Abstract
4-Phosphoryloxy-N,N-dimethyltryptamine (psilocybin) is a naturally occurring tertiary amine found in many mushroom species. Psilocybin is a prodrug for 4-hydroxy-N,N-dimethyltryptamine (psilocin), which induces psychedelic effects via agonist activity at the serotonin (5-HT) 2A receptor (5-HT2A). Several other 4-position ring-substituted tryptamines are present in psilocybin-containing mushrooms, including the secondary amine 4-phosphoryloxy-N-methyltryptamine (baeocystin) and the quaternary ammonium 4-phosphoryloxy-N,N,N-trimethyltryptamine (aeruginascin), but these compounds are not well studied. Here, we investigated the structure–activity relationships for psilocybin, baeocystin, and aeruginascin, as compared to their 4-acetoxy and 4-hydroxy analogues, using in vitro and in vivo methods. Broad receptor screening using radioligand binding assays in transfected cells revealed that secondary and tertiary tryptamines with either 4-acetoxy or 4-hydroxy substitutions display nanomolar affinity for most human 5-HT receptor subtypes tested, including the 5-HT2A and the serotonin 1A receptor (5-HT1A). The same compounds displayed affinity for 5-HT2A and 5-HT1A in mouse brain tissue in vitro and exhibited agonist efficacy in assays examining 5-HT2A-mediated calcium mobilization and β-arrestin 2 recruitment. In mouse experiments, only the tertiary amines psilocin, psilocybin, and 4-acetoxy-N,N-dimethyltryptamine (psilacetin) induced head twitch responses (ED50 0.11–0.29 mg/kg) indicative of psychedelic-like activity. Head twitches were blocked by 5-HT2A antagonist pretreatment, supporting 5-HT2A involvement. Both secondary and tertiary amines decreased body temperature and locomotor activity at higher doses, the effects of which were blocked by 5-HT1A antagonist pretreatment. Across all assays, the pharmacological effects of 4-acetoxy and 4-hydroxy compounds were similar, and these compounds were more potent than their 4-phosphoryloxy counterparts. Importantly, psilacetin appears to be a prodrug for psilocin that displays substantial serotonin receptor activities of its own.
Keywords: psilocybin, baeocystin, aeruginascin, head twitch response, hypothermia, mice
4-Phosphoryloxy-N,N-dimethyltryptamine (psilocybin) is a naturally occurring tertiary amine found in diverse mushroom species throughout the world.1−4 Psilocybin-containing mushrooms, sometimes colloquially called “magic mushrooms”, have been used for centuries as religious sacraments to facilitate spiritual or mystical-type subjective experiences.2−5 Additionally, psilocybin-containing mushrooms are used in various recreational contexts, which can pose risks to public health.1,6 Psychedelics like psilocybin,7−10 its active metabolite 4-hydroxy-N,N-dimethyltryptamine (psilocin),11−14 and some other related compounds are designated as controlled substances internationally. Legal restrictions on psychedelic compounds have significantly hindered research efforts aimed at understanding their complex pharmacology.3,4,15 However, in recent times, there is renewed interest in the therapeutic potential of psychedelics, and psilocybin has been investigated as an adjunct to psychotherapy in the treatment of psychological distress in end stage cancer, depression, substance use disorders, chronic pain, and obsessive-compulsive disorders.3,16−20
The subjective effects of psilocybin administration in humans are blocked by pretreatment with antagonists of the serotonin (5-HT) 2A receptor (5-HT2A) and are positively correlated with occupancy of this receptor in the brain,11,21,22 supporting a primary role in mediating psychedelic subjective effects. Likewise, in rodent behavioral models, many effects of psilocybin and psilocin are reversed by 5-HT2A antagonists or genetic deletion of the receptor.23−25 On the other hand, it is well known that psilocin acts as a nonselective serotonin receptor agonist.26 Indeed, some effects of psilocybin in humans and rodents are modulated by actions at non-5HT2A sites, like the serotonin 1A receptor (5-HT1A) and the serotonin 2C receptor (5-HT2C).23,26−30 Thus, some effects of psilocybin and related tryptamine compounds likely involve multiple serotonergic receptor sites of action.
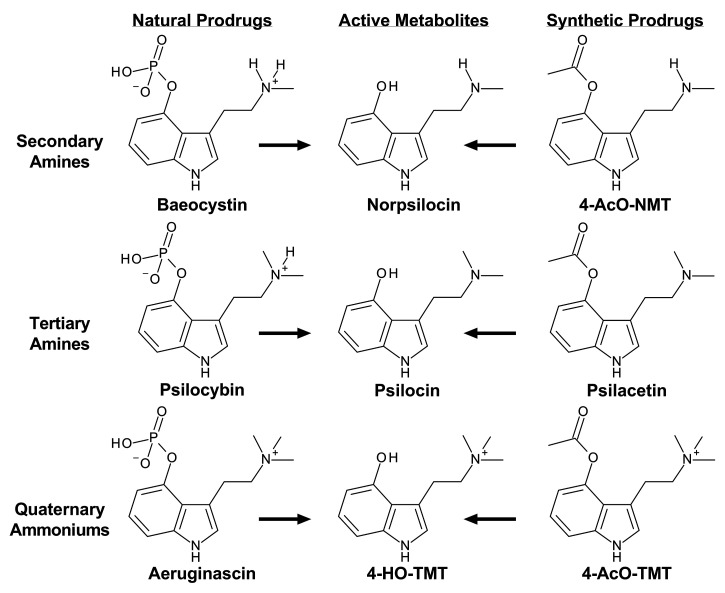
Some psychoactive mushroom species contain other potentially bioactive compounds in addition to psilocybin, including various tryptamines, β-carbolines, and terpenes.5,31,32 Underexplored tryptamines present in psilocybin-containing mushrooms include 4-phosphoryloxy-N-methyltryptamine (baeocystin), 4-phosphoryloxy-N,N,N-trimethyltryptamine (aeruginascin), 4-hydroxy-N-methyltryptamine (norpsilocin), and 4-phosphoryloxytryptamine (norbaeocystin)33−39 (see Figure 1). The precise amounts of these tryptamines relative to that of psilocybin vary across mushroom species, but the compounds are found in mushroom species commonly ingested by humans, such as Psilocybe cubensis.33 There is scant information about the pharmacological effects of most tryptamines found in psilocybin-containing mushrooms, with the exception of psilocybin. Preclinical evaluation of baeocystin in the mouse head twitch response (HTR) assay, which predicts psychedelic subjective effects in humans,40 shows that the substance does not produce effects in vivo.41 Pharmacological evaluations of tryptamines other than psilocybin and psilocin are lacking, despite initiatives in the USA to legalize or decriminalize psilocybin-containing mushrooms,42 many of which contain the aforementioned psilocybin analogues.5,33,35−38
Figure 1.
Chemical structures of the natural prodrugs baeocystin, psilocybin, and aeruginascin as well as their 4-hydroxy active metabolites and synthetic 4-acetoxy analogues.
Beyond naturally occurring psilocybin analogues, synthetic 4-acetoxy derivatives of psilocybin and aeruginascin have been previously synthesized by members of our team and are thought to be prodrugs for corresponding 4-hydroxy active metabolites.43,44 The first synthetic prodrug identified was 4-acetoxy-N,N-dimethyltryptamine (psilacetin), which was originally reported by Hofmann and Troxler,45 discussed by the Shulgins in TiHKAL as a likely psilocin prodrug ester,46 and suggested as an alternative to psilocybin based on a more efficient synthesis route by Nichols and Frescas.47 More recently, psilacetin has emerged as a popular new psychoactive substance (NPS) on recreational drug markets, where it is sold as a psilocybin alternative.48−52 Analogous to the metabolism of psilocybin to psilocin, 4-acetoxy compounds are thought to hydrolyze to their corresponding 4-hydroxy compounds.43,44,51 While definitive biotransformation studies in animals are lacking, in vitro evidence from liver microsomes supports the metabolic conversion of 4-acetoxy compounds to their 4-hydroxy analogues.53 This biotransformation reaction probably also occurs with secondary amines like 4-acetoxy-N-methyltryptamine (4-AcO-NMT) and quaternary ammoniums like 4-acetoxy-N,N,N-trimethyltryptamine (4-AcO-TMT), the synthetic analogues of baeocystin and aeruginascin.41,43,54 Importantly, psilacetin is less potent than psilocin in producing psychedelic-like effects in rodents,52,55 which is consistent with the expected profile of a prodrug. As with the other constituents of psilocybin-containing mushrooms, the pharmacology of 4-acetoxy analogues of psilocybin, baeocystin, and aeruginascin is largely uncharacterized, and a side-by-side comparison of the natural products and their corresponding active metabolites has not been performed.
Herein, we investigated the receptor binding profiles and 5-HT2 receptor functional activities for a series of psilocybin analogues which vary in their degree of N-methylation and 4-position indole ring substitution. Specifically, we examined the in vitro effects of naturally occurring 4-phosphoryloxy prodrugs, synthetic 4-acetoxy prodrugs, and their shared 4-hydroxy metabolites (see Figure 1 for chemical structures). We further tested the ability of these compounds to compete for 5-HT2A and 5-HT1A binding in mouse brain, as well as to produce behavioral and physiological effects in mice. Lastly, we determined the role of 5-HT2A and 5-HT1A in mediating behavioral and physiological effects using antagonist reversal studies with M100907 and WAY100635, respectively. Overall, the data reveal that the degree of N-methylation and 4-position ring substitution powerfully influence receptor binding profiles and the potential for inducing psychedelic-like effects in mice.
Results and Discussion
Radioligand Binding Assays in Cells Transfected with Human Receptors
At a 10 μM screening concentration, two of three secondary (norpsilocin, 4-AcO-NMT) and tertiary amines (psilocin, psilacetin) displayed >50% inhibition of radioligand binding at all human 5-HT receptor subtypes tested, except serotonin 3 receptors (5-HT3). The 4-position constituent impacted receptor affinities, with the 4-phosphoryloxy compounds binding to fewer targets than 4-hydroxy and 4-acetoxy analogues. Notably, psilocybin did not exhibit >50% inhibition of radioligand binding at 5-HT2A, while psilocin and psilacetin did. For secondary and tertiary amine compounds, non-5-HT receptor targets included alpha adrenergic receptors (alpha2A and alpha2C), the dopamine transporter (DAT), the histamine receptor 1 (H1), and the serotonin transporter (SERT). Quaternary ammonium compounds (4-HO-TMT, aeruginascin, 4-AcO-TMT) displayed little activity at 10 μM in receptor assays and only displayed >50% inhibition of radioligand binding at serotonin 2B (5-HT2B), the serotonin 6 receptor (5-HT6), the sigma 1 receptor, and SERT.
Compounds that displayed >50% inhibition for any potential target at 10 μM were further assessed to determine binding affinities at these sites. Affinities of all 9 compounds for human 5-HT receptor subtypes are listed in Table 1, while affinities for non-5-HT human targets are listed in Table S1. For secondary amines, the screening data revealed that norpsilocin and 4-AcO-NMT share similar 5-HT receptor targets. However, when compared to that of norpsilocin, the affinity of 4-AcO-NMT was weaker for the serotonin 1B receptor (5-HT1B), the serotonin 1e receptor (5ht1e), 5-HT2A, 5-HT2C, and the serotonin 7A receptor (5-HT7a), with high nanomolar to low micromolar affinities. Baeocystin inhibited binding to fewer 5-HT receptors than either norpsilocin or 4-AcO-NMT, displaying variable affinities for 5-HT1B, the serotonin 1D receptor (5-HT1D), 5-ht1e, 5-HT2B, and 5-HT7a. Regarding non-5-HT receptors, norpsilocin displayed low micromolar affinity for alpha2A and SERT. Overall, the 4-acetoxy prodrug had a receptor binding profile more similar to norpsilocin than the 4-phosphoryloxy prodrug, and all three compounds were nonselective 5-HT receptor ligands.
Table 1. Affinities of Psilocybin Analogues in Radioligand Binding Assays for Human 5-HT Receptorsa.
| 5-HT1A | 5-HT1B | 5-HT1D | 5-ht1e | 5-HT2A | 5-HT2B | 5-HT2C | 5-HT5A | 5-HT6 | 5-HT7a | |
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Ki (nM) | Ki (nM) | Ki (nM) | Ki (nM) | Ki (nM) | Ki (nM) | Ki (nM) | Ki (nM) | Ki (nM) | Ki (nM) |
| norpsilocin | 86 | 99 | 194 | 161 | 391 | 57 | 243 | 365 | 54 | 68 |
| baeocystin | 370 | 394 | 1,352 | 134 | 117 | |||||
| 4-AcO-NMT | 141 | 994 | 150 | 522 | 1,512 | 76 | 729 | 389 | 82 | 1,766 |
| psilocin | 164 | 580 | 130 | 155 | 180 | 8 | 175 | 116 | 38 | 75 |
| psilocybin | 5,284 | 4,983 | 186 | 601 | 259 | 808 | 89 | 138 | ||
| psilacetin | 337 | 1,178 | 108 | 335 | 395 | 9 | 210 | 350 | 112 | 157 |
| 4-HO-TMT | 720 | 2,267 | ||||||||
| aeruginascin | ||||||||||
| 4-AcO-TMT | 891 | |||||||||
| DOI | 607 | 713 | 11 | 5 | 41 | 2,254 | 1,857 |
Radioligands used, reference control compound used, and control Ki values for each 5-HT receptor were 5-HT1A = [3H]WAY100635 vs. 8-HO-DPAT (Ki = 0.6 nM), 5-HT1B = [3H]GR125743 vs. ergotamine tartrate (Ki = 4 nM), 5-HT1D = [3H]GR125743 vs. ergotamine tartrate (Ki = 4 nM), 5-ht1e = [3H]5-HT vs. 5-HT (Ki = 2 nM), 5-HT2A = [3H]ketanserin vs. clozapine (Ki = 4 nM), 5-HT2B = [3H]LSD vs. SB206553 (Ki = 18 nM), 5-HT2C = [3H]mesulergine vs. ritanserin (Ki = 1 nM), 5-HT5A = [3H]LSD vs. ergotamine tartrate (Ki = 12 nM), 5-HT6 = [3H]LSD vs. clozapine (Ki = 7 nM), 5-HT7a = [3H]LSD vs. clozapine (Ki = 20 nM). No value = <50% inhibition of radioligand binding in the 10 μM primary screening. DOI = (±)-2,5-dimethoxy-4-iodoamphetamine.
Tertiary amine compounds displayed near identical target profiles to secondary amines across human 5-HT receptor subtypes. For tertiary amine compounds, psilocybin did not compete for binding to 5-HT2A or serotonin 5A (5-HT5A) receptors in primary screens, in contrast to psilocin and psilacetin. In addition, psilocybin generally had weaker affinity across human 5-HT receptor subtypes compared to both psilocin and psilacetin. Beyond these differences with psilocin and psilacetin, psilocybin displayed nearly equivalent binding affinities for 5-HT6 and 5-HT7a receptors. Psilocin displayed binding affinities of <200 nM across nearly all 5-HT receptor subtypes examined. Affinities for psilacetin at 5-HT receptor subtypes were nearly all <400 nM, and psilacetin had no additional receptor binding activity in the target validation screen. Outside of 5-HT receptors, psilocin displayed high nanomolar affinity at H1 and low micromolar affinity at SERT, but affinity at SERT was >100 times weaker than the highest affinity displayed by psilocin at 5-HT2B. Similar to secondary amines, tertiary amines were overall nonselective 5-HT receptor ligands with nanomolar affinities and limited non-5-HT receptor binding activities. 4-Phosphoryloxy substitution was associated with fewer 5-HT receptor targets and decreased affinity at those receptor subtypes.
Quaternary ammonium compounds interacted with few receptor targets in primary screens, and secondary binding analysis revealed that only 4-HO-TMT and 4-AcO-TMT displayed 5-HT receptor activity, with high nanomolar affinities for both compounds at 5-HT2B and low micromolar affinity for 4-HO-TMT at 5-HT6. Beyond the 5-HT receptors, quaternary ammonium compounds 4-HO-TMT and aeruginascin had micromolar affinities at SERT and sigma 1 receptors, respectively. The standard 5-HT2 ligand, DOI, displayed expected high affinity and selectivity at 5-HT2 receptors relative to most other targets tested. Despite some other listed affinities of DOI across 5-HT receptor subtypes and other targets, only alpha2A binding was <100 times lower than the affinity of DOI at 5-HT2 receptors.
A separate radioligand binding screen was utilized to assess competition binding of all the compounds using agonist radioligands (data shown in Table S2). Compounds were screened using 100 nM and 10 μM concentrations to assess percent reduction of agonist radioligand binding at several 5-HT receptors, monoamine transporters, and opioid receptors. Norpsilocin, psilocin, and psilacetin were the only compounds assessed at 100 nM to display >50% reduction in radioligand binding at 5-HT1A, 5-HT2B, and/or 5-HT2C receptors. At 10 μM, secondary amines displayed nonselective 5-HT receptor activity, with norpsilocin showing additional activity at SERT at this concentration. Psilacetin and psilocybin also displayed nonselective 5-HT receptor activity at this concentration, while quaternary ammonium 4-HO-TMT showed 5-HT2A, 5-HT2B, and SERT activity. The quaternary ammonium prodrugs showed reduced receptor binding targets, with only 4-AcO-TMT showing weak 5-HT2B activity at the high 10 μM screening concentration. In general, the findings from the additional agonist radioligand binding screen agreed with the findings from the more comprehensive target screening.
Radioligand Binding at 5-HT1A and 5-HT2A Receptors in Mouse Brain
As a means to characterize the receptor binding of psilocybin analogues in native tissue preparations, we examined binding affinities of test compounds at 5-HT1A ([3H]8-OH-DPAT binding) and 5-HT2A ([3H]M100907 binding) in mouse brain membranes. Affinity values at mouse 5-HT2A are shown in Table 2, whereas affinity values at mouse 5-HT1A can be found in Table 3. Binding curves for 5-HT2A and 5-HT1A assays are depicted in Figure S1. At 5-HT1A receptors, secondary amine compounds displayed high affinities for [3H]8-OH-DPAT-labeled binding sites (25–50 nM). Tertiary amine compounds also competed for [3H]8-OH-DPAT binding in mouse brain, but affinities were right-shifted when compared to the secondary amines (118–299 nM). Quaternary ammonium compounds displayed little competition for [3H]8-OH-DPAT binding in mouse brain up to 10 μM. As expected, standard reference compounds 5-HT and WAY-100635 displayed high affinity for 5-HT1A binding in mouse brain (i.e., 3.2–4.4 nM).
Table 2. Affinity (Ki), Potency (EC50 or ED50), and Maximum Efficacy (Emax) of Psilocybin Analogues in 5-HT2A Binding Assays, 5-HT2A Functional Assays, and In Vivo Tests in Micea.
| affinity—mouse brain | potency and efficacy—cells |
potency and efficacy—mice |
||||
|---|---|---|---|---|---|---|
| [3H]M100907 binding m5-HT2A | calcium mobilization h5-HT2A | β-arrestin 2 recruitment h5-HT2A | HTR | hypolocomotion | temperature Δ | |
| ligand | Ki (nM) | EC50 (nM) Emax (% 5-HT) | EC50 (nM)Emax (% LSD) | ED50 (mg/kg s.c.) | ED50 (mg/kg s.c.) | ED50 (mg/kg s.c.) |
| norpsilocin | 706 | 22 | 140 | >30 | 7.6 | 13.7 |
| (525–948) | (17–28) | (92–228) | (7.3–14.1) | (10.9–16.5) | ||
| Emax = 89 | Emax = 85 | Emax = 585 cm | Emax = −2.8 °C | |||
| baeocystin | >5,000 | n.d. | 1,202 | >30 | 18.5 | 20.9 |
| (575–8,276) | (14.9–20.9) | (10.4–31.5) | ||||
| Emax = 82 | Emax = 506 cm | Emax = −2.5 °C | ||||
| 4-AcO-NMT | 1,334 | 312 | 408 | >30 | 11.7 | 17.7 |
| (1,019–1,773) | (214–456) | (297–562) | (10.7–21.5) | (13.5–23.7) | ||
| Emax = 96 | Emax = 74 | Emax = 843 cm | Emax = −3.9 °C | |||
| psilocin | 235 | 13 | 81 | 0.11 | 1.8 | 2.5 |
| (187–296) | (10–17) | (54–123) | (0.09–0.19) | (0.9–3.5) | (1.9–3.5) | |
| Emax = 67 | Emax = 76 | Emax = 23 HTR events | Emax = 303 cm | Emax = −5.6 °C | ||
| psilocybin | 2,096 | 2,132 | 1,242 | 0.29 | 2.8 | 5.4 |
| (1,209–3,635) | (1,786–2545) | (771–2,538) | (0.17–0.53) | (1.5–5.6) | (2.8–10.9) | |
| Emax = 88 | Emax = 74 | Emax = 30 HTR events | Emax = 605 cm | Emax = −6.3 °C | ||
| psilacetin | 649 | 328 | 95 | 0.21 | 1.4 | 5.0 |
| (430–980) | (227–474) | (66–138) | (0.08–0.34) | (0.7–2.9) | (2.9–9.2) | |
| Emax = 54 | Emax = 74 | Emax = 27 HTR events | Emax = 670 cm | Emax = −6.1 °C | ||
| 4-HO-TMT | >5,000 | 2,312 | 2,506 | >30 | >30 | >30 |
| (1,754–3,048) | (1,010–6,216) | |||||
| Emax = 86 | Emax = 46 | |||||
| aeruginascin | >5,000 | n.d. | >10,000 | >30 | >30 | >30 |
| 4-AcO-TMT | >5,000 | n.d. | 6,564 | >30 | >30 | >30 |
| (3,687–18,350) | ||||||
| Emax = 65 | ||||||
m5-HT2A and h5-HT2A = mouse and human 5-HT2A. N.d. = not determined. ED50 values for hypolocomotion and temperature change for secondary amines are approximate as full sigmoidal curves were not exhibited up to 30 mg/kg for these compounds. Potency and affinity values are shown with 95% confidence intervals noted below in parentheses. Affinities and potencies of reference compounds in binding and functional assays are listed in the text of the Results and Discussion section.
Table 3. Affinity of Psilocybin Analogues at 5-HT1A Receptors in Mouse Braina.
| ligand | affinity—mouse brain |
|---|---|
| [3H]8-OH-DPAT binding m5-HT1A | |
| Ki (nM) | |
| norpsilocin | 29 |
| (22–39) | |
| baeocystin | 25 |
| (21–29) | |
| 4-AcO-NMT | 50 |
| (39–65) | |
| psilocin | 118 |
| (84–165) | |
| psilocybin | 197 |
| (118–328) | |
| psilacetin | 299 |
| (205–438) | |
| 4-HO-TMT | >10,000 |
| aeruginascin | >10,000 |
| 4-AcO-TMT | >10,000 |
Reference compounds WAY100635 and 5-HT displayed affinities of 4.4 (3.3–5.8) nM and 3.2 (2.4–4.1) nM, respectively. Affinity values are accompanied by 95% CI in parentheses below.
At 5-HT2A receptors in mouse brain, psilocin displayed the highest affinity for [3H]M100907-labeled sites, with a Ki in the low nanomolar range. Psilocybin, psilacetin, norpsilocin, and 4-AcO-NMT all displayed high nanomolar to low micromolar affinities at 5-HT2A. The affinity of psilocybin for 5-HT2A binding was 3–4-fold weaker than that of psilacetin. Baeocystin and the quaternary ammonium analogues showed little competition for [3H]M100907 binding up to 5 μM. Standard reference compounds M100907 and DOI displayed the expected high affinities (0.7 and 12.5 nM, respectively). Affinities for all compounds at 5-HT2A in mouse brain were relatively similar to results from transfected cells reported in Table 1. For example, the reference compounds DOI displayed an affinity of 12 nM at mouse 5-HT2A labeled with [3H]M100907, whereas this same compound displayed an affinity of 11 nM at human 5-HT2A labeled with [3H]ketanserin. Regardless of any differences in absolute Ki values between radioligands used in tissue-based and cell-based assays, the rank order of drug potencies at 5-HT2A was similar for both methods.
Functional Activities at Identified 5-HT Receptor Targets
Calcium mobilization assays were also carried out to assess functional potency (EC50) and efficacy (Emax) for six of the nine compounds at 5-HT2A, 5-HT2B, and 5-HT2C. Potency values for 5-HT2A receptor assays are listed in Table 2, while potency values for 5-HT2B and 5-HT2C assays are listed in Table 4. Concentration–response curves for 5-HT2 subtypes are shown in Figure S2. The reference agonist used for each assay was 5-HT (Emax = 100%), which had EC50 values of 2.4, 0.9, and 0.4 nM at 5-HT2A, 5-HT2B, and 5-HT2C, respectively. Psilocin and norpsilocin were the most potent ligands for calcium mobilization at 5-HT2A (EC50 = 13 and 22 nM). Secondary amines displayed Emax values more akin to full agonists (89–96%), while tertiary amines exhibited partial agonist Emax values (54–88%). 4-HO-TMT displayed weak agonist potency for calcium mobilization at 5-HT2A (EC50 = 2,300 nM, Emax = 86%).
Table 4. Functional Activity at Human 5-HT2B and 5-HT2C Receptors in Cellular Assaysa.
| potency and efficacy—cells |
||
|---|---|---|
| calcium mobilization h5-HT2B | calcium mobilization h5-HT2C | |
| ligand | EC50 (nM) Emax(% 5-HT) | EC50 (nM) Emax(% 5-HT) |
| norpsilocin | 13 | 32 |
| (12–15) | (28–37) | |
| Emax = 82 | Emax = 96 | |
| 4-AcO-NMT | 153 | 296 |
| (135–173) | (254–345) | |
| Emax = 74 | Emax = 84 | |
| psilocin | 8 | 34 |
| (7–10) | (28–40) | |
| Emax = 38 | Emax = 84 | |
| psilocybin | 612 | 3,741 |
| (504–743) | (3,120–4,486) | |
| Emax = 38 | Emax = 88 | |
| psilacetin | 88 | 408 |
| (69–114) | (318–525) | |
| Emax = 29 | Emax = 32 | |
| 4-HO-TMT | 845 | 8,940 |
| (675–1,059) | (7,592–10,520) | |
| Emax = 32 | Emax = 64 | |
Potency (EC50) with 95% CI values (parentheses) and maximum efficacy (Emax) for each are listed. Reference compound 5-HT displayed potency values of 0.9 nM (0.7–1.1) and 0.4 nM (0.3–0.5) at 5-HT2B and 5-HT2C, respectively.
Norpsilocin and psilocin exhibited low nanomolar potencies for calcium mobilization at 5-HT2B (EC50 = 8–13 nM). Psilocin was a weak partial agonist (Emax = 38%), while norpsilocin displayed the highest partial agonist activity (Emax = 82%). Similar to psilocin, psilacetin and psilocybin displayed weak partial agonist efficacy at 5-HT2B (Emax = 29 and 38%) for calcium mobilization with potencies in the low or mid nanomolar range (EC50 = 88 and 612 nM, respectively). 4-AcO-NMT had similar efficacy and reduced potency compared to norpsilocin (EC50 = 153 nM, Emax = 74%). 4-HO-TMT displayed high nanomolar potency (EC50 = 845 nM) and partial agonist efficacy (Emax = 32%) for calcium mobilization at 5-HT2B.
In calcium mobilization assays at 5-HT2C, norpsilocin and psilocin again had similar potencies (EC50 = 32 and 34 nM), but norpsilocin produced full agonist efficacy (Emax = 96%), whereas psilocin had slightly lower efficacy (Emax = 84%). Both 4-AcO-NMT and psilacetin had similar potencies for calcium mobilization at 5-HT2C (EC50 = 296 and 408 nM), but psilacetin was a weak partial agonist (Emax = 32%) in contrast to 4-AcO-NMT (Emax = 84%) and psilocybin (EC50 = 3,741 nM, Emax = 88%). 4-HO-TMT also displayed weak partial agonist potency and efficacy for calcium mobilization at 5-HT2C (EC50 = 8,940 nM, Emax = 64%).
Next, six of the nine compounds were also screened for potential functional activities for β-arrestin translocation across various 5-HT receptors (Table S3). In the Tango G protein-independent β-arrestin recruitment assay, all compounds except psilocybin and the quaternary ammoniums displayed agonist-like activity at most 5-HT receptors tested. None of the compounds displayed agonist-like activity at 5-hydroxytryptamine 4 receptors (5-HT4), and as expected, DOI exhibited little to no agonist-like activity at these receptors, consistent with its known selectivity at 5-HT2 receptor subtypes. Interestingly, only norpsilocin and 4-AcO-NMT displayed weak 5-HT1A agonist-like activity (34–39% of 5-HT response) at the highest concentration tested.
Functional activity of psilocybin analogues was further examined in a NanoBiT system-based assay measuring 5-HT2A-mediated β-arrestin 2 recruitment in vitro. Potency (EC50) and efficacy (Emax) values for all test compounds and controls are listed in Table 2, while concentration–response curves can be found in Figure S3. Lysergic acid diethylamide (LSD) was used as the reference ligand (Emax = 100%) for the assays to determine the relative efficacy of the test compounds and displayed a potency of 7.7 nM. The positive control compound DOI also displayed potent (EC50 = 5.5 nM) stimulation of β-arrestin 2 recruitment in vitro, with maximal efficacy similar to LSD (106%). Secondary amines all induced β-arrestin 2 recruitment, with norpsilocin and 4-AcO-NMT exhibiting higher potencies (EC50 = 140 and 408 nM) than baeocystin (EC50 = 1.20 μM). Tertiary amines similarly all induced β-arrestin 2 recruitment, with psilocybin producing the weakest potency (EC50 = 1.24 μM) versus psilocin and psilacetin, which displayed similar potencies (EC50 = 81 and 95 nM). Quaternary ammoniums displayed weak micromolar potencies for β-arrestin 2 recruitment. 4-HO-TMT and 4-AcO-TMT exhibited EC50 values of 2.5 and 6.6 μM, while aeruginascin did not produce a sigmoidal concentration effect curve up to 10 μM. Lastly, secondary and tertiary amines tested were all partial agonists, with Emax values of 73.5–85.3% relative to LSD, whereas quaternary compounds were partial agonists with low potencies.
Dose–Response Effects of Psilocybin Analogues on HTR, Locomotor Activity, and Body Temperature in Mice
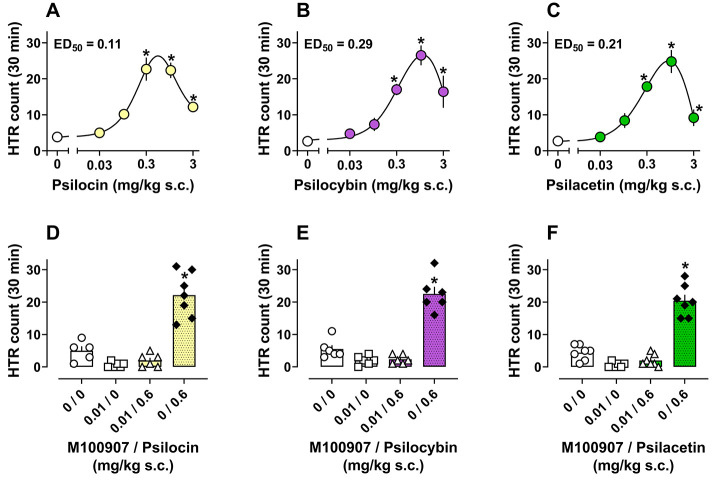
Based on our in vitro pharmacological data and the known behavioral effects of psilocybin, we sought to examine SAR for psilocybin analogues to produce psychedelic-like, as well as other effects, in mice. To test for pharmacological effects in vivo, each compound was administered subcutaneously (s.c.) to mice at doses ranging from 0.03 to 30 mg/kg to measure HTR, temperature change, and locomotor activity over a 30 min testing session. Dose–response data for HTR induced by psilocin, psilocybin, and psilacetin over the testing session are shown in Figure 2A–C while time-course data are shown in Figure S4. Potency (ED50) values are shown in Table 2, while descriptive statistics and additional details of statistical comparisons can be found in Table S4. Tertiary amines displayed roughly similar potencies and efficacies for producing HTR in mice, with the rank order of potencies being psilocin > psilacetin > psilocybin (Figure 2A–C). This is further demonstrated when considering the salt used: psilocin (0.54 μmol/kg) > psilacetin (0.58 μmol/kg) > psilocybin (1.0 μmol/kg). In contrast to tertiary amines, secondary amines and quaternary ammoniums produced little to no HTR in mice (Figure S5). Total HTR counts over the session for psilocin, psilocybin, and psilacetin were significantly increased versus vehicle controls at 0.3–3 mg/kg (F5,30 = 19.09, p < 0.0001; F5,27 = 15.16, p < 0.0001; F5,29 = 22.30, p < 0.0001). All three tertiary amine compounds produced peak effects on HTR within the first 5–10 min after administration that waned to vehicle control levels by the end of the session. Inverted U dose-related effects were evident for psilocin, psilocybin, and psilacetin, with a downward slope in activity at doses above 1 mg/kg. At the highest doses tested (3–30 mg/kg), HTR was rapidly reduced to vehicle control levels within the first 10–15 min for all three tertiary amine compounds.
Figure 2.
Dose–response curves (A–C) and antagonist reversal plots (D–F) for a total number of HTR produced by psilocin, psilocybin, and psilacetin in mice. *p < 0.05 vs vehicle control group (0 or 0, 0). All values are mean ± SEM of n = 4–6 (A–C) and n = 5–7 (D–F) per condition.
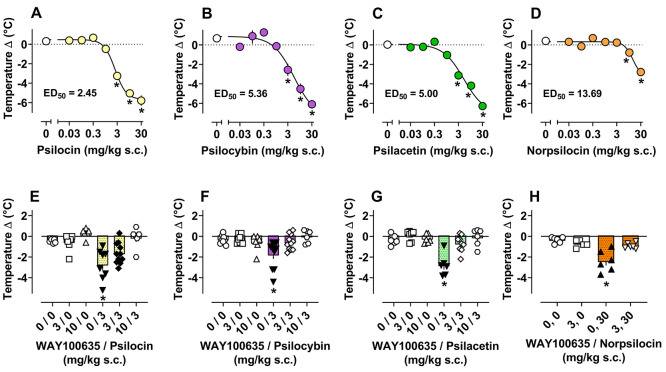
In conjunction with measuring HTR, we monitored body temperature change and locomotor activity during all experimental drug administration sessions. Dose–response effects for tertiary amines and norpsilocin to affect body temperature are depicted in Figure 3A–D, whereas temperature data for other compounds can be found in Figure S6 and Table 2. Dose–response and time-course of effects for all compounds tested to affect locomotor activity are shown in Figures S7–S9 and Table 2. Psilocin, psilocybin, and psilacetin displayed the most potent effects on body temperature (F7,40 = 58.19, p < 0.0001; F7,37 = 33.63, p < 0.0001; F7,36 = 53.73, p < 0.0001) and locomotor activity (F7,40 = 14.90, p < 0.0001; F7,36 = 11.20, p < 0.0001; F7,38 = 15.93, p < 0.0001), decreasing both measures significantly versus vehicle controls from 3 to 30 mg/kg. It is noteworthy that the potencies of psilocin, psilocybin, and psilacetin to reduce body temperature and locomotor activity were ∼20-fold weaker than their potencies to elicit HTR. Time-course data for locomotor effects of tertiary amines showed that at the same doses that reduced HTR, mice displayed reduced forward locomotion relative to vehicle controls over the last 15–20 min of the session. Secondary amines displayed weak potencies for reducing body temperature (14–21 mg/kg) and locomotor activity (8–19 mg/kg), while quaternary ammonium compounds had little to no effects on these measures. Overall, dose–response mouse studies revealed that only tertiary amines produced measurable effects on HTR in mice, whereas both secondary and tertiary amines reduced body temperature and locomotor activity at sufficient doses. By contrast, quaternary ammonium psilocybin analogues were essentially inactive in vivo.
Figure 3.
Dose–response curves (A–D) and antagonist reversal plots (E–H) of pre- vs. post-session temperature change produced by psilocin, psilocybin, psilacetin, and norpsilocin in mice. *p < 0.05 vs. vehicle control group (0 or 0, 0). All values are mean ± SEM of n = 4–6 (A–D), n = 6–11 (E–G), and n = 5–6 (H) per condition.
5-HT2A Antagonist Pretreatment Effects in Mice
Since the effects of psilocybin and related psychedelics to produce HTR in rodents depend on 5-HT2A activation, we hypothesized that HTR produced by psilocybin analogues would be attenuated by the blockade of this receptor. To test whether the HTR produced by psilocin, psilocybin, and psilacetin was mediated by actions at 5-HT2A receptors, we pretreated mice with 5-HT2A antagonist M100907 prior to administration of each compound. The dose of M100907 was chosen based on a small pilot experiment showing no reduction of locomotor activity by 0.01 or 0.1 mg/kg doses relative to 1 mg/kg (F3,16 = 4.69, p = 0.015, Figure S10). The 0.01 mg/kg dose of M100907 did not significantly change body temperature or locomotor activity relative to vehicle controls (Figure S11).
Overall, treatment differences were observed in M100907 pretreatment experiments with psilocin, psilocybin, and psilacetin (F3,20 = 37.33, p < 0.0001; F3,20 = 54.64, p < 0.0001; F3,23 = 67.86, p < 0.0001). Specifically, the HTR produced by psilocin, psilocybin, and psilacetin at a near maximal dose of 0.6 mg/kg was blocked by M100907 pretreatment as this condition was not significantly different from vehicle controls in contrast to the vehicle + drug conditions (Figure 2D,E, Table S5). These results demonstrate that drug-induced HTR is dependent upon 5-HT2A activation.
5-HT1A Antagonist Pretreatment Effects in Mice
Given that the highest doses of psilocybin analogues tested (3–30 mg/kg) produced reductions in HTR and induced physiological (body temperature decrease) and behavioral (locomotor activity decrease) responses indicative of the 5-HT1A receptor agonist-induced syndrome, we surmised that these effects might be reversible by pretreatment with a 5-HT1A receptor antagonist. To evaluate this hypothesis, we pretreated mice with the 5-HT1A antagonist WAY100635 prior to administration of psilocin, psilocybin, and psilacetin. Mean effects of WAY100635 pretreatment on body temperature changes induced by psilocybin analogues are shown in Figure 3E–H and Table S6, while effects on HTR and locomotor activity are shown in Figure S12 and Table S6. Overall, for psilocin, psilocybin, and psilacetin, significant main effects were observed for HTR (F5,39 = 13.99, p < 0.0001; F5,43 = 32.95, p < 0.0001; F5,35 = 15.43, p < 0.0001; F3,19 = 5.12, p = 0.0092), body temperature (F5,39 = 10.24, p < 0.0001; F5,43 = 4.88, p = 0.0013; F5,36 = 14.37, p < 0.0001), and locomotor activity (F5,43 = 5.03, p = 0.001; F5,42 = 4.15, p = 0.0037; F5,37 = 3.72, p = 0.0080) measures in WAY100635 experiments. Main effects were also observed for norpsilocin in locomotor activity (F3,19 = 4.59, p = 0.0139) and body temperature change (F3,19 = 14.26, p < 0.0001) data sets from WAY100635 experiments. Specifically, the effects of psilocin, psilocybin, psilacetin, and norpsilocin on body temperature and locomotor activity were blocked by WAY100635 treatment, suggesting a 5-HT1A-mediated mechanism of action. Psilocin required the highest dose of WAY100635 (10 mg/kg) to block reductions of body temperature and locomotor activity, while the effects of the other compounds were blocked by 3 and 10 mg/kg WAY1003635. WAY100635 pretreatment seemed to slightly increase the HTR produced by psilocin, psilocybin, and psilacetin at 3 mg/kg; however, the currently employed design did not assess these potential treatment effects. WAY100635 pretreatment prior to norpsilocin and norpsilocin alone significantly reduced basal vehicle-induced HTR (Figure S12D).
Discussion
In the present study, we examined the preclinical pharmacology of naturally occurring and synthetic psilocybin analogues with varying degrees of N-methylation and differing 4-position ring substitutions. Determining the biological effects of psilocybin and its analogues is critical for understanding their potential therapeutic utility and possible health risks. Our in vitro data show that secondary (i.e., norpsilocin, baeocystin, 4-AcO-NMT) and tertiary amines (i.e., psilocin, psilocybin, psilacetin) display nanomolar affinity for most 5-HT receptor subtypes, while quaternary ammoniums (i.e., 4-HO-TMT, aeruginascin, 4-AcO-TMT) do not. In mouse brain tissue, secondary and tertiary amines inhibited radioligand binding at 5-HT2A and 5-HT1A, analogous to their effects in transfected cells. Secondary and tertiary amine compounds also displayed agonist activity at 5-HT2A in functional assays assessing Gαq-dependent calcium mobilization and β-arrestin 2 recruitment in vitro. Importantly, only the tertiary amine compounds produced 5-HT2A-mediated HTR, illustrating the importance of an N,N-dimethyl moiety for enabling psychedelic-like effects of these compounds in vivo.23,40,41,55 Both secondary and tertiary amines caused 5-HT1A-mediated suppression of locomotion and body temperature in vivo, but these effects required high drug doses. 4-Acetoxy compounds, regardless of the degree of N-methylation, had effects that were more similar to 4-hydroxy compounds when compared to 4-phosphoryloxy compounds. In this regard, our findings support the notion that psilacetin is a prodrug which displays its own agonist activity at 5-HT receptors.46,47 Overall, the results suggest that secondary amines and quaternary ammoniums tested in the present study may lack psychoactive effects in humans, in contrast to the established psychedelic effects of tertiary amines like psilocin and its prodrugs.
We provide the first comprehensive receptor binding screen comparing the effects of psilocybin, baeocystin, aeruginascin, and their analogues. Secondary and tertiary amine compounds displayed nanomolar affinities for most 5-HT receptor subtypes, while quaternary ammonium compounds had weak affinities for only 5-HT2B and SERT, as previously reported.56 Our findings with 4-HO-TMT might seem inconsistent with a previous study which showed that this compound has affinity (i.e., 670 nM) for 5-HT2A in transfected cells..43 However, the present study used the antagonist [3H]ketanserin to label 5-HT2A, while the prior work used the agonist [125I]DOI. It is well known that agonists preferentially bind to the high affinity state of the receptor compared to antagonists, and radiolabeled agonists will yield Ki values for the high affinity state.57 4-Hydroxy and 4-acetoxy compounds displayed higher affinities across all 5-HT receptors when compared to their 4-phosphoryloxy counterparts. Notably, our binding data agree with previous studies showing that psilocin is a nonselective 5-HT receptor ligand.26,58 Drug binding affinities at human 5-HT2A and 5-HT1A in transfected cells were subsequently verified in native tissue preparations using radioligand binding assays in mouse brain membranes consistent with another recent study.59 The present studies also determined the potency and efficacy of psilocybin analogues for 5-HT2A-mediated calcium mobilization and β-arrestin 2 recruitment in vitro. Importantly, the rank order of drug potencies in the 5-HT2A functional assays was similar to the rank order of affinities in 5-HT2A binding at human and mouse receptors. In both functional assays, secondary and tertiary amine compounds displayed agonist efficacies relative to those of 5-HT and LSD.
5-HT2A and other 5-HT receptors couple to many canonical and noncanonical intracellular signaling pathways, depending on biological context, which could mediate distinct pharmacological effects of psychedelics.60,61 In cells transfected with rat 5-HT2A, drug potencies in the 5-HT2A calcium mobilization assay associated with Gαq signaling have been shown to predict potency for phenethylamine psychedelic-induced HTR in mice.57 However, a previous mouse study showed that genetic deletion of Gαq only partially reduces drug-induced HTR,62 while another mouse study pointed to a possible role for Gαs in producing psychedelic-like drug effects.63 We found that psilocin and norpsilocin were both potent agonists in the calcium mobilization assay, and our results generally agree with those of Sherwood and colleagues.41 Although many assays can be used to study the functional potency and efficacy of psychedelics at 5-HT2A,60 it is unclear which signaling pathway(s) are predictive for therapeutic or other effects. The usefulness of in vitro functional potencies may be limited as these values do not always relate to binding affinities at 5-HT2A3,64−66 or in vivo potencies in rodent behavioral models.67 On the other hand, the binding affinities of various 5-HT2A agonists at [3H]ketanserin-labeled 5-HT2A are positively correlated with bioactive doses of psychedelics in humans68−70 and the potencies for inducing effects in rodents.66,71,72 Such observations support the idea that 5-HT2A binding affinity is at least one useful metric for predicting possible psychedelic activity of novel compounds.
5-HT2A is known to form complexes with β-arrestin 2 in cortical neurons in vivo.73 Here, we demonstrate that both secondary and tertiary amines act as potent partial agonists in an assay measuring 5-HT2A-mediated β-arrestin 2 recruitment. Prior studies show that genetic deletion of β-arrestin 2 in mice reduces HTR induced by 5-hydroxytryptophan (5-HTP) and LSD, but not DOI.74,75 Paradoxically, the HTR activities of 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) and 5-HT are reportedly enhanced in β-arrestin 2 knockout mice.76 Despite the uncertain role of β-arrestin 2 signaling for psychedelic drug action in mice, potencies for 30 phenethylamine psychedelics in the β-arrestin 2 assay used here were shown to positively correlate with doses that produce psychedelic subjective effects in humans.77 Whether 5-HT2A-mediated β-arrestin recruitment underlies the psychedelic effects of tryptamine or LSD-like compounds warrants further investigation. Some data indicate that LSD and related psychedelics exhibit preferential β-arrestin 2 bias at 5-HT2A receptors,78,79 but other investigators found no evidence for such bias when directly comparing the effects of compounds in assays measuring 5-HT2A-mediated β-arrestin 2 versus mini-Gαq recruitment.60,80 Unraveling which in vitro assay(s) and receptor signaling pathway(s) are predictive for pharmacological and potential clinical effects of psychedelics will be crucial for future drug development efforts.
The HTR is a widely used behavioral proxy for 5-HT2A-mediated psychedelic-like drug activity in rodent models.40,61,81 Here, we quantified HTR in mice using a novel automated computer software-based scoring method recently validated in our laboratory.82 We found that tertiary amines—psilocin, psilocybin, and psilacetin—induce dose-related increases in HTR, whereas secondary amines and quaternary ammoniums do not. The HTR produced by tertiary amines was completely blocked by pretreatment with the 5-HT2A antagonist M100907. Our in vivo findings with psilocybin and psilocin agree with previous results showing that these compounds, and related N,N-disubstituted tryptamines, induce HTR in mice.23,40,41,55,59 It is noteworthy that ED50 potency values reported here for psilocybin (i.e., 0.29 mg/kg, s.c.) and psilocin (i.e., 0.11 mg/kg, s.c.) to induce HTR are nearly identical to those reported by others who used magnetometer-based scoring methods to quantify HTR.40,55,83 It is not surprising that quaternary ammoniums failed to induce HTR because these compounds display weak or no affinity for 5-HT2A. By contrast, the secondary amines norpsilocin and 4-AcO-NMT are potent agonists at 5-HT2Ain vitro, yet they did not induce HTR at any dose tested. Possible reasons for the lack of in vivo activity for secondary amines could be poor blood–brain barrier penetration or rapid metabolism by monoamine oxidase.84,85 Another feasible explanation is that norpsilocin and 4-AcO-NMT exhibit agonist effects at non-5-HT2A sites which serve to dampen the expression of HTR. Indeed, there are many examples of 5-HT2A agonists that do not produce psychedelic-like effects in rodents,24,86−88 suggesting the possibility that competing receptor actions, or perhaps biased agonism at 5-HT2A,89 could underlie the lack of HTR associated with these compounds.3,26,81,90
An important non-5HT2A site of action for psilocybin analogues and other tryptamine psychedelics is the 5-HT1A receptor.3,70,91 It is well established that 5-HT1A ligands can modulate 5-HT2A-mediated pharmacological effects. For example, 5-HT1A agonist pretreatment reduces 5-HT2A-mediated effects of psychedelics in rodents and humans, while 5-HT1A antagonists have the opposite effect.27,92−96 Here, we show that secondary and tertiary amine psilocybin analogues produce 5-HT1A-mediated suppression of locomotor activity and body temperature. These effects are consistent with other studies showing similar effects of psilocybin and psilocin at high doses in rodents that were reversed by 5-HT1A antagonist pretreatment.59,97 In the case of tertiary amines, the induction of 5-HT1A effects in vivo required ∼10-fold higher doses than those eliciting 5-HT2A-mediated HTR. In fact, the descending limb of the HTR dose–response curve for all drugs coincided with the expression of 5-HT1A-mediated hypolocomotion and hypothermia, suggesting that 5-HT1A activation counteracted HTR. In the case of secondary amines, the induction of 5-HT1A effects in vivo required even higher doses than those observed with the tertiary amines. It is tempting to speculate that the low in vivo potency of secondary amines could result from poor brain penetrance or rapid metabolism, as noted above. Given that 5-HT1A-mediated effects were only observed at high drug doses in mice, these effects might only be relevant to overdose situations in humans. However, the ability of psychedelic compounds to cause long-term changes in brain neuronal structure (i.e., neuroplasticity) is thought to be mediated in part by 5-HT1A receptors.29,98 Future preclinical and clinical investigations should examine the role of 5-HT1A and other 5-HT receptor sites in modulating the pharmacological effects of psychedelic compounds at clinically relevant doses.
Conclusions
In summary, the SAR findings reported here show that the degree of N-methylation and 4-position ring substitution can powerfully influence pharmacological effects of psilocybin analogues. Secondary and tertiary amine compounds are nonselective 5-HT receptor ligands in vitro, whereas quaternary ammonium compounds bind to few receptor targets. Secondary and tertiary amines act as potent partial agonists at 5-HT2Ain vitro, but only tertiary amine compounds produce 5-HT2A-mediated HTR in vivo. Secondary and tertiary amine compounds also induce 5-HT1A-mediated suppression of locomotion and body temperature, but these effects require high drug doses. While our studies focused on the role of 5-HT2A and 5-HT1A, further research is warranted to examine the role of other 5-HT receptors in modulating effect of psilocybin analogues. Across all assays, the effects of 4-acetoxy and 4-hydroxy compounds were more potent than those of 4-phosphoryloxy compounds. In this regard, psilacetin appears to be a potential prodrug which displays its own serotonergic receptor activities. Future studies should directly test the hypothesis that psilacetin is hydrolyzed to psilocin in vivo. Importantly, our data indicate that the naturally occurring mushroom constituents, baeocystin and aeruginascin, are devoid of in vivo psychedelic-like effects in mice, but the pharmacology of other compounds from psilocybin-containing mushrooms or combinations thereof remain unexplored. As decriminalization or legalization efforts continue to allow personal use of psilocybin-containing mushrooms, examining the pharmacology of different constituents will be crucial for understanding their potential therapeutic and adverse effects.
Materials and Methods
Drugs
4-AcO-NMT hydrochloride was synthesized and structurally characterized using the scheme described in Supporting Information (Figure S13, Tables S7–S11). Baeocystin (zwitterion) and aeruginascin were synthesized as previously described.41 The solid-state structure of synthesized aeruginascin was determined by single-crystal X-ray diffraction, revealing stoichiometric hydration and ammonium iodide co-crystallization for a bis(aeruginascin) bis(ammonium) iodide salt; thus, the corrected aeruginascin molecular weight (379.26 g/mol) was calculated for the lot used in these experiments. Structural confirmation of baeocystin has been reported by members of our team previously,99 and structural data for aeruginascin are described in Supporting Information (Figure S14, Tables 12–16). Psilacetin hydrofumarate, norpsilocin hydrofumarate, 4-HO-TMT iodide, and 4-AcO-TMT iodide were synthesized previously.43,44,54 Psilocin freebase and psilocybin (zwitterion) were generously provided by the National Institute on Drug Abuse (NIDA), Drug Supply Program (Rockville, MD, USA). For β-arrestin functional assays, psilocybin was obtained from Cayman Chemical (Ann Arbor, MI, USA), whereas psilocin freebase was obtained from Chiron (Trondheim, Norway). DOI hydrochloride and WAY100635 maleate (WAY100635) were purchased from Cayman Chemical. DOI and LSD for functional studies were purchased from Chiron. 5-HT hydrochloride was purchased from MilliporeSigma (St. Louis, MO, USA). (+)-M100907 freebase (M100907) was generously provided by Kenner Rice, Ph.D., and Agnieszka Sulima, Ph.D. (NIDA). For all in vitro and mouse brain tissue studies, compounds were initially dissolved in 100% DMSO and were subsequently diluted in respective assay buffers. The nonenzymatic hydrolysis rates for the stability of psilocybin and psilacetin in aqueous solution were determined to address concerns of background hydrolysis rates, and results are shown in Figure S15. For mouse behavior studies, all drug doses represent the weight of the salt dissolved in 0.9% saline vehicle.
Animals
Mice (C57BL/6J males) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) at 5–6 weeks of age and allowed at least 1–2 weeks to acclimate to the NIDA, Intramural Research Program (IRP), animal research facility in Baltimore, MD, USA. The animal facility is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, and all procedures were approved by the NIDA IRP Animal Care and Use Committee. Mice were initially group housed 3–5 per cage during acclimation and housed in a 12 h light–dark cycle throughout the study, with lights on at 0700 h. Food and water were available ad libitum except during testing.
Receptor Binding and Functional Screening
Receptor binding and functional screening assays were generously conducted by the National Institute of Mental Health (NIMH) Psychoactive Drug Screening Program (PDSP) in the laboratory of Dr. Bryan Roth (University of North Carolina, Chapel Hill, NC, USA). All 9 test compounds (depicted in Figure 1) were screened at 46 potential binding sites using radioligand binding assays in cells transfected with human G protein-coupled receptors (GPCRs). Initial screening was carried out at a fixed 10 μM concentration using quadruplicate determinations from a single experiment for each compound. For any receptor site where binding was inhibited by 50% or greater in the 10 μM screen, full concentration–effect curves were constructed using triplicate determinations from 1 to 2 independent experiments, and binding affinities (i.e., Ki) were calculated. Primary functional studies were conducted for six of the nine compounds at 3 and 10 μM concentrations, based on the receptors identified from the binding screens, using the GPCR Tango assay for G-protein-independent β-arrestin translocation.78,100 Lastly, secondary full concentration effect curves were also generated for the same compounds at selected identified targets in primary functional screens using a calcium mobilization assay. Relevant details about the assay procedures, including radioligands used, are provided in the text or table legends. Further details (cell types used, etc.) about the binding and functional assays carried out by PDSP can be found online.101
Additional receptor screening was conducted by the Addiction Treatment Discovery Program of NIDA (Rockville, MD, USA). Briefly, all nine compounds were screened at two concentrations (100 nM and 10 μM) to determine % inhibition of agonist radioligand binding at the following sites: 5-HT1A, 5-HT2A, serotonin 2B receptor (5-HT2B), 5-HT2C, DAT, norepinephrine transporter (NET), SERT, mu-opioid receptor (MOR), delta opioid receptor (DOR), and kappa opioid receptor (KOR). Radioligand binding methods are similar to those previously described for [3H]8-OH-DPAT,102 [3H]5-HT,103 [3H]RTI-55,104 [3H]DAMGO, [3H]DPDPE, and [3H]U69,593.105 Data represent mean inhibition of binding, as assessed by triplicate determinations from two independent experiments.
Radioligand Binding Assays in Mouse Brain
Dose–response curves for inhibition of radioligand binding in mouse brain tissue were constructed using triplicate determinations from three independent experiments. Effects of test compounds on [3H]8-OH-DPAT or [3H]M100907 binding are expressed as percent inhibition of specific binding versus log of compound concentration in graphical plots and as affinity values in relevant tables.
Inhibition of [3H]8-OH-DPAT Binding to 5-HT1A
Radioligand binding to mouse brain 5-HT1A was performed as previously described with minor modifications.106 Briefly, for each experiment, two frozen mouse brains (C57BL/6 strain, BioIVT, Westbury, NY, USA) were each homogenized in 10 mL of ice cold 50 mM Tris–HCl pH 7.7 using a polytron at 15,000 rpm for 7 s. Homogenates were centrifuged at 40,000g for 10 min at 4 °C, the supernatants were discarded, and each pellet was resuspended in 10 mL of 50 mM Tris–HCl pH 7.7 by vigorous vortexing. The tissue suspensions were incubated at 37 °C for 10 min and were then centrifuged at 40,000g for 10 min at 4 °C. Each pellet was resuspended in 10 mL assay buffer (50 mM Tris–HCl, 4 mM CaCl2, 10 μM pargyline, 0.1% ascorbic acid, pH 7.7), and the tissue suspensions were combined, diluted to 150 mL in assay buffer (22 °C), and used immediately.
Binding assays were performed in 12 × 75 mm polystyrene test tubes that contained 800 μL of tissue prep, 100 μL of 5 nM [3H]8-OH-DPAT (129 Ci/mmol, PerkinElmer, Boston, MA, USA) diluted in assay buffer, and 100 μL of the test compound diluted in assay buffer. Assays were initiated by the addition of tissue and were terminated after 30 min at 22 °C by rapid vacuum filtration through GF/B filter paper (Brandel, Gaithersburg, MD, USA) mounted on a cell harvester (Brandel). Filters were rinsed with 5 mL of ice cold wash buffer (50 mM Tris–HCl, pH 7.7) and placed into 24-well flex plates (PerkinElmer) with 500 μL/well CytoScint (MP Biomedical, Irvine, California, USA). After an overnight incubation, radioactivity was quantitated using a MicroBeta2 liquid scintillation counter (PerkinElmer) at 40% efficiency. Nonspecific binding was determined in the presence of 10 μM serotonin, and maximal binding was determined in the absence of any competing drug.
Inhibition of [3H]M100907 Binding to 5-HT2A
Radioligand binding to mouse brain 5-HT2A was performed as previously described with minor modifications.66 For each experiment, two frozen mouse brains (C57BL/6 strain, BioIVT) were each homogenized in 10 mL of ice cold 0.25 M sucrose using a polytron at 15,000 rpm for 7 s. Homogenates were centrifuged at 40,000g for 10 min at 4 °C, the supernatants were discarded, and each pellet was resuspended in 10 mL of ice cold 50 mM Tris–HCl pH 7.0 by vigorous vortexing. The tissue suspensions were incubated at 37 °C for 10 min and were then centrifuged at 40,000g for 10 min at 4 °C. Pellets were resuspended to a final volume of 16 mL in assay buffer (50 mM Tris–HCl, 0.5 mM EDTA, 10 mM MgSO4, 0.1% ascorbic acid, pH 7.4) and were used immediately.
Binding assays were performed in 12 × 75 mm polystyrene test tubes that contained 700 μL of assay buffer, 100 μL of 10 nM [3H]M100,907 (87 Ci/mmol, Novandi, Sodertalje, Sweden) diluted in assay buffer, 100 μL of the test drug diluted in assay buffer, and 100 μL of tissue preparation for a total volume of 1 mL. Assays were initiated by the addition of tissue and were terminated after 30 min at 37 °C by rapid vacuum filtration as described above. Filters were rinsed with 5 mL of ice cold wash buffer (10 mM Tris–HCl, pH 7.0) and processed as described above. Nonspecific binding was determined in the presence of 10 μM ketanserin, and maximal binding was determined in the absence of any competing drug.
5-HT2A-Mediated β-Arrestin 2 Recruitment
β-Arrestin 2 recruitment at the human 5-HT2A was assessed as previously described using Human Embryonic Kidney (HEK) 293T cells stably expressing the SmBiT-βarr2 and 5-HT2A-LgBiT constructs.60,77 Briefly, the dilutions for functional activity assessments were made in Hank’s buffered saline solution (HBSS) by means of serial dilution, while keeping the solvent concentration constant, yielding in-well concentration ranges from 10 μM to 10–11 M. One day prior to the experiment, cultured cells stably expressing 5-HT2A-LgBiT and SmBiT-β-arrestin 2 (LgBiT and SmBiT being the enzyme fragments of the NanoBiT system, initially developed by Promega)107 were seeded at a density of 50,000 cells per well in 96-well plates coated with poly-D-lysine for optimal cell adhesion. Plates were incubated at 37 °C and 5% CO2 until the readout on day 2. On the second day, the cells were washed twice with 150 μL of HBSS to remove all remaining medium/serum, and 100 μL of HBSS was pipetted into each well. Next, 25 μL of the NanoGlo Live Cell substrate was added (1/20 diluted in the LCS dilution buffer provided by the manufacturer), and plates were transferred to a plate reader (Tristar2—Berthold Technologies GmbH and Co, Germany). After equilibration of the luminescent signal, 10 μL of each agonist solution was added, and the signal was monitored for 2 h. All concentrations were tested in duplicate in three independent experiments, and solvent controls were run for all conditions. LSD was used as the reference compound, and DOI was also used as a standard comparator positive control.
Mouse Studies
Details and Design
For the mouse studies, cohorts of 9–12 mice were used for each test drug. The mice were subjected to experimental testing once every 1–2 weeks for 2–3 months to complete dose–effect curves and antagonist experiments. A minimum of 7 days between treatments was utilized to avoid any tolerance to effects of repeated drug administration.81,108 Mice were tested first in dose–response studies to assess the effects of each compound at doses from 0.03 to 30 mg/kg s.c. and were subsequently tested in antagonist reversal studies utilizing pretreatment with M100907 and WAY100635. All experiments were conducted from 0900 to 1700 local time during the light phase, as sensitivity of rodents to other tryptamine psychedelics is diurnal, with maximal HTR observed in the middle of the light phase.109,110 Experiments were run during the light phase also to avoid any potential influence of melatonin receptor activity on HTR as melatonin and related agonists are known to reduce HTR induced by DOI in rats.111,112
For each experiment, mice were acclimated to the testing room in their home cage for at least 1 h prior to experimental sessions. Behavioral test sessions were carried out in Tru Scan mouse locomotor arenas equipped with photobeam arrays (Coulbourn Instruments, Holliston, MA, USA), which were modified with cylindrical inserts and transparent floors useful in detecting mouse HTR as described previously.82
Subcutaneous Temperature Transponder Implants
At least 1 week prior to the start of the experiments, mice received s.c. implanted temperature transponders (14 × 2 mm, model IPTT-300, Bio Medic Data Systems, Inc., Seaford, DE, USA) under brief isoflurane anesthesia as previously described.113 Mice were single housed post implant for the remainder of the study to protect the transponder from removal by cage mates.Temperature was determined noninvasively using a handheld receiver that is sensitive to signals emitted from the implanted transponders.
Behavioral Testing
Prior to each experiment, mouse body weight and temperature were recorded. Mice were then placed into testing chambers for acclimation. In dose–response studies, after a brief 5 min acclimation, mouse body temperature was recorded for baseline measurement, mice received s.c. injection of test substance or vehicle, and animals were returned to the testing arena for 30 min. During the session, locomotor activity was monitored via photobeam tracking of movements in the horizontal plane to yield distance traveled in centimeter. HTR was monitored by the analysis of GoPro Hero Black 7 video recordings (120 frames per sec and 960p resolution) using a commercially available software package from Clever Sys Inc. (Reston, VA, USA).82 Post-treatment body temperature values were also recorded, and temperature data are represented as change from pretreatment baseline.
Antagonist Studies
In antagonist reversal experiments, mice received a s.c. injection of either receptor antagonists or vehicle and were returned to the testing chamber for 30 min. During this period, locomotor activity was monitored to examine the potential effects of antagonist treatment on general behavior or movement. At 30 min after antagonist administration, mice were given test drug or vehicle and returned to the chambers for an additional 30 min of video recording used for analyses.
Data Analyses
All statistical analyses were conducted using GraphPad Prism 9 (La Jolla, CA, USA). Nonlinear regression was utilized to determine receptor affinity (Ki), potency (EC50 or ED50), and efficacy (Emax) for in vitro and in vivo experiments. Ki values were determined in mouse brain experiments using previously reported Kd values for these radioligands in mouse brain.106,114 For the β-arrestin recruitment assays, the full 2 h time–luminescence profile was corrected for inter-well variability and used to calculate the area under the curve (AUC). The AUC of the corresponding solvent control was subtracted, and the obtained values were used to fit sigmoidal concentration–response curves as well as to determine EC50 and Emax values relative to the Emax of LSD (set at 100%). Dose–response data from mouse experiments were analyzed using nonlinear regression, and potency values were determined from the rising phase of the curves for HTR measures. For mouse studies, one-way ANOVA with Dunnett’s post hoc test was used to compare all conditions to vehicle controls (0 or 0,0) in dose–response and antagonist experiments. Time-course drug effects for all parameters in mouse studies are shown for reference. Mean HTR count, distance traveled, and temperature change for each condition were used for statistical comparisons. Alpha was set at 0.05 for all analyses.
Glossary
Abbreviations
- 5-HT
serotonin
- 5-HT1A
serotonin 1A receptor
- 5-HT1B
serotonin 1B receptor
- 5-HT1D
serotonin 1D receptor
- 5-ht1e
serotonin 1e receptor
- 5-HT2A
serotonin 2A receptor
- 5-HT2B
serotonin 2B receptor
- 5-HT2C
serotonin 2C receptor
- 5-HT3
serotonin 3 receptor
- 5-HT4
serotonin 4 receptor
- 5-HT5A
serotonin 5A receptor
- 5-HT6
serotonin 6 receptor
- 5-HT7a
serotonin 7a receptor
- Alpha2A
alpha 2A receptor
- Alpha2C
alpha 2C receptor
- DAT
dopamine transporter
- SERT
serotonin transporter
- H1
histamine H1 receptor
- norbaeocystin
4-phosphoryloxytryptamine
- baeocystin
4-phosphoryloxy-N-methyltryptamine
- psilocybin
4-phosphoryloxy-N,N-dimethyltryptamine
- aeruginascin
4-phosphoryloxy-N,N,N-trimethyltryptamine
- norpsilocin
4-hydroxy-N-methyltryptamine
- psilocin
4-hydroxy-N,N-dimethyltryptamine
- 4-HO-TMT
4-hydroxy-N,N,N-trimethyltryptamine
- 4-AcO-NMT
4-acetoxy-N-methyltryptamine
- psilacetin or 4-AcO-DMT
4-acetoxy-N,N-dimethyltryptamine
- 4-AcO-TMT
4-acetoxy-N,N,N-trimethyltryptamine
- M100907
(+)MDL 100907
- WAY100635
WAY 100653
- DOI
(±)-2,5-dimethoxy-4-iodoamphetamine
- LSD
lysergic acid diethylamide
- 5-MeO-DMT
5-methoxy-N,N-dimethyltryptamine
- 5-HTP
5-hydroxytryptophan
- NIMH PDSP
National Institute of Mental Health Psychoactive Drug Screening Program
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.2c00177.
Affinity values of various psilocybin analogues for sites other than 5-HT receptors; competition of psilocybin analogues for agonist radioligand binding in vitro; concentration effect curves of psilocybin analogues in mouse brain; concentration–response curves for psilocybin analogues in 5-HT2 receptor calcium mobilization assays; functional agonist activity screening for psilocybin analogues across 5-HT receptors; concentration effect curves of psilocybin analogues at 5-HT2A receptors in β arrestin 2 functional assays; time-course for HTR by psilocin, psilocybin, psilacetin, and norpsilocin in mice; statistical information and descriptive statistics for dose–response mouse studies; dose–response curves of secondary amine and quaternary ammonium psilocybin analogues for HTR count in mice; dose–response curves of psilocybin analogues for temperature change in mice; psilocin, psilocybin, and psilacetin locomotor plots; secondary amine locomotor plots; locomotor plots for quaternary ammonium psilocybin analogues; dose-related effects of M100907 on locomotor activity in mice; M100907 experiment control plots; descriptive statistics for HTR data from M100907 experiments; descriptive statistics for all data from WAY100635 experiments; WAY100635 effects on HTR and locomotor activity in mice; X-ray crystal structures of salts used of 4-AcO-NMT and aeruginascin; extended information and data related to crystallographic analyses of 4-AcO-NMT and aeruginascin; and nonenzymatic hydrolysis rates of psilocybin and psilacetin in aqueous buffer (PDF)
Author Contributions
Study design: G.C.G. and M.H.B. Chemical synthesis and analysis: D.R.M., J.A.G., A.M.S., D.N.K.P., V.R.S., and M.N. Hydrolysis experiments: S.D. and E.B.H. Mouse brain binding assays: J.P. 5-HT2A β-arrestin 2 assays: E.P. Mouse experiments: G.C.G. Manuscript was drafted by G.C.G. and critically reviewed by M.H.B., A.R.C., D.R.M., A.M.S., K.K., E.B.H., E.P., and C.P.S., and final version approved by all authors.
This work was supported in part by collaborative research funds to UMass Dartmouth provided by CaaMTech, Inc., and crystallographic and NMR data were collected on NSF-funded instruments (CHE-1229339, CHE-1429086). This work was additionally supported by the NIDA Intramural Research Program grant number DA-000522-13 and Cooperative Research and Development Agreement between NIDA and CaaMTech (M.H.B.). We acknowledge the NIMH PDSP (Contract # HHSN-271-2018-00023 C) for providing Ki determinations, receptor binding profiles, and functional potency as well as efficacy data. The NIMH PDSP is directed by Bryan L. Roth M.D., Ph.D., at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda MD, USA. We lastly thank David White, Ph.D., of the NIDA Division of Therapeutics and Medical Consequences Addiction Treatment Discovery Program for preclinical testing by the laboratory of Aaron J. Janowsky Ph.D. (Oregon Health & Science University) which is a laboratory in an existing framework of contracts maintained by NIDA. The authors acknowledge Bob Discordia, Ph.D., for helpful comments on some portions of the manuscript.
The authors declare the following competing financial interest(s): A.R.C. has an ownership stake in CaaMTech, Inc., which owns patent applications covering new tryptamine compounds, their compositions, formulations, novel crystalline forms, methods of treatment, and methods for synthesis. No other authors report any competing financial interests related to the present work.
Supplementary Material
References
- Geiger H. A.; Wurst M. G.; Daniels R. N. DARK Classics in Chemical Neuroscience: Psilocybin. ACS Chem. Neurosci. 2018, 9, 2438–2447. 10.1021/acschemneuro.8b00186. [DOI] [PubMed] [Google Scholar]
- Nichols D. E. Psilocybin: from ancient magic to modern medicine. J. Antibiot. 2020, 73, 679–686. 10.1038/s41429-020-0311-8. [DOI] [PubMed] [Google Scholar]
- Nichols D. E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. 10.1124/pr.115.011478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D. E. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Van Court R. C.; Wiseman M. S.; Meyer K. W.; Ballhorn D. J.; Amses K. R.; Slot J. C.; Dentinger B. T. M.; Garibay-Orijel R.; Uehling J. K. Diversity, biology, and history of psilocybin-containing fungi: Suggestions for research and technological development. Fungal Biol. 2022, 126, 308–319. 10.1016/j.funbio.2022.01.003. [DOI] [PubMed] [Google Scholar]
- Carbonaro T. M.; Bradstreet M. P.; Barrett F. S.; MacLean K. A.; Jesse R.; Johnson M. W.; Griffiths R. R. Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. J. Psychopharmacol. 2016, 30, 1268–1278. 10.1177/0269881116662634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland J. C.; Johnson M. W. Human behavioral pharmacology of psychedelics. Adv. Pharmacol. 2022, 93, 105–132. 10.1016/bs.apha.2021.10.003. [DOI] [PubMed] [Google Scholar]
- Carbonaro T. M.; Johnson M. W.; Griffiths R. R. Subjective features of the psilocybin experience that may account for its self-administration by humans: a double-blind comparison of psilocybin and dextromethorphan. Psychopharmacology 2020, 237, 2293–2304. 10.1007/s00213-020-05533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro T. M.; Johnson M. W.; Hurwitz E.; Griffiths R. R. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: similarities and differences in subjective experiences. Psychopharmacology 2018, 235, 521–534. 10.1007/s00213-017-4769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E.; Kometer M.; Hasler F.; Vollenweider F. X. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J. Psychopharmacol. 2010, 25, 1434–1452. 10.1177/0269881110382466. [DOI] [PubMed] [Google Scholar]
- Madsen M. K.; Fisher P. M.; Burmester D.; Dyssegaard A.; Stenbæk D. S.; Kristiansen S.; Johansen S. S.; Lehel S.; Linnet K.; Svarer C.; Erritzoe D.; Ozenne B.; Knudsen G. M. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 2019, 44, 1328–1334. 10.1038/s41386-019-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita A.; Weber L. J. The enzymic dephosphorylation and oxidation of psilocybin and psilocin by mammalian tissue homogenates. Biochem. Pharmacol. 1961, 7, 47–54. 10.1016/0006-2952(61)90124-1. [DOI] [PubMed] [Google Scholar]
- Horita A.; Weber L. J. Dephosphorylation of psilocybin to psilocin by alkaline phosphatase. Proc. Soc. Exp. Biol. Med. 1961, 106, 32–34. 10.3181/00379727-106-26228. [DOI] [PubMed] [Google Scholar]
- Horita A.; Weber L. J. Dephosphorylation of psilocybin in the intact mouse. Toxicol. Appl. Pharmacol. 1962, 4, 730–737. 10.1016/0041-008x(62)90102-3. [DOI] [PubMed] [Google Scholar]
- Nutt D. J.; King L. A.; Nichols D. E. Effects of Schedule I drug laws on neuroscience research and treatment innovation. Nat. Rev. Neurosci. 2013, 14, 577–585. 10.1038/nrn3530. [DOI] [PubMed] [Google Scholar]
- Nutt D.; Carhart-Harris R. The Current Status of Psychedelics in Psychiatry. JAMA Psychiatry 2021, 78, 121–122. 10.1001/jamapsychiatry.2020.2171. [DOI] [PubMed] [Google Scholar]
- Nichols D. E.; Johnson M. W.; Nichols C. D. Psychedelics as Medicines: An Emerging New Paradigm. Clin. Pharmacol. Ther. 2017, 101, 209–219. 10.1002/cpt.557. [DOI] [PubMed] [Google Scholar]
- McClure-Begley T. D.; Roth B. L. The promises and perils of psychedelic pharmacology for psychiatry. Nat. Rev. Drug Discovery 2022, 21, 463. 10.1038/s41573-022-00421-7. [DOI] [PubMed] [Google Scholar]
- Nicholas C. R.; Henriquez K. M.; Gassman M. C.; Cooper K. M.; Muller D.; Hetzel S.; Brown R. T.; Cozzi N. V.; Thomas C.; Hutson P. R. High dose psilocybin is associated with positive subjective effects in healthy volunteers. J. Psychopharmacol. 2018, 32, 770–778. 10.1177/0269881118780713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler E. A. D. Psychedelics as preventive treatment in headache and chronic pain disorders. Neuropharmacology 2022, 215, 109166. 10.1016/j.neuropharm.2022.109166. [DOI] [PubMed] [Google Scholar]
- Kometer M.; Schmidt A.; Jancke L.; Vollenweider F. X. Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J. Neurosci. 2013, 33, 10544–10551. 10.1523/jneurosci.3007-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider F. X.; Vollenweider-Scherpenhuyzen M. F.; Bäbler A.; Vogel H.; Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 1998, 9, 3897–3902. 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Koedood L.; Powell S. B.; Geyer M. A. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol. 2011, 25, 1548–1561. 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J.; Weisstaub N. V.; Zhou M.; Chan P.; Ivic L.; Ang R.; Lira A.; Bradley-Moore M.; Ge Y.; Zhou Q.; Sealfon S. C.; Gingrich J. A. Hallucinogens Recruit Specific Cortical 5-HT2A Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 2007, 53, 439–452. 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Winter J. C.; Rice K. C.; Amorosi D. J.; Rabin R. A. Psilocybin-induced stimulus control in the rat. Pharmacol., Biochem. Behav. 2007, 87, 472–480. 10.1016/j.pbb.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L.; Geyer M. A. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 2011, 61, 364–381. 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorny T.; Preller K. H.; Kraehenmann R.; Vollenweider F. X. Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur. Neuropsychopharmacol. 2016, 26, 756–766. 10.1016/j.euroneuro.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Carter O. L.; Burr D. C.; Pettigrew J. D.; Wallis G. M.; Hasler F.; Vollenweider F. X. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J. Cognit. Neurosci. 2005, 17, 1497–1508. 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- Slocum S. T.; DiBerto J. F.; Roth B. L. Molecular insights into psychedelic drug action. J. Neurochem. 2021, 162, 24. 10.1111/jnc.15540. [DOI] [PubMed] [Google Scholar]
- Winter J. C. Hallucinogens as discriminative stimuli in animals: LSD, phenethylamines, and tryptamines. Psychopharmacology 2009, 203, 251–263. 10.1007/s00213-008-1356-8. [DOI] [PubMed] [Google Scholar]
- Blei F.; Dörner S.; Fricke J.; Baldeweg F.; Trottmann F.; Komor A.; Meyer F.; Hertweck C.; Hoffmeister D. Simultaneous Production of Psilocybin and a Cocktail of β-Carboline Monoamine Oxidase Inhibitors in “Magic” Mushrooms. Chemistry 2020, 26, 729–734. 10.1002/chem.201904363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörner S.; Rogge K.; Fricke J.; Schäfer T.; Wurlitzer J. M.; Gressler M.; Pham D. N. K.; Manke D. R.; Chadeayne A. R.; Hoffmeister D. Genetic Survey of Psilocybe Natural Products. Chembiochem 2022, 23, e202200249 10.1002/cbic.202200249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotvaldová K.; Hájková K.; Borovička J.; Jurok R.; Cihlářová P.; Kuchař M. Stability of psilocybin and its four analogs in the biomass of the psychotropic mushroom Psilocybe cubensis. Drug Test. Anal. 2021, 13, 439–446. 10.1002/dta.2950. [DOI] [PubMed] [Google Scholar]
- Leung A. Y.; Paul A. G. Baeocystin, a mono-methyl analog of psilocybin from Psilocybe baeocystis saprophytic culture. J. Pharm. Sci. 1967, 56, 146. 10.1002/jps.2600560132. [DOI] [PubMed] [Google Scholar]
- Leung A. Y.; Paul A. G. Baeocystin and Norbaeocystin: New Analogs of Psilocybin from Psilocybe baeocystis. J. Pharm. Sci. 1968, 57, 1667–1671. 10.1002/jps.2600571007. [DOI] [PubMed] [Google Scholar]
- Lenz C.; Wick J.; Hoffmeister D. Identification of ω-N-Methyl-4-hydroxytryptamine (Norpsilocin) as a Psilocybe Natural Product. J. Nat. Prod. 2017, 80, 2835–2838. 10.1021/acs.jnatprod.7b00407. [DOI] [PubMed] [Google Scholar]
- Gartz J. Variation of the Amount of Alkaloids in Fruit Bodies of Inocybe aeruginascens. Planta Med. 1987, 53, 539–541. 10.1055/s-2006-962805. [DOI] [PubMed] [Google Scholar]
- Jensen N.; Gartz J.; Laatsch H. Aeruginascin, a trimethylammonium analogue of psilocybin from the hallucinogenic mushroom Inocybe aeruginascens. Planta Med. 2006, 72, 665–666. 10.1055/s-2006-931576. [DOI] [PubMed] [Google Scholar]
- Fricke J.; Blei F.; Hoffmeister D. Enzymatic Synthesis of Psilocybin. Angew. Chem., Int. Ed. Engl. 2017, 56, 12352–12355. 10.1002/anie.201705489. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Chatha M.; Klein A. K.; Wallach J.; Brandt S. D. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 2020, 167, 107933. 10.1016/j.neuropharm.2019.107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A. M.; Halberstadt A. L.; Klein A. K.; McCorvy J. D.; Kaylo K. W.; Kargbo R. B.; Meisenheimer P. Synthesis and Biological Evaluation of Tryptamines Found in Hallucinogenic Mushrooms: Norbaeocystin, Baeocystin, Norpsilocin, and Aeruginascin. J. Nat. Prod. 2020, 83, 461–467. 10.1021/acs.jnatprod.9b01061. [DOI] [PubMed] [Google Scholar]
- Sheppard B. A Trip Through Employment Law: Protecting Therapeutic Psilocybin Users in the Workplace. J. Law Health 2021, 35, 146–180. [PubMed] [Google Scholar]
- Chadeayne A. R.; Pham D. N. K.; Reid B. G.; Golen J. A.; Manke D. R. Active Metabolite of Aeruginascin (4-Hydroxy-N,N,N-trimethyltryptamine): Synthesis, Structure, and Serotonergic Binding Affinity. ACS Omega 2020, 5, 16940–16943. 10.1021/acsomega.0c02208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadeayne A. R.; Golen J. A.; Manke D. R. Bis(4-acet-oxy-N,N-di-methyl-tryptammonium) fumarate: a new crystalline form of psilacetin, an alternative to psilocybin as a psilocin prodrug. Acta Crystallogr., Sect. E: Crystallogr. Commun. 2019, 75, 900–902. 10.1107/s2056989019007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A.; Troxler F.. Esters of Indoles. U.S. Patent 3,075,992 A, 1963.
- Shulgin A. T.; Shulgin A.. TiHKAL: The Continuation; Transform Press, 1997. [Google Scholar]
- Nichols D. E.; Frescas S. Improvements to the Synthesis of Psilocybin and a Facile Method for Preparing the O-Acetyl Prodrug of Psilocin. Synthesis 1999, 1999, 935–938. 10.1055/s-1999-3490. [DOI] [Google Scholar]
- Palamar J. J.; Barratt M. J.; Ferris J. A.; Winstock A. R. Correlates of new psychoactive substance use among a self-selected sample of nightclub attendees in the United States. Am. J. Addict. 2016, 25, 400–407. 10.1111/ajad.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma-Conesa J.; Ventura M.; Galindo L.; Fonseca F.; Grifell M.; Quintana P.; Fornís I.; Gil C.; Farré M.; Torrens M. Something New about Something Old: A 10-Year Follow-Up on Classical and New Psychoactive Tryptamines and Results of Analysis. J. Psychoact. Drugs 2017, 49, 297–305. 10.1080/02791072.2017.1320732. [DOI] [PubMed] [Google Scholar]
- Palamar J. J.; Acosta P. A qualitative descriptive analysis of effects of psychedelic phenethylamines and tryptamines. Hum. Psychopharmacol. 2020, 35, e2719 10.1002/hup.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S. P.; Holdbrook T.; Brandt S. D. Prodrugs of New Psychoactive Substances (NPS): A New Challenge. J. Forensic Sci. 2020, 65, 913–920. 10.1111/1556-4029.14268. [DOI] [PubMed] [Google Scholar]
- Gatch M. B.; Hoch A.; Carbonaro T. M. Discriminative Stimulus Effects of Substituted Tryptamines in Rats. ACS Pharmacol. Transl. Sci. 2021, 4, 467–471. 10.1021/acsptsci.0c00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai W.; Li L.; Zhao J.; Xiang P.; Liu M.; Shi Y.; Dang Y. Tentative identification of in vitro metabolites of O-acetylpsilocin (psilacetin, 4-AcO-DMT) by UHPLC-Q-Orbitrap MS. Drug Test. Anal. 2022, 14, 1300. 10.1002/dta.3255. [DOI] [PubMed] [Google Scholar]
- Chadeayne A. R.; Pham D. N. K.; Golen J. A.; Manke D. R. Norpsilocin: freebase and fumarate salt. Acta Crystallogr., Sect. E: Crystallogr. Commun. 2020, 76, 589–593. 10.1107/s2056989020004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. K.; Chatha M.; Laskowski L. J.; Anderson E. I.; Brandt S. D.; Chapman S. J.; McCorvy J. D.; Halberstadt A. L. Investigation of the Structure-Activity Relationships of Psilocybin Analogues. ACS Pharmacol. Transl. Sci. 2021, 4, 533–542. 10.1021/acsptsci.0c00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatfelter G. C.; Pham D. N. K.; Walther D.; Golen J. A.; Chadeayne A. R.; Baumann M. H.; Manke D. R. Synthesis, Structural Characterization, and Pharmacological Activity of Novel Quaternary Salts of 4-Substituted Tryptamines. ACS Omega 2022, 7, 24888–24894. 10.1021/acsomega.2c03476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D. E.; Sassano M. F.; Halberstadt A. L.; Klein L. M.; Brandt S. D.; Elliott S. P.; Fiedler W. J. N-Benzyl-5-methoxytryptamines as Potent Serotonin 5-HT2 Receptor Family Agonists and Comparison with a Series of Phenethylamine Analogues. ACS Chem. Neurosci. 2015, 6, 1165–1175. 10.1021/cn500292d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. S. Psychedelics and the human receptorome. PLoS One 2010, 5, e9019 10.1371/journal.pone.0009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkizia-Santamaría I.; Alles-Pascual R.; Horrillo I.; Meana J. J.; Ortega J. E. Serotonin 5-HT2A, 5-HT2c and 5-HT1A receptor involvement in the acute effects of psilocybin in mice. In vitro pharmacological profile and modulation of thermoregulation and head-twich response. Biomed. Pharmacother. 2022, 154, 113612. 10.1016/j.biopha.2022.113612. [DOI] [PubMed] [Google Scholar]
- Pottie E.; Stove C. P. In vitro assays for the functional characterization of (psychedelic) substances at the serotonin receptor 5-HT(2A) R. J. Neurochem. 2022, 162, 39. 10.1111/jnc.15570. [DOI] [PubMed] [Google Scholar]
- Canal C. E. Serotonergic Psychedelics: Experimental Approaches for Assessing Mechanisms of Action. Handb. Exp. Pharmacol. 2018, 252, 227–260. 10.1007/164_2018_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E. E.; Smith R. L.; Sanders-Bush E. Role of Gq protein in behavioral effects of the hallucinogenic drug 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology 2007, 52, 1671–1677. 10.1016/j.neuropharm.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Zhu H.; Gao H.; Tian X.; Tan B.; Su R. Gs signaling pathway distinguishes hallucinogenic and nonhallucinogenic 5-HT2AR agonists induced head twitch response in mice. Biochem. Biophys. Res. Commun. 2022, 598, 20–25. 10.1016/j.bbrc.2022.01.113. [DOI] [PubMed] [Google Scholar]
- Egan C.; Grinde E.; Dupre A.; Roth B. L.; Hake M.; Teitler M.; Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT(2A) and 5-HT(2C) receptors. Synapse 2000, 35, 144–150. . [DOI] [PubMed] [Google Scholar]
- Roth B. L.; Choudhary M. S.; Khan N.; Uluer A. Z. High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model. J. Pharmacol. Exp. Ther. 1997, 280, 576–583. [PubMed] [Google Scholar]
- Elmore J. S.; Decker A. M.; Sulima A.; Rice K. C.; Partilla J. S.; Blough B. E.; Baumann M. H. Comparative neuropharmacology of N-(2-methoxybenzyl)-2,5-dimethoxyphenethylamine (NBOMe) hallucinogens and their 2C counterparts in male rats. Neuropharmacology 2018, 142, 240–250. 10.1016/j.neuropharm.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin R. A.; Regina M.; Doat M.; Winter J. C. 5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens. Pharmacol., Biochem. Behav. 2002, 72, 29–37. 10.1016/s0091-3057(01)00720-1. [DOI] [PubMed] [Google Scholar]
- Titeler M.; Lyon R. A.; Glennon R. A. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology 1988, 94, 213–216. 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- Luethi D.; Liechti M. E. Monoamine Transporter and Receptor Interaction Profiles in Vitro Predict Reported Human Doses of Novel Psychoactive Stimulants and Psychedelics. Int. J. Neuropsychopharmacol. 2018, 21, 926–931. 10.1093/ijnp/pyy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A.; Moning O. D.; Hoener M. C.; Liechti M. E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol. 2016, 26, 1327–1337. 10.1016/j.euroneuro.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Glennon R. A.; Titeler M.; McKenney J. D. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984, 35, 2505–2511. 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Glennon R. A.; Titeler M.; Young R. Structure-activity relationships and mechanism of action of hallucinogenic agents based on drug discrimination and radioligand binding studies. Psychopharmacol. Bull. 1986, 22, 953. [PubMed] [Google Scholar]
- Gelber E. I.; Kroeze W. K.; Roth D. L.; Gray J. A.; Sinar C. A.; Hyde E. G.; Gurevich V.; Benovic J.; Roth B. L. Structure and function of the third intracellular loop of the 5-hydroxytryptamine2A receptor: the third intracellular loop is alpha-helical and binds purified arrestins. J. Neurochem. 1999, 72, 2206–2214. 10.1046/j.1471-4159.1999.0722206.x. [DOI] [PubMed] [Google Scholar]
- Schmid C. L.; Raehal K. M.; Bohn L. M. Agonist-directed signaling of the serotonin 2A receptor depends on β-arrestin-2 interactions in vivo. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 1079–1084. 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguiz R. M.; Nadkarni V.; Means C. R.; Pogorelov V. M.; Chiu Y.-T.; Roth B. L.; Wetsel W. C. LSD-stimulated behaviors in mice require β-arrestin 2 but not β-arrestin 1. Sci. Rep. 2021, 11, 17690. 10.1038/s41598-021-96736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. L.; Bohn L. M. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ß-arrestin2/Src/Akt signaling complex in vivo. J. Neurosci. 2010, 30, 13513–13524. 10.1523/jneurosci.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottie E.; Cannaert A.; Stove C. P. In vitro structure-activity relationship determination of 30 psychedelic new psychoactive substances by means of β-arrestin 2 recruitment to the serotonin 2A receptor. Arch. Toxicol. 2020, 94, 3449–3460. 10.1007/s00204-020-02836-w. [DOI] [PubMed] [Google Scholar]
- Kroeze W. K.; Sassano M. F.; Huang X. P.; Lansu K.; McCorvy J. D.; Giguère P. M.; Sciaky N.; Roth B. L. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol. 2015, 22, 362–369. 10.1038/nsmb.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.; Che T.; Panova O.; DiBerto J. F.; Lyu J.; Krumm B. E.; Wacker D.; Robertson M. J.; Seven A. B.; Nichols D. E.; Shoichet B. K.; Skiniotis G.; Roth B. L. Structure of a Hallucinogen-Activated Gq-Coupled 5-HT(2A) Serotonin Receptor. Cell 2020, 182, 1574–1588. 10.1016/j.cell.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottie E.; Dedecker P.; Stove C. P. Identification of psychedelic new psychoactive substances (NPS) showing biased agonism at the 5-HT(2A)R through simultaneous use of β-arrestin 2 and miniGα(q) bioassays. Biochem. Pharmacol. 2020, 182, 114251. 10.1016/j.bcp.2020.114251. [DOI] [PubMed] [Google Scholar]
- Canal C. E.; Morgan D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test. Anal. 2012, 4, 556–576. 10.1002/dta.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatfelter G. C.; Chojnacki M. R.; McGriff S. A.; Wang T.; Baumann M. H. Automated Computer Software Assessment of 5-Hydroxytryptamine 2A Receptor-Mediated Head Twitch Responses from Video Recordings of Mice. ACS Pharmacol. Transl. Sci. 2022, 5, 321. 10.1021/acsptsci.1c00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A. M.; Halberstadt A. L.; Klein A. K.; McCorvy J. D.; Kaylo K. W.; Kargbo R. B.; Meisenheimer P. Synthesis and Biological Evaluation of Tryptamines Found in Hallucinogenic Mushrooms: Norbaeocystin, Baeocystin, Norpsilocin, and Aeruginascin. J. Nat. Prod. 2020, 83, 461–467. 10.1021/acs.jnatprod.9b01061. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L. Behavioral and pharmacokinetic interactions between monoamine oxidase inhibitors and the hallucinogen 5-methoxy-N,N-dimethyltryptamine. Pharmacol., Biochem. Behav. 2016, 143, 1–10. 10.1016/j.pbb.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt A. L.; Nichols D. E.; Geyer M. A. Behavioral effects of α,α,β,β-tetradeutero-5-MeO-DMT in rats: comparison with 5-MeO-DMT administered in combination with a monoamine oxidase inhibitor. Psychopharmacology 2012, 221, 709–718. 10.1007/s00213-011-2616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron L. P.; Tombari R. J.; Lu J.; Pell A. J.; Hurley Z. Q.; Ehinger Y.; Vargas M. V.; McCarroll M. N.; Taylor J. C.; Myers-Turnbull D.; Liu T.; Yaghoobi B.; Laskowski L. J.; Anderson E. I.; Zhang G.; Viswanathan J.; Brown B. M.; Tjia M.; Dunlap L. E.; Rabow Z. T.; Fiehn O.; Wulff H.; McCorvy J. D.; Lein P. J.; Kokel D.; Ron D.; Peters J.; Zuo Y.; Olson D. E. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 2021, 589, 474–479. 10.1038/s41586-020-3008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D.; Yu J.; Wang H.; Luo Z.; Liu X.; He L.; Qi J.; Fan L.; Tang L.; Chen Z.; Li J.; Cheng J.; Wang S. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 2022, 375, 403–411. 10.1126/science.abl8615. [DOI] [PubMed] [Google Scholar]
- Halberstadt A. L.; Geyer M. A. Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology 2013, 227, 727–739. 10.1007/s00213-013-3006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T.; Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat. Rev. Drug Discovery 2013, 12, 205–216. 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D.; Kurrasch-Orbaugh D. M.; Selken J. R.; Cumbay M. G.; Lisnicchia J. G.; Nichols D. E. Re-evaluation of lisuride pharmacology: 5-hydroxytryptamine1A receptor-mediated behavioral effects overlap its other properties in rats. Psychopharmacology 2002, 164, 93–107. 10.1007/s00213-002-1141-z. [DOI] [PubMed] [Google Scholar]
- Blough B. E.; Landavazo A.; Decker A. M.; Partilla J. S.; Baumann M. H.; Rothman R. B. Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes. Psychopharmacology 2014, 231, 4135–4144. 10.1007/s00213-014-3557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S. D.; Kavanagh P. V.; Twamley B.; Westphal F.; Elliott S. P.; Wallach J.; Stratford A.; Klein L. M.; McCorvy J. D.; Nichols D. E.; Halberstadt A. L. Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM-775). Drug Test. Anal. 2018, 10, 310–322. 10.1002/dta.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani N. A.; Martin B. R.; Pandey U.; Glennon R. A. Do functional relationships exist between 5-HT1A and 5-HT2 receptors?. Pharmacol., Biochem. Behav. 1990, 36, 901–906. 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Fox M. A.; Stein A. R.; French H. T.; Murphy D. L. Functional interactions between 5-HT2A and presynaptic 5-HT1A receptor-based responses in mice genetically deficient in the serotonin 5-HT transporter (SERT). Br. J. Pharmacol. 2010, 159, 879–887. 10.1111/j.1476-5381.2009.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L. M.; Cozzi N. V.; Daley P. F.; Brandt S. D.; Halberstadt A. L. Receptor binding profiles and behavioral pharmacology of ring-substituted N,N-diallyltryptamine analogs. Neuropharmacology 2018, 142, 231–239. 10.1016/j.neuropharm.2018.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R.; Brocco M.; Audinot V.; Gobert A.; Veiga S.; Millan M. J. 1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J. Pharmacol. Exp. Ther. 1995, 273, 101. [PubMed] [Google Scholar]
- Tylš F.; Páleníček T.; Kadeřábek L.; Lipski M.; Kubešová A.; Horáček J. Sex differences and serotonergic mechanisms in the behavioural effects of psilocin. Behav. Pharmacol. 2016, 27, 309–320. 10.1097/fbp.0000000000000198. [DOI] [PubMed] [Google Scholar]
- Savalia N. K.; Shao L. X.; Kwan A. C. A Dendrite-Focused Framework for Understanding the Actions of Ketamine and Psychedelics. Trends Neurosci. 2021, 44, 260–275. 10.1016/j.tins.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem M.; Sherwood A. M.; Chadeayne A. R.; Golen J. A.; Manke D. R. The crystal structure of baeocystin. Acta Crystallogr., Sect. E: Crystallogr. Commun. 2022, 78, 550. 10.1107/S2056989022004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea G.; Strapps W.; Herrada G.; Berman Y.; Ong J.; Kloss B.; Axel R.; Lee K. J. The genetic design of signaling cascades to record receptor activation. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 64–69. 10.1073/pnas.0710487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth B. L.National Institute of Mental Health Psychoactive Drug Screening Program (NIMH PDSP): ASSAY PROTOCOL BOOK Version III, 2018. https://pdsp.unc.edu/pdspweb/content/UNC-CH%20Protocol%20Book.pdf [Google Scholar]
- Eshleman A. J.; Wolfrum K. M.; Hatfield M. G.; Johnson R. A.; Murphy K. V.; Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem. Pharmacol. 2013, 85, 1803–1815. 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman A. J.; Wolfrum K. M.; Reed J. F.; Kim S. O.; Johnson R. A.; Janowsky A. Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors. Biochem. Pharmacol. 2018, 158, 27–34. 10.1016/j.bcp.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman A. J.; Carmolli M.; Cumbay M.; Martens C. R.; Neve K. A.; Janowsky A. Characteristics of Drug Interactions with Recombinant Biogenic Amine Transporters Expressed in the Same Cell Type. J. Pharmacol. Exp. Ther. 1999, 289, 877–885. [PubMed] [Google Scholar]
- Eshleman A. J.; Nagarajan S.; Wolfrum K. M.; Reed J. F.; Nilsen A.; Torralva R.; Janowsky A. Affinity, potency, efficacy, selectivity, and molecular modeling of substituted fentanyls at opioid receptors. Biochem. Pharmacol. 2020, 182, 114293. 10.1016/j.bcp.2020.114293. [DOI] [PubMed] [Google Scholar]
- Ulrichsen J.; Partilla J. S.; Dax E. M. Long-term administration of m-chlorophenylpiperazine (mCPP) to rats induces changes in serotonin receptor binding, dopamine levels and locomotor activity without altering prolactin and corticosterone secretion. Psychopharmacology 1992, 107, 229–235. 10.1007/BF02245142. [DOI] [PubMed] [Google Scholar]
- Dixon A. S.; Schwinn M. K.; Hall M. P.; Zimmerman K.; Otto P.; Lubben T. H.; Butler B. L.; Binkowski B. F.; Machleidt T.; Kirkland T. A.; Wood M. G.; Eggers C. T.; Encell L. P.; Wood K. V. NanoLuc Complementation Reporter Optimized for Accurate Measurement of Protein Interactions in Cells. ACS Chem. Biol. 2016, 11, 400–408. 10.1021/acschembio.5b00753. [DOI] [PubMed] [Google Scholar]
- Darmani N. A.; Martin B. R.; Glennon R. A. Withdrawal from chronic treatment with (+/-)-DOI causes super-sensitivity to 5-HT2 receptor-induced head-twitch behaviour in mice. Eur. J. Pharmacol. 1990, 186, 115–118. 10.1016/0014-2999(90)94066-7. [DOI] [PubMed] [Google Scholar]
- Moser P. C.; Redfern P. H. Circadian variation in behavioural responses to central 5-HT receptor stimulation in the mouse. Psychopharmacology 1985, 86, 223–227. 10.1007/bf00431714. [DOI] [PubMed] [Google Scholar]
- Nagayama H.; Lu J. Q. Circadian rhythm in the responsiveness of central 5-HT2A receptor to DOI in rats. Psychopharmacology 1996, 127, 113–116. 10.1007/bf02805983. [DOI] [PubMed] [Google Scholar]
- Eison A. S.; Freeman R. P.; Guss V. B.; Mullins U. L.; Wright R. N. Melatonin agonists modulate 5-HT2A receptor-mediated neurotransmission: behavioral and biochemical studies in the rat. J. Pharmacol. Exp. Ther. 1995, 273, 304–308. [PubMed] [Google Scholar]
- Eison A. S.; Mullins U. L. Regulation of central 5-HT2A receptors: a review of in vivo studies. Behav. Brain Res. 1996, 73, 177–181. 10.1016/0166-4328(96)00092-7. [DOI] [PubMed] [Google Scholar]
- Glatfelter G. C.; Partilla J. S.; Baumann M. H. Structure-activity relationships for 5F-MDMB-PICA and its 5F-pentylindole analogs to induce cannabinoid-like effects in mice. Neuropsychopharmacology 2022, 47, 924–932. 10.1038/s41386-021-01227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux A.; Fabre V.; Peter Lesch K. P.; Moessner R.; Murphy D. L.; Lanfumey L.; Hamon M.; Martres M. P. Adaptive changes of serotonin 5-HT2A receptors in mice lacking the serotonin transporter. Neurosci. Lett. 1999, 262, 113–116. 10.1016/s0304-3940(99)00049-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.