Abstract

Developing methyltransferase inhibitors is challenging, since most of the currently used assays are time-consuming and cost-intensive. Therefore, efficient, fast, and reliable methods for screenings and affinity determinations are of utmost importance. Starting from a literature-known fluorescent S-adenosylhomocysteine derivative, 5-FAM-triazolyl-adenosyl-Dab, developed for a fluorescence polarization assay to investigate the histone methyltransferase mixed-lineage leukemia 1, we herein describe the applicability of this compound as a fluorescent tracer for the investigation of DNA-methyltransferase 2 (DNMT2), a human RNA methyltransferase. Based on these findings, we established a microscale thermophoresis (MST) assay for DNMT2. This displacement assay can circumvent various problems inherent to this method. Furthermore, we optimized a screening method via MST which even indicates if the detected binding is competitive and gives the opportunity to estimate the potency of a ligand, both of which are not possible with a direct binding assay.
Keywords: Drug discovery, High-throughput-screening, Microscale thermophoresis, RNA methyltransferase DNMT2, Fluorescein-labeling
Originally the human DNA methyltransferase 2 (DNMT2) was considered to be a DNA-modifying enzyme, as it shares most of its sequence with other members of the DNMT family (DNMT1, DNMT3A, DNMT3B).1,2 However, in 2006, Goll et al. discovered that the main substrate of DNMT2 is tRNA. DNMT2 catalyzes the methylation of a cytosine at position 38 in the anticodon loop of tRNAAsp. During this modification, the cofactor S-adenosylmethionine (SAM) is converted to S-adenosylhomocysteine (SAH), a known product inhibitor of DNMT2.3 Subsequently, tRNAVal and tRNAGly were also found to be substrates of DNMT2, underlining its role as an RNA methyltransferase, especially in humans.4 Since then, a lot of possible physiological roles of DNMT2 were discussed. It was proven that DNMT2 together with other RNA-methyltransferases like NSUN2 has a critical influence on tRNA stability and protein translation in cells.5−7 During oxidative stress, an up-regulation of DNMT2 could be detected, leading to the assumption that DNMT2 also helps cells coping with exogenous stress factors.8−10 DNMT2 was as well found to be overexpressed in cancer cells, where mutations on DNMT2 are assumed to play a functional role in tumorigenesis.11,12 Furthermore, DNMT2 is also involved in the epigenetic inheritance process.13 Taken all this into account, DNMT2 appears to be a promising drug target for medicinal chemistry. Therefore, reliable methods to identify potent binders are required.
Tritium incorporation assays using 3H-SAM are still the most reliable and therefore often used methods to assay methyltransferases like DNMT2, despite all the disadvantages, such as time consumption and high costs.14,15 Other methods, like LC-MS, still require a lot of working steps and time.16 Therefore, fast, reliable, and cost-efficient screening methods are needed to reduce the number of compounds subjected to tritium incorporation or LC-MS assays to a minimum. At present, various biophysical methods are available for affinity-based screening assays such as isothermal titration calorimetry (ITC),17 differential scanning fluorimetry (DSF),18 surface plasmon resonance (SPR),19 or microscale thermophoresis (MST).20 All have their advantages but also their disadvantages. ITC measurements not only determine ligand affinities, by measuring the binding enthalpy, but also reveal thermodynamic data of the binding as well as binding stoichiometry. Although this is a very robust method, the enormous amounts of protein and ligand needed can be a major obstacle.21−23 DSF is a fast-screening method based on the measurement of protein melting curves in the presence of a fluorescent dye.18,24,25 A disadvantage is the fact that some ligands induce only small thermal shifts, leading to a false negative result in a screening campaign.26 Today, SPR is one key technology applied in pharmaceutical research.27 Although it offers several advantages, such as fast, reliable, and label-free measurements, one cannot ignore that the necessity of surface immobilization remains a major challenge. Besides SPR, the significance of MST also emerges.28−31 In contrast to SPR or ITC, thermodynamic properties cannot be analyzed using MST with a single measurement but require several measurements at different temperatures.32 However, especially in early drug discovery, this sample-saving method can be of great benefit. It is based on the principle that molecules migrate in a temperature gradient. This so-called thermophoresis is reproducible at given conditions, but even small changes such as ligand binding can alter that behavior.32 Furthermore, the change in the thermophoresis behavior correlates with the extent of this interaction. This allows an affinity determination of a protein–ligand interaction by observing fluorescence changes.20,32,33 Besides thermophoresis, other effects can cause these changes, e.g., temperature differences and changes in the local environment of the fluorophore.34,35 The advantages of this method are certainly its sensitivity and scalability, but also the fact that screenings can be performed directly with cell lysates or blood sera, which makes it a powerful method for drug discovery.20,26,32,36−40 Determining binding affinity by MST, however, comes with some inherent problems. In most cases, the protein of interest must be fluorescently labeled, either in a covalent manner, by thiol or amide coupling, or in a non-covalent manner, by His-tag labeling with a fluorescent dye.32,33 Direct measuring of protein–ligand interactions by MST only shows binding events but cannot reveal if the detected binding is competitive with respect to the active site of the protein, or if the ligand binding occurs on an allosteric site. It can also be very challenging to reach saturation conditions for the bound protein–ligand complex, especially if ligands only have binding affinities in the low single-digit micromolar range like the currently known inhibitors of DNMT2. Achieving saturation conditions often needs high protein and ligand concentrations resulting in protein and/or ligand aggregation and/or unspecific binding. Furthermore, the maximal effect of a ligand on the thermophoresis behavior of the protein–ligand complex is not predictable. Therefore, it is not uncommon, that low-affinity ligands have a stronger influence on the thermophoresis behavior than high-affinity ones.
The main cause of these problems is notably due to the necessity of fluorescent-labeling of the protein. If the fluorescent dye is not attached to the enzyme but rather a known ligand (fluorescence tracer)—especially one with a strong thermophoresis behavior—a displacement assay could be established to circumvent a lot of this problems. In this paper, we introduce an optimized MST method with excellent suitability for high-throughput-screening of DNMT2 ligands. We developed a displacement assay, using a literature-known fluorescein-based tool compound 5-FAM-triazolyl-adenosyl-Dab (6, FTAD, see Scheme 1), that originally was applied by Luan et al. in a fluorescence polarization (FP) assay for the histone methyltransferase mixed-lineage leukemia 1 (MLL1).41 Our method circumvents the inherent problems previously described, and provides the following improvements:
reduced aggregation due to lower concentrations of ligand
half-quantitative screenings outcomes
determination of active site interactions
no enzyme labeling required
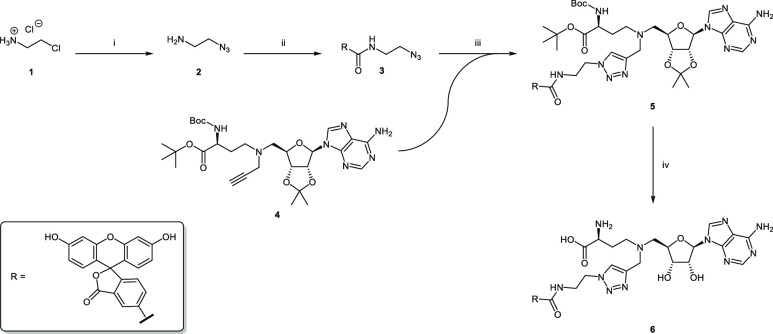
Scheme 1. Synthesis of FTAD (6).
Reagents and conditions: (i) NaN3, H2O, 85 °C, 24 h, 67%; (ii) 5-FAM, TPTU, DIPEA, DMF, 0–20 °C, 48 h, 96%; (iii) CuSO4, sodium ascorbate, MeOH/H2O, 16 h, 61%; (iv) 1. TFA, DCM, 5 °C; 2. TFA, H2O, 5 °C, 99%.
Results and Discussion
Chemistry
For the preparation of the tool compound, we followed a synthetic procedure similar to the one used by Luan et al. (Scheme 1).41 First the azide 2 was prepared in a nucleophilic substitution reaction of 2-chloroethylamine hydrochloride 1 and sodium azide. This product was then coupled to 5-carboxyfluorescein (5-FAM) using O-(2-oxo-1(2H)pyridyl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate (TPTU) and N-ethyldiisopropylamine (DIPEA), yielding compound 3. In the subsequent copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC), the alkyne 4 was connected to the azide 3, forming the triazole product 5. The synthesis of building block 4 was carried out according to literature.42 In the final step, all protecting groups were cleaved using a two-step procedure. First, 50% (v/v) trifluoroacetic acid (TFA in DCM was used at 5 °C, then 14% TFA in water at 5 °C to finally yield FTAD (6) as its trifluoroacetate salt.
Establishment of DNMT2 Fluorescence Polarization Assay
Binding affinity of FTAD (6) to DNMT2 was investigated using the fluorescence polarization assay protocol described for the histone methyltransferase MLL1.41 Therefore, a saturation curve was measured, which resulted in a KD value of 2.4 μM. These results indicated that this fluorescent probe is not only suitable to assay MLL1 but also DNMT2. To check the quality of this assay, the Z-factor was evaluated. The obtained value of 0.92 for the Z-factor is very good and sufficient to establish an assay for the determination of binding affinities.43 Next, the literature known binding affinities of S-adenosylhomocysteine (7, SAH) and sinefungin (8, SFG) could be confirmed. KD values of 12.2 μM and 6.5 μM for SAH and SFG were measured, which are quite similar to reported values from literature (KD = 13.6 μM and KD = 7.5 μM).42 Furthermore, the binding affinity of a recently published DNMT2 inhibitor (9) was determined, revealing a KD of 7.8 μM which was also found to fit to the published value of 8.1 μM.42
To investigate if this tool compound may be suitable for pan-methyltransferase assays, binding toward the human tRNA methyltransferases NSUN2 and NSUN6 as well as the histone methyltransferase EHMT2 of the KMT family was tested. Unfortunately, FTAD showed only weak affinities to those methyltransferases with approximate KD values in the higher two-digit or the three-digit micromolar range (data not shown).
Nevertheless, these findings proved that this tool compound which originally was designed to assay MLL1 via FP was also suitable for DNMT2.
Establishment of DNMT2 Microscale Thermophoresis Assay
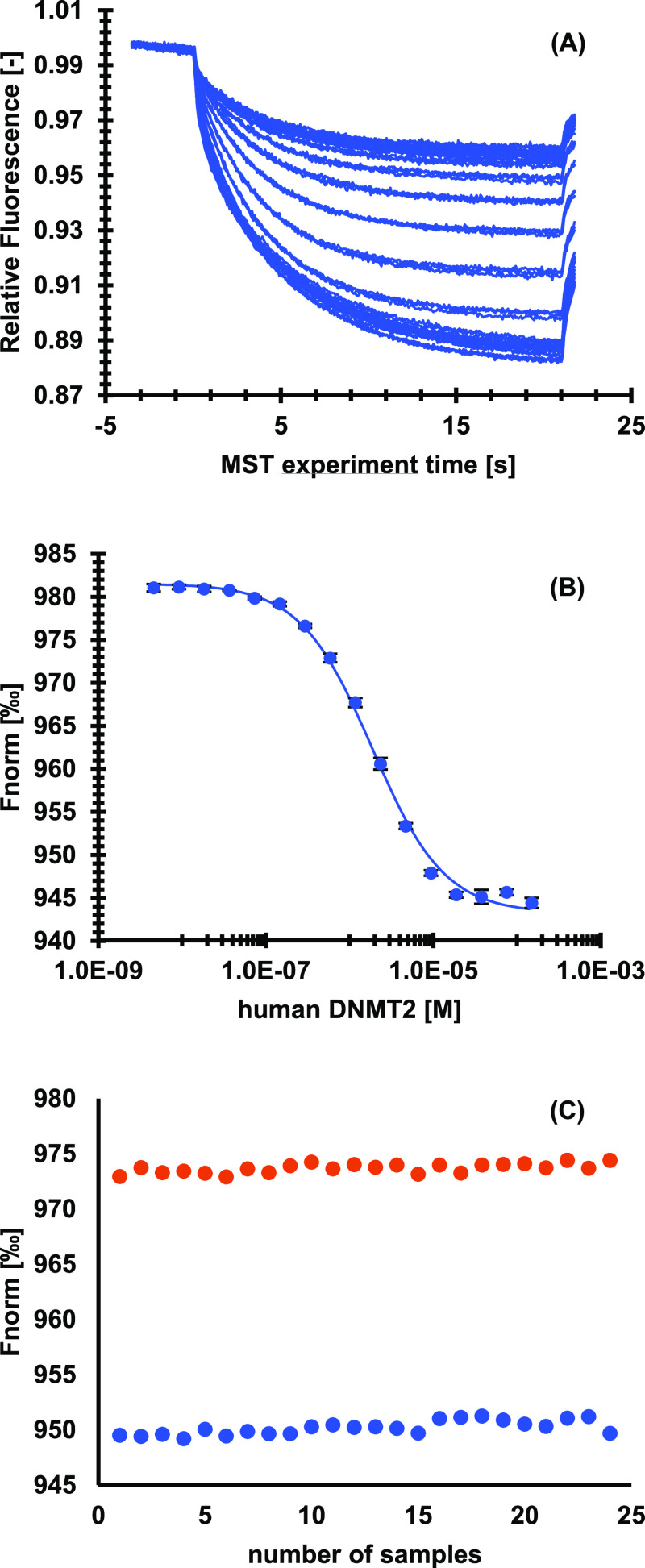
In a next step, FTAD (6) was used as a fluorescence tracer for a microscale thermophoresis (MST) displacement assay. The assay is based on the formation of a FTAD-DNMT2 complex, which allows fluorescence tracing. By adding a ligand of interest FTAD is displaced, which results in an altered fluorescence signal, since FTAD is no longer bound to DNMT2 (Scheme 2). Excitation and emission wavelengths of FTAD were sufficient for MST measurements with blue light settings (excitation: 465–490 nm and emission: 500–550 nm). At first, an assay concentration for FTAD was evaluated, starting with 50 nM, as described in the FP protocol. Since the fluorescence signal was not optimal, the concentration was increased. Finally, a concentration of 100 nM was chosen, which allowed measurements with sufficient fluorescence signal, at the lowest possible concentration. Furthermore, no surface absorption or bleaching was detected in standard capillaries. In a dilution series of DNMT2 at constant FTAD concentrations, binding affinity of FTAD against DNMT2 was investigated using MST, revealing a KD value of 1.8 μM, which is in very good accordance with the binding affinity determined using FP (Figure 1A,B). Furthermore, a Z-factor of 0.90 (Figure 1C) for this assay indicated that this assay had a very good quality. In the next step, the binding affinities of SAH and SFG were verified by MST. Since displacement assays only provide EC50 values, those results were corrected for the binding competition with the fluorescence tracer according to the instructions of Nanotemper Technologies to receive KD values. For SAH, KD = 6.5 μM was determined, while for SFG, KD = 9.2 μM was found. Both values were in high accordance with literature, where KD values of 13.6 μM and 7.5 μM, respectively, were described (Table 1). For compound 9, a KD value of 5.2 μM was found, which correlates quite well with the literature value (KD = 8.1 μM42). Interestingly, also for compound 10, a literature known non-inhibitor of DNMT2,42 a very slight protein–ligand interaction could be detected in this assay with an EC50 of >100 μM, this indicates that the method is even capable to identify very low affinity binders if needed. To further confirm the potential of this assay for affinity determination, three additional SAH-derived DNMT2 inhibitors, 11–13,42 were selected and the KD values were determined by the MST displacement assay and by ITC as an orthogonal method.
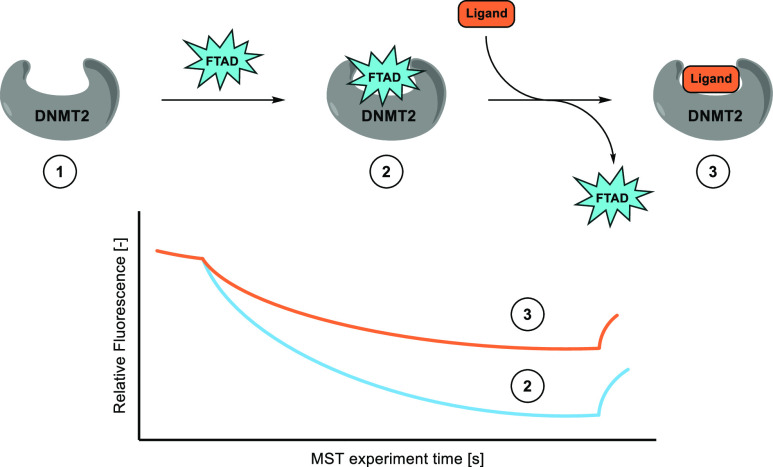
Scheme 2. Establishment of DNMT2 Microscale Thermophoresis Assay.
Steps: (1) Unlabeled DNMT2 cannot be observed by MST. (2) DNMT2 is pre-incubated with FTAD; the FTAD-DNMT2 complex can be observed by MST. (3) Displacement of FTAD with ligand of interest; free FTAD can be observed by MST.
Figure 1.
Microscale thermophoresis assay for FTAD against a dilution series of human DNMT2. (A) Raw traces of runs. (B) Normalized fluorescence plotted against concentration of human DNMT2. Data is given as mean ± SD of triplicates. KD of FTAD against DNMT2 was found to be 1.8 μM ± 0.1 μM. (C) Z-factor determination of microscale thermophoresis assay. In orange, free FTAD without DNMT2; in blue, FTAD in the presence of 2 μM human DNMT2.
Table 1. Binding Affinities of Different Ligands toward DNMT2 Measured by Orthogonal Biophysical Methods.
KD values from ITC experiments; shown are mean values ± SD of n = 3 experiments.
Mean values ± SD of n = 6 experiments.
KD values from FP assays; shown are mean values ± SD of n = 3 experiments.
KD values from MST displacement assays; shown are mean values ± SD of n = 4 experiments. *n.d. = could not be determined due to the high concentrations of enzyme and ligand required to quantify low-affinity binding.
The data are in very good agreement, proving the reliability of the assay. Moreover, in comparison to the ITC method, the amounts of samples, especially of protein, are much lower.
One of the most considerable findings was the fact that with this displacement method the plateaus for total unbound and bound protein could be resolved very well (Figure 2). Since the maxima of the shifts can easily be measured by control runs, the fits derived from those measurements are far more reliable than those of direct binding assays based on MST. Aggregation, up to concentrations needed to reach a plateau, was not observed in any run. Furthermore, the signal-to-noise ratio was highly increased compared to direct measurements of labeled DNMT2. This may derive from the largely increased shift, which is probably due to the fact, that the large DNMT2 molecule influences the thermophoresis behavior of the small fluorescence tracer much more, than vice versa a small molecular ligand a >40 kDa protein like DNMT2.
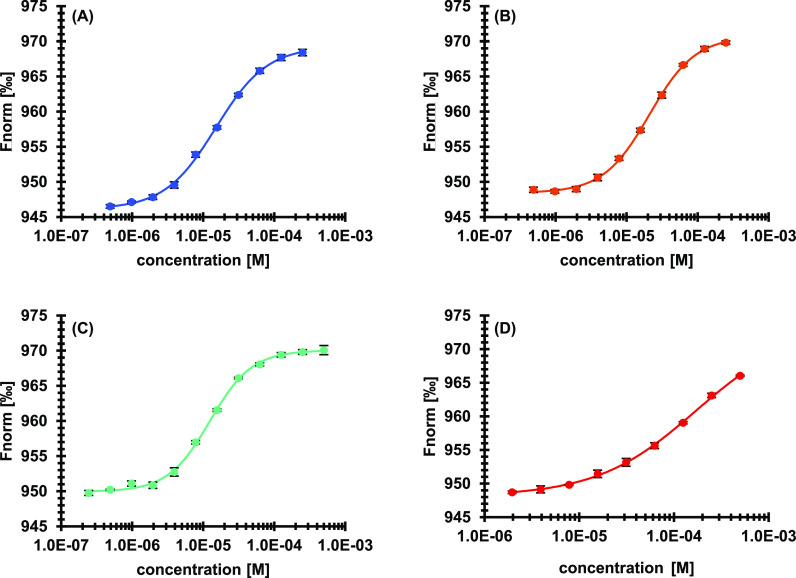
Figure 2.
Displacement of FTAD by literature known inhibitors in a microscale thermophoresis assay. Data is given as mean ± SD of quadruplicates. (A) SAH (7), EC50 = 15.0 ± 0.7 μM; (B) SFG (8), EC50 = 20.9 ± 0.7 μM; (C) compound 9, EC50 = 12.4 ± 0.5 μM; and (D) compound 10, EC50 = > 100 μM.
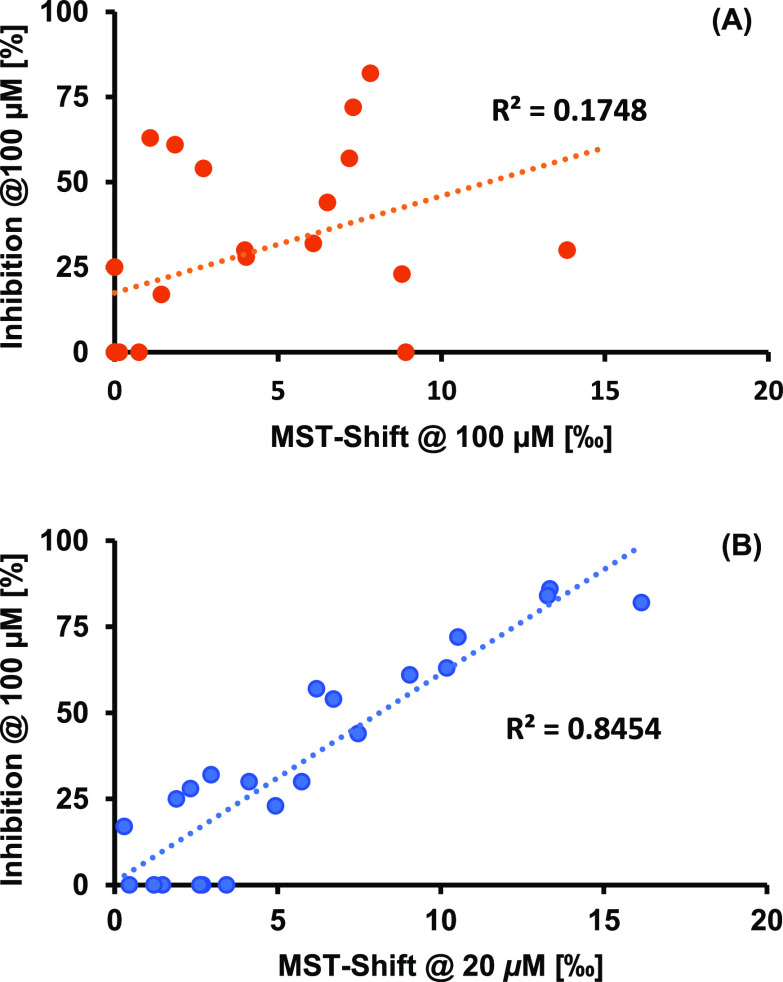
MST can be used as a fast and efficient screening method.42 Although common MST assays using labeled protein are highly sensitive screening methods that can detect binders with almost negligible affinity, there remain some problems. One is for sure the comparability of the detected shifts. The extent of the included shifts in thermophoresis does not correlate very well with the affinity of different ligands; i.e., a less affine ligand can induce a larger shift than a ligand with higher affinity. In our recently published structure–activity relationship (SAR) study for SAH-derived DNMT2 inhibitors, MST was used as an initial screening method. Some ligands were found to induce noteworthy shifts in the thermophoretic behavior, but a tritium incorporation assay revealed that a lot of those ligands showed only poor inhibitory properties.42 By plotting the induced shifts against the found inhibition, no correlation could be detected (Figure 3A).
Figure 3.
Correlation of MST shifts at 20 μM compound concentrations and inhibition at 100 μM in a tritium incorporation assay for literature-known compounds: (A) for direct MST assay and (B) for MST displacement assay.
Therefore, a screening with our novel displacement method was performed for a set of binders and non-binders of this SAR study. A ligand concentration of 20 μM was found to be suitable for this screening. Since for a displacement assay the detectable shift is known from the beginning and depends only on the fraction of bound and unbound fluorescence tracer, this seemed to open the possibility to achieve a correlation between the thermophoresis shifts and the actual inhibition of DNMT2. Indeed, a correlation between the thermophoresis shifts in this displacement assay and the measured inhibition from the tritium incorporation assay could be found (Figure 3B). This led to the conclusion that ligands inducing a thermophoretic shift <5‰ should not be considered for further measurements, while ligands inducing shifts ∼10‰ and higher are likely to be very promising DNMT2 inhibitors. The displacement assay not only allows researchers to discriminate good and poor binders. Given the fact that FTAD binds to the active site of DNMT2, this assay also indicates if the ligand is active-site directed, which can be very helpful for the screening of DNMT2 inhibitors that structurally do not resemble the natural ligand. It is noteworthy that the displacement assay can be performed at decreased ligand concentrations in a range of 20 μM instead of 100 μM, which can prevent unspecific binding to the protein and therefore misleading hits from the beginning.
Conclusion
Within this study we present a novel method for a fast and reliable ligand screening for the human RNA methyltransferase DNMT2 using microscale thermophoresis. The assay can easily be extended to a high-throughput screening. This displacement assay allows screenings at low ligand, dye, and protein concentrations. Together with the small volumes required for microscale thermophoresis, this results in a cost-effective but still robust method. Under the conditions described, no adsorption, bleaching, or aggregation was detected, which usually can cause severe interferences in direct microscale thermophoresis assays. Furthermore, this assay reveals accurate screening results, which facilitates early-stage drug discovery in the field of DNMT2. Due to the fact that the fluorescence tracer is bound to the active site of DNMT2, thermophoresis shifts detected by this method always indicate active-site targeting, making further binding-site verification unnecessary. As a result of the increased thermophoresis shifts compared to direct microscale thermophoresis assays with labeled protein, this method can be used for reliable binding affinity determination up to the low micromolar range. In summary, we are highly convinced that this method can be a powerful tool accelerating the search for potent DNMT2 inhibitors.
Glossary
Abbreviations
- CCR2
CC chemokine receptor 2
- CCL2
CC chemokine ligand 2
- CCR5
CC chemokine receptor 5
- Dab
2,4-diaminobutyric acid
- 5-FAM
5-carboxyfluorescein
- FTAD
5-FAM-triazolyl-adenosyl-Dab
- TLC
thin-layer chromatography
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.2c00175.
Microscale thermophoresis raw traces, NMR spectra, and chromatograms (PDF)
Author Contributions
‡ R.A.Z., M.S., and J.L.M. contributed equally to the project.
Financial support by the DFG (Deutsche Forschungsgemeinschaft) in the framework of the Transregio Collaborative Research Center TRR 319 (RMaP, RNA Modification and Processing), projects A01 (T.S.), C01, and C03 (M.H.), is gratefully acknowledged. Z.N. gratefully acknowledges financial support by the Volkswagen Stiftung.
The authors declare no competing financial interest.
Supplementary Material
References
- Van den Wyngaert I.; Sprengel J.; Kass S. U.; Luyten W. H. Cloning and Analysis of a Novel Human Putative DNA Methyltransferase. FEBS Lett. 1998, 426 (2), 283–289. 10.1016/S0014-5793(98)00362-7. [DOI] [PubMed] [Google Scholar]
- Dong A. Structure of Human DNMT2, an Enigmatic DNA Methyltransferase Homolog That Displays Denaturant-Resistant Binding to DNA. Nucleic Acids Res. 2001, 29 (2), 439–448. 10.1093/nar/29.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll M. G.; Kirpekar F.; Maggert K. A.; Yoder J. A.; Hsieh C. L.; Zhang X.; Golic K. G.; Jacobsen S. E.; Bestor T. H. Methylation of TRNAAsp by the DNA Methyltransferase Homolog Dnmt2. Science 2006, 311 (5759), 395–398. 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- Schaefer M.; Pollex T.; Hanna K.; Tuorto F.; Meusburger M.; Helm M.; Lyko F. RNA Methylation by Dnmt2 Protects Transfer RNAs against Stress-Induced Cleavage. Genes Dev. 2010, 24 (15), 1590–1595. 10.1101/gad.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuorto F.; Liebers R.; Musch T.; Schaefer M.; Hofmann S.; Kellner S.; Frye M.; Helm M.; Stoecklin G.; Lyko F. RNA Cytosine Methylation by Dnmt2 and NSun2 Promotes TRNA Stability and Protein Synthesis. Nat. Struct. Mol. Biol. 2012, 19 (9), 900–905. 10.1038/nsmb.2357. [DOI] [PubMed] [Google Scholar]
- Shanmugam R.; Fierer J.; Kaiser S.; Helm M.; Jurkowski T. P.; Jeltsch A. Cytosine Methylation of TRNA-Asp by DNMT2 Has a Role in Translation of Proteins Containing Poly-Asp Sequences. Cell Discovery 2015, 1, 15010. 10.1038/celldisc.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuorto F.; Herbst F.; Alerasool N.; Bender S.; Popp O.; Federico G.; Reitter S.; Liebers R.; Stoecklin G.; Gröne H.; Dittmar G.; Glimm H.; Lyko F. The TRNA Methyltransferase Dnmt2 Is Required for Accurate Polypeptide Synthesis during Haematopoiesis. EMBO J. 2015, 34 (18), 2350–2362. 10.15252/embj.201591382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M.; Müller S.; Nellen W.; Jurkowski T. P.; Jeltsch A.; Ehrenhofer-Murray A. E. Pmt1, a Dnmt2 Homolog in Schizosaccharomyces Pombe, Mediates TRNA Methylation in Response to Nutrient Signaling. Nucleic Acids Res. 2012, 40 (22), 11648–11658. 10.1093/nar/gks956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M.-J.; Tang L.-Y.; Reddy M. N.; Shen C.-K. J. DNA Methyltransferase Gene DDnmt2 and Longevity of Drosophila. J. Biol. Chem. 2005, 280 (2), 861–864. 10.1074/jbc.C400477200. [DOI] [PubMed] [Google Scholar]
- Kaul G.; Thippeswamy H. Role of Heat Shock Proteins in Diseases and Their Therapeutic Potential. Indian J. Microbiol. 2011, 51 (2), 124–131. 10.1007/s12088-011-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes S. A.; Beare D.; Gunasekaran P.; Leung K.; Bindal N.; Boutselakis H.; Ding M.; Bamford S.; Cole C.; Ward S.; Kok C. Y.; Jia M.; De T.; Teague J. W.; Stratton M. R.; McDermott U.; Campbell P. J. COSMIC: Exploring the World’s Knowledge of Somatic Mutations in Human Cancer. Nucleic Acids Res. 2015, 43, D805–11. 10.1093/nar/gku1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhardt W.; Shanmugam R.; Jurkowski T. P.; Jeltsch A. Somatic Cancer Mutations in the DNMT2 TRNA Methyltransferase Alter Its Catalytic Properties. Biochimie 2015, 112, 66–72. 10.1016/j.biochi.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zhang X.; Shi J.; Tuorto F.; Li X.; Liu Y.; Liebers R.; Zhang L.; Qu Y.; Qian J.; Pahima M.; Liu Y.; Yan M.; Cao Z.; Lei X.; Cao Y.; Peng H.; Liu S.; Wang Y.; Zheng H.; Woolsey R.; Quilici D.; Zhai Q.; Li L.; Zhou T.; Yan W.; Lyko F.; Zhang Y.; Zhou Q.; Duan E.; Chen Q. Dnmt2Mediates Intergenerational Transmission of Paternally Acquired Metabolic Disorders through Sperm Small Non-Coding RNAs. Nat. Cell Biol. 2018, 20 (5), 535–540. 10.1038/s41556-018-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkowski T. P.; Meusburger M.; Phalke S.; Helm M.; Nellen W.; Reuter G.; Jeltsch A. Human DNMT2Methylates TRNA(Asp) Molecules Using a DNA Methyltransferase-like Catalytic Mechanism. RNA 2008, 14 (8), 1663–1670. 10.1261/rna.970408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S.; Windhof I. M.; Maximov V.; Jurkowski T.; Jeltsch A.; Förstner K. U.; Sharma C. M.; Gräf R.; Nellen W. Target Recognition, RNA Methylation Activity and Transcriptional Regulation of the Dictyostelium Discoideum Dnmt2-Homologue (DnmA). Nucleic Acids Res. 2013, 41 (18), 8615–8627. 10.1093/nar/gkt634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyan M. E. K.; Pedicord D. L.; Bergeron L.; Mintier G. A.; Hunihan L.; Kuit K.; Balanda L. A.; Robertson B. J.; Feder J. N.; Westphal R.; Shipkova P. A.; Blat Y. A General Liquid Chromatography/Mass Spectroscopy-Based Assay for Detection and Quantitation of Methyltransferase Activity. Anal. Biochem. 2006, 349 (1), 112–117. 10.1016/j.ab.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Szlag V. M.; Jung S.; Rodriguez R. S.; Bourgeois M.; Bryson S.; Schatz G. C.; Reineke T. M.; Haynes C. L. Isothermal Titration Calorimetry for the Screening of Aflatoxin B1 Surface-Enhanced Raman Scattering Sensor Affinity Agents. Anal. Chem. 2018, 90 (22), 13409–13418. 10.1021/acs.analchem.8b03221. [DOI] [PubMed] [Google Scholar]
- Gao K.; Oerlemans R.; Groves M. R. Theory and Applications of Differential Scanning Fluorimetry in Early-Stage Drug Discovery. Biophys. Rev. 2020, 12 (1), 85–104. 10.1007/s12551-020-00619-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaru A.; Bala C.; Jaffrezic-Renault N.; Aboul-Enein H. Y. Surface Plasmon Resonance (SPR) Biosensors in Pharmaceutical Analysis. Crit. Rev. Anal. Chem. 2015, 45 (2), 97–105. 10.1080/10408347.2014.881250. [DOI] [PubMed] [Google Scholar]
- Jerabek-Willemsen M.; Wienken C. J.; Braun D.; Baaske P.; Duhr S. Molecular Interaction Studies Using Microscale Thermophoresis. Assay Drug Dev. Technol. 2011, 9 (4), 342–353. 10.1089/adt.2011.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos M.; Velazquez-Campoy A. Isothermal Titration Calorimetry (ITC): A Standard Operating Procedure (SOP). Eur. Biophys. J. 2021, 50 (3–4), 363–371. 10.1007/s00249-021-01509-5. [DOI] [PubMed] [Google Scholar]
- Baranauskiene L.; Kuo T.-C.; Chen W.-Y.; Matulis D. Isothermal Titration Calorimetry for Characterization of Recombinant Proteins. Curr. Opin. Biotechnol. 2019, 55, 9–15. 10.1016/j.copbio.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Kabiri M.; Unsworth L. D. Application of Isothermal Titration Calorimetry for Characterizing Thermodynamic Parameters of Biomolecular Interactions: Peptide Self-Assembly and Protein Adsorption Case Studies. Biomacromolecules 2014, 15 (10), 3463–3473. 10.1021/bm5004515. [DOI] [PubMed] [Google Scholar]
- Niesen F. H.; Berglund H.; Vedadi M. The Use of Differential Scanning Fluorimetry to Detect Ligand Interactions That Promote Protein Stability. Nat. Protoc. 2007, 2 (9), 2212–2221. 10.1038/nprot.2007.321. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Monsma F. Fluorescence-Based Thermal Shift Assays. Curr. Opin. Drug Discovery Devel. 2010, 13 (4), 389–402. [PubMed] [Google Scholar]
- Linke P.; Amaning K.; Maschberger M.; Vallee F.; Steier V.; Baaske P.; Duhr S.; Breitsprecher D.; Rak A. An Automated Microscale Thermophoresis Screening Approach for Fragment-Based Lead Discovery. J. Biomol. Screen. 2016, 21 (4), 414–421. 10.1177/1087057115618347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman S. S.; McKeating K. S.; Cheng Q. Surface Plasmon Resonance: Material and Interface Design for Universal Accessibility. Anal. Chem. 2018, 90 (1), 19–39. 10.1021/acs.analchem.7b04251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainard J. M.; Pandarakalam G. C.; McElroy S. P. Using Microscale Thermophoresis to Characterize Hits from High-Throughput Screening: A European Lead Factory Perspective. SLAS Discovery Adv. life Sci. R D 2018, 23 (3), 225–241. 10.1177/2472555217744728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milite C.; Feoli A.; Horton J. R.; Rescigno D.; Cipriano A.; Pisapia V.; Viviano M.; Pepe G.; Amendola G.; Novellino E.; Cosconati S.; Cheng X.; Castellano S.; Sbardella G. Discovery of a Novel Chemotype of Histone Lysine Methyltransferase EHMT1/2 (GLP/G9a) Inhibitors: Rational Design, Synthesis, Biological Evaluation, and Co-Crystal Structure. J. Med. Chem. 2019, 62 (5), 2666–2689. 10.1021/acs.jmedchem.8b02008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozielski F.; Sele C.; Talibov V. O.; Lou J.; Dong D.; Wang Q.; Shi X.; Nyblom M.; Rogstam A.; Krojer T.; Fisher Z.; Knecht W. Identification of Fragments Binding to SARS-CoV-2 Nsp10 Reveals Ligand-Binding Sites in Conserved Interfaces between Nsp10 and Nsp14/Nsp16. RSC Chem. Biol. 2022, 3 (1), 44–55. 10.1039/D1CB00135C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoli A.; Pisapia V.; Viviano M.; Castellano S.; Bartoschik T.; Sbardella G. Development of a Microscale Thermophoresis-Based Method for Screening and Characterizing Inhibitors of the Methyl-Lysine Reader Protein MRG15. SLAS Discovery Adv. life Sci. R D 2021, 26 (1), 77–87. 10.1177/2472555220949166. [DOI] [PubMed] [Google Scholar]
- Seidel S. A. I.; Dijkman P. M.; Lea W. A.; van den Bogaart G.; Jerabek-Willemsen M.; Lazic A.; Joseph J. S.; Srinivasan P.; Baaske P.; Simeonov A.; Katritch I.; Melo F. A.; Ladbury J. E.; Schreiber G.; Watts A.; Braun D.; Duhr S. Microscale Thermophoresis Quantifies Biomolecular Interactions under Previously Challenging Conditions. Methods 2013, 59 (3), 301–315. 10.1016/j.ymeth.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoschik T.; Gupta A.; Kern B.; Hitchcock A.; Adams N. B. P.; Tschammer N. Quantifying the Interaction of Phosphite with ABC Transporters: MicroScale Thermophoresis and a Novel His-Tag Labeling Approach. Methods Mol. Biol. 2020, 2168, 51–62. 10.1007/978-1-0716-0724-4_2. [DOI] [PubMed] [Google Scholar]
- López-Méndez B.; Uebel S.; Lundgren L. P.; Sedivy A. Microscale Thermophoresis and Additional Effects Measured in NanoTemper Monolith Instruments. Eur. Biophys. J. 2021, 50 (3–4), 653–660. 10.1007/s00249-021-01529-1. [DOI] [PubMed] [Google Scholar]
- Royer C. A. Probing Protein Folding and Conformational Transitions with Fluorescence. Chem. Rev. 2006, 106 (5), 1769–1784. 10.1021/cr0404390. [DOI] [PubMed] [Google Scholar]
- Wienken C. J.; Baaske P.; Rothbauer U.; Braun D.; Duhr S. Protein-Binding Assays in Biological Liquids Using Microscale Thermophoresis. Nat. Commun. 2010, 1, 100. 10.1038/ncomms1093. [DOI] [PubMed] [Google Scholar]
- Seidel S. A. I.; Wienken C. J.; Geissler S.; Jerabek-Willemsen M.; Duhr S.; Reiter A.; Trauner D.; Braun D.; Baaske P. Label-Free Microscale Thermophoresis Discriminates Sites and Affinity of Protein-Ligand Binding. Angew. Chem., Int. Ed. Engl. 2012, 51 (42), 10656–10659. 10.1002/anie.201204268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerabek-Willemsen M.; André T.; Wanner R.; Roth H. M.; Duhr S.; Baaske P.; Breitsprecher D. MicroScale Thermophoresis: Interaction Analysis and Beyond. J. Mol. Struct. 2014, 1077, 101–113. 10.1016/j.molstruc.2014.03.009. [DOI] [Google Scholar]
- Bartoschik T.; Galinec S.; Kleusch C.; Walkiewicz K.; Breitsprecher D.; Weigert S.; Muller Y. A.; You C.; Piehler J.; Vercruysse T.; Daelemans D.; Tschammer N. Near-Native, Site-Specific and Purification-Free Protein Labeling for Quantitative Protein Interaction Analysis by MicroScale Thermophoresis. Sci. Rep. 2018, 8 (1), 4977. 10.1038/s41598-018-23154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnez R.; Thiroux B.; Taront S.; Segaoula Z.; Quesnel B.; Thuru X. PD-1/PD-L1 Binding Studies Using Microscale Thermophoresis. Sci. Rep. 2017, 7 (1), 17623. 10.1038/s41598-017-17963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan Y.; Blazer L. L.; Hu H.; Hajian T.; Zhang J.; Wu H.; Houliston S.; Arrowsmith C. H.; Vedadi M.; Zheng Y. G. Design of a Fluorescent Ligand Targeting the S-Adenosylmethionine Binding Site of the Histone Methyltransferase MLL1. Org. Biomol. Chem. 2016, 14 (2), 631–638. 10.1039/C5OB01794G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickert M.; Fischer T. R.; Zimmermann R. A.; Hoba S. N.; Meidner J. L.; Weber M.; Weber M.; Stark M. M.; Koch J.; Jung N.; Kersten C.; Windbergs M.; Lyko F.; Helm M.; Schirmeister T. Discovery of Inhibitors of DNA Methyltransferase 2, an Epitranscriptomic Modulator and Potential Target for Cancer Treatment. J. Med. Chem. 2022, 65 (14), 9750–9788. 10.1021/acs.jmedchem.2c00388. [DOI] [PubMed] [Google Scholar]
- Zhang J. H.; Chung T. D.; Oldenburg K. R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4 (2), 67–73. 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.