Abstract
Introduction
The increasing incidence of pathogen transmission from animals to humans (zoonotic spillover events) has been attributed to behavioural practices and ecological and socioeconomic change. As these events sometimes involve pathogens with epidemic or pandemic potential, they pose a serious threat to population health. Public policies may play a key role in preventing these events. The aim of this review is to identify evaluations of public policies that target the determinants of zoonotic spillover, examining approaches taken to evaluation, choice of outcomes measures and evidence of effectiveness. Our approach to identifying and analysing this literature will be informed by a One Health lens, acknowledging the interconnectedness of human, animal and environmental health.
Methods and analysis
A systematic scoping review methodology will be used. To identify articles, we will search Medline, SCOPUS, Web of Science and Global Health in May 2021 using search terms combining animal health and the animal–human interface, public policy, prevention and zoonoses. We will screen titles and abstracts and extract data according to published guidelines for scoping reviews. All evaluations of public policies aiming to prevent zoonotic spillover events will be eligible for inclusion. We will summarise key data from each study, mapping policies along the spillover pathway and outlining the range of policies, approaches to evaluation and outcome measures. Review findings will provide a useful reference for researchers and practitioners, outlining the state of the evaluative evidence around policies to prevent zoonotic spillover.
Ethics and dissemination
Formal ethical approval is not required, because the study does not involve primary data collection. The findings of this study will be disseminated through a peer-reviewed publication, presentations and summaries for key stakeholders.
Keywords: Public health, Health policy, PUBLIC HEALTH
Strengths and limitations of this study.
This scoping review protocol outlines the first piece of work to systematically identify and review evaluations of public policies designed to prevent zoonotic spillover, and will be undertaken in line with published guidelines for best practice in scoping reviews.
The review will be informed by a One Health lens, encompassing distal determinants and risk factors for spillover events and acknowledging the interconnectedness of human, animal and environmental health.
Due to the complex drivers of spillover events, some potentially relevant policy evaluations may not be identified where outcome measures are too far removed from zoonotic spillover.
Introduction
The increasing incidence of zoonotic emerging infectious diseases (EIDs) has been attributed to behavioural practices and ecological and socioeconomic change, and is predicted to continue in the coming years.1 Higher levels of anthropogenic activity, including agricultural intensification, urbanisation and other forms of land use change, have led to increased interactions between wildlife, humans and livestock, increasing the risk of cross-species transmission.2 3 In response, a call has been issued by leading organisations and experts, including the United Nations Environment Programme, the International Livestock Research Institute and the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, to complement reactive policy responses with policies that prevent zoonotic EIDs.1 4–7
Preventing zoonotic spillover from a One Health perspective
Zoonotic spillover, defined as the transmission of a pathogen from an animal to a human, depends on the alignment of ecological, epidemiological and behavioural factors.8 Zoonotic pathogens must meet a series of conditions in order to induce spillover infections in humans, including appropriate density and distribution of reservoir hosts, pathogen prevalence, infection intensity and human exposure.8 Across this transmission pathway, a number of drivers of zoonotic spillover have been identified, including changes in wildlife and livestock populations;9 deforestation, urbanisation and other forms of land use change;10 and a variety of human practices including hunting, farming, animal husbandry, keeping of exotic pets and trade.6 7 11 12 These large-scale changes have on multiple occasions given rise to spillover events, sometimes involving pathogens with epidemic or pandemic potential.
A One Health perspective, which recognises the health of humans, animals and ecosystems as being closely linked and interdependent,13 can be useful in conceptualising a range of potential determinants of spillover events. From this perspective, interventions could include surveillance of pools of viruses in wildlife and management of wildlife populations;14 enhanced food safety measures in both the wildlife and livestock value chain, prefarm and postfarm gate;12 15–17 replacement of traditional ‘wet’ markets with supermarkets;18 controls on wildlife hunting, trade and consumption;11 19 20 and phasing out of unsustainable agriculture practices.6 21
While some evaluative evidence exists around the effectiveness of interventions,22–25 they have often been implemented as short-term to medium-term programmes or academic investigations.6 In some cases, zoonoses have re-emerged after successful programmes have ended.25 As a result, experts have argued for the incorporation of successful interventions into policy frameworks, providing interventions with the sustainability required for long-term disease control.6
Governance, systems and the role of multisectoral actors
Public policy is ‘a set of interrelated decisions taken by a political actor or group of actors concerning the selection of goals and the means of achieving them’.26 Public policy decisions are ultimately in the hands of government and supranational governing bodies, and have greater longevity compared with many programmes, which are often implemented for a fixed term. Non-government actors, including vested interest stakeholders, can also play a powerful role in shaping government decisions.27 28
Although the longevity and scope of government actions may make policy an effective vehicle for prevention of emergent diseases, implementing policy is a complex process involving numerous stakeholders with competing views and interests.29 The responsibility for addressing zoonotic disease frequently spans multiple sectors of governance due to its relevance for both animals and humans. Where relevant policies are designed and implemented in isolation, opportunities for synergy may be missed and efforts may even be counter-productive.
Successful policy measures require both a sound evidence base, and also governance structures that enable action to be taken. Given the range of possible risk factors that might contribute to emerging zoonoses, and the possible impacts of policies to prevent zoonotic spillover, a One Health response has been advocated, requiring coordination between institutions and government departments involved in human and animal health, trade, agriculture and the environment.30 At the international level, the WHO, the Food and Agriculture Organisation and the World Organisation for Animal Health have endorsed a One Health policy framework to respond to zoonotic infectious diseases, emphasising collaboration between agencies.31 Within countries, national and local governments have also emphasised the need for multisectoral efforts, although many report that further integration is still required.32
Furthermore, given the complex social–ecological systems within which policies to prevent zoonotic spillover are implemented, the risk of unintended consequences is high. For example, region-specific closures of live animal markets have been shown to spread pathogens further afield as vendors seek new venues to sell their animals.33 Meanwhile, attempts to manage populations of wild animals may alter pathogen dynamics, unintentionally increasing the risk of spillover into livestock or people.34
Given these particular characteristics of policy development and implementation, they may be usefully considered as a particular case of intervention, and the evidence around them assessed accordingly. Different types of interventions might be more or less feasibly implemented by governments (or their partners), and their impacts might be different given potentially more complex implementation contexts, longer timespans and broader geographic ranges. Evaluations of these policies should also include consideration and monitoring of potential unintended consequences. In order to facilitate this, multisectoral involvement in both policy development and evaluation may be required.
Aims and scope
Approaches to managing epidemic and pandemic infectious pathogens once they have entered human populations have been systematically catalogued in the medical literature.35–41 These measures include hand washing, face masks, school closures and contact tracing and case isolation. Further upstream, systematic reviews of interventions targeting the spillover pathway have predominantly focused on programmes rather than policies, and have been restricted by various characteristics such as geographic region24 or pathogen type,25 or focused on programmes with an explicit endorsement of a One Health approach.23 In consequence, a comprehensive understanding of how policies to prevent zoonotic spillover have been evaluated, and what evidence there is of their effectiveness, is lacking. To address these research gaps, our objectives are to do the following:
Identify evaluations of policies that target the determinants of zoonotic spillover included in the spillover pathway8 (ie, human and animal health and interactions).
Identify insights around policy success and failure, and unintended consequences of policy implementation.
Describe approaches to evaluation and key barriers and facilitators to evaluating policies to reduce the risk of zoonotic spillover.
Our approach to identifying and analysing this literature will be informed by a One Health lens, acknowledging the interconnectedness of human, animal and environmental health.
Methods and analysis
We will conduct a systematic scoping review of evaluations of policies aimed at preventing zoonotic spillover events. The scoping review will be conducted in line with guidelines published by Arksey and O’Malley and refined by Levac and colleagues,42–44 which emphasise an iterative approach suited to an exploratory research question.
Stage 1: identifying the research question
The aim of this review is to use a One Health lens to identify and describe the range of policies that have been evaluated, the approaches to evaluation and the evaluative evidence. Informed by this aim, our research questions are as follows:
-
What policies aimed at preventing zoonotic spillover have been evaluated?
What are the types of policies?
Which policy actors (single department, multisectoral, whole of government) are engaged?
What are the reasons for policy success and failure, and the unintended consequences of implementing these policies?
-
How has evaluation of these policies been approached in the literature?
What are the methods or study designs used?
What are the outcomes?
What are the barriers and facilitators to evaluation?
Stage 2: identifying relevant studies
We searched four electronic databases (Medline, Scopus, Web of Science and Global Health) in May 2021. The search strategy is organised by the main concepts in our research question: the spillover pathway; public policy; prevention and zoonotic pathogens. The search strategy was developed iteratively, informed by existing systematic reviews focused on related concepts24 45–49 and known indicator papers meeting inclusion criteria. We also searched the websites of 18 organisations involved in the prevention of zoonotic spillover to identify relevant grey literature. See online supplemental file 1 for details of search strategy and websites searched.
bmjopen-2021-058437supp001.pdf (58.5KB, pdf)
Stage 3: study selection
Records identified through the searches will be collated and double screened using the online platform Covidence.50 Studies will be included where they meet all of the following criteria:
Primary empirical study from any country or region with English-language abstracts.
Report empirical findings from an evaluation of any sort.
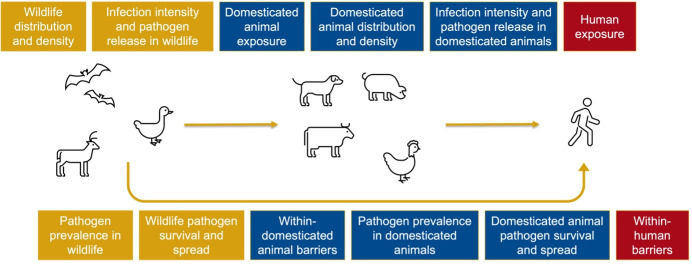
Focus on a policy implemented by government that targets a determinant of zoonotic spillover located on the spillover pathway (see figure 1).
Figure 1.
Spillover pathway adapted from Plowright et al.8 22
Titles and abstracts will initially be screened, followed by full-text screening. Title and abstract screening of an initial set of 100 papers will be undertaken by two independent researchers. Results will be compared in order to ensure consistency in decisions around study eligibility, and discrepancies resolved through discussion of the inclusion criteria. This process will be repeated until an acceptable level of agreement (>90%) is reached. The remaining papers will then be screened by one of the two reviewers. Full-text screening will be undertaken by two independent researchers and discrepancies will be resolved by discussing reasons for inclusion or exclusions among the screeners. Studies with full-texts in languages other than English will be eligible for inclusion if they include an English-language abstract. Full-text studies published in French, Spanish or Chinese will be single-screened by a member of the research team fluent in that language. Studies published in other languages will be translated as necessary.
In line with published guidelines, the approach to study selection may be refined iteratively when reviewing articles for inclusion.42–44 Reporting on the search and screening process will follow the guidelines provided in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews.51
Stage 4: charting the data
Data charting will be conducted using a data charting form designed to identify the information required to answer the research question and subresearch questions (see online supplemental file 2). Data charting focused on characteristics of the study, the policy and the evaluation. For each policy, this included identifying which determinant of zoonotic spillover situated along the spillover pathway was being targeted. For the purpose of this study, we used a model of the spillover pathway adapted from Plowright et al.’s work,8 22 in which we differentiated between wildlife and domesticated animals (figure 1). This differentiation is important in the policy context, as the wildlife-domesticated animal interface is an important site for intervention, as well as the human–animal interface.
bmjopen-2021-058437supp002.pdf (37.2KB, pdf)
As recommended, the data charting form will be piloted with 10 records to ensure that it is consistent with the research question, and the data charting form will be revised iteratively in order to ensure the purpose of the research is being met.42–44
Stage 5: collating, summarising and reporting the results
We will undertake quality assessment of the included studies using the Quality Assessment Tool for Quantitative Studies developed by the Effective Public Health Practice Project,52 which has previously been used to assess the quality of natural experiments including public policy evaluations.53
We will analyse the extracted data, presenting a numerical summary of the included studies in table form, allowing us to describe the range of policy interventions that have been evaluated, approaches to evaluation and evidence of effectiveness. We will also conduct a thematic analysis of the contents of the included articles in order to identify, if possible, barriers and facilitators to implementing and evaluating these policies, as well as insights into why policies succeeded or failed in achieving their aims.
Patient and public involvement
This scoping review is being undertaken as part of a larger project involving policy actors at national and international levels as research team members, knowledge users and participants. Insights from the project have informed protocol development and stakeholders are able to provide input and perspectives on the results of the review. Project-level dissemination events involving policy stakeholders are also planned, where findings from the proposed review will be shared.
Strengths and weaknesses of the study
To our knowledge, this is the first attempt to systematically identify and document evaluations of policies aiming to prevent the spillover of zoonoses into human populations. However, because of the complex drivers of spillover events, some potentially relevant policy evaluations may be excluded where their outcome measures are too far removed from zoonotic spillover. For example, it has been hypothesised that declines in vulture populations may increase the risk of pathogen transmission by increasing the number of uneaten carcasses, as well as, potentially, the population of feral dogs.54 In 2006, India, Pakistan and Nepal implemented a ban on the veterinary drug diclofenac, which had been identified as a driver of declining vulture populations. While policy evaluations suggest that this ban has resulted in a resurgence of vultures,55–58 the knock-on effects of this on zoonotic pathogen transmission risk have not been included in these evaluations. While relevant, such evaluations will be difficult to systematically identify as they make no reference to zoonotic disease.
In addition, this review will focus on policy evaluations that have been reported in the peer-reviewed and grey literature. Policies that have been implemented but not evaluated, or evaluated but not reported in the literature, will therefore be excluded from this review. As a result, potentially effective and important policies in the prevention of zoonotic spillover events may not be identified. However, we hope that the findings from this review will highlight these gaps in the evaluative evidence. We also hope that this review, by extracting practical dimensions such as study design, outcome measures and the challenges encountered in the evaluation process, will support policymakers and researchers in carrying out policy evaluations in this space.
Ethics and dissemination
Formal ethical approval is not required, because the study does not involve primary data collection. The findings of this study will be disseminated through a peer-reviewed publication, presentations and summaries for key stakeholders.
Supplementary Material
Footnotes
Twitter: @chloecastbury, @LabonteRonald, @TarraPenney
Contributors: CCA, KML and TLP conceived and designed the study. CCA prepared the manuscript. KML, TLP, RA, AA, MB, JC, RL, AR, KCT, AMV, MW and AY provided critical input on the manuscript and methods and have read and approved the final manuscript.
Funding: CCA, JC and TLP acknowledge internal research support from York University. This review will be undertaken as part of a project funded by the Canadian Institutes of Health Research, Grant Reference Number VR5-172686. The funder had no role in developing the protocol.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Morse SS, Mazet JAK, Woolhouse M, et al. Prediction and prevention of the next pandemic zoonosis. Lancet 2012;380:1956–65. 10.1016/S0140-6736(12)61684-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulliam JRC, Epstein JH, Dushoff J, et al. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. J R Soc Interface 2012;9:89–101. 10.1098/rsif.2011.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IPCC . Climate Change 2022: Impacts, Adaptation and Vulnerability [Internet]. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In: Pörtner H-O, Roberts DC, Tignor M, eds. Cambridge University Press, 2022. https://www.ipcc.ch/report/ar6/wg2/ [Google Scholar]
- 4.Di Marco M, Baker ML, Daszak P, et al. Opinion: sustainable development must account for pandemic risk. Proc Natl Acad Sci U S A 2020;117:3888–92. 10.1073/pnas.2001655117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heymann DL, Dixon M. Infections at the Animal/Human Interface: Shifting the Paradigm from Emergency Response to Prevention at Source. In: Mackenzie JS, Jeggo M, Daszak P, et al., eds. One health: the Human-Animal-Environment interfaces in emerging infectious diseases: food safety and security, and international and national plans for implementation of one health activities. Berlin, Heidelberg: Springer, 2013: 207–15. [Google Scholar]
- 6.United Nations Environment Programme, International Livestock Research Institute . Preventing the next pandemic: zoonotic diseases and how to break the chain of transmission. Nairobi, Kenya, 2020: 82. [Google Scholar]
- 7.Intergovernmental Science-Policy Platform On Biodiversity And Ecosystem Services (IPBES) . Workshop Report on Biodiversity and Pandemics of the Intergovernmental Platform on Biodiversity and Ecosystem Services (IPBES) [Internet]. Zenodo, 2020. Available: https://zenodo.org/record/4147317 [Accessed 7 Jan 2021].
- 8.Plowright RK, Parrish CR, McCallum H, et al. Pathways to zoonotic spillover. Nat Rev Microbiol 2017;15:502–10. 10.1038/nrmicro.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson CK, Hitchens PL, Pandit PS, et al. Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proc Biol Sci 2020;287:20192736. 10.1098/rspb.2019.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen T, Murray KA, Zambrana-Torrelio C, et al. Global hotspots and correlates of emerging zoonotic diseases. Nat Commun 2017;8:1124. 10.1038/s41467-017-00923-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguirre AA, Catherina R, Frye H, et al. Illicit wildlife trade, wet markets, and COVID-19: preventing future pandemics. World Med Health Policy 2020;12:256–65. 10.1002/wmh3.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadimpalli ML, Pickering AJ. A call for global monitoring of wash in wet markets. Lancet Planet Health 2020;4:e439–40. 10.1016/S2542-5196(20)30204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joint Tripartite (FAO, OIE, WHO) and UNEP Statement . Tripartite and UNEP support OHHLEP’s definition of “One Health” [Internet]. OIE - World Organisation for Animal Health, 2021. Available: https://www.oie.int/en/tripartite-and-unep-support-ohhleps-definition-of-one-health/ [Accessed 18 Feb 2022].
- 14.Kelly TR, Karesh WB, Johnson CK, et al. One health proof of concept: bringing a transdisciplinary approach to surveillance for zoonotic viruses at the human-wild animal interface. Prev Vet Med 2017;137:112–8. 10.1016/j.prevetmed.2016.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aiyar A, Pingali P. Pandemics and food systems - towards a proactive food safety approach to disease prevention & management. Food Secur 2020;12:749–56. 10.1007/s12571-020-01074-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki Y, Furutani A, Furuichi T, et al. Development of a biosecurity assessment tool and the assessment of biosecurity levels by this tool on Japanese commercial swine farms. Prev Vet Med 2020;175:104848. 10.1016/j.prevetmed.2019.104848 [DOI] [PubMed] [Google Scholar]
- 17.Soon JM, Abdul Wahab IR. On-Site hygiene and biosecurity assessment: a new tool to assess live bird stalls in wet markets. Food Control 2021;127:108108. 10.1016/j.foodcont.2021.108108 [DOI] [Google Scholar]
- 18.Maruyama M, Wu L, Huang L. The modernization of fresh food retailing in China: the role of consumers. Journal of Retailing and Consumer Services 2016;30:33–9. 10.1016/j.jretconser.2015.12.006 [DOI] [Google Scholar]
- 19.Challender DWS, Harrop SR, MacMillan DC. Towards informed and multi-faceted wildlife trade interventions. Glob Ecol Conserv 2015;3:129–48. 10.1016/j.gecco.2014.11.010 [DOI] [Google Scholar]
- 20.Gómez A, Aguirre AA. Infectious diseases and the illegal wildlife trade. Ann N Y Acad Sci 2008;1149:16–19. 10.1196/annals.1428.046 [DOI] [PubMed] [Google Scholar]
- 21.Kessler MK, Becker DJ, Peel AJ, et al. Changing resource landscapes and spillover of henipaviruses. Ann N Y Acad Sci 2018;1429:78–99. 10.1111/nyas.13910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokolow SH, Nova N, Pepin KM, et al. Ecological interventions to prevent and manage zoonotic pathogen spillover. Philos Trans R Soc Lond B Biol Sci 2019;374:20180342. 10.1098/rstb.2018.0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baum SE, Machalaba C, Daszak P, et al. Evaluating one health: are we demonstrating effectiveness? One Health 2017;3:5–10. 10.1016/j.onehlt.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halton K, Sarna M, Barnett A, et al. A systematic review of community-based interventions for emerging zoonotic infectious diseases in Southeast Asia. JBI Database System Rev Implement Rep 2013;11:1–235. 10.11124/01938924-201311020-00001 [DOI] [Google Scholar]
- 25.Meyer A, Holt HR, Selby R, et al. Past and ongoing tsetse and animal trypanosomiasis control operations in five African countries: a systematic review. PLoS Negl Trop Dis 2016;10:e0005247. 10.1371/journal.pntd.0005247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkins WI. Policy analysis: a political and organisational perspective. M. Robertson, 1978: 278.p. [Google Scholar]
- 27.Renn O, Schweizer P-J. Inclusive risk governance: concepts and application to environmental policy making. Environmental Policy and Governance 2009;19:174–85. 10.1002/eet.507 [DOI] [Google Scholar]
- 28.Keck ME, Sikkink K. Transnational advocacy networks in international and regional politics. Int Soc Sci J 1999;51:89–101. 10.1111/1468-2451.00179 [DOI] [Google Scholar]
- 29.Howlett M, Cashore B. Conceptualizing Public Policy. In: Engeli I, Allison CR, eds. Comparative policy studies: conceptual and methodological challenges. London: Palgrave Macmillan UK, 2014: 17–33. 10.1057/9781137314154_2 [DOI] [Google Scholar]
- 30.Mazet J, Uhart MM, Keyyu JD. Stakeholders in one health. Rev. Sci. Tech. OIE 2014;33:443–52. 10.20506/rst.33.2.2295 [DOI] [PubMed] [Google Scholar]
- 31.Chien Y-J. How did international agencies perceive the avian influenza problem? The adoption and manufacture of the 'One World, One Health' framework. Sociol Health Illn 2013;35:213–26. 10.1111/j.1467-9566.2012.01534.x [DOI] [PubMed] [Google Scholar]
- 32.Maxwell MJ, Freire de Carvalho MH, Hoet AE, et al. Building the road to a regional zoonoses strategy: a survey of zoonoses programmes in the Americas. PLoS One 2017;12:e0174175. 10.1371/journal.pone.0174175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Wang Y, Shen C, et al. Closure of live bird markets leads to the spread of H7N9 influenza in China. PLoS One 2018;13:e0208884. 10.1371/journal.pone.0208884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cotterill GG, Cross PC, Cole EK, et al. Winter feeding of elk in the greater Yellowstone ecosystem and its effects on disease dynamics. Philos Trans R Soc Lond B Biol Sci 2018;373:20170093. 10.1098/rstb.2017.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saunders-Hastings P, Crispo JAG, Sikora L, et al. Effectiveness of personal protective measures in reducing pandemic influenza transmission: a systematic review and meta-analysis. Epidemics 2017;20:1–20. 10.1016/j.epidem.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 36.Bin Nafisah S, Alamery AH, Al Nafesa A, et al. School closure during novel influenza: a systematic review. J Infect Public Health 2018;11:657–61. 10.1016/j.jiph.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 37.Viner RM, Russell SJ, Croker H, et al. School closure and management practices during coronavirus outbreaks including COVID-19: a rapid systematic review. Lancet Child Adolesc Health 2020;4:397–404. 10.1016/S2352-4642(20)30095-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juneau C-E, Pueyo T, Bell M. Evidence-Based, cost-effective interventions to suppress the COVID-19 pandemic: a systematic review. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacIntyre CR, Chughtai AA. Facemasks for the prevention of infection in healthcare and community settings. BMJ 2015;350:h694. 10.1136/bmj.h694 [DOI] [PubMed] [Google Scholar]
- 40.Smith SMS, Sonego S, Wallen GR, et al. Use of non-pharmaceutical interventions to reduce the transmission of influenza in adults: a systematic review. Respirology 2015;20:896–903. 10.1111/resp.12541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jefferson T, Del Mar CB DL, Ferroni E. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev 2011;7:CD006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 43.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5:69. 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colquhoun HL, Levac D, O'Brien KK, et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol 2014;67:1291–4. 10.1016/j.jclinepi.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 45.Zhou X, Wang Y, Liu H, et al. Effectiveness of Market-Level biosecurity at reducing exposure of poultry and humans to avian influenza: a systematic review and meta-analysis. J Infect Dis 2018;218:1861–75. 10.1093/infdis/jiy400 [DOI] [PubMed] [Google Scholar]
- 46.Conan A, Goutard FL, Sorn S, et al. Biosecurity measures for backyard poultry in developing countries: a systematic review. BMC Vet Res 2012;8:240. 10.1186/1746-6148-8-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Youssef DM, Wieland B, Knight GM, et al. The effectiveness of biosecurity interventions in reducing the transmission of bacteria from livestock to humans at the farm level: a systematic literature review. Zoonoses Public Health 2021;68:549–62. 10.1111/zph.12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi N, Huang J, Zhang X, et al. Interventions in live poultry markets for the control of avian influenza: a systematic review and meta-analysis. J Infect Dis 2020;221:553–60. 10.1093/infdis/jiz372 [DOI] [PubMed] [Google Scholar]
- 49.Cupertino M, Resende M, Mayer N, et al. Emerging and re-emerging human infectious diseases: a systematic review of the role of wild animals with a focus on public health impact. Asian Pac J Trop Med 2020;13:99. 10.4103/1995-7645.277535 [DOI] [Google Scholar]
- 50.Covidence - Better systematic review management [Internet]. Available: https://www.covidence.org/home [Accessed 17 Jul 2020].
- 51.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 52.Effective Public Health Practice Project . Quality assessment tool for quantitative studies, 2009. Available: https://merst.ca/wp-content/uploads/2018/02/quality-assessment-tool_2010.pdf [Accessed 17 May 2021].
- 53.Bramante CT, Thornton RLJ, Bennett WL, et al. Systematic review of natural experiments for childhood obesity prevention and control. Am J Prev Med 2019;56:147–58. 10.1016/j.amepre.2018.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogada DL, Torchin ME, Kinnaird MF, et al. Effects of vulture declines on facultative scavengers and potential implications for mammalian disease transmission. Conserv Biol 2012;26:453–60. 10.1111/j.1523-1739.2012.01827.x [DOI] [PubMed] [Google Scholar]
- 55.Chaudhry MJI, Ogada DL, Malik RN, et al. First evidence that populations of the critically endangered Long-billed Vulture Gyps indicus in Pakistan have increased following the ban of the toxic veterinary drug diclofenac in south Asia. Bird Conserv Int 2012;22:389–97. 10.1017/S0959270912000445 [DOI] [Google Scholar]
- 56.Cuthbert R, Taggart MA, Prakash V, et al. Effectiveness of action in India to reduce exposure of Gyps vultures to the toxic veterinary drug diclofenac. PLoS One 2011;6:e19069. 10.1371/journal.pone.0019069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nambirajan K, Muralidharan S, Roy AA, et al. Residues of diclofenac in tissues of Vultures in India: a Post-ban scenario. Arch Environ Contam Toxicol 2018;74:292–7. 10.1007/s00244-017-0480-z [DOI] [PubMed] [Google Scholar]
- 58.Prakash V, Bishwakarma MC, Chaudhary A, et al. The population decline of Gyps vultures in India and Nepal has slowed since veterinary use of diclofenac was banned. PLoS One 2012;7:e49118. 10.1371/journal.pone.0049118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058437supp001.pdf (58.5KB, pdf)
bmjopen-2021-058437supp002.pdf (37.2KB, pdf)