Abstract
Various reports have been published based on covalently attaching biomolecules to polyaniline (PANI). The functional groups connected to the surface of polymeric units determine the immobilization method as well as the method of detection. The present mini-review aims at covering recent advances in the field of protein binding and detection using PANI. Several proteins have been attached to the polymer using different immobilization techniques. The application of PANI in protein detection has also been discussed along with the future scope of these materials in diagnosis and detection.
1. Introduction
Polyaniline (PANI) was discovered during the late 19th century, as a semi-flexible conducting polymer, which established itself as one of the versatile materials in all well-known areas of science and technology including the electrochromic devices,1,2 bio-actuators3,4 solar cells,5,6 tissue engineering7,8 and biosensors.9,10 Its advantages, studied so far, include multiple color transitions, depending upon the pH of its synthesis and its oxidation states; its tunable conductivity and maintenance of electrochemical behavior by monitoring the surrounding pH, type of dopant and doping intensity, oxidation state of PANI, its morphology, thickness, design; electrochemical, chemical, and environmental stability due to strong and stable heterocyclic aromatic backbone; ease of processability due to its simple synthetic methodology and its high solubility in innumerable solvents; and capability to fabricate versatile composites, nanocomposites, nano-biocomposites in view of its chemical skeleton rich of functionalities and low cost. These advantages make PANI a material known for wide spectrum of applications. All of these features are related to its chemical structure. Chemically, this polymer comprises of “n” number of reduced benzenoid diamine and “m” number of oxidized quinoid diamine repeating units, where the oxidation state of polyaniline (PANI) depends on the value of “m.” The three different redox forms of PANI are: leucoemeraldine, emeraldine, and pernigraniline the having m : n ratio of 0 : 1, 1 : 1, and 1 : 0, respectively, Fig. 1. Besides the imine groups, the amine groups in the polymer chains can further be protonated in the presence of H+ (acidic) ion to generate cationic defects (polarons and bipolarons) that are responsible for the conductivity and redox behavior of the polymer. The two forms i.e. the unprotonated and protonated forms of PANI are known as base and salt, respectively. Conclusively, the conductivity of PANI can be tuned by the use of different doping agents, varying the extent of doping, and also by controlling the chain length and morphology including the porosity and dimensions.
Fig. 1. Different redox states of PANI.
2. Synthesis techniques of PANI
PANIs are synthesized using both chemical and electrochemical oxidative polymerization techniques in acidic medium.11,12 Usually, the electrochemical method is used for the small scale synthesis, whereas the chemical method allows large-scale preparation of the polymer and/or the corresponding nanocomposites. The electrochemical methods include the electrode coating and co-deposition approaches. In the electrode coating method, reference, working, and counter electrodes are used in a one compartment cell containing the electrolyte and the monomer solution.13,14 In the co-deposition method, an insulating polymer host is dissolved in an electrolyte solution comprising the monomer of the conductive polymer. Potentiodynamic and galvanostatic procedures have been used to prepare PANI. The electrochemical method has a number of advantages over the chemical method such as easy approach, attainment of homogeneous films, purity of the end product. In the process of chemical oxidation polymerization, the polymer PANI is synthesized by utilizing HCl/H2SO4 as a dopant and ammonium persulfate (APS)/FeCl3 as an oxidant under aqueous environmental conditions.15,16 Afterwards, a proton can be removed by means of an oxidant from the monomer of aniline entity without creating a new bond, and with the absolute product. Ammonium sulfate ((NH4)2S2O8), sodium vanadate (NaVO3), hydrogen peroxide (H2O2), potassium dichromate (K2Cr2O7), cerium sulfate (Ce(SO4)2), potassium free cyanide (K3[Fe(CN)6] and potassium iodate (KIO3) can be used as oxidants.17,18
3. Applications in protein detection
PANI is highly efficient in detection and sensing due to its redox behaviour and the capacity to mediate electron transfer between the reaction site and the electrode surface via biomolecules. The existence of redox couples in PANI facilitates charge transfer activities, making it an excellent option for electrochemical biosensor development.19–25 The consistent and sensitive interdependence between the electrochemical response and the pH of the electrolytic solution opens up new possibilities for developing pH sensitive electrochemical biosensors for analytes that generate either acidic or basic moieties as end products of any biochemical reaction, such as triglycerides. The amine enriched chemical backbone of PANI offers a wide range of possibilities for binding/immobilizing biomolecules. Several studies have been reported on the design and fabrication of PANI based protein sensors as summarized in Table 1.
PANI based protein sensors with their detection limits.
| Sensor composition | Method of detection | Analyte | Detection limit |
|---|---|---|---|
| Single PANI nanowire19 | Conductance | Immunoglobulin G (IgG) | 3 ng mL−1 |
| Myoglobin (Myo) | 1.4 ng mL−1 | ||
| PANI molecularly imprinted polymers (MIPs)20 | Luminescence quenching | Horseradish peroxidase (HRP) | 1.00 on glass slips 0.07 ng mL−1 on polycapillaries |
| Molecular imprinted MIP-PANI21 | Electrochemical | Ovalbumin (OVA) | 10−12 mg mL−1 |
| Aptamer functionalized PANI nanowire22 | Electrochemical | Immunoglobulin E (IgE) | 0.56 pg mL−1 (signal-to-noise ratio of 3) |
| Whatman filter paper coated with PANI23 | Impedance | Myoglobin (Myo), Myeloperoxidase (MPO) | 500 ng mL−1 |
| PANI and electro-deposited gold (Au) nanocrystals (NCs)24 | Impedance | Human serum albumin (HSA) | 300 μg mL−1 cell lysate and actin protein |
| Single site-specific polyaniline (PANI) nanowire25 | Conductometric sensing | Cardiac troponin-I (cTnI), myoglobin (Myo), creatine kinase-MB (CK-MB), and type-b natriuretic peptide (BNP) | cTnI (250 fg mL−1), Myo (100 pg mL−1), CK-MB (150 fg mL−1), BNP (50 fg mL−1) |
| 2-D PANI nanostructures on both non-flexible (SiO2) and polyethylene terephthalate and polyimide flexible substrates26 | Electrochemical | B-type natriuretic peptide (BNP) | 100 pg mL−1 |
| PANI-Au modified paper working electrodes27 | Electrochemical | Carcinoembryonic antigen (CEA) and fetoprotein (AFP) | 0.5 and 0.8 pg mL−1 |
| Electrospun polystyrene (PS)/PANI nanofiber28 | Electrochemical | C-reactive protein (CRP) | 100 fg mL−1 |
| PANI electrochemically deposited on Si29 | Electrochemical | Human IgG antigen | 5 μg mL−1 |
| Acrylic acid (AAc) is grafted PANI30 | Surface adsorption studies | ICHA antigen | 50 μg mL ICHA concentration surface covering achieved |
| PANI nanowires31 | Electrochemical | Immunoglobulin G (IgG) | 0.27 ng mL−1 |
| MoS2–PANI gold nanoparticles32 | Electrochemical | C-reactive protein (CRP) | 40 pg mL−1 |
| PANI films33 | Electrochemical | Cholesterol oxidase (CHOx), cholesterol esterase (ChEt) | 25 mg dL−1 |
| Nanostructured PANI sheets34 | Electrochemical | Cholesterol oxidase | 25–500 mg dL−1 |
| PANI nanospheres35 | Electrochemical | Cholesterol oxidase | 25–500 mg dL−1 |
| Polyaniline nanospheres36 | Electrochemical | DENV NS1 antibody | 0.33 ng mL−1 |
| PANI film38 | Electrochemical | Cholesterol oxidase, cholesterol peroxidase, and cholesterol esterase | 25 mg dL−1 |
| PANI nanowire and hyaluronic acid39 | Electrochemical | Carcinoembryonic antigen (CEA) | 0.0075 pg mL−1 |
| PANI–Au nanocomposite40 | Electrochemical | Cholesterol oxidase | 37.89 mg dL−1 |
| PANI and carboxylated PANI on graphene oxide (GO)41 | Electrochemical | cTnT for cardiac troponin T probe | 0.008 ng mL−1 |
| Manganese sulfide nanoparticles/graphene oxide/polyaniline (MnS/GO/PANI) and magnetite incorporated gold nanoparticles (AuNPs@Fe3O4)42 | Electrochemical | Tau protein | 10−14 M |
| PANI/CNT43 | Electrochemical | Vascular endothelial growth factor (VEGF165) | 0.4 pg mL−1 |
| PANI nanowires44 | Electrochemical | COVID-19 N-gene | 3.5 fM |
Proteins can be immobilized showed that the detection/sensing of proteins like immunoglobulin G (IgG) and myoglobin (Myo) is based on measuring the conductance changes of PANI in response to target proteins and their various concentrations, Fig. 2.
Fig. 2. (A) SEM image of non-functionalized single PANI nanowire. (B) SEM image of functionalized single PANI nanowire with IgG mAbs. (C) Measurement of conductance changes on single non-functionalized PANI nanowire (a: PBS; b: BSA, 10 μg mL−1; and c: IgG, 3.3 μg mL−1). (D) Specificity test of functionalized single PANI nanowire with IgG mAbs (a: PBS; b: BSA, 50 ng mL−1; and c: IgG, 33 ng mL−1) (reprinted with permission from Elsevier, ref. 19 Lee et al., (2011)).
The Fig. 2(A) shows non-functionalized single PANI of width 105.4 nm while the Fig. 2(B) revealed the deposition of few particles ranging between 10 nm to 15 nm after functionalization of PANI nanowire which were speculated to be immobilized IgG mAbs. The IgG protein was examined and the feasibility of a single PANI nanowire biosensor was investigated via with a conductometric mechanism, Fig. 2(C) and (D). There were no changes in the non-functionalized PANI nanowire biosensor. The conductance of the functionalized single PANI nanowire biosensor with IgG towards monoclonal antibodies (mAbs) increased with PBS injection due to a change in the surface charge generated by the net electrical field. The unique IgG (33 ng mL−1) response after BSA injection, demonstrated the biosensor's high sensitivity for IgG detection. IgG mAbs exhibited stepwise changes upon varying the concentrations of IgG. The lowest limit of detection was 3 ng mL−1 for IgG and 1.4 ng mL−1 for Myo, while a clear non-response towards BSA was observed at concentrations as high as 5 μg mL−1.
Pidenko et al.20 proposed a facile and fast approach for the synthesis of PANI molecularly imprinted polymers (MIPs) based on oxidative chemical polymerization of aniline monomers that were used for protein recognition. It was for the first time that the strategy of surface imprinting was implemented for the synthesis of PANI-MIPs on the inner surface of glass polycapillaries (PC) having 2237 number of microcapillaries. Uniform and stable PANI films were synthesized by means of oxidative polymerization at around pH less than 1. PANI MIP nanowires were however synthesized at “milder” conditions (pH greater than 4.5) to keep the protein template activity. The binding ability of horseradish peroxidase (HRP) molecules on the as-synthesized PANI-MIP selective sites was confirmed by means of photometry, FTIR spectroscopy and SEM images. The as developed PANI-MIPs enabled HRP determination with low limit of detection (LOD) i.e. 1.00 on glass slips and 0.07 ng mL−1 on PC. Luo et al.21 used ovalbumin (OVA) as a template protein which was trapped inside the PANI nanoparticles (NPs), resulting in the production of molecular imprinted MIP-PANI, Fig. 3(a).
Fig. 3. (a) Schematic illustration of the synthesis of MIP-PANI particles and the fabrication of MIP-PANI sensor, (b) EIS curves and (c) CV curves of MIP-PANI particles modified electrode in [Fe(CN)6]3−/4− solution before extraction, after extraction and after rebinding OVA by incubation in 0.5 mg mL−1 OVA solution (reprinted with permission from Elsevier, ref. 21 Luo et al., (2017)).
The PANI sensor not only showed good selectivity towards template protein (the imprinting factor was 5.31), but also significantly lower detection limit of 10−12 mg mL−1, outperforming most of the reported OVA detecting methods. Furthermore, a response time of less than 3 minutes was demonstrated. The water compatibility as well as large specific surface area of PANI particles, and electrical conductivity of PANI, provided a direct channel for electron conduction from the imprinting sites to the electrode surface, Fig. 3(b) and (c). Based on the immobilisation of the immunoglobulin-E (IgE) aptamer onto a single PANI nanowire electrochemically produced in a facile and controllable method, Luo et al.22 synthesized a highly sensitive, conductometric, and label-free biosensor for the detection of IgE. Myoglobin (Myo) and Myeloperoxidase (MPO) cardiac biomarkers were detected using a disposable, label-free impedance-based biosensor. Mondal et al.23 fabricated a biosensor out of Whatman filter paper that was coated with PANI, Fig. 4. Prior to the covalent attachment of antibodies unique to each biomarker protein, the PANI-coated paper was functionalized with glutaraldehyde. Treatment with BSA blocked the non-specific active areas of the sensor. Myo and MPO in buffer solution were detected at concentrations ranging between 100 ng mL−1 and 50 g mL−1. For these cardiac biomarkers spiked in human serum, the detection limit was 1 g mL−1. The lowest detection limit was improved to 500 ng mL−1 after further blocking with human serum albumin (HSA). The developed biosensor was appropriate for diagnostic applications due to its low-cost fabrication procedure, detection of cardiac biomarkers in clinically relevant quantities quickly (i.e., within 20 minutes), and environmentally acceptable disposable nature. The presence of minute levels of HSA in urine, also known as microalbuminuria (30–300 g mL−1) was utilized as a useful clinical diagnostic method used for detecting chronic kidney disease (CKD). Shiakh et al.24 provided a report in which they had used electrochemical impedance spectroscopy for the creation of a low-cost, disposable immunosensor for the specific, sensitive, and label-free detection of HSA, Fig. 5.
Fig. 4. Functionalization of PANI coated filter paper. The amine groups of PAni bonds with the one of the two aldehyde (–CHO) groups of Glut. The other –CHO group is available for covalent bonding with the amine groups of the antibodies. A wash step is present between each modification step (reprinted with permission from IEEE, ref. 23 Mondal et al., (2017)).
Fig. 5. Schematic of the systematic protocol for SPCE surface modification and immunosensing. PANI – polyaniline; AuNCs – gold nanocrystals; HSA – human serum albumin; Ab-HSA – anti-human, serum albumin antibody; BSA – bovine serum albumin; EIS – electrochemical impedance spectroscopy (reprinted with permission from MDPI, ref. 24 Shaikh et al., (2019)).
In order to develop the carbon-based tri-electrode system on some flexible plastic substrates, a straightforward one-step screen-printing process was utilized. The carbon working electrode was later on modified with some electro-polymerized PANI and electro-deposited gold (Au) nanocrystals (NCs) to enable an effective antibody immobilization and increased sensitivity. The PANI matrix functioned as a nanostructured scaffold for uniform distribution of the Au NCs, resulting in an increased immunosensor response. SEM was used to study the PANI/Au NCs-modified working electrode surface, and electrochemical impedance spectroscopy (EIS) was used to investigate the electrochemical response at every step in a probe solution of ferri/ferrocyanide. The immunosensor showed good selectivity as normalized impedance variation of 3.6% ± 0.6% and 4.7% ± 1% was observed for immunosensing with 300 μg mL−1 cell lysate and actin protein, and was lower for the same concentration of HSA. In order to diagnose the cardiovascular disorders, proteins like cardiac troponin-I (cTnI), myoglobin (Myo), creatine kinase-MB (CK-MB), and type-b natriuretic peptide (BNP) must be detected. Lee et al.25 showed that single-site-specific PANI nanowire biosensors had ultra-high sensitivity and specificity for cardiac biomarkers, Fig. 6. Very low amounts of Myo (100 pg mL−1), cTnI (250 fg mL−1), CK-MB (150 fg mL−1), and BNP (50 fg mL−1) were detected using single PANI nanowire-based biosensors connected with microfluidic channels. For concentrations ranging from a few hundreds (fg mL−1) to tens of hundreds (ng mL−1), the single PANI nanowire-based biosensors showed linear sensing characteristics.
Fig. 6. An illustration and the experimental set-up of the single polyaniline (PANI) nanowire biosensor to detect cardiac biomarkers. (a) The experimental setup; the microfluidic channel is adhered on the nanowire biosensor and the nanowire biosensor chip is mounted on a probe station connected to the semiconductor analyzer and syringe pump with inlet and outlet; (b) the conductance change in the single PANI nanowire-based biosensor is monitored. The injection of PBS (mark a), BSA (mark b), and cardiac biomarker (mark c) shows the different changes of conductance (reprinted with permission from MDPI, ref. 25 Lee et al., (2017)).
In addition to that, the devices demonstrated a quick response of minutes that met the reference conditions for Myo, CK-MB, BNP and cTnI diagnosis of heart failure, as well as determining the severity of heart failure. PANI also showed strong biocompatibility with monoclonal antibodies, due to which its nanowire-based biosensor displayed superior biosensing reliability, label-free detection, and highly improved processing charge efficiency.
Liu et al.,26 designed field-effect transistor (FET) biosensors based on two-dimensional (2-D) PANI nanostructures on both non-flexible (SiO2) and polyethylene terephthalate and polyimide flexible substrates. The biosensors were made using a simple and low-cost technology that combined top-down and bottom-up methods. The development of a low-temperature bilayer method greatly enhanced the yield of flexible electronics. Chemically produced PANI nanostructures demonstrated outstanding p-type semiconductor characteristics as well as flexibility in design. The produced biosensors displayed remarkable sensing capability in detecting B-type natriuretic peptide (BNP) biomarkers, with the 2-D PANI nanostructure being as thin as 80 nm and an extraordinarily large surface-area-to-volume (SA/V) ratio due to the intrinsic features of PANI chemical production. Both designs showed excellent repeatability and high specificity, with detection limits as low as 100 pg mL−1. The controllable conductance fluctuations of less than 20% with good restorability of the PANI nanostructure under bending conditions were also examined. Li et al.27 carried out the simultaneous measurement of two tumour markers, carcinoembryonic antigen (CEA) and α-fetoprotein (AFP), in actual human serum samples using PANI-AuNP-modified paper working electrodes (PWEs). PANI/Au-PWE exhibited good biocompatibility and retained good stability for the sandwich-type immunoassay. The multiplex immunoassay showed linear ranges of over 4 orders of magnitude with the detection limits being down to 0.5 and 0.8 pg mL−1 respectively for CEA and α-fetoprotein (AFP).
Anwar et al.28 designed a “label-free” biosensor for detecting the cardiovascular risk biomarker C-reactive protein (CRP). An electrospun polystyrene (PS)/PANI nanofiber was used as the biosensor's sensing element, which was coupled to an electrical measurement apparatus. Size-matched confinement for micro biomolecule immobilization was possible using nanoscale fibers with a high aspect ratio and porosity, resulting in faster transmission and higher signal intensity. At a concentration of 100 fg mL−1, CRP was successfully detected in human serum, demonstrating that this lab-on-chip technique could be used to detect cardiovascular risk early. Less than 10% cross-reactivity with human albumin demonstrates the suggested biosensor's selective detection capabilities. PANI was electrochemically deposited on a silicon (Si) substrate by Deep et al.29 to form thin nanostructured films with a thickness of around 50 nm. These films were coated with avidin and subsequently immobilised with biotinylated anti-human IgG to generate a sensing bioelectrode. The deposited PANI thin layers on the Si substrate were examined using atomic force microscopy (AFM), Fig. 7(a).
Fig. 7. (a) AFM topography of the electrodeposited PANi film on Si surface; line (left image) and region (right image) analysis to estimate the average height of the deposited film. Data suggest the formation of thin film of 45–50 nm with globular PANi structures, (b) current–voltage characteristic (regression data) of the PAni–Si substrate and the formed biosensor ‘anti-human IgG–PAni–Si’. Ohmic conduction characteristics are evident with average conductivities of 45 μS for the PAni–Si substrate and 32 μS for the formed bioelectrode (reprinted with permission from Elsevier, ref. 29 Deep et al., (2012)).
AFM revealed globular PAni nanostructures and the film thickness was investigated to be around 45–50 nm. Growth of PAni as globular nanostructures on the p-Si(1 1 1) wafers absorbed atmospheric O2 that caused an inhomogeneous thin oxide layer with number of defects serving as the active sites for the assembly of PANI in nanostructured pattern. The biomolecules were successfully immobilized, according to Raman spectrometric studies. The current–voltage characteristics of the built sensor were utilized to calculate the concentration of human IgG antigen in the range of 5–550 μg mL−1, Fig. 7(b). The suggested biosensor's detection limit and sensitivity are 5 μg mL−1 and 0.15 S ppm−1 human IgG, respectively.
Crombrugghe30 synthesized PANI grafted transducer. Bioreceptors were covalently bonded to the polymeric surface to achieve selective and efficient bioanalyte detection. Using typical coupling agents, carboxylic groups can be used to covalently immobilise proteins like the ICHA antigen (carbodiimide and succinimide). A surface covering of 50 μg mL−1 ICHA concentration, was attained which did not form a compact monolayer. Li et al.31 fabricated a highly sensitive and antifouling biosensing interface based on the completely cost-effective and inert protein bovine serum albumin (BSA) cross-linked with PANI nanowires (NWs), Fig. 8.
Fig. 8. Schematic illustration of the fabrication process of the electrochemical IgG biosensor based on a BSA/PANI-NW antifouling interface (reprinted with permission from American Chemical Society, ref. 31 Li et al., (2021)).
The cross-linking of BSA demonstrated a relatively significant antifouling capacity when compared to physically adsorbed BSA, which was widely utilized to inhibit nonspecific adsorption/binding of proteins. An electrochemical biosensor with outstanding sensitivity and selectivity was produced using a suitable immobilisation of the peptide aptamer for protein immunoglobulin G (IgG) recognition onto the BSA in PANI-NW interface. The IgG biosensor that was developed had a linear range of 1.0 ng mL−1 to 10 g mL−1 and a very low detection limit of 0.27 ng mL−1, and was capable of accurately assaying IgG in complex human serum samples. The IgG biosensor showed a linear range of 1.0 ng mL−1 to 10 g mL−1 and a low detection limit of 0.27 ng mL−1 as compared to the findings obtained using commercial enzyme-linked immunosorbent test kits.
Molybdenum sulphide PANI gold nanoparticles (MoS2–PANI–GNPs) with a large surface area and many adsorbing sites significantly increased antibody loading amounts and hence improved biosensing performance. The proposed immunosensor was used to determine C-reactive protein (CRP) in HSA with success.32 The sensing technique had a linear dynamic range of 0.2 to 80 ng mL−1 and a detection limit of 40 pg mL−1. Singh et al.33 used cholesterol oxidase (CHOx), cholesterol esterase (ChEt), and HRP enzymes to modify electrochemically produced PANI films and examine their optical and electrochemical biosensing response to total cholesterol. PANI-based cholesterol oxidase/cholesterol esterase films showed a detection limit of 25 mg dL−1 with sensitivity of 0.042 μA mg dL−1. Dhand et al.34 proposed the use of electrophoretically deposited nanostructured PANI sheets in the production of cholesterol biosensors. For assessing the photometric response of the created bioelectrodes, o-dianisidine was used. Electrophoretically synthesized PANI matrices improved cholesterol oxidase–cholesterol interactions. The PANI based bioelectrode detected cholesterol in a wide range of 25–500 mg dL−1 and a high sensitivity of 3.57 × 10−4 mA mg dL−1 was attained with a low response time of 30 s high electroactive behaviour of PANI matrix. PANI nanospheres (NS) were manufactured utilizing an ethylene glycol-mediated morphological transformation approach and investigated for cholesterol biosensing in another interesting research by Dhand et al.35 The nanospheres film was deposited onto an indium-tin-oxide (ITO) coated glass plate by the solution casting method and was utilized for covalent immobilization of cholesterol oxidase (ChOx) via N-ethyl-N′-(3-dimethylaminopropyl) carbodiimide (EDC) and Nhydroxysuccinimide (NHS). The ChOx/PANI-NS/ITO bioelectrode was able to detect cholesterol in the concentration range of 25 to 500 mg dL−1 with sensitivity of 1.3 × 10−3 mA mg dL−1.
Dutta et al.36 suggested a label-free electrochemical immunosensor based on a PANI-modified GC electrode for the detection of dengue NS1 protein, Fig. 9. The accuracy of NS1 determination was tested using an ELISA dengue analysis kit to compare the results obtained from the immunosensor. The immunosensor detected in-house expressed, commercially acquired, spiked, and blood NS1 samples, demonstrating its sensitivity across a diverse set of samples. The limit of detection was investigated to be 0.33 ng mL−1.
Fig. 9. Schematic representation of construction and development of the immunosensor (reprinted with permission from Avicenna Journal of Medical Biotechnology, ref. 36 Dutta et al., (2020)).
Langer et al.37 used PANI layers with regulated porosity to immobilise choline oxidase in a choline sensor. The obtained sensor was found to be extremely stable, and the electrical response of the sensor stayed constant for more than a month. The electrical response was found to be dependent on choline concentration showing a sensitivity of 5 μA mM−1 in the amperometric mode and of 10 mV mM−1 in the potentiometric mode of measurements.
Singh et al.38 suggested a biosensor for determining cholesterol levels that uses electrochemically produced PANI-enzyme film. Cholesterol oxidase, cholesterol peroxidase, and cholesterol esterase were all co-immobilized to create these films. The biosensor had a 240-second response time, a 42-day shelf life, a sensitivity of 0.042 A mg dL−1, and a detection limit of 25 mg dL−1. Wang and his colleagues39 created a non-fouling electrochemical immunosensor using a hybrid structure made of PANI nanowire and hyaluronic acid (HA) which was then immobilized with carcinoembryonic antigen (CEA) antibodies, Fig. 10.
Fig. 10. Stepwise fabrication process of the immunosensor. (a) The bare GCE, (b) PANI nanowires electrodeposited on the GCE, (c) the formation of HA/PANI composite wires, (d) CEA antibody immobilization, (e) specific CEA capturing and the antifouling towards nonspecific protein and (f) DPV current signal recording (reprinted with permission from Springer, ref. 39 Wang et al., (2018)).
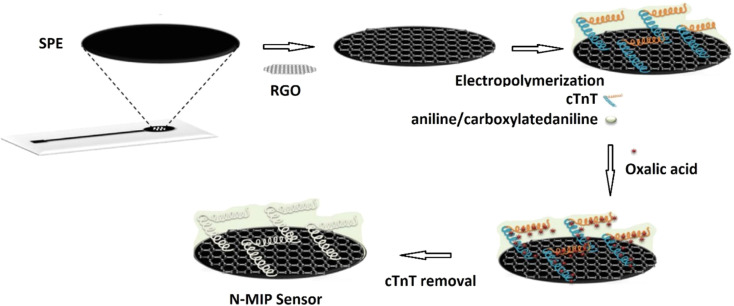
The antifouling efficacy of the PANI/HA surface was evaluated and the detection limit of the immunoelectrode was 0.0075 pg mL−1, with a linear response of 0.01 to 10 000 pg mL−1. Srivastava et al.40 used chitosan (CH) and PANI–Au nanocomposite to manufacture a biosensor with good sensitivity and accuracy for cholesterol detection. Chemically produced PANI/Au nanocomposite was used as a matrix for immobilisation of cholesterol oxidase (ChOx) enzyme on the modified electrode surface, together with CH. The linearity of this enzymatic biosensor was 50–500 mg dL−1, with a detection limit of 37.89 mg dL−1 and a sensitivity of 0.86 mA mg dL−1. It was inferred that this matrix outperforms other cholesterol sensors due to its good immobilisation and enzyme–substrate reactions. Karimi et al.41 created a biomimetic nano-molecularly imprinted polymer (N-MIP) electrode for the ultrasensitive detection of cardiac troponin T based on a graphene screen-printed electrode (cTnT). By electropolymerizing conductive co-polymer matrix of aniline and carboxylated aniline on the graphene oxide (GO) electrode in the presence of template protein (cTnT for cardiac troponin T probe) and cyclic voltammetry (CV), biomimetic cavities for targeted analyte sensing were produced, Fig. 11. The detection limit of the cTnT probe was 0.008 ng mL−1, with a linear range of 0.02 to 0.09 ng mL−1. Lately a tau protein immunosensor based on manganese sulphide (MS) graphene oxide (GO) and PANI (MnS/GO/PANI) with magnetite-incorporated gold nanoparticles immunosensor has been developed for the electrochemical detection of tau protein which exhibited superior selectivity, sensitivity, and environmental compatibility.42 The sensor demonstrated the a detection limit of 1.0 × 10−14 M which could be utilized for diagnosis of Alzheimers disease. Park et al.43 designed an aptasensor based on PANI and carbon nanotube (CNT) for detecting VEGF165 as a tumor marker with a limit of detection of 0.4 pg mL−1 and showed high selectivity in presence of other proteins. Song et al.44 formulated PANI nanowires immobilized with an inverted Y-shaped peptide as an antifouling agent and the specific N-gene capture probe (an antisense oligonucleotide) as a recognition element for the detection of the COVID-19 N-gene (nucleocapsid phosphoprotein gene). The detection limit for the COVID-19 N-gene, reached as low as 3.5 fM even in complex human serum samples which showed immense potential for early detection of COVID-19 viral infection.
Fig. 11. Schematic of the N-MIP sensor (reprinted with permission from Elsevier, ref. 41 Karimi et al., (2019)).
4. Conclusion and future aspects
PANI-based biosensors have received special attention during the past several decades. Different nanostructured PANI composites, PANI 2D films, and PANI nanowires have been investigated and have shown immense selectivity as well as sensitivity towards a variety of protein analytes. PANI based selective probes, including metal oxides and carbon nanotubes have been developed for efficient biosensing platform. Nanostructured PANI shows increased sensitivity and faster response time due to changes in the electrostatic charges from surface adsorption of various protein molecules, which causes accumulation/depletion of charge carriers in PANI. The functionalization of the PANI nanowires could be utilized to improve resolution and good specificity of target detection. The construction of sensing interfaces with protein-cross-linked conducting polymers can be extended for the development of biosensors with high selectivity for assaying of different targets in biological media.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors wish to acknowledge National Science Foundation (Award # 2122044), the NSF PREM for Hybrid Nanoscale Systems between NCCU and Penn State for providing financial assistance.
Biographies
Biography
Ufana Riaz.

Dr Ufana Riaz has lately joined as Assistant Professor at Department of Chemistry and Biochemistry, North Carolina Central University, USA in 2022. She is also an Assistant Professor at Jamia Millia Islamia, New Delhi-110025, India since 2009. She obtained her PhD in 2007. Her research interests include: Development of conducting polymers with outstanding opto-electronic properties for their potential application in photocatalysis, drug delivery, bioimaging and corrosion protective coatings.
Biography
Nuzhat Nabi.

Nuzhat Nabi is presently a doctoral student in her second year at Jamia Millia Islamia, New Delhi in the department of chemistry. Her research dissertation, “Synthesis, Characterization and Biophysical Interaction of Amino Acid Modified Conducting Polymers” focuses mainly on the synthesis of some highly polar conducting polymers and their derivatives that show good binding affinities towards various carrier proteins in the human blood. She has received her M.Phil. Degree in chemistry from Jiwaji University, Gwalior in 2020 and holds a Masters degree in chemistry from Lovely Professional University, Jalandhar in 2019.
Biography
India Pointer.

India Pointer is currently pursuing a Bachelor of Science degree in Chemistry and her undergraduate thesis under the guidance of Prof. Darlene K. Taylor at North Carolina Central University. Her current research is focused on the design and synthesis of semiconducting polymers for organic solar cell devices.
Biography
Amit Kumar.

Dr Amit Kumar is currently Assistant Professor at Department of Chemistry, Jamia Millia Islamia (A Central University), New Delhi (India). He obtained his Ph.D. degree from University of Delhi, Delhi in 2014. His field of specialization is Theoretical Physical Chemistry. His research interests include: Statistical Theory of Complex Polymeric System: Dynamics of Branched Polymers and Polymer Networks along with their Rheological behaviour, Statistical Mechanics, Molecular Dynamics Simulation of biopolymers: proteins, DNA and membranes.
Biography
Darlene. K. Taylor.

Dr Darlene Taylor received her PhD degree from the University of North Carolina at Chapel Hill in Physical Chemistry (1998) and currently holds the rank of Professor in the Department of Chemistry and Biochemistry at North Carolina Central University. She has an adjunct appointment in the Department of Obstetrics and Gynecology at Duke University. Her research focuses on the design and characterization of stimuli responsive materials for potential applications in technologies such as solar cells, membranes, and drug delivery systems.
References
- Turner A. P. Biosensors: sense and sensibility. Chem. Soc. Rev. 2013;42:3184–3196. doi: 10.1039/C3CS35528D. [DOI] [PubMed] [Google Scholar]
- Kuila T. Bose S. Khanra P. Mishra A. K. Kim N. H. Lee J. H. Recent advances in graphene-based biosensors. Biosens. Bioelectron. 2011;26:4637–4648. doi: 10.1016/j.bios.2011.05.039. [DOI] [PubMed] [Google Scholar]
- Clark L. C. Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962;102:29–45. doi: 10.1111/j.1749-6632.1962.tb13623.x. [DOI] [PubMed] [Google Scholar]
- Dhand C. Das M. Datta M. Malhotra B. D. Recent advances in polyaniline based biosensors. Biosens. Bioelectron. 2011;26:2811–2821. doi: 10.1016/j.bios.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Shi L. Xiao Y. Willner I. Electrical contacting of glucose oxidase by DNA-templated polyaniline wires on surfaces. Electrochem. Commun. 2006:1057–1060. [Google Scholar]
- Deepa M. Awadhia A. Bhandari S. Electrochemistry of poly(3,4-ethylenedioxythiophene)-polyaniline/Prussian blue electrochromic devices containing an ionic liquid based gel electrolyte film. Phys. Chem. Chem. Phys. 2009;11:5674–5685. doi: 10.1039/B900091G. [DOI] [PubMed] [Google Scholar]
- Sansinena J. M. Gao J. Wang H. L. High performance, monolithic polyaniline electrochemical actuators. Adv. Funct. Mater. 2003;13:703–709. doi: 10.1002/adfm.200304347. [DOI] [Google Scholar]
- Liu Z. Zhou J. Xue H. Shen L. Zang H. Chen W. Polyaniline/TiO2 solar cells. Synth. Met. 2006;156:721–723. doi: 10.1016/j.synthmet.2006.04.001. [DOI] [Google Scholar]
- Li M. Guo Yi. Wei Y. Macdiarmid A. G. Lelkes P. I. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials. 2006;27:2705–2715. doi: 10.1016/j.biomaterials.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Song E. Choi J. W. Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterials. 2011;11:102–203. doi: 10.3390/nano3030498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhand C. Solanki P. R. Sood K. N. Datta M. Malhotra B. D. Polyaniline nanotubes for impedimetric triglyceride detection. Electrochem. Commun. 2009;11:1482–1486. doi: 10.1016/j.elecom.2009.05.034. [DOI] [Google Scholar]
- Ciríc-Marjanovíc G. Recent advances in polyaniline research: polymerization mechanisms, structural aspects, properties and applications. Synth. Met. 2013;177:1–47. doi: 10.1016/j.synthmet.2013.06.004. [DOI] [Google Scholar]
- Tan S. Zhai J. Xue B. Wan M. Meng Q. Li Y. Jiang L. Zhu D. Property Influence of Polyanilines on Photovoltaic Behaviors of Dye-Sensitized Solar Cells. Langmuir. 2004;20:2934–2937. doi: 10.1021/la036260m. [DOI] [PubMed] [Google Scholar]
- Kumari Jangid N. Jadoun S. Kaur N. A review on high-throughput synthesis, deposition of thin films and properties of polyaniline. Eur. Polym. J. 2020;125:109485. doi: 10.1016/j.eurpolymj.2020.109485. [DOI] [Google Scholar]
- Geethalakshmi D. Muthukumarasamy N. Balasundaraprabhu R. Measurement on the structural, morphological, electrical and optical properties of PANI-CSA nanofilms. Measurement. 2016;92:446–452. doi: 10.1016/j.measurement.2016.06.054. [DOI] [Google Scholar]
- Aldissi M., Intrinsically Conducting Polymers: An Emerging Technology, Springer Science & Business Media, Berlin, Germany, 2013, vol. 246, ISBN 9401719527 [Google Scholar]
- Borole D. D. Kapadi U. R. Mahulikar P. P. Hundiwale D. G. Synthesis and characterization of conducting homopolymers and copolymers of o-anisidine and o-toluidine in inorganic and organic supporting electrolytes. J. Appl. Polym. Sci. 2003;90:2634–2642. doi: 10.1002/app.12899. [DOI] [Google Scholar]
- Koval E. P. Whittingham S. Skolozdra O. M. Zavalij P. Y. Zavaliy I. Y. Reshetnyak O. V. Seledets M. Co-polymers of aniline and nitroanilines, Part I. Mechanism of aniline oxidation polycondensation. Mater. Chem. Phys. 2001;69:154–162. doi: 10.1016/S0254-0584(00)00393-X. [DOI] [Google Scholar]
- Lee I. Luo X. Cui X. T. Yun M. Highly sensitive single polyaniline nanowire biosensor for the detection of immunoglobulin G and myoglobin. Biosens. Bioelectron. 2011;26(7):3297–3302. doi: 10.1016/j.bios.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidenko P. S. Pidenko S. A. Skibina Y. S. Zacharevich A. M. Drozd D. D. Yu Goryacheva I. Burmistrova N. A. Molecularly imprinted polyaniline for detection of horseradish peroxidase. Anal. Bioanal. Chem. 2020;412:6509–6517. doi: 10.1007/s00216-020-02689-3. [DOI] [PubMed] [Google Scholar]
- Luo J. Huang J. Wu Y. Sun J. Wei W. Liu X. Synthesis of hydrophilic and conductive molecularly imprinted polyaniline particles for the sensitive and selective protein detection. Biosens. Bioelectron. 2017;94:39–46. doi: 10.1016/j.bios.2017.02.035. [DOI] [PubMed] [Google Scholar]
- Luo X. Lee I. Huang J. Yun M. Cui X. T. Ultrasensitive protein detection using an aptamer-functionalized single polyaniline nanowire. Chem. Commun. 2011;47:6368–6370. doi: 10.1039/C1CC11353D. [DOI] [PubMed] [Google Scholar]
- Mondal D. Paul D. Mukherji S. Impedance spectroscopy based detection of cardiac biomarkers on polyaniline coated filter paper. IEEE Sens. J. 2016;c:1558–1748. [Google Scholar]
- Shaikh M. O. Srikanth B. Zhu P.-Y. Chuang C. H. Impedimetric Immunosensor Utilizing Polyaniline/Gold Nanocomposite-Modified Screen-Printed Electrodes for Early Detection of Chronic Kidney Disease. Sensors. 2019;19(18):3990. doi: 10.3390/s19183990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I. Luo X. Huang J. Cui X. T. Yun M. Detection of Cardiac Biomarkers Using Single Polyaniline Nanowire-Based Conductometric. Biosensors. 2012;2:205–220. doi: 10.3390/bios2020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Dissertation, University of Pittsburgh, 2017 [Google Scholar]
- Li L. Li W. Yang H. Ma C. Yu J. Yan M. Sensitive origami dual-analyte electrochemical immunodevice based on polyaniline/Au-paper electrode and multi-labeled 3D graphene sheets. Electrochim. Acta. 2014;120:102–109. doi: 10.1016/j.electacta.2013.12.076. [DOI] [Google Scholar]
- Anwar M. R., Electrospun nanofiber based biosensor for ultrasensitive protein detection, thesis, Bangladesh University of Engineering & Technology, 2006 [Google Scholar]
- Deep A. Sharma A. L. Kumar P. Bharadwaj L. M. Nanostructured polyaniline–silicon substrate for protein biosensing. Sens. Actuators. 2012;171:210–215. doi: 10.1016/j.snb.2012.03.014. [DOI] [Google Scholar]
- Crombrugghe A. de. Yunus S. Bertrand P. Grafting and characterization of protein on polyaniline surface for biosensor applications. Surf. Interface Anal. 2008;40:404–407. doi: 10.1002/sia.2792. [DOI] [Google Scholar]
- Li Y. Han R. Chen M. Zhang L. Wang G. Luo X. Bovine Serum Albumin-Cross-Linked Polyaniline Nanowires for Ultralow Fouling and Highly Sensitive Electrochemical Protein Quantification in Human Serum Samples. Anal. Chem. 2021;93:4326–4333. doi: 10.1021/acs.analchem.1c00089. [DOI] [PubMed] [Google Scholar]
- Zhang X. Hu R. Zhang K. Bai R. Li D. Yang Y. Ultrasensitive Label-Free Immunoassay for C-reactive protein Detection in Human Serum based on the electron transfer. Anal. Methods. 2011;20:192–201. [Google Scholar]
- Singh S. Solanki P. R. Pandey M. K. Malhotra B. D. Cholesterol biosensor based on cholesterol esterase, cholesterol oxidase and peroxidase immobilized onto conducting polyaniline films. Sens. Actuators, B. 2006;115:534–541. doi: 10.1016/j.snb.2005.10.025. [DOI] [Google Scholar]
- Dhand C. Solanki P. R. Pandey M. K. Datta M. Malhotra B. D. Electrophoretically deposited polyaniline nanotubes based film for cholesterol detection. Electrophoresis. 2010;31:3754–3762. doi: 10.1002/elps.201000321. [DOI] [PubMed] [Google Scholar]
- Dhand C. Das M. Sumana G. et al., Preparation, characterization and application of polyaniline nanospheres to biosensing. Nanoscale. 2010;2:747–754. doi: 10.1039/B9NR00346K. [DOI] [PubMed] [Google Scholar]
- Dutta R. Thangapandi K. Mondal S. Nanda A. Bose S. Sanyal S. Jana S. K. Ghorai S. Polyaniline Based Electrochemical Sensor for the Detection of Dengue Virus Infection. Avicenna J. Med. Biotechnol. 2020;12(2):77–84. [PMC free article] [PubMed] [Google Scholar]
- Langer J. J. Filipiak M. Ke J. Jasnowska J. Włodarczak J. Buładowski B. Polyaniline Biosensor for Choline Determination. Surf. Sci. 2004;573:140–145. doi: 10.1016/j.susc.2004.05.140. [DOI] [Google Scholar]
- Singh S. Solanki P. R. Pandey M. K. Malhotra B. D. Cholesterol Biosensor Based on Cholesterol Esterase, Cholesterol Oxidase and Peroxidase Immobilized onto Conducting Polyaniline Films. Sens. Actuators, B. 2006;115:534–541. doi: 10.1016/j.snb.2005.10.025. [DOI] [Google Scholar]
- Wang J. Hui N. A nonfouling voltammetric immunosensor for the carcinoembryonic antigen based on the use of polyaniline nanowires wrapped with hyaluronic acid. Microchim. Acta. 2018;185:329. doi: 10.1007/s00604-018-2854-y. [DOI] [PubMed] [Google Scholar]
- Srivastava M. Srivastava S. Nirala N. Prakash R. A chitosan-based polyaniline–Au nanocomposite biosensor for determination of cholesterol. Anal. Methods. 2014;6:817–824. doi: 10.1039/C3AY41812J. [DOI] [Google Scholar]
- Karimi M. Rabiee M. Tahriri M. R. Salarian R. Tayebi L. A graphene based–biomimetic molecularly imprinted polyaniline sensor for ultrasensitive detection of human cardiac troponin T (cTnT) Synth. Met. 2019;256:116136. doi: 10.1016/j.synthmet.2019.116136. [DOI] [Google Scholar]
- Yola B. B. Karaman C. Özcan N. Atar N. Polat I. Yola M. L. Electrochemical Tau Protein Immunosensor Based on MnS/GO/PANI and Magnetite-incorporated Gold Nanoparticles. Electroanalysis. 2022;34:1–11. doi: 10.1002/elan.202200159. [DOI] [Google Scholar]
- Park Y. Hong M.-S. Lee W.-H. Kim J.-G. Kim K. Highly Sensitive Electrochemical Aptasensor for Detecting the VEGF165 Tumor Marker with PANI/CNT Nanocomposites. Biosensors. 2021;114:1–11. doi: 10.3390/bios11040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z. Ma Y. Chen M. Ambrosi A. Ding C. Luo X. Electrochemical Biosensor with Enhanced Antifouling Capability of COVID-19 Nucleic Acid Detection in Complex Biological Media. Anal. Chem. 2021;93(14):5963–5971. doi: 10.1021/acs.analchem.1c00724. [DOI] [PubMed] [Google Scholar]