulate, preventing CaOx precipitation in the kidneys and the formation of kidney stones and end-stage renal diseases.37 Blocking LDH can reduce accumulation of oxalate caused by the genetic mutations in the genes associated with all three types of PH. The drug nedosiran goes through a series of events at cellular and molecular levels in hepatocytes (Figure 3). Once nedosiran is subcutaneously injected and reaches the liver, GalNAc-conjugated nedosiran is preferentially taken into hepatocytes via the asialoglycoprotein receptor (ASGPR).36,37 ASGPR is very abundant in hepatocyte cells with roughly 500 000 copies per cell. Among these, 5%–10% are presented at the sinusoidal surface of hepatocytes at any given time.35 Binding to ASGPR by GalNAc residues occurs at the sinusoidal surface, which initiates monomeric ASGPR to diffuse, followed by endocytosis or internalization.35 Internalization of nedosiran occurs by rapid local aggregation of ligand bound ASGPR receptors, followed by larger scale aggregation in clathrin coated pits, leading to endocytosis.35 Once in the cytoplasm, acidification during endosomal maturation results in release of GalNAc-conjugated nedosiran from ASGPR into the cytoplasm. GalNAc is then degraded in the lysosomes while ASGPR is recycled to the cell surface.35 Nedosiran is loaded into the RNA-induced silencing complex (RISC) containing Dicer, transactivating response RNA-binding protein (TRBP), and Argonaute 2 (AGO2). The sense strand is removed by AGO2, leaving the antisense strand still bound. Then AGO2 with help from TRBP provides antisense strand structural context for the initial binding of the seed region to the target LDH mRNA to exploits its endogenous RNAi regulatory mechanism to degrade LDH mRNA, thereby reducing production of the LDH protein.35,36 Reduction of LDH protein leads to decreased production and accumulation of oxalate.36
Figure 3.
Principle of drug action of nedosiran. After subcutaneous administration, nedosiran is transported across the interstitial space into the blood and then to the liver via asialoglycoprotein receptor (ASGPR)-mediated uptake. The GalNAc moiety of nedosiran binds to the ASGPR receptor expressed on the surface of hepatocytes followed by internalization via endocytosis. After endocytosis, nedosiran is trapped in endocytic vesicles, which fuse with endosomes/lysosomes. The GalNAc ligand of nedosiran is then degraded and the double stranded siRNA is slowly released into cytoplasm from endosomes/lysosomes, while ASGPR is recycled to the cell surface. The double-stranded siRNA component of nedosiran is then loaded into the RNA-induced silencing complex (RISC), containing Dicer, transactivating response RNA-binding protein (TRBP), and Argonaute 2 (AGO2), which removes the sense strand. The antisense strand retained in the RISC scans and binds to the complementary sequence in its target LDH mRNA. The RISC utilizes the catalytic slicer activity that degrades LDH mRNA, resulting in less mRNA available for translation. As a result, less LDH enzyme is available to convert glyoxylate to oxalate in hepatocytes and leading to less accumulation of CaOx in the kidneys for the PH progress.
Preclinical Studies of Nedosiran
Pharmacological efficacy and safety of nedosiran were first evaluated by preclinical studies with in vitro and in vivo models (Figure 4). In vitro studies addressed the synthesis of oxalate from glyoxylate with the one key enzyme being LDH.38 Other systems tested were GO and xanthine oxidase. Xanthine oxidases were determined to have in vitro but not in vivo activity. On the other hand, GO and LDH were studied for their in vitro activities.39 These two systems are currently the targets of siRNA therapeutics for PH. Of the two enzymatic systems, LDH demonstrated wide distribution and high activity triggering further investigation of in vivo studies.39
Figure 4.
Preclinical studies of nedosiran. (1) In vitro studies demonstrated that nedosiran could effectively reduce production of oxalate by inhibition of LDH. (2) A mouse PH1 model was used to prove the reduction of urinary oxalate (UOx) levels by treatment with nedosiran. (3) Nedosiran treatment with GRHPR-knockdown PH2 mice (HP2 model) showed increased oxalate clearance. (4) Transferability to humans was tested in non-human primates and humanized mice with human hepatocyte replacement.
In mouse models, GO was reported as a safe and efficient target for substrate reduction therapy in PH1.40 siRNA targeting GO was previously shown to reduce UOx levels in mouse models with PH1.41 siRNA targeting LDHA in mouse models showed reduced urinary oxalate excretion and also significant changes in a number of glycolytic and tricarboxylic acid (TCA) cycle metabolites secondary to hepatic LDHA inhibition.42 Therefore, using GalNAc–siRNA targeting either LDH or GO is a suitable comparison to test the efficacy of the interference on the LDH reduction to UOx production. GalNAc-siRNA targeting LDH was shown to significantly reduce UOx concentration in mouse models with a single subcutaneous (SQ) injection of 5 mg/kg dose.37 This in vivo study was the first to confirm the potential of the LDH targeting in oxalate reduction.
Kidney damage as a result of PH1 is also a main concern. Using mouse models fed with ethylene glycol (EG) to elicit kidney CaOx, LDH target inhibition by siRNA was evaluated. After eight weekly doses of GalNAc–siRNA conjugate targeting LDH, histological characterization of kidney tissue demonstrated reduced kidney CaOx deposition.37
Although both GO and LDH play a role in oxalate conversion, LDH was shown to be the key enzyme in catalyzing the conversion.37 Reduction of UOx levels in siRNA-mediated inhibition of LDH was observed in the condition of high glyoxylate concentration with a functional GO enzyme.37
Since PH2 is another genetic subtype of PH that differs from PH1, mouse genetically engineered with PH2 were tested with GalNAc–siRNA targeting hepatic LDH. Reduced UOx levels and increased oxalate clearance occurred when siRNA targeted LDH, as compared to GO. In another experiment, investigating the role of LDH in oxalate production under hyperoxaluric conditions, siRNA targeting LDH resulted in reduced UOx levels to near baseline levels in GRHPR-knockdown PH2 mice fed with EG.37 These studies validated hepatic LDH as a suitable target in oxalate conversion.
A common issue with in vivo experiments is transferability of findings to human models. To test this, inhibition of LDH target mRNA in non-human primates and chimeric mice with humanized hepatocyte content was evaluated. Liver biopsies were collected across time points and screened for LDH mRNA, protein levels, and activity. Expected gene inhibition and delivery to liver hepatocytes were demonstrated in non-human primates and chimeric mouse models, suggesting translatable results. Hepatic LDH as an efficient target for reducing oxalate production was demonstrated in animal models with both PH1 and PH2. LDH RNAi conjugates were shown to result in potent and lasting reduction in LDH protein and enzyme activity in mouse models and non-human primates.37
Pharmacokinetics and Metabolism of Nedosiran
Preclinical studies with animal models proved that siRNAs targeting LDH could decrease oxalate production in PH without affecting nontargeted tissues. In mouse and non-human primate models, GalNAc conjugated siRNAs were found to knockdown the LDH enzyme specifically in liver cells, with no consequence in other tissues.37 Normally, LDH will convert lactate to pyruvate for gluconeogenesis. Inhibition of LDH should therefore lead to an accumulation of lactate and possible lactic acidosis. However, there were no elevated lactate levels observed in the mice injected with LDHA targeted siRNAs or with LDHA knockdown. LDH inhibition may also decrease pyruvate levels, which could lead to adverse effects such as myopathy. However, the mice in the study treated with LDHA targeted siRNAs showed normal muscle function and even a transient elevation of pyruvate levels. This proves that hepatic-specific LDH inhibition does not interfere with gluconeogenesis.36 To further support this claim, there were no signs of increased plasma lactate and pyruvate levels in mouse and non-human primate models, proving that the GalNAc conjugated siRNAs had no other systemic effects than the inhibition of hepatic LDH.37 The lack of unintended consequences in nonhepatic tissues in animal models suggested a good safety profile for nedosiran in humans, which proved to be true in clinical trials.
Clinical studies aimed to establish a dose that could yield a useful reduction of UOx level within a 24 h period. Weight-based dosing and other dose related adjustments were utilized to determine this dosage. From the clinical trial PHYOX1, it took an average of 6–12 h for nedosiran to reach peak plasma concentrations. The elimination half-life of a single dose was also found to be between 4.6 and 13.8 h.36 In part two of this study, an increase of nedosiran dose resulted in an equal increase in peak plasma nedosiran concentrations and an equal increase in the area under the concentration–time curve. This indicates a linear pharmacokinetic profile for nedosiran.36
Furthermore, the study also showed a trend of greater reduction of UOx with increasing doses of nedosiran. It was determined that a fixed dose of 160 mg of nedosiran once a month was most effective for optimizing the greatest reduction of UOx. Weight-based dosing, different dosing intervals, and different loading regimens were not as effective in helping patients to reach normal or near-normal UOx excretion within a 24 h period. During the second part of the study, 10 of 18 patients (55.6%) were able to achieve normal 24 h UOx excretion (<0.46 mmol per 24 h) and four more patients were able to achieve near-normal 24 h UOx excretion (<0.6 mmol per 24 h).36
Clinical Studies
Nedosiran has completed phase I and II clinical trials and is currently in phase II and III clinical trials (Table 1). PHYOX1 (ClinicalTrials.gov Identifier: NCT03392896) was a placebo-controlled, single-blind phase I study that was completed in 2019. The main purpose of this study was to evaluate the safety profile, tolerability, pharmacokinetics, and pharmacodynamics of nedosiran. Safety was measured in group A with 25 healthy volunteers using methods that included adverse drug reactions (ADRs), physical examination, laboratory testing, concomitant medications, vital signs, and electrocardiograms.34 No significant drug related ADRs were observed during the study.36 Most of the ADRs were mild to moderate injection site reactions that were resolved spontaneously within a few days. Four patients experienced severe ADRs, such as pyelonephritis, ureterolithiasis, and appendicitis. However, they were not considered to be a result of the nedosiran treatment and therefore not considered as treatment related. Additionally, it was determined in the study in group B with 18 PH1 or PH2 patients that a fixed dose of 160 mg of nedosiran once monthly was the most effective dose for optimizing 24 h UOx excretion.36 PHYOX4 (ClinicalTrials.gov Identifier: NCT04555486) was another phase I placebo-controlled, double-blind, multicenter study with a purpose of evaluating nedosiran safety and efficacy in 6 PH3 patients who had at least one kidney stone in the past year. The study was completed in September 2021 with initial data showing results that were consistent with the trials that have already been conducted. PHYOX4 met the primary end point, in which nedosiran demonstrated a good safety profile in patients with PH3. All ADRs reported were mild, with the most common being back pain. The study’s secondary end point was at least a 30% decrease in a 24 h UOx excretion from baseline. These levels must also be observed on at least two consecutive visits. Although this end point was not achieved in this study, the patients all showed decreased levels of UOx, demonstrating the efficacy of nedosiran. Nedosiran has not been shown to cause any immune responses though nedosiran’s ability to cause immune responses has not been examined in animal models.
Table 1. Summary of the Clinical Trials of Nedosiran (DCR-PHXC).
| phase | clinicaltrials.gov identifier | recruitment status | actual or estimated enrollment | type of study | summary of major findings |
|---|---|---|---|---|---|
| 1 | PHYOX1, NCT03392896 | completed 11/19/2019 | 25 normal healthy volunteers; 18 PH1 or PH2 patients | placebo-controlled, single-blind, single-center study to evaluate the safety, pharmacokinetics, and tolerability; open-label multicenter study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of single ascending doses | no significant drug related ADRs were observed; the most common ADRs were mild to moderate injection site reactions that were resolved spontaneously; four patients experienced severe ADRs that were not considered treatment-related and have since resolved |
| 1 | PHYOX4, NCT04555486 | completed 09/07/2021 | 6 PH3 patients | placebo-controlled, double-blind, multicenter study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of a single dose | primary end point was met with nedosiran, demonstrating a good safety profile in patients with PH3; all ADRs reported were mild, with the most common being back pain; the secondary end point was not met; however, all patients showed decreased levels of UOx compared to baseline levels, demonstrating the efficacy of nedosiran |
| 2 | PHYOX2, NCT03847909 | completed 06/29/2021 | 35 PH1 or PH2 patients | placebo-controlled, double-blind, multicenter study to evaluate the efficacy, safety, and tolerability | the primary end point was met with patients who received nedosiran having a 57.5% greater daily reduction in UOx compared to placebo; the secondary end point was also met with 50% of the patients given nedosiran achieving and maintaining a normal or near-normal UOx level compared to placebo; nedosiran was generally well tolerated, with the most common ADRs being injection site reactions that resolved spontaneously; three patients who received nedosiran reported kidney stones |
| 2 | PHYOX7, NCT04580420 | estimated completion 05/2025 | 12 PH1 or PH2 patients with severe renal impairment with or without dialysis | open-label study to evaluate the safety and efficacy | not reported yet |
| 2 | PHYOX8, NCT05001269 | estimated completion 12/2022 | 10 HP1 or HP2 pediatric patients from birth to 5 years and relatively intact renal function | open-label multicenter study to evaluate the safety, pharmacokinetics, and efficacy | not reported yet |
| 3 | PHYOX3, NCT04042402 | estimated completion 12/2023 | 50 PH1 or PH2 patients from the previous phase I and II studies | open-label roll-over study to evaluate the long-term safety and efficacy | an interim data report shows that nedosiran is generally well tolerated, and there have been no serious adverse events thus far; all participants who have received nedosiran achieved normal or near-normal UOx by day 180 |
PHYOX2 (ClinicalTrials.gov Identifier: NCT03847909) was a placebo-controlled, double-blind, randomized phase II study in children and adults with 35 PH1 or PH2 patients that was completed in 2021. The purpose of this study was to evaluate the safety and efficacy of a monthly subcutaneous injection of nedosiran. In PHYOX2, patients who received nedosiran had a 57.5% greater daily reduction in UOx compared to placebo. Additionally, among those who were given nedosiran, 50% were able to achieve and maintain a normal or near-normal UOx level compared to placebo. Results in PH2 patients varied because only two out of the five patients that were given nedosiran showed reduction in UOx levels between day 90 and day 180.43 Nedosiran was well tolerated, similar to PHYOX1. The most common ADRs were injection site reactions that resolved spontaneously. However, there were reports of kidney stones in PHYOX2 as well in three patients who received nedosiran and five who received placebo.43
There are currently two other ongoing phase II clinical trials for testing the safety and efficacy of nedosiran. PHYOX7 (ClinicalTrials.gov Identifier: NCT04580420) has the purpose of evaluating nedosiran safety and efficacy in 12 PH1 and PH2 patients with severe renal impairment, who may or may not be undergoing dialysis. The study is estimated to be completed in May 2025. PHYOX8 (ClinicalTrials.gov Identifier: NCT05001269) is a study being planned with the purpose of evaluating nedosiran safety and efficacy in 10 pediatric patients having an age of five years or younger with intact renal function. This study is expected to be completed in December 2022.
Phase III trial PHYOX3 (ClinicalTrials.gov Identifier: NCT04042402) is a currently ongoing study. It is a multidose, open-label trial with the purpose of further evaluating the long-term safety and efficacy of nedosiran for 50 PH1 and PH2 patients who were enrolled in the previous two clinical trials. The primary end point of PHYOX3 is to evaluate nedosiran’s ability to preserve kidney function by measuring the annual rate of decline in estimated glomerular filtration rate (eGFR). No results on the primary end point have been published so far. All 13 participants in this study who have reached day 180 have achieved normal or near-normal UOx levels. The secondary end point of PHYOX3 is to evaluate the incidence and severity of treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs). The most common ADRs, as in previous clinical trials, were injection site reactions. However, there were two serious TEAEs, pyelonephritis and nephrolithiasis, but they were determined to be not caused by nedosiran.
The results and data available from three completed clinical trials and three ongoing clinical trials show that nedosiran has a good safety profile and is effective in significantly reducing UOx in PH patients. However, phase III clinical trials are still ongoing, and nedosiran needs to be tested in more PH2 and PH3 patients, pediatric patients, and patients with renal impairment before the drug can be approved by the FDA.
A compassionate use case report showed that nedosiran dramatically reduced serum oxalate in a dialysis dependent PH1 patient.44 The study concluded that nedosiran represents a novel and impactful potential therapeutic for PH patients with end-stage renal diseases.
Safety and Toxicity of Nedosiran
Dicerna Pharmaceuticals initiated the PHYOX clinical trials in 2017 to assess nedosiran’s safety, tolerability, pharmacokinetics, and efficacy in patients. PHYOX1 phase 1 clinical trial, which started in late 2017, was conducted with two distinct groups. Group A consisted of healthy individuals, and group B had patients with either PH1 or PH2. PHYOX1 phase 1 study showed no significant ADRs or dose-limiting toxicity.36 PHYOX2 clinical trial included patients with PH1 and PH2. In PHYOX2 clinical trial, nedosiran achieved primary end point, producing clinically significant reductions in UOx levels. No significant or new ADRs were reported compared to the previous study. PHYOX3 is an ongoing long-term study for participants who had completed any of the previous PHYOX trials. PHYOX4 was a randomized, placebo-controlled, double-blind, multicenter clinical study that evaluated the safety and tolerability of nedosiran in patients with PH3. No serious ADRs were reported for this study. All ADRs that were reported were mild and irrelevant to the drug with the most common event of back pain. Dicerna is currently enrolling for 3 ongoing clinical trials that evaluate nedosiran’s usage in comorbid conditions with patients of all three types of PH.
Potential Drug Interactions
GalNAc conjugated siRNAs have limited ability to influence metabolism of small chemical drugs mediated by cytochrome P450s (CYPs) for potential drug–drug interactions (DDIs). GalNAc conjugated siRNAs have been studied non-clinically for DDIs as substrates, inhibitors, or inducers of CYPs.45 The study found that CYP2C8 was inhibited at high concentrations while CYP2B6 was mildly inhibited. However, overall data showed a low risk of DDIs with GalNAc conjugated siRNAs. As this study was conducted in vitro, no conclusions can be drawn about nedosiran’s compatibility with other drugs clinically.45
Conclusion
PH is a group of rare genetic disorders without therapeutic treatment options for a long time. Recent development of siRNA-based therapeutics has provided opportunity to manage the disease by therapeutic options. By targeting LDH in liver, nedosiran has shown promising clinical outcomes for management of PH1 with tolerable ADRs and limited potential DDIs. More PH2 and PH3 patients are needed to be tested for efficacy and ADRs. The siRNA drug nedosiran may receive FDA approval in the near future to treat various PH patients.
Acknowledgments
This study was partly supported by the National Institutes of Health (NIH), National Institute of General Medical Sciences [Grant R35GM140862 to X.B.Z.].
Glossary
Abbreviations
- 2′-F
2′-fluoro-ribonucleotide
- 2′-OMe
2-O-methyl-ribonucleotide
- AGO2
Argonaute 2
- AGT
alanine–glycine aminotransferase
- ASGPR
asialoglycoprotein receptor
- CaOx
calcium oxalate
- CYP
cytochrome P450
- DDI
drug–drug interaction
- EG
ethylene glycol
- FDA
Food and Drug Administration
- GalNAc
N-acetyl-d-galactosamine amino sugar residue
- GO
glycolate oxidase
- GR
glyoxylate reductase
- HAO1
hydroxyacid oxidase 1
- HOGA1
4-hydroxy-2-oxoglutarate aldolase
- LDH
lactate dehydrogenase
- PH
primary hyperoxaluria
- RISC
RNA-induced silencing complex
- siRNA
small interfering RNA
- TRBP
transactivating response RNA-binding protein
- UOx
urinary oxalate
Author Contributions
A.L., J.Z., M.S., J.M.M., and S.M.T. wrote the manuscript, and R.B., T.P.R., J.E.M., and X.B.Z. provided comments for revision.
The authors declare no competing financial interest.
References
- van Woerden C. S.; Groothoff J. W.; Wanders R. J. A.; Davin J.-C.; Wijburg F. A. Primary Hyperoxaluria Type 1 in The Netherlands: Prevalence and Outcome. Nephrol Dial Transplant 2003, 18 (2), 273–279. 10.1093/ndt/18.2.273. [DOI] [PubMed] [Google Scholar]
- Kopp N.; Leumann E. Changing Pattern of Primary Hyperoxaluria in Switzerland. Nephrology Dialysis Transplantation 1995, 10 (12), 2224–2227. 10.1093/ndt/10.12.2224. [DOI] [PubMed] [Google Scholar]
- Cochat P.; Deloraine A.; Rotily M.; Olive F.; Liponski I.; Deries N. Epidemiology of Primary Hyperoxaluria Type 1. Société de Néphrologie and the Société de Néphrologie Pédiatrique. Nephrol Dial Transplant 1995, 10 (Suppl 8), 3–7. 10.1093/ndt/10.supp8.3. [DOI] [PubMed] [Google Scholar]
- Shah A.; Leslie S. W.; Ramakrishnan S.. Hyperoxaluria; StatPearls, 2022. [PubMed] [Google Scholar]
- Viggiano D.; Simonelli F.; Capasso G.; de Santo N. SP771HISTORY OF RENAL AND OCULAR FINDINGS IN PRIMARY HYPEROXALURIA: FROM OXALIS ACETOSELLA TO THE MULBERRY CALCULUS, CKD, RETINOPATHY AND SYSTEMIC OXALOSIS. Nephrology Dialysis Transplantation 2018, 33 (suppl_1), i608–i608. 10.1093/ndt/gfy104.SP771. [DOI] [Google Scholar]
- Zhang M. M.; Bahal R.; Rasmussen T. P.; Manautou J. E.; Zhong X. The Growth of SiRNA-Based Therapeutics: Updated Clinical Studies. Biochem. Pharmacol. 2021, 189, 114432. 10.1016/j.bcp.2021.114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebow A.; Li X.; Racie T.; Hettinger J.; Bettencourt B. R.; Najafian N.; Haslett P.; Fitzgerald K.; Holmes R. P.; Erbe D.; Querbes W.; Knight J. An Investigational RNAi Therapeutic Targeting Glycolate Oxidase Reduces Oxalate Production in Models of Primary Hyperoxaluria. J. Am. Soc. Nephrol 2017, 28 (2), 494–503. 10.1681/ASN.2016030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes T. A.; Brown B. D.; Lai C. Therapeutic RNA Interference: A Novel Approach to the Treatment of Primary Hyperoxaluria. Br. J. Clin. Pharmacol. 2022, 88, 2525. 10.1111/bcp.14925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejban P.; Lieske J. C. New Therapeutics for Primary Hyperoxaluria Type 1. Curr. Opin Nephrol Hypertens 2022, 31, 344. 10.1097/MNH.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal D.; Su W. S.; DaBreo D.; Puglia M.; Gregor L.; Gangji A. S. Liver-Kidney Transplantation in Primary Hyperoxaluria Type-1: Case Report and Literature Review. Int. J. Organ Transplant Med. 2011, 2 (3), 126–132. [PMC free article] [PubMed] [Google Scholar]
- Xie X.; Zhang X. Primary Hyperoxaluria. New England Journal of Medicine 2022, 386 (10), 976–976. 10.1056/NEJMicm2113369. [DOI] [PubMed] [Google Scholar]
- Soliman N. A.; Nabhan M. M.; Abdelrahman S. M.; Abdelaziz H.; Helmy R.; Ghanim K.; Bazaraa H. M.; Badr A. M.; Tolba O. A.; Kotb M. A.; Eweeda K. M.; Fayez A. Clinical Spectrum of Primary Hyperoxaluria Type 1: Experience of a Tertiary Center. Nephrol Ther 2017, 13 (3), 176–182. 10.1016/j.nephro.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe B. Evidence of True Genotype–Phenotype Correlation in Primary Hyperoxaluria Type 1. Kidney Int. 2010, 77 (5), 383–385. 10.1038/ki.2009.471. [DOI] [PubMed] [Google Scholar]
- Lage M. D.; Pittman A. M. C.; Roncador A.; Cellini B.; Tucker C. L. Allele-Specific Characterization of Alanine: Glyoxylate Aminotransferase Variants Associated with Primary Hyperoxaluria. PLoS One 2014, 9 (4), e94338. 10.1371/journal.pone.0094338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams E. L.; Acquaviva C.; Amoroso A.; Chevalier F.; Coulter-Mackie M.; Monico C. G.; Giachino D.; Owen T.; Robbiano A.; Salido E.; Waterham H.; Rumsby G. Primary Hyperoxaluria Type 1: Update and Additional Mutation Analysis of the AGXT Gene. Hum Mutat 2009, 30 (6), 910–917. 10.1002/humu.21021. [DOI] [PubMed] [Google Scholar]
- Amoroso A.; Pirulli D.; Florian F.; Puzzer D.; Boniotto M.; Crovella S.; Zezlina S.; Spanò A.; Mazzola G.; Savoldi S.; Ferrettini C.; Berutti S.; Petrarulo M.; Marangella M. AGXT Gene Mutations and Their Influence on Clinical Heterogeneity of Type 1 Primary Hyperoxaluria. J. Am. Soc. Nephrol 2001, 12 (10), 2072–2079. 10.1681/ASN.V12102072. [DOI] [PubMed] [Google Scholar]
- Cregeen D. P.; Williams E. L.; Hulton S.; Rumsby G. Molecular Analysis of the Glyoxylate Reductase (GRHPR) Gene and Description of Mutations Underlying Primary Hyperoxaluria Type 2. Hum Mutat 2003, 22 (6), 497. 10.1002/humu.9200. [DOI] [PubMed] [Google Scholar]
- Bhat S.; Williams E. L.; Rumsby G. Tissue Differences in the Expression of Mutations and Polymorphisms in the GRHPR Gene and Implications for Diagnosis of Primary Hyperoxaluria Type 2. Clin Chem. 2005, 51 (12), 2423–2425. 10.1373/clinchem.2005.058305. [DOI] [PubMed] [Google Scholar]
- Riedel T. J.; Knight J.; Murray M. S.; Milliner D. S.; Holmes R. P.; Lowther W. T. 4-Hydroxy-2-Oxoglutarate Aldolase Inactivity in Primary Hyperoxaluria Type 3 and Glyoxylate Reductase Inhibition. Biochim. Biophys. Acta 2012, 1822 (10), 1544–1552. 10.1016/j.bbadis.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel T. J.; Johnson L. C.; Knight J.; Hantgan R. R.; Holmes R. P.; Lowther W. T. Structural and Biochemical Studies of Human 4-Hydroxy-2-Oxoglutarate Aldolase: Implications for Hydroxyproline Metabolism in Primary Hyperoxaluria. PLoS One 2011, 6 (10), e26021. 10.1371/journal.pone.0026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belostotsky R.; Seboun E.; Idelson G. H.; Milliner D. S.; Becker-Cohen R.; Rinat C.; Monico C. G.; Feinstein S.; Ben-Shalom E.; Magen D.; Weissman I.; Charon C.; Frishberg Y. Mutations in DHDPSL Are Responsible for Primary Hyperoxaluria Type III. Am. J. Hum. Genet. 2010, 87 (3), 392–399. 10.1016/j.ajhg.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.; Liu Y.; Kang L.; He R.; Song J.; Li Y.; Li J.; Yang Y. Mutation Hot Spot Region in the HOGA1 Gene Associated with Primary Hyperoxaluria Type 3 in the Chinese Population. Kidney Blood Press Res. 2019, 44 (4), 743–753. 10.1159/000501458. [DOI] [PubMed] [Google Scholar]
- M’dimegh S.; Aquaviva-Bourdain C.; Omezzine A.; Souche G.; M’barek I.; Abidi K.; Gargah T.; Abroug S.; Bouslama A. HOGA1 Gene Mutations of Primary Hyperoxaluria Type 3 in Tunisian Patients. J. Clin Lab Anal 2017, 31 (3), e22053. 10.1002/jcla.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monico C. G.; Rossetti S.; Belostotsky R.; Cogal A. G.; Herges R. M.; Seide B. M.; Olson J. B.; Bergstrahl E. J.; Williams H. J.; Haley W. E.; Frishberg Y.; Milliner D. S. Primary Hyperoxaluria Type III Gene HOGA1 (Formerly DHDPSL) as a Possible Risk Factor for Idiopathic Calcium Oxalate Urolithiasis. Clin J. Am. Soc. Nephrol 2011, 6 (9), 2289–2295. 10.2215/CJN.02760311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariceta G.; Barrios K.; Brown B. D.; Hoppe B.; Rosskamp R.; Langman C. B. Hepatic Lactate Dehydrogenase A: An RNA Interference Target for the Treatment of All Known Types of Primary Hyperoxaluria. Kidney Int. Rep 2021, 6 (4), 1088–1098. 10.1016/j.ekir.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L. J.; Keam S. J. Lumasiran: First Approval. Drugs 2021, 81 (2), 277–282. 10.1007/s40265-020-01463-0. [DOI] [PubMed] [Google Scholar]
- Shah V. N.; Pyle L. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N Engl J. Med. 2021, 385 (20), e69. 10.1056/NEJMc2107661. [DOI] [PubMed] [Google Scholar]
- Hulton S. A.; Groothoff J. W.; Frishberg Y.; Koren M. J.; Overcash J. S.; Sellier-Leclerc A.-L.; Shasha-Lavsky H.; Saland J. M.; Hayes W.; Magen D.; Moochhala S. H.; Coenen M.; Simkova E.; Garrelfs S. F.; Sas D. J.; Meliambro K. A.; Ngo T.; Sweetser M. T.; Habtemariam B. A.; Gansner J. M.; McGregor T. L.; Lieske J. C. Randomized Clinical Trial on the Long-Term Efficacy and Safety of Lumasiran in Patients With Primary Hyperoxaluria Type 1. Kidney Int. Rep 2022, 7 (3), 494–506. 10.1016/j.ekir.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sas D. J.; Magen D.; Hayes W.; Shasha-Lavsky H.; Michael M.; Schulte I.; Sellier-Leclerc A.-L.; Lu J.; Seddighzadeh A.; Habtemariam B.; McGregor T. L.; Fujita K. P.; Frishberg Y.; et al. Phase 3 Trial of Lumasiran for Primary Hyperoxaluria Type 1: A New RNAi Therapeutic in Infants and Young Children. Genet Med. 2022, 24 (3), 654–662. 10.1016/j.gim.2021.10.024. [DOI] [PubMed] [Google Scholar]
- Frishberg Y.; Deschênes G.; Groothoff J. W.; Hulton S.-A.; Magen D.; Harambat J.; Van’t Hoff W. G.; Lorch U.; Milliner D. S.; Lieske J. C.; Haslett P.; Garg P. P.; Vaishnaw A. K.; Talamudupula S.; Lu J.; Habtemariam B. A.; Erbe D. v; McGregor T. L.; Cochat P. study collaborators. Phase 1/2 Study of Lumasiran for Treatment of Primary Hyperoxaluria Type 1: A Placebo-Controlled Randomized Clinical Trial. Clin J. Am. Soc. Nephrol 2021, 16 (7), 1025–1036. 10.2215/CJN.14730920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrelfs S. F.; Frishberg Y.; Hulton S. A.; Koren M. J.; O’Riordan W. D.; Cochat P.; Deschênes G.; Shasha-Lavsky H.; Saland J. M.; van’t Hoff W. G.; Fuster D. G.; Magen D.; Moochhala S. H.; Schalk G.; Simkova E.; Groothoff J. W.; Sas D. J.; Meliambro K. A.; Lu J.; Sweetser M. T.; Garg P. P.; Vaishnaw A. K.; Gansner J. M.; McGregor T. L.; Lieske J. C. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. New England Journal of Medicine 2021, 384 (13), 1216–1226. 10.1056/NEJMoa2021712. [DOI] [PubMed] [Google Scholar]
- Méaux M.-N.; Sellier-Leclerc A.-L.; Acquaviva-Bourdain C.; Harambat J.; Allard L.; Bacchetta J. The Effect of Lumasiran Therapy for Primary Hyperoxaluria Type 1 in Small Infants. Pediatr Nephrol 2022, 37 (4), 907–911. 10.1007/s00467-021-05393-1. [DOI] [PubMed] [Google Scholar]
- Aldabek K.; Grossman O. K.; Al-Omar O.; Fox J. A.; Moritz M. L. Infantile Primary Hyperoxaluria Type 1 Treated With Lumasiran in Twin Males. Cureus 2022, 14 (1), e21673. 10.7759/cureus.21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariceta G.; Barrios K.; Brown B. D.; Hoppe B.; Rosskamp R.; Langman C. B. Hepatic Lactate Dehydrogenase A: An RNA Interference Target for the Treatment of All Known Types of Primary Hyperoxaluria. Kidney Int. Rep 2021, 6 (4), 1088–1098. 10.1016/j.ekir.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer A. D.; Dowdy S. F. GalNAc-SiRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther 2018, 28 (3), 109–118. 10.1089/nat.2018.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe B.; Koch A.; Cochat P.; Garrelfs S. F.; Baum M. A.; Groothoff J. W.; Lipkin G.; Coenen M.; Schalk G.; Amrite A.; McDougall D.; Barrios K.; Langman C. B. Safety, Pharmacodynamics, and Exposure-Response Modeling Results from a First-in-Human Phase 1 Study of Nedosiran (PHYOX1) in Primary Hyperoxaluria. Kidney Int. 2022, 101 (3), 626–634. 10.1016/j.kint.2021.08.015. [DOI] [PubMed] [Google Scholar]
- Lai C.; Pursell N.; Gierut J.; Saxena U.; Zhou W.; Dills M.; Diwanji R.; Dutta C.; Koser M.; Nazef N.; Storr R.; Kim B.; Martin-Higueras C.; Salido E.; Wang W.; Abrams M.; Dudek H.; Brown B. D. Specific Inhibition of Hepatic Lactate Dehydrogenase Reduces Oxalate Production in Mouse Models of Primary Hyperoxaluria. Molecular Therapy 2018, 26 (8), 1983–1995. 10.1016/j.ymthe.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V.; Schwille P. O. Oxalate Production from Glyoxylate by Lactate Dehydrogenase in Vitro: Inhibition by Reduced Glutathione, Cysteine, Cysteamine. Biochem Int. 1992, 27 (3), 431–438. [PubMed] [Google Scholar]
- Smith L. H.; Bauer R. L.; Craig J. C.; Chan R. P. K.; Williams H. E. Inhibition of Oxalate Synthesis: In Vitro Studies Using Analogues of Oxalate and Glycolate. Biochem Med. 1972, 6 (4), 317–332. 10.1016/0006-2944(72)90018-X. [DOI] [PubMed] [Google Scholar]
- Martin-Higueras C.; Luis-Lima S.; Salido E. Glycolate Oxidase Is a Safe and Efficient Target for Substrate Reduction Therapy in a Mouse Model of Primary Hyperoxaluria Type I. Molecular Therapy 2016, 24 (4), 719–725. 10.1038/mt.2015.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebow A.; Li X.; Racie T.; Hettinger J.; Bettencourt B. R.; Najafian N.; Haslett P.; Fitzgerald K.; Holmes R. P.; Erbe D.; Querbes W.; Knight J. An Investigational RNAi Therapeutic Targeting Glycolate Oxidase Reduces Oxalate Production in Models of Primary Hyperoxaluria. Journal of the American Society of Nephrology 2017, 28 (2), 494–503. 10.1681/ASN.2016030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. D.; Holmes R. P.; Erbe D.; Liebow A.; Fargue S.; Knight J. Reduction in Urinary Oxalate Excretion in Mouse Models of Primary Hyperoxaluria by RNA Interference Inhibition of Liver Lactate Dehydrogenase Activity. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2019, 1865 (9), 2203–2209. 10.1016/j.bbadis.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum M. A.; et al. Nedosiran Reduced Urinary Oxalate Excretion in Patients with Primary Hyperoxaluria. Am. Soc. Nephrol 2021, PO2538. [Google Scholar]
- Shee K.; Ahn J.; Hamouche F.; Mena J.; Chi T.; Stoller M. L. Nedosiran Dramatically Reduces Serum Oxalate in Dialysis-Dependent Primary Hyperoxaluria 1: A Compassionate Use Case Report. Urology 2021, 156, e147–e149. 10.1016/j.urology.2021.03.014. [DOI] [PubMed] [Google Scholar]
- Ramsden D.; Wu J.-T.; Zerler B.; Iqbal S.; Jiang J.; Clausen V.; Aluri K.; Gu Y.; Dennin S.; Kim J.; Chong S. In Vitro Drug-Drug Interaction Evaluation of GalNAc Conjugated SiRNAs Against CYP450 Enzymes and Transporters. Drug Metab. Dispos. 2019, 47 (10), 1183–1194. 10.1124/dmd.119.087098. [DOI] [PubMed] [Google Scholar]