To the Editor:
Light’s criteria remains the standard for pleural fluid analysis (1). Light’s criteria has superb sensitivity but less robust specificity, particularly in certain populations (2). Hematopoietic stem cell transplant (HSCT) recipients and patients with hematologic malignancies often develop pleural effusions (3, 4). Cohort studies within these populations have used Light’s criteria as the reference standard for case adjudication; however, the majority of effusions were classified as transudate or exudate without a specific etiology (3–6). Light’s criteria may misclassify effusions in patients with neutropenia, particularly in processes like infection where neutrophils are thought to play a role in pleural pathology, thereby leading to diagnostic uncertainty. This study examines the test characteristics of Light’s criteria and other pleural fluid tests that help to identify exudative effusions in a cohort of patients with neutropenia.
Methods
The study was approved by University of Virginia (UVA) Health-Sciences Research Institutional Review Board with approval number 22067. This study is retrospective with a waiver of patient consent. This convenience cohort was created by querying the UVA data warehouse for thoracenteses performed between March 2011 and June 2020 on patients who were neutropenic at least 24 hours prior to the thoracentesis. Neutropenia was defined by an absolute neutrophil count of less than 1,000 cells/μl. All etiologies of neutropenia and indications for thoracentesis were included in this study. Clinical data, pleural fluid data, serum lab data, and imaging studies were extracted from the electronic medical record and stored in a REDCap database (7, 8).
Case Adjudication
A summary of the index hospitalization was generated by authors I.M.B. and S.F.O. Two board-certified pulmonologists (A.J.B. and J.M.S.) independently reviewed the available data, including the index test results, and case summary to determine the etiology of the effusion. After the independent review, the outcomes were compared and a consensus outcome was obtained. Categories for final diagnosis included exudate due to infection, exudate due to malignancy, exudate due to other, and transudate. Case definitions were established prior to adjudication and are outlined in Methods in the online supplement.
Data Analysis
Determining if an effusion was an exudate was the outcome of interest. Case adjudication was the reference standard. Sensitivity, specificity, positive likelihood ratio (LR+), and negative likelihood ratio (LR−) were determined for each of the following: Light’s criteria, two-test rule, three-test rule, and the individual components of these tests as previously described (1, 9). Analysis of pleural fluid cholesterol was not completed due to the limited number of samples in this cohort.
Pleural fluid data from the exudates due to infection and exudates due to malignancy were compared. The variables of interest included pleural fluid values of pH, lactate dehydrogenase (LDH), protein, glucose, total cell count, and cell count differential. Mean values and standard deviations were calculated for these variables. An independent sample t test assuming unequal variances was used for comparison of mean values. Two-tail P values were reported with each variable of interest. A Bonferroni correction was performed to correct for multiplicity with a familywise error rate of 0.05.
Results
Baseline Demographics
The search query identified 83 thoracenteses from 80 patients (Table E1). Median age was 61.6 years old and 48.8% were male. The cohort included individuals with acute or chronic leukemia (26.3%), lymphoma of unspecified subtype (18.8%), nonpulmonary solid tumors (21.3%), pulmonary solid tumors (13.8%), HSCT recipients (11.3%), solid organ transplant recipients (11.3%), individuals with heart failure (12.0%), chronic kidney disease (7.2%), and decompensated cirrhosis (8.4%). Regarding duration of neutropenia, 30.1% of thoracenteses occurred with neutropenia of less than 48 hours, 15.7% with neutropenia of less than 7 but greater than 2 days, 28.9% with neutropenia of less than 30 but greater than 7 days, and 25.3% with neutropenia of greater than 30 days. The pleural effusions were determined by case adjudication to be transudates in 33.7% of cases, exudates due to infection in 37.3%, exudates due to malignancy in 25.3%, and exudates due to other etiology in 3.6%.
Test Characteristics
Light’s criteria had a sensitivity of 92% and specificity of 55% with an associated LR+ 2.07 and LR− 0.14 (Table 1). Pleural fluid/serum protein ratio >0.5 had a sensitivity of 58% and specificity of 89% with an associated LR+ 5.22 and LR− 0.47 (Table 1). Pleural fluid protein values >2.9 g/dl had a sensitivity of 42% and a specificity of 96% with a corresponding LR+ 11.12 and LR− 0.61 (Table 1). Analyses stratified by both duration (Table E2) and severity (Table E3) of neutropenia show that both factors appear to further negatively impact Light’s criteria’s specificity and positively impact the specificity of the other aforementioned tests.
Table 1.
Neutropenic pleural fluid test characteristics
| Parameter | Sample Size, n | Sensitivity, % | Specificity, % | LR+ | LR− |
|---|---|---|---|---|---|
| Light’s criteria | 78 | 92 | 55 | 2.07 | 0.14 |
| Pleural fluid protein/serum ratio >0.5 | 77 | 58 | 89 | 5.22 | 0.47 |
| Pleural fluid LDH/serum LDH ratio >0.6 | 67 | 68 | 59 | 1.67 | 0.54 |
| Pleural fluid LDH >⅔ ULN | 76 | 60 | 73 | 2.23 | 0.55 |
| Two-test rule* | 77 | 84 | 38 | 1.37 | 0.41 |
| Three-test rule† | 77 | 90 | 40 | 1.51 | 0.24 |
| Pleural fluid protein >2.9 | 78 | 42 | 96 | 11.12 | 0.61 |
| Pleural fluid/serum protein gradient >3.1 g/dl | 77 | 52 | 82 | 2.82 | 0.59 |
| Pleural fluid LDH >0.45 ULN | 76 | 83 | 42 | 1.42 | 0.42 |
Definition of abbreviations: LDH = lactate dehydrogenase; ULN = upper limit of normal.
Two-test rule identifies an exudate by meeting at least one of the following criteria: either pleural fluid cholesterol >45 mg/dl or pleural fluid LDH >0.45 × ULN for serum LDH.
Three-test rule identifies an exudate by meeting at least one of the following criteria: either pleural fluid protein >2.9 g/dl, pleural fluid cholesterol >45 mg/dl, or pleural fluid LDH >0.45 × ULN for serum LDH.
Exudative Effusions
Pleural fluid studies from the exudates due to infection versus exudates due to malignancy were compared (Table E4). The percentage of neutrophils within the pleural fluid was significantly higher in the exudates due to infection as compared with exudates due to malignancy (Figure 1). None of the other variables of interest had a statistically significant difference between groups (Figure 1). Finally, of the samples sent for culture, 20% (6/30) were positive for microorganisms, comparable to prior studies (Table E5) (10, 11). Of the samples sent for cytology, 36% (4/11) were positive for malignancy (Table E5).
Figure 1.
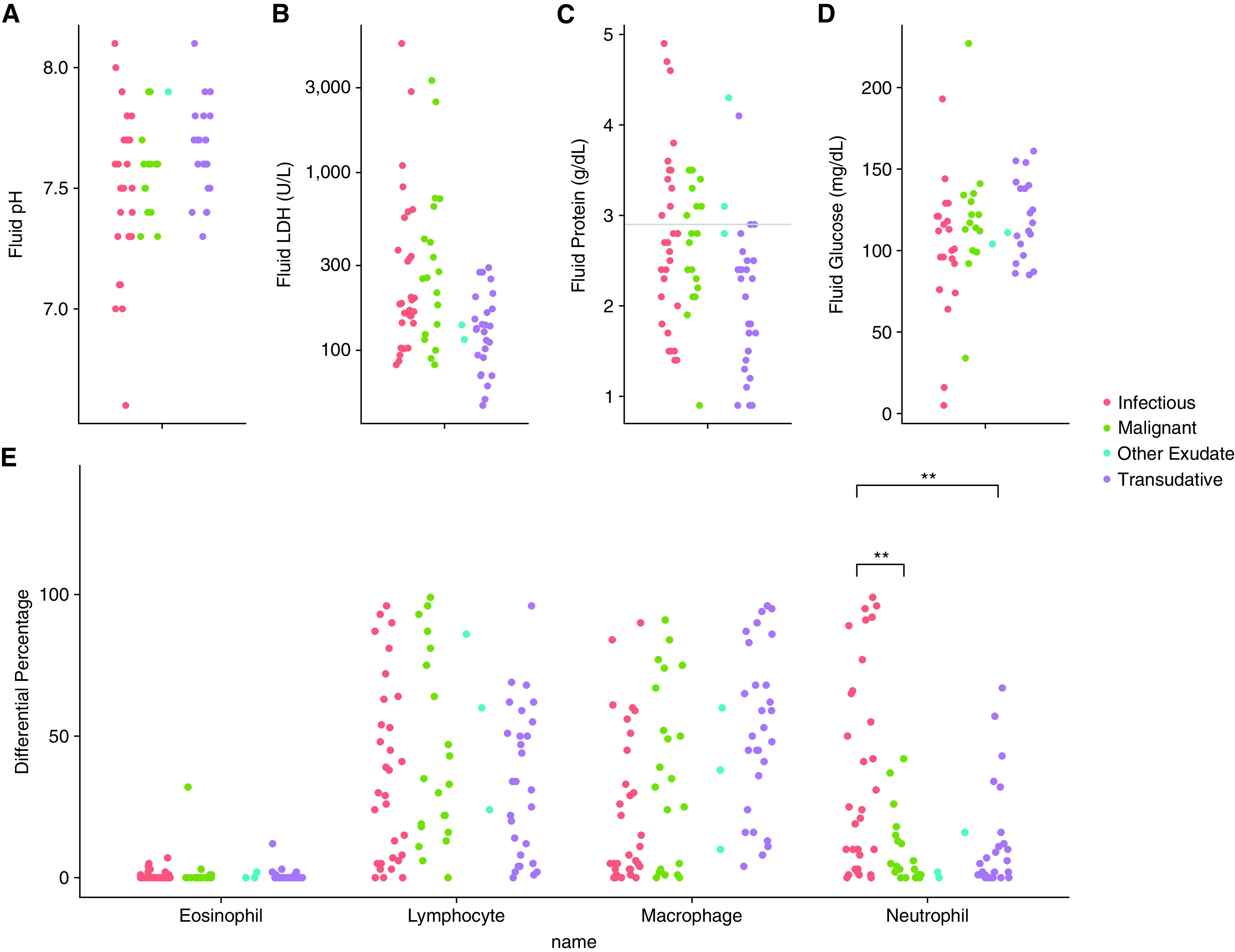
Neutropenic pleural fluid laboratory results. (A–D) Comparison of fluid pH, LDH, protein, and glucose, respectively, between all four case categories. Horizontal line in (C) represents pleural fluid protein 2.9 as referenced in Table 1. (E) Comparison of fluid cell profile between all four case categories. **P value determined by Bonferroni correction with familywise error rate of 0.05. LDH = lactate dehydrogenase.
Discussion
This study is the first to analyze the test characteristics of Light’s criteria in a cohort of neutropenic patients using the clinical diagnosis as the reference standard. Light’s criteria demonstrated similar sensitivity but worse specificity compared with prior studies (2, 12), particularly with more profound and longer duration neutropenia. Specificity could suffer in particular due to nonspecific systemic LDH elevations in inflammatory conditions wherein neutropenia is common (12). Pleural fluid protein >2.9 g/dl and pleural fluid/serum protein >0.5 had robust LR+ in this population, which is similar to other studies (7). Within exudates, pleural fluid neutrophil percentage distinguished those due to infection versus malignancy despite the presence of peripheral neutropenia. In clinical practice, clinicians should feel confident diagnosing a transudate in a patient with neutropenia when none of Light’s criteria are met; however, when Light’s criteria are met, we suggest additionally using pleural fluid protein >2.9 g/dl or a pleural fluid/serum protein ratio >0.5 as more specific indicators of an exudative effusion.
Strengths of this study include independent case adjudication and clinical diagnosis as case definition. Limitations include cohort size, variable etiologies and duration of neutropenia, and limited pleural cholesterol values. Future studies could utilize a similar approach but focus on a particular etiology of neutropenia.
Footnotes
Supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR003015 and KL2TR003016 (J.M.S.).
Author Contributions: J.M.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. J.M.S., I.M.B., S.F.O., and A.J.B. made substantial contributions to the study design, data acquisition, data analysis and interpretation, figure design and creation, and manuscript writing. J.M.S., I.M.B., S.F.O., and A.J.B. have all given final approval for publication of this version of the manuscript and are accountable for all aspects of the work.
This letter has an online data supplement, which is accessible from this issue’s table of content online at www.atsjournals.org.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Light RW, Macgregor MI, Luchsinger PC, Ball WC., Jr Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med . 1972;77:507–513. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 2. Porcel JM. Identifying transudates misclassified by Light’s criteria. Curr Opin Pulm Med . 2013;19:362–367. doi: 10.1097/MCP.0b013e32836022dc. [DOI] [PubMed] [Google Scholar]
- 3. Modi D, Jang H, Kim S, Deol A, Ayash L, Bhutani D, et al. Incidence, etiology, and outcome of pleural effusions in allogeneic hematopoietic stem cell transplantation. Am J Hematol . 2016;91:E341–E347. doi: 10.1002/ajh.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adam AK, Zamlut M, Soubani AO. The yield and safety of thoracentesis in hematopoietic stem cell transplantation recipients. Lung . 2007;185:257–262. doi: 10.1007/s00408-007-9025-y. [DOI] [PubMed] [Google Scholar]
- 5. Faiz SA, Bashoura L, Lei X, Sampat KR, Brown TC, Eapen GA, et al. Pleural effusions in patients with acute leukemia and myelodysplastic syndrome. Leuk Lymphoma . 2013;54:329–335. doi: 10.3109/10428194.2012.713478. [DOI] [PubMed] [Google Scholar]
- 6. Bass J, White DA. Thoracentesis in patients with hematologic malignancy: yield and safety. Chest . 2005;127:2101–2105. doi: 10.1378/chest.127.6.2101. [DOI] [PubMed] [Google Scholar]
- 7. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform . 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. REDCap Consortium The REDCap consortium: building an international community of software platform partners. J Biomed Inform . 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilcox ME, Chong CA, Stanbrook MB, Tricco AC, Wong C, Straus SE. Does this patient have an exudative pleural effusion? The Rational Clinical Examination systematic review. JAMA . 2014;311:2422–2431. doi: 10.1001/jama.2014.5552. [DOI] [PubMed] [Google Scholar]
- 10. Ferrer A, Osset J, Alegre J, Suriñach JM, Crespo E, Fernández de Sevilla T, et al. Prospective clinical and microbiological study of pleural effusions. Eur J Clin Microbiol Infect Dis . 1999;18:237–241. doi: 10.1007/s100960050270. [DOI] [PubMed] [Google Scholar]
- 11. Porcel JM. Pleural fluid tests to identify complicated parapneumonic effusions. Curr Opin Pulm Med . 2010;16:357–361. doi: 10.1097/MCP.0b013e328338a108. [DOI] [PubMed] [Google Scholar]
- 12. Chubb SP, Williams RA. Biochemical analysis of pleural fluid and ascites. Clin Biochem Rev . 2018;39:39–50. [PMC free article] [PubMed] [Google Scholar]


