Abstract
This study evaluated the reinforcing effects of fentanyl, alone or in combination with the benzodiazepine alprazolam, in rhesus monkeys (3 females, 3 males). Subjects were trained to self-administer the opioid remifentanil (0.3 µg/kg/injection) under a progressive-ratio schedule of reinforcement. The reinforcing effects of fentanyl (0.1–10 µg/kg/injection) or alprazolam (1.0–100 µg/kg/injection) alone, or in combinations of fixed proportions (1:1, 1:3, and 3:1 fentanyl:alprazolam, with 1:1 based on the potencies of drugs alone) were evaluated in single-day test sessions (with double determinations). Dose-equivalence analysis was used to determine the extent to which fentanyl and alprazolam combinations differed from additivity. Fentanyl functioned as a positive reinforcer in all monkeys, while alprazolam was a reinforcer in 3 of 6 monkeys only. Therefore, drug combination data were grouped as “alprazolam-taking” and “non-alprazolam-taking” monkeys. For alprazolam-taking monkeys, we observed additive effects for the 3:1 and 1:3 combinations, and a significant supra-additive interaction for the 1:1 combination of fentanyl and alprazolam. For 2 of the 3 non-alprazolam-taking monkeys, the combination of fentanyl and alprazolam resulted in enhanced reinforcing effects relative to either drug alone. However, the one monkey showed primarily inhibitory, or suppressive effects, with the 3:1 dose combination resulting in a relatively modest rightward shift in the fentanyl dose-response function. In summary, our findings show that combining fentanyl and alprazolam generally result in proportion-dependent additive or supra-additive enhancements. These data raise the possibility that the prevalence of opioid-benzodiazepine polydrug abuse may reflect a unique enhancement of these drugs’ reinforcing effects, although individual differences may exist.
SIGNIFICANCE STATEMENT
Addressing the critical question of the degree to which benzodiazepines can modulate the abuse-related effects of opioids may provide improved pathways to treatment of this common form of polydrug addiction. In the present study, we show that combinations of the opioid fentanyl and the benzodiazepine alprazolam can be more reinforcing than either drug alone in a rhesus monkey model, suggesting that enhancement of reinforcement processes may underlie this prevalent form of polydrug use disorder.
Introduction
The co-abuse of opioids and benzodiazepines has been recognized since the 1970s, and recent studies show that use of benzodiazepines continues to be prevalent in patients with opioid use disorder (OUD; Brands, et al., 2008; Lavie et al., 2009; Stein et al., 2016). In the U.S., nearly 70% of patients with OUD report lifetime use of illicit benzodiazepines (Votaw et al., 2019), while up to 17% of patients with tranquilizer (predominantly benzodiazepine) misuse also report opioid co-abuse (Votaw et al., 2020). In addition, up to 20% of individuals prescribed a benzodiazepine or benzodiazepine-type drug (e.g., zolpidem) for an extended period in the U.S. are co-prescribed an opioid (Moore and Mattison, 2017). Alarmingly, non-medical use of benzodiazepines is associated with greater negative consequences of drug use compared with sedative use as prescribed among heroin users (Moses et al., 2018; Moses and Greenwald, 2019). In fact, overdose deaths due to concurrent opioid and benzodiazepine use have increased at least 2-fold since 1999 (Paulozzi et al., 2015), and for 2015–2016, young adults were the most likely of all age groups to engage in opioid and benzodiazepine misuse (Schepis et al., 2018). According to the most recent data, nearly 93% of benzodiazepine-involved deaths between January and June 2020 also involved opioids, with close to 67% of those involving illicitly manufactured fentanyl-type drugs (Liu et al., 2021).
Many factors influence the rise in opioid-benzodiazepine co-abuse. Stein and colleagues (2016) have shown that among patients with OUD seeking detoxification, 40% of surveyed individuals used a benzodiazepine in the month prior to admission, and 25% of these met criteria for benzodiazepine use disorder. More importantly, the reason for benzodiazepine use was significantly associated with the benzodiazepine source. For example, prescription benzodiazepine use was more likely to be reported to manage anxiety (Stein et al., 2016), and the frequency of illicit benzodiazepine use has been associated with significantly higher anxiety sensitivity (McHugh et al., 2017). On the other hand, individuals reporting buying benzodiazepines from illicit sources are more likely to report using benzodiazepines to get or enhance feeling “high” (Stein et al., 2016), suggesting that benzodiazepines may enhance the abuse-related effects of opioids.
While animal models can address these questions, relatively few laboratory studies have investigated this phenomenon systematically. Weed et al. (2017) found that in a concurrent choice procedure, rhesus monkeys preferred intravenous (i.v.) injections of the combination of the opioid remifentanil and the benzodiazepine midazolam over injections of remifentanil alone. Ator et al. (2005) also showed that chronic oral administration of the opioid agonist methadone enhanced the reinforcing effects of the benzodiazepine flunitrazepam in baboons. Together, these findings further corroborate the idea that opioids when combined with benzodiazepines may be more reinforcing relative to either drug alone, which may contribute to the prevalence of this type of polydrug abuse in patients with OUD.
The aim of the present study was to evaluate the reinforcing effects of alprazolam, alone and in combination with the opioid fentanyl, in opioid-experienced rhesus monkeys. Alprazolam (Xanax) is the most widely prescribed benzodiazepine in the U.S. (Moore and Mattison, 2017), and fentanyl-alprazolam combinations are used often by drug users (Kuczyńska et al., 2018). We used a progressive-ratio (PR) schedule of reinforcement, which has been employed previously to study benzodiazepine and opioid reinforcement (e.g., Woolverton et al., 2008a; Berro and Rowlett, 2020) and allows for the direct measurement of both potency and strength as a reinforcer of drugs alone or combined as a mixture (e.g., Griffiths et al., 1975; Rowlett et al., 1996). We have used this approach previously to quantify the reinforcing effects of drug combinations from different classes (e.g., Rowlett and Woolverton, 1997; Rowlett et al., 2007; Fischer and Rowlett 2011). Dose equivalence analysis (Grabovsky and Tallarida, 2004; Woolverton et al., 2008b; Minervini et al., 2018) was used to evaluate the extent to which self-administration of fentanyl-alprazolam combinations were additive, supra-additive, or infra-additive.
Material and Methods
Subjects and Surgery
Three adult male and three adult female rhesus monkeys (Macaca mulatta) ranging from 8–14 kg were chosen as subjects for the study. The three females were experimentally naïve prior to the initiation of the studies, and the three males had a previous history of opioid/benzodiazepine self-administration (Berro and Rowlett, 2020). Subjects were housed individually with access to chew toys and a mirror in their cage, and had visual, auditory and olfactory contact with other monkeys throughout the duration of the study. The room was maintained on a 12 h light/12 h dark cycle (lights on at 0600 hours) at a temperature of 21±1°C, with water available ad libitum. Monkeys were weighed every other week and given physical examinations. The amount of chow for each monkey was determined in consultation with veterinary staff to be that which maintains healthy weights in rhesus monkeys. Because sessions could last more than 10 hours, animals were fed in the morning before the beginning of their self-administration sessions to avoid any feeding influences on self-administration behavior. In addition to chow, monkeys were given fruit/forage twice a day, in the morning and afternoon, with the afternoon fruit potentially being given during the self-administration session if the session was still ongoing (see Self-Administration Training and Testing for further details on session duration).
The monkeys were prepared with a chronic indwelling venous catheter (femoral, brachial, or jugular vein) according to the procedures described by Platt et al. (2011). The catheter was protected by a stainless steel tether and cloth jacket (Lomir Biomedical, Inc., Malone, NY, USA) and flushed daily with heparinized saline (100–150 U/ml). All procedures and animal maintenance was in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011), with review and approval via the Institutional Animal Care and Use Committees of the University of Mississippi Medical Center.
Drugs
Remifentanil hydrochloride (ChemPartner Co., Ltd., Shangai, China) and fentanyl citrate (Sigma-Aldrich, St Louis, MO) were prepared using 0.9% saline. Alprazolam (Sigma-Aldrich, St Louis, MO) was prepared in 100% propylene glycol and diluted using 25% propylene glycol and sterile water solutions. Fentanyl-alprazolam combinations were prepared from a fentanyl stock prepared in saline and an alprazolam stock prepared in 100% propylene glycol, and diluted using 25% propylene glycol and sterile water solutions. New remifentanil solutions were prepared every 7 days, and fentanyl, alprazolam, and fentanyl-alprazolam solutions were used for up to 30 days before a new solution and stock were prepared.
Self-Administration Training and Testing
Drug self-administration training and testing were conducted as previously described (Berro and Rowlett, 2020). Sessions occurred in each monkey’s home cage, which was custom-modified for these studies (Carter2 Systems, Beaverton, OR). In this apparatus, the self-administration panel is inserted into one side of the cage. Self-administration sessions (Monday through Friday) started at 0900 h, and access to the room was restricted to research staff. Monkeys were given the opportunity to self-administer the opioid remifentanil (0.3 μg/kg/injection) under a PR schedule of reinforcement. Remifentanil was chosen as the training drug because it maintains robust i.v. self-administration in rhesus monkeys under conditions used in this study (Woolverton et al., 2008b; Berro and Rowlett, 2020) and has an “ultra-short” elimination half-life, which minimizes the likelihood of physical dependence as a potential experimental factor (Stroumpos et al., 2010).
At the beginning of each session, one white stimulus light above a response lever was illuminated (Med Associates, St. Albans, VT). Upon completion of a response requirement, the white light was extinguished, and a red stimulus light was illuminated for 3 seconds, coinciding with a 3-second infusion. All sessions consisted of five components made up of four trials each. Each trial ended with either an injection or the expiration of a 30-minute limited hold, and trials were separated by a 30-minute timeout period. The response requirement remained constant for each of the four trials within a component and doubled during each successive component. The session ended when a monkey self-administered a maximum of 20 injections or when the response requirement was not completed for two consecutive trials. The PR schedule consisted of a sequence of response requirements: either 60, 120, 240, 480, and 960 (subject M-98-007) or 100, 200, 400, 800, and 1600 (all other subjects) responses per injection. Once performance was stable under these conditions (i.e., no increasing or decreasing trends in the number of injections per session for three consecutive sessions), remifentanil or vehicle (saline) was made available on alternating days. Alternating self-administration sessions for remifentanil and vehicle (saline) were conducted until a consistent difference between drug and saline self-administration was obtained (number of injections during saline sessions ≤ 35% of remifentanil sessions). The test phase began immediately after reaching these criteria.
Test sessions (T) were added to the alternating sequence of remifentanil (R) and saline (S) sessions according to the following sequence: RTXST or STXRT, in which X represents a random R or S day or a physical exam day (during which experiments were not conducted). Our studies followed the general design of (1) determination of dose-response functions to calculate potencies (i.e., ED50 values; described below) for alprazolam and fentanyl alone, then (2) compared self-administration of alprazolam-fentanyl combinations to self-administration of the ligands alone. During test (T) sessions, a dose of fentanyl or alprazolam alone was made available in random order and alternating with its vehicle. Doses of fentanyl (0.1–1.0 μg/kg/injection) and alprazolam (10.0–100.0 μg/kg/injection) were chosen based on their ability to maintain self-administration in rhesus monkeys in preliminary studies from our laboratory. Potencies (ED50 values) for fentanyl and alprazolam were estimated using linear regression analysis. ED50s for drugs alone were then averaged to determine means and 95% confidence limits. To assess the effects of combining alprazolam with fentanyl, dose-response functions for fentanyl were re-determined mixed with proportional doses of alprazolam (estimated fixed proportions of fentanyl:alprazolam for combination tests: 3:1, 1:1 and 1:3, with 1:1 being based on the ED50 values for each drug). For instance, if the ED50 of fentanyl was X and the ED50 for alprazolam was Y, the “relative potency” between fentanyl and alprazolam was calculated as Y/X. A dose-range for fentanyl was then determined based on the fentanyl dose-response function, and each dose of alprazolam in each combination was determined as follows: 1:1 ratio: dose of alprazolam = dose of fentanyl * relative potency; 3:1 ratio: dose of alprazolam = (dose of fentanyl * relative potency)/3; 1:3 ratio: dose of alprazolam = (dose of fentanyl * relative potency)*3. For subjects who did not self-administer alprazolam alone (and, therefore, an ED50 for alprazolam could not be determined), doses determined for the other subjects from the same sex were used for drug combination studies. On intervening (non-test) days, sessions alternated between saline and remifentanil. Each test condition (each dose of fentanyl, alprazolam and combinations, plus vehicles) was determined at least twice, once after a saline maintenance day, and once after a remifentanil maintenance day, and results of the two determinations for each test condition were averaged together for analysis.
Determinations of dose-response functions for fentanyl and alprazolam alone were repeated after completion of all drug combination test conditions to control for baseline changes, as changes in baseline opioid self-administration have been reported in previous combination studies (Rowlett and Woolverton, 1997).
Data Analysis
Because of individual variability in the peak dose, for fentanyl and alprazolam, when the drug was self-administered above vehicle levels, the maximally effective unit dose of each drug (EDMax, i.e., the unit dose that engendered highest number of injections/session) and one-half log-step unit dose below (−0.5 EDMax) and above (+0.5 EDMax) the EDMax dose were identified. The injections/session data were analyzed by one-way repeated-measures analysis of variance (ANOVA) with dose as the within-subject factor. A dose of drug was determined to be self-administered significantly above vehicle levels by comparing mean injections/session for each dose to the corresponding vehicle control value (Bonferroni t test, alpha level equal to P ≤ 0.05).
Potency (dose engendering 50% of the maximum effect, ED50) for fentanyl, alprazolam, and each of the combinations was estimated using linear regression analysis for individual monkey’s dose-response functions. Because only N = 3 subjects self-administered alprazolam above vehicle levels, drug combination data were grouped as alprazolam-taking (N = 3, 2 males, 1 female) and non-alprazolam-taking monkeys (N = 3, 1 male, 2 females). To adjust for individual variability in potencies, “dose ratios” also were computed, in which the ED50 values for the combination were divided by the ED50 for fentanyl alone for each monkey. For those analyses, ED50s for drugs alone and drug combinations were compared using mixed-effects model repeated measures ANOVA, with Dunnett’s tests for comparing ED50s of drug combinations to the ED50s of fentanyl alone.
Individually, fentanyl and alprazolam produced different maximum effects in mean number of injections/session across all monkeys, including the alprazolam-taking monkeys (see Fig. 2). To account for differences in maximum effect, the combinations of fentanyl and alprazolam were examined using dose-equivalence analyses as described previously (Grabovsky and Tallarida 2004; Tallarida 2006; Tallarida 2011; Woolverton et al., 2008b). These analyses were conducted in the alprazolam-taking monkeys only, since ED50s and slope values were required for alprazolam alone for the analysis. Briefly, for each alprazolam-taking monkey, data were converted to the percent of each individual’s maximum number of injections/session for fentanyl, and then a regression line was fit to the linear portion of the dose-response function, which encompassed doses ranging from <20% to >80% of the maximum effect for each drug. The dose of alprazolam (b) in the mixture was converted to fentanyl dose (a) equivalence [represented as “beq(a)”] according to the following equation:
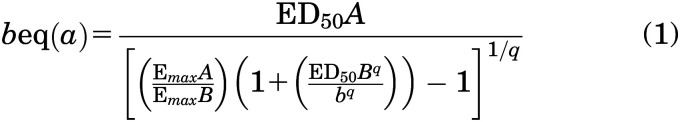 |
where ED50A and ED50B were the potencies of fentanyl and alprazolam alone, respectively; EmaxA and EmaxB were maximum effect levels for fentanyl and alprazolam alone, respectively; and q was the slope derived from the linear regression analyses of fentanyl.
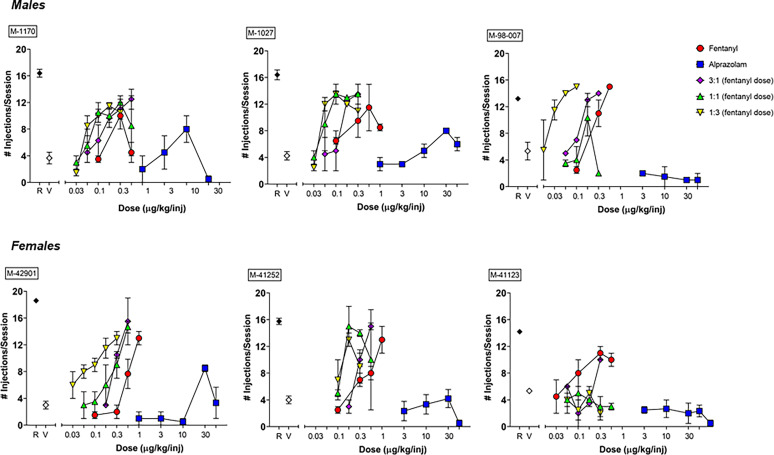
Fig. 2.
Individual subject data (top panel: males; bottom panel: females) showing self-administration of fentanyl, alprazolam and 3 different ratios of fentanyl-alprazolam combinations (1:1, 1:3 and 3:1 fentanyl:alprazolam, with 1:1 being based on the ED50s of drugs alone). Monkeys were trained under a progressive-ratio schedule of i.v. remifentanil (R, 0.3 μg/kg/injection) injection, and self-administration sessions alternating remifentanil and its vehicle (saline) were conducted until a consistent difference between drug and saline self-administration was obtained. Once self-administration was stable, test sessions with fentanyl, alprazolam or fentanyl-alprazolam combinations and their vehicle (V) were added to an alternating sequence of remifentanil and saline sessions. For drug combination tests, data are plotted as a function of the fentanyl dose included in each combination. Data are represented as mean number of injections/session ± S.E.M., out of a total of 20 injections available in a daily session. Note that symbols obscure error bars in some instances.
The total additive dose of fentanyl plus alprazolam in fentanyl equivalence was calculated using the following equation:
and this value, along with the slope for the respective alprazolam function (p), was used to determine the predicted effects for individual subjects using the following equation:
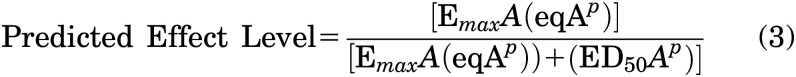 |
To compare predicted effects to observed effects, we used the approach of Minervini et al. (2018), in which linear regressions were fit to all data (i.e., from all subjects not averaged) between the largest dose that produced <20% and the smallest dose that produced >80% of the predicted and observed effects. To determine deviations from additivity, we used a best-fit approach, in which we compared slope and y-intercept for a single function (i.e., additivity) to fit both data sets versus two functions (one for predicted, one for observed) using an extra sum-of-squares F test, with the simpler model chosen based on P > 0.05. If the F test was not significant, then a single function described the two data sets, and the conclusion was an additive effect. If two functions achieved the best fit, supra-additive effects were concluded if the slope for the observed values was less than predicted values, whereas infra-additive effects were concluded if the slope for observed values was greater than predicted values (Grabovsky and Tallarida 2004; Minervini et al., 2018). All analyses were conducted using GraphPad Prism (GraphPad Software, Inc., Release 9.1.2., La Jolla, CA).
Results
Fentanyl and Alprazolam Dose-Response Functions.
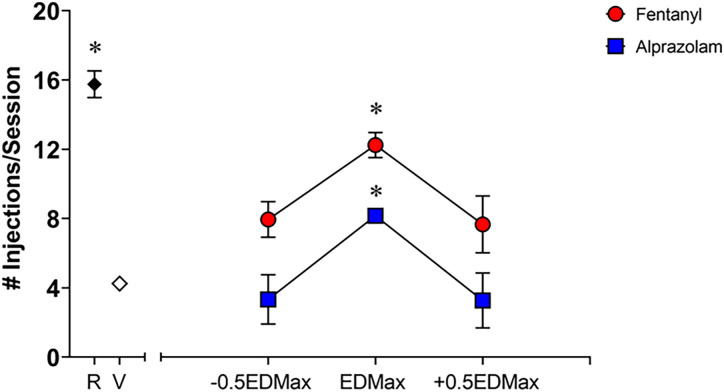
Under remifentanil maintenance conditions, the dose of 0.3 μg/kg/injection maintained an average of 15.76 injections/session (S.E.M. = 0.77), whereas saline availability resulted in an average of 4.3 injections/session (S.E.M. = 0.37). No substantial changes in baseline (maintenance) responding occurred during the ∼1.5-year duration of this study. Fig. 1 illustrates the average number of injections per session for fentanyl and alprazolam, as a function of EDMax ± 0.5EDMax. Fentanyl was self-administered significantly above vehicle levels in all six subjects. The EDMax dose for fentanyl varied between subjects, with the dose of 0.3 μg/kg/injection being the EDMax dose for two subjects, the dose of 0.56 μg/kg/injection being the EDMax for two subjects, and the dose of 1.0 μg/kg/injection being the EDMax dose for two remaining subjects. Due to concerns with using high doses of fentanyl during self-administration sessions, we capped our fentanyl dose at 1.0 μg/kg/injection. Therefore, some of the subjects were not assigned a +0.5EDMax dose. Alprazolam, on the other hand, was self-administered significantly above vehicle levels in only three subjects. The EDMax dose for alprazolam also varied between subjects, with the dose of 10.0 μg/kg/injection being the EDMax dose for one subject, and the dose of 30.0 μg/kg/injection being the EDMax for the other two subjects. Grouped data show that remifentanil, the EDMax dose of fentanyl, and the EDMax dose of alprazolam maintained a mean number of injections/session that was significantly above vehicle (Fig. 1, [F (7, 23) = 25.8, P < 0.0001], Bonferroni t tests versus vehicle, P < 0.05).
Fig. 1.
Self-administration of fentanyl and alprazolam by rhesus monkeys (N = 6, 3 males, 3 females) trained under a progressive-ratio schedule of i.v. remifentanil (R, 0.3 μg/kg/injection) injection. Self-administration maintenance sessions alternating remifentanil and its vehicle (saline) were conducted until a consistent difference between drug and saline self-administration was obtained. Once self-administration was stable, test sessions with fentanyl or alprazolam and their vehicle (V) were added to an alternating sequence of remifentanil and saline sessions. Grouped data are expressed as the unit dose that engendered highest number of injections/session (EDMax) and one-half log-step unit dose below (−0.5 EDMax) and above (+0.5 EDMax) the EDMax dose. Data are represented as mean number of injections/session ± S.E.M., out of a total of 20 injections available in a daily session. Note that symbols obscure error bars in some instances. *P < 0.05 versus vehicle (V) (Bonferroni t tests).
Fentanyl-Alprazolam Combinations.
Individual subject data for the number of injections/session are shown in Fig. 2, with data for fentanyl-alprazolam combinations being plotted as a function of the fentanyl dose. For the majority of the subjects (M-1170, M-1027, M-98-007, M-42901, and M-41252), combining alprazolam with fentanyl shifted the fentanyl dose-response function to the left for at least one of the fentanyl:alprazolam proportions tested (3:1, 1:1, and 1:3, with 1:1 being based on the ED50 values for each drug alone). For one of the subjects, however, combining alprazolam with fentanyl shifted the fentanyl dose-response function mostly down (M-41123). Because only three subjects self-administered alprazolam above vehicle levels, for statistical analyses, the data were grouped as alprazolam-taking (M-1170, M-1027 and M-42901) and non-alprazolam-taking monkeys (M-98-007, M-41123, and M-41252).
Analysis of ED50 values for the combinations compared with fentanyl alone are shown in Table 1. For both alprazolam-taking and non-alprazolam-taking monkeys, no significant effects of fentanyl alone versus combinations was evident for the raw ED50 values (ANOVA, p’s> 0.05). For the dose-ratio analysis, mixed effects ANOVAs revealed significant effects for alprazolam-taking monkeys, consistent with the combinations increasing potencies relative to fentanyl alone [F(3,6) = 19.09, P = 0.032]. Non-alprazolam-taking monkeys showed a mixture of an apparent increase in dose ratio (3:1) and decreases in dose-ratios similar in magnitude with the alprazolam-taking monkeys, but the overall analysis for non-alprazolam-taking monkeys was not significant [F(3,6) = 1.88, P = 0.33]. No additional analyses for non-alprazolam-taking monkeys were conducted due to N = 2 for the 1:1 and 1:3 combinations. For the alprazolam-taking monkeys, Dunnett’s tests revealed significantly lower average ratios for the 1:1 and 1:3 ratios versus fentanyl, consistent with significant increases in potency relative to fentanyl alone (Table 1).
TABLE 1.
Potencies, expressed as ED50 or dose ratio (ED50 of combination of fentanyl and alprazolam/ED50 of fentanyl alone), in rhesus monkeys responding under a progressive-ratio schedule (N=6).
| ED50 (μg/kg/injection) | Dose Ratio (Combination ED50/Fentanyl Alone ED50) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alprazolam-Taking (N=3)a | ||||||||||||
| M-1170b | M-1027 | M-42901 | mean | S.E.M. | M-1170 | M-1027 | M-42901 | mean | S.E.M. | |||
| Fentanyl alone | 0.17 | 0.22 | 0.55 | 0.31 | 0.12 | 1.00 | 1.00 | 1.00 | 1.00 | 0.00 | ||
| 3:1 | 0.13 | 0.14 | 0.28 | 0.18 | 0.05 | 0.76 | 0.64 | 0.51 | 0.64 | 0.07 | ||
| 1:1 | 0.071 | 0.036 | 0.25 | 0.12 | 0.07 | 0.42 | 0.16 | 0.45 | 0.35*,c | 0.09 | ||
| 1:3 | 0.044 | 0.012 | 0.25 | 0.10 | 0.07 | 0.26 | 0.05 | 0.45 | 0.26* | 0.12 | ||
| Non-Alprazolam-Taking (N=3) | ||||||||||||
| M-98-007 | M-41123 | M-42901 | mean | S.E.M. | M-98-007 | M-41123 | M-42901 | mean | S.E.M. | |||
| Fentanyl alone | 0.20 | 0.092 | 0.38 | 0.22 | 0.08 | 1.00 | 1.00 | 1.00 | 1.00 | 0.00 | ||
| 3:1 | 0.12 | 0.22 | 0.28 | 0.21 | 0.05 | 0.60 | 2.39 | 0.74 | 1.24 | 0.58 | ||
| 1:1 | 0.026 | –d | 0.13 | 0.08 | 0.05 | 0.13 | – | 0.13 | 0.13 | 0.00 | ||
| 1:3 | 0.072 | – | 0.38 | 0.23 | 0.15 | 0.36 | – | 0.38 | 0.37 | 0.01 | ||
aPotencies are grouped into monkeys that self-administered alprazolam alone above vehicle levels (“Alprazolam-Taking”) and those that did not (“Non-Alprazolam-Taking”).
bMonkey identification numbers.
cNote that *P < 0.05 versus Fentanyl alone, Dunnett’s test.
dDashes indicate potency could not be calculated due to no significant self-administration above vehicle levels.
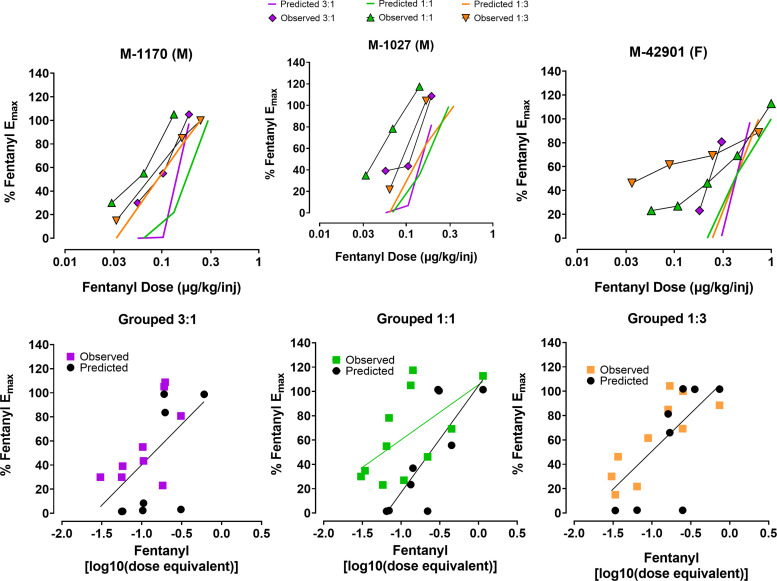
A more in-depth analysis of changes in potency associated with combining fentanyl and alprazolam was conducted using dose-equivalence analysis (Grabovsky and Tallarida 2004; Woolverton et al., 2008b; Minervini et al., 2018). This analysis involved calculating fentanyl dose equivalence values based on predicted effects of the combination and statistically comparing predicted versus observed values via linear regression. As noted above, this requires potency and slope values for both drugs in the combination, restricting the analysis to the alprazolam-taking monkeys only. The top row of Fig. 3 shows predicted (lines only) and observed (lines and symbols) for the three alprazolam-taking monkeys, and in the majority of cases, the observed functions are shifted rightward and upward, or mostly rightward, relative to predicted functions for all monkeys. The bottom row of Fig. 3 plots all data for the respective conditions as a function of log fentanyl equivalent doses and tests whether the data were best described by a single linear function or two linear functions. The equations and results of best-fit F-tests are shown in Table 2, and for the 1:1 combination, the observed values were statistically different from the predicted values. Because the slope was lower for observed versus predicted, the conclusion is a supra-additive interaction. For the other two combinations, the observed and predicted values were not different (i.e., a single function fit both data sets), indicating additive effects for 3:1 and 1:3 combinations (Table 2).
Fig. 3.
The predicted additive effects and the observed (empirically determined) effects for self-administration of fentanyl:alprazolam combinations in rhesus monkeys that self-administered alprazolam alone under a progressive-ratio procedure. Top graphs: Individual subject graphs with monkey identification (and sex in parentheses) at the top of each panel. Bottom graphs: Regression analyses for the 3:1, 1:1, and 1:3 combinations, shown in individual panels. Data are individual observed and predicted points for each monkey, not averaged. Regression lines represent the best-fit analysis for either 2 linear functions for observed versus predicted, or a single function as the default if 2 functions did not fit significantly (see Table 2 for statistics).
TABLE 2.
Dose equivalence analysis of predicted versus observed effects of fentanyl-alprazolam combinations in rhesus monkeys that self-administered alprazolam (“alprazolam-taking subjects”, N=3).
| Parameters | Test of Fit for 2 Linear Functions | |||||
|---|---|---|---|---|---|---|
| Ratio | Best Fit Model | Equation | F (df) | p | Goodness-of-fit (R2) | |
| 3:1 | One Function | y = 67.2x + 107.5 | 2.08 (2, 13) | 0.16 | 0.31 | |
| 1:1 | Two Functions | Observed | y = 45.3x + 105.5 | 3.72 (2, 16) | 0.047 | 0.30 |
| Predicted | y = 87.9x + 104.7 | 0.62 | ||||
| 1:3 | One Function | y = 62.3x + 113.1 | 1.51 (2, 14) | 0.26 | 0.50 | |
Ratio refers to proportions of fentanyl:alprazolam.
Equation refers to linear regression in the form of y = slope * x + y-intercept, where y = % of each monkey’s maximum number of injections/session and x = log10 (fentanyl dose equivalent). Note that the y-intercept is the value of y when log(x) = 0.
Redetermination of Fentanyl and Alprazolam Dose-Response Functions.
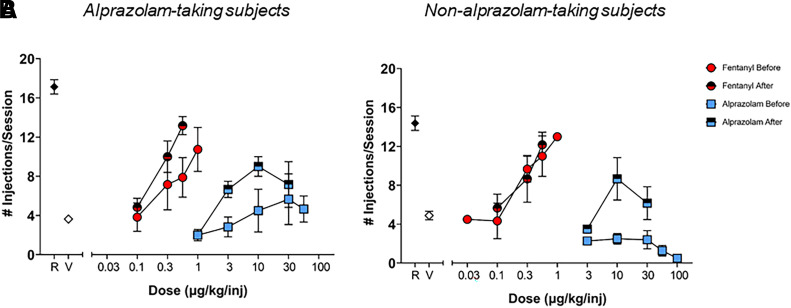
To control for baseline changes, determinations of dose-response functions for fentanyl and alprazolam alone were repeated after completion of all drug combination test conditions. Dose-response functions before and after drug combination tests were grouped for alprazolam-taking and non-alprazolam-taking subjects, and are shown in Fig. 4. For the three alprazolam-taking monkeys, the initial determination of fentanyl and alprazolam resulted in average ED50 values (± 95% confidence interval) for fentanyl and alprazolam of 0.31 μg/kg/injection (0.08–0.55) and 12.0 μg/kg/injection (2.1–22.0), respectively. The redetermination resulted in similar average ED50 values for fentanyl and alprazolam of 0.26 μg/kg/injection (0.11–0.37) and 7.5 μg/kg/injection (1.1–13.8), respectively. No significant differences were observed between the ED50 values for the original dose-response function versus the redetermination (fentanyl: t(2) = 0.3705, P = 0.7466; alprazolam: t(2) = 0.8248, P = 0.4962) in alprazolam-taking monkeys.
Fig. 4.
Grouped dose-response functions before and after drug combination tests for alprazolam-taking subjects (A) and non-alprazolam-taking subjects (B). Data are mean number of injections/session ± S.E.M., from at least 2 determinations. Note that symbols obscure error bars in some instances. R = remifentanil maintenance sessions; V = vehicle maintenance sessions.
The initially-determined average ED50 value (±95% confidence interval) of fentanyl for the three non-alprazolam-taking monkeys was 0.27 μg/kg/injection (0.058 – 0.38), with the re-determined ED50 value of 0.30 μg/kg/injection (0.11–0.48). Again, no significant differences were observed between the ED50 values for the original fentanyl dose-response function versus the redetermination (fentanyl: t(2) = 0.5032, P = 0.6647) in non-alprazolam-taking monkeys. Interestingly, while two of those subjects (M-41123 and M-41252) continued to not self-administer alprazolam during redetermination tests, one subject (M-98-007) did reliably self-administer alprazolam above vehicle levels after fentanyl-alprazolam combination tests, showing an ED50 of 5.5 μg/kg/injection.
Discussion
The negative consequences of opioid-benzodiazepine co-abuse are clear (Moses et al., 2018; Moses and Greenwald, 2019). Yet, the determining factors that give rise to this form of polydrug abuse are understood poorly. In the present study, we investigated the reinforcing effects of fentanyl and alprazolam, alone and in combination, in opioid-experienced rhesus monkeys using a PR self-administration procedure. Alprazolam was chosen for this study given its high degree of co-abuse with fentanyl (Kuczyńska et al., 2018) and because it is one of the most widely prescribed and misused benzodiazepines (Moore and Mattison, 2017; Ait-Daoud et al., 2018).
Alprazolam has a distinct pharmacokinetic and pharmacodynamic profile that may promote its abuse potential, including rapid absorption, relatively short half-life, and a putatively unique potentiating action at dopaminergic neurons in the striatum compared with other benzodiazepines (for review, see Ait-Daoud et al., 2018). In the present study, however, alprazolam appeared to have lower reinforcing effectiveness than other benzodiazepines. In this regard, only three of the six monkeys self-administered alprazolam above vehicle levels, which is in contrast to our prior findings of benzodiazepine-type positive modulators consistently self-administered in all subjects (e.g., Fischer and Rowlett, 2011; Shinday et al., 2013; Berro and Rowlett, 2020). The factors underlying this finding are unknown, although alprazolam demonstrates plasma level/pharmacological effect hysteresis in healthy human volunteers when administered intravenously, i.e., a delay is observed between peak drug serum concentrations and peak drug effect (Venkatakrishnan et al., 2005). This property of alprazolam (presumably a delay to reinforcing effects following administration) has been shown to influence its reinforcing effects in rats (Lau and Heatherington, 1997) and may have influenced alprazolam self-administration in the present study. In addition, rodent studies have shown that chronic treatment with morphine (Sim et al., 1996) and a history of heroin self-administration (Sim-Selley et al., 2000) decreased mu opioid receptor functionality. Therefore, a history of opioid self-administration also may alter endogenous opioid transmission in rhesus monkeys. This possibility is important to consider, since some studies indicate that endogenous opioid peptides modulate the rewarding and motivational effects of benzodiazepines. For instance, opioid antagonists reverse benzodiazepine-induced hyperphagia and palatability enhancement (Cooper, 1983; Richardson et al., 2005), anxiolysis (Billingsley and Kubena, 1978), as well as conditioned place preference (Spyraki et al., 1985). These findings suggest that an alteration in endogenous opioid neurotransmission, as a consequence of a history of opioid self-administration, also may modify the reinforcing effects of benzodiazepines. Indirect support for this idea from the present results is the observation that one monkey that initially did not self-administer alprazolam did so when it was re-determined after exposures to the combinations, and overall, there was an approximately 2-fold increase (albeit not significant) in the potency of alprazolam alone following re-determination in the alprazolam-taking monkeys. However, it is important to note that, in the present study, fentanyl dose-response functions and potency and remifentanil baseline responses were unchanged before versus after combination tests. These possibilities clearly warrant further investigation under conditions of self-administration trained and maintained by opioids in monkeys.
Regardless of self-administration of either fentanyl or alprazolam alone, at least one combination of fentanyl and alprazolam was self-administered above vehicle levels in all six monkeys. In fact, for five out of six monkeys, the dose-response functions for fentanyl were shifted to the left in an approximately parallel fashion, indicative of an enhancement of the reinforcing potency of fentanyl by alprazolam combination, as supported by analysis of potency values (ED50s). Interestingly, the monkey that was the exception (M-41123) demonstrated rightward, but mostly downward shifts in the fentanyl dose-response function. The converse effects were observed as well, with fentanyl mostly enhancing the reinforcing effects of alprazolam. However, it is noteworthy that for most of the monkeys, combination with fentanyl resulted in an enhancement of the maximum reinforcing effects of alprazolam in addition to an increase in reinforcing potency. Again, the exception was M-41123, in which alprazolam mostly did not function as a reinforcer, either alone or combined with fentanyl. Overall, these results clearly suggest that combining fentanyl and alprazolam results in an overall increase in reinforcing effects compared with either drug alone in the majority of subjects.
To quantitatively test the hypothesis that fentanyl-alprazolam combinations were additive or supra-additive (i.e., synergistic), we used dose-equivalence analyses as developed by Grabovsky and Tallarida (2004). For the individual alprazolam-taking monkeys, the majority of observed functions for the combinations were shifted to the left of the predicted functions, suggesting the possibility of supra-additive interactions across individual subjects. However, when analyzed as a group, we observed additive effects for the 3:1 (fentanyl:alprazolam) and 1:3 combinations, and a supra-additive interaction for the 1:1 combination of fentanyl and alprazolam. Overall, these findings clearly suggest that combining fentanyl and alprazolam can mutually enhance the reinforcing effects of the two drugs, with supra-additive interactions present, but likely restricted to a relatively narrow range of dose combinations.
For monkeys that did not self-administer alprazolam, the results are more complicated, although for two of the three monkeys, the combination of fentanyl and alprazolam resulted in enhanced reinforcing effects relative to either drug alone. These interactions may be classified as “potentiation”, i.e., one drug of the combination lacks the studied effect, yet enhances the effects of the other drug when combined. However, the one outlier monkey (M-41123) showed primarily inhibitory, or suppressive effects, with the 3:1 dose combination resulting in a relatively modest rightward shift in the fentanyl dose-response function, suggesting that alprazolam in some fashion inhibits responding maintained by fentanyl in this subject. When taken as a whole, our results extend previous studies showing that rhesus monkeys prefer an opioid-benzodiazepine combination over an opioid alone in a choice procedure (Weed et al., 2017), and that chronic opioid administration may enhance the reinforcing effects of benzodiazepines (Ator et al., 2005). Collectively, these findings align with previous studies showing that patients with OUD purchasing benzodiazepines for recreational purposes report using these drugs to enhance feelings of “high” (Stein et al., 2016).
Rodent studies also have demonstrated that while alprazolam did not induce conditioned place preference itself, treatment with alprazolam enhanced intravenous heroin-induced conditioned place preference (Walker and Ettenberg, 2003). The same findings were observed when heroin was administered directly into the ventral tegmental area (VTA; Walker and Ettenberg, 2005), corroborating evidence that the VTA seems to be a major site for opioid and benzodiazepine reward (Tan et al., 2011; Jones et al., 2012). Benzodiazepines exert their effects by binding at GABAA receptors, which are co-localized with µ opioid receptors on inhibitory interneurons within the VTA (Xi and Stein, 1998). Activation of both GABAA and µ opioid receptors leads to decreased GABA release within the VTA and, consequently, increased (or “disinhibition” of) dopamine neurotransmission from the VTA to the nucleus accumbens (Tan et al., 2011; Jones et al., 2012), a structure that has been proposed as a major modulator of drug reward and reinforcement (Volkow et al., 2016). Therefore, benzodiazepines and opioids could enhance each other’s effects at the dopaminergic mesolimbic system, which would contribute to their synergistic and additive reinforcing effects and co-abuse.
Although the majority of monkeys in this study demonstrated mutual enhancement of the reinforcing effects of fentanyl and alprazolam when combined, there were notable individual differences in magnitude of effect, with one monkey showing a suppression of behavior rather than enhancement. Benzodiazepines may induce paradoxical reactions, which happens in less than 1% of patients and may be associated with aversive effects (Mancuso et al., 2004). While the mechanisms underlying these reactions remain unclear, predisposing factors may include genetic variability leading to altered pharmacodynamic and/or pharmacokinetic responses to benzodiazepines (Hall and Zisook, 1981; Short et al., 1987; Weinbroum et al., 2001). The extent to which genotypic variance may account for the results with subject M-41123, and rhesus macaques in general, remains unknown.
In summary, our findings show that combining fentanyl and alprazolam can result in proportion-dependent additive or supra-additive enhancements. Most of the combined effects were additive, but a significant supra-additive interaction was observed between fentanyl and alprazolam for the 1:1 proportion in alprazolam-taking taking monkeys. For monkeys that did not self-administer alprazolam, the combined effects were more variable but in many cases resulted in a potentiation of the reinforcing effects of fentanyl by alprazolam. Together, these findings suggest that the abuse-related effects of opioids when combined with benzodiazepines may be more reinforcing than the single drugs, which may play an important role in the prevalence of this type of polydrug abuse in patients with opioid use disorders. The U.S. opioid crisis has occurred in conjunction with an increase in benzodiazepine use disorders, resulting in a treatment population with a “secondary” addictive disorder and a poorer prognosis for recovery. Given the high prevalence of co-abuse between benzodiazepines and methadone/buprenorphine (Limandri, 2018), it is feasible that additive and/or supra-additive reinforcing effects of benzodiazepines combined with methadone or buprenorphine might occur. Considering that Ator et al. (2005) have shown that chronic treatment with methadone may increase the reinforcing effects of a benzodiazepine in primates, investigating whether supra-additivity also would be observed with methadone and/or buprenorphine combined with benzodiazepines is important, as that could represent a significant barrier to successful OUD treatment.
Acknowledgments
The authors thank the Veterinary staff from the UMMC Center for Comparative Research for their exceptional care of our animals. The authors also thank Meagan Follett and Tanya Pareek for technical assistance.
Abbreviations
- OUD
opioid use disorder
- PR
progressive ratio
Authorship Contributions
Participated in research design: Berro, Rowlett.
Conducted experiments: Berro.
Performed data analysis: Berro, Zamarripa, Rowlett.
Wrote or contributed to the writing of the manuscript: Berro, Zamarripa, Rowlett.
Footnotes
This study was funded by an ALKERMES PATHWAYS RESEARCH AWARDS grant, an independent competitive grants program supported by Alkermes (to L.F.B.). This work also was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA011792, DA043204, DA046778, DA049886, and DA048586].
The authors report no conflicts of interest.
References
- Ait-Daoud N, Hamby AS, Sharma S, Blevins D (2018) A Review of Alprazolam Use, Misuse, and Withdrawal. J Addict Med 12:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR, Weerts EM (2005) Self-injection of flunitrazepam alone and in the context of methadone maintenance in baboons. Drug Alcohol Depend 78:113–123. [DOI] [PubMed] [Google Scholar]
- Berro LF, Rowlett JK (2020) GABAA Receptor Subtypes and the Reinforcing Effects of Benzodiazepines in Remifentanil-Experienced Rhesus Monkeys. Drug Alcohol Depend 213:108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingsley ML, Kubena RK (1978) The effects of naloxone and picrotoxin on the sedative and anticonflict effects of benzodiazepines. Life Sci 22:897–906. [DOI] [PubMed] [Google Scholar]
- Brands B, Blake J, Marsh DC, Sproule B, Jeyapalan R, Li S (2008) The impact of benzodiazepine use on methadone maintenance treatment outcomes. J Addict Dis 27:37–48. [DOI] [PubMed] [Google Scholar]
- Cooper SJ (1983) Minireview. Benzodiazepine-opiate antagonist interactions in relation to feeding and drinking behavior. Life Sci 32:1043–1051. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Rowlett JK (2011) Anticonflict and reinforcing effects of triazolam + pregnanolone combinations in rhesus monkeys. J Pharmacol Exp Ther 337:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabovsky Y, Tallarida RJ (2004) Isobolographic analysis for combinations of a full and partial agonist: curved isoboles. J Pharmacol Exp Ther 310:981–986. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Findley JD, Brady JV, Dolan-Gutcher K, Robinson WW(1975) Comparison of progressive-ratio performance maintained by cocaine, methylphenidate and secobarbital. Psychopharmacologia 43:81–83 DOI: 10.1007/BF00437619. [DOI] [PubMed] [Google Scholar]
- Hall RC, Zisook S (1981) Paradoxical reactions to benzodiazepines. Br J Clin Pharmacol 11 (Suppl 1):99S–104S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Mogali S, Comer SD (2012) Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend 125:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczyńska K, Grzonkowski P, Kacprzak Ł, Zawilska JB (2018) Abuse of fentanyl: An emerging problem to face. Forensic Sci Int 289:207–214. [DOI] [PubMed] [Google Scholar]
- Lau CE, Heatherington AC (1997) Pharmacokinetic-pharmacodynamic modeling of stimulatory and sedative effects of alprazolam: timing performance deficits. J Pharmacol Exp Ther 283:1119–1129. [PubMed] [Google Scholar]
- Lavie E, Fatséas M, Denis C, Auriacombe M (2009) Benzodiazepine use among opiate-dependent subjects in buprenorphine maintenance treatment: correlates of use, abuse and dependence. Drug Alcohol Depend 99:338–344. [DOI] [PubMed] [Google Scholar]
- Limandri BJ (2018) Benzodiazepine Use: The Underbelly of the Opioid Epidemic. J Psychosoc Nurs Ment Health Serv 56:11–15. [DOI] [PubMed] [Google Scholar]
- Liu S, O’Donnell J, Gladden RM, McGlone L, Chowdhury F (2021) Trends in Nonfatal and Fatal Overdoses Involving Benzodiazepines—38 States and the District of Columbia, 2019–2020. MMWR Morb Mortal Wkly Rep 70:1136–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso CE, Tanzi MG, Gabay M (2004) Paradoxical reactions to benzodiazepines: literature review and treatment options. Pharmacotherapy 24:1177–1185. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Votaw VR, Bogunovic O, Karakula SL, Griffin ML, Weiss RD (2017) Anxiety sensitivity and nonmedical benzodiazepine use among adults with opioid use disorder. Addict Behav 65:283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minervini V, Lu HY, Padarti J, Osteicoechea DC, France CP(2018) Interactions between kappa and mu opioid receptor agonists: effects of the ratio of drugs in mixtures. Psychopharmacology (Berl) 235:2245–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TJ, Mattison DR (2017) Adult Utilization of Psychiatric Drugs and Differences by Sex, Age, and Race. JAMA Intern Med 177:274–275. [DOI] [PubMed] [Google Scholar]
- Moses TEH, Lundahl LH, Greenwald MK (2018) Factors associated with sedative use and misuse among heroin users. Drug Alcohol Depend 185:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses TEH, Greenwald MK (2019) History of regular nonmedical sedative and/or alcohol use differentiates substance-use patterns and consequences among chronic heroin users. Addict Behav 97:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals: Eighth Edition. The National Academies Press, Washington, DC DOI: 10.17226/12910. [DOI] [Google Scholar]
- Paulozzi LJ, Strickler GK, Kreiner PW, Koris CM; Centers for Disease Control and Prevention (CDC) (2015) Controlled Substance Prescribing Patterns--Prescription Behavior Surveillance System, Eight States, 2013. MMWR Surveill Summ 64:1–14. [DOI] [PubMed] [Google Scholar]
- Platt DM, Carey G, Spealman RD(2011) Models of neurological disease (substance abuse): self-administration in monkeys. Curr Protoc Pharmacol Chapter 10: Unit10.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DK, Reynolds SM, Cooper SJ, Berridge KC (2005) Endogenous opioids are necessary for benzodiazepine palatability enhancement: naltrexone blocks diazepam-induced increase of sucrose-‘liking’. Pharmacol Biochem Behav 81:657–663. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Massey BW, Kleven MS, Woolverton WL(1996) Parametric analysis of cocaine self-administration under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 125:361–370. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL (1997) Self-administration of cocaine and heroin combinations by rhesus monkeys responding under a progressive-ratio schedule. Psychopharmacology (Berl) 133:363–371. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Yao W-D, Spealman RD (2007) Modulation of heroin and cocaine self-administration by dopamine D1- and D2-like receptor agonists in rhesus monkeys. J Pharmacol Exp Ther 321:1135–1143. [DOI] [PubMed] [Google Scholar]
- Schepis TS, Teter CJ, Simoni-Wastila L, McCabe SE (2018) Prescription tranquilizer/sedative misuse prevalence and correlates across age cohorts in the US. Addict Behav 87:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinday NM, Sawyer EK, Fischer BD, Platt DM, Licata SC, Atack JR, Dawson GR, Reynolds DS, Rowlett JK (2013) Reinforcing effects of compounds lacking intrinsic efficacy at α1 subunit-containing GABAA receptor subtypes in midazolam- but not cocaine-experienced rhesus monkeys. Neuropsychopharmacology 38:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short TG, Forrest P, Galletly DC (1987) Paradoxical reactions to benzodiazepines--a genetically determined phenomenon? Anaesth Intensive Care 15:330–331. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR (1996) Effects of chronic morphine administration on mu opioid receptor-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci 16:2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ (2000) Chronic heroin self-administration desensitizes mu opioid receptor-activated G-proteins in specific regions of rat brain. J Neurosci 20:4555–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyraki C, Kazandjian A, Varonos D (1985) Diazepam-induced place preference conditioning: appetitive and antiaversive properties. Psychopharmacology (Berl) 87:225–232. [DOI] [PubMed] [Google Scholar]
- Stein MD, Kanabar M, Anderson BJ, Lembke A, Bailey GL (2016) Reasons for Benzodiazepine Use Among Persons Seeking Opioid Detoxification. J Subst Abuse Treat 68:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroumpos C, Manolaraki M, Paspatis GA (2010) Remifentanil, a different opioid: potential clinical applications and safety aspects. Expert Opin Drug Saf 9:355–364. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ (2006) An overview of drug combination analysis with isobolograms. J Pharmacol Exp Ther 319:1–7. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ (2011) Quantitative methods for assessing drug synergism. Genes Cancer 2:1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Rudolph U, Lüscher C (2011) Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci 34:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatakrishnan K, Culm KE, Ehrenberg BL, Harmatz JS, Corbett KE, Fleishaker JC, Greenblatt DJ (2005) Kinetics and dynamics of intravenous adinazolam, N-desmethyl adinazolam, and alprazolam in healthy volunteers. J Clin Pharmacol 45:529–537. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT (2016) Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med 374:363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votaw VR, McHugh RK, Vowles KE, Witkiewitz K (2020) Patterns of Polysubstance Use among Adults with Tranquilizer Misuse. Subst Use Misuse 55:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votaw VR, Witkiewitz K, Valeri L, Bogunovic O, McHugh RK (2019) Nonmedical prescription sedative/tranquilizer use in alcohol and opioid use disorders. Addict Behav 88:48–55. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ettenberg A (2003) The effects of alprazolam on conditioned place preferences produced by intravenous heroin. Pharmacol Biochem Behav 75:75–80. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ettenberg A (2005) Intra-ventral tegmental area heroin-induced place preferences in rats are potentiated by peripherally administered alprazolam. Pharmacol Biochem Behav 82:470–477. [DOI] [PubMed] [Google Scholar]
- Weed PF, France CP, Gerak LR (2017) Preference for an Opioid/Benzodiazepine Mixture over an Opioid Alone Using a Concurrent Choice Procedure in Rhesus Monkeys. J Pharmacol Exp Ther 362:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbroum AA, Szold O, Ogorek D, Flaishon R (2001) The midazolam-induced paradox phenomenon is reversible by flumazenil. Epidemiology, patient characteristics and review of the literature. Eur J Anaesthesiol 18:789–797. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z, Vasterling T, Tallarida R (2008b) Self-administration of cocaine-remifentanil mixtures by monkeys: an isobolographic analysis. Psychopharmacology (Berl) 198:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z, Vasterling T, Carroll FI, Tallarida R (2008a) Self-administration of drug mixtures by monkeys: combining drugs with comparable mechanisms of action. Psychopharmacology (Berl) 196:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Stein EA (1998) Nucleus accumbens dopamine release modulation by mesolimbic GABAA receptors-an in vivo electrochemical study. Brain Res 798:156–165. [DOI] [PubMed] [Google Scholar]