Abstract
Objective:
Growing use of clinical exome sequencing (CES) has led to an increased burden of genomic education. Self-guided educational tools can minimize the educational burden for genetic counselors (GCs). The effectiveness of these tools must be evaluated.
Methods:
Parents of patients offered CES were randomized to watch educational videos before their visit or to receive routine care. Parents and GCs were surveyed about their experiences following the sessions. The responses of the video (n=102) and no-video (n=105) groups were compared.
Results:
GCs reported no significant differences between parents in the video and no-video groups on genetics knowledge or CES knowledge. In contrast, parents’ scores on genetics knowledge questions were lower in the video than no-video group (p=0.007). Most parents reported the videos were informative, and the groups did not differ in satisfaction with GCs or decisions to have CES.
Conclusion:
GCs and parents perceived the videos to be beneficial. However, lower scores on genetics knowledge questions highlight the need for careful development of educational tools.
Practice Implications:
Educational tools should be developed and assessed for effectiveness with the input of all stakeholders before widespread implementation. Better measures of the effectiveness of these educational tools are needed.
Keywords: exome sequencing, video education, genetic counselor, patient experience
1. INTRODUCTION
An important role of genetic counselors (GCs) is to educate patients about genetic testing to facilitate informed decisions [1,2]. To aid them in this goal, GCs often use supplemental educational tools such as visual aids or printed fact sheets. As clinical exome sequencing (CES) becomes more widely used, educational tools also need to evolve. The overall high diagnostic yield of CES has led to increased utilization of the test [3,4], resulting in an increased educational burden on both GCs and patients [5]. At the same time, GCs are a limited resource [6] increasing the need for effective educational tools that allow GCs to focus on the specific concerns of the family.
Education for CES has several specific challenges including the complexity of the number of conditions tested, potential for uncertain results, residual risk of a genetic disorder in the event of a negative result, the possibility of secondary findings (SF; medically actionable genetic variations unrelated to the primary purpose for testing, such as BRCA1 mutations), and difficulties in managing patient expectations due to changing estimates of the yield of testing as the field evolves [7–9]. While education is an important component of the GC session, it is just one of many goals. Others include addressing psychosocial needs, developing an appropriate genetic testing plan, and receipt of and adherence to appropriate medical recommendations [10,11]. Meeting the psychosocial needs of patients is particularly important. When psychosocial counseling is emphasized over teaching within a session, patients have better medical management adherence [11], improved information retention, and greater satisfaction [12–14]. Despite this, GCs report addressing education over psychosocial needs when time is constrained [15]. Given increasing time constraints in clinics and the complexity of CES, education and consenting for this test can limit the amount of time available to address these important psychosocial issues [16,17].
Self-guided supplementary educational tools have the potential to prepare patients for more meaningful discussions, improve consistency of education, and offset some of the in-session educational burden. Despite the general potential positive effects of supplementary educational tools [18–20], most have been developed only for cancer genetics. Understanding how educational tools affect GC sessions from both patients’ and GCs’ perspectives is important in assessing the effectiveness of the tool. GCs have reported that use of pre-session video educational materials led to improved initial levels of genetic knowledge and retention of education provided during cancer GC sessions [20]. In a study in which women used a guided, personalized cancer genetics education web-portal before a GC appointment, GCs were able to provide greater patient-tailored care by addressing questions specific to the patient compared to standard sessions (i.e., no pre-session educational materials) [15]. The patients in this study also had improved knowledge about hereditary causes of breast cancer and improved satisfaction with the GC experience [21,22], both of which are important outcomes of a GC session [10,23–25]. One study in the cancer setting showed that women who viewed an educational video before meeting with the surgeon had shorter consultations and better understanding of their risk estimate. Additionally, women who viewed the videos had greater satisfaction with information given in the session [26]. In another study, patients who received carrier results through a web-based platform did not differ in genetics knowledge or test-related distress from patients who received results from a GC [27]. One study of the lay population demonstrated improved genetics knowledge after video education on genomic sequencing [28].
We developed a pre-session, self-guided video educational tool for CES and conducted a randomized controlled trial of the tool compared to standard care genetic counseling for parents of minors having CES. We explored the GCs’ perceptions of the videos’ impact on GC sessions and parental experience including genetics knowledge, parental satisfaction with genetic counseling, regret about the decision to have testing, psychological impact of the testing, and understanding of their child’s CES results. Parents’ and GCs’ perspectives and experiences of educational tools are critical to guide improvement of those tools and to support clinical implementation and patient engagement.
2. METHODS & MATERIALS
2.1. Educational Videos
Six educational videos reviewing basic information about genes, inheritance, chromosome and microarray analyses, CES, and the benefits and limitations of genetic testing were developed for this study. The videos were created by a team that included GCs, geneticists, psychiatrists, epidemiologists, social scientists, medical students, and research coordinators over a period of eighteen months. In the first phase, video scripts and proposed pictures were drafted by a GC and research assistant. Drafts were reviewed by the group, and modifications to pictures and text were made to improve clarity and reduce length. Animation was added. There were two additional rounds of reviews and edits including a final review that included non-medical professionals. The final videos (Supplemental Materials A) were approved by the development team. The videos are available at www.learninggenetics.org.
2.2. Parent Participants
Potential participants were identified from patients scheduled in the Division of Clinical Genetics at The Children’s Hospital of New York from April, 2016 until December, 2017. Patients were screened for all of the following eligibility requirements: 1) patient was a minor or adult with a medical guardian, 2) patient was being evaluated in an initial appointment for a neurodevelopmental disorder or was being seen in a follow-up appointment after non-diagnostic genetic testing for any indication except non-syndromic cancer, cardiomyopathy or cardiac arrhythmia, 3) patient had not previously had CES and did not have a genetically confirmed diagnosis, and 4) parent/guardian was able to speak and read English. The parents of the patients were randomized either to watch the educational videos (video group) or to receive standard care (no-video group). A full description of the randomization is in the supplemental methods and materials. Those in the video group were invited by phone and email to view the videos before the appointment and again on the day of the appointment but were not required to do so.
When CES was offered as part of the patient’s clinical evaluation (regardless of whether or not they pursued CES), the parent was then invited to the study by the research coordinator on the day of the visit or by phone within five days following the appointment. Following verbal consent, the survey was emailed or mailed (parent’s preference). A follow-up survey was sent a month after the results of the CES were disclosed to the parent by the treating GC or geneticist.
2.3. GC Participants
The GC participants in this study included the twelve GCs who provided clinical care for the eligible patients. All GCs viewed the videos before initiation of the study. GCs were blinded to the patient’s video group randomization. The treating GC was emailed an invitation to complete the GC study survey within five days following the patient’s appointment for all patients who provided consent to the study.
2.4. Surveys
The parental surveys were developed by the core research team and underwent four revisions. The initial survey (Appendix A) was completed following the initial genetics consultation. A follow-up survey was administered following the disclosure of the CES results (Appendix B). The GC survey was also completed following the initial consultation (Appendix C). Details of the development and administration of the surveys are in the supplemental methods and materials.
Consent was obtained at the beginning of each survey. This study was approved by the Columbia University Medical Center and Sarah Lawrence College institutional review boards.
2.5. Statistical Analysis
Descriptive statistics of the patient sessions and demographics are presented as frequencies and percentages for categorical variables and means, standard deviations, and confidence intervals for continuous variables. Chi-squared tests and two sample t-tests were used to assess differences between the no-video cohort and video cohort for binary and continuous variables, respectively. An intent-to-treat analysis was conducted to maintain the randomization of the video education intervention. Per-protocol analysis is presented in supplemental tables and figures. Analyses were completed in SAS 9.4 [29].
3. RESULTS
3.1. Participants
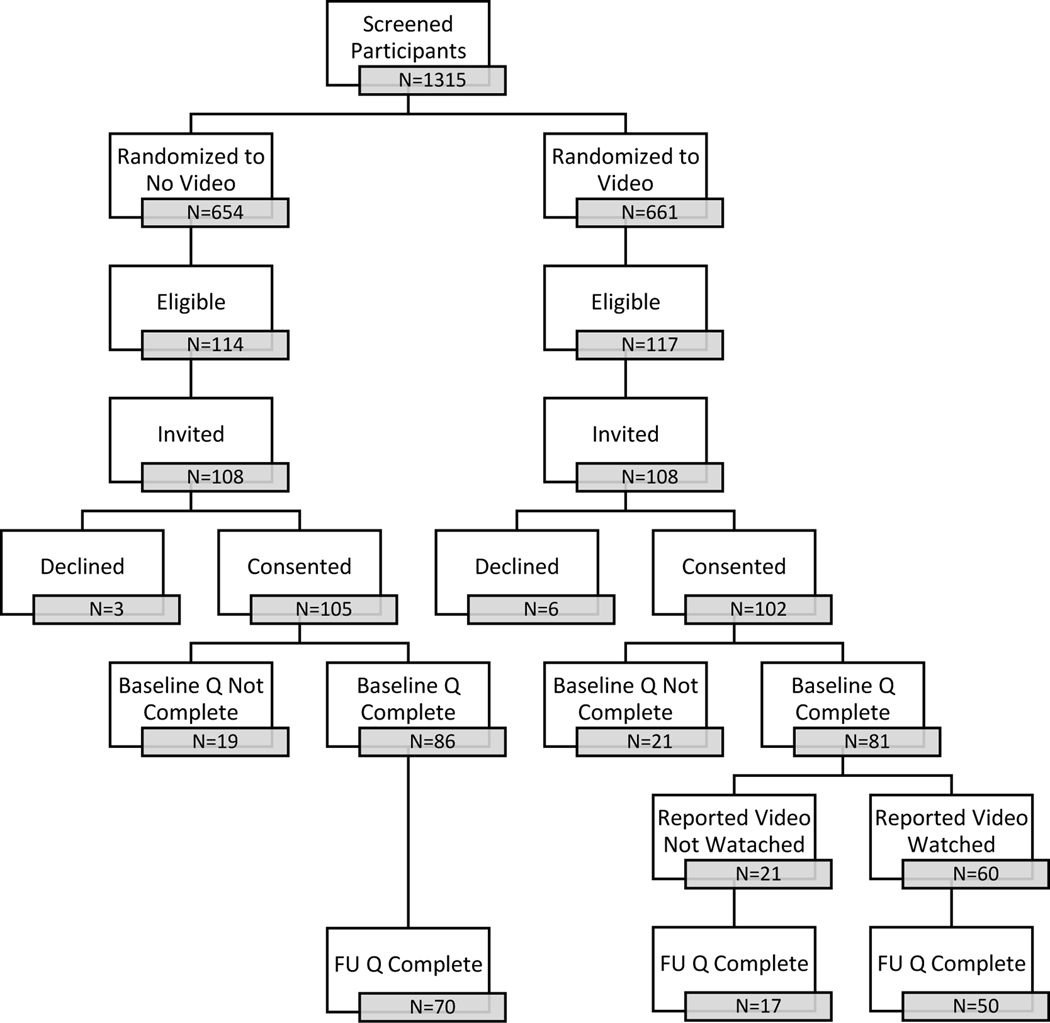
Enrollment and retention of the parent participants are presented in Figure 1. The attrition in the two groups was similar at each step from the original, randomized groups to the final study groups (Table S1). A total of 207 parents consented to the study (105 no-video group, 102 video group) and 167 (81%) completed the baseline survey (86 no-video group, 81 video group). The GC survey was completed for 205 (99%) of the patients (103 no-video group, 102 video group). Sixty of the 81 (74%) parents in the video group who completed the baseline survey reported they watched one or more of the educational videos. Among the remaining 21 parents who reported they did not watch a video, nine did not recall being invited, seven indicated they did not have time, and five indicated that they already knew the information or would have preferred to learn information via website or brochure.
Figure 1.
Flow diagram of study participants. Prospective patients were screened prior to their genetic visit and randomized to be invited or not to watch the videos. Parents of patients who were offered and consented to clinical exome sequencing as part of their standard of care pre-test GC session and could read and speak English were eligible. Fifteen eligible participants were not invited at the request of the healthcare provider. Participants who consented to the study were sent a survey (S) by email or paper, according to their preference. Participants who were randomized to be invited to watch the video reported on the questionnaire whether or not they actually watched the videos. Participants were invited to complete the post-results disclosure survey one month after results were provided.
Four of the 12 GCs each saw between 12–17% of the patients, and the remaining GCs cared for fewer than 10% of the patients. Patients were evenly distributed between GCs with more than five years of experience and those with less than five years of experience. Two physicians treated a majority of the patients (81%) while three additional physicians treated the others. The most common indications for genetic evaluation were developmental delay/intellectual disability (38%) and birth defects/dysmorphic features (24%); however, patients were evaluated for over 15 different medical indications. A majority of patients were male (62%), and the mean age was 8.4 years (Table 1).
Table 1.
Characteristics of baseline survey respondents stratified by intervention group. N=167 (81% of those who initally consented to participate)
| Total | No Video | Video | p-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | Freq | % | Freq | % | ||
| TOTAL | 167 | 86 | 81 | ||||
|
| |||||||
| Patient and Visit Demographics | |||||||
|
| |||||||
| Male | 102 | 61% | 52 | 60% | 50 | 62% | 0.87 |
| Ageb | 8.4 | 6.7 | 8.7 | 7.1 | 8.0 | 6.3 | 0.46 |
| Indication | 0.57 | ||||||
| Developmental Delay/ Intellectual Disability | 46 | 28% | 23 | 27% | 23 | 28% | |
| Birth Defects/ Dysmorphic | 30 | 18% | 12 | 14% | 18 | 22% | |
| Seizures | 14 | 8% | 6 | 7% | 8 | 10% | |
| Autism | 41 | 25% | 25 | 29% | 16 | 20% | |
| Dermatology | 8 | 5% | 4 | 5% | 4 | 5% | |
| Otherc | 28 | 17% | 16 | 19% | 12 | 15% | |
| Initial visit | 110 | 66% | 59 | 69% | 51 | 63% | 0.47 |
| Follow up visit | 57 | 34% | 27 | 31% | 30 | 37% | |
| Counselor | |||||||
| 1 | 19 | 11% | 8 | 9% | 11 | 14% | |
| 2 | 7 | 4% | 3 | 3% | 4 | 5% | |
| 3 | 4 | 2% | 2 | 2% | 2 | 2% | |
| 4 | 16 | 10% | 10 | 12% | 6 | 7% | |
| 5 | 6 | 4% | 3 | 3% | 3 | 4% | |
| 6 | 10 | 6% | 5 | 6% | 5 | 6% | |
| 7 | 20 | 12% | 10 | 12% | 10 | 12% | |
| 8 | 38 | 23% | 24 | 28% | 14 | 17% | |
| 9 | 19 | 11% | 9 | 10% | 10 | 12% | |
| 10 | 10 | 6% | 4 | 5% | 6 | 7% | |
| 11 | 8 | 5% | 3 | 3% | 5 | 6% | |
| 12 | 6 | 4% | 3 | 3% | 3 | 4% | |
| 13 | 4 | 2% | 2 | 2% | 2 | 2% | |
| GC < 5yr | 99 | 59% | 54 | 63% | 45 | 56% | 0.34 |
| GC > 5yr | 68 | 41% | 32 | 37% | 36 | 44% | |
| Physician | 0.75 | ||||||
| 1 | 60 | 36% | 31 | 36% | 29 | 36% | |
| 2 | 14 | 8% | 6 | 7% | 8 | 10% | |
| 3 | 4 | 2% | 2 | 2% | 2 | 2% | |
| 4 | 17 | 10% | 11 | 13% | 6 | 7% | |
| 5 | 72 | 43% | 36 | 42% | 36 | 44% | |
| CES Complete | 157 | 94% | 79 | 92% | 78 | 96% | 0.22 |
| CES Result (n=157) | |||||||
| Pathogenic (P) or Likely Pathogenic (LP) variant | 43 | 27% | 21 | 26% | 22 | 28% | 0.92 |
| Variant of Uncertain Signficance (VUS) | 25 | 16% | 12 | 15% | 13 | 17% | |
| No P/LP variant or VUS | 89 | 57% | 46 | 57% | 43 | 55% | |
| GC survey completion (days) b | 5.7 | 7.0 | 5.1 | 5.3 | 6.3 | 8.3 | 0.31 |
| Male | 34 | 20% | 16 | 19% | 18 | 22% | 0.56 |
| Female | 132 | 79% | 70 | 81% | 62 | 77% | |
| Race | 0.50 | ||||||
| White, Non-Hispanic | 92 | 55% | 49 | 57% | 43 | 53% | |
| All other races | 73 | 44% | 35 | 41% | 38 | 47% | |
| NR | 2 | 1% | 2 | 2% | 0 | 0% | |
| Education | 0.71 | ||||||
| < College | 49 | 29% | 24 | 28% | 25 | 31% | |
| ≥ College | 117 | 70% | 61 | 71% | 56 | 69% | |
| NR | 1 | 1% | 1 | 1% | 0 | 0% | |
| Age (n=142)b | 40.5 | 8.5 | 40.8 | 8.9 | 40.3 | 8.2 | 0.73 |
| Employment | 0.50 | ||||||
| Employed | 107 | 64% | 53 | 62% | 54 | 67% | |
| Unemployed, homemaker, retired, disabled | 60 | 36% | 33 | 38% | 27 | 33% | |
| NR | 0 | 0% | 0 | 0% | |||
| Not Married | 40 | 24% | 24 | 28% | 16 | 20% | 0.20 |
| Married | 126 | 75% | 61 | 71% | 65 | 80% | |
| NR | 1 | 1% | 1 | 1% | 0 | 0% | |
| Depression, PHQ-9 | 21 | 13% | 11 | 13% | 10 | 12% | 0.96 |
| NM | 3 | 2% | 1 | 1% | 2 | 2% | |
| Anxiety, GAD-7 | 20 | 12% | 14 | 16% | 6 | 7% | 0.07 |
| NM | 5 | 3% | 3 | 3% | 2 | 2% | |
| Time from appointment to survey (days) b | 22.8 | 34.0 | 20.6 | 37.2 | 25.2 | 30.5 | 0.38 |
| Time from results disclosure to FU survey (days) b | 68.0 | 49.2 | 70.6 | 30.4 | 65.5 | 34.0 | 0.56 |
Abbreviations: not reported (NR), not measured (NM), follow up (FU), personal health questionnaire 9-item (PHQ-9), generalized anxiety disorder 7-item (GAD-7)
p-value calculated by chi square or Fisher exact analysis for categorical variables and two sample t-test for continuous variables
mean and standard deviation
hearing loss, ophthalmological, myopathy, skeletal dysplasia, connective tissue disorder, metabolic
Of the 167 parents who completed the parental survey, 55% were white, non-Latino/a, 70% college educated, 79% mothers; mean age was 41. The no-video and video groups did not differ in parent demographics or visit characteristics (Table 1). For the 81 parents in the video group who reported whether they watched the videos, demographic characteristics did not differ significantly between those who did and did not watch. (Table S2). The average time between a GC session and completion of the parent baseline survey was 23 days; 78% completed it within 30 days. The average time between the GC session and completion of the GC survey was six days; 70% completed it within seven days. The follow-up survey was sent a month after results were disclosed; the average time between results disclosure and completion of the survey was 67 days, with 80% completing it within 90 days of results disclosure.
3.2. Initial Parental Survey
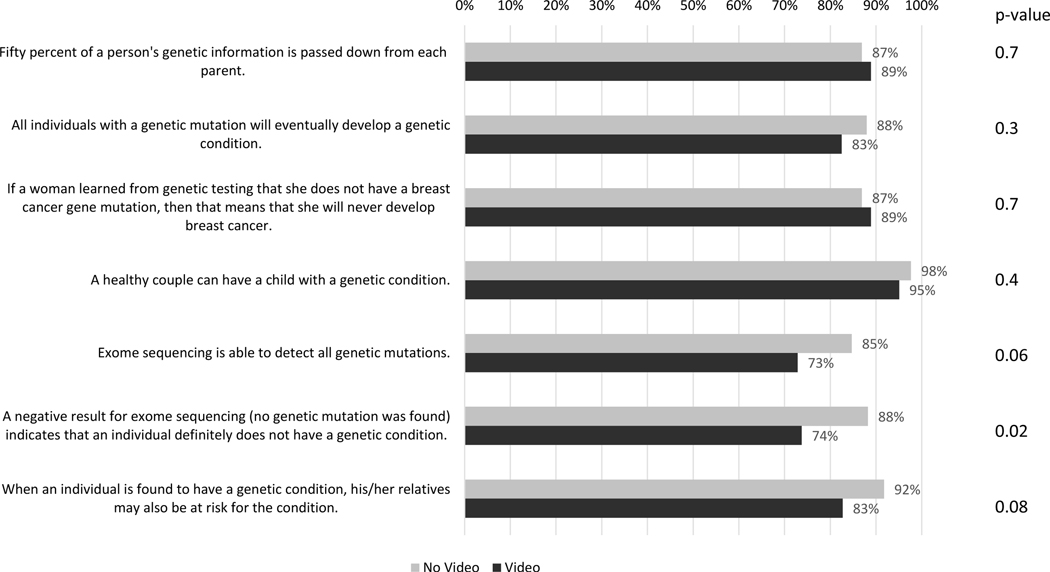
The majority of the parents did well on the genetic knowledge quiz (GKQ) with a mean score of 6.1 correct answers out of 7, SD 1.2 (95% CI 5.9–6.3). Parents assigned to the video group performed slightly worse on the GKQ (mean 5.9, SD 1.3 (95% CI 5.6–6.1)) than those assigned to the no-video group (mean 6.4, SD 1.0 (95% CI 5 6.1–6.9)) (p value 0.007). The difference in performance was most pronounced for questions about CES, including the question regarding the meaning of a negative result (% correct: video 74%, no-video 88%, p-value 0.02) (Figure 2). These differences were also present in the per-protocol analysis (Figure S1). Parents in both groups had a high level of satisfaction with the GC sessions (mean 6.9, SD 3.0; scale range 5 – 25 with lower scores indicating greater satisfaction), and satisfaction did not differ between the two groups (Table 2). Per-protocol analysis was similar (Table S3).
Figure 2.
Proportion of participants who correctly answered each genetic knowledge question stratified by those who were randomized to not watch the video (n=81) or watch the video (n=80). Mean summed score was 6.4 (95% CI 6.1–6.6; SD 1.0) for the no video group and 5.9 (95% CI 5.6–6.1; SD 1.3) for the video group (p-value 0.007). Intent to treat analysis. Chi squared analysis.
Table 2.
Genetic Counselor Statisfication (GCS), Decision Regret Scale (DRS), ammended Multidimensional Impact of Cancer Risk Assessment (MICRA) total score and domain scores stratifed by video intervention group. The maximum range of the scale and interpretation. Intent to treat analysis. Two sample t-test.
| N | Mean | 95% CI L | 95% CI L | SD | p-value | Range | Interpretation | Cronbach α | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| GCS | 5–25 | Lower = More Satisfaction | 0.89 | ||||||
| No Video | 83 | 6.7 | 6.1 | 7.3 | 2.7 | 0.40 | |||
| Video | 84 | 7.1 | 6.3 | 7.8 | 3.3 | ||||
|
| |||||||||
| DRS | 5–25 | Lower = More Satisfaction | 0.72 | ||||||
| No Video | 65 | 7.9 | 7.3 | 8.5 | 2.4 | 0.29 | |||
| Video | 62 | 8.4 | 7.7 | 9.1 | 2.6 | ||||
|
| |||||||||
| MICRA | 0–95 | Lower = Less Impact | 0.88 | ||||||
| No Video | 60 | 25.6 | 21.0 | 30.3 | 18.0 | 0.14 | |||
| Video | 62 | 21.2 | 17.5 | 24.9 | 14.5 | ||||
|
| |||||||||
| MICRA Uncertain | 0–30 | Lower = Less Uncertainty | |||||||
| No Video | 64 | 11.0 | 8.8 | 13.2 | 8.9 | 0.07 | |||
| Video | 64 | 8.3 | 6.5 | 10.1 | 7.2 | ||||
|
| |||||||||
| MICRA Positive | 0–25 | Lower = More Positivity | |||||||
| No Video | 66 | 6.7 | 5.5 | 7.9 | 4.8 | 0.80 | |||
| Video | 65 | 6.9 | 5.6 | 8.2 | 5.3 | ||||
|
| |||||||||
| MICRA Distress | 0–40 | Lower = Less Distress | |||||||
| No Video | 68 | 7.7 | 5.8 | 9.5 | 7.7 | 0.17 | |||
| Video | 65 | 6.0 | 4.4 | 7.5 | 6.4 | ||||
Abbreviations: confidence interval (CI), lower (L), upper (U), standard deviation (SD)
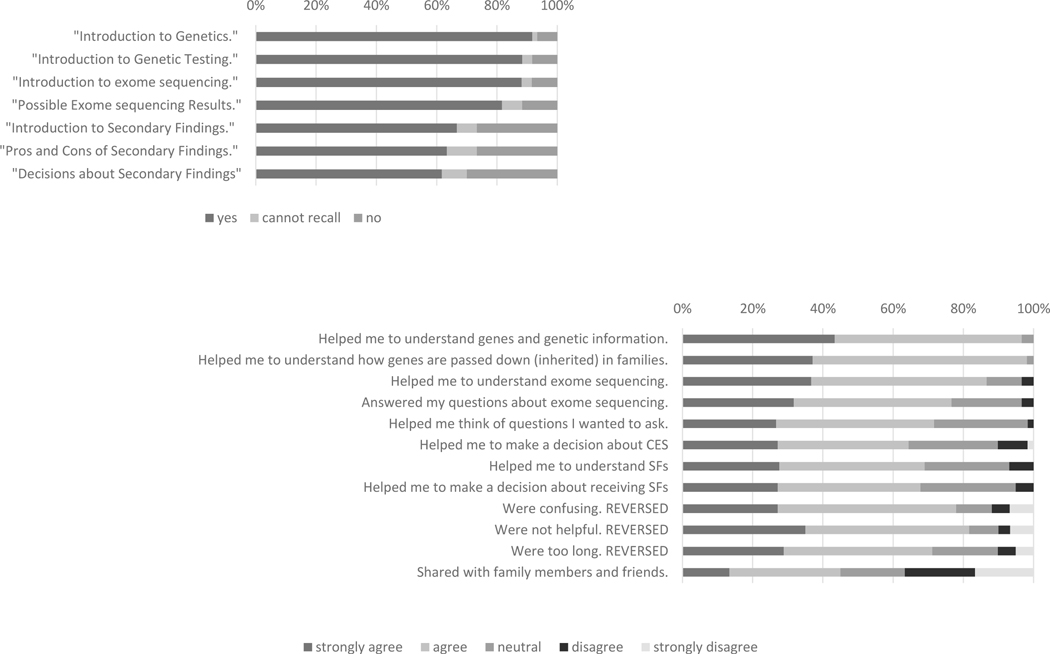
The 60 parents who reported watching at least one video most often watched the videos in advance of their child’s appointment (n=49), as opposed to in the waiting room (n=10) or after the appointment (n=4). Four parents reported they viewed the videos at all three time points and one viewed the videos only after the session. The introductory video was the most commonly watched video, with a decreasing number of reported views as the videos advanced (Figure 3a). The majority of the reviews of the videos were positive. Parents reported the videos helped their understanding of genetic concepts and exome sequencing testing. Few parents reported that the videos helped them make a decision about whether to pursue testing for their child (Figure 3b), though four parents said that it affected their decision (all pursued CES) and 11 parents said that it affected the decision to receive SF (all 11 elected to receive SF).
Figure 3.
Reported number of educational videos watched by the participants randomized to the Video intervention (a). Participant reported benefits of the educational videos (restricted to those participants who indicated they watched at least one video) (b).
Abbreviations: whole exome sequencing (WES), secondary findings (SF)
3.3. Parental Post-Results Survey
Parental interpretation of their child’s CES results did not differ between the two groups, with 16% of the parents in the video group and 19% of the parents in the no-video group incorrectly interpreting the results (p-value 0.73). In most cases of misinterpretation, the parent incorrectly interpreted a laboratory reported variant of uncertain significance (VUS) as uncertain when the clinician interpreted and reported to the family the result as benign. Levels of regret regarding the decision to have CES for their child were minimal (mean 8.2, SD2.5; scale range 5–25, with lower scores indicating less regret) and did not differ between the two groups (Table 2). Overall psychological impact of testing and test-related distress, uncertainty and positivity measured by the adapted MICRA, also did not differ between the groups (Table 2). The results of the per-protocol analysis were similar (Table S3)
3.4. GC Survey
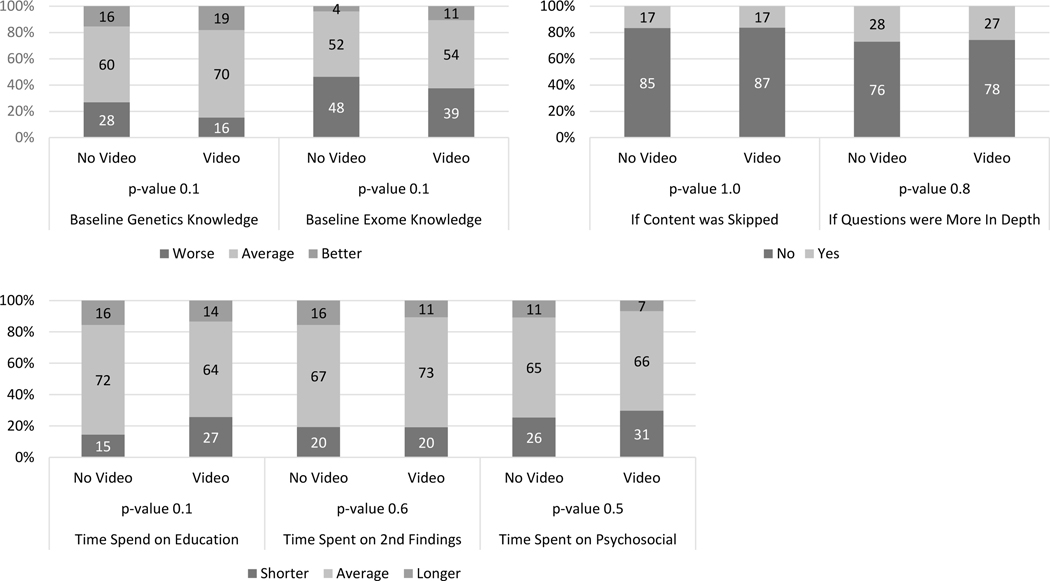
For GCs’ ratings of parents’ baseline understanding of general genetics and CES, GCs reported a higher proportion having “greater-than-average” understanding in the video group than in the no-video group, but the difference was not significant. GC’s ratings of whether parents’ questions regarding CES conveyed a deeper-than-average understanding of the material did not differ between the two groups (Figure 4a). Additionally, GCs seldom reported skipping any parts of genetics education within the session. GCs were asked about allocation of time within a session (Figure 4b) and their reports of “less-than-average” time spent on genetic education did not differ significantly between the video and no-video groups (Figure 4c). The GC-reported time spent on education about SF and counseling for psychosocial issues did not differ between the two groups (Figure 4c). The per-protocol analysis produced similar results (Figure S2a-c).
Figure 4.
Genetic counselors’ assessment of the parents’ knowledge and session content stratified by intervention group. Intent to treat analysis. (a) Genetic counselors’ assessment of parents’ genetic and exome knowledge compared to a typical patient. (b) Genetic counselors’ report of skipping session content and the depth of parental questions. (c) Genetic counselors’ estimate of time spent on different aspects of the session. Chi-squared test.
4. DISCUSSION AND CONCLUSION
4.1. Discussion
Historically, genetic counseling has been a time-intensive, in-person process [30,31]. As testing has become more accessible and applicable to many and varied medical specialties, the field has grown, and the need for innovative service delivery models has become apparent [32]. As education is a large part of the GC session, educational tools have been created to facilitate better understanding and more efficient and effective learning for patients and their families. These tools have often been handouts and visual aids, however, the use of video is not widely employed. Videos provide alternative modalities of learning for patients and can be more accessible in the digital era [28,33,34].
Similar to prior studies of alternative education platforms, the participants in the video group reported the videos helped them to understand genetics and specific genetic concepts [35,36]. Participants in the two groups did not differ in satisfaction with their GC, regret regarding the decision to have CES for their child, or the amount of test-related distress, uncertainty or positivity. Other studies have also demonstrated no impact on these factors and, in one case, participants who received the tool reported more positive genetic counseling experiences [22,27]. These findings continue to suggest that video intervention does not have a negative impact on the GC session and is beneficial for some. Somewhat different from prior studies, in the blinded GC assessment of the session, there were no significant differences between the video and no-video groups. This differs from other studies that have reported pre-session educational tools resulted in greater focus on psychosocial issues during the subsequent genetic counseling session [20,26]. One explanation may be differences in study design. One of the previously studied tools was an interactive program that required an hour to complete in a cancer genetics clinic. For our study, the videos were short modules (7 videos, each 2–4 minutes long) that could be viewed at home or on a tablet at the visit. Furthermore, in our study the GCs were blinded to patient randomization. The degree of GC un-blinding was not measured, but anecdotally was minimal. GCs were instructed not to ask parents if they watched the video, though parents were not explicitly instructed not to share this information. While this method was used to prevent biases, it is likely that it also prevented modification of the sessions because GCs did not know if the parent had received prior education and could not readily assess parents’ knowledge and tailor the session without un-blinding themselves. Finally, though the time spent on psychosocial issues may not have been different, the depth and quality of the psychosocial counseling, which was not measured, may have differed.
A significant difference in our study compared to prior studies was the poorer performance of the video group on the GKQ while prior studies have found improved or non-inferior genetic knowledge in the intervention group compared to traditional education [21,27,36]. The overall score of the video group was significantly lower as were the scores for the questions about the ability of exome sequencing to detect all mutations and the interpretation of a negative CES result. In an attempt to explain this finding, the authors (JW, RH) carried out a post hoc line-by-line review of the video narration and quiz questions. From this review, we identified potential areas of misunderstanding that may have limited the ability of the quiz to measure the impact of the videos. For example, we stated in the videos “Now we have the ability to look at almost all of our 20,000 genes all at one time using powerful computers. This is called exome sequencing or whole exome sequencing,” and included a quiz question: “Exome sequencing is able to detect all genetic mutations” (correct answer: false). In this example, the video does not explicitly state the answer to the quiz question but rather requires the participant to infer it from the information provided (i.e., use of the word “almost”). This poor design of the educational video on this one point may explain the differences in the GC surveys and the parent surveys. The most frequent incorrect answers on the GKQ were in the more complicated questions about CES. Baseline genetic knowledge as assessed by GCs may have been more general and may not have addressed more nuanced details. Therefore, it is possible that GCs correctly assessed the parents in the video group as having similar general genetics knowledge while the more nuanced details for CES were not assessed by the GCs. Future educational tools may be more effective if they explicitly state the concluding message. For example, we should have made the important point in the video that exome sequencing is NOT able to detect all genetic mutations and therefore a negative result does not exclude the possibility of a genetic diagnosis. Follow up interviews with both parents and GCs would be helpful to improve future versions of the videos. Collaboration with medical education experts and more extensive testing with the target population will also help to ensure that educational tools iteratively improve and adequately deliver the desired content. Finally, studies that collect baseline knowledge, as many prior studies have done, provide a better assessment of if and how an intervention changes knowledge.
Our randomized design is both a strength and a limitation of the study. Randomization reduces potential known (e.g., parental education) and unknown biases of the measured relationship between the intervention (video) and the outcomes (surveys). However, biases are introduced when there is non-random compliance with the intervention; there was 26% non-compliance (n=21) in the video cohort. In this analysis, we attributed differences between the two groups to the act of watching the videos as opposed to the act of asking parents to watch a video; however, our design does not allow us to make this distinction. Parents self-reported if and when they watched the videos. Additionally, the timing of watching the video (before coming to the appointment, in the waiting room, or after the appointment) varied and may have affected the outcomes. We presented the intent-to-treat (ITT) analysis, the accepted analytic strategy for randomized studies, above. We also provide the per-protocol (PP) analysis in the supplementary documents which showed similar findings to the ITT. The PP analysis needs to be interpreted cautiously, as the two groups being compared no longer reflect the original randomization. Our cohorts may not reflect true randomization as participants were enrolled after randomization to allow the intervention to take place before the clinical visit; which is when eligibility was determined. However, the proportions eligible were the same in the video and no-video groups, and the proportions of those who declined were also the same. Despite these limitations, a strength of our study is the high proportion of families who consented and the low rate of attrition. Additionally, we had almost 100% completion for the GC surveys.
GCs were asked to compare each session to a typical session, something that is dependent on the individual GC’s style and experience. Total time of the GC session and time spent on specific topics was not measured quantitatively, and studies employing these methods compared to the GC report of this study may reveal different findings. Validated measures were used on the parent surveys, but two were tailored to our patient population. For the GCSS, the question “I felt better about my health after meeting with my genetic counselor” was removed and for the MICRA, references to cancer were changed to “genetic test.” These changes may have altered the validity of the scales, though the Cronbach’s alphas for our scales were similar to those published in previous validation studies. The study was restricted to parents who were able to read English. Parents may have had higher levels of education than the average patient, and a higher baseline knowledge of genetics and genetic testing. Finally, both the GC and parent surveys rely on self-reported measures, and responses could have been affected by recall bias and variation of time from survey invitation to completion.
4.2. Conclusion
The further development of effective and efficient alternative genomic education tools is critical to meet the growing demands for genomics education. However, creating tools that provides the necessary education clearly while engaging the patient is challenging. Additionally, there are no standardized measures for the effectiveness of these tools. Our study demonstrates the challenges of developing and assessing novel educational videos for CES.
The findings presented here are based on self-reported data from GCs and parent participants. Qualitative studies of recorded GC sessions could provide additional insight into the impact of pre-test educational tools on the content, length and quality of a session. Future studies are needed to evaluate educational tools in more diverse patient populations, including non-English-speaking patients, adult patients, and patients evaluated for predictive genomic screening. There is a need for standardized outcome measures, evaluating all potential impacts and not just genetic knowledge, to assess the efficacy of the educational tools. Finally, studies supporting the benefits of educational tools for the patient and providers may help to increase GC confidence in these tools and increase overall use which is critical for the success of these tools to improve practice efficiently.
4.3. Practice Implications
Genomic education for CES is complex and can take significant time in a genetic counseling session. Identifying alternative methods of education will help to minimize this burden and free time for other components of the session. Our findings indicate the potential benefits but also the challenges of developing and assessing these tools. Production of these tools is an iterative process, and development of future tools should improve them. Better tools will be developed with more user testing of the instruments with a diverse population and standardized measures that accurately assess their effectiveness. GC training and continued education about the importance of and methods to assess a patient’s baseline knowledge and then adjust education accordingly will also be beneficial. Finally, we must also be aware that one tool will not fit all; different tools may be needed for different patient populations and testing indications, and traditional models may be the best model for certain populations and clinical scenarios.
Supplementary Material
Highlights.
Parents reported that educational videos were helpful in understanding genetics.
Satisfaction with genetic counseling was equivalent for standard care and videos.
Genetic counselors did not report significant differences between the two groups.
Creating effective and efficient educational tools is challenging.
Standardized measures of educational tools need to be developed and validated.
Acknowledgements
We would like to thank the patients who participated in this study and the staff of the Department of Clinical Genetics at Columbia University Medical Center.
We confirm all patient/personal identifiers have been removed or disguised so the patient/person(s) described are not identifiable and cannot be identified through the details of the story.
Funding
This study was funded by NSGC JEMF grant (PI: J. Wynn) and National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873 (PI: P. Appelbaum).
Footnotes
Human Studies and Informed Consent Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and nation) and with the Helsinski Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants in the study.
Animal Studies
No animal studies were carried out by the authors for this article.
Compliance with Ethical Standards Conflict of Interest
Megan T. Cho is currently an employee at National Human Genome Research Institute. The other authors have no conflicts of interest to declare.
References
- [1].Ormond KE, Wheeler MT, Hudgins L, Klein TE, Butte AJ, Altman RB, Ashley EA, Greely HT, Challenges in the clinical application of whole-genome sequencing, Lancet. 375 (2010) 1749–1751. doi: 10.1016/S0140-6736(10)60599-5. [DOI] [PubMed] [Google Scholar]
- [2].Ropers H-H, On the future of genetic risk assessment, J. Community Genet. 3 (2012) 229–236. doi: 10.1007/s12687-012-0092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Seaby EG, Pengelly RJ, Ennis S, Exome sequencing explained: a practical guide to its clinical application, Brief. Funct. Genomics. 15 (2016) 374–384. doi: 10.1093/bfgp/elv054. [DOI] [PubMed] [Google Scholar]
- [4].Need AC, Shashi V, Hitomi Y, Schoch K, V Shianna K, McDonald MT, Meisler MH, Goldstein DB, Clinical application of exome sequencing in undiagnosed genetic conditions., J. Med. Genet. 49 (2012) 353–61. doi: 10.1136/jmedgenet-2012-100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wynn J, Ottman R, Duong J, Wilson AL, Ahimaz P, Martinez J, Rabin R, Rosen E, Webster R, Au C, Cho MT, Egan C, Guzman E, Primiano M, Shaw JE, Sisson R, Klitzman RL, Appelbaum PS, Lichter-Konecki U, Anyane-Yeboa K, Iglesias A, Chung WK, Diagnostic exome sequencing in children: A survey of parental understanding, experience and psychological impact, Clin. Genet. 93 (2018) 1039–1048. doi: 10.1111/cge.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hoskovec JM, Bennett RL, Carey ME, DaVanzo JE, Dougherty M, Hahn SE, LeRoy BS, O’Neal S, Richardson JG, Wicklund CA, Projecting the Supply and Demand for Certified Genetic Counselors: a Workforce Study, J. Genet. Couns. 27 (2018) 16–20. doi: 10.1007/s10897-017-0158-8. [DOI] [PubMed] [Google Scholar]
- [7].Amendola LM, Lautenbach D, Scollon S, Bernhardt B, East K, Everett J, Gilmore MJ, Himes P, Victoria M, Illustrative case studies in the return of exome and genome sequencing results, Per. Med. 12 (2015) 283–295. doi: 10.2217/pme.14.89.Illustrative. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tomlinson AN, Skinner D, Perry DL, Scollon SR, Roche MI, Bernhardt BA, “Not Tied Up Neatly with a Bow”: Professionals’ Challenging Cases in Informed Consent for Genomic Sequencing, J. Genet. Couns. 25 (2016) 62–72. doi: 10.1007/s10897-015-9842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hooker GW, Ormond KE, Sweet K, Biesecker BB, Teaching genomic counseling: Preparing the genetic counseling workforce for the genomic era, J. Genet. Couns. 23 (2014) 445–451. doi: 10.1007/s10897-014-9689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zierhut HA, Shannon KM, Cragun DL, Cohen SA, Elucidating Genetic Counseling Outcomes from the Perspective of Genetic Counselors, J. Genet. Couns. Oct;25 (2016) 993–1001. doi: 10.1007/s10897-015-9930-9. [DOI] [PubMed] [Google Scholar]
- [11].Rutherford S, Zhang X, Atzinger C, Ruschman J, Myers MF, Medical management adherence as an outcome of genetic counseling in a pediatric setting., Genet. Med. 16 (2014) 157–63. doi: 10.1038/gim.2013.90. [DOI] [PubMed] [Google Scholar]
- [12].Austin J, Semaka A, Hadjipavlou G, Conceptualizing genetic counseling as psychotherapy in the era of genomic medicine., J. Genet. Couns. 23 (2014) 903–9. doi: 10.1007/s10897-014-9728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Overby CL, Chung WK, Hripcsak G, Kukafka R, Cancer Genetic Counselor Information Needs for Risk Communication: A Qualitative Evaluation of Interview Transcripts., J. Pers. Med. 3 (2013) 238–250. doi: 10.3390/jpm3030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roter DL, Erby P, Lori H, Larson M, Susan P. Ellington, Lee, Assessing the Oral Literacy Burden in Genetic Counseling Dialogue, Soc. Sci. Med. 65 (2007) 1442–1457. doi: 10.1038/nature13314.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hartmann JE, Veach PM, MacFarlane IM, LeRoy BS, Genetic Counselor Perceptions of Genetic Counseling Session Goals: A Validation Study of the Reciprocal-Engagement Model, J. Genet. Couns. 24 (2015) 225–237. doi: 10.1007/s10897-013-9647-6. [DOI] [PubMed] [Google Scholar]
- [16].Mills R, Haga SB, Genomic counseling: Next generation counseling, J. Genet. Couns. 23 (2014) 689–692. doi: 10.1007/s10897-013-9641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wynn J, Genomic Testing: a Genetic Counselor’s Personal Reflection on Three Years of Consenting and Testing, J. Genet. Couns. Aug;25 (2016) 691–7. doi: 10.1007/s10897-015-9868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Axilbund JE, Hamby LA, Thompson DB, Olsen SJ, Griffin CA, Assessment of the use and feasibility of video to supplement the genetic counseling process: A cancer genetic counseling perspective, J. Genet. Couns. 14 (2005) 235–243. doi: 10.1007/s10897-005-4065-z. [DOI] [PubMed] [Google Scholar]
- [19].Machini K, Douglas J, Braxton A, Tsipis J, Kramer K, Genetic counselors’ views and experiences with the clinical integration of genome sequencing, J. Genet. Couns. 23 (2014) 496–505. doi: 10.1007/s10897-014-9709-4. [DOI] [PubMed] [Google Scholar]
- [20].Green MJ, Peterson SK, Baker MW, Friedman LC, Harper GR, Rubinstein WS, a Peters J, Mauger DT, Use of an educational computer program before genetic counseling for breast cancer susceptibility: effects on duration and content of counseling sessions., Genet. Med. 7 (2005) 221–229. doi: 10.1097/01.GIM.0000159905.13125.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Albada A, van Dulmen S, Ausems MGEM, Bensing JM, A pre-visit website with question prompt sheet for counselees facilitates communication in the first consultation for breast cancer genetic counseling: findings from a randomized controlled trial, Genet. Med. 14 (2012) 535–542. doi: 10.1038/gim.2011.42. [DOI] [PubMed] [Google Scholar]
- [22].Albada A, van Dulmen S, Spreeuwenberg P, Ausems MGEM, Follow-up effects of a tailored pre-counseling website with question prompt in breast cancer genetic counseling, Patient Educ. Couns. 98 (2015) 69–76. doi: 10.1016/j.pec.2014.10.005. [DOI] [PubMed] [Google Scholar]
- [23].Redlinger-Grosse K, Veach PMC, Cohen S, LeRoy BS, MacFarlane IM, Zierhut H, Defining Our Clinical Practice: The Identification of Genetic Counseling Outcomes Utilizing the Reciprocal Engagement Model, J. Genet. Couns. 25 (2016) 239–257. doi: 10.1007/s10897-015-9864-2. [DOI] [PubMed] [Google Scholar]
- [24].Bernhardt BA, Biesecker BB, Mastromarino CL, Goals, Benefits, and Outcomes of Genetic Counseling: Client and Genetic Counselor Assessment, 2000. https://onlinelibrary.wiley.com/doi/pdf/10.1002/1096-8628(20000918)94:3%3C189::AID-AJMG3%3E3.0.CO;2-E (accessed December 7, 2018). [DOI] [PubMed]
- [25].Biesecker BB, Goals of genetic counseling, Clin. Genet. 60 (2001) 323–330. [DOI] [PubMed] [Google Scholar]
- [26].Cull A, Miller H, Porterfield T, Mackay J, Anderson ED, Steel CM, Elton RA, The use of videotaped information in cancer genetic counselling: a randomized evaluation study., Br. J. Cancer. 77 (1998) 830–837. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2149970&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Biesecker BB, Lewis KL, Umstead KL, Johnston JJ, Turbitt E, Fishler KP, Patton JH, Miller IM, Heidlebaugh AR, Biesecker LG, Web Platform vs In-Person Genetic Counselor for Return of Carrier Results From Exome Sequencing, JAMA Intern. Med. 178 (2018) 338–346. doi: 10.1001/jamainternmed.2017.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sanderson SC, Suckiel SA, Zweig M, Bottinger EP, Jabs EW, Richardson LD, Development and preliminary evaluation of an online educational video about whole-genome sequencing for research participants, patients, and the general public., Genet. Med. 18 (2016) 501–12. doi: 10.1038/gim.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].SAS Institute Inc. SAS 9.4 [computer program], (2014). [Google Scholar]
- [30].McPherson E, Zaleski C, Benishek K, McCarty CA, Giampietro PF, Reynolds K, Rasmussen K, Clinical genetics provider real-time workflow study., Genet. Med. 10 (2008) 699–706. doi: 10.1097/GIM.0b013e318182206f. [DOI] [PubMed] [Google Scholar]
- [31].Sukenik-Halevy R, Ludman MD, Ben-Shachar S, Raas-Rothschild A, The time-consuming demands of the practice of medical genetics in the era of advanced genomic testing, Genet. Med. 18 (2016) 372–377. doi: 10.1038/gim.2015.96. [DOI] [PubMed] [Google Scholar]
- [32].Stoll K, Kubendran S, Cohen SA, The past, present and future of service delivery in genetic counseling: Keeping up in the era of precision medicine, Am. J. Med. Genet. Part C Semin. Med. Genet. 178C (2018) 24–37. doi: 10.1002/ajmg.c.31602. [DOI] [PubMed] [Google Scholar]
- [33].Temme R, Gruber A, Johnson M, Read L, Lu Y, McNamara J, Assessment of Parental Understanding of Positive Newborn Screening Results and Carrier Status for Cystic Fibrosis with the use of a Short Educational Video, J. Genet. Couns. 24 (2015) 473–481. doi: 10.1007/s10897-014-9767-7. [DOI] [PubMed] [Google Scholar]
- [34].Björklund U, Marsk A, Levin C, Öhman SG, Audiovisual information affects informed choice and experience of information in antenatal Down syndrome screening – A randomized controlled trial, Patient Educ. Couns. 86 (2012) 390–395. doi: 10.1016/J.PEC.2011.07.004. [DOI] [PubMed] [Google Scholar]
- [35].Green MJ, McInerney AM, Biesecker BB, Fost N, Education about genetic testing for breast cancer susceptibility: Patient preferences for a computer program or genetic counselor, Am. J. Med. Genet. 103 (2001) 24–31. doi: 10.1002/ajmg.1501. [DOI] [PubMed] [Google Scholar]
- [36].Green MJ, Biesecker BB, Mcinerney AM, Mauger D, Fost N, An Interactive Computer Program Can Effectively Educate Patients About Genetic Testing for Breast Cancer Susceptibility, 2001. https://onlinelibrary.wiley.com/doi/pdf/10.1002/ajmg.1500 (accessed November 15, 2018). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.