Abstract
Candida infection represents a global threat with associated high resistance and mortality rate. Azoles such as the triazole drug fluconazole are the frontline therapy against invasive fungal infections; however, the emerging multidrug-resistant strains limit their use. Therefore, a series of novel azole UOSO1–15 derivatives were developed based on a modified natural scaffold to combat the evolved resistance mechanism and to provide improved safety and target selectivity. The antifungal screening against C. albicans and C. auris showed that UOSO10 and 12–14 compounds were the most potent derivatives. Among them, UOSO13 exhibited superior potent activity with MIC50 values of 0.5 and 0.8 μg mL−1 against C. albicans and C. auris compared to 25 and 600 μg mL−1 for fluconazole, respectively. UOSO13 displayed significant CaCYP51 enzyme inhibition activity in a concentration-dependent manner with an IC50 10-fold that of fluconazole, while exhibiting no activity against human CYP50 enzyme or toxicity to human cells. Furthermore, UOSO13 caused a significant reduction of Candida ergosterol content by 70.3% compared to a 35.6% reduction by fluconazole. Homology modeling, molecular docking, and molecular dynamics simulations of C. auris CYP51 enzyme indicated the stability and superiority of UOSO13. ADME prediction indicated that UOSO13 fulfils the drug-likeness criteria with good physicochemical properties.
Effective targeting of Candida CYP51 enzyme by oxadiazole derivatives following iterated lead optimization using plant cuminaldehyde as a scaffold.
Introduction
Candidiasis is a severe worldwide invasive fungal infection (IFI). Candida spp. including in particular C. albicans and the emerging C. auris are considered as a severe provoking threat associated with high mortality rates, especially in immunocompromised patients mainly because of multidrug resistance.1 Further, chronic candidiasis requires a long-term treatment that may develop drug resistance.2 The limited tolerability, efficacy and high resistance profile of the available antifungal drugs highlight the challenge to discover and develop novel antifungal agents.3 The mechanisms of antifungal drugs include the targeting of (i) lanosterol 14-α-demethylase, (ii) beta-d-glucan synthesis, (iii) squalene epoxidase, (iv) ergosterol binding, (v) aminoacyl transferase, (vi) microtubule aggregation, or (vii) chitin synthesis.3 Among the limited FDA-approved antifungal agents in clinics are azoles, well-known CYP51 inhibitors.4 Azoles are a crucial scaffold with electron-rich property that enables their hydrogen bond interactions with the therapeutic target enzymes which accounts for their unique antifungal activity.5 Azole-based antifungal drugs include oxazole, imidazole, benzimidazole, triazole, benzotriazole, pyrazole, thiazole, oxadiazole, carbazole, and tetrazole.6 The first generation with the imidazole scaffold includes miconazole and clotrimazole, which are employed as topical treatment.7 The second generation with the imidazole-based scaffold such as ketoconazole was the first orally active antifungal that showed toxicity due to the lack of selectivity for fungal enzymes over human enzymes.8 The third generation with the triazole scaffold showed improved physiochemical properties including fluconazole, itraconazole and tetraconazole. Although triazole showed better selectivity, its toxicity limits its use.9 The fourth generation with the tetrazole scaffold instead of triazole showed enhanced physiochemical properties and promising efficacy against resistant strains, while having no effect on human CYP51 (CYP3A4) such as VT-1161, VT-1129 and VT-1598 under clinical trials.10 Oteseconazole (VT-1161) and quilseconazole (VT-1129) are orally active fluconazole derivatives11 with the replacement of triazole rings by tetrazole and halogen substituted pyridine rings, which potently inhibits C. albicans CYP51.11 Despite the special characteristics and superior clinical applications of azoles, fungi including Candida evolved significant resistance. Therefore, there is a continuous interest in the design of novel azoles with proper structure modification that enhances the efficacy and can evade the emerging resistance.4
The 1,3,4-oxadiazole moiety has been employed to improve the pharmacokinetics properties of the antifungal azole drugs including polarity, flexibility, ligand binding and metabolic profile.12 1,3,4-Oxadiazoles were reported in many natural compounds that inspired the potential design of novel azoles.13,14 1,3,4-Oxadiazoles exhibit diverse biological activities including antifungal activity.12,15–19 Furthermore, many marketed drugs containing a 1,3,4-oxadiazole core were reported including furamizole,20 oxadiazon,21 tiodazosin,22 nesapidil,23 and zibotentan.23 A fluconazole derivative with a 1,3,4-oxadiazole moiety showed significant antifungal activity when compared to the parent fluconazole. Incorporation of oxadiazole enhanced the binding affinity of fluconazole to lanosterol-14α-demethylase (CYP51) enzyme.12 Collectively, this indicates the potential use of the 1,3,4-oxadiazole scaffold as a promising pharmacophore in antifungal drug design.
Candida lanosterol-14α-demethylase (CYP51) enzyme, a hydrophobic membrane bound protein, is the selective target of azole drugs.24 It is a critical enzyme in the oxidative demethylation of lanosterol to desaturated intermediates for the biosynthesis of ergosterol, a key component of the fungal cell membrane, which regulates its permeability and fluidity.25 CYP51, a cytochrome P450 metalloenzyme, is known as the major target of azole derivatives.26 The high homology of CYP metalloenzyme with the common heme iron motif presented a challenge to discover selective target inhibitors.27 Azole with different metal binding groups binds to CYP51 and blocks ergosterol biosynthesis with subsequent cell damage.9 The magnitude of inhibitor binding to heme determined its potency and toxicity; tetrazole followed by triazole showed a lower binding affinity to the heme iron and an improved safety profile when compared to imidazole.28 However, the structural insight into azole binding to the active site of CYP51 showed its strong reversible interaction with heme,29 which is responsible for the off-targeting and binding to the host enzyme. Azole drugs attenuate the binding affinity to heme iron with molecular scaffold modifications that enhance the potency and selectivity (Fig. 1). Besides other mechanisms responsible for the evolved resistance of Candida to azoles, mutations in the target enzyme-coding gene ERG11 is considered as the primary mechanism of resistance,30 which subsequently hinders the binding capacity of azoles to the target enzyme.
Fig. 1. Major azole drugs in the market and under clinical trials.
The availability of the 3D crystal structure of C. albicans CYP51 (PDB: 5FSA) and homology modeling construction of the 3D crystal structure of C. auris alongside the human CYP3A4 (PDB: 4D7D) provide an excellent opportunity to design and discover a selective, safe and potent antifungal agent.33 CYP3A4 was employed as a CYP51 human counterpart for designing a selective CYP51 inhibitor.38 For example, VT-1129 has been used as a powerful inhibitor for CYP51, while it weakly inhibits the human CYP3A4.39 Analysis of the common pharmacophoric features of CACYP51's active binding site revealed its hydrophobic amino acid residues (Phe-126, Phe-228, Phe-233, Leu-376 and Pro-375),31 non-ionized polar amino acid residues (Tyr-118, Thr-122, Thr-132, Gly-303 and Ser-378), one basic amino acid residue (His-310), and the heme axial ligand. Previous molecular modeling studies, in vitro mutagenesis studies, and CACYP51 homology models30,32,33 reported the importance of key active site residues (Tyr-118, Leu-376, His-377, Phe-380, Met-508, and Val-509) for CACYP51 inhibition.34 Interestingly, the conservative Tyr-118 was responsible for the selectivity to azole drugs,35 while His-310 and Ser-378 might play an important role in mutational drug resistance.36 This motivates the possibility to improve the structural features of azole drugs to boost their potency and selectivity.37 Therefore, we have designed novel oxadiazole derivatives with favorable features that enhance the selectivity and efficacy against CYP51 enzyme by increasing the binding affinity to the hydrophobic pocket active site rather than the metal ion motif. Fortunately, the hydrophobic active site of the human CYP3A4 is different from the fungal CYP51, thus providing a potential opportunity to selectively target the fungus.40
Results
Rational design of oxadiazole UOSO1–15 compounds
Previously we have reported the efficient use of cuminaldehyde as a lead structure to develop synthetic triazole analogues UOST with improved antifungal activities.41 Here, we have designed a novel series of azoles by incorporating bioactive oxadiazoles as bio-isosteres of triazoles UOST to develop UOSO series with enhanced efficacy and safety. Primarily, a significant conversion of cuminaldehyde (1) to cumnic acid (2) was achieved using iodine, NaOH, and TBHP as the catalyst. Following this, the acids (2, 3) were converted to the corresponding acid chlorides using oxalyl chloride, DCM, and DMF under nitrogen at 0 °C. The acid hydrazide derivatives (4, 5) were synthesized in considerable yields by refluxing the acid chlorides with hydrazine hydrate. Substituted phenyl isothiocyanates 6–16 were then used in the synthesis of thiosemicarbazides H (1–11) from the acid hydrazide derivatives. Subsequently, they were cyclized to the title oxadiazole UOSO (1–15) compounds in moderate yields (60–80%) using 2 N NaOH and DBDMH (Fig. 2 and 3).
Fig. 2. Synthesis of UOSO1–15 compounds. Reagents and conditions: i) NaOH–I2/TBHP, 80 °C, 16–18 h; ii) a-oxalyl chloride/anhydrous DCM/DMF, b-NH2NH2·H2O/ethanol; iii) ethanol/reflux 3 h; iv) DBDMH, 2 N NaOH, 5 °C.
Fig. 3. Design rationale for oxadiazole-based CYP51-inhibitory antifungal agents.
The new structures were proposed to overcome the resistance mechanism of C. albicans and C. auris by interacting with the conservative hydrophobic binding site of the fungal CYP51 enzyme and weak interaction with heme that is responsible for the toxicity and off-target effect. Targeting the key amino acids of CYP51 enzyme that are responsible for triazole sensitivity of mutated strains would provide an opportunity to discover a safe, selective, and potent antifungal towards mutated strains.
UOSO10, 12, 13 and 14 showed potent anti-Candida activity
The anti-Candida activity of compounds UOSO1–15 was screened against C. albicans and C. auris. The results showed that compounds UOSO10, 12, 13 and 14 at the screening concentration (100 μg ml−1) caused significant inhibition of Candida growth by 57%, 83%, 99.3% and 80% against C. albicans, respectively, and 86%, 85%, 99% and 40% against C. auris, respectively (Fig. 4). Furthermore, the micro-dilution assay of the selected compounds showed the superior activity of the UOSO13 compound with an MIC50 of 0.5 ± 0.01 and 0.8 ± 0.04 μg ml−1 against C. albicans and C. auris, respectively (Fig. 5A and B, Table 1). Fluconazole as a positive control showed an MIC50 of 25 and 600 μg ml−1 against C. albicans and C. auris, respectively (Fig. 5C and D and Table 1). Compound UOSO13 showed 50 and 750 times less MIC50 compared to fluconazole against C. albicans and C. auris, respectively.
Fig. 4. Screening of UOSO1–15 compounds at 100 μg mL−1 against Candida strains. (A) C. albicans. (B) C. auris. The data were analyzed using one-way ANOVA and were calculated with the Bonferroni multiple comparisons test and the significance level indicated by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). The data display the mean ± standard error (SEM) of three replicates.
Fig. 5. MIC50 calculations. (A and B) MIC50 of UOSO10, 12, 13 and 14 against (A) C. albicans and (B) C. auris. (C and D) MIC50 of fluconazole against (C) C. albicans and (D) C. auris.
MIC50 (μM) of UOSO10, 12, 13 and 14.
| Compound | C. albicans | C. auris |
|---|---|---|
| UOSO10 | 5 ± 0.6 | 30 ± 0.46 |
| UOSO12 | 10 ± 0.09 | 12 ± 0.55 |
| UOSO13 | 0.5 ± 0.01 | 0.8 ± 0.04 |
| UOSO14 | 3 ± 0.05 | 2.08 ± 0.016 |
| Fluconazole | 25 ± 0.12 | 600 ± 0.45 |
UOSO13 significantly inhibited the fungal CYP51 without affecting the human CYP3A4
The superior inhibition activity of UOSO13 was validated by evaluating its binding inhibition effect on C. albicans sterol 14-α demethylase (CaCYP51) enzyme. The results indicated that UOSO13 inhibited the purified C. albicans CaCYP51 enzyme at IC50 of ∼1 μg mL−1, which is 10 times less than that of fluconazole (Fig. 6A and B). Importantly, UOSO13 selectively inhibited the fungal enzyme without a significant effect on the human enzyme with more than 10 000 times higher IC50 value (Fig. 6C).
Fig. 6. The selective inhibition of ergosterol biosynthesis. (A) IC50 of UOSO13 inhibition of CaCYP51. (B) IC50 of fluconazole inhibition of CaCYP51. (C) IC50 of UOSO13 inhibition against CYP3A4 enzyme. (D) The ergosterol percentage following the treatment of C. albicans by UOSO13 and fluconazole.
UOSO13 caused significant reduction in the fungal ergosterol content
UOSO13 at its MIC50 caused 70.3% inhibition of fungal ergosterol content, the product of Candida CYP51 enzyme activity, compared to 35.6% inhibition due to fluconazole (Fig. 6D). The reduction of ergosterol content indicated the disruption of its biosynthetic pathway due to the inhibition of CYP51 activity.
Molecular docking indicated the superiority and selectivity of UOSO13 against Candida CYP51 enzyme over the human homologue
The UOSO13 compound showed strong interaction with the fungal CYP51 pocket site of C. albicans (PDB: 5V5Z). It showed stable interaction with H-bond interaction of NH with Met-508 and pi–pi stacking interactions between oxadiazole and the phenyl ring and Tyr-118 amino acid (Table 2, Fig. 7A), in addition to hydrophobic interaction of the tert-butyl group with heme-601 and phenyl interaction with Pro-230, Phe-233, Leu-376 and Tyr-132. On the other hand, fluconazole showed only hydrophobic interaction of the triazole ring with heme-601 without interaction with the pocket site amino acid residues (Table 2, Fig. 7B). Furthermore, the native ligand (1YN, itraconazole) showed hydrophobic interactions of the triazole ring with heme-601, and the phenyl ring with Leu-376, Pro-230, Phe-233, Tyr-132, Phe-126 and Tyr-118 (Table 2, Fig. 7C). This indicated that the replacement of the triazole ring with the tert-butyl group caused weak interaction with heme that would expect fewer side effects. Besides, UOSO13 showed stronger interaction compared to fluconazole and slightly better interaction than the co-crystalized ligand.
Molecular modeling of UOSO13 compared to fluconazole within the binding active site of C. albicans (PDB: 5V5Z).
| Compound | Moiety | Interaction | Amino acid residue |
|---|---|---|---|
| UOSO13 | NH | H-bond | Met-508 |
| Oxadiazole | Pi–pi stacking bond | Tyr-118 | |
| Phenyl ring | Pi–pi stacking bond | Tyr-118 | |
| tert-Butyl group | Hydrophobic interaction | Heme-601 | |
| CH | Hydrophobic interaction | Leu-376 and Tyr-132 | |
| Phenyl ring | Hydrophobic interaction | Pro-230 and Phe-233 | |
| Fluconazole | Triazole | Coordination bond | Heme-601 |
| Native ligand | Phenyl ring | Pi–pi stacking bond | Tyr-118 |
| Triazole ring | Hydrophobic bond | Heme-601 | |
| Phenyl ring | Hydrophobic bond | Leu-376, Phe-233, Pro-230, Tyr-132 and Tyr-118 and Phe-126 | |
| Cl | Hydrophobic bond |
Fig. 7. Molecular docking studies against C. albicans CYP51 (PDB: 5FSA). (A) Binding mode of UOSO13. (B) Binding mode of fluconazole. (C) Binding mode of the native ligand.
C. albicans CYP51 (PDB: 5FSA) lacked the first 44 residues at the N-terminus and had six mutations compared to the wild type sequence8 at Lys-16, Thr-16, Pro-16, Glu-76, Tyr-136 and Leu-256.42 To understand the effect of the mutation of C. albicans CYP51 (PDB: 5FSA) on the active site interaction, the binding of UOSO-13 was also calculated.
UOSO-13 showed strong interaction with the key amino acids without interaction with the heme residue. It showed three pi–pi stacking interactions including the phenyl ring with His-377, oxadiazole with Tyr-118, and the phenyl ring with Phe-233, and hydrophobic interaction of the tert-butyl group with Tyr-118, Tyr-122, Tyr-132 and Leu-121, and Cl with Leu-88 and Tyr-118 alongside the phenyl ring interaction with Leu-88, Tyr-118, Pro-230, Phe-228, Phe-380, Met-508 and Tyr-132 (Table 3, Fig. 8A). Fluconazole showed H-bond interaction with Tyr-132 and hydrophobic interaction with Heme-580 and Tyr-132 (Table 3, Fig. 8B). The native ligand showed pi–pi stacking interaction of phenyl and triazole rings with Heme-580 alongside hydrophobic interaction of the phenyl ring with Phe-58, Tyr-64, Phe-126, Leu-376, Phe-233, Pro-230 and Heme-580 (Table 3, Fig. 8C).
Molecular modeling of UOSO13 compared to fluconazole within the binding active site of mutated C. albicans (PDB: 5FSA).
| Compound | Moiety | Interaction | Amino acid residue |
|---|---|---|---|
| UOSO13 | Phenyl ring | Pi–pi stacking bond | His-377 |
| Oxadiazole | Pi–pi stacking bond | Tyr-118 | |
| Phenyl ring | Pi–pi stacking bond | Phe-233 | |
| tert-Butyl group | Hydrophobic interaction | Tyr-122, Tyr-118, Tyr-132 and Leu-121 | |
| Phenyl ring | Hydrophobic interaction | Leu-88, Tyr-118, Pro-230, Phe-228, Phe-380, Met-508 and Tyr-132 | |
| Cl | Hydrophobic interaction | Leu-88 and Tyr-118 | |
| Fluconazole | OH | H-bond | Tyr-132 |
| Triazole | Coordinate interaction | Heme-580 | |
| Phenyl ring | Hydrophobic interaction | Tyr-132 | |
| Native ligand | Phenyl ring | Pi–pi stacking bond | Heme-580 |
| Triazole ring | Pi–pi stacking bond | Heme-580 | |
| Phenyl ring | Hydrophobic bond | Phe-58, Tyr-64, Phe-126, Leu-376, Phe-233, Pro-230 and Heme-580 |
Fig. 8. Molecular docking studies against C. albicans CYP51 (PDB: 5V5Z). (A) Binding mode of UOSO13. (B) Binding mode of fluconazole. (C) Binding mode of the native ligand.
The constructed 3D crystal structure of the C. auris model showed strong bonding with the UOSO13 compound with various interactions. It showed pi–pi stacking interaction between phenyl and oxadiazole rings with Tyr-118 and His-377, respectively, in addition to H-bond interaction of NH with His-377, and the aromatic H-bond of the phenyl ring with Met-504. It exhibited several hydrophobic interactions including the phenyl ring with Leu-376, the tert-butyl group with Heme-500, Tyr-118, Phe-233 and Tyr-132, the phenyl ring with Tyr-64 and Phe-380, and Cl with Leu-88 and Met-87 (Table 4, Fig. 9A), while fluconazole showed H-bond interaction of OH with Tyr-132 and hydrophobic interaction of the phenyl ring with Heme-500 and Tyr-132 (Table 4, Fig. 9B). The native ligand showed pi–pi stacking interaction of triazole and phenyl rings with Heme-500 and Tyr-1118, respectively, and hydrophobic interaction with Leu-88, Leu-376, Phe-223 and Pro-380 (Table 4, Fig. 9B). Together, these results confirmed the superior binding affinity of UOSO13 over fluconazole to the fungal enzyme of both Candida strains by targeting the key amino acids responsible for azole sensitivity.
Molecular modeling of UOSO13 compared to fluconazole within the binding active site of the constructed 3D-crystal structure of C. auris.
| Compound | Moiety | Interaction | Amino acid residue |
|---|---|---|---|
| UOSO13 | Phenyl ring | Pi–pi stacking bond | Tyr-118 |
| Oxadiazole | Pi–pi stacking bond | His-377 | |
| NH | H-bond | His-377 | |
| Phenyl ring | Hydrophobic interaction | Leu-376 | |
| tert-Butyl group | Hydrophobic interaction | Tyr-118, Tyr-132, Hem-500 and Phe-233 | |
| Phenyl ring | Hydrophobic interaction | Tyr-64 and Phe-380 | |
| Cl | Hydrophobic interaction | Leu-88 and Met-87 | |
| Fluconazole | OH | H-bond | Tyr-132 |
| Phenyl ring | Coordinate interaction | Heme-500 | |
| Hydrophobic interaction | Tyr-132 | ||
| Native ligand | Phenyl ring | Pi–pi stacking bond | Tyr-118 |
| Triazole ring | Pi–pi stacking bond | Heme-500 | |
| Phenyl ring | Hydrophobic bond | Leu-88, Leu-376, Phe-233 and Pro-380 |
Fig. 9. Molecular docking studies against the C. auris CYP51 constructed model. (A) Binding mode of UOSO13. (B) Binding mode of fluconazole. (C) Binding mode of the native ligand.
The free binding energies of the active UOSO10, 12, 13 and 14 compounds were higher than the corresponding azole (fluconazole), indicating the stability of the formed complexes within the fungal CYP51 pocket site (Table 5). Furthermore, compound UOSO13 showed excellent binding affinity when compared to UOSO10, 12, 14 and fluconazole. On the other side, they all showed lower binding affinity with higher energy to the human enzyme, confirming the selectivity of the newly designed compounds particularly UOSO13 to the Candida CYP51 enzyme over the human enzyme.
Calculated binding energies of active oxadiazoles with 14 cytochrome P450 (14 α-sterol demethylase, CYP51).
| Compound | Binding affinity (kcal mol−1) | |
|---|---|---|
| C. albicans CYP51 (PDB: 5FSA) | Human CYP34A (PDB: 4D7D) | |
| UOSO10 | −8.5 | −5.9 |
| UOSO12 | −8.4 | −5.4 |
| UOSO13 | −9.3 | −4.9 |
| UOSO14 | −8.9 | −5.3 |
| Fluconazole | −6.7 | −7.1 |
MD simulation validates the stability of the complex formed by UOSO compounds with the target C. albicans and C. auris CYP51 enzymes
MD of the complex of UOSO13 with C. albicans CYP51 enzyme indicates the stability of the complex during the MD simulation that reached an equilibrium within 20 ns and a low RMSD fluctuation value of 1.5 Å (Fig. 10A). It showed water bridge interaction with Ala-117, Tyr-118, His-377, Ser-378, Ser-507 and Met-508; H-bond interaction with Met-508 and hydrophobic contacts with Tyr-64, Tyr-118, Leu-121, Phe-126, Tyr-132, Phe-228, Pro-230, Phe-233, His-310, Leu-376, His-377, Phe-380, Met-508 and Val-509 (Fig. 10B). The UOSO13 binding pose displayed H-bond interaction of NH with Met-508 (47%) and pi–pi stacking interaction of the phenyl ring with two residues Phe-228 (38%) and Tyr-118 (45%) (Fig. 10C). Moreover, the MD of UOSO13 with C. auris CYP51 enzyme revealed complex stability with an RMSD value of 2 Å (Fig. 11A), in addition to hydrophobic interaction with Met-87, Met-92, Tyr-118, Leu-121, Phe-126, Tyr-132, Phe-228, Pro-230, Phe-233, Leu-376, His-377, Phe-380, Met-403, Met-504 and Val-505; H-bond interactions with Tyr-64, His-377 and Ser-378; and water bridge interaction with Tyr-64, Tyr-118, Pro-375, His-377, Ser-378, Ser-503 and Met-504 (Fig. 11B). The binding mode showed pi–pi stacking interaction of the phenyl ring with Tyr-118 (61%) and H-bond interactions of oxadiazole N with His-377 (30%) and Ser-378 (42%) (Fig. 11C). The lower fluctuation range of the UOSO10 ligand over the simulation period indicates the stability of the formed complex with C. albicans CYP51 enzyme (Fig. S6A†). Ligand contact showed hydrophobic interaction with Leu-87, Tyr-118, Leu-121, Phe-126, Ile-131, Tyr-132, Leu-139, Phe-228, Leu-300, Ile-304, His-310, Leu-376, Ile-379, Phe-380, Ile-471 and Met-504; and H-bond interaction with Tyr-132 (Fig. S6B†). UOSO10 interaction with C. albicans CYP51 exhibited pi–pi stacking of the phenyl ring with Tyr-118 (81%) and H-bond interaction of NH with Tyr-132 (92%) (Fig. S6C†). The UOSO12 complex with C. albicans CYP51 enzyme showed a conformational change with a higher RMSD value than within the accepted range of 3 Å (Fig. S7A†). It exhibited water bridge interaction with Tyr-64, Tyr-118, Leu-121, Phe-228, Ser-378, Ser-507 and Met-508; H-bonding with Ser-378; and hydrophobic contact with Leu-87, Leu-88, Met-92, Tyr-118, Leu-121, Phe-126, Ile-131, Tyr-132, Phe-228, Pro-230, Phe-233, His-310, Leu-376, His-377, Phe-380, Leu-403 and Met-508 residues (Fig. S7B†). Moreover, it displayed interaction with C. albicans CYP51 enzyme such as water bridge H-bond interaction of NH with Ser-378 (62%, 62%) and pi–pi stacking interaction of the phenyl ring with Tyr-118 (32%) (Fig. S7C†). MD analysis of the UOSO14 complex with C. albicans showed a fluctuation that lasted for a longer time until reaching the equilibrium with an RMSD value of 3.2 Å (Fig. S8A†). The ligand interaction showed hydrophobic contact with Tyr-64, Leu-87, Leu-88, Met-92, Tyr-118, Leu-121, Phe-126, Ile-131, Tyr-132, Phe-228, Pro-230, Phe-233, His-310, Leu-376, His-377, Phe-380, Leu-403 and Met-508 residues; water bridge with Tyr-118, Leu-121, His-310, His-377, Ser-378, Ser-507 and Met-508; and H-bond interaction with His-310, Ser-378 and Met-508 (Fig. S8B†). UOSO14 showed water bridge H-bond interactions between NH and Ser-378 residues (31%, 31%) (Fig. S8C†). The binding mode of UOSO10 with C. auris CYP51 enzyme showed fluctuation for 20 ns until reaching an equilibrium with a low RMSD value of 1.8 3 Å (Fig. S9A†). It also showed hydrophobic contacts with Met-87, Leu-88, Met-92, Tyr-118, Leu-121, Phe-126, Val-130, Ile-131, Tyr-132, Pro-230, Phe-233, Val-234, Leu-300, Val-304, Leu-376, Phe-380 and Met-504; water bridge with Ala-117, Tyr-118, Leu-121, Tyr-132, Gly-307, Gly-308, Thr-311, Ser-378 and Met-504; and H-bond interaction with Thr-122 and Tyr-132 residues (Fig. S9B†). The UOSO10 pose exhibited pi-pi stacking interaction of the oxadiazole ring with Tyr-132 (76%), H-bond interaction of oxadiazole N with Thr-122 (65%), and pi–pi stacking interaction of the phenyl ring with Phe-126 (48%) (Fig. S9C†). MD analysis of UOSO12 with C. auris CYP51 enzyme showed relative stability with an RMSD value of 2.4 Å with conformational changes that lasted for a few seconds (Fig. S10A†). The ligand showed hydrophobic contacts with Tyr-118, Leu-121, Phe-126, Ile-131, Leu-139, Phe-228, Leu-300, Val-304, Leu-376, Phe-380, Met-504 and Val-505; water bridge interaction with Thr-122, Tyr-132, Gln-142, Lys-143, Gly-303, Met-306, Gly-307 and His-310; and H-bond interaction with Tyr-132 and Lys-143 (Fig. S10B†). The docked pose exhibited water bridge interaction between oxadiazole N and Gly-303 (42%, 42%); and pi–pi stacking interaction of phenyl rings with Phe-126 (46%), Tyr-118 (36%), Phe-228 (30%) residues (Fig. S10C†). MD of UOSO14 with C. auris takes a longer time of 45 ns to reach an equilibrium with a slightly high RMSD fluctuation value of 3.5 Å (Fig. S11A†). The ligand exhibited hydrophobic contacts with Tyr-64, Met-87, Tyr-118, Leu-121, Tyr-132, Phe-228, Pro-230, Phe-233, His-310, Leu-376, His-377, Phe-380 and Met-504; H-bond interaction with Tyr-64, His-377, Ser-378 and Met-504; and water bridge interaction with Tyr-64, Pro-375, His-377, Ser-378, Tyr-501, Gln-502, Ser-503, Met-504 and Thr-506 (Fig. S11B†). The docked posed showed pi–pi stacking interaction between oxadiazole and Phe-380 (45%), and H-bond interaction of oxadiazole N and His-377 (40%) (Fig. S11C†). Together, the MD simulations revealed the relative stability of UOSO compounds in particular UOSO13 with both C. albicans and C. auris CYP51 enzymes during the simulation period with a low RMSD value and stable binding with the key amino acid residues that may contribute to the CYP51 azole mutations in resistant strains by establishing alternative contacts with Tyr-118 and Met-508 amino acid residues.
Fig. 10. Molecular dynamics simulation of UOSO13 with C. albicans CYP51 (PDB: 5V5Z). (A) The root mean standard deviation (RMSD) plot of UOSO13 with C. albicans CYP51. (B) Ligand UOSO13 interacts with the active site residues during MD simulation. (C) Schematic interaction diagram of UOSO13 with C. albicans CYP51.
Fig. 11. Molecular dynamics simulation of UOSO13 with the C. auris CYP51 model (A) the root mean standard deviation (RMSD) plot of UOSO13 with the C. auris CYP51 model. (B) Ligand UOSO13 contacts with the active site residues during MD simulation. (C) Schematic interaction diagram of UOSO13 with C. auris CYP51.
ADME indicated the drug likeness of UOSO compounds
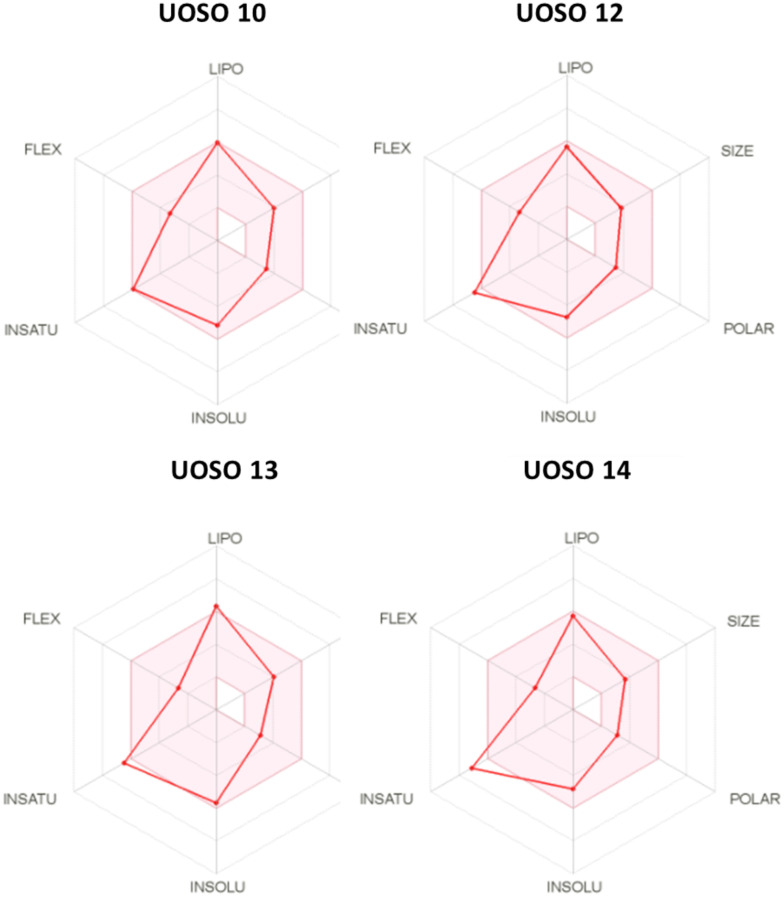
Physicochemical properties were used to identify the critical aspects that control the biological activities. In silico ADME properties provide accurate pharmacokinetics and guidance on oral bioavailability as the preferred route of administration.43 The results showed that most of the synthesized UOSO compounds including UOSO10, 12, 13, and 15 comply with the Lipinski rule of 5 (DHB less than 5, log p less than 5, AHB less than 10, and Mw less than 500), which reflected their ability to be transported, diffused, and absorbed easily. The log S-values were between 5 and 6, indicating that they showed moderate solubility and good absorbance. The solvent-accessible surface area (SASA) values revealed their cellular bioavailability and reflected the compound transport properties, indicating promising candidates (Table 6). The bioavailability radars of the active compounds UOSO10, 12, 13, 14 are represented in Fig. 12. The pink areas identify the optimum range of six properties such as lipophilicity (LIPO) with log p −0.7 and +5, flexibility (FLEX); rotatable bonds less than 9, instauration (INSATU); sp3 not less than 0.25, insolubility (INSOLU); log S less than 6, polarity (POLAR); TPSA 20–130 Å, and size; Mwt 150–500 g mol−1 (ref. 44). The four active compounds were within the conformity range and with acceptable parameters for further development. The optimization of physicochemical properties through salt formation can be developed, while maintaining the potent activity.
Prediction of ADME properties of UOSO1–15.
| Compound | MW | AHB | DHB | SASA | log P | log s | HOA | Role of 5 violations |
|---|---|---|---|---|---|---|---|---|
| UOSO10 | 323.39 | 3.75 | 1 | 634.76 | 3.64 | −5.93 | 3 | 0 |
| UOSO12 | 309.36 | 3.75 | 1 | 633.41 | 3.46 | −5.37 | 3 | 0 |
| UOSO13 | 327.81 | 3 | 1 | 620.67 | 3.71 | −5.68 | 1 | 0 |
| UOSO14 | 297.33 | 3 | 1 | 594.67 | 3.36 | −5.30 | 3 | 0 |
Fig. 12. The bioavailability radars of UOSO10, 12, 13 and 14 compounds. The identified pink areas are the preferred property ranges with accepted pharmacokinetics.
Compound UOSO13 is safe on normal human cells
The fibroblast F-180 normal cell line was used to measure the toxicity of the active compounds.45 Compounds UOSO13 and 14 showed no toxicity on normal cells at 60 and 10 times their MIC50 against C. albicans and 37.5 and 15 times their MIC50 against C. auris. Compound UOSO12 showed no toxicity at 3 and 2.3 times its MIC50, while compound UOSO10 showed no toxicity at 6 and 1 times its MIC50 against C. albicans and C. auris, respectively (Fig. 13).
Fig. 13. Cytotoxic effect of UOSO10, 12, 13 and 14 compounds on the survival of normal mammalian cells (F-180). The data were analyzed using two-way ANOVA and statistical significance was calculated with the Bonferroni multiple comparison test and the significance level indicated by asterisks (*). The data display the mean ± SEM of 6 replicates.
R-group analysis confirmed the uniqueness of the newly designed UOSO compounds
This analysis revealed that the minor structural modification such as ring substitution resulted in a remarkable influence of the biological activity and binding affinity. The different R-groups showed different binding affinities and exhibited different experimental PIC50. SAR heat map analysis showed different colours ranging from red to blue correlating the activity. The good activity was indicated with methoxy substitution at the meta-position of UOSO10 and 12, while the para-methoxy substitution of UOSO7 showed a drop of the activity. However, the halogen substitution at the para position showed potent antifungal activity. Replacement of the small halogen moiety such as fluorine of UOSO14 with a large one such as chlorine of UOSO13 enhanced the antifungal activity against Candida spp., indicating that the large size of the chlorine atom appeared to be important for the activity. It played a role in the binding interaction and stabilization of the complex that can significantly increase the activity (Fig. 14). Therefore, the compounds with potent activity have pharmacophoric features of being negatively ionic and a hydrogen bond acceptor.
Fig. 14. R-group analysis heat map. PIC50 values displayed as color scheme from blue to red as shown in the key.
Discussion
The oxadiazole compounds UOSO10, 12, 13 and 14 showed potent in vitro antifungal activity against Candida spp. compared to fluconazole. The rationale design of the new oxadiazole UOSO compounds was based on the CYP51 common pharmacophoric features such as the substitution of the hydrophobic substituted-aromatic moiety and aliphatic groups (isopropyl or tert-butyl)-phenyl at positions 3 and 5 of the oxadiazoles, respectively. The NH linker as a hydrogen bond acceptor also affected the spatial orientation for the enzyme binding. Subsequently, substitution with various electronegative groups enhanced the biological activity, while the atom size appeared to be crucial for the antifungal activity through additional contact with fungal CYP51 enzyme. Thus, the efficacy of UOSO13 was higher because of its relatively large chlorine atom compared to the small fluorine in UOSO 14. Moreover, 3-OCH3 substitution of UOSO10 and 12 showed a drop in the activity compared to the halogen substitution.
Collectively, this explained the robust potency of the UOSO13 compound since it exhibited the optimal orientation pose and high binding affinity to the critical amino acid residues of CYP51 that mediated its antifungal activity. UOSO13's oxadiazole ring interacted with the conservative Tyr-118 residue that is responsible for azole sensitivity with stable interaction during MD simulation, while demonstrating an alternative stable interaction with non-mutational amino acid (Phe-228, His-377, and Met-508) residues during the MD simulation period. Therefore the UOSO13 compound satisfied the chemical criteria for CaCYP51 inhibition as an effective strategy to tackle azole resistance in Candida spp. The UOSO13 compound deserves further investigation as a novel inhibitor of fungal sterol 14α-demethylase CYP51.
Further experiments including molecular docking binding studies (e.g. by surface plasmon resonance, isothermal calorimetry or microscale thermophoresis) and selectivity studies using other human CYP51 crystal structures will be performed for future validation and optimization.
Experimental
Chemistry
Reactions were monitored by thin-layer chromatography (TLC) using pre-coated silica gel plates (Kieselgel 60F254, BDH, Taufkirchen, Germany), and the identity/purity of synthesized products was visualized with UV light at 254 nm. Melting points (mp) were determined using a Gallenkamp melting point apparatus (London, UK). 1HNMR spectra were recorded on a Bruker spectrometer at 500 MHz. Chemical shifts were expressed in parts per million (ppm) relative to TMS, coupling constant (J) values were represented in hertz (Hz) and the signals were designated as follows: s, singlet; d, doublet; t, triplet; m, multiplet. Mass spectroscopic data were obtained through positive electrospray ionization (ESI) mass spectroscopy (Bruker Daltonics mass spectrometer, Bremen, Germany).
Synthesis of UOSO1–15 compounds
The synthesis of oxadiazoles UOSO1–15 was achieved through slight modification of a previously reported method.46 The refluxing of 4-substituted benzoic acid hydrazides (4, 5) with substituted phenyl isothiocyanates (6–16) in ethanol was performed to afford the thiosemicarbazide derivatives H (1–15).47 This is followed by the oxidative cyclodesulfuration with 1,3-dibromo-5,5 dimethylhydantoin (DBDMH) in basic solution46 to afford the corresponding oxadiazoles UOSO1–15 (Fig. 1).
Synthesis of cuminic acid 2
Cuminaldehyde 1 (10 mmol) in water was mixed with iodine and NaOH (10 mmol) and TBHP (20 mmol) was employed as a catalyst.41 Yield 62%. 1H-NMR (DMSO-d6): δ 1.21 (d, 6H, 2CH3 of isopropyl), 2.90 (m, 1H, CH of isopropyl), 7.34 (d, 2H, J = 8, ArH), 7.52 (d, 2H, J = 7.5, ArH).
Synthesis of 4-substituted benzoic acid hydrazides 4, 5 (ref. 48)
Oxalyl chloride (12 mmol) was added dropwise to a solution of cuminic acid 2 and 4-tert-butyl benzoic acid 3 (10 mmol) in anhydrous DCM (30 mL) and anhydrous DMF (10 μL) under inert nitrogen at 0 °C. The reaction mixture was stirred at room temperature for additional 3 h until the reaction was completed. The redistillation with anhydrous DCM was performed to remove the excess oxalyl chloride. The resulting oily residue was dissolved in 10 mL ethanol and treated with 5 mL hydrazine hydrate. The reaction mixture was refluxed for 24 h and left to cool at room temperature to afford the corresponding acid hydrazide 4, 5.
4-Isopropyl-benzoic acid hydrazide (4)
Yield 75%, 1H-NMR (DMSO-d6): δ 1.21 (d, 6H, 2 CH3 of isopropyl), 2.93 (m, 1H, CH of isopropyl), 4.45 (s, 2H, NH2), 7.31 (d, 2H, J = 8, ArH), 7.54 (d, 2H, J = 6.5, ArH), 9.67 (s, 1H, NH).
4-tert-Butyl-benzoic acid hydrazide (5)
Yield 76%, 1H-NMR (DMSO-d6): δ 1.29 (s, 9H, tert-butyl), 4.45 (s, 2H, NH2), 7.45 (d, 2H, J = 8.5, ArH), 7.75 (d, 2H, J = 6.5, ArH), 9.68 (s, 1H, NH).
Synthesis of 4-substituted benzoyl-N-substituted phenyl thiosemicarbazides (H1–15)
The 4-substituted benzoyl-N-substituted phenyl thiosemicarbazide derivatives (H1–15) were synthesized as previously described.41 To a solution of 4-substituted benzoic acid hydrazide, 4 and 5 (5 mmol) in 15 mL ethanol and 5 mmol substituted phenyl isothiocyanate (6–16) in 10 mL ethanol were mixed. The reaction mixture was refluxed for 3 h. The formed precipitate after adding water was collected by filtration to give the corresponding thiosemicarbazide, yield 60–79%.
4-tert-Butyl-benzoyl-N-(phenyl) hydrazine carbothioamide (H1)
Yield 67%, 1H-NMR (DMSO-d6): δ 1.31 (s, 9H, tert-butyl), 7.15 (t, 1H, J = 7.5, ArH), 7.32 (t, 2H, J = 7.5, ArH), 7.34 (d, 2H, J = 6.5, ArH), 7.52 (d, 2H, J = 2, ArH), 7.90 (d, 2H, J = 8, ArH), 9.6 (s, 1H, NH), 9.76 (s, 1H, NH), 10.46 (s, 1H, NH).
4-tert-Butyl-benzoyl-N-(3-bromophenyl)hydrazine carbothioamide (H2)
Yield 70%, 1H-NMR (DMSO-d6): δ 1.31 (s, 9H, tert-butyl), 6.64 (dd, 2H, J = 6.5, ArH), 6.98 (d, 1H, J = 6, ArH), 7.29 (d, 2H, J = 8.5, ArH), 7.48 (d, 1H, J = 8, ArH), 7.59 (d, 1H, J = 7, ArH), 7.82 (s, 1H, ArH), 9.68 (s, 1H, NH), 9.86 (s, 1H, NH), 10.50 (s, 1H, NH).
4-tert-Butyl-benzoyl-N-(3-fluorophenyl)hydrazine carbothioamide (H3)
Yield 64%, 1H-NMR (DMSO-d6): δ 1.30 (s, 9H, tert-butyl), 6.97 (d, 1H, J = 7.5, ArH), 7.23 (d, 1H, J = 6.5, ArH), 7.34 (m, 3H, ArH), 7.84 (d, 2H, J = 7.25, ArH), 7.82 (s, 1H, ArH), 9.86 (s, 1H, NH), 10.31 (s, 1H, NH), 10.47 (s, 1H, NH).
4-Isopropyl-benzoyl-N-(3-chlorophenyl)hydrazine carbothioamide (H4)
Yield 75%, 1H-NMR (DMSO-d6): δ 1.24 (d, 6H, 2CH3 of isopropyl), 2.93 (m, 1H, CH of isopropyl), 7.25 (d, 2H, J = 8.50, ArH), 7.45 (m, 4H, ArH), 9.78 (d, 2H, J = 6.5, ArH), 9.87 (s, 1H, NH), 10.23 (s, 1H, NH), 10.47 (s, 1H, NH).
4-tert-Butyl-benzoyl-N-(3-chlorophenyl)hydrazine carbothioamide (H5)
Yield 64%, 1H-NMR (DMSO-d6): δ 1.31 (s, 9H, tert-butyl), 7.2 (d, 1H, J = 7.5, ArH), 7.35 (t, 1H, J = 8, ArH), 7.48 (d, 1H, J = 7, ArH), 7.53 (d, 2H, J = 7, ArH), 7.61 (s, 1H, ArH), 7.90 (d, 2H, J = 8.5, ArH), 9.83 (s, 1H, NH), 9.89 (s, 1H, NH), 10.50 (s, 1H, NH).
4-tert-Butyl-benzoyl-N-(p-tolyl) hydrazine carbothioamide (H6)
Yield 69%, 1H-NMR (DMSO-d6): δ 1.31 (s, 9H, tert-butyl), 2.28 (s, 3H, CH3), 7.12 (d, 2H, J = 8.5 ArH), 7.31 (d, 2H, J = 6.5, ArH), 7.51 (d, 2H, J = 8.5, ArH), 7.89 (d, 2H, J = 8, ArH), 9.62 (s, 1H, NH), 9.69 (s, 1H, NH), 10.44 (s, 1H, NH).
4-tert-Butyl-benzoyl-N-(4-methoxyphenyl)hydrazine carbothioamide (H7)
Yield 64%, 1H-NMR (DMSO-d6): δ 1.31 (s, 9H, tert-butyl), 3.75 (s, 3H, OCH3), 6.89 (d, 2H, J = 9, ArH), 7.28 (d, 2H, J = 6.5, ArH), 7.50 (d, 2H, J = 4.5, ArH), 7.89 (d, 2H, J = 6, ArH), 9.58 (s, 1H, NH), 9.66 (s, 1H, NH), 10.43 (s, 1H, NH).
4-Isopropyl-benzoyl-N-(3-bromophenyl)hydrazine carbothioamide (H8)
Yield 65%, 1H-NMR (DMSO-d6): δ 1.23 (d, 6H, 2 CH3 of isopropyl), 2.94 (m, 1H, CH of isopropyl), 7.31 (m, 2H, J = 8, ArH), 7.32 (d, 1H, J = 4, ArH), 7.39 (d, 1H, J = 8.5, ArH), 7.52 (d, 1H, J = 8.5, ArH), 7.72 (s, 1H, ArH), 7.89 (d, 2H, J = 8, ArH), 9.83 (s, 1H, NH), 9.87 (s, 1H, NH), 10.49 (s, 1H, NH).
4-tert-Butyl-benzoyl-N-(4-fluorophenyl)hydrazine carbothioamide (H9)
Yield 67%, 1H-NMR (DMSO-d6): δ 1.31 (s, 9H, tert-butyl), 7.16 (t, 2H, J = 9, ArH), 7.42 (d, 2H, J = 7, ArH), 7.53 (d, 2H, J = 5.5, ArH), 7.90 (d, 2H, J = 8.5, ArH), 9.73 (s, 1H, NH), 9.77 (s, 1H, NH), 10.47 (s, 1H, NH).
4-tert-Butyl-benzoyl-N-(3-methoxyphenyl)hydrazine carbothioamide (H10)
Yield 64%, 1H-NMR (DMSO-d6): δ 1.33 (s, 9H, tert-butyl), 3.83 (s, 3H, OCH3), 6.76 (d, 2H, J = 8, ArH), 7.12 (d, 2H, J = 7.5, ArH), 7.34 (d, 2H, J = 6.5, ArH), 7.59 (s, 1H, ArH), 7.89 (d, 1H, J = 5, ArH), 9.50 (s, 1H, NH), 9.86 (s, 1H, NH), 10.45 (s, 1H, NH).
4-tert-Butyl-benzoyl-N-(4-trifluoromethylphenyl)hydrazine carbothioamide (H11)
Yield 67%, 1H-NMR (DMSO-d6): δ 1.31 (s, 9H, tert-butyl), 7.52 (d, 2H, J = 7, ArH), 7.68 (d, 2H, J = 8.5, ArH), 7.76 (d, 2H, J = 2, ArH), 7.90 (d, 2H, J = 8.5, ArH), 9.96 (s, 2H, NH), 10.53 (s, 1H, NH).
4-Isopropyl-benzoyl-N-(3-methoxyphenyl)hydrazine carbothioamide (H12)
Yield 60%, 1H-NMR (DMSO-d6): δ 1.12 (d, 6H, 2 CH3 of isopropyl), 2.93 (m, 1H, CH of isopropyl), 3.68 (s, 3H, OCH3), 6.72 (dd, 1H, J = 8, ArH), 7.02 (d, 1H, J = 8, ArH), 7.10 (s, 1H, ArH), 7.10 (t, 1H, J = 8.5, ArH), 7.24 (d, 2H, J = 8, ArH), 7.85 (d, 2H, J = 8, ArH), 9.65 (s, 2H, NH), 10.43 (s, 1H, NH).
4-tert-Butyl-benzoyl-N-(4-chlorophenyl)hydrazine carbothioamide (H13)
Yield 64%, 1H-NMR (DMSO-d6): δ 1.31 (s, 9H, tert-butyl), 7.37 (d, 2H, J = 9, ArH), 7.53 (m, 4H, ArH), 7.89 (d, 2H, J = 8, ArH), 9.80 (s, 2H, 2 NH), 10.48 (s, 1H, NH).
4-Isopropyl-benzoyl-N-(4-fluorophenyl)hydrazine carbothioamide (H14)
Yield 63%, 1H-NMR (DMSO-d6): δ 1.23 (d, 6H, 2 CH3 of isopropyl), 2.97 (m, 1H, CH of isopropyl), 7.37 (dd, 2H, J = 8, ArH), 7.68 (d, 2H, J = 8.5, ArH), 7.74 (d, 2H, J = 6.5, ArH), 7.88 (d, 2H, J = 8, ArH), 9.74 (s, 1H, NH), 9.77 (s, 1H, NH), 10.45 (s, 1H, NH).
4-Isopropyl-benzoyl-N-(p-tolyl) hydrazine carbothioamide (H15)
Yield 61%, 1H-NMR (DMSO-d6): δ 1.12 (d, 6H, 2 CH3 of isopropyl), 2.28 (s, 3H, CH3), 2.93 (m, 1H, CH of isopropyl), 7.32 (d, 2H, J = 8, ArH), 7.67 (d, 2H, J = 8.5, ArH), 7.74 (d, 2H, J = 8.5, ArH), 7.88 (d, 2H, J = 8, ArH), 9.95 (s, 2H, NH), 10.51 (s, 1H, NH).
Synthesis of [5-(4-substituted phenyl)-[1,3,4]-oxadiazol-2-yl]-substituted phenyl amine (UOSO1–15)
To a suspension of 4-substituted benzoyl-N-substituted phenyl thiosemicarbazides 6–16 (10 mmol) in isopropanol (10 mL), potassium iodide in 10 mL of 5 N NaOH was added under nitrogen at 5 °C, and the mixture was stirred until a clear solution was formed. A solution of DBDMH (30 mmol) in acetonitrile (10 mL) was added over 1 h whilst maintaining the temperature below 10 °C. The mixture was stirred overnight, then quenched with aqueous NaHCO3 solution and the crude product was collected by filtration. The corresponding oxadiazoles UOSO1–15 compounds were then recrystallized from ethanol.46
[5-(4-tert-Butyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-phenyl-amine UOSO1
Yield 79%, 1H-NMR (DMSO-d6): δ 1.32 (s, 9H, tert-butyl), 7.02 (t, 1H, J = 7.5, ArH), 7.37 (t, 2H, J = 7.5, ArH), 7.62 (m, 4H, ArH), 7.83 (d, 2H, J = 6.5, ArH), 10.65 (s, 1H, NH). 13C-NMR (DMSO-d6): δ 28.02, 34.63, 116.77, 120.67, 121.61, 125.06, 126.12, 129.35, 138.92, 154.50, 157.12, 159.34. MS analysis for C18H19N3O: calcd mass: 293.15, found (m/z, ES+): 293.91.
(3-Bromo-phenyl)-[5-(4-tert-butyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-amine UOSO2
Yield 72%, 1H-NMR (DMSO-d6): δ 1.32 (s, 9H, tert-butyl), 6.83 (t, 1H, J = 2.5, ArH), 7.37 (m, 2H, ArH), 7.56 (d, 1H, J = 6.5, ArH), 7.62 (d, 2H, J = 9.5, ArH), 7.83 (d, 2H, J = 9.5, ArH), 10.92 (s, 1H, NH). 13C-NMR (DMSO-d6): δ 30.86, 34.74, 116.01, 119.27, 120.99, 121.99, 124.40, 125.52, 126.22, 131.08, 140.29, 154.03, 159.34. MS analysis for C18H18BrN3O: calcd mass: 372.06, found (m/z, ES+): 374.12.
[5-(4-tert-Butyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-(3-fluoro-phenyl)-amine UOSO3
Yield 69%, 1H-NMR (DMSO-d6): δ 1.32 (s, 9H, tert-butyl), 6.83 (t, 1H, J = 2.5, ArH), 7.37 (m, 2H, ArH), 7.56 (d, 1H, J = 6.5, ArH), 7.62 (d, 2H, J = 9.5, ArH), 7.83 (d, 2H, J = 9.5, ArH), 10.92 (s, 1H, NH). 13C-NMR (DMSO-d6): δ 30.94, 34.84, 103.96, 104.17, 108.27, 113.29, 121.11, 125.59, 126.28, 130.80, 140.87, 154.07, 158.03, 161.73. MS analysis for C18H18FN3O: calcd mass: 311.14, found (m/z, ES+): 312.06.
(3-Chloro-phenyl)-[5-(4-isopropyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-amine UOSO4
Yield 66%, 1H-NMR (DMSO-d6): δ 1.24 (d, 6H, 2 CH3 of isopropyl), 2.93 (m, 1H, CH of isopropyl), 7.06 (d, 1H, J = 8, ArH), 7.39 (t, 1H, J = 8, ArH), 7.49 (m, 3H, ArH), 7.78 (t, 1H, J = 4, ArH), 7.83 (d, 2H, J = 8.5, ArH), 10.51 (s, 1H, NH). 13C-NMR (DMSO-d6): δ 23.92, 33.83, 116.09, 116.87, 121.523, 122.07, 126.24, 127.75, 131.24, 133.97, 140.52, 152.41, 158.58, 159.85. MS analysis for C17H16ClN3O: calcd mass: 313.10, found (m/z, ES+): 314.26.
[5-(4-tert-Butyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-(3-chloro-phenyl)-amine UOSO5
Yield 71%, 1H-NMR (DMSO-d6): δ 1.32 (s, 9H, tert-butyl), 7.08 (d, 1H, J = 6.5, ArH), 7.53 (t, 1H, J = 8.5, ArH), 7.60 (d, 1H, J = 6.5, ArH), 7.62 (d, 2H, J = 7.5, ArH), 7.78 (t, 1H, J = 4.5, ArH), 7.85 (d, 2H, J = 6.5, ArH), 10.93 (s, 1H, NH). 13C-NMR (DMSO-d6): δ 30.69, 34.77, 115.65, 116.44, 121.00, 121.49, 125.59, 126.20, 130.80, 133.52, 140.18, 154.01, 158.02, 159.73. MS analysis for C18H18ClN3O: calcd mass: 327.11, found (m/z, ES+): 327.68.
[5-(4-tert-Butyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-p-tolyl-amine UOSO6
Yield 78%, 1H-NMR (DMSO-d6): δ 1.31 (s, 9H, tert-butyl), 2.26 (s, 3H, CH3), 7.15 (d, 2H, J = 8, ArH), 7.51 (d, 2H, J = 6, ArH), 7.58 (d, 2H, J = 8.5, ArH), 7.83 (d, 2H, J = 8.5, ArH), 10.53 (s, 1H, NH). 13C-NMR (DMSO-d6): δ 20.31, 30.84, 34.71, 117.06, 121.20, 125.37, 126.13, 129.46, 130.68, 136.20, 153.73, 157.63, 159.83. MS analysis for C19H21N3O: calcd mass: 307.11, found (m/z, ES+): 308.02.
[5-(4-tert-Butyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-(4-methoxy-phenyl)-amine UOSO7
Yield 68%, 1H-NMR (DMSO-d6): δ 1.32 (s, 9H, tert-butyl), 3.77 (s, 3H, OCH3), 6.95 (d, 2H, J = 8, ArH), 7.14 (t, 1H, J = 7.5, ArH), 7.53 (d, 2H, J = 6.5, ArH), 7.60 (d, 1H, J = 4.5, ArH), 7.79 (d, 2H, J = 8.5, ArH), 10.49 (s, 1H, NH). 13C-NMR (DMSO-d6): δ 30.76, 34.65, 55.13, 104.11, 108.18, 110.58, 121.23, 125.17, 127.10, 130.96, 139.73, 152.96, 157.98, 159.89. MS analysis for C19H21N3O2: calcd mass: 323.16, found (m/z, ES+): 324.48.
(3-Bromo-phenyl)-[5-(4-isopropyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-amine UOSO8
Yield 67%, 1H-NMR (DMSO-d6): δ 1.24 (d, 6H, 2 CH3 of isopropyl), 2.93 (m, 1H, CH of isopropyl), 7.20 (dd, 1H, J = 8, ArH), 7.34 (t, 1H, J = 8.25, ArH), 7.47 (d, 2H, J = 8.5, ArH), 7.55 (dd, 1H, J = 2, ArH), 7.83 (d, 2H, J = 6.5, ArH), 7.92 (t, 1H, J = 2, ArH), 10.49 (s, 1H, NH). 13C-NMR (DMSO-d6): δ 23.60, 33.46, 116.15, 119.38, 121.43, 122.02, 124.27, 125.78, 127.36, 131.04, 140.62, 151.71, 158.08, 159.53. MS analysis for C17H16BrN3O: calcd mass: 357.05, found (m/z, ES+): 358.30.
[5-(4-tert-Butyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-(4-fluoro-phenyl)-amine UOSO9
Yield 65%, 1H-NMR (DMSO-d6): δ 1.31 (s, 9H, tert-butyl), 7.21 (t, 2H, J = 9, ArH), 7.63 (m, 4H, ArH), 7.85 (d, 2H, J = 8.5, ArH), 10.58 (s, 1H, NH). 13C-NMR (DMSO-d6): δ 30.73, 34.78, 115.68, 118.56, 121.14, 125.44, 126.19, 135.22, 153.85, 156.35, 156.34, 159.78. MS analysis for C18H18FN3O: calcd mass: 311.14, found (m/z, ES+): 312.12
[5-(4-tert-Butyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-(3-methoxy-phenyl)-amine UOSO10
Yield 68%, 1H-NMR (DMSO-d6): δ 1.32 (s, 9H, tert-butyl), 3.77 (s, 3H, OCH3), 6.65 (d, 1H, J = 7, ArH), 7.14 (d, 1H, J = 8, ArH), 7.26 (t, 1H, J = 8.5, ArH), 7.33 (t, 1H, J = 4.5, ArH), 7.59 (d, 2H, J = 8.5, ArH), 7.83 (d, 2H, J = 8.5, ArH), 10.73 (s, 1H, NH). 13C-NMR (DMSO-d6): δ 30.86, 34.75, 55.03, 103.11, 107.18, 109.58, 115.34, 121.13, 125.47, 126.18, 129.96, 139.83, 153.86, 157.78, 159.69. MS analysis for C19H21N3O2: calcd mass: 323.16, found (m/z, ES+): 324.28.
[5-(4-tert-Butyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-(4-trifluoromethyl-phenyl)-amine UOSO11
Yield 64%, 1H-NMR (DMSO-d6): δ 1.31 (s, 9H, tert-butyl), 7.32 (d, 2H, J = 8, ArH), 7.53 (m, 4H, ArH), 7.75 (d, 2H, J = 7.5, ArH), 10.78 (s, 1H, NH). 13C-NMR (DMSO-d6): δ 30.63, 33.78, 36.55, 113.68, 115.56, 120.14, 124.44, 126.89, 135.12, 139.30, 152.85, 156.35, 159.78. MS analysis for C19H18F3N3O: calcd mass: 361.14, found (m/z, ES+): 362.21.
[5-(4-Isopropyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-(3-methoxy-phenyl)-amine UOSO12
Yield 75%, 1H-NMR (DMSO-d6): δ 1.20 (d, 6H, 2 CH3 of isopropyl), 2.92 (m, 1H, CH of isopropyl), 3.74 (s, 3H, OCH3), 6.61 (dd, 1H, J = 7.5, ArH), 7.09 (dd, 1H, J = 8, ArH), 7.24 (m, 2H, ArH), 7.42 (d, 2H, J = 8.85, ArH), 7.83 (d, 2H, J = 8, ArH), 10.51 (s, 1H, NH). 13C-NMR (DMSO-d6): δ 23.75, 33.80, 55.34, 115.29, 116.30, 120.92, 122.37, 126.04, 127.15, 130.94, 134.27, 139.52, 151.41, 157.58, 159.65. MS analysis for C18H19N3O2: calcd mass: 309.15, found (m/z, ES+): 310.60.
[5-(4-tert-Butyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-(4-chloro-phenyl)-amine UOSO13
Yield 60%, 1H-NMR (DMSO-d6): δ 1.29 (s, 9H, tert-butyl), 7.44 (d, 2H, J = 9, ArH), 7.64 (m, 4H, ArH), 7.85 (d, 2H, J = 9, ArH), 10. 78 (s, 1H, NH). 13C-NMR (DMSO-d6): δ 23.63, 33.78, 118.98, 121.61, 124.23, 125.86, 127.48, 129.08, 139.73, 151.85, 158.01, 160.06. MS analysis for C19H18ClN3O: calcd mass: 327.11, found (m/z, ES+): 328.
(4-Fluoro-phenyl)-[5-(4-isopropyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-amine UOSO14
Yield 56%, 1H-NMR (DMSO-d6): δ 1.20 (d, 6H, 2 CH3 of isopropyl), 2.92 (m, 1H, CH of isopropyl), 7.22 (t, 2H, J = 8.5, ArH), 7.46 (d, 2H, J = 8.5, ArH), 7.62 (m, 2H, ArH), 7.81 (d, 2H, J = 8.5, ArH). 13C-NMR (DMSO-d6): δ 23.65, 32.80, 116.30, 121.92, 123.37, 126.40, 127.56, 132.27, 139.50, 150.41, 157.58, 159.65. MS analysis for C17H16FN3O: calcd mass: 297.13, found (m/z, ES+): 298.
[5-(4-Isopropyl-phenyl)-[1,3,4]-oxadiazol-2-yl]-p-tolyl-amine UOSO15
Yield 73%, 1H-NMR (DMSO-d6): δ 1.25 (d, 6H, 2 CH3 of isopropyl), 2.29 (s, 3H, CH3), 2.97 (m, 1H, CH of isopropyl), 7.16 (d, 2H, J = 8, ArH), 7.45 (d, 2H, J = 7, ArH), 7.50 (d, 2 H, J = 8, ArH), 7.80 (d, 2H, J = 6.5, ArH). 13C-NMR (DMSO-d6): δ 20.37, 23.62, 33.46, 117.21, 121.65, 125.65, 127.34, 129.50, 130.58, 136.58, 151.54, 157.62, 160.06. MS analysis for C18H19N3O: calcd mass: 293.15, found (m/z, ES+): 294.26.
Biology
Candida strains and cell line growth conditions
The Candida strains employed in this study were C. albicans (SC5314) and C. auris (clinical isolate). The clinical isolates were obtained from the Centers for Disease Control and Prevention (CDC), USA. Both Candida strains were maintained on Sabouraud dextrose agar (SDA) and incubated for 24 h at 37 °C. The mammalian normal human skin fibroblast cell line (F-180) was acquired from the Radiobiology and Experimental Radio Oncology lab, University Cancer Center, Hamburg University. F-180 cells were grown in Dulbecco's modified Eagle medium (DMEM) (Sigma-Aldrich) with 15% FBS (Sigma-Aldrich) and 1% penicillin–streptomycin (Sigma-Aldrich) and the cultures were maintained at 37 °C in an atmosphere of 5% CO2.
Anti-Candida activity of UOSO1–15
Antimicrobial susceptibility testing using the spotting assay and micro-dilution assay
The anti-Candida activity of UOSO1–15 compounds was screened using the spotting assay and micro-dilution broth assay according to the CLSI method.49 Briefly, SDA plates were streaked with 0.1 ml culture containing 104 CFU ml−1. The plates were then treated with 5 μl of the tested compounds for 24 h at 37 °C. For the micro-dilution assay, each Candida strain was inoculated at a concentration of 105 CFU mL−1 in 96-well microplates and incubated at 37 °C for 24 h with 100 μg mL−1 of each of the tested compounds. The promising candidate was selected to measure the MIC50 values. Fluconazole was employed as a positive control at its predetermined MIC50 value. Culture media without drugs were used as the negative control. The microbial growth (turbidity) was measured at λmax 570 nm by a microplate reader (LT-4500, Labtech, Pocklington, York, UK).
Minimum inhibitory concentration (MIC50) assay
MIC50 values of the active compounds UOSO10, 12, 13 and 14 that showed significant anti-Candida activity were evaluated. Briefly, 100 μl of each Candida strain was inoculated at a concentration of 105 CFU mL−1 and 100 μl of the tested compounds and fluconazole at different concentrations (1, 3, 5, 10, 25, 50, 100 and 200 μg ml−1) were added to 96-well plates and incubated at 37 ° C for 24 h. The microbial growth turbidity was measured by a microplate reader at λmax 570 nm. MIC50, which is the concentration that showed 50% growth inhibition, was calculated using GraphPad prism. All experiments were tested in triplicate.
Lanosterol 14-α demethylase (CaCYP51) inhibition assay
The inhibitory effect of USOS13 against C. albicans sterol 14α-demethylase enzyme (CaCYP51) was evaluated according to previously reported methods with slight modification.50 The fluorescent substrate 7-ethoxyresorufin (7-ER), from which resorufin was produced after enzymatic cleavage, was utilized to measure the enzymatic activity. The maximum excitation and emission wavelengths for 7-ER and resorufin (both purchased from Sigma-Aldrich, St. Louis, MO, USA) are 490, 580 and 572, 604 nm, respectively. This procedure was carried out under yellow light to preserve the integrity of the stock solutions.51 Briefly, UOSO13 at different concentrations (0, 0.1, 1, 10, 100 μg ml−1) was incubated in 96-black well plates with 7-ER as the substrate and 1 mM CaCYP51 bactosomes in pH 7.4 phosphate buffer containing 5 mM MgCl2. The substrate 7-ER and its metabolite resorufin are both available from Cypex.3, Dundee, Scotland, UK. The reaction was initiated with the addition of 4 mM NADPH and shaking for 10 min at 37 °C. The formation of resorufin was measured fluorometrically every 30 s using a visible spectroscopy microtiter plate-reader (Biotek Ltd, Synergy H1, Winooski, VT, USA) at an excitation and emission wavelength of 572 and 604 nm, respectively to minimize the interference from NADPH and 7ER.52 Fluconazole was used as a positive control, while the reaction without an inhibitor was used as the negative control. The inhibitor concentration required for 50% inhibition of the CYP51 (IC50) activity was determined using GraphPad prism.
Human CYP3A4 inhibition assay
The P450-Glo™ CYP3A4 assay kit (Cat# 9001) was used to evaluate CYP3A4 activity inhibition using luciferin-IPA according to the manufacturer's procedure.53 The CYP3A4 enzyme can convert the luminogenic substrate luciferin-IPA to a light-producing luciferin product. Briefly, the reaction was started by incubating 12.5 μl of CYP3A4 enzyme with the test compound and luciferin-IPA substrate at 37 °C for 10 min. As positive and negative controls, 12.5 μl ketoconazole and luciferin-free vehicle were used respectively. The initiation of the reaction was then started by the addition of 25 μl NADPH as a regeneration reagent. After 30 min, 50 μl of reconstituted luciferin detection reagent was added to the reaction mixture. Using a plate shaker or tapping the plate, mix briefly. The plate was then incubated at room temperature for 20 min to stabilize the luminescent signal. The luminescence signal was then measured by an Infinite 200 PRO NanoQuant (Tecan Group Ltd. Switzerland).
Measurement of ergosterol content
The mode of action of UOSO13 as an inhibitor of lanosterol 14α-demethylase enzyme was confirmed by the determination of ergosterol content following the treatment of C. albicans cells with UOSO13 at its MIC50 compared to untreated C. albicans and fluconazole treatment as negative and positive controls, respectively. Briefly, a Candida colony was inoculated in 20 mL SDB and incubated for 18 h at 37 °C. The cells were centrifuged and the harvested cells were washed with PBS and normalized prior to sterol extraction as previously described.28 The cells were then suspended in 2 mL alcoholic potassium hydroxide (60%) and heated at 80 °C for 1 h. Sterols were then extracted with hexane and derivatized with N-trimethylsilyl-N-methyl trifluoroacetamide and trimethylchlorosilane, followed by gas chromatography-mass spectrometry (GC-MS) analysis using ergosterol as a reference standard for the retention time. The ergosterol content was determined as % area under the curve compared to untreated cells as the negative control.Ergosterol reduction = (AUC treated)/(AUC control) × 100
Cytotoxicity using the sulforhodamine B assay
The cytotoxic activity of UOSO10, 12, 13 and 14 compounds was tested using the sulforhodamine B assay as previously described.54,55 Briefly, F-180 cells were cultured at a density of 20 000 cells per well in a 24-well plate. After overnight incubation, cells were treated with different concentrations of the compounds (10, 20 and 30 μg ml−1) for 48 h. DMSO (vehicle) was used as the control in a concentration not exceeding 0.1%. The cells were then fixed with 50% trichloroacetic acid for 1 h at 4 °C. The plates were then washed with water, dried, and stained with 0.4% sulforhodamine B (SRB). The retained dye was solubilized with 400 μL of 10 mM Tris base for 10 min. The optical density (OD) was measured at 492 nm with the microplate reader Varioskan™ Flash (Thermo Fisher scientific-Massachusetts-USA). A curve was constructed to relate the concentration of the compound to the cell survival. All experiments were performed six times. Statistical analysis and curve fittings were done using GraphPad Prism 5 software (GraphPad Software, California, USA).
Computational study
Molecular docking of active oxadiazoles with 4 cytochrome P450 (14 α-sterol demethylase, CYP51)
Protein and ligand preparation
The crystal structures of C. albicans CYP51 (PDB: 5FSA, 5V5Z) and human CYP3A4 (PDB: 4D7D) were downloaded from the Protein Data Bank at https://www.rcsb.org, prepared and refined using the Protein Preparation Wizard.56 Crystallographic water molecules beyond 5 Å were removed. All hydrogen atoms were added at pH 7.4. Finally, the energy minimization was done using OPLS-4 to relieve the steric clashes.57
Ligand preparation
The 2D structures of the generated library were converted to 3D structures using LigPrep, Schrodinger.58 Hydrogen atoms were added, and the salt ions were removed. The most probable ionization states were calculated at pH 7.3 using the Epik module.59,60 During the ligand preparation, the specified chirality of the 3D crystal structure was retained. The subsequent energy minimization of each structure was carried out using the OPLS4 force field57 and filtered through a relative energy tool to exclude the high energy structures from the given input. Any errors in the ligands were eradicated in order to enhance the accuracy of the molecular docking.61
Homology modelling
Homology modelling was performed to build a model for the unknown 3D crystal structure of C. auris CYP51. Alignment and sequence analysis using ClustalW Mastero prime Schrödinger62 of the FASTA amino acid sequence of C. auris lanosterol 14-α demethylase (accession # PIS56902) is available at https://www.ncbi.nlm.nih.gov. Prime software was used to select and identify the globally conserved residue. The selection of the 3D crystal structure was carried out by targeting with the highest similarity, positivity, and identity using prime software. The model generated using a knowledge-based tool and validation of the generated model after solving and adding the missing side chain and backbone residues was performed with the Ramachandran plot, protein structure alignment and protein reliability report.62 The alignment and sequence analysis showed high homology of C. auris protein to C. albicans CYP51 crystal structures (PDB: 5V5Z, 5FSA and 5TZ1). The globally conserved residue search using C. albicans CYP51 crystal structures (PDB: 5V5Z) as a template identify 70% identity, 83% positives at alignment score of 2079 (Fig. S1†). ClustalW was used to align the C. auris sequence with the template sequence, revealing the matching and mismatching amino acids (Fig. S1).
Grid generation and molecular docking
The ligand with the crystal structure of C. albicans CYP51 (PDB: 5FSA and 5V5Z), homology constructed model of C. auris and human CYP3A4 (PDB: 4D7D) were used for grid generation. A grid box was generated at the centroid of the active site for docking studies and the active site was defined around the ligand crystal structure. Molecular docking was performed for the prepared UOSO13 within the grid generated63 binding site of C. albicans CYP51 (PDB: 5FSA and 5V5Z), generated C. auris model and human CYP3A4 (PDB: 4D7D) enzymes using the standard precision (SP) mode of Glide64,65 without applying any constraints. The DockScore (DSore) representing the affinity of the docked ligands to enzymes was obtained from the project table file of docked complexes.
Molecular dynamics (MD) simulation
The stability and compatibility of the active UOSO10, 12, 13 and 14-ligand enzyme complexes were monitored with molecular dynamics (MD) simulations using Desmond software.66 Prime67 was used to fill in the missing loop and side chains in the protein. The docked complexes of the UOSO10, 12, 13 and 14 active compounds with the protein were then employed as the starting point for MD simulations. The MD system was created using the Desmond builder system.60 The orthorhombic box with periodic boundary conditions was created using the TIP4P solvent model with enzyme–ligand complexes as the solute, and the system was neutralized by introducing the appropriate amount of counter ions. An energy minimization was applied to the basic ligand–enzyme complex system. The simulation was run for 100 ns under the NPT (constant number of atoms, constant pressure, and constant temperature) ensemble using Desmond's MD option. The simulated trajectory of ligand–enzyme complexes yielded detailed information such as protein and ligand root mean square deviation (RMSD) in relation to the starting frame backbone, root mean square fluctuation (RMSF), and ligand interaction profile. Throughout the MD simulation, RMSD gives insight into the complicated structural conformation. The RMSF showed the variation in the protein chain.
R-group analysis
Structure–activity relationships (SAR) of the shortlisted compounds UOSO10, 12, 13, and 14 were studied using the R-group analysis tool.68 The R-group mapping analysis was performed by Schrödinger. First, the input LigPrep structure with an IC50 value was converted to PIC50 values. The maximum common core was defined with Combi-Glide bond labelling and the alignment of structure for fingerprint similarity of the side chain was performed to minimize the number of attached R-groups. The heat map analysis displayed the effect of different functional group positions with different color ranges that reflect the PIC50 activity. The QSAR model was generated based on the pharmacophoric features (D, A, H, N, R and P).
Physiochemical, pharmacokinetic and drug likeness properties of the oxadiazoles UOSO1–15
The ADME properties of the synthesized oxadiazoles UOSO1–15 were predicted with the Qikprop tool in Maestro QikProp.69 It predicts the physical appropriate descriptors including the hydrogen bond donor, hydrogen bond acceptor, solvent-accessible surface area (SASA), absorption, bioavailability, rule of five that followed the standard methods and drug likeness properties for the discovery and development of novel drug candidates.
Statistical analysis
GraphPad Prism was used to analyze the data (5.04, GraphPad Inc., La Jolla, CA, USA). The cytotoxic effects and enzyme inhibition of the compounds were assessed by one-way analysis of variance (ANOVA) using Bonferroni's multiple comparison test. The significance level was set at P < 0.05. The data display the mean ± SEM of 3–6 replicas.
Conclusion
A series of oxadiazoles UOSO1–15 were designed, synthesized, and evaluated for anti-Candida activity. The novel series inhibited C. albicans and C. auris at sub-micromolar MIC50 concentration. UOSO13 was the most active analogue with selective MIC50 values of 0.5 and 0.8 μg ml−1 against C. albicans and C. auris, respectively, and without showing toxicity on normal mammalian cells. The CaCYP51 inhibitory assay revealed that UOSO13 showed a potent binding activity with an IC50 value of 0.9 μg ml−1 compared to 8.87 μg ml−1 of fluconazole as a positive control and with no binding activity to the human enzyme. UOSO13 showed 70% reduction of the fungal ergosterol content. Moreover, molecular modelling studies indicated its strong interaction and complex stability with the key CYP51 amino acid residues involved in azole sensitivity of the mutant strains. Furthermore, it fulfils the drug likeness criteria as a promising antifungal candidate. Taken together, UOSO13 could be a promising antifungal agent against C. albicans and C. auris strains and deserves further investigation.
Abbreviations
- ADME
Absorption distribution metabolism and excretion
- FASTA
Format for nucleotide sequences
- C
Candida
- CDC
Centers for Disease Control and Prevention
- CFU
Colony forming unit
- CLSI
Clinical and Laboratory Standards Institute
- CYP51
Candida lanosterol-14α-demethylase
- DBDMH
1,3-Dibromo-5,5-dimethylhydantoin
- DMEM
Dulbecco's modified Eagle medium
- 7-ER
7-Ethoxyresorufin
- FBS
Fetal bovine serum
- GC-MS
Gas chromatography-mass spectrometry
- H
Hydrophobic group
- HBA
Hydrogen bond acceptor
- H-bond
Hydrogen bond
- IC50
50% inhibitory concentration
- IFIs
Invasive fungal infections
- Ligprep
Ligand preparation
- MD
Molecular dynamics
- MTT
3-(4,5-Dimethylthiazolyl-2-yl)-2,5 diphenyl tetrazolium bromide
- NPT
Constant number of atoms constant pressure and constant temperature
- N
Negatively ionisable
- OD
Optical density
- PBS
Phosphate buffered saline
- PDB
Protein Data Bank
- QSAR
Quantitative structure–activity relationship
- R
Aromatic ring
- RCSB
Research Collaboratory for Structural Bioinformatics
- RMSF
Root mean square fluctuation
- RMSD
Root mean square deviation
- SAR
Structure–activity relationships
- SASA
Solvent-accessible surface area
- SDA
Sabouraud dextrose agar
- SP
Standard precision
- SRB
Sulforhodamine B
- TLC
Thin-layer chromatography
- UV
Ultraviolet
Author contributions
RH developed the rationale and conducted the compound synthesis, all computational modelling, data analysis and interpretation. AH run all microbiology assays and ergosterol content determination, and MK helped in the purification of the compounds. VM and RA helped with the cytotoxicity assay. SS developed the idea, supervised the project, obtained the fund, and helped in data analysis and interpretation. RH, AH and SS wrote the first draft and final version. All the authors revised the manuscript and agreed on publication.
Conflicts of interest
All the authors declare that there is no conflict of interest.
Supplementary Material
Acknowledgments
The authors acknowledge the generous support received from the University of Sharjah to SSMS. The authors also acknowledge the technical support provided by Dr. Pritesh Bhat, Principal Scientist, Schrödinger, India.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2md00196a
References
- Du H. Bing J. Hu T. Ennis C. L. Nobile C. J. Huang G. PLoS Pathog. 2020;16:e1008921. doi: 10.1371/journal.ppat.1008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler J. Stevens D. A. Clin. Infect. Dis. 2003;36:S31–S41. doi: 10.1086/344658. [DOI] [PubMed] [Google Scholar]
- Soontorngun N., Somboon P. and Watchaputi K., in Non-conventional Yeasts: from Basic Research to Application, Springer, 2019, pp. 453–476 [Google Scholar]
- Aigner M. Lass-Flörl C. Expert Opin. Pharmacother. 2015;16:2267–2270. doi: 10.1517/14656566.2015.1083976. [DOI] [PubMed] [Google Scholar]
- Bodey G. P. Clin. Infect. Dis. 1992;14:S161–S169. doi: 10.1093/clinids/14.Supplement_1.S161. [DOI] [PubMed] [Google Scholar]
- Peng X.-M. Cai G.-X. Zhou C.-H. Curr. Top. Med. Chem. 2013;13:1963–2010. doi: 10.2174/15680266113139990125. [DOI] [PubMed] [Google Scholar]
- Maertens J. Clin. Microbiol. Infect. 2004;10:1–10. doi: 10.1111/j.1470-9465.2004.00841.x. [DOI] [PubMed] [Google Scholar]
- Chen A. Sobel J. D. Expert Opin. Emerging Drugs. 2005;10:21–33. doi: 10.1517/14728214.10.1.21. [DOI] [PubMed] [Google Scholar]
- Han G. Liu N. Li C. Tu J. Li Z. Sheng C. J. Med. Chem. 2020;63:5341–5359. doi: 10.1021/acs.jmedchem.0c00102. [DOI] [PubMed] [Google Scholar]
- Girmenia C. Expert Opin. Invest. Drugs. 2009;18:1279–1295. doi: 10.1517/13543780903176407. [DOI] [PubMed] [Google Scholar]
- Zou Y. Liu L. Liu J. Liu G. Future Sci. 2020;12:91–93. doi: 10.4155/fmc-2019-0288. [DOI] [PubMed] [Google Scholar]
- Liao J. Yang F. Zhang L. Chai X. Zhao Q. Yu S. Zou Y. Meng Q. Wu Q. Arch. Pharmacal Res. 2015;38:470–479. doi: 10.1007/s12272-014-0378-5. [DOI] [PubMed] [Google Scholar]
- Maftei C. V. Fodor E. Jones P. G. Daniliuc C. G. Franz M. H. Kelter G. Fiebig H.-H. Tamm M. Neda I. Tetrahedron. 2016;72:1185–1199. doi: 10.1016/j.tet.2016.01.011. [DOI] [Google Scholar]
- Rajak H. Thakur B. S. Singh A. Raghuvanshi K. Sah A. K. Veerasamy R. Sharma P. C. Pawar R. S. Kharya M. D. Bioorg. Med. Chem. Lett. 2013;23:864–868. doi: 10.1016/j.bmcl.2012.11.051. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Vendramini F. A. V. Faria D. R. Arita G. S. Capoci I. R. G. Sakita K. M. Caparroz-Assef S. M. Becker T. C. A. de Souza Bonfim-Mendonça P. Felipe M. S. Svidzinski T. I. E. PLoS Neglected Trop. Dis. 2019;13:e0007441. doi: 10.1371/journal.pntd.0007441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-J. Song B.-A. Yang S. Xu G.-F. Bhadury P. S. Jin L.-H. Hu D.-Y. Li Q.-Z. Liu F. Xue W. Bioorg. Med. Chem. 2007;15:3981–3989. doi: 10.1016/j.bmc.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Faria D. R. Sakita K. M. Capoci I. R. G. Arita G. S. Rodrigues-Vendramini F. A. V. de Oliveira Junior A. G. Soares Felipe M. S. Bonfim de Mendonça P. d. S. Svidzinski T. I. E. Kioshima E. S. PLoS One. 2020;15:e0227876. doi: 10.1371/journal.pone.0227876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subhashini N. Bhadraiah B. Janaki P. Russ. J. Gen. Chem. 2017;87:550–553. doi: 10.1134/S1070363217030276. [DOI] [Google Scholar]
- Zhu H.-H. Zeng D. Wang M.-W. Wang P.-Y. Wu Y.-Y. Liu L.-W. Yang S. J. Saudi Chem. Soc. 2020;24:127–138. doi: 10.1016/j.jscs.2019.10.002. [DOI] [Google Scholar]
- Kaur R. Kaur P. Eur. J. Biomed. Pharm. Sci. 2018;5:865–877. [Google Scholar]
- Zhang M.-Z. Mulholland N. Beattie D. Irwin D. Gu Y.-C. Chen Q. Yang G.-F. Clough J. Eur. J. Med. Chem. 2013;63:22–32. doi: 10.1016/j.ejmech.2013.01.038. [DOI] [PubMed] [Google Scholar]
- Paruch K. Popiołek Ł. Wujec M. Med. Chem. Res. 2020;29:1–16. doi: 10.1007/s00044-019-02463-w. [DOI] [Google Scholar]
- Lelyukh M. Martynets M. Kalytovska M. Drapak I. Harkov S. Chaban T. Chaban I. Matiychuk V. J. Appl. Pharm. Sci. 2020;10:151–165. [Google Scholar]
- Friggeri L. Hargrove T. Y. Wawrzak Z. Blobaum A. L. Rachakonda G. Lindsley C. W. Villalta F. Nes W. D. Botta M. Guengerich F. P. J. Med. Chem. 2018;61:5679–5691. doi: 10.1021/acs.jmedchem.8b00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher R. Wirsel S. G. Appl. Microbiol. Biotechnol. 2012;95:825–840. doi: 10.1007/s00253-012-4195-9. [DOI] [PubMed] [Google Scholar]
- Lepesheva G. I. Hargrove T. Y. Kleshchenko Y. Nes W. D. Villalta F. Waterman M. R. Lipids. 2008;43:1117–1125. doi: 10.1007/s11745-008-3225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafferty S. W. Eisner J. R. Moore W. R. Schotzinger R. J. Hoekstra W. J. Bioorg. Med. Chem. Lett. 2014;24:2444–2447. doi: 10.1016/j.bmcl.2014.04.024. [DOI] [PubMed] [Google Scholar]
- Warrilow A. Hull C. Parker J. Garvey E. Hoekstra W. Moore W. Schotzinger R. Kelly D. Kelly S. Antimicrob. Agents Chemother. 2014;58:7121–7127. doi: 10.1128/AAC.03707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Cantero A. Lopez-Fernandez L. Guarro J. Capilla J. Int. J. Antimicrob. Agents. 2020;55:105807. doi: 10.1016/j.ijantimicag.2019.09.011. [DOI] [PubMed] [Google Scholar]
- Flowers S. A. Colón B. Whaley S. G. Schuler M. A. Rogers P. D. Antimicrob. Agents Chemother. 2015;59:450–460. doi: 10.1128/AAC.03470-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshkumar A. Suvaithenamudhan S. Rajaram R. Curr. Microbiol. 2021;78:292–302. doi: 10.1007/s00284-020-02269-9. [DOI] [PubMed] [Google Scholar]
- Doğan İ. S. Sarac S. Sari S. Kart D. Gökhan Ş. E. Vural I. Dalkara S. Eur. J. Med. Chem. 2017;130:124–138. doi: 10.1016/j.ejmech.2017.02.035. [DOI] [PubMed] [Google Scholar]
- Hoekstra W. J. Garvey E. P. Moore W. R. Rafferty S. W. Yates C. M. Schotzinger R. J. Bioorg. Med. Chem. Lett. 2014;24:3455–3458. doi: 10.1016/j.bmcl.2014.05.068. [DOI] [PubMed] [Google Scholar]
- Morio F. Loge C. Besse B. Hennequin C. Le Pape P. Diagn. Microbiol. Infect. Dis. 2010;66:373–384. doi: 10.1016/j.diagmicrobio.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Chen S.-H. Sheng C.-Q. Xu X.-H. Jiang Y.-Y. Zhang W.-N. He C. Biol. Pharm. Bull. 2007;30:1246–1253. doi: 10.1248/bpb.30.1246. [DOI] [PubMed] [Google Scholar]
- Sun B. Huang W. Liu M. J. Mol. Graphics Modell. 2017;73:157–165. doi: 10.1016/j.jmgm.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Revie N. M. Iyer K. R. Robbins N. Cowen L. E. Curr. Opin. Microbiol. 2018;45:70–76. doi: 10.1016/j.mib.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates C. M. Garvey E. P. Shaver S. R. Schotzinger R. J. Hoekstra W. J. Bioorg. Med. Chem. Lett. 2017;27:3243–3248. doi: 10.1016/j.bmcl.2017.06.037. [DOI] [PubMed] [Google Scholar]
- Warrilow A. G. Parker J. E. Price C. L. Nes W. D. Garvey E. P. Hoekstra W. J. Schotzinger R. J. Kelly D. E. Kelly S. L. Antimicrob. Agents Chemother. 2016;60:4530–4538. doi: 10.1128/AAC.00349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb D. C. Kelly D. E. Baldwin B. C. Kelly S. L. Chem.-Biol. Interact. 2000;125:165–175. doi: 10.1016/S0009-2797(99)00169-6. [DOI] [PubMed] [Google Scholar]
- Hamdy R. Fayed B. Hamoda A. M. Rawas-Qalaji M. Haider M. Soliman S. S. Molecules. 2020;25:1463. doi: 10.3390/molecules25061463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S. M. U. Nazeer Y. Jayanthi S. Kabir M. A. Mol. Biol. Res. Commun. 2020;9:155. doi: 10.22099/mbrc.2020.36298.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honorio K. M. Moda T. L. Andricopulo A. D. Med. Chem. 2013;9:163–176. doi: 10.2174/1573406411309020002. [DOI] [PubMed] [Google Scholar]
- Zveaghintseva M. Stingaci E. Pogrebnoi S. Smetanscaia A. Valica V. Uncu L. Kravtsov V. Ch. Melnic E. Petrou A. Glamočlija J. Molecules. 2021;26:4304. doi: 10.3390/molecules26144304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponec M. Haverkort M. Soei Y. L. Kempenaar J. Bodde H. J. Pharm. Sci. 1990;79:312–316. doi: 10.1002/jps.2600790408. [DOI] [PubMed] [Google Scholar]
- Hamdy R. Ziedan N. I. Ali S. Bordoni C. El-Sadek M. Lashin E. Brancale A. Jones A. T. Westwell A. D. Bioorg. Med. Chem. Lett. 2017;27:1037–1040. doi: 10.1016/j.bmcl.2016.12.061. [DOI] [PubMed] [Google Scholar]
- Hamdy R. Ziedan N. Ali S. El-Sadek M. Lashin E. Brancale A. Jones A. T. Westwell A. D. Bioorg. Med. Chem. Lett. 2013;23:2391–2394. doi: 10.1016/j.bmcl.2013.02.029. [DOI] [PubMed] [Google Scholar]
- Soliman S. S. Hamdy R. Elseginy S. A. Gebremariam T. Hamoda A. M. Madkour M. Venkatachalam T. Ershaid M. N. Mohammad M. G. Chamilos G. Biochem. J. 2020;477:2489–2507. doi: 10.1042/BCJ20200310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy R. Soliman S. S. Alsaadi A. I. Fayed B. Hamoda A. M. Elseginy S. A. Husseiny M. I. Ibrahim A. S. Eur. J. Pharm. Sci. 2020;148:105327. doi: 10.1016/j.ejps.2020.105327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. E. Warrilow A. G. Cools H. J. Fraaije B. A. Lucas J. A. Rigdova K. Griffiths W. J. Kelly D. E. Kelly S. L. Appl. Environ. Microbiol. 2013;79:1639–1645. doi: 10.1128/AEM.03246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcoss M. M. El Shimaa M. Ibrahem R. A. Abdel-Rahman H. M. Abdel-Aziz M. Abou El-Ella D. A. Bioorg. Chem. 2020;101:103956. doi: 10.1016/j.bioorg.2020.103956. [DOI] [PubMed] [Google Scholar]
- Hassan N. H. El-Hawary S. S. Emam M. Rabeh M. A. Abdelmohsen U. R. Selim N. M. ACS Omega. 2022;7:13808–13817. doi: 10.1021/acsomega.2c00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X. Pan L. Wang J. Lu L. Yan W. Zhu Y. Xu Y. Guo M. Zhuang S. Environ. Pollut. 2017;222:504–512. doi: 10.1016/j.envpol.2016.11.051. [DOI] [PubMed] [Google Scholar]
- El-Awady R. A. Semreen M. H. Saber-Ayad M. M. Cyprian F. Menon V. Al-Tel T. H. DNA Repair. 2016;37:1–11. doi: 10.1016/j.dnarep.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Elseginy S. A. Hamdy R. Menon V. Almehdi A. M. El-Awady R. Soliman S. S. M. Bioorg. Med. Chem. Lett. 2020;30:127658. doi: 10.1016/j.bmcl.2020.127658. [DOI] [PubMed] [Google Scholar]
- Sastry G. M. Adzhigirey M. Day T. Annabhimoju R. Sherman W. J. Comput.-Aided Mol. Des. 2013;27:221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Harder E. Damm W. Maple J. Wu C. Reboul M. Xiang J. Y. Wang L. Lupyan D. Dahlgren M. K. Knight J. L. J. Chem. Theory Comput. 2016;12:281–296. doi: 10.1021/acs.jctc.5b00864. [DOI] [PubMed] [Google Scholar]
- Giardina S. F. Werner D. S. Pingle M. Feinberg P. B. Foreman K. W. Bergstrom D. E. Arnold L. D. Barany F. J. Med. Chem. 2020;63:3004–3027. doi: 10.1021/acs.jmedchem.9b01689. [DOI] [PubMed] [Google Scholar]
- Shelley J. C. Cholleti A. Frye L. L. Greenwood J. R. Timlin M. R. Uchimaya M. J. Comput.-Aided Mol. Des. 2007;21:681–691. doi: 10.1007/s10822-007-9133-z. [DOI] [PubMed] [Google Scholar]
- Greenwood J. R. Calkins D. Sullivan A. P. Shelley J. C. J. Comput.-Aided Mol. Des. 2010;24:591–604. doi: 10.1007/s10822-010-9349-1. [DOI] [PubMed] [Google Scholar]
- Ganai S. A. Abdullah E. Rashid R. Altaf M. Front. Mol. Neurosci. 2017;10:357. doi: 10.3389/fnmol.2017.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammed M. T. Aki-Yalcin E. Chem. Biol. Drug Des. 2019;93:12–20. doi: 10.1111/cbdd.13388. [DOI] [PubMed] [Google Scholar]
- Friesner R. A. Murphy R. B. Repasky M. P. Frye L. L. Greenwood J. R. Halgren T. A. Sanschagrin P. C. Mainz D. T. J. Med. Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Halgren T. A. Murphy R. B. Friesner R. A. Beard H. S. Frye L. L. Pollard W. T. Banks J. L. J. Med. Chem. 2004;47:1750–1759. doi: 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
- Friesner R. A. Banks J. L. Murphy R. B. Halgren T. A. Klicic J. J. Mainz D. T. Repasky M. P. Knoll E. H. Shelley M. Perry J. K. J. Med. Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- Chow E., Rendleman C. A., Bowers K. J., Dror R. O., Hughes D. H., Gullingsrud J., Sacerdoti F. D. and Shaw D. E., DE Shaw Research Technical Report DESRES/TR--2008-01, 2008
- Jacobson M. P. Pincus D. L. Rapp C. S. Day T. J. Honig B. Shaw D. E. Friesner R. A. Proteins: Struct., Funct., Bioinf. 2004;55:351–367. doi: 10.1002/prot.10613. [DOI] [PubMed] [Google Scholar]
- Hamdy R. Fayed B. Mostafa A. Shama N. M. A. Mahmoud S. H. Mehta C. H. Nayak Y. Soliman S. S. M. Int. J. Mol. Sci. 2021;22:9057. doi: 10.3390/ijms22169057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L.-C. Liang Y.-F. Huang Y. Yang G.-X. Zheng L.-L. Sun J.-M. Li Y. Zhu F.-L. Qian H.-W. Wang R. Eur. J. Med. Chem. 2021;219:113426. doi: 10.1016/j.ejmech.2021.113426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.