Abstract
Introduction:
Health state valuation and diagnostic-therapeutic pathways at the junction between non-metastatic and metastatic castration-resistant prostate cancer (CRPC) are not well documented. We aimed at: (i) estimating the disability weights (DWs) for health states across a continuum of disease from asymptomatic non-metastatic (nmCRPC) to symptomatic metastatic state (mCRPC); (ii) mapping the diagnostic-therapeutic pathway of nmCRPC in Italy.
Methods:
Structured qualitative interviews were performed with clinical experts to gather information on nmCRPC clinical pathway. An online survey was administered to clinical experts to estimate DWs for four CRPC health states defined from interviews and literature review (i.e., nmCRPC, asymptomatic mCRPC, symptomatic mCRPC, mCRPC in progression during or after chemotherapy). Clinicians’ preferences for health states were elicited using the Person-Trade-Off (PTO) and Visual Analogue Scale (VAS) methods. DWs associated with each health state, from 0 (best imaginable health state) and 1 (worst imaginable health state), were estimated.
Results:
We found that the management of nmCRPC is heterogeneous across Italian regions and hospitals, especially with respect to diagnostic imaging techniques. DWs for PTO ranged from 0.415 (95% confidence interval [CI] 0.208-0.623) in nmCRPC to 0.740 (95% CI 0.560-0.920) in mCRPC, in progression during or after chemotherapy. DWs for VAS ranged between 0.246 (95% CI 0.131-0.361) in nmCRPC to 0.689 (95% CI 0.583-0.795) in mCRPC, in progression during or after chemotherapy.
Conclusions:
Estimated DWs suggest that delaying transition to a metastatic state might ease the disease burden at both patient and societal levels.
Keywords: Castration-resistant prostate cancer, Disability weights, Disease burden, Person-trade-off, Visual analogue scale
Introduction
Health care needs and demand run always ahead of the supply of health care resources. To tackle this issue, policy and decision-makers are increasingly compelled to use evidence-based tools to achieve a more rational and efficient distribution of health care resources. The Disability-Adjusted Life Year (DALY) has gradually emerged as an aid in setting priorities in resource allocation, identifying the targets that are most relevant in terms of public health (1). The DALY aims at quantifying the burden of disease in terms of healthy life years lost at population level by summarizing the years of life lost due to premature death (YLLs) and the years lived with disability (YLDs), with one DALY representing one lost year of healthy life (2). The disability weight (DW) is an essential factor for estimating the YLDs: it represents the proportion of health lost due to an adverse outcome and can assume values from 0 (perfect health) to 1 (death or worst possible health state) (3). DWs reflect social values and are based on preferences for health states expressed by a panel of individuals, which can be composed by the general population, patients, medical experts or other professionals (e.g., policy-makers) (4). Since the 1996 Global Burden of Disease (GBD) study (5), a milestone for population health studies, several authors have investigated DWs for specific diseases or for several health states within one disease (6). DWs should be constantly updated to take into consideration changes in disease characteristics, treatments and the onset of new conditions or health states (7).
Prostate cancer (PC) is a major public health problem, being the second most commonly diagnosed cancer and the fifth most common cause of cancer death in men worldwide (8). In Italy, PC is the most commonly diagnosed cancer in men, with a higher incidence in people aged 50-69 years, and the third cause of cancer death in men (9). Castration-resistant PC (CRPC), defined by disease progression despite androgen deprivation therapy (ADT), is a continuum of disease ranging from patients without metastases or symptoms (non-metastatic CRPC, nmCRPC) to patients with metastases and cancer-related complications (metastatic CRPC, mCRPC) (10,11). Variations in the management of the disease in clinical practice may significantly impact on metastases onset, affecting patients’ survival and health-related quality of life (HRQoL) (12,13). However, at present clinical management of nmCRPC patients is patchy and not well documented (14,15). Moreover, although several studies have revealed that metastases onset is associated with higher DWs (16,17), there is no empirical evidence within the CRPC setting. This study aims at estimating the DWs for health states associated with a CRPC disease based on a survey of health care professionals in Italy. As a secondary objective, we aimed at mapping the diagnostic and therapeutic pathway of nmCRPC disease in the country.
Methods
The study developed through two key methodological steps: structured qualitative interviews and an online survey. The study was conducted in accordance with the Declaration of Helsinki (18) and ethical approval for this research was obtained.
Structured qualitative interviews
We carried out structured, individual qualitative interviews with a purposive sample of Italian medical oncologists and urologists, selected to maximize representativeness of clinical practice across different Italian regions and on the basis of their recognized expertise in the management of nmCRPC (e.g., authors of peer-reviewed publication on nmCRPC). Although purposive sampling can be subject to selection bias (19), it is a technique widely used in qualitative research for the identification and selection of information-rich cases, that is, individuals who are especially knowledgeable about or experienced with the central phenomenon in the study (20).
The objectives of the interviews were to characterize different health states within the spectrum of CRPC disease (from nmCRPC to mCRPC), and to gather qualitative information on the diagnostic and treatment pathways of nmCRPC patients. Experts were invited via email to partake in the project, and an informed consent form was sent to those who agreed to participate. The interview questions (Supplementary file S1) were shared in advance. We carried out a pilot phase with two clinicians in order to validate the interview guide. Interviews were conducted in Italian either by phone or face-to-face, recorded and transcribed. Transcripts were analyzed qualitatively and synthesized using standard thematic analysis (21), and results were reported in an anonymized format. Quotes from the experts interviewed were included to illustrate key points, and a numerical code reported in parentheses was used to refer to different responders (i.e., experts were labelled from I to X). Descriptors were used in the text to inform readers on the degree of agreement of clinicians interviewed on a certain topic (see Supplementary Table A for interpretation of these descriptors).
Survey on DWs
An online survey addressed to urologists and medical oncologists was carried out in order to estimate DWs for CRPC.
The information collected during the interviews and the evidence from the literature (22) contributed to the drafting of the health states of CRPC. Four health states and their respective labels were identified, each assumed to be internally homogeneous in terms of disease severity, disability, treatment and prognosis. In line with several studies on DWs elicitation (23-25), also a description of the health states through EQ-5D-3L descriptive system was provided on five different dimensions: mobility, self-care, usual activities, pain/discomfort, anxiety/depression (26). Each dimension presents three levels of severity: no problems, moderate problems and severe problems. The EQ-5D descriptions for the health states were drafted using the results of qualitative interviews, where clinicians were asked to describe how a nmCRPC and mCRPC patient feels, and the published literature on mCRPC (27). For each health state, a standardized vignette was prepared, reporting the disease label, a brief clinical description and the generic description through EQ-5D dimensions (Figure 1). The vignettes were validated by one clinician and two health economists. We decided to use the 3-level version of the EQ-5D as, during the validation step of the vignettes, it resulted easier to implement than the 5-level version and was judged as being more easily comprehensible.
Fig. 1 -.
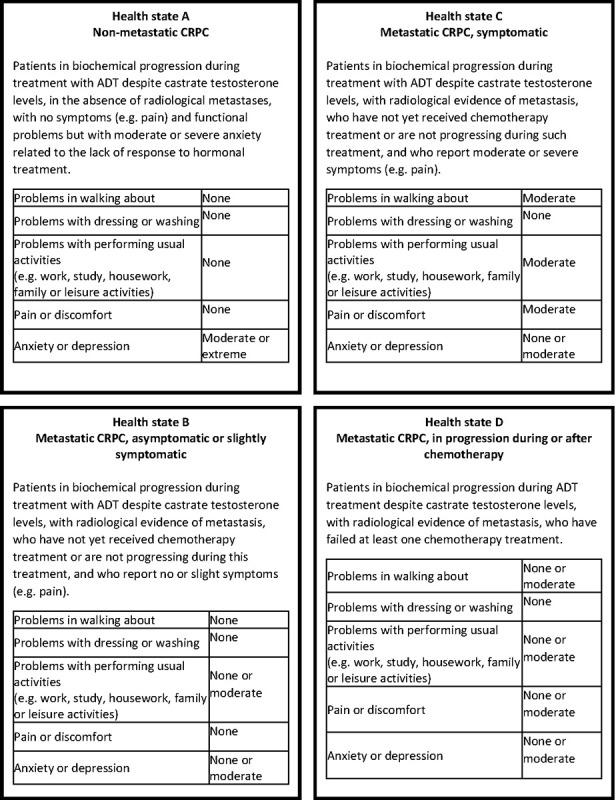
Vignettes for health states. ADT = androgen deprivation therapy; CRPC = castration-resistant prostate cancer.
An online questionnaire was designed through Qualtrics XM and administered to clinicians, whose preferences on the health states were elicited using two valuation methods, the Person-Trade-Off (PTO) and the Visual Analogue Scale (VAS). With the PTO method, experts were asked to trade-off a number of individuals with perfect health and a number of individuals in the health state to be assessed (Supplementary File S2) (23,28). Due to the complexity of PTO exercise, respondents were given the possibility to check their answers and revise them if necessary. The PTO method attempts to measure social preference instead of individual preferences, as interviewees are asked to state preferences between people (28). Therefore, the estimates derived from PTO should reflect the perspective of a policy-maker, and PTO may represent the preferred method for estimating DWs for burden of disease studies. With the VAS method, instead, experts were asked to give a direct ranking of the health states in two steps: (1) by ordering the health states from the best (I) to the worst (IV); (2) by assigning a score to each health state through a graduated scale, which assumes values from 0 (worst score = death or worst possible health status) to 100 (best score = perfect health) (Supplementary File S2) (23). The VAS exercise is cognitively simpler than the PTO (29) as it does not entail a trade-off feature, and it was used to double-check the reliability of DWs obtained through the PTO method (23). The VAS scores provide information about the relative desirability of health states rather than giving evidence on the trade-offs that people are willing to make (30). All health states were valued independently according to both methods. The DWs were derived according to the following formulas (23):
DW (PTO) = 1 − (1,000/N)
DW (VAS) = 1 − (VAS score/100)
where N is the number of subjects in a certain health state for which 1 year of life extension is equivalent to the extension of 1 year of life for 1,000 healthy individuals. Data analyses were carried out using Stata® (v16; StataCorp LLC, College Station, TX). A complete case analysis was carried out, that is, only complete questionnaires were considered (31). Individual DWs for both PTO and VAS were averaged across responders to obtain the DWs for the whole sample. Means and corresponding confidence intervals were computed using the command ci means in Stata. To establish whether the disease health states were valued in a consistent manner by individuals between the two valuation methods, the DWs obtained from the PTO and VAS methods were compared using Kendall rank correlation test, which is intended for use on small- and moderate-sized datasets (32). Kendall tau-a and tau-b statistics can assume values from −1 to 1, where −1 means perfectly negative correlation and 1 means perfectly positive correlation. A p-value <0.05 was considered significant.
In the absence of a gold standard to evaluate the validity of DWs (6), we first compared the results obtained with the rankings of matching diseases drawn the GBD 2013 study (33), a web-based survey of participants aged 18-65 years in four European countries (Hungary, Italy, the Netherlands and Sweden). More specifically, we considered the DWs for the following cancer health states: (i) diagnosis and primary therapy; (ii) metastatic cancer; (iii) terminal phase with medication; (iv) terminal phase without medication. Then, we compared the DWs for objectively less severe and more severe health states.
A pilot of the survey was conducted by recruiting a sample of clinicians during two national scientific conferences of urologists in Italy. Clinicians were randomly identified and interviewed by one researcher at the congress venues. Following the pilot phase, a distribution list of urologists was created ad hoc by mapping all the urology units in Italy. A search on the hospitals’ website and on Google was performed to retrieve the email address of urologists operating in each unit. A snowballing procedure led to further enrich this list with Italian clinical experts in CRPC. Similarly to what discussed for the interviews, also in this case the purposive sampling approach adopted may have led to selection bias (19). However, due to the lack of a publicly available and comprehensive database on Italian clinicians dealing with nmCRPC patients, a purposive sampling represented the only doable and most cost-effective approach to recruit responders.
The survey was sent at the end of September 2020. Three reminders were sent through Qualtrics XM, one every 2 weeks, before closing the survey at the beginning of November 2020.
Results
Ten clinical experts over thirty-two invited (31%), four urologists and six oncologists operating in eight different Italian regions with an average experience of 13 years in the management of PC patients, agreed to be interviewed and partake in the project (Supplementary Fig. A).
Health states description and HRQoL for CRPC patients
With respect to HRQoL, clinicians agreed that nmCRPC patients usually do not experience pain due to the disease (i.e., are asymptomatic) nor difficulties in performing daily activities. The main impact of the disease is on the emotional and psychological sphere, as «(…) it is not easy to live in a state in which the [Prostate-Specific Antigen] PSA continues to rise and you know that the disease will become metastatic sooner or later. The patient knows that he has a sword of Damocles above his head (…)» (III). mCRPC patients can be asymptomatic, symptomatic or heavily symptomatic. Generally, the presence and severity of pain is due to metastases and «(…) depends also from the site of metastases. Pain may become extremely relevant if the patient has diffused bone metastases and problems in lymph nodes» (I). For symptomatic or heavily symptomatic patients, the impact of the disease on the ability to perform daily activities and on the social sphere may be substantial, and can vary according to the patients’ age (e.g., older patients usually have a less aggressive disease and more leniently accept their conditions). The anxiety may be more pronounced when the patient receives the first diagnosis of metastatic disease, as he knows that his health state has changed, but usually «(..) the passage from first to second treatment line is not lived as a drama» (X). In some cases, the mCRPC patient is less worried and anxious than the nmCRPC one because he is more “prepared” («Sometimes the certainty, although negative, is better than the doubt» (VII)).
On the basis of the interviews and published literature (22), four health states for CRPC patients were identified: nmCRPC, asymptomatic mCRPC, symptomatic mCRPC, mCRPC in progression during or after chemotherapy. For these health states, DWs were estimated through a survey addressed to clinical experts (see the “Disability weights” section).
Diagnostic and treatment pathway of CRPC patients
nmCRPC patients usually face a long history of disease, and, according to the majority of clinicians interviewed, their clinical pathway is currently «not well defined» (I) and «needs to be ameliorated and codified» (X).
The diagnosis is made with a combination of laboratory tests (PSA) and imaging. Computed tomography (CT) scan and bone scan «are the gold standards for diagnosis» (VI) and, with very few exceptions, are always used for the diagnosis of nmCRPC, as indicated by clinical guidelines (34). Moreover, their use is compulsory to administer next-generation androgen receptors (e.g., apalutamide, enzalutamide, darolutamide), also for compassionate use. Despite the presence of a standard approach, however, nmCRPC patients «are diagnosed in a very heterogeneous manner» (IX) and «very often the choice of imaging is discretional» (X). Currently, prostate-specific membrane antigen (PSMA) positron emission tomography (PET) or choline PET is available, characterized by higher sensitivity and specificity. Sometimes diagnosis is performed through PET only, in contrast to clinical guidelines. According to some clinicians, the use of PET varies widely across different hospitals, «on the basis of the possibility of the center to perform these exams» (IV) and may depend on the preferences of clinician who is in charge of patient care. Despite the use of new-generation imaging, some responders recognize that this approach might not be appropriate in this setting «because in the phase III trials of the new molecules (…) the patient was diagnosed on the basis of a negative CT scan and bone scan» (X) and «in theory the staging in real life should follow the one performed in the trials, i.e., traditional imaging» (IX), to replicate similar results.
After diagnosis, nmCRPC patients treated with ADT «are always followed in outpatient setting» (III), «continue to do visits and exams with increasing frequency and continue to receive the therapy that was already ongoing» (II). The clinician in charge of nmCRPC patients’ care is mainly represented by the urologist, the oncologist or the radiotherapist. The prominence of one figure over the other may vary across different hospitals, as the management of nmCRPC is extremely heterogeneous in Italy, and may depend on the type of primary treatment, for example, urologist for patients who underwent surgical treatment or radiotherapist for those treated with radiotherapy. Despite the presence of a main responsible figure in the patient pathway, all clinicians interviewed declare that in their hospitals there is a multidisciplinary team for the management of PC patients, thus treatment decisions for nmCRPC patients are taken collectively and responsibility is shared. In the majority of hospitals, however, only some selected nmCRPC cases are discussed by the multidisciplinary team, usually identified by the main responsible clinician and limited to the most complex patients. The majority of responders report that there are no codified criteria that drive the choice of which cases should be discussed. The specialists usually involved in the multidisciplinary teams are urologists, medical oncologists, radiotherapists and pathologists. In some cases, also radiologists and nuclear medicine clinicians are involved to interpret the imaging results, as well as palliative care clinicians, endocrinologists, physiatrists and psycho-oncologists. Overall, clinicians believe that the discussion of cases in multidisciplinary teams should follow a generalized approach as «it ensures quality and gives a holistic vision of the patient. (…) This multidisciplinary approach is a guarantee of clinical pathway’s speed, from diagnosis to restaging to therapy choice» (I). The formal recognition of multidisciplinary teams through integrated care pathway, with either hospital or regional accreditation, is heterogeneous across Italian hospitals and in place in 50% of the organizations represented by interviewees.
At the time of the interviews, the reimbursement of next-generation androgen inhibitors (e.g., apalutamide, enzalutamide, darolutamide) in Italy was still under negotiation, the standard of care for nmCRPC patients was therefore ADT and active follow-up, although the majority of clinicians declared to administer the next-generation androgen inhibitors for compassionate use. Clinicians cited several factors, besides effectiveness, that may influence the choice of one treatment over the others, among which were the availability of alternative therapies, the patient’s profile, the impact of treatment on HRQoL, its cost, clinician’s preferences and also an explicit request coming from the patient. Clinicians agree that only one line (maximum two lines) of hormonal manipulation should be performed in this patient population, and that the use of successive manipulations, very common in the past, should be abandoned as they do not provide any added value for a nmCRPC patient (34). Clinicians expect that, with the diffusion of next-generation androgen inhibitors in clinical practice, «(…) the overall management of these patients will change both in terms of referral and in terms of more homogeneity in patients’ selection, follow-up and treatment» (X).
Disability weights
The online survey on DWs for CRPC health states was sent to 1,323 clinicians (of whom 1,227 were urologists). Among the experts invited, 54 started the online survey and 21 completed it (response rate = 1.6%; completion rate = 39%). Respondents reported an average experience in managing PC patients of approximately 16 years (standard deviation [SD] = 10.1), and the majority were urologists (66.7%) (Tab. I). They operated in 10 different Italian regions, and the majority in Northern Italy (66.7%).
Table I -.
Background characteristics of respondents
|
Respondents’ characteristics
N = 21 |
|
|---|---|
| Professional figure, n (%) | |
| Oncologist | 6 (28.6%) |
| Urologist | 14 (66.7%) |
| Radiotherapist | 1 (4.8%) |
| Gender, n (%) | |
| Male | 16 (76.2%) |
| Female | 5 (23.8%) |
| Years of experience, mean (SD) | 15.7 (10.1) |
| Years of experience by professional figure, mean (SD) | |
| Oncologist | 15.1 (9.4) |
| Urologist | 15.2 (10.8) |
| Radiotherapist | 25.0 (0.0) |
| Geographical region in which they operate, n (%) | |
| North | 14 (66.7%) |
| Centre | 4 (19.0%) |
| South | 3 (14.3%) |
SD = standard deviation.
According to the ranking of the health states provided in the VAS exercise, experts considered nmCRPC as the best state (with one exception), while responses were more heterogeneous as regards symptomatic and progressive metastatic CRPC (Tab. II).
Table II -.
Visual Analogue Scale (VAS) results (ordering)
| Health states | VAS order n (%) | VAS order mean (SD) | |||
|---|---|---|---|---|---|
| I (best) | II | III | IV (worst) | ||
| Non-metastatic CRPC | 20 (95.2%) | 1 (4.8%) | 0 (0.0%) | 0 (0.0%) | 1.0 (0.2) |
| Metastatic CRPC, asymptomatic or slightly symptomatic | 1 (4.8%) | 20 (95.2%) | 0 (0.0%) | 0 (0.0%) | 2.0 (0.2) |
| Metastatic CRPC, symptomatic | 0 (0.0%) | 0 (0.0%) | 10 (47.6%) | 11 (52.4%) | 3.5 (0.5) |
| Metastatic CRPC, in progression during or after chemotherapy | 0 (0.0%) | 0 (0.0%) | 11 (52.4%) | 10 (47.6%) | 3.5 (0.5) |
CRPC = castration-resistant prostate cancer; SD = standard deviation.
The estimates (Tab. III, Fig. 2) clearly show that the DWs for metastatic disease states are higher than the non-metastatic one, and increase with disease progression. The absolute values of the weights for PTO ranged from 0.415 (95% confidence interval [CI], 0.208-0.623) in nmCRPC to 0.740 (95% CI, 0.560-0.920) in mCRPC, in progression during or after chemotherapy. As for the VAS method, weights ranged between 0.246 (95% CI 0.131-0.361) in nmCRPC and 0.689 (95% CI, 0.583-0.795) in mCRPC, in progression during or after chemotherapy. The strength and direction of association that exists between PTO and VAS values for each health state, as assessed by Kendall rank correlation test, was weak and not statistically significant (Tab. III). The lack of statistical significance might be partly explained by the limited sample size analyzed. However, at the aggregate level, the ranking of PTO and VAS is consistent (i.e., monotonically increasing), suggesting some internal coherence of data. Exploratory analyses indicate that in the PTO exercise higher experience corresponds to lower values assigned to the DW (negative correlation, although statistically significant only for the nmCRPC state).
Table III -.
Disability weights according to Person-Trade-Off (PTO) and Visual Analogue Scale (VAS)
| Health states | Disability weights | Kendall rank correlation * | ||||
|---|---|---|---|---|---|---|
| PTO | VAS | |||||
| Mean | 95% CI | Mean | 95% CI | tau-a | tau-b | |
| Non-metastatic CRPC | 0.415 | 0.208-0.623 | 0.246 | 0.131-0.361 | 0.2238
n.s. |
0.2859
n.s. |
| Metastatic CRPC, asymptomatic or slightly symptomatic | 0.474 | 0.261-0.688 | 0.375 | 0.282-0.469 | 0.1286
n.s. |
0.1463
n.s. |
| Metastatic CRPC, symptomatic | 0.681 | 0.494-0.868 | 0.605 | 0.522-0.690 | 0.0333
n.s. |
0.0370
n.s. |
| Metastatic CRPC, in progression during or after chemotherapy | 0.740 | 0.560-0.920 | 0.689 | 0.583-0.795 | 0.1048
n.s. |
0.1145
n.s. |
CRPC = castration-resistant prostate cancer.
*Kendall tau-a and tau-b statistics are reported, as well as the statistical significance of the correlation (n.s. means not significant).
Fig. 2 -.
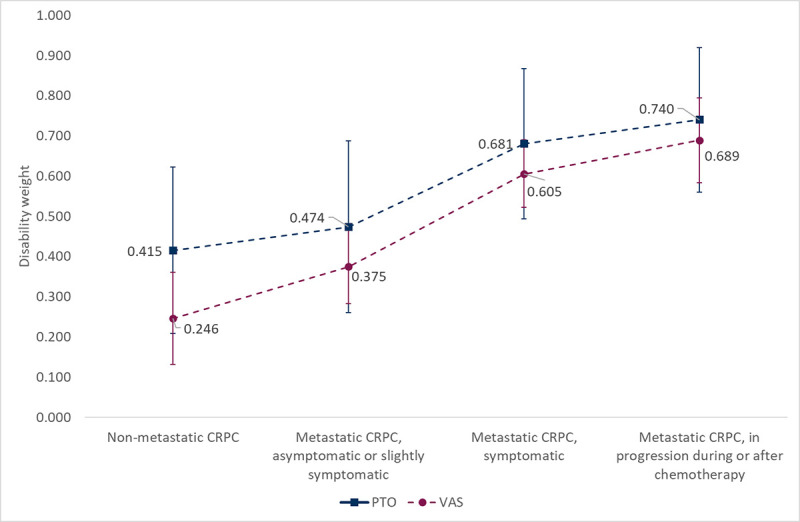
Disability weights according to Person-Trade-Off (PTO) and Visual Analogue Scale (VAS). CRPC = castration-resistant prostate cancer.
Discussion
Over the years, resource allocation decisions have been increasingly supported by information on the distribution of population health. To this end, investigating aspects related to the burden of disease are essential to guarantee an equitable access to care to all patients. This is particularly relevant for relatively new subpopulations, whose health needs have not been fully assessed and addressed.
The interest toward nmCRPC is growing, due to the advancements in treatment and in diagnosis, with the approval of next-generation androgen inhibitors and new imaging modalities respectively (14,15). However, there are still many concerns regarding the management of patients with nmCRPC (35). For example, it is currently unclear whether treatment decisions in a CRPC setting should change based on new imaging techniques, or which next-generation androgen inhibitor is to be chosen due to the lack of direct comparisons among agents (36). This lack of clarity is likely to translate into heterogeneity in the management of nmCRPC in clinical practice. Moreover, to date, the evidence on the burden of the disease is scant.
This study, by means of a mixed-methods approach that combines qualitative and quantitative evidence, aimed at estimating the DWs of CRPC health states and investigating the diagnostic and clinical pathway of CRPC patients in the Italian National Healthcare System.
Individual, qualitative interviews with 10 clinical experts revealed that the management of nmCRPC is heterogeneous across Italian regions and hospitals, especially with respect to the imaging techniques used for the diagnosis of nmCRPC. Due to disease complexity, these patients are usually followed by multidisciplinary teams, which, according to experts, have become more widespread in Italy in the last years as they ensure a comprehensive vision on the patients’ condition, leading to the definition of a more appropriate clinical pathway. Despite the relevance of several factors in treatment decisions, clinicians recognized that treatment choices may be constrained by the availability of alternative therapies. Ultimately, this may have a substantial impact also on patients’ quality of life, as clinicians agreed that metastatic patients have a more impaired quality of life than non-metastatic ones.
These insights were confirmed by the DWs for CRPC health states estimated using two different valuation methods, PTO and VAS, through an online survey addressed to clinical experts in Italy. According to both VAS and PTO, metastatic disease states are associated with higher disability than the non-metastatic state. Our results revealed that the DWs based on the VAS were lower than those based on PTO. This is in line with the findings of a study conducted by Stouthard and colleagues (23), who revealed that VAS weights were lower than PTO ones for a substantial number of conditions. This discrepancy may be partially explained by the difference in the information provided by VAS and PTO. The former reveals the relative desirability of a health state compared to others, rather than the preference for it (37). The latter, instead, by forcing respondents to make trade-offs (e.g., decide how many healthy life years of individuals they are willing to sacrifice), is recommended to assess the preference for a specific health state (28).
The face validity of these results was assessed in comparison with estimates provided by the GBD 2013 study (33) about the following cancer DWs: (i) diagnosis and primary therapy: 0.288; (ii) metastatic: 0.451; (iii) terminal phase with medication: 0.540; (iv) terminal phase without medication: 0.569. Although the health states of the GBD study do not perfectly match with the health states evaluated in our study, the clinical setting and disease severity are comparable. Our estimated DWs are generally higher; however, the ranking is in line with that described in the GBD study. A second approach to assess validity is to compare the DWs of disease stages within a certain disease, that is, compare less severe and more severe health states. According to our results, the more severe is the health state the higher is the DW.
This study has some limitations. First, due to the limited sample of respondents, the opinions collected from experts may not be fully representative of the Italian clinical practice. However, as regards individual interviews, clinicians were carefully identified on the basis of their recognized expertise in the management of nmCRPC patients and taking into account possible heterogeneity in clinical practice due to different geographical distribution. With respect to valuation exercise, the low response rate could be mainly attributed to the ongoing Covid-19 pandemic at the time of survey administration. Moreover, the unfamiliarity of clinicians with this type of valuation exercise may have discouraged them to access the survey. According to a literature review by Haagsma and colleagues (6), however, the panel of medical experts involved in DWs studies varied from 9 to 49 members. Therefore, although limited, the size of our expert panel is in line with published literature. Future research might involve a higher number of respondents in order to improve the external validity of DWs estimates.
Second, the survey completion rate was low. This may be explained by the complexity of the valuation exercise, especially the one regarding PTO. The perception of complexity might have been enhanced by the fact that the survey was self-administered, that is, the respondent could not receive immediate support from the researchers during completion. The majority of respondents who did not complete the questionnaire stopped at the beginning of the valuation exercise, which started with PTO. The complexity of the exercise may therefore have caused data to be missing not at random. As a consequence, the use of a complete case analysis might have negatively impacted the robustness of estimates (38,39). Future research might address the complexity issue by administering the survey in person rather than online, in order to better support the respondents in the completion of the valuation exercise.
Third, in the valuation exercise we did not include indicator conditions to be valued, preventing us from assessing the correlation between the DWs estimated by our experts and the corresponding DWs established in previous studies (i.e., external consistency of the panel). However, based on the pilot phase, we realized that adding one or more indicator conditions would have increased the burden for respondents, therefore we decided to focus the valuation only on the health states of interest.
Conclusions
This study provides insights about the current diagnostic and therapeutic pathway of nmCRPC patients, highlighting challenges associated with the management of this patient population. Our findings contribute for the first time to estimate the DWs associated with different health states of CRPC, based on two different approaches. Despite the differences in the absolute values of DWs between PTO and VAS, our results suggest that delaying transition to a metastatic state would ease the disease burden, with a positive impact both on patients and on the health care system as a whole. Further research involving a larger and/or different sample of respondents (e.g., general population, policy-makers) is needed in order to confirm our preliminary estimates of DWs for CRPC. Delaying metastases onset might be a therapeutic goal in clinical decision-making at the individual level, and knowledge of health states DWs may help establish priority setting in health care funding at the population level.
Disclosures
Conflict of interest: LB, OC, RDV and GIR declare no potential conflicts of interest with respect to the research, authorship and publication of this study. MS declares the following competing interests: Janssen-Cilag SpA (consultations), Takeda (consultations). CDF, PB and IL are employees of Janssen-Cilag SpA.
Financial support: CERGAS SDA Bocconi received an unrestricted grant from Janssen-Cilag SpA for this study. The funders had no access to the dataset and had no role in study design, data collection or analysis.
Author contributions: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, writing - original draft: LB and OC; Funding acquisition, supervision: OC; Visualization: LB; Writing – review and editing: LB, OC, RDV, GIR, MS, CDF, PB and IL.
Research ethics: The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Università Commerciale Luigi Bocconi (prot n. 14354-2, September 16, 2019).
Data Availability Statement: All relevant data can be found in the manuscript or in the supplementary materials provided online.
This article includes supplementary material
References
- 1.World Bank. World Development Report 1993: Investing in Health. [(Accessed May; 2022 )]. Online [Google Scholar]
- 2.Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72(3):429–445. PubMed [PMC free article] [PubMed] [Google Scholar]
- 3.Devleesschauwer B,, Havelaar AH,, Maertens de Noordhout C, et al. Calculating disability-adjusted life years to quantify burden of disease. Int J Public Health. 2014;59(3):565–569. doi: 10.1007/s00038-014-0552-z. PubMed [DOI] [PubMed] [Google Scholar]
- 4.Essink-Bot ML,, Bonsel G. How to derive disability weights (Chapter 9.1). J Immunol. 2002;01(01):449–465. [Google Scholar]
- 5.Murray CJL,, Lopez AD; In: The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020: Christopher J. L. Murray,, Alan D. Lopez., editors. World Health Organization.; [(Accessed May; 2022 )]. World Health Organization, World Bank & Harvard School of Public Health. (1996).Online summary/edited by. [Google Scholar]
- 6.Haagsma JA,, Polinder S,, Cassini A,, Colzani E,, Havelaar AH. Review of disability weight studies: comparison of methodological choices and values. Popul Health Metr. 2014;12(1):20. doi: 10.1186/s12963-014-0020-2. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ock M,, Park B,, Park H, et al. Disability weights measurement for 289 causes of disease considering disease severity in Korea. J Korean Med Sci. 2019;34(suppl 1):e60. doi: 10.3346/jkms.2019.34.e60. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray F,, Ferlay J,, Soerjomataram I,, Siegel RL,, Torre LA,, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. PubMed [DOI] [PubMed] [Google Scholar]
- 9.AIOM-AIRTUM. [(Accessed May; 2022 )]. The numbers of cancer in Italy. 2019 [in Italian].Online [Google Scholar]
- 10.Saad F,, Hotte SJ. Guidelines for the management of castrate-resistant prostate cancer. Can Urol Assoc J. 2010;4(6):380–384. doi: 10.5489/cuaj.10167. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotte SJ,, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010;17(12)(suppl 2):S72–S79. doi: 10.3747/co.v17i0.718. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie W,, Regan MM,, Buyse M, et al. ICECaP Working Group. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017;35(27):3097–3104. doi: 10.1200/JCO.2017.73.9987. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halabi S,, Kelly WK,, Ma H, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34(14):1652–1659. doi: 10.1200/JCO.2015.65.7270. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Campos F,, Conde-Moreno A,, Barrado Los Arcos M,, Gómez-Caamaño A,, García-Gómez R,, Hervás Morón A. treatment landscape of nonmetastatic castration-resistant prostate cancer: a window of opportunity. J Pers Med. 2021;11(11):1190. doi: 10.3390/jpm11111190. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mateo J,, Fizazi K,, Gillessen S, et al. Managing nonmetastatic castration-resistant prostate cancer. Eur Urol. 2019 Feb;75(2):285–293. doi: 10.1016/j.eururo.2018.07.035. PubMed [DOI] [PubMed] [Google Scholar]
- 16.Fitzmaurice C,, Abate D,, Abbasi N, et al. Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768. doi: 10.1001/jamaoncol.2019.2996. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haagsma JA,, Maertens de Noordhout C,, Polinder S, et al. Assessing disability weights based on the responses of 30,660 people from four European countries. Popul Health Metr. 2015;13(1):10. doi: 10.1186/s12963-015-0042-4. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. PubMed [DOI] [PubMed] [Google Scholar]
- 19.Lohr SL. 3rd ed. CRC Press/Chapman & Hall;; 2022. Sampling. Design and analysis. [Google Scholar]
- 20.Creswell JW,, Poth CN. SAGE Publications;; 2016. Qualitative inquiry and research design: choosing among five approaches. [Google Scholar]
- 21.Castleberry A,, Nolen A. Thematic analysis of qualitative research data: is it as easy as it sounds? Curr Pharm Teach Learn. 2018;10(6):807–815. doi: 10.1016/j.cptl.2018.03.019. PubMed [DOI] [PubMed] [Google Scholar]
- 22.Scher HI,, Solo K,, Valant J,, Todd MB,, Mehra M. Prevalence of prostate cancer clinical states and mortality in the United States: estimates using a dynamic progression model. PLoS One. 2015;10(10):e0139440. doi: 10.1371/journal.pone.0139440. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stouthard M,, Essink-Bot M-L,, Bonsel G, et al. Disability weights for diseases in The Netherlands. Tijdschr Gerontol Geriatr. 1997;01(01) [Google Scholar]
- 24.Schwarzinger M,, Stouthard ME,, Burström K,, Nord E. Cross-national agreement on disability weights: the European Disability Weights Project. Popul Health Metr. 2003;1(1):9. doi: 10.1186/1478-7954-1-9. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haagsma JA,, Havelaar AH,, Janssen BM,, Bonsel GJ. Disability adjusted life years and minimal disease: application of a preference-based relevance criterion to rank enteric pathogens. Popul Health Metr. 2008;6(1):7. doi: 10.1186/1478-7954-6-7. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. PubMed [DOI] [PubMed] [Google Scholar]
- 27.Lloyd AJ,, Kerr C,, Penton J,, Knerer G. Health-related quality of life and health utilities in metastatic castrate-resistant prostate cancer: a survey capturing experiences from a diverse sample of UK patients. Value Health. 2015;18(8):1152–1157. doi: 10.1016/j.jval.2015.08.012. PubMed [DOI] [PubMed] [Google Scholar]
- 28.Nord E. The person-trade-off approach to valuing health care programs. Med Decis Making. 1995;15(3):201–208. doi: 10.1177/0272989X9501500302. PubMed [DOI] [PubMed] [Google Scholar]
- 29.van Spijker BA,, van Straten A,, Kerkhof AJ,, Hoeymans N,, Smit F. Disability weights for suicidal thoughts and non-fatal suicide attempts. J Affect Disord. 2011 Nov;134(1-3):341–347. doi: 10.1016/j.jad.2011.05.020. PubMed [DOI] [PubMed] [Google Scholar]
- 30.Froberg DG,, Kane RL. Methodology for measuring health-state preferences—I: measurement strategies. J Clin Epidemiol. 1989;42(4):345–354. doi: 10.1016/0895-4356(89)90039-5. PubMed [DOI] [PubMed] [Google Scholar]
- 31.Little RJ,, Carpenter JR,, Lee KJ. Sociol Methods Res. 2022. A comparison of three popular methods for handling missing data: complete-case analysis, inverse probability weighting, and multiple imputation. [DOI] [Google Scholar]
- 32.Kendall MG. A new measure of rank correlation. Biometrika. 1938;30(1/2):81–93. doi: 10.2307/2332226. [DOI] [Google Scholar]
- 33.Salomon JA,, Haagsma JA,, Davis A, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3(11):e712–e723. doi: 10.1016/S2214-109X(15)00069-8. PubMed [DOI] [PubMed] [Google Scholar]
- 34.Associazione Italiana di Oncologia Medica (AIOM). [(Accessed May; 2022 )]. Guidelines – Prostate cancer. 2019 [in Italian].Online [Google Scholar]
- 35.Tartarone A,, Lerose R,, Tartarone M. Decisions and dilemmas in non-metastatic castration-resistant prostate cancer management. Med Oncol. 2022;39(7):107. doi: 10.1007/s12032-022-01743-7. PubMed [DOI] [PubMed] [Google Scholar]
- 36.Cattrini C,, Caffo O,, De Giorgi U, et al. Apalutamide, darolutamide and enzalutamide for nonmetastatic castration-resistant prostate cancer (nmCRPC): a critical review. Cancers (Basel). 2022;14(7):1792. doi: 10.3390/cancers14071792. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brazier J,, Deverill M,, Green C. A review of the use of health status measures in economic evaluation. J Health Serv Res Policy. 1999;4(3):174–184. doi: 10.1177/135581969900400310. PubMed [DOI] [PubMed] [Google Scholar]
- 38.Allison PD. Sage Publications;; 2002. Missing data. [DOI] [Google Scholar]
- 39.Little RJ,, Rubin DB. John Wiley & Sons;; 2020. Statistical analysis with missing data. [Google Scholar]


