Abstract
In both acute and chronic disease, functional differences in host immune responses arise from a multitude of intrinsic and extrinsic factors. Two of the most important factors affecting the immune response are biological sex and aging. Ischemic stroke is a debilitating disease that predominately affects older individuals. Epidemiological studies have shown that older women have poorer functional outcomes compared to men, in part due to the older age at which they experience their first stroke and the increased co-morbidities seen with aging. The immune response also differs in men and women, which could lead to altered inflammatory events that contribute to sex differences in post-stroke recovery. Intrinsic factors including host genetics and chromosomal sex play a crucial role both in shaping the host immune system and in the neuroimmune response to brain injury. Ischemic stroke leads to altered intracellular communication between astrocytes, neurons, and resident immune cells in the central nervous system (CNS). Increased production of cytokines and chemokines orchestrate the infiltration of peripheral immune cells and promote neuroinflammation. To maintain immunosurveillance, the host immune and CNS are highly regulated by a diverse population of immune cells which are strategically distributed within the neuro-vascular unit and become activated with injury. In this review, we provide a comprehensive overview of sex-specific host immune responses in ischemic stroke.
Introduction:
Over the past decade, many studies have highlighted the importance of clinical and public health initiatives directed at addressing stroke disparities in women1,2. Stroke incidence is higher in men as compared to women throughout most of the lifespan, however, the prevalence of stroke in women is significantly higher due to their increased longevity3. A multitude of pre-clinical and clinical studies have shown that post-stroke outcomes (i.e., functional recovery, post-stroke inflammatory conditions, quality of life, and depression) are worse in women compared to men4. Interestingly, recent retrospective studies using a claimed database of insured Americans found that in the 25 to 34 and 35 to 44-year age groups, more women had strokes than men (incidence rate ratio: men: women, 0.70 [95% confidence interval (CI), 0.57-0.86]; and 0.87 [95% CI, 0.78-0.98], respectively). However, younger women had better outcomes compared with age-matched men5. In contrast, in the 45 to-74-year-old age group, more men had strokes. A recent systematic review confirmed these findings, but noted the risk was highest in women ≤35 years (44% more women with ischemic strokes6). This suggests that factors such as pregnancy (a time of high risk for women) or other non-atherosclerotic risk factors may be contributing to the increase in incidence in younger women. Sex differences in both risk factors and in post-stroke outcomes may be secondary to chronic hormonal effects from life exposure to sex hormones, or from acute effects of circulatory hormones primarily during the reproductive years. Most studies suggest that older post-menopausal women have poorer outcomes after stroke compared to older men, suggesting potential contributions from factors beyond gonadal hormone exposure7. More recently, sex chromosomal effects (XX vs. XY) have been recognized in pre-clinical models, and X-linked genes can regulate the neuroinflammatory response to stroke8. As the presence of sex differences are apparent in the context of ischemic stroke, recent studies have focused on the mechanistic pathways that may play a contributory role9.
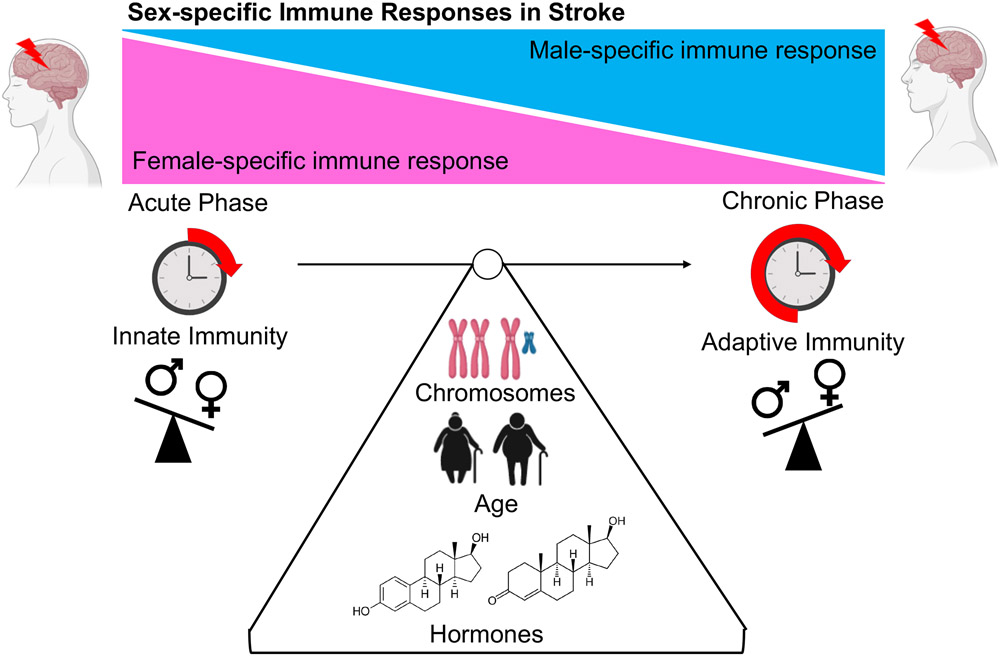
Aging contributes to an enhanced basal state of inflammation in the host, often referred to as “inflammaging”. This also contributes to a functional decline in the immune system or immunosenescence10. Many independent reports have shown that the host immune response towards foreign or self-antigens are distinct among males and females11,12. Following ischemic stroke, the brain is exposed to a myriad of inflammatory signals leading to neuroinflammation. The protective effects of the blood brain barrier (BBB) in the naïve non-diseased brain actively protects the host from pathological immune cell infiltration into the brain13. Impairment in the BBB enhances ischemic injury, allowing for the infiltration of immune cells and serum factors into the brain, contributing to secondary ischemic damage14, edema, hemorrhagic transformation, and disruption of the neurovascular unit15. Mechanisms through which sex and sex hormones influence the integrity of the BBB may potentially play a contributory role in the sex-specific differences observed in peripheral infiltration of immune cells post-stroke16 and in the extent of ischemic damage. Sex differences in the activation states of both the innate and adaptive immune compartments, regulated by many physiological factors, may lead to differences in post-stroke outcomes (Figure 1). Over the past decade, studies have shed light on how the host immune system responds in a sex-specific manner following acute ischemic injury11 which will be the focus of this review.
Figure 1.
Physiological factors that influence sex-specific immune responses after ischemic stroke. Figure made in ©BioRender - biorender.com.
Innate and Adaptive Immunity in Stroke
Mechanisms of Innate Immunity and Stroke
Innate immunity is the host’s first line of defense to prevent invading pathogens at physical (i.e., primarily tight junctions and secretive mucin located on the skin or mucosal surfaces) and anatomical barriers (i.e., skin, epithelial barriers, etc.). Phagocytes (i.e., monocytes, neutrophils, etc.), and a wide array of transient receptor-mediated immune activation pathways (i.e., Toll-like-receptors (TLRs), pattern recognition receptors (PRRs), Purinergic receptors (PRs), etc.)17 are components of innate immunity. In contrast to adaptive immunity, innate immunity relies on rapid activation through transient and ubiquitous receptors on innate immune cells that sense the host environment for foreign- and self-antigens referred to as damage-associated molecular patterns (DAMPs)18. Innate immune cells include dendritic cells, macrophages, and monocytes along with unconventional subsets of lymphocytes such as gamma-delta (γδ) T cells which can be activated independently of primary innate immune activation. These cells are found in higher quantities in the blood of women and decline with age in both sexes19. Upon antigenic challenge, females respond more efficiently for the clearance of pathogens compared to males, however, females have significantly higher incidence of activation-induced immunopathology and autoimmune diseases20. Activation of specific genes associated with TLR pathways and antiviral type I interferon (IFN) responses differ between males and females. Females express significantly higher levels of TLRs contributing to increased activation of pathogen-mediated immunity and pathogen clearance21. Gonadal steroids can cause direct effects on immune cell function and development, however, sex differences may arise from inherit imbalance in the expression of genes encoded on the X and Y chromosomes. Multiple genes related to the immune activation and TLR-mediated signaling pathways are encoded on the X chromosome leading to higher expression levels in females22.
Microglia
Microglia drive the infiltration of systemic immune cells towards the site of brain injury through cytokine-mediated recruitment, initiating neuroinflammation. There are confirmed sex differences in microglia numbers and transcriptomic signatures early in development and in neuroinflammatory environments23. For example, endogenous processing of antigen presentation and higher expression of major histocompatibility complex (MHC I) and MHCII are more potently activated in males24. There are sex specific effects on microglial development that begin in the neonatal period suggesting that early life development of innate immunity potentially dictates immune-associated disease outcomes later in life25. There are large number of studies showing the influence of sex-specific microglial responses following experimental stroke26. Young male rodents have larger infarcts compared to young females, an effect primarily mediated by estrogen27 as it is lost with ovariectomy and with aging (reproductive senescence)28. Interestingly, this reversal in outcomes with aging may be mediated in part by microglia as basal immune cell activation and inflammation are significantly higher in aged females29,30.
Sex hormones contribute to both sex-specific proliferation of microglia and the sex-specific chemotactic signals produced from microglia, resulting in differential immune cell infiltration into the brain in males and females31. Specific chemotactic surface ligands such as chemokine ligand (CCL4), (CCL20), and CD206 differ in males and females within the hippocampus, amygdala, and the cortex after stroke, suggesting differences in systemic immune cell recruitment32. In addition, levels of specific cytokines including interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and C-X-C motif (CXC)L10 differ in males and females further supporting the presence of sex differences in microglial-specific inflammation33. Interestingly, one study found that ovariectomy (with the subsequent loss of circulating estrogen) did not significantly alter microglial gene expression patterns in the brains of adult female mice. Further studies investigating how the sex-specific landscape of the brain shapes basal and injury-induced activation states of microglia are needed.
Neutrophils
Neutrophils increase in the brain following ischemic stroke, and positively correlate with stroke severity, infarction volume, and post-stroke outcomes34,35. Most studies have highlighted the detrimental role of neutrophils in ischemic stroke including disruption of the BBB, enhanced cerebral edema, and increased brain injury36. However, potential beneficial effects of neutrophil infiltration, such as enhanced clearance of necrotic cells have also been reported37. One hallmark feature of neutrophils is their ability to produce neutrophil extracellular traps (NETs), implicated in clot formation, thrombosis38 and reduced effectiveness of tissue-type plasminogen (tPA)-induced thrombolysis. Microbial cues and endogenous danger signals can also potentiate the highly regulated process of NETosis39. Neutrophils isolated from females undergo greater NETosis after calcium induced ex vivo stimulation compared to males40.
Recent advancements in single-cell technology have highlighted sex-specific transcriptional profiles of circulating neutrophils in young adult men and women. Circulating neutrophils in males were more developmentally immature and had a higher threshold for activation compared to those from age-matched females, leading to a distinct neutrophil-specific immune-metabolic signatures41. Genes specific to neutrophil activation and migration were both time and sex-specific; females showed differentially expressed genes acutely following cardioembolic stroke, however, this was not seen in males42. In contrast, in experimental stroke models, aged males had greater brain infiltration of neutrophils compared to age-matched females. This was further corroborated by the significantly higher levels of neutrophil specific cytokines, monocyte chemoattractant protein-1 (MCP-1) and granulocyte colony stimulating factor (G-CSF) in the circulation of aged males, potentially contributing to an increased incidence of hemorrhagic transformation43. The discrepancies among these studies may be due to the progression of aging and its effects on neutrophil biology, or in the clinical vs. pre-clinical models examined (as humans have significantly more neutrophils than rodents). Although multiple studies have highlighted the presence of sex differences in the neutrophilic response to stroke, mechanistic approaches incorporating both sexes, and deeper consideration of biological variables such as hormonal or chromosomal influences on sex-specific neutrophilic signatures are needed.
Mechanisms of Adaptive Immunity and Stroke
The components of the adaptive immune system that have been the most well studied in stroke include T and B lymphocytes44. Cell-mediated responses are primarily comprised of the activation of T cells toward a cytotoxic phenotype, or differentiation into specific T cell subtypes (i.e., T-helper (TH)) to modulate the immune response through the production of subset specific cytokines45. In contrast, “unconventional” T cell subsets, including TH17 and γδ T cells are activated and differentiated independently of antigen presentation from peripheral tissues46. Once activated, B cells differentiate into antibody producers to promote complement activation, antigen neutralization, antigen opsonization, and the apoptosis of other phagocytic immune cells for DAMP or PAMP clearance47. T and B cell deficient mice have less stroke induced inflammation and better outcomes48. Sex differences in lymphocyte subset diversity have been extensively documented in adult humans. In non-diseased states, males show significantly lower numbers of TH cells in the circulation compared to age-matched females, however, males have higher numbers of cytotoxic T cells49. In contrast to confirmed sex differences in T cell biology, sex-specific B cell immunity has been less studied. A few studies have reported that B cells numbers and immunoglobulin levels are higher in females50.
Dendritic Cells
Dendritic Cells (DCs) act as sentinels of the host innate immune system51, they are professional antigen presenting cells (APCs) expressing MHCII and are considered to bridge the gap among innate and adaptive immune systems. During homeostatic conditions DCs are positioned strategically near the cerebral spinal fluid (CSF)-blood barrier. They can migrate towards draining cervical lymph nodes and activate immunogenic T cell responses or promote host tolerance by exhibiting tolerogenic characteristics52. With stroke there is a temporally regulated migration and maturation of peripheral DCs, primarily from the bone marrow, into the ischemic area53. DC activation state, efficacy of antigen presentation, and surface marker phenotypes are distinct between the sexes. Plasmacytoid DCs (pDCs) from females produce significantly higher levels of IFN-α after TLR activation compared to males54. In multiple pre-clinical studies females exhibit greater expression of TLRs on DCs compared to age-matched males, leading to more effective antigen sensing, processing, and presentation55,56. The differences in TLR expression seen between males and females may be X chromosome mediated as many classes of TLRs, including TLR2, TLR3, and TLR7, are on the X chromosome21.
The specific lineage of DCs (i.e., CD11b and CD103) is highly sexually dimorphic in peripheral tissues (i.e., spleen) with advancing age in mice57. A hallmark feature of DCs is their ability to perform antigen presentation to initiate T-cell mediated immune activation in the host58. However, downstream sex-specific DC-mediated T cell activation may differ between the sexes. For example, Cytomegalovirus (CMV) infection in females elicited a profound TLR specific-DC response resulting in an IFN-γ mediated TH1 response compared to males, which resulted in faster and more effective viral clearance59. TH1 responses in the host are protective in the acute phases of infection and enhance effective viral removal, however, these may be detrimental chronically, as reflected by the higher incidence of auto-immune diseases in women60. Studies investigating the specific role of DCs in ischemic stroke are needed.
T cells
The infiltration of lymphocytes, specifically T cells, in the brain after stroke has been extensively documented. During acute stroke, infiltrating T cells orchestrate the adhesion of platelets to the cerebral endothelium resulting in thrombo-inflammation and larger infarcts61. Sex differences in leukocyte-platelet interactions in patients with atherosclerosis, a major risk factor for stroke, have been documented. Females had significantly more leukocyte-platelet aggregates which were detrimental to recovery62. Conway et al. demonstrated IL-10, an anti-inflammatory cytokine, secreted by regulatory T cells (Treg) and TH2 cells differs by sex. The exacerbated IL-10 production seen in females was associated with poorer recovery and immunosuppression which was not evident in males63. The effects of excessive IL-10 production have been associated with post-stroke immunosuppression, potentially increasing the incidence of post-stroke infections64. IL-10 production from cytotoxic T cells was significantly elevated after stroke in female compared male mice65. Separately, Ahnstedt et al. demonstrated that although there was temporal elevation of TH and T-cytotoxic cells in the brain across both sexes at post-stroke day 15, the relative frequencies of T-cytotoxic cells and Treg were significantly higher in males4, as was hemorrhagic transformation. These sex-specific differences in adaptive immunity after stroke were correlated with greater stroke induced cognitive deficits. Future studies investigating how chromosomal and hormonal effects influence T cell transcriptomics, the TCR repertoire, and T cell signaling in the context of stroke in both sexes are warranted.
Unconventional T Cells: γδ T Cells
Unconventional innate T cells characterized by an invariant T cell receptor in γδ T cells have become increasingly recognized as a contributor to ischemic damage66,67. These cells produce IL-17 which exacerbates neuroinflammation through induction of G-CSF and other chemokines which foster the recruitment of pro-inflammatory immune cells such as neutrophils into the brain. γδ T cells are a relatively minor subset of T lymphocytes in the peripheral blood (PB), comprising only 1–5% of the circulating lymphocytes68. However, γδ T cells are abundant at barrier sites such as the skin, gut, lung, and reproductive tract and up to 20% of intraepithelial lymphocytes in the human colon express the γδ TCRs69. IL-17 plays a key role in infection and autoimmunity70 and meningeal IL-17 producing γδ T cells induce anxiety-like behavior in mice71. In clinical studies, post-mortem brains from stroke patients had higher IL-17 positive lymphocytes72. Zhang et al. found that γδ T cells and a specific subset of IL-17 producing TH cells (TH17) were significantly increased in the circulation of stroke patients suggesting that these cells traffic from peripheral tissues73. Notably, γδ T cells are highly abundant in the lamina propria of the gut and migrate toward the leptomeninges following stroke74. Several studies have highlighted the importance of commensal-derived signals from the gut microbiome that regulate the migration of IL-17 producing γδ T cells from the gut to the brain that increase post stroke inflammation67,75. Various extrinsic factors including host genetics, environmental conditions, and sex have the potential to shape the composition of the gut microbiome. Future studies are warranted to investigate if sex-specific differences in the host microbiome can influence the migration of IL-17 producing γδ T cells into the brain after stroke.
B cells
B cells are classified as effector cells and are involved in antigen presentation and antibody production. Early studies documented the presence of immunoglobulins within the CSF of human stroke survivors at chronic timepoints after stroke76,77. Selective depletion of B cells led to larger infarct volumes, poorer recovery, and higher overall mortality48. The early infiltration of B cells may be beneficial acutely after stroke by promoting immunosuppression and production of neurotrophins, but detrimental chronically, due to enhanced autoantibody production. However, future studies are essential to investigate the diminishing effect of these protective neurotrophic B cells in the chronic phases of stroke78. Aging and sex can both cause a decline of B cell lymphopoiesis resulting in a decrease in systemic B cells, leading to a reduction in B cell-specific neurotrophic signaling and an augmentation of the detrimental effects of B cell antibody production and cognitive decline79. B Cell Maturation Antigen Protein (BCMA) regulates B cell proliferation, survival, and plasma cell formation80. As sex differences have been documented in antibody production, recent studies have manipulated BCMA to investigate sex specific B cell activation and antibody production in experimental autoimmune encephalomyelitis (EAE) models. Male mice lacking BCMA had significantly worse disease with increased demyelination, higher infiltration of inflammatory T cells and macrophages, and enhanced detrimental neuroinflammation compared to females81. These findings suggest that mechanisms that regulate B cell proliferation, survival, and differentiation are highly sex-specific in their immunogenic potential in the context of neuroinflammation. It has been well documented that IL-10 producing regulatory B cells (Breg) cells play a protective role following ischemic stroke, although sex-specific effects were not investigated82. Females had a significant increase in overall B cell numbers after stroke, but a specific decrease in Breg numbers in the spleen compared to males, possibly due to differential migration into the brain83. Similarly, Benedek et al. showed an estrogen-mediated increase in Breg numbers in the female brain after EAE84. As aged females are disproportionately affected by stroke, future studies investigating how sex and aging alter B cells are needed.
Conclusions
The neuroimmune landscape encompasses tissue-resident microglia which play a role in immune surveillance and in maintenance of the neurovascular unit. In addition, a wide array of immune cell types resides within the leptomeninges, the choroid plexus, and the cerebral endothelia. With ischemia, a culmination of pro-inflammatory events results in the activation of innate and adaptive immunity. Accordingly, mounting evidence in pre-clinical (Table 1) and clinical studies (Table 2) demonstrate that sex differences in immune responses following stroke are evident and contribute to post-stroke outcomes.
Table 1.
Pre-clinical studies examining sex-specific responses in ischemic stroke.
| Study | Species and Strain |
Age | Key Findings |
|---|---|---|---|
| Villapol et al.85 | C57BL6/J Mice | Post-natal day 9 (P9) | Neonatal ischemia models showed that female mice had significantly smaller infarcts compared to males as far as 3 months post-ischemia. Increased activation of microglia/macrophages and higher myeloid-specific brain infiltrates were observed in males. |
| Borgus et al.86 | Sprague-Dawley Rats | Not reported | Sex and region-specific differences in adenosine, a ubiquitous neuromodulator, were seen. |
| Ahnstedt et al.87 | C57BL/6N Mice | 20-22 months | Independent of ischemic damage, aged males had significantly increased peripheral immune responses in the gut and exacerbated neuroinflammation in the sub-acute phase of stroke. |
| Dotson et al.88 | C57BL/6J or PPARα knockout (KO) Mice (C57BL/6J background) | 2-4 months | PPARα, a nuclear receptor transcription factor, plays a key role in perpetuating sex-specific peripheral immune responses following ischemic stroke. |
| Ahnstedt et al.89 | C57BL/6J Mice | Young (3-4 months) and middle-aged (15 - 16 months) | Female mice had elevated levels of pro-inflammatory T cells and suppressed levels of anti-inflammatory Tregs in adipose tissue. The degree of adiposity, a major risk for ischemic stroke, may be phenotypically distinct across sexes. |
| Manwani et al.90 | C57BL/6 Mice | Young (5-6 months), middle aged (14-15 months), and aged (20-22 months) | Heterogenic age- and sex-specific changes were evident in the post-stroke inflammatory milieu. Higher inflammatory macrophages and less lymphocytic infiltration in cycling females. Exacerbated splenic contraction in aged females. Age-specific increases in pro-inflammatory T cells, DCs, and microglia in the brain. |
| Davis et al.91 | Sprague-Dawley Rats | 18 months | Sex-specific effects of leukemia inhibitory factor treatment in mice seen following ischemic stroke. Sex differences in peripheral splenic responses and production of pro-inflammatory cytokine including IL-3 in females. |
| Jackson et al.92 | Wister Rats | 2-3 months | Sex differences in diabetes, a major risk factor for ischemic stroke. Higher pro-inflammatory TH17 in female brains after stroke. Expansion of T cell subsets was sex-dependent and regulated by the sexual dimorphic effects of diabetes. |
| McCullough et al.93 | C57BL/6J XYM and wild-type C57BL/6J Mice | 18-20 months | X chromosome compliment (XX) regulates ischemic damage in aged animals (worse in females). Sex-specific effects of innate immune responses following ischemic stroke. |
| Mirza et al.94 | C57BL/6 Mice | Post-natal day 10 (P10) | Sex-based innate immune response differed with increase microglial activation and infiltration of peripheral leukocytes following HIE in males. |
| Xiong et al.95 | BALB/cJ and IL-4 KO (BALB/c-IL-4tm2Nnt/J) Mice | 2-3 months | Neuroprotective mechanisms after stroke in female mice are mediated by IL-4. Females exhibit activation of anti-inflammatory M2 microglia as well as reduced in infiltration of peripheral leukocytes. |
| Nguyen et al.96 | C57BL/6 and BALB/c Mice | Young (3-4 months) and aged (18 months) | Sex-based differences in cytokines and chemokines within the infarct area at the liquefactive stage of necrosis after stroke. |
| Dotson et al.97 | C57BL/6J Mice | Not reported | Splenectomy prior to experimental stroke abolished sex differences in infarct volume and stroke-induced monocyte and microglia activation. |
| Wang et al.98 | C57BL/6J Mice | 2-3 months | Adoptive transfer of peripheral immune cells (CD4/ CD8/ CD11b), prior to stroke had no effects on post-stroke inflammation. Suggested that other immunoregulatory factors exert sex-based effects of the spleen on post-stroke outcomes. |
| Raval et al.99 | Sprague-Dawley Rats | Young (6-7 months) and retried breeder (9-13 months) | Sex differences in the inflammasome related to the reproductive hormones may contribute to sex-based differences in regulation of inflammatory states, seen in microglia activation and immune cell infiltration in the brain post-stroke. |
| Liu et al.100 | CaMKK β knockout (KO) and CaMK IV KO Mice (C57BL/6J background) | 3-4 months | Pharmacological inhibition of CaMMK downstream signaling exacerbated stroke outcomes as characterized by BBB impairment and activation of pro-inflammatory responses seen only in females following stroke. |
| Li et al.101 | Fischer Rats | 2 months | Female rats exhibited a significantly higher inflammatory response following stroke in infarcts across all severities. However, severe infarcts within the cerebral regions in males were significantly larger compared to female counterparts. |
Table 2.
Clinical studies examining sex-specific responses in ischemic stroke.
| Study | Male/Female Ratio | Mean Age | Comorbidities | Key Findings |
|---|---|---|---|---|
| Ross et al.102 | Males: 96 Females: 96 |
Males: 73.2 Females: 77.3 |
Males: Hypertension: 78 Diabetes: 39 Hyperlipidemia: 57 Smoking: 44 TIA/stroke: 38 Females: Hypertension: 83 Diabetes: 27 Hyperlipidemia: 58 Smoking: 28 TIA/stroke: 44 |
Significant age- and sex-based changes were seen in circulating lymphocytes and myeloid cells after stroke. |
| Xu et al.103 | Males: 10 Females: 10 |
Males: 60 Females: 60 |
Not reported | CCL20, ICAM1, and PTGS2 identified as sex specific targets in ischemic stroke patients. Males showed a CD8+ T cell response; females had a more monocytic response to stroke. |
| Zhu et al.104 | Males: 10 Females: 10 |
Males: 60.2 Females: 60.2 |
Not reported | IL-1α, IL-1β, IL-6, IL-8, CXCL1, CXCL2, CXCL20, CCL4, ICAM1, and PTGS2 were linked to protective effects in female stroke patients and contributed to post-stroke immune and apoptotic sex differences. |
| Lasek-Bal et al.105 | Males: 63 Females: 75 |
Males: 70.4 Females: 75.4 |
Males: Hypertension: 59 Atrial Fibrillation: 21 Carotid Artery Stenosis: 7 Coronary Disease: 33 Diabetes: 23 Hemorrhagic Transformation: 2 Lipid Disorders: 21 Females: Hypertension: 68 Atrial Fibrillation: 23 Carotid Artery Stenosis: 7 Coronary Disease: 38 Diabetes: 26 Hemorrhagic Transformation: 3 Lipid Disorders: 23 |
Sex differences in WBC, platelet counts, CRP, S100B and IL-6 levels seen 24 hours after stroke. |
| Trott et al.106 | Males: 70 Females: 59 |
Males: 62 Females: 62 |
Not reported | A positive correlation was observed in WBC change and absolute NIHSS was seen in female ischemic stroke patients. |
| Aleksandrova et al.107 | Quartiles of FABP4 in plasma Q1 (7.8-10.2 ng/mL): Males: 37.1% Females: 62.9% Q2 (12.0-14.5 ng/mL): Males: 36.6% Females: 63.4% Q3 (15.3-19.5 ng/mL) Males: 36.8% Females: 63.2% Q4 (22.4-32.7 ng/mL) Males: 36.3% Females: 63.7% |
Quartiles (Q) of FABP4 in plasma Q1 Males and Females: 560 Q2 Males and Females: 544 Q3 Males and Females: 544 Q4 Males and Females: 546 |
Quartiles (Q) of FABP4 in plasma Q1 Males and Females Physically active: 21.6% Self-reported Hypertension: 29.6% Current smoker: 25.9% Q2 Males and Females Physically active: 17.1% Self-reported Hypertension: 41.0% Current smoker: 20.4% Q3 Males and Females Physically active: 14.7% Self-reported Hypertension: 52.2% Current smoker: 18.9% Q4 Males and Females Physically active: 11.4% Self-reported Hypertension: 63.7% Current smoker: 16.7% |
FABP4 and T2D were significantly associated with higher risk of stroke in males vs. females. |
| Åkerblom et al.108 | Quartiles (Q) of IL-18 in plasma Q1 (<180.0 ng/L): Males: 2,613 Females: 1,563 Q2 (180-237.0 ng/L) Males: 2,963 Females: 1,211 Q3 (237.0-311.0 ng/L) Males: 3,099 Females: 1019 Q4 (>311.0 ng/L) Males: 3,204 Females: 964 |
Quartiles (Q) of IL-18 in plasma Q1 Males and Females: 63 Q2 Males and Females: 62 Q3 Males and Females: 61 Q4 Males and Females: 61 |
Quartiles (Q) of IL-18 in plasma Q1 Males and Females Smoker: 1338 Hypertension: 2749 Dyslipidemia: 2018 Diabetes: 866 Q2 Males and Females Smoker: 1484 Hypertension: 2670 Dyslipidemia: 2030 Diabetes: 985 Q3 Males and Females Smoker: 1526 Hypertension: 2725 Dyslipidemia: 1917 Diabetes: 1045 Q4 Males and Females Smoker: 1561 Hypertension: 2734 Dyslipidemia: 1886 Diabetes: 1256 |
A positive correlation with circulating plasma levels of IL-18 in ACS men was an independent risk factor for ischemic stroke. |
| Stamova et al.109 |
Vascular Risk Factor Controls: Males: 12 Females: 11 Embolic Stroke: Males: 12 Females: 11 |
Vascular Risk Factor Controls: Males: 56.8 Females: 59 Embolic Stroke: Males: 72.1 Females: 71.3 |
Vascular Risk Factor Controls: Hyperlipidemia: Males: 9 Females: 7 Hypertension: Males: 9 Females: 7 Diabetes: Males: 3 Females: 2 Atrial fibrillation: Males: 0 Females: 0 Cardioembolic Stroke: Hyperlipidemia: Males: 3 Females: 3 Hypertension: Males: 8 Females: 8 Diabetes: Males: 2 Females: 2 Atrial fibrillation: Males: 3 Females: 6 |
Female cardioembolic stroke patients had significantly more differentially expressed genes in the blood linked to cell death and cell-cell inflammatory signaling. Circulatory neutrophil-specific genes were identified as early as 3 hours in females with cardioembolic stroke. |
| Nguyen et al.110 |
Normal controls: Males: 15 Females: 8 Acute stroke: Males: 3 Females: 2 Liquefactive necrosis Males: 8 Females: 8 Encephalomalacia: Males: 2 Females: 7 |
Normal controls: Males and Females: 77.4 Acute stroke: Males and Females: 76.2 Liquefactive necrosis Males and Females: 86.8 Encephalomalacia: Males and Females: 79.8 |
Not reported | A wide array of sex-based cytokines and chemokines were identified within the infarct at the liquefactive stage of necrosis post-stroke. |
| Stamova et al.111 |
Controls: Males: 41 Females: 68 Ischemic Stroke Males: 35 Females: 26 |
Controls: Males: 50.2 Females: 49 Ischemic Stroke Males: 67.1 Females: 67 |
Not reported | Distinct classes of X-chromosome genes were altered across male and female patients after stroke. Male-specific genes regulated pathways involved in cellular movement, development, cell-trafficking, and cell death while female-specific genes included post-translational modification, small-molecule biochemistry, and cell-cell signaling. |
| Tian et al.112 |
Controls: Males: 28 Females: 24 Ischemic stroke: Males: 27 Females: 24 |
Controls: Males: 60.3 Females: 60.6 Ischemic stroke: Males: 63.6 Females: 65.1 |
Controls: Hyperlipidemia: Males: 12 Females: 16 Hypertension: Males: 13 Females: 13 Diabetes: Males: 4 Females: 1 Ischemic stroke: Hyperlipidemia: Males: 10 Females: 6 Hypertension: Males: 17 Females: 9 Diabetes: Males: 6 Females: 5 |
Sex differences in gene patterns in the blood after ischemic stroke. These contributed to sexually dimorphic immune, inflammatory, and cell death responses after stroke. |
Advancements in single-cell sequencing technologies will clarify hormonal and chromosomal effects in the regulation of immune sex-specific transcriptomics and intra-immune cell communication within the CNS. Despite the growing recognition of the importance of sex and gender in diseases such as stroke113, women remain underrepresented in clinical trials114, and females remain underutilized in experimental studies. Pre-clinical studies should utilize both sexes to investigate mechanisms of immune activation and immune cell transcriptomic signatures in the context of ischemic stroke. Unfortunately, despite repeated recommendations, the sex and age of animals used in preclinical research remains underreported115, and little progress has been made116. Clinical studies should include comprehensive documentation of sex-specific co-morbidities (e.g., obesity, cardiovascular disease, diabetes mellitus, etc.) and social factors (isolation and pre-stroke functional status) that may contribute to stroke incidence and outcomes.
Acknowledgements
Sources of Funding
This work was supported by NIH/NINDS R01 NS08779 (Dr. McCullough) and NIH/NINDS R01 NS103592 (Dr. McCullough).
Non-standard Abbreviations and Acronyms
- CI
Confidence Interval
- BBB
Blood Brain Barrier
- TLRs
Toll-Like Receptors
- PRRs
Pattern Recognition Receptors
- PRs
Purinergic Receptors
- DAMPs
Damage-associated Molecular Patterns
- (γδ) T Cells
Gamma-delta T Cells
- TCR
T cells receptors
- CD
Cluster of Differentiation
- IFN
Type I Interferon
- MHC
Major Histocompatibility Complex
- CCL
Chemokine Ligand
- IL
Interleukin
- TNF
Tumor Necrosis Factor
- CXCL
(C-X-C) Motif Ligand
- NETs
Neutrophil Extracellular Traps
- tPA
Tissue-type Plasminogen
- MCP-1
Monocyte Chemoattactant-1
- G-CSF
Granulocyte Colony Stimulating Factor
- TH
T-helper
- DCs
Dendritic Cells
- CNS
Central Nervous System
- CSF
Cerebral Spinal Fluid
- pDCs
Plasmacytoid DCs
- CMV
Cytomegalovirus
- Treg
Regulatory T Cells
- PB
Peripheral Blood
- BCMA
B Cell Maturation Antigen Protein
- EAE
Experimental Autoimmune Encephalomyelitis
- Breg
Regulatory B Cells
- PPAR
Peroxisome proliferator-activated receptor
- CaMMK
Ca2+/calmodulin-dependent protein kinase
- ICAM
Intracellular Adhesion Molecules
- PTGS2
Prostaglandin-endoperoxide synthase 2
- WBC
White Blood Cells
- CRP
C-reactive Protein
- S100B
S100 Calcium Binding Protein B
- NIHSS
NIH Stroke Scale/Score
- FABP4
Fatty Acid Binding Protein 4
- T2D
Type 2 Diabetes
- ACS
Acute Coronary Syndrome
Footnotes
Disclosures
None
References
- 1.Bushnell CD, Chaturvedi S, Gage KR, Herson PS, Hurn PD, Jiménez MC, Kittner SJ, Madsen TE, McCullough LD, McDermott M, et al. Sex differences in stroke: Challenges and opportunities. Journal of Cerebral Blood Flow & Metabolism [Internet]. 2018. [cited 2022 Jan 22];38:2179. Available from: /labs/pmc/articles/PMC6282222/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girijala RL, Sohrabji F, Bush RL. Sex differences in stroke: Review of current knowledge and evidence. Vascular Medicine (United Kingdom) [Internet]. 2017. [cited 2022 Jan 22];22:135–145. Available from: https://journals.sagepub.com/doi/full/10.1177/1358863X16668263 [DOI] [PubMed] [Google Scholar]
- 3.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet neurology [Internet]. 2008. [cited 2022 Jan 22];7:915. Available from: /labs/pmc/articles/PMC2665267/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahnstedt H, Patrizz A, Chauhan A, Roy-O’Reilly M, Furr JW, Spychala MS, D’Aigle J, Blixt FW, Zhu L, Bravo Alegria J, et al. Sex differences in T cell immune responses, gut permeability and outcome after ischemic stroke in aged mice. Brain, Behavior, and Immunity. 2020;87:556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekker MS, de Leeuw FE. Higher incidence of ischemic stroke in young women than in young men: Mind the gap. Stroke [Internet]. 2020. [cited 2022 Jan 22];3195–3196. Available from: https://www.ahajournals.org/doi/abs/10.1161/STROKEAHA.120.032062 [DOI] [PubMed] [Google Scholar]
- 6.Leppert MH, Burke JF, Lisabeth LD, Madsen TE, Kleindorfer DO, Sillau S, Schwamm LH, Daugherty SL, Bradley CJ, Ho PM, et al. Systematic Review of Sex Differences in Ischemic Strokes Among Young Adults: Are Young Women Disproportionately at Risk? Stroke [Internet]. 2022. [cited 2022 Feb 23];53:319–327. Available from: https://www.ahajournals.org/doi/abs/10.1161/STROKEAHA.121.037117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahnstedt H, McCullough LD. The impact of sex and age on T cell immunity and ischemic stroke outcomes. Cellular Immunology. 2019;345:103960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCullough LD, Mirza MA, Xu Y, Bentivegna K, Steffens EB, Ritzel R, Liu F. Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. Aging [Internet]. 2016. [cited 2022 Jan 22];8:1432–1441. Available from: https://www.aging-us.com/article/100997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi S, al Mamun A, Ngwa C, Romana S, Ritzel R, Arnold AP, McCullough LD, Liu F. X chromosome escapee genes are involved in ischemic sexual dimorphism through epigenetic modification of inflammatory signals. Journal of Neuroinflammation [Internet]. 2021. [cited 2022 Feb 4];18:1–16. Available from: https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-021-02120-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teissier T, Boulanger E, Cox LS. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells [Internet]. 2022. [cited 2022 Feb 23];11:359. Available from: https://pubmed.ncbi.nlm.nih.gov/35159168/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamova B, Jickling GC, Ander BP, Zhan X, Liu DZ, Turner R, Ho C, Khoury JC, Bushnell C, Pancioli A, et al. Gene Expression in Peripheral Immune Cells following Cardioembolic Stroke Is Sexually Dimorphic. PLOS ONE [Internet]. 2014. [cited 2022 Jan 22];9:e102550. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0102550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dotson AL, Offner H. Sex Differences in the Immune Response to Experimental Stroke: Implications for Translational Research. Journal of neuroscience research [Internet]. 2017. [cited 2022 Jan 22];95:437. Available from: /labs/pmc/articles/PMC5120653/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kam A, Li MK, Razmovski-Naumovski V, Nammi S, Chan K, Li Y, Li GQ. The protective effects of natural products on blood-brain barrier breakdown. Current medicinal chemistry [Internet]. 2012. [cited 2022 Jan 22];19:1830–1845. Available from: https://pubmed.ncbi.nlm.nih.gov/22376038/ [DOI] [PubMed] [Google Scholar]
- 14.Prakash R, Carmichael ST. Blood–brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Current opinion in neurology [Internet]. 2015. [cited 2022 Jan 22];28:556. Available from: /labs/pmc/articles/PMC5267616/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nian K, Harding IC, Herman IM, Ebong EE. Blood-Brain Barrier Damage in Ischemic Stroke and Its Regulation by Endothelial Mechanotransduction. Frontiers in Physiology. 2020;11:1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber CM, Clyne AM. Sex differences in the blood–brain barrier and neurodegenerative diseases. APL Bioengineering [Internet]. 2021. [cited 2022 Feb 6];5. Available from: /labs/pmc/articles/PMC7968933/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Innate Immunity. 2002. [cited 2022 Jan 22];Available from: https://www.ncbi.nlm.nih.gov/books/NBK26846/ [Google Scholar]
- 18.Jentho E, Weis S. DAMPs and Innate Immune Training. Frontiers in Immunology. 2021;12:3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenzie DR, Hart R, Bah N, Ushakov DS, Muñoz-Ruiz M, Feederle R, Hayday AC. Normality sensing licenses local T cells for innate-like tissue surveillance. Nature Immunology 2022 23:3 [Internet]. 2022. [cited 2022 Mar 8];23:411–422. Available from: https://www.nature.com/articles/s41590-021-01124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn E Why autoimmunity is most common in women. Nature. 2021;595:S51–S53. [Google Scholar]
- 21.dela Justina V, Giachini FR, Sullivan JC, Webb RC. Toll-Like Receptors Contribute to Sex Differences in Blood Pressure Regulation. Journal of cardiovascular pharmacology [Internet]. 2020. [cited 2022 Mar 8];76:255. Available from: /labs/pmc/articles/PMC7751064/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCullough LD, Mirza MA, Xu Y, Bentivegna K, Steffens EB, Ritzel R, Liu F. Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. Aging (Albany NY) [Internet]. 2016. [cited 2022 Feb 14];8:1432. Available from: /labs/pmc/articles/PMC4993340/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han J, Fan Y, Zhou K, Blomgren K, Harris RA. Uncovering sex differences of rodent microglia. Journal of Neuroinflammation [Internet]. 2021. [cited 2022 Jan 22];18:1–11. Available from: https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-021-02124-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barton EA, Baker C, Leigh Leasure J. Investigation of Sex Differences in the Microglial Response to Binge Ethanol and Exercise. Brain Sciences [Internet]. 2017. [cited 2022 Jan 22];7. Available from: /labs/pmc/articles/PMC5664066/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osborne BF, Turano A, Caulfield JI, Schwarz JM. Sex- and region-specific differences in microglia phenotype and characterization of the peripheral immune response following early-life infection in neonatal male and female rats. Neuroscience letters [Internet]. 2019. [cited 2022 Jan 22];692:1. Available from: /labs/pmc/articles/PMC6351186/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerr N, Dietrich DW, Bramlett HM, Raval AP. Sexually dimorphic microglia and ischemic stroke. CNS Neuroscience & Therapeutics [Internet]. 2019. [cited 2022 Jan 22];25:1308. Available from: /labs/pmc/articles/PMC6887716/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends in endocrinology and metabolism: TEM [Internet]. 2003. [cited 2022 Feb 23];14:228–235. Available from: https://pubmed.ncbi.nlm.nih.gov/12826329/ [DOI] [PubMed] [Google Scholar]
- 28.Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Experimental neurology [Internet]. 2013. [cited 2022 Feb 23];249:120–131. Available from: https://pubmed.ncbi.nlm.nih.gov/23994069/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castelo-Branco C, Soveral I. The immune system and aging: a review. Gynecol. Endocrinol 2014;30:16–22. [DOI] [PubMed] [Google Scholar]
- 30.Márquez EJ, han Chung C, Marches R, Rossi RJ, Nehar-Belaid D, Eroglu A, Mellert DJ, Kuchel GA, Banchereau J, Ucar D. Sexual-dimorphism in human immune system aging. Nature Communications 2020 11:1 [Internet]. 2020. [cited 2022 Jan 22];11:1–17. Available from: https://www.nature.com/articles/s41467-020-14396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doran SJ, Ritzel RM, Glaser EP, Henry RJ, Faden AI, Loane DJ. Sex differences in acute neuroinflammation after experimental traumatic brain injury are mediated by infiltrating myeloid cells. Journal of Neurotrauma [Internet]. 2019. [cited 2022 Jan 22];36:1040–1053. Available from: https://www.liebertpub.com/doi/abs/10.1089/neu.2018.6019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lenz KM, McCarthy MM. A Starring Role for Microglia in Brain Sex Differences. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry [Internet]. 2015. [cited 2022 Jan 22];21:306. Available from: /labs/pmc/articles/PMC5742269/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hui CW, Vecchiarelli HA, Gervais É, Luo X, Michaud F, Scheefhals L, Bisht K, Sharma K, Topolnik L, Tremblay MÈ. Sex Differences of Microglia and Synapses in the Hippocampal Dentate Gyrus of Adult Mouse Offspring Exposed to Maternal Immune Activation. Frontiers in Cellular Neuroscience. 2020;14:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue J, Huang W, Chen X, Li Q, Cai Z, Yu T, Shao B. Neutrophil-to-Lymphocyte Ratio Is a Prognostic Marker in Acute Ischemic Stroke. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association [Internet]. 2017. [cited 2022 Jan 22];26:650–657. Available from: https://pubmed.ncbi.nlm.nih.gov/27955949/ [DOI] [PubMed] [Google Scholar]
- 35.Roy-O’Reilly MA, Ahnstedt H, Spychala MS, Munshi Y, Aronowski J, Sansing LH, McCullough LD. Aging exacerbates neutrophil pathogenicity in ischemic stroke. Aging (Albany NY) [Internet]. 2020. [cited 2022 Jan 22];12:436. Available from: /labs/pmc/articles/PMC6977697/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perl M, Chung CS, Perl U, Biffl WL, Cioffi WG, Ayala A. Beneficial versus detrimental effects of neutrophils are determined by the nature of the insult. Journal of the American College of Surgeons [Internet]. 2007. [cited 2022 Jan 22];204:840–852. Available from: https://pubmed.ncbi.nlm.nih.gov/17481496/ [DOI] [PubMed] [Google Scholar]
- 37.Jickling GC, Liu DZ, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. Journal of Cerebral Blood Flow & Metabolism [Internet]. 2015. [cited 2022 Jan 22];35:888. Available from: /labs/pmc/articles/PMC4640255/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laridan E, Denorme F, Desender L, François O, Andersson T, Deckmyn H, Vanhoorelbeke K, de Meyer SF. Neutrophil extracellular traps in ischemic stroke thrombi. Annals of neurology [Internet]. 2017. [cited 2022 Jan 22];82:223–232. Available from: https://pubmed.ncbi.nlm.nih.gov/28696508/ [DOI] [PubMed] [Google Scholar]
- 39.Papayannopoulos V Neutrophil extracellular traps in immunity and disease. Nature Reviews Immunology 2017 18:2 [Internet]. 2017. [cited 2022 Mar 17];18:134–147. Available from: https://www.nature.com/articles/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- 40.Yuan XZ. Sex differences in neutrophil extracellular trap formation. 2018. [cited 2022 Feb 15];Available from: https://tspace.library.utoronto.ca/handle/1807/91738
- 41.Gupta S, Nakabo S, Blanco LP, O’Neil LJ, Wigerblad G, Goel RR, Mistry P, Jiang K, Carmona-Rivera C, Chan DW, et al. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proceedings of the National Academy of Sciences of the United States of America [Internet]. 2020. [cited 2022 Jan 22];117:16481–16491. Available from: https://www.pnas.org/content/117/28/16481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamova B, Jickling GC, Ander BP, Zhan X, Liu DZ, Turner R, Ho C, Khoury JC, Bushnell C, Pancioli A, et al. Gene Expression in Peripheral Immune Cells following Cardioembolic Stroke Is Sexually Dimorphic. PLoS ONE [Internet]. 2014. [cited 2022 Jan 22];9. Available from: /labs/pmc/articles/PMC4103830/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahnstedt H, Patrizz A, Roy-O’Reilly M, Spychala M, Bravo Alegria J, Chauhan A, McCullough LD. Abstract TMP36: Sex Differences in Neutrophil-T Cell Immune Responses and Outcome After Ischemic Stroke in Aged Mice. Stroke [Internet]. 2018. [cited 2022 Jan 22];49. Available from: https://www.researchgate.net/publication/328157225_Abstract_TMP36_Sex_Differences_in_Neutrophil-T_Cell_Immune_Responses_and_Outcome_After_Ischemic_Stroke_in_Aged_Mice [Google Scholar]
- 44.Qin X, Akter F, Qin L, Cheng J, Guo M, Yao S, Jian Z, Liu R, Wu S. Adaptive Immunity Regulation and Cerebral Ischemia. Frontiers in Immunology. 2020;11:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu X, Zhu J. CD4 T Helper Cell Subsets and Related Human Immunological Disorders. International journal of molecular sciences [Internet]. 2020. [cited 2022 Jan 22];21:1–26. Available from: https://pubmed.ncbi.nlm.nih.gov/33126494/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shekhar S, Milling S, Yang X. Migration of γδ T cells in steady-state conditions. Veterinary immunology and immunopathology [Internet]. 2012. [cited 2022 Jan 22];147:1–5. Available from: https://pubmed.ncbi.nlm.nih.gov/22520944/ [DOI] [PubMed] [Google Scholar]
- 47.Wienands J, Engels N. The Memory Function of the B Cell Antigen Receptor. Current topics in microbiology and immunology [Internet]. 2016. [cited 2022 Jan 22];393:107–121. Available from: https://pubmed.ncbi.nlm.nih.gov/26362935/ [DOI] [PubMed] [Google Scholar]
- 48.Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism [Internet]. 2007. [cited 2022 Jan 22];27:1798. Available from: /labs/pmc/articles/PMC2592689/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdullah M, Chai PS, Chong MY, Tohit ERM, Ramasamy R, Pei CP, Vidyadaran S. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cellular immunology [Internet]. 2012. [cited 2022 Jan 22];272:214–219. Available from: https://pubmed.ncbi.nlm.nih.gov/22078320/ [DOI] [PubMed] [Google Scholar]
- 50.Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine-induced humoral immunity. Seminars in Immunopathology [Internet]. 2019. [cited 2022 Jan 22];41:239. Available from: /labs/pmc/articles/PMC6373179/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature [Internet]. 1998. [cited 2022 Jan 22];392:245–252. Available from: https://pubmed.ncbi.nlm.nih.gov/9521319/ [DOI] [PubMed] [Google Scholar]
- 52.Pashenkov M, Huang YM, Kostulas V, Haglund M, Söderström M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain [Internet]. 2001. [cited 2022 Jan 22];124:480–492. Available from: https://academic.oup.com/brain/article/124/3/480/334334 [DOI] [PubMed] [Google Scholar]
- 53.Dai H, Thomson AW, Rogers NM. Dendritic Cells as Sensors, Mediators, and Regulators of Ischemic Injury. Frontiers in Immunology [Internet]. 2019. [cited 2022 Jan 22];10. Available from: /labs/pmc/articles/PMC6803430/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griesbeck M, Ziegler S, Laffont S, Smith N, Chauveau L, Tomezsko P, Sharei A, Kourjian G, Porichis F, Hart M, et al. Sex Differences in Plasmacytoid Dendritic Cell Levels of IRF5 Drive Higher IFN- Production in Women. The Journal of Immunology [Internet]. 2015. [cited 2022 Feb 15];195:5327–5336. Available from: /labs/pmc/articles/PMC4654231/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.dela Justina V, Giachini FR, Sullivan JC, Webb RC. Toll-Like Receptors Contribute to Sex Differences in Blood Pressure Regulation. Journal of cardiovascular pharmacology [Internet]. 2020. [cited 2022 Jan 22];76:255. Available from: /labs/pmc/articles/PMC7751064/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischer H-G, Reichmann G Brain dendritic cells and macrophages/microglia in central nervous system inflammation. Journal of immunology (Baltimore, Md. : 1950) [Internet]. 2001. [cited 2022 Jan 22];166:2717–2726. Available from: https://pubmed.ncbi.nlm.nih.gov/11160337/ [DOI] [PubMed] [Google Scholar]
- 57.Honarpisheh P, Blixt FW, Blasco Conesa MP, Won W, d’Aigle J, Munshi Y, Hudobenko J, Furr JW, Mobley A, Lee J, et al. Peripherally-sourced myeloid antigen presenting cells increase with advanced aging. Brain, behavior, and immunity [Internet]. 2020. [cited 2022 Jan 22];90:235–247. Available from: https://pubmed.ncbi.nlm.nih.gov/32861719/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tai Y, Wang Q, Korner H, Zhang L, Wei W. Molecular Mechanisms of T Cells Activation by Dendritic Cells in Autoimmune Diseases. Frontiers in Pharmacology [Internet]. 2018. [cited 2022 Jan 22];9. Available from: /labs/pmc/articles/PMC6028573/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, et al. Sex differences in the TLR-mediated response of pDCs to HIV-1 are associated with higher immune activation in infected women. Nature medicine [Internet]. 2009. [cited 2022 Jan 22];15:955. Available from: /labs/pmc/articles/PMC2821111/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sohn E Why autoimmunity is most common in women. Nature. 2021;595:S51–S53. [Google Scholar]
- 61.Salas-Perdomo A, Miró-Mur F, Urra X, Justicia C, Gallizioli M, Zhao Y, Brait VH, Laredo C, Tudela R, Hidalgo A, et al. T Cells Prevent Hemorrhagic Transformation in Ischemic Stroke by P-Selectin Binding. Arteriosclerosis, thrombosis, and vascular biology [Internet]. 2018. [cited 2022 Jan 22];38:1761–1771. Available from: https://pubmed.ncbi.nlm.nih.gov/29903733/ [DOI] [PubMed] [Google Scholar]
- 62.Gremmel T, Kopp CW, Eichelberger B, Koppensteiner R, Panzer S. Sex differences of leukocyte-platelet interactions and on-treatment platelet reactivity in patients with atherosclerosis. Atherosclerosis [Internet]. 2014. [cited 2022 Jan 22];237:692–695. Available from: https://pubmed.ncbi.nlm.nih.gov/25463107/ [DOI] [PubMed] [Google Scholar]
- 63.Conway SE, Roy-O’Reilly M, Friedler B, Staff I, Fortunato G, McCullough LD. Sex differences and the role of IL-10 in ischemic stroke recovery. Biology of Sex Differences [Internet]. 2015. [cited 2022 Jan 22];6. Available from: /labs/pmc/articles/PMC4601121/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. Journal of immunology (Baltimore, Md. : 1950) [Internet]. 2002. [cited 2022 Jan 22];168:1968–1977. Available from: https://pubmed.ncbi.nlm.nih.gov/11823533/ [DOI] [PubMed] [Google Scholar]
- 65.Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Translational stroke research [Internet]. 2013. [cited 2022 Jan 22];4:554–563. Available from: https://pubmed.ncbi.nlm.nih.gov/24187596/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faura J, Bustamante A, Miró-Mur F, Montaner J. Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. Journal of Neuroinflammation. 2021;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nature Medicine 2016 22:5 [Internet]. 2016. [cited 2022 Jan 22];22:516–523. Available from: https://www.nature.com/articles/nm.4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wo J, Zhang F, Li Z, Sun C, Zhang W, Sun G. The Role of Gamma-Delta T Cells in Diseases of the Central Nervous System. Frontiers in Immunology. 2020;11:2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCarthy NE, Eberl M. Human γδ T-cell control of mucosal immunity and inflammation. Frontiers in Immunology. 2018;9:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.AL-Ayadhi LY, Mostafa GA. Elevated serum levels of interleukin-17A in children with autism. Journal of Neuroinflammation [Internet]. 2012. [cited 2022 Jan 22];9:158. Available from: /labs/pmc/articles/PMC3410815/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alves de Lima K, Rustenhoven J, da Mesquita S, Wall M, Salvador AF, Smirnov I, Martelossi Cebinelli G, Mamuladze T, Baker W, Papadopoulos Z, et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nature Immunology 2020 21:11 [Internet]. 2020. [cited 2022 Jan 22];21:1421–1429. Available from: https://www.nature.com/articles/s41590-020-0776-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Milovanovic J, Arsenijevic A, Stojanovic B, Kanjevac T, Arsenijevic D, Radosavljevic G, Milovanovic M, Arsenijevic N. Interleukin-17 in Chronic Inflammatory Neurological Diseases. Frontiers in Immunology [Internet]. 2020. [cited 2022 Jan 22];11. Available from: /labs/pmc/articles/PMC7283538/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Q, Liao Y, Liu Z, Dai Y, Li Y, Li Y, Tang Y. Interleukin-17 and ischaemic stroke. Immunology [Internet]. 2021. [cited 2022 Jan 22];162:179–193. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/imm.13265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benakis C, Llovera G, Liesz A. The meningeal and choroidal infiltration routes for leukocytes in stroke. Therapeutic advances in neurological disorders [Internet]. 2018. [cited 2022 Jan 22];11:1756286418783708. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29977343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee J, D’Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, Hassan A, Graf J, Petrosino J, Putluri N, et al. Gut Microbiota-Derived Short-Chain Fatty Acids Promote Poststroke Recovery in Aged Mice. Circulation Research [Internet]. 2020. [cited 2022 Jan 22];127:453–465. Available from: https://www.ahajournals.org/doi/abs/10.1161/CIRCRESAHA.119.316448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roström B, Link H. Oligoclonal immunoglobulins in cerebrospinal fluid in acute cerebrovascular disease. Neurology [Internet]. 1981. [cited 2022 Jan 22];31:590–596. Available from: https://pubmed.ncbi.nlm.nih.gov/6785663/ [DOI] [PubMed] [Google Scholar]
- 77.Tsementzis SA, Chao SW, Hitchcock ER, Gill JS, Beevers DG. Oligoclonal immunoglobulin G in acute subarachnoid hemorrhage and stroke. Neurology [Internet]. 1986. [cited 2022 Jan 22];36:395–397. Available from: https://pubmed.ncbi.nlm.nih.gov/3951707/ [DOI] [PubMed] [Google Scholar]
- 78.Ortega SB, Torres VO, Latchney SE, Whoolery CW, Noorbhai IZ, Poinsatte K, Selvaraj UM, Benson MA, Meeuwissen AJM, Plautz EJ, et al. B cells migrate into remote brain areas and support neurogenesis and functional recovery after focal stroke in mice. Proceedings of the National Academy of Sciences of the United States of America [Internet]. 2020. [cited 2022 Jan 22];117:4983–4993. Available from: https://www.pnas.org/content/117/9/4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Engler-Chiurazzi EB, Monaghan KL, Wan ECK, Ren X. Role of B cells and the aging brain in stroke recovery and treatment. GeroScience [Internet]. 2020. [cited 2022 Jan 22];42:1199. Available from: /labs/pmc/articles/PMC7525651/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shah N, Chari A, Scott E, Mezzi K, Usmani SZ. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia 2020 34:4 [Internet]. 2020. [cited 2022 Mar 9];34:985–1005. Available from: https://www.nature.com/articles/s41375-020-0734-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumar G, Ko RM, Axtell RC. Deficiency in B Cell Maturation Antigen reveals gender differences in Experimental Autoimmune Encephalomyelitis. The Journal of Immunology. 2019;202. [Google Scholar]
- 82.Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H. Regulatory B Cells Limit CNS Inflammation and Neurologic Deficits in Murine Experimental Stroke. Journal of Neuroscience [Internet]. 2011. [cited 2022 Jan 22];31:8556–8563. Available from: https://www.jneurosci.org/content/31/23/8556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seifert HA, Benedek G, Liang J, Nguyen H, Kent G, Vandenbark AA, Saugstad JA, Offner H. Sex Differences in Regulatory Cells in Experimental Stroke. Cellular immunology [Internet]. 2017. [cited 2022 Jan 22];318:49. Available from: /labs/pmc/articles/PMC5551457/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benedek G, Zhang J, Bodhankar S, Nguyen H, Kent G, Jordan K, Manning D, Vandenbark AA, Offner H. Estrogen induces multiple regulatory B cell subtypes and promotes M2 microglia and neuroprotection during experimental autoimmune encephalomyelitis. Journal of neuroimmunology [Internet]. 2016. [cited 2022 Jan 22];293:45. Available from: /labs/pmc/articles/PMC4824954/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Villapol S, Faivre V, Joshi P, Moretti R, Besson VC, Charriaut-Marlangue C. Early Sex Differences in the Immune-Inflammatory Responses to Neonatal Ischemic Stroke. International Journal of Molecular Sciences [Internet]. 2019. [cited 2022 Feb 15];20. Available from: /labs/pmc/articles/PMC6695584/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Borgus JR, Puthongkham P, Venton BJ. Complex Sex and Estrous Cycle Differences in Spontaneous Transient Adenosine. Journal of neurochemistry [Internet]. 2020. [cited 2022 Feb 15];153:216. Available from: /labs/pmc/articles/PMC7310595/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahnstedt H, Patrizz A, Chauhan A, Roy-O’Reilly M, Furr JW, Spychala MS, D’Aigle J, Blixt FW, Zhu L, Bravo Alegria J, et al. Sex differences in T cell immune responses, gut permeability and outcome after ischemic stroke in aged mice. Brain, behavior, and immunity [Internet]. 2020. [cited 2022 Feb 15];87:556–567. Available from: https://pubmed.ncbi.nlm.nih.gov/32058038/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dotson AL, Wang J, Liang J, Nguyen H, Manning D, Saugstad JA, Offner H. Loss of PPARα perpetuates sex differences in stroke reflected by peripheral immune mechanisms. Metabolic brain disease [Internet]. 2016. [cited 2022 Feb 15];31:683–692. Available from: https://pubmed.ncbi.nlm.nih.gov/26868919/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahnstedt H, Roy-O’Reilly M, Spychala MS, Mobley AS, Bravo-Alegria J, Chauhan A, Aronowski J, Marrelli SP, McCullough LD. Sex Differences in Adipose Tissue CD8 + T Cells and Regulatory T Cells in Middle-Aged Mice. Frontiers in immunology [Internet]. 2018. [cited 2022 Feb 15];9. Available from: https://pubmed.ncbi.nlm.nih.gov/29670627/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Experimental neurology [Internet]. 2013. [cited 2022 Feb 15];249:120–131. Available from: https://pubmed.ncbi.nlm.nih.gov/23994069/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davis SM, Collier LA, Messmer SJ, Pennypacker KR. The Poststroke Peripheral Immune Response Is Differentially Regulated by Leukemia Inhibitory Factor in Aged Male and Female Rodents. Oxidative medicine and cellular longevity [Internet]. 2020. [cited 2022 Feb 15];2020. Available from: https://pubmed.ncbi.nlm.nih.gov/33376583/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jackson L, Li W, Abdul Y, Dong G, Baban B, Ergul A. Diabetic Stroke Promotes a Sexually Dimorphic Expansion of T Cells. Neuromolecular medicine [Internet]. 2019. [cited 2022 Feb 15];21:445–453. Available from: https://pubmed.ncbi.nlm.nih.gov/31197651/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCullough LD, Mirza MA, Xu Y, Bentivegna K, Steffens EB, Ritzel R, Liu F. Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. Aging [Internet]. 2016. [cited 2022 Feb 15];8:1432–1441. Available from: https://pubmed.ncbi.nlm.nih.gov/27405096/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mirza MA, Ritzel R, Xu Y, McCullough LD, Liu F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. Journal of neuroinflammation [Internet]. 2015. [cited 2022 Feb 15];12. Available from: https://pubmed.ncbi.nlm.nih.gov/25889641/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xiong X, Xu L, Wei L, White RE, Ouyang YB, Giffard RG. IL-4 Is Required for Sex Differences in Vulnerability to Focal Ischemia in Mice. Stroke [Internet]. 2015. [cited 2022 Feb 15];46:2271–2276. Available from: https://pubmed.ncbi.nlm.nih.gov/26130091/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nguyen TV v., Frye JB, Zbesko JC, Stepanovic K, Hayes M, Urzua A, Serrano G, Beach TG, Doyle KP. Multiplex immunoassay characterization and species comparison of inflammation in acute and non-acute ischemic infarcts in human and mouse brain tissue. Acta neuropathologica communications [Internet]. 2016. [cited 2022 Feb 15];4:100. Available from: https://pubmed.ncbi.nlm.nih.gov/27600707/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dotson AL, Wang J, Saugstad J, Murphy SJ, Offner H. Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. Journal of neuroimmunology [Internet]. 2015. [cited 2022 Feb 15];278:289–298. Available from: https://pubmed.ncbi.nlm.nih.gov/25434281/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang J, Dotson AL, Murphy SJ., Offner H, Saugstad JA. Adoptive transfer of immune subsets prior to MCAO does not exacerbate stroke outcome in splenectomized mice. Journal of systems and integrative neuroscience [Internet]. 2015. [cited 2022 Feb 15];1:20–28. Available from: https://pubmed.ncbi.nlm.nih.gov/26634148/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raval AP, Martinez CC, Mejias NH, de Rivero Vaccari JP. Sexual dimorphism in inflammasome-containing extracellular vesicles and the regulation of innate immunity in the brain of reproductive senescent females. Neurochemistry international [Internet]. 2019. [cited 2022 Feb 15];127:29–37. Available from: https://pubmed.ncbi.nlm.nih.gov/30500463/ [DOI] [PubMed] [Google Scholar]
- 100.Liu L, McCullough L, Li J. Genetic deletion of calcium/calmodulin-dependent protein kinase kinase β (CaMKK β) or CaMK IV exacerbates stroke outcomes in ovariectomized (OVXed) female mice. BMC neuroscience [Internet]. 2014. [cited 2022 Feb 15];15. Available from: https://pubmed.ncbi.nlm.nih.gov/25331941/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li K, Futrell N, Tovar JS, Wang LJC, Wang DZ, Schultz LR. Gender influences the magnitude of the inflammatory response within embolic cerebral infarcts in young rats. Stroke [Internet]. 1996. [cited 2022 Feb 15];27:498–503. Available from: https://pubmed.ncbi.nlm.nih.gov/8610320/ [DOI] [PubMed] [Google Scholar]
- 102.Ross AM, Lee CS, Lutsep H. Influence of Gender and Age on the Peripheral Immune Response in Stroke. The Journal of cardiovascular nursing [Internet]. 2016. [cited 2022 Feb 15];31:331–335. Available from: https://pubmed.ncbi.nlm.nih.gov/25774839/ [DOI] [PubMed] [Google Scholar]
- 103.Xu H, Ge Y, Liu Y, Zheng Y, Hu R, Ren C, Liu Q. Identification of the key genes and immune infiltrating cells determined by sex differences in ischaemic stroke through co-expression network module. IET systems biology [Internet]. 2022. [cited 2022 Feb 15];16:28–41. Available from: https://pubmed.ncbi.nlm.nih.gov/34792838/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu W, Nan Y, Wang S, Liu W. Bioinformatics Analysis of Gene Expression Profiles of Sex Differences in Ischemic Stroke. BioMed research international [Internet]. 2019. [cited 2022 Feb 15];2019. Available from: https://pubmed.ncbi.nlm.nih.gov/31183363/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lasek-Bal A, Jedrzejowska-Szypulka H, Student S, Warsz-Wianecka A, Zareba K, Puz P, Bal W, Pawletko K, Lewin-Kowalik J. The importance of selected markers of inflammation and blood-brain barrier damage for short-term ischemic stroke prognosis. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society [Internet]. 2019. [cited 2022 Feb 15];70. Available from: https://pubmed.ncbi.nlm.nih.gov/31356182/ [DOI] [PubMed] [Google Scholar]
- 106.Trott S, Vsevolozhskaya O, Pennypacker K, Alhajeri A, Fraser JF. Immune System Activation in Perioperative Thrombectomy Patients: Preliminary Retrospective Study. World neurosurgery [Internet]. 2019. [cited 2022 Feb 15];128:e966–e969. Available from: https://pubmed.ncbi.nlm.nih.gov/31100531/ [DOI] [PubMed] [Google Scholar]
- 107.Aleksandrova K, Drogan D, Weikert C, Schulze MB, Fritsche A, Boeing H, Pischon T. Fatty Acid-Binding Protein 4 and Risk of Type 2 Diabetes, Myocardial Infarction, and Stroke: A Prospective Cohort Study. The Journal of clinical endocrinology and metabolism [Internet]. 2019. [cited 2022 Feb 15];104:5991–6002. Available from: https://pubmed.ncbi.nlm.nih.gov/31211381/ [DOI] [PubMed] [Google Scholar]
- 108.Åkerblom A, James SK, Lakic TG, Becker RC, Cannon CP, Steg PG, Himmelmann A, Katus HA, Storey RF, Wallentin L, et al. Interleukin-18 in patients with acute coronary syndromes. Clinical cardiology [Internet]. 2019. [cited 2022 Feb 15];42:1202–1209. Available from: https://pubmed.ncbi.nlm.nih.gov/31596518/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stamova B, Jickling GC, Ander BP, Zhan X, Liu DZ, Turner R, Ho C, Khoury JC, Bushnell C, Pancioli A, et al. Gene expression in peripheral immune cells following cardioembolic stroke is sexually dimorphic. PloS one [Internet]. 2014. [cited 2022 Feb 15];9. Available from: https://pubmed.ncbi.nlm.nih.gov/25036109/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nguyen TV v., Frye JB, Zbesko JC, Stepanovic K, Hayes M, Urzua A, Serrano G, Beach TG, Doyle KP. Multiplex immunoassay characterization and species comparison of inflammation in acute and non-acute ischemic infarcts in human and mouse brain tissue. Acta neuropathologica communications [Internet]. 2016. [cited 2022 Feb 15];4:100. Available from: https://pubmed.ncbi.nlm.nih.gov/27600707/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stamova B, Tian Y, Jickling G, Bushnell C, Zhan X, Liu D, Ander BP, Verro P, Patel V, Pevec WC, et al. The X-chromosome has a different pattern of gene expression in women compared with men with ischemic stroke. Stroke [Internet]. 2012. [cited 2022 Feb 15];43:326–334. Available from: https://pubmed.ncbi.nlm.nih.gov/22052522/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tian Y, Stamova B, Jickling GC, Liu D, Ander BP, Bushnell C, Zhan X, Davis RR, Verro P, Pevec WC, et al. Effects of gender on gene expression in the blood of ischemic stroke patients. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism [Internet]. 2012. [cited 2022 Feb 15];32:780–791. Available from: https://pubmed.ncbi.nlm.nih.gov/22167233/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, de Vries GJ, Epperson CN, Govindan R, Klein SL, et al. Sex and gender: modifiers of health, disease, and medicine. The Lancet [Internet]. 2020. [cited 2022 Mar 18];396:565–582. Available from: http://www.thelancet.com/article/S0140673620315610/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carcel C, Reeves M. Under-Enrollment of Women in Stroke Clinical Trials: What Are the Causes and What Should Be Done About It? Stroke [Internet]. 2021. [cited 2022 Mar 18];52:452–457. Available from: https://pubmed.ncbi.nlm.nih.gov/33493049/ [DOI] [PubMed] [Google Scholar]
- 115.du Sert NP, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLOS Biology [Internet]. 2020. [cited 2022 Mar 18];18:e3000410. Available from: https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Daniel Ramirez F, Motazedian P, Jung RG, di Santo P, Macdonald Z, Simard T, Clancy AA, Russo JJ, Welch V, Wells GA, et al. Sex Bias Is Increasingly Prevalent in Preclinical Cardiovascular Research: Implications for Translational Medicine and Health Equity for Women: A Systematic Assessment of Leading Cardiovascular Journals over a 10-Year Period. Circulation [Internet]. 2017. [cited 2022 Mar 18];135:625–626. Available from: https://www.ahajournals.org/doi/abs/10.1161/CIRCULATIONAHA.116.026668 [DOI] [PubMed] [Google Scholar]