Abstract
Background:
Comorbidities influence the outcomes of injured patients, yet a lack of consensus exists regarding how to quantify that association. This study details the development and internal validation of a trauma comorbidity index (TCI) designed for use with trauma registry data and compares its performance to other existing measures to estimate the association between comorbidities and mortality.
Methods:
Indiana state trauma registry data (2013–2015) were used to compare the TCI with the Charlson and Elixhauser comorbidity indices, a count of comorbidities, and comorbidities as separate variables. The TCI approach utilized a randomly selected training cohort and was internally validated in a distinct testing cohort. The C-statistic of the adjusted models was tested using each comorbidity measure in the testing cohort to assess model discrimination. C-statistics were compared using a Wald test, and stratified analyses were performed based on predicted risk of mortality. Multiple imputation was used to address missing data.
Results:
The study included 84,903 patients (50% each in training and testing cohorts). The Indiana TCI model demonstrated no significant difference between testing and training cohorts (p = 0.33). It produced a C-statistic of 0.924 in the testing cohort, which was significantly greater than that of models using the other indices (p < 0.05). The C-statistics of models using the Indiana TCI and the inclusion of comorbidities as separate variables—the method used by the American College of Surgeons Trauma Quality Improvement Program—were comparable (p = 0.11) but use of the TCI approach reduced the number of comorbidity-related variables in the mortality model from 19 to one.
Conclusions:
When examining trauma mortality, the TCI approach using Indiana state trauma registry data demonstrated superior model discrimination and/or parsimony compared to other measures of comorbidities.
INTRODUCTION
Comorbidities influence the detection, prognosis, treatment, and outcomes of disease.1,2 As the U.S. population continues to age and cases of geriatric trauma become more prevalent, the influence of comorbidities on the outcomes of the trauma population is likely to grow.3 Studies of trauma patient outcomes have long advocated for specific clinical practices, such as the transfer of certain patients to highly specialized trauma centers based on the presence of comorbid conditions.4,5 Moreover, quality improvement efforts, such as those of the American College of Surgeons Trauma Quality Improvement Program (ACS TQIP), routinely include comorbidities in the risk-adjusted models used to report patient outcomes and evaluate hospital quality.6
Despite widespread recognition that comorbidities influence trauma care and outcomes, a lack of consensus exists regarding how best to measure that influence. Virtually all U. S. trauma centers and many nontrauma hospitals maintain detailed clinical registries, which are the predominant data source for trauma quality improvement initiatives.6,7 Yet neither of the two most prevalent composite indices of comorbidities, the Charlson and Elixhauser comorbidity indices (CCI and ECI, respectively), were designed to leverage trauma registry data; the former was developed using clinical registry data from patients with nontrauma diagnoses, while the latter employed administrative data. Current statistical models employed by ACS TQIP include each comorbidity as a separate variable, an approach that requires considerable statistical power and consumes valuable degrees of freedom when investigating low-prevalence outcomes such as mortality.6
We postulate that a comorbidity index specifically developed for use with trauma registry data would improve the predictive modelling of trauma mortality, particularly for hospitals and patient cohorts whose case volumes cannot support the statistical demands of the ACS TQIP approach. To test that hypothesis, in this study, we describe an approach to develop and internally validate such an index, and we compare the model discrimination of that measure with other, existing comorbidities measures when evaluating the mortality of injured patients.
METHODS
Study design
We conducted a retrospective cohort study of trauma patients using data from the Indiana State Trauma Registry. Primary exposure variables included four different measures of comorbid disease burden, and the outcome of interest was in-hospital mortality. The study consisted of three stages: first, we developed and internally validated the trauma comorbidity index (TCI) in “training” and “testing” cohorts, respectively; second, we compared the predictive value of the TCI with other comorbidity measures using the testing cohort; and third, we compared model specification attributable to the TCI and two other comorbidity indices using the testing cohort, stratified by predicted risk of mortality.
Data source and study population
The study cohort consisted of all patient data (ages ≥ 16 years) collected in the state trauma registry by the Indiana State Department of Health (ISDH) from 2013 through 2015. All diagnoses are encoded using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, because ICD-10-CM codes were not included in the Indiana trauma registry until 2016.8 The registry includes all data fields of the National Trauma Data Standards set by the ACS Committee on Trauma, and it consists of data from all hospitals that submit data in compliance with state rule 410 IAC 34 of the ISDH Trauma Care Committee.9,10 The Indiana trauma registry is inclusive, since the rule applies to all hospitals, including both trauma centers and nontrauma hospitals. To populate the registry, hospital personnel collect detailed prehospital, emergency department (ED), operative, intensive care, and hospital data for all patients with diagnoses encoded as injury and poisoning.8 These data are provided in an encrypted fashion through collaboration with ISDH to ensure compliance with the Health Insurance Portability and Accountability Act. We excluded patients who presented to EDs without signs of life, defined as an initial systolic blood pressure of 0 mm Hg, heart rate of 0 beats/min, and Glasgow Coma Scale motor score of 1.11 Selection of the study cohort is summarized in Data Supplement S1, Figure S1 (available as supporting information in the online version of this paper, which is available at http://onlinelibrary.wiley.com/doi/10.1111/acem.14270/full).
We supplemented the data from the trauma registry with hospital-level data—number of hospital beds, teaching status, and profit status—obtained from the American Hospital Association (AHA) by linking the data sets using the name of each hospital identified in both data sets.12 For hospitals that lacked AHA data, ISDH conducted a hospital survey to directly acquire that information so that we had comprehensive hospital data from all hospitals included in the study.
Preexisting comorbid conditions and comorbidity indices
The ISDH trauma registry provides a list of comorbid factors defined by ICD-9-CM/ICD-10-CM codes consistent with the National Trauma Data Standards.13 To conduct this study, we used four different measures of preexisting comorbid conditions to model trauma outcomes: the CCI,14 the ECI,15 a count of comorbidities, all comorbidities included as separate variables (the method used by ACS TQIP),6 and the TCI. We accounted for changes that occurred to the comorbidity data collected during the study period with the following two steps: 1) “pulmonary disease” was changed to “chronic obstructive pulmonary disease,” so we classified both diseases as “chronic obstructive pulmonary disease,” and 2) the ACS COT omitted the variable “prehospital cardiac arrest” from the National Trauma Data Standard as a preexisting comorbid condition in 2015, so we omitted that variable from the analyses.”16,17
CCI
First described in 1987, the CCI was developed in a training cohort of 559 patients admitted to the medical service of a single hospital and externally validated in a testing cohort of 685 patients admitted to the medical service in another hospital.14 The CCI consists of 16 diagnoses that are weighted (1, 2, 3, and 6) based on association of the comorbidity with 1-year mortality. Greater weights, therefore, represent an increased association with mortality. To generate CCI scores using trauma registry data, we identified all available comorbidity diagnoses included in the CCI and weighted them accordingly. Five comorbidities included in the CCI were not present in the trauma registry, and those diagnoses are listed in Data Supplement S1, Table S2 (available as supporting information in the online version of this paper, which is available at http://onlinelibrary.wiley.com/doi/10.1111/acem.14270/full). The missing comorbidities were assigned with zero weights to compute CCI.
ECI
The ECI was first described in 1998, and it was developed using administrative data from 439 hospitals in California.15 The ECI consists of 30 diagnoses associated with increased hospital length of stay, charges, and in-patient mortality. In the scoring system, all diagnoses are weighted equally and tabulated to determine a single score.18 We identified all available comorbidity diagnoses in the trauma registry that are included in the ECI. Fourteen variables included in the ECI were not included in trauma registry, and those diagnoses are listed in the Table S2. The missing comorbidities were assigned with zero weights to compute ECI.
Trauma count of comorbidities (TCC)
We calculated a TCC by testing the unadjusted association between each comorbidity included in the trauma registry and mortality through bivariate logistic regression. We then tabulated all comorbidities with a p-value of ≤0.25. We based this cutoff on previously published methods for the development of forward stepwise regression models.19–21
Comorbidities included separately
We identified comorbidities that met a minimum threshold association with mortality (p ≤ 0.25) through bivariate analysis, and we included each variable separately in the mortality model, an approach consistent with the method used by ACS TQIP.6
TCI
The TCI approach used a three-step process:
Identify comorbidities associated with mortality (p ≤ 0.25) based on bivariate analysis.
Obtain coefficients for each comorbidity through multivariable regression models.
Sum the comorbidity coefficients to create coefficient weighted TCI for each patient.
Details of the multivariable model are provided below under “Risk adjustment.” Positive coefficients derived in Step 2 denote that a comorbidity has an association with increased mortality, and negative values denote an association with decreased mortality.
Risk adjustment
When modeling the association between comorbidities and trauma outcomes (in-hospital mortality and length of stay), we included the following patient-level covariates: Injury Severity Score, Glasgow Coma Scale, age, gender, race, initial systolic blood pressure and pulse rate in the ED, mechanism of injury, payer type, and transfer status. Additionally, we controlled for the following hospital-level covariates: American College of Surgeons trauma verification level, number of hospital beds, teaching status, and profit status. We directly selected the variables listed above to develop the risk-adjusted mortality models for this study, since they have been used previously by ACS TQIP as part of its established practice for risk adjustment.6
Data analysis
We began by inspecting the graphic distribution of continuous variables (patient age, systolic blood pressure, and heart rate) and found no skewness of the data. Additionally, we checked for statistically significant outlying observations and influential data points using the Pregibon’s beta test and found no evidence of influential observations or data points that may significantly alter our conclusions.22 To reduce bias and preserve statistical power to compare the comorbidity indices, we performed multiple imputation using chained equation algorithm (20 iterations) to address missing values.23 We evaluated the results of imputation by examining trace plots of the imputed values (means and standard deviations) and found no evidence of violation of convergence. A summary of the missingness of variables is available in Data AQ3 Supplement S1, Table S1 (available as supporting information in the online version of this paper, which is available at http://onlinelibrary.wiley.com/doi/10.1111/acem.14270/full).
To develop and internally validate the TCI, we randomly divided the entire 2013 to 2015 cohort into training (50%) and testing cohorts (50%). We performed descriptive statistics to characterize the study cohort using chi-square and t-test to calculate p-values for categorical and continuous variables, respectively. We elected to use this split-sample approach to validation, because the size of our training and testing cohorts was large enough (>42,000 patients in each cohort) so the model would not suffer from unmeasured biases.24
In the first stage of analysis, we established a baseline estimate of the mortality model by performing multivariable logistic regression—omitting any comorbidity measure—using both training and testing cohorts. We clustered at the hospital level to account for any hospital-level association with mortality and to derive robust standard errors, and we calculated the C-statistic for the mortality model in each cohort. We tested for difference between the C-statistics of the two cohorts using the Wald test.25 We then examined how the inclusion of the comorbidity measures impacted the predictive value of the mortality model. We calculated the TCI using the method detailed above using the training cohort, included it in the adjusted model, and calculated the C-statistic. We evaluated the internal validity of the TCI by calculating it in the testing cohort, taking care to apply the coefficients derived from the training cohort, and we tested for difference between the C-statistics of the two cohorts using the Wald test. Next, we repeated these steps, substituting the TCI for each of the other measures of comorbidities. In the second stage of analysis, we used the Wald test to compare the C-statistics of the respective mortality models with each comorbidity measure in the testing cohort.
In the third stage of analysis, we compared the model specification attributable to the CCI, ECI, and TCI in two different ways, given the prevalence of the two former indices in existing literature. First, using the testing cohort, we calculated the number of deaths accurately predicted by each mortality model by 1) calculating the sensitivity of the mortality models for each dataset (the original and imputed ones) using a posterior predictive command that defines “sensitivity” as true positives (accurately predicted deaths) divided by all positives, 2) deriving the mean sensitivity of the data sets, and 3) multiplying that mean value by the total number of deaths in the unimputed testing cohort. Second, we examined how closely each comorbidity index score corresponded to observed and expected mortality. We did so by 1) calculating the predicted (i.e., expected) mortality for each patient using the three mortality models, 2) dividing patients into deciles of predicted risk, 3) calculating the percentage of actual (i.e., observed) deaths per decile, and 4) calculating the mean comorbidity index score within each decile.
The study was approved by the Indiana University Institutional Review Board, and all analyses were performed using Stata 15 software (StataCorp LLC, College Station, TX).
RESULTS
The cohort consisted of 84,903 patients admitted to 109 hospitals over the study period. The hospitals included three Level I trauma centers, six Level II trauma centers, 10 Level III trauma centers, and 90 nontrauma centers. All trauma centers had ACS verification for their respective levels. Patients were predominantly elderly, White, and male, and the most commonly identified mechanism of injury was falls. Patient data—demographics and injury characteristics— are summarized in Table 1. Approximately 65% of the patient cohort had at least one comorbidity, including conditions such as “drug abuse disorder” and “current smoker,” and the maximum number of comorbidities was nine (median = 1, interquartile range [IQR] = 0–2). Table 2 summarizes patient comorbidities. The incidence of in-hospital mortality was 2.8%.
TABLE 1.
Patient characteristics
| All patients (N = 84,903) | Training cohort (n = 42,451) | Testing cohort (n = 42,452) | p-valuea | |
|---|---|---|---|---|
| Age (y), % | 0.37 | |||
| 16–24 | 10.77 | 10.69 | 10.85 | |
| 25–34 | 10.30 | 10.30 | 10.30 | |
| 35–44 | 9.17 | 9.07 | 9.26 | |
| 45–54 | 11.34 | 11.41 | 11.27 | |
| 55–64 | 13.19 | 13.15 | 13.23 | |
| 65–74 | 13.03 | 13.14 | 12.92 | |
| ≥75 | 32.18 | 32.21 | 32.16 | |
| Race, % | 0.20 | |||
| White | 84.88 | 84.77 | 84.98 | |
| Black | 8.98 | 8.92 | 9.03 | |
| Other | 1.97 | 1.98 | 1.96 | |
| NA/not known | 3.55 | 3.66 | 3.45 | |
| Female, % | 47.05 | 46.97 | 47.12 | 0.06 |
| Payer type, % | 0.22 | |||
| Private/commercial | 25.29 | 25.53 | 25.06 | |
| Medicaid | 6.70 | 6.55 | 6.86 | |
| Medicare | 39.61 | 39.70 | 39.51 | |
| Other | 20.13 | 19.98 | 20.28 | |
| NA/not known | 8.17 | 8.16 | 8.18 | |
| Mechanism, % | 0.40 | |||
| Adverse reaction/overdose/poisoning | 0.54 | 0.55 | 0.53 | |
| Assault | 6.30 | 6.29 | 6.30 | |
| Burn/electrocution | 1.90 | 1.88 | 1.92 | |
| Cut/pierce | 1.56 | 1.53 | 1.59 | |
| Fall | 53.74 | 53.71 | 53.77 | |
| Firearm | 1.11 | 1.08 | 1.14 | |
| Hanging/asphyxiation/drowning | 0.14 | 0.13 | 0.15 | |
| Machinery | 0.96 | 0.95 | 0.96 | |
| Motor vehicle collision | 22.11 | 22.01 | 22.20 | |
| Natural | 0.04 | 0.05 | 0.02 | |
| Other/not known | 2.68 | 2.65 | 2.70 | |
| Overexertion | 0.26 | 0.29 | 0.24 | |
| Pedestrian/pedestrian cyclist/pedestrian struck | 2.83 | 2.92 | 2.74 | |
| Struck by/against | 2.70 | 2.81 | 2.60 | |
| Transport | 0.68 | 0.69 | 0.68 | |
| Injury Severity Score, mean (±SD) | 8 (±7) | 8 (±7) | 8 (±7) | 0.33 |
| Initial systolic blood pressure (mm Hg), mean (±SD) | 142 (±27) | 142 (±27) | 142 (±27) | 0.17 |
| Initial heart rate (beats/min), mean (±SD) | 86 (±19) | 86 (±19) | 86 (±19) | 0.70 |
| Glasgow Coma Scale, mean (±SD) | 14 (±3) | 14 (±3) | 14 (±3) | 0.44 |
| Interhospital transfer, % | 18.99 | 19.05 | 19.94 | 0.19 |
| American College of Surgeons trauma verification level, % | 0.93 | |||
| I | 16.60 | 16.61 | 16.60 | |
| II | 30.32 | 30.27 | 30.36 | |
| III | 7.24 | 7.19 | 7.28 | |
| Nontrauma center | 45.84 | 45.93 | 45.76 | |
| Hospital beds | 0.28 | |||
| <200 | 56.35 | 56.02 | 56.64 | |
| 201–400 | 25.77 | 25.87 | 25.66 | |
| 401–600 | 6.41 | 6.49 | 6.33 | |
| >600 | 11.49 | 11.62 | 11.36 | |
| Teaching | 69.42 | 69.60 | 69.25 | 0.28 |
| Nonprofit | 91.92 | 91.95 | 91.88 | 0.64 |
Chi-square used to calculate p-values for categorical variables, and t-test used to calculate p-values for continuous variables.
TABLE 2.
Prevalence of comorbidities used to develop TCI and comparison between training and testing cohorts (%)
| All patients(N = 84,903) | Training cohort (n = 42,451) | Testing cohort (n = 42,452) | p-valuea | |
|---|---|---|---|---|
| Advanced directive | 1.23 | 1.16 | 1.30 | 0.06 |
| Bleeding disorder | 6.96 | 7.03 | 6.89 | 0.42 |
| Chemotherapy | 0.36 | 0.35 | 0.36 | 0.86 |
| Chronic obstructive pulmonary disease | 8.08 | 8.01 | 8.15 | 0.48 |
| Chronic renal failure | 2.08 | 2.00 | 2.15 | 0.14 |
| Cirrhosis | 0.62 | 0.62 | 0.62 | 1.00 |
| Congestive heart failure | 5.70 | 5.66 | 5.74 | 0.65 |
| Current smoker | 20.21 | 19.97 | 20.46 | 0.08 |
| Dementia | 5.75 | 5.86 | 5.63 | 0.17 |
| Diabetes mellitus | 15.84 | 15.89 | 15.79 | 0.69 |
| Disseminated cancer | 0.89 | 0.86 | 0.93 | 0.29 |
| Drug use disorder | 2.55 | 2.60 | 2.49 | 0.31 |
| Functionally dependent | 3.74 | 3.72 | 3.75 | 0.82 |
| History of myocardial infarction | 14.01 | 13.86 | 14.15 | 0.21 |
| History of myocardial infarct within past 6 months | 0.85 | 0.90 | 0.81 | 0.18 |
| History of peripheral vascular disease | 0.63 | 0.62 | 0.63 | 0.83 |
| Hypertension | 24.80 | 24.96 | 24.63 | 0.28 |
| Major psychiatric illness | 4.69 | 4.60 | 4.78 | 0.21 |
| Steroid use | 0.61 | 0.62 | 0.60 | 0.79 |
Abbreviation: TCI, trauma comorbidity index.
Chi-square used to calculate p-value.
When divided into training and testing cohorts, demographic, injury, and comorbidity characteristics were evenly distributed between the two groups (p > 0.05).26 The distribution of demographic and injury characteristics between the cohorts is summarized in Table 1, and Table 2 summarizes the distribution of comorbid conditions used to develop the TCI. Of note, mortality was also evenly distributed between the training and testing cohorts (2.9% and 2.8%, respectively; p = 0.82).
In the training cohort, we identified 19 comorbidities that met the minimum threshold association with mortality using bivariate analysis (p ≤ 0.25), and the coefficients derived from the multivariable models ranged from –1.0 (drug use disorder) to 1.2 (presence of an advanced directive limiting care). The TCI ranged from –1.8 to 5.1, with negative values representing a decreased association with risk-adjusted mortality, relative to a TCI of zero. The p-values and coefficients for each comorbidity used to develop the TCI, along with the corresponding coefficient in the CCI and ECI are summarized in Table 3. Comorbidities included in the trauma registry but not incorporated in the TCI are listed in Table S2.
TABLE 3.
p-values from bivariate regression and risk-adjusted coefficients used to develop the TCI with mortality as the outcome and coefficients for the CCI and ECI for corresponding comorbidities
| Coefficienta |
||||
|---|---|---|---|---|
| p-value | TCI | CCI | ECI | |
| Advanced directive | <0.001 | 1.24 | — | — |
| Bleeding disorder | <0.001 | 0.86 | — | 1 |
| Chemotherapy | 0.02 | 1.02 | — | — |
| Chronic obstructive pulmonary disease | <0.001 | 0.45 | 1 | 1 |
| Chronic renal failure | 0.07 | 0.44 | 2 | 1 |
| Cirrhosis | 0.05 | 0.91 | 3 | 1 |
| Congestive heart disease | <0.001 | 0.87 | 1 | 1 |
| Current smoker | <0.001 | − 0.41 | — | — |
| Dementia | 0.003 | − 0.01 | 1 | — |
| Diabetes mellitus | 0.05 | 0.22 | 1 | 1 |
| Disseminated cancer | 0.003 | 0.75 | 6 | 1 |
| Drug use disorder | 0.17 | − 1.04 | — | 1 |
| Functionally dependent | <0.001 | 0.32 | — | — |
| History of myocardial infarction | 0.05 | 0.15 | 1 | — |
| History of myocardial infarct within past 6 months | 0.20 | 0.53 | 1 | — |
| History of peripheral vascular disease | 0.20 | 0.75 | 1 | 1 |
| Hypertension | 0.11 | 0.09 | — | 1 |
| Major psychiatric illness | 0.22 | − 0.31 | — | — |
| Steroid use | 0.002 | 0.78 | — | — |
Note: — = not included in index
Abbreviations: CCI, Charlson Comorbidity Index; ECI, Elixhauser Comorbidity Index; TCI, trauma comorbidity index.
Coefficients with positive values denote an association with increased mortality and negative values indicate an association with decreased mortality.
Regarding internal validation of the TCI, we found no significant difference (p = 0.33) between the C-statistics of the training (0.918) and testing (0.924) cohorts when we included the TCI in the mortality model. Similarly, we found no significant difference between cohorts when using no measure of comorbidities and the alternative comorbidity measures (Table 4).
TABLE 4.
Comparison of mortality models with different comorbidity measures between training and testing cohorts
| C-statistic |
|||
|---|---|---|---|
| Method of measurement | Training cohort | Testing cohort | p-value |
| NCI | 0.909 | 0.915 | 0.32 |
| CCI | 0.913 | 0.921 | 0.16 |
| ECI | 0.914 | 0.920 | 0.29 |
| TCC | 0.914 | 0.920 | 0.25 |
| CIS | 0.918 | 0.925 | 0.23 |
| TCI | 0.918 | 0.924 | 0.33 |
Abbreviations: CCI, Charlson Comorbidity Index; CIS, comorbidities included separately; ECI, Elixhauser Comorbidity Index; NCI, no comorbidities included; TCC, cumulative count of trauma comorbidities; TCI, trauma comorbidity index.
In the testing cohort, all methods of comorbidity measurement significantly increased the C-statistic above a mortality model that lack any comorbidity measure (0.915). Inclusion of the CCI and ECI produced C-statistics of 0.921 and 0.920, respectively, which were statistically comparable to each other (p = 0.27). The C-statistic of the TCI model was significantly greater than models with the CCI and ECI (p < 0.05). Models that included the TCI and all 19 comorbidities included separately (CIS) yielded the greatest C-statistics, 0.924 and 0.925, respectively. Those C-statistics were comparable (p = 0.11), but the CIS model included 18 more variables than the TCI model. A summary of the C-statistics for mortality models with each of the comorbidity measures is summarized in Table 5.
TABLE 5.
Comparison of C-statistic of mortality models with different comorbidity measures in the testing cohort, p-value
| Method of measurement (C-statistic) | NCI (0.915) | CCI (0.921) | ECI (0.920) | TCC (0.921) | CIS (0.925) | TCI (0.924) |
|---|---|---|---|---|---|---|
| NCI (0.915) | — | |||||
| CCI (0.921) | <0.001 | — | ||||
| ECI (0.920) | <0.001 | 0.27 | — | |||
| TCC (0.921) | <0.001 | 0.42 | 0.72 | — | ||
| CIS (0.925) | <0.001 | 0.005 | 0.001 | 0.001 | — | |
| TCI (0.924) | <0.001 | 0.03 | 0.003 | 0.003 | 0.11 | — |
Abbreviations: CCI, Charlson Comorbidity Index; CIS, comorbidities included separately; ECI, Elixhauser Comorbidity Index; NCI, no comorbidities included; TCC, cumulative count of trauma comorbidities; TCI, trauma comorbidity index.
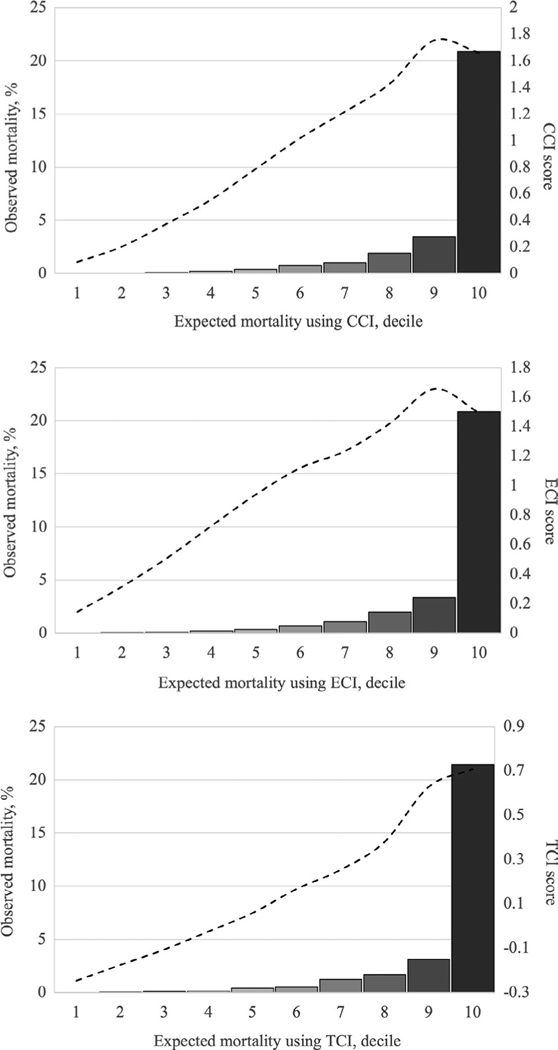
When comparing the model specification attributable to the comorbidity indices—CCI, ECI, and TCI—in the testing cohort, sensitivity was greatest for the model with the TCI (91.1%), whereas the models with the CCI and ECI had sensitivities of 90.9% and 90.8%, respectively, and the model that lacked any measure of comorbidity had a sensitivity of 90.3%. Accordingly, of 1,201 deaths in the testing cohort, the TCI model accurately predicted 1,094; the CCI and ECI models predicted 1,091 and 1,090 deaths, respectively; and the model without a comorbidity measure predicted 1,084 deaths. When risk stratified, each mortality model demonstrated that observed mortality progressively increased across decile of expected mortality (Figure 1). However, only the TCI score peaked in the 10th decile (that with the greatest mortality), whereas the CCI and ECI scores peaked in the ninth decile and decreased in the 10th decile.
FIGURE 1.
Comparison of observed and expected mortality using different comorbidity indices with corresponding comorbidity index scores. Expected mortality is stratified by decile of risk-adjusted, predicted mortality - - - - = Comorbidity index score, mean calculated per decile of expected mortality. CCI, Charlson Comorbidity Index; ECI, Elixhauser Comorbidity Index; TCI, trauma comorbidity index
DISCUSSION
In this study of Indiana state adult trauma patients, we found that comorbidities, as defined by the ACS National Trauma Data Standard, were exceedingly prevalent (65%), and the measurement of comorbidities using any method significantly improved the statistical modelling of in-hospital mortality. Inclusion of the TCI increased calibration of the mortality model in a manner similar to the CCI and ECI, providing concurrent validity to the TCI approach. Although the previously developed indices accounted for mortality risk associated with comorbidities, the TCI improved the model discrimination of that relationship, albeit slightly. That improvement was evidenced by the increased number of deaths accurately predicted by the TCI model in comparison to models with the other indices. Moreover, although the benefit of using the TCI approach over other comorbidity indices was slight, it is notable that only the TCI score corresponded directly with mortality among patients with the greatest risk, whereas the mean scores of other comorbidity indices actually decreased from the ninth to the 10th decile of expected mortality (Figure 1). This finding indicates that the TCI is calibrated so its score reflects risk of mortality more closely than those of CCI and ECI. Therefore, we submit that, at the very least, investigators should consider the TCI approach to be a viable alternative to develop a trauma-specific comorbidity index rather than use more general comorbidity indices when performing risk adjustment to examine trauma mortality.
The TCI and CIS (the method currently employed by ACS TQIP) estimated the mortality risk associated with comorbidities comparably, but the TCI afforded substantially more parsimony, reducing the required number of comorbidity-related variables from 19 to one. These findings have notable implications for risk adjustment when examining both rare outcomes and small patient cohorts, instances when degrees of freedom must be used sparingly to preserve statistical power. Whereas in this study, we divided the overall study cohort into training and testing cohorts, future studies need not perform this separate step of internal validation if the Indiana weights for TCI externally validate in a national data set. Therefore, future studies that incorporate the TCI approach could retain all of the statistical power imparted by the full size of their study cohort.
Unlike previously described comorbidity indices, the TCI uses comorbidity selection specific to trauma registry data sets. As a result, the TCI potentially identifies preexisting conditions that one may not consider to be comorbidities in a conventional sense, such as the presence of an advanced directive limiting care. However, we submit that such diagnoses are both clinically relevant and designated as comorbidities by the ACS National Trauma Data Standard. Conversely, the TCI does not include certain well-recognized comorbidities, such as human immunodeficiency virus (HIV), if they are not included in the data or do not meet a minimum threshold association with mortality. Specifically, regarding missing comorbidities, the CCI and ECI are widely used for risk adjustment in trauma outcomes research; however, those indices include diagnoses such as HIV that are not included in the ACS National Trauma Data Standard. This discrepancy between the scoring systems and trauma registry data inherently limits the performance of the scoring systems themselves as they were originally derived and validated.
The flexibility of comorbidity selection of the TCI approach is particularly advantageous for the study of clinical registry data, which is subject to change over time or vary depending on whether or not an institution adheres to the ACS National Trauma Data Standard. Moreover, the TCI approach accounts for potential lapses in data quality, since it would exclude variables with fields that are consistently omitted, as they would be unlikely to meet the minimum statistical threshold of association with an outcome. As with other indices, the TCI approach achieves parsimony by estimating the cumulative effect of multiple factors—comorbidities, in this case—as a single value. The combination of these attributes (model flexibility and parsimony) make the TCI approach uniquely well suited for the study of trauma subpopulations such as patients with specific mechanisms of injury or hospitals that treat small numbers of injured patients, such as nontrauma hospitals.
LIMITATIONS
Although the TCI has certain advantages over other comorbidity indices, it also has limitations. Like other comorbidity indices, the TCI has no role in prospectively determining the expected outcomes of a given patient. Rather, the TCI was designed to enhance risk-adjusted models used to examine trauma mortality retrospectively using clinical registry data. If the Indiana-derived weights for TCI externally validate on a national dataset, then the Indiana TCI can be used for future trauma registry risk-adjusted modeling.
If the Indiana TCI does not externally validate, we provide detailed methods of how to conduct the TCI approach to either derive and validate more generalizable TCI weights using a national data set or use the TCI approach for project-specific derivation and internal validation. Compared with other fixed-weight comorbidity indices, the TCI approach requires additional steps for its calculation, specifically, the identification of statistically relevant comorbidities and the estimated association between those comorbidities and mortality. As a result, the TCI approach does not assign fixed coefficients to comorbidities. Instead, the coefficients can be derived from the particular data set. Further, the TCI does not test for interaction effects or collinearity between comorbidities and assumes a cumulative relationship between comorbidities and mortality. Alternative methods, such as random-forest regression, may address those shortcoming but would also add complexity to the calculation of a comorbidity index.27 Despite the limitations of the TCI, it is notable for its improved predictive modelling compared with previously described comorbidity indices.
The results of this study should be interpreted in the context of its limitations. First, the trauma population in Indiana may not be representative of the national trauma population. As stated, we do not propose to apply the coefficients for comorbidities reported in this study to other populations without external validation. Instead, the purpose of this study was to detail the approach for deriving the TCI. Further study, using national data, is necessary to externally validate the Indiana-derived TCI weights or derive nationally representative TCI coefficients for comorbidities that can be applied more broadly. Second, the analyses are limited to in-hospital mortality, a short-term outcome. In the process of deriving the TCI, we found that certain comorbidities—current smoker, dementia, drug abuse disorder, and major psychiatric illness—were actually associated with decreased mortality. Since this study is retrospective, the results do not connote mechanisms for these relationships, and we do not intend to suggest that smoking, for example, is protective overall, but simply associated with lower in-hospital mortality after traumatic injury. The cumulative, long-term sequelae of smoking (e.g., peripheral vascular disease, respiratory disease, and myocardial infarction) are clearly associated with an increased risk of mortality.28,29 Regarding the association between psychiatric illnesses and decreased mortality, our findings are consistent with other previously published work, but the influence of psychiatric illnesses on long-term mortality following trauma is still unclear.30
CONCLUSION
In conclusion, this study provides a critical analysis of several methods previously used to measure the association of comorbidities and trauma outcomes, and it identifies limitations of those methods when applied to trauma registry data. In response to those shortcomings, this study details the development of the trauma comorbidity index approach, a method of measurement specifically designed for use with clinical registry data. When compared with other methods of measuring the clinical impact of comorbidities, the trauma comorbidity index approach demonstrated superior model discrimination and/or parsimony for estimating the risk of trauma mortality using Indiana state trauma registry data.
Supplementary Material
ACKNOWLEDGMENTS
The Indiana State Department of Health, Division of Trauma and Injury Prevention, provided access to data in support of this study.
Funding information Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1TR002529 and the Indiana State Department of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Indiana State Department of Health.
Presented at the 78th Annual Meeting of American Association for the Surgery of Trauma, Dallas, TX, September 2019.
Funding information
National Center for Advancing Translational Sciences, Grant/Award Number: UL1TR002529
Footnotes
CONFLICT OF INTEREST
The authors have no potential conflicts to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbiditya critical review of available methods. J Clin Epidemiol. 2003;56(3):221–229. [DOI] [PubMed] [Google Scholar]
- 2.Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chron Dis. 1970;23(7):455–468. [DOI] [PubMed] [Google Scholar]
- 3.Hospital resources for optimal care of the injured patient. Prepared by a Task Force of the Committee on Trauma of the American College of Surgeons. Bull Am Coll Surg. 1979;64(8):43–48. [PubMed] [Google Scholar]
- 4.Morris JA Jr, MacKenzie EJ, Edelstein SL. The effect of preexisting conditions on mortality in trauma patients. JAMA. 1990;263(14):1942–1946. [PubMed] [Google Scholar]
- 5.Ohmori T, Kitamura T, Ishihara J, et al. Early predictors for massive transfusion in older adult severe trauma patients. Injury. 2017;48(5):1006–1012. [DOI] [PubMed] [Google Scholar]
- 6.Newgard CD, Fildes JJ, Wu L, et al. Methodology and analytic rationale for the American College of Surgeons Trauma quality improvement program. J Am Coll Surg. 2013;216(1):147–157. [DOI] [PubMed] [Google Scholar]
- 7.MacKenzie EJ, Hoyt DB, Sacra JC, et al. National inventory of hospital trauma centers. JAMA. 2003;289(12):1515–1522. [DOI] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). 2015. Accessed November 1, 2018. Available from: https://www.cdc.gov/nchs/icd/icd9cm.htm
- 9.Article 34. State Trauma Registry 2013. Indiana State Department of Health. 2021. Accessed November 1, 2018. http://www.in.gov/legislative/iac/20131120-IR-410120617FRA.xml.pdf
- 10.Indiana Patient Registry. Indiana State Department of Health. c2021. Accessed November 1, 2019. https://www.in.gov/isdh/25407.htm
- 11.Calland JF, Nathens AB, Young JS, et al. The effect of dead-on-arrival and emergency department death classification on risk-adjusted performance in the American College of Surgeons Trauma Quality Improvement Program. J Trauma Acute Care Surg. 2012;73(5):1086–1092. [DOI] [PubMed] [Google Scholar]
- 12.AHA Annual Survey of Hospitals. 2013–2016. Dallas, TX: American Heart Association; 2016. [Google Scholar]
- 13.State of Indiana Trauma Registry Data Dictionary. Indiana State Department of Health. 2015. Accessed January 19, 2019. https://www.in.gov/isdh/files/NEW_VERSION_2015_Indiana_Data_Dictionary.pdf
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 15.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 16.ACS NTDB, National Trauma Data Standard: Data Dictionary. 2014 Admissions. American College of Surgeons. 2014. Accessed March 2021. https://www.dshs.texas.gov/injury/registry/Data-Dictionaries/2014NTDSDataDictionary.pdf [Google Scholar]
- 17.ACS NTDB, National Trauma Data Standard: Data Dictionary. 2015 Admissions. American College of Surgeons. 2015. Accessed March 2021. https://www.facs.org/-/media/files/quality-programs/trauma/ntdb/ntds/data-dictionaries/ntds-data-dictionary-2015.ashx
- 18.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633. [DOI] [PubMed] [Google Scholar]
- 19.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z. Model building strategy for logistic regression: purposeful selection. Ann Transl Med. 2016;4(6):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bendal RB, Afifi AA. Comparison of stopping rules in forward “step-wise” regression. J Am Stat Assoc. 1977;72(357):46–53. [Google Scholar]
- 22.Pregibon D. Logistic regression diagnostics. Ann Statist. 1981;9(4):705–724. [Google Scholar]
- 23.Buuren SV, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 24.Xu Y, Goodacre R. On splitting training and validation set: a comparative study of cross-validation, bootstrap and systematic sampling for estimating the generalization performance of supervised learning. J Anal Test. 2018;2(3):249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 26.Buis ML. Discrete uses for uniform. Stata J. 2007;7(3):434–435. [Google Scholar]
- 27.Bertsimas D, Dunn J, Velmahos GC, Kaafarani HM. Surgical risk is not linear: derivation and validation of a novel, user-friendly, and machine-learning-based predictive optimal trees in emergency surgery risk (POTTER) calculator. Ann Surg. 2018;268(4):574–583. [DOI] [PubMed] [Google Scholar]
- 28.Trap-Jensen J. Effects of smoking on the heart and peripheral circulation. Am Heart J. 1988;115:263–267. [DOI] [PubMed] [Google Scholar]
- 29.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131(5):1557–1566. [DOI] [PubMed] [Google Scholar]
- 30.Townsend LL, Esquivel MM, Uribe-Leitz T, et al. The prevalence of psychiatric diagnoses and associated mortality in hospitalized US trauma patients. J Surg Res. 2017;213:171–176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.