Abstract
Despite the advantages of getting access to the coronavirus disease 2019 (COVID-19) vaccines, their potential ability to induce severe adverse events (AEs) has been a significant concern. Neurological complications are significant among the various adverse events following immunization (AEFI) due to their likely durability and debilitating sequelae. Neurological AEs following COVID-19 vaccination can either exacerbate or induce new-onset neuro-immunologic diseases, such as myasthenia gravis (MG) and Guillain–Barre syndrome (GBS). The more severe spectrum of AEs post-COVID19 vaccines has included seizures, reactivation of the varicella-zoster virus, strokes, GBS, Bell’s palsy, transverse myelitis (TM), and acute disseminated encephalomyelitis (ADEM). Here, we discuss each of these neurological adverse effects separately.
Keywords: COVID-19, Vaccine, Adverse event, Neurologic, SARS-CoV-2
Introduction
Despite the advantages of getting access to the coronavirus disease 2019 (COVID-19) vaccines, their potential ability to induce severe adverse events (AEs) has been a significant concern. Neurological complications are significant among the various adverse events following immunization (AEFI) due to their likely durability and debilitating sequelae [1, 2]. Neurological AEs following COVID-19 vaccination can either exacerbate or induce new-onset neuro-immunologic diseases, such as myasthenia gravis (MG), and Guillain–Barre syndrome (GBS) [3–5]. In addition, after vaccination, hypercoagulability and a pro-thrombotic state may further increase cerebrovascular events [6, 7]. The Centers for Disease Control (CDC) Vaccine Adverse Event Reporting System (VAERS) has announced several neurological complications following COVID-19 vaccines [8]. The most common neurological symptoms following COVID-19 vaccines have included headache, anosmia, dysgeusia, myalgia, paresthesia, weakness, and dizziness [9]. Several rare side effects, including tremor, diplopia, tinnitus, dysphonia, delirium, and syncope, have also been observed that are significant to note [10, 11]. The more severe spectrum of AEs post-COVID19 vaccines has included seizures, reactivation of the varicella-zoster virus, strokes, GBS, Bell’s palsy, transverse myelitis (TM), and acute disseminated encephalomyelitis (ADEM). Here, we discuss each of these neurological adverse effects separately.
Bells palsy
Bell’S palsy (BP), known as idiopathic facial paralysis, is an acute unilateral peripheral facial nerve palsy [12]. This condition is, in fact, an idiopathic facial palsy of spontaneous origin, although a causal association with the herpes simplex virus has been considered [13]. However, in the current pandemic, studies revealed abundant cases of BP following SARS-CoV-2 infection [14–16]. Several vaccines, including influenza, hepatitis B, and meningococcal conjugate vaccines, have been associated with BP [17–19]. With the development of COVID-19 vaccines, significant concerns have arisen about their potential to trigger the onset of Bell’s palsy. Despite being less prevalent than expected, this complication has still been abundantly reported following these vaccines [12, 20, 21]. Nonetheless, the US Food and Drug Administration (FDA) announced that the frequency of Bell’s palsy cases following vaccination is not more unusual than in the general population [22]. Despite the inability to confirm the causal relationship between the vaccines and this complication, the timing of onset following vaccination can suggest the association. Confirmation would need to be studied in larger populations [23]. This reaction could be either immune-mediated or induced by viral reactivation [24], but the latter does not seem valid for COVID-19 vaccines since no live attenuated COVID-19 vaccine platform has yet been introduced.
The immune-mediated mechanism for this complication is thought to be through host molecules’ mimicry of the vaccine’s antigens or by eliciting a type I interferons response [25, 26]. The timing of BP onset in relationship to vaccination is unclear, although most of the cases have occurred in an average 4-week interval after vaccination [27]. Moreover, there has been a case of sequential contralateral facial nerve palsies following each dose of COVID-19 vaccines reported recently [27]. Up to now, BP has been reported following various COVID-19 vaccine types, including Pfizer-BioNTech, Janssen, CoronaVac, Moderna, and Oxford-AstraZeneca vaccines [12, 23, 28–30]. Nonetheless, the risk of developing facial nerve palsy has been estimated to be higher with mRNA vaccines than with other vaccine platforms; this fact can help us decide the choice of COVID-19 vaccine in individuals with a history of BP [31]. It is vital to note that most cases of BP, regardless of etiology, are self-limiting and subside within a few months [32]. However, antiviral agents and steroids are frequently tried as a treatment and hasten recovery [32].
Guillain–Barré Syndrome
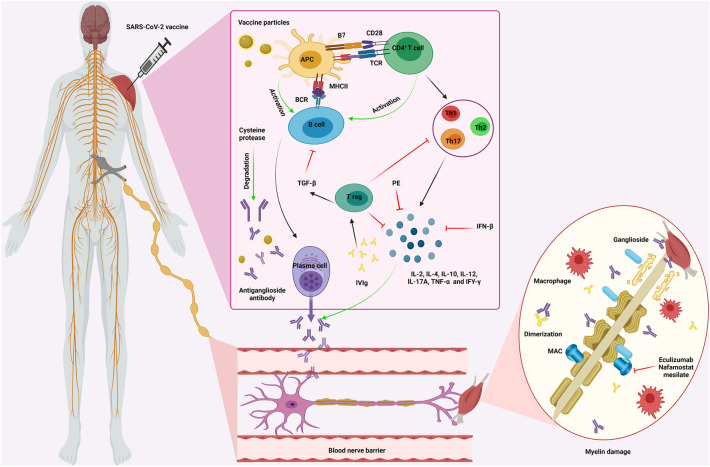
Guillain–Barré Syndrome (GBS) is defined as an inflammatory ascending polyradiculoneuropathy. The underlying triggers for this neurological disorder include infections and vaccines on an autoimmune basis [33] (Fig. 1). The introduction of COVID vaccines has arisen the concerns of developing GBS following vaccination [34] since GBS had been previously observed in individuals who received the meningococcal, tetanus-toxoid, human papillomavirus (HPV), and most prominently, the influenza vaccines [35–39]. This complication has been reported following Pfizer-BioNTech, Johnson & Johnson, and ChAdOx1 nCoV-19 COVID-19 vaccines [1, 40–42]. Moreover, one case of isolated bilateral facial diplegia with paresthesias (BFP), an uncommon variant of GBS, has been reported following the Janssen COVID-19 vaccination [43]. It is believed that vaccine-induced immune responses may trigger autoimmune reactions, resulting in autoantibody production against myelin, resulting in GBS [40]. The diagnosis of vaccine-induced GBS is the same as that of other causes, through clinical and paraclinical findings such as cerebrospinal fluid (CSF) analysis and electromyography and nerve conduction velocity (EMG/NCV) studies, in addition to considering the temporal relationship between the event and vaccination [44]. Intravenous immune globulin (IVIg) (0.4 g per kg body weight every day for 5 days) and plasma exchange (200–250 ml plasma kg body weight in 5 sessions) can be considered as efficient treatments for GBS [45].
Fig. 1.
COVID-19 vaccine-induced Guillain–Barré syndrome. After the administration of the COVID-19 vaccine, the vaccine particles enter the body and activate APCs, which could trigger B cells and CD4+ T cells activation. Naïve CD4+ T cells are then differentiated into three subgroups, Th1, Th2, and Th17, producing cytokines, such as IL-2, IL-4, IL-10, IL-12, IL-17A, TNF-α, and IFY-γ. Moreover, B cells are converted to plasma cells, secreting antiganglioside antibodies. These antibodies are then gone through the blood-nerve barrier, binding to the ganglioside of the myelinated motor neurons or attaching to the neuromuscular junction. As a result of forming gangliosides-antiganglioside antibody complexes, MAC and macrophages are activated, attacking and destroying the myelin. Such demyelination would decrease the speed of action potential transmission through these nerves, causing an inflammatory ascending polyradiculoneuropathy. IVIg administration could reverse these mechanisms via 2 main pathways: triggering Treg cells, inhibiting B cells and inflammatory cytokines, and dimerizing with antiganglioside antibodies. Moreover, plasmapheresis could alleviate the symptoms via actively depleting inflammatory cytokines from patients’ bloodstream. Furthermore, cysteine proteases can degrade antiganglioside antibodies, inhibiting this inflammatory neuropathy. Eculizumab and nafamostat mesylate would also inhibit MAC, alleviating the demyelination. Abbreviations: APC Antigen-presenting cell, TCR T cell receptor, MHC II Major histocompatibility complex II, BCR B cell receptor, Th T helper cell, IL-2 Interleukin-2, IL-4 Interleukin-4, IL-10 Interleukin-10, IL-12 Interleukin-12, IL-17A Interleukin-17A, TNF-α Tumor necrosis factor-α, IFY-γ Interferon-γ, GBS Guillain-Barré syndrome, IVIg Intravenous immune globulin, Treg regulatory T cell, TGF-β Transforming growth factor-β, PP Plasmapheresis, IFN-β Interferon-β, MAC Membrane attack complex
Transverse myelitis
Transverse myelitis (TM) is a condition where spinal cord segments may become inflamed, resulting in significant motor, autonomic and sensory deficits. [46]. Since the beginning of the current pandemic, several cases of TM have been reported in SARS-CoV-2-infected patients [47, 48]. Apart from infections as a cause of TM, vaccines are of great importance in the evolution of this neurological condition [49]. TM following vaccination had previously been observed with various vaccines, including tetanus, measles–mumps–rubella, influenza (H1N1), hepatitis B, polio, and Japanese B encephalitis vaccines [50–54]. This neurological complication has also been reported following COVID-19 vaccination with viral vector-based and mRNA-based COVID-19 vaccines. However, their association has not been confirmed in a number of trials [2, 55–57]. Moreover, a rare subtype of TM, known as longitudinally extensive transverse myelitis (LETM), has been reported following COVID-19 vaccination [58]. The pathophysiology of TM in the settings of SARS-CoV-2 infection is thought to be either via direct viral neuro-invasion or immune-mediated, while the mechanism by which COVID-19 vaccines might trigger TM may be immune and inflammatory reactions [59, 60]. The diagnosis is made by typical clinical evidence of bilateral sensory, motor, or autonomic dysfunction with an established spinal cord defect origin in magnetic resonance imaging (MRI) [61]. Unfortunately, TM is a neurological condition with unfavorable outcomes [62]. Although there is insufficient evidence, high-dose IV methylprednisolone (1 g per day for 3–7 days) has to be started immediately for all TM cases to improve neurological function and accelerate recovery [63, 64]. In a case report study, a COVID-19 patient with acute TM treated with high-dose IV methylprednisolone (1 g daily for 3 days) showed improved neurological symptoms immediately after receiving IV corticosteroid therapy [65].
Cerebrovascular events
Since the beginning of the COVID-19 pandemic, a significant increase in stroke rates was observed, later discovered to be due to SARS-CoV-2 infection [66–68]. Although not as common as SARS-CoV-2 infection, COVID-19 vaccination is also suspected to increase the risk of cerebrovascular events [69]. It is unknown whether the cerebrovascular events, including ischemic/hemorrhagic strokes and cerebral venous sinus thrombosis, are related to COVID-19 vaccination. If they are related, it also remains unclear how the vaccines may contribute–by causing arterial hypertension, worsening thrombocytopenia, or exacerbating a hypercoagulable state [70, 71]. Previously, a stroke took place following vaccination with various other vaccines, including diphtheria, measles–mumps–rubella, and influenza vaccines [72–74]. The probability and reports of systemic thrombotic thrombocytopenic events following COVID-19 vaccination have caused a great deal of concern and hesitancy worldwide [71]. The Oxford-AstraZeneca (ChAdOx1 nCoV-19) vaccine had been the most notorious for this complication that many countries suspended its use [75]. However, thrombotic events have not been uncommon following the Johnson & Johnson COVID-19 vaccine [76, 77]. Cerebrovascular events, including hemorrhagic and ischemic strokes, from venous and arterial etiologies and an increased thrombotic and embolic risk, have been noted [78–81]. There have been reports of thrombosis in the cortical veins, transverse sinus, sigmoid sinus, inferior sagittal sinus, the vein of Galen, and the straight sinus that have all presented with intracranial hemorrhage (ICH) and subarachnoid hemorrhage (SAH), shortly after COVID-19 vaccination [80–83].
However, we should take into account that individuals with risk factors of thromboembolic events, such as pregnancy, postpartum state, oral contraceptives use, surgery, trauma, immobilization, malignancies, and thrombophilic genetic or autoimmune conditions including anti-thrombin, protein C, and protein S deficiency, factor V Leiden mutation, antiphospholipid antibodies, and hyperhomocysteinemia, are more prone to vaccine-induced cerebrovascular complications [84–87]. It is important to note that anti-CXCL4 antibodies are responsible for most cases of vaccine‐induced immune thrombotic thrombocytopenia (VITT); this is similar to what happens with heparin-induced thrombocytopenia (HIT) [71, 88]. Depending on which vessel is involved, clinical manifestations may range from a simple headache, nausea, vomiting, and diplopia to focal neurologic signs, altered consciousness, and coma. The diagnosis of cerebrovascular AEs is generally made with comprehensive imaging, including brain computed tomography (CT), venogram, angiography, and MRI [89]. Management of cerebrovascular events following vaccination is generally the same as with any other cause, with the goal being assessment and management of risk factors and secondary stroke prevention. Heparin and platelet transfusions should be avoided until VITT has been excluded [90, 91]. In cases of systemic thrombotic thrombocytopenic events, IVIg, high-dose glucocorticoids, and plasmapheresis are recommended when indicated to restore platelet counts and address the autoimmune phenomenon [79, 92].
Encephalopathy
Acute encephalopathy has been attributed to various etiologies, including toxins, infections, and vaccines. One of the most prevalent neurological sequelae of COVID-19 has been encephalopathy which presents with cognitive impairment, altered consciousness, and even seizures [93–96]. However, the condition has been observed much less frequently following COVID-19 vaccination [94, 97–100]. In the past, several cases of encephalopathy had been reported after various vaccines, including hepatitis B, rabies, pertussis, measles, influenza, and HPV vaccines [101–107]. The pathophysiologic mechanism for vaccine-induced acute disseminated encephalomyelitis (ADEM) seems to be the inflammatory cascade or the cytokine storm triggered by the production of spike protein from translated mRNA in the vaccines [108, 109]. The diagnosis of ADEM in the settings of COVID-19 vaccination is similar to that of other causes and is accomplished through clinical and cerebrospinal fluid (CSF) findings and imaging modalities such as brain MRI. The treatment consists of corticosteroids and sometimes IVIg and plasmapheresis [110]. Fortunately, ADEM has a favorable outcome if conservative support is satisfactory [111].
New-onset seizures
The pathophysiologic mechanisms of seizures during a SARS-CoV-2 infection differ significantly from those following COVID-19 vaccination. In the former, specific antibiotic therapies, cerebral hypoxemia, acute renal failure, and electrolyte impairment can be the underlying reasons [112–114]. The latter can happen in the settings of vaccine-induced encephalopathy or venous occlusion [97]. Before the current pandemic, HPV and H1N1 vaccinations had been related to functional (non-epileptic) seizures, which were believed to be psychogenic attacks [115, 116]. The association of febrile seizure with the measles–mumps–rubella–varicella vaccine has long been well established [117]. It is unknown whether non-motor seizures are related to COVID-19 vaccines or only a coincidence [118]. Patients with a known history of epilepsy or prior history of seizures may have a decreased threshold in the post-vaccine period due to the symptoms and illness. Rare attacks have included new-onset refractory status epilepticus that require further assessment and follow-up [119]. The diagnosis is based on clinical history, physical examinations, brain imaging (CT scan and MRI), electroencephalography, and serum prolactin level measurement. There are a variety of antiepileptic drugs (Table 1) that can be administered as first-line monotherapy in adults with epilepsy [120].
Table 1.
Summary of the proposed diagnosis and management of neurological adverse events following COVID-19 vaccination
| Neurological adverse event | Diagnosis | Management |
|---|---|---|
| Bell’s palsy | History and physical examination: Rapid-onset (less than 72 h) unilateral paralysis of the facial nerve (weakness or complete loss of movement) with no defined reason | Oral corticosteroids (prednisolone) and antiviral agents (acyclovir and valacyclovir) |
| Guillain–Barré Syndrome | Clinical and paraclinical findings such as cerebrospinal fluid (CSF) analysis and electromyography and nerve conduction velocity (EMG/NCV) studies, in addition to considering the temporal relationship between the event and vaccination | IVIg (0.4 g per kg body weight every day for 5 days) and plasma exchange (200–250 mL plasma per kg body weight in 5 sessions) are similarly efficient remedies for GBS |
| Transverse myelitis | Typical clinical evidence of bilateral sensory, motor, or autonomic dysfunction with an established spinal cord defect origin in magnetic resonance imaging (MRI) | High-dose IV methylprednisolone (1 g daily for 3–7 days) |
| Cerebrovascular events | Brain computed tomography (CT), venogram, angiography, and MRI |
IVIg High-dose glucocorticoids Plasmapheresis Non-heparin anticoagulants (like fondaparinux and argatroban) |
| Encephalopathy | Clinical and cerebrospinal fluid (CSF) findings and imaging modalities such as brain MRI | Corticosteroids and sometimes IVIg and plasmapheresis |
| New-onset seizures | Clinical history, physical examinations, brain imaging (CT scan and MRI), electroencephalography, and serum prolactin level measurement |
Narrow-spectrum drugs (focal seizure) Carbamazepine Eslicarbazepine Gabapentin Lacosamide Oxcarbazepine Phenytoin Broad-spectrum drugs (focal and almost all generalized seizures) Lamotrigine Levetiracetam Topiramate Valproate Zonisamide |
| Myasthenia gravis exacerbation | Suspected through compatible signs and symptoms of fatigable muscle weakness and confirmed by EMG studies, pharmacologic testing, and serum Ab assay |
Pyridostigmine (30 mg 3–4 times a day, then can be increased to 60 mg 4 times a day) Oral prednisone (0.75–1 mg per kg daily) Azathioprine Cyclosporine Tacrolimus Rituximab |
| Varicella-zoster virus reactivation | Clinical manifestations (dermatomal rash, pain, paresthesia, dysesthesia, allodynia, pruritus), IF test for VZV antigen, PCR test for VZV DNA |
Acyclovir (800 mg orally 5 times a day for 7–10 days) Valacyclovir (1 g orally 3 times a day for 7 days) Famciclovir (500 mg orally 3 times a day for 7 days) |
Varicella-zoster virus reactivation
Since the beginning of the COVID-19 pandemic, several cases of herpes zoster have been reported in SARS-CoV-2 infected patients, even in immunocompetent individuals [121, 122]. The potential mechanism for this event is suggested to be COVID-19-induced lymphopenia and CD4+ T cell dysfunction [123]. Nonetheless, immunomodulation, immune dysregulation, and attenuated alloreactivity are believed to be the underlying pathophysiology for vaccine-induced herpes zoster reactivation [124, 125]. Herpes zoster reactivation was previously reported following yellow fever, influenza, hepatitis A, and rabies vaccines [126, 127]. Up to the present time, this neurological complication has been reported following various COVID-19 vaccines, including mRNA-based (Pfizer-BioNTech, Moderna), viral vector (Oxford ChAdOx1-S or AZD1222), and inactivated vaccines (COVAXIN) [125, 128–133]. Moreover, a case of varicella-zoster virus-induced small vessel vasculitis following the first dose of the Pfizer-BioNTech COVID-19 vaccine has been reported [134]. It should be noted that in all suspected cases of vaccine-induced herpes zoster reactivation, a de novo SARS-CoV-2 infection should be ruled out. The diagnosis is made with clinical manifestations (dermatomal rash, pain, paresthesia, dysesthesia, allodynia, and pruritus), immunofluorescence (IF) test for VZV antigen, and PCR test for VZV DNA. acyclovir, valacyclovir, and famciclovir (guanosine analogs) are recommended for VZV treatment [135].
Miscellaneous neurological adverse events
Apart from the aforementioned neurological side effects, some other AEs, including narcolepsy, small fiber neuropathy, neuroleptic malignant syndrome (NMS), and multiple sclerosis flare-ups, have been reported following COVID-19 vaccination, although their causal relationships are not confirmed [4, 136–139].
Relative risks of neurological adverse events in SARS-CoV-2 infection vs. post-COVID-19 vaccination
Many studies have reported various neurological disorders associated with COVID-19 infection and vaccines [80, 140–143]. However, given the shortage of comprehensive, prospective studies, it is still far too difficult to establish cause-effect relations between these factors. Therefore, future studies should determine the real risk of these adverse events following COVID-19 vaccination. Until then, it does not seem reasonable to limit vaccine administration. Nonetheless, considering the existing data, in this section, we have summarized some studies reporting cases of neurological adverse events associated with SARS-CoV-2 infection or vaccination (Table 2).
Table 2.
Summary of the reported cases of neurological adverse events following SARS-CoV-2 infection vs post-COVID-19 vaccination
| Case number | Reference | Neurological disorder | Sex/Age (years) | Country | Clinical manifestation | Diagnosis | Treatment | Associated with SARS-CoV-2 infection vs post-COVID-19 vaccination |
|---|---|---|---|---|---|---|---|---|
| 1 | Afshar et al. [144]/2021 | Bell’s palsy | F/64 | Iran | Left side facial nerve palsy |
History and physical examination, brain CT scan and MRI, pulmonary CT scan, RT-PCR for SARS-CoV-2, and anti-SARS-CoV-2 IgM test |
lopinavir/ritonavir (Kaletra) + dexamethasone | Associated with COVID-19 infection |
| 2 | Dahl et al. [145]/2021 | Bell’s palsy | M/37 | Norway | Right side facial nerve palsy |
History and physical examination, cerebral CT scan, spinal fluid examination, spinal fluid PCR, anti-SARS-CoV-2 IgG antibodies test |
– | Associated with COVID-19 infection |
| 3 | Bastola et al.[146]/2021 | Bell’s palsy | M/48 | India | Left side facial nerve palsy |
History and physical examination, chest HRCT and PCR for SARS-CoV-2 |
Prednisolone | Associated with COVID-19 infection |
| 4 | Al-Mashdali et al.[147]/2021 | Bell’s palsy | M/21 | Qatar | Right side facial nerve palsy |
History and physical examination, chest CT scan and RT-PCR for SARS-CoV-2 |
Prednisolone and eye lubricant | Associated with COVID-19 infection |
| 5 | Hasibi et al.[148]/2021 | Bell’s palsy | M/52 | Iran | Right side facial nerve palsy |
History and physical examination, RT-PCR for SARS-CoV-2 and spiral chest CT scan |
Prednisolone and favipiravir Then: remdesivir and IV dexamethasone |
Associated with COVID-19 infection |
| 6 | Ferreira et al.[149]/2022 | Bell’s palsy | M/11 | Portugal | Right side peripheral facial paralysis |
History and physical examination, PCR for SARS-CoV-2, cranial CT, and MRI |
Prednisolone | Associated with COVID-19 infection |
| 7 | Iacono et al.[150]/2022 | Bell’s palsy | M/5 | Italy | Right side facial nerve palsy |
History and physical examination, brain MRI and serological tests for SARS-CoV-2 |
Prednisolone and eye lubricant | Associated with COVID-19 infection |
| 8 | Kaplan[151]/2021 | Bell’s palsy | F/48 | USA | Left side facial nerve palsy |
History and physical examination, PCR for SARS-CoV-2 and chest CT scan |
Prednisone, valacyclovir, and doxycycline (the doxycycline was discontinued after Lyme disease titers became negative) | Associated with COVID-19 infection |
| 9 | Szewczyk et al. [152]/2021 | Bilateral facial nerve palsy | M/70 | Poland | Bilateral facial nerve palsy |
History and physical examination, brain CT scan and MRI and serological tests for SARS-CoV-2 |
Intravenous immunoglobulins (IVIg) | Associated with COVID-19 infection |
| 10 | Kumar et al. [153]/2021 | Bell’s palsy | F/28 | India | Right side lower motor neuron facial nerve palsy |
History and physical examination, RT-PCR for SARS-CoV-2 |
Prednisone and valacyclovir | Associated with COVID-19 infection |
| 11 | Neo et al. [154]/2021 | Bell’s palsy | M/25 | Singapore | Left side facial weakness/palsy | History and physical examination, RT-PCR for SARS-CoV-2, and serological tests for SARS-CoV-2 | Oral corticosteroids, valacyclovir, and eye care advice | Associated with COVID-19 infection |
| 12 | Neo et al. [154]/2021 | Bell’s palsy | M/34 | Singapore | Right side facial weakness | History and physical examination, RT-PCR for SARS-CoV-2, and serological tests for SARS-CoV-2 | Oral corticosteroids, valacyclovir, and eye care advice | Associated with COVID-19 infection |
| 13 | Khaja et al. [155]/2020 | Guillain-Barré Syndrome and Bell’s Palsy | M,44 | USA | Bilateral facial weakness | History and physical examination, RT-PCR for SARS-CoV-2, serological tests for SARS-CoV-2, and brain MRI | IVIg | Associated with COVID-19 infection |
| 14 | Theophanous et al. [156]/2021 | Bell’s palsy | M/6 | USA | Right side facial nerve palsy | History and physical examination, RT-PCR for SARS-CoV-2 |
IV acyclovir and IVIg infusion (because of agammaglobulinemia) Then discharged with prednisolone and acyclovir |
Associated with COVID-19 infection |
| 15 | Wan et al. [157]/2020 | Bell’s palsy | F/65 | China | Left side facial nerve palsy | History and physical examination, RT-PCR for SARS-CoV-2, and brain MRI | Arbidol and ribavirin | Associated with COVID-19 infection |
| 16 | Bohania et al. [158]/2021 | Bell’s palsy | F/18 | – | Right side facial nerve palsy | History and physical examination, COVID-19 antigen testing | Steroids, eye taping during sleep, and methylcellulose eye drops | Associated with COVID-19 infection |
| 17 | Burrows et al. [159]/2021 | Bell’s palsy | M/61 | UK | Right side lower motor neuron facial palsy | History and physical examination and head CT scan | Prednisolone | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 18 | Cellina et al. [160]/2022 | Bell’s palsy | F/35 | Italy | Left side facial nerve palsy | History and physical examination and brain MRI | Prednisone | Associated with COVID-19 vaccination (Moderna) |
| 19 | Iftikhar et al .[12]/2021 | Bell’s palsy | M/36 | Qatar | Left side facial nerve palsy + left upper limb numbness and weakness | History and physical examination, brain CT scan, and MRI | Prednisolone and eye lubricant | Associated with COVID-19 vaccination (Moderna) |
| 20 | Mussatto et al. [161]/2022 | Bell’s palsy (he was also a 20-year-prior case of HIV) | M/60 | USA | Left side facial nerve palsy | History and physical examination | Prednisone and valacyclovir | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 21 | Repajic et al. [28]/2021 | Bell’s palsy | F/57 | USA | Left side facial nerve palsy | History and physical examination | Prednisone and an antiviral agent | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 22 | Yu et al. [162]/2021 | Bell’s palsy | F/36 | China | Right side facial nerve palsy | History and physical examination, serological tests for SARS-CoV-2, and brain CT scan | Prednisone and artificial tear | Associated with COVID-19 vaccination (Sinovac Life Sciences inactivated COVID-19 vaccine) |
| 23 | Mason et al. [163]/2021 | Bell’s palsy | F/35 | USA | Bilateral facial nerve palsy | History and physical examination, brain MRI and CT angiography | Methylprednisolone (IV) and acyclovir | Associated with COVID-19 vaccination (Moderna) |
| 24 | Mirmosayyeb et al. [164]/2022 | Bell’s palsy | F/27 | Iran | Left side facial nerve palsy | History and physical examination and brain MRI | Prednisone and valacyclovir | Associated with COVID-19 vaccination (Russian Sputnik V) |
| 25 | Mirmosayyeb et al. [164]/2022 | Bell’s palsy | M/58 | Iran | Left side facial nerve palsy | History and physical examination | Prednisone and valacyclovir | Associated with COVID-19 vaccination (Russian Sputnik V) |
| 26 | Pothiawala[165]/ 2021 | Bell’s palsy | M/46 | Singapore | Right side facial nerve palsy | History and physical examination | Prednisone and acyclovir | Associated with COVID-19 vaccination (Moderna) |
| 27 | Kundi et al.[166]/2022 | Bell’s palsy | F/66 | USA | Right side facial nerve palsy | History and physical examination and brain CT | Prednisone, acyclovir, meclizine, and ondansetron | Associated with COVID-19 vaccination (Ad26.COV2.S vaccine) |
| 28 | Nishizawa et al. [167]/2021 | Bell’s palsy | F/62 | Japan | Right side facial nerve palsy | History and physical examination, head CT and brain MRI | – | Associated with COVID-19 vaccination (Ad26.COV2.S vaccine) |
| 29 | Colella et al. [20]/2021 | Bell’s palsy | M/37 | Italy | Left side facial nerve palsy | History and physical examination | Prednisone and artificial tear | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 30 | Martin-Villares et al. [168]/2022 | Bell’s palsy | F/34 | Spain | Right side facial nerve palsy | History and physical examination and brain MRI | – | Associated with COVID-19 vaccination (Moderna) |
| 31 | Scheidl et al. [169]/2020 | Guillain-Barré Syndrome | F/54 | Germany | Acute, proximally pronounced, moderate, symmetric paraparesis, areflexia, numbness, and tingling of all extremities | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis and EMG and NCV studies) | IVIg (0.4 g per kg body weight every day for 5 days) | Associated with COVID-19 infection |
| 32 | Bueso et al. [170]/2021 | Guillain-Barré Syndrome | F/60 | USA | Symmetrical weakness of both the lower and upper extremities | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis) | IVIg (0.4 g per kg body weight every day for 5 days) + enoxaparin 30 mg twice a day | Associated with COVID-19 infection |
| 33 | Sedaghat and Karimi [171]/2020 | Guillain-Barré Syndrome | M/65 | Iran | Acute progressive weakness of distal lower extremities, quadriplegia, and bilateral facial paresis | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis and EMG and NCV studies) | IVIg (0.4 g per kg body weight every day for 5 days) | Associated with COVID-19 infection |
| 34 | Agosti et al. [172]/2021 | Guillain-Barré Syndrome | M/68 | Italy | Acute progressive symmetric ascending flaccid tetraparesis, bifacial nerve palsy, and muscular weakness | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis and NCV studies) | IVIg (0.4 g per kg body weight every day for 5 days) | Associated with COVID-19 infection |
| 35 | Paybast et al. [173]/2020 | Guillain–Barré Syndrome | M/38 | Iran | Acute symmetric progressive ascending paresthesia of both lower and upper extremities and bilateral facial droop | clinical and paraclinical findings (such as CSF analysis and EMG and NCV studies) | Therapeutic plasma exchange for 5 sessions | Associated with COVID-19 infection |
| 36 | Paybast et al. [173]/2020 | Guillain-Barré Syndrome | F/14 | Iran | Progressive ascending quadripareshtesia and mild lower limb weakness | Clinical and paraclinical findings (such as CSF analysis) | IVIg (20 g daily for 5 d) | Associated with COVID-19 infection |
| 37 | Dufour et al. [174]/2021 | Guillain-Barré Syndrome | F/36 | USA | Progressive ascending weakness | RT-PCR for SARS-CoV-2, history and physical examinations | IVIg (0.4 g per kg body weight every day for 5 days) | Associated with COVID-19 infection |
| 38 | Toscano et al. [175]/2020 | Guillain-Barré Syndrome | – | Italy | Flaccid areflexic tetraplegia evolving to facial weakness, upper-limb paresthesia | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis and head and spine MRI) | 2 IVIg cycles | Associated with COVID-19 infection |
| 39 | Toscano et al. [175]/2020 | Guillain-Barré Syndrome | – | Italy | Facial diplegia and generalized areflexia evolving to paresthesia of lower limbs with ataxia | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis and head and spine MRI) | IVIg | Associated with COVID-19 infection |
| 40 | Toscano et al. [175]/2020 | Guillain-Barré Syndrome | – | Italy | Flaccid tetraparesis and facial weakness evolving to areflexia | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis and head and spine MRI) | 2 IVIg cycles | Associated with COVID-19 infection |
| 41 | Toscano et al. [175]/2020 | Guillain-Barré Syndrome | – | Italy | Flaccid areflexic tetraparesis and ataxia | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis and head and spine MRI) | IVIg | Associated with COVID-19 infection |
| 42 | Toscano et al. [175]/2020 | Guillain-Barré Syndrome | – | Italy | Facial weakness, flaccid areflexic paraplegia | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis and head and spine MRI) | IVIg and plasma exchange | Associated with COVID-19 infection |
| 43 | El Otmani et al. [176]/2020 | A subtype of GBS: Acute Motor and Sensory Axonal Neuropathy (AMSAN) | F/70 | Morocco | Bilateral weakness and paresthesia in all four extremities | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis and EMG and NCV studies) | IVIg (2 g/kg for 5 days), Hydroxychloroquine (600 mg/day), and Azithromycin (500 mg on the first day, then 250 mg/day) | Associated with COVID-19 infection |
| 44 | Khan et al. [177]/2021 | Guillain-Barré Syndrome | M/27 | India | Myalgia, weakness of the lower limb (then it involved the upper limb), and generalized hypotonia | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis and NCV studies) | IVIg (0.4 g per kg body weight every day for 5 days) | Associated with COVID-19 infection |
| 45 | Khan et al. [177]/2021 | Guillain-Barré Syndrome | F/35 | India | Paresthesia in both lower limbs followed by mild weakness | COVID-19 testing (she was positive for COVID‐19), clinical and paraclinical findings (such as CSF analysis and NCV studies) | Managed as a case of COVID‐19 (Supportive) | Associated with COVID-19 infection |
| 46 | Khan et al. [177]/2021 | Guillain-Barré Syndrome | F/40 | India | Lower-limb paresthesia is associated with weakness rapidly progressing from lower to upper limbs, respiratory muscles weakness | Clinical and paraclinical findings (such as CSF analysis and NCV studies) | IVIg (0.4 g per kg body weight every day for 5 days) | Associated with COVID-19 infection |
| 47 | Khan et al. [177]/2021 | Guillain-Barré Syndrome | F/48 | India | Paresthesia in both lower limbs and weakness | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis and NCV studies) | IVIg (0.4 g per kg body weight every day for 5 days) | Associated with COVID-19 infection |
| 48 | Khan et al. [177]/2021 | Guillain-Barré Syndrome | M/50 | India | Paresthesia in both lower limbs (then it involved upper limbs) and weakness | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis) | IVIg (0.4 g per kg body weight every day for 5 days) | Associated with COVID-19 infection |
| 49 | Su et al. [178]/2020 | Guillain-Barré Syndrome with dysautonomia | M/72 | USA | Symmetric paresthesias and ascending appendicular weakness | SARS-CoV-2 PCR, clinical and paraclinical findings (such as CSF analysis and EMG and NCV studies) | IVIg (2 g/kg between days 3 and 6) | Associated with COVID-19 infection |
| 50 | Darvishi et al. [179]/2021 | Guillain-Barré Syndrome | M/56 | Iran | Subacute progressive lower limbs weakness, paresthesia, and pain (then it progressed to severe, flaccid paraparesis) | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis) | IVIg (0.5 g/kg/d for 5 days) | Associated with COVID-19 infection |
| 51 | Zhao et al. [180]/ 2020 | Guillain-Barré Syndrome | F/61 | China | Symmetric weakness and areflexia in both lower limbs | RT-PCR for SARS-CoV-2, clinical and paraclinical findings (such as CSF analysis and NCV studies) | IVIg | Associated with COVID-19 infection |
| 52 | Mackenzie et al. [181]/2021 | Guillain-Barré Syndrome | F/39 | Colombia | Progressive generalized weakness of lower limbs | SARS-CoV-2 PCR, clinical and paraclinical findings (such as CSF analysis and EMG and NCV studies) | Supportive care, enoxaparin, losartan, meperidine IV for muscle pain, hydroxychloroquine, and dexamethasone | Associated with COVID-19 infection |
| 53 | Mostel et al. [182]/2021 | Guillain-Barré Syndrome | F/69 | USA | Progressive motor weakness and sensation loss in extremities, numbness and paresthesia in the right hand and leg, numbness and paresthesia in the left limb | SARS-COV-2 antibodies, clinical and paraclinical findings (such as EMG studies) | IVIg (2 g/kg for 5 days) | Associated with COVID-19 infection |
| 54 | Farzi et al. [183]/2020 | Guillain-Barré Syndrome | M/41 | Iran | Ascending paresthesia and paralysis | SARS-CoV-2 PCR, clinical and paraclinical findings (such as EMG and NCV studies) | IVIg (0.4 g per kg body weight every day for 5 days) | Associated with COVID-19 infection |
| 55 | Nejad et al. [184]/2021 | Guillain-Barré Syndrome | M/70 | Iran | Symmetric weakness and arefexia in both lower limbs | SARS-CoV-2 PCR, clinical and paraclinical findings (such as CSF analysis) | IVIg | Associated with COVID-19 infection |
| 56 | McKean et al. [185]/ 2021 | Guillain-Barré Syndrome | M/48 | Malta | Bilateral facial weakness, ascending paraesthesia, and bilateral progressive lower limb weakness | Clinical and paraclinical findings (such as CSF analysis and NCV studies),brain CT and MRI | IVIg (2 g/kg for 5 days) | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 57 | Hasan et al. [186]/2021 | Guillain-Barré Syndrome | F/62 | UK | Paraesthesia and progressive weakness of both lower limbs | Clinical and paraclinical findings (such as CSF analysis and NCV studies),brain CT and MRI | IVIg (2 g/kg for 5 days) | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 58 | Allen et al. [187]/2021 | Guillain–Barré Syndrome | M/54 | UK | Bilateral facial weakness and distal dysesthesia in hands and feet | Clinical and paraclinical findings (such as CSF analysis and NCV studies),brain MRI | Oral prednisolone (60mg for 5 days) | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 59 | Allen et al. [187]/2021 | Guillain–Barré Syndrome | M/20 | UK | Bilateral facial weakness and distal dysesthesia in feet | Clinical and paraclinical findings (such as CSF analysis and NCV studies),brain MRI | Oral prednisolone (60 mg for 5 days) | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 60 | Allen et al. [187]/2021 | Guillain-Barré Syndrome | M/57 | UK | Dysarthria and facial weakness, distal dysesthesia in feet, and proximal leg weakness |
Clinical and paraclinical findings (such as CSF analysis and NCV studies), and brain MRI |
IVIg | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 61 | Allen et al. [187]/2021 | Guillain–Barré Syndrome | M/55 | UK | Bilateral thigh paresthesia, bilateral facial weakness | Clinical and paraclinical findings (such as CSF analysis) and brain MRI | No treatment | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 62 | Min et al. [188]/2021 | Sensory Guillain–Barré Syndrome | M/58 | Republic of Korea | Severe paresthesia on both feet, mild hypoesthesia in vibration, temperature, and pain on both feet |
SARS-CoV-2 PCR, Clinical and paraclinical findings (such as CSF analysis, NCV studies, and skin biopsy), and MRI |
– | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 63 | Min et al. [188]/2021 | Sensory Guillain-Barré Syndrome | F/37 | Republic of Korea | Paresthesia in both lower limbs |
SARS-CoV-2 PCR, Clinical and paraclinical findings (such as CSF analysis, NCV studies, and skin biopsy), and MRI |
– | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 64 | Scendoni et al. [189]/2021 | Guillain-Barré Syndrome | F/82 | Italy | Progressively worsening of walking, weakness, lack of sensitivity in both lower limbs, and areflexia | Clinical and paraclinical findings (such as CSF analysis and EMG and NCV studies) | IVIg (0.4 g per kg body weight every day for 5 days) | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 65 | Maramattom et al. [190]/2021 | Guillain–Barré Syndrome | F/43 | India | Areflexic quadriparesis, facial diplegia, and respiratory failure | Clinical and paraclinical findings (such as CSF analysis and NCV studies) | IVIg | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 66 | Maramattom et al. [190]/2021 | Guillain-Barré Syndrome | F/67 | India | Distal paraesthesia in all the extremities, bilateral facial weakness, dysphagia, and increasing limb weakness | – | IVIg and plasmapheresis | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 67 | Maramattom et al. [190]/2021 | Guillain-Barré Syndrome | F/53 | India | Bilateral lower limb numbness and weakness, right-sided facial and tongue numbness (then it progressed to bilateral lower motor neuron facial palsy and Areflexic flaccid quadriplegia) | – | Mechanical ventilation | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 68 | Introna et al. [191]/2021 | Guillain-Barré Syndrome | M/62 | Italy | Absent deep tendon reflexes, severe bilateral optic disk edema, progressively worsening sensory ataxia and ascending quadriparesis | Clinical and paraclinical findings (such as CSF analysis and EMG and NCV studies), brain CT and MRI | IVIg (2 g/kg for 5 days) | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 69 | Razok et al. [192]/2021 | Guillain-Barré Syndrome | M/73 | Qatar | Progressive bilateral lower limb weakness | Clinical and paraclinical findings (such as CSF analysis and EMG and NCV studies), brain CT and MRI | IVIg (0.4 g per kg body weight every day for 5 days) | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 70 | Rao et al. [193]/2021 | Guillain-Barré Syndrome | F/42 | USA | Progressive ascending weakness and paresthesias | Clinical and paraclinical findings (such as CSF analysis and NCV studies), brain and cervical MRI | IVIg (total of 2 g/kg in four divided doses) | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 71 | Moreno-Escobar et al. [194] | Transverse myelitis | M/41 | – | Bilateral paresthesia in upper and lower limbs, along with urinary and fecal retention | Serological study and CSF analysis, imaging studies | IV methylprednisolone | Associated with COVID-19 infection |
| 72 | Qazi et al. [195]/2021 | Transverse myelitis | F/35 | Pakistan | Abrupt bilateral lower limb weakness, paresthesia, and urinary retention | Serological study and CSF analysis, imaging studies | IV methylprednisolone (1 g/day for 7 days) | Associated with COVID-19 infection |
| 73 | Chow et al. [196]/2020 | Transverse myelitis | M/60 | Australia | Bilateral lower limb weakness, urinary retention, and constipation | Serological study and CSF analysis, imaging studies | IV methylprednisolone (1 g/day for 3 days) | Associated with COVID-19 infection |
| 74 | Sarma et al.[197]/2020 | Transverse myelitis | F/28 | USA | Lower back pain, bilateral symmetric upper and lower extremity numbness, and urinary retention | Serological study and CSF analysis, imaging studies | Prednisolone and plasma exchange | Associated with COVID-19 infection |
| 75 | Ahmad et al. [198]/2021 | Transverse myelitis | F/34 | Iraq | Progressive intermittent leg pain, paresthesia, and weakness on both sides | Serological study and CSF analysis, imaging studies | IV methylprednisolone (500 mg 1 × 1 for 5 days) | Associated with COVID-19 infection |
| 76 | Nejad Biglari et al.[199]/2021 | Transverse myelitis | F/11 | Iran | Acute paresis in the lower limbs, urinary and fecal retention | Serological study and CSF analysis, imaging studies | IVIg (0.4 g per kg body weight every day for 5 days) + pulse of methylprednisolone 30 mg/ Kg for 3 days | Associated with COVID-19 infection |
| 77 | Palahuta et al. [200]/2021 | Transverse myelitis | M/23 | Ukraine | Acute-onset non-compressive myelitis with bilateral paresthesia | Serological study and CSF analysis, imaging studies | IV methylprednisolone (1 g/day for 5 days) | Associated with COVID-19 infection |
| 78 | Lingas [201]/2022 | Transverse myelitis | M/70 | USA | Numbness on both lower limbs | Serological study and CSF analysis, imaging studies | IV methylprednisolone (high dose), IVIg, ceftriaxone, ampicillin, and acyclovir | Associated with COVID-19 infection |
| 79 | Prete [202]/2022 | Transverse myelitis | F/43 | USA | Progressive numbness and tingling in lower limbs and complete quadriplegia | Serological study and CSF analysis, imaging studies | Long-term steroid regimen and plasmapheresis | Associated with COVID-19 infection |
| 80 | Shahali [203]/2021 | Transverse myelitis | M/63 | Iran | Weakness and immobility in lower extremities, constipation, and urinary retention | Serological study and CSF analysis, imaging studies | IV methylprednisolone (1 g/day for 3 days) | Associated with COVID-19 infection |
| 81 | Hsiao et al. [204] /2021 | Transverse myelitis | M/41 | Taiwan | Progressive paresthesia below T4, lower-limb weakness | Serological study and CSF analysis, imaging studies | IV methylprednisolone (1000 mg/day for 5 days) | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 82 | Tan et al. [205]/ 2021 | Transverse myelitis | F/25 | Malaysia | Bilateral lower-limb weakness and impaired walking | Serological study and CSF analysis, imaging studies | IV methylprednisolone (1000 mg/day for 5 days) | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 83 | Notghi et al. [206]/2021 | Transverse myelitis | M/58 | UK | Progressive numbness in lower limbs, allodynia up to chest level, genital dysaesthesia, and an episode of urinary incontinence | Serological study and CSF analysis, imaging studies | IV methylprednisolone (1 g/day for 5 days) | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 84 | Pagenkopf et al. [58]/2021 | Transverse myelitis | M/45 | Germany | Acute flaccid tetraparesis (especially in the lower limbs) and urinary retention | Serological study and CSF analysis, imaging studies | IV methylprednisolone (1 g/day for 5 days) | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 85 | Eom et al. [207]/ 2022 | Transverse myelitis | M/81 | Republic of Korea | Bilateral hand weakness and numbness | Serological study and CSF analysis, imaging studies | IV methylprednisolone (1 g/day for 5 days) | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 86 | Eom et al. [207]/2022 | Transverse myelitis | F/23 | Republic of Korea | Bilateral paresthesia and weakness in the lower limbs | Serological study and CSF analysis, imaging studies | IV methylprednisolone (1 g/day for 5 days) | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 87 | Miyaue et al. [208]/2022 | Transverse myelitis | M/75 | Japan | Total sensory loss below the umbilicus and complete paralysis in both lower limbs | Serological study and CSF analysis, imaging studies | IV methylprednisolone (1 g/day for 3 days), then oral prednisolone (initial dose of 1 mg/kg/day) | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 88 | Maroufi et al. [209]/2022 | Transverse myelitis | F/31 | Iran | Progressive lower limbs paraparesis and paresthesia, urinary retention, and fecal incontinence | Serological study and CSF analysis, imaging studies |
IV methylprednisolone (1 g/day for 7 days); Then, oral prednisolone 50 mg daily |
Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 89 | Tahir et al. [210]/2021 | Transverse myelitis | F/44 | USA | Numbness and weakness in the lower extremities, urinary retention, and back pain | Serological study and CSF analysis, imaging studies | IV methylprednisolone and plasma exchange | Associated with COVID-19 vaccination (Johnson and Johnson COVID-19 vaccine) |
| 90 | Hirose et al. [211]/2021 | Transverse myelitis | M/70 | Japan | Progressive sensorimotor dysfunction of both lower limbs | Serological study and CSF analysis, imaging studies | IV methylprednisolone (1000 mg/day for 5 days); then oral prednisolone (30 mg/day with gradual tapering) | Associated with COVID-19 vaccination (Moderna) |
| 91 | Rajae et al. [212]/2021 | Cerebrovascular events (ischemic stroke) | M/68 | Morocco | Left hemiparesis with dysarthria and left facial paralysis | Serological study and imaging studies (brain CT scan and MRI) | Thrombolysis | Associated with COVID-19 infection |
| 92 | Avvantaggiato et al. [213]/2021 | Cerebrovascular events (ischemic stroke) | F/29 | Italy | Left hemiplegia, left-sided central facial palsy, dysarthria, facial drop, and complete paralysis of the ipsilateral upper and lower limbs | Serological study and imaging studies (brain CT scan and MRI) | – | Associated with COVID-19 infection |
| 93 | Bigliardi et al. [214]/2020 | Cerebrovascular events (ischemic stroke) | M/62 | Italy | Left hemiplegia, left hemianopsia, and forced right deviation of gaze | Serological study and imaging studies (brain CT scan and chest CT angiography) | anticoagulant (LMWH) | Associated with COVID-19 infection |
| 94 | Zhai et al. [215]/2020 | Cerebrovascular events (ischemic stroke) | M/79 | China | Right limb weakness and non-fluent speech | Serological study and imaging studies (brain CT scan) | Clopidogrel (75 mg) and atorvastatin (20 mg) | Associated with COVID-19 infection |
| 95 | Farooque et al. [216]/2020 | Cerebrovascular events (ischemic stroke) | M/70 | Pakistan | Right-sided weakness and sensory loss in both upper and lower limbs | Serological study and imaging studies (brain CT scan and MRI) | Aspirin. (150 mg twice a day), LMWH (0.6 ml twice a day), and IV dexamethasone (1cc twice a day) | Associated with COVID-19 infection |
| 96 | Owolabi et al. [217]/2021 | Cerebrovascular events (hemorrhagic stroke) | M/59 | Saudi Arabia | Right-sided incoordination, weakness, facial deviation, and altered level of consciousness | Serological study and imaging studies (brain CT scan) | Hydroxychloroquine, dexamethasone, remdesivir, and antibiotics | Associated with COVID-19 infection |
| 97 | Owolabi et al. [217]/2021 | Cerebrovascular events (hemorrhagic stroke) | M/51 | Saudi Arabia | Left-sided limb and facial weakness | Serological study and imaging studies (brain CT scan) | Hydroxychloroquine, dexamethasone, and antibiotics | Associated with COVID-19 infection |
| 98 | Fraiman et al. [218]/2020 | Cerebrovascular events (hemorrhagic stroke) | F/38 | Brazil | Acute alteration in the level of consciousness | Serological study and imaging studies (brain CT scan and MRI) | – | Associated with COVID-19 infection |
| 99 | Flores et al. [219]/2020 | Cerebrovascular events (hemorrhagic stroke) | M/40 | USA | Pinpoint, minimally reactive pupils, withdrawal to painful stimuli in the right side of the body, left hemiparesis | Serological study and imaging studies (brain CT scan and MRI) | – | Associated with COVID-19 infection |
| 100 | Dakay et al. [220]/2021 | Cerebrovascular events (cerebral venous sinus thrombosis) | M/17 | USA | Left-sided headaches and occasional emesis | Serological study and imaging studies (MRI brain with MR venography) | Anticoagulation | Associated with COVID-19 infection |
| 101 | Dakay et al. [220]/2021 | Cerebrovascular events (cerebral venous sinus thrombosis) | F/72 | USA | Dyspnea and generalized weakness | Serological study and imaging studies (CT angiogram) | – | Associated with COVID-19 infection |
| 102 | Dakay et al. [220]/2021 | Cerebrovascular events (cerebral venous sinus thrombosis) | M/26 | USA | Acute left-sided hemiparesis followed by severe headache, nausea, and dizziness | Serological study and imaging studies (MRI brain with MR venography, brain CT scan, cerebral angiography, and CT angiogram) | – | Associated with COVID-19 infection |
| 103 | Anipindi et al. [221]/2021 | Cerebrovascular events (cerebral venous sinus thrombosis) | M/66 | USA | Severe headaches, palpitations, dizziness, and diaphoresis | Serological study and imaging studies (MRI and CT brain along with CT venogram) | Rivaroxaban 20 mg (6 months) | Associated with COVID-19 infection |
| 104 | Tu et al. [222]/2020 | Cerebrovascular events (cerebral venous sinus thrombosis) | M/ mid-thirties | Singapore | Generalized non-remitting headache | Serological study and imaging studies (MRI and CT brain along with CT venogram and MR venogram) | Dabigatran | Associated with COVID-19 infection |
| 105 | Tu et al. [222]/2020 | Cerebrovascular events (cerebral venous sinus thrombosis) | M/the late thirties | Singapore | First-onset seizure (generalized tonic–clonic convulsion) | Serological study and imaging studies (CT brain along with CT venogram) | IV heparin, IV levetiracetam, and cobalamin replacement | Associated with COVID-19 infection |
| 106 | Blauenfeldt et al. [78]/2021 | Cerebrovascular events (ischemic stroke) | F/60 | Denmark | Strong, persistent abdominal pain, headache | Serological study and imaging studies (CT brain) | Hemicraniectomy + postoperative dalteparin 5000 IU daily | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 107 | Elaidouni et al. [223]/2022 | Cerebrovascular events (ischemic stroke) | M/36 | Morocco | Numbness in left hemibody, headaches (24 h after the vaccine injection), | Serological study and imaging studies (CT brain, MRI brain, MRI angiography of supra-aortic trunks) | Aspirin and Enoxaparin (100 UI/kg/12 h) | Associated with COVID-19 vaccination (Sinopharm) |
| 108 | Kenda et al. [224]/ 2021 | Cerebrovascular events (ischemic stroke) | F/51 | Slovenia | Acute-onset global aphasia, right-sided hemiplegia, and hemianopsia | Serological study and imaging studies (CT/MRI brain and CT angiography) | Mechanical thrombectomy + high-dose IVIg (1 g/kg for 2 consecutive days) | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 109 | Al-Mayhani et al. [225]/2021 | Cerebrovascular events (ischemic stroke) | F/35 | UK | Right temporal and periorbital headache | Serological study and imaging studies (CT brain and CT angiography) | Urgent decompressive hemicraniectomy, IVIg, plasmapheresis, and fondaparinux | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 110 | Al-Mayhani et al. [225]/2021 | Cerebrovascular events (ischemic stroke) | F/37 | UK | Diffused headache, left visual field loss, confusion, and left arm weakness | Serological study and imaging studies (diffusion-weighted MRI and CT angiography) | IVIg, IV methylprednisolone, plasmapheresis, and fondaparinux | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 111 | de Mélo Silva et al. [226]/2021 | Cerebrovascular events (hemorrhagic stroke) | F/57 | Brazil | Acute-onset sweating and paleness, followed by left-sided hemiparesis, vomiting, and somnolence | Serological study and imaging studies (CT brain) | Hematoma drainage, external ventricular drain, and decompressive craniectomy | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 112 | Takeyama et al. [227]/2022 | Cerebrovascular events (hemorrhagic stroke) | F/48 | Japan | Gradually progressing left-sided hemiparesis | Serological study and imaging studies (CT/MRI brain and CT angiography) | Right frontotemporal craniotomy | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 113 | Dias et al. [228]/2021 | Cerebrovascular events (cerebral venous sinus thrombosis) | F/47 | Portugal | The sudden left-sided motor deficit, papilledema, left visual extinction, right gaze deviation, and left hemiparesis | Serological study and imaging studies (MRI brain, MRI venography) | Acetazolamide and enoxaparin 60 mg twice a day (later changed to warfarin) | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 114 | Dias et al. [228]/2021 | Cerebrovascular events (cerebral venous sinus thrombosis) | F/67 | Portugal | Sudden right-sided lower limb clonic movements, motor deficit, loss of consciousness, and headache | Serological study and imaging studies (MRI brain) and Electroencephalography | Levetiracetam (500 mg twice a day) and enoxaparin (80 mg twice a day); then switched to dabigatran (150 mg twice a day) | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 115 | Zakaria et al. [229]/2021 | Cerebrovascular events (cerebral venous sinus thrombosis) | M/49 | Malaysia | A new-onset headache and giddiness | Serological study and imaging studies (CT brain and CT cerebral venogram) | Subcutaneous Clexane (1 mg/kg twice a day) and clopidogrel (75 mg) | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 116 | D'Agostino et al. [230]/2021 | Cerebrovascular events (cerebral venous sinus thrombosis) | F/54 | Italy | Left-sided signs | Serological study and imaging studies (CT/MRI brain and CT/MRI angiography) | – | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 117 | Atta et al. [231]/2021 | Cerebrovascular events (cerebral venous sinus thrombosis) | F/48 | UK | Right-sided headache, | Serological study and imaging studies (CT cerebral venogram) | Fondaparinux (7.5 mg), IVIg (1 g/kg) and dexamethasone (20 mg/day) | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 118 | Delorme et al. [232]/2020 | Encephalopathy | M/72 | France | Acute psychomotor agitation, cognitive and behavioral frontal lobe syndrome, upper limbs myoclonus, and cerebellar ataxia | CSF analysis, electroencephalogram (EEG), brain MRI, and brain FDG-PET/CT imaging | IVIg (2 g/kg) | Associated with COVID-19 infection |
| 119 | Delorme et al. [232]/2020 | Encephalopathy | F/66 | France | Acute cognitive impairment, psychomotor slowing, cognitive and behavioral frontal lobe syndrome, and severe apraxia | CSF analysis, EEG, brain MRI, and brain FDG-PET/CT imaging | IVIg; then, due to persisting severe cognitive impairment, IV pulse corticosteroids (2 mg/kg/day 3 days then 1 g/day 3 days) started | Associated with COVID-19 infection |
| 120 | Delorme et al. [232]/2020 | Encephalopathy | F/60 | France | Acute anxiety, depressed mood, akathisia, gait imbalance, psychomotor agitation, dysexecutive syndrome, and cerebellar ataxia | CSF analysis, EEG, brain MRI, and brain FDG-PET/CT imaging | Pulse corticosteroids (2 mg/kg/day 3 days) | Associated with COVID-19 infection |
| 122 | Delorme et al. [232]/2020 | Encephalopathy | M/69 | France | Generalized convulsive status epilepticus, fever, fatigue, anosmia, and ageusia | CSF analysis, EEG, brain MRI, and brain FDG-PET/CT imaging | Pulse corticosteroids (1 g/day 5 days) | Associated with COVID-19 infection |
| 123 | Lazraq et al. [233]/2021 | Encephalopathy | M/79 | Morocco | Mental confusion, sudden-onset dysarthria | The serological study, CSF analysis, EEG, brain CT, MRI, and MR angiography | Sodium valproate | Associated with COVID-19 infection |
| 124 | Goodloe et al. [234]/2021 | Encephalopathy | M/52 | USA | Altered mental status, fever, and severe agitation | The serological study, CSF analysis, EEG, brain CT, and MRI | Vancomycin, ceftriaxone, azithromycin, acyclovir, and clevidipine | Associated with COVID-19 infection |
| 125 | Teimouri-Jervekani et al. [235]/2021 | Encephalopathy | M/53 | Iran | Severe headache and bizarre behavior | The serological study, brain CT and MRI | Hydroxychloroquine (200 mg twice a day for 5 days) | Associated with COVID-19 infection |
| 126 | Al-Mashdali et al. [236]/ 2021 | Encephalopathy | M/32 | Qatar | Acute confusion, disturbed memory, and auditory hallucination | The serological study, CSF analysis, EEG, and brain MRI | Methylprednisolone | Associated with COVID-19 vaccination (Moderna) |
| 127 | Liu et al. [237]/2021 | Encephalopathy | F/86 | USA | Acute confusion with visual hallucinations and left frontal headache | The serological study, CSF analysis, EEG, brain CT, and MRI | Lorazepam, fosphenytoin, and discharged with levetiracetam | Associated with COVID-19 vaccination (Moderna) |
| 128 | Liu et al. [237]/2021 | Encephalopathy | M/73 | USA | Cognitive deficits, hallucinations, and periods of unresponsiveness | The serological study, CSF analysis, EEG, brain CT, and MRI | Lorazepam and levetiracetam | Associated with COVID-19 vaccination (Moderna) |
| 129 | Baldelli et al. [108]/2021 | Encephalopathy | M/77 | Italy | Confusion, agitation, and delirium | The serological study, CSF analysis, EEG, brain CT, and MRI | Oral prednisone (50 mg per day) | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 130 | Bensaidane et al. [238]/2022 | Encephalopathy | M/56 | Canada | Altered mental status | The serological study, CSF analysis, EEG, brain CT angiography, and MRI | High-dose IV methylprednisolone (1 g/day for 7 days) | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 131 | Monti et al. [239]/2020 | New-onset seizures | M/50 | Italy | Acute onset of psychiatric symptoms (confabulations and delirious ideas), focal motor seizures, and impaired awareness | The serological study, CSF analysis, EEG, brain MRI, and total-body CT and PET | Diazepam, valproic acid, lacosamide, methylprednisolone, IVIg, plasma-exchange | Associated with COVID-19 infection |
| 132 | Bhatta et al. [240]/2020 | New-onset seizures | M/11 | USA | Acute-onset seizure for 2 min | The serological study, EEG, and brain CT | Levetiracetam (500 mg twice a day) | Associated with COVID-19 infection |
| 133 | Park et al. [241]/2021 | New-onset seizures | F/45 | USA | Focal to bilateral tonic–clonic seizure, loss of consciousness, and urinary incontinence | The serological study, CSF analysis, EEG, brain MRI, and CT | Oxcarbazepine (600 mg twice a day); then lacosamide (200 mg twice a day) | Associated with COVID-19 infection |
| 134 | Dono et al. [242]/2021 | New-onset seizuresa | M/81 | Italy | Non-convulsive status epilepticus with coma | The serological study, CSF analysis, EEG, brain MRI, and CT |
Lorazepam (4 mg two IV boluses), levetiracetam (2000 mg IV), methylprednisolone (1 g/daily IV for 5 days); then, oral prednisolone (60 mg/day for 10 days) and IVIg (160 g over 5 days) |
Associated with COVID-19 infection |
| 135 | Cho et al. [243]/2022 | New-onset seizures | M/84 | Korea | Myoclonic seizures (Myoclonic status epilepticus) | The serological study, EEG, brain Diffusion-weighted MRI |
Sedative medication: Midazolam Antiseizure medication: Lorazepam and Levetiracetam |
Associated with COVID-19 infection |
| 136 | Cho et al. [243]/2022 | New-onset seizures | M/45 | Korea | Focal to bilateral tonic–clonic seizures (2 times) | Serological study and EEG | Sedative medication: Remifentanil and Dexmedetomidine Antiseizure medication: Lorazepam and Levetiracetam | Associated with COVID-19 infection |
| 137 | Cho et al. [243]/2022 | New-onset seizures | M/63 | Korea | Focal impaired aware seizures (several times) | Serological study and EEG | Sedative medication: Dexmedetomidine Antiseizure medication: Phenobarbital, Levetiracetam, Topiramate, and Perampanel | Associated with COVID-19 infection |
| 138 | Cho et al. [243]/2022 | New-onset seizures | M/72 | Korea | Myoclonic seizure, generalized tonic–clonic seizures (several times) | Serological study and EEG | Sedative medication: Remifentanil and Dexmedetomidine Antiseizure medication: Levetiracetam and Topiramate | Associated with COVID-19 infection |
| 139 | Cho et al. [243]/2022 | New-onset seizures | M/73 | Korea | Myoclonic seizures (several times) | Serological study and EEG |
Sedative medication: Remifentanil and Propofol Antiseizure medication: Levetiracetam and Valproic acid |
Associated with COVID-19 infection |
| 140 | Cho et al. [243]/2022 | New-onset seizures | F/39 | Korea | Generalized tonic–clonic seizures (3 times) | The serological study, EEG, brain CT | Sedative medication:—Antiseizure medication: Levetiracetam | Associated with COVID-19 infection |
| 141 | Aladdin et al. [119]/2021 | New-onset seizures | F/42 | Saudi Arabia | Generalized tonic–clonic seizure | The serological study, CSF analysis, EEG, and brain MRI | Lorazepam, phenytoin, levetiracetam, and lacosamide | Associated with COVID-19 vaccination (Oxford/AstraZeneca) |
| 142 | Bauman et al. [244] | New-onset seizures | M/56 | USA | New-onset refractory status epilepticus | The serological study, CSF analysis, EEG, and brain MRI | Corticosteroids, plasmapheresis, IVIg, rituximab, midazolam, propofol, ketamine, levetiracetam, lacosamide, phenobarbital, clobazam, zonisamide, oxcarbazepine, and perampanel | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 143 | Desai et al. [245]/2021 | Varicella-zoster virus reactivation | F/62 | India | Painful blisters and fluid-filled bubble-form rashes | Serological studies | Oral and topical Acyclovir | Associated with COVID-19 infection |
| 144 | Saleh et al. [246]/2021 | Varicella-zoster virus reactivation | F/49 | Egypt | Unilateral fluid-filled vesicles and painful erythematous areas over the hard palate | Serological studies | Oral acyclovir, topical antiseptics, chlorhexidine, and paracetamol | Associated with COVID-19 infection |
| 145 | Saati et al. [122]/2020 | Varicella-zoster virus reactivation | M/57 | Saudi Arabia | Fluid-filled bubble-form rashes and vesicles with surrounding erythematous areas over the right nipple | Serological studies | Famciclovir | Associated with COVID-19 infection |
| 146 | Van Dam et al. [129]/2021 | Varicella-zoster virus reactivation | F/29 | The Netherlands | Painful multiple vesicles on the left side of the ox coccyges | Clinically diagnosed | – | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 147 | Van Dam et al. [247]/2021 | Varicella-zoster virus reactivation | M/34 | The Netherlands | Swollen, painful inguinal lymph nodes and a rash on the right lower limb | Serological studies and PCR tests over vesical fluid for VZV | Valacyclovir | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 148 | Rodríguez-Jiménez et al. [248]/2021 | Varicella-zoster virus reactivation | M/58 | Spain | Asymptomatic herpes-form umbilicated vesicles and lymphadenopathy in the cervical area | PCR | – | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 149 | Rodríguez-Jiménez et al. [248]/2021 | Varicella-zoster virus reactivation | F/47 | Spain | Herpes-form umbilicated vesicles and dysesthesia | PCR | – | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 150 | Rodríguez-Jiménez et al. [248]/2021 | Varicella-zoster virus reactivation | M/39 | Spain | Painful herpes-form umbilicated vesicles | – | – | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 151 | Rodríguez-Jiménez et al. [248]/2021 | Varicella-zoster virus reactivation | F/56 | Spain | Herpes-form umbilicated vesicles | PCR | – | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 152 | Rodríguez-Jiménez et al. [248]/2021 | Varicella-zoster virus reactivation | F/41 | Spain | Herpes-form umbilicated vesicles and dysesthesia | – | – | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
| 153 | Santovito et al. (249)/2021 | Varicella-zoster virus reactivation | M/27 | USA | Popular rashes over the left upper limb | – | – | Associated with COVID-19 vaccination (Pfizer-BioNTech) |
Conclusion
The present review can help healthcare workers and also the general population by emphasizing these points: any neurological symptom after COVID-19 vaccination can be potentially critical and needs to be cautiously evaluated; for any suspected adverse event following vaccination, we should initially exclude current or recent SARS-CoV-2 infection; and despite the current literature on serious complications imposed by COVID-19 vaccines, the benefits of vaccination outweigh the risks in ending the current pandemic since all of these complications can occur with the infection itself.
Acknowledgments
The authors would like to thank the clinical research development center of Imam Reza Hospital, Kermanshah University of Medical Sciences, for their kind support.
Authors’ Contributions
ZMA: Data collection and writing the manuscript. AS: Data collection and contributed substantial revisions to the manuscript’s content. AB: Data collection and writing the manuscript. AAK: Data collection and helped with manuscript writing. TTS: Contributed substantial revisions to the manuscript’s content. MATM: Data collection and helped with manuscript writing. ATP: Data collection and helped with manuscript writing. AM: Data collection and helped with manuscript writing. RH: Helped with manuscript writing and visualization. MB: Data collection, helped with manuscript writing, and contributed substantial revisions to the manuscript’s content. SE: Design of the research study and supervision.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
Terence T. Sio reports that he provides strategic and scientific recommendations as a member of the Advisory Board and speaker for Novocure, Inc. and also as a member of the Advisory Board to Galera Therapeutics, which are not in any way associated with the content or disease site as presented in this manuscript. All other authors have no relevant financial interests to be declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammad Barary, Email: m.barary@mubabol.ac.ir.
Soheil Ebrahimpour, Email: drsoheil1503@yahoo.com.
References
- 1.Goss AL, Samudralwar RD, Das RR, Nath A. ANA investigates: neurological complications of COVID-19 vaccines. Ann Neurol. 2021;89(5):856–857. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhotra HS, Gupta P, Prabhu V, Kumar Garg R, Dandu H, Agarwal V. COVID-19 vaccination-associated myelitis. QJM: An International Journal of Medicine. 2021 Aug;114(8):591-3. [DOI] [PMC free article] [PubMed]
- 3.von Csefalvay C. A case-control study of autoimmune AEFIs following COVID-19 vaccination reported to VAERS. medRxiv. 2021 Jan 1.
- 4.Al Battah A, Hammamy R. Multiple sclerosis flare secondary to COVID-19 vaccine, a case report. Authorea Preprints. 2021 Jul 25.
- 5.Finsterer J, Scorza FA, Fiorini AC. SARS-CoV-2 infection/vaccination associated new or exacerbating immune-mediated disease. J Med Res Health Sci. 2021;4(6):1302–1304. [Google Scholar]
- 6.Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mrna/dna sars-cov-2 vaccination. Vaccines (Basel) 2021;9(5):435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Althaus K, Moller P, Uzun G, Singh A, Beck A, Bettag M, et al. Antibody-mediated procoagulant platelets in SARS-CoV-2-vaccination associated immune thrombotic thrombocytopenia. Haematologica. 2021;106(8):2170–2179. doi: 10.3324/haematol.2021.279000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hause AM, Gee J, Baggs J, Abara WE, Marquez P, Thompson D, et al. COVID-19 vaccine safety in adolescents aged 12–17 years-United States, december 14, 2020-july 16, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(31):1053–1058. doi: 10.15585/mmwr.mm7031e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogidi OI, Berefagha WL, Okara E. Covid-19 vaccination: the pros and cons. World J Biol Pharm Health Sci. 2021;7(1):015–22. doi: 10.30574/wjbphs.2021.7.1.0072. [DOI] [Google Scholar]
- 10.García-Grimshaw M, Hernández-Vanegas LE, Núñez I, Hernández-Valdivia N, Carrillo-García DA, Michel-Chávez A, Galnares-Olalde JA, Carbajal-Sandoval G, del Mar Saniger-Alba M, Carrillo-Mezo RA, Fragoso-Saavedra S. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: a nationwide descriptive study. Clinical Immunology. 2021 Aug 1;229:108786. [DOI] [PMC free article] [PubMed]
- 11.Zavala-Jonguitud LF, Perez-Garcia CC. Delirium triggered by COVID-19 vaccine in an elderly patient. Geriatr Gerontol Int. 2021;21(6):540. doi: 10.1111/ggi.14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iftikhar H, Noor SMU, Masood M, Bashir K. Bell's Palsy after 24 hours of mrna-1273 sars-cov-2 vaccine. Cureus. 2021;13(6):e15935. doi: 10.7759/cureus.15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCormick DP, Spruance SL. Herpes simplex virus as a cause of Bell's palsy. Rev Med Virol. 2000;10(5):285. doi: 10.1002/1099-1654(200009/10)10:5<285::AID-RMV269>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 14.Tamaki A, Cabrera CI, Li S, Rabbani C, Thuener JE, Rezaee RP, et al. Incidence of Bell Palsy in patients with covid-19. JAMA Otolaryngol Head Neck Surg. 2021;147(8):767–768. doi: 10.1001/jamaoto.2021.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oke IO, Oladunjoye OO, Oladunjoye AO, Paudel A, Zimmerman R. Bell's Palsy as a late neurologic manifestation of covid-19 infection. Cureus. 2021 doi: 10.7759/cureus.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastola A, Sah R, Nepal G, Gajurel BP, Rajbhandari SK, Chalise BS, et al. Bell’s palsy as a possible neurological complication of COVID-19: a case report. Clinical Case Reports. 2021;9(2):747–750. doi: 10.1002/ccr3.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alp H, Tan H, Orbak Z. Bell's palsy as a possible complication of hepatitis B vaccination in a child. J Health Popul Nutr. 2009;27(5):707. doi: 10.3329/jhpn.v27i5.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou CH, Liou WP, Hu KI, Loh CH, Chou CC, Chen YH. Bell's palsy associated with influenza vaccination: two case reports. Vaccine. 2007;25(15):2839–2841. doi: 10.1016/j.vaccine.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Tseng HF, Sy LS, Ackerson BK, Hechter RC, Tartof SY, Haag M, et al. Safety of Quadrivalent Meningococcal Conjugate Vaccine in 11- to 21-Year-Olds. Pediatrics. 2017 doi: 10.1542/peds.2016-2084. [DOI] [PubMed] [Google Scholar]
- 20.Colella G, Orlandi M, Cirillo N. Bell's palsy following COVID-19 vaccination. J Neurol. 2021;268(10):3589–3591. doi: 10.1007/s00415-021-10462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Villares C, Vazquez-Feito A, Gonzalez-Gimeno MJ, de la Nogal-Fernandez B. Bell’s palsy following a single dose of mRNA SARS-CoV-2 vaccine: a case report. Journal of Neurology. 2022 Jan;269(1):47-8. [DOI] [PMC free article] [PubMed]
- 22.Ledford H. US authorization of first COVID vaccine marks new phase in safety monitoring. Nature. 2020;588(7838):377–378. doi: 10.1038/d41586-020-03542-4. [DOI] [PubMed] [Google Scholar]
- 23.Pothiawala S. Bell’s Palsy after second dose of moderna COVID-19 Vaccine: coincidence or causation? Acta medica Lituanica. 2021;28(2):7. doi: 10.15388/Amed.2021.28.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamath A, Maity N, Nayak MA. Facial paralysis following influenza vaccination: a disproportionality analysis using the vaccine adverse event reporting system database. Clin Drug Investig. 2020;40(9):883–889. doi: 10.1007/s40261-020-00952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Principi N, Esposito S. Do vaccines have a role as a cause of autoimmune neurological syndromes?. Frontiers in Public Health. 2020 Jul 28;8:361. [DOI] [PMC free article] [PubMed]
- 26.Soeiro T, Salvo F, Pariente A, Grandvuillemin A, Jonville-Béra A-P, Micallef J. Type I interferons as the potential mechanism linking mRNA COVID-19 vaccines to Bell’s palsy. Therapie. 2021 doi: 10.1016/j.therap.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burrows A, Bartholomew T, Rudd J, Walker D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Reports CP. 2021;14(7):e243829. doi: 10.1136/bcr-2021-243829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Repajic M, Lai XL, Xu P, Liu A. Bell's Palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell's palsy. Brain Behav Immun Health. 2021;13:100217. doi: 10.1016/j.bbih.2021.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan EYF, Chui CSL, Lai FTT, Chan EWY, Li X, Yan VKC, et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Ostropolets A, Makadia R, Shoaibi A, Rao G, Sena AG, Martinez-Hernandez E, Delmestri A, Verhamme K, Rijnbeek PR, Duarte-Salles T. Characterising the background incidence rates of adverse events of special interest for covid-19 vaccines in eight countries: multinational network cohort study. bmj. 2021 Jun 14;373. [DOI] [PMC free article] [PubMed]
- 31.Cirillo N, Doan R. Bell's palsy and SARS-CoV-2 vaccines-an unfolding story. Lancet Infect Dis. 2021;21(9):1210–1211. doi: 10.1016/S1473-3099(21)00273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baugh RF, Basura GJ, Ishii LE, Schwartz SR, Drumheller CM, Burkholder R, et al. Clinical practice guideline: Bell’s palsy. Otolaryngology-Head Neck Surg. 2013 doi: 10.1177/0194599813505967. [DOI] [PubMed] [Google Scholar]
- 33.Haber P, Sejvar J, Mikaeloff Y, DeStefano F. Vaccines and Guillain-Barre syndrome. Drug Saf. 2009;32(4):309–323. doi: 10.2165/00002018-200932040-00005. [DOI] [PubMed] [Google Scholar]
- 34.Loza AMM, Holroyd KB, Johnson SA, Pilgrim DM, Amato AA. Guillain-Barré syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: temporal associations do not imply causality. Neurology. 2021;96(22):1052–1054. doi: 10.1212/WNL.0000000000011881. [DOI] [PubMed] [Google Scholar]
- 35.Babazadeh A, Mohseni Afshar Z, Javanian M, Mohammadnia-Afrouzi M, Karkhah A, Masrour-Roudsari J, et al. Influenza vaccination and guillain-barre syndrome: reality or fear. J Transl Int Med. 2019;7(4):137–142. doi: 10.2478/jtim-2019-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juurlink DN, Stukel TA, Kwong J, Kopp A, McGeer A, Upshur RE, et al. Guillain-Barre syndrome after influenza vaccination in adults: a population-based study. Arch Intern Med. 2006;166(20):2217–2221. doi: 10.1001/archinte.166.20.2217. [DOI] [PubMed] [Google Scholar]
- 37.Souayah N, Michas-Martin PA, Nasar A, Krivitskaya N, Yacoub HA, Khan H, et al. Guillain-Barre syndrome after Gardasil vaccination: data from vaccine adverse event reporting system 2006–2009. Vaccine. 2011;29(5):886–889. doi: 10.1016/j.vaccine.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 38.Tuttle J, Chen RT, Rantala H, Cherry JD, Rhodes PH, Hadler S. The risk of Guillain-Barre syndrome after tetanus-toxoid-containing vaccines in adults and children in the United States. Am J Public Health. 1997;87(12):2045–2048. doi: 10.2105/AJPH.87.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC. Update: Guillain-Barré syndrome among recipients of Menactra meningococcal conjugate vaccine--United States, June 2005-September 2006. MMWR. Morbidity and mortality weekly report. 2006 Oct 20;55(41):1120-4. [PubMed]
- 40.Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021 doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.James J, Jose J, Gafoor VA, Smita B, Balaram N. Guillain-Barre syndrome following ChAdOx1 nCoV-19 COVID-19 vaccination: a case series. Neurol Clin Neurosci. 2021;9(5):402–405. doi: 10.1111/ncn3.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel SU, Khurram R, Lakhani A, Quirk B. Guillain-Barre syndrome following the first dose of the chimpanzee adenovirus-vectored COVID-19 vaccine, ChAdOx1. BMJ Case Rep. 2021;14(4):e242956. doi: 10.1136/bcr-2021-242956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad A, Hurlburt G, Podury S, Tandon M, Kingree S, Sriwastava S. A Novel case of bifacial diplegia variant of guillain-barre syndrome following janssen covid-19 vaccination. Neurol Int. 2021;13(3):404–409. doi: 10.3390/neurolint13030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leonhard SE, Mandarakas MR, Gondim FAA, Bateman K, Ferreira MLB, Cornblath DR, et al. Diagnosis and management of Guillain-Barre syndrome in ten steps. Nat Rev Neurol. 2019;15(11):671–683. doi: 10.1038/s41582-019-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leonhard SE, Mandarakas MR, Gondim FA, Bateman K, Ferreira ML, Cornblath DR, et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol. 2019;15(11):671–683. doi: 10.1038/s41582-019-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.West TW. Transverse myelitis–a review of the presentation, diagnosis, and initial management. Discov Med. 2013;16(88):167–177. [PubMed] [Google Scholar]
- 47.Munz M, Wessendorf S, Koretsis G, Tewald F, Baegi R, Kramer S, et al. Acute transverse myelitis after COVID-19 pneumonia. J Neurol. 2020;267(8):2196–2197. doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zachariadis A, Tulbu A, Strambo D, Dumoulin A, Di Virgilio G. Transverse myelitis related to COVID-19 infection. J Neurol. 2020;267(12):3459–3461. doi: 10.1007/s00415-020-09997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agmon-Levin N, Kivity S, Szyper-Kravitz M, Shoenfeld Y. Transverse myelitis and vaccines: a multi-analysis. Lupus. 2009;18(13):1198–1204. doi: 10.1177/0961203309345730. [DOI] [PubMed] [Google Scholar]
- 50.Akkad W, Salem B, Freeman JW, Huntington MK. Longitudinally extensive transverse myelitis following vaccination with nasal attenuated novel influenza A(H1N1) vaccine. Arch Neurol. 2010;67(8):1018–1020. doi: 10.1001/archneurol.2010.167. [DOI] [PubMed] [Google Scholar]
- 51.Kelly H. Evidence for a causal association between oral polio vaccine and transverse myelitis: a case history and review of the Literature. J Paediatr Child Health. 2006;42(4):155–159. doi: 10.1111/j.1440-1754.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 52.Iniguez C, Mauri JA, Larrode P, Lopez del Val J, Jerico I, Morales F. Acute transverse myelitis secondary to hepatitis B vaccination. Rev Neurol. 2000;31(5):430–432. [PubMed] [Google Scholar]
- 53.Joyce KA, Rees JE. Transverse myelitis after measles, mumps, and rubella vaccine. BMJ. 1995;311(7002):422. doi: 10.1136/bmj.311.7002.422a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Read SJ, Schapel GJ, Pender MP. Acute transverse myelitis after tetanus toxoid vaccination. Lancet. 1992;339(8801):1111–1112. doi: 10.1016/0140-6736(92)90703-6. [DOI] [PubMed] [Google Scholar]
- 55.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narasimhalu K, Lee WC, Salkade PR, De Silva DA. Trigeminal and cervical radiculitis after tozinameran vaccination against COVID-19. BMJ Case Rep. 2021;14(6):e242344. doi: 10.1136/bcr-2021-242344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evans SJW, Day SJ. Medicines and Healthcare Products Regulatory Agency (MHRA) (Formerly MCA). In: Armitage P, Colton T, editors. Encyclopedia of Biostatistics, 2005
- 58.Pagenkopf C, Sudmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J Neuroimmunol. 2021;358:577606. doi: 10.1016/j.jneuroim.2021.577606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021;218(3):e20202135. doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vera-Lastra O, Medina G, Cruz-Dominguez Mdel P, Jara LJ, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld's syndrome): clinical and immunological spectrum. Expert Rev Clin Immunol. 2013;9(4):361–373. doi: 10.1586/eci.13.2. [DOI] [PubMed] [Google Scholar]
- 61.Roman GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute Transverse Myelitis (ATM): clinical review of 43 patients with COVID-19-associated atm and 3 post-vaccination atm serious adverse events with the chadox1 ncov-19 vaccine (AZD1222) Front Immunol. 2021;12:653786. doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calvo ÁC, Martínez MAM, Alentorn-Palau A, Escuer JB, Pinel LR, Martínez-Yélamos S. Idiopathic acute transverse myelitis: outcome and conversion to multiple sclerosis in a large series. BMC Neurol. 2013;13(1):1–8. doi: 10.1186/1471-2377-13-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beh SC, Greenberg BM, Frohman T, Frohman EM. Transverse myelitis. Neurol Clin. 2013;31(1):79–138. doi: 10.1016/j.ncl.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scott T, Frohman E, De Seze J, Gronseth G, Weinshenker B. Evidence-based guideline: clinical evaluation and treatment of transverse myelitis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011 doi: 10.1212/WNL.0b013e31823dc535. [DOI] [PubMed] [Google Scholar]
- 65.Chow CCN, Magnussen J, Ip J, Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Reports CP. 2020;13(8):e236720. doi: 10.1136/bcr-2020-236720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shahjouei S, Naderi S, Li J, Khan A, Chaudhary D, Farahmand G, et al. Risk of stroke in hospitalized SARS-CoV-2 infected patients: a multinational study. EBioMedicine. 2020;59:102939. doi: 10.1016/j.ebiom.2020.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qureshi AI, Baskett WI, Huang W, Shyu D, Myers D, Raju M, et al. Acute ischemic stroke and COVID-19: an analysis of 27 676 patients. Stroke. 2021;52(3):905–912. doi: 10.1161/STROKEAHA.120.031786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gunduz ZB. Venous sinus thrombosis during COVID-19 infection in pregnancy: a case report. Sao Paulo Med J. 2021;139(2):190–195. doi: 10.1590/1516-3180.2020.0659.r1.08122020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Markus HS. Ischaemic stroke can follow COVID-19 vaccination but is much more common with COVID-19 infection itself. BMJ Publishing Group Ltd. 2021 doi: 10.1136/jnnp-2021-327057. [DOI] [PubMed] [Google Scholar]
- 70.Finsterer J, Korn M. Aphasia seven days after second dose of an mRNA-based SARS-CoV-2 vaccine. Brain Hemorrhages. 2021 doi: 10.1016/j.hest.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mohseni Afshar Z, Babazadeh A, Janbakhsh A, Afsharian M, Saleki K, Barary M, et al. Vaccine-induced immune thrombotic thrombocytopenia after vaccination against Covid-19: A clinical dilemma for clinicians and patients. Rev Med Virol. 2021 doi: 10.1002/rmv.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mittelmeier H. Generalized anaphylactic toxic blood vessel wall injury with sinus thrombosis after active diphtheria vaccination with a study on the etiology and pathogenesis of organic blood vessel diseases. Monatsschr Kinderheilkd. 1959;107(6):288–293. [PubMed] [Google Scholar]
- 73.Nieminen U, Peltola H, Syrjala MT, Makipernaa A, Kekomaki R. Acute thrombocytopenic purpura following measles, mumps and rubella vaccination. A report on 23 patients. Acta Paediatr. 1993;82(3):267–270. doi: 10.1111/j.1651-2227.1993.tb12657.x. [DOI] [PubMed] [Google Scholar]
- 74.Vickers ER, McClure DL, Naleway AL, Jacobsen SJ, Klein NP, Glanz JM, et al. Risk of venous thromboembolism following influenza vaccination in adults aged 50 years and older in the vaccine safety datalink. Vaccine. 2017;35(43):5872–5877. doi: 10.1016/j.vaccine.2017.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oldenburg J, Klamroth R, Langer F, Albisetti M, von Auer C, Ay C, et al. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021 doi: 10.1055/a-1469-7481. [DOI] [PubMed] [Google Scholar]
- 76.See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al. 2021 US case reports of cerebral venous sinus Thrombosis with Thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21. JAMA. 2021;325(24):2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic Thrombocytopenia after Ad26.COV.2S Vaccination. N Engl J Med. 2021 doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost. 2021;19(7):1771–1775. doi: 10.1111/jth.15347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Mayhani T, Saber S, Stubbs MJ, Losseff NA, Perry RJ, Simister RJ, et al. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopaenia. J Neurol Neurosurg Psychiatry. 2021 doi: 10.1136/jnnp-2021-326984. [DOI] [PubMed] [Google Scholar]
- 80.Mehta PR, Apap Mangion S, Benger M, Stanton BR, Czuprynska J, Arya R, et al. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination - a report of two UK cases. Brain Behav Immun. 2021;95:514–517. doi: 10.1016/j.bbi.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after “COVID-19 vaccine AstraZeneca” exposure. J Clin Med. 2021;10(8):1599. doi: 10.3390/jcm10081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schultz NH, Sorvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bjørnstad-Tuveng TH, Rudjord A, Anker P. Fatal cerebral haemorrhage after COVID-19 vaccine. Tidsskr Nor Laegeforen. 2021 Apr 29;141. [DOI] [PubMed]
- 84.Mahmoodi BK, Brouwer J-LP, Veeger NJ, van der Meer J. Hereditary deficiency of protein C or protein S confers increased risk of arterial thromboembolic events at a young age: results from a large family cohort study. Circulation. 2008;118(16):1659–1667. doi: 10.1161/CIRCULATIONAHA.108.780759. [DOI] [PubMed] [Google Scholar]
- 85.Simpson EL, Lawrenson RA, Nightingale AL, Farmer RD. Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. BJOG. 2001;108(1):56–60. doi: 10.1111/j.1471-0528.2001.00004.x. [DOI] [PubMed] [Google Scholar]
- 86.Lin J, Wakefield TW, Henke PK. Risk factors associated with venous thromboembolic events in patients with malignancy. Blood Coagul Fibrinolysis. 2006;17(4):265–270. doi: 10.1097/01.mbc.0000224845.27378.c3. [DOI] [PubMed] [Google Scholar]
- 87.Ageno W, Squizzato A, Garcia D, Imberti D. Epidemiology and risk factors of venous thromboembolism. Semin Thromb Hemost. 2006;32(7):651-8. [DOI] [PubMed]
- 88.Dutta A, Ghosh R, Bhattacharya D, Bhat S, Ray A, Pandit A, et al. Anti-PF4 antibody negative cerebral venous sinus thrombosis without thrombocytopenia following immunization with COVID-19 vaccine in an elderly non-comorbid Indian male, managed with conventional heparin-warfarin based anticoagulation. Diabetes Metab Syndr. 2021;15(4):102184. doi: 10.1016/j.dsx.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shakibajahromi B, Haghighi AB, Salehi A, Vardanjani HM, Ghaedian M, Safari A, et al. Clinical and radiological characteristics and predictors of outcome of cerebral venous sinus thrombosis, a hospital-based study. Acta Neurol Belg. 2020;120(4):845–852. doi: 10.1007/s13760-018-1009-6. [DOI] [PubMed] [Google Scholar]
- 90.McCrae KR. Thrombotic thrombocytopenia due to SARS-CoV-2 vaccination. Cleveland Clin J Med. 2021 doi: 10.3949/ccjm.88a.ccc078. [DOI] [PubMed] [Google Scholar]
- 91.Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cines DB, Bussel JB. SARS-CoV-2 vaccine–induced immune thrombotic thrombocytopenia. Mass Medical Soc. 2021 doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vogrig A, Janes F, Gigli GL, Curcio F, Negro ID, D'Agostini S, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg. 2021;208:106839. doi: 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Manzano GS, McEntire CRS, Martinez-Lage M, Mateen FJ, Hutto SK. Acute disseminated encephalomyelitis and acute hemorrhagic leukoencephalitis following covid-19: systematic review and meta-synthesis. Neurol Neuroimmunol Neuroinflamm. 2021 doi: 10.1212/NXI.0000000000001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Utukuri PS, Bautista A, Lignelli A, Moonis G. Possible acute disseminated encephalomyelitis related to severe acute respiratory syndrome coronavirus 2 infection. AJNR Am J Neuroradiol. 2020;41(9):E82–E83. doi: 10.3174/ajnr.A6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu BD, Ugolini C, Jha P. Two cases of post-moderna COVID-19 vaccine encephalopathy associated with nonconvulsive status epilepticus. Cureus. 2021 doi: 10.7759/cureus.16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cao L, Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Belg. 2021 doi: 10.1007/s13760-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kenangil GO, Ari BC, Guler C, Demir MK. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine. Acta Neurol Belg. 2021 doi: 10.1007/s13760-021-01855-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raknuzzaman M, Jannaty T, Hossain MB, Saha B, Dey SK, Shahidullah M. Post covid19 vaccination acute disseminated encephalomyelitis: a case report in bangladesh. Int J Med Sci Clin Res Stud. 2021;1(03):31–36. [Google Scholar]
- 101.Byers RK, Moll FC. Encephalopathies following prophylactic pertussis vaccine. Pediatrics. 1948;1(4):437–457. doi: 10.1542/peds.1.4.437. [DOI] [PubMed] [Google Scholar]
- 102.Weibel RE, Caserta V, Benor DE, Evans G. Acute encephalopathy followed by permanent brain injury or death associated with further attenuated measles vaccines: a review of claims submitted to the National vaccine injury compensation program. Pediatrics. 1998;101(3):383–387. doi: 10.1542/peds.101.3.383. [DOI] [PubMed] [Google Scholar]
- 103.Souayah S, Thepmankorn P, Jedidi N, Nasar A, Souayah N. Encephalopathy after Gardasil Vaccination: A Vaccine Adverse Event Reporting System Study 2006–2019 (3038). Neurology. 2021;96(15 Supplement):3038.
- 104.Sugaya N. Influenza-associated encephalopathy in Japan. Semin Pediatr Infect Dis. 2002;13(2):79-84. [DOI] [PubMed]
- 105.Denholm JT, Neal A, Yan B, Petty S, Knox J, French C, et al. Acute encephalomyelitis syndromes associated with H1N1 09 influenza vaccination. Neurology. 2010;75(24):2246–2248. doi: 10.1212/WNL.0b013e3182020307. [DOI] [PubMed] [Google Scholar]
- 106.Cavanagh L, Strutt AM, Schulz PE. Acute Demyelinating Encephalomyelitis (ADEM) following rabies vaccination. International Journal of Case Reports. 2021 Jun 18;5:206-206.
- 107.Tourbah A, Gout O, Liblau R, Lyon-Caen O, Bougniot C, Iba-Zizen MT, et al. Encephalitis after hepatitis B vaccination: recurrent disseminated encephalitis or MS? Neurology. 1999;53(2):396–401. doi: 10.1212/WNL.53.2.396. [DOI] [PubMed] [Google Scholar]
- 108.Baldelli L, Amore G, Montini A, Panzera I, Rossi S, Cortelli P, et al. Hyperacute reversible encephalopathy related to cytokine storm following COVID-19 vaccine. J Neuroimmunol. 2021;358:577661. doi: 10.1016/j.jneuroim.2021.577661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alnefeesi Y, Siegel A, Lui LMW, Teopiz KM, Ho RCM, Lee Y, et al. Impact of SARS-CoV-2 infection on cognitive function: a systematic review. Front Psychiatry. 2020;11:621773. doi: 10.3389/fpsyt.2020.621773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chahil M, Pillainayagam C, Schulz P. Treatment of Post-Influenza Vaccination Induced Acute Disseminated Encephalomyelitis (ADEM) with Plasma Exchange - A Case Report (P4.036). Neurology. 2014;82(10 Supplement):P4.036
- 111.Iype M, Kunju PAM, Saradakutty G, Anish TS, Sreedharan M, Ahamed SM. Short term outcome of ADEM: results from a retrospective cohort study from South India. Mult Scler Relat Disord. 2017;18:128–134. doi: 10.1016/j.msard.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 112.Ebrahimpour S, Mohseni Afshar Z, Mohseni S, Masrour-Roudsari J, Oladzade S, Bayani M, et al. Neurologic manifestations in patients with COVID-19: a case report. Caspian J Intern Med. 2020;11(Suppl 1):557–560. doi: 10.22088/cjim.11.0.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Habib MB, Hamad MK, Kalash T, Ahmed A, Mohamed MF. COVID-19 Pneumonia complicated by seizure due to severe hyponatremia. Cureus. 2021 doi: 10.7759/cureus.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fasano A, Cavallieri F, Canali E, Valzania F. First motor seizure as presenting symptom of SARS-CoV-2 infection. Neurol Sci. 2020;41:1651–1653. doi: 10.1007/s10072-020-04460-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marchetti RL, Gallucci-Neto J, Kurcgant D, Proença ICGF, Valiengo LdCL, Fiore LA, et al. Immunization stress-related responses presenting as psychogenic non-epileptic seizures following HPV vaccination in Rio Branco. Brazil. Vaccine. 2020;38(43):6714–6720. doi: 10.1016/j.vaccine.2020.08.044. [DOI] [PubMed] [Google Scholar]
- 116.Lin CY, Peng CC, Liu HC, Chiu NC. Psychogenic movement disorder after H1N1 influenza vaccination. J Neuropsychiatry Clin Neurosci. 2011;23(3):E37–E38. doi: 10.1176/jnp.23.3.jnpe37. [DOI] [PubMed] [Google Scholar]
- 117.Ma SJ, Xiong YQ, Jiang LN, Chen Q. Risk of febrile seizure after measles-mumps-rubella-varicella vaccine: a systematic review and meta-analysis. Vaccine. 2015;33(31):3636–3649. doi: 10.1016/j.vaccine.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 118.Ghosh R, Dubey S, Roy D, Mandal A, Naga D, Benito-Leon J. Focal onset non-motor seizure following COVID-19 vaccination: a mere coincidence? Diabetes Metab Syndr. 2021;15(3):1023–1024. doi: 10.1016/j.dsx.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aladdin Y, Shirah B. New-onset refractory status epilepticus following the ChAdOx1 nCoV-19 vaccine. J Neuroimmunol. 2021;357:577629. doi: 10.1016/j.jneuroim.2021.577629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gavvala JR, Schuele SU. New-onset seizure in adults and adolescents: a review. JAMA. 2016;316(24):2657–2668. doi: 10.1001/jama.2016.18625. [DOI] [PubMed] [Google Scholar]
- 121.Brambilla L, Maronese CA, Tourlaki A, Veraldi S. Herpes zoster following COVID-19: a report of three cases. Eur J Dermatol. 2020;30(6):754–756. doi: 10.1684/ejd.2020.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saati A, Al-Husayni F, Malibari AA, Bogari AA, Alharbi M. Herpes Zoster co-Infection in an immunocompetent patient with COVID-19. Cureus. 2020;12(7):e8998. doi: 10.7759/cureus.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tartari F, Spadotto A, Zengarini C, Zanoni R, Guglielmo A, Adorno A, et al. Herpes zoster in COVID-19-positive patients. Int J Dermatol. 2020;59(8):1028–1029. doi: 10.1111/ijd.15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ruder H, Kerling F, Daniel V, Korn K, Wassmuth R. Decreased alloreactivity after vaccination against hepatitis B. Transplantation. 1995;59(9):1339–1342. doi: 10.1097/00007890-199505150-00020. [DOI] [PubMed] [Google Scholar]
- 125.Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20(6):1566–1567. doi: 10.1111/jocd.14035. [DOI] [PubMed] [Google Scholar]
- 126.Bayas JM, Gonzalez-Alvarez R, Guinovart C. Herpes zoster after yellow fever vaccination. J Travel Med. 2007;14(1):65–66. doi: 10.1111/j.1708-8305.2006.00098.x. [DOI] [PubMed] [Google Scholar]
- 127.Walter R, Hartmann K, Fleisch F, Reinhart WH, Kuhn M. Reactivation of herpesvirus infections after vaccinations? Lancet. 1999;353(9155):810. doi: 10.1016/S0140-6736(99)00623-6. [DOI] [PubMed] [Google Scholar]
- 128.Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA Covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford, England). 2021 [DOI] [PMC free article] [PubMed]
- 129.van Dam CS, Lede I, Schaar J, Al-Dulaimy M, Rosken R, Smits M. Herpes zoster after COVID vaccination. Int J Infect Dis. 2021;111:169–171. doi: 10.1016/j.ijid.2021.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Channa L, Torre K, Rothe M. Herpes zoster reactivation after mRNA-1273 (Moderna) SARS-CoV-2 vaccination. JAAD Case Rep. 2021;15:60–61. doi: 10.1016/j.jdcr.2021.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vastarella M, Picone V, Martora F, Fabbrocini G. Herpes zoster after ChAdOx1 nCoV-19 vaccine: a case series. J Eur Acad Dermatol Venereol. 2021 Dec;35(12):e845-e846. [DOI] [PMC free article] [PubMed]
- 132.Arora P, Sardana K, Mathachan SR, Malhotra P. Herpes zoster after inactivated COVID-19 vaccine: A cutaneous adverse effect of the vaccine. J Cosmet Dermatol. 2021 Nov;20(11):3389-3390. [DOI] [PMC free article] [PubMed]
- 133.Chiu HH, Wei KC, Chen A, Wang WH. Herpes zoster following COVID-19 vaccine: A report of three cases. QJM: An International Journal of Medicine. 2021 Jul;114(7):531-2. [DOI] [PMC free article] [PubMed]
- 134.Nastro F, Fabbrocini G, di Vico F, Marasca C. Small vessel vasculitis related to varicella-zoster virus after Pfizer-BioNTech COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35(11):e745–e747. doi: 10.1111/jdv.17550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cohen JI. Clinical practice: Herpes zoster. N Engl J Med. 2013;369(3):255–263. doi: 10.1056/NEJMcp1302674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li X, Ostropolets A, Makadia R, Shaoibi A, Rao G, Sena AG, et al. 2021 Characterizing the incidence of adverse events of special interest for COVID-19 vaccines across eight countries: a multinational network cohort study. medRxiv. 22: 553 [DOI] [PMC free article] [PubMed]
- 137.Tagliaferri AR, Narvaneni S, Azzam MH, Grist W. A case of COVID-19 vaccine causing a myasthenia gravis crisis. Cureus. 2021;13(6):e15581. doi: 10.7759/cureus.15581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alfishawy M, Bitar Z, Elgazzar A, Elzoueiry M. Neuroleptic malignant syndrome following COVID-19 vaccination. Am J Emerg Med. 2021 doi: 10.1016/j.ajem.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Waheed W, Carey ME, Tandan SR, Tandan R. Post COVID-19 vaccine small fiber neuropathy. Muscle Nerve. 2021;64(1):E1–E2. doi: 10.1002/mus.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wan EYF, Chui CSL, Lai FTT, Chan EWY, Li X, Yan VKC, et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22(1):64–72. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim JE, Min YG, Shin JY, Kwon YN, Bae JS, Sung JJ, et al. Guillain-Barré Syndrome and variants following covid-19 vaccination: report of 13 cases. Front Neurol. 2021;12:820723. doi: 10.3389/fneur.2021.820723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Khan E, Shrestha AK, Colantonio MA, Liberio RN, Sriwastava S. Acute transverse myelitis following SARS-CoV-2 vaccination: a case report and review of literature. J Neurol. 2022;269(3):1121–1132. doi: 10.1007/s00415-021-10785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang YJ, Huang CS. 2022 Postvaccinal Encephalopathy Presenting with Amnesia and Seizure After ChAdOx1 nCov-19 Vaccination: A Case Report. Acta Neurol Taiwan [PubMed]
- 144.Afshar ZM, Babazadeh A, Afsharian M, Vaziri S, Ebrahimpour S. Bell's palsy associated with covid-19 infection: a case report. Oman Med J. 2021;36(5):e313. doi: 10.5001/omj.2022.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dahl EH, Mosevoll KA, Cramariuc D, Vedeler CA, Blomberg B. COVID-19 myocarditis and postinfection Bell's palsy. BMJ Case Rep. 2021;14(1):e240095. doi: 10.1136/bcr-2020-240095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bastola A, Sah R, Nepal G, Gajurel BP, Rajbhandari SK, Chalise BS, et al. Bell's palsy as a possible neurological complication of COVID-19: a case report. Clin Case Rep. 2021;9(2):747–750. doi: 10.1002/ccr3.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Al-Mashdali AF, Al Samawi MS. A case of post COVID-19 multisystem inflammatory syndrome and Bell's palsy in a young adult. Clin Case Rep. 2021;9(9):e04801. doi: 10.1002/ccr3.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hasibi M, Seyed Ahadi M, Abdollahi H, Jafari M. Protracted COVID-19 during treatment of facial palsy. Case Rep Neurol Med. 2021;2021:5569841. doi: 10.1155/2021/5569841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ferreira EF, Portugal D, Silva N, Peixoto C, Matos C, Pereira I, et al. Rehabilitation of peripheral facial palsy associated with COVID-19 in a child: a case report. Ann Phys Rehabil Med. 2022;65(1):101600. doi: 10.1016/j.rehab.2021.101600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Iacono A, Pennisi E, Benincasa C, Marchetti F. A case of facial nerve palsy in a pediatric patient associated with Covid-19. Ital J Pediatr. 2022;48(1):75. doi: 10.1186/s13052-022-01263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kaplan AC. Noteworthy neurological manifestations associated with covid-19 infection. Cureus. 2021;13(4):e14391. doi: 10.7759/cureus.14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Szewczyk AK, Skrobas U, Jamroz-Wisniewska A, Mitosek-Szewczyk K, Rejdak K. Facial diplegia-complication or manifestation of SARS-cov-2 Infection? a Case report and systemic literature review. Healthcare (Basel). 2021;9(11):1492. doi: 10.3390/healthcare9111492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kumar V, Narayanan P, Shetty S, Mohammed AP. Lower motor neuron facial palsy in a postnatal mother with COVID-19. BMJ Case Rep. 2021;14(3):e240267. doi: 10.1136/bcr-2020-240267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Neo WL, Ng JCF, Iyer NG. The great pretender-Bell's palsy secondary to SARS-CoV-2? Clin Case Rep. 2021;9(3):1175–1177. doi: 10.1002/ccr3.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Khaja M, Gomez GPR, Santana Y, Hernandez N, Haider A, Lara JLP, et al. A 44-year-old Hispanic man with loss of taste and bilateral facial weakness diagnosed with Guillain-Barre syndrome and bell's palsy associated with sars-cov-2 infection treated with intravenous immunoglobulin. Am J Case Rep. 2020;21:e927956. doi: 10.12659/AJCR.927956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Theophanous C, Santoro JD, Itani R. Bell's palsy in a pediatric patient with hyper IgM syndrome and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Brain Dev. 2021;43(2):357–359. doi: 10.1016/j.braindev.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yue Wan, Shugang Cao, Qi Fang et al. Coronavirus disease 2019 complicated with Bell’s palsy: a case report, 16 April 2020, PREPRINT (Version 1) available at Research Square [10.21203/rs.3.rs-23216/v1]
- 158.Bohania N, Ish P, Nune A, Iyengar KP. Cranial neuropathy in COVID-19: a case series and review of literature. Infez Med. 2021;29(4):609–613. doi: 10.53854/liim-2904-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Burrows A, Bartholomew T, Rudd J, Walker D. Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses. BMJ Case Rep. 2021;14(7):e243829. doi: 10.1136/bcr-2021-243829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cellina M, D'Arrigo A, Floridi C, Oliva G, Carrafiello G. Left Bell's palsy following the first dose of mRNA-1273 SARS-CoV-2 vaccine: a case report. Clin Imaging. 2022;82:1–4. doi: 10.1016/j.clinimag.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mussatto CC, Sokol J, Alapati N. Bell's palsy following COVID-19 vaccine administration in HIV+ patient. Am J Ophthalmol Case Rep. 2022;25:101259. doi: 10.1016/j.ajoc.2022.101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yu BY, Cen LS, Chen T, Yang TH. Bell's palsy after inactivated COVID-19 vaccination in a patient with history of recurrent Bell's palsy: a case report. World J Clin Cases. 2021;9(27):8274–8279. doi: 10.12998/wjcc.v9.i27.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mason MC, Liaqat A, Morrow J, Basso R, Gujrati Y. Bilateral facial nerve palsy and covid-19 vaccination: causation or coincidence? Cureus. 2021;13(8):e17602. doi: 10.7759/cureus.17602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mirmosayyeb O, Barzegar M, Rezaei M, Baharlouie N, Shaygannejad V. Bell's palsy after Sputnik V COVID-19 (Gam-COVID-Vac) vaccination. Clin Case Rep. 2022;10(2):e05468. doi: 10.1002/ccr3.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pothiawala S. Bell's palsy after second dose of moderna covid-19 vaccine: coincidence or causation? Acta Med Litu. 2021;28(2):298–301. doi: 10.15388/Amed.2021.28.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kundi M, Montgomery S, Mao S, Asghar S. Not all that is droopy post ad26cov2s (jnj) vaccine is bell's palsy: a rare case of isolated dorsal pontine stroke causing ipsilateral complete hemi-facial palsy. Cureus. 2022;14(3):e23195. doi: 10.7759/cureus.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nishizawa Y, Hoshina Y, Baker V. Bell's palsy following the Ad26.COV2.S COVID-19 vaccination. QJM. 2021;114(9):657–658. doi: 10.1093/qjmed/hcab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Martin-Villares C, Vazquez-Feito A, Gonzalez-Gimeno MJ, de la Nogal-Fernandez B. Bell's palsy following a single dose of mRNA SARS-CoV-2 vaccine: a case report. J Neurol. 2022;269(1):47–48. doi: 10.1007/s00415-021-10617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Scheidl E, Canseco DD, Hadji-Naumov A, Bereznai B. Guillain-Barre syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. 2020;25(2):204–207. doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bueso T, Montalvan V, Lee J, Gomez J, Ball S, Shoustari A, et al. Guillain-Barre Syndrome and COVID-19: a case report. Clin Neurol Neurosurg. 2021;200:106413. doi: 10.1016/j.clineuro.2020.106413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Agosti E, Giorgianni A, D'Amore F, Vinacci G, Balbi S, Locatelli D. Is Guillain-Barre syndrome triggered by SARS-CoV-2? Case report and literature review. Neurol Sci. 2021;42(2):607–612. doi: 10.1007/s10072-020-04553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Paybast S, Gorji R, Mavandadi S. Guillain-Barre syndrome as a neurological complication of novel covid-19 infection: a case report and review of the literature. Neurologist. 2020;25(4):101–103. doi: 10.1097/NRL.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dufour C, Co TK, Liu A. GM1 ganglioside antibody and COVID-19 related Guillain Barre Syndrome - a case report, systemic review and implication for vaccine development. Brain Behav Immun Health. 2021;12:100203. doi: 10.1016/j.bbih.2021.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain-Barre syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.El Otmani H, El Moutawakil B, Rafai MA, El Benna N, El Kettani C, Soussi M, et al. Covid-19 and Guillain-Barre syndrome: more than a coincidence! Rev Neurol (Paris) 2020;176(6):518–519. doi: 10.1016/j.neurol.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Khan F, Sharma P, Pandey S, Sharma D, V V, Kumar N,, et al. COVID-19-associated Guillain-Barre syndrome: Postinfectious alone or neuroinvasive too? J Med Virol. 2021;93(10):6045–6049. doi: 10.1002/jmv.27159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Su XW, Palka SV, Rao RR, Chen FS, Brackney CR, Cambi F. SARS-CoV-2-associated Guillain-Barre syndrome with dysautonomia. Muscle Nerve. 2020;62(2):E48–E49. doi: 10.1002/mus.26988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Darvishi M, Shahali H, Farahani AA. Guillain-Barre syndrome associated with sars-cov-2 infection: a case report. Eur J Transl Myol. 2021 doi: 10.4081/ejtm.2021.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mackenzie N, Lopez-Coronel E, Dau A, Maloof D, Mattar S, Garcia JT, et al. Concomitant Guillain-Barre syndrome with COVID-19: a case report. BMC Neurol. 2021;21(1):135. doi: 10.1186/s12883-021-02162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mostel Z, Ayat P, Capric V, Trimmingham A, McFarlane SI. Guillain-Barre syndrome in a covid-19 patient: a case report and review of management strategies. Am J Med Case Rep. 2021;9(3):198–200. doi: 10.12691/ajmcr-9-3-16. [DOI] [Google Scholar]
- 183.Farzi MA, Ayromlou H, Jahanbakhsh N, Bavil PH, Janzadeh A, Shayan FK. Guillain-Barre syndrome in a patient infected with SARS-CoV-2, a case report. J Neuroimmunol. 2020;346:577294. doi: 10.1016/j.jneuroim.2020.577294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nejad JH, Heiat M, Hosseini MJ, Allahyari F, Lashkari A, Torabi R, et al. Guillain-Barre syndrome associated with COVID-19: a case report study. J Neurovirol. 2021;27(5):802–805. doi: 10.1007/s13365-021-00984-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.McKean N, Chircop C. Guillain-Barre syndrome after COVID-19 vaccination. BMJ Case Rep. 2021;14(7):e244125. doi: 10.1136/bcr-2021-244125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hasan T, Khan M, Khan F, Hamza G. Case of Guillain-Barre syndrome following COVID-19 vaccine. BMJ Case Rep. 2021;14(6):e243629. doi: 10.1136/bcr-2021-243629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Allen CM, Ramsamy S, Tarr AW, Tighe PJ, Irving WL, Tanasescu R, et al. Guillain-Barre syndrome variant occurring after sars-cov-2 vaccination. Ann Neurol. 2021;90(2):315–318. doi: 10.1002/ana.26144. [DOI] [PubMed] [Google Scholar]
- 188.Min YG, Ju W, Ha YE, Ban JJ, Lee SA, Sung JJ, et al. Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine: report of two cases and review of literature. J Neuroimmunol. 2021;359:577691. doi: 10.1016/j.jneuroim.2021.577691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Scendoni R, Petrelli C, Scaloni G, Logullo FO. Electromyoneurography and laboratory findings in a case of Guillain-Barre syndrome after second dose of Pfizer COVID-19 vaccine. Hum Vaccin Immunother. 2021;17(11):4093–4096. doi: 10.1080/21645515.2021.1954826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Maramattom BV, Krishnan P, Paul R, Padmanabhan S, Cherukudal Vishnu Nampoothiri S, Syed AA, et al. Guillain-Barre Syndrome following ChAdOx1-S/nCoV-19 Vaccine. Ann Neurol. 2021;90(2):312–314. doi: 10.1002/ana.26143. [DOI] [PubMed] [Google Scholar]
- 191.Introna A, Caputo F, Santoro C, Guerra T, Ucci M, Mezzapesa DM, et al. Guillain-Barre syndrome after AstraZeneca COVID-19-vaccination: a causal or casual association? Clin Neurol Neurosurg. 2021;208:106887. doi: 10.1016/j.clineuro.2021.106887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Razok A, Shams A, Almeer A, Zahid M. Post-COVID-19 vaccine Guillain-Barre syndrome; first reported case from Qatar. Ann Med Surg (Lond) 2021;67:102540. doi: 10.1016/j.amsu.2021.102540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rao SJ, Khurana S, Murthy G, Dawson ET, Jazebi N, Haas CJ. A case of Guillain-Barre syndrome following Pfizer COVID-19 vaccine. J Community Hosp Intern Med Perspect. 2021;11(5):597–600. doi: 10.1080/20009666.2021.1954284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Moreno-Escobar MC, Kataria S, Khan E, Subedi R, Tandon M, Peshwe K, et al. Acute transverse myelitis with dysautonomia following sars-cov-2 infection: a case report and review of literature. J Neuroimmunol. 2021;353:577523. doi: 10.1016/j.jneuroim.2021.577523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Qazi R, Memon A, Mohamed AS, Ali M, Singh R. Post-COVID-19 acute transverse myelitis: a case report and literature review. Cureus. 2021;13(12):e20628. doi: 10.7759/cureus.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chow CCN, Magnussen J, Ip J, Su Y. 2020 Acute transverse myelitis in COVID-19 infection. BMJ Case Rep.;13(8) [DOI] [PMC free article] [PubMed]
- 197.Sarma D, Bilello LA. A case report of acute transverse myelitis following novel coronavirus infection. Clin Pract Cases Emerg Med. 2020;4(3):321–323. doi: 10.5811/cpcem.2020.5.47937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ahmad SA, Salih KH, Ahmed SF, Kakamad FH, Salh AM, Hassan MN, et al. Post COVID-19 transverse myelitis; a case report with review of literature. Ann Med Surg (Lond) 2021;69:102749. doi: 10.1016/j.amsu.2021.102749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nejad Biglari H, Sinaei R, Pezeshki S, Khajeh HF. Acute transverse myelitis of childhood due to novel coronavirus disease 2019: The first pediatric case report and review of literature. Iran J Child Neurol. 2021;15(1):107–112. doi: 10.22037/ijcn.v15i1.31579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Palahuta HV, Fartushna OY, Yevtushenko SK, Hnepa YY. Acute transverse myelitis as a neurological complication of covid-19: a case report. Wiad Lek. 2021;74(4):1045–1049. doi: 10.36740/WLek202104144. [DOI] [PubMed] [Google Scholar]
- 201.Lingas EC. A case of acute transverse myelitis in a mildly symptomatic patient: an emerging and serious neurological manifestation of COVID-19. Cureus. 2022;14(4):e24222. doi: 10.7759/cureus.24222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Prete S, McShannic JD, Fertel BS, Simon EL. Acute transverse myelitis progressing to permanent quadriplegia following COVID-19 infection. Am J Emerg Med. 2022;56(391):e1–e3. doi: 10.1016/j.ajem.2022.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shahali H, Ghasemi A, Farahani RH, Nezami Asl A, Hazrati E. Acute transverse myelitis after SARS-CoV-2 infection: a rare complicated case of rapid onset paraplegia. J Neurovirol. 2021;27(2):354–358. doi: 10.1007/s13365-021-00957-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hsiao YT, Tsai MJ, Chen YH, Hsu CF. Acute transverse myelitis after COVID-19 vaccination. Medicina. 2021 Sep 25;57(10):1010. [DOI] [PMC free article] [PubMed]
- 205.Tan WY, Yusof Khan AHK, Mohd Yaakob MN, Abdul Rashid AM, Loh WC, Baharin J, et al. Longitudinal extensive transverse myelitis following ChAdOx1 nCOV-19 vaccine: a case report. BMC Neurol. 2021;21(1):395. doi: 10.1186/s12883-021-02427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Notghi AA, Atley J, Silva M. Lessons of the month 1: longitudinal extensive transverse myelitis following astrazeneca covid-19 vaccination. Clin Med (Lond) 2021;21(5):e535–e538. doi: 10.7861/clinmed.2021-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Eom H, Kim SW, Kim M, Kim YE, Kim JH, Shin HY, et al. Case reports of acute transverse myelitis associated with mrna vaccine for COVID-19. J Korean Med Sci. 2022;37(7):e52. doi: 10.3346/jkms.2022.37.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Miyaue N, Yoshida A, Yamanishi Y, Tada S, Ando R, Hosokawa Y, et al. Refractory longitudinally extensive transverse myelitis after severe acute respiratory syndrome coronavirus 2 vaccination in a Japanese man. Intern Med. 2022;61(5):739–742. doi: 10.2169/internalmedicine.8747-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Maroufi SF, Naderi Behdani F, Rezania F, Tanhapour Khotbehsara S, Mirzaasgari Z. Longitudinally extensive transverse myelitis after Covid-19 vaccination: case report and review of literature. Hum Vaccin Immunother. 2022;18(1):2040239. doi: 10.1080/21645515.2022.2040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tahir N, Koorapati G, Prasad S, Jeelani HM, Sherchan R, Shrestha J, et al. SARS-CoV-2 vaccination-induced transverse myelitis. Cureus. 2021;13(7):e16624. doi: 10.7759/cureus.16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hirose S, Hara M, Koda K, Natori N, Yokota Y, Ninomiya S, et al. Acute autoimmune transverse myelitis following COVID-19 vaccination: a case report. Medicine (Baltimore) 2021;100(51):e28423. doi: 10.1097/MD.0000000000028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rajae A, Manal M, Ghizlane EA, Amine B, Zaid I, Houssam B, et al. Ischemic stroke revealing covid-19 infection: case report. Ann Med Surg (Lond) 2021;71:102912. doi: 10.1016/j.amsu.2021.102912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Avvantaggiato C, Amoruso L, Lo Muzio MP, Mimmo MA, Delli Bergoli M, Cinone N, et al. Ischemic stroke in a 29-year-old patient with covid-19: a case report. Case Rep Neurol. 2021;13(2):334–340. doi: 10.1159/000515457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bigliardi G, Ciolli L, Giovannini G, Vandelli L, Dell'Acqua ML, Borzi GM, et al. Middle cerebral artery ischemic stroke and COVID-19: a case report. J Neurovirol. 2020;26(6):967–969. doi: 10.1007/s13365-020-00898-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhai P, Ding Y, Li Y. The impact of COVID-19 on ischemic stroke. Diagn Pathol. 2020;15(1):78. doi: 10.1186/s13000-020-00994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Farooque U, Shabih S, Karimi S, Lohano AK, Kataria S. Coronavirus disease 2019-related acute ischemic stroke: a case report. Cureus. 2020;12(9):e10310. doi: 10.7759/cureus.10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Owolabi LF, Raafat A, Enwere OO, Mustapha AF, Adamu B, AlGhamdi M. Hemorrhagic infarctive stroke in COVID-19 patients: report of two cases and review of the literature. J Community Hosp Intern Med Perspect. 2021;11(3):322–326. doi: 10.1080/20009666.2021.1883814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fraiman P, Freire M, Moreira-Neto M, Godeiro-Junior C. Hemorrhagic stroke and COVID-19 infection: coincidence or causality. eNeurologicalSci. 2020 doi: 10.1016/j.ensci.2020.100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Flores G, Kumar JI, Pressman E, Sack J, Alikhani P. Spontaneous brainstem hemorrhagic stroke in the setting of novel coronavirus disease 2019 - a case report. Cureus. 2020;12(10):e10809. doi: 10.7759/cureus.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dakay K, Cooper J, Bloomfield J, Overby P, Mayer SA, Nuoman R, et al. Cerebral venous sinus thrombosis in COVID-19 Infection: a case series and review of the literature. J Stroke Cerebrovasc Dis. 2021;30(1):105434. doi: 10.1016/j.jstrokecerebrovasdis.2020.105434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Anipindi M, Scott A, Joyce L, Wali S, Morginstin M. Case report: cerebral venous sinus thrombosis and COVID-19 infection. Front Med (Lausanne) 2021;8:741594. doi: 10.3389/fmed.2021.741594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tu TM, Goh C, Tan YK, Leow AS, Pang YZ, Chien J, et al. Cerebral venous thrombosis in patients with COVID-19 infection: a case series and systematic review. J Stroke Cerebrovasc Dis. 2020;29(12):105379. doi: 10.1016/j.jstrokecerebrovasdis.2020.105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Elaidouni G, Chetouani Z, Manal Merbouh CB, Bkiyar H, Housni B. Acute ischemic stroke after first dose of inactivated COVID-19 vaccine: a case report. Radiol Case Rep. 2022;17(6):1942–1945. doi: 10.1016/j.radcr.2022.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kenda J, Lovric D, Skerget M, Milivojevic N. Treatment of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia related acute ischemic stroke. J Stroke Cerebrovasc Dis. 2021;30(11):106072. doi: 10.1016/j.jstrokecerebrovasdis.2021.106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Al-Mayhani T, Saber S, Stubbs MJ, Losseff NA, Perry RJ, Simister RJ, et al. Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. J Neurol Neurosurg Psychiatry. 2021;92(11):1247–1248. doi: 10.1136/jnnp-2021-326984. [DOI] [PubMed] [Google Scholar]
- 226.de Melo Silva ML, Jr., Lopes DP. Large hemorrhagic stroke after ChAdOx1 nCoV-19 vaccination: a case report. Acta Neurol Scand. 2021;144(6):717–718. doi: 10.1111/ane.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Takeyama R, Fukuda K, Kouzaki Y, Koga T, Hayashi S, Ohtani H, et al. Intracerebral hemorrhage due to vasculitis following COVID-19 vaccination: a case report. Acta Neurochir. 2022;164(2):543–547. doi: 10.1007/s00701-021-05038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dias L, Soares-Dos-Reis R, Meira J, Ferrao D, Soares PR, Pastor A, et al. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J Stroke Cerebrovasc Dis. 2021;30(8):105906. doi: 10.1016/j.jstrokecerebrovasdis.2021.105906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zakaria Z, Sapiai NA, Ghani ARI. Cerebral venous sinus thrombosis 2 weeks after the first dose of mRNA SARS-CoV-2 vaccine. Acta Neurochir (Wien) 2021;163(8):2359–2362. doi: 10.1007/s00701-021-04860-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.D'Agostino V, Caranci F, Negro A, Piscitelli V, Tuccillo B, Fasano F, et al. A rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated to the COVID-19 vaccine administration. J Pers Med. 2021;11(4):285. doi: 10.3390/jpm11040285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Atta SN, Othman N, Babar M. Cerebral venous sinus thrombosis secondary to ChAdOx-1 nCov-19 vaccine. BMJ Case Rep. 2021 doi: 10.1136/bcr-2021-246200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Delorme C, Paccoud O, Kas A, Hesters A, Bombois S, Shambrook P, et al. COVID-19-related encephalopathy: a case series with brain FDG-positron-emission tomography/computed tomography findings. Eur J Neurol. 2020;27(12):2651–2657. doi: 10.1111/ene.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lazraq M, Benhamza S, Saadaoui S, Hayar S, Louardi M, Moujahid H, et al. Encephalopathy and COVID-19: a case report. Pan Afr Med J. 2021;38:139. doi: 10.11604/pamj.2021.38.139.27845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Goodloe TB, 3rd, Walter LA. COVID-19 presenting as encephalopathy in the emergency department: a case report. Clin Pract Cases Emerg Med. 2021;5(1):26–29. doi: 10.5811/cpcem.2020.12.50036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Teimouri-Jervekani Z, Salmasi M. Presentation of COVID-19 infection with bizarre behavior and encephalopathy: a case report. J Med Case Rep. 2021;15(1):220. doi: 10.1186/s13256-021-02851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Al-Mashdali AF, Ata YM, Sadik N. Post-COVID-19 vaccine acute hyperactive encephalopathy with dramatic response to methylprednisolone: a case report. Ann Med Surg (Lond) 2021;69:102803. doi: 10.1016/j.amsu.2021.102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Liu BD, Ugolini C, Jha P. Two Cases of Post-Moderna COVID-19 Vaccine Encephalopathy Associated With Nonconvulsive Status Epilepticus. Cureus. 2021;13(7):e16172. doi: 10.7759/cureus.16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bensaidane MR, Picher-Martel V, Emond F, De Serres G, Dupre N, Beauchemin P. Case report: acute necrotizing encephalopathy following COVID-19 vaccine. Front Neurol. 2022;13:872734. doi: 10.3389/fneur.2022.872734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Monti G, Giovannini G, Marudi A, Bedin R, Melegari A, Simone AM, et al. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure. 2020;81:18–20. doi: 10.1016/j.seizure.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bhatta S, Sayed A, Ranabhat B, Bhatta RK, Acharya Y. New-onset seizure as the only presentation in a child with COVID-19. Cureus. 2020;12(6):e8820. doi: 10.7759/cureus.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Park S, Majoka H, Sheikh A, Ali I. A presumed case of new-onset focal seizures as a delayed complication of COVID-19 infection. Epilepsy Behav Rep. 2021;16:100447. doi: 10.1016/j.ebr.2021.100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dono F, Carrarini C, Russo M, De Angelis MV, Anzellotti F, Onofrj M, et al. New-onset refractory status epilepticus (NORSE) in post SARS-CoV-2 autoimmune encephalitis: a case report. Neurol Sci. 2021;42(1):35–38. doi: 10.1007/s10072-020-04846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Cho YJ, Kim HK. New-onset seizures in patients with COVID-19: A case series from a single public hospital in Korea. J Korean Med Sci. 2022;37(12):e97. doi: 10.3346/jkms.2022.37.e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bauman K, Rosenthal J, Lin J. New-Onset Refractory Status Epilepticus After Pfizer-BioNTech COVID-19 Vaccination (P3-8.006). Neurology. 2022;98(18 Supplement):3631.
- 245.Desai HD, Sharma K, Patoliya JV, Ahadov E, Patel NN. A rare case of Varicella-Zoster virus reactivation following recovery from COVID-19. Cureus. 2021;13(1):e12423. doi: 10.7759/cureus.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Saleh W, Ata F, Elashry MM. Is COVID-19 infection triggering oral herpes zoster? A case report. SAGE Open Med Case Rep. 2021 doi: 10.1177/2050313X211065793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.van Dam CS, Lede I, Schaar J, Al-Dulaimy M, Rösken R, Smits M. Herpes zoster after COVID vaccination. Int J Infect Dis. 2021;111:169–171. doi: 10.1016/j.ijid.2021.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rodriguez-Jimenez P, Chicharro P, Cabrera LM, Segui M, Morales-Caballero A, Llamas-Velasco M, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58–59. doi: 10.1016/j.jdcr.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Santovito LS, Pinna G. A case of reactivation of varicella-zoster virus after BNT162b2 vaccine second dose? Inflamm Res. 2021;70(9):935–937. doi: 10.1007/s00011-021-01491-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.