Abstract
Objective
Dengue shock syndrome (DSS) is a serious health condition leading to paediatric intensive care unit (PICU) admissions and deaths in tropical countries. Acute respiratory failure (ARF) is associated with DSS and is a major cause of dengue deaths. We aimed to identify risk factors associated with ARF in children with DSS.
Methods
We retrospectively reviewed children with DSS admitted to a PICU from 2010 to 2020 at a tertiary level hospital in Bangkok, Thailand. Patient characteristics, clinical parameters and laboratory data were collected. Multivariable logistic regression analysis was used to identify factors associated with ARF.
Results
Twenty-six (43.3%) of 60 children with DSS developed ARF and 6 did not survive to day 28. The median (IQR) age was 8.1 years (IQR 4.0–11.0). Fluid accumulation during the first 72 hours of PICU admission was greater in the ARF group compared with the non-ARF group (12.2% (IQR 7.6–21.7) vs 8.3% (IQR 4.4–13.3), p=0.009). In a multivariate analysis at 72 hours post PICU admission, the presence of ˃15% fluid accumulation was independently associated with ARF (adjusted OR 5.67, 95% CI 1.24 to 25.89, p=0.025).
Conclusion
ARF is an important complication in children with DSS. A close assessment of patient fluid status is essential to identify patients at risk of ARF. Once the patient is haemodynamically stable and leakage slows, judicious fluid management is required to prevent ARF.
Keywords: Resuscitation
Key messages.
Acute respiratory failure (ARF) is not an uncommon complication in paediatric dengue shock syndrome (DSS).
This study demonstrated that fluid accumulation is a strong risk factor for developing ARF among children with DSS.
Once shock stabilised, early recognition of fluid accumulation and prompt management of fluid removal are needed to prevent unfavourable respiratory outcomes.
However, further larger prospective cohort studies are required to establish evidence for the causal relationship.
Introduction
Dengue infections, a mosquito-borne viral disease, range from subclinical illness to fatal outcomes. Dengue shock syndrome (DSS) is a dangerous complication of dengue infection that is associated with admission to the paediatric intensive care unit (PICU) and mortality rates of 1%–26%.1–4 In addition to shock, uncontrolled bleeding, and multiple organ failure, acute respiratory failure (ARF) is an important complication that can lead to death.5 6 An improved understanding of risk factors for ARF in paediatric DSS will help improve patient care and clinical outcomes. We aimed to identify factors associated with ARF in children with DSS admitted to a PICU in a Thai tertiary university referral hospital.
Methods
Study design, setting and patients
This retrospective study was conducted at a PICU in a tertiary university referral hospital in Bangkok, Thailand. All children aged 1 month to 18 years with DSS admitted from January 2010 to December 2020 were included. The primary outcome was ARF.
Data collection
Data were obtained from medical records, nursing flow sheets, physician notes and physician orders. The patients were divided into ARF and non-ARF groups. The data include demographic characteristics, underlying complex chronic conditions,7 8 severity at the time of admission represented by the Pediatric Risk of Mortality III score9, features of severe dengue,6 10 multiple organ dysfunction syndrome defined as the presence of two or more dysfunctional organs at any time during PICU admission according to the International Pediatric Sepsis Consensus Conference definition,11 12 clinical variables (table 1) and laboratory results (table 2). Percentage of fluid accumulation adjusted for body weight (table 3), and therapeutic interventions and PICU resources used were also recorded. These include fluid administration, vasoactive/inotropic agents, continuous renal replacement therapy, blood component transfusion, haemodynamic monitoring, length of PICU stay, length of hospital stay and 28-days mortality. The vasoactive/inotropic score obtained during the PICU stay was calculated based on the following formula13 14:
Table 1.
Demographic and clinical characteristic
| Variables | Total patients (n=60) | Acute respiratory failure | P value‡ | |
| Absent (n=34) | Present (n=26) | |||
| Age, year, median (IQR) | 8.1 (4.0–11.0) | 8.3 (4.3–11.1) | 7.9 (3.4–11.1) | 0.602 |
| Male gender, n (%) | 33 (55.0) | 21 (61.8) | 12 (46.2) | 0.297 |
| BMI, kg/m2, median (IQR) | 16.0 (13.9–21.0) | 15.8 (13.6–20.8) | 16.6 (14.1–21.6) | 0.864 |
| Complex chronic conditions, n (%) | 18 (30.0) | 11 (32.4) | 7 (26.9) | 0.779 |
| PRISM III score at PICU admission, median (IQR) | 10.0 (7.0–12.0) | 9.0 (5.0–11.3) | 12.0 (8.8–18.5) | 0.015 |
| Numbers of patients with MODS on day 1, n (%) | 43 (71.7) | 20 (58.8) | 23 (88.5) | 0.019 |
| Features of severe dengue* | ||||
| Severe bleeding (required transfusion), n (%) | 22 (36.7) | 7 (20.6) | 15 (57.7) | 0.006 |
| AST or ALT≥1000 U/L, n (%) | 16 (26.7) | 5 (14.7) | 11 (42.3) | 0.021 |
| Impaired consciousness, n (%) | 17 (28.3) | 3 (8.8) | 14 (53.8) | < 0.001 |
| Acute kidney injury†, n (%) | 25 (41.7) | 12 (35.3) | 13 (50.0) | 0.298 |
| Complications during PICU admission | ||||
| Evidence of fluid leakage | 43 (71.1) | 21 (61.8) | 22 (84.6) | 0.082 |
| Acute liver failure, n (%) | 8 (13.3) | 1 (2.9) | 7 (26.9) | 0.016 |
| Haemophagocytic syndrome, n (%) | 2 (3.3) | 1 (2.9) | 1 (3.8) | 1.000 |
| Coinfection (pathogen identified), n (%) | 11 (18.3) | 2 (5.9) | 9 (34.6) | 0.006 |
| 28-day mortality, n (%) | 6 (10.0) | 0 (0) | 6 (23.1) | 0.005 |
| PICU stay, days, median (IQR) | 3.8 (2.3–5.8) | 3.0 (2.1–5.0) | 5.2 (3.4–8.5) | 0.004 |
| Hospital stay, days, median (IQR) | 7.3 (4.8–10.8) | 6.7 (4.4–10.6) | 8.8 (6.2–13.7) | 0.081 |
*Features of severe dengue are defined by 2009 WHO guidelines.
†Acute kidney injury is defined according to 2012 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines for acute kidney injury.
‡Pearson’s χ2 test or Fisher’s exact test for qualitative data whereas Mann-Whitney U test for quantitative data.
§MODS = multiple organ dysfunction syndrome, defined by 2 or more organ dysfunction. Organ dysfunction, defined according to IPSCC definitions for sepsis and organ dysfunction in paediatrics.
¶
ALT, Alanine transaminase; AST, Aspartate transaminase; BMI, body mass index; CCC, complex chronic conditions; KDIGO, Kidney Disease: Improving Global Outcomes; PICU, paediatric intensive care unit; PRISM III, Paediatric Risk of Mortality Score.
Table 2.
Laboratory results during the first 24 hours after PICU admission
| Total patients (n=60) |
Acute respiratory failure | P value‡ | ||
| Absent (n=34) | Present (n=26) | |||
| Haemoglobin, g/dL | 13.0 (10.4–15.0) | 12.7 (10.0–15.1) | 13.4 (10.7–14.9) | 0.665 |
| Haematocrit, % | 38.3 (31.7–44.7) | 37.6 (30.7–44.9) | 39.8 (33.7–44.1) | 0.743 |
| White blood cell count, 103/mm3 | 6.2 (3.1–9.2) | 4.6 (2.9–8.0) | 8.0 (4.4–16.0) | 0.022 |
| Neutrophil, % | 50.3 (31.3–63.3) | 44.5 (18.8–59.5) | 56.4 (46.0–64.2) | 0.023 |
| Lymphocyte, % | 27.0 (14.3–42.1) | 31.9 (16.8–51.5) | 18.5 (14.0–35.7) | 0.205 |
| Band form, % | 2.3 (0.0–11.3) | 2.7 (0.0–10.7) | 1.0 (0.0–13.3) | 0.880 |
| Atypical lymphocyte, % | 4.5 (1.3–8.8) | 4.9 (1.3–12.6) | 4.2 (1.3–6.8) | 0.361 |
| Platelet, 103/mm3 | 22.0 (15.0–46.0) | 21.5 (13.0–46.0) | 23.5 (15.0–49.3) | 0.571 |
| Prothrombin time, s | 14.6 (12.7–16.5) | 14.0 (12.5–15.2) | 16.2 (13.8–25.6) | 0.007 |
| INR, s | 1.3 (1.1–1.5) | 1.3 (1.1–1.3) | 1.4 (1.2–2.3) | 0.345 |
| Partial thromboplastin time, s | 42.5 (35.6–54.5) | 38.5 (32.1–43.3) | 57.0 (42.7–60.5) | < 0.001 |
| Fibrinogen, mg/dL | 117.4 (87.2–171.4) | 130.0 (82.1–172.7) | 113.8 (87.4–170.2) | 0.687 |
| Albumin, mg/dL | 2.8 (2.3–3.4) | 2.8 (2.2–3.5) | 2.8 (2.3–3.4) | 0.811 |
| AST, U/L | 215.5 (105.3–1253.3) | 185.5 (90.5–433.3) | 446.5 (113.5–3292.0) | 0.080 |
| ALT, U/L | 73.0 (37.0–296.5) | 63.5 (28.8–146.5) | 120.0 (37.0–894.8) | 0.187 |
| ALP, U/L | 102.0 (69.0–125.0) | 100.0 (68.0–121.0) | 115.5 (73.5–142.5) | 0.303 |
| Total bilirubin, mg/dL | 0.40 (0.21–1.33) | 0.31 (0.20–0.78) | 0.63 (0.22–2.58) | 0.136 |
| Direct bilirubin, mg/dL | 0.20 (0.10–0.59) | 0.17 (0.10–0.41) | 0.23 (0.10–1.86) | 0.135 |
| BUN, mg/dL | 10.8 (7.5–19.3) | 9.9 (7.2–15.8) | 11.4 (7.5–24.9) | 0.447 |
| Creatinine, mg/dL | 0.52 (0.36–0.85) | 0.49 (0.38–0.82) | 0.57 (0.36–1.02) | 0.660 |
| Estimated GFR, mL/min/1.73 m2* | 86.4 (69.5–124.2) | 95.0 (71.0–125.2) | 82.9 (57.2–117.8) | 0.257 |
| Bicarbonate, mmol/L | 17.0 (14.0–19.0) | 17.5 (14.0–20.0) | 16.0 (13.0–18.0) | 0.096 |
| Lactate, mmol/L (N1=13/34, N2=12/26)† | 3.3 (2.2–5.8) | 2.5 (1.6–3.9) | 4.7 (2.4–8.7) | 0.087 |
| Arterial pH (N1=17/34, N2=22/26)† | 7.40 (7.34–7.42) | 7.42 (7.38–7.46) | 7.36 (7.25–7.41) | 0.006 |
*Estimated GFR is calculated using modified Schwartz formular.
†N1=number of patients who did not require respiratory support. N2=number of patients who were supported with NIV/MV.
‡Pearson’s χ2 test or Fisher’s exact test for qualitative data whereas Mann-Whitney U test for quantitative data.
§
ALP, Alkaline phosphatase; BUN, blood urea nitrogen; GFR, glomerulous filtration rate; MV, mechanical ventilation; NIV, non-invasive ventilation; PICU, paediatric intensive care unit.
Table 3.
Interventions and resource utilisation
| Total patients (n=60) |
Acute respiratory failure | P value§ | ||
| Absent (n=34) | Present (n=26) | |||
| Fluid accumulation*, (%) | ||||
| At 24 hours | 11.1 (5.6–16.8) | 9.3 (4.8–14.8) | 14.2 (8.2–22.6) | 0.018 |
| At 48 hours | 10.4 (5.9–15.3) | 8.8 (4.4–14.0) | 11.7 (6.9–23.7) | 0.077 |
| At 72 hours | 10.5 (5.6–15.1) | 8.3 (4.4–13.3) | 12.2 (7.6–21.7) | 0.009 |
| Early fluid accumulation†, n (%) | 33 (55.0) | 15 (44.1) | 18 (69.2) | 0.069 |
| Patients with>15% fluid accumulation at 72 hours*, (N1=33/34, N2=25/26)‡ | 14 (24.1) | 3 (9.1) | 11 (44.0) | 0.004 |
| Fluid removal, n (%) | ||||
| None, n (%) | 20 (33.3) | 15 (44.1) | 5 (19.2) | 0.004 |
| Diuretic administration only, n (%) | 31 (51.7) | 18 (52.9) | 13 (50.0) | |
| Renal replacement therapy, n (%) | 9 (15.0) | 1 (2.9) | 8 (30.8) | |
| Vasoactive-inotropic agent administration | ||||
| ≥ 1 agent (s), n (%) | 23 (38.3) | 10 (29.4) | 13 (50.0) | 0.118 |
| ≥ 2 agents, n (%) | 10 (16.7) | 3 (8.8) | 7 (26.9) | 0.085 |
| Duration of vasoactive-inotropic administration (hours), median (IQR) | 47.0 (22.7–93.2) | 46.0 (28.9–82.7) | 51.0 (17.2–94.5) | 0.976 |
| Maximum VI scores, median (IQR) | 12.5 (8.0–26.0) | 10.0 (8.0–20.0) | 20.0 (10.0–70.0) | 0.131 |
| Blood and blood component transfusion | ||||
| PRC transfusion (mL/kg), median (IQR) | 17.0 (9.3–31.6) | 10.0 (7.8–24.6) | 20.5 (10.0–43.1) | 0.146 |
| Platelet transfusion (mL/kg), median (IQR) | 11.3 (5.3–25.5) | 9.0 (5.3–20.2) | 18.7 (5.3–41.3) | 0.370 |
| FFP transfusion (mL/kg), median (IQR) | 16.6 (10.0–25.6) | 10.9 (7.7–20.5) | 18.8 (10.0–28.3) | 0.220 |
| Cryoprecipitate transfusion (mL/kg), median (IQR) | 6.7 (3.8–13.6) | 5.7 (4.5–8.5) | 6.8 (3.2–18.3) | 0.799 |
| Antibiotic administration, n (%) | 57 (95.0) | 31 (91.2) | 26 (100) | 0.251 |
| Other interventions | ||||
| Thoracentesis, n (%) | 3 (5.0) | 0 (0) | 3 (11.5) | 0.076 |
| Chest tube placement, n (%) | 2 (3.3) | 0 (0) | 2 (7.7) | 0.184 |
| Invasive monitoring | ||||
| Arterial blood pressure monitoring, n (%) | 12 (20.0) | 4 (11.8) | 8 (30.8) | 0.104 |
| Central venous access, n (%) | 37 (61.7) | 16 (47.1) | 21 (80.8) | 0.015 |
| Intra-abdominal pressure, n (%) | 2 (3.4) | 1 (2.9) | 1 (4.2) | 1.000 |
*Fluid accumulation (%) was calculated by the formula ((fluid intake – fluid output)/BW at PICU admission)×100.
†Early fluid accumulation was defined as fluid accumulation>10% on the first 24 hours after PICU admission.
‡N1=number of patients who did not require respiratory support. N2=number of patients who were supported with NIV/MV.
§Pearson’s χ2 test or Fisher’s exact test for qualitative data whereas Mann-Whitney U test for quantitative data.
FFP, Fresh frozen plasma; MV, mechanical ventilation; NIV, non-invasive ventilation; PICU, paediatric intensive care unit; PRC, Packed red cells.
*The units are measured in μg/kg/min.
Definition
The case definition was based on the WHO classification.6 15 DSS was defined as Dengue haemorrhagic fever (DHF) or laboratory-confirmed dengue infection with evidence of circulatory failure described as rapid, and/ or weak pulse with narrow pulse pressure (≤ 20 mm Hg), or hypotension for age with cold, clammy skin with restlessness.
In terms of fluid assessment, fluid accumulation was measured as the net balance between fluid intake and output. Intake fluid were measured by volume in mL, which included intravascular fluid, water and liquid formula feeding either via oral route or tube feeding. Routine output measurement in PICU included urine output, NG content and stool volume in case of watery stool. Insensible fluid loss during respiration could not be estimated, so, it was not taken into account.
The degree of fluid accumulation was calculated using the formula16 17
Early fluid accumulation was defined as≥10% in the first 24 hours after PICU admission.18
ARF was diagnosed when a patient developed clinical symptoms of severe acute respiratory distress, either hypoxaemic or hypercapnic, that could not be maintained with oxygen therapy (e.g. low-flow oxygen cannula, non-rebreathing mask, and high-flow oxygen cannula), and required an escalation of respiratory support including non-invasive ventilation (NIV) and mechanical ventilation (MV), either conventional or high-frequency oscillatory ventilation.
The decisions in the utilisation of either NIV or MV and fluid administration were made by an attending physician.
Statistical analysis
Data were analysed using SPSS V.20.0 (IBM). Data were presented as numbers and percentages for categorical variables and as the median and IQR for continuous variables. Quantile normal plots and density plots were used to assess normality and they showed non-normal distributions. Comparison between patient groups was performed using the χ2 test or the Fisher’s exact test for categorical variables, and the Mann-Whitney U test for continuous variables based on the distribution of data. Not all laboratory results were available for all patients. No data imputation was performed for missing data. For a bivariate analysis, simple logistic regression was performed to identify any potential predictor variables. Variables with a bivariate p value<0.1 and variables considered to be clinically relevant were included in the multivariate analysis to identify factors independently associated with ARF.
Results
Sixty patients were included and 33 (55%) were male. The median (IQR) age was 8.1 (4.0–11.0) years, and the overall 28-day mortality rate was 10%. Twenty-six (43%) patients had ARF. Among patients who developed ARF, NIV was used in 6 patients, while MV was used in the remaining 20 patients. Overall, the median (IQR) time of ventilatory support was 2.8 days (IQR 1.8–4.9), and no patient required supplemental oxygen at the time of hospital discharge. Among patients using NIV, median expiratory positive airway pressure (EPAP) used was 5.0 cmH2O (IQR 4.8–6.5). Among patients using MV, median PEEP used was 7.5 cmH2O (IQR 5.3–10.0) and median MAP was 12.6 cmH2O (IQR 10.9–17.8). Dengue NS1 Ag were tested in 52 patients, and 28 of them were positive. Dengue IgM were tested in 50 patients, and 32 of them were positive. There were missing data in 2 variables: 21/60 missing for arterial pH and 35/60 missing for lactate, which may contribute to interpretative limitation of the results.
The demographic and clinical characteristics are presented in table 1. All patients who died had ARF. Patients in the ARF group exhibited higher white blood cell counts (p=0.022) and neutrophils percentages (p=0.023), more prolonged prothrombin time (p=0.007), more prolonged partial thromboplastin time (p<0.001) and lower blood pH (p=0.006). (table 2) Two patients had no data on fluid balance at 48 hours and 72 hours due to a short PICU stay. One patient clinically improved was transferred to the general floor, and then discharged the following day. The other died within 24 hours of PICU admission.
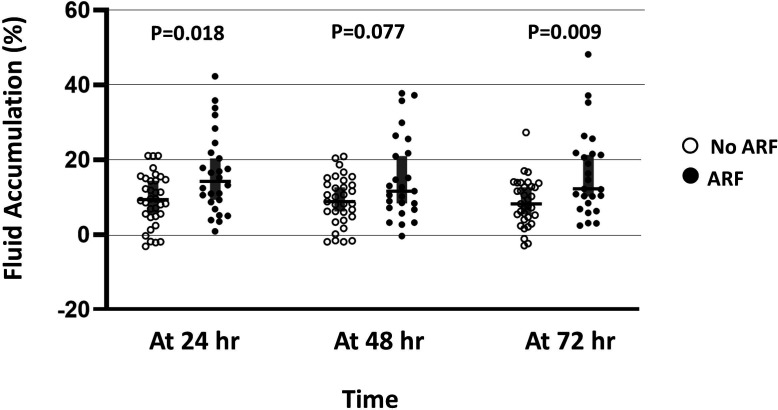
The median fluid accumulation during the first 72 hours of PICU admission was greater in the ARF group compared with the non-ARF group (12.2%, IQR 7.6–21.7 vs 8.3%, IQR 4.4–13.3, p=0.009). (figure 1) The proportion of patients in the ARF group having a cumulative fluid balance of>15% at 72 hours after PICU admission was higher compared with patients in the non-ARF group (44.0% vs 9.1%, p=0.004, table 3). Multivariate logistic regression analysis (table 4) revealed that a presence of>15% fluid accumulation at 72 hours after PICU admission was independently associated with ARF in paediatric patients with DSS (aOR 5.67, 95% CI 1.24 to 25.89, p=0.025).
Figure 1.
Percentages of fluid accumulation at 24, 48, and 72 hours, median (IQR). ARF, acute respiratory failure.
Table 4.
Factors associated with acute respiratory failure in paediatric dengue shock syndrome
| Factors | Bivariate analysis | Multivariate analysis | ||
| P value | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | |
| Age | 0.540 | 0.97 (0.86 to 1.08) | ||
| BMI (kg/m2) | 0.413 | 1.04 (0.95 to 1.13) | ||
| Presence of complex chronic conditions | 0.650 | 1.30 (0.42 to 4.00) | ||
| PRISM III | 0.013 | 1.16 (1.03 to 1.30) | ||
| Presence of MODS (≥ 2 ODs) | 0.017 | 5.37 (1.35 to 21.41) | 0.201 | 3.14 (0.54 to 18.06) |
| Prothrombin time, s | 0.031 | 1.15 (1.01 to 1.30) | 0.128 | 1.10 (0.97 to 1.25) |
| Estimated GFR (mL/min/1.73 m2) | 0.404 | 0.99 (0.98 to 1.01) | ||
| Presence of>15% fluid accumulation at 72 hours | 0.005 | 7.86 (1.89 to 32.69) | 0.025 | 5.67 (1.24 to 25.89) |
| Blood transfusion (mL/kg) | 0.207 | 1.02 (0.99 to 1.06) | ||
| Platelet transfusion (mL/kg) | 0.176 | 1.03 (0.99 to 1.06) | ||
| FFP transfusion (mL/kg) | 0.317 | 1.02 (0.98 to 1.06) | ||
BMI, body mass index; MODS, multiple organ dysfunction syndrome; PRISM III, Pediatric Risk of Mortality III Score.
Discussion
The 43% incidence of ARF in our cohort was much higher compared with other reports in Vietnam (17.1%)19 and from another study in Thailand (18.8%)5. This may be explained by many reasons. First, case definitions for acute respiratory distress and ARF vary between studies. Second, by defining ARF as requiring the use of respiratory support including NIV which is increasingly used as an alternative to MV, this may affect the overall numbers of patients having of ARF in different cohorts. Finally, because our facility is a tertiary referral hospital, our cohort may have more severe disease than in other studies. Unfortunately, illness severity was not assessed in the two previous studies, making direct comparisons difficult.
An increase in capillary permeability contributing to plasma leakage makes prompt fluid resuscitation a key DSS management strategy that aims to restabilise circulation in these haemodynamically unstable patients.20 21 Nevertheless, excessive fluid often accumulates in the extravascular space leading to unfavourable clinical outcomes including ARF. We focused on the first 72 hours after admission because shock episodes in DSS generally last 24–48 hours22 and the probability of fluid accumulation is highest during that period.
We found that more than 15% fluid accumulation at 72 hours after PICU admission was strongly associated with ARF in children with DSS. Consistent results were reported in a prospective interventional study conducted by Ranjit et al.23 In that study, restrictive fluid resuscitation and fluid removal were included in targeted interventions. They found lower degrees of positive fluid balance during the first 3 days, and fewer patients required positive pressure ventilation in the targeted intervention group compared with standard treatment. Finally, the study demonstrated a lower incidence of organ dysfunction and mortality in the intervention arm.
Few published articles have addressed DSS and adverse respiratory outcomes. However, a number of previous studies have reported associations between fluid overload and adverse respiratory outcomes in patients with critical illness. Septic shock, a condition that also involves massive capillary leakage, also requires careful fluid management. Our findings are consistent with other studies that showed that higher fluid accumulation, ranging from more than 10% to 20%, is associated with a higher proportion of patients requiring MV and a longer duration of MV.24–28
Variable aetiologies of ARF have been reported to concomitantly occur with features of severe dengue. These include significant pleural effusion from fluid leakage, pulmonary haemorrhage from thrombocytopenia and coagulopathy, transfusion-related acute lung injury from massive blood product transfusions, acute lung injury/ARDS and from hepatic dysfunction, and decreased level of consciousness and airway protective reflexes in dengue encephalopathy. In our cohort, we found the association between ARF and some features of severe dengue/complications including evidence of fluid leakage, severe bleeding, acute liver injury and impaired consciousness. However, those factors were not proven independent risk factors according to multivariable logistic regression analysis.29–32
Our study had certain limitations. First, this is a retrospective study in a single centre which may limit the generalisability of our findings. Second, a larger prospective cohort study is needed to provide better evidence of a causal relationship. Finally, we decided to use the presence of ARF as an outcome instead of mortality because of our small sample and the low fatality rate in our cohort. A larger sample size could overcome this limitation with respect to mortality.
Conclusion
The incidence of ARF among children with DSS in our cohort was high (43%). A presence of more than 15% fluid accumulation during the initial 72 hours after PICU admission was an independent risk factor for ARF. A careful assessment of the patient’s fluid status and fluid balance is necessary. Attention must be focused not only on fluid intake, but also on excessive fluid removal to mitigate the risk of devastating fluid accumulation and ARF in paediatric DSS.
Supplementary Material
Acknowledgments
The authors would like to thank the staff of the Division of Clinical Epidemiology for their assistance with the statistical analysis.
Footnotes
NP contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript. SP supervised and critically reviewd the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available. Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Institutional Review Board of Siriraj Hospital, Bangkok, Thailand with an exemption for informed consent (approval number: Si 073/2021).
References
- 1.Anders KL, Nguyet NM, Chau NVV, et al. Epidemiological factors associated with dengue shock syndrome and mortality in hospitalized dengue patients in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg 2011;84:127–34. 10.4269/ajtmh.2011.10-0476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta V, Yadav TP, Pandey RM, et al. Risk factors of dengue shock syndrome in children. J Trop Pediatr 2011;57:451–6. 10.1093/tropej/fmr020 [DOI] [PubMed] [Google Scholar]
- 3.Branco MdosRFC, Luna EJdeA, Braga Júnior LL, et al. Risk factors associated with death in Brazilian children with severe dengue: a case-control study. Clinics 2014;69:55–60. 10.6061/clinics/2014(01)08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catharina S, Tatty ES, Van Gorp ECM, et al. Risk factors for mortality in dengue shock syndrome (DSS). Media Medika Indonesiana 2009;43:213–9. [Google Scholar]
- 5.Laoprasopwattana K, Chaimongkol W, Pruekprasert P, et al. Acute respiratory failure and active bleeding are the important fatality predictive factors for severe dengue viral infection. PLoS One 2014;9:e114499. 10.1371/journal.pone.0114499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . Special programme for research and training in tropical diseases. dengue guidelines for diagnosis, treatment, prevention and control. Geneva: World Health Organization, 2009. [Google Scholar]
- 7.Feudtner C, Hays RM, Haynes G, et al. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics 2001;107:E99. 10.1542/peds.107.6.e99 [DOI] [PubMed] [Google Scholar]
- 8.Feudtner C, Feinstein JA, Zhong W, et al. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr 2014;14:199. 10.1186/1471-2431-14-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollack MM, Patel KM, Ruttimann UE. Prism III: an updated pediatric risk of mortality score. Crit Care Med 1996;24:743–52. 10.1097/00003246-199605000-00004 [DOI] [PubMed] [Google Scholar]
- 10.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–84. 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 11.Goldstein SL, Somers MJG, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int 2005;67:653–8. 10.1111/j.1523-1755.2005.67121.x [DOI] [PubMed] [Google Scholar]
- 12.Typpo KV, Petersen NJ, Hallman DM, et al. Day 1 multiple organ dysfunction syndrome is associated with poor functional outcome and mortality in the pediatric intensive care unit. Pediatr Crit Care Med 2009;10:562–70. 10.1097/PCC.0b013e3181a64be1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010;11:234–8. 10.1097/PCC.0b013e3181b806fc [DOI] [PubMed] [Google Scholar]
- 14.Gaies MG, Jeffries HE, Niebler RA, et al. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the pediatric cardiac critical care consortium and virtual PICU system registries. Pediatr Crit Care Med 2014;15:529–37. 10.1097/PCC.0000000000000153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . Dengue hemorrhagic fever: diagnosis, treatment, prevention and control. 2nd edn. Geneva: World Health Organization, 1997. [Google Scholar]
- 16.Goldstein SL, Currier H, Graf Cd C, et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 2001;107:1309–12. 10.1542/peds.107.6.1309 [DOI] [PubMed] [Google Scholar]
- 17.Selewski DT, Cornell TT, Lombel RM, et al. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med 2011;37:1166–73. 10.1007/s00134-011-2231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L, Shi T, Quan W, et al. Assessment of early renal angina index for prediction of subsequent severe acute kidney injury during septic shock in children. BMC Nephrol 2020;21:358. 10.1186/s12882-020-02023-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran DT, Nguyen The Nguyen Phung TTKP . Respiratory distress associated with dengue hemorrhagic fever on paediatric patients: learning from a provincial hospital in southern Vietnam. Arch Pharma Pract. 2019;10:92–7. [Google Scholar]
- 20.Halstead SB. Dengue. Lancet 2007;370:1644–52. 10.1016/S0140-6736(07)61687-0 [DOI] [PubMed] [Google Scholar]
- 21.Wills BA, Oragui EE, Dung NM, et al. Size and charge characteristics of the protein leak in dengue shock syndrome. J Infect Dis 2004;190:810–8. 10.1086/422754 [DOI] [PubMed] [Google Scholar]
- 22.Lee TH, Lee LK, Lye DC, et al. Current management of severe dengue infection. Expert Rev Anti Infect Ther 2017;15:67–78. 10.1080/14787210.2017.1248405 [DOI] [PubMed] [Google Scholar]
- 23.Ranjit S, Ramanathan G, Ramakrishnan B, et al. Targeted interventions in critically ill children with severe dengue. Indian J Crit Care Med 2018;22:154–61. 10.4103/ijccm.IJCCM_413_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinitsky L, Walls D, Nadel S, et al. Fluid overload at 48 hours is associated with respiratory morbidity but not mortality in a general PICU: retrospective cohort study. Pediatr Crit Care Med 2015;16:205–9. 10.1097/PCC.0000000000000318 [DOI] [PubMed] [Google Scholar]
- 25.Laroque Sinott Lopes C, Unchalo Eckert G, Sica dA Rocha T, Fontela PS, Pedro PIVA J. early fluid overload was associated with prolonged mechanical ventilation and more aggressive parameters in critically ill paediatric patients. Acta Paediatr 2020;109:557–64. [DOI] [PubMed] [Google Scholar]
- 26.Bhaskar P, Dhar AV, Thompson M, et al. Early fluid accumulation in children with shock and ICU mortality: a matched case-control study. Intensive Care Med 2015;41:1445–53. 10.1007/s00134-015-3851-9 [DOI] [PubMed] [Google Scholar]
- 27.Alobaidi R, Morgan C, Basu RK, et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr 2018;172:257–68. 10.1001/jamapediatrics.2017.4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arikan AA, Zappitelli M, Goldstein SL, et al. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med 2012;13:253–8. 10.1097/PCC.0b013e31822882a3 [DOI] [PubMed] [Google Scholar]
- 29.Tangsathapornpong A. Comparison of the 1997 and 2009 who classifications for determining dengue severity in Thai patients. Southeast Asian J Trop Med Public Health. 2017;48. [Google Scholar]
- 30.de Almeida RR, Paim B, de Oliveira SA, et al. Dengue hemorrhagic fever: a state-of-the-art review focused in pulmonary involvement. Lung 2017;195:389–95. 10.1007/s00408-017-0021-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrero R, Sánchez G, Asensio I, et al. Liver-lung interactions in acute respiratory distress syndrome. Intensive Care Med Exp 2020;8:48. 10.1186/s40635-020-00337-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C-C, Liu S-F, Liao S-C, et al. Acute respiratory failure in adult patients with dengue virus infection. Am J Trop Med Hyg 2007;77:151–8. 10.4269/ajtmh.2007.77.151 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data are available. Not applicable.