Abstract
Cell penetrating peptides (CPPs) are known to possess a unique capacity to penetrate biological membranes and translocate various molecules into the cells. Therefore, porphyrin–CPP conjugates could be envisaged to boost the intracellular delivery of porphyrins thereby providing an improved tool for the development of agents for multi-modal applications for cancer management. Working in this direction, an unsymmetrically substituted porphyrin derivative was conjugated with a transactivating transcriptional activator peptide (TAT) and various in vitro and in vivo studies were carried out in order to study the effect of adding a CPP to the porphyrin derivative. MTT assay revealed the preferential light dependent toxicity of the porphyrin derivative which was further enhanced upon peptide conjugation. Fluorescence and flow cytometry studies revealed the relatively higher cellular internalization of the porphyrin–TAT conjugate in comparison with the porphyrin derivative. The elevated light dependent cell toxicity of the porphyrin–TAT conjugate along with its capability of generating cytotoxic singlet oxygen indicated the advantages of using the porphyrin–TAT conjugate for PDT applications. Also, porphyrin and the porphyrin–peptide conjugate were radiolabelled with 68Ga to investigate their possible potential as PET agents. In vivo biodistribution studies revealed a higher tumor uptake for the 68Ga-porphyrin–TAT conjugate (6.32 ± 1.24% IA per g) than for 68Ga-porphyrin (2.45 ± 0.88% IA per g) at 60 min post-administration. However, the observation of a higher non-target retention of the radiolabelled agents during in vivo studies might pose a limitation on their possible application in PET imaging.
Demonstration of the effect of conjugation of a cell penetrating peptide towards enhancing the in vitro and in vivo tumor targeting potential of porphyrin derivatives.
Introduction
Porphyrin and its analogues in conjunction with a radiometal have potential applications in imaging and therapy of cancerous tumors.1–5 The cavity size (1.92 to 2.10 Å) and the four pyrrolic nitrogens of the porphyrin core provide a suitable environment for coordination with radiometals (68Ga, 64Cu, 52Mn, 111In) having smaller ionic radii for nuclear medicine imaging (PET/SPECT).6–10 However, the porphyrin core does not stably coordinate with larger therapeutic radiometals (177Lu, 90Y) requiring peripheral derivatization of porphyrin to introduce bifunctional chelating agents (BFCAs).6–10 Therapeutic application of porphyrin compounds is governed by lipophilicity, water solubility, cellular penetration and selective accumulation in tumor cells. Transportation of molecules inside the tumor tissue is a typical phenomenon controlled by the semi-permeable nature of the plasma membrane. Its lipophilic character drives molecules across the lipid bilayer and facilitates intracellular localization. However, compounds also need to express hydrophilic behavior for rapid clearance and favorable pharmacokinetics. Hence, a proper balance between hydrophilicity/lipophilicity is an important criterion for biological applications. Porphyrin compounds exhibit high tumor avidity but their poor pharmacokinetics (non-specific uptake in blood, the liver and lungs) poses limitations on their use in clinical settings.5,7,11,12
The chemical and biological characteristics of porphyrin compounds can be tuned by introduction of appropriate substituents at their periphery. Intracellular delivery of porphyrins can be facilitated by peripheral tagging of specific vectors capable of traversing membranes. Cell penetrating peptides (CPP) are such transporter peptides with a unique capacity to penetrate biological membranes and translocate different molecules into the cell.13–15 CPPs are short peptides consisting of 5–30 amino acids and are classified as cationic, amphipathic and hydrophobic peptides based on amino acid chains and physical–chemical characteristics.16,17 One of the well exploited CPPs is the cationic, arginine-rich peptide known as TAT (transactivator of transcription), obtained from human immunodeficiency virus (HIV).18,19 There are several reports where conjugation with the polyarginine peptide TAT has led to increased cellular uptake and enhanced delivery of drugs into the cell membrane.20–26 Besides channelizing their entry into the tumor cells, conjugation of CPPs also mediates the rapid clearance of porphyrins and improves the pharmacokinetics by imparting hydrophilicity.
The aim of the present work was to enhance the cell permeability of an unsymmetrically substituted porphyrin derivative (5,10,15-tris(4-carboxyphenyl)-20-(4-hydroxyphenyl)-porphyrin) by coupling the peptide (TAT) at the periphery and thereby augment its applications such as in photodynamic therapy (PDT) (when utilized in the free base form) as well as positron emission tomography (PET) (after radiolabelling with a suitable PET radionuclide). An unsymmetrically substituted porphyrin containing peripheral carboxy and hydroxyl groups at meso positions was synthesized by a two-step reaction procedure. The presence of carboxy groups at the peripheral position in porphyrin derivatives is reported to facilitate enhanced accumulation in solid tumors owing to the intracellular acidic environment whereas the hydroxyl group at the single peripheral position provides selectivity in the present case towards further functionalization.27 The cell penetrating peptide, TAT, having a peptide sequence of RKKRRQRRR, was synthesized using solid phase peptide synthesis which was then conjugated with porphyrin. The porphyrin and the corresponding porphyrin–peptide conjugate were evaluated in vitro in cancer cells (A549) to compare the cell internalization behavior. Cellular toxicity, both in the dark as well as in the presence of light, for both compounds was also studied in cancer cell lines.
For in vivo investigations, porphyrin and the porphyrin–peptide conjugate were radiolabeled with 68GaCl3. Gallium-68 is a PET radionuclide [T1/2 = 68 min, β+(max) = 1.89 MeV (89%)] and unlike various other PET radionuclides, it is available from a 68Ge–68Ga generator system thereby making it feasible to carry out PET studies at centres which lack proximity to a cyclotron facility. Another advantage of using 68Ga is its direct complexation in the porphyrin core which provides the flexibility to exclude the use of any BFCA. In the present study, the in vivo tumor targeting potential of 68Ga-labeled porphyrin and a porphyrin–TAT conjugate was evaluated by performing bio-distribution studies in a fibrosarcoma bearing Swiss mice model.
Experimental
Materials and methods
5,10,15,20-Tetrakis-(4-hydroxyphenyl)porphyrin, bromoacetic acid, bromo-tert-butylacetate, trifluoroacetic acid (TFA) and diphenylisobenzofuran (DPBF) were procured from Sigma-Aldrich (USA). Various other chemicals and reagents such as, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), sodium acetate, sodium chloride, sodium bicarbonate, DMSO, HEPES, and DMEM were procured from Sigma Chemical Inc. (USA). Common solvents utilized in the present study, such as acetone, methanol, and chloroform, were procured from Merck (India). The silica gel 60 F254 coated aluminium sheets utilized for performing thin layer chromatography (TLC) were procured from Merck (India). DAPI (4′,6-diamidino-2-phenylindole) was procured from Merck. Fetal Bovine Serum (FBS) was purchased from GIBCO Laboratories (USA).
A Bruker spectrophotometer (Germany) was employed for recording Fourier transform infrared (FT-IR) spectra. A Labindia Analytical (UV 3092) UV-vis spectrophotometer and an FP-8500 spectrofluorometer (JASCO) were employed for recording UV-vis absorption and emission spectra for porphyrin derivatives, respectively. Mass analyses of the samples were carried out using a Varian ProStar 410 Binary LC mass spectrometer (USA) employing the electron spray ionization (ESI) technique as well as a MALDI-TOF mass spectrometer (UltrafleXtreme, Bruker, Germany). The nuclear magnetic resonance (NMR) spectra for the compounds were recorded using either a Bruker 300 MHz (Bruker Avance II) (Germany) or an 800 MHz NMR (Bruker, 800 US) spectrometer (Switzerland). A POLARstar Omega plate reader spectrophotometer [(BMG LABTECH (Germany)] was used to the measure spectrometric readings associated with MTT assays.
Photocytotoxicity experiments were carried out using a LED (light emitting diode) bulb (9 W Philips, India) as a light source. Light doses received by the cells were measured as W m−2 using an irradiance meter (Megger PVM210, Taiwan) and were expressed as kJ cm−2 after multiplying irradiance by the time of light exposure. Cell imaging studies were performed using an Olympus IX83 inverted microscope with the laser beams focused to the back focal plane of a 10 × 1.40 NA apochromatic objective lens.
The Gallium-68 used in the present study was eluted from a 68Ge/68Ga radionuclide generator [1.11 GBq, (30 mCi)], which was purchased from ITG (Germany). C-18 reversed phase Sep-Pak® cartridges were procured from Waters (India). 69/71Ga as gallium nitrate (99.9% chemically pure), used for the preparation of non-radioactive metal complexes of porphyrin derivatives, was procured from Thermo Fisher Scientific (USA). The High-Performance Liquid Chromatography (HPLC) system (PU 1580) was purchased from JASCO (Japan). HPLC studies were performed using a C-18 reverse phase HiQSil (250 × 4 mm) column and the elution profile was monitored by detecting the radioactivity signal using a NaI(Tl) detector (Raytest, Germany) coupled with the HPLC system. All solvents used for the HPLC studies were filtered prior to use and of HPLC grade. Durapore® polyvinylidene fluoride (PVDF) membrane filters (47 mm, 0.22 μm), used for the filtration of HPLC solvents, were procured from Merck (India). All radioactive countings were performed using a well-type NaI(Tl) scintillation detector, procured from the Electronic Corporation of India Limited (India), unless mentioned otherwise. The baseline of the detector was kept at 450 keV and a window of 100 keV was used in order to utilize the 511 keV annihilation photo-peak of 68Ga.
A549 human lung adenocarcinoma cells were procured from the National Centre for Cell Sciences (India). Fibrosarcoma bearing Swiss mice (4–6 weeks age, 20–25 g weight) were used as animal models for the biological studies. The HSDM1C1 murine fibrosarcoma cell line, used for raising the tumors in the animals, was also purchased from the National Centre for Cell Sciences (India). All the animals used in the bio-distribution studies were bred and reared in the laboratory animal house facility at Bhabha Atomic Research Centre (BARC) following the standardized protocol. Radioactive counting associated with the animal studies was performed using a flat-type NaI(Tl) scintillation detector, obtained from the Electronic Corporation of India Limited (India), using the same counting set-up mentioned earlier. The animal studies reported in the present article were approved by the Institutional Animal Ethics Committee (IAEC) of BARC and all the animal experiments were carried out in strict compliance with institutional (IAEC) guidelines following the relevant national laws related to the conduct of animal experimentation (Prevention of Cruelty to Animals Act, 1960).
The data in the present study are presented as mean ± standard deviation. Biological experiments were performed twice with each sample in triplicate. The differences between two groups were analyzed by using the Student's t-test. ANOVA was used for comparisons among multiple groups to compare between different pairs of treatments and for determining the significance value; p < 0.05 was considered to be statistically significant.
Synthesis of the unsymmetrical water soluble porphyrin derivative
An unsymmetrically substituted porphyrin derivative, namely, 5,10,15-tris(4-carboxyphenyl)-20-(4-hydroxyphenyl)-porphyrin (UTriCOOHPhOH), was synthesized by following the two-step synthetic procedure described below:
Synthesis of 5,10,15-tris(4-tert-butoxycarbomethyleneoxyphenyl)-20-(4-hydroxyphenyl)-porphyrin (1)
As a first step, 5,10,15,20-tetrakis-(4-hydroxyphenyl)porphyrin (200 mg, 0.294 mmol) was allowed to react with tert-butylbromoacetate (0.13 mL, 0.882 mmol) in a molar ratio of 1 : 3 by carrying out refluxing in dry acetone in the presence of anhydrous potassium carbonate for 8 hours. The reaction mixture was subsequently left overnight at room temperature with continuous magnetic stirring. The progress of the reaction was monitored by thin layer chromatography using 3% methanol in chloroform as the mobile phase (Rf = 0.33). The reaction mixture was dried and extracted with a chloroform : water mixture (1 : 1, v/v) using a separating funnel. The organic layer was evaporated under reduced pressure and the solid residue, thus obtained, was subjected to column purification using 0.5–1% methanol in chloroform as the eluting solvent. The purified product (110 mg, yield 36.5%) was characterized by various spectroscopic techniques such as UV-Vis, FT-IR, ESI-MS and 1H-NMR and 13C-NMR spectroscopy.
UV-Vis (CHCl3, λmax, nm, log ε): 422 (4.47), 518 (3.88), 554 (3.75), 592 (3.49), 648 (3.49). FT-IR (CHCl3, neat, , cm−1): 3420, 2927, 2854, 1750, 1606, 1509, 1369, 1217, 1155, 997. ESI-MS: C62H60N4O10, theoretical: 1021.16, observed: 1022.0 [M + H]. 1H-NMR (CDCl3, δ, ppm): 1.68 (s, 27H), 4.83 (s, 6H), 7.26–7.36 (m, 8H), 8.15–8.23 (m, 8H), 9.03–9.08 (m, 8H). 13C-NMR (CDCl3, δ, ppm): 28.30, 66.19, 82.68, 112.91, 113.70, 120.68, 132.02, 135.49, 150.56, 155.82, 157.97, 168.43.
Synthesis of 5,10,15-tris(4-carboxymethyleneoxyphenyl)-20-(4-hydroxyphenyl)-porphyrin (UTriCOOHPhOH) (2)
The esterified derivative (compound 1) (90 mg, 0.08 mmol) was hydrolyzed using a 1 : 1 mixture of trifluoroacetic acid and dichloromethane (1 : 1 v/v) by room temperature stirring for 6 h. The unreacted starting product was removed by repetitive extractions using dichloromethane. The removal of the reactant was assured by thin layer chromatography using 5% methanol in chloroform as the mobile phase. The final porphyrin derivative (UTriCOOHPhOH) obtained post acid hydrolysis (67 mg, yield, 90%) was characterized by employing the spectroscopic techniques mentioned above.
UV-Vis (CHCl3, λmax, nm, log ε): 425 (4.60), 520 (3.35), 560 (2.34), 654 (2.07). FT-IR (MeOH, neat, , cm−1): 1681, 1599, 1482, 1210, 1175, 1139, 805, 727. MALDI-TOF: C50H36N4O10, theoretical: 852.24, observed 853.04 [M + H]. 1H-NMR (DMSO-d6, δ, ppm): 4.96 (s, 6H), 6.54 (s, 1H), 7.19–7.20 (d, J = 8 Hz, 2H), 7.34–7.35 (d, J = 8 Hz, 6H), 7.98–7.99 (d, J = 8 Hz, 2H), 8.11–8.12 (d, J = 8 Hz, 6H), 8.82–8.87 (m, 8H). 13C-NMR (DMSO-d6, δ, ppm): 65.32, 113.54, 114.44, 116.22, 120.00, 132.27, 134.45, 135.84, 136.03, 157.94, 158.21, 170.80.
Synthesis of TAT (transactivator of transcription) using manual solid phase peptide synthesis and the porphyrin–TAT conjugate (UTriCOOHPhO–TAT) (3)
The cell penetrating peptide TAT with side-chain protecting groups Arg(Pbf)-Lys(Boc)-Lys(Boc)-Arg(Pbf)-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-NH2 was constructed manually on a 4-methylbenzhydrylamine (MBHA) resin by the Fmoc solid phase peptide synthesis (SPPS) methodology. Coupling reactions were performed in DMF for 2 h using Fmoc-protected amino acid (3 eq.), N,N,N′,N′-tetramethyluroniumhexafluorophosphate (HATU, 3 eq.) and N,N′-diisopropylethylamine (DIPEA, 6 eq.). The coupling efficiency was determined by performing the trinitrobenzene sulphonic acid (TNBS) color test. Fmoc groups were deprotected using 20% piperidine in DMF (2 × 10 min). Sequential steps of coupling and deprotection were repeated till the completion of the TAT peptide chain. The N-terminus of the TAT peptide was conjugated with bromoacetic acid (5 eq.) in the presence of diisopropyl carbodiimide (DIC, 5 eq.) in DMF for 2 h. The coupling procedure was repeated and successful completion of the reaction was ascertained by the colorimetric TNBS (2,4,6-trinitrobenzene sulfonic acid) test. The bromo functionalized resin was then allowed to react overnight with 5,10,15-tris(4-tert-butoxycarbomethyleneoxyphenyl)-20-(4-hydroxyphenyl)-porphyrin (compound 1, 2 eq.) dissolved in DMF in the presence of DIPEA. Finally, the peptide was cleaved from the solid support with a cocktail mixture of TFA : H2O : TIPS (95 : 2.5 : 2.5, v/v) with simultaneous removal of side chain protecting groups. The filtrate was concentrated and the crude TAT–porphyrin conjugate (UTriCOOHPhO–TAT) was isolated as a green precipitate on addition of diethyl ether. The crude conjugate was subsequently purified by semi-preparative HPLC and characterized by mass spectroscopy.
UV-Vis (CHCl3, λmax, nm, log ε): 422 (5.26), 521(3.86), 559 (3.80), 597 (3.17), 654 (3.02). MALDI-TOF: C107H146N33O21, theoretical: 2230.510, observed: 2230.484 [M].
MTT assay
For the MTT cytotoxicity assay, A549 cells were cultured in DMEM medium containing 10% FBS and grown up to 60–70% confluence at 37 °C and 5% CO2 in an incubator. After trypsinization, 7 × 103 cells were seeded in 96-well tissue culture plates and allowed to grow a monolayer after overnight incubation at 37 °C in the presence of 5% CO2. A549 cells were treated with different concentrations of both compounds (2 and 3) ranging from 0.5 to 10 μM (viz. 0.5, 1, 2.5, 5, and 10 μM). Control cells were set up wherein cells were treated with buffer. All treatments were carried out in sextuplicate (n = 6). After 24 h, MTT solution (5 mg mL−1 in PBS) was added to the cells at a final concentration of 0.5 mg mL−1 and incubated for 4 h at 37 °C in the presence of 5% CO2. After incubation, the supernatant was removed from the wells. 50 μL of DMSO was added to each well to dissolve the formazan crystals. The plate was kept on the plate shaker in the dark. After 30 min, the absorbance was measured at 570 nm which corresponds to the viability of cells. The absorbance at 660 nm was used as a reference wavelength. The percentage of dead cells was calculated as ([optical density (OD) of the vehicle control sample − OD of the treated sample/OD of the control sample] × 100) and presented as mean ± SD (n = 6). These data were used to plot the percentage cell proliferation at different concentrations for both the porphyrin derivative and its peptide conjugate.
Determination of the comparative photo-cytotoxicity of UTriCOOHPhOH and UTriCOOHPhO–TAT in A549 cell lines
Photocytotoxicity studies were carried out at two different concentrations of compounds 1 and 3viz. 0.5 and 1 μM using a single light dose (0.02 kJ cm−2). A549 cells were plated as described earlier and three sets of cells were studied for percentage cell proliferation under the conditions described below. The first set of cells labeled as ‘vehicle control-1’ was not incubated with any porphyrin derivative and was exposed to light; the second set of cells labeled as ‘vehicle control-2’ was incubated with either UTriCOOHPhOH or UTriCOOHPhO–TAT, but not exposed to light; meanwhile, the third set of cells labeled as ‘treated’ was incubated with either UTriCOOHPhOH or UTriCOOHPhO–TAT and was exposed to light too. The vehicle control-2 and treated sets were incubated with either porphyrin or the porphyrin–TAT conjugate for 6 h and subsequently the treated set was exposed to light. The cell lines were incubated for another 18 h at room temperature after the completion of irradiation. Each set of cells was then subjected to MTT assay (as described earlier) to determine the percentage cell proliferation. The irradiations were carried out at a radiance flux of 42 W m−2 and were continued for 90 min resulting in a cumulative light dose of 0.02 kJ cm−2.
Determination of the IC50 value
For the determination of IC50 values, the photocytotoxicity associated with both compounds (UTriCOOHPhOH and UTriCOOHPhO–TAT) was determined at different ligand concentrations viz. 0.1, 0.5, 1, 2.5, 5.0, 10.0, and 20.0 μM. The light dose was kept fixed at 0.02 kJ cm−2. The experiment was performed by following the procedure mentioned above. The percentage cell death was calculated and was plotted against the logarithm of the ligand concentration (in nanomolar). The graphs were analyzed to calculate the IC50 value for the respective ligands using GraphPad Prism 6 software.
Internalization assay using confocal microscopy
A549 cells were cultured as mentioned earlier and 5 × 103 cells were seeded in a 96-well black polystyrene plate. After overnight incubation, the cells were treated with two different concentrations (1 and 10 μM) of UTriCOOHPhOH and UTriCOOHPhO–TAT. After treatment for 24 h, the cells were washed twice with PBS (pH = 7.4). The fluorescence associated with both UTriCOOHPhOH and UTriCOOHPhO–TAT was detected using a 485/630 nm excitation/emission wavelength. To study the nuclear localization of UTriCOOHPhOH and UTriCOOHPhO–TAT, the cells pre-treated with a 10 μM concentration of both compounds for a period of 24 h and then stained with DAPI (1 μg mL−1). Excess DAPI was removed after 30 minutes of staining by washing the cells with PBS and this step was repeated twice. The fluorescence associated with DAPI was detected using a filter utilizing a 402/460 nm (excitation/emission) wavelength, respectively, and the corresponding images were processed using cellSens software and Adobe Photoshop 7.0.
Internalization assay using flow cytometry
A549 cells were cultured as described previously and about 5 × 103cells were seeded in a 96-well black polystyrene plate for the experiment. After overnight incubation, the cells were treated separately with a 10 μM concentration of UTriCOOHPhOH and UTriCOOHPhO–TAT. After treatment for 24 h, the cells were washed twice with PBS (pH = 7.4) to remove the excess of either UTriCOOHPhOH or UTriCOOHPhO–TAT. Subsequently, the cells were harvested and images were acquired using the InCyte software module in a Guava® easyCyte™ Flow Cytometer and analyzed using Cyflogic software for internalization of UTriCOOHPhOH and UTriCOOHPhO–TAT.
Determination of singlet oxygen formation by UTriCOOHPhOH and UTriCOOHPhO–TAT)
Stock solutions of UTriCOOHPhOH (1 mM), UTriCOOHPhO–TAT (1 mM) and DPBF (10 mM) were prepared in DMF. The UV-visible absorption spectra were recorded for all the compounds in DMF. For the present experiment, irradiations were carried out at 519 and 521 nm for UTriCOOHPhOH and UTriCOOHPhO–TAT, respectively. For the irradiation, an aliquot of 4 μL, withdrawn from the stock solution of either of the compounds, was added with 5 μL of DPBF in DMF, such that the final volume in the cuvette becomes 2 mL and the final concentrations corresponding to porphyrin and DPBF were 2 μM and 25 μM, respectively. Irradiation was continued for different time points ranging from 1 min to 14 min and the decline in the absorbance at 325 nm (corresponding to DPBF) was monitored after each cycle of irradiation as none of the compounds exhibited significant absorption at this wavelength. The results of these studies were plotted as line graphs by taking the ln(OD0/ODt) values along the Y-axis and the time of irradiation (in min) along the X-axis, where OD0 is the optical density at 325 nm at ‘zero’ time of irradiation, whereas ODt represents the optical density after ‘t’ time of irradiation).
Synthesis of non-radioactive gallium complexes of UTriCOOHPhOH and UTriCOOHPhO–TAT
Syntheses of the corresponding non-radioactive gallium complexes of both UTriCOOHPhOH and UTriCOOHPhO–TAT were carried out using natural gallium metal (natGa or 67/71Ga) following the procedure mentioned here. About 1.0 mg of UTriCOOHPhOH (1.17 μmol) was dissolved in DMSO (1.0 mL) and was allowed to react with 0.49 mg of natGa (7.03 μmol, 50 μL) dissolved in 0.5 M sodium acetate buffer (pH = 4.8) at 80 °C for 16 h. For the synthesis of the non-radioactive gallium complex of UTriCOOHPhO–TAT, about 1.0 mg UTriCOOHPhO–TAT (0.428 μmol) dissolved in 0.5 M sodium acetate buffer (pH = 4.8) was incubated with 0.49 mg of natGa (7.03 μmol, 50 μL) under refluxing conditions in an overnight reaction. The formation of natural-gallium complexes of UTriCOOHPhOH and UTriCOOHPhO–TAT was confirmed by HPLC as well as UV-Vis spectrophotometry.
Radiolabeling of UTriCOOHPhOH and UTriCOOHPhO–TAT with 68Ga
Radiolabeling of UTriCOOHPhOH and UTriCOOHPhO–TAT with 68Ga was carried out by following the procedure mentioned below. 68Ga (9 mCi, 333 MBq, 4 mL) was eluted from a 68Ge/68Ga generator using 0.05 M HCl. For radiolabeling, about 1 mCi (37 MBq) of 68Ga was added to 0.5 mg of UTriCOOHPhOH (0.586 μmol) or UTriCOOHPhO–TAT (0.214 μmol) dissolved in Milli-Q water (0.5 mL). The pH of the reaction mixture was adjusted to 5.5 using 2 N aqueous sodium acetate solution prior to incubation in a boiling water bath for 30 min. The percentage radiochemical yield (% RCY) corresponding to each radiolabeled preparation was determined by injecting an aliquot (5–10 μL) of the preparation into an RP-HPLC system. Further purification of the radiolabeled preparations was attempted by passing the radiolabeled preparations through a pre-conditioned C-18 reversed phase Sep-Pak® cartridge. Pre-conditioning of the cartridge was carried out by passing 4.0 mL of ethanol followed by 2.0 mL of double distilled water. The radiolabeled preparations were loaded separately into the column and unlabeled/free 68GaCl3 was eluted using 5.0 mL of saline followed by extraction of the radiolabeled product using 1.0 mL of ethanol. The ethanol present in the purified preparation was removed by gentle warming and the preparation was reconstituted with normal saline. The final percentage radiochemical purity corresponding to both radiolabeled preparations was determined by RP-HPLC using a gradient solvent system comprising water with 0.1% TFA as solvent ‘A’ and acetonitrile with 0.1% TFA as solvent ‘B’ and maintaining a flow rate of 1.0 mL minute−1 (0.0 min 10% B, 0.0–28.0 min 90% B, 28.0–30.0 min 90% B, 30.0–32.0 min 10% B).
Determination of the partition coefficient (log Po/w)
In the present experiment, about 100 μL of either of the radiolabeled preparations was added to a solution consisting of octanol (1.0 mL) and water (900 μL). The solution was mixed thoroughly and subsequently centrifuged at 2500 rpm for 5 minutes to enable the separation between aqueous and organic layers. An aliquot (100 μL) of both layers was withdrawn and was counted separately using a well-type NaI(Tl) counter. The partition coefficient was calculated by taking the logarithm of the ratio between the counts observed in the organic layer and the aqueous layer.
Bio-distribution studies
Bio-distribution studies for the 68Ga-labeled UTriCOOHPhOH and UTriCOOHPhOH–TAT complexes were carried out on a fibrosarcoma bearing Swiss mice model. To grow fibrosarcoma tumors in the animals, ∼106 cells of HSDM1C1 murine fibrosarcoma (100 μL) were injected subcutaneously on the dorsum of each animal and the animals were maintained in a normal laboratory atmosphere with an adequate supply of food and water. The animals were used for the experiment when the tumor size became ∼1 cm in diameter. Each animal, weighing 20–25 g, was intravenously injected with 150 μL of either of the radiolabeled preparations (∼100 μCi, 3.7 MBq) through one of the lateral tail veins. The biological distribution of both radiolabeled complexes was studied at two different time-points, viz. 30 and 60 min, post-administration utilizing three animals for each complex. After the lapse of the designated time, the animals were sacrificed through CO2 asphyxia. Blood samples were collected from each animal by cardiac puncture immediately after sacrificing the animals. The organs/tissues were excised, washed with normal saline, dried and weighed on a weighing balance. The radioactivity associated with each organ/tissue was measured using a flat-type NaI(Tl) counter. The activity accumulated in various organs/tissue was calculated from the above data and expressed as the percentage injected activity per gram (% IA per g) of organ/tissue.
Results
Synthesis of porphyrin (UTriCOOHPhOH) and the porphyrin–TAT (UTriCOOHPhOH–TAT) conjugate
An unsymmetrically substituted porphyrin derivative (compound 1) was synthesized by carrying out O-alkylation of terminal hydroxyl groups of a commercially available symmetrical porphyrin derivative (Scheme 1). The first indication of the formation of the desired unsymmetrically alkylated porphyrin derivative was obtained from thin layer chromatography wherein other by-products corresponding to tetra-, di- and mono-O-alkylation were also observed to be co-produced along with the tri-O-alkylated porphyrin derivative. The desired porphyrin derivative was purified by repeated silica-based glass column chromatography and characterized using standard spectroscopic techniques. Attachment of an ester moiety was indicated in FT-IR spectroscopy, where a sharp band was observed at 1750 cm−1 (Fig. S1, ESI†) and confirmed by 1H- and 13C-NMR spectroscopy, where a singlet corresponding to 27 hydrogens of the tertiary ester group was observed at 1.68 ppm in the former whereas three carbons belonging to this moiety exhibited signals at 28.30, 82.68 and 168.43 ppm in the latter. The formation of compound 1 was further confirmed by mass spectroscopy where a signal corresponding to m/z of [M + H] was observed at 1022.0 (Fig. S2, ESI†). Compound 1 was utilized for the synthesis of compound 2 (UTriCOOHPhOH) as well as for conjugation with the peptide (TAT), which, post-hydrolysis, led to the formation of compound 3 (UTriCOOHPhO–TAT) (Scheme 1). The formation of compound 2 was confirmed using FT-IR spectroscopy where the signal corresponding to the ester group disappeared and a new signal corresponding to the carboxylic acid moiety was observed at 1681 cm−1 (Fig. S3, ESI†). Additionally, the 1H- and 13C-NMR spectra recorded for compound 2 indicated the disappearance of peaks specific to the aliphatic region (ester peaks). The molecular ion peak observed at m/z 853.04 for [M + H] in the mass spectrum of compound 2 provided further confirmatory evidence in favor of its formation (Fig. S4, ESI†).
Scheme 1. Schematic representation of the two-step reaction for the syntheses of compounds 1 and 2.
The cell penetrating peptide, TAT, was synthesized using solid phase peptide synthesis by an Fmoc-based protocol. Subsequent to the assembly of the peptide sequence RKKRRQRRR with side chain protecting groups in the solid phase, the N-terminus was coupled with bromoacetic acid to introduce a terminal bromo-functional group in the peptide moiety (Br-TAT). The bromo functionality was utilized for conjugating the peptide moiety with the porphyrin derivative (compound 1) bearing a phenolic group at one of the four meso positions in the solid phase. The porphyrin–peptide conjugate was cleaved from the resin using TFA wherein side chain protecting groups of the peptide as well as of the porphyrin also got removed. The crude porphyrin–TAT conjugate (compound 3) was purified by semi-preparative HPLC and lyophilized to obtain a green colored powder (Scheme 2). The formation of compound 3 (UTriCOOHPhO–TAT) was confirmed by mass spectrometry where a mass peak at m/z 2230.484 [M] was observed (Fig. S5, ESI†).
Scheme 2. Schematic representation of the steps involved in the synthesis of the peptide–porphyrin conjugate, UTriCOOHPhO–TAT (3).
MTT assay for the determination of dark cytotoxicity
The MTT assay performed in the A549 cell line using different concentrations of compound 2 and compound 3 revealed a steady decrease in percentage cell proliferation with increasing concentrations of both ligands (Fig. 1). However, no significant difference in percentage cell proliferation was observed at all concentrations for both ligands (p < 0.05).
Fig. 1. Percentage cell proliferation (A549 cell line) observed corresponding to different concentrations (0.5, 1, 2.5, 5 and 10 μM) of UTriCOOHPhOH and UTriCOOHPhO–TAT.
Determination of comparative photocytotoxicity associated with UTriCOOHPhOH and UTriCOOHPhO–TAT
Unlike in previous experiments, UTriCOOHPhOH and UTriCOOHPhO–TAT exhibited a differential decline in percentage cell proliferation when exposed to light under the same set of conditions. A similar cell proliferation pattern was observed at both concentrations (0.5 and 1.0 μM) of UTriCOOHPhOH and UTriCOOHPhO–TAT (Fig. 2). It is evident from Fig. 2 that in comparison with porphyrin (UTriCOOHPhOH), the porphyrin–TAT conjugate (UTriCOOHPhO–TAT) exhibited a higher light dependent toxicity under the same conditions. This observation may be attributed to the cell internalizing capacity of TAT conjugated with the porphyrin derivative, which might have led to the relatively higher photocytotoxicity of UTriCOOHPhO–TAT than that of UTriCOOHPhOH (Fig. S6, ESI†).
Fig. 2. Graph depicting the dark and photocytotoxicity in terms of percentage cell proliferation in A549 cell lines corresponding to two different concentrations (0.5 and 1.0 μM) of UTriCOOHPhOH and UTriCOOHPhO–TAT under a light dose of 0.02 kJ cm−2.
Determination of the IC50 value
The IC50 value was determined for both UTriCOOHPhOH and UTriCOOHPhO–TAT in A549 cells under light exposure and the values were found to be 5653 ± 40.87 and 1362 ± 46.47 nM for UTriCOOHPhOH and UTriCOOHPhO–TAT, respectively (Fig. 3). These data indicate the higher potency of the TAT conjugated porphyrin towards exerting light dependent toxicity in cancer cells.
Fig. 3. Graphs of the percentage cell survival versus the logarithm of the ligand concentration (in nanomolar) for the determination of IC50 values for (a) UTriCOOHPhOH and (b) UTriCOOHPhO–TAT, respectively.
Internalization assay using confocal microscopy and flow cytometry
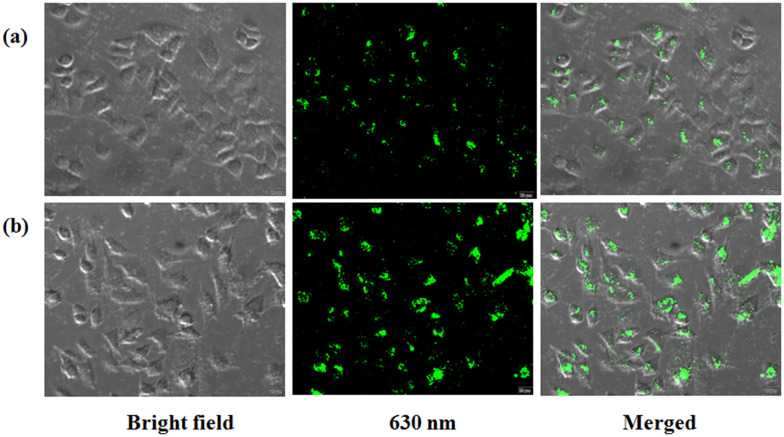
The cell internalizing behavior of the porphyrin derivative (UTriCOOHPhOH) as well as the porphyrin–peptide conjugate (UTriCOOHPhO–TAT) was investigated using confocal microscopy and flow cytometry. Both techniques revealed the internalization of porphyrin as well as of the porphyrin–TAT conjugate in the cells. The cell imaging using confocal microscopy was performed at two different concentrations of UTriCOOHPhOH and UTriCOOHPhO–TAT, namely, 1 and 10 μM (Fig. S7, ESI† and 4). This study revealed a higher cell internalization for UTriCOOHPhO–TAT compared to UTriCOOHPhOH at similar concentrations (Fig. 4). The results obtained in fluorescence microscopy were further confirmed by the results of the flow cytometry (Fig. 5). These results support the findings of the photocytotoxicity experiment performed in the same cell line, where a higher cell death was observed to be associated with UTriCOOHPhO–TAT, thereby pointing towards the ability of TAT to facilitate the greater internalization of porphyrin upon conjugation. However, DAPI staining revealed that neither UTriCOOHPhOH nor UTriCOOHPhO–TAT could exhibit accumulation in the nucleus of the cancer cells, which is evident from Fig. 6.
Fig. 4. Cell images depicting the cellular internalization of (a) UTriCOOHPhOH and (b) UTriCOOHPhO–TAT (10 μM) in A549 cell lines. The images were captured by recording the emission at 630 nm (λex = 485 nm) for both ligands.
Fig. 5. Graph depicting the higher fluorescence intensity associated with UTriCOOHPhO–TAT compared with UTriCOOHPhOH confirming the relatively higher internalization corresponding to the former [black: untreated cells, green: porphyrin (10 μM), red: UTriCOOHPhO–TAT (10 μM), X-axis: fluorescence intensity, Y-axis: counts (cells)].
Fig. 6. Fluorescence images depicting the nuclear exclusion for (a) UTriCOOHPhOH and (b) UTriCOOHPhO–TAT.
Determination of singlet oxygen generation
The porphyrin derivative, UTriCOOHPhOH, and its peptide conjugate i.e. UTriCOOHPhO–TAT were investigated for their ability to generate singlet oxygen after observing their ability to exert light dependent cytotoxicity in A549 cell lines (described in the previous section). The generation of singlet oxygen was indicated by the decrease in absorbance of DPBF, which was due to the decomposition of DPBF upon interaction with singlet oxygen. The decrease in absorption at a wavelength specific to DPBF with time of irradiation {corresponding to UTriCOOHPhOH and UTriCOOHPhO–TAT, (Fig. S8 and S9, ESI†)} was plotted and the results are shown in Fig. 7(a)–(d), respectively. These figures indicate that both UTriCOOHPhOH and UTriCOOHPhO–TAT have the ability to generate singlet oxygen.
Fig. 7. Graphical representation of the singlet oxygen generation ability of UTriCOOHPhOH [(a) and (b)] and UTriCOOHPhO–TAT [(c) and (d)] in terms of the decline in absorbance of DPBF (25 μM) upon irradiation, at a wavelength specific to the porphyrin moiety [λ = 519 and 521 nm for UTriCOOHPhOH (2 μM) and UTriCOOHPhO–TAT (2 μM), respectively], at different time points ranging from 0 to 14 minutes.
Radiolabeling studies
Radiolabeling of UTriCOOHPhOH and UTriCOOHPhO–TAT was carried out with 68Ga in order to evaluate their ability to target the tumorous lesions in the small animal model. The radiolabeling was monitored using RP-HPLC, where free 68Ga exhibited a retention time (Rt) of = 3.9 min [Fig. 8(a)]. The UV signals corresponding to UTriCOOHPhOH and UTriCOOHPhO–TAT were observed at Rt = 25.4 and 19.9 min, respectively. The percentage radiochemical yields for 68Ga-labeled-UTriCOOHPhOH and UTriCOOHPhO–TAT were found to be 89.23 ± 1.50 and 22.20 ± 1.69, respectively. However, post-radiolabeling purification, both 68Ga-labeled UTriCOOHPhOH and 68Ga-labeled UTriCOOHPhO–TAT could be prepared with a radiochemical purity >95%.
Fig. 8. Profiles depicting the retention times (x-axis) for (a) radio-HPLC: free/unlabeled 68GaCl3, (b) radio-HPLC: 68Ga-labeled UTriCOOHPhOH, (c) UV-HPLC: natGa–UTriCOOHPhOH, (d) radio-HPLC: 68Ga-labeled UTriCOOHPhO–TAT, and (e) UV-HPLC: natGa-UTriCOOHPhO–TAT.
Synthesis of non-radioactive gallium complexes of UTriCOOHPhOH and UTriCOOHPhO–TAT
The formation of natGa complexes of UTriCOOHPhOH and UTriCOOHPhO–TAT was confirmed by HPLC as well as UV-Vis spectrophotometry. In HPLC, the retention times of natGa-UTriCOOHPhOH and UTriCOOHPhO–TAT [(19.6 and 17.8 min, Fig. 8(c) and (e), respectively] were found to be in close agreement with those obtained for the corresponding 68Ga-labeled complexes (21.1 and 18.2 min) [Fig. 8(b) and (d), respectively]. The HPLC profiles indicated the quantitative formation of natGa-UTriCOOHPhOH whereas the signature of un-complexed UTriCOOHPhO–TAT was also observed along with that of the natGa-UTriCOOHPhO–TAT complex [Fig. 8(e)]. Additionally, in UV-Vis spectrophotometry, an alteration in the Q-band pattern of the absorption spectra confirmed the formation of natGa-complexes using core protons of the porphyrin ring (Fig. S10, ESI†).
Determination of the partition coefficient (log Po/w)
The partition coefficient for 68Ga-UTriCOOHPhOH was found to be −1.54 ± 0.01, whereas that for 68Ga-UTriCOOHPhO–TAT was determined to be −2.0 ± 0.05. These values indicate that the hydrophilicity of the porphyrin derivative was enhanced further after its coupling with TAT.
Bio-distribution studies
The bio-distribution studies performed in the tumor bearing small animal model revealed the differential in vivo behaviour of 68Ga-UTriCOOHPhOH and 68Ga-UTriCOOHPhO–TAT (Fig. 9). 68Ga-UTriCOOHPhO–TAT exhibited a substantially higher uptake in tissues and organs, such as blood, lungs, the liver, spleen and kidney (21.85 ± 2.06 and 16.15 ± 0.99, 16.18 ± 2.98 and 15.51 ± 2.34, 22.82 ± 1.26 and 21.79 ± 1.44, 10.74 ± 1.11 and 10.91 ± 0.14 and 8.20 ± 1.78 and 8.58 ± 0.53% IA per g, respectively) compared to that exhibited by 68Ga-UTriCOOHPhOH (9.97 ± 2.97 and 6.87 ± 0.36, 8.87 ± 1.13 and 5.33 ± 0.61, 23.46 ± 0.70 and 15.11 ± 1.48, 5.52 ± 1.19 and 3.23 ± 0.35 and 4.78 ± 0.95 and 3.35 ± 0.35% IA per g, respectively) at 30 and 60 min post-administration, respectively. It is evident from Fig. 9 that 68Ga-UTriCOOHPhO–TAT exhibited a relatively slower clearance from the majority of organs as compared to 68Ga-UTriCOOHPhOH. However, 68Ga-UTriCOOHPhO–TAT exhibited a much higher tumor uptake (3.91 ± 0.66 and 6.32 ± 1.24% IA per g) compared to 68Ga-UTriCOOHPhOH (2.53 ± 0.58 and 2.45 ± 0.88% IA per g) at both post-administration time points.
Fig. 9. Bio-distribution patterns of 68Ga-UTriCOOHPhOH and 68Ga-UTriCOOHPhO–TAT in fibrosarcoma tumor bearing Swiss mice at 30 and 60 min, post-administration (n = 3).
Discussion
In an attempt towards enhancing the cell permeability of the porphyrin derivative, a porphyrin–cell penetrating peptide conjugate (UTriCOOHPhO–TAT) was synthesized to carry out a systemic study to compare the cell internalizing behavior of the porphyrin derivative and its peptide conjugate. For this, an unsymmetrically substituted porphyrin derivative (UTriCOOHPhOH) was synthesized from a commercially available symmetrical porphyrin and was subsequently conjugated with a cell penetrating peptide (TAT), prepared by the Fmoc-solid phase peptide synthesis method. The cell penetrating peptide selected in the present study is a cationic CPP which is known to have affinity towards overall negatively charged cell membranes and helps to facilitate the cell penetration of macromolecules/drugs.24 The formation of the unsymmetrical porphyrin derivative (UTriCOOHPhOH), peptide (TAT) and desired porphyrin–peptide conjugate (UTriCOOHPhO–TAT) was confirmed by various standard spectroscopic and spectrometric techniques. The behavioural pattern of porphyrin (UTriCOOHPhOH) and its peptide conjugate (UTriCOOHPhO–TAT) with respect to cell cytotoxicity, photocytotoxicity, cell internalization, singlet oxygen generation and tumor uptake was investigated and compared through a series of in vitro and in vivo experiments.
Our studies showed that both porphyrin (UTriCOOHPhOH) and the porphyrin–peptide conjugate (UTriCOOHPhO–TAT) have similar ‘dark toxicities’ at all the studied concentrations (p > 0.05). However, light dependent toxicity was observed to be higher for the porphyrin–peptide conjugate (UTriCOOHPhO–TAT) compared to non-conjugated porphyrin (UTriCOOHPhOH) (p < 0.05). The aforementioned finding could probably be attributed to the higher cellular internalization observed for the porphyrin–peptide conjugate. Though both UTriCOOHPhOH and UTriCOOHPhO–TAT demonstrated cell internalizing properties, none of them could exhibit localization in the cell nucleus. This observation is at par with the earlier results reported by Patel, et al. where nuclear localization was observed to be absent for cationic CPPs.25
Radio-complexation of UTriCOOHPhOH and UTriCOOHPhO–TAT with 68Ga resulted in the formation of 68Ga-UTriCOOHPhOH and 68Ga-UTriCOOHPhO–TAT with different radiochemical yields. A comparison between the radiochemical conditions utilized for 68Ga-labeling in the porphyrin core in the present study and those reported in the literature is shown in Table 1. Both conventional heating and microwave reaction conditions have been employed for enabling 68Ga labelling in the porphyrin core.28,29 Bryden et al. reported lower radiolabelling yields for the porphyrin–peptide conjugate in their study under conventional heating and an increase in percentage radiolabelling yields upon shifting to microwave heating. Fazaeli et al. reported a higher yield (>98%) for 68Ga-labeling of a porphyrin moiety having a hydrophobic core under a conventional heating environment. In the present study, a high radiolabelling yield was achieved (89.23 ± 1.50%) within 30 min when the porphyrin derivative was heated with 68Ga in a water bath. However, a substantially low radiolabelling yield was achieved when the same porphyrin derivative was allowed to react with 68Ga under the same set of conditions, after peptide conjugation. This could be attributed to the lesser availability of the porphyrin core for metal insertion due to the possibility of some non-covalent interactions between the porphyrin and peptide in UTRiCOOHPhO–TAT.30
Comparison of the radiolabeling parameters employed for 68Ga-labeling of the porphyrin core in the present study with those reported in the literature.
| Reaction conditions | Present study | Fazaeli et al. (2012)28 | Zoller et al. (2013)5 | Bryden et al. (2015)29 |
|---|---|---|---|---|
| Conventional/microwave | Conventional | Conventional | Conventional and microwave | Microwave |
| 68Ga | 0.05 M HCl | Acidic | 0.05 M HCl in acetone (2.4%) | 0.6 M HCl |
| pH (reaction mixture) | 5.6 | 5.5 | — | Highly acidic |
| Temperature of incubation | 100 °C/boiling | 100 °C/boiling | 170 °C, 150 W (microwave) & 90 °C (conventional) | 110 °C, 100 W |
| Time of incubation | 30 min | 60 min | 15 min (conventional) >3 min (microwave) | 5 min |
| Radiochemical yield (RCY) | 89.23 ± 1.50 22.20 ± 1.69 for 68Ga-UtriCOOHPhOH and 68Ga-UTriCOOHPhO–TAT, respectively | >98% | <20% (conventional) & 49 and 69% (microwave) in an aqueous medium | >95% |
It is worth mentioning that there are limited literature reports which have documented the in vivo studies of radiolabeled TAT conjugates.25,26 In the present work, bio-distribution studies in tumor bearing mice demonstrated a higher accumulation of activity in tumorous lesions for the 68Ga-UTriCOOHPhO–TAT complex compared to that observed for the 68Ga-UTriCOOHPhOH complex. However, the former also exhibited a higher uptake in the majority of non-target tissues and organs (blood, lungs, the liver, spleen and kidneys), which could be attributed to the influence of the CPP (TAT) towards enhancing the uptake in the organs/tissue. Another possible reason for the observed higher uptake for the radiolabeled porphyrin–peptide conjugate (68Ga-UTriCOOHPhO–TAT) in lungs, the liver and spleen could either be the immunogenicity associated with the cationic cell penetrating peptides, as reported in the contemporary literature or the enhanced tissue permeability reported to be associated with such peptides.25 However, the aforementioned observations are contrary to the results published by Sarko et al., wherein relatively lower organ uptakes for 111In-DOTA–TAT in nu/nu female mice bearing PC3 tumors were reported. Sarko et al. employed a hydrophilic DOTA chelator for radiometal complexation which resulted in faster plasma clearance whereas in the present study no additional bifunctional chelator was used as the porphyrin skeleton is known to form a stable 68Ga-chelate. Our present study demonstrated that the potency of a CPP towards enhancing the efficacy of a porphyrin derivative is not only towards expediting higher photocytotoxic effects but also promoting its uptake in tumorous mass.26 This approach can be exploited for designing and developing porphyrin-based photosensitizing agents for theranostic applications. However, it should be noted that the attachment of a CPP also increases the non-specific organ uptake of the porphyrin–TAT conjugate. This indicates the necessity of further tailoring the structure of the porphyrin–peptide conjugate, so as to maneuver its hydrophilicity/lipophilicity and obtain the desired in-vivo outcome.
Conclusion
The results of the present study revealed that there was a definite advantage of CPP conjugation with porphyrin towards greater cellular internalization enabling a relatively higher photocytotoxicity in cancer cell lines relative to bare porphyrin. The effect of the presence of TAT as a CPP in the 68Ga-labeled porphyrin–peptide conjugate was reflected during in vivo studies wherein greater tumor localization was observed compared to that obtained corresponding to 68Ga-labeled porphyrin alone. Though the promising results of in vitro investigation accompanied by higher tumor uptake may promote further investigation by attaching different porphyrin derivatives, the un-desired uptake in non-target organs will be an issue. Also, the preparation of such radiolabeled CPP–porphyrin conjugates using relatively long-lived radionuclides will help to obtain data towards their long-term distribution and retention characteristics.
Disclosure
Bhabha Atomic Research Centre is a constituent unit of the Department of Atomic Energy (DAE), Government of India and all the research activities carried out at this Institute are fully funded by the Government of India.
Conflicts of interest
The authors declare that they have no conflict of interests.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Dr. S. Kannan, Director, Radiochemistry and Isotope Group, Bhabha Atomic Research Centre (BARC) for his constant support and encouragement. The authors are thankful to Dr. Ankona Dutta, Ms. Geetanjali Dhotre and the other staff members of the MALDI-TOF facility of the Tata Institute of Fundamental Research (TIFR), Mumbai for allowing and helping us to record the mass spectra of the compounds using MALDI-TOF MS. The authors also express their gratitude to Ms. Mamata Joshi of the National NMR Facility, TIFR, Mumbai for providing the facility for recording the 1H- and 13C-NMR spectra reported in the article. The authors also thank Mr. Umesh Kumar of the Radiopharmaceuticals Division, BARC and all the staff members of the Animal House Facility, Radiation Biology and Health Sciences Division, BARC for the help they provided during the course of the present study.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2md00097k
References
- Waghorn P. A. Radiolabeled porphyrins in nuclear medicine. J. Labelled Compd. Radiopharm. 2014;57:304–309. doi: 10.1002/jlcr.3166. [DOI] [PubMed] [Google Scholar]
- Zhou Y. Liang X. Dai Z. Porphyrin-loaded Nanoparticles for cancer theranostics. Nanoscale. 2017;8:12394–12405. doi: 10.1039/C5NR07849K. [DOI] [PubMed] [Google Scholar]
- Rai P. Mallidi S. Zheng X. Rahmanzadeh R. Mir Y. Elrington S. Khurshid A. Hasan T. Development and applications of photo-triggered theranostic agents. Adv. Drug Delivery Rev. 2010;62:1094–1124. doi: 10.1016/j.addr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões A. V. C. Pinto S. M. A. Calvete M. J. F. Gomes C. M. F. Ferreira N. C. Castelo-Brancocd M. Llope J. Pereira M. M. Abrunhosa A. J. Synthesis of a new 18F labeled porphyrin for potential application in positron emission tomography: In vivo imaging and cellular uptake. RSC Adv. 2015;5:99540–99546. doi: 10.1039/C5RA16103G. [DOI] [Google Scholar]
- Zoller F. Riss P. J. Montforts F. P. Kelleher D. K. Eppard E. Rösch F. Radiolabelling and preliminary evaluation of 68Ga-tetrapyrrole derivatives as potential tracers for PET. Nucl. Med. Biol. 2013;40:280–288. doi: 10.1016/j.nucmedbio.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Bhadwal M. Mittal S. Das T. Sarma H. D. Chakraborty S. Banerjee S. Pillai M. R. A. Synthesis and biological evaluation of 177Lu-DOTA-porphyrin conjugate: a potential agent for targeted tumor radiotherapy detection. Q. J. Nucl. Med. Mol. Imaging. 2014;58:224–233. [PubMed] [Google Scholar]
- Bhadwal M. Das T. Sarma H. D. Banerjee S. Radiosynthesis and bioevaluation of 68Ga-labeled 5,10,15,20-tetra(4-methylpyridyl)-porphyrin for possible application as a PET radiotracer for tumor imaging. Mol. Imaging Biol. 2015;17:111–118. doi: 10.1007/s11307-014-0760-1. [DOI] [PubMed] [Google Scholar]
- Mittal S. Bhadwal M. Das T. Sarma H. D. Chakravarty R. Dash A. Banerjee S. Pillai M. R. A. Synthesis and biological evaluation of 90Y-labeled DOTA-Porphyrin conjugate: A potent molecule for targeted tumor therapy. Cancer Biother. Radiopharm. 2013;28:651–656. doi: 10.1089/cbr.2013.1512. [DOI] [PubMed] [Google Scholar]
- Das T. Chakraborty S. Sarma H. D. Banerjee S. Radiochim. Acta. 2012;96:427–433. doi: 10.1524/ract.2008.1505. [DOI] [Google Scholar]
- Thomas G. S. Resonance Raman Spectroscopy as a Probe of Heme Protein Structure and Dynamics. Adv. Protein Chem. 1985;37:111–159. doi: 10.1016/S0065-3233(08)60064-9. [DOI] [PubMed] [Google Scholar]
- Qi Z. L. Cheng Y. H. Xu Z. Chen M. L. Recent Advances in Porphyrin-Based Materials for Metal Ions Detection. Int. J. Mol. Sci. 2020;21:5839. doi: 10.3390/ijms21165839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh J. Vicente M. G. H. Mechanisms of porphyrinoid localization in tumors. J. Porphyrins Phthalocyanines. 2002;5:305–324. doi: 10.1142/S1088424602000373. [DOI] [Google Scholar]
- Rizzuti M. Nizzardo M. Zanetta C. Ramirez A. Corti S. Therapeutic applications of the cell-penetrating HIV-1 Tat peptide. Drug Discovery Today. 2015;20:76–85. doi: 10.1016/j.drudis.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Jing X. Ye B. Huan Z. Shiyan D. Lesheng T. Lee Robert J. Zhaogang Y. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020;11:697. doi: 10.3389/fphar.2020.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari S. Dizaj S. M. Adibkia K. Cell-penetrating peptides and their as novel nanocarriers for drug delivery. BioImpacts. 2015;5:103. doi: 10.15171/bi.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshankhah H. Jafari S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed. Pharmacother. 2018;108:1090–1096. doi: 10.1016/j.biopha.2018.09.097. [DOI] [PubMed] [Google Scholar]
- Heitz F. Morris M. C. Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooga M. Langel U. Classes of Cell-Penetrating Peptides. Methods Mol. Biol. 2015;1324:3–28. doi: 10.1007/978-1-4939-2806-4_1. [DOI] [PubMed] [Google Scholar]
- Frankel A. D. Pabo C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Koren E. Apte A. Sawant R. R. Grunwald J. Torchilin V. P. Cell-penetrating TAT peptide in drug delivery systems: proteolytic stability requirements. Drug Delivery. 2011;18:377–384. doi: 10.3109/10717544.2011.567310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno J. C. Ngwa V. M. Nowak S. J. Chrestensen C. A. Healey A. N. McMurry J. L. Novel cell-penetrating peptide-adaptors effect intracellular delivery and endosomal escape of protein cargos. J. Cell Sci. 2016;129:893–897. doi: 10.1242/jcs.192666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant R. Torchilin V. Intracellular delivery of nanoparticles with CPPs. Methods Mol. Biol. 2011;683:431–451. doi: 10.1007/978-1-60761-919-2_31. [DOI] [PubMed] [Google Scholar]
- Srinivasan D. Muthukrishnan N. Johnson G. A. Erazo-Oliveras A. Lim J. Simanek E. E. Pellois J. P. Conjugation to the cell-penetrating peptide TAT potentiates the photodynamic effect of carboxytetramethylrhodamine. PLoS One. 2011;14:e17732. doi: 10.1371/journal.pone.0017732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J. Bi Y. Zhang H. Dong S. Teng L. Lee R. J. Yang Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020;11:697. doi: 10.3389/fphar.2020.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. G. Sayers E. J. He L. Narayan R. Williams T. L. Mills E. M. Allemann R. K. Luk L. Y. Jones A. T. Tsai Y. H. Cell-penetrating peptide sequence and modification dependent uptake and subcellular distribution of green florescent protein in different cell lines. Sci. Rep. 2019;9:6298. doi: 10.1038/s41598-019-42456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarko D. Beijer B. Garcia Boy R. Nothelfer E. M. Leotta K. Eisenhut M. Altmann A. Haberkorn U. Mier W. The Pharmacokinetics of Cell-Penetrating Peptides. Mol. Pharmaceutics. 2010;7:2224–2231. doi: 10.1021/mp100223d. [DOI] [PubMed] [Google Scholar]
- Shirasu N. Nam S. O. Kuroki M. Tumor-targeted photodynamic therapy. Anticancer Res. 2013;33:2823–2832. [PubMed] [Google Scholar]
- Fazaeli Y. Jalilian A. R. Amini M. M. Ardaneh K. Rahiminejad A. Bolourinovin F. Moradkhani S. Majdabadi A. Development of a 68Ga-fluorinated porphyrin complex as a possible PET imaging agent. Nucl. Med. Mol. Imaging. 2012;46:20–26. doi: 10.1007/s13139-011-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden F. Savoie H. Rosca E. V. Boyle R. W. PET/PDT theranostics: synthesis and biological evaluation of a peptide-targeted gallium porphyrin. Dalton Trans. 2015;44:4925–4932. doi: 10.1039/C4DT02949F. [DOI] [PubMed] [Google Scholar]
- Kovaric B. C. Kokona B. Schwab A. D. Twomey M. A. de Paula J. C. Fairman R. Self-Assembly of Peptide Porphyrin Complexes: Toward the Development of Smart Biomaterials. J. Am. Chem. Soc. 2006;128:4166–4167. doi: 10.1021/ja056357q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.