Abstract
Objectives
Mobile health applications are instrumental in the self-management of chronic diseases like diabetes. Technology acceptance models such as Unified Theory of Acceptance and Use of Technology 2 (UTAUT2) have proven essential for predicting the acceptance of information technology. However, earlier research has found that the constructs “perceived disease threat” and “trust” should be added to UTAUT2 in the mHealth acceptance context. This study aims to evaluate the extended UTAUT2 model for predicting mHealth acceptance, represented by behavioral intention, using mobile diabetes applications as an example.
Methods
We extended UTAUT2 with the additional constructs “perceived disease threat” and “trust”. We conducted a web-based survey in German-speaking countries focusing on patients with diabetes and their relatives who have been using mobile diabetes applications for at least 3 months. We analysed 413 completed questionnaires by structural equation modelling.
Results
We could confirm that the newly added constructs “perceived disease threat” and “trust” indeed predict behavioural intention to use mobile diabetes applications. We could also confirm the UTAUT2 constructs “performance expectancy” and “habit” to predict behavioural intention to use mobile diabetes applications. The results show that the extended UTAUT2 model could explain 35.0% of the variance in behavioural intention.
Discussion
Even if UTAUT2 is well established in the information technologies sector to predict technology acceptance, our results reveal that the original UTAUT2 should be extended by “perceived disease threat” and “trust” to better predict mHealth acceptance.
Conclusion
Despite the newly added constructs, UTAUT2 can only partially predict mHealth acceptance. Future research should investigate additional mHealth acceptance factors, including how patients perceive trust in mHealth applications.
Keywords: Information Technology, Medical Informatics
WHAT IS ALREADY KNOWN ON THIS TOPIC
mHealth applications are essential for comprehensive self-management of chronic diseases.
The use of mHealth applications depends significantly on their acceptance, which can be predicted using technology acceptance models.
The UTAUT2 model has proven suitable for predicting technology acceptance in various information technology domains.
WHAT THIS STUDY ADDS
“Perceived disease threat” and ”trust” are relevant in predicting the acceptance of mobile diabetes applications and should be added to Unified Theory of Acceptance and Use of Technology 2 (UTAUT2) when used in this context.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
“Performance expectancy”, “habit”, “perceived disease threat” and “trust” are relevant for accepting mobile diabetes applications. Therefore, these factors should be considered when developing new mHealth applications in this context.
External conditions, such as country-specific financial support for mHealth users from, for example, statutory health insurances, which helps to fund the required mHealth applications, should be addressed in future mHealth acceptance studies.
Introduction
mHealth acceptance
Mobile health (mHealth) applications, especially the so-called lifestyle apps such as fitness apps, have become increasingly popular, specifically among younger people, due to growing health awareness.1 2 Besides lifestyle apps, mHealth applications such as continuous glucose monitoring systems (CGMs) are instrumental for the self-management of chronic diseases like diabetes mellitus.3 4 Different studies have shown that using mHealth applications leads to improved self-management and better health among people with chronic diseases.5 6 This is especially true in the case of diabetes, which is one of the most frequently occurring chronic diseases worldwide.7 mHealth applications for patients with diabetes can support sustained self-management and help maintain lower long-term glucose levels.3 6 8
Despite the benefits associated with mHealth applications, there are several reasons why they are not used, such as difficulties in their control9 or acceptance problems.10–12 User acceptance can be described as ‘the demonstrable willingness within a user group to employ information technology for the tasks it is designed to support’.13 Several studies have shown that, especially for people with type 2 diabetes, the acceptance of mHealth self-management applications is noticeably low.8 14
Theoretical background
Technology acceptance models such as the Unified Theory of Acceptance and Use of Technology 2 (UTAUT2) have been developed to predict the acceptance of information technologies in health informatics and other fields of application.15–17
The UTAUT2 model was established in 2012 for use in a consumer context.18 In contrast to the previous technology acceptance models, UTAUT2 used additional exogenous constructs “habit”, “hedonic motivation” and “price value” to predict the endogenous construct “behavioral intention”, which is understood as an expression of technology acceptance.18 With the focus on the individuals and their needs, UTAUT2 is particularly suitable for predicting the acceptance of mHealth applications such as mobile diabetes applications.18 19 However, it is still not as widely used in mHealth acceptance studies as other technology acceptance models. Some studies using UTAUT2 have pointed out that essential aspects such as health-related factors19 20 or factors related to trust in the data collected2 21 are missing. In a previous qualitative study, we could confirm the general suitability of the UTAUT2 model in the field of mHealth self-management applications but identified some missing aspects, such as the awareness of the perceived threat of disease and credibility in the data collected by the application for predicting mHealth acceptance.22 Therefore, we proposed adding the following constructs to the UTAUT2 model: “perceived disease threat” and “trust”. The construct “trust” is associated with the belief that people accept uncertainties due to positive expectations.2 It is used to determine the data credibility and trustworthiness of the mobile health application, which is particularly important for behavioural intention and long-term use of mHealth applications.2 22
Furthermore, when patients face health-threatening situations, they are more open to new health technologies.11 12 Especially with chronic diseases like diabetes, the individual awareness of the risk and limitations for their health, reflected by the construct “perceived disease threat”, is a significant driver for acceptance and use of mHealth applications.20 22 Few studies have used the UTAUT2 model to predict mHealth acceptance to date, and these studies have not yet considered the two constructs of “perceived disease threat” and “trust”.19 22
Study objectives
This study aims to validate whether the exogenous UTAUT2 constructs, combined with the additional constructs “perceived disease threat” and “trust”, can predict mHealth acceptance using mobile diabetes applications as an example.
Hypotheses development and proposed research model
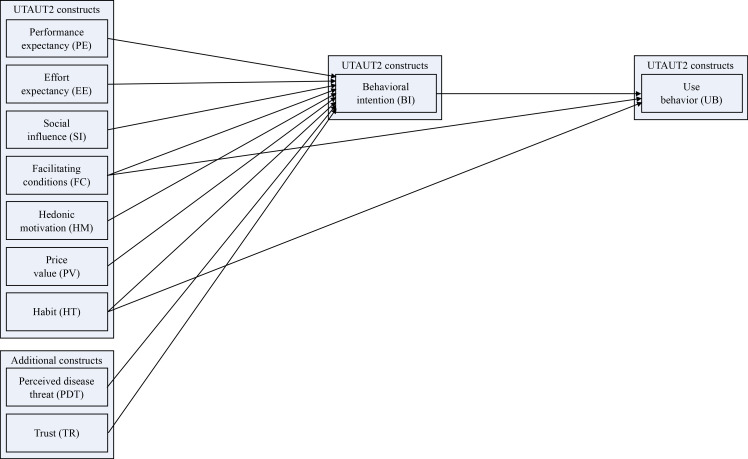
Figure 1 shows the proposed research model using the exogenous UTAUT2 constructs and additional constructs “perceived disease threat” (PDT) and “trust” (TR) for predicting the endogenous construct “behavioral intention” (BI) to use mHealth applications. Although this study focused on the acceptance (BI) of mobile diabetes applications, we also included the endogenous construct “use behavior” (UB) in the analysis to validate the exogenous constructs in the complete UTAUT2 model.
Figure 1.
Research model based on Unified Theory of Acceptance and Use of Technology 2 (UTAUT2) and additional constructs for predicting mHealth acceptance.18
Based on the existing UTAUT2 model, we adopted the relationships between the exogenous constructs “performance expectancy” (PE), “effort expectancy” (EE), “social influence” (SI), “facilitating conditions” (FC), “hedonic motivation” (HM), “price value” (PV) and “habit” (HT) and the endogenous construct “behavioral intention” (BI).18 In addition, the factors PDT and TR have been shown in various studies to predict the acceptance of mHealth applications.1 2 11 12 20 22 This leads to the following hypothesis: PE, EE, SI, FC, HM, PV, HT, PDT and TR affect the BI to use mobile diabetes applications.
Methods
Study design
To validate the proposed extension of the UTAUT2 model, we adopted a cross-sectional study design based on data from active mHealth users collected in an online survey.
Questionnaire
We used a web-based questionnaire based on a previously validated German translation of the UTAUT2 questionnaire.23 We slightly adapted the wording of the items to match the area of mobile diabetes applications.
We extended the questionnaire with already validated English items for the constructs PDT24 and TR.25 We used back-to-back translation by two independent translators fluent in English and German to translate these items, as no German translation was available.
The central part of the questionnaire consisted of the validated UTAUT2 items combined with the validated items from the constructs PDT and TR to predict mHealth acceptance (see table 1).
Table 1.
Constructs and items used in the web-based questionnaire
| Construct | Items | Cronbach’s alpha | Source adapted from |
| Performance expectancy (PE) | PE1: I find mobile diabetes applications useful in my daily life. PE2: Using mobile diabetes applications increases my chances of achieving things that are important to me. PE3: Using mobile diabetes applications helps me accomplish things more quickly. PE4: Using mobile diabetes applications increases my productivity. |
0.951* | Venkatesh et al.18, Harborth and Pape23 |
| Effort expectancy (EE) | EE1: Learning how to use mobile diabetes applications is easy for me. EE2: My interaction with mobile diabetes applications is clear and understandable. EE3: I find mobile diabetes applications easy to use. EE4: It is easy for me to become skillful at using mobile diabetes applications. |
0.922* | Venkatesh et al.18, Harborth and Pape23 |
| Social influence (SI) | SI1: People who are important to me think that I should use mobile diabetes applications. SI2: People who influence my behavior think that I should use mobile diabetes applications. SI3: People whose opinions that I value prefer that I use mobile diabetes applications. |
0.948* | Venkatesh et al.18, Harborth and Pape23 |
| Facilitating conditions (FC) | FC1: I have the resources necessary to use mobile diabetes applications. FC2: I have the knowledge necessary to use mobile diabetes applications. FC3: Mobile diabetes applications are compatible with other technologies I use. FC4: I can get help from others when I have difficulties using mobile diabetes applications. |
0.733* | Venkatesh et al.18, Harborth and Pape23 |
| Hedonic motivation (HM) | HM1: Using mobile diabetes applications is fun. HM2: Using mobile diabetes applications is enjoyable. HM3: Using mobile diabetes applications is very entertaining. |
0.937* | Venkatesh et al.18, Harborth and Pape23 |
| Price value (PV) | PV1: Mobile diabetes applications are reasonably priced. PV2: Mobile diabetes applications are a good value for the money. PV3: At the current price, mobile diabetes applications provide a good value. |
0.867* | Venkatesh et al.18, Harborth and Pape23 |
| Habit (HT) | HT1: The use of mobile diabetes applications has become a habit for me. HT2: I am addicted to using mobile diabetes applications. HT3: I must use mobile diabetes applications. |
0.879* | Venkatesh et al.18, Harborth and Pape23 |
| Behavioral intention (BI) | BI1: I intend to continue using mobile diabetes applications in the future. BI2: I will always try to use mobile diabetes applications in my daily life. BI3: I plan to continue to use mobile diabetes applications frequently. |
0.898* | Venkatesh et al.18, Harborth and Pape23 |
| Use behavior (UB) | Please choose your usage frequency for mobile diabetes applications: Never Once a month Several times a month Once a week Several times a week Once a day Several times a day Once an hour Several times an hour All the time |
1.000* | Venkatesh et al.18, Harborth and Pape23 |
| Perceived disease threat (PDT)† | PDT1: I am aware that my blood sugar control is not optimal. PDT2: I am very concerned about my blood sugar. PDT3: I am very concerned about diabetes-associated complications. |
0.743‡ | Zhang et al.24 |
| Trust (TR)† | TR1: I trust my mobile diabetes application. TR2: I find mobile diabetes applications reliable in conducting health services. TR3: I feel that mobile diabetes applications are safe for receiving reliable medical information. TR4: I trust mobile diabetes applications’ commitment to satisfy my medical information needs. |
0.869§ | Lee et al.25 |
We used a seven-point Likert scale ranging from 1 ‘strongly disagree’ to 7 ‘strongly agree’ to measure the items of the reflective measurement models.
In addition, we assessed information such as users’ experience with mobile diabetes applications, type of diabetes, use behaviour and sociodemographic data using single-item measurement. Overall, the questionnaire comprised 42 items.
We conducted a qualitative pretest to confirm content validity and understandability of the translated questionnaire. We discussed the questionnaire with five academic experts from the field of quantitative research and five users of mobile diabetes applications. We used the think-aloud method and captured all feedback. The results from this qualitative pretest were collected and discussed with all coauthors to reach a shared consensus before implementation. We slightly revised the questionnaire, for example, by adding some examples.
Participants
We used convenience sampling to recruit mobile diabetes application users from Austria and Germany. We included persons with diabetes type 1, type 2, and others or persons caring for relatives (eg, child) with diabetes who were 18 years or older and used a mobile diabetes application (eg, CGMs) for at least 3 months. We only included active mobile diabetes application users, as some constructs, such as HT, require current use.2 18 We primarily used social media to recruit participants for our web-based questionnaire. In addition, we teamed up with gatekeepers in diabetes associations, support groups and direct contacts to medical staff in diabetes outpatient clinics to encourage patients to participate in the online survey.
Participation in the online survey was voluntary, anonymous and could be discontinued anytime. We used browser session ID blocking and cookie settings to prevent multiple participation from the same individuals.
The inverse square root method was used to calculate the required sample size.26 Using a power of 80%, significance level p<0.05, and a minimum path coefficient of pmin=0.185 based on studies with similar complexity,2 the calculated minimum sample size was 181 participants.
Data analysis
We used partial least squares structural equation modelling (PLS-SEM) for data analysis because this method is particularly suitable for exploratory research with high model complexity and has already been used and established in similar studies.1 2 27 We used SmartPLS3 software for structural equation modelling.
We followed the data analysis approach described by Hair et al,28 divided into measurement and structural model evaluation. After completing the measurement and structural model evaluation, we conducted an additional moderator analysis using the UTAUT2 moderators ‘age’, ‘gender’ and ‘experience’ to evaluate any potential moderator effect.
Results
Descriptive data
Overall, 514 persons participated in the web-based survey, of which only 413 gave their consent, completed the questionnaire, and met the inclusion criteria. The sample demographic characteristics are shown in table 2.
Table 2.
Sample demographic characteristics
| Characteristics | Mobile diabetes app users (n=413) |
| Gender, n (%) | |
| Female | 256 (62.0%) |
| Male | 152 (36.8%) |
| Diverse | 0 (0.0%) |
| Not mentioned | 5 (1.2%) |
| Age, n (%) | |
| 18–24 years | 15 (3.6%) |
| 25–34 years | 68 (16.5%) |
| 35–44 years | 102 (24.7%) |
| 45–54 years | 108 (26.2%) |
| 55–64 years | 84 (20.3%) |
| 65 years and older | 32 (7.7%) |
| Not mentioned | 4 (1.0%) |
| Type of diabetes, n (%) | |
| Type 1 | 344 (83.3%) |
| Type 2 | 64 (15.5%) |
| Others | 5 (1.2%) |
| Disease duration, years | |
| Mean (SD) | 18.17 (SD 14.77) |
| Range (median) | 0.25–63.00 (15.00) |
| Duration of use, months | |
| Mean (SD) | 31.30 (SD 26.18) |
| Range (median) | 3.00–240.00 (24.00) |
| Type of mobile diabetes app, n (%) | |
| Smartphone app (eg, diabetes diary) | 199 (48.18%) |
| Continuous glucose measurement system | 383 (92.7%) |
| Smart insulin pump (eg, closed-loop system) | 79 (19.1%) |
| Others | 0 (0.0%) |
| Frequency of use, n (%) | |
| Never | 0 (0.0%) |
| Once a month | 1 (0.2%) |
| Several times a month | 3 (0.7%) |
| Once a week | 2 (0.5%) |
| Several times a week | 6 (1.5%) |
| Once a day | 3 (0.7%) |
| Several times a day | 154 (37.3%) |
| Once an hour | 25 (6.1%) |
| Several times an hour | 59 (14.3%) |
| All the time | 160 (38.7%) |
Measurement model evaluation
We started evaluating the reflective measurement model’s convergent validity by checking the indicators’ loadings to assess indicator reliability. However, the loadings of the items FC4, HT2, PDT1 and PV1 did not satisfy the required value of 0.708.27 Based on Hair et al,28 items with loadings below 0.40 should be eliminated. Therefore, we deleted PDT1 with a loading of 0.156 from the measurement model for the following investigations. In addition, items with loadings between 0.40 and 0.70 should only be deleted if this will increase composite reliability, which was not the case for FC4, HT2 and PV1.28 We assessed internal consistency reliability by checking composite reliability and Cronbach’s alpha in the second step, both in the recommended range for exploratory research, as shown in table 3. In the third step, we evaluated convergent validity by assessing the average variance extracted (AVE). The values for AVE were above the required 0.50.27 In the last step, we assessed discriminant validity by checking the heterotrait–monotrait (HTMT) ratio of the correlations for which all values were in the interval (0.028, 0.747), satisfying the HTMT requirements to be below 0.9027 (see online supplemental material 1). Thus, the evaluation of the measurement model proved that our data satisfied the requirements for reliability and validity. However, UB was excluded from the measurement model evaluation since the test criteria do not apply to single-item constructs.28
Table 3.
Evaluation of the measurement model
| Construct | Item | Convergent validity | Internal consistency reliability | Discriminant validity | |||
| Loadings | Indicator reliability | AVE | Composite reliability | Cronbach’s alpha | HTMT ratio below 0.90 | ||
| > 0.708 | > 0.50 | > 0.50 | > 0.70 | 0.60–0.95 | |||
| BI | BI1 | 0.872 | 0.760 | 0.835 | 0.938 | 0.901 | Yes |
| BI2 | 0.938 | 0.880 | |||||
| BI3 | 0.929 | 0.863 | |||||
| EE | EE1 | 0.899 | 0.808 | 0.773 | 0.931 | 0.902 | Yes |
| EE2 | 0.909 | 0.826 | |||||
| EE3 | 0.901 | 0.812 | |||||
| EE4 | 0.804 | 0.646 | |||||
| FC | FC1 | 0.776 | 0.602 | 0.525 | 0.812 | 0.695 | Yes |
| FC2 | 0.757 | 0.573 | |||||
| FC3 | 0.809 | 0.654 | |||||
| FC4 | 0.521 | 0.271 | |||||
| HM | HM1 | 0.891 | 0.794 | 0.705 | 0.876 | 0.807 | Yes |
| HM2 | 0.899 | 0.808 | |||||
| HM3 | 0.716 | 0.513 | |||||
| HT | HT1 | 0.798 | 0.637 | 0.561 | 0.792 | 0.628 | Yes |
| HT2 | 0.672 | 0.452 | |||||
| HT3 | 0.771 | 0.594 | |||||
| PDT | PDT2 | 0.876 | 0.767 | 0.844 | 0.915 | 0.817 | Yes |
| PDT3 | 0.936 | 0.876 | |||||
| PE | PE1 | 0.789 | 0.623 | 0.666 | 0.888 | 0.833 | Yes |
| PE2 | 0.801 | 0.642 | |||||
| PE3 | 0.835 | 0.697 | |||||
| PE4 | 0.838 | 0.702 | |||||
| PV | PV1 | 0.663 | 0.440 | 0.706 | 0.876 | 0.789 | Yes |
| PV2 | 0.910 | 0.828 | |||||
| PV3 | 0.922 | 0.850 | |||||
| SI | SI1 | 0.875 | 0.766 | 0.768 | 0.909 | 0.850 | Yes |
| SI2 | 0.887 | 0.787 | |||||
| SI3 | 0.867 | 0.752 | |||||
| TR | TR1 | 0.788 | 0.621 | 0.662 | 0.887 | 0.830 | Yes |
| TR2 | 0.784 | 0.615 | |||||
| TR3 | 0.817 | 0.667 | |||||
| TR4 | 0.864 | 0.746 | |||||
BI, behavioural intention; EE, effort expectancy; FC, facilitating conditions; HM, hedonic motivation; HT, habit; PDT, perceived disease threat; PE, performance expectancy; PV, price value; SI, social influence; TR, trust.
bmjhci-2022-100640supp001.pdf (12.1KB, pdf)
Structural model evaluation
In the first step of structural model evaluation, we assessed collinearity to ensure no bias in the regression results using the variance inflation factor (VIF). The values for VIF ideally should be below 3,27 which could be verified by our data with values in the interval (1.061, 1.741) (see online supplemental material 2). We assessed the path coefficients by checking their p values using the bootstrapping method (see figure 2). We applied a significance level of p<0.05, where we could identify that only PDT (0.091, p=0.019, f²=0.012), TR (0.145, p=0.034, f²=0.022), PE (0.285, p<0.001, f²=0.076) and HT (0.171, p=0.025, f²=0.027), had a significant impact on BI (see online supplemental material 3). In addition, we could identify a significant impact of HT (0.362, p<0.001, f²=0.113) and FC (−0.110, p=0.049, f²=0.012) on UB (see online supplemental material 4). Next, we assessed the adjusted coefficient of determination (), which measures the models’ predictive power and explains the variance of the endogenous construct.27 Our extended UTAUT2 model could explain 35.0% of the variance in BI and 14.4% in UB (see figure 2) compared with the original UTAUT2 model without the constructs PDT and TR, which explained 32.8% of the variance in BI and 14.4% in UB (see online supplemental material 5). In addition, we assessed the predictive relevance (Q²) of the extended UTAUT2 model for BI and UB using blindfolding procedures. Our model achieved a Q²=0.274 for BI, implying medium predictive accuracy, and a Q²=0.129 for UB, implying low predictive accuracy.27 Thus, our results indicate that the extended UTAUT2 model is suitable for predicting the acceptance of mobile diabetes applications. The additional moderator analysis revealed no significant effect of the UTAUT2 moderators on the relationships between endogenous and exogenous constructs (see online supplemental material 6).
Figure 2.
Extended Unified Theory of Acceptance and Use of Technology 2 (UTAUT2) model, including path coefficients and adjusted coefficient of determination.
bmjhci-2022-100640supp002.pdf (12.5KB, pdf)
bmjhci-2022-100640supp003.pdf (15KB, pdf)
bmjhci-2022-100640supp004.pdf (14.6KB, pdf)
bmjhci-2022-100640supp005.pdf (503.7KB, pdf)
bmjhci-2022-100640supp006.pdf (16.5KB, pdf)
Discussion
Principal findings and comparison with prior work
As a starting point for such an extension, we could confirm the relevance of the newly added constructs PDT and TR for predicting mHealth acceptance. Both constructs have already been individually highlighted as relevant in earlier mHealth acceptance studies.1 2 20 In particular, TR has been emphasised as a significant driver for accepting mHealth applications as they need to be reliable and trustworthy.2 12 21 Several studies have shown that TR is crucial for BI, especially for applications that impact personal health, such as mobile diabetes applications.1 2 12 PDT has also been identified as significant to the acceptance of mHealth applications, particularly for chronic diseases.12 19 24 Various studies have emphasised that people who are aware and concerned about their poor health conditions are more open to new technologies.20 24 29 To summarise, PDT and TR need to be considered for a UTAUT2 extension to predict mHealth acceptance better.
We also identified a significant impact of the UTAUT2 constructs, PE and HT, in predicting mHealth acceptance, where PE showed the strongest effect, which is in line with several mHealth acceptance studies.20 30
In contrast to the previous qualitative study,22 we could not confirm the significance of the remaining exogenous UTAUT2 constructs EE, SI, FC, HM and PV for predicting mHealth acceptance. These findings are consistent with other mHealth acceptance studies using UTAUT2 that also showed no significant impact of those constructs on BI.12 20 30
There could be different reasons why proven UTAUT2 constructs are not showing significance in this study. First, the motivation of people with chronic diseases to use mobile applications is probably different from those of the average technology consumer. While aspects such as fun (HM), convenience (EE), low price (PV), social influence (SI) and support (FC) are more relevant in the consumer context, the focus for mHealth applications is on the actual health benefit (PE) and the possibility of integrating the applications into everyday life (HT).11 12 18 30 Second, the relevance of these aspects might depend on the mHealth application and how it is provided to the patient. For example, according to the ‘National Association of Health Insurers’ (German: GKV-Spitzenverband) in Germany and the ‘Main Association of Austrian Social Insurance Institutions’ (German: Hauptverband der oesterreichischen Sozialversicherungstraeger), CGM systems are considered medical aids that physicians usually prescribe, and whose costs are covered by statutory health insurances.22 Thus, the application used is mainly specified by the statutory health insurance, which makes factors such as the cost (PV) and personal recommendations (SI) less relevant for patients. As 92.7% of participants used CGMs, this could explain the lack of influence of several UTAUT2 constructs in this study. Additionally, the German Federal Institute for Drugs and Medical Devices (BfArM) has released the so-called digital health applications (DiGA) registry, containing digital medical products (web applications, native apps) which have undergone an appropriate evaluation procedure and can be prescribed by physicians and psychotherapists at the expense of the statutory health insurances.20 Third, from the perspective of several authors of mHealth acceptance studies using UTAUT2, constructs such as PV and HM are not applicable.20 21 30 In summary, all this may explain why not all UTAUT2 constructs are relevant in the context of mHealth acceptance. Thus, this points to the need for a larger UTAUT2 extension for mHealth acceptance.
Using our extended UTAUT2 model, we increased the explained variance in BI from 32.8% in the original UTAUT2 model to 35.0%. This aligns with other mHealth acceptance studies extending UTAUT2, where the explained variance in BI ranged from 19.4%2 to 56.0%.20 Thus, the explained variance in BI of R² = 0.350 is comparable to results from other studies and is within the medium range.
Finally, the additional moderator analysis revealed no significant moderating effect, which aligns with other mHealth acceptance studies not showing a moderating effect.1 29
Strengths and limitations
With our quantitative study design, we validated the proposed extension of the UTAUT2 model for predicting mHealth acceptance of mobile diabetes applications. Using validated scales combined with a qualitative pretest, we could ensure the quality of our web-based questionnaire.
The focus of this study was to validate the constructs relevant for predicting mHealth acceptance (BI) in the UTAUT2 model. We thus only briefly touched on the construct of UB. UB is subject to some weaknesses due to its operationalisation as an ordinal-scaled single item in the validated German translation of the UTAUT2 questionnaire. In addition, several UTAUT and UTAUT2 studies focusing on mHealth acceptance have operationalised BI only and have not considered UB.2 20 31 However, in mHealth acceptance studies that operationalised UB, the values for the explained variance (R²) in UB were also relatively low, for example, R² = 0.111 in Dou et al29 or R² = 0.320 in Fitrianie et al,21 which is consistent with our results of R² = 0.144. We thus concentrated on BI in our statistical analysis.
Using a convenience sample-based recruitment process via social media and directly through, for example, diabetes outpatient clinics, we recruited a diverse sample of different ages, genders, diabetes types and experiences. Using these different recruitment channels, we reduced the risk of sampling bias, thus ensuring that we reached all relevant user groups. However, since all participants used the same link to access the web-based questionnaire (regardless of whether they accessed it via social media or another recruitment channel), it was impossible to distinguish which proportion of the sample derived from social media platforms and which, for example, from diabetes outpatient clinics.
With 413 participants, our study achieved sufficient power to perform PLS-SEM and predict mHealth acceptance. Our study only involved active and experienced mobile diabetes application users. Thus, we did not include non-users or seldom users, making our result generalisable to real mHealth users who use the mHealth application as part of their chronic disease self-management, have similar levels of experience with mHealth applications, and come from countries with comparable healthcare systems, technical infrastructure, cultural and socioeconomic background like the convenience sample used. In particular, this applies to mHealth self-management applications, such as diet or exercise apps designed for various chronic diseases, for example, type 2 diabetes, hypertension, obesity and others.32
Implications for future research
Our results showed the relevance of PE, HT, PDT and TR for accepting mobile diabetes applications. Therefore, mHealth providers should consider addressing and implementing these factors when developing new mHealth applications in this context.
Although we could verify two new relevant constructs, our extended UTAUT2 model still explained only 35.0% of the variance in BI. Further research thus needs to identify additional factors for predicting mHealth acceptance that have not yet been identified and considered in the extended UTAUT2 model.
In addition, future mHealth acceptance research in chronic diseases should consider external conditions, such as country-specific financial support for mHealth users from, for example, statutory health insurances, which helps to fund the required mHealth applications.
Future research may also investigate if considering additional dimensions to our extended UTAUT2 model, for example, from the diffusion of innovations theory, could contribute to the explained variance in BI.
Our study focused on active and experienced mobile diabetes application users. Future research may also involve patients with diabetes who do not use mobile diabetes applications. This could help understanding barriers and facilitators to accept and use mobile diabetes applications and identify potential additional predictors.
Since other studies have identified previous use as a strong predictor of future behaviour,20 this predictor should be considered in future mHealth acceptance studies using UTAUT2.
Conclusions
We could confirm that the additional constructs PDT and TR need to be added to UTAUT2 to predict mHealth acceptance, especially for chronic diseases such as diabetes. However, our results also show that not all constructs of the UTAUT2 model can predict mHealth acceptance.
Despite the newly added constructs, UTAUT2 can only partially predict mHealth acceptance. Future research should investigate additional mHealth acceptance factors, including how patients perceive trust in mHealth applications.
Acknowledgments
We gratefully acknowledge the Austrian Diabetes Association, Diabetics Lower Saxony e.V., Tirol Clinics GmbH, and Dr. Stefan Richter for their help with mobile diabetes app user recruitment. In addition, we would like to thank all online survey participants.
Footnotes
Contributors: PS and EA planned the study. PS conducted the data collection and analysis and drafted and revised the paper. PS and AH planned the statistical analysis. EA and AH helped in reviewing the results of the pretests, in data analysis, and in drafting the paper. PS acts as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by research committee for scientific, ethical questions of the UMIT TIROL - Private University for Health Sciences and Health Technology (RCSEQ 2805/20). Participants gave informed consent to participate in the study before taking part.
References
- 1.Akdur G, Aydin MN, Akdur G. Adoption of mobile health apps in dietetic practice: case study of diyetkolik. JMIR Mhealth Uhealth 2020;8:e16911. 10.2196/16911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schomakers E-M, Lidynia C, Vervier LS, et al. Applying an extended UTAUT2 model to explain user acceptance of lifestyle and therapy mobile health apps: survey study. JMIR Mhealth Uhealth 2022;10:e27095. 10.2196/27095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hood M, Wilson R, Corsica J, et al. What do we know about mobile applications for diabetes self-management? A review of reviews. J Behav Med 2016;39:981–94. 10.1007/s10865-016-9765-3 [DOI] [PubMed] [Google Scholar]
- 4.Virella Pérez YI, Medlow S, Ho J, et al. Mobile and web-based Apps that support self-management and transition in young people with chronic illness: systematic review. J Med Internet Res 2019;21:e13579. 10.2196/13579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H, Long H. The effect of smartphone app-based interventions for patients with hypertension: systematic review and meta-analysis. JMIR Mhealth Uhealth 2020;8:e21759. 10.2196/21759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, Guo X, Zhang Z. The efficacy of mobile phone apps for lifestyle modification in diabetes: systematic review and meta-analysis. JMIR Mhealth Uhealth 2019;7:e12297. 10.2196/12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Diabetes Federation . IDF diabetes atlas. 10th edition, 2021. [Google Scholar]
- 8.Trawley S, Baptista S, Browne JL, et al. The use of mobile applications among adults with type 1 and type 2 diabetes: results from the second Miles-Australia (Miles-2) study. Diabetes Technol Ther 2017;19:730–8. 10.1089/dia.2017.0235 [DOI] [PubMed] [Google Scholar]
- 9.Bentley CL, Powell L, Potter S, et al. The use of a smartphone app and an activity tracker to promote physical activity in the management of chronic obstructive pulmonary disease: randomized controlled feasibility study. JMIR Mhealth Uhealth 2020;8:e16203. 10.2196/16203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheibe M, Reichelt J, Bellmann M, et al. Acceptance factors of mobile apps for diabetes by patients aged 50 or older: a qualitative study. Med 2 0 2015;4:e1. 10.2196/med20.3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breil B, Kremer L, Hennemann S, et al. Acceptance of mHealth apps for self-management among people with hypertension. Stud Health Technol Inform 2019;267:282–8. 10.3233/SHTI190839 [DOI] [PubMed] [Google Scholar]
- 12.Jacob C, Sezgin E, Sanchez-Vazquez A, et al. Sociotechnical factors affecting patients' adoption of mobile health tools: systematic literature review and narrative synthesis. JMIR Mhealth Uhealth 2022;10:e36284. 10.2196/36284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillon A. User acceptance of information technology. London: Taylor and Francis; 2001: 10. [Google Scholar]
- 14.Bults M, van Leersum CM, Olthuis TJJ, et al. Barriers and drivers regarding the use of mobile health apps among patients with type 2 diabetes mellitus in the Netherlands: explanatory sequential design study. JMIR Diabetes 2022;7:e31451. 10.2196/31451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatesh V, Morris MG, Davis GB, et al. User acceptance of information technology: toward a unified view. MIS Quarterly 2003;27:425–78. 10.2307/30036540 [DOI] [Google Scholar]
- 16.Davis FD. User acceptance of information technology: system characteristics, user perceptions and behavioral impacts. Int J Man Mach Stud 1993;38:475–87. 10.1006/imms.1993.1022 [DOI] [Google Scholar]
- 17.Ammenwerth E. Technology acceptance models in health informatics: TAM and UTAUT. Stud Health Technol Inform 2019;263:64–71. 10.3233/SHTI190111 [DOI] [PubMed] [Google Scholar]
- 18.Venkatesh V, Thong J, Xu X. Consumer acceptance and use of information technology: extending the unified theory of acceptance and use of technology. MIS Quarterly 2012;36:157–78. 10.2307/41410412 [DOI] [Google Scholar]
- 19.Alam MMD, Alam MZ, Rahman SA, et al. Factors influencing mHealth adoption and its impact on mental well-being during COVID-19 pandemic: a SEM-ANN approach. J Biomed Inform 2021;116:103722. 10.1016/j.jbi.2021.103722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breil B, Salewski C, Apolinário-Hagen J. Comparing the acceptance of mobile hypertension apps for disease management among patients versus clinical use among physicians: cross-sectional survey. JMIR Cardio 2022;6:e31617. 10.2196/31617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitrianie S, Horsch C, Beun RJ, et al. Factors affecting user's behavioral intention and use of a mobile-phone-delivered cognitive behavioral therapy for insomnia: a small-scale UTAUT analysis. J Med Syst 2021;45:110. 10.1007/s10916-021-01785-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schretzlmaier P, Hecker A, Ammenwerth E. Suitability of the unified theory of acceptance and use of technology 2 model for predicting mHealth acceptance using diabetes as an example: qualitative methods triangulation study. JMIR Hum Factors 2022;9:e34918. 10.2196/34918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harborth D, Pape S. German translation of the unified theory of acceptance and use of technology 2 (UTAUT2) questionnaire. SSRN Journal 2018;116. 10.2139/ssrn.3147708 [DOI] [Google Scholar]
- 24.Zhang Y, Liu C, Luo S, et al. Factors influencing patients' intentions to use diabetes management apps based on an extended unified theory of acceptance and use of technology model: web-based survey. J Med Internet Res 2019;21:e15023. 10.2196/15023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee W-I, Fu H-P, Mendoza N, et al. Determinants impacting user behavior towards emergency use intentions of m-Health services in Taiwan. Healthcare 2021;9. 10.3390/healthcare9050535. [Epub ahead of print: 03 05 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kock N. Minimum Sample Size Estimation in PLS-SEM: An Application in Tourism and Hospitality Research. In: Ali F, ed. Applying partial least squares in tourism and hospitality research. Bingley: Emerald Publishing Limited, 2019: 1–16. [Google Scholar]
- 27.Hair JF, Risher JJ, Sarstedt M, et al. When to use and how to report the results of PLS-SEM. EBR 2019;31:2–24. 10.1108/EBR-11-2018-0203 [DOI] [Google Scholar]
- 28.Hair JF, Hult GTM, Ringle CM. A primer on partial least squares structural equation modeling (PLS-SEM). Los Angeles: Sage Publ, 2014. [Google Scholar]
- 29.Dou K, Yu P, Deng N, et al. Patients' acceptance of smartphone health technology for chronic disease management: a theoretical model and empirical test. JMIR Mhealth Uhealth 2017;5:e177. 10.2196/mhealth.7886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salgado T, Tavares J, Oliveira T. Drivers of mobile health acceptance and use from the patient perspective: survey study and quantitative model development. JMIR Mhealth Uhealth 2020;8:e17588. 10.2196/17588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apolinário-Hagen J, Menzel M, Hennemann S, et al. Acceptance of mobile health Apps for disease management among people with multiple sclerosis: web-based survey study. JMIR Form Res 2018;2:e11977. 10.2196/11977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debon R, Coleone JD, Bellei EA, et al. Mobile health applications for chronic diseases: a systematic review of features for lifestyle improvement. Diabetes Metab Syndr 2019;13:2507–12. 10.1016/j.dsx.2019.07.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjhci-2022-100640supp001.pdf (12.1KB, pdf)
bmjhci-2022-100640supp002.pdf (12.5KB, pdf)
bmjhci-2022-100640supp003.pdf (15KB, pdf)
bmjhci-2022-100640supp004.pdf (14.6KB, pdf)
bmjhci-2022-100640supp005.pdf (503.7KB, pdf)
bmjhci-2022-100640supp006.pdf (16.5KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.