To the Editor:
Interstitial lung disease (ILD) comprises diffuse pulmonary parenchymal disorders, with fibrotic ILD leading to poor outcomes when progressive (1). Though reliably detected by high-resolution computed tomography (HRCT), diagnostic delays remain common in patients with ILD and are associated with disease progression (2–4). Immunosuppressive and antifibrotic therapies have been shown to slow lung function decline in patients with fibrotic ILD (5, 6), underscoring the importance of early detection.
While delays in ILD diagnosis are multifactorial (3), diagnostic testing decisions may contribute. The chest radiograph is commonly used to evaluate dyspnea and cough in the primary care setting. While this may sufficiently evaluate some conditions (7–9), HRCT provides a higher degree of confidence when diagnosing diffuse parenchymal lung disease, including fibrotic ILD (10). Despite this observation, the diagnostic utility of chest radiography remains unclear. In this investigation, we conducted a retrospective analysis to determine test performance characteristics of chest radiography for detecting ILD, confirmed by contemporaneous HRCT.
Methods
This study was approved by the University of California at Davis (UC-Davis) Institutional Review Board (#875917). Adult patients receiving primary care at UC-Davis from 2014 to 2021 with a multidisciplinary diagnosis of fibrotic ILD, including idiopathic pulmonary fibrosis, connective tissue disease-associated ILD, chronic hypersensitivity pneumonitis, and unclassifiable ILD were identified using the UC-Davis ILD registry. Those with incident ILD who underwent chest radiography up to 12 months before the first HRCT confirming ILD were eligible for inclusion.
The presence of fibrotic ILD on HRCT was confirmed by a chest radiologist (A.G. or M.K.) during multidisciplinary evaluation and was defined as fibrotic changes (reticular opacities, traction bronchiectasis, and/or honeycombing) affecting >10% of the lung parenchyma, consistent with prior therapeutic trials in ILD (11). HRCT pattern was further characterized as usual interstitial pneumonia (UIP), probable UIP, indeterminate, or alternate pattern on the basis of guideline criteria (12). Chest radiograph reports were reviewed to determine whether features of ILD were mentioned and were considered present when any of the following were mentioned: reticulation, reticular, fibrosis, fibrotic, or interstitial.
Consecutive age- and sex-matched control subjects without ILD, ascertained by review of radiology report and secondary visual assessment by an ILD-trained pulmonologist (J.V.P.), were identified over the same timeframe and matched 1:1 with ILD cases to determine test performance characteristics.
Statistical Analysis
Continuous variables are reported as means ± standard deviation and compared using a student’s t test. Categorical variables are reported as counts and percentages and compared using a chi-square test. Time to pulmonologist evaluation was compared between ILD cases according to whether ILD features were mentioned on chest radiograph using Cox proportional hazards regression and plotted using the Kaplan-Meier estimator. Statistical analysis was performed using Stata, Release 16.1 (StataCorp).
Results
Of 264 eligible patients, 76 (29%) were excluded because of lack of prior chest radiography and 8 (3%) because of prevalent ILD, leaving 180 ILD cases (Figure 1A). Case characteristics are shown in Table 1. Idiopathic pulmonary fibrosis was the most common diagnosis (36%), followed by connective tissue disease associated-ILD (26%). A chest radiologist interpreted roughly half of the chest radiographs. When comparing patients with and without mention of ILD features on chest radiography (Table 1), few differences were observed, suggesting ILD severity did not influence the mention of ILD features.
Figure 1.
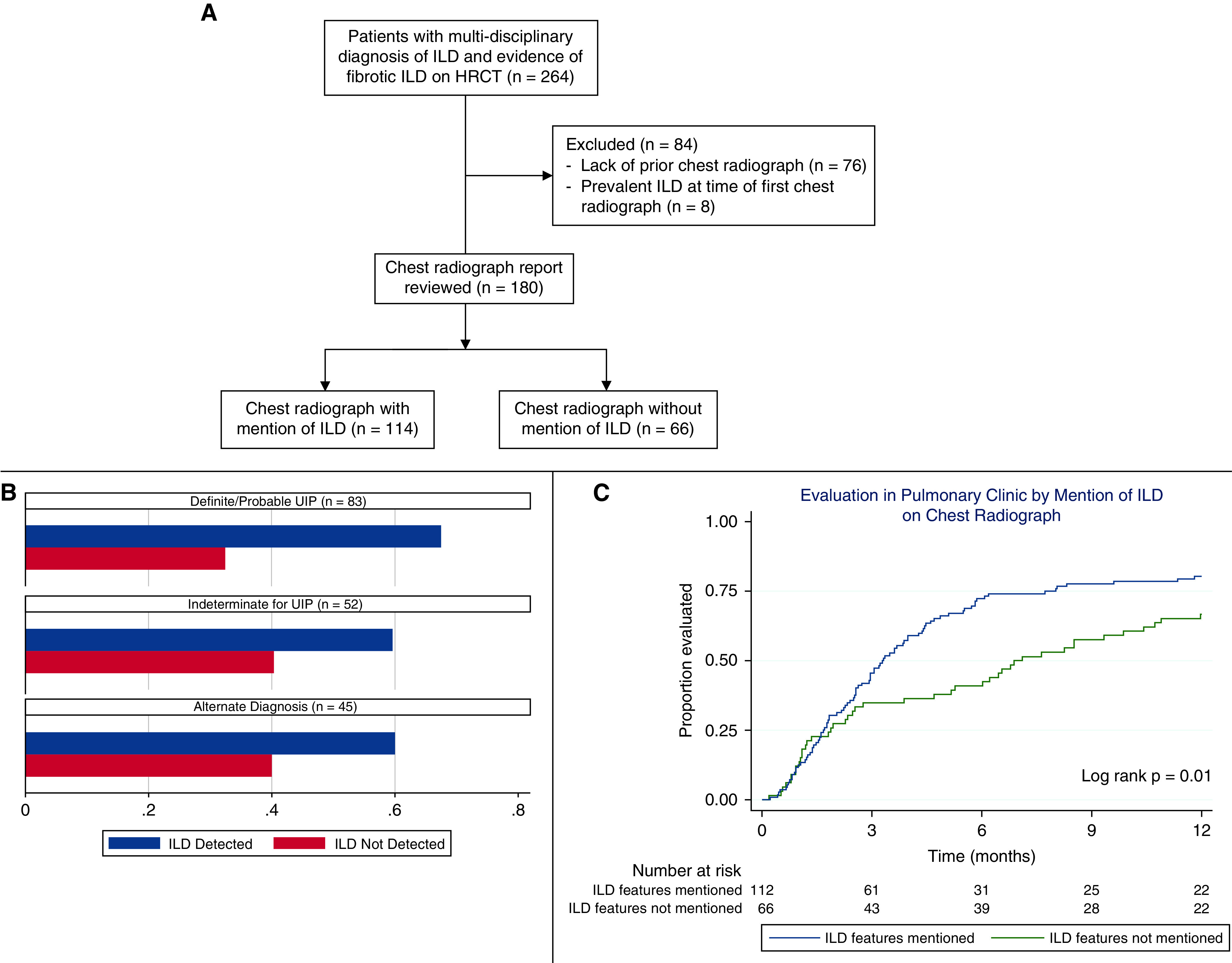
(A) Flow diagram of eligible and included interstitial lung disease (ILD) cases. (B) Percentage of cases with ILD mentioned on chest radiography, stratified by high-resolution computed tomography pattern. (C) Time to pulmonary evaluation stratified by the mention of ILD on chest radiography. HRCT = high-resolution computed tomography; UIP = usual interstitial pneumonia.
Table 1.
Baseline characteristics for interstitial lung disease cases with and without mention of interstitial lung disease features on chest radiography
| Characteristic | Patients with ILD (n = 180) | ILD Features Mentioned (n = 114) | ILD Features not Mentioned (n = 66) | P Value |
|---|---|---|---|---|
| Age (yr), mean (±SD) | 69.7 (11.4) | 70.9 (10.2) | 67.7 (13.1) | 0.073 |
| Male sex, n (%) | 108 (60.0) | 70 (61.4) | 38 (57.6) | 0.61 |
| Race/ethnicity, n (%) | — | — | — | 0.65 |
| White | 130 (72.2) | 81 (71.1) | 49 (74.2) | — |
| Black | 10 (5.6) | 5 (4.4) | 5 (7.6) | — |
| Asian | 19 (10.6) | 14 (12.3) | 5 (7.6) | — |
| Hispanic | 19 (10.6) | 12 (10.5) | 7 (10.6) | — |
| Other/unknown | 2 (1.1) | 2 (1.8) | 0 (0) | — |
| Ever-smoker, n (%) | 108 (60.0) | 70 (61.4) | 38 (57.6) | 0.64 |
| Radiograph interpreted by chest radiologist, n (%) | 88 (48.9) | 56 (49.1) | 32 (48.5) | 0.93 |
| ILD classification, n (%) | — | — | — | 0.76 |
| IPF | 64 (35.6) | 43 (37.7) | 21 (31.8) | — |
| CTD-ILD | 46 (25.6) | 28 (24.6) | 18 (27.3) | — |
| uILD | 40 (22.2) | 25 (21.9) | 15 (22.7) | — |
| CHP | 23 (12.8) | 15 (13.2) | 8 (12.1) | — |
| Other non-IPF IIP | 7 (3.9) | 3 (2.6) | 4 (6.1) | — |
| Pulmonary function, mean (±SD) | — | — | — | — |
| FVC% predicted | 80.5 (18.5) | 79.9 (17.2) | 81.4 (20.6) | 0.61 |
| DLCO% predicted | 54.9 (16.6) | 53.2 (15.1) | 57.7 (18.5) | 0.085 |
Definition of abbreviations: CHP = chronic hypersensitivity pneumonitis; CTD-ILD = connective tissue disease-associated interstitial lung disease; DLCO = diffusion capacity of the lung for carbon monoxide; FVC = forced vital capacity; ILD = interstitial lung disease; IIP = idiopathic interstitial pneumonia; IPF = idiopathic pulmonary fibrosis; SD = standard deviation; uILD = unclassifiable interstitial lung disease.
Compared with the HRCT gold standard, the chest radiograph demonstrated a sensitivity of 63% and specificity of 93%, with a positive predictive value of 90% and a negative predictive value of 72% (Table 2). After stratification by HRCT pattern, the chest radiograph detected a higher percentage of cases with definite or probable UIP compared with other patterns (Figure 1B). Those for whom ILD was mentioned had a significantly shorter time to pulmonologist evaluation (Figure 1C), with a 40% higher likelihood of pulmonologist evaluation (hazard ratio, 1.40; 95% confidence interval, 1.03–1.91; P = 0.013).
Table 2.
Chest radiograph test performance characteristics
| Mention of ILD on chest radiograph | ILD by HRCT |
||
|---|---|---|---|
| (+) | (−) | ||
| (+) | 114 | 13 | |
| (−) | 66 | 167 | |
| Test characteristic (95% CI) | |||
| Sensitivity | 0.63 (0.56–0.70) | ||
| Specificity | 0.93 (0.88–0.96) | ||
| Receiver–operator curve area | 0.78 (0.74–0.82) | ||
| Positive predictive value | 0.90 (0.83–0.94) | ||
| Negative predictive value | 0.72 (0.65–0.77) | ||
| Positive likelihood ratio | 8.77 (5.13–14.98) | ||
| Negative likelihood ratio | 0.40 (0.32–0.48) | ||
Definition of abbreviations: CI = confidence interval; HRCT = high-resolution computed tomography; ILD = interstitial lung disease.
Discussion
In this study, we found that the chest radiograph had good specificity but modest sensitivity for detecting ILD. These data suggest that using chest radiography to screen for fibrotic ILD will miss nearly 30% of cases. Furthermore, baseline lung function was similar between those with and without ILD mentioned, suggesting that chest radiography does not simply select for more advanced ILD. Our findings also suggest that failure to mention ILD features on chest radiography is associated with a delay in pulmonology evaluation, underscoring the importance of reporting ILD features. Together, these findings highlight the limited utility of the chest radiograph for diagnosing ILD.
To our knowledge, our study is among the first to assess the test performance of chest radiography for detecting common fibrotic ILDs. This work corroborates two previous investigations showing HRCT to be superior to chest radiography in reaching a confident diagnosis among patients with diffuse parenchymal lung disease, including fibrotic ILD, nonfibrotic ILD, and other non-ILD processes (10, 13). However, these studies predated our current radiologic schema of understanding and classifying ILD and included large numbers of rare lung diseases, limiting the interpretation of results in the context of fibrotic ILD. Our data build on these findings and suggest that the chest radiograph will miss a large proportion of a potentially deadly disease. A study by Afzal and colleagues suggested that chest radiography had 80% sensitivity for diagnosing ILD (14). However, patients were aged 20–50, and ILD diagnoses were not specified, suggesting that this study population was systematically different from ours, as many common fibrotic ILDs are associated with older age (15).
Limitations
Our study has several limitations. First, this is a single-center, retrospective study of patients with both chest radiography and computed tomography, which may have introduced selection bias and limited generalizability. Furthermore, reliance on existing radiology interpretations may not accurately capture the true performance of the chest radiograph, though this approach is more likely to reflect real-life primary care settings.
Conclusions
Chest radiography is likely to miss a large proportion of patients with fibrotic ILD, but ILD is likely when features are detected. A chest radiograph showing features of ILD should be followed up with HRCT, while ILD should remain among the differential diagnoses in those without ILD mentioned. Further research is needed to validate these findings and further elucidate factors underpinni ng diagnostic delays in patients with fibrotic ILD.
Footnotes
Supported by the National Heart, Lung, and Blood Institute (K23HL138190 [J.M.O.] and T32HL007013 [J.V.P.]).
Author Contributions: Study design: S.G., J.V.P., and J.M.O. Data analysis: J.V.P. and J.M.O. Interpretation of results: S.G., J.V.P., M.A.K., A.G., and J.M.O. Manuscript preparation: S.G., J.V.P., M.A.K., A.G., and J.M.O. J.V.P. takes responsibility for (is the guarantor of) the content of the manuscript, including the data and analysis.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Kolb M, Vašáková M. The natural history of progressive fibrosing interstitial lung diseases. Respir Res . 2019;20:57. doi: 10.1186/s12931-019-1022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pritchard D, Adegunsoye A, Lafond E, Pugashetti JV, DiGeronimo R, Boctor N, et al. Diagnostic test interpretation and referral delay in patients with interstitial lung disease. Respir Res . 2019;20:253. doi: 10.1186/s12931-019-1228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoyer N, Prior TS, Bendstrup E, Wilcke T, Shaker SB. Risk factors for diagnostic delay in idiopathic pulmonary fibrosis. Respir Res. 2019;20:103. doi: 10.1186/s12931-019-1076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med . 2011;184:842–847. doi: 10.1164/rccm.201104-0668OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet . 2012;380:689–698. doi: 10.1016/S0140-6736(12)61079-4. [DOI] [PubMed] [Google Scholar]
- 6. Maher TM, Strek ME. Antifibrotic therapy for idiopathic pulmonary fibrosis: time to treat. Respir Res . 2019;20:205. doi: 10.1186/s12931-019-1161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Emamian SA, Kaasbol MA, Olsen JF, Pedersen JF. Accuracy of the diagnosis of pleural effusion on supine chest X-ray. Eur Radiol . 1997;7:57–60. doi: 10.1007/s003300050109. [DOI] [PubMed] [Google Scholar]
- 8. Glazer HS, Anderson DJ, Wilson BS, Molina PL, Sagel SS. Pneumothorax: appearance on lateral chest radiographs. Radiology . 1989;173:707–711. doi: 10.1148/radiology.173.3.2813774. [DOI] [PubMed] [Google Scholar]
- 9. Albaum MN, Hill LC, Murphy M, Li YH, Fuhrman CR, Britton CA, et al. PORT Investigators Interobserver reliability of the chest radiograph in community-acquired pneumonia. Chest . 1996;110:343–350. doi: 10.1378/chest.110.2.343. [DOI] [PubMed] [Google Scholar]
- 10. Grenier P, Valeyre D, Cluzel P, Brauner MW, Lenoir S, Chastang C. Chronic diffuse interstitial lung disease: diagnostic value of chest radiography and high-resolution CT. Radiology . 1991;179:123–132. doi: 10.1148/radiology.179.1.2006262. [DOI] [PubMed] [Google Scholar]
- 11. Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. INBUILD Trial Investigators Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med . 2019;381:1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 12. Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society Diagnosis of idiopathic pulmonary fibrosis. An Official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med . 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 13. Padley SP, Hansell DM, Flower CD, Jennings P. Comparative accuracy of high resolution computed tomography and chest radiography in the diagnosis of chronic diffuse infiltrative lung disease. Clin Radiol . 1991;44:222–226. doi: 10.1016/s0009-9260(05)80183-7. [DOI] [PubMed] [Google Scholar]
- 14. Afzal F, Raza S, Shafique M. Diagnostic accuracy of X-ray chest in interstitial lung disease as confirmed by high resolution computed tomography (HRCT) chest. Pak Armed Forces Med. . 2017;67:593–598. [Google Scholar]
- 15. Patterson KC, Shah RJ, Porteous MK, Christie JD, D’Errico CA, Chadwick M, et al. Interstitial lung disease in the elderly. Chest . 2017;151:838–844. doi: 10.1016/j.chest.2016.11.003. [DOI] [PubMed] [Google Scholar]


