Abstract
Crohn’s disease (CD) and ulcerative colitis (UC) are relapsing and remitting chronic inflammatory diseases of the gastrointestinal tract. Although surgery for UC can provide a cure, surgery for CD is rarely curative. In the past few decades, research has identified risk factors for postsurgical CD recurrence, enabling patient risk stratification to guide monitoring and prophylactic treatment to prevent CD recurrence. A MEDLINE literature review identified articles regarding post-operative monitoring of CD recurrence after resection surgery. In this review, we discuss the evidence on risk factors for post-operative CD recurrence as well as suggestions on post-operative management.
Keywords: post-operative, Crohn’s, management, recurrence, biologics
Introduction
Crohn’s disease (CD) management has advanced rapidly with the advent of biologics and small molecule inhibitors. Despite these advancements, around half of patients with CD require surgery within the first decade of their diagnosis [1]. Patients with CD may receive a variety of different surgeries, including ileostomy, colostomy, ileocolonic resection, diverted bowel, and stricturotomy. Most commonly, patients undergo surgical resection [2]. Although surgery for ulcerative colitis can be curative, surgery in CD is rarely curative, and most patients experience post-operative CD recurrence. Post-operative CD recurrence rates will vary based on the modality and definition of assessing recurrence in each study. Put simply, post-operative CD recurrence is the reappearance of CD lesions after resection, and this is assessed clinically, endoscopically, radiologically, or surgically [3, 4]. Clinical recurrence is the reappearance of CD symptoms, and this should be carefully assessed to ensure the symptoms are not due to other malabsorptive or motility issues post-operatively [4]. Clinical recurrence rates after resection surgery have been reported from 36% to 86% at ≤10 years of follow-up [5, 6]. Various scoring systems exist for monitoring endoscopic CD recurrence, and endoscopic recurrence rates after resection surgery have been reported even higher at ≤70% within 6 months [6–8]. Post-operative CD recurrence typically presents on a continuum from histologic findings to endoscopic findings to clinical presentation [1, 9]. Hence, early endoscopic monitoring and prophylactic pharmacological therapy are key tenets of the post-operative management of patients with CD [1]. Additionally, accumulating evidence has identified individual risk factors for post-operative disease recurrence, allowing for risk stratification to help gastroenterologists balance the risks and benefits of pharmacologic prophylaxis, timing, and escalation for individual patients.
Methods
The MEDLINE (via PubMed) database was searched on 8 July 2022 by a medical librarian (S.C.) in order to facilitate this narrative review, using a combination of keywords and database-specific subject headings for the following concepts: Crohn's, ileostomy, colostomy, ileocolonic resection, diverted bowel, stricturotomy. Animal-only studies were excluded, as were non-English articles (Table 1). Additional references were identified by hand-searching reference lists of included articles.
Table 1.
Search strategy
| Search set plus description | Search strategy | Results |
|---|---|---|
| #1 Crohn's Disease | “Crohn Disease”[Mesh] OR Crohn[tiab] OR Crohns[tiab] OR Crohn’s[tiab] | 62,604 |
| #2 Ileocolonic Resection | “ileocolonic resection”[tiab] OR “ileocolonic resections”[tiab] OR “ileo-colonic resection”[tiab] OR “ileo-colonic resections”[tiab] | 212 |
| #3 Ileostomy | “Ileostomy”[Mesh] OR ileostomy[tiab] OR ileostomies[tiab] | 10,742 |
| #4 Colostomy | “Colostomy”[Mesh] OR colostomy[tiab] OR colostomies[tiab] | 13,947 |
| #5 Diverted bowel | “diverted bowel”[tiab] OR “diverted bowels”[tiab] OR “fecal diversion”[tiab] OR “fecal diversions”[tiab] OR “faecal diversion”[tiab] OR “faecal diversions”[tiab] OR “diverted large bowel”[tiab] OR “diverted large bowels”[tiab] | 756 |
| #6 Stricturotomy | stricturotomy[tiab] OR stricturotomies[tiab] | 62 |
| #7 Combining procedures w/OR | #2 OR #3 OR #4 OR #5 OR #6 | 22,930 |
| #8 Combining Crohn's w/procedures | #1 AND #7 | 1,796 |
| #9 Eliminate animal studies | #8 NOT (animals[MeSH Terms] NOT humans[MeSH Terms]) | 1,794 |
| #10 Limit to English language articles | #9 AND English[lang] | 1,542 |
Database: MEDLINE [via PubMed]; search date: 7/8/2022. Articles were exported from MEDLINE to an EndNote 20 Library; only citations from 2000 to the present (n = 843 citations) were then sent to Covidence, screening software, for review.
The search yielded a total of 1,542 citations. Articles were exported from MEDLINE to an EndNote 20 Library; only citations from 2000 to the present (n = 843 citations) were uploaded to Covidence for screening (Covidence systematic review software, Veritas Health Innnovation, Melbourne, Australia). One author (K.E.L.) reviewed the citations at the title/abstract level, followed by full-text review. A total of 43 citations were found after screening. The reference lists of these citations were hand-searched, and 47 additional citations were identified, for a total of 90 references included in the final analysis.
Results
Common surgery in CD
The most common surgeries for CD include ileocolonic resection and ileocolonic or ileorectal anastomosis, strictureplasty, diverting ostomy, and bowel bypass [10]. Surgeries can be divided into bowel-resecting and bowel-sparing options. Resection surgeries include ileocolonic resection and ileocolonic or ileorectal anastomosis, small-bowel resection with anastomosis, and partial colectomy with anastomosis based on the affected bowel segments. If the small bowel is inflamed or perforated, it can be resected. If the terminal ileum is involved, an ileocecal resection can be performed. If the patient has Crohn’s colitis or proctitis, colorectal resection can be performed. The surgeon should spare as much bowel as possible; a randomized–controlled trial by Fazio et al. of 152 patients showed no difference in CD recurrence, defined in this study as reoperation for recurrent pre-anastomotic disease, between 2 and 12 cm from the macroscopically involved area [11]. Bowel resection can improve health and quality of life [12]. However, resection results in loss of absorptive surface area, and consequently further nutritional deficiencies in the patient with CD [13]. Resection of the ileum can cause severe nutritional deficiencies as the ileum is responsible for absorbing fats bound to bile salts, fat-soluble vitamins, and vitamin B12 [14].
Although resection surgeries are the most commonly performed, there are diverting procedures, strictureplasty, and bypass surgery. Diverting surgery includes fecal diversion with ileostomy, jejunostomy, and colostomy. Fecal diversion can move the fecal stream away from affected bowel, and the fecal stream is thought to trigger tissue damage in CD [15]. It can be used in instances of refractory perianal fistulas or abscesses, surgical complication, or for disease control [10, 15]. Fecal diversion can be temporary or permanent, but the likelihood of restoring bowel continuity is low [15]. Strictureplasty is a surgery that can address fibrostenotic obstructive disease, especially in cases where bowel preservation is more crucial, e.g. the patient has recurrent disease after small-bowel resection, or resection would be extensive [13, 16]. When patients have strictures in areas of the duodenum, duodenal bypass surgery is one option, though less common, in which affected areas of bowel are bypassed and unaffected areas are connected [13, 17].
Recurrence by type of surgery
Most of the literature centers on post-operative recurrence after resection surgery. Reports of clinical recurrence rates after resection surgery have ranged from 36% to 86% at ≤10 years of follow-up [5, 6]. A recent meta-analysis found the rate of clinical recurrence (as defined in individual studies) after subtotal or total colectomy or proctocolectomy with permanent ileostomy to be 28.0% (95% confidence interval [CI], 21.7–35.3; 14 studies, 260 of 1,004 patients), with considerable heterogeneity (I2 = 80%), and 5- and 10-year median cumulative clinical recurrence rates of 23.5% and 40%, respectively [1]. This meta-analysis found similar rates of clinical recurrence in studies from the pre-biological <1998 vs the biological >1998 era (26.9% vs 30.0%, P = 0.64) [1]. Endoscopic recurrence precedes clinical recurrence. A recent study reported the rate of endoscopic recurrence (Rutgeerts score > i1) after ileocolic resection with anastomosis as 70% at median time to ileocolonoscopy of 6.2 (interquartile range [IQR], 5.4–7.8) months [18]. Generally, studies have shown endoscopic recurrence rates after resection surgery at ≤70% within 6 months, ∼73%–95% at 1 year, and 83%–100% at 3 years [6–8, 19]. Sometimes, temporary fecal diversion is used for perianal CD; a systematic review and meta-analysis found that studies reported early clinical response (usually defined clinically within 3–6 months after fecal diversion) in 63.8% of patients [20]. However, this study showed low rates of success in bowel restoration at 16.6%, and in those with attempted restorations, 26.5% had severe relapse and required re-diversion for symptom management [20]. Overall, 41.6% of patients required proctectomy due to lack of clinical improvement after initial diversion or relapse of perianal disease after attempted restoration of the bowel [20]. As for strictureplasty, recent data from a retrospective study on patients treated with strictureplasty for CD showed rates of site-specific recurrence (defined as the reappearance of CD at the site of a strictureplasty requiring surgery) were 12.2% at 5 and 25.7% at 10 years with a median follow-up time of 96 months and median time to recurrence at 62.5 months [21]. Another study looking at patients who received side-to-side isoperistaltic strictureplasty from 1996 to 2010 found that 44.6% experienced a recurrence (defined as relapse of CD symptoms with radiological and/or endoscopic confirmation of lesions requiring medical treatment or surgery) at a mean of 55.46 (standard deviation 36.79) months after surgery [22]. The authors concluded that this recurrence rate was acceptable and side-to-side isoperistaltic strictureplasty can be a useful alternative to resection, especially in patients with multiple operations with risk of short-bowel syndrome [22].
General principles of monitoring for disease recurrence
Regardless of the type of surgery, postsurgical patients should be monitored clinically and endoscopically, with the use of laboratory and imaging studies. The specifics of disease monitoring by patient risk are outlined below; however, general principles are as follows. Ileocolonoscopy is the most accurate for monitoring for CD recurrence, and has a Grade A recommendation for monitoring for disease at 6–12 months post-ileocolectomy [10]. The endoscopic monitoring of postsurgical CD can be complicated by varying anatomy based on procedure, loss of bowel, obstruction, poor nutritional status, and immunosuppression [10].
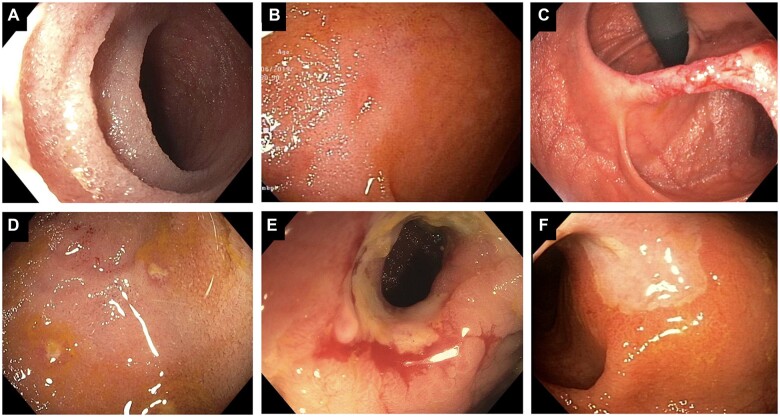
In ileocolonic resection and ileocolonic or ileorectal anastomosis, the Rutgeerts score has been developed specifically for monitoring of disease recurrence in the post-operative setting [10, 23, 24]. The Rutgeerts scoring system categorizes endoscopic findings into five categories (i0–i4) based on extent and severity of lesions: i0—no lesions; i1—up to five aphthous ulcers; i2—more than five aphthous ulcers with normal intervening mucosa or skip lesions or lesions confined to the ileocolic anastomosis; i3—diffuse aphthous ileitis and diffusely inflamed mucosa; i4—diffuse ileal inflammation with large ulcers, nodules, and/or narrowing (Figure 1A–F) [23, 24]. Low-grade mucosal inflammation (i0 and i1) has been found to correlate with a low symptomatic recurrence rate of 9% at 7 years, and high-grade disease (i3–i4) with almost 100% symptomatic recurrence rate at 4 years [23]. A score of i2 and higher defines endoscopic recurrence [23, 24].
Figure 1.
Lesions in the neo-terminal ileum associated with Rutgeerts ratings i0 to i3. (A) normal i0; (B) i1 lesion; (C) i2A lesion; (D) i2B lesion with erosion; (E) i3 stricturing lesion; (F) i3 large ulcer. Figures reprinted with permission of Bo Shen, MD.
In more recent years, a modified endoscopy scoring system with improved predictive ability for post-operative recurrence was developed by Hammoudi et al. in the groupe de REcherche sur les Maladies INflammatoires Digestives (REMIND group) [25]. This group scored anastomotic and ileal lesions separately, finding that clinical recurrence was mainly determined by ileal lesions, not anastomotic lesions; anastomotic lesions were associated only with occlusive complications [25]. The REMIND group defined clinical recurrence as “(i) CD-related clinical manifestations confirmed either by ileocolonoscopy, imaging (active lesions of the small bowel or colon confirmed by the center referring radiologist) or therapeutic intensification (treatment optimization or drug switch), (ii) CD-related complications (intra-abdominal abscess or occlusive manifestation), or (iii) CD-related subsequent surgery” [25]. Ileal lesions were scored using the original Rutgeerts score (i0 to i4) while anastomotic lesions (<1 cm after anastomosis) were scored as follows: A(0)—no lesion; A(1)—ulcerations covering <50% of anastomotic circumference; A(2)—ulcerations covering >50% of anastomotic circumference; A(3)—anastomotic stenosis [25]. In ileostomy, anatomic landmarks include stoma, jejunum, ileum, and colon [10]. The endoscopist can look for disease including stomal stenosis, stomal fistula, peristomal pyoderma gangrenosum, and CD in the neo-small bowel [10]. In strictureplasty, anatomic landmarks include proximal and distal small bowel, strictureplasty lumen, and the inlet and outlet [10]. Disease recurrence can manifest with inlet and outlet stricture [10]. There is currently no consensus on the definition of CD recurrence in strictureplasty [10]. In the endoscopic monitoring of diverted colon and rectum, the anatomic landmarks include the diverted area and diverted ileal pouch, and disease can include diversion colitis or diversion-associated stricture [10]. There are few data on the need, frequency, and techniques regarding endoscopy in diverted bowel; in addition, grading of inflammation is difficult due to friable mucosa [10].
Risk stratification
Several risk factors have been identified that make individuals at high or low risk for recurrence after resection surgery. These factors are often divided into patient-related, disease-related, and surgery-related risk factors.
The main patient-related risk factor is smoking. Studies have repeatedly identified smoking to increase the risk of post-operative recurrence, and a meta-analysis of 16 studies showed an odds ratio of between 2 and 3 for clinical or surgical recurrence at 10-year follow-up [8, 26–33]. Other patient-related factors including age, sex, and age at disease onset have inconclusive evidence, and genetic factors have not been well studied [34–36]. However, it is important to note that older patients have been found to have the same risk of post-operative recurrence as younger patients [37].
Disease-related factors include duration of CD prior to first surgery, history of previous CD surgery, extent of gastrointestinal (GI) tract involvement, and complications of CD including penetrating, fistulizing, and stricturing CD [36]. Although disease duration prior to surgery is not a consistent risk factor for post-operative CD recurrence, there is some evidence suggesting that shorter durations to surgery increase recurrence rate [28, 36]. A study from almost 40 years ago showed that patients with shorter CD duration of <10 years had a relative risk for recurrence of 1.5 times that of patients with CD duration of >10 years [38]. A more recent study found that on multivariable analysis, duration of disease before first surgery of <9.5 months (P = 0.048) was an independent factor for clinical relapse [28]. Studies generally list prior CD surgery as a risk factor for further CD surgery [36, 39, 40]. The extent of bowel involvement, such as CD that involves more of the small bowel, has been found to be associated with CD recurrence [41–43]. CD has multiple complications including penetrating disease, fistulizing disease, and stricturing disease. All three of these complications have been associated in the literature with increased risk of post-operative CD recurrence [34, 44–46]. One recent meta-analysis found that penetrating disease conferred a hazard ratio of ∼1.5 for recurrence compared with non-penetrating disease, in both a high-quality study subset and recent study subset [40]. However, this meta-analysis noticed high heterogeneity in their studies [40]. In one study, fistulizing disease conferred an odds ratio of 4 for post-operative CD recurrence, and was also associated with earlier recurrence [47].
Surgery-related risk factors that have been studied in the literature include length of resection, anastomosis type, and myenteric plexitis in the resected specimen [31, 36, 48–51]. Multiple studies have shown the presence of myenteric plexitis in resected specimens to be independently predictive of post-operative recurrence [31, 48–51]. Studies regarding length of resection and type of anastomosis have not been consistently shown to increase the risk of CD recurrence, although there is some evidence to suggest that end-to-end anastomosis increases recurrence rates compared to side-to-side anastomosis in ileocolic resection, as does ileorectal anastomosis compared to end ileostomy or subtotal colectomy [36, 52, 53]. Overall, the more consistent risk factors identified in the literature for post-operative CD recurrence are smoking, penetrating disease, history of prior resection surgery, and presence of myenteric plexitis on the resected specimen [26, 31, 36, 40].
Risk stratification is important in clinical decision-making regarding post-operative patients with CD. The post-operative Crohn's endoscopic recurrence (POCER) trial stratified individuals after resection surgery into low or high risk of post-operative CD recurrence by categorizing individuals who smoked (any number of cigarettes at study entry), had perforating disease (abscess, enteric fistula, free perforation), or had history of at least one previous resection as high-risk [54]. Those who did not have these risk factors were deemed low-risk [54]. All patients were treated with metronidazole, while high-risk patients were treated with thiopurine or adalimumab (if thiopurine intolerant) [54]. Active care was defined as colonoscopy at 6 months, with step-up therapy if recurrence (addition of adalimumab for patients at high risk initially receiving a thiopurine, and weekly adalimumab for patients at high risk initially on fortnightly adalimumab) [54]. This study found that 51% of high-risk patients treated with active care were in endoscopic remission (i0–i1) vs 30% of high-risk patients treated with standard care [54]. Meanwhile, 50% of high-risk patients treated with active care had endoscopic recurrence compared with 70% treated with standard care [54]. Overall, the POCER data support early endoscopic monitoring and endoscopically tailored therapy, and have significantly impacted the post-operative management of CD. They strongly suggest that early ileocolonoscopy and step-up therapy for recurrence prevent post-operative CD recurrence. The authors of the POCER study noted that some patients at low risk did have endoscopic disease recurrence, so all patients should receive follow-up and monitoring [54].
Patients at low risk of post-operative recurrence
Patients with CD at lower risk of post-operative recurrence would be non-smokers with no prior CD surgery, and only shorter strictures. They may have more long-standing CD before their first surgery. All patients, even those at lower risk, should be monitored endoscopically at 6–12 months post-surgery with an ileocolonoscopy [55]. The endoscopic findings then inform step-up therapy. All patients should be followed clinically for any symptoms that may prompt earlier ileocolonoscopy. Fecal calprotectin and C-reactive protein (CRP) are non-invasive markers that assess disease activity. These can be tracked every 6 months for the first 2 years after surgery and afterwards every year. Abnormal values of fecal calprotectin should prompt ileocolonoscopy. Low levels of fecal calprotectin (<65 μg/g) at 3 months post-surgery have predicted endoscopic remission at 1 year [56]. One study found that levels of fecal calprotectin correlated with endoscopic remission (using Crohn's Disease Endoscopic Activity Index of Severity [CDEIS] < 3) with point-of-care quantitative fecal calprotectin levels of <272 μg/g showing an area under the receiver-operating characteristic (ROC) curve of 0.933 [57]. Another study assessing fecal calprotectin levels for predicting endoscopic remission vs recurrence by the Rutgeerts score found that a level of 100 μg/g had a sensitivity, specificity, positive, and negative predictive values, as well as overall accuracy, of 95%, 54%, 69%, 93%, and 77%, respectively [58]. CRP can support suspicion of recurrence alongside the fecal calprotectin, and has been shown to have an area under the ROC curve to discriminate between remission and recurrence of 0.70 [58]. In general, CRP values of >45 mg/L in patients with inflammatory bowel disease (IBD) have been found to predict need for colectomy and reflect severe gut inflammation [59].
Initially, low-risk patients should be given a 3-month course of metronidazole [36, 54, 60–63]. Metronidazole and ornidazole have been shown to help prevent post-operative clinical and endoscopic recurrence [64, 65]. One trial studied whether metronidazole for 3 months with azathioprine (AZA) for 12 months was superior to metronidazole alone to reduce post-operative CD in high-risk patients, and attributed overall low recurrence rates throughout the study to the widespread metronidazole use [66]. A retrospective case–control study of 35 patients receiving post-operative low-dose metronidazole therapy vs 35 patients not receiving metronidazole therapy found that endoscopic recurrence at 1 year was significantly lower in the metronidazole group vs the control group (20% vs 54%, P = 0.006). Due to side effects of metronidazole (metallic taste, GI discomfort, paresthesia, peripheral neuropathy), patients may not tolerate the drug or decline it; these patients can be monitored clinically and with endoscopy at 6–12 months. At the 6- to 12-month ileocolonoscopy, if there is evidence of endoscopic recurrence, patients should receive further medical therapy with anti-TNF agents or AZA or 6-mercaptopurine (6-MP). If there is endoscopic remission, patients may be scheduled for their next ileocolonoscopy in 1–3 years.
Patients at high risk of post-operative recurrence
Patients at high risk of post-operative CD recurrence would include those who smoke any number of cigarettes and have had prior CD surgery and penetrating or fistulizing disease. Medical therapy options include antitumor necrosis factor (anti-TNF) agents, or a combination of 6-MP or AZA with metronidazole. These agents are specifically first-line in the post-operative setting because post-operative anti-TNF or AZA/6-MP has been shown to reduce the risk of clinical recurrence.
As with any patient, high-risk patients should be monitored endoscopically at 6–12 months, and fecal calprotectin and CRP should be checked as in low-risk patients discussed above. If there is endoscopic recurrence, medical therapy should be modified or intensified. If there is endoscopic remission, the patient should continue their medical therapy and undergo ileocolonoscopy at 1–3 years.
The efficacy of post-operative thiopurines such as AZA and 6-MP have been well studied. Trials have compared thiopurine with placebo and found that endoscopic recurrence was lower in the thiopurine arm at 1 year [66, 67]. Trials have shown the superiority of thiopurine to mesalamine [67–69]. In one randomized double-blind clinical trial, there were significantly lower rates of clinical recurrence after AZA vs mesalazine (0% vs 10.8%, P = 0.03) [70]. However, AZA was associated with higher rates of drug discontinuation due to side effects than mesalazine (22% vs 0%, P = 0.002) [70]. Patients may not tolerate AZA due to side effects including leukopenia, elevated liver enzymes, arthralgia/myalgia, vomiting, and abdominal pain [69, 70].
When deciding on initial biologic therapy, patients who had not failed treatment with AZA or 6-MP or anti-TNF prior to surgery should begin AZA or 6-MP within 2–8 weeks of resection surgery. This should be ideally in combination with 3 months of metronidazole. If the patient had been previously treated with AZA or 6-MP or anti-TNF, they should be started on an anti-TNF agent within 4–8 weeks of resection surgery. Infliximab has been studied extensively in the post-operative setting. Studies have shown that infliximab was superior to placebo, mesalamine, and thiopurines for the prevention of post-operative CD. A randomized trial by Regueiro et al. [71] comparing infliximab to placebo showed that 1-year endoscopic recurrence was significantly lower in patients on infliximab vs placebo (9.1% vs 84.6%, P = 0.0006). Additionally, 1-year histologic recurrence was lower in patients on infliximab than in those on placebo (27.3% vs 84.6%, P = 0.01) [71]. However, the trial was unable to show a significant decrease in clinical remission in patients on infliximab vs placebo (80% vs 54%, P = 0.38) [71]. A follow-up trial by Regueiro et al. then gave patients previously randomly assigned to receive infliximab for 1 year after resection the option to continue, stop, or start infliximab therapy and followed patients for 5 years; they found that patients originally assigned to the infliximab group in the first year after surgery had a longer mean time to first endoscopic recurrence as well as longer mean time to surgery than patients originally assigned to placebo [72]. Additionally, the rate of requiring additional surgery was significantly lower in patients who received infliximab for most of the 5-year follow-up period than in those who received it for shorter periods (20% vs 64%, P = 0.047) [72]. A larger trial by Regueiro et al. looked at post-operative recurrence rates in 297 patients randomized to receive infliximab vs placebo and found lower endoscopic rates in the infliximab arm compared to placebo (31% vs 60%, P < 0.001) but no significant difference in clinical recurrence rates in the infliximab arm vs placebo (13% vs 20%, P = 0.097) [73]. However, in a randomized trial by Yoshida et al., infliximab was shown to be superior to placebo in clinical remission rate at 1 year based on the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) score (100% vs 68%, P = 0.02) [74]. In terms of endoscopic remission, the infliximab group also had higher rates at 1 year compared with placebo (78% vs 18%, P = 0.004) [74]. Data are accumulating for adalimumab in the post-operative setting. Analyses from the POCER trial data showed that adalimumab was superior to thiopurines for preventing early disease recurrence in high-risk patients; endoscopic recurrence occurred in 45% of thiopurine-treated patients vs 21% of adalimumab-treated patients (intention-to-treat [ITT]; P = 0.028) [60].
If there is endoscopic recurrence upon the 6- to 12-month ileocolonoscopy, medical therapy should be modified or intensified. Patients who were receiving AZA or 6-MP can be switched to an anti-TNF agent or an anti-TNF agent can be added to their regimen. Patients who were already taking anti-TNF or other biologic can be increased on their anti-TNF dose, switched to another anti-TNF agent, or can continue their current anti-TNF with the addition of AZA or 6-MP.
Newer biologics in prevention of post-operative recurrence
In recent years, there has been greater use and interest in newer biologics, e.g. vedolizumab and ustekinumab, in the post-operative setting to prevent CD recurrence. A survey study of gastroenterologists attending the 2019 European Crohn’s and Colitis Organisation (ECCO) congress found that 62% and 56% of gastroenterologists with access to vedolizumab and ustekinumab, respectively, would prescribe these drugs in the post-operative setting [75]. The authors of this report note that this was an “unexpectedly high” percentage who would already consider these biologics as reliable treatment despite little published data [75]. Generally, these newer biologics are reserved for patients who have failed anti-TNFα therapy or have contraindications to anti-TNFα agents [76]. Yamada et al. published a retrospective study looking at vedolizumab in the post-operative setting, finding that at rates of clinical (Harvey–Bradshaw index ≤ 4) remission were similar between patients receiving vedolizumab or anti-TNFα therapy at 6–12 months of follow-up, whereas rates of endoscopic remission, defined by simple endoscopic score for CD (SES-CD) of 0, was significantly decreased in patients receiving vedolizumab as compared with anti-TNFα therapy (25% vs 66%, P = 0.01) [77]. In a retrospective study looking at patients with CD receiving vedolizumab or ustekinumab after curative intestinal resection with ileocolonic anastomosis in the ENEIDA registry (Nationwide study on genetic and environmental determinants of inflammatory bowel disease) by GETECCU, authors found that 40% of patients on vedolizumab and 42% of patients on ustekinumab had post-operative endoscopic recurrence (defined by Rutgeerts score > i1, with endoscopy within 18 months post-surgery) [78]. These rates are similar to those reported with anti-TNFα agents, e.g. 40% for infliximab and 30% for adalimumab-treated patients, using the same definition as the study looking at vedolizumab/ustekinumab, endoscopic post-operative recurrence as Rutgeerts score of >i1 within the first 18 months after surgery [79]. Several other retrospective studies looked at the utility of ustekinumab or vedolizumab in the post-operative setting. A retrospective multicenter study found rates of endoscopic post-operative recurrence, defined as Rutgeerts score of >i1 or colonic-segmental-SES-CD of >5 within 1 year, as 33% for vedolizumab and 61.8% for ustekinumab; the authors also found that patients treated with vedolizumab or ustekinumab tended to have more experience with prior biologics and surgery [80]. A small retrospective study defining endoscopic recurrence as Rutgeerts score of >i1 at 6 months found that after inversed probability of treatment weighting, endoscopic recurrence was lower with ustekinumab compared with AZA (28.0% vs 54.5%, P = 0.029) [81]. Further study using randomized–controlled trials comparing these newer biologics with prior biologics would be helpful in determining the optimal biologic regimen to prevent post-operative CD recurrence.
Emerging imaging modalities for post-operative Crohn’s recurrence
Recently, there has been interest in using non-invasive imaging modalities such as ultrasound (US), computed tomography enterography (CTE), and magnetic resonance enterography (MRE) to assess for post-operative CD recurrence. A recent systematic review and meta-analysis found that US performed very well to detect post-operative recurrence defined in individual studies: pooled sensitivity 0.94 (95% CI, 0.86–0.97) and pooled specificity 0.84 (95% CI, 0.62–0.94) [82]. In US, oftentimes bowel wall thickness (BWT) is used as an indicator for recurrence, with most studies using BWT of ≤3 mm to define the US examination as normal [82]. This study found that a BWT of ≥5.5 mm was optimal to predict the presence of severe post-operative recurrence (Rutgeerts ≥ 3), with sensitivity of 83.8% (95% CI, 73.6%–90.6%) and specificity of 97.7% (95% CI, 93%–99%) [82]. As for the particular type of US, bowel sonography had pooled sensitivity and specificity of 0.82 and 0.88, respectively, whereas small-intestine contrast ultrasound had pooled sensitivity and specificity of 0.99 and 0.74, respectively [82]. Based on such emerging data, a recent international panel of experts have stated that bowel US performed well in measures of sensitivity and specificity, and is a good tool to evaluate for recurrence and response to medical therapy in the post-operative setting [83]. The use of CTE and MRE has also been studied in the post-operative setting. A recent systematic review and meta-analysis found high pooled sensitivity and specificity for MRE at 97% and 84%, respectively, with very high accuracy with area under the curve of 0.98 [84]. Several studies have reported good correlation between endoscopic and CTE recurrence (r = 0.782, P < 0.0001) and high sensitivity and specificity for CD recurrence at 92% and 83%, respectively [85, 86]. More and larger studies need to further assess the use of these non-invasive imaging modalities in the post-operative setting.
No recurrence after ileocolonic resection
Although we focus on postsurgical CD recurrence in this review, it is worth reminding that surgery is effective for CD remission. For example, a post-operative symptomatic recurrence rate of 40%–80% also means that 20%–60% did not recur in the study periods [36, 53, 87–89]. The Laparoscopic Ileocecal Resection vs Infliximab for terminal ileitis in Crohn's disease (LIRIC) trial assessed whether surgery was effective in treating patients with ileocecal Crohn's disease who did not respond to conventional therapy (corticosteroids, thiopurines, or methotrexate) compared with escalated medical therapy [90]. The authors found that endoscopic recurrence occurred in 21% of patients who underwent ileocecal resection compared with 16% of those who received infliximab [90]. The authors concluded that the low rates of endoscopic recurrence after ileocecal resection suggested that early resection in terminal ileitis could be a good alternative to long-term infliximab therapy [90].
Summary of post-operative management and general considerations for all patients
To summarize the post-operative management of CD, all patients regardless of risk should be monitored endoscopically, and based on risk should receive certain medical therapy (Table 2, for summary). In all patients, ileocolonoscopy should be performed at 6–12 months following surgery, and if there is endoscopic remission, at 1–3 years after that. In all patients, fecal calprotectin and CRP can be used to track disease every 6 months for the first 2 years and every year afterwards. When monitoring endoscopic recurrence, the modified version of the Rutgeerts scoring system developed by the REMIND group can be used, where the original Rutgeerts score is used for ileal lesions and a modified score is used for anastomotic lesions separately. Risk stratification can be completed based on patient-related, disease-related, and surgery-related risk factors. Low-risk patients can be given a short course of metronidazole, and at the 6- to 12-month ileocolonoscopy, those with recurrence can receive step-up therapy with anti-TNF agents or AZA or 6-MP. High-risk patients can be given medical therapy with anti-TNF agents, or a combination of 6-MP or AZA with metronidazole. Those with recurrence at the 6- to 12-month ileocolonoscopy should receive step-up therapy intensifying or modifying their medical regimen. Currently there is increasing evidence for the post-operative use of newer biologics such as vedolizumab and ustekinumab, as well as for non-invasive imaging such as US or CTE/MRE.
Table 2.
Recommendations on the post-operative management of Crohn’s disease (CD)
| Recommendations in the literature | Main source |
|---|---|
| In all patients, ileocolonoscopy should be used to monitor for disease at 6–12 months post-ileocolectomy. Fecal calprotectin and C-reactive protein can be tracked every 6 months for the first 2 years after surgery and afterwards every year | [10, 55–59] |
| Endoscopic recurrence can be assessed using a scoring system developed by the REMIND group that modifies the Rutgeerts scoring system: ileal lesions can be scored using the original Rutgeerts score (i0—no lesions; i1—up to five aphthous ulcers; i2—more than five aphthous ulcers with normal intervening mucosa or skip lesions or lesions confined to the ileocolic anastomosis; i3—diffuse aphthous ileitis and diffusely inflamed mucosa; i4—diffuse ileal inflammation with large ulcers, nodules, and/or narrowing). Anastomotic lesions (<1 cm after anastomosis) can be scored: A(0)—no lesion; A(1)—ulcerations covering <50% of anastomotic circumference; A(2)—ulcerations covering >50% of anastomotic circumference; A(3)—anastomotic stenosis | [23–25] |
| During ileocolonoscopy in the post-operative patient, the gastroenterologist should look for anatomic landmarks based on type of surgical procedure | [10] |
| Patients should be stratified based on risk of recurrence. There are patient-related (e.g. smoking), disease-related (e.g. duration of CD prior to first surgery, history of previous CD surgery, extent of gastrointestinal [GI] tract involvement, and complications of CD including penetrating, fistulizing, and stricturing CD), and surgery-related (e.g. length of resection, anastomosis type, and myenteric plexitis in resected specimen) risk factors. Patients should be given smoking-cessation counseling | [54, 55]; smoking [8, 26–33]; disease-related risk factors [36]; surgery-related risk factors [31, 36, 48–51] |
| Low-risk patients may be given a 3-month course of metronidazole. At the 6- to 12-month ileocolonoscopy, if there is evidence of endoscopic recurrence, patients should receive further medical therapy with anti-TNF agents or azathioprine (AZA) or 6-mercaptopurine (6-MP). If there is endoscopic remission, patients may be scheduled for their next ileocolonoscopy in 1–3 years | [54, 55] |
| High-risk patients may be given medical therapy with (i) anti-TNF agents or (ii) a combination of 6-MP or AZA with metronidazole, all after carefully assessing prior medications and treatment failure. If there is endoscopic recurrence at 6–12 months, medical therapy should be modified or intensified. If there is endoscopic remission, the patient should continue their medical therapy and undergo ileocolonoscopy at 1–3 years | [54, 66–74] |
| Updates | |
| There is not enough evidence yet to definitively recommend use of vedolizumab and ustekinumab in the post-operative setting to prevent CD recurrence. However, there is very high interest. These newer biologics are reserved for patients who have failed anti-TNFα therapy or have contraindications to anti-TNFα agents. Further study using randomized–controlled trials comparing these newer biologics with prior biologics would be helpful in determining optimal biologic regimen to prevent post-operative CD recurrence | [75–81] |
| There is limited but promising evidence supporting the use of non-invasive imaging modalities such as ultrasound, computed tomography enterography, and magnetic resonance enterography, in the post-operative setting to monitor for CD recurrence. Current data suggest high sensitivity and specificity for each of these modalities. Further larger studies should be done to evaluate the optimal use of imaging post-operatively | [82–86] |
All patients should be counseled that surgery for CD is not curative, and there is a high chance of both initial endoscopic remission and subsequent recurrence. While many risk factors for recurrence are not modifiable, smoking is a modifiable patient-related risk factor, hence all gastroenterologists should counsel all post-operative CD patients regarding tobacco cessation. As surgery does not cure CD, patients should undergo general health and IBD maintenance including nutritional assessment and screening for metabolic bone disease.
SUMMARY AND RECOMMENDATIONS
Post-operative CD recurrence typically presents on a continuum from histologic findings to endoscopic findings to clinical presentation [1, 9]. This biological reason as well as the POCER trial data underscore the need for early monitoring at 6–12 months post-surgery with ileocolonoscopy, and tailored pharmacologic therapy based on endoscopic findings. There are several risk factors that can help stratify patients as low- or high-risk; high-risk includes patients who smoke tobacco, have had prior CD surgery, or have penetrating disease. Low-risk patients should receive antibiotic therapy usually with metronidazole. High-risk patients should receive anti-TNF therapy, or 6-MP/AZA therapy alongside metronidazole. All patients regardless of risk should undergo their 6- to 12-month postsurgical ileocolonoscopy and adjust their therapy based on evidence of endoscopic recurrence.
Acknowledgements
None.
Contributor Information
Kate E Lee, Department of Medicine, Duke University Medical Center, Durham, NC, USA.
Sarah Cantrell, Duke University Medical Center Library & Archives, Duke University School of Medicine, Durham, NC, USA.
Bo Shen, Center for Inflammatory Bowel Diseases, NewYork-Presbyterian/Columbia University Irving Medical Center, New York, NY, USA.
Adam S Faye, Division of Gastroenterology, NYU Grossman School of Medicine, New York, NY, USA.
Funding
None.
Authors' Contributions
All authors have made substantial contributions to all of the following: (i) the conception and design of the article and interpretation of the relevant literature, (ii) drafting of the article or critical revision of important intellectual content, and (iii) final approval of the version to be submitted.
Conflict of interest statement
The authors report no conflict of interest regarding this work. B.S. has received educational grants and personal fees from Abbvie and Janssen, personal fees from Takeda, and consulting fees from Abbvie, Janssen, and Takeda. A.S.F. has received consulting fees from GLG, M3, Janssen, Guidepoint, and research support from the Crohn’s and Colitis Foundation.
References
- 1. Fumery M, Dulai PS, Meirick P. et al. Systematic review with meta-analysis: recurrence of Crohn's disease after total colectomy with permanent ileostomy. Aliment Pharmacol Ther 2017;45:381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamamoto T, Watanabe T.. Surgery for luminal Crohn's disease. World J Gastroenterol 2014;20:78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nos P, Domenech E.. Postoperative Crohn's disease recurrence: a practical approach. World J Gastroenterol 2008;14:5540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spinelli A, Sacchi M, Fiorino G. et al. Risk of postoperative recurrence and postoperative management of Crohn's disease. World J Gastroenterol 2011;17:3213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernell O, Lapidus A, Hellers G.. Risk factors for surgery and recurrence in 907 patients with primary ileocaecal Crohn's disease. Br J Surg 2000;87:1697–701. [DOI] [PubMed] [Google Scholar]
- 6. Olaison G, Smedh K, Sjodahl R.. Natural course of Crohn's disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut 1992;33:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rutgeerts P, Geboes K, Vantrappen G. et al. Natural history of recurrent Crohn's disease at the ileocolonic anastomosis after curative surgery. Gut 1984;25:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joustra V, Duijvestein M, Mookhoek A. et al. Natural history and risk stratification of recurrent Crohn's disease after ileocolonic resection: a multicenter retrospective cohort study. Inflamm Bowel Dis 2022;28:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghosh S, D'Haens G.. Is an ounce of prevention worth a pound of cure: postoperative recurrence of Crohn's disease? Gastroenterology 2016;150:1521–4. [DOI] [PubMed] [Google Scholar]
- 10. Shen B, Kochhar GS, Navaneethan U. et al. Endoscopic evaluation of surgically altered bowel in inflammatory bowel disease: a consensus guideline from the Global Interventional Inflammatory Bowel Disease Group. Lancet Gastroenterol Hepatol 2021;6:482–97. [DOI] [PubMed] [Google Scholar]
- 11. Fazio VW, Marchetti F, Church M. et al. Effect of resection margins on the recurrence of Crohn's disease in the small bowel: a randomized controlled trial. Ann Surg 1996;224:563–71. discussion 571–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delaney CP, Kiran RP, Senagore AJ. et al. Quality of life improves within 30 days of surgery for Crohn's disease. J Am Coll Surg 2003;196:714–21. [DOI] [PubMed] [Google Scholar]
- 13. Jobanputra S, Weiss EG.. Strictureplasty. Clin Colon Rectal Surg 2007;20:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeejeebhoy KN. Short bowel syndrome: a nutritional and medical approach. CMAJ 2002;166:1297–302. [PMC free article] [PubMed] [Google Scholar]
- 15. Burke JP. Role of fecal diversion in complex Crohn's disease. Clin Colon Rectal Surg 2019;32:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis RT, Maron DJ.. Efficacy and complications of surgery for Crohn's disease. Gastroenterol Hepatol (N Y) 2010;6:587–96. [PMC free article] [PubMed] [Google Scholar]
- 17. Racz JM, Davies W.. Severe stricturing Crohn's disease of the duodenum: a case report and review of surgical options. Int J Surg Case Rep 2012;3:242–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riviere P, Vermeire S, Irles-Depe M. et al. Rates of postoperative recurrence of crohn's disease and effects of immunosuppressive and biologic therapies. Clin Gastroenterol Hepatol 2021;19:713–20.e1. [DOI] [PubMed] [Google Scholar]
- 19. Swoger JM, Regueiro M.. Evaluation for postoperative recurrence of Crohn disease. Gastroenterol Clin North Am 2012;41:303–14. [DOI] [PubMed] [Google Scholar]
- 20. Singh S, Ding NS, Mathis KL. et al. Systematic review with meta-analysis: faecal diversion for management of perianal Crohn's disease. Aliment Pharmacol Ther 2015;42:783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rottoli M, Tanzanu M, Manzo CA. et al. Strictureplasty for Crohn's disease of the small bowel in the biologic era: long-term outcomes and risk factors for recurrence. Tech Coloproctol 2020;24:711–20. [DOI] [PubMed] [Google Scholar]
- 22. Fazi M, Giudici F, Luceri C. et al. Long-term results and recurrence-related risk factors for Crohn disease in patients undergoing side-to-side isoperistaltic strictureplasty. JAMA Surg 2016;151:452–60. [DOI] [PubMed] [Google Scholar]
- 23. Chongthammakun V, Fialho A, Fialho A. et al. Correlation of the Rutgeerts score and recurrence of Crohn's disease in patients with end ileostomy. Gastroenterol Rep (Oxf) 2017;5:271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rutgeerts P, Geboes K, Vantrappen G. et al. Predictability of the postoperative course of Crohn's disease. Gastroenterology 1990;99:956–63. [DOI] [PubMed] [Google Scholar]
- 25. Hammoudi N, Auzolle C, Tran Minh ML. et al. Postoperative endoscopic recurrence on the neoterminal ileum but not on the anastomosis is mainly driving long-term outcomes in Crohn's disease. Am J Gastroenterol 2020;115:1084–93. [DOI] [PubMed] [Google Scholar]
- 26. Reese GE, Nanidis T, Borysiewicz C. et al. The effect of smoking after surgery for Crohn's disease: a meta-analysis of observational studies. Int J Colorectal Dis 2008;23:1213–21. [DOI] [PubMed] [Google Scholar]
- 27. Coletta M, Zefelippo A, Mazza S. et al. Previous colonic resection is a risk factor for surgical relapse in Crohn's disease. Dig Liver Dis 2019;51:206–11. [DOI] [PubMed] [Google Scholar]
- 28. Hammami A, Harbi R, Elleuch N. et al. Predictors of postoperative recurrence in a cohort of Tunisian patients with Crohn's disease. Ther Adv Gastrointest Endosc 2022;15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maggiori L, Brouquet A, Zerbib P. et al. ; GETAID Chirurgie Group. Penetrating Crohn disease is not associated with a higher risk of recurrence after surgery: a prospective nationwide cohort conducted by the GETAID Chirurgie Group. Ann Surg 2019;270:827–34. [DOI] [PubMed] [Google Scholar]
- 30. Orlando A, Mocciaro F, Ventimiglia M. et al. Azathioprine for prevention of clinical recurrence in Crohn's disease patients with severe endoscopic recurrence: an IG-IBD randomized double-blind trial. Eur Rev Med Pharmacol Sci 2020;24:11356–64. [DOI] [PubMed] [Google Scholar]
- 31. Sokol H, Polin V, Lavergne-Slove A. et al. Plexitis as a predictive factor of early postoperative clinical recurrence in Crohn's disease. Gut 2009;58:1218–25. [DOI] [PubMed] [Google Scholar]
- 32. Tang S, Liu W, Qi W. et al. Real-world experience with AGA guidelines in the management of Crohn's disease following ileocolonic resection: a retrospective cohort study. Gastroenterol Res Pract 2020;2020:8618574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamamoto T, Watanabe T.. Strategies for the prevention of postoperative recurrence of Crohn's disease. Colorectal Dis 2013;15:1471–80. [DOI] [PubMed] [Google Scholar]
- 34. Alvarez-Lobos M, Arostegui JI, Sans M. et al. Crohn's disease patients carrying Nod2/CARD15 gene variants have an increased and early need for first surgery due to stricturing disease and higher rate of surgical recurrence. Ann Surg 2005;242:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borowiec AM, Fedorak RN.. Predicting, treating and preventing postoperative recurrence of Crohn's disease: the state of the field. Can J Gastroenterol 2011;25:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vaughn BP, Moss AC.. Prevention of post-operative recurrence of Crohn's disease. World J Gastroenterol 2014;20:1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. El Halabi J, Bs Shah R, Joseph A. et al. Chronologic age is not associated with risk of post-operative recurrence in Crohn’s disease. Presented at: DDW 2022, 21–24 May 2022. [Google Scholar]
- 38. Sachar DB, Wolfson DM, Greenstein AJ. et al. Risk factors for postoperative recurrence of Crohn's disease. Gastroenterology 1983;85:917–21. [PubMed] [Google Scholar]
- 39. Hollis RH, Smith N, Sapci I. et al. Small bowel Crohn's disease recurrence is common after total proctocolectomy for Crohn's colitis. Dis Colon Rectum 2022;65:390–8. [DOI] [PubMed] [Google Scholar]
- 40. Simillis C, Yamamoto T, Reese GE. et al. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn's disease. Am J Gastroenterol 2008;103:196–205. [DOI] [PubMed] [Google Scholar]
- 41. Calabrese E, Petruzziello C, Onali S. et al. Severity of postoperative recurrence in Crohn's disease: correlation between endoscopic and sonographic findings. Inflamm Bowel Dis 2009;15:1635–42. [DOI] [PubMed] [Google Scholar]
- 42. Raab Y, Bergstrom R, Ejerblad S. et al. Factors influencing recurrence in Crohn's disease: an analysis of a consecutive series of 353 patients treated with primary surgery. Dis Colon Rectum 1996;39:918–25. [DOI] [PubMed] [Google Scholar]
- 43. Bernell O, Lapidus A, Hellers G.. Risk factors for surgery and postoperative recurrence in Crohn's disease. Ann Surg 2000;231:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Amiot A, Gornet JM, Baudry C. et al. Crohn's disease recurrence after total proctocolectomy with definitive ileostomy. Dig Liver Dis 2011;43:698–702. [DOI] [PubMed] [Google Scholar]
- 45. Beelen EMJ, Nieboer D, Arkenbosch JHC. et al. Risk prediction and comparative efficacy of Anti-TNF vs thiopurines, for preventing postoperative recurrence in Crohn's disease: a pooled analysis of 6 trials. Clin Gastroenterol Hepatol 2021;S1542-3565(21)01134-4. 10.1016/j.cgh.2021.10.021. [DOI] [PubMed] [Google Scholar]
- 46. Li Y, Ge Y, Gong J. et al. Mesenteric lymphatic vessel density is associated with disease behavior and postoperative recurrence in Crohn's disease. J Gastrointest Surg 2018;22:2125–32. [DOI] [PubMed] [Google Scholar]
- 47. Avidan B, Sakhnini E, Lahat A. et al. Risk factors regarding the need for a second operation in patients with Crohn's disease. Digestion 2005;72:248–53. [DOI] [PubMed] [Google Scholar]
- 48. Decousus S, Boucher AL, Joubert J. et al. Myenteric plexitis is a risk factor for endoscopic and clinical postoperative recurrence after ileocolonic resection in Crohn's disease. Dig Liver Dis 2016;48:753–8. [DOI] [PubMed] [Google Scholar]
- 49. Ferrante M, de Hertogh G, Hlavaty T. et al. The value of myenteric plexitis to predict early postoperative Crohn's disease recurrence. Gastroenterology 2006;130:1595–606. [DOI] [PubMed] [Google Scholar]
- 50. Lemmens B, de Buck van Overstraeten A, Arijs I. et al. Submucosal plexitis as a predictive factor for postoperative endoscopic recurrence in patients with Crohn's disease undergoing a resection with ileocolonic anastomosis: results from a prospective single-centre study. J Crohns Colitis 2017;11:212–20. [DOI] [PubMed] [Google Scholar]
- 51. Bressenot A, Chevaux JB, Williet N. et al. Submucosal plexitis as a predictor of postoperative surgical recurrence in Crohn's disease. Inflamm Bowel Dis 2013;19:1654–61. [DOI] [PubMed] [Google Scholar]
- 52. Scarpa M, Ruffolo C, Bertin E. et al. Surgical predictors of recurrence of Crohn's disease after ileocolonic resection. Int J Colorectal Dis 2007;22:1061–9. [DOI] [PubMed] [Google Scholar]
- 53. Bernell O, Lapidus A, Hellers G.. Recurrence after colectomy in Crohn's colitis. Dis Colon Rectum 2001;44:647–54; discussion 654. [DOI] [PubMed] [Google Scholar]
- 54. De Cruz P, Kamm MA, Hamilton AL. et al. Crohn's disease management after intestinal resection: a randomised trial. Lancet 2015;385:1406–17. [DOI] [PubMed] [Google Scholar]
- 55. Nguyen GC, Loftus EV Jr, Hirano I. et al. ; AGA Institute Clinical Guidelines Committee. American gastroenterological association institute guideline on the management of Crohn's disease after surgical resection. Gastroenterology 2017;152:271–5. [DOI] [PubMed] [Google Scholar]
- 56. Veyre F, Boschetti G, Meunier C. et al. Low levels of fecal calprotectin 3 months after surgery predict subsequent endoscopic postoperative remission in Crohn's disease. Dig Dis Sci 2021;66:4429–35. [DOI] [PubMed] [Google Scholar]
- 57. Lobatón T, López-García A, Rodríguez-Moranta F. et al. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn's disease. J Crohns Colitis 2013;7:e641–51. [DOI] [PubMed] [Google Scholar]
- 58. Boschetti G, Laidet M, Moussata D. et al. Levels of fecal calprotectin are associated with the severity of postoperative endoscopic recurrence in asymptomatic patients with Crohn's disease. Am J Gastroenterol 2015;110:865–72. [DOI] [PubMed] [Google Scholar]
- 59. Vermeire S, Van Assche G, Rutgeerts P.. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis 2004;10:661–5. [DOI] [PubMed] [Google Scholar]
- 60. De Cruz P, Kamm MA, Hamilton AL. et al. Efficacy of thiopurines and adalimumab in preventing Crohn's disease recurrence in high-risk patients: a POCER study analysis. Aliment Pharmacol Ther 2015;42:867–79. [DOI] [PubMed] [Google Scholar]
- 61. Glick LR, Sossenheimer PH, Ollech JE. et al. Low-dose metronidazole is associated with a decreased rate of endoscopic recurrence of Crohn's disease after ileal resection: a retrospective cohort study. J Crohns Colitis 2019;13:1158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. López-Sanromán A, Vera-Mendoza I, Domènech E. et al. ; Spanish GETECCU group [APPRECIA study]. Adalimumab vs azathioprine in the prevention of postoperative Crohn's disease recurrence: a GETECCU randomised trial. J Crohns Colitis 2017;11:1293–301. [DOI] [PubMed] [Google Scholar]
- 63. Thukral C, Travassos WJ, Peppercorn MA.. The role of antibiotics in inflammatory bowel disease. Curr Treat Options Gastroenterol 2005;8:223–8. [DOI] [PubMed] [Google Scholar]
- 64. Regueiro M, Velayos F, Greer JB. et al. American gastroenterological association institute technical review on the management of Crohn's disease after surgical resection. Gastroenterology 2017;152:277–95.e3. [DOI] [PubMed] [Google Scholar]
- 65. Rutgeerts P, Van Assche G, Vermeire S. et al. Ornidazole for prophylaxis of postoperative Crohn's disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2005;128:856–61. [DOI] [PubMed] [Google Scholar]
- 66. D'Haens GR, Vermeire S, Van Assche G. et al. Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn's disease: a controlled randomized trial. Gastroenterology 2008;135:1123–9. [DOI] [PubMed] [Google Scholar]
- 67. Hanauer SB, Korelitz BI, Rutgeerts P. et al. Postoperative maintenance of Crohn's disease remission with 6-mercaptopurine, mesalamine, or placebo: a 2-year trial. Gastroenterology 2004;127:723–9. [DOI] [PubMed] [Google Scholar]
- 68. Ardizzone S, Maconi G, Sampietro GM. et al. Azathioprine and mesalamine for prevention of relapse after conservative surgery for Crohn's disease. Gastroenterology 2004;127:730–40. [DOI] [PubMed] [Google Scholar]
- 69. Herfarth H, Tjaden C, Lukas M. et al. ; Z T-1 Study Group. Adverse events in clinical trials with azathioprine and mesalamine for prevention of postoperative recurrence of Crohn's disease. Gut 2006;55:1525–6. [PMC free article] [PubMed] [Google Scholar]
- 70. Reinisch W, Angelberger S, Petritsch W. et al. ; International AZT-2 Study Group. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn's disease with endoscopic recurrence: efficacy and safety results of a randomised, double-blind, double-dummy, multicentre trial. Gut 2010;59:752–9. [DOI] [PubMed] [Google Scholar]
- 71. Regueiro M, Schraut W, Baidoo L. et al. Infliximab prevents Crohn's disease recurrence after ileal resection. Gastroenterology 2009;136:441–50.e1; quiz 716. [DOI] [PubMed] [Google Scholar]
- 72. Regueiro M, Kip KE, Baidoo L. et al. Postoperative therapy with infliximab prevents long-term Crohn's disease recurrence. Clin Gastroenterol Hepatol 2014;12:1494–502.e1. [DOI] [PubMed] [Google Scholar]
- 73. Regueiro M, Feagan BG, Zou B. et al. ; PREVENT Study Group. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn's disease after ileocolonic resection. Gastroenterology 2016;150:1568–78. [DOI] [PubMed] [Google Scholar]
- 74. Yoshida K, Fukunaga K, Ikeuchi H. et al. Scheduled infliximab monotherapy to prevent recurrence of Crohn's disease following ileocolic or ileal resection: a 3-year prospective randomized open trial. Inflamm Bowel Dis 2012;18:1617–23. [DOI] [PubMed] [Google Scholar]
- 75. Dragoni G, Ding N, Gecse KB. et al. ; Clinical Research Committee (ClinCom) of ECCO and Young ECCO Committee (Y-ECCO). The prevention and management of Crohn's disease postoperative recurrence: results from the Y-ECCO/ClinCom 2019 Survey. Eur J Gastroenterol Hepatol 2020;32:1062–6. [DOI] [PubMed] [Google Scholar]
- 76. Barnes EL, Lightner AL, Regueiro M.. Perioperative and postoperative management of patients with Crohn's disease and ulcerative colitis. Clin Gastroenterol Hepatol 2020;18:1356–66. [DOI] [PubMed] [Google Scholar]
- 77. Yamada A, Komaki Y, Patel N. et al. ; The use of vedolizumab in preventing postoperative recurrence of Crohn's disease. Inflamm Bowel Dis 2018;24:502–9. [DOI] [PubMed] [Google Scholar]
- 78. Manosa M, Fernandez-Clotet A, Nos P. et al. Ustekinumab and vedolizumab for the prevention of postoperative recurrence of Crohn's disease: results from the ENEIDA registry. Dig Liver Dis 2022. 10.1016/j.dld.2022.07.013. [DOI] [PubMed] [Google Scholar]
- 79. Canete F, Manosa M, Casanova MJ. et al. ; ENEIDA registry by GETECCU. Adalimumab or infliximab for the prevention of early postoperative recurrence of Crohn disease: results from the ENEIDA registry. Inflamm Bowel Dis 2019;25:1862–70. [DOI] [PubMed] [Google Scholar]
- 80. Yanai H, Kagramanova A, Knyazev O. et al. Endoscopic postoperative recurrence in Crohn's disease after curative ileocecal resection with early prophylaxis by Anti-Tnf, Vedolizumab or Ustekinumab: a real-world multicenter European study. J Crohns Colitis 2022. 10.1093/ecco-jcc/jjac100. [DOI] [PubMed] [Google Scholar]
- 81. Buisson A, Nancey S, Manlay L. et al. ; USTEK Post-Op Study Group. Ustekinumab is more effective than azathioprine to prevent endoscopic postoperative recurrence in Crohn's disease. United European Gastroenterol J 2021;9:552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rispo A, Imperatore N, Testa A. et al. Diagnostic accuracy of ultrasonography in the detection of postsurgical recurrence in Crohn's disease: a systematic review with meta-analysis. Inflamm Bowel Dis 2018;24:977–88. [DOI] [PubMed] [Google Scholar]
- 83. Calabrese E, Maaser C, Zorzi F. et al. Bowel ultrasonography in the management of Crohn's disease: a review with recommendations of an international panel of experts. Inflamm Bowel Dis 2016;22:1168–83. [DOI] [PubMed] [Google Scholar]
- 84. Yung DE, Har-Noy O, Tham YS. et al. Capsule endoscopy, magnetic resonance enterography, and small bowel ultrasound for evaluation of postoperative recurrence in Crohn's disease: systematic review and meta-analysis. Inflamm Bowel Dis 2017;24:93–100. 10.1093/ibd/izx027. [DOI] [PubMed] [Google Scholar]
- 85. Choi IY, Park SH, Park SH. et al. CT enterography for surveillance of anastomotic recurrence within 12 months of bowel resection in patients with Crohn's disease: an observational study using an 8-year registry. Korean J Radiol 2017;18:906–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mao R, Gao X, Zhu ZH. et al. CT enterography in evaluating postoperative recurrence of Crohn's disease after ileocolic resection: complementary role to endoscopy. Inflamm Bowel Dis 2013;19:977–82. [DOI] [PubMed] [Google Scholar]
- 87. Lopez J, Konijeti GG, Nguyen DD. et al. Natural history of Crohn's disease following total colectomy and end ileostomy. Inflamm Bowel Dis 2014;20:1236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lightner AL, Steele SR, Delaney CP. et al. Colonic disease recurrence following proctectomy with end colostomy for anorectal Crohn's disease. Colorectal Dis 2021;23:2425–35. [DOI] [PubMed] [Google Scholar]
- 89. Ng SC, Lied GA, Arebi N. et al. Clinical and surgical recurrence of Crohn's disease after ileocolonic resection in a specialist unit. Eur J Gastroenterol Hepatol 2009;21:551–7. [DOI] [PubMed] [Google Scholar]
- 90. Ponsioen CY, de Groof EJ, Eshuis EJ. et al. ; LIR!C study group. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn's disease: a randomised controlled, open-label, multicentre trial. Lancet Gastroenterol Hepatol 2017;2:785–92. [DOI] [PubMed] [Google Scholar]