Abstract
Rationale
The etiology of cystic fibrosis (CF) pulmonary exacerbations (PEx) is likely multifactorial with viral, bacterial, and non-infectious pathways contributing.
Objectives
To determine whether viral infection status and CRP (C-reactive protein) can classify subphenotypes of PEx that differ in outcomes and biomarker profiles.
Methods
Patients were recruited at time of admission for a PEx. Nasal swabs and sputum samples were collected and processed using the respiratory panel of the FilmArray multiplex polymerase chain reaction (PCR). Serum and plasma biomarkers were measured. PEx were classified using serum CRP and viral PCR: “pauci-inflammatory” if CRP < 5 mg/L, “non-viral with systemic inflammation” if CRP ⩾ 5 mg/L and no viral infection detected by PCR and “viral with systemic inflammation” if CRP ⩾ 5 mg/L and viral infection detected by PCR.
Results
Discovery cohort (n = 59) subphenotype frequencies were 1) pauci-inflammatory (37%); 2) non-viral with systemic inflammation (41%); and 3) viral with systemic inflammation (22%). Immunoglobulin G, immunoglobulin M, interleukin-10, interleukin-13, serum calprotectin, and CRP levels differed across phenotypes. Reduction from baseline in forced expiratory volume in 1 second as percent predicted (FEV1pp) at onset of exacerbation differed between non-viral with systemic inflammation and viral with systemic inflammation (−6.73 ± 1.78 vs. −13.5 ± 2.32%; P = 0.025). Non-viral with systemic inflammation PEx had a trend toward longer duration of intravenous antibiotics versus pauci-inflammation (18.1 ± 1.17 vs. 14.8 ± 1.19 days, P = 0.057). There were no differences in percent with lung function recovery to <10% of baseline FEV1pp. Similar results were seen in local and external validation cohorts comparing a pauci-inflammatory to viral/non-viral inflammatory exacerbation phenotypes.
Conclusions
Subphenotypes of CF PEx exist with differences in biomarker profile, clinical presentation, and outcomes.
Keywords: cystic fibrosis, viral infection, exacerbation, phenotype, pauci-inflammatory
Cystic fibrosis (CF) pulmonary exacerbations (PEx) are a common cause of morbidity in CF and are associated with impaired quality of life and increased mortality (1, 2). The etiology of CF PEx is complex and multifactorial. Viral, bacterial, allergic, and inflammatory pathways have been implicated in the pathophysiology of these events (3–6). CF PEx have a significant negative impact on five-year survival, lung function, quality of life, and healthcare cost (1, 2, 7, 8). Despite this, there is still a lack of consensus on the diagnosis and management of PEx.
It is likely that CF PEx exhibit different subphenotypes and that all exacerbations are not the same. Exacerbation subtypes have been identified in COPD and asthma using serum and sputum biomarkers (9, 10). In CF, it is likely that bacterial and viral infection are critical to the development of exacerbations but it is possible that non-infectious factors (poor adherence, allergy, reduced exercise and/or airway clearance) are also important (11). Characterizing subphenotypes of CF PEx is important as different therapeutic approaches may be tailored to specific exacerbation subtypes.
The interplay between bacterial and viral infection in CF PEx is complex. Respiratory viruses are detected in 30–72% of PEx in children and 10–68% of PEx in adults (3–6). Viral infections have been associated with increased risk of PEx and increased Pseudomonas aeruginosa density, acquisition, and adherence (3, 12–15), yet viral infection is commonly seen in patients with CF who are clinically well with no features of exacerbation (4, 16). Infection with P. aeruginosa is also associated with an increased frequency of PEx and lower lung function; however, the role of bacterial infection in the development of CF PEx is not fully understood. P. aeruginosa bacterial burden, as measured by sputum density of organisms, does not increase at time of exacerbation (17, 18) though it does fall with intravenous antibiotics (i.v.) (19, 20). In addition, microbiome studies have generally not shown that new strains of bacteria appear, or that there are consistent and major changes in the sputum microbiota composition during exacerbations (21–23). A recent case study raises the possibility that increases in the abundance of hyper-virulent mutants in P. aeruginosa could cause an acute and sustained lung function decline (24). Despite these different suspected mechanisms of CF exacerbations, i.v., airway clearance, and optimization of nutrition are all recommended treatments, regardless of etiology (11, 25).
In this observational cohort study, we hypothesize that subphenotypes of PEx exist, and these can be identified through assessment of viral infection status and the widely used marker of systemic inflammation, CRP (C-reactive protein). CRP has been shown to differ between stable and exacerbations states and is higher in those with lower lung function (26–30). Using these easily measured markers, we chose to categorize exacerbation subphenotypes into 1) pauci-inflammatory; 2) non-viral with systemic inflammation; and 3) viral with systemic inflammation, to determine if these were associated with differences in clinical presentation and outcomes.
Methods
Irish Discovery Cohort
This was a prospective observational cohort study based at the National Referral Center for Adult Cystic Fibrosis at St. Vincent’s University Hospital, Dublin, between 2016 and 2017. Patients were recruited within 24 hours of admission for i.v. antibiotics with a physician-diagnosed CF pulmonary exacerbation. Patients were recruited from the outpatient clinics, inpatient and day wards, and all patients admitted with an exacerbation were eligible for inclusion in this study. Each patient contributed a single exacerbation. Exacerbation onset was defined as starting at the time of hospital admission. Patients were excluded if they required oral or i.v. antibiotics in the past 14 days. Long-term azithromycin was not an exclusion criterion. Patients in whom a CRP or FEV1 could not be collected within 24 hours were also excluded; for example, patients admitted over weekends (>24 h before consent and sampling) or with hemoptysis/chest pain (no spirometry). Exacerbations were defined by the admitting physician and the decision to stop treatment was also decided by the treating physician who were blinded to the viral swab/sputum results and biomarkers results but not the CRP, white cell count, or spirometry results. Ethical approval was obtained from St. Vincent’s University Hospital research and ethics committee.
Clinical data, and biological (sputum/nasal swabs and blood) samples were collected at the onset of exacerbation. Retrospective information regarding exacerbation frequency and previous measures of lung function (forced expiratory volume in one second as percent predicted [FEV1pp]) was extracted from clinical records. Baseline FEV1pp was defined as the highest FEV1 value in percent predicted recorded in the preceding six months consistent with previous studies of exacerbation (31–33). Absolute drop in FEV1 was defined as baseline FEV1pp minus admission FEV1pp. Patients with frequent exacerbations were defined as those with two or more exacerbations requiring i.v. antibiotics in the preceding 12 months.
Mild cystic fibrosis transmembrane conductance regulator (CFTR) genotype was defined at least one allele in class IV or V. Severe CFTR genotype was defined as both alleles in class I-III. Cystic fibrosis related diabetes (CFRD) was defined as per the last documented oral glucose tolerance test. In those with known diabetes, use of insulin was used as an alternative indicator of diabetes (where glucose tolerance test may not have been performed recently). The diagnostic cutoff for 2-hour oral glucose tolerance test was a glucose level greater than 11.1 mmol/L or a fasting blood glucose greater than 7 mmol/L. Cystic fibrosis related liver disease was defined by the presence of cirrhosis and/or splenomegaly on most recent abdominal ultrasound.
Viral Analysis
Nasal swabs were obtained using nasal FLOQ SwabsTM. Swabs were taken in accordance manufacturer’s instructions and placed in Universal Transport Medium (UTMTM-RT) and stored at −80°C until processing. 200 μl aliquot of a larger spontaneously expectorated sputum sample was stored at −80°C. Sputum samples were subsequently thawed and homogenized in 200 μl of Copan SL solution at room temperature and were vortexed intermittently over 15 minutes. Thawed homogenized sputum samples and nasal swabs were transported at 4°C to the National Virus Reference Laboratory for same day batch processing using the respiratory panel of the FilmArray multiplex polymerase chain reaction (PCR) system for Adenovirus, Coronavirus HKU1, Coronavirus NL63, Coronavirus 229E, Coronavirus OC43, Human Metapneumovirus, Human Rhinovirus/Enterovirus, Influenza A, Influenza A/H1, Influenza A/H1 2009, Influenza A/H3, Influenza B, Parainfluenza 1–4, RSV.
Bacterial Analysis
Spontaneously expectorated sputum samples were collected within 24 hours of exacerbation onset and maintained at −4°C for less than 24 hours before culture. Sputum was liquified and homogenized by diluting it 1:5 in cold 0.1% solution of dithiothreitol (DTT) followed by vortexing. Samples were then plated in duplicate on MacConkey Agar, incubated at 37°C for 24–72 hours, and colony-forming units (cfus) enumerated.
Biomarker Analysis
Blood results were obtained from routine hospital sampling on the day of testing: total white blood cell count, neutrophil count, lymphocyte count, eosinophil count, immunoglobulin A (IgA), immunoglobulin E (IgE), immunoglobulin G (IgG), immunoglobulin M (IgM), and CRP. Plasma and serum were extracted from blood samples and processed for biomarkers (see online supplement for biomarker methodology).
Statistical Methods
A priori, we divided exacerbations into three phenotypes. This classification was based on the observation that some exacerbations were associated with different clinical presentations including those with a viral-illness type presentation, those with a pauci-inflammatory blood response at time of exacerbation onset, and the remainder which were non-viral and assumed to be related to increased activity of their chronic bacterial infection.
Exacerbation phenotype was determined by 1) viral swab status and 2) plasma CRP above or below the upper limit of normal for our laboratory (5 mg/l).
-
i)
Viral with Systemic Inflammation (V): Viral infection with evidence of systemic inflammation: Positive viral PCR and elevated CRP ⩾ ULN 5 mg/l.
-
ii)
Non-viral with Systemic Inflammation (NV): No evidence of viral infection with systemic inflammation: Negative viral PCR and elevated CRP ⩾ ULN 5 mg/l.
-
iii)
Pauci-Inflammatory (P): Normal CRP < ULN 5 mg/l regardless of viral PCR status.
The chi-square test was used to determine significant differences in categorical variables between the three predefined phenotypes. Analysis of variance was used to test for trends between each variable across the three exacerbation phenotypes. Heteroscedasticity of residuals was assessed using hettest in Stata and Cooks distance was used to identify any influential outliers. Patients with missing data were excluded from each analysis. Statistical analyses were performed using Stata 11.0.
Validation Cohort Analysis
To validate the discovery cohort results, we also studied exacerbation phenotypes in two independent validation cohorts. As viral swabs were not routinely collected at onset of exacerbation, viral infection status was not available for the validation cohorts. Non-viral with systemic inflammation and viral with systemic inflammation phenotypes were combined into a non-viral/viral with inflammation (NV/V) phenotype based solely on an elevated serum CRP and this was compared with the pauci-inflammatory exacerbation phenotype with a normal CRP.
Local Irish validation cohort
The local internal validation cohort was selected using a retrospective cohort study of patients admitted with CF PEx to St. Vincent’s University Hospital between October 2012 and July 2014 and a second cohort admitted with CF PEx between March 2020 and July 2020. These cohorts are part of a separate study of CF pulmonary exacerbations. Exacerbation data were extracted from the patients’ charts. Variables collected included patient demographics, measure of lung function (FEV1pp) at baseline (pre-exacerbation), onset and end of exacerbation, duration of i.v. treatment, and laboratory test results. Absolute and relative change in lung function (FEV1pp) at onset of exacerbation, as well as duration of i.v. antibiotic treatment for exacerbation, were outcomes of interest. In addition, for this cohort, PEx response was dichotomized into a composite outcome measure of “responders” versus “slow or incomplete-responders.” Responders were defined by two criteria: 1) recovery of post-exacerbation FEV1 to a relative change of <10% of pre-exacerbation baseline and 2) i.v. antibiotic duration of 14 days or less. “Slow or incomplete-responders” either needed i.v. antibiotics for greater that 14 days and/or failed to recover to within 10% of baseline pre-exacerbation FEV1pp irrespective of i.v. antibiotic duration.
Statistical analysis for local Irish validation cohort
Mixed-effects linear regression for repeated measures was used to compare exacerbation outcomes and lung function changes, adjusting for baseline FEV1pp using xtmixed, with post-regression estimates calculated using adjust function in Stata. For the responder analysis, odds ratios of response to treatment were determined using logistic regression.
External validation cohort
As these results could reflect local exacerbation practice, an external validation cohort was selected from the Adult CF Unit, St. Paul’s Hospital (Vancouver, Canada). Data from physician-diagnosed exacerbations admitted to St. Paul’s Hospital for i.v. antibiotics between April 2013 and March 2015 (34) were included if: 1) CRP levels were measured within 24 hours of admission and 2) FEV1pp was measured at onset of exacerbation and within 48 hours of i.v. antibiotic completion. Transplant recipients and subjects receiving chronic immunosuppressive therapy were excluded. Analysis was completed using R Studio v1.1.463 running R v4.0.4 (the R Foundation for Statistical Computing) using t test, linear regression with robust estimates, and chi-square.
Results
The Irish Discovery Cohort patient demographics are shown in Table 1. The meanage of the sample population was 31.3 ± 10.1 years. Mean baseline FEV1pp was 58.7 ± 26.9%. Sputum microbiology was predominantly positive for P. aeruginosa (68%) with Methicillin-sensitive Staphylococcus aureus (MSSA) second most common at 33%. No patients had Burkholderia cepacia complex. Eight patients had neither Pseudomonas nor Staph aureus identified on sputum culture at onset of exacerbation. Eleven patients were on CFTR modulator therapy at time of recruitment: six patients were on lumacaftor/ivacaftor, and five patients were on ivacaftor alone.
Table 1.
Exacerbation discovery cohort: demographics (n = 59)
| Male, n (%) | 33 (56%) |
| Age (yr) | 31.3 (10.2) |
| Severe CFTR genotype (class I-III) | 46 (78%) |
| Baseline FEV1 (% predicted) | 59.1 (27.0) |
| Baseline FEV1 (L) | 2.1 (1.0) |
| Weight (kg) | 63.1 (12.7) |
| BMI (kg/m2) | 22.3 (3.7) |
| CF related diabetes | 19 (32%) |
| CF liver disease | 9 (15%) |
| CFTR modulator use | 11 (18.6%) |
| Frequent exacerbation (⩾2) in preceding year | 30 (51%) |
| Chronic Pseudomonas aeruginosa | 40 (68%) |
| Chronic MSSA | 20 (33%) |
| Viral infection detected (any method) | 18 (32%) |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; CFTR = cystic fibrosis transmembrane conductance regulator; FEV1 = forced expiratory volume in 1 second; MSSA = methicillin-sensitive Staphylococcus aureus.
Mean (standard deviation) unless otherwise stated. Lung function and BMI are at baseline.
At the onset of PEx, 58% of subjects had a significant fall (⩾10% relative drop from baseline FEV1pp) in lung function. Respiratory viruses were detected by PCR in nasal swab, sputum, or both in 33% of exacerbations.
Cytokine levels measured fell within human physiologic range for all biomarkers (see Table E2 in the online supplement). Mean levels of the antiinflammatory cytokine interleukin-10 (IL-10) (mean 15.2 ± 10.9 pg/mL) were higher than reported in previous exacerbation studies. Mean CRP levels at onset of exacerbation were 27.8 ± 42.5 mg/l. Only two patients had an elevated eosinophil count greater than 0.5 × 109/l, the upper limit of normal for our laboratory. No patient had a high eosinophil count with a high IgE.
Exacerbation Phenotypes
The most common phenotype category was “non-viral with systemic inflammation” followed by “pauci-inflammatory” and “viral with systemic inflammation” (41% vs. 37% vs. 22%, respectively). Demographic variables including baseline lung function, nutritional, microbiological status, and exacerbation history did not differ significantly between these three exacerbation groups at onset of exacerbation (Table 2). Thirteen subjects (22%) classified as having a pauci-inflammatory exacerbation tested positive for a viral infection.
Table 2.
Discovery cohort: demographics by exacerbation phenotype (n = 59)
| Variable | Pauci-Inflammatory (n = 22) | Non-viral with Inflammation (n = 24) | Viral with Inflammation (n = 13) |
|---|---|---|---|
| Age (yr) | 29.2 (9.8) | 31.3 (8.9) | 34.8 (12.6) |
| Male, n (%) | 11 (50%) | 15 (63%) | 7 (54%) |
| Severe CFTR genotype | 16 (73%) | 20 (83%) | 11 (79%) |
| Baseline FEV1 (% predicted) | 64.7 (26.9) | 52.9 (28.6) | 61.0 (23.3) |
| Baseline FEV1 (L) | 2.3 (1.0) | 2.0 (1.1) | 2.1 (0.8) |
| Frequent exacerbations, n (%) | 9 (41%) | 15 (62%) | 6 (46%) |
| Weight (kg) | 63.8 (14.3) | 61.0 (11.2) | 65.5 (12.6) |
| BMI (kg/m2) | 22.9 (4.5) | 21.2 (2.8) | 23.2 (3.5) |
| CFRD, n (%) | 7 (32%) | 6 (25%) | 6 (50%) |
| CFLD, n (%) | 3 (14%) | 4 (17%) | 2 (15%) |
| Chronic PA, n (%) | 14 (64%) | 18 (75%) | 8 (62%) |
| PA (cfu/mL) | 1.06 × 108 (3.3 × 108) | 3.6 × 107 (6.1 × 107) | 2.7 × 107 (5.3 × 107) |
| Chronic MSSA, n (%) | 9 (40%) | 7 (29%) | 4 (29%) |
Definition of abbreviations: BMI = body mass index; CFLD = cystic fibrosis-related liver disease; CFRD = cystic fibrosis-related diabetes; CFTR = cystic fibrosis transmembrane conductance regulator; cfu: colony-forming unit; FEV1 = forced expiratory volume in 1 second; MSSA = methicillin-sensitive Staphylococcus aureus; PA = Pseudomonas aeruginosa.
Mean (standard deviation) unless otherwise stated.
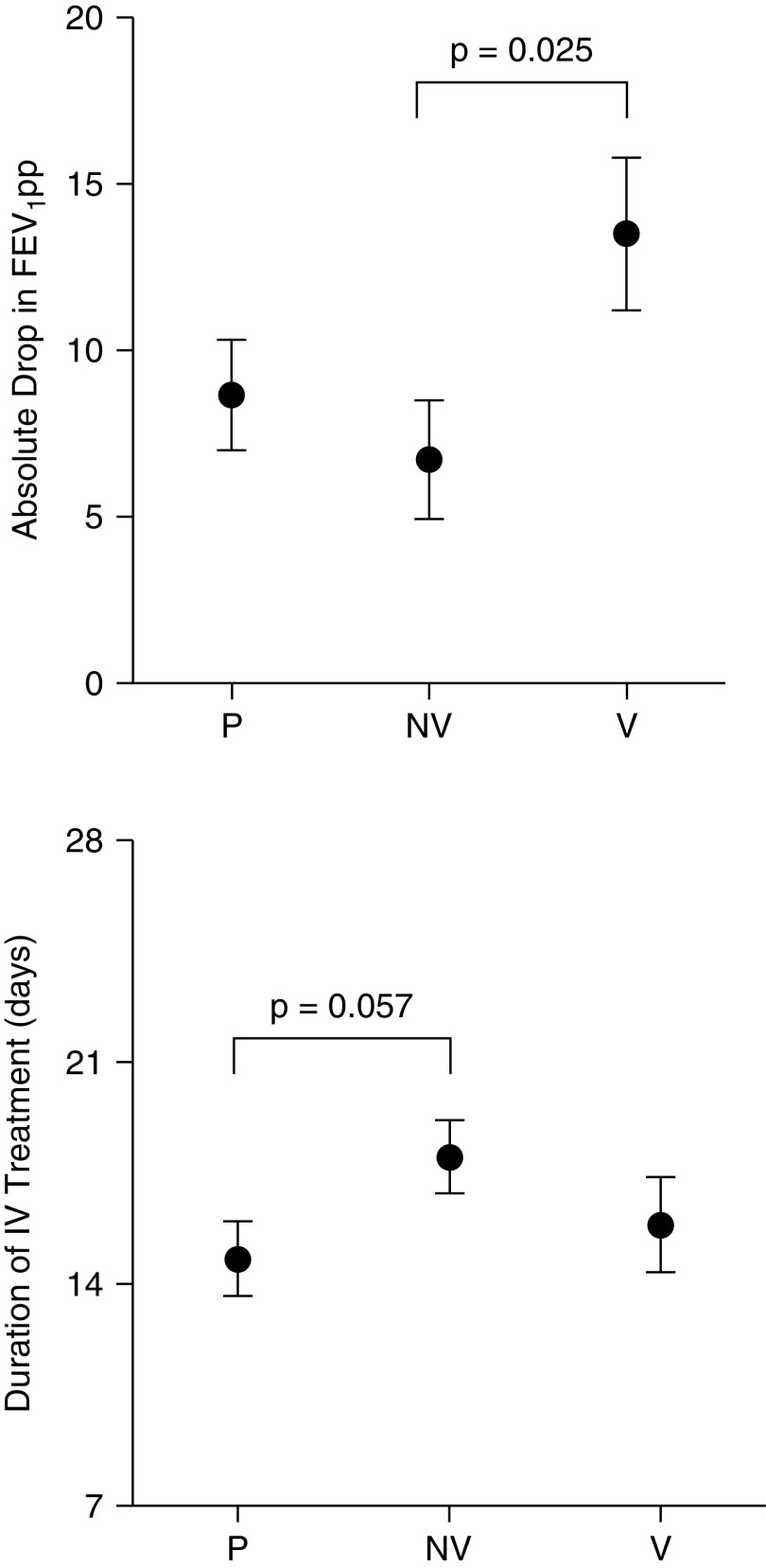
Absolute drop from baseline in lung function (FEV1pp) at onset of exacerbation was similar for pauci-inflammatory and non-viral with systemic inflammation, however viral with systemic inflammation exacerbation phenotype had a greater drop in FEV1pp than non-viral with inflammation (−13.5 ± 2.32% vs. −6.72 ± 1.77; P < 0.025) (Figure 1). Non-viral with systemic inflammation exacerbations had a trend toward a longer duration of intravenous antibiotics when compared with pauci-inflammatory exacerbations (18.1 ± 1.17 vs. 14.8 ± 1.20 days, P = 0.057) (Figure 1). There was no significant difference between the exacerbation categories in the proportion of individuals experiencing recovery of FEV1pp to within 10% of baseline: (pauci-inflammatory (72%); non-viral with systemic inflammation (69%); and viral with systemic inflammation (77%), P = 0.75).
Figure 1.
Differences in lung function change (mean ± SEM) at onset of exacerbation and duration of intravenous treatment by exacerbation phenotype: local discovery cohort. FEV1pp = forced expiratory volume in 1 second as percent predicted; IV = intravenous; P = pauci-inflammatory; NV = non-viral with inflammation; V = viral with inflammation.
Of the blood biomarkers, there were significant differences between exacerbation phenotypes in IgG, IgM, IL-10, IL-13, and serum calprotectin as well as an expected difference in CRP, confirming that CRP was a useful marker of systemic inflammation in CF exacerbations. There was a stepwise increase in CRP across pauci-inflammatory, non-viral with systemic inflammation and viral with systemic inflammation phenotypes (2.4 ± 3.7 vs. 26.2 ± 3.72 vs. 45.5 ± 4.9 mg/l, P < 0.001). This is shown in Figure 2 and in Table E1. Reproducibility and missingness of blood biomarkers are shown in Table E2. Serum calprotectin was only available on 37 subjects.
Figure 2.
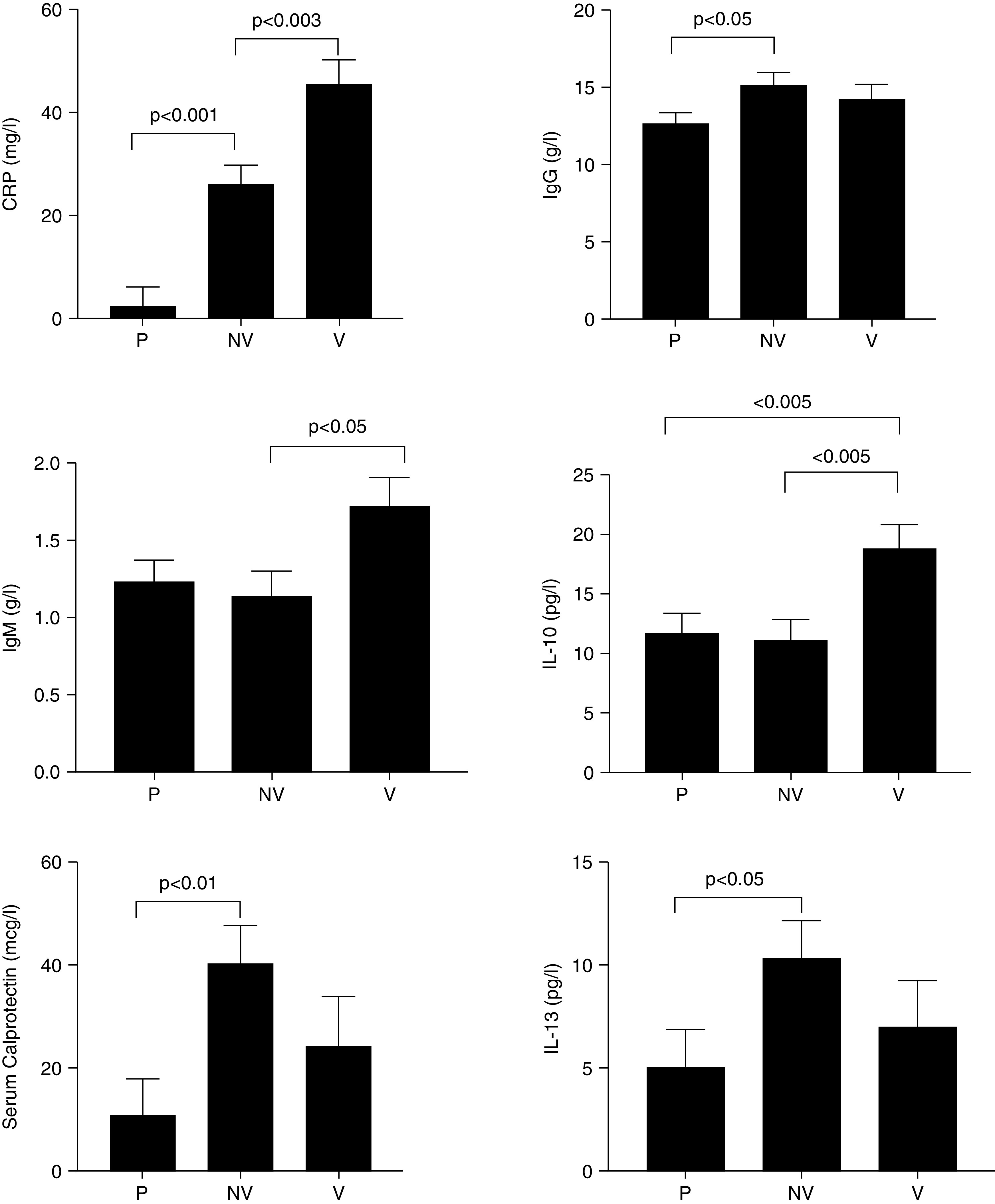
Differences in biomarkers (mean ± SEM) by exacerbation phenotypes: local discovery cohort. CRP = C-reactive protein; P = pauci-inflammatory; NV = non-viral with inflammation; V = viral with inflammation.
Internal Validation of the Pauci-inflammatory Exacerbation Phenotype
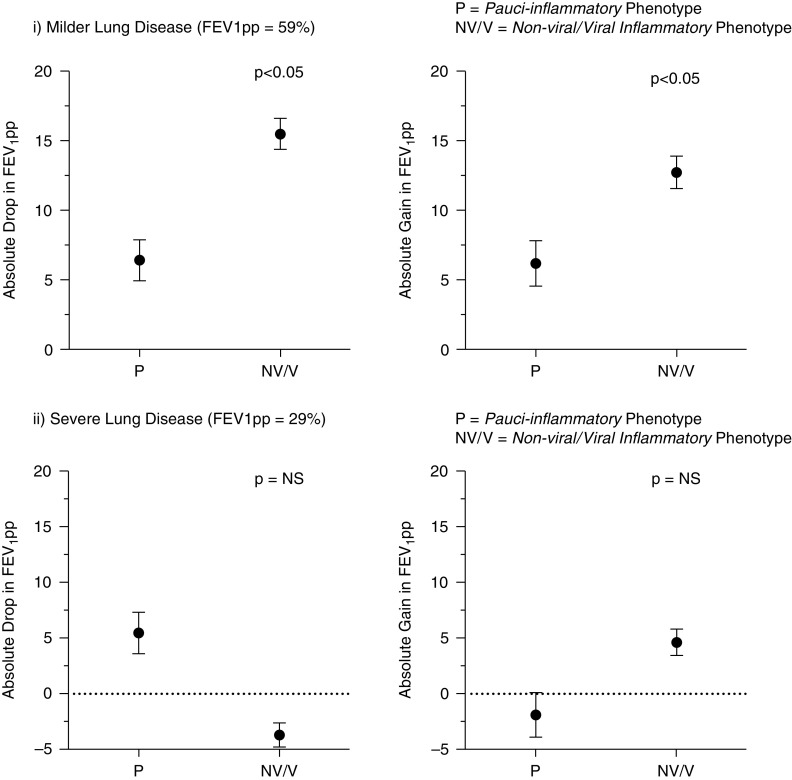
In the local Irish validation cohort, there were 197 exacerbation events in 80 patients with CF. Nine exacerbations had no recorded CRP at onset and were excluded. Demographics for the remaining 188 exacerbations are shown in Table 3. Forty-nine patients had more than one exacerbation, of which fifteen patients had an exacerbation that was classified as pauci-inflammatory (P) and non-viral/viral with systemic inflammation (N/NV) at different time points. Baseline FEV1pp values were lower in the non-viral/viral with systemic inflammation phenotype than in the pauci-inflammatory exacerbation phenotype (mean 41.2 ± 2.2 vs. 63.3 ± 4.9; P < 0.001). After repeated measures analysis and adjusting for differences in baseline FEV1pp, the pauci-inflammatory exacerbation phenotype had a lower but non-significant difference in length of i.v. antibiotics than the non-viral/viral with systemic inflammation exacerbation (17.2 ± 2.8 vs. 22.2 ± 1.4 days, P = 0.242). Lung function changes at onset and after exacerbation treatment differed between exacerbation phenotypes, and this difference was dependent on baseline lung function. Figure 3 shows the changes in FEV1pp for different exacerbation phenotypes at onset and after treatment in patients in the highest and lowest quartile for baseline FEV1pp (median baseline FEV1pp = 43%; interquartile range 29–59) for the group. Non-viral/viral with systemic inflammation exacerbation phenotype demonstrated a significantly greater drop and better recovery of FEV1pp than the pauci-inflammatory exacerbation phenotype group, and these differences were only seen in patients with higher baseline lung function.
Table 3.
Demographics of internal Irish validation cohort
| All (n = 80) | Pauci-Inflammatory (n = 15) | Inflammatory (Viral/Non-viral) (n = 65) | |
|---|---|---|---|
| Exacerbations (n) | 188 | 28 | 160 |
| Male, n (%) | 41 (51%) | 8 (50%) | 33 (52%) |
| Age (yr) | 31.0 (9.1) | 29.5 (9.4) | 31.7 (9.4) |
| FEV1 (% predicted) | 45.5 (19.6) | 63.3 (19.1) | 41.2 (17.3) |
| FEV1 (L) | 1.89 (0.93) | 2.64 (0.87) | 1.71 (0.85) |
| BMI (kg/m2) | 20.9 (3.21) | 21.2 (2.3) | 20.8 (3.5) |
| CFRD, n (%) | 30 (37.5) | 5 (31%) | 25 (39%) |
| Chronic PA | 48 (60%) | 11 (69%) | 37 (58%) |
Definition of abbreviations: BMI = body mass index; CFRD = cystic fibrosis-related diabetes; FEV1 = forced expiratory volume in 1 second; PA = Pseudomonas aeruginosa.
Mean (standard deviation) unless otherwise stated.
Figure 3.
Differences in lung function change (mean ± SEM) at onset of exacerbation and duration of i.v. treatment (pauci-inflammatory vs. other [non-viral/viral] in validation cohort) stratified by severity of underlying lung function: local validation cohort. FEV1pp = forced expiratory volume in 1 second as percent predicted; NS = not significant.
Univariate logistic regression responder analysis identified almost a 3-fold increased odds of lung function recovery to within 10% (relative) of baseline FEV1pp within 14 days in the pauci-inflammatory exacerbation phenotype group compared with the non-viral/viral with systemic inflammation exacerbation phenotype (odds ratio of recovery [ORR]: 2.74 [95% confidence interval (CI), 1.11–6.77]; P = 0.029).
External Validation of the Pauci-inflammatory Exacerbation Phenotype
There were 83 exacerbation events in 43 patients with CF in the external validation cohort. Demographics for this external validation cohort are shown in Table 4. Similar to the Irish Validation cohort, there were significant differences in baseline FEV1pp with mean values for non-viral/viral with systemic inflammation exacerbation lower than pauci-inflammatory phenotype group. The pauci-inflammatory exacerbation phenotype had a shorter duration of i.v. antibiotics compared with the non-viral/viral with systemic inflammation phenotype (mean difference 13.9 ± 1.9 vs. 16.3 ± 4.1 days; P = 0.012). After adjusting for baseline lung function, improvement within 14 days of lung function to within 10% (relative) of baseline was more common in the pauci-inflammatory phenotype than non-viral/viral with systemic inflammation phenotype (ORR: 3.9 [CI, 0.95–15.8]; P = 0.058).
Table 4.
Demographics of external Canadian validation cohort
| Pauci-Inflammatory (n = 21) | Inflammatory (n = 62) | |
|---|---|---|
| Male, n (%) | 8 (38) | 29 (47) |
| Age (yr) | 40.4 (14.6) | 33.7 (12.6) |
| FEV1 (% predicted) | 79.1 (20.8) | 48.4 (19.1) |
| BMI, (kg/m2) | 21.8 (2.1) | 21.7 (3.6) |
| CF-related diabetes, n (%) | 9 (43) | 24 (39) |
| Chronic Pseudomonas aeruginosa, n (%) | 12 (57) | 44 (71) |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; FEV1 = forced expiratory volume in 1 second.
Mean (standard deviation) unless otherwise stated.
Discussion
Using a predefined method of classification for CF pulmonary exacerbations, differences in clinical outcomes and inflammatory profiles were detected in three cohorts of patients with CF with acute pulmonary exacerbations. For the first time, we describe the pauci-inflammatory exacerbation subphenotype of CF exacerbation associated with reduced markers of systemic inflammation and lower loss of lung function at exacerbation onset, and more rapid response to treatment. Viral and non-viral inflammatory exacerbation subphenotypes also exist with differences in CRP and lung function drop at onset of exacerbation but similar responses to treatment.
Pulmonary exacerbation remains a major challenge when managing patients with CF (11, 25). Although there are several ways of defining an acute CF pulmonary exacerbation, once the threshold for exacerbation is met, the management is largely the same irrespective of etiology; focusing on systemic antibiotic use and increased exercise/airway clearance in the hospital or home setting.
CF pulmonary exacerbations may be due to a variety of causes with viral or changes in chronic bacterial infection being the most common (3, 5, 18, 35, 36). The use of antibiotics in this setting is important to prevent progression of the exacerbation and permanent loss of lung function. However, non-infectious causes of pulmonary exacerbation are also important. Allergy (e.g., asthma or allergic bronchopulmonary aspergillosis), progressive mucus retention, non-adherence with airway clearance/exercise can all lead to increased pulmonary symptoms and a drop in lung function suggestive of an exacerbation. In these settings, antibiotic treatment may not be required and, in an era of increased antibiotics stewardship, identifying situations where antibiotic usage may not be required is an important goal.
It can be difficult to distinguish non-infectious exacerbations from bacterial or viral infections. Using CRP, we have characterized a novel pauci-inflammatory exacerbation phenotype that may not always require antibiotics and that an initial approach focusing on increased exercise/airway clearance and/or treatment of asthma or allergy may be more appropriate, with antibiotics only introduced if there is early evidence of symptom progression or failure to improve lung function. Clinical trials looking at a different treatment approaches to pauci-inflammatory exacerbation phenotypes may be of value.
Limitations
There are a number of limitations to our study. To define a pauci-inflammatory phenotype we used the absence of viral infection and an elevation of CRP. Elevation in CRP may occur with exacerbation, but we acknowledge that CRP may not rise in some patients with acute infection (27, 34). Likewise, in some patients with more advanced CF lung disease, CRP may be elevated even when the patient is well and not exacerbating (27). The presence of reduced levels of additional markers of infection/inflammation, serum calprotectin, IgG, and IL-10, IL-13 in the pauci-inflammatory phenotype group support our use of CRP as a marker of low levels of systemic inflammation. Also, in our non-viral/viral inflammation cohorts, we used viral infection as a single criterion to distinguish between viral versus non-viral (presumed bacterial) inflammatory phenotype. Quantifying cfu/ml of P. aeruginosa at the onset of exacerbation did not assist in characterizing exacerbation subphenotypes, and the cytokine profile was largely similar between the viral versus non-viral infection with systemic inflammation cohorts, other than IL-10 which was elevated in the viral cohort and, as a cytokine predominantly associated with viral infection (37), is supportive of our classification method.
We defined exacerbation onset as the date treatment of the exacerbation started. It is likely that patients may have been symptomatic for many days before treatment started, that the timing of symptom onset may have affected the levels of CRP observed on Day 1. Also, in the discovery cohort, we excluded those who had received oral antibiotics. This was to minimize the impact of oral antibiotics on CRP at time of recruitment. Use of oral antibiotics was not an exclusion criterion in the validation cohorts. The observation that there were similar results in the validation cohorts suggest that this approach was valid but may explain some of the differences in results that were seen between discovery and validation cohorts.
Analysis of the discovery cohort was limited to those with available biomarker data. The biomarker with the lowest yield from the multiplex platform was IL-2. A final sample of 59 patients was included in the analysis after 3 were removed due to missing data for biomarkers (>50% of values missing). The reason for absent data for the multiplex panel results was low bead counts when reading the plates. Also, serum calprotectin levels were not processed in all cases due to the difficulty in processing and the need to ship samples overseas for analysis. A subset of randomly selected exacerbations was shipped to Edinburgh and the only prerequisite was availability of adequate quantity of 500 μl serum for analysis. Caution must be observed in interpretation of the findings for biomarkers with a high degree of missing values (Table E2) such as serum and sputum calprotectin. Serum and sputum calprotectin levels during exacerbations were comparable with the levels observed by Gray and colleagues in previous studies of CF exacerbations (38). Data on biomarkers IL-6, IL-8, IL-10, IgG, and CRP were available in ∼80% of patients supporting the validity of the finding that levels for these biomarkers differ across phenotypes. These biomarkers could also be used with CRP to help to differentiate viral and bacterial exacerbations from other causes of exacerbation. The lack of serial longitudinal sampling of the same individual is a limitation of this study as this would be a means of detecting the duration of viral carriage in well CF patients and study the impact of this on exacerbation phenotypes. In addition, some patients in the study were on CFTR modulator therapy, which at the time of recruitment, would have been ivacaftor ± tezacaftor or lumacaftor. With the advent of highly effective CFTR modulation with elexacaftor/tezacaftor/ivacaftor (ETI), further work will be needed to look at how ETI impacts the presentation and response to treatment of CF PEx and how exacerbation phenotypes are impacted by improved CFTR function.
Conclusions
Using a predefined method of classification for CF pulmonary exacerbations, differences in clinical outcomes were detected. Comparing biomarkers across exacerbation categories to define exacerbation subphenotypes may help to explain some of the variation in exacerbation outcomes. Exacerbation duration is also associated with category of exacerbation, with the longest duration of antibiotics seen in the non-viral with systemic inflammation subphenotype. Drop and recovery of lung function during exacerbations are also associated with exacerbation subphenotype with recovery within a shorter time frame more common in the pauci-inflammatory exacerbation group.
The results of this study would suggest that subcategorization of exacerbations could be useful for future studies looking at management strategies for CF pulmonary exacerbations as well as how we classify CF pulmonary exacerbations as an outcome measure. The identification of pauci-inflammatory exacerbation phenotypes is an important area for future research as current treatment approaches are not tailored to exacerbation subphenotypes. In an era of increasing antimicrobial resistance and new CF therapeutics, a more personalized approach to treating CF pulmonary exacerbations may help reduce the significant morbidity and mortality associated with these events.
Acknowledgments
Acknowledgment
The authors thank the people with CF and their families for participation in this study.
Footnotes
Supported by a 2015 Cystic Fibrosis Ireland/Irish Thoracic Society/Gilead Research Fellowship and by the Cystic Fibrosis Research Fund, St. Vincent’s Hospital Foundation (S.C.C., Ireland); a Michael Smith Foundation for Health Research Trainee Award (#RT-2020–0493) (A.N.F., Canada); a Michael Smith Foundation for Health Research Scholar Award (#16414) (B.S.Q., Canada); a Wellcome Trust Clinical Research Training Fellowship (201,246/Z/16/Z) (S.M.L., Scotland); and an NRS Senior Clinical Fellowship (SCAF/16/02) (R.D.G., Scotland).
Author Contributions: S.C.C., E.F.M, S.C, C.F.D.G., G.C, K.B.H, and P.K.S planned the study. S.C.C, B.K, P.O’R., S.M.L., and R.D.G performed laboratory analysis. C.G.G and T.T.N provided clinical care and were involved in making clinical diagnosis of exacerbations. E.F.M, A.N.F, K.M.O’S., A.B., A.S., B.G., O.M.O’C., and B.S.Q gathered and analyzed data for validation cohorts. S.C.C, E.F.M, A.N.F, and B.S.Q wrote the first draft of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest . 2002;121:64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 2. Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol . 2001;153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chin M, De Zoysa M, Slinger R, Gaudet E, Vandemheen KL, Chan F, et al. Acute effects of viral respiratory tract infections on sputum bacterial density during CF pulmonary exacerbations. J Cyst Fibros . 2015;14:482–489. doi: 10.1016/j.jcf.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flight WG, Bright-Thomas RJ, Sarran C, Mutton KJ, Morris J, Webb AK, et al. The effect of the weather on pulmonary exacerbations and viral infections among adults with cystic fibrosis. Int J Biometeorol . 2014;58:1845–1851. doi: 10.1007/s00484-013-0786-0. [DOI] [PubMed] [Google Scholar]
- 5. Hoek RA, Paats MS, Pas SD, Bakker M, Hoogsteden HC, Boucher CA, et al. Incidence of viral respiratory pathogens causing exacerbations in adult cystic fibrosis patients. Scand J Infect Dis . 2013;45:65–69. doi: 10.3109/00365548.2012.708942. [DOI] [PubMed] [Google Scholar]
- 6. Punch G, Syrmis MW, Rose BR, Harbour C, Bye PT, Nissen MD, et al. Method for detection of respiratory viruses in the sputa of patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis . 2005;24:54–57. doi: 10.1007/s10096-004-1273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jackson AD, Jackson AL, Fletcher G, Doyle G, Harrington M, Zhou S, et al. Estimating direct cost of cystic fibrosis care using irish registry healthcare resource utilisation data, 2008-2012. PharmacoEconomics . 2017;35:1087–1101. doi: 10.1007/s40273-017-0530-4. [DOI] [PubMed] [Google Scholar]
- 8. Parkins MD, Rendall JC, Elborn JS. Incidence and risk factors for pulmonary exacerbation treatment failures in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa. Chest . 2012;141:485–493. doi: 10.1378/chest.11-0917. [DOI] [PubMed] [Google Scholar]
- 9. Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med . 2011;184:662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 10. Ghebre MA, Pang PH, Diver S, Desai D, Bafadhel M, Haldar K, et al. Biological exacerbation clusters demonstrate asthma and chronic obstructive pulmonary disease overlap with distinct mediator and microbiome profiles. J Allergy Clin Immunol . 2018;141:2027–2036.e12. doi: 10.1016/j.jaci.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goss CH. Acute pulmonary exacerbations in cystic fibrosis. Semin Respir Crit Care Med . 2019;40:792–803. doi: 10.1055/s-0039-1697975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bucher J, Boelle PY, Hubert D, Lebourgeois M, Stremler N, Durieu I, et al. Lessons from a French collaborative case-control study in cystic fibrosis patients during the 2009 A/H1N1 influenza pandemy. BMC Infect Dis . 2016;16:55. doi: 10.1186/s12879-016-1352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Ewijk BE, Wolfs TF, Aerts PC, Van Kessel KP, Fleer A, Kimpen JL, et al. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr Res . 2007;61:398–403. doi: 10.1203/pdr.0b013e3180332d1c. [DOI] [PubMed] [Google Scholar]
- 14. Wark PA, Tooze M, Cheese L, Whitehead B, Gibson PG, Wark KF, et al. Viral infections trigger exacerbations of cystic fibrosis in adults and children. Eur Respir J . 2012;40:510–512. doi: 10.1183/09031936.00202311. [DOI] [PubMed] [Google Scholar]
- 15. Waters V, Ratjen F. Pulmonary exacerbations in children with cystic fibrosis. Ann Am Thorac Soc . 2015;12:S200–S206. doi: 10.1513/AnnalsATS.201502-098AW. [DOI] [PubMed] [Google Scholar]
- 16. Goffard A, Lambert V, Salleron J, Herwegh S, Engelmann I, Pinel C, et al. Virus and cystic fibrosis: rhinoviruses are associated with exacerbations in adult patients. J Clin Virol . 2014;60:147–153. doi: 10.1016/j.jcv.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carmody LA, Zhao J, Schloss PD, Petrosino JF, Murray S, Young VB, et al. Changes in cystic fibrosis airway microbiota at pulmonary exacerbation. Ann Am Thorac Soc . 2013;10:179–187. doi: 10.1513/AnnalsATS.201211-107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stressmann FA, Rogers GB, Marsh P, Lilley AK, Daniels TW, Carroll MP, et al. Does bacterial density in cystic fibrosis sputum increase prior to pulmonary exacerbation? J Cyst Fibros . 2011;10:357–365. doi: 10.1016/j.jcf.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 19. Mayer-Hamblett N, Kronmal RA, Gibson RL, Rosenfeld M, Retsch-Bogart G, Treggiari MM, et al. EPIC Investigators Initial Pseudomonas aeruginosa treatment failure is associated with exacerbations in cystic fibrosis. Pediatr Pulmonol . 2012;47:125–134. doi: 10.1002/ppul.21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sawicki GS, Ayyagari R, Zhang J, Signorovitch JE, Fan L, Swallow E, et al. A pulmonary exacerbation risk score among cystic fibrosis patients not receiving recommended care. Pediatr Pulmonol . 2013;48:954–961. doi: 10.1002/ppul.22741. [DOI] [PubMed] [Google Scholar]
- 21. Aaron SD, Ramotar K, Ferris W, Vandemheen K, Saginur R, Tullis E, et al. Adult cystic fibrosis exacerbations and new strains of Pseudomonas aeruginosa. Am J Respir Crit Care Med . 2004;169:811–815. doi: 10.1164/rccm.200309-1306OC. [DOI] [PubMed] [Google Scholar]
- 22. Fodor AA, Klem ER, Gilpin DF, Elborn JS, Boucher RC, Tunney MM, et al. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS One . 2012;7:e45001. doi: 10.1371/journal.pone.0045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Price KE, Hampton TH, Gifford AH, Dolben EL, Hogan DA, Morrison HG, et al. Unique microbial communities persist in individual cystic fibrosis patients throughout a clinical exacerbation. Microbiome . 2013;1:27. doi: 10.1186/2049-2618-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jorth P, Durfey S, Rezayat A, Garudathri J, Ratjen A, Staudinger BJ, et al. Cystic fibrosis lung function decline after within-host evolution increases virulence of infecting Pseudomonas aeruginosa. Am J Respir Crit Care Med . 2021;203:637–640. doi: 10.1164/rccm.202007-2735LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flume PA, Mogayzel PJ, Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, et al. Clinical Practice Guidelines for Pulmonary Therapies Committee Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med . 2009;180:802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 26. Jain R, Baines A, Khan U, Wagner BD, Sagel SD. Evaluation of airway and circulating inflammatory biomarkers for cystic fibrosis drug development. J Cyst Fibros . 2021;20:50–56. doi: 10.1016/j.jcf.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jung D, Dong K, Jang J, Lam GY, Wilcox PG, Quon BS. Circulating CRP and calprotectin to diagnose CF pulmonary exacerbations. J Cyst Fibros . 2021;20:46–49. doi: 10.1016/j.jcf.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 28. Liou TG, Adler FR, Keogh RH, Li Y, Jensen JL, Walsh W, et al. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PLoS One . 2012;7:e42748. doi: 10.1371/journal.pone.0042748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loh G, Ryaboy I, Skabelund A, French A. Procalcitonin, erythrocyte sedimentation rate and C-reactive protein in acute pulmonary exacerbations of cystic fibrosis. Clin Respir J. 2018;12:1545–1549. doi: 10.1111/crj.12703. [DOI] [PubMed] [Google Scholar]
- 30. Paats MS, Bergen IM, Bakker M, Hoek RA, Nietzman-Lammering KJ, Hoogsteden HC, et al. Cytokines in nasal lavages and plasma and their correlation with clinical parameters in cystic fibrosis. J Cyst Fibros . 2013;12:623–629. doi: 10.1016/j.jcf.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 31. Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med . 2010;182:627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanders DB, Li Z, Zhao Q, Farrell PM. Poor recovery from a pulmonary exacerbation does not lead to accelerated FEV1 decline. J Cyst Fibros . 2018;17:492–495. doi: 10.1016/j.jcf.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanders DB, Solomon GM, Beckett VV, West NE, Daines CL, Heltshe SL, et al. STOP Study Group Standardized Treatment of Pulmonary Exacerbations (STOP) study: observations at the initiation of intravenous antibiotics for cystic fibrosis pulmonary exacerbations. J Cyst Fibros . 2017;16:592–599. doi: 10.1016/j.jcf.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharma A, Kirkpatrick G, Chen V, Skolnik K, Hollander Z, Wilcox P, et al. Clinical utility of C-reactive protein to predict treatment response during cystic fibrosis pulmonary exacerbations. PLoS One . 2017;12:e0171229. doi: 10.1371/journal.pone.0171229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Regelmann WE, Elliott GR, Warwick WJ, Clawson CC. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. Am Rev Respir Dis . 1990;141:914–921. doi: 10.1164/ajrccm/141.4_Pt_1.914. [DOI] [PubMed] [Google Scholar]
- 36. Smith AL, Redding G, Doershuk C, Goldmann D, Gore E, Hilman B, et al. Sputum changes associated with therapy for endobronchial exacerbation in cystic fibrosis. J Pediatr . 1988;112:547–554. doi: 10.1016/s0022-3476(88)80165-3. [DOI] [PubMed] [Google Scholar]
- 37. Rojas JM, Avia M, Martín V, Sevilla N. IL-10: a multifunctional cytokine in viral infections. J Immunol Res . 2017;2017:6104054. doi: 10.1155/2017/6104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gray RD, Imrie M, Boyd AC, Porteous D, Innes JA, Greening AP. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J Cyst Fibros . 2010;9:193–198. doi: 10.1016/j.jcf.2010.01.005. [DOI] [PubMed] [Google Scholar]