Abstract
Background
Tinnitus is a symptom defined as the perception of sound in the absence of an external source. In England alone there are an estimated ¾ million general practice consultations every year where the primary complaint is tinnitus, equating to a major burden on healthcare services. Clinical management strategies include education and advice, relaxation therapy, tinnitus retraining therapy (TRT), cognitive behavioural therapy (CBT), sound enrichment using ear‐level sound generators or hearing aids, and drug therapies to manage co‐morbid symptoms such as insomnia, anxiety or depression.
Objectives
To assess the effects of Ginkgo biloba for tinnitus in adults and children.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Register; CENTRAL (2022, Issue 6); Ovid MEDLINE; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 7 June 2022.
Selection criteria
Randomised controlled trials (RCTs) recruiting adults and children with acute or chronic subjective tinnitus. We included studies where the intervention involved Ginkgo biloba and this was compared to placebo, no intervention, or education and information. Concurrent use of other medication or other treatment was acceptable if used equally in each group. Where an additional intervention was used equally in both groups, we analysed this as a separate comparison. The review included all courses of Ginkgo biloba, regardless of dose regimens or formulations, and for any duration of treatment.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were tinnitus symptom severity measured as a global score on a multi‐item tinnitus questionnaire and serious adverse effects (bleeding, seizures). Our secondary outcomes were tinnitus loudness (change in subjective perception), tinnitus intrusiveness, generalised depression, generalised anxiety, health‐related quality of life and other adverse effects (gastrointestinal upset, headache, allergic reaction). We used GRADE to assess the certainty of the evidence for each outcome.
Main results
This review included 12 studies (with a total of 1915 participants). Eleven studies compared the effects of Ginkgo biloba with placebo and one study compared the effects of Ginkgo biloba with hearing aids to hearing aids alone. All included studies were parallel‐group RCTs. In general, risk of bias was high or unclear due to selection bias and poor reporting of allocation concealment and blinding of participants, personnel and outcome assessments. Due to heterogeneity in the outcomes measured and measurement methods used, only limited data pooling was possible.
Ginkgo biloba versus placebo
When we pooled data from two studies for the primary outcome tinnitus symptom severity, we found that Ginkgo biloba may have little to no effect (Tinnitus Handicap Inventory scores) at three to six months compared to placebo, but the evidence is very uncertain (mean difference (MD) ‐1.35 (scale 0 to 100), 95% confidence interval (CI) ‐8.26 to 5.55; 2 studies; 85 participants) (very low‐certainty). Ginkgo biloba may result in little to no difference in the risk of bleeding or seizures, with no serious adverse effects reported in either group (4 studies; 1154 participants; low‐certainty).
For the secondary outcomes, one study found that there may be little to no difference between the effects of Ginkgo biloba and placebo on tinnitus loudness measured with audiometric loudness matching at 12 weeks, but the evidence is very uncertain (MD ‐4.00 (scale ‐10 to 140 dB), 95% CI ‐13.33 to 5.33; 1 study; 73 participants) (very low‐certainty). One study found that there may be little to no difference between the effects of Ginkgo biloba and placebo on health‐related quality of life measured with the Glasgow Health Status Inventory at three months (MD ‐0.58 (scale 0 to 100), 95% CI ‐4.67 to 3.51; 1 study; 60 participants) (low‐certainty). Ginkgo biloba may not increase the frequency of other adverse effects (gastrointestinal upset, headache, allergic reaction) at three months compared to placebo (risk ratio 0.91, 95% CI 0.52 to 1.60; 4 studies; 1175 participants) (low‐certainty). None of the studies reported the other secondary outcomes of tinnitus intrusiveness or changes in depressive symptoms or depression, anxiety symptoms or generalised anxiety.
Gingko biloba with concurrent intervention versus concurrent intervention only
One study compared Ginkgo biloba with hearing aids to hearing aids only. It assessed the mean difference in the change in Tinnitus Handicap Inventory scores and tinnitus loudness using a 10‐point visual analogue scale (VAS) at three months. The study did not report adverse effects, tinnitus intrusiveness, changes in depressive symptoms or depression, anxiety symptoms or generalised anxiety, or health‐related quality of life. This was a single, very small study (22 participants) and for all outcomes the certainty of the evidence was very low. We were unable to draw meaningful conclusions from the numerical results.
Authors' conclusions
There is uncertainty about the benefits and harms of Ginkgo biloba for the treatment of tinnitus when compared to placebo. We were unable to draw meaningful conclusions regarding the benefits and harms of Ginkgo biloba when used with concurrent intervention (hearing aids). The certainty of the evidence for the reported outcomes, assessed using GRADE, ranged from low to very low. Future research into the effectiveness of Ginkgo biloba in patients with tinnitus should use rigorous methodology. Randomisation and blinding should be of the highest quality, given the subjective nature of tinnitus and the strong likelihood of a placebo response. The CONSORT statement should be used in the design and reporting of future studies. We also recommend the use of validated, patient‐centred outcome measures for research in the field of tinnitus.
Plain language summary
Ginkgo biloba herbal supplement for tinnitus
What is tinnitus?
Tinnitus is a symptom where people have a perception of sound without there being an external source. It is often described as a ringing, hissing, buzzing or whooshing sound. It is common, affecting between 5% and 43% of the general population, and its prevalence increases with age. For some people tinnitus is persistent and troublesome, and it may lead to sleep problems (insomnia), difficulty concentrating, difficulties in communication and social interaction, and anxiety and depression. Management can include education and advice, relaxation therapy, tinnitus retraining therapy (TRT), cognitive behavioural therapy (CBT), sound generators or hearing aids, and drug therapies. The herbal supplement Ginkgo biloba has also been used.
What did we want to find out?
We wanted to find out whether Ginkgo biloba reduces tinnitus severity and whether it has any unwanted or harmful effects.
What did we do?
We searched for studies that looked at Ginkgo biloba compared to placebo ('dummy' treatment), no treatment or education/information alone in adults and children with tinnitus. We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as how the studies had been done and how many people were involved.
What did we find?
We found 12 studies (with a total of 1915 people who participated). Eleven studies compared the effects of Ginkgo biloba with placebo. One study compared the effects of Ginkgo biloba combined with hearing aids to hearing aids alone.
Main results
When we combined the results of two studies that measured tinnitus severity in the same way we found that Ginkgo biloba may have little to no effect compared to placebo, but the evidence is very uncertain. We looked at four studies that recorded any serious harmful effects, all of which reported none, so Ginkgo probably does not result in any difference in risk compared to placebo. However, the included studies did not look at the potentially harmful effects of Ginkgo biloba when used alongside other drugs. There may not be any difference between Ginkgo biloba and placebo in the effect on tinnitus loudness, but this is very uncertain. We also found that there may not be any difference in other outcomes (health‐related quality of life and minor unwanted effects such as gastrointestinal upset, headache and allergic reaction). There is no evidence to suggest that Ginkgo biloba has an effect on tinnitus when compared to placebo.
We looked at the study that compared Ginkgo biloba combined with hearing aids to hearing aids alone. It assessed the difference in the change in tinnitus severity and loudness using a scale at three months. The study did not report any of the other outcomes we were interested in. This was a single, very small study (22 people) and the evidence was very uncertain. We were unable to draw meaningful conclusions from the findings of this study.
What are the limitations of the evidence?
Although we found 12 studies, half of them did not report outcomes that we were interested in. We were not able to combine the results from many of the remaining studies. We are not confident in the evidence for the effect on tinnitus severity of Ginkgo biloba compared to placebo. This is because some people dropped out of one study, only people over 60 were included, the studies were small and very few studies reported this important outcome. We have little confidence in the evidence about serious harmful effects because none were reported in either group and the studies may have had some problems in the way they were done. For tinnitus loudness we are not confident in the evidence because the study that measured this was very small, some people dropped out and only this one study reported this important outcome. We have little confidence in the evidence for health‐related quality of life and minor unwanted effects because the studies were small and may have had problems with the way they were done.
We are not confident in the evidence for the effects of Ginkgo in combination with hearing aids because the number of participants in the study was very small.
How up to date is this evidence?
The evidence is up to date to June 2022.
Summary of findings
Summary of findings 1. Ginkgo biloba compared to placebo for tinnitus.
| Ginkgo biloba compared to placebo for tinnitus | ||||||
| Patient or population: adults with tinnitus Setting: departments of otorhinolaryngology in Brazil, Germany and Turkey and one study conducted by telephone/email Intervention: Ginkgo biloba Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Ginkgo biloba | |||||
| Tinnitus symptom severity Assessed with: THI (range: 0 to 100) Follow‐up: 3 to 6 months |
The mean tinnitus symptom severity at 3 to 6 months was 4 | MD 1.35 lower (8.26 lower to 5.55 higher) | — | 85 (2 RCTs) | ⊕⊝⊝⊝ very low1,2,3,4 | Ginkgo biloba may have little to no effect on tinnitus severity compared to placebo, but the evidence is very uncertain. |
| Serious adverse effects (bleeding or seizures) Yes or no Follow‐up: at 3 months |
Study population | — | 1154 (4 RCTs) | ⊕⊕⊝⊝ low5,7 | Ginkgo biloba may result in little to no difference in the risk of serious adverse effects (bleeding or seizures), with zero cases reported in either group. | |
| Zero events in the placebo group | Zero events in the Ginkgo biloba group | |||||
| Tinnitus loudness Assessed with: audiometric loudness matching Follow‐up: at 12 weeks |
The mean tinnitus loudness was 0.8 | MD 4 lower (13.33 lower to 5.33 higher) | — | 73 (1 RCT) | ⊕⊝⊝⊝ very low3,4,6 | Ginkgo biloba may result in little to no difference in tinnitus loudness compared to placebo. |
| Health‐related quality of life Assessed with: GHSI (range: 0 to 100) Follow‐up: 3 months |
The mean quality of life at 3 months was 2.52 | MD 0.58 lower (4.67 lower to 3.51 higher) | — | 60 (1 RCT) | ⊕⊕⊝⊝ low3,5 | Ginkgo biloba may result in little to no difference in quality of life compared to placebo. |
| Other adverse effects (gastrointestinal upset, headache, allergic reaction) Yes or no Follow‐up: at 3 months |
Study population | RR 0.91 (0.52 to 1.60) | 1175 (4 RCTs) | ⊕⊕⊝⊝ low3,5 | Ginkgo biloba may not increase the frequency of other adverse effects (gastrointestinal upset, headache, allergic reaction) compared to placebo. | |
| 41 per 1000 | 37 per 1000 (21 to 66) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GHSI: Glasgow Health Status Inventory; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; THI: Tinnitus Handicap Inventory | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1One of the two studies omitted primary outcome data, and had dropout without reason; downgraded by 0.5 level for study limitations. 2Only people over the age of 60 are included in this body of evidence; downgraded by 0.5 level for indirectness. 3Wide confidence interval around the point estimate; small sample size; downgraded by 1 level for imprecision. 4Only a small proportion of the included studies reported on this critical outcome: publication bias is suspected; downgraded by 1 level for publication bias. 5Some of the studies have multiple domains of unclear or high risk of bias; downgraded by 1 level for study limitations. 6There are no data regarding the primary outcome measure for 14 participants in the intervention group and 12 participants in the control group at 12 weeks. Dropout is not explained. Almost all of the bias domains are rated as either unclear or high risk; downgraded by 1 level for study limitations. 7Zero events in either group; downgraded by 1 level for imprecision.
Summary of findings 2. Gingko biloba with concurrent intervention versus concurrent intervention only.
| Gingko biloba with concurrent intervention versus concurrent intervention only | ||||||
| Patient or population: adults with tinnitus Setting: audiology centre in Brazil Intervention: Ginkgo biloba plus hearing aids Comparison: hearing aids | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with hearing aids | Risk with Ginkgo biloba plus hearing aids | |||||
| Tinnitus symptom severity Assessed with: THI (range: 0 to 100) Follow‐up: at 3 months |
The mean tinnitus symptom severity at 3 months was ‐40 | MD 7.5 higher (2.83 higher to 12.17 higher) | — | 22 (1 RCT) | ⊕⊝⊝⊝ very low1,2,3 | When Ginkgo biloba was used in combination with hearing aids, the reduction in tinnitus symptom severity at 3 months was not as great as when hearing aids were used alone, but the evidence is very uncertain. |
| Serious adverse effects (bleeding or seizures) | The study did not report serious adverse effects. | |||||
| Tinnitus loudness Assessed with: 10‐point VAS (range: 0 to 10) Follow‐up: at 3 months |
The mean tinnitus loudness at 3 months was ‐3.2 | MD 1 higher (0.52 higher to 1.48 higher) | — | 22 (1 RCT) | ⊕⊝⊝⊝ very low1,2,3 | When Ginkgo biloba was used in combination with hearing aids, the reduction in tinnitus loudness at 3 months was not as great as when hearing aids were used alone, but the evidence is very uncertain. |
| Health‐related quality of life | The study did not include measures of health‐related quality of life. | |||||
| Other adverse effects (gastrointestinal upset, headache, allergic reaction) | The study did not reported adverse effects. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; SD: standard deviation; THI: Tinnitus Handicap Inventory; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Not downgraded for indirectness, but it is worth noting that the study only included adult participants (mean age 56.3 years, SD 16.8 years). 2Very small sample size, below the requirement for optimal information size; downgraded by two levels for imprecision. 3Downgraded by one level for study limitations (high risk of bias) due to the lack of blinding during the conduct of the trial.
Background
This review supersedes the Cochrane Review 'Ginkgo biloba for tinnitus', which was first published in the Cochrane Library in Issue 3, 2013. The following paragraphs and Description of the condition are based on the Cochrane Review 'Amplification with hearing aids for patients with tinnitus and co‐existing hearing loss' and are reproduced with permission (Hoare 2014). Description of the intervention and How the intervention might work are based on the Cochrane Review 'Ginkgo biloba for tinnitus' and are reproduced with permission (Hilton 2013).
Description of the condition
Tinnitus is defined as the perception of sound in the absence of an external source (Jastreboff 2004). It is typically described by those who experience it as a ringing, hissing, buzzing or whooshing sound and is thought to result from abnormal neural activity at some point or points in the auditory pathway, which is erroneously interpreted by the brain as sound. Tinnitus can be either objective or subjective. Objective tinnitus refers to the perception of sound that can be also heard by the examiner and is usually due to turbulent blood flow or muscular contraction (Roberts 2010). Most commonly, however, tinnitus is subjective; the sound is only heard by the person experiencing it and no source of the sound is identified (Jastreboff 1988). Tinnitus affects between 5% and 43% of the general population and prevalence increases with age (McCormack 2016). It can be experienced acutely, recovering spontaneously within minutes to weeks, but is considered chronic and unlikely to resolve spontaneously when experienced for more than three months (Gallus 2015; Hall 2011). For many people tinnitus is persistent and troublesome, and has disabling effects such as insomnia, difficulty concentrating, difficulties in communication and social interaction, and negative emotional responses such as anxiety and depression (Hall 2018). In approximately 90% of cases, chronic tinnitus is co‐morbid with some degree of measurable hearing loss, which may confound these disabling effects (Fowler 1944; Sanchez 2002). Nevertheless, the association between hearing loss and tinnitus is not simple or straightforward; not all people with hearing loss experience tinnitus, and conversely some people with clinically normal hearing have tinnitus (Baguley 2013). It has been reported that 40% of patients are unable to identify what health condition is associated with their tinnitus onset, i.e. the tinnitus is idiopathic (Henry 2005). An important implication in clinical research is that outcome measures need to distinguish benefits specific to improved hearing from those specific to improvement in the psychological aspects of tinnitus.
Diagnosis and clinical management of tinnitus
There is no standard procedure for the diagnosis or management of tinnitus. Practice guidelines and the approaches described in studies of usual clinical practice typically reflect differences between the clinical specialisms of the authors or differences in the clinical specialisms charged with meeting tinnitus patients' needs (medical, audiology/hearing therapy, clinical psychology, psychiatry), or the available resources of a particular country or region (access to clinicians or devices, for example) (Biesinger 2010; Cima 2012; Department of Health 2009; Hall 2011; Henry 2008; Hoare 2011). Common across all these documents, however, is the use or recommendation of written questionnaires to assess tinnitus and its impact on patients and their families by measuring tinnitus symptom severity (e.g. impact of tinnitus on quality of life, activities of daily living or sleep), and a judgement about patients who are experiencing a degree of psychological distress (depression or anxiety). Assessment of the perceptual characteristics of tinnitus (pitch, loudness, minimum masking level) and residual inhibition are also recommended (Cima 2019). Although these measures do not correlate well with tinnitus symptom severity (Hiller 2006), they can prove useful in patient counselling (Henry 2004), as a baseline before start of treatment (El Refaie 2004), or by demonstrating stability of the tinnitus percept over time (Department of Health 2009).
Clinical management strategies include education and advice, relaxation therapy, tinnitus retraining therapy (TRT), cognitive behavioural therapy (CBT), sound enrichment using ear‐level sound generators or hearing aids, and drug therapies to manage co‐morbid symptoms such as insomnia, anxiety or depression (for example, Department of Health 2009; Tunkel 2014). As yet, no drug has been approved for tinnitus by a regulatory body (e.g. the European Medicines Agency or US Food and Drug Administration).
Pathophysiology
Most people with chronic tinnitus have some degree of measurable hearing loss (Ratnayake 2009), and the prevalence of tinnitus increases with greater hearing loss (Han 2009; Martines 2010). The varying theories of tinnitus generation involve changes in either function or activity of the peripheral (cochlea and auditory nerve) or central auditory nervous systems (Henry 2005). Theories involving the peripheral systems include the discordant damage theory, which predicts that the loss of outer hair cell function, where inner hair cell function is left intact, leads to a release from inhibition of inner hair cells and aberrant activity (typically hyperactivity) in the auditory nerve (Jastreboff 1990). Such aberrant auditory nerve activity can also have a biochemical basis, resulting from excitotoxicity or stress‐induced enhancement of inner hair cell glutamate release with upregulation of N‐methyl‐D‐aspartate (NMDA) receptors (Guitton 2003; Sahley 2001).
In the central auditory system, structures implicated as possible sites of tinnitus generation include the dorsal cochlear nucleus (Middleton 2011; Pilati 2012), the inferior colliculus (Dong 2010; Mulders 2010), and the auditory and non‐auditory cortex (discussed further below). There is a strong rationale that tinnitus is a direct consequence of maladaptive neuroplastic responses to hearing loss (Moller 2000; Muhlnickel 1998). This process is triggered by sensory deafferentation and a release from lateral inhibition in the central auditory system allowing irregular spontaneous hyperactivity within the central neuronal networks involved in sound processing (Eggermont 2004; Rauschecker 1999; Seki 2003). As a consequence of this hyperactivity, a further physiological change noted in tinnitus patients is increased spontaneous synchronous activity occurring at the subcortical and cortical level, measurable using electroencephalography (EEG) or magnetoencephalography (MEG) (Dietrich 2001; Tass 2012; Weisz 2005). Another physiological change thought to be involved in tinnitus generation is a process of functional reorganisation, which amounts to a change in the response properties of neurons within the primary auditory cortex to external sounds. This effect is well demonstrated physiologically in animal models of hearing loss (Engineer 2011; Norena 2005). Evidence in humans, however, is limited to behavioural evidence of cortical reorganisation after hearing loss, demonstrating improved frequency discrimination ability at the audiometric edge (Kluk 2006; McDermott 1998; Moore 2009; Thai‐Van 2002; Thai‐Van 2003), although Buss 1998 did not find this effect. For comprehensive reviews of these physiological models, see Adjamian 2009 and Norena 2011.
It is also proposed that spontaneous hyperactivity could cause an increase in sensitivity or 'gain' at the level of the cortex, whereby neural sensitivity adapts to the reduced sensory inputs, in effect stabilising mean firing and neural coding efficiency (Norena 2011; Schaette 2006; Schaette 2011). Such adaptive changes would be achieved at the cost of amplifying 'neural noise' due to the overall increase in sensitivity, ultimately resulting in the generation of tinnitus.
Increasingly, non‐auditory areas of the brain, particularly areas associated with emotional processing, are also implicated in bothersome tinnitus (Rauschecker 2010; Vanneste 2012). Vanneste 2012 describes tinnitus as "an emergent property of multiple parallel dynamically changing and partially overlapping sub‐networks", implicating the involvement of many structures of the brain more associated with memory and emotional processing in tinnitus generation. However, identification of the structural components of individual neural networks responsible for either tinnitus generation or tinnitus intrusiveness, which are independent of those for hearing loss, remains open to future research (Melcher 2013). One further complication in understanding the pathophysiology of tinnitus is that not all people with hearing loss have tinnitus and not all people with tinnitus have a clinically significant and measurable hearing loss. Other variables, such as the profile of a person's hearing loss, may account for differences in their tinnitus report. For example, König 2006 found that the maximum slope within audiograms was higher in people with tinnitus than in people with hearing loss who do not have tinnitus, despite the 'non‐tinnitus' group having the greater mean hearing loss. This suggests that a contrast in sensory inputs between regions of normal and elevated threshold may be more likely to result in tinnitus. However, this finding is not consistent across the literature (Sereda 2011; Sereda 2015).
Description of the intervention
Extracts of Ginkgo biloba leaves have been used for medicinal purposes for at least 5000 years in China, where they form an important component of the traditional Chinese pharmacopoeia (a book which lists drugs and instructions for their use). More recently Ginkgo biloba extracts have been used in Western countries. In the USA, Canada and the UK extracts are widely available as non‐prescription food supplements (Diamond 2013; Mei 2017; Ude 2013). In France and Germany a standardised dry leaf extract is registered as a drug and is commonly prescribed for tinnitus (Hall 2011; Ude 2013). However, there are several components in the available Gingko biloba preparations. A purified and enriched liquid extract is prepared from dried leaves of the maidenhair plant. The liquid extract is dried to give one part extract from 50 raw leaves. The most important active chemical compounds are flavonoids (ginkgo‐flavone glycosides) and terpenoids (ginkgolides A, B, C, J and bilobalide). Ginkgolides appear to be unique to Ginkgo biloba and have not been isolated from any other plant species. Standardised Ginkgo leaf extracts have been used in clinical trials for tinnitus, and cognitive and cardiovascular disorders, at daily doses of 60 mg to 450 mg (Mei 2017; Yang 2011). These preparations contain standardised amounts of the above compounds. EGb761 (Tebonin, Tanakan, Rökan) contains 24% ginkgo‐flavone glycosides and 6% terpenoids, and LI 1370 (Kaveri) contains 25% ginkgo flavone glycosides and 6% terpenoids (Blumenthal 1998; Mei 2017). Although the quantities are standardised, the manufacturing process is different and the ratio of active ingredients within each sub‐class may be different. There is no standardisation for food supplement preparations (Mei 2017).
The most commonly reported adverse effect of Ginkgo biloba is mild gastrointestinal disturbance (e.g. stomach pain, change in bowel habit). Serious adverse effects are rare, but include bleeding problems, interaction with anticoagulant medication and seizures (Ernst 2002; Mei 2017; Rajarajan 2018).
How the intervention might work
Several mechanisms of action of Ginkgo biloba have been proposed in the light of its many active ingredients. Human, animal and in vitro studies indicate the following effects:
A vasoregulatory effect (altering the tone of blood vessels) promoting increased blood flow (Diamond 2013; Lichota 2019; Nuhu 2014; Shu 2019; Xia 2007; Zhou 2004). Animal and human studies have shown that Ginkgo biloba can increase skin (Boelsma 2004; Jung 1990; Koltinger 1989), cardiac (Xiao 2019), and cerebral blood flow (Li 2018; Mashayekh 2011).
Antagonism of platelet activating factor (PAF) (Diamond 2013; Nash 2015; Xia 2007; Zhou 2004). This effect is specific to the ginkgolides (predominantly B). PAF causes platelet (a blood constituent involved in blood clot formation) aggregation, neutrophil degranulation (activation of immune cells within the blood stream) and oxygen radical production. Ginkgolides appear to protect against the effects of hypoxic brain injury from cerebral ischaemia (permanent brain damage caused by insufficient blood and oxygen supply) in laboratory animals (Braquet 1991; DeFeudis 1991; Li 2018; Smith 1996), and humans (Oskouei 2013).
Antioxidant activity including scavenging of free radicals, indirectly inhibiting formation of free radicals, regulation of oxidative stress and anti‐lipid peroxidation (Lichota 2019; Mahadevan 2008; Singh 2019; Zhou 2004; Zuo 2017).
Changes in the metabolism of neurons (Blecharz‐Klin 2009; DeFeudis 2000; Eckert 2005), and restoration of age‐related deficiencies in central neurotransmitter systems (Blecharz‐Klin 2009; DeFeudis 2000; Fehske 2009).
Enhancement of neuronal plasticity including increased long‐term potentiation, spine density, neuritogenesis and neurogenesis, as shown in pre‐clinical reports (Müller 2012).
Anti‐inflammatory effects and protective actions against brain damage, possibly through its terpenoids and ginkgolides (Cheng 2003; Lichota 2019; Nuhu 2014; Shu 2019; Xia 2007; Zhang 2016). The Ginkgo biloba leaf extract has been shown to reduce the level of cytokines and inflammatory factors such as tumour necrosis factor alpha (TNF‐α), interleukin 6 (Il‐6), interleukin 1 beta (Il 1‐β) and matrix metalloproteinase 9 (Omidkhoda 2019; Zhang 2016).
These mechanisms may treat tinnitus by preventing free‐radical damage to the cochlea, or increasing blood flow, improving the health of the inner ear (Didier 1996; Smith 2013). Tziridis 2014 tested the effectiveness of prophylactic treatment with EGb 761 for noise‐induced hearing loss and development of tinnitus after noise trauma in an animal model. Based on the results they suggested significant neuroplastic effects of EGb 761 on auditory processing at the peripheral and central level of the auditory pathway as measured with behavioural and electrophysiological approaches. They proposed a model of the effects of EGb 761 on auditory processing with two main effects: 1) an increase in auditory brainstem activity leading to an increased thalamic input to the primary auditory cortex; and 2) an asymmetric effect on lateral inhibition in the primary auditory cortex.
A study by Krauss 2016 examined the therapeutic effects of EGb 761 after the formation of permanent noise‐induced hearing loss and tinnitus in an animal model. They found that treatment with EGb 761 led to recovery of auditory thresholds and reduced behavioural signs of tinnitus. Interestingly, while the auditory thresholds were maintained, behavioural signs of tinnitus reappeared after discontinuation of treatment. An analysis of the auditory brainstem responses (ABRs) showed changes in ABR wave amplitude and latency at different levels of the auditory pathway (increase of response to low stimulus intensities and decrease at high intensities) rather than restoration of the auditory processing back to pre‐trauma conditions. Based on that result, the authors suggested a global inhibitory mechanism that counteracts tinnitus. The EGb 761 extract was also shown to protect against noise‐induced hearing loss by inhibiting the expression of proinflammatory cytokines and cyclooxygenase 2 (COX‐2), and increasing values of heat shock proteins in the rat cochlea (Dogan 2018). It was also shown to protect against cisplatin‐ and gentamicin‐induced hair cell loss, and subsequent changes in brain activity in animal models (Huang 2007; Yang 2011).
Why it is important to do this review
In England alone there are an estimated ¾ million general practice consultations every year where the primary complaint is tinnitus (El Shunnar 2011), equating to a major burden on healthcare services. Use of Gingko biloba for tinnitus is currently recommended against in the European tinnitus guideline (Cima 2019) and the American Academy of Otolaryngology Clinical Practice Guideline (Tunkel 2014). Both guidelines conclude that there is no proven efficacy of Gingko biloba and that there is potential for harm. There is evidence that Ginkgo biloba interacts with antithrombotic drugs to cause serious bleeding and increases bleeding risk in clotting disorders (Posadzki 2012). A worldwide survey of dietary supplements used to treat tinnitus reported that Ginkgo biloba was the most cited supplement resulting in adverse effects (diarrhoea, nausea, hearing, dizziness, headache, bleeding, blood pressure changes, chest pain, palpitation and increased urination) (Coelho 2016). Despite this, Ginkgo biloba is commonly used for tinnitus (Hall 2011). A survey of treatment options for subjective tinnitus showed that Ginkgo biloba was a popular first‐line treatment prescribed by general practitioners (GPs) and ENT physicians across Europe, with a proportion of patients prescribed Ginkgo biloba as high as 71% in some countries (Hall 2011). Ginkgo biloba is freely available for purchase in health food stores across Europe and America (Chan 2007). A recent survey showed that 1 in 10 people with tinnitus in the UK use alternative therapies, including Gingko biloba (McFerran 2018).
The previous Cochrane Review on this question concluded that there was no evidence that Gingko biloba was effective in patients with a primary complaint of tinnitus (Hilton 2013). However, the methods and searches used in that review now require updating.
Objectives
To assess the effects of Ginkgo biloba for tinnitus in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
We included studies with following design characteristics:
randomised controlled trials, including cluster‐randomised (cross‐over trials were eligible if data from before the cross‐over could be extracted, to avoid the potential for a carry‐over phenomenon).
We excluded studies with the following design characteristics:
quasi‐randomised controlled studies.
We applied no restrictions on language, year of publication or publication status.
Types of participants
Adults and children with acute (≤ 3 months) or chronic (> 3 months) subjective tinnitus.
Types of interventions
The review included all courses of Ginkgo biloba, regardless of dose regimens or formulations, and for any duration of treatment.
The main comparison was:
Ginkgo biloba versus placebo.
Other possible comparison pairs included:
Ginkgo biloba versus no intervention;
Ginkgo biloba versus education and information only.
Concurrent use of other medication or other treatment was acceptable if used equally in each group. For example, Ginkgo biloba with an additional intervention versus placebo with an identical intervention. Where an additional intervention was used equally in both groups, we analysed this as a separate comparison.
Types of outcome measures
We analysed the following outcomes in the review, but did not use them as a basis for including or excluding studies.
Primary outcomes
-
Tinnitus symptom severity (such as the impact of tinnitus on quality of life, activities of daily living and sleep), as measured by the global score on a multi‐item tinnitus questionnaire (Table 3). These include:
Tinnitus Questionnaire (Hallam 1996; Hiller 1992);
Tinnitus Functional Index (Meikle 2012);
Tinnitus Handicap Inventory (Newman 1996);
Tinnitus Handicap Questionnaire (Kuk 1990);
Tinnitus Reaction Questionnaire (Wilson 1991);
Tinnitus Severity Scale (Sweetow 1990).
Serious adverse effects: bleeding, seizures.
1. Examples of questionnaires measuring tinnitus symptom severity.
| Measurement instrument (author, year) | Number of items and subscales | Internal consistency (Cronbach’s alpha for global score) |
| Tinnitus Functional Index (Meikle 2012) | 25 items, 8 subscales | a = 0.97 |
| Tinnitus Handicap Inventory (Newman 1996) | 25 items, 3 subscales | a = 0.93 |
| Tinnitus Handicap Questionnaire (Kuk 1990) | 27 items, 3 subscales | a = 0.94 |
| Tinnitus Questionnaire (Hallam 1996) | 52 items, 5 subscales | a = 0.94 |
| Tinnitus Reaction Questionnaire (Wilson 1991) | 26 items, 4 subscales | a = 0.96 |
| Tinnitus Severity Scale (Sweetow 1990) | 15 items | Not reported |
Secondary outcomes
Tinnitus loudness (a change in subjective perception) measured using either patient‐reported instruments (including visual analogue scales or numerical rating scales) or performance‐based procedures (including tinnitus loudness matching or minimum masking level).
Tinnitus intrusiveness measured using a self‐report multi‐item questionnaire or validated subscale (Hall 2018a).
Generalised depression as measured by validated questionnaires, such as the Beck Depression Inventory II (Beck 1996), the depression scale of the Hospital Anxiety and Depression Scale (HADS; Zigmond 1983), and the Hamilton Rating Scale for Depression (Hamilton 1960).
Generalised anxiety as measured by a validated scale, for example the anxiety scale of the HADS or Beck Anxiety Inventory (Beck 1988) or the Anxiety Sensitivity Index (Reiss 1986).
Health‐related quality of life as measured by a validated scale, for example the Short‐Form 36 (Hays 1993), WHOQoLBREF (Skevington 2004), and other WHOQoL versions, and the Health Utilities Index (Furlong 2001).
Other adverse effects: gastrointestinal upset, headache, allergic reaction.
We assessed outcomes as short‐term (less than three months) and long‐term (three to six months). We also considered whether these outcomes are sustained beyond six months.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 7 June 2022.
Electronic searches
The Information Specialist searched:
the Cochrane ENT Register (searched via the Cochrane Register of Studies 7 June 2022);
the Cochrane Central Register of Controlled Trials (CENTRAL 2022, Issue 6) (searched via the Cochrane Register of Studies);
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 7 June 2022);
Ovid EMBASE (1974 to 7 June 2022);
Ovid AMED (1985 to 7 June 2022);
Web of Science, Web of Science (1945 to 7 June 2022);
EBSCO CINAHL (1982 to 7 June 2022);
LILACS (Latin American and Caribbean Health Science Information database), lilacs.bvsalud.org (searched 7 June 2022);
CNKI, www.cnki.com.cn (searched via Google Scholar 1999 to 7 June 2022);
ClinicalTrials.gov, (searched via clinicaltrials.gov to 7 June 2022);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (search via https://trialsearch.who.int 7 June 2022).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
We did not perform a separate search for adverse effects. We considered adverse effects described in the included studies only.
Data collection and analysis
Selection of studies
Two authors (MS, MH, AER and DJH) independently reviewed all records retrieved to determine their eligibility for inclusion in the review. Two authors (MS, MH, AER and DJH) reviewed the full‐text articles of the retrieved studies and independently applied the inclusion criteria. Any disagreements were discussed, involving a third author if necessary until a consensus was reached.
Data extraction and management
MS, MH, AER and DJH independently extracted data using a purposefully designed data extraction form. We piloted the data extraction form on a subset of articles and revised it as indicated before formal data extraction began. Where necessary or where insufficient data were provided for the study, we contacted study authors for further information.
Information extracted included: study design, setting, methods or randomisation and blinding, power, inclusion and exclusion criteria, type of intervention and control, treatment duration, treatment fidelity, type and duration of follow‐up, and outcome measures and statistical tests.
Data extracted included: baseline characteristics of participants (age, sex, duration of tinnitus, tinnitus symptom severity, tinnitus loudness and pitch estimates, details of co‐morbid hearing loss, anxiety or depression) and details of any attrition or exclusion.
Outcome data included: group mean and standard deviation at pre‐ and post‐intervention and follow‐up, and results of any statistical tests of between‐group comparisons.
Where not reported or provided by the authors we estimated standard deviations in RevMan 5.3 (RevMan 2014) using the available data, such as standard errors, confidence intervals, P values and t values. Where data were only available in graph form, we made and agreed numeric estimates.
After independent data extraction by MS, MH, AER and DJH, authors reviewed the extracted data for disagreements, and revisited and discussed the relevant studies as required to reach a final consensus.
Assessment of risk of bias in included studies
MS, MH, AER and DJH independently assessed risk of bias of the included studies, with the following taken into consideration, as guided by the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2019):
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
We used the Cochrane risk of bias tool in RevMan 5.3 (RevMan 2014), which involves describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias. We resolved differences of opinion by discussion.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RR) with 95% confidence intervals (CIs). We summarised continuous outcomes as mean differences (MD) with 95% CI, provided that the selected studies used the same scale of measurement. We had planned to use standardised mean differences (SMD) when different scales of measurement had been used to measure the same outcome but the studies all used the same scale.
Unit of analysis issues
For parallel‐group RCTs the unit of analysis was the group mean. We planned to use alternative analyses for cluster‐randomised trials but none were identified. For studies with more than two intervention groups, we planned to either combine groups to create a single pair‐wise comparison or, if this was not appropriate, select the most relevant pair of interventions for comparison. We selected the most relevant pair of interventions for comparison for studies with more than two arms, in line with the comparison of interest for this review.
Dealing with missing data
We planned that where necessary and where sufficient data from the study were not provided, we would contact the authors of the study requesting further details about missing data and reasons for the incompleteness of the data. We were alert to potential mislabelling or non‐identification of standard errors and standard deviations. Our method for imputation would have been according to chapter Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2019). Other missing data would not have been imputed. The studies identified did not have missing data so we performed complete case analyses and no sensitivity analysis was used.
Assessment of heterogeneity
We determined whether the selected studies suffered from clinical, statistical and methodological heterogeneity. We quantified statistical heterogeneity using the I2 statistic and the Chi2 test. With respect to the I2 statistic, an approximate guide to interpretation is provided in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2019). If the I2 value was 50% or higher, we considered the data to suffer from substantial or considerable heterogeneity. For the Chi2 test, we used the indicator that where the Chi2 is greater than the degrees of freedom (where the degrees of freedom are the number of studies K minus 1), then heterogeneity is likely to be present. We considered heterogeneity to be statistically significant if the P value was less than 0.10. Subsequently, in the absence of heterogeneity (I2 = 0%, P = 0.41) we performed the meta‐analysis using a fixed‐effect model.
Assessment of reporting biases
We searched for and requested study protocols for the included studies and, where available, we evaluated whether there was evidence of selective reporting. We planned to assess publication bias using a funnel plot and Egger's test if a meta‐analysis contained at least 10 studies. Unfortunately, none of the meta‐analyses contained more than two studies.
Data synthesis
We planned to perform meta‐analyses using RevMan 5.3 if more than one study was identified for a given outcome (RevMan 2014). Two studies were identified for our main outcome of tinnitus symptom severity and four for other adverse effects for the comparison of Ginkgo biloba versus placebo For other comparisons there was not data from more than one study so it was not possible to calculate a pooled estimate.
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analyses to explore the potential effect modifiers of hearing loss, baseline tinnitus symptom severity and baseline anxiety or depression. However, insufficient data were available.
Sensitivity analysis
We planned to conduct a sensitivity analysis by excluding those studies with a high risk of bias, thereby checking the robustness of the conclusion from the studies included in the meta‐analysis. However, only one to four studies were included in the meta‐analyses, all with similar, non‐significant estimates of effect. Moreover, all of the included studies carried a high or unclear risk of bias.
Summary of findings and assessment of the certainty of the evidence
Two authors (MS and JX) used the GRADE approach to rate the overall certainty of evidence using GRADEpro GDT (https://gradepro.org/). The certainty of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high certainty of evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of very low certainty implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high certainty. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision; and
publication bias.
We planned to include a summary of findings table, constructed according to the recommendations described in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2019), for the following comparison:
Ginkgo biloba versus placebo.
In addition we included a summary of findings table for the comparison:
Gingko biloba with concurrent intervention versus concurrent intervention only.
We included the following outcomes in the summary of findings tables:
tinnitus symptom severity;
serious adverse effects (bleeding disorders, seizures);
tinnitus loudness;
health‐related quality of life;
other adverse effects (gastrointestinal upset, headache, allergic reaction).
Results
Description of studies
Results of the search
Our electronic database search on 7 June 2022 identified 1036 records, of which 492 remained after removing duplicates. We discarded 455 records based on title and/or abstract. We retrieved 37 articles for full‐text screening. We excluded 17 studies because they had the wrong study design (n = 11), had the wrong intervention or comparator (n = 4), had the wrong patient population (n = 1) or were a trial that was terminated before obtaining evaluable data (n = 1) (see Characteristics of excluded studies). Two records are awaiting further classification as full texts could not be obtained (Characteristics of studies awaiting classification). Five records supplemented the methodological information that was extracted for three included studies. We identified one ongoing study (Characteristics of ongoing studies).
In total, 12 completed studies met our inclusion criteria (Cekkayan 1996; Drew 2001; Fucci 1992; Halama 1988; Meyer 1986; Morgenstern 1997; Napryeyenko 2009; Nishad 2019; Polanski 2016; Radnuz 2019; Rejali 2004; Yarmohammadi 2007). Five of these studies reported quantitative data that we included in quantitative synthesis (Cekkayan 1996; Drew 2001; Morgenstern 1997; Polanski 2016; Rejali 2004).
We identified no additional records from other sources. A flowchart of study retrieval and selection is provided in Figure 1.
1.
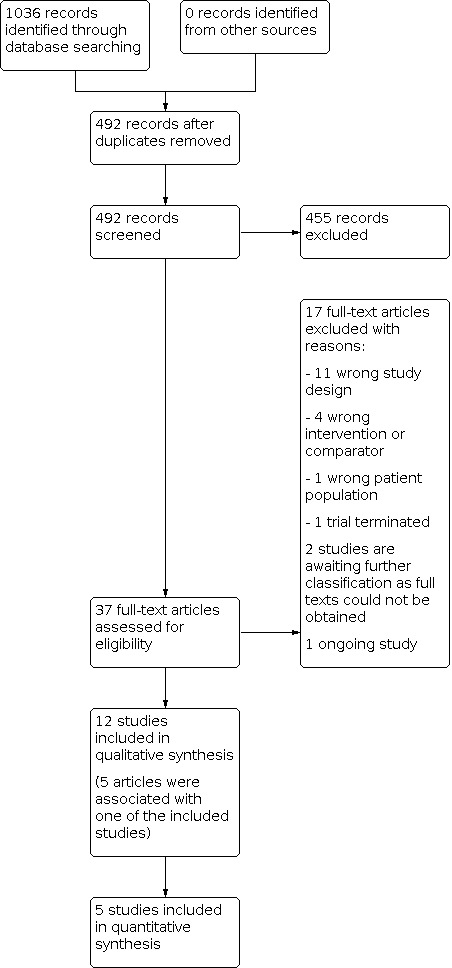
Included studies
See Characteristics of included studies.
We included 12 published studies (Cekkayan 1996; Drew 2001; Fucci 1992; Halama 1988; Meyer 1986; Morgenstern 1997; Napryeyenko 2009; Nishad 2019; Polanski 2016; Radnuz 2019; Rejali 2004; Yarmohammadi 2007).
Design
All included studies were parallel‐group RCTs. Four of the included studies had more than two treatment arms. Cekkayan 1996 was a three‐arm trial comparing Ginkgo biloba, betahistine and placebo. Nishad 2019 was a four‐arm trial comparing Ginkgo biloba to placebo and caroverine to placebo. Radnuz 2019 was a three‐arm trial comparing Ginkgo biloba, hearing aids and Ginkgo biloba with hearing aids. Polanski 2016 was a four‐arm trial comparing Gingko biloba, α‐lipoic acid plus vitamin C, papaverine hydrochloride plus vitamin E and placebo.
Sample sizes
The total sample size for all included studies was 1915 (range 22 to 978 participants).
Setting
Seven studies were set in ENT departments in Brazil, France, Germany, India, Iran, Turkey and the UK (Cekkayan 1996; Meyer 1986; Morgenstern 1997; Nishad 2019; Polanski 2016; Rejali 2004; Yarmohammadi 2007), one in an audiology centre in Brazil (Radnuz 2019), one in a neurology practice in Germany (Halama 1988), one in psychiatric or neurological hospitals in Ukraine (Napryeyenko 2009), and one was conducted completely by email and telephone (Drew 2001). One study did not provide setting details (Fucci 1992).
Participants
Eight studies included participants with tinnitus as a main complaint. Two studies included participants with neurological conditions who also had tinnitus; in Napryeyenko 2009 all participants had either Alzheimer's disease or vascular dementia and in Halama 1988 all patients had "mild cerebral insufficiency of vascular origin". The participant groups in these two studies may not fully represent the tinnitus population (Halama 1988; Napryeyenko 2009). Polanski 2016 included participants with sensorineural hearing loss who also complained of tinnitus.
Eleven studies recruited adult participants (18 years or over). In one study the age range was 14 to 70 years (Cekkayan 1996). Three studies recruited older participants: Halama 1988 recruited participants over 55 years of age, Napryeyenko 2009 50 years or older and Polanski 2016 60 years and over. Nishad 2019 had an upper age cut‐off for participants of 60 years and Drew 2001 of 70 years. The mean age of participants in the included studies ranged from 38.8 to 66.6 years. Mean age was not reported in two studies (Fucci 1992; Nishad 2019).
Three studies did not report the gender of the participants (Fucci 1992; Halama 1988; Morgenstern 1997), and three did not report gender separately for the arms of interest, only for all participants in all study arms (Nishad 2019; Polanski 2016; Radnuz 2019). In the remaining studies 56% of participants were men and 44% of participants were women. Men accounted for between 28% and 69% of participants, depending on the study. One study had a markedly larger proportion of women than men (Napryeyenko 2009; 72%) and two studies had a markedly larger proportion of men than women (Drew 2001; Nishad 2019; 69% and 67% respectively).
Baseline hearing level was rarely reported in the included studies. Only one study reported baseline hearing thresholds for participants (Rejali 2004), with averaged air‐conduction thresholds at 0.5 kHz, 1 kHz, 2 kHz and 4 kHz for both ears of 26.8 dB in the intervention group and 35.2 dB in the placebo group. Participants in Cekkayan 1996 had various degrees of uni‐ or bilateral sensorineural hearing loss varying from mild to profound. In Morgenstern 1997, all participants had normal hearing at three neighbouring frequencies in the audiogram. Nishad 2019 performed audiometry (0 kHz to 16,000 Hz) for all participants and had an inclusion criterion of "high frequency loss and sensorineural hearing loss is consistent with the diagnosis of cochlear synaptic tinnitus". Participants in Polanski 2016 had a variable degree of sensorineural hearing loss confirmed by previous audiometric testing. Yarmohammadi 2007 recruited participants with normal hearing. Five studies did not report the baseline hearing level (Drew 2001; Fucci 1992; Halama 1988; Meyer 1986; Napryeyenko 2009; Radnuz 2019).
Individual tinnitus duration ranged from four months to over 10 years. Meyer 1986 recruited participants with recent tinnitus, i.e. tinnitus that had appeared within less than one year and Radnuz 2019 with a complaint of tinnitus for at least three months. Drew 2001 excluded patients with a tinnitus duration of less than 12 months. Tinnitus duration was not reported in Cekkayan 1996, Fucci 1992, Halama 1988, Napryeyenko 2009, Nishad 2019 or Polanski 2016.
The majority of included studies did not specify inclusion criteria based on tinnitus symptom severity, presence of tinnitus being sufficient. Polanski 2016 recruited participants with "clinical complaints of tinnitus", Radnuz 2019 with "complaint of tinnitus" and Rejali 2004 with "tinnitus as only or main presenting complaint". Five studies reported baseline tinnitus symptom severity. In Meyer 1986, the average tinnitus severity measure on the 1 to 4 scale (mild to severe) was 2.46 (standard deviation (SD) 0.9) in the intervention group and 2.47 (SD 0.6) in the control group. In Napryeyenko 2009, baseline tinnitus symptom severity in the whole study sample of participants with dementia measured on the 11‐point box scale (from 0 indicating absence to 10 indicating extreme severity of the symptom) was 2.1 (SD 2.3) in the Ginkgo biloba group and 2.1 (SD 2.2) in the placebo group. From 400 participants enrolled in the study 204 had tinnitus (101 in the Ginkgo biloba group and 103 in the placebo group). Baseline symptom severity for participants with tinnitus was 4.02 in the Ginkgo biloba group and 3.90 in the placebo group. Three studies reported baseline tinnitus symptom severity measured with the THI (Polanski 2016; Radnuz 2019; Rejali 2004). In Polanski 2016, baseline tinnitus symptom severity measured with the THI was 32.8 (SD 19.9; range 12 to 80) in the Ginkgo biloba group and 28.2 (SD 25.1; range 2 to 72) in the placebo group. In Radnuz 2019, the baseline THI scores were 57.5 (SD 7.5) in the Ginkgo biloba plus hearing aids group and 52.5 (SD 5) in the hearing aid group. In Rejali 2004, these were 37.5 (SD 20.5) in the intervention group and 50.7 (SD 23.3) in the placebo group. Baseline tinnitus symptom severity was not reported in seven studies (Cekkayan 1996; Drew 2001; Fucci 1992; Halama 1988; Morgenstern 1997; Nishad 2019; Yarmohammadi 2007).
Baseline anxiety and/or depression scores were not reported in any of the included studies. In Napryeyenko 2009, participants with severe depression were excluded by requiring a score below 20 on the 17‐item Hamilton Rating Scale for Depression (HAMD).
Interventions and comparisons
Eleven studies evaluated the effects of Ginkgo biloba (Cekkayan 1996; Drew 2001; Fucci 1992; Halama 1988; Meyer 1986; Morgenstern 1997; Napryeyenko 2009; Nishad 2019; Polanski 2016; Rejali 2004; Yarmohammadi 2007), and one study the effects of Ginkgo biloba with concurrent intervention (digital Beltone hearing aids) (Radnuz 2019).
The daily dosage of ginkgolides varied from 80 mg daily to 240 mg daily. Daily dosage was not reported in Fucci 1992. Duration of treatment varied from two weeks to six months.
The comparator in nine studies was a placebo not otherwise specified (Cekkayan 1996; Drew 2001; Fucci 1992; Halama 1988; Meyer 1986; Morgenstern 1997; Napryeyenko 2009; Nishad 2019; Rejali 2004; Yarmohammadi 2007). In Polanski 2016, starch capsules were used as a placebo. In Radnuz 2019, the comparator was the digital Beltone hearing aid.
Outcomes
Seven included studies used one of the pre‐specified outcome measures (Types of outcome measures) (Cekkayan 1996; Drew 2001; Fucci 1992; Morgenstern 1997; Polanski 2016; Radnuz 2019; Rejali 2004).
Primary outcomes
Three studies reported changes in tinnitus symptom severity before and after treatment, as measured by the THI (Polanski 2016; Radnuz 2019; Rejali 2004). Four studies assessed the frequency of serious adverse events (Cekkayan 1996; Drew 2001; Morgenstern 1997; Rejali 2004). Outcomes were measured at three months (Cekkayan 1996; Drew 2001; Morgenstern 1997; Radnuz 2019; Rejali 2004), and six months (Polanski 2016).
Secondary outcomes
Three studies assessed tinnitus loudness before and after treatment (Fucci 1992; Morgenstern 1997; Radnuz 2019). Fucci 1992 assessed tinnitus loudness measured with loudness matching and minimum masking levels, however it did not provide before and after data for those measures. Morgenstern 1997 assessed loudness measured with audiometric loudness matching. Radnuz 2019 assessed loudness using a visual analogue scale (VAS) (range 0 to 10). One study reported change in quality of life before and after treatment, as measured by the Glasgow Health Status Inventory (GHSI) (Rejali 2004). Four studies assessed other adverse effects (Cekkayan 1996; Drew 2001; Morgenstern 1997; Rejali 2004).
Outcomes were measured at three months (Cekkayan 1996; Drew 2001; Morgenstern 1997; Radnuz 2019; Rejali 2004).
None of the studies reported changes in depressive symptoms or depression, anxiety symptoms or generalised anxiety. None of the studies included measures of tinnitus intrusiveness.
Other outcomes
Cekkayan 1996 reported change in tinnitus symptom severity using a single‐item five‐point Likert scale: 0 = the tinnitus disappeared completely; 1 = great relief, but the complaint was still ongoing; 2 = relieved by 50%; 3 = relief was very small; 4 = no changes were noticed. Drew 2001 reported change in loudness measured with a categorical rating scale (‐6 to 6) and change in troublesome nature of tinnitus measured with a categorical rating scale (‐4 to 4). Halama 1988 reported change in the severity of tinnitus assessed on a four‐point rating scale. Meyer 1986 reported change in tinnitus severity on a scale from 1 to 7 (1 to 2 = deterioration; 3 = no change; 4 = slight improvement; 5 = improvement; 6 = major improvement; 7 = discontinued due to intolerance). Napryeyenko 2009 reported change in tinnitus severity, using an 11‐point box scale, 0 representing absence and 10 indicating extreme severity of a symptom. Yarmohammadi 2007 reported change in tinnitus severity measured with an undefined questionnaire. Outcomes were collected at three months.
Excluded studies
We excluded 16 studies after reviewing the full‐text paper. We excluded 11 studies because they were not RCTs (Abascal 2012; Ahsan 2017; Coles 1988; Dau 2000; Feinberg 2003; Hajna 1999; Holgers 1994; Novotny 2000; Sadner 2017; Schneider 2000; von Wedel 1995). We excluded four studies because of the intervention or control they used (Claussen 1988; Kiefer 2019; Plath 1995; Walger 1993), and one study because of the patient population (Bruchert 1991). See Characteristics of excluded studies for details.
Two studies are awaiting classification as full texts could not be obtained (Fandriantika 2017; Rogowski 2001).
We identified one ongoing study comparing oral administration of Ginkgo biloba extract tablets with medication combined with music in people with tinnitus (Characteristics of ongoing studies).
Risk of bias in included studies
We assessed risk of bias based on the information provided in the published reports. See Figure 2 and Figure 3 for a graph and summary of risk of bias across studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We considered the risk of selection bias due to inadequate sequence generation to be high in two studies (Fucci 1992; Yarmohammadi 2007), unclear in three studies (Cekkayan 1996; Meyer 1986; Morgenstern 1997), and low in seven studies (Drew 2001; Halama 1988; Napryeyenko 2009; Nishad 2019; Polanski 2016; Radnuz 2019; Rejali 2004). In Fucci 1992, an author confirmed the study was randomised but disclosed that there was a potential bias in the selection of participants. In Yarmohammadi 2007, Ginkgo and placebo tablets were placed in an equal number of paper bags and the doctor randomly administered the content of the bags to the patient. Four studies reported that participants had been randomised to the treatment arms, but did not provide further information on the method of sequence generation (Cekkayan 1996; Meyer 1986; Morgenstern 1997). Drew 2001 paired participants according to pre‐defined criteria and each pair was then allocated two numbers from a randomly arranged code; one number corresponded to placebo treatment and one to active treatment. In Halama 1988, intervention or placebo were randomly assigned by a computer program to participant enrolment numbers. In Napryeyenko 2009, centre‐stratified randomisation (drug–placebo ratio 1:1) in blocks of four was performed by the sponsor's biometrics unit using a validated computer program that linked ascending drug numbers to active drug or placebo, respectively. Nishad 2019 used computer‐generated block randomisation to randomise participants into four groups. Polanski 2016 used http://www.randomization.com for distribution and randomisation of participants. In Radnuz 2019, the random allocation process consisted of generating a random sequence using an Excel file and used the random allocation rule, which chooses at random one of the possible balanced assignments of the given number of participants per treatment. In Rejali 2004, randomisation was carried out by an independent third party using a card from bag system.
Allocation concealment
We judged three studies to have low risk of bias for allocation concealment (Drew 2001; Napryeyenko 2009; Rejali 2004). In Drew 2001, placebo tablets of identical size and colour were dispensed in coded bottles, and treatment allocation was masked. The allocation procedure ensured that all matched participants received active or placebo tablets without the code being identified. In Napryeyenko 2009, the randomisation list was sealed and stored safely at the sponsor's biometrics unit, and block length was not disclosed to investigators. In Rejali 2004, randomisation was carried out by an independent third party using a card from bag system. For the remaining nine studies, risk of bias due to allocation concealment was unclear as the information was not reported (Cekkayan 1996; Fucci 1992; Halama 1988; Meyer 1986; Morgenstern 1997; Nishad 2019; Polanski 2016; Radnuz 2019; Yarmohammadi 2007).
Blinding
Blinding of participants and personnel
We judged the risk of performance bias to be low in six studies (Drew 2001; Halama 1988; Napryeyenko 2009; Polanski 2016; Rejali 2004; Yarmohammadi 2007). In Drew 2001, placebo tablets were identical to the active tablets in shape, size, colour and packaging, and provided by the third party (Lichtwer Pharma). The allocation procedure ensured that all matched participants received active or placebo tablets without the code being identified. Halama 1988 used identical tablets for intervention and placebo; neither the patient nor the examining physician knew the assignment and the code was only opened after the data collection had been completed at the beginning of the evaluation. In Napryeyenko 2009, drug and placebo tablets were indistinguishable by appearance, packaging and labelling. Polanski 2016 stated that the substances were not identified by name in the containers into which they were packed, but rather through symbols defined by a professional who did not participate in the research, as a way of blinding investigators and participants. In Rejali 2004, all tablets were provided by third party (Lambert’s Health Care, Tunbridge Wells, UK). Yarmohammadi 2007 stated that the doctor and patients were unaware of the type of medication, with only the second co‐researcher in charge of numbering and packaging the bags being aware. We judged the risk of performance bias to be unclear in five of the included studies, as the information regarding blinding was not provided in the records (Cekkayan 1996; Fucci 1992; Meyer 1986; Morgenstern 1997; Nishad 2019), and high in one study as due to the nature of the intervention (hearing aids) participants were not blinded (Radnuz 2019). However, the examiners responsible for applying the questionnaires during the study were blinded to the intervention (Radnuz 2019).
Blinding of outcome assessment
The risk of detection bias was low in three studies (Drew 2001; Halama 1988; Radnuz 2019). In Drew 2001, data entry and initial analyses were carried out by a researcher blinded to the participant's allocation. In Halama 1988, neither the patient nor the examining physician knew the assignment; the code was only opened after the data collection had been completed at the beginning of the evaluation. In Radnuz 2019, the examiners responsible for applying the questionnaires during the study were blinded to the intervention. In addition, an employee outside the research team inserted data into the computer in separate data sheets so that the researchers could analyse data without having access to information about the allocation. The risk of detection bias was unclear in the remaining nine studies as the information was not provided (Cekkayan 1996; Fucci 1992; Meyer 1986; Morgenstern 1997; Napryeyenko 2009; Nishad 2019; Polanski 2016; Rejali 2004; Yarmohammadi 2007).
Incomplete outcome data
We judged four studies to have high risk of bias due to incomplete outcome data (Cekkayan 1996; Fucci 1992; Morgenstern 1997; Polanski 2016). Cekkayan 1996 reported data on changes in tinnitus symptom severity only for 8 out of 15 participants, without explaining reasons for dropout; all missing data were for participants in the placebo group. For Fucci 1992 only a brief abstract was available and we were not able to obtain additional information from the authors. No information was provided on the number of participants who completed the study and completeness of outcome measures. In Morgenstern 1997, there were missing data for the primary outcome measure for 14 (out of 49) participants in the intervention group and 12 (out of 50) in the control group at 12 weeks; this dropout was not explained. In Polanski 2016, 15 placebo and 14 test participants who had tinnitus participated in the trial, however data for only 12 and 13 participants respectively were included in the report without reasons for dropout/exclusion given. We judged Drew 2001 to have an unclear risk of bias. The study paired participants according to pre‐defined criteria, however only 956 out of 1121 participants were paired. The remaining 165 participants were also randomised and analysed, however analyses on unmatched data were not reported, except the statement that: "Unmatched analyses did not provide any additional information and have therefore been excluded from this paper". We judged seven studies to have low risk of bias due to incomplete outcome data as all participant data were reported or reasons for dropout were explained (Halama 1988; Meyer 1986; Napryeyenko 2009; Nishad 2019; Radnuz 2019; Rejali 2004; Yarmohammadi 2007).
Selective reporting
We judged seven studies to have high risk of bias due to selective reporting (Cekkayan 1996; Fucci 1992; Halama 1988; Napryeyenko 2009; Nishad 2019; Polanski 2016; Yarmohammadi 2007). In Cekkayan 1996, routine and audiological examinations were performed every 15 days, however those data were not reported. For Fucci 1992 it was not clear whether all primary outcomes were reported. In addition, no data on secondary outcomes were reported. In Halama 1988, the data for most secondary outcome measures were not reported. For Napryeyenko 2009 there were two reports from the study, one reporting the tinnitus score in the whole study population (from 0 ‐ absence of tinnitus to 10 ‐ extreme severity) and one brief report reporting tinnitus scores only in those participants with tinnitus. No additional data were reported specifically for participants with tinnitus. Nishad 2019 specified quality of life as a primary outcome measure but no results were reported. The manuscript mentioned that "tinnitus matching" improved but this was not pre‐defined as an outcome measure. The trial registration for Polanski 2016 lists hearing improvement in an audiometric test as a primary outcome, however this outcome was not described. In Yarmohammadi 2007, a questionnaire of tinnitus severity was used as an outcome measure but not described; the authors interpreted the result as no change, improvement and exacerbation but it is unclear what were the criteria for such classifications. We judged two studies to have an unclear risk of bias (Meyer 1986; Morgenstern 1997). Meyer 1986 did not specify outcomes. In Morgenstern 1997, no values for secondary outcome measures were reported, only brief statements regarding whether those improved or not were included. Drew 2001, Radnuz 2019 and Rejali 2004 reported all pre‐specified outcome measures, therefore we judged the risk of bias to be low.
Other potential sources of bias
Conflict of interest was not reported in eight studies (Cekkayan 1996; Fucci 1992; Halama 1988; Meyer 1986; Morgenstern 1997; Napryeyenko 2009; Rejali 2004; Yarmohammadi 2007), and funding was not disclosed in seven studies (Cekkayan 1996; Fucci 1992; Halama 1988; Meyer 1986; Morgenstern 1997; Nishad 2019; Polanski 2016). For nine studies there was no prospective protocol or trial registration available (Cekkayan 1996; Fucci 1992; Halama 1988; Meyer 1986; Napryeyenko 2009; Nishad 2019; Radnuz 2019; Rejali 2004; Yarmohammadi 2007).
Cekkayan 1996 did not report the statistical tests used, therefore it was not possible to judge whether the analysis of the results was appropriate. In Drew 2001, there was limited reporting of baseline data regarding tinnitus characteristics and severity. Participants were matched for age, sex and duration of tinnitus and stated cause of tinnitus but not severity. No data regarding 'unmatched' participant characteristics (i.e. was a specific group excluded from the analysis) were reported. In Fucci 1992, when contacted, one of the authors expressed concerns regarding the quality of the study, however no additional details were provided. Halama 1988 was a trial with patients with "mild cerebral insufficiency of vascular origin" and tinnitus was a secondary outcome measure. No study registration or protocol was available. For Meyer 1986 only an abbreviated version of the full study report was available. Napryeyenko 2009 was a study of the effects of Ginkgo biloba on Alzheimer's disease or vascular dementia and tinnitus was a secondary outcome measure. In Nishad 2019, there were no criteria for defining tinnitus improvement, no statistical analyses were described and the results did not justify the conclusions. Audiological selection criteria were complex and of uncertain significance in selecting the stated aetiological group. Polanski 2016 was a trial concentrating on hearing loss, with tinnitus as a secondary outcome. In Rejali 2004, baseline tinnitus severity was significantly higher in the control group than in the intervention group. No other sources of bias were identified for Radnuz 2019.
Effects of interventions
Ginkgo biloba versus placebo
Primary outcomes
Tinnitus symptom severity
Two studies measured tinnitus symptom severity using a multi‐item questionnaire (Polanski 2016; Rejali 2004). Both studies used the Tinnitus Handicap Inventory (THI). Outcomes were measured at 12 weeks (Rejali 2004) and six months (Polanski 2016). Ginkgo biloba may have little to no effect on THI scores compared to placebo, but the evidence is very uncertain. The pooled mean difference (MD) was ‐1.35 (scale 0 to 100; 95% confidence interval (CI) ‐8.26 to 5.55; 2 studies; 85 participants) (Analysis 1.1) (GRADE: very low‐certainty).
1.1. Analysis.

Comparison 1: Ginkgo biloba versus placebo, Outcome 1: Tinnitus symptom severity (THI) at 3 to 6 months
Serious adverse effects
Four studies reported adverse effects at three months follow‐up (Cekkayan 1996; Drew 2001; Morgenstern 1997; Rejali 2004). Ginkgo biloba may results in little to no difference in the risk of bleeding or seizures, with no serious adverse effects reported in either the Ginkgo biloba or placebo group (4 studies; 1154 participants; low‐certainty) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Ginkgo biloba versus placebo, Outcome 2: Serious adverse effects at 3 months (yes or no)
Secondary outcomes
Tinnitus loudness
Morgenstern 1997 found that there may be little to no difference between the effect of Ginkgo biloba or placebo on tinnitus loudness measured with audiometric loudness matching at 12 weeks, but the evidence is very uncertain (MD ‐4.00 (scale ‐10 to 140 dB), 95% CI ‐13.33 to 5.33; 1 study; 73 participants) (Analysis 1.3) (GRADE: very low‐certainty).
1.3. Analysis.

Comparison 1: Ginkgo biloba versus placebo, Outcome 3: Tinnitus loudness measured by audiometric loudness matching at 12 weeks
Tinnitus intrusiveness
None of the studies included measures of tinnitus intrusiveness.
Generalised depression
None of the studies included measures of generalised depression.
Generalised anxiety
None of the studies included measures of generalised anxiety.
Health‐related quality of life
Rejali 2004 found that there may be little to no difference in health‐related quality of life measured with the Glasgow Health Status Inventory (GHSI) at three months (MD ‐0.58 (scale 0 to 100), 95% CI ‐4.67 to 3.51; 1 study; 60 participants) (Analysis 1.4) (GRADE: low‐certainty).
1.4. Analysis.

Comparison 1: Ginkgo biloba versus placebo, Outcome 4: Health‐related quality of life at 3 months
Other adverse effects
Four studies reported adverse effects at three months follow‐up (Cekkayan 1996; Drew 2001; Morgenstern 1997; Rejali 2004). None of the participants reported adverse effects in Cekkayan 1996 and Morgenstern 1997. In Drew 2001, participants reported gastrointestinal upset (14 in the intervention group and 14 in the placebo group), headache (4 and 4 respectively) and allergic skin reaction (2 and 3 respectively) and in Rejali 2004 participants reported gastrointestinal upset (2 in the intervention group and 1 in the placebo group) and headache (1 in each group). When we pooled the data we found that Ginkgo biloba may not increase the frequency of other adverse effects compared to placebo (risk ratio 0.91, 95% CI 0.52 to 1.60; 4 studies; 1175 participants) (Analysis 1.5) (GRADE: low‐certainty).
1.5. Analysis.

Comparison 1: Ginkgo biloba versus placebo, Outcome 5: Other adverse effects at 3 months (yes or no)
Gingko biloba with concurrent intervention versus concurrent intervention only
Primary outcomes
Tinnitus symptom severity
Radnuz 2019 measured tinnitus symptom severity with the THI at three months. We found that when Ginkgo biloba was used alongside hearing aids, the reduction in tinnitus symptom severity (as measured by the THI) at three months was not as great as when hearing aids were used alone, but the evidence is very uncertain (MD 7.50 (scale 0 to 100), 95% CI 2.83 to 12.17; 1 study; 22 participants) (Analysis 2.1) (GRADE: very low‐certainty).
2.1. Analysis.

Comparison 2: Gingko biloba with concurrent intervention versus concurrent intervention only, Outcome 1: Tinnitus symptom severity (THI) at 3 months
Serious adverse effects
The study did not report serious adverse effects.
Secondary outcomes
Tinnitus loudness
Radnuz 2019 measured tinnitus loudness using a visual analogue scale (VAS) at three months. We found that when Ginkgo biloba was used alongside hearing aids, the reduction in tinnitus loudness (as measured by VAS) at three months was not as great as when hearing aids were used alone, but the evidence is very uncertain (MD 1.00 (scale 0 to 10), 95% CI 0.52 to 1.48; 1 study; 22 participants) (Analysis 2.2) (GRADE: very low‐certainty).
2.2. Analysis.

Comparison 2: Gingko biloba with concurrent intervention versus concurrent intervention only, Outcome 2: Tinnitus loudness (VAS) at 3 months (range 0 to 10)
Tinnitus intrusiveness
The study did not include measures of tinnitus intrusiveness.
Generalised depression
The study did not include measures of generalised depression.
Generalised anxiety
The study did not include measures of generalised anxiety.
Health‐related quality of life
The study did not include measures of health‐related quality of life.
Other adverse effects
The study did not report other adverse effects.
Discussion
Summary of main results
The objective of this review was to assess the effects of Ginkgo biloba for subjective tinnitus in adults and children. This review included 12 studies (1915 participants). Eleven studies investigated the effects of Ginkgo biloba compared to placebo (Cekkayan 1996; Drew 2001; Fucci 1992; Halama 1988; Meyer 1986; Morgenstern 1997; Napryeyenko 2009; Nishad 2019; Polanski 2016; Rejali 2004; Yarmohammadi 2007), and one study investigated the effects of Ginkgo biloba plus hearing aids compared to hearing aids alone (Radnuz 2019). Only six studies reported outcomes of interest to this review.
Ginkgo biloba versus placebo
At three to six months, Ginkgo biloba may have little to no effect on Tinnitus Handicap Inventory (THI) scores but the evidence is very uncertain. No participants in either the Ginkgo biloba or placebo groups reported serious adverse effects (bleeding, seizures) in the included studies. One study found that there may be little to no difference between Ginkgo biloba and placebo in tinnitus loudness at 12 weeks. One study found that there may be little to no difference between Ginkgo biloba and placebo in health‐related quality of life at three months. Ginkgo biloba may not increase the frequency of other adverse effects (such as gastrointestinal upset, headache, allergic reaction) at three months compared to placebo.
None of the studies reported the other secondary outcomes of changes in depressive symptoms or depression, anxiety symptoms or generalised anxiety, or tinnitus intrusiveness.
There is insufficient evidence to support the superiority or inferiority of Ginkgo biloba over placebo. See Table 1.
Gingko biloba with concurrent intervention versus concurrent intervention only
The use of Ginkgo biloba alongside hearing aids may result in a smaller reduction in tinnitus symptom severity (measured with the THI) and tinnitus loudness (measured with a visual analogue scale) at three months compared to when hearing aids are used alone but the evidence is very uncertain.
The study did not report tinnitus intrusiveness, or changes in depressive symptoms or depression, anxiety symptoms or generalised anxiety as measured by a validated instrument.
The evidence is very uncertain about the effect of Ginkgo biloba on the reduction in tinnitus symptom severity when used alongside hearing aids.
See Table 2.
Overall completeness and applicability of evidence
Only six studies reported outcomes of interest to this review and contributed to the meta‐analyses (Cekkayan 1996; Drew 2001; Morgenstern 1997; Polanski 2016; Radnuz 2019; Rejali 2004). Nine studies included participants with subjective idiopathic tinnitus as a main complaint. Two studies included participants with neurological conditions who also had tinnitus; in Napryeyenko 2009 all participants had either Alzheimer's disease or vascular dementia and in Halama 1988 all patients had "mild cerebral insufficiency of vascular origin". The participant groups in these two studies may not fully represent the tinnitus population.
Only one study recruited participants with recent onset of tinnitus (< 12 months; Meyer 1986), therefore we were not able to compare the effects of Ginkgo biloba for acute (≤ 3 months) versus chronic (> 3 months) tinnitus. Eleven out of 12 included studies included adult participants, with only one study recruiting patients from 14 years. Three studies recruited older participants (Halama 1988; Napryeyenko 2009; Polanski 2016), while two studies had an upper age cut‐off of 60 or 70 years (Drew 2001; Nishad 2019). There were no studies assessing the effects of Ginkgo biloba for tinnitus in children as a standalone group. One study had a markedly larger proportion of women than men (Napryeyenko 2009; 72%) and two studies had a markedly larger proportion of men than women (Drew 2001; Nishad 2019; 69% and 67% respectively). Baseline hearing level was rarely reported in the included studies. Only one study reported baseline hearing thresholds for participants (Rejali 2004). Yarmohammadi 2007 recruited participants with normal hearing. Most included studies did not specify inclusion criteria based on tinnitus symptom severity, presence of tinnitus being sufficient. Therefore, it was not possible to judge whether different study populations were representative of the whole tinnitus population with respect to hearing loss and tinnitus symptom severity. One study excluded participants with severe depression (Napryeyenko 2009).
Eleven studies evaluated the effects of Ginkgo biloba (Cekkayan 1996; Drew 2001; Fucci 1992; Halama 1988; Meyer 1986; Morgenstern 1997; Napryeyenko 2009; Nishad 2019; Polanski 2016; Rejali 2004; Yarmohammadi 2007), and one study evaluated the effects of Ginkgo biloba with digital Beltone hearing aids (Radnuz 2019). The daily dosage varied from 80 mg daily to 240 mg daily and the duration of treatment varied from two weeks to six months.
The comparator in nine studies was a placebo not otherwise specified (Cekkayan 1996; Drew 2001; Fucci 1992; Halama 1988; Meyer 1986; Morgenstern 1997; Napryeyenko 2009; Nishad 2019; Rejali 2004; Yarmohammadi 2007). In Polanski 2016, starch capsules were used as a placebo. In the analyses we pooled the different dosages and types of placebo together. In Radnuz 2019, the comparator was a digital Beltone hearing aid.
Quality of the evidence
The certainty of the evidence ranged from very low to low. We are uncertain about the estimates for change in tinnitus symptom severity, tinnitus loudness assessed with audiometric matching or visual analogue scale, quality of life and other adverse effects. The certainty of the evidence was affected by several issues, including multiple domains of high or unclear risk of bias, indirectness of evidence, imprecision and possible publication bias. Allocation concealment and blinding of participants, personnel and outcome assessments were poorly reported. Most studies were characterised by high or unclear risk of selection bias and multiple other sources of bias were identified. Only three of the included studies had a pre‐published protocol available.
Potential biases in the review process
Our searches of the electronic databases were comprehensive. We also searched the reference lists of the included studies and previous Cochrane Review (Hilton 2013). Language was not a barrier to inclusion and we obtained translations of the articles in French, German, Turkish and Persian. All author roles were pre‐defined in the review process. Two authors selected studies for inclusion and judged risk of bias independently, with recourse to a third author for resolution of any disagreement or uncertainty. Two authors independently extracted data to minimise personal bias. We adhered to a pre‐published protocol and no post hoc decisions or changes were made (Sereda 2019).
Agreements and disagreements with other studies or reviews
This is a new review, superseding the previous Cochrane Review on Ginkgo biloba for tinnitus (Hilton 2013). Hilton 2013 included three studies, all considering patients with tinnitus as a primary complaint. All three trials included in Hilton 2013 were also included in the current review. We also included two studies that had been excluded from Hilton 2013 due to lack of preferred outcomes (Halama 1988) and differences in baseline tinnitus severity between intervention and placebo groups (Meyer 1986), as those reasons are no longer considered sufficient for exclusion (Handbook 2019). In the current review we identified and included an additional seven studies with both participants with a primary complaint of tinnitus as well as tinnitus associated with neurological conditions. Similarly to the current review Hilton 2013 concluded that there was no evidence that Ginkgo biloba was effective for tinnitus, with a relatively small incidence of adverse effects that was similar between the intervention and placebo groups.
A search for systematic reviews of the effectiveness of Ginkgo biloba for tinnitus identified three reviews (Ernst 1999; Hilton 2013; von Boetticher 2011) referring to four randomised trials (Drew 2001; Meyer 1986; Morgenstern 1997; Rejali 2004), which included 1246 patients (Kramer 2018). The authors concluded that Ginkgo biloba probably did not decrease the severity of tinnitus, did not improve quality of life and did not reduce the intensity of tinnitus. Additionally, Ginkgo biloba was not associated with adverse effects in patients with tinnitus, namely there was no difference in the type and frequency of adverse effects between the intervention and placebo groups (Kramer 2018).
A review looking at the effects of Ginkgo biloba on neurosensory symptoms (tinnitus and dizziness) in patients with dementia concluded that Ginkgo biloba was effective in alleviating tinnitus in those patients (Spiegel 2018). It is interesting to hypothesise why there might be a favourable response to Ginkgo biloba when tinnitus is associated with cognitive insufficiency. The aetiology of tinnitus in cognitive insufficiency is different from primary tinnitus. For example, tinnitus in cognitive insufficiency may be caused by central vascular insufficiency or a neural metabolic disorder, whereas in primary tinnitus the initiating pathology is a cochlear disorder. Changes in vascular perfusion and neuronal metabolism are well‐documented effects of Ginkgo biloba in animal and human studies. Improved cognitive functioning with Ginkgo biloba may also allow habituation to tinnitus. Habituation to repetitive presentation of a sensory stimulus or perception is the norm. There is a strong psychological component in appropriate habituation to tinnitus. If Ginkgo biloba causes a significant improvement in overall cognitive functioning then a positive effect on tinnitus may be real, but non‐specific. A Cochrane Review, however, concluded that the evidence that Ginkgo biloba has a predictable and clinically significant benefit for people with dementia or cognitive impairment is inconsistent and unreliable (Birks 2009). In the current review we included the studies Napryeyenko 2009, where all participants had either Alzheimer's disease or vascular dementia, and Halama 1988, where all patients had "mild cerebral insufficiency of vascular origin". However, neither of the studies included outcomes of interest to the current review.
Several reviews reported side effects of Ginkgo biloba such as gastrointestinal problems, headache, nausea and vomiting, which were usually mild and reversible (Ernst 2002; Mei 2017; Rajarajan 2018). Serious adverse effects were rare but these can include bleeding problems and seizures. The most serious adverse effects reported relate to the interactions of Ginkgo biloba with other drugs, such as anticoagulant medications, which results in the reports of haemorrhage, haematoma, apraxia, permanent neurological deficit and death (Posadzki 2012). Only four studies in the current review reported adverse effects (Cekkayan 1996; Drew 2001; Morgenstern 1997; Rejali 2004). They included gastrointestinal upset, headache and allergic skin reaction. None of the studies reported any serious adverse effects, however it is worth noting that six studies excluded patients using concurrent anticoagulant medications (Drew 2001; Halama 1988; Napryeyenko 2009; Nishad 2019; Polanski 2016; Rejali 2004). The frequency of adverse effects was relatively small and similar in the intervention and placebo groups.
Authors' conclusions
Implications for practice.
Ginkgo biloba is commonly used as a food supplement for tinnitus and is also a popular first‐line treatment prescribed by general practitioners (GPs) and ENT physicians across Europe (Hall 2011). However, we did not find any evidence to support or refute the prescription of Ginkgo biloba for subjective tinnitus. There is uncertainty about the benefits and harms of Ginkgo biloba for the treatment of tinnitus when compared to placebo. The included studies did not investigate the potentially harmful interactions of Ginkgo biloba with other drugs (Posadzki 2012).
In line with the lack of evidence for the effectiveness of Ginkgo biloba and potentially harmful interactions with other drugs, current tinnitus management guidelines recommend against its use for tinnitus treatment (Cima 2019; Tunkel 2014). The Multidisciplinary European Guideline for Tinnitus (Cima 2019) based their recommendation on the results of several systematic reviews, which either concluded that Ginkgo biloba was not effective (Hilton 2013; Rejali 2004), or highlighted the low methodological rigour of the included trials (von Boetticher 2011). The authors also highlighted that there was evidence that Ginkgo biloba can interact with other anticoagulant drugs to cause serious bleeding and worsen bleeding risk in patients with underlying clotting disorders (Posadzki 2012). Similarly, the American Academy of Audiology Clinical Practice Guideline recommended against the use of Ginkgo biloba for treating patients with persistent, bothersome tinnitus (Tunkel 2014). The above recommendation was based on variation in conclusions, methodological limitations and heterogeneity in the study protocols of the randomised controlled trials (RCTs) in addition to conflicting conclusions from systematic reviews. The guideline also referenced the evidence for serious side effects, which involve platelet inhibitory actions of Ginkgo biloba, particularly if taken along with other medications that impair coagulation (Posadzki 2012), with the recommendation to avoid use of Ginkgo biloba in older adults, in which the use of anticoagulants and analgesics is widespread. Other mentioned herb‐drug interactions included thiazide diuretics, which resulted in increased blood pressure, and trazodone, which results in increased sedation (Tunkel 2014).
It is unlikely that the aetiology of tinnitus is the same for every tinnitus sufferer. Ginkgo biloba has been shown to affect vascular permeability and neuronal metabolism. If a greater level of understanding and diagnostic accuracy can be reached about the different aetiologies of tinnitus, this may naturally highlight subgroups of tinnitus patients in whom further controlled trials of Ginkgo biloba are worth considering.
Implications for research.
Future research into the effectiveness of Ginkgo biloba in patients with tinnitus should use rigorous methodology. Randomisation and blinding should be of the highest quality, given the subjective nature of tinnitus and the strong likelihood of a placebo response. The CONSORT statement should be used in the design and reporting of future studies (CONSORT 2010).
We also recommend the development of validated, patient‐centred outcome measures for research in the field of tinnitus. Visual analogue scales have limited value in this regard because quantifying change using only a single item has inadequate measurement properties (e.g. internal consistency cannot be established and test‐retest scores are at greater risk of instability). Only three out of 12 studies included in this review used multi‐item questionnaires of tinnitus symptom severity, and only one used a measure of heath‐related quality of life. Other outcomes such as depression and anxiety were not measured. Only four of the studies reported adverse effects. In future trials, multi‐item questionnaires of tinnitus symptom severity, validated instruments measuring depressive symptoms or depression, anxiety symptoms or generalised anxiety and health‐related quality of life should also be used.
Core outcome measures for adults with subjective tinnitus have been identified (Hall 2018a). For pharmacological interventions, these are tinnitus intrusiveness and loudness. None of the included studies assessed tinnitus intrusiveness, and while loudness was assessed in two out of the five included studies, the measurement methods varied. There is no standard test for tinnitus loudness, and psychoacoustic loudness matching and subjective rating methods are equally common. These measurement methods are generally applied, and findings interpreted, with the assumption that they measure the same underlying construct (i.e. that they have convergent validity). However, retrospective analysis of one randomised placebo‐controlled trial in 91 participants with subjective idiopathic tinnitus indicates otherwise (Hall 2017). Use of the core outcome set as a minimum standard for what should be assessed and reported in RCTs will facilitate comparison between studies and meta‐analyses (Tunis 2016).
Given the heterogeneity of tinnitus patients, future trials should assess and report baseline characteristics so that the risk of potential confounding factors can be better understood. Examples include tinnitus duration, tinnitus symptom severity, age, hearing loss and co‐morbidities since these might reasonably modify treatment success. Future trials might also consider, as a subgroup analysis, the differential effect of Ginkgo biloba on acute (i.e. less than three months duration) versus chronic (more than three months duration) subjective idiopathic tinnitus. Individual participant characteristics were poorly described in the included studies. All studies included in the current review recruited chronic tinnitus patients (more than three months). Only three included studies performed a sample size estimation (Drew 2001; Halama 1988; Rejali 2004). Future studies should seek to recruit an adequate sample size based on an appropriate power calculation for the primary outcome.
It was not possible to address the question of the effectiveness of Ginkgo biloba for tinnitus associated with cognitive insufficiency as only two studies included such groups of patients (Halama 1988; Napryeyenko 2009), with neither study including outcomes of interest to the current review, but it is a key area to consider for future development. Therefore, any future research into the effect of Ginkgo biloba on tinnitus must be careful to define its patient population with respect to cerebral function. It would be beneficial if further trials in cognitive insufficiency explicitly report the results for the subgroup of patients who have tinnitus at the outset of the study.
What's new
| Date | Event | Description |
|---|---|---|
| 9 January 2023 | Amended | Award number for University of Maryland School of Medicine/Cochrane Complementary Medicine Field bursary added (Sources of support and Acknowledgements). |
History
Protocol first published: Issue 12, 2019 Review first published: Issue 11, 2022
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, NIHR, NHS or the Department of Health.The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
This review was also supported by a bursary from the University of Maryland School of Medicine/Cochrane Complementary Medicine Field (award number: R24 AT001293).
We are grateful to Dr Sujana Chandrasekhar for peer reviewing the draft protocol and to Elizabeth Doney, Information Specialist with the Cochrane Skin Group, for providing peer review comments on the draft search methods. Thanks to Sandy Walsh for her consumer referee comments on the protocol. We are also grateful to Mr Don McFerran for peer reviewing the draft review and Brian Duncan for his consumer referee comments.
Samantha Cox, Cochrane Information Specialist, designed the search strategy for the review.
We are also indebted to Luma Haj Kassem for initial translation of text from a Persian study.
Editorial and peer reviewer contributions
Cochrane ENT supported the authors in the development of this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Professor Martin Burton (Co‐ordinating Editor, Cochrane ENT) and Mr Samuel MacKeith (Assistant Co‐ordinating Editor, Cochrane ENT).
Managing Editor (selected peer reviewers, collated peer reviewer comments, provided editorial guidance to authors, edited the article): Jenny Bellorini, Cochrane ENT.
Copy Editor (copy editing and production): Jenny Bellorini, Cochrane ENT.
Peer reviewers (provided comments and recommended an editorial decision): Mr DJ McFerran MA FRCS (British Tinnitus Association, UK) (clinical/content review); Professor Adrian James, Cochrane ENT Editor (clinical/content review), Brian Duncan (consumer review), Nuala Livingstone, Cochrane Central Editorial Service (methods review).
Appendices
Appendix 1. Search strategies
| CENTRAL and Cochrane ENT Register (CRS) | MEDLINE (Ovid) | Embase (Ovid) | AMED (Ovid) |
| 1 MESH DESCRIPTOR Tinnitus EXPLODE ALL 2 (zumbido or tinnit*):AB,EH,KW,KY,MC,MH,TI,TO 3 MESH DESCRIPTOR Ginkgo biloba EXPLODE ALL 4 (Ginkgo* or Gingko* or Ginko* or Maidenhair):AB,EH,KW,KY,MC,MH,TI,TO 5(Egb 761 or Egb761 or EGb‐761 or GBE 761 or GBE761 or GBE‐761):AB,EH,KW,KY,MC,MH,TI,TO 6 (GBE50 or EGB50):AB,EH,KW,KY,MC,MH,TI,TO 7 (rokan or tanakan or tebofortran or tebokan or tebonin or Kavari):AB,EH,KW,KY,MC,MH,TI,TO 8 (LI 1370 or LI1370 or LI‐1370):AB,EH,KW,KY,MC,MH,TI,TO 9 (flavanoid* or terpenoid* or bioflavanoid*):AB,EH,KW,KY,MC,MH,TI,TO 10 (gingkco* or gingho* or ginosan* or bilobalid* or tanakene or supergin*):AB,EH,KW,KY,MC,MH,TI,TO 11 (biloba):AB,EH,KW,KY,MC,MH,TI,TO 12 (Eun‐haeng* OR Fossil Tree* OR Ginkyo* OR Icho* OR Ityo* OR Japanese Apricot* OR Kew Tree* OR Salisburia* OR Silver Apricot* OR Pterophyllus salisburiensis OR Yinxingye* OR Prunus Ume OR Prunus mume):AB,EH,KW,KY,MC,MH,TI,TO 13 #1 OR #2 14 #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 15 #13 AND #14 | 1 exp Tinnitus/ 2 (tinnit* or zumbido).ti,ab. 3 1 or 2 4 exp Ginkgo biloba/ 5 (Ginkgo* or Gingko* or Ginko* or Maidenhair).ab,ti. 6 (Egb 761 or Egb761 or EGb‐761 or GBE 761 or GBE761 or GBE‐761).ab,ti. 7 (GBE50 or EGB50).ab,ti. 8 (rokan or tanakan or tebofortran or tebokan or tebonin or Kavari).ab,ti. 9 (LI 1370 or LI1370 or LI‐1370).ab,ti. 10 (flavanoid* or terpenoid* or bioflavanoid*).ab,ti. 11 (gingkco* or gingho* or ginosan* or bilobalid* or tanakene or supergin*).ab,ti. 12 (ginkgo* or "LI 1370").nm. 13 biloba.ab,ti. 14 (Eun‐haeng* or Fossil Tree* or Ginkyo* or Icho* or Ityo* or Japanese Apricot* or Kew Tree* or Salisburia* or Silver Apricot* or Pterophyllus salisburiensis or Yinxingye* or Prunus Ume or Prunus mume).ab,ti. 15 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 16 3 and 15 |
1 exp tinnitus/ 2 (tinnit* or zumbido).ab,ti. 3 1 or 2 4 exp Ginkgo biloba/ 5 (Ginkgo* or Gingko* or Ginko* or Maidenhair).ab,ti. 6 (Egb 761 or Egb761 or EGb‐761 or GBE 761 or GBE761 or GBE‐761).ab,ti. 7 (GBE50 or EGB50).ab,ti. 8 (rokan or tanakan or tebofortran or tebokan or tebonin or Kavari).ab,ti. 9 (LI 1370 or LI1370 or LI‐1370).ab,ti. 10 (flavanoid* or terpenoid* or bioflavanoid*).ab,ti. 11 (gingkco* or gingho* or ginosan* or bilobalid* or tanakene or supergin*).ab,ti. 12 biloba.ab,ti. 13 (Eun‐haeng* or Fossil Tree* or Ginkyo* or Icho* or Ityo* or Japanese Apricot* or Kew Tree* or Salisburia* or Silver Apricot* or Pterophyllus salisburiensis or Yinxingye* or Prunus Ume or Prunus mume).ab,ti. 14 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 3 and 14 |
1 exp Tinnitus/ 2 (tinnit* or zumbido).ti,ab. 3 1 or 2 4 exp Ginkgo biloba/ 5 (Ginkgo* or Gingko* or Ginko* or Maidenhair).ab,ti. 6 (Egb 761 or Egb761 or EGb‐761 or GBE 761 or GBE761 or GBE‐761).ab,ti. 7 (GBE50 or EGB50).ab,ti. 8 (rokan or tanakan or tebofortran or tebokan or tebonin or Kavari).ab,ti. 9 (LI 1370 or LI1370 or LI‐1370).ab,ti. 10 (flavanoid* or terpenoid* or bioflavanoid*).ab,ti. 11 (gingkco* or gingho* or ginosan* or bilobalid* or tanakene or supergin*).ab,ti. 12 biloba.ab,ti. 13 (Eun‐haeng* or Fossil Tree* or Ginkyo* or Icho* or Ityo* or Japanese Apricot* or Kew Tree* or Salisburia* or Silver Apricot* or Pterophyllus salisburiensis or Yinxingye* or Prunus Ume or Prunus mume).ab,ti. 14 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 3 and 14 |
| CINAHL (EBSCO) | Web of Science (Web of Knowledge) | Trial registries | Other |
| S15 S3 AND S14 S14 S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S13 TX Eun‐haeng* or Fossil Tree* or Ginkyo* or Icho* or Ityo* or Japanese Apricot* or Kew Tree* or Salisburia* or Silver Apricot* or Pterophyllus salisburiensis or Yinxingye* or Prunus Ume or Prunus mume S12 TX biloba S11 TX gingkco* or gingho* or ginosan* or bilobalid* or tanakene or supergin* S10 TX flavanoid* or terpenoid* or bioflavanoid* S9 TX LI 1370 or LI1370 or LI‐1370 S8 TX rokan or tanakan or tebofortran or tebokan or tebonin or Kavari S7 TX GBE50 or EGB50 S6 TX Egb 761 or Egb761 or EGb‐761 or GBE 761 or GBE761 or GBE‐761 S5 TX Ginkgo* or Gingko* or Ginko* or Maidenhair S4 (MH "Ginkgo Biloba") S3 S1 OR S2 S2 TX tinnit* or zumbido S1 (MH "Tinnitus") |
# 12 #11 AND #1 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years # 11 #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years # 10 TOPIC: (Eun‐haeng* or Fossil Tree* or Ginkyo* or Icho* or Ityo* or Japanese Apricot* or Kew Tree* or Salisburia* or Silver Apricot* or Pterophyllus salisburiensis or Yinxingye* or Prunus Ume or Prunus mume) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years # 9 TOPIC: (biloba) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years # 8 TOPIC: (gingkco* or gingho* or ginosan* or bilobalid* or tanakene or supergin*) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years # 7 TOPIC: (flavanoid* or terpenoid* or bioflavanoid*) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years # 6 TOPIC: (LI 1370 or LI1370 or LI‐1370) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years # 5 TOPIC: (rokan or tanakan or tebofortran or tebokan or tebonin or Kavari) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years # 4 TOPIC: (GBE50 or EGB50) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years # 3 TOPIC: (Egb 761 or Egb761 or EGb‐761 or GBE 761 or GBE761 or GBE‐761) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years #2 TOPIC: (Ginkgo* or Gingko* or Ginko* or Maidenhair) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years # 1 TOPIC: (tinnit* or zumbido) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years |
ClinicalTrials.gov tinnitus | ( Ginkgo OR Gingko OR Ginko OR gingkco OR gingho OR maidenhair ) ICTRP tinnitus AND ginkgo OR tinnitus AND gingko OR tinnitus AND ginko OR tinnitus AND gingkco OR tinnitus AND gingho OR tinnitus AND maidenhair |
LILACS tw:(tinnit* OR zumbido) AND ( db:("LILACS") AND type_of_study:("clinical_trials")) CNKI via Google Scholar site:en.cnki.com.cn tinnitus ( Ginkgo OR Gingko OR Ginko OR gingkco OR gingho OR maidenhair ) Google Scholar allintitle: tinnitus ( Ginkgo OR Gingko OR Ginko OR gingkco OR gingho OR maidenhair ) |
Data and analyses
Comparison 1. Ginkgo biloba versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Tinnitus symptom severity (THI) at 3 to 6 months | 2 | 85 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐8.26, 5.55] |
| 1.2 Serious adverse effects at 3 months (yes or no) | 4 | 1154 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.3 Tinnitus loudness measured by audiometric loudness matching at 12 weeks | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐4.00 [‐13.33, 5.33] |
| 1.4 Health‐related quality of life at 3 months | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐4.67, 3.51] |
| 1.5 Other adverse effects at 3 months (yes or no) | 4 | 1175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.52, 1.60] |
Comparison 2. Gingko biloba with concurrent intervention versus concurrent intervention only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Tinnitus symptom severity (THI) at 3 months | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 7.50 [2.83, 12.17] |
| 2.2 Tinnitus loudness (VAS) at 3 months (range 0 to 10) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [0.52, 1.48] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cekkayan 1996.
| Study characteristics | ||
| Methods | A 3‐armed (betahistine, Gingko biloba and placebo), parallel‐group randomised controlled trial with 3 months duration of treatment and 3 months duration of follow‐up | |
| Participants |
Location: Malatya, Turkey Setting: single‐centre study, Department of Otorhinolaryngology, Inönü University, from September 1993 to April 1994 Sample size:
Participant baseline characteristics:
Inclusion criteria: patients with subjective tinnitus Exclusion criteria: none |
|
| Interventions |
Intervention group: Ginkgo biloba tablets (9.6 mg ginkgo glycoside/tablet; Tebokan Fort tbl./Rökan/Tebonin), 1 tablet 3 times a day, 3 months Comparator group: placebo capsules, 1 capsule 3 times a day, 3 months Use of additional interventions: none reported |
|
| Outcomes | Primary outcome: change in tinnitus severity scale (range of 0 to 4) at 3 months Secondary outcome: adverse effects at 3 months | |
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors stated that participants were randomly allocated to groups but did not provide any details on methods Quote: "Randomised groups were created (...)" |
| Allocation concealment (selection bias) | Unclear risk | Information not reported in the manuscript. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information not reported in the manuscript. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not reported in the manuscript. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data on changes in tinnitus severity categories are reported only for 8 out of 13 participants (Table 3) but dropout and reasons are not mentioned. It seems that data for 5 participants in the placebo group are missing. |
| Selective reporting (reporting bias) | High risk | Routine and audiological examinations were performed every 15 days, however those data are not reported. Only data regarding tinnitus severity categories are reported at 3 months. |
| Other bias | Unclear risk | No prospective protocol available. No funding sources and declarations of interest reported. Statistical tests not reported ‐ not clear whether they were appropriate. |
Drew 2001.
| Study characteristics | ||
| Methods | A 2‐armed (Gingko biloba and placebo), parallel‐group randomised controlled trial with 12 weeks duration of treatment and 4, 12 and 14 weeks duration of follow‐up | |
| Participants |
Location: Birmingham, UK Setting: carried out entirely by mail and telephone Sample size:
Participant baseline characteristics:
Inclusion criteria: participants with tinnitus Exclusion criteria: age < 18 years or > 70 years old, pregnant or trying to get pregnant, previously taken Ginkgo biloba extract, had had tinnitus for < 12 months, reported that their tinnitus had varied greatly in the 6 months before the screening questionnaire, tried any treatment for tinnitus in the 6 months before completing the screening questionnaire (for example, acupuncture, homoeopathy, hypnotherapy, etc.), not generally in good health, taking anticoagulant drugs or antidepressants, abnormal blood pressure |
|
| Interventions |
Intervention group: Ginkgo biloba tablets (50 mg of Ginkgo biloba standardised extract LI 1370 containing 25% flavonoids, 3% ginkgolides and 5% bilobalides), 3 tablets daily for 12 weeks Comparator group: placebo tablets, 3 tablets daily for 12 weeks Use of additional interventions: none reported |
|
| Outcomes |
Primary outcomes: change in loudness measured with a categorical rating scale (‐6 to 6) and change in troublesome nature of tinnitus measured with a categorical rating scale (‐4 to 4) at 4, 12 and 14 weeks Secondary outcomes: non‐standard questionnaire created for this study: loudness questions, awareness/ability to ignore tinnitus, impact questions, tinnitus variability, cerebral insufficiency, compliance and side effects at 4, 12 and 14 weeks |
|
| Funding sources | This work was funded by the British Tinnitus Association in conjunction with Lichtwer Pharma UK, manufacturer of the extract used in the study | |
| Declarations of interest | The study was financed (2 years' salary for SD and running costs) by a contract between the British Tinnitus Association and Lichtwer Pharma GmbH, Berlin, who also supplied the Ginkgo biloba extract and placebo tablets | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were paired according to the criteria described. Each pair was then allocated two numbers from a randomly arranged code. One number corresponded to placebo treatment and one to active treatment." |
| Allocation concealment (selection bias) | Low risk | Quote: "Placebo tablets were identical to the active tablets in shape, size, colour, and packaging. The tablets were dispensed in coded bottles, and treatment allocation was masked. The allocation procedure ensured that all matched participants received active or placebo tablets without the code being identified." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo tablets were identical to the active tablets in shape, size, colour, and packaging. The tablets were dispensed in coded bottles, and treatment allocation was masked. The allocation procedure ensured that all matched participants received active or placebo tablets without the code being identified." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Data entry and initial analyses were carried out by a researcher blinded to the participant’s allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analyses on unmatched data were performed but not reported, except statement that: Quote: "Unmatched analyses did not provide any additional information and have therefore been excluded from this paper." |
| Selective reporting (reporting bias) | Low risk | All stated outcome measures reported. |
| Other bias | Unclear risk | Limited reporting of baseline data regarding tinnitus characteristics and severity. Participants were matched for age, sex and duration of tinnitus and stated cause of tinnitus but not severity. No data regarding ‘unmatched’ participants characteristics (i.e. was a specific group excluded from the analysis). |
Fucci 1992.
| Study characteristics | ||
| Methods | A 2‐armed (Gingko biloba and placebo), parallel‐group randomised controlled trial with 6 months duration of treatment and 3 and 6 months duration of follow‐up | |
| Participants |
Location: Philadelphia, USA Setting: Department of Otorhinolaryngology, Temple University Medical School, Philadelphia, USA Sample size:
Participant baseline characteristics:
Inclusion criteria: participants with tinnitus Exclusion criteria: not reported |
|
| Interventions |
Intervention group: Ginkgo biloba extract (EGb, bioflavonoid) for 6 months Comparator group: placebo for 6 months Use of additional interventions: none reported |
|
| Outcomes |
Primary outcomes: tinnitus loudness and annoyance measured with numerical rating scales (1 to 10) at 3 and 6 months Secondary outcomes: pure tone air and bone conduction thresholds, speech discrimination scores, speech reception thresholds, impedance audiometry, tinnitus loudness matching levels, tinnitus minimum masking levels and tinnitus pitch matching frequencies at 3 and 6 months |
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | Only conference abstract was available for the study. Author was contacted but did not provide additional data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Author confirmed that study was randomised but did not provide further details. Methods not described. Author disclosed that there was a potential bias in the selection of participants. |
| Allocation concealment (selection bias) | Unclear risk | Only brief abstract available. Information not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Only brief abstract available. Information not provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only brief abstract available. Information not provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only brief abstract available. No information provided regarding number of participants who completed the study and completeness of outcome measures. |
| Selective reporting (reporting bias) | High risk | Only brief abstract available. Not clear if all outcomes were reported. No data for secondary outcomes reported. |
| Other bias | High risk | Author expressed concerns regarding quality of the study; no additional details provided. No prospective protocol available. No funding sources and declarations of interest reported. |
Halama 1988.
| Study characteristics | ||
| Methods | A 2‐armed (Gingko biloba and placebo), parallel‐group randomised controlled trial with 12 weeks duration of treatment and 4, 12 and 14 weeks duration of follow‐up | |
| Participants |
Location: Hamburg, Germany Setting: outpatients in the neurology practice Sample size:
Participant baseline characteristics:
Inclusion criteria: outpatients over 55 years of age from a neurology practice with diagnosis of "mild to mass cerebral insufficiency of vascular origin", the diagnosis was based on the clinical findings and the results of the psychometric tests performed and scale ratings (Hachinski: > 7, Crichton: levels 1 to 3). Exclusion criteria: patients with psychoses or neuroses, with primary degenerative dementia, with secondary brain impairment (treatable underlying disease, e.g. intoxication, metabolic disorder, alcoholism, etc.), epilepsy or other serious illnesses (e.g. myocardial infarction in the last 6 months, severe kidney failure, severe, life‐threatening) were excluded cardiac arrhythmias, malignancy) and those who were not sufficiently capable of co‐operating. In addition, clonidine, reserpine, antihistamines, anti‐Parkinson drugs, anticoagulants, platelet aggregation inhibitors, psychotropic drugs, centrally acting stimulants or drugs that influence the hormone metabolism or blood flow were not allowed to be taken. |
|
| Interventions |
Intervention group: Gingko biloba extract (Tebonin forte film tablets) 3 x 1 per day, corresponding to a daily dose of 120 mg Gingko biloba extract for 12 weeks Comparator group: placebo tablets, 3 x 1 per day for 12 weeks Use of additional interventions: none reported |
|
| Outcomes |
Primary outcomes: Sandoz Clinical Assessment Geriatric (SCAG) scale at 12 weeks Secondary outcomes: Crichton scale (CRBRS), background interference method (HIV), short syndrome test (SKT), craniocorpography (CCG). The severity of dizziness symptoms, noises in the ears, headaches and reduced hormones was assessed with the help of a 4‐point rating scale at 12 weeks. |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Intervention or placebo randomly assigned by computer program to participant enrolment numbers. |
| Allocation concealment (selection bias) | Unclear risk | Information not provided in the manuscript. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither the patient nor the examining physician knew the assignment; the code was only opened after the data collection had been completed at the beginning of the evaluation. Identical tablets. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neither the patient nor the examining physician knew this assignment; the code was only opened after the data collection had been completed at the beginning of the evaluation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients who were randomised were assessed. |
| Selective reporting (reporting bias) | High risk | Data for the majority of secondary outcome measures were not reported. |
| Other bias | High risk | This was a trial with patients with "mild cerebral insufficiency of vascular origin"; tinnitus was a secondary outcome measure. No prospective protocol available. No funding sources and declarations of interest reported. |
Meyer 1986.
| Study characteristics | ||
| Methods | A 2‐armed (Ginkgo biloba and placebo), parallel‐group randomised controlled trial with 3 months duration of treatment and 3 months duration of follow‐up | |
| Participants |
Location: Paris, France Setting: single‐centre study, ENT Department, Saint‐Antoine Hospital, Paris Sample size:
Participant baseline characteristics:
Inclusion criteria: recent tinnitus, i.e. tinnitus that had appeared within less than 1 year Exclusion criteria: patients undergoing surgical or anti‐infectious treatment, those affected by acute outer or middle ear illnesses (otitis, tubal catarrh etc.), or associated pathological issues that could potentially skew the analysis of the results. The study also excluded patients who were undergoing treatment and for whom development was favourable as well as those requiring medical treatment that could interfere with the assessment of the results. |
|
| Interventions |
Intervention group: Ginkgo biloba extract (GbE 761 Tanakan – I.P.S.E.N Institute), 4 mL daily, taken in 2 doses for 3 months Comparator group: placebo, 4 mL daily, taken in 2 doses for 3 months Use of additional interventions: none reported |
|
| Outcomes |
Primary outcome: change in tinnitus on a scale from 1 to 7 (1 to 2 = deterioration; 3 = no change; 4 = slight improvement; 5 = improvement; 6 = major improvement; 7 = discontinued due to intolerance) at 3 months Secondary outcomes: time to tinnitus disappearance or clear improvement, change of tinnitus intensity, change in tinnitus discomfort at 3 months |
|
| Funding sources | Not reported | |
| Declarations of interest | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information on methods of random sequence generation not reported in the manuscript. |
| Allocation concealment (selection bias) | Unclear risk | Information not reported in the manuscript. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinding stated but no details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not reported in the manuscript. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants dropped out due to intolerance. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not specified in the methods section |
| Other bias | High risk | No prospective protocol available. No funding sources and declarations of interest reported. Scales for intensity, discomfort, time to disappear or clear improvement are not defined; unclear what numbers 0 to 3 mean. 1 participant dropped out due to intolerance in the placebo group – unclear whether placebo was a real placebo. |
Morgenstern 1997.
| Study characteristics | ||
| Methods | A 2‐armed (Gingko biloba and placebo), parallel‐group randomised controlled trial with 12 weeks duration of treatment and 4, 8 and 12 weeks duration of follow‐up | |
| Participants |
Location: Hamburg, Germany Setting: single‐centre study, St. Georg Hospital, Hamburg, Germany Sample size:
Participant baseline characteristics:
Inclusion criteria: over 18 years old and chronic subjective tinnitus, normal hearing at 3 neighbouring frequencies in the audiogram Exclusion criteria: objective tinnitus, middle ear pathology, reproducibility of the transient evoked otoacoustic emissions at the intake examination < 85%, latency extensions or pathological wave models of earlier brain stem auditory evoked potentials (BAEPs), severe organic diseases, alcohol or drug abuse, pregnancy |
|
| Interventions |
Intervention group: Ginkgo biloba coated tablets (40 mg of Gingko special extract EGb 761, a dry extract made from gingko biloba leaves (35 to 67:1), set to 9.6 mg gingko flavone glycoside and 2.4 mg of terpene lactone), 1 tablet 3 x day (120 mg), 12 weeks Comparator group: placebo coated tablets, 1 tablet 3 x day, 12 weeks Use of additional interventions: none reported |
|
| Outcomes |
Primary outcome: change in tinnitus loudness measured with audiometric methods in the ear with louder tinnitus at 4, 8 and 12 weeks Secondary outcomes: change in tinnitus intensity measured with a scale of Subjective Evaluation of Tinnitus Intensity (0 to 5), click‐evoked otoacoustic emissions (OAEs) at 4, 8 and 12 weeks |
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information on methods of random sequence generation not reported in the manuscript. |
| Allocation concealment (selection bias) | Unclear risk | Information not reported in the manuscript. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinding stated, methods not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not reported in the manuscript. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There are no data regarding the primary outcome measure for 14 patients in the intervention group and 12 in the control group at 12 weeks. Dropout is not explained. |
| Selective reporting (reporting bias) | Unclear risk | No values for secondary outcome measures are reported. Only brief statements regarding whether those improved or not. |
| Other bias | Unclear risk | This is an abbreviated version of the full study report, which is not available. No prospective protocol available. No funding sources and declarations of interest reported. |
Napryeyenko 2009.
| Study characteristics | ||
| Methods | A 2‐armed (Ginkgo biloba and placebo), parallel‐group randomised controlled trial with 22 weeks duration of treatment and 12 and 22 weeks duration of follow‐up | |
| Participants |
Location: Kyiv, Ukraine Setting: multi‐centre study, outpatient clinics of 16 psychiatric or neurological hospitals Sample size:
Participant baseline characteristics:
Inclusion criteria: participants were eligible for the study if they were at least 50 years of age and diagnosed with AD (probable AD or possible AD with cerebrovascular disease) or VaD. Diagnoses were established employing the criteria specified by the National Institute for Neurological and Communicative Disorders and Stroke (NINCDS) together with the Alzheimer's Disease and Related Disorders Association (ADRDA) and by the National Institute of Neurological Disorders and Stroke (NINDS) together with the Association Internationale pour la Recherche et l'Enseignement en Neurosciences (AIREN) as appropriate. A CT or MRI scan, no more than 1 year old, had to be consistent with the inclusion diagnosis. The Test for Early Detection of Dementia with Discrimination from Depression (TE4D) was used as a screening instrument and to verify the presence of cognitive impairment in at least 2 domains. A total cognitive score of no more than 35 was required for inclusion. Patients had to have mild to moderate dementia as evidenced by a total score from 9 to 23 (both inclusive) on the SKT test battery, which roughly corresponds to a range from 14 to 25 on the MMSE or 17 to 35 on the cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS‐cog). The Clock‐Drawing Test (CDT) after Sunderland et al was employed as a second screening instrument, the score of which had to be below 6. Patients had to score at least 5 on the 12‐item Neuropsychiatric Inventory (NPI), with at least one item score (other than delusions or hallucinations) being 3 or higher. Severe depression was excluded by requiring a score below 20 on the 17‐item Hamilton Rating Scale for Depression (HAMD). The presence of a caregiver was required who was able and willing to provide information on the patient's behaviour and competence to perform activities of daily living. Exclusion criteria: patients were excluded from the study if they had any other type of dementia or neurological disorder, current or recent major depression or other psychiatric disorder, severe or insufficiently controlled cardiovascular, renal or hepatic disorder, diabetes, anaemia or thyroid dysfunction. Active malignant disease, HIV or lues infection and gastrointestinal diseases with uncertain absorption were not accepted. Treatment with other anti‐dementia drugs, cognitive enhancers, cholinergic, anti‐cholinergic or haemo‐rheologically active drugs, anti‐Parkinson drugs or Ginkgo supplements was prohibited during the study and at least 8 weeks preceding randomisation. |
|
| Interventions |
Intervention group: EGb 761 dry extract from Ginkgo biloba leaves (35 to 67:1), extraction solvent: acetone 60% (w/w). The extract was adjusted to 22.0% to 27.0% Ginkgo flavonoids, calculated as Ginkgo flavone glycosides, and 5.0% to 7.0% terpene lactones consisting of 2.8% to 3.4% ginkgolides A, B, C and 2.6% to 3.2% bilobalide, with a content of ginkgolic acids below 5 ppm. Daily dose of 240 mg (2 × 120 mg), 22 weeks. Comparator group: placebo, 22 weeks Use of additional interventions: none reported |
|
| Outcomes |
Primary outcome: the SKT, a 9‐item cognitive test battery with scores ranging from 0 to 27 at 12 and 22 weeks Secondary outcomes: 12‐item Neuropsychiatric Inventory (NPI), which assesses frequency and severity of neuropsychiatric symptoms (composite score range 0 to 144) and caregiver distress caused by such symptoms (score range 0 to 60); the activities‐of‐daily living (ADL) subscale of the Gottfries–Bråne–Steen (GBS) overall geriatric assessment scale; the total score of the GBS (comprised by the intellectual “I”, the emotional “E”, and ADL subscores, but not the behavioural “S” subscore which was not documented to avoid redundancy); the Verbal Fluency Test (animal fluency); the Clock‐Drawing Test and the Hamilton Rating Scale for Depression. Patient self‐ratings of presence and severity of dizziness and tinnitus, symptoms often associated with old age, were documented using 11‐point box scales, 0 representing absence and 10 indicating extreme severity of a symptom. All measures were collected at baseline, 12 and 22 weeks. Safety was assessed by documentation of adverse events at 6, 12, 17 and 22 weeks and physical examination, electrocardiography and laboratory tests at 22 weeks. |
|
| Funding sources | The clinical trial was sponsored by Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Centre‐stratified randomisation (drug–placebo ratio 1:1) in blocks of four was performed by the sponsor's biometrics unit using a validated computer program that linked ascending drug numbers to active drug or placebo, respectively." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation list was sealed and stored safely at the sponsor's biometrics unit, and block length was not disclosed to investigators." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The multi‐centre trial with two parallel treatment arms was carried out in a double‐blind manner. Drug and placebo tablets were indistinguishable by appearance, packaging and labelling." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not reported in the manuscript. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 214 of 218 patients with Alzheimer's and 181 of 182 patients with vascular dementia with full data for analysis. |
| Selective reporting (reporting bias) | High risk | There are 2 reports from this study, one reporting tinnitus score in the whole study population (from 0 ‐ absence of tinnitus to 10 ‐ extreme severity) and one brief report reporting tinnitus scores only in those participants with tinnitus. |
| Other bias | High risk | No prospective protocol available. No declarations of interest reported. This was a study of effects of Ginkgo biloba on Alzheimer's disease or vascular dementia. Tinnitus was secondary outcome measure. |
Nishad 2019.
| Study characteristics | ||
| Methods | A 4‐armed (Gingko biloba and placebo; caroverine and placebo), parallel‐group randomised controlled trial with 3 months duration of treatment and 4 weeks, 12 weeks and 6 months duration of follow‐up | |
| Participants |
Location: India Setting: single‐centre study, Otolaryngology Department, India Sample size:
Participant baseline characteristics:
Inclusion criteria: age between 18 and 60 years, diagnosis of tinnitus, unilateral or bilateral, cochlear hearing defect, reflex audiometry confirming cochlear‐synaptic tinnitus and excluding middle ear tinnitus, written consent after explanation by consultant in charge Exclusion criteria: patients not willing to give written consent after receiving due instructions, reasonable doubts as to the patient’s co‐operation, severe secondary disorders (i.e. any acute/chronic illness), contra‐indication for caroverine therapy (e.g. severe hypertension), concomitant symptoms, pregnancy or plan for having children, hydrops cochlea or Ménière's disease, retrocochlear hearing defect, blast injury, status post psychiatrist therapy, status post operation of the middle ear, pulse‐synchronous tinnitus, excessive consumption of alcohol, drug or nicotine, known to intolerance to caroverine, concomitant medication, medicative tinnitus therapy within 1 week prior to enrolment, masticatory movements influencing subjective tinnitus sensation |
|
| Interventions |
Intervention group: Ginkgo biloba (60 mg of Ginkgo biloba extract) 12‐hourly for a period of 3 months Comparator group: placebo (60 mg of placebo) 12‐hourly for a period of 3 months Use of additional interventions: none reported |
|
| Outcomes | Primary outcome: change in tinnitus grading measured with unspecified non‐standard questionnaire at 4 weeks, 12 weeks and 6 months Secondary outcome: quality of life (method not described) and tinnitus matching (method not described) at 4 weeks, 12 weeks and 6 months | |
| Funding sources | None specified | |
| Declarations of interest | The authors declare that they have no conflict of interest | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By using a computer‐generated block randomisation method participants were randomised into 4 groups. |
| Allocation concealment (selection bias) | Unclear risk | Information not reported in the manuscript. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information not reported in the manuscript. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not reported in the manuscript. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants seemed to complete the study, no dropout reported. |
| Selective reporting (reporting bias) | High risk | Quality of life stated as primary outcome but no results were reported. Report mentions "tinnitus matching" improved but this was not pre‐defined as an outcome measure. |
| Other bias | High risk | Lack of criteria for defining improvement, no statistical analyses, results do not justify conclusions. Audiological selection criteria complex and of uncertain significance in selecting stated aetiological group. No prospective protocol available. No funding sources reported. |
Polanski 2016.
| Study characteristics | ||
| Methods | A 4‐armed (Gingko biloba; α‐lipoic acid plus vitamin C; papaverine hydrochloride plus vitamin E; placebo (starch capsules)), parallel‐group randomised controlled trial with 6 months duration of treatment and 6 months duration of follow‐up | |
| Participants |
Location: São Paulo, Brazil Setting: single‐centre study, Department of Otorhinolaryngology and Head and Neck Surgery, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil Sample size:
Participant baseline characteristics:
Inclusion criteria: aged 60 years or older with clinical complaints of tinnitus associated with a variable degree of sensorineural hearing loss confirmed by previous audiometric testing Exclusion criteria: known allergy to any substance to be tested or with clinical contraindications to the use of these substances; anticoagulant users or subjects with coagulopathy, as well as diabetics |
|
| Interventions |
Intervention group: Ginkgo biloba dry extract (120 mg/day) for a period of 6 months Comparator group: placebo (starch capsules) for a period of 6 months Use of additional interventions: none reported |
|
| Outcomes | Primary outcome: hearing improvement measured with audiometry at 250, 500, 1000, 2000, 3000, 4000, 6000 and 8000 Hz at 6 months Secondary outcome: change in tinnitus symptom severity measured with the THI at 6 months | |
| Funding sources | None specified | |
| Declarations of interest | The authors declare no conflict of interest | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For distribution and randomization of participants, the resources available at http://www.randomization.com were used." |
| Allocation concealment (selection bias) | Unclear risk | Information not reported in the manuscript. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The substances were not identified by name in the containers into which they were packed, but rather through symbols defined by a professional who did not participate in the research, as a way of blinding investigators and patients." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not reported in the manuscript. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "In the original trial paper there were 15 placebo and 14 test participants who had tinnitus. Data for only 12 and 13 participants are included in this report. So some tinnitus participants were excluded without reason given." |
| Selective reporting (reporting bias) | High risk | Trial registration lists primary outcome: hearing improvement in an audiometric test as primary outcome, which is not described in this report. |
| Other bias | High risk | Funding not disclosed. This was a hearing loss trial with tinnitus as secondary outcome. |
Radnuz 2019.
| Study characteristics | ||
| Methods | A 3‐armed (Ginkgo biloba, hearing aid, Ginkgo biloba plus hearing aid), parallel‐group randomised controlled trial with 90 days duration of treatment and 90 days duration of follow‐up | |
| Participants |
Location: São Paulo, Brazil Setting: single‐centre study, outpatient clinic of the audiology centre in Rondonópolis (MT), Brazil Sample size:
The sample size (n = 35) was determined by the number of individuals submitted to medical consultation between July and August 2015 at an audiological centre in Rondonópolis (MT), Brazil Participant baseline characteristics:
Inclusion criteria: individuals over 18 years of age; complaint of tinnitus (uni‐ or bilateral) for at least 3 months; (single or bilateral) sensorineural or mixed hearing loss independent of degree and configuration Exclusion criteria: having used aspirin or acetylsalicylic acid in the last month, having used Ginkgo biloba in the last 3 months, on antidepressants, diagnosed with compromised middle ear (otitis or tubal dysfunction) at the time of evaluation |
|
| Interventions |
Intervention group: Ginkgo biloba (EGb 761 Equitam leaf extract, patented/trademarked by Eurofarma Laboratorios S.A.), 240 mg/day plus digital Beltone hearing aid for a period of 3 months Comparator group: digital Beltone hearing aid for a period of 3 months Use of additional interventions: none |
|
| Outcomes |
Primary outcome: change in tinnitus symptom severity measured using the THI and change in tinnitus loudness measured using a visual analogue scale (range of 0 to 10) at 3 months Secondary outcomes: percentage of variation of the THI score before and after treatment in relation to the tinnitus onset time at 3 months |
|
| Funding sources | Quote: "S.N.D is grateful to Anhanguera University of São Paulo(UNIAN) for providing financial support and Momenta Farmacêutica Ltd for provide the EGb 761 Equitam® Ginkgo biloba extract." | |
| Declarations of interest | The authors declared no conflicts of interest | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random allocation process has consisted in generating a random sequence using an Excel file for randomization and used the random allocation rule, which chooses at random one of possible balanced assignments of the given number of subjects per treatment." |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The examiners responsible for applying the questionnaires during the study were blinded to the intervention. Due to the nature of the intervention participants were not blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The examiners responsible for applying the questionnaires during the study were blinded to the intervention. In addition, an employee outside the research team inserted data into the computer in separate data sheets so that the researchers can analyze data without having access to information about the allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients' data were analysed. |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported. |
| Other bias | Low risk | No prospective protocol available. |
Rejali 2004.
| Study characteristics | ||
| Methods | A 2‐armed (Gingko biloba and placebo), parallel‐group randomised controlled trial with 12 weeks duration of treatment and 12 weeks duration of follow‐up | |
| Participants |
Location: Airdrie, UK Setting: single‐centre study, Department of Otolaryngology, Monklands Hospital Sample size:
Participant baseline characteristics:
Inclusion criteria: adult patients recruited from general otolaryngology outpatient clinics with tinnitus as their only or main presenting complaint Exclusion criteria: patients with active middle or external ear disease, known hypersensitivity to Ginkgo biloba and pregnant patients, and patients already on Ginkgo biloba, warfarin or with bleeding diatheses were excluded from the study |
|
| Interventions |
Intervention group: Ginkgo biloba tablets, 120 mg once a day, 12 weeks Comparator group: placebo tablets, 1 tablet a day, 12 weeks Use of additional interventions: none reported |
|
| Outcomes |
Primary outcome: change in tinnitus symptom severity measured with THI at 12 weeks Secondary outcomes: change in handicap caused by otolaryngological disease measured with Glasgow Health Status Inventory (GHSI) at 12 weeks; change in hearing determined by averaging air conduction thresholds for both ears at 0.5 kHz, 1 kHz, 2 kHz and 4 kHz at 12 weeks; adverse effects |
|
| Funding sources | Quote: "We acknowledge Lamberts Healthcare for the supply of G. biloba and placebo medication." | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out by an independent third party using a card from bag system. |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out by an independent third party using a card from bag system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was double‐blind. The patients received either 120 mg once daily sustained release formulation of Ginkgo biloba or placebo (all tablets provided by Lambert’s Health Care, TunbridgeWells, UK) for 12 weeks. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not reported in the manuscript. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small dropout (3 participants: 1 in intervention and 2 in placebo group); reasons for dropout explained. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | No prospective protocol available. No declarations of interest reported. Baseline tinnitus severity higher in the control group. |
Yarmohammadi 2007.
| Study characteristics | ||
| Methods | A 2‐armed (Ginkgo biloba and placebo), parallel‐group randomised controlled trial with 2 weeks duration of treatment and 2 weeks duration of follow‐up | |
| Participants |
Location: Teheran, Iran Setting: single‐centre study, otolaryngology clinic Sample size:
Participant baseline characteristics:
Inclusion criteria: patients diagnosed with tinnitus and with no reduction of hearing who had a normal MRI were included in the study Exclusion criteria: none |
|
| Interventions |
Intervention group: Ginkgo T.D. tablets (Tolid Darou Pharmaceutical Company), 40 mg, twice daily for 2 weeks Comparator group: placebo tablets, 40 mg, twice daily for 2 weeks Use of additional interventions: none reported |
|
| Outcomes |
Primary outcome: improvement in severity of tinnitus measured with undefined questionnaire at 2 weeks Secondary outcomes: none |
|
| Funding sources | Quote: "The authors would like to thank Tolid Darou Pharmaceutical Company for providing the medication and the placebo." | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “The ginkgo T.D. tablets and the placebos were placed in an equal number of paper bags—30 bags containing ginkgo T.D. and 30 bags containing the placebo, labelling the bags with numbers. The doctor randomly administered the contents of the bags to the patients.” |
| Allocation concealment (selection bias) | Unclear risk | Bags with tablets but concealment methods not described (doctor randomly administered the contents of the bags to the patients). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "In this study, the doctor and patients were unaware of the type of medication, with only the second co‐researcher in charge of numbering and packaging the bags being aware." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information not reported in the manuscript. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all participants reported. |
| Selective reporting (reporting bias) | High risk | Questionnaire for tinnitus severity was not described; authors interpreted the result as no change, improvement and exacerbation but it is unclear what the criteria were for such classification. |
| Other bias | Unclear risk | No prospective protocol available. No declarations of interest reported. |
AD: Alzheimer's disease; CT: computerised tomography; MRI: magnetic resonance imaging; SD: standard deviation; SKT: Short Cognitive Performance Test (Syndrom‐Kurztest); THI: Tinnitus Handicap Inventory; VaD: vascular dementia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abascal 2012 | ALLOCATION: not randomised; review |
| Ahsan 2017 | ALLOCATION: not randomised; review |
| Bruchert 1991 | ALLOCATION: randomised controlled trial POPULATION: patients with cognitive insufficiency not with tinnitus |
| Claussen 1988 | ALLOCATION: randomised trial POPULATION: patients with vertigo, nausea and tinnitus INTERVENTION: combined therapy, Ginkgo biloba and other medications |
| Coles 1988 | ALLOCATION: not randomised; uncontrolled before‐and‐after study |
| Dau 2000 | ALLOCATION: not randomised; uncontrolled before and after study |
| Feinberg 2003 | ALLOCATION: not randomised; Q&A |
| Hajna 1999 | ALLOCATION: not randomised; uncontrolled before‐and‐after study |
| Holgers 1994 | ALLOCATION: the study was divided into two steps: an open phase where all participants were offered Ginkgo biloba extract, followed by a randomised cross‐over study in patients who reported benefit of the extract for tinnitus |
| ISRCTN38408464 | The trial stopped on 11 September 2015 due to participant recruitment issues. No evaluable data available. |
| Kiefer 2019 | ALLOCATION: randomised controlled trial POPULATION: adults with tinnitus INTERVENTION: the study compared Ginkgo biloba extract with pentoxifylline; there was no placebo group |
| Novotny 2000 | ALLOCATION: non‐randomised controlled trial |
| Plath 1995 | ALLOCATION: randomised controlled trial POPULATION: adults with chronic tinnitus INTERVENTION: the study compared a low‐power laser and Ginkgo biloba extract with sham laser and Ginkgo biloba extract |
| Sadner 2017 | ALLOCATION: not randomised; review |
| Schneider 2000 | ALLOCATION: not randomised; uncontrolled before‐and‐after study |
| von Wedel 1995 | ALLOCATION: non‐randomised controlled study |
| Walger 1993 | ALLOCATION: randomised controlled trial POPULATION: adults with chronic tinnitus INTERVENTION: the study compared a low‐power laser with Ginkgo biloba |
Characteristics of studies awaiting classification [ordered by study ID]
Fandriantika 2017.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | Thesis under embargo, request for accessing full text refused. |
Rogowski 2001.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | No full text available: contacted authors, no response. |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR2000038850.
| Study name | 'A randomized controlled clinical study of sound therapy combined with drug therapy for tinnitus' |
| Methods | RCT |
| Participants | n = 44 Inclusion criteria: 1) patients with tinnitus frequency between 125 Hz and 8000 Hz; 2) patients with chronic tinnitus (Otolaryngology ‐ Head and Neck Surgery 2014 tinnitus clinical guidelines: chronic tinnitus is defined as continuous tinnitus lasting more than 6 months at the time of examination); 3) patients without tinnitus treatment; 4) patients who did not take stimulants and ototoxic drugs; 5) patients who are willing to follow up Exclusion criteria: 1) pulsatile tinnitus; 2) patients with a history of auditory trauma; 3) patients with middle or inner ear diseases (such as otosclerosis, chronic suppurative otitis media or endolymphatic hydrops); 4) severe hearing asymmetry between ears, patients with post cochlear lesions, tinnitus induced by previous ear surgery and other diseases; 5) patients with head and neck discomfort or tinnitus caused by temporomandibular or cranial neck; 6) have used or are using other oral drugs to improve microcirculation within 3 months; 7) women who are pregnant or prepare for pregnancy within 12 months; 8) patients with severe chronic diseases not under control |
| Interventions | Oral administration of Ginkgo biloba extract tablets vs music combined with medication |
| Outcomes | Tinnitus Handicap Inventory, Zung Self‐Rating Anxiety Scale (SAS), electroencephalogram (EEG) |
| Starting date | 1 December 2020 |
| Contact information | Jianlong Wu |
| Notes | Status: recruiting |
RCT: randomised controlled trial
Differences between protocol and review
In the protocol, under Electronic searches we planned to search ClinicalTrials.gov and ICTRP via the Cochrane Register of Studies. The Information Specialist determined that these searches were not necessary because they would be entirely covered by the searches run in CENTRAL and the Cochrane ENT Register.
We have changed the wording of the outcome 'Significant adverse effects' to 'Serious adverse effects' to avoid any confusion with statistical significance.
Contributions of authors
MS and DJH conceived and all authors contributed to the design of the study. MS drafted the protocol. All authors critically revised the protocol for important intellectual content.
Author contributions to the full review:
The Cochrane ENT Information Specialist developed and ran the search strategy.
MS obtained copies of studies with the assistance of the University of Nottingham library.
MS, MH, AER and DJH were responsible for selection of studies.
MS, MH, AER and DJH were responsible for data extraction.
MS, MH, AER, DJH and JX were responsible for assessing risk of bias.
MS entered data into RevMan.
MS and PS conducted the analysis.
MS, PS and JX interpreted the analysis.
MS drafted the final review.
MS and DJH will be responsible for updating the review.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute for Health Research, UK
Infrastructure funding for Cochrane ENT
-
University of Maryland School of Medicine/Cochrane Complementary Medicine Field Bursary, USA
Cochrane Complementary Medicine Field Bursary; award number: R24 AT001293
Declarations of interest
Magdalena Sereda: MS is a member of the Steering Committee for the British Society of Audiology Tinnitus and Hyperacusis Special Interest Group and Associate Editor for the International Journal of Audiology and BMC Health Services Research. She is funded by the NIHR Nottingham Biomedical Research Centre. She has received tinnitus research funding from the British Tinnitus Association, the British Society of Audiology and the NIHR.
Jun Xia: none known.
Polly Scutt: none known.
Malcolm Hilton: none known.
Amr El Refaie: none known.
Derek J Hoare: DJH is Editor‐at‐large for Ear and Hearing and Chair of the British Society of Audiology. He is funded by the NIHR and research lead for hearing at the NIHR Nottingham Biomedical Research Centre. He has received tinnitus research funding from the British Society of Audiology, the British Tinnitus Association, RNID, Horizon 2020, Royal British Legion, Help Musicians UK and the NIHR.
Edited (no change to conclusions)
References
References to studies included in this review
Cekkayan 1996 {published data only}
- Cekkayan S, Ozlüoğlu L, Yoloğlu S, Söylemezoğlu S, Erpek G. Comparison of the efficiency of betahistine hydrochloride and gingko biloba extract in tinnitus patients [Tinnituslu hastalarda betahistin ve gingko biloba ekstresinin etkinliginin karsilastirilmasi]. K.B.B. ve Baş Boyun Cerrahisi Dergisi 1996;4:19-22. [Google Scholar]
Drew 2001 {published data only}
- Drew S, Davies E. Effectiveness of Ginkgo biloba in treating tinnitus: double blind, placebo controlled trial. BMJ 2001;322(7278):73. [DOI: 10.1136/bmj.322.7278.73] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew SJ, Davies WE. Ginkgo biloba in the treatment of tinnitus: preliminary results of a match-paired, double-blinded placebo-controlled trial involving 1115 subjects. In: Proceedings of the Sixth International Tinnitus Seminar. Cambridge, UK, 1999:261-5.
Fucci 1992 {published data only}
- Fucci MJ, Martin WH, Foster WP, Schwegler JW, Ronis ML. Effects of Ginkgo biloba extract on tinnitus: a double blind study. In: Association for Research in Otolaryngology (HRSG). 15th Midwinter Research Meeting. 1992:112 .
Halama 1988 {published data only}
- Halama P, Bartsch G, Meng G. Cerebrovascular insufficiency - placebo-controlled, randomized, double blind-trial on the effect of Gingko biloba-extract [Hirnleistungsstörungen vaskulärer Genese. Randomisierte Doppelblindstudie zur Wirkamskeit von Gingko-biloba-Extrakt]. Fortschritte der Medizin 1988;106(19):54-60. [PubMed] [Google Scholar]
Meyer 1986 {published data only}
- Meyer B. A multicentre, randomized, double-blind drug versus placebo study of Ginkgo biloba extract in the treatment of tinnitus [Etude multicentrique randomisee a double insu faceau placebo du traitmement des acouphens par l'extrait de Ginkgo biloba]. Presse Medicale 1986;15:1562-4. [PubMed] [Google Scholar]
Morgenstern 1997 {published data only}
- Morgenstern C, Biermann E. Long term therapy of tinnitus with Gingko biloba extract EGb 761 [Tinnitus-langzeittherapiemit Ginkgo-spezialextrakt EGb 761]. Fortschritte der Medizin 1997;115(29):57-8. [Google Scholar]
Napryeyenko 2009 {published data only}
- Hoerr R, Napryeyenko O, Borzenko I. Effects of ginkgo biloba extract EGb 761 on dizziness and tinnitus in patients with dementia. Focus on Alternative and Complementary Therapies 2007;12:26-7. [Google Scholar]
- Napryeyenko O, Borzenko I, for the GINDEM-NP Study Group. Ginkgo biloba special extract in dementia with neuropsychiatric features. Arzneimittel-Forschung (Drug Research) 2007;57(1):4-11. [DOI] [PubMed] [Google Scholar]
- Napryeyenko O, Sonnik G, Tartakovsky I. Efficacy and tolerability of Ginkgo biloba extract EGb 761 by type of dementia: analyses of a randomised controlled trial. Neurological Sciences 2009;283(1-2):224-9. [DOI] [PubMed] [Google Scholar]
Nishad 2019 {published data only}
- Nishad RK, Jain AK, Singh M, Verma R, Jain S. Randomised controlled clinical study of injection caroverine and Ginkgo biloba extract in cochlear synaptic tinnitus. Indian Journal of Otolaryngology & Head & Neck Surgery 2019;71(Suppl 2):1523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polanski 2016 {published data only}
- Polanski JF, Cruz OL. Evaluation of antioxidant treatment in presbycusis: prospective, placebo-controlled, double-blind, randomised trial. Journal of Laryngology & Otology 2013;127:134-41. [DOI] [PubMed] [Google Scholar]
- Polanski JF, Soares AD, Mendonça Cruz OL. Antioxidant therapy in the elderly with tinnitus. Brazilian Journal of Otorhinolaryngology 2016;82(3):269-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Radnuz 2019 {published data only}
- Radunz CL, Okuyama CE, Branco-Barreiro FCA, Pereira RMS, Diniz SN. Clinical randomized trial study of hearing aids effectiveness in association with Ginkgo biloba extract (EGb 761) on tinnitus improvement. Brazilian Journal of Otorhinolaryngology 2020;86(6):734-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rejali 2004 {published data only}
- Rejali D, Sivakumar A, Balaji N. Ginkgo biloba does not benefit patients with tinnitus: a randomized placebo-controlled double-blind trial and meta-analysis of randomized trials. Clinical Otolaryngology and Allied Sciences 2004;29(3):226-31. [DOI] [PubMed] [Google Scholar]
Yarmohammadi 2007 {published data only}
- Yarmoammadi ME, Naseri M, Nabavi SM, Zanganeh A, Zayeri F. The efficacy of Gingko-TD on amelioration of subjective tinnitus. Bimonthly Scientific Research Journal of Shahed University 2007;14(67):67-71. [Google Scholar]
References to studies excluded from this review
Abascal 2012 {published data only}
- Abascal K, Yarnell E. Herbal formulas for alleviating symptoms of tinnitus. Alternative and Complementary Therapies 2012;18(2):74-7. [Google Scholar]
Ahsan 2017 {published data only}
- Ahsan SF. Alternative medical management of tinnitus. Brain Injury Professional 2017;13(1):30-3. [Google Scholar]
Bruchert 1991 {published data only}
- Bruchert E, Heinrich SE, Ruf-Kohler P. Effectiveness of LI 1370 in patients with cognitive insufficiency. Munchener Medizinische Wochenschrift 1991;133(Suppl 1):S9-14. [Google Scholar]
Claussen 1988 {published data only}
- Claussen CF, Claussen E, Bocking HH, Patil NP. Extractum Ginkgo biloba in the combined treatment of vertigo, nausea and tinnitus. In: Claussen CF, Kirtane MV, Schlitter K, editors(s). Vertigo, Nausea, Tinnitus and Hypoacusia in Metabolic Disorders. Elsevier Science Publishers B.V. (Biomedical Division), 1988:583-9. [Google Scholar]
Coles 1988 {published data only}
- Coles R. Trial of an extract of Ginkgo biloba (EGB) for tinnitus and hearing loss. Clinical Otolaryngology 1988;13:501-4. [PubMed] [Google Scholar]
Dau 2000 {published data only}
- Dau J. Rationale Phytotherapie: tinnitus zahlt zu den Indikationen von Ginkgo biloba. Natura Med 2000;15(12):29-31. [Google Scholar]
Feinberg 2003 {published data only}
- Feinberg AW. Are there any studies showing whether ginkgo biloba is effective for tinnitus (ringing in the ears)? Health News (Waltham, Mass.) 2003;9(1):12. [PubMed] [Google Scholar]
Hajna 1999 {published data only}
- Hajna A, Sejna I. Ginkgo biloba in the treatment of tinnitus, vertigo and hypacusis. Prakticky Lekar 1999;79(10):588-9. [Google Scholar]
Holgers 1994 {published data only}
- Holgers KM, Axelsson A, Pringle I. Ginkgo biloba extract for the treatment of tinnitus. Audiology 1994;33:85-92. [DOI] [PubMed] [Google Scholar]
ISRCTN38408464 {published data only}
- ISRCTN38408464. Pragmatic trial to find out if the additional use of Ginkgo biloba extract EGb 761® in patients with chronic tinnitus may influence the properties of the tinnitus retraining therapy positively. https://www.isrctn.com/ISRCTN38408464 (first received 6 June 2014).
Kiefer 2019 {published data only}
- Kiefer D. Gingko benefits for tinnitus? Integrative Medicine Alert 2019;22(10):1-9. [Google Scholar]
Novotny 2000 {published data only}
- Novotny M, Ditmarova J, Hahn A, Ošťádalová I. Tinnitus therapy with Tebokan (R) and hyperbaric oxygenation. In: Ošťádalová I, editors(s). Equilibrium Research, Clinical Equilibriometry and Modern Treatment. 1st edition. Amsterdam, Lausanne, New York, Oxford: Elsevier, 1999:635-7. [Google Scholar]
Plath 1995 {published data only}
- Plath P, Olivier J. Results of combined low-power laser therapy and extracts of ginkgo biloba in cases of sensorineural hearing loss and tinnitus. Advances in Otorhinolaryngology 1995;49:101-4. [DOI] [PubMed] [Google Scholar]
Sadner 2017 {published data only}
- Sandner F. With Ginkgo biloba dry leaf extract against the tinnitus (ear) orchestra in the head. Journal fur Pharmakologie und Therapie 2017;26(2):54-5. [Google Scholar]
Schneider 2000 {published data only}
- Schneider D, Schneider L, Shulman A, Claussen CF, Just E, Koltchev C, et al. Gingko biloba (Rokan) therapy in tinnitus patients and measurable interactions between tinnitus and vestibular disturbances. International Tinnitus Journal 2000;6(1):56-62. [PubMed] [Google Scholar]
von Wedel 1995 {published data only}
- Wedel H, Calero L, Walger M, Hoenen S, Rutwalt D. Soft-laser/ginkgo therapy in chronic tinnitus. Advances in Otorhinolaryngology 1995;49:105-8. [PubMed] [Google Scholar]
Walger 1993 {published data only}
- Walger M, Wedel H, Calero L, Hoenen S, Rutwalt D. Ergegnisse einer studie zur effektivitat einer kombinierten low-power-laser und gingko-therapie auf den chronischen tinnitus. HNO - Informationen 1993;4:35-6. [Google Scholar]
References to studies awaiting assessment
Fandriantika 2017 {published data only}
- Fandriantika DR. Penanganan Tinnitus Dengan Titik Akupunktur Yifeng (SJ17), Tinggong (SI19), Taixi (KI3) Serta Pemberian Herba Ginkgo Biloba [Thesis]. Universitas Airlangga, 2017. [REPOSITORY WEBSITE: http://repository.unair.ac.id/id/eprint/65655] [Google Scholar]
Rogowski 2001 {published data only}
- Rogowski M, Bartnik G, Fabijanska A. Randomized double-blind drug/retraining vs placebo/retraining study of the treatment of tinnitus with Ginkgo biloba extract (EGb 761). European Archives of Oto-rhino-laryngology 2001;258(8):4222001. [Google Scholar]
References to ongoing studies
ChiCTR2000038850 {published data only}
- ChiCTR2000038850. A randomized controlled clinical study of sound therapy combined with drug therapy for tinnitus. https://www.chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR2000038850 (first received 28 December 2020). [ICTRP: ChiCTR2000038850]
Additional references
Adjamian 2009
- Adjamian P, Sereda M, Hall DA. The mechanisms of tinnitus: perspectives from human functional neuroimaging. Hearing Research 2009;253(1-2):15-31. [DOI] [PubMed] [Google Scholar]
Baguley 2013
- Baguley D, McFerran D, Hall D. Tinnitus. Lancet 2013;382(9904):1600-7. [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988;56(6):893-7. [DOI] [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation, 1996. [Google Scholar]
Biesinger 2010
- Biesinger E, Del Bo L, De Ridder D, Goodey R, Herraiz C, Kleinjung T, et al. Algorithm for the diagnostic & therapeutic management of tinnitus. Tinnitus Clinic Network, Tinnitus Research Initiative. Available at: www.tinnitus research.org/en/documents/downloads/TRI_Tinnitus_Flowchart.pdf (accessed 9 August 2010) 2010.
Birks 2009
- Birks J, Grimley Evans J . Ginkgo biloba for cognitive impairment and dementia. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD003120. [DOI: 10.1002/14651858.CD003120.pub3] [DOI] [PubMed] [Google Scholar]
Blecharz‐Klin 2009
- Blecharz-Klin K, Piechal A, Joniec I, Pyrzanowska J, Widy-Tyszkiewicz E. Pharmacological and biochemical effects of Ginkgo biloba extract on learning, memory consolidation and motor activity in old rats. Acta Neurobiologiae Experimentalis 2009;69(2):217-31. [DOI] [PubMed] [Google Scholar]
Blumenthal 1998
- Blumenthal M, Busse W, Goldberg A, Gruenwald J, Hall T, Riggins C. The Complete German Commission EMonographs: Therapeutic Guide to Herbal Medicines. Austin, Texas: American Botanical Council, 1998. [Google Scholar]
Boelsma 2004
- Boelsma E, Lamers RJ, Hendriks HF, Nesselrooij JH, Roza L. Evidence of the regulatory effect of Ginkgo biloba extract on skin blood flow and study of its effects on urinary metabolites in healthy humans. Planta Medica 2004;70(11):1052-7. [DOI] [PubMed] [Google Scholar]
Braquet 1991
- Braquet P, Hosford D. Ethnopharmacology and the development of natural PAF antagonists as therapeutic agents. Journal of Ethnopharmacology 1991;32(1-3):135-9. [DOI] [PubMed] [Google Scholar]
Buss 1998
- Buss E, Hall JW, Grose JH, Hatch DR. Perceptual consequences of peripheral hearing loss: do edge effects exist for abrupt cochlear lesions? Hearing Research 1998;125(1-2):98-108. [DOI] [PubMed] [Google Scholar]
Chan 2007
- Chan PC, Xia Q, Fu PP. Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. Journal of Environmental Science and Health. Part C, Environmental Carcinogenesis & Ecotoxicology Reviews 2007;25(3):211-44. [DOI] [PubMed] [Google Scholar]
Cheng 2003
- Cheng SM, Yang SP, Ho LJ, Tsao TP, Juan TY, Chang DM, et al. Down-regulation of c-jun N-terminal kinase-activator protein-1 signalling pathway by Ginkgo biloba extract in human peripheral blood T cells. Biochemical Pharmacology 2003;66(4):679-89. [DOI] [PubMed] [Google Scholar]
Cima 2012
- Cima RF, Maes IH, Joore MA, Scheyen DJ, El Refaie A, Baguley DM, et al. Specialised treatment based on cognitive behaviour therapy versus usual care for tinnitus: a randomised controlled trial. Lancet 2012;379(9830):1951-9. [DOI] [PubMed] [Google Scholar]
Cima 2019
- Cima RFF, Mazurek B, Haider H, Kikidis D, Lapira A, Noreña A, et al. A multidisciplinary European guideline for tinnitus: diagnostics, assessment, and treatment. HNO 2019;67(Suppl 1):10-42. [DOI] [PubMed] [Google Scholar]
Coelho 2016
- Coelho C, Tyler R, Ji H, Rojas-Roncancio E, Witt S, Tao P, et al. Survey of effectiveness of dietary supplements to treat tinnitus. American Journal of Audiology 2016;25:184-205. [DOI] [PubMed] [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeFeudis 1991
- DeFeudis FV. Ginkgo biloba extract (EGb 761): pharmacological activities and clinical applications. Editions Scientifiques. In: Editions Scientifiques Elseviere. Elseviere, 1991:68-73. [Google Scholar]
DeFeudis 2000
- DeFeudis FV, Drieu K. Ginkgo biloba extract (EGb 761) and CNS functions: basic studies and clinical applications. Current Drug Targets 2000;1(1):25-58. [DOI] [PubMed] [Google Scholar]
Department of Health 2009
- Department of Health. Provision of Services for Adults with Tinnitus. A Good Practice Guide. London: Central Office of Information, 2009. [Google Scholar]
Diamond 2013
- Diamond BJ, Bailey MR. Ginkgo biloba: indications, mechanisms, and safety. Psychiatric Clinics of North America 2013;36(1):73-83. [DOI] [PubMed] [Google Scholar]
Didier 1996
- Didier A, Droy-Lefaix MT, Aurousseau C, Cazals Y. Effects of Ginkgo biloba extract (EGb 761) on cochlear vasculature in the guinea pig: morphometric measurements and laser Doppler flowmetry. European Archives of Oto-rhino-laryngology 1996;253(1-2):25-30. [DOI] [PubMed] [Google Scholar]
Dietrich 2001
- Dietrich V, Nieschalk M, Stoll W, Rajan R, Pantev C. Cortical reorganization in patients with high frequency cochlear hearing loss. Hearing Research 2001;158(1-2):95-101. [DOI] [PubMed] [Google Scholar]
Dogan 2018
- Dogan R, Sjostrand AP, Yenıgun A, Karatas E, Kocyigit A, Ozturan O. Influence of Ginkgo Biloba extract (EGb 761) on expression of IL-1 beta, IL-6, TNF-alfa, HSP-70, HSF-1 and COX-2 after noise exposure in the rat cochlea. Auris Nasus Larynx 2018;45(4):680-5. [DOI] [PubMed] [Google Scholar]
Dong 2010
- Dong S, Rodger J, Mulders WH, Robertson D. Tonotopic changes in GABA receptor expression in guinea pig inferior colliculus after partial unilateral hearing loss. Brain Research 2010;1342:24-32. [DOI] [PubMed] [Google Scholar]
Eckert 2005
- Eckert A, Keil U, Scherping I, Hauptmann S, Müller WE. Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by Ginkgo biloba extract EGb 761. Annals of the New York Academy of Sciences 2005;1056:474-85. [DOI] [PubMed] [Google Scholar]
Eggermont 2004
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends in Neurosciences 2004;27(11):676-82. [DOI] [PubMed] [Google Scholar]
El Refaie 2004
- El Refaie A, Davis A, Kayan A, Baskill J, Lovell E, Owen V. A questionnaire study of the quality of life and quality of family life of individuals complaining of tinnitus pre- and post-attendance at a tinnitus clinic. International Journal of Audiology 2004;43(7):410-6. [DOI] [PubMed] [Google Scholar]
El Shunnar 2011
- El-Shunnar SK, Hoare DJ, Smith S, Gander PE, Kang S, Fackrell K, et al. Primary care for tinnitus: practice and opinion among GPs in England. Journal of Evaluation in Clinical Practice 2011;17(4):684-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engineer 2011
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature 2011;470(7332):101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ernst 1999
- Ernst E, Stevinson C . Ginkgo biloba for tinnitus: a review. Clinical Otolaryngology and Allied Sciences 1999;24(3):164-7. [DOI] [PubMed] [Google Scholar]
Ernst 2002
- Ernst E. The risk-benefit profile of commonly used herbal therapies: Ginkgo, St. John’s wort, ginseng, echinacea, saw palmetto, and kava. Annals of Internal Medicine 2002;136:42-53. [DOI] [PubMed] [Google Scholar]
Fehske 2009
- Fehske CJ, Leuner K, Müller WE. Ginkgo biloba extract (EGb761) influences monoaminergic neurotransmission via inhibition of NE uptake, but not MAO activity after chronic treatment. Pharmacological Research 2009;60(1):68-73. [DOI] [PubMed] [Google Scholar]
Fowler 1944
- Fowler EP. Head noises in normal and in disordered ears: significance, measurement, differentiation and treatment. Archives of Otolaryngology 1944;39(6):498-503. [Google Scholar]
Furlong 2001
- Furlong WJ, Feeny DH, Torrance GW, Barr RD. The Health Utilities Index (HUI®) system for assessing health-related quality of life in clinical studies. Annals of Medicine 2001;33:375-84. [DOI] [PubMed] [Google Scholar]
Gallus 2015
- Gallus S, Lugo A, Garavello W, Bosetti C, Santoro E, Colombo P, et al. Prevalence and determinants of tinnitus in the Italian adult population. Neuroepidemiology 2015;45(1):12-9. [DOI] [PubMed] [Google Scholar]
Guitton 2003
- Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL. Salicylate induces tinnitus through activation of cochlear NMDA receptors. Journal of Neuroscience 2003;23(9):3944-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2011
- Hall DA, Lainez MJ, Newman CW, Sanchez TG, Egler M, Tennigkeit F, et al. Treatment options for subjective tinnitus: self reports from a sample of general practitioners and ENT physicians within Europe and the USA. BMC Health Services Research 2011;11:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2017
- Hall DA, Mehta RL, Fackrell K. How to choose between measures of tinnitus loudness for clinical research? A report on the reliability and validity of an investigator-administered test and a patient-reported measure using baseline data collected in a phase IIa drug trial. American Journal of Audiology 2017;26(3):338-46. [DOI] [PubMed] [Google Scholar]
Hall 2018
- Hall DA, Fackrell K, Li AB, Thavayogan R, Smith S, Kennedy V, et al. A narrative synthesis of research evidence for tinnitus-related complaints as reported by patients and their significant others. Health and Quality of Life Outcomes 2018;16(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2018a
- Hall DA, Smith H, Hibbert A, Colley V, Haider HF, Horobin A, et al, Core Outcome Measures in Tinnitus (COMiT) initiative. The COMiT'ID Study: Developing core outcome domains sets for clinical trials of sound-, psychology-, and pharmacology-based interventions for chronic subjective tinnitus in adults. Trends in Hearing 2018;22:2331216518814384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallam 1996
- Hallam RS. Manual of the Tinnitus Questionnaire (TQ). London: The Psychological Corporation, Brace & Co, 1996. [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry 1960;23(1):56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2009
- Han BI, Lee HW, Kim TY, Lim JS, Shin KS. Tinnitus: characteristics, causes, mechanisms, and treatments. Journal of Clinical Neurology 2009;5(1):11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Handbook 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hays 1993
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Economics 1993;2(3):217-27. [DOI] [PubMed] [Google Scholar]
Henry 2004
- Henry JA, Snow JB (editors). Tinnitus: Theory and Management. Ontario: BC Becker Inc, 2004. [Google Scholar]
Henry 2005
- Henry JA, Dennis KC, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. Journal of Speech, Language, and Hearing Research 2005;48(5):1204-35. [DOI] [PubMed] [Google Scholar]
Henry 2008
- Henry JA, Zaugg TL, Myers PJ, Schechter MA. The role of audiologic evaluation in progressive audiologic tinnitus management. Trends in Amplification 2008;12(3):170-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hiller 1992
- Hiller W, Goebel G. A psychometric study of complaints in chronic tinnitus. Journal of Psychosomatic Research 1992;36(4):337-48. [DOI] [PubMed] [Google Scholar]
Hiller 2006
- Hiller W, Goebel G. Factors influencing tinnitus loudness and annoyance. Archives of Otolaryngology-–Head & Neck Surgery 2006;132(12):1323-30. [DOI] [PubMed] [Google Scholar]
Hilton 2013
- Hilton MP, Zimmermann EF, Hunt WT. Ginkgo biloba for tinnitus. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD003852. [DOI: 10.1002/14651858.CD003852.pub3] [DOI] [PubMed] [Google Scholar]
Hoare 2011
- Hoare DJ, Hall DA. Clinical guidelines and practice: a commentary on the complexity of tinnitus management. Evaluation & the Health Professions 2011;34(4):413-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoare 2014
- Hoare DJ, Edmondson-Jones M, Sereda M, Akeroyd MA, Hall D. Amplification with hearing aids for patients with tinnitus and co-existing hearing loss. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD010151. [DOI: 10.1002/14651858.CD010151.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2007
- Huang X, Whitworth CA, Rybak LP. Ginkgo biloba extract (EGb 761) protects against cisplatin-induced ototoxicity in rats. Otology and Neurotology 2007;28(6):828-33. [DOI] [PubMed] [Google Scholar]
Jastreboff 1988
- Jastreboff PJ, Brennan JF, Coleman JK, Sasaki CT. Phantom auditory sensation in rats: an animal model for tinnitus. Behavioral Neuroscience 1988;102(6):811-22. [DOI] [PubMed] [Google Scholar]
Jastreboff 1990
- Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neuroscience Research 1990;8(4):221-54. [DOI] [PubMed] [Google Scholar]
Jastreboff 2004
- Jastreboff PJ, Hazell JWP. Tinnitus Retraining Therapy: Implementing the Neurophysiological Model. New York: Cambridge University Press, 2004. [Google Scholar]
Jung 1990
- Jung F, Mrowietz C, Kiesewetter H, Wenzel E. Effect of Ginkgo biloba on fluidity of blood and peripheral microcirculation in volunteers. Arzneimittelforschung 1990;40(5):589-93. [PubMed] [Google Scholar]
Kluk 2006
- Kluk K, Moore BC. Dead regions in the cochlea and enhancement of frequency discrimination: effects of audiogram slope, unilateral versus bilateral loss, and hearing-aid use. Hearing Research 2006;222(1-2):1-15. [DOI] [PubMed] [Google Scholar]
Koltinger 1989
- Koltringer P, Eber O, Klima G, Rothlauer W, Wakonig P, Langsteger W, et al. Microcirculation in parenteral Ginkgo biloba extract therapy [Die Mikrozirkulation unterparenteraler Ginkgo–biloba–Extrakt–Therapie]. WienerKlinische Wochenschrift 1989;101(6):198-200. [PubMed] [Google Scholar]
König 2006
- König O, Schaette R, Kempter R, Gross M. Course of hearing loss and occurrence of tinnitus. Hearing Research 2006;221:59-64. [DOI] [PubMed] [Google Scholar]
Kramer 2018
- Kramer F, Ortigoza Á. Ginkgo biloba for the treatment of tinnitus. Medwave 2018;18(6):e7294. [DOI] [PubMed] [Google Scholar]
Krauss 2016
- Krauss P, Tziridis K, Buerbank S, Schilling A, Schulze H. Therapeutic value of Ginkgo biloba extract EGb 761® in an animal model (Meriones unguiculatus) for noise trauma induced hearing loss and tinnitus. PLOS One 2016;11(6):e0157574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuk 1990
- Kuk FK, Tyler RS, Russell D, Jordan H. The psychometric properties of a tinnitus handicap questionnaire. Ear and Hearing 1990;11:434-45. [DOI] [PubMed] [Google Scholar]
Li 2018
- Li MZ, Zhang Y, Zou HY, Ouyang JY, Zhan Y, Yang L, et al. Investigation of Ginkgo biloba extract (EGb 761) promotes neurovascular restoration and axonal remodeling after embolic stroke in rat using magnetic resonance imaging and histopathological analysis. Biomedicine & Pharmacotherapy 2018;103:989-1001. [DOI] [PubMed] [Google Scholar]
Lichota 2019
- Lichota A, Gwozdzinski L, Gwozdzinski K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. European Journal of Medicinal Chemistry 2019;176:68-91. [DOI] [PubMed] [Google Scholar]
Mahadevan 2008
- Mahadevan S, Park Y. Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. Journal of Food Science 2008;73(1):R14-9. [DOI] [PubMed] [Google Scholar]
Martines 2010
- Martines F, Bentivegna D, Di Piazza F, Martines E, Sciacca V, Martinciglio G. Investigation of tinnitus patients in Italy: clinical and audiological characteristics. International Journal of Otolaryngology 2010;2010:265861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mashayekh 2011
- Mashayekh A, Pham DL, Yousem DM, Dizon M, Barker PB, Lin DD. Effects of Ginkgo biloba on cerebral blood flow assessed by quantitative MR perfusion imaging: a pilot study. Neuroradiology 2011;53(3):185-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCormack 2016
- McCormack A, Edmondson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hearing Research 2016;337:70-9. [DOI] [PubMed] [Google Scholar]
McDermott 1998
- McDermott HJ, Lech M, Kornblum MS, Irvine DR. Loudness perception and frequency discrimination in subjects with steeply sloping hearing loss: possible correlates of neural plasticity. Journal of the Acoustical Society of America 1998;104(4):2314-25. [DOI] [PubMed] [Google Scholar]
McFerran 2018
- McFerran D, Hoare DJ, Carr S, Ray J, Stockdale D. Tinnitus services in the United Kingdom: a survey of patient experiences. BMC Health Services Research 2018;18:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mei 2017
- Mei N, Guo X, Ren Z, Kobayashi D, Wada K, Guo L. Review of Ginkgo biloba-induced toxicity, from experimental studies to human case reports. Journal of Environmental Science and Health. Part C, Environmental Carcinogenesis & Ecotoxicology Reviews 2017;35(1):1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meikle 2012
- Meikle MB, Henry JA, Griest SE, Stewart BJ, Abrams HB, McArdle R, et al. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear and Hearing 2012;33:153-76. [DOI] [PubMed] [Google Scholar]
Melcher 2013
- Melcher JR, Knudson IM, Levine RA. Subcallosal brain structure: correlation with hearing threshold at supra-clinical frequencies (>8 kHz), but not with tinnitus. Hearing Research 2013;295:79-86. [DOI] [PubMed] [Google Scholar]
Middleton 2011
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proceedings of the National Academy of Sciences of the United States of America 2011;108(18):7601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moller 2000
- Moller AR. Similarities between severe tinnitus and chronic pain. Journal of the American Academy of Audiology 2000;11(3):115-24. [PubMed] [Google Scholar]
Moore 2009
- Moore BC, Vinay SN. Enhanced discrimination of low-frequency sounds for subjects with high-frequency dead regions. Brain 2009;132(Pt 2):524-36. [DOI] [PubMed] [Google Scholar]
Muhlnickel 1998
- Muhlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proceedings of the National Academy of Sciences of the United States of America 1998;95(17):10340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulders 2010
- Mulders WH, Seluakumaran K, Robertson D. Efferent pathways modulate hyperactivity in inferior colliculus. Journal of Neuroscience 2010;30(28):9578-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Müller 2012
- Müller WE, Heiser J, Leuner K. Effects of the standardized Ginkgo biloba extract EGb 761® on neuroplasticity. International Psychogeriatrics 2012;Suppl 1:S21-4. [DOI] [PubMed] [Google Scholar]
Nash 2015
- Nash KM, Shah ZA. Current perspectives on the beneficial role of Ginkgo biloba in neurological and cerebrovascular disorders. Integrative Medicine Insights 2015;10:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newman 1996
- Newman CW, Jacobson GP, Spitzer JB. Development of the tinnitus handicap inventory. Archives of Otolaryngology--Head and Neck Surgery 1996;122:143-8. [DOI] [PubMed] [Google Scholar]
Norena 2005
- Norena AJ, Eggermont JJ. Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. Journal of Neuroscience 2005;25(3):699-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norena 2011
- Norena AJ. An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neuroscience and Biobehavioral Reviews 2011;35(5):1089-109. [DOI] [PubMed] [Google Scholar]
Nuhu 2014
- Nuhu AA. Ginkgo biloba: A ‘living fossil’ with modern day phytomedicinal applications. Journal of Applied Pharmaceutical Science 2014;4(3):96-103. [Google Scholar]
Omidkhoda 2019
Oskouei 2013
- Oskouei DS, Rikhtegar R, Hashemilar M, Sadeghi-Bazargani H, Sharifi-Bonab M, Sadeghi-Hokmabadi E, et al. The effect of Ginkgo biloba on functional outcome of patients with acute ischemic stroke: a double-blind, placebo-controlled, randomized clinical trial. Journal of Stroke and Cerebrovascular Diseases 2013;22(8):e557-63. [DOI] [PubMed] [Google Scholar]
Pilati 2012
- Pilati N, Large C, Forsythe ID, Hamann M. Acoustic overexposure triggers burst firing in dorsal cochlear nucleus fusiform cells. Hearing Research 2012;283(1-2):98-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Posadzki 2012
- Posadzki P, Watson L, Ernst E. Herb-drug interaction: an overview of systematic reviews. British Journal of Clinical Pharmacology 2012;75(3):603-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rajarajan 2018
- Rajarajan G, Pavithra Priyadorshoni SP, Geetha RV. A review on the medicinal properties of Gingko biloba. Drug Invention Today 2018;10(3):3420-3. [Google Scholar]
Ratnayake 2009
- Ratnayake SA, Jayarajan V, Bartlett J. Could an underlying hearing loss be a significant factor in the handicap caused by tinnitus? Noise & Health 2009;11(44):156-60. [DOI] [PubMed] [Google Scholar]
Rauschecker 1999
- Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems. Trends in Neurosciences 1999;22(2):74-80. [DOI] [PubMed] [Google Scholar]
Rauschecker 2010
- Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 2010;66(6):819-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reiss 1986
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy 1986;24(1):1-8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2010
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. Journal of Neuroscience 2010;30(45):14972-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sahley 2001
- Sahley TL, Nodar RH. A biochemical model of peripheral tinnitus. Hearing Research 2001;152(1-2):43-54. [DOI] [PubMed] [Google Scholar]
Sanchez 2002
- Sanchez TG, Ferrari GMS. The control of tinnitus through hearing aids: suggestions for optimal use. Pró-Fono Revista de Atualização Científica 2002;14:111-8. [Google Scholar]
Schaette 2006
- Schaette R, Kempter R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: a computational model. European Journal of Neuroscience 2006;23(11):3124-38. [DOI] [PubMed] [Google Scholar]
Schaette 2011
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. Journal of Neuroscience 2011;31(38):13452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seki 2003
- Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hearing Research 2003;180(1-2):28-38. [DOI] [PubMed] [Google Scholar]
Sereda 2011
- Sereda M, Hall DA, Bosnyak DJ, Edmondson-Jones M, Roberts LE, Adjamian P, et al. Re-examining the relationship between audiometric profile and tinnitus pitch. International Journal of Audiology 2011;50(5):303-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sereda 2015
- Sereda M, Edmondson-Jones M, Hall DA. Relationship between tinnitus pitch and edge of hearing loss in individuals with a narrow tinnitus bandwidth. International Journal of Audiology 2015;54(4):249-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shu 2019
- Shu Z, Shar AH, Shahen M, Wang H, Alagawany M, Abd El-Hack ME, et al. Pharmacological uses of Ginkgo biloba extracts for cardiovascular disease and coronary heart diseases. International Journal of Pharmacology 2019;15:1-9. [Google Scholar]
Singh 2019
- Singh SK, Srivastav S, Castellani RJ, Plascencia-Villa G, Perry G. Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics 2019;16(3):666-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skevington 2004
- Skevington SM, Lotfy M, O'Connell KA. The World Health Organization's WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Quality of Life Research 2004;13(2):299-310. [DOI] [PubMed] [Google Scholar]
Smith 1996
- Smith PF, Maclennan K, Darlington CL. The neuroprotective properties of the Ginkgo biloba leaf: a review of the possible relationship to platelet-activating factor (PAF). Journal of Ethnopharmacology 1996;50(3):131-9. [DOI] [PubMed] [Google Scholar]
Smith 2013
- Smith GS, Romanelli-Gobbi M, Gray-Karagrigoriou E, Artz GJ. Complementary and integrative treatments: tinnitus. Otolaryngologic Clinics of North America 2013;46:389-408. [DOI] [PubMed] [Google Scholar]
Spiegel 2018
- Spiegel R, Kalla R, Maire R, Mueller H, Hoerr R, Ihl R. Ginkgo biloba extract EGb 761® alleviates neurosensory symptoms in patients with dementia: a meta-analysis of treatment effects on tinnitus and dizziness in randomized, placebo-controlled trials. Clinical Interventions in Aging 2018;13:1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sweetow 1990
- Sweetow RW, Levy MC. Tinnitus severity scaling for diagnostic/therapeutic usage. Hearing Instruments 1990;41:20-1. [Google Scholar]
Tass 2012
- Tass PA, Adamchic I, Freund HJ, Stackelberg T, Hauptmann C. Counteracting tinnitus by acoustic coordinated reset neuromodulation. Restorative Neurology and Neuroscience 2012;30(2):137-59. [DOI] [PubMed] [Google Scholar]
Thai‐Van 2002
- Thai-Van H, Micheyl C, Norena A, Collet L. Local improvement in auditory frequency discrimination is associated with hearing-loss slope in subjects with cochlear damage. Brain 2002;125(Pt 3):524-37. [DOI] [PubMed] [Google Scholar]
Thai‐Van 2003
- Thai-Van H, Micheyl C, Moore BC, Collet L. Enhanced frequency discrimination near the hearing loss cut-off: a consequence of central auditory plasticity induced by cochlear damage? Brain 2003;126(Pt 10):2235-45. [DOI] [PubMed] [Google Scholar]
Tunis 2016
- Tunis SR, Clarke M, Gorst SL, Gargon E, Blazeby JM, Altman DG, et al. Improving the relevance and consistency of outcomes in comparative effectiveness research. Journal of Comparative Effectiveness Research 2016;5(2):193-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tunkel 2014
- Tunkel DE, Bauer CA, Sun GH, Rosenfeld RM, Chandrasekhar SS, Cunningham ER Jr, et al. Clinical practice guideline: tinnitus executive summary. Otolaryngology--Head and Neck Surgery 2014;151(4):533-41. [DOI] [PubMed] [Google Scholar]
Tziridis 2014
- Tziridis K, Korn S, Ahlf S, Schulze H. Protective effects of Ginkgo biloba extract EGb 761 against noise trauma-induced hearing loss and tinnitus development. Neural Plasticity 2014;2014:427298. [DOI: 10.1155/2014/427298] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ude 2013
- Ude C, Schubert-Zsilavecz M, Wurglics M. Ginkgo biloba extracts: a review of the pharmacokinetics of the active ingredients. Clinical Pharmacokinetics 2013;52(9):727-49. [DOI] [PubMed] [Google Scholar]
Vanneste 2012
- Vanneste S, De Ridder D. The auditory and non-auditory brain areas involved in tinnitus. An emergent property of multiple parallel overlapping subnetworks. Frontiers in Systems Neuroscience 2012;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Boetticher 2011
- Boetticher A. Ginkgo biloba extract in the treatment of tinnitus: a systematic review. Neuropsychiatric Disease and Treatment 2011;7:441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weisz 2005
- Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLOS Medicine 2005;2(6):e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 1991
- Wilson PH, Henry J, Bowen M, Haralambous G. Tinnitus reaction questionnaire: psychometric properties of a measure of distress associated with tinnitus. Journal of Speech and Hearing Research 1991;34:197-201. [PubMed] [Google Scholar]
Xia 2007
- Xia SH, Fang DC. Pharmacological action and mechanisms of ginkgolide B. Chinese Medical Journal 2007;120(10):922-8. [PubMed] [Google Scholar]
Xiao 2019
- Xiao G, Lyu M, Wang Y, He S, Liu X, Ni J, et al. Ginkgo flavonol glycosides or ginkgolides tend to differentially protect myocardial or cerebral ischemia-reperfusion injury via regulation of TWEAK-Fn14 signaling in heart and brain. Frontiers in Pharmacology 2019;10:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2011
- Yang TH, Young YH, Liu SH. EGb 761 (Ginkgo biloba) protects cochlear hair cells against ototoxicity induced by gentamicin via reducing reactive oxygen species and nitric oxide-related apoptosis. Journal of Nutritional Biochemistry 2011;22(9):886-94. [DOI] [PubMed] [Google Scholar]
Zhang 2016
- Zhang W, Tao Q, Guo Z, Fu Y, Chen X, Shar PA, et al. Systems pharmacology dissection of the integrated treatment for cardiovascular and gastrointestinal disorders by traditional Chinese medicine. Scientific Reports 2016;6:32400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2004
- Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Clinical use and molecular mechanisms of action of extract of Ginkgo biloba leaves in cardiovascular diseases. Cardiovascular Drug Reviews 2004;22(4):309-19. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67:361-70. [DOI] [PubMed] [Google Scholar]
Zuo 2017
- Zuo W, Yan F, Zhang B, Li J, Mei D. Advances in the studies of Ginkgo biloba leaves extract on aging-related diseases. Aging and Disease 2017;8(6):812-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Sereda 2019
- Sereda M, Xia J, Scutt P, Hilton MP, El Refaie A, Hoare DJ. Ginkgo biloba for tinnitus. Cochrane Database of Systematic Reviews 2019, Issue 12. Art. No: CD013514. [DOI: 10.1002/14651858.CD013514] [DOI] [PMC free article] [PubMed] [Google Scholar]


