Abstract

PROteolysis-TArgeting Chimeras (PROTACs) are a powerful class of drugs that selectively degrade the proteins of interest (POIs) through cellular ubiquitination mechanisms. Estrogen receptor α (ERα) plays a vital role in the pathogenesis and treatment of breast cancer. In this work, the DNA-binding domain (DBD) of ERα was selected as the target to avoid drug resistance caused by the ligand-binding domain (LBD) of ERα. The estrogen response element (ERE), a natural DNA sequence binding with DBD of ERα, was chosen as a recognized unit of PROTAC. Therefore, we designed a nucleic acid-conjugated PROTAC, ERE-PROTAC, via a click reaction, in which the ERE sequence recruits ERα and the typical small molecule VH032 recruits the von Hippel–Lindau (VHL) E3 ligase. The proposed ERE-PROTAC showed to efficiently and reversibly degrade ERα in different breast cancer cells by targeting the DBD, indicating its potential to overcome the current resistance caused by LBD mutations.
Keywords: PROTAC, breast cancer, estrogen receptor α, DNA-binding domain, estrogen response element, VHL E3 ligase
Breast cancer affects about 2 million people each year worldwide and is the highest incidence of cancer among women, accounting for 15% of all cancer-related deaths.1,2 There are currently three main subtypes of breast cancer: hormone receptor-positive/ERBB2-negative (HR+/ERBB2−), ERBB2-positive (ERBB2+), and triple-negative.3 The two subtypes of the estrogen receptor (ER) include ERα and ERβ, whereby ERα is overexpressed in 70% of breast cancers and is a significant breast cancer treatment target.4 ERα acts as a transcription factor in physiological processes by binding with estrogen and as a ligand-dependent nuclear hormone receptor, which is one of the primary targets of breast cancer.5,6 Therefore, estrogen suppression and ERα antagonists have been utilized in the main treatments of ERα+ breast cancer for decades. The ERα protein shares a core modular structure consisting of a central DNA-binding domain (DBD) flanked by an N-terminal transactivation domain (NTD) and a C-terminal ligand-binding domain (LBD) (Figure 1A).7−9 Currently, there are three types of approved drugs targeting ERα, namely, aromatase inhibitors (AIs) that prevent estrogen production, selective ERα modulators (SERMs), and selective ERα downregulators/degraders (SERDs).3 The latter two both compete with estrogen to bind with the LBD of ERα, which hinders ER transcription activity or induces ERα degradation to make it possible to effectively treat ERα+ breast cancer.10 While the currently used breast cancer treatments, such as tamoxifen, have achieved effective results, the targeted tumors often exhibit drug resistance due to gene mutations.11 Inevitably, acquired mutations in the LBD of ERα are a common driving factor for drug resistance in ERα+ breast cancer, which is one of the biggest barriers to overcome in current therapies. Therefore, it is urgent to develop new breast cancer treatments following different mechanisms.10
Figure 1.
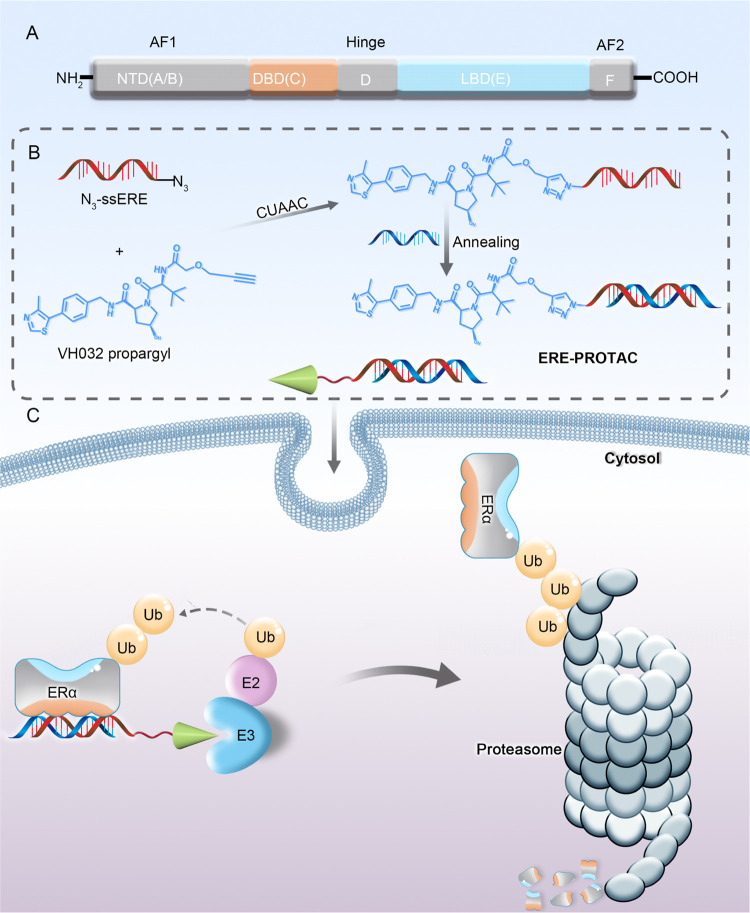
(A) A–F domains that constitute the estrogen receptor structure, including the activation function 1 (AF1) domain, the DNA-binding domain (DBD), the hinge region, and the ligand-binding domain (LBD)/activation function 2 (AF2 domain). (B) Synthesis of ERE-PROTAC using copper-catalyzed azide-alkynyl reaction (CUAAC). (C) ERE-PROTAC degrades ERα through the ubiquitin–proteasome system by targeting the DBD of ERα.
In 2001, Sakamoto et al. first introduced the concept of PROTAC, a heterobifunctional molecule that can promote the degradation of target proteins.12 This heterobifunctional molecule is usually combined with two recognition units via a suitable linker, whereby one unit hijacks the endogenous E3 ligase and the other unit hijacks the target protein.13 In proximity space, the target protein will be labeled with polyubiquitin by the E3 ligase, subsequently inducing the recognition and degradation of the protein via cellular ubiquitination.14 Compared with traditional small-molecule inhibitor strategies, PROTAC technology focuses on binding to and targeting any cellular POI for proteasomal degradation, including undruggable targets, and offers higher selectivity and efficiency, low toxicity, and avoidance of drug resistance.15−17 So far, more than 100 targets degraded by PROTACs have been reported, while some PROTACs are currently in clinical trials, such as ARV110 and ARV766 that target the androgen receptor (AR).18,19 This shows that PROTAC technology is a more promising treatment method, especially for the development of cancer drugs. PROTACs based on small molecules and peptides have also been successfully developed.20 Recently, researchers have turned their attention to developing nucleic acid-based PROTACs, in which the binding sequence is usually used as one unit of PROTAC for recruiting the corresponding protein. In 2020, RNA-PROTAC for degradation of the RNA-binding protein (RBP) was reported for the first time.21 Comparatively, DNA oligomer-based PROTAC strategies have demonstrated superior stability. For instance, TRAFTAC was the first DNA-based PROTAC proposed by the Craws group, which provides a generalizable strategy for targeted transcription factor degradation requiring the ectopic expression of Cas-9 protein in the cell based on CRISPR technology.22 Subsequently, two other DNA-based PROTACs, namely, TF-PROTACs and O PROTACs, were developed, which can directly recruit transcription factors and E3 enzymes to achieve the degradation of target proteins.23,24 Based on TF-PROTACs, decoy-based chimeric degraders have been developed, which can involve different kinds of E3 ligands.25 Moreover, G4-PROTAC and aptamer-PROTAC were raised as novel strategies to target proteins because G-quadruplexes and aptamers can serve as high-quality warheads.26,27 Overall, these works fully illustrate the feasibility of DNA-based PROTACs.
Additionally, PROTACs show potential for nondruggable targets and to overcome drug resistance.28 Most of the currently approved drugs and the existing ERα PROTACs concentrate on the LBD of ERα, the main cause of drug resistance.29 It is pertinent to note that ERα is a transcription factor consisting of a DNA-binding domain.30,31 Thus, in this work, we designed an ERE-PROTAC via a click reaction, selecting the DBD of ERα as the target of PROTAC and estrogen response element (ERE) as a recognized unit of PROTAC, a DNA sequence in cells that binds with the DBD of ERα in the nanomolar range.25,32 The 5′-terminus of the sense sequence of ERE (ssERE), which was modified with an azide group (named N3-ssERE), and VH032, which was modified with a terminal alkyne (VH032-propargyl), were linked by the copper-catalyzed azide–alkyne cycloaddition (CUAAC) reaction to form ERE-PROTAC (Figure 1B). Specifically, the ERE sequence recruited ERα, while the typical small molecule VH032 recruited the endogenous von Hippel–Lindau (VHL) E3 ligase.32−34 The designed ERE-PROTAC can induce the ubiquitination of ERα, which is subsequently degraded by the proteasome (Figure 1C). This PROTAC targeting DBD of ERα was first proposed that efficiently and controllably degraded ERα in different breast cancer cells and could potentially overcome the current drug resistance caused by LBD mutations of ERα.
Results
Synthesis, Characterization, and Cell Permeability of ERE-PROTAC
In the proposed PROTAC design, the ERE sequence is a conserved double-stranded DNA sequence in the promoter of estrogen target genes, which can bind to the estrogen receptor for transcriptional regulation. The sense sequence of the estrogen response element (ssERE), 5′-GTCCAAAGTCAGGTCA-CAGTGACCTGATCAAAGT-3′,35,36 and VH032, a typical small molecule, were chosen as the two recognized units. To synthesize the ERE-PROTAC, we adopted a robust biorthogonal reaction named the copper-catalyzed azide–alkyne cycloaddition (CUAAC) reaction.37,38 The 5′-end of the ssERE was modified with an azide group (named N3-ssERE), and the VH032 was modified with the terminal alkyne (VH032-propargyl) (Figure S1).39
To evaluate the efficiency of the CUAAC reaction, we monitored the amount of product produced by the click reaction under different reaction conditions. After incorporating various amounts of VH032-propargyl into N3-ssERE, the amount of N3-ssERE with enhanced molecular weight increased and could be clearly separated by 15% urea polyacrylamide gel electrophoresis (urea PAGE, Figure S2A).40,41 As expected, after incubation with a tenfold excess of VH032-propargyl for 24 h at a temperature of 310 K, a higher reaction efficiency was achieved (Figure S2B). According to the reversed-phase high-performance liquid chromatography–mass spectrometry (LC–MS) spectrum of the purified product, the ssERE–VH032 complex was successfully synthesized (Figure S3). After annealing the purified ssERE-PROTAC with its complementary chain, ERE-PROTAC was formed for subsequent experiments.
In addition, to explore the ability of ERE-PROTAC to degrade ERα, we verified its cell membrane permeability.42 The 3′-end of the antisense strand of ssERE was modified with fluorescent molecule CUAAC to form FITCERE-PROTAC. After various concentrations of FITCERE-PROTAC were incubated in MCF-7 cells for 12 h, the fluorescein isothiocyanate (FITC) fluorescent signal increased significantly, indicating that this PROTAC performs good cell permeability (Figure 2A).43 After that, we also verified that it could successfully bind with ERα after entering the cell based on immunofluorescence analysis. Compared with the control group that was not incubated with PROTAC, the green fluorescence of FITCERE-PROTAC coincided with the red fluorescence of the ERα secondary antibody labeled with Cy5 in the nucleus after 12 h of incubation with 5 μM FITCERE-PROTAC, demonstrating that ERE-PROTAC could successfully target the ERα (Figure 2B).44 The above experimental results showed that ERE-PROTAC has good cell permeability and can specifically bind with intracellular ERα.
Figure 2.
Good cell permeability of ERE-PROTAC in MCF-7. (A) Effect of different concentrations of FITCERE-PROTAC on the uptake of MCF-7 cells by flow cytometry detection. The data are expressed as the mean ± standard error of the mean (SEM) of the triple-independent experiments. *P < 0.05. **P < 0.01. (B) MCF-7 cells were treated with 5 μM FITCERE-PROTAC at 310 K for 12 h and stained with ERα antibody (scale bar, 50 μm); the image was acquired by a confocal microscope.
Degradation of ERα by ERE-PROTAC
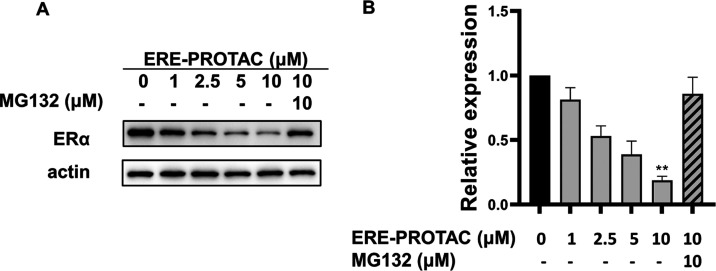
To evaluate the degradation ability mediated by ERE-PROTAC, we analyzed the protein level of ERα by immunoblotting.45 We found that ERE-PROTAC induced ERα degradation in MCF-7 cells in a dose-dependent manner, for which DC50 (the concentration at which the drug causes 50% protein degradation) was less than 5 μM (Figure 3A,B). Besides, 3T-ERE-PROTAC, 6T-ERE-PROTAC, and PEG-ERE-PROTAC were synthesized to study the effect of the linker (Table S1 and Figure S3). After these structures were incubated with MCF-7 cells for 24 h, respectively, Figure S4 shows that the ERE-PROTAC without a linker had the best degradation efficiency of ERα. Next, we tested the degradation efficiency of ERα at a concentration of 5 μM ERE-PROTAC under different incubation times. The results showed that ERα reached the maximum degradation efficiency after 4 h under 5 μM PROTAC treatment (Figure 3C,D).
Figure 3.
ERE-PROTAC degrades ERα in MCF-7 cells in a proteasome-dependent manner. (A) Dose effect of ERE-PROTAC on ERα degradation. (C) Time course of 5 μM ERE-PROTAC for ERα degradation. (E) MG132, individual VH032-propargyl, or ERE sequence inhibited the degradation of ERα, which indicated ERα degradation in a proteasome-dependent manner. (B), (D), and (F) Quantification of the changes in ERα levels under the (A), (C), and (E) conditions, respectively. The data is presented as the mean ± SEM values of triple-independent experiments; n = 3. *P < 0.05, **P < 0.01, and ***P < 0.005 vs the control group.
We further explored the mechanism of ERα degradation induced by ERE-PROTAC. When 5 μM ERE-PROTAC was incubated with MCF-7, MG132, VH032-propargyl, and the ERE sequence were added for 24 h incubation, respectively, and then the ERα protein degradation level was analyzed by immunoblotting. As shown in Figure 3E,F, the excess individual VH032-propargyl or ERE sequence inhibited the degradation of ERα, competing with PROTAC to bind with VHL or ERα, respectively. In addition, the degradation was blocked by proteasome inhibitor MG132, confirming that the protein degradation induced by PROTAC was proteasome-dependent.46 Based on the results, ERE-PROTAC-mediated degradation of ERα was realized by the ubiquitin–proteasome system after the formation of the POI-PROTAC-E3 ligase ternary complex. To test the dependence of ERα degradation on VHL, we prepared Chiral-ERE-PROTAC (Table S1), an epimer structure of ERE-PROTAC possessing the reversed (S) stereochemistry at the proline 4-position. As shown in Figure S5, the Chiral-ERE-PROTAC molecule, which has a weak binding ability to VHL,47 exhibited no significant degradation of ERα, confirming VHL’s role in the degradation of ERα by ERE-PROTAC.
Apoptosis of Breast Cancer Cells Induced by ERE-PROTAC
We further studied the effect of ERE-PROTAC on the proliferation of MCF-7 cells.48 First, we evaluated different concentrations of ERE-PROTAC to test the cytotoxicity of MCF-7 cells using Cell Counting Kit 8.49,50 Experimental results showed that ERE-PROTAC has a significant growth inhibitory effect on MCF-7 cells with an IC50 of 6.106 μM (Figure 4A). Then, we conducted an apoptosis detection experiment to examine the effect of ERE-PROTAC on the cytotoxicity of cancer cells.51 After treating MCF-7 cells with 2.5 and 5 μM ERE-PROTAC for 24 h, the cells were collected and stained with Annexin V and propidium iodide (PI).52 The flow cytometry results revealed 49.65% apoptotic cells at 5 μM ERE-PROTAC compared to only 32.95% apoptotic cells at 2.5 μM ERE-PROTAC, indicating the dose-dependent apoptosis of MCF-7 cells. Furthermore, the proportion of cells exhibiting features of early apoptosis increased immediately with increasing concentrations of ERE-PROTAC (Figure 4B,D). Next, through PI staining, the effect of 2.5 and 5 μM ERE-PROTAC on the MCF-7 cell cycle was analyzed by flow cytometry. After 24 h of treatment, as the concentration of ERE-PROTAC increased, more cells stagnated in the S phase, indicating that ERE-PROTAC inhibits proliferation by inducing S phase arrest (Figures 4C and S6). These results confirm that ERE-PROTAC can effectively kill breast cancer cells.
Figure 4.

ERE-PROTAC treatment induced cell death in MCF-7 cells. (A) After different concentrations of ERE-PROTAC acted on MCF-7 cells for 24 h, the CCK-8 method was used to detect the inhibitory effect on the proliferation. (B) and (D) Treatment with different concentrations of ERE-PROTAC for 24 h; cell apoptosis was analyzed by Annexin V/PI staining. (C) Treated MCF-7 with ERE-PROTAC for 24 h and analyzed the cell cycle through PI staining analysis; the data was the average value of three independent cell cycle experiments. The data are expressed as the mean ± SEM of triple-independent experiments. *P < 0.05. **P < 0.01, and ***P < 0.005 vs the control group.
Reversibility of ERα Degradation
ERE is a natural DNA sequence derived from cells. Considering the variety of nucleases existing in cells, it can be conjectured that synthetic ERE-PROTAC entering cells will be degraded by nucleases after some time.53,54 This degradation prevents the sustained effects of ERE-PROTAC on cells, and the decomposed oligonucleotides could be utilized in other physiological processes. We hypothesize that after PROTAC is enzymatically hydrolyzed, the protein expression level could be restored. To validate this hypothesis, we designed a fluorescence analysis experiment to track the changes of ERα and ERE-PROTAC in MCF-7 cells by confocal microscopy imaging.42,55,56 As shown in Figure 5A, when FITCERE-PROTAC was incubated with cells for 24 h, the red fluorescence of ERα secondary antibody specifically labeled Cy5 was absent and the green fluorescence of FITC diffused from the nucleus into the cytoplasm, indicating that most of the ERα was degraded. After 48 h of incubation with FITCERE-PROTAC, a small amount of red fluorescence of ERα was recovered as ERE-PROTAC was progressively broken down by intracellular nucleases. Subsequently, after incubation with ERE-PROTAC for 24 h, the medium was replaced with the fresh complete growth medium. After another 24 h of incubation, we observed obvious recovered red fluorescence of ERα. Next, we performed western blotting to determine the expression level of ERα in cells, which revealed that about 66% of the ERα protein was recovered after 24 h of washing out ERE-PROTAC (Figure 5B,C). These results suggested that ERα degradation can be reversible by adjusting the amount and dose time of ERE-PROTAC.
Figure 5.
Reversibility degradation of ERα by ERE-PROTAC. (A) MCF-7 cells were cultured with 5 μM FITCERE-PROTAC, characterized by ERα fluorescent secondary antibody and photographed by a confocal microscope at different times and culture conditions (scale bar, 50 μm). (B) After being cultured with 5 μM ERE-PROTAC for 24 h, ERE-PROTAC was washed off and then MCF-7 cells were cultured for 24 h. (C) Western blotting was used to characterize the ERα level, and actin was used as a control group for quantification. The data are presented as the mean ± SEM values of triple-independent experiments; n = 3. *P < 0.05, **P < 0.01, and ***P < 0.005 vs the control group.
ERE-PROTAC Degrades ERα in T47D Cells
It is well known that ERα is overexpressed in 70% of breast cancer cells. To expand the application of ERE-PROTAC, we further incubated different concentrations of ERE-PROTAC with T47D cells for 24 h and verified the expression of ERα by immunoblotting experiments.57,58 The results showed that ERE-PROTAC could also successfully degrade ERα in T47D cells in a dose-dependent manner. Coincubation of 10 μM MG132 and 10 μM ERE-PROTAC in T47D cells prevented ERα degradation, which indicated that ERE-PROTAC induced ERα degradation through the ubiquitin–proteasome system (Figure 6). The results showed that ERE-PROTAC could be effective on other types of ERα-positive breast cancers.
Figure 6.
ERE-PROTAC degraded ERα in T47D cells in a proteasome-dependent manner. (A) After incubating with different concentrations of ERE-PROTAC and 10 μM MG132 in T47D cells for 24 h, the protein level of ERα was analyzed by western blotting. (B) Quantitative analysis of the relative protein content of ERα. The data are presented as the mean ± SEM values of triple-independent experiments; n = 3. *P < 0.05, **P < 0.01, and ***P < 0.005 vs the control group. ****P < 0.001 vs the control group.
Discussion
In summary, we developed a new strategy for the treatment of breast cancer with high ERα expression. The current mainstream ERα therapeutic drugs all target the LBD of ERα, which often exhibits drug resistance.59 Therefore, we designed a novel ERE-PROTAC that targets the DBD of ERα using the natural DNA-binding sequence ERE as the recognizing unit to degrade ERα and efficiently kill ERα+ breast cancer cells. This design successfully provides a novel target for ERα and a new way to solve the problem of drug resistance. Importantly, due to the significance of DNA-binding proteins (DBPs) in cancer treatments, especially transcription factors, the proposed strategy based on natural DNA modification PROTAC may have a wide range of applications in targeted protein degradation. Our future work will focus on exploring new PROTAC molecules targeting different DBPs to obtain better clinical efficacy.
Methods
Synthesis of ERE-PROTAC
The 5′ end of the ERE sense sequence (Sangon Biotech, Shanghai) was modified with an azide group and then conjugated with VH032-propargyl (HY-126465, MedChemExpress) through a copper-catalyzed azide–alkyne cycloaddition (CUAAC) reaction. After the reaction, the product was precipitated by ethanol and dried in a vacuum concentrator. The dried product was diluted with water to 20 μM. Mass and purity (>95%) were confirmed by liquid chromatography–mass spectrometry (LC–MS) (Thermo Fisher’s Q-Exactive Focus) on a Waters Acquity OST C-18 column, 2.1 × 50 mm, 1.7 μm at 338 K. Buffer A was 10 mM triethylamine, buffer B was acetonitrile, the gradient was 5–50% B for 15 min, and the flow rate was 0.3 mL/min. The sense and antisense ERE oligonucleotides were mixed in a ratio of 1:1, heated to 368 K for 10 min, and then cooled to room temperature. In this way, the ERE was annealed into a double strand. 3T-ERE-PROTAC, 6T-ERE-PROTAC, and PEG-ERE-PROTAC were synthesized by different modified ERE sequences with VH032-propargyl through the above method. Chiral-ERE-PROTAC was synthesized by an ERE sequence conjugated with (S,S,S)-AHPC-propargyl (MedChemExpress).
Urea DNA Polyacrylamide Gel Electrophoresis (Urea PAGE)
The unreacted ERE and the reacted ERE can be separated by 15% urea polyacrylamide gel electrophoresis (urea PAGE).60 The specific operation process was electrophoresis in 1× tris-borate-EDTA buffer (TBE, pH = 8.3) at 120 V for 2 h. The gel after electrophoresis was incubated in 0.2% SYBR Gold nucleic acid gel stain (S11494, Thermo Fisher Scientific) for 15 min and imaged using Molecular Imager PharosFX (Bio-Rad).
Western Blot Assay
A total of 1 × 105 adherent cells were seeded in a 12-well plate and grown in Dulbecco’s modified Eagle’s medium (DMEM) for 24 h. After that, a specified concentration of ERE-PROTAC was incubated with the cells in a complete medium for a specified time. To separate the protein, the cells were trypsinized and lysed in RIPA buffer for 30 min and centrifuged at 12,000g for 3 min. The lysate (15 μg protein) was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 120 V for 80 min. Next, the electrophoresed protein was transferred to the poly(vinylidene fluoride) (PVDF) membrane, immunoblotting with the anti-ERα and antiactin antibodies at 277 K overnight. Next, the PVDF membrane was washed three times with TBST (containing 0.1% Tween-20) for 10 min each time. Afterward, the PVDF membrane was incubated with the secondary antibody at room temperature for 1 h and washed three times with TBST (containing 0.1% Tween-20) for 10 min each time. The anti-ERα (#8644s 1:1000) antibody was purchased from Cell Signaling Technology. Antiactin (#AC026, 1:1000) was purchased from ABclonal. A peroxidase-conjugated antirabbit secondary antibody (A-0208, 1:1000) was purchased from Beyotime Biotechnology. All primary antibodies were diluted with 5% bovine serum albumin in TBST buffer, and secondary antibodies were diluted with 5% skimmed milk in TBST buffer. A gel imager (Tanon 5200) was used to display the image.
Protocol of Ethanol Precipitation of DNA
A one-tenth volume of sodium acetate (3 M, pH = 5.2) was added and mixed thoroughly in the DNA solution after the reaction. Twice the volume of precooled ethanol was mixed in the solution, and then, it was placed at 253 K overnight and centrifuged at 12,000g for 10 min to remove the supernatant. Ethanol (70%) was added to wash DNA with 1/2 the centrifuge tube volume, and finally, the DNA was dried at 318 K for 1 h with a rotary evaporator.61,62
Confocal Microscopy
A total of 5 × 104 MCF-7 cells were seeded in a confocal dish and cultured in DMEM for 24 h. After the cells were fully attached, an appropriate amount of FITC-labeled ERE-PROTAC was added to the medium and incubated with the MCF-7 cells at 310 K for 24 h. The cells were washed with PBS, fixed in 4% paraformaldehyde (PFA) (in PBS) at room temperature for 15 min, and incubated with 0.25% Triton X-100 for 5 min. Then, the cells were blocked at 310 K for 30 min by a blocking solution (containing 1% BSA in PBST) and washed with PBST. Next, the anti-ERα antibody (8644S; Cell Signaling Technology) was dissolved in the blocking solution at a dilution rate of 1:300 and incubated with the cells at 310 K for 1 h. The Cy5-conjugated goat antirabbit IgG antibody (#550083, Zen Bioscience) was dissolved in PBST at a dilution rate of 1:1000 and incubated with the cells at 310 K for 40 min. Finally, the cells were stained with DAPI for 5 min at 310 K, washed three times with PBST for 10 min each time, and imaged by a confocal laser scanning microscope (NIKON A1 HD25).
Fluorescence-Activated Cell Sorting (FACS)
MCF-7 cells were incubated with 2.5, 5, and 10 μM FITC-modified ERE-PROTAC for 24 h. The cells were digested with 0.25% trypsin (Gibco) for 3 min, collected, and then centrifuged at 1000g for 3 min to remove the supernatant. The cells were resuspended in PBS. Next, the FITC fluorescence in the cells was analyzed by flow cytometry (Beckman Coulter). The experiment was repeated three times, and at least 10,000 gated events were collected and analyzed each time.
Apoptosis and Cell Cycle Analysis
MCF-7 cells were cultured in DMEM with different concentrations of ERE-PROTAC for 24 h. The cells were washed with PBS and digested with 0.25% trypsin (Gibco) for 3 min. The digested cells were collected and centrifuged at 1000g for 5 min to remove the supernatant. For apoptosis analysis, the cells were processed according to the protocol of the FITC Annexin V Apoptosis Detection Kit (40302ES20, Yeason Biosciences). For cell cycle analysis, the collected cells were resuspended in 70% precooled ethanol, placed at 277 K overnight, centrifuged to remove the ethanol in the supernatant, and processed according to the protocol of Cell Cycle and Apoptosis Analysis Kit (40301ES50, Yeason Biosciences). The samples were tested on a flow cytometer (Beckman Coulter) and analyzed using Modfit software. The experiments were repeated three times, and at least 10,000 gated events were collected and analyzed each time.
Cell Culture and Treatment
MCF-7 cells and T47D cells were cultured in DMEM containing 10% fetal bovine serum (FBS), 100 units/mL of penicillin, and 100 μg/mL streptomycin.
Protocol for Click Chemistry
According to the protocol of the 1000 μL CUAAC reaction system, this research designed a 200 μL reaction system.63,64 N3-ssERE was added to a final concentration of 50 μM, and VH032-propargyl was added to a final concentration of 500 μM in 0.1 M potassium phosphate (pH 7.0) buffer. Next, 10 mM CuSO4 and ligand 20 mM THPTA were premixed and added such that the concentration ratio of THPTA was 15 mM and the concentration ratio of CuSO4 was 2.5 mM. Finally, 25 mM sodium ascorbate was added to a final concentration of 2.5 mM. The compounds reacted at 310 K for 24 h.
CCK-8 Cell Proliferation Test
MCF-7 cells were spread evenly in a 96-well plate. After adhering to the wall, the cells were incubated with different concentrations of ERE-PROTAC for 24 h. CCK-8 (10 μL; Cell Counting Kit-8) was added to each well and incubated for 2 h; then, the optical density was measured at 450 nm by employing a microplate reader (Tecan Infinite).
Acknowledgments
The authors acknowledge the financial support from the State Key Laboratory of Chemical Oncogenomics and the National Key R&D Program of China, Synthetic Biology Research (2019YFA0905900).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.2c00109.
Synthesis of ERE-PROTAC, result of urea DNA polyacrylamide gel electrophoresis, LC–MS chromatograms of all synthesized PROTACs, ERα protein degradation efficiency induced by different PROTACs, effect of ERE-PROTAC on MCF-7 cell cycle, and structures of different PROTACs (PDF)
Author Contributions
† X.Z. and Z.Z. contributed equally to this work. X.Z., Y.J., and Y.T. proposed the idea. X.Z., C.T., and Y.T. designed the experiments. Z.Z. and X.X. helped with the LC–MS experiments. F.L. and F.T. also provided help in the FACS experiments. Y.J., X.Z., and Y.J. wrote the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Bray F.; Ferlay J.; Soerjomataram I.; Siegel L.; Torre A.; Ahmedin D. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 2020, 71, 209–249. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Siegel R. L.; Miller Kimberly D.; Jemal A. Cancer statistics, 2019. Ca-Cancer J. Clin. 2019, 69, 7–34. 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- Waks A. G.; Winer E. P. Breast cancer treatment. J. Am. Med. Assoc. 2019, 321, 316. 10.1001/jama.2018.20751. [DOI] [PubMed] [Google Scholar]
- Siersbæk R.; Kumar S.; Carroll J. S. Signaling pathways and steroid receptors modulating estrogen receptor α function in breast cancer. Genes Dev. 2018, 32, 1141–1154. 10.1101/gad.316646.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V.; Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell 1988, 55, 145–156. 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Pawlak M.; Lefebvre P.; Staels B. General molecular biology and architecture of nuclear receptors. Curr. Top. Med. Chem. 2012, 12, 486–504. 10.2174/156802612799436641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.; Thompson E. B. The structure of the nuclear hormone receptors. Steroids 1999, 64, 310–319. 10.1016/S0039-128X(99)00014-8. [DOI] [PubMed] [Google Scholar]
- Kumar V.; Green S.; Stack G.; Berry M.; Jin J.-R.; Chambon P. Functional domains of the human estrogen receptor. Cell 1987, 51, 941–951. 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- McEwan I. J.Nuclear Receptors: One Big Family. In Methods in Molecular Biology; Springer, 2009; Vol. 505, pp 3–18. [DOI] [PubMed] [Google Scholar]
- Hanker A. B.; Sudhan D. R.; Arteaga C. L. Overcoming endocrine resistance in breast cancer. Cancer Cell 2020, 37, 496–513. 10.1016/j.ccell.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J.; Massarweh S.; Osborne C. K.; Wakeling A. E.; Ali S.; Weiss H.; Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2–positive breast cancer. J. Natl. Cancer Inst. 2004, 96, 926–935. 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- Sakamoto K. M.; Kim K. B.; Kumagai A.; Mercurio F.; Crews C. M.; Deshaies R. J. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 8554–8559. 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Song Y. Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. J. Hematol. Oncol. 2020, 13, 50 10.1186/s13045-020-00885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.; Gao H.; Yang Y.; He M.; Wu Y.; Song Y.; Tong Y.; Rao Y. PROTACs: great opportunities for academia and industry. Signal Transduction Targeted Ther. 2019, 4, 1–33. 10.1038/s41392-019-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.; Zhang X.; Lv D.; Zhang Q.; He Y.; Zhang P.; Liu X.; Thummuri D.; Yuan Y.; Wiegand J. S.; et al. A selective BCL-XL PROTAC degrader achieves safe and potent antitumor activity. Nat. Med. 2019, 25, 1938–1947. 10.1038/s41591-019-0668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhimschi A. D.; Armstrong H. A.; Toure M.; Jaime-Figueroa S.; Chen T. L.; Lehman A. M.; Woyach J. A.; Johnson A. J.; Byrd J. C.; Crews C. M. Targeting the C481S ibrutinib-resistance mutation in Bruton’s tyrosine kinase using PROTAC-mediated degradation. Biochemistry 2018, 57, 3564–3575. 10.1021/acs.biochem.8b00391. [DOI] [PubMed] [Google Scholar]
- Burslem G. M.; Smith B. E.; Lai A. C.; Jaime-Figueroa S.; McQuaid D. C.; Bondeson D. P.; Toure M.; Dong H.; Qian Y.; Wang J.; et al. The advantages of targeted protein degradation over inhibition: an RTK case study. Cell Chem. Biol. 2018, 25, 67–77.e3. 10.1016/j.chembiol.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burslem G. M.; Crews C. M. Proteolysis-targeting chimeras as therapeutics and tools for biological discovery. Cell 2020, 181, 102–114. 10.1016/j.cell.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullard A. Targeted protein degraders crowd into the clinic. Nat. Rev. Drug Discovery 2021, 20, 247–250. 10.1038/d41573-021-00052-4. [DOI] [PubMed] [Google Scholar]
- Smith B. E.; Wang S. L.; Jaime-Figueroa S.; Harbin A.; Wang J.; Hamman B. D.; Crews C. M. Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase. Nat. Commun. 2019, 10, 131 10.1038/s41467-018-08027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghidini A.; Cléry A.; Halloy F.; Allain F. H.; Hall J. RNA-PROTACs: degraders of RNA-binding proteins. Angew. Chem., Int. Ed. 2021, 133, 3200–3206. 10.1002/ange.202012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarasinghe K. T.; Jaime-Figueroa S.; Burgess M.; Nalawansha D. A.; Dai K.; Hu Z.; Bebenek A.; Holley S. A.; Crews C. M. Targeted degradation of transcription factors by TRAFTACs: transcription factor targeting chimeras. Cell Chem. Biol. 2021, 28, 648–661. e5. 10.1016/j.chembiol.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J.; Yan Y.; Ding D.; Wang D.; He Y.; Pan Y.; Yan W.; Kharbanda A. Li, H. y.; Huang, H., Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O’PROTAC): Effective Targeting of LEF1 and ERG (Adv. Sci. 20/2021). Adv. Sci. 2021, 8, 2170136 10.1002/advs.202170136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Chen H.; Kaniskan H. U.; Xie L.; Chen X.; Jin J.; Wei W. TF-PROTACs enable targeted degradation of transcription factors. J. Am. Chem. Soc. 2021, 143, 8902–8910. 10.1021/jacs.1c03852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma M.; Ohoka N.; Tsuji G.; Tsujimura H.; Matsuno K.; Inoue T.; Naito M.; Demizu Y. Development of chimeric molecules that degrade the estrogen receptor using decoy oligonucleotide ligands. ACS Med. Chem. Lett. 2022, 13, 134–139. 10.1021/acsmedchemlett.1c00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S.; Gao F.; Ma J.; Ma H.; Dong G.; Sheng C. Aptamer-protac conjugates (apcs) for tumor-specific targeting in breast cancer. Angew. Chem., Int. Ed. 2021, 60, 23299–23305. 10.1002/anie.202107347. [DOI] [PubMed] [Google Scholar]
- Patil K. M.; Chin D.; Seah H. L.; Shi Q.; Lim K. W.; Phan A. T. G4-PROTAC: targeted degradation of a G-quadruplex binding protein. Chem. Commun. 2021, 57, 12816–12819. 10.1039/D1CC05025G. [DOI] [PubMed] [Google Scholar]
- Samarasinghe K. T.; Crews C. M. Targeted protein degradation: a promise for undruggable proteins. Cell Chem. Biol. 2021, 28, 934–951. 10.1016/j.chembiol.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X.; Xiang H.; Luo G. Targeting estrogen receptor α for degradation with PROTACs: A promising approach to overcome endocrine resistance. Eur. J. Med. Chem. 2020, 206, 112689 10.1016/j.ejmech.2020.112689. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J.; Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science 1989, 245, 371–378. 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J.; Thummel C.; Beato M.; Herrlich P.; Schütz G.; Umesono K.; Blumberg B.; Kastner P.; Mark M.; Chambon P.; Evans R. M. The nuclear receptor superfamily: the second decade. Cell 1995, 83, 835–839. 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge C. M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001, 29, 2905–2919. 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley D. L.; Gustafson J. L.; Van Molle I.; Roth A. G.; Tae H. S.; Gareiss P. C.; Jorgensen W. L.; Ciulli A.; Crews C. M. Small-Molecule Inhibitors of the Interaction between the E3 Ligase VHL and HIF1α. Angew. Chem., Int. Ed. 2012, 51, 11463–11467. 10.1002/anie.201206231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley D. L.; Van Molle I.; Gareiss P. C.; Tae H. S.; Michel J.; Noblin D. J.; Jorgensen W. L.; Ciulli A.; Crews C. M. Targeting the von Hippel–Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1α interaction. J. Am. Chem. Soc. 2012, 134, 4465–4468. 10.1021/ja209924v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. N.; Su X.; Liu E. T.; Thomsen J. S. Gold-nanoparticle-based assay for instantaneous detection of nuclear hormone receptor– response elements interactions. Anal. Chem. 2010, 82, 2759–2765. 10.1021/ac9026498. [DOI] [PubMed] [Google Scholar]
- Uliana C. V.; Peverari C. R.; Afonso A. S.; Cominetti M. R.; Faria R. C. Fully disposable microfluidic electrochemical device for detection of estrogen receptor alpha breast cancer biomarker. Biosens. Bioelectron. 2018, 99, 156–162. 10.1016/j.bios.2017.07.043. [DOI] [PubMed] [Google Scholar]
- Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. A stepwise huisgen cycloaddition process: copper (I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem., Int. Ed. 2002, 114, 2708–2711. . [DOI] [PubMed] [Google Scholar]
- Tornøe C. W.; Christensen C.; Meldal M. Peptidotriazoles on solid phase:[1, 2, 3]-triazoles by regiospecific copper (I)-catalyzed 1, 3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- Wurz R. P.; Dellamaggiore K.; Dou H.; Javier N.; Lo M.-C.; McCarter J. D.; Mohl D.; Sastri C.; Lipford J. R.; Cee V. J. A “click chemistry platform” for the rapid synthesis of bispecific molecules for inducing protein degradation. J. Med. Chem. 2018, 61, 453–461. 10.1021/acs.jmedchem.6b01781. [DOI] [PubMed] [Google Scholar]
- Fried M.; Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981, 9, 6505–6525. 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E.; Givel F.; Wahli W. The estrogen-responsive element as an inducible enhancer: DNA sequence requirements and conversion to a glucocorticoid-responsive element. EMBO J. 1987, 6, 3719–3727. 10.1002/j.1460-2075.1987.tb02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; Deng Q.; Zhao H.; Xie M.; Chen L.; Yin F.; Qin X.; Zheng W.; Zhao Y.; Li Z. Development of stabilized peptide-based PROTACs against estrogen receptor α. ACS Chem. Biol. 2018, 13, 628–635. 10.1021/acschembio.7b00985. [DOI] [PubMed] [Google Scholar]
- Rejman J.; Bragonzi A.; Conese M. Role of clathrin-and caveolae-mediated endocytosis in gene transfer mediated by lipo-and polyplexes. Mol. Ther. 2005, 12, 468–474. 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Phillips C.; Roberts L. R.; Schade M.; Bazin R.; Bent A.; Davies N. L.; Moore R.; Pannifer A. D.; Pickford A. R.; Prior S. H.; et al. Design and structure of stapled peptides binding to estrogen receptors. J. Am. Chem. Soc. 2011, 133, 9696–9699. 10.1021/ja202946k. [DOI] [PubMed] [Google Scholar]
- Gallagher S.; Winston S. E.; Fuller S. A.; Hurrell J. G. Immunoblotting and immunodetection. Curr. Protoc. Cell Biol. 2011, 52, 6.2.1–6.2.28. 10.1002/0471143030.cb0602s52. [DOI] [PubMed] [Google Scholar]
- Rana S.; Bendjennat M.; Kour S.; King H. M.; Kizhake S.; Zahid M.; Natarajan A. Selective degradation of CDK6 by a palbociclib based PROTAC. Bioorg. Med. Chem. Lett. 2019, 29, 1375–1379. 10.1016/j.bmcl.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew A. P.; Raina K.; Dong H.; Qian Y.; Wang J.; Vigil D.; Serebrenik Y. V.; Hamman B. D.; Morgan A.; Ferraro C.; et al. Identification and characterization of Von Hippel-Lindau-recruiting proteolysis targeting chimeras (PROTACs) of TANK-binding kinase 1. J. Med. Chem. 2018, 61, 583–598. 10.1021/acs.jmedchem.7b00635. [DOI] [PubMed] [Google Scholar]
- Kerr J. F. R.; Wyllie A. H.; Currie A. R. Apoptosis: a basic biological phenomenon with wideranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.; Yang Y.; Liu Q.; Wang J. Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells. Med. Oncol. 2011, 28, 105–111. 10.1007/s12032-009-9397-3. [DOI] [PubMed] [Google Scholar]
- Niu Y.; Ma F.; Huang W.; Fang S.; Li M.; Wei T.; Guo L. Long non-coding RNA TUG1 is involved in cell growth and chemoresistance of small cell lung cancer by regulating LIMK2b via EZH2. Mol. Cancer 2017, 16, 1–13. 10.1186/s12943-016-0575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Cell 1993, 75, 641–652. 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Nicoletti I.; Migliorati G.; Pagliacci M.; Grignani F.; Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. 10.1016/0022-1759(91)90198-O. [DOI] [PubMed] [Google Scholar]
- Chen J. S.; Ma E.; Harrington L. B.; Da Costa M.; Tian X.; Palefsky J. M.; Doudna J. A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. 10.1126/science.aar6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z.; Chung W.-H.; Shim E. Y.; Lee S. E.; Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 2008, 134, 981–994. 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korlach J.; Schwille P.; Webb W. W.; Feigenson G. W. Characterization of lipid bilayer phases by confocal microscopy and fluorescence correlation spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 8461–8466. 10.1073/pnas.96.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell S.; Reiner G.; Cremer C.; Stelzer E. H. Aberrations in confocal fluorescence microscopy induced by mismatches in refractive index. J. Microsc. 1993, 169, 391–405. 10.1111/j.1365-2818.1993.tb03315.x. [DOI] [Google Scholar]
- Rusnak D. W.; Lackey K.; Affleck K.; Wood E. R.; Alligood K. J.; Rhodes N.; Keith B. R.; Murray D. M.; Knight W. B.; Mullin R. J. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol. Cancer Ther. 2001, 1, 85–94. 10.1097/00008390-200112000-00011. [DOI] [PubMed] [Google Scholar]
- Li Y.; Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science 1996, 274, 246–248. 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- Cortazar P.; Zhang L.; Untch M.; Mehta K.; Costantino J. P.; Wolmark N.; Bonnefoi H.; Cameron D.; Gianni L.; Valagussa P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- Lopez-Gomollon S.; Nicolas F. E.. Purification of DNA Oligos by Denaturing Polyacrylamide Gel Electrophoresis (PAGE). In Methods in Enzymology; Elsevier, 2013; Vol. 529, pp 65–83. 10.1016/B978-0-12-418687-3.00006-9. [DOI] [PubMed] [Google Scholar]
- Burger R. A.; Monk B. J.; Kurosaki T.; Anton-Culver H.; Vasilev S. A.; Berman M. L.; Wilczynski S. P. Human papillomavirus type 18: association with poor prognosis in early stage cervical cancer. J. Natl. Cancer Inst. 1996, 88, 1361–1368. 10.1093/jnci/88.19.1361. [DOI] [PubMed] [Google Scholar]
- Hallaj-Nezhadi S.; Valizadeh H.; Baradaran B.; Dobakhti F.; Lotfipour F. Preparation and characterization of gelatin nanoparticles containing pDNA encoding IL-12 and their expression in CT-26 carcinoma cells. Future Oncol. 2013, 9, 1195–1206. 10.2217/fon.13.82. [DOI] [PubMed] [Google Scholar]
- Besanceney-Webler C.; Jiang H.; Zheng T.; Feng L.; Soriano del Amo D.; Wang W.; Klivansky L. M.; Marlow F. L.; Liu Y.; Wu P. Increasing the efficacy of bioorthogonal click reactions for bioconjugation: a comparative study. Angew. Chem., Int. Ed. 2011, 50, 8051–8056. 10.1002/anie.201101817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogoi K.; Mane M. V.; Kunte S. S.; Kumar V. A. A versatile method for the preparation of conjugates of peptides with DNA/PNA/analog by employing chemo-selective click reaction in water. Nucleic Acids Res. 2007, 35, e139 10.1093/nar/gkm935. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.