Abstract
The emergence and spread of multidrug-resistant bacteria highlight the need for new antibacterial interventions. A screening of 24 newly synthesized dibenzoxazepines identified a small molecule compound, SW14, with potent inhibitory activity against intracellular multidrug-resistant and fluoroquinolone-resistant strains of S. typhimurium in macrophages and epithelial cells. Moreover, intra-macrophagic Salmonella typhi, Yersinia enterocolitica, and Listeria monocytogenes and methicillin-resistant Staphylococcus aureus are also susceptible to SW14. Overall, our findings suggest that SW14 has a broad-spectrum activity against intracellular bacteria.
The emergence and spread of multidrug-resistant bacteria highlight the need for new antibacterial interventions.
Introduction
Salmonella enterica is a facultative intracellular pathogen, and more than 2600 serotypes have been identified.1,2 According to its pathogenicity, Salmonella enterica is generally divided into typhoid and non-typhoidal Salmonella.1 Typhoidal Salmonella, including S. enterica serovar Typhi and S. enterica serovar Paratyphi, are serotypes that mainly cause typhoid fever in humans.1 Among the types of non-typhoidal Salmonella, S. enterica serovar Typhimurium (hereafter S. typhimurium) is the most common and normally causes gut-restricted food poisoning.3 However, S. typhimurium infections in immunocompromised patients can lead to lethal invasive non-typhoidal salmonellosis (iNTS), which is responsible for approximately 77 500 deaths per year.4 During infection, S. typhimurium can invade both phagocytic and non-phagocytic cells (such as intestinal epithelial tissue) using effectors secreted by the type III secretion system 1 (T3SS-1), which is encoded on Salmonella pathogenicity island 1 (SPI-1).5 After entering the cells, S. typhimurium resides and replicates in Salmonella-containing vacuoles (SCVs) and then moves to the vicinity of the Golgi apparatus, and this process is mediated by the effectors of SPI-2-encoded T3SS-2.6 The host cell membranes and SCV provide a barrier for intracellular S. typhimurium from host extracellular defences and antibiotics with low membrane permeability.7 In addition, the inappropriate use of antibiotics has led to increasing incidences of infections caused by S. typhimurium that is resistant to multiple antibiotics.8 Thus, the WHO has included antibiotic-resistant S. enterica on the list of pathogens in which new antibiotics are greatly needed.9
Previously, we found that the antipsychotic drug loxapine exhibited certain suppressive activity against intracellular S. typhimurium in murine RAW264.7 macrophages.10 Loxapine is a first-generation tricyclic antipsychotic drug with a dibenzoxazepine structure and is mainly used to treat schizophrenia.11 Its mechanism of action involves the antagonism of postsynaptic dopamine D2 receptors (D2R), which is the major drawback of using loxapine as a therapeutic agent for Salmonella infections.11 Thus, the focus of this study was to dissociate the anti-intracellular Salmonella activity from the antipsychotic activity of loxapine by rational design and to screen newly synthesized loxapine derivatives using image-based high-content analysis. Furthermore, we evaluated the activity of the lead compound against intracellular multidrug-resistant (MDR) S. typhimurium, as well as other facultative intracellular bacteria, and examined the antagonistic effect of the compound on D2R.
Results and discussion
Design, synthesis, and anti-intracellular Salmonella activity of loxapine derivatives
The molecular dynamics analysis of loxapine and D2R indicates that the piperazine ring on the loxapine structure forms an ionic bond with aspartic acid 114 on transmembrane helices (TMH) 3 of D2R. Certain interactions were observed between the chlorine atom and serine 194 of TMH 5, as well as between the benzene ring structure and phenylalanine 389 of TMH 6.12 Previously, Liegeois et al. reported that dibenzoxazepine, which possesses a side chain from the aliphatic ring that extends the alkyl group to various lengths, had a sharp drop in dopamine and serotonin receptor binding affinity.13 Accordingly, to reduce the major binding force of the new compound to D2R, we employed a long carbon chain with different lengths, a non-aromatic ring, or an aromatic ring to replace the piperazine ring of loxapine. The synthesis of loxapine derivatives was performed following the procedure reported by Jain et al. with some modifications.14
As shown in Scheme 1, methyl 5-chloro-2-hydroxybenzoate was considered a starting backbone of loxapine (A). For a convenient ring closure of compound A, 2-fluoro-nitrobenzene was chosen as a reagent and coupled with A with an addition–elimination reaction under basic conditions. The nitro group in the resulting product compound C was further reduced to an amino group in the presence of SnCl2 to obtain compound D. Next, the process of ring closure for compound D proceeded under acidic conditions to generate compound E. Finally, the amide group in compound E was converted to formimidoyl chloride by phosphonyl trichloride and subsequently reacted with alkyl amine groups of different lengths or alkyl amines conjugated with versatile rings, including heterorings and aromatic rings. In addition, to facilitate the assessment of the antibacterial activity of the newly synthesized compounds against intracellular S. typhimurium ATCC14028 and the cytotoxicity toward infected murine macrophage RAW264.7 cells, we utilized an image-based high-content analysis (HCA) to simultaneously assess the number of intracellular S. typhimurium on the basis of RFP and CellTracker signals and the number of cells via nuclear staining by 4′,6-diamidino-2-phenylindole (DAPI) (ESI† Fig. S1A).
Scheme 1. General synthetic procedure of SW compounds.
First, we designed and synthesized derivatives SW2, 3, 4, 6, and 14 to investigate whether the compounds that contained alkyl-aliphatic ring substituents connected to the loxapine backbone remain active against intracellular Salmonella. As shown in Table 1, the anti-intracellular Salmonella activity of SW2 and SW3 is close to that of loxapine, with EC50 values of 4.89 μM, 4.34 μM and 5.74 μM, respectively. Furthermore, though the cytotoxicity of SW6 and SW14 was higher than loxapine, they exhibited superior antibacterial activity in inhibiting intracellular Salmonella, resulting in a selectivity ratio (CC50/EC50) of 18.9 and 60.0, respectively. Notably, SW14, which contained the piperazine ring in the side chain, was the most potent, but if the piperazine ring was changed to a morpholine ring (SW4), the antibacterial ability against intracellular Salmonella was greatly attenuated. The data suggested that compared to the ring with the hydrogen acceptor, the ring with a hydrogen donor ability more potently inhibited intracellular Salmonella.
Structures, anti-intracellular S. typhimurium activities and cytotoxicity of loxapine derivatives.

| ||||
|---|---|---|---|---|
| Cpd. | R group | EC50 (μM), p | CC50 (μM), p | Selectivity ratio (CC50/EC50) |
| S. typhimurium ATCC14028 | RAW264.7 | |||
| Loxapine |

|
7.25 ± 1.5 | 107.1 ± 3.1 | 14.8 |
| SW1 |

|
3.65 ± 1.0, * | 34.6 ± 4.4, *** | 9.5 |
| SW2 |

|
4.89 ± 0.3, ns | 26.6 ± 0.4, *** | 5.4 |
| SW3 |

|
4.34 ± 0.3, * | 22.2 ± 0.3, *** | 5.1 |
| SW4 |
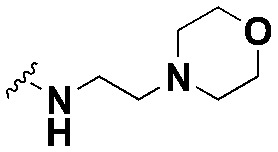
|
31.98 ± 3.2, *** | 84.7 ± 3.4, *** | 2.6 |
| SW5 |
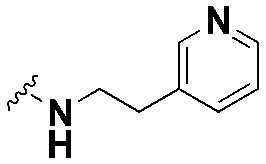
|
38.17 ± 5.6, *** | 74.0 ± 1.0, *** | 1.9 |
| SW6 |

|
1.22 ± 0.5, ** | 23.0 ± 1.5, *** | 18.9 |
| SW7 |

|
21.23 ± 6.1, * | 56.3 ± 1.6, *** | 2.7 |
| SW8 |

|
54.11 ± 5.5, *** | 72.7 ± 3.5, *** | 1.3 |
| SW9 |

|
19.66 ± 3.2, ** | 60.8 ± 1.2, * | 3.1 |
| SW10 |
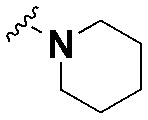
|
ND | ND | — |
| SW11 |
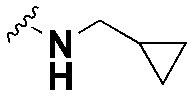
|
44.00 ± 3.7, *** | 80.3 ± 3.6, *** | 1.8 |
| SW12 |

|
33.51 ± 4.7, *** | 52.1 ± 2.0, *** | 1.6 |
| SW13 |
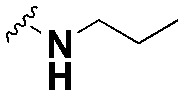
|
55.97 ± 4.5, *** | 111.7 ± 4.2, ns | 2.0 |
| SW14 |

|
0.39 ± 0.03, ** | 23.4 ± 0.2, *** | 60.0 |
| SW15 |

|
31.08 ± 2.0, *** | 82.7 ± 1.8, *** | 2.7 |
| SW16 |

|
18.51 ± 2.1, ** | 51.1 ± 1.4, *** | 2.8 |
| SW17 |

|
28.51 ± 0.7, *** | 72.8 ± 0.9, *** | 2.6 |
| SW18 |

|
55.11 ± 6.1, *** | 70.1 ± 0.2, *** | 1.3 |
| SW19 |

|
45.16 ± 8.19, ** | >90 | 2.0 |
| SW20 |
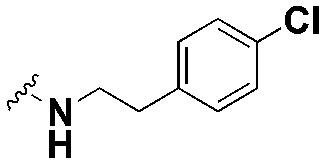
|
53.46 ± 3.2, *** | >90 | 1.7 |
| SW22 |

|
1.12 ± 0.1, ** | 16.3 ± 3.0, *** | 14.6 |
| SW23 |

|
>90 | >90 | — |
| SW24 |

|
>90 | >90 | — |
| SW26 |

|
1.12 ± 0.1, ns | 19.6 ± 0.2, *** | 17.5 |
| AR12 | ND | 7.70 ± 1.66 | — | |
| CIP | 0.15 ± 0.03 | ND | — | |
Next, we sought to investigate the effect of the chain length on the anti-intracellular bacterial activity of loxapine derivatives. We first changed the lateral chain length of SW3 and synthesized SW10 and SW26, which have a piperidine ring that is directly connected to the main backbone or is linked by an alkyl chain with three carbon atoms, respectively. However, due to its poor solubility in DMSO, we were unable to evaluate the antibacterial activity of SW10. In contrast, SW26 exhibited similar cytotoxic effects to SW3, but it was more potent than SW3 against intracellular Salmonella (Table 1). Similar results were observed when comparing SW22 and SW2. As shown, compared to SW2 with a two-carbon lateral chain, SW22 with a pyrrolidine that is connected to the backbone by a three-carbon alkyl chain is more potent in suppressing intracellular Salmonella (Table 1). Moreover, compounds with an amide linkage in the carbon chain or pyrrolidine, SW23 and SW24, respectively, have no detectable activity against intracellular Salmonella (Table 1). Based on our findings, the chain length and the terminal bulky group without an amide linkage play important roles in the antibacterial activity of loxapine derivatives with alkyl–aliphatic ring substituents.
We further synthesized a set of analogues including SW8, 11, 13, 17, and 18, in which alkyl groups with various lengths and bulky sizes were connected to the tricycle fused-ring backbone. The results indicated that these compounds exhibited only a weak suppressive effect on intracellular Salmonella (Table 1). Subsequently, we examined the antibacterial activity of the compounds with substituents of alkyl–aromatic rings, such as SW7 and SW9. Both compounds exhibited a higher potency in inhibiting intracellular Salmonella than those with alkyl substituents (Table 1). However, extending the phenyl ring in SW7 with hydroxyl, chloride, and fluoride groups resulted in SW12, 15, 19, and 20, respectively, which did not exhibit an enhancement in anti-intracellular Salmonella activity (Table 1). Additionally, compounds SW5 and SW16, which replaced the phenyl ring with a pyridine ring, did not exhibit a significant improvement in antibacterial activity compared to that of compounds with a phenyl substituent.
SW14 does not suppress bacterial growth in broth and does not bind to the recombinant dopamine D2 receptor
The above HCA identified SW14 as the most potent loxapine derivative, with an EC50 of 0.39 μM in suppressing intracellular S. typhimurium; however, SW14 caused no obvious toxicity to the host cells (CC50 = 23.4 μM), resulting in a selectivity index (CC50/EC50) nearly three times that of loxapine (Table 1 and ESI† Fig. S1B). To validate the HCA results, we further assessed the effect of SW14 on intracellular S. typhimurium and RAW264.7 cells using the colony-forming unit (CFU) assay and MTT cell viability assay, respectively. As shown in Fig. 1A and B, the viability of intracellular Salmonella and RAW264.7 cells decreased with increasing SW14 concentration, indicating that the suppressive effect of SW14 on intracellular Salmonella and infected cells observed in HCA was not due to interference with RFP and DAPI fluorescence.
Fig. 1. SW14 does not suppress Salmonella growth in broth and does not bind to dopamine D2 receptors. (A) S. typhimurium-infected RAW264.7 cells were treated with various doses of SW14 in the presence of 20 mg L−1 gentamicin for 24 h. Intracellular bacterial survival was assayed using HCA or the CFU assay. The data are expressed as percentages relative to the untreated control and are presented as mean ± SD (n = 3/group). (B) The viability of RAW264.7 cells treated with a range of SW14 was evaluated using HCA or the MTT cell viability assay. The data are expressed as percentages relative to the untreated control and are presented as mean ± SD (n = 3/group). (C) The growth of S. typhimurium in CAMH broth (CAMHB) or cell culture medium (CCM) containing 0, 0.5, or 64 μM SW14 was monitored by measuring the OD600 of each bacterial culture at designated times for a total of 24 h. The data are presented as mean ± SD (n = 3/group). (D) The binding of isotope-labelled spiperone to recombinant D2R in the presence of various concentrations of loxapine or SW14 was assessed. The data are expressed as percentages of inhibition relative to the untreated control and are presented as mean ± SD (n = 3/group).
To determine the mechanism of action of SW14, we first assessed the influence of SW14 on S. typhimurium growth in cation-adjusted Mueller Hinton broth (CAMHB), a rich broth that is often used to evaluate the antibiotic susceptibility of bacteria. As shown in Fig. 1C, we did not observe any significant difference in the growth of bacteria that were treated with SW14 at a concentration up to 64 μM. Next, we further evaluated the growth of S. typhimurium in the cell culture medium (DMEM supplemented with 10% FBS) of RAW264.7 cells. Again, no obvious change in bacterial growth was observed, suggesting that the anti-intracellular bacterial activity of SW14 is not mediated by directly suppressing bacterial growth (Fig. 1C).
As loxapine can bind to D2R and has a certain anti-intracellular Salmonella activity, we then wondered if the antibacterial activity of SW14 occurred through interactions with D2R. To address this possibility, we assessed the competitive inhibitory effect of loxapine and SW14 on the binding of spiperone, a dopamine D2 receptor antagonist, to recombinant D2R. As shown in the results, we found that loxapine suppressed the binding of spiperone to D2R in a dose-dependent manner (Fig. 1D). In contrast, even at a concentration higher than its EC50, SW14 exhibited no detectable repressive effect on the binding of spiperone to D2R against intracellular Salmonella (Fig. 1D). Our findings suggest that the anti-intracellular bacterial activity of SW14 does not function through interactions with D2R.
SW14 suppresses intracellular S. typhimurium in both phagocytic and non-phagocytic cells
Given that Salmonella can invade and proliferate in both macrophages and intestinal epithelial cells when infecting hosts, we sought to evaluate the efficacy of SW14 against intracellular S. typhimurium in other cell lines, including J774.1 murine macrophagic cells, HT-29 human colon epithelial cells, HCT-116 human colon epithelial cells and INT-407 human intestinal epithelial. However, we observed that Salmonella in macrophagic cells was more susceptible to the antibacterial activity of SW14, as the EC50 of SW14 against intracellular Salmonella in RAW264.7 and J774.1 cells was much lower than that in HT-29, HCT-116 and INT-407 cells (0.24 μM and 0.22 μM versus 4.05 μM, 2.47 μM and 3.0 μM; Fig. 1A and 2A–D). Next, we further investigated the activity of SW14 against intracellular Salmonella in THP-1 human monocytic cells with and without phorbol 12-myristate-13-acetate (PMA) treatment, which can induce the differentiation of THP-1 cells into macrophage-like cells. Again, we observed that Salmonella in macrophage-like THP-1 cells (EC50 = 0.22) is more vulnerable to the antibacterial activity of SW14 than that in monocytic THP-1 cells (EC50 = 1.78 μM; Fig. 2E and F). Thus, the findings indicated that SW14 is more active in suppressing Salmonella infection in macrophagic cells.
Fig. 2. SW14 inhibits S. typhimurium infection in both phagocytic and epithelial cells. (A–F) The Salmonella-infected J774.1 cells (A), HT-29 cells (B), HCT116 cells (C), INT-407 cells (D), monocytic THP-1 cells (E), or macrophagic THP-1 cells (F) were treated with different doses of SW14 combined with 20 mg L−1 gentamicin for 24 h. The viability of intracellular S. typhimurium (icSTM) was evaluated using the CFU assay. In addition, the viability of individual cell lines after exposure to SW14 combined with 20 mg L−1 gentamicin for 24 h was evaluated using the MTT cell viability assay. The data are expressed as percentages relative to the untreated control and are presented as mean ± SD (n = 3/group). ns, not significant; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 for the comparison of the anti-intracellular Salmonella activity and cytotoxicity of SW14 in individual cell lines with that of RAW264.87 cells.
SW14 is effective against intracellular antibiotic-resistant S. typhimurium
The emergence and spread of antibiotic-resistant S. typhimurium poses a serious threat to public health worldwide.8 To investigate whether SW14 can overcome the antibiotic resistance of intra-macrophagic Salmonella, we assessed the suppressive effects of SW14 on the infection of an MDR-S. typhimurium isolate, CGC18, in RAW264.7 cells. Although this MDR-S. typhimurium strain is resistant to multiple classes of antibiotics (ESI† Table S1), it remained susceptible to the suppressive activity of SW14 (Fig. 3A). Next, we further investigated the activity of SW14 against another S. typhimurium clinical isolate that is resistant to ciprofloxacin, the first-line antibiotic for salmonellosis (ESI† Table S1).15 Similar to the MDR clinical isolate, the ciprofloxacin-resistant isolate NL10 was also sensitive to the antibacterial activity of SW14 (Fig. 3A). These results suggest that the resistance mechanisms of S. typhimurium toward conventional antibiotics do not provide cross-resistance to SW14.
Fig. 3. SW14 is effective against MDR-S. typhimurium and other pathogenic bacteria in macrophages. (A) RAW264.7 cells were infected with ATCC14028, MDR isolate CGC18 or the ciprofloxacin-resistant isolate NL10 of S. typhimurium and were then treated with ciprofloxacin (CIP; H: 0.125 mg L−1; L: 0.016 mg L−1), rifampicin (RIF; 0.031 mg L−1) or various doses of SW14 combined with 20 mg L−1 gentamicin for 24 h. The relative numbers of intracellular bacteria were determined using the CFU assay, and the results are expressed as percentages relative to the untreated control. The data are presented as mean ± SD (n = 3/group). ns, non-significant; ***, P < 0.001; ****, P < 0.0001. (B–E) RAW264.7 cells were infected with S. typhi (B), Y. enterocolitica (C), L. monocytogenes (D), or MRSA USA300 (E) and then treated with SW14 at concentrations ranging from 0–1 μM for 24 h. Then, the number of viable intracellular bacteria was assessed using the CFU assay. The data are presented as mean ± SD (n = 3/group). ns, not significant; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
SW14 exerts broad-spectrum activity against other pathogenic bacteria in macrophages
In addition to S. typhimurium, many other pathogenic bacteria can also inhabit and proliferate inside macrophages.16 To determine whether SW14 can inhibit the infection of other pathogenic bacteria in macrophages, we infected RAW264.7 cells with S. typhi, Yersinia enterocolitica, Listeria monocytogenes, and MRSA USA300, treated the infected cells with various doses of SW14 for 24 h and measured the number of intracellular bacteria using a CFU assay. As shown in the results, SW14 reduced the bacterial burden in the macrophages that were infected by either pathogenic bacterium, of which S. typhi exerted the highest susceptibility to SW14 compared with the other three bacteria (Fig. 3B–E). Thus, SW14 is a broad-spectrum antibacterial agent that effectively against different pathogenic bacteria in macrophages.
Conclusions
Due to the misuse of antibiotics, antibiotic-resistant Salmonella typhimurium strains are spreading more and are increasingly prevalent. In contrast, the supply of newly developed or discovered antibiotics is shrinking.17 Therefore, we urgently need a new therapeutic strategy for controlling Salmonella infections without promoting antibiotic resistance. Here, we provide a proof of concept that the antibacterial activity of loxapine can be disassociated from its antipsychotic activity, and our efforts led to the discovery of a novel antibacterial agent, SW14. SW14 exerted potent antibacterial activity against intracellular S. typhimurium, including those resistant to multiple antibiotics, but had no suppressive effect on bacterial growth in the medium. This finding suggested that SW14 might act via a host-targeted or virulence-targeted mechanism and may be used in combination with conventional antibiotics. Antibacterial agents that target host or bacterial virulence factors are considered to exert a less selective pressure on bacteria and are less likely to induce resistance.18,19 As SW14 also exhibits suppressive activity against other intracellular bacteria, its structure represents a promising scaffold for developing a new type of antibacterial drug.
Author contributions
HCL and YLW contributed equally to this work. YLW and MYL designed and synthesized all the compounds. HCL, CYH and LHC performed the biological studies. CWS and HCC conceptualized the work, designed the experiments, and supervised the whole project. The manuscript was written through the contributions of all the authors. All the authors have given approval to the final version of the manuscript.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the Ministry of Science and Technology, Taiwan (grant numbers: MOST 110-2628-B-002-038), and National Taiwan University (grant number: 111L7827).
Electronic supplementary information (ESI) available: Fig. S1, Table S1, experimental, structures and 1H NMR, 13C NMR, and mass (HRMS) spectroscopy data. See DOI: https://doi.org/10.1039/d2md00182a
References
- Gal-Mor O. Boyle E. C. Grassl G. A. Front. Microbiol. 2014;5:391. doi: 10.3389/fmicb.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M. Wain J. Weill F. X. Nair S. Zhou Z. M. Sangal V. Krauland M. G. Hale J. L. Harbottle H. Uesbeck A. Dougan G. Harrison L. H. Brisse S. Grp S. E. M. S. PLoS Pathog. 2012;8:e1002776. doi: 10.1371/journal.ppat.1002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis E. Wong D. M. A. L. F. Patrick M. E. Binsztein N. Cieslik A. Chalermchaikit T. Aidara-Kane A. Ellis A. Angulo F. J. Wegener H. C. Surv W. G. S. Emerging Infect. Dis. 2006;12:381–388. doi: 10.3201/eid1205.050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2017 Non-Typhoidal Salmonella Invasive Disease Collaborators Lancet Infect. Dis. 2019;19:1312–1324. doi: 10.1016/S1473-3099(19)30418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. J. Kendall M. M. Front. Microbiol. 2017;8:1983. doi: 10.3389/fmicb.2017.01983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowski M. A. Braun V. Brumell J. H. Traffic. 2008;9:2022–2031. doi: 10.1111/j.1600-0854.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- Watson K. G. Holden D. W. Cell. Microbiol. 2010;12:1389–1397. doi: 10.1111/j.1462-5822.2010.01511.x. [DOI] [PubMed] [Google Scholar]
- McDermott P. F. Zhao S. Tate H. Microbiol. Spectrum. 2018;6 doi: 10.1128/microbiolspec.ARBA-0014-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconelli E. Carrara E. Savoldi A. Harbarth S. Mendelson M. Monnet D. L. Pulcini C. Kahlmeter G. Kluytmans J. Carmeli Y. Ouellette M. Outterson K. Patel J. Cavaleri M. Cox E. M. Houchens C. R. Grayson M. L. Hansen P. Singh N. Theuretzbacher U. Magrini N. Workin W. P. P. L. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- Yang C. Y. Hsu C. Y. Fang C. S. Shiau C. W. Chen C. S. Chiu H. C. J. Microbiol., Immunol. Infect. 2019;52:638–647. doi: 10.1016/j.jmii.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Popovic D. Nuss P. Vieta E. Ann. Gen. Psychiatry. 2015;14:15. doi: 10.1186/s12991-015-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjerde E. Dahl S. G. Sylte I. Eur. J. Med. Chem. 2005;40:185–194. doi: 10.1016/j.ejmech.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Liegeois J. F. Eyrolles L. Ellenbroek B. A. Lejeune C. Carato P. Bruhwyler J. Geczy J. Damas J. Delarge J. J. Med. Chem. 2002;45:5136–5149. doi: 10.1021/jm0104825. [DOI] [PubMed] [Google Scholar]
- Jain M. S. Surana S. J. Arabian J. Chem. 2017;10:S2032–S2039. doi: 10.1016/j.arabjc.2013.07.033. [DOI] [Google Scholar]
- Crump J. A. Sjolund-Karlsson M. Gordon M. A. Parry C. M. Clin. Microbiol. Rev. 2015;28:901–937. doi: 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T. Front. Microbiol. 2012;3:71. doi: 10.3389/fmicb.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luepke K. H. Mohr J. F. Expert Rev. Anti-infect. Ther. 2017;15:425–433. doi: 10.1080/14787210.2017.1308251. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. E. Dorhoi A. Hotchkiss R. S. Bartenschlager R. Nat. Rev. Drug Discovery. 2018;17:35–56. doi: 10.1038/nrd.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy A. E. Pierson E. Hung D. T. Nat. Chem. Biol. 2007;3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






