SUMMARY
New tuberculosis (TB) vaccine candidates in the development pipeline need to be studied in people with HIV, who are at high risk of developing Mycobacterium tuberculosis (Mtb) infection and TB disease and tend to develop less robust vaccine induced immune responses. Many questions in the development of a TB vaccine for people with HIV remain unanswered. To address the gaps in developing TB vaccines for people with HIV, a series of symposia was held that posed framing questions to a panel of international experts. Framing questions specific to developing TB vaccines for people with HIV included: 1) What is the use case or rationale for developing TB vaccines? 2) What is the landscape of TB vaccines? 3) Which vaccine candidates should be prioritized? 4) What are the TB vaccine trial design considerations? 5) What is the role of immunological correlates of protection? and 6) What are the gaps in preclinical models for studying TB vaccines? The international expert panel formulated consensus statements to each of the framing questions, with the intention of informing TB vaccine development and the prioritization of clinical trials for inclusion of people with HIV.
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), was responsible for 1·5 million deaths in 2020 and continues to pose a threat to global health, particularly to those who live in high TB burden nations. The World Health Organisation (WHO) estimated that 9·9 million people developed TB in 2020, 8% of whom were coinfected with HIV (1). Almost 800,000 people with HIV (PWHIV) were diagnosed with TB in 2020 leading to 214,000 deaths (1).
PWHIV have a 15-21-fold higher risk of developing TB disease and succumbing to death compared to their uninfected counterparts (1–3). HIV infection results in T-cell immune dysfunction, including in the lung (4–6). Although the risk of TB in PWHIV may be substantially reduced by antiretroviral therapy (ART) and TB preventive treatment (TPT) (7, 8), ART does not fully reconstitute HIV-induced immune suppression, which may compromise immune-dependent TB clearance (9).
Developing TB vaccines for people with HIV
A comprehensive roadmap including short and long term goals for TB vaccine research and development (Global Roadmap for Research and Development of Tuberculosis Vaccines) was recently developed by the Amsterdam Institute for Global Health & Development in cooperation with the European & Developing Countries Clinical Trials Partnership, but it does not specifically address TB vaccines in PWHIV (10). We therefore convened an international panel of experts to make strategic recommendations to address key gaps and priorities in the development of TB vaccines for PWHIV with respect to 1) basic and translational studies, 2) pre-clinical models, 3) vaccine candidate selection, and 4) clinical trial design considerations.
TB vaccines
Bacillus Calmette-Guérin (BCG)
BCG, a live attenuated vaccine first used in 1921, remains the only vaccine for the prevention of TB. BCG is effective in preventing severe forms of TB in children, particularly TB meningitis and miliary TB, and in 2004 the WHO recommended a single dose of BCG be given to infants at birth in high TB burden countries. In 2007, WHO provided additional guidance that infants and children with HIV not on ART should not be given BCG due to an increased risk of disseminated BCG disease (11). More recent evidence, however, suggests that HIV-infected infants and children who initiate ART early prior to immunological or clinical progression have a reduced risk of developing BCG-IRIS (immune reconstitution inflammatory syndrome) regional lymphadenitis (12). The WHO SAGE Working Group on BCG vaccination in 2017 therefore recommended that BCG administration can be considered in PWHIV that are clinically well and immunologically stable, especially those living in high burden countries (13).
TB vaccine pipeline
There are 10 vaccine candidates currently in Phase 1-Phase 3 clinical trials and several more in various stages of planning (Figure 1) (14–17). Vaccine candidates in development include live attenuated (n=3), viral vector (n=1), protein subunit (n=4), and whole cell/inactivated (n=2) that may be used for the prevention of infection (POI), prevention of disease (POD), prevention of recurrence (POR), and adjunctively with TB treatment (therapeutic vaccines). So far, no DNA or mRNA-based TB vaccines are being tested in humans, although an mRNA based vaccine is in the planning stages (18).
Figure 1. TB vaccine pipeline in 2021.
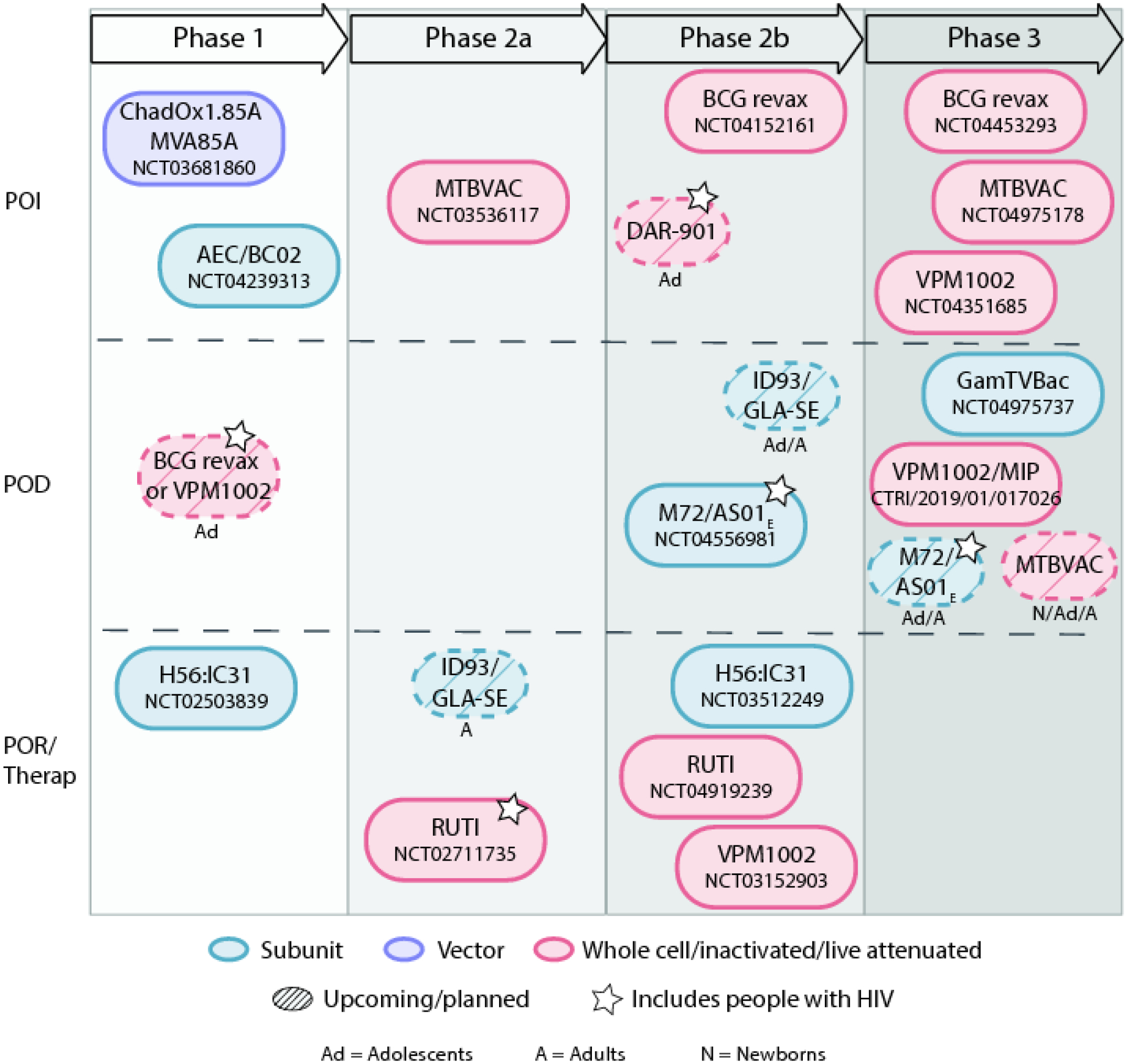
Ongoing trials were identified through clinicaltrials.gov, WHO International Clinical Trials Registry, and Clinical Trials Registry of India. Upcoming or planned trials were identified by references 14–16. Figure adapted from reference 17.
TB vaccine trials in people with HIV
Justification
Due to HIV-associated immunosuppression, TB vaccines in PWHIV may have lower immunogenicity and efficacy (19). PWHIV have historically been excluded from TB vaccine trials to maximize the ability to demonstrate immunogenicity and efficacy. There have been concerns with using live attenuated vaccines, such as BCG, in PWHIV, particularly those not on ART, due to possible dissemination of live bacteria.
Modelling suggests that exclusion of PWHIV from mass POD vaccination campaigns targeting adolescents and adults in high HIV prevalence communities reduces the ability to control TB transmission at a population level (20). As PWHIV are a large subpopulation of persons at high risk of TB infection and disease, it is crucial that TB vaccine trials include them. Additional evidence is required to optimize vaccine safety, immunogenicity and efficacy in PWHIV. Additionally, there is a substantial population of PWHIV who do not know they are seropositive, a majority of whom live in TB endemic regions, and would be recipients of any mass vaccination rollout. It is therefore imperative PWHIV are included in trials of any potential vaccine for widespread use.
Experience
To date, nine completed studies involving six TB vaccine candidates have included PWHIV: two viral vectored (MVA85A, Aeras-402), two subunit (H1:IC31, M72/AS01E), and two whole cell inactivated bacterial vaccines (RUTI, M. obuense) (19, 21–32). Overall, TB vaccines in PWHIV are safe, induce cellular immunity, and have variable durability. Key findings from these trials are summarised in Table 1 and Supplementary Appendix pg 1–2. Several trials are in the planning and development stages, and a subset of these will include PWHIV (Figure 1).
Table 1.
TB vaccine trials conducted in people with HIV.
| MVA85A (TB011) | MVA85A (AERAS-485) | Ad35/85A85BT B10.4 (Aeras-402) | M72/AS01E | M72/AS01E | H1:IC31 | RUTI | M. obuense (DARDAR)1 | M. obuense (DAR901) | |
|---|---|---|---|---|---|---|---|---|---|
| Product type | Viral vector | Viral vector | Viral vector | Subunit/adj | Subunit/adj | Subunit/adj | FCMtb2 | WC inact. | WC inact. |
| Phase | 2a | 2 | 2 | 2 | 1/2 | 2 | 2 | 3 | 1 |
| Participants (n) | |||||||||
| HIV+ | 12 | 136 | 26 | 80 | 48 | 47 | 2000 | ||
| HIV+ ART | 12 | 513 | 80 | 37 | 6 | ||||
| TB+ | 12 | ||||||||
| HIV/TB+ | 12 | ||||||||
| HIV− | 80 | 48 | 53 | ||||||
| Safety | Safe in all | Safe in all | Safe in all | Safe in all | Safe in all | Safe in all | Mild local nodules & abscesses | Safe in all | Safe in all |
| T-cell responses | HIV+ ART similar to uninfected with 85A-specific CD4 durable to 3 years; HIV+ no CD4 durable responses | Mostly monofunctional 85A-specific CD4 and low CD8; No difference between HIV+ ART and HIV+ | Mixed CD4 & CD8 to 85A and 85B, which decreased by 6 months; mostly bi and polyfunctional | HIV+ ART higher M72-specific CD4 than uninfected or HIV+ out to 3 years; mostly polyfunctional; no CD8 detected | M72-specific CD4 peaked one month post 2nd dose but durable to 7 months; mostly polyfunctional; no CD8 detected | H1-specific CD4 peaked one month post 2nd dose but durable to 6 months; mostly bi and polyfunctional; no CD8 detected | Polyantigenic IFN-γ highest with 25 μg; uninfected higher than HIV+ | Polyantigenic IFN-γ and proliferation increased at 2 months post last dose | No difference between uninfected and HIV+ ART |
| Humoral responses | Not measured | Not measured | Binding Ab to 85A and 85B | Binding Ab to M72 peaked one month post 2nd dose but durable to 3 years; uninfected ≈ HIV+ART>HIV+ | Binding Ab to M72 peaked one month post 2nd dose but durable to 7 months | Not measured | Not measured | Binding Ab to lipoarabinomannan increased at 2 months post last dose | No difference between uninfected and HIV+ ART |
| Reference and trial ID | [19,23] NCT00480558 |
[21] NCT01151189 |
[26] NCT01017536/DOH-27-0809-2497 |
[27,28] NCT01262976 |
[32] NCT00707967 |
[31] PACTR201105000289276 |
[22] NCT01136161 |
[25,29] NCT00052195 |
[24] NCT02063555 |
39% efficacy for secondary endpoint of definite TB.
FCMtb = fragmented, detoxified, heat inactivated (FCMtb) and liposomed Mtb
METHODS
In 2019, the U.S. National Institute of Allergy and Infectious Diseases (NIAID) established a Cross-Network TB Vaccine Working Group comprised of members from the AIDS Clinical Trials Group (ACTG), HIV Vaccine Trials Network (HVTN), International Maternal Paediatric Adolescent AIDS Clinical Trials (IMPAACT) Network, and NIAID. The Working Group was tasked to develop consensus statements to help guide the prioritization of candidate vaccines for study in PWHIV. Between January and March 2021, the Cross-Network Working Group convened an international panel of recognized experts in TB and HIV epidemiology, modelling, clinical care, immunology, vaccinology, ethics, community engagement, and regulatory affairs to participate in a virtually held workshop. We also invited members of the TB vaccine working groups from the three networks, opinion leaders, representatives from the global community of HIV and TB, vaccine developers, funders, and other TB research networks. Subject matter experts were identified based on review of published work as well as those known to be working in the field of TB vaccines. Organizers and panellists were tasked with generating consensus statements supporting priorities and pathways for inclusion of PWHIV in trials of novel TB vaccine candidates and strategies. Discussions were framed by six guiding questions (Box 1) developed a priori by the organizing members (GC, AG, JGK) with input from participating experts (see Acknowledgements). A series of presentations by subject matter experts was followed by discussion sessions based on the six framing questions developed by the symposium organizers and experts. The workshop was conducted virtually comprising a total of six sessions (see Supplementary Appendix pg 3–10 for full agenda). A written draft of the summary discussions of the framing questions and consensus statements was developed by a core group (GC, AG, JGK, MDM) and additional comments to the context and consensus statements were then sought by participating subject matter experts.
Box 1. Framing Questions.
What is the use case or rationale for developing TB vaccines for people with HIV?
What is the landscape of TB vaccine candidates for people with HIV?
Which vaccine candidates should be prioritized for study in people with HIV (infants, children, adolescents, and adults)?
What are the trial design considerations of TB vaccine trials that include people with HIV?
What is the role of immunological correlates of protection in people with HIV?
What are the gaps in preclinical models for studying TB vaccines in people with HIV?
RESULTS
Framing questions and consensus statements
For each framing question, the context for each question is provided followed by the consensus statement.
1. What is the use case or rationale for developing TB vaccines for PWHIV?
Context: TB remains the leading cause of morbidity and mortality in PWHIV, and persons with advanced HIV have the highest risk for TB disease (1). Despite ART lowering viral load to undetectable levels and effective TPT reducing the risk of TB, PWHIV remain at significantly higher risk of developing TB and having poorer outcomes than the general population (33, 34). As HIV results in innate and adaptive immune response dysfunction, both safety and immunogenicity findings from studies conducted in persons without HIV cannot be assumed to be replicated in PWHIV. Reduced immunogenicity has been observed in virologically suppressed and unsuppressed persons including those with in utero HIV exposure (35). Therefore, it is imperative to include PWHIV in upcoming vaccine trials to determine potential differences in safety and immunogenicity. Models clearly show the importance of vaccines to reduce TB incidence, but these models require refinement as they have not included all the relevant parameters specific to PWHIV or those exposed (36). As we have seen with SARS-CoV-2, having data from PWHIV in vaccine trials is necessary to make any real-world recommendations for that population. As this population exceeds 20% of some African populations, being able to vaccinate this group has not only local but global ramifications (37). Delaying inclusion of PWHIV in TB vaccine trials results in unnecessary morbidity and mortality.
Consensus statements: There is a higher burden of TB among PWHIV and infants exposed to HIV than the general population. There are also potentially different risk-benefit profiles that must be carefully studied to generate relevant evidence for vaccine strategies among PWHIV across the TB disease and HIV spectrum. The potential individual and population level impact of novel TB vaccines targeting PWHIV should be further modelled. Mathematical modelling should also be used to develop a target product profile for TB vaccines for PWHIV and particular sub-populations (e.g., by CD4 T-cell count, age group, and TPT and ART history), and to estimate cost effectiveness and budget impact.
2. What is the landscape of TB vaccine candidates for people with HIV?
Context: A variety of TB vaccines are being tested in early and late phase clinical trials. However, landscape assessments to date have not focused specifically on PWHIV. Certain vaccine candidates, such as live or vectored vaccines, need special assessment of safety profiles in PWHIV. Including PWHIV beginning early in clinical development avoids unnecessary delays for this population accessing vaccine products. All vaccine approaches, including POI, POD, POR and therapeutic vaccines, should include PWHIV given their higher TB incidence, higher recurrence, and poorer treatment outcomes. PWHIV can be categorized by age group into adults/adolescents and infants/children and the strategies for TB vaccines may differ for each population. As most adolescents/adults living with HIV in TB endemic countries will have received BCG at birth, a new vaccine would be considered a booster to the BCG ‘prime.’ For example, pre-exposure/POI vaccines could target newborns/infants/children as a prime while pre- and post-exposure/POD strategies may be more appropriate for adolescents/adults as a booster strategy in TB endemic countries. According to the WHO preferred product characteristics, a TB vaccine for adolescents/adults should show ≥50% efficacy in preventing confirmed pulmonary TB, protect participants with or without past Mtb infection, and be protective in many geographical regions (38). For infants/children, the efficacy of a pre-exposure TB vaccine should be 80% or higher compared to baseline incidence or superior to BCG with equal or improved safety. Additionally, reduction of injection site swelling, pain, drainage, scarring and local lymphadenopathy would be improvements over BCG. As described earlier, Figure 1 highlights the current TB vaccine pipeline and shows which vaccine candidates are being evaluated in PWHIV.
Consensus statement: Trials of TB vaccine candidates should include PWHIV with careful assessment of safety, immunogenicity and efficacy specific to this group.
3. Which vaccine candidates should be prioritized for study in PWHIV on ART?
Context: As only a fraction of Mtb infected persons goes on to develop clinical disease, there are two critical time points for prevention using vaccines: pre-infection or post-infection. Pre-infection (POI) vaccine strategies are appropriate for use in newborns in endemic settings or slightly older adolescents in lower burden regions. Post-infection vaccine strategies include POD in Mtb infected persons, therapeutic vaccination in those with TB disease to reduce the proportion of TB patients with unfavourable treatment outcomes, and POR in TB patients who have been successfully treated (39). TB vaccine candidates evaluated in PWHIV are summarized in Table 1 and Supplementary Appendix pg 1–2. Viral vectored, subunit protein adjuvanted and whole cell (killed) TB vaccines induce variable humoral and cellular immunity in PWHIV, although responses in ART naïve persons tend to be poorer.
Consensus statement: For adolescents/adults with HIV balancing potential safety, immunogenicity and efficacy, subunit protein/adjuvanted TB vaccines and inactivated mycobacterial vaccines should be prioritised for development in people with HIV, followed by non-replicating viral vectored vaccines. Similarly, for infants/children with HIV, subunit protein/adjuvanted, inactivated and viral vectored vaccines should be evaluated in this population. As live attenuated vaccines are being developed for infants, it will be important to know the safety, immunogenicity, and efficacy of these vaccines in infants with HIV on ART. We encourage the evaluation of immunogenicity and safety of novel live attenuated vaccines early in development, considering possible risks and benefits for each candidate vaccine (in each age group) in people with HIV on ART. Novel vaccine platforms such as mRNA and DNA should be prioritized for evaluation among PWHIV, including infants/children.
4. What are the trial design considerations of TB vaccine trials that include PWHIV?
Including PWHIV in TB vaccine trials raises many important design issues that should be considered. These trial design considerations can be divided into 8 sub-considerations: 1) participant characteristics; 2) standard of care (SOC); 3) eligibility criteria; 4) efficacy endpoints; 5) statistics; 6) ethics; 7) regulatory policies; and 8) community involvement. We have provided context and consensus statements for each sub-consideration below.
4.1: When should PWHIV be included in TB vaccine trials?
Context: PWHIV are at high risk of TB disease and would benefit from participating in TB vaccine trials as soon as safely possible to minimize the time to accessing effective TB vaccines that come to market.
Consensus statement: Among adolescents/adults and infants/children with HIV:
Subunit, viral vectored, inactivated, and novel mRNA or DNA TB vaccines, once developed, may be evaluated in Phase 1b trials, depending on the preclinical safety profile of the candidate vaccine, and then in Phase 2, Phase 3 and post-licensure trials.
BCG and new live attenuated vaccines may be evaluated in Phase 2, Phase 3, and post-licensure trials, depending on CD4 count and viral load and if there is prospect for more benefit than harm. That is, the safety and efficacy signal in PWHIV supports further development.
Pregnant women with HIV on ART:
May be included in Phase 2, Phase 3, and post-licensure trials of subunit, viral vectored, and inactivated vaccines.
Should not be considered for planned trials of BCG and new live attenuated vaccines, as WHO does not recommend BCG for pregnant women.
4.2: What should the SOC be for PWHIV in TB vaccine trials?
Context: An effective TB vaccine for PWHIV would complement existing tools for TB prevention in PWHIV, which includes early disease detection, prompt diagnosis and treatment, infection prevention and control, and TPT.
TPT is the WHO standard of prevention for PWHIV (1). Isoniazid preventive treatment in conjunction with ART is more effective in reducing the risk of TB than ART alone (40). An extended duration of isoniazid TPT was found to be equally effective as short-term rifamycin and isoniazid-based therapy in reducing TB risk in PWHIV (41). As the combined effect of TPT with immune modulation is greater than either intervention alone, it is reasonable to assume that TPT with TB vaccines may have a synergistic effect on reducing the risk of developing TB disease. However, offering TPT to eligible participants with HIV in TB vaccine trials may reduce the apparent effectiveness of TB vaccines. This confounder is not unlike offering pre-exposure prophylaxis (PrEP) to participants in HIV vaccine clinical trials; ethically, it is the right thing to do but does reduce the power to observe potential vaccine efficacy. Thus, next generation HIV vaccine and other preventative trials are being designed to allow for a lower incidence due to PrEP uptake (42).
Consensus statement: All PWHIV participating in TB vaccine trials must be on ART. As WHO recommends TPT as SOC for people with HIV regardless of Mtb infection status, TB vaccine trial participants with HIV (on ART), regardless of age, Mtb infection status, phase of trial (1–3) or mechanism of action (POI, POD, POR), should either previously have completed a course of TPT prior to enrolment or be offered TPT during the study if they previously have not completed a course of TPT and have no evidence of active TB disease. TPT should not be provided in trials of live attenuated TB vaccines, as it may reduce the activity of live attenuated TB vaccines. Persons eligible for TPT who have not previously taken TPT should be advised to complete a course of TPT prior to enrolling in the trial.
4.3: What are the HIV-specific eligibility criteria?
Context: As CD4+ T-cell count and viral load are predictive of developing opportunistic infections, survival, and vaccine responses, these clinical characteristics should be included as eligibility criteria in TB vaccine trials that include participants with HIV. PWHIV receiving ART should therefore only be considered for inclusion in TB vaccine trials if viremia and CD4+ T-cell counts meet pre-specified thresholds.
Consensus statement: Eligibility criteria for people with HIV on ART differ depending on CD4+ T-cell count. Participants with HIV with CD4+ T-cell counts <100 cells/mm3 or HIV RNA >200 copies/mL:
Should be excluded from trials of BCG and live attenuated vaccines
May be included in Phase 1b/2 trials of subunit, viral vectored and inactivated TB vaccines
May be included in Phase 3 trials if vaccines are shown to be safe and immunogenic in Phase 2 trials
Participants with HIV with CD4+ T-cell counts ≥100 cells/mm3 or HIV RNA <200 copies/mL may be included in:
Phase 1b/2 trials of subunit, viral vectored, and inactivated TB vaccines
Phase 2 trials of live attenuated TB vaccines
Phase 3 trials of subunit, viral vectored, inactivated, and live attenuated TB vaccines, if shown to be safe and immunogenic in Phase 2 trials.
4.4: What are the HIV-specific efficacy endpoints for PWHIV?
Context: TB among PWHIV is often paucibacillary, extrapulmonary or subclinical, particularly among those with marked immunosuppression (43, 44). POI vaccine trials in infants, uninfected adolescents or adults evaluate Mtb infection as the endpoint. The gold standard diagnostic for Mtb infection is the interferon gamma release assay (IGRA), which measures cytokine production from Mtb antigen stimulated blood cells. Also, it has been shown that higher IGRA levels or sustained conversion predicts a greater risk of TB disease progression. Whether this holds true for PWHIV is currently unknown, as is how accurate IGRA is in this population. POD vaccine trials typically evaluate clinical bacteriologically confirmed pulmonary TB disease as a highly specific endpoint using solid and liquid culture methods and nucleic acid amplification assays.
Subclinical TB occurs frequently in PWHIV and may have a role in Mtb transmission. A benefit of including subclinical TB as an endpoint in POD/POR/therapeutic vaccine studies is that it may decrease the sample size and reduce the duration of follow-up, as subclinical TB would contribute to the number of endpoints and occurs earlier than clinical TB disease. The decrease in sample size, however, assumes that the vaccine will be equally efficacious at preventing clinical and subclinical TB. It’s unclear whether prevention of subclinical TB should be a priority for POD, POR and therapeutic TB vaccines for the following reasons: preventing subclinical TB would be a higher bar for the vaccine to achieve; the evidence that subclinical TB substantially contributes to TB transmission is still circumstantial; identifying and treating subclinical TB disease may compromise the ability to show efficacy against clinical TB.
Both POR and therapeutic TB vaccine trials evaluate clinical bacteriologically confirmed recurrent pulmonary TB disease as a highly specific endpoint using solid or liquid sputum culture; therapeutic trials additionally consider treatment failure and TB-related deaths as unfavourable outcomes in a trial. Isolates of Mtb should undergo whole genome sequencing to characterize recurrent TB as relapse or reinfection TB.
Consensus statement: Efficacy endpoints for participants with HIV overall should be the same as for people without HIV in POI, POD, POR and therapeutic TB vaccine trials. As paucibacillary, extrapulmonary or subclinical TB occurs more commonly in PWHIV, consideration should be given to also include these as endpoints in TB vaccine trials among PWHIV. So as not to compromise evaluation of efficacy in preventing clinical (symptomatic) TB disease, subclinical TB should ideally only be assessed at the end of follow-up. As sustained Mtb infection is used as an endpoint in POI trials, the risk of TB among PWHIV with sustained TB infection should be established.
4.5: What are the trial design and statistical considerations?
Context: Statistical considerations for TB vaccine trials involving PWHIV include comparator arms, immune-bridging, and sample size. TPT history, participant preferences and values, and local policy should also be considered when designing POD TB vaccine efficacy trials.
Consensus statements: As a comparator arm, placebo gives the best chance of minimizing bias and is the preferred choice, except in infants for whom BCG is licensed and has shown efficacy. Therefore, a placebo should not be used in BCG-naïve infants who are well controlled on ART; rather, BCG should serve as the SOC comparator. Similarly, the comparator arms for testing safety and efficacy of live attenuated vaccines in older children, adolescents and adults who are well controlled on ART could include BCG revaccination in addition to placebo to enable comparison with BCG if a new vaccine is shown to be efficacious in this age group.
We recommend using immune-bridging studies, which measure participant immune responses to vaccines rather than waiting for efficacy endpoints, for PWHIV if a correlate of protection (CoP) has been identified and PWHIV are not a sufficiently large subgroup in Phase 3 trials to permit precise estimation of efficacy. Even without an established CoP, immunogenicity endpoints will be beneficial.
4.6: What are the ethical considerations?
Context: PWHIV have a more urgent need for TB vaccines than the general population given their significantly higher risks of developing TB disease, drug-drug interactions, and poorer TB treatment outcomes (1–3). Consequently, delays in developing an effective TB vaccine for PWHIV would have greater individual-level consequences than for the general population. Excluding PWHIV from TB vaccine trials would also worsen existing health disparities. The differentially higher burden of TB among PWHIV justifies their inclusion in TB vaccine trials with some degree of greater in-trial risk compared to participants from the general population.
Consensus statement: An equity-oriented research agenda that seeks to reduce disparities between PWHIV and the general population should be adopted. The timing of when to include PWHIV in TB vaccine trials should be based on consideration of risks (safety) versus the need to reduce the “time-to-evidence” for PWHIV.
4.7: What are the regulatory considerations?
Context: In order to increase enrolment of underrepresented populations including PWHIV in later phase clinical trials, sponsors can follow the U.S. FDA Guidance for Industry (45). Sponsors developing a TB vaccine are encouraged to submit an Investigational New Drug Application even if the U.S. market for that vaccine is limited and the primary target population is outside of the U.S. (46, 47). Expedited program designations are available to facilitate development of qualifying TB vaccines for PWHIV (48). TB is on the list of qualifying tropical diseases eligible for a Tropical Disease Priority Review Voucher, which includes TB vaccines developed for PWHIV (49). Furthermore, sponsors can use the European Union-Medicines for all (EU-M4all) procedure, which aims to facilitate prequalification by the WHO and registration by national regulatory authorities by providing a scientific opinion of the benefit-risk balance of the product, as well as the African Vaccine Regulatory Forum.
Consensus statement: Communication with regulatory authorities should occur early and throughout the development process.
4.8: How should community be involved?
Context: In the past few years, HIV vaccine efficacy trial design has been modified to account for volunteer willingness to take PrEP (42, 50). This newer trial design was implemented after extensive community engagement and deliberations with community advisory boards (CABs) and other local leaders (51). This type of creative next-generation trial design can be applied to the TB vaccine field to ensure PWHIV are included safely. Additionally, CABs and other community stakeholders significantly enhance enrolment and retention of participants in clinical trials, especially in underserved populations (52, 53).
Consensus statement: Community stakeholders of PWHIV should be engaged early in the process to ensure best outcomes and to provide input into study design, trial conduct, and results dissemination.
5. What is the role of immunological correlates of protection in PWHIV?
Context: Currently, there are no CoPs accepted by regulatory authorities for TB vaccines. Concerted efforts are being made to analyse immune responses from TB vaccine clinical trials that have shown some measure of efficacy (54, 55). CoPs identified in these trials will require validation in larger Phase 3 or implementation studies. Ultimately, establishing CoPs for specific classes of vaccines could enable immune-bridging of vaccines to more inclusive populations; this could help accelerate licensure and broaden indication for these populations, even if they are not adequately represented in the efficacy trials. One other avenue for TB vaccine trials is the human infection challenge platform, where volunteers are vaccinated and challenged with either BCG or another attenuated mycobacterial strain. Done with strict regulatory and safety oversight, these studies could help down select potential immune correlates and help inform future studies in PWHIV.
A more detailed immunological characterization of PWHIV at baseline may be required, as the quality and quantity of innate and adaptive immune responses of virally suppressed individuals may vary (56–58).
Consensus statement: CoPs and other immunogenicity endpoints identified in PWHIV should be applied to and evaluated in people with HIV using immune-bridging studies. Collection of standardized sets of samples across trials is essential to enable such immune-bridging studies. Immunogenicity trials (Phase 1b/2) should include PWHIV to maximize the chance of identifying a CoP that could enable immune-bridging.
6. What are the gaps in preclinical models for studying TB vaccines in PWHIV?
Context: The nonhuman primate (NHP) model of simian and simian/HIV immunodeficiency virus (SIV/SHIV) infection recapitulates many aspects of HIV acquisition and pathogenesis. As such, it remains a valuable research tool to aid in assessing the immunogenicity and efficacy of candidate TB vaccines to model what happens in PWHIV (59).
These SIV/SHIV NHP models can help tailor preclinical studies to be relevant to PWHIV. Importantly, SIV/SHIV NHP models provide an opportunity to look at possible effects of ART and TPT co-administration; study correlates in an unbiased fashion; and further understand the impact of HIV acquisition on memory immune responses from infant BCG vaccination. This platform would also be ideal for testing new vaccine regimens before doing Phase 1 studies with participants with HIV, although NHP models have not yet been shown to be predictive of protection from TB in humans. The NHP model can also help identify tissue-specific correlates that can then be measured in human trials and subsequently modify the tissue-specific assays to those that can use plasma or sputum samples. The NIH recently funded several centres to focus on preclinical models for identifying vaccine CoP (60).
Consensus statement: It is necessary to invest in NHP SIV/SHIV models (with and without ART) for TB vaccine studies. Novel vaccine platforms, such as mRNA and DNA TB vaccines, should be evaluated in NHP SIV/SHIV models, keeping in mind that NHP models have not yet been validated as predictive of protection from TB in humans.
CONCLUSIONS
We developed consensus statements to accelerate the development of TB vaccines for PWHIV. The consensus statements address a number of strategic questions that make the case for including PWHIV as early as possible in clinical development of TB vaccines and also addresses gaps in preclinical models that may portend challenges in future development of a variety of vaccine candidates. The safety and efficacy of TB vaccines in PWHIV needs to be optimized to maximize individual benefit and population level impact.
Supplementary Material
ACKNOWLEDGEMENTS
The U.S. National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Division of AIDS (DAIDS), Cross-Network TB Vaccine Working Group report on “Developing TB Vaccines for People Living with HIV: consensus statements from an international expert panel” was led and written by Gavin Churchyard (ACTG), James Kublin (HVTN), Amita Gupta (IMPAACT) and Maurine Miner (HVTN), with support from Austin Van Grack (Social & Scientific Systems) under the overall direction of Judith Currier and Joseph Eron (ACTG), Glenda Gray (HVTN), Sharon Nachman (IMPAACT) and Peter Kim and Sarah Read (NIAID DAIDS). The ACTG, HVTN and IMPAACT provided support for developing the consensus statements. Funding was provided by NIH grants UM1 AI 068636, UM1 AI068614, 5UM1AI154463, UM1 AI 068636 (GC); UM1 AI068614-14 (JK); NIH UM1 AI069465 (AG).
The contribution of the following people who participated in various sessions is gratefully acknowledged: Austin Van Grack (DLH Corporation), Abdou Fofana (Boston University), Adrienne Shapiro (University of Washington), Ann Ginsberg (Gates Foundation), Catherine Yen (NIAID Division of AIDS [DAIDS]), Chandler Church (University of Washington), César Boggiano (NIAID DAIDS), Chetan Seshadri (University of Washington), Corey Casper (AAHI), Dale Hu (NIAID DAIDS), Debra Benator (Washington DC Veterans Affairs Medical Center), Deepak Kaushal (Texas BioMedical Research Institute), Dereck Tait (IAVI), Richard Chaisson (Johns Hopkins University), Emily Douglass (Rutgers University), Georgia Tomaras (Duke University Medical Center), Gerald Voss (TuBerculosis Vaccine Initiative), Hans Spiegel (Contractor to NIAID), Justin Shenje (South African Tuberculosis Vaccine Initiative), Katrin Eichelberg (NIAID Division of Microbiology and Infectious Diseases [DMID]), Yasmin Mejia-Guevara (DLH Corporation), Lakshmi Ramachandra (NIH), Lesley de Armas (University of Miami), Mamodikoe Makhene (NIAID DMID), Mark Harrington (Treatment Action Group), Meg Trahey (Fred Hutchinson Cancer Research Center), Michael Saag (University of Alabama at Birmingham), Michael W. Dunne (Gates MRI), Moises Huaman (University of Cincinnati), One Dintwe (Fred Hutchinson Cancer Research Center), Patrick Jean-Philippe (NIAID DAIDS), Payam Nahid (University of California at San Francisco), Que Dang (NIAID DAIDS), Rada Savic (UCSF School of Pharmacy and Medicine), Richard Hafner (NIAID DAIDS), Savita Pahwa (University of Miami), Sai Majji (NIH National Institute of Child Health and Human Development), Simon Mallal (Vanderbilt Therapeutics), Stephen Carpenter (Case Western), Steve De Rosa (Fred Hutchinson Cancer Research Center), Susan Swindells (University of Nebraska), Teri Roberts (Campaign for Access to Essential Medicines), Vicki Godleski (Providence/Boston Center for AIDS Research), and Wolfgang Leitner (NIAID DAIT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors claim no conflicts of interest
SEARCH STRATEGY AND SELECTION CRITERIA
References for this Review were identified through searches of PubMed with the search terms “TB/tuberculosis/Mycobacterium tuberculosis,” “vaccine,” “people with HIV/PWH/PLWH,” “HIV,” and “clinical trial” up until November 1, 2021. We also identified ongoing TB vaccine clinical trials involving people with HIV by searching clinicaltrials.gov, WHO International Clinical Trials Registry, and Clinical Trials Registry of India. Studies related to TB vaccine clinical trials among the general public and people with HIV were included if they were peer-reviewed and written in English. The final reference list was generated on the basis of relevance to this Review.
SUPPLEMENTARY APPENDIX
Summary of TB vaccines evaluated in people with HIV.
Symposium Agenda. Agenda for Developing a TB Vaccine Roadmap for People with HIV.
REFERENCES
- 1.WHO. WHO Global TB Report 2021. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021; 2021.
- 2.Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev. 2011;24(2):351–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell LCK, Noursadeghi M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat Rev Microbiol. 2018;16(2):80–90. [DOI] [PubMed] [Google Scholar]
- 4.Bunjun R, Soares AP, Thawer N, Muller TL, Kiravu A, Ginbot Z, et al. Dysregulation of the Immune Environment in the Airways During HIV Infection. Front Immunol. 2021;12:707355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corleis B, Bucsan AN, Deruaz M, Vrbanac VD, Lisanti-Park AC, Gates SJ, et al. HIV-1 and SIV Infection Are Associated with Early Loss of Lung Interstitial CD4+ T Cells and Dissemination of Pulmonary Tuberculosis. Cell Rep. 2019;26(6):1409–18 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin S, Vodovotz L, Zamora R, Fitzpatrick M, Kessinger C, Kingsley L, et al. Association Between Inflammatory Pathways and Phenotypes of Pulmonary Dysfunction Using Cluster Analysis in Persons Living With HIV and HIV-Uninfected Individuals. J Acquir Immune Defic Syndr. 2020;83(2):189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodd PJ, Knight GM, Lawn SD, Corbett EL, White RG. Predicting the long-term impact of antiretroviral therapy scale-up on population incidence of tuberculosis. PLoS One. 2013;8(9):e75466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Group TAS, Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015;373(9):808–22. [DOI] [PubMed] [Google Scholar]
- 9.Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev. 2013;254(1):326–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EDCTP. Global roadmap for research and development of tuberculosis vaccines. http://www.edctp.org/web/app/uploads/2021/04/EDCTP-TB-vaccine-roadmap-full-version-final.pdf; 2021.
- 11.Nuttall JJ, Eley BS. BCG Vaccination in HIV-Infected Children. Tuberc Res Treat. 2011;2011:712736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabie H, Violari A, Duong T, Madhi SA, Josipovic D, Innes S, et al. Early antiretroviral treatment reduces risk of bacille Calmette-Guerin immune reconstitution adenitis. Int J Tuberc Lung Dis. 2011;15(9):1194–200, i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Report on BCG vaccine use for protection against mycobacterial infections including tuberculosis, leprosy, and other nontuberculous mycobacteria (NTM) infections. https://www.who.int/immunization/sage/meetings/2017/october/1_BCG_report_revised_version_online.pdf; 2017.
- 14.Spanish pharmaceutical company Zendal and IAVI partner to advance the tuberculosis vaccine candidate MTBVAC into efficacy trials [press release]. https://www.iavi.org/news-resources/press-releases/2021/zendal-and-iavi-partner-to-advance-the-tuberculosis-vaccine-candidate-mtbvac-into-efficacy-trials. 2021.
- 15.Suliman S, Pelzer PT, Shaku M, Rozot V, Mendelsohn SC. Meeting report: Virtual Global Forum on Tuberculosis Vaccines, 20–22 April 2021. Vaccine. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treatment Action Group T. Tuberculosis Vaccines 2021 Pipeline Report. https://www.treatmentactiongroup.org/wp-content/uploads/2021/10/2021_pipeline_TB_vaccines_final.pdf; 2021.
- 17.TuBerculosis Vaccine Initiative. Pipeline of vaccines https://www.tbvi.eu/what-we-do/pipeline-of-vaccines/2021 [
- 18.BioNTech Provides Update on Plans to Develop Sustainable Solutions to Address Infectious Diseases on the African Continent [press release]. Mainz, Germany, July 26, 2021. 2021. [Google Scholar]
- 19.Scriba TJ, Tameris M, Smit E, van der Merwe L, Hughes EJ, Kadira B, et al. A phase IIa trial of the new tuberculosis vaccine, MVA85A, in HIV− and/or Mycobacterium tuberculosis-infected adults. Am J Respir Crit Care Med. 2012;185(7):769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris RC, Sumner T, Knight GM, Zhang H, White RG. Potential impact of tuberculosis vaccines in China, South Africa, and India. Sci Transl Med. 2020;12(564). [DOI] [PubMed] [Google Scholar]
- 21.Ndiaye BP, Thienemann F, Ota M, Landry BS, Camara M, Dieye S, et al. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2015;3(3):190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nell AS, D’Lom E, Bouic P, Sabate M, Bosser R, Picas J, et al. Safety, tolerability, and immunogenicity of the novel antituberculous vaccine RUTI: randomized, placebo-controlled phase II clinical trial in patients with latent tuberculosis infection. PLoS One. 2014;9(2):e89612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tameris M, Geldenhuys H, Luabeya AK, Smit E, Hughes JE, Vermaak S, et al. The candidate TB vaccine, MVA85A, induces highly durable Th1 responses. PLoS One. 2014;9(2):e87340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Reyn CF, Lahey T, Arbeit RD, Landry B, Kailani L, Adams LV, et al. Safety and immunogenicity of an inactivated whole cell tuberculosis vaccine booster in adults primed with BCG: A randomized, controlled trial of DAR-901. PLoS One. 2017;12(5):e0175215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Reyn CF, Mtei L, Arbeit RD, Waddell R, Cole B, Mackenzie T, et al. Prevention of tuberculosis in Bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS. 2010;24(5):675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Churchyard GJ, Snowden MA, Hokey D, Dheenadhayalan V, McClain JB, Douoguih M, et al. The safety and immunogenicity of an adenovirus type 35-vectored TB vaccine in HIV-infected, BCG-vaccinated adults with CD4(+) T cell counts >350 cells/mm(3). Vaccine. 2015;33(15):1890–6. [DOI] [PubMed] [Google Scholar]
- 27.Kumarasamy N, Poongulali S, Beulah FE, Akite EJ, Ayuk LN, Bollaerts A, et al. Long-term safety and immunogenicity of the M72/AS01E candidate tuberculosis vaccine in HIV-positive and -negative Indian adults: Results from a phase II randomized controlled trial. Medicine (Baltimore). 2018;97(45):e13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumarasamy N, Poongulali S, Bollaerts A, Moris P, Beulah FE, Ayuk LN, et al. A Randomized, Controlled Safety, and Immunogenicity Trial of the M72/AS01 Candidate Tuberculosis Vaccine in HIV-Positive Indian Adults. Medicine (Baltimore). 2016;95(3):e2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahey T, Arbeit RD, Bakari M, Horsburgh CR, Matee M, Waddell R, et al. Immunogenicity of a protective whole cell mycobacterial vaccine in HIV-infected adults: a phase III study in Tanzania. Vaccine. 2010;28(48):7652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenz N, Schindler T, Kagina BM, Zhang JD, Lukindo T, Mpina M, et al. Antiviral Innate Immune Activation in HIV-Infected Adults Negatively Affects H1/IC31-Induced Vaccine-Specific Memory CD4+ T Cells. Clin Vaccine Immunol. 2015;22(7):688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reither K, Katsoulis L, Beattie T, Gardiner N, Lenz N, Said K, et al. Safety and immunogenicity of H1/IC31(R), an adjuvanted TB subunit vaccine, in HIV-infected adults with CD4+ lymphocyte counts greater than 350 cells/mm3: a phase II, multi-centre, double-blind, randomized, placebo-controlled trial. PLoS One. 2014;9(12):e114602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thacher EG, Cavassini M, Audran R, Thierry AC, Bollaerts A, Cohen J, et al. Safety and immunogenicity of the M72/AS01 candidate tuberculosis vaccine in HIV-infected adults on combination antiretroviral therapy: a phase I/II, randomized trial. AIDS. 2014;28(12):1769–81. [DOI] [PubMed] [Google Scholar]
- 33.Ogyiri L, Lartey M, Ojewale O, Adjei AA, Kwara A, Adanu RM, et al. Effect of HIV infection on TB treatment outcomes and time to mortality in two urban hospitals in Ghana-a retrospective cohort study. Pan Afr Med J. 2019;32:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO. Tuberculosis Fact Sheet https://www.who.int/news-room/fact-sheets/detail/tuberculosis2021 [
- 35.Cranmer LM, Draper HR, Mandalakas AM, Kim S, McSherry G, Krezinski E, et al. High Incidence of Tuberculosis Infection in HIV-exposed Children Exiting an Isoniazid Preventive Therapy Trial. Pediatr Infect Dis J. 2018;37(10):e254–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weerasuriya CK, Clark RA, White RG, Harris RC. New tuberculosis vaccines: advances in clinical development and modelling. J Intern Med. 2020;288(6):661–81. [DOI] [PubMed] [Google Scholar]
- 37.Corey L, Corbett-Detig R, Beyrer C. Expanding Efforts and Support to Respond to the HIV and COVID-19 Intersecting Pandemics. JAMA. 2022;327(13):1227–8. [DOI] [PubMed] [Google Scholar]
- 38.WHO. WHO preferred product characteristics for new tuberculosis vaccines. https://apps.who.int/iris/bitstream/handle/10665/273089/WHO-IVB-18.06-eng.pdf?sequence=1&isAllowed=y; 2018. Contract No.: WHO/IVB/18.06. [DOI] [PubMed]
- 39.Hatherill M, White RG, Hawn TR. Clinical Development of New TB Vaccines: Recent Advances and Next Steps. Front Microbiol. 2019;10:3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross JM, Badje A, Rangaka MX, Walker AS, Shapiro AE, Thomas KK, et al. Isoniazid preventive therapy plus antiretroviral therapy for the prevention of tuberculosis: a systematic review and meta-analysis of individual participant data. Lancet HIV. 2021;8(1):e8–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinson NA, Barnes GL, Moulton LH, Msandiwa R, Hausler H, Ram M, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janes H, Donnell D, Gilbert PB, Brown ER, Nason M. Taking stock of the present and looking ahead: envisioning challenges in the design of future HIV prevention efficacy trials. Lancet HIV. 2019;6(7):e475–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bajema KL, Bassett IV, Coleman SM, Ross D, Freedberg KA, Wald A, et al. Subclinical tuberculosis among adults with HIV: clinical features and outcomes in a South African cohort. BMC Infect Dis. 2019;19(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bock P, Jennings K, Vermaak R, Cox H, Meintjes G, Fatti G, et al. Incidence of Tuberculosis Among HIV-Positive Individuals Initiating Antiretroviral Treatment at Higher CD4 Counts in the HPTN 071 (PopART) Trial in South Africa. J Acquir Immune Defic Syndr. 2018;77(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.FDA. Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrolment Practices, and Trial Designs Silver Spring, MD: FDA; 2020. [Google Scholar]
- 46.FDA. FDA Guidance for Industry: General Principles for the Development of Vaccines to Protect Against Global Infectious Diseases. https://www.fda.gov/media/82306/download2011.
- 47.FDA. FDA Acceptance of Foreign Clinical Studies Not Conducted Under IND: Frequently Asked Questions https://www.fda.gov/media/83209/download2012.
- 48.FDA. FDA Guidance for Industry: Expedited Programs for Serious Conditions – Drugs and Biologics. Silver Spring, MD: FDA; 2014. [Google Scholar]
- 49.FDA. Tropical Disease Priority Review Vouchers Silver Spring, MD: FDA; 2016. [Google Scholar]
- 50.Miner MD, Bekker LG, Kredo T, Bhagwandin N, Corey L, Gray GE. Meeting report: South African Medical Research Council Standard of Care in Clinical Research in Low- And Middle-Income Settings Summit, November 2017. Trials. 2021;22(1):778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dawson L, Garner S, Anude C, Ndebele P, Karuna S, Holt R, et al. Testing the waters: Ethical considerations for including PrEP in a phase IIb HIV vaccine efficacy trial. Clin Trials. 2015;12(4):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Broder GB, Lucas JP, Davis J, Wallace SE, Luthuli N, Baepanye K, et al. Standardized metrics can reveal region-specific opportunities in community engagement to aid recruitment in HIV prevention trials. PLoS One. 2020;15(9):e0239276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andrasik MP, Broder GB, Wallace SE, Chaturvedi R, Michael NL, Bock S, et al. Increasing Black, Indigenous and People of Color participation in clinical trials through community engagement and recruitment goal establishment. PLoS One. 2021;16(10):e0258858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, et al. Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N Engl J Med. 2018;379(2):138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tait DR, Hatherill M, Van Der Meeren O, Ginsberg AM, Van Brakel E, Salaun B, et al. Final Analysis of a Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N Engl J Med. 2019;381(25):2429–39. [DOI] [PubMed] [Google Scholar]
- 56.Nabatanzi R, Bayigga L, Ssinabulya I, Kiragga A, Kambugu A, Olobo J, et al. Low antigen-specific CD4 T-cell immune responses despite normal absolute CD4 counts after long-term antiretroviral therapy an African cohort. Immunol Lett. 2014;162(2 Pt B):264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scully EP, Lockhart A, Garcia-Beltran W, Palmer CD, Musante C, Rosenberg E, et al. Innate immune reconstitution with suppression of HIV-1. JCI Insight. 2016;1(3):e85433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carrasco I, Tarancon-Diez L, Vazquez-Alejo E, Jimenez de Ory S, Sainz T, Apilanez M, et al. Innate and adaptive abnormalities in youth with vertically acquired HIV through a multicentre cohort in Spain. J Int AIDS Soc. 2021;24(10):e25804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ganatra SR, Bucsan AN, Alvarez X, Kumar S, Chatterjee A, Quezada M, et al. Antiretroviral therapy does not reduce tuberculosis reactivation in a tuberculosis-HIV coinfection model. J Clin Invest. 2020;130(10):5171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.NIH awards contracts to advance tuberculosis immunology research [press release]. Bethesda, MD: NIAID; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


