Abstract
The choriocapillaris, a dense capillary network located at the posterior pole of the eye, is essential for supporting normal vision, supplying nutrients, and removing waste products from photoreceptor cells and the RPE. The anatomical location, heterogeneity, and homeostatic interactions with surrounding cell types makes the choroid complex to study both in vivo and in vitro. Recent advances in single-cell RNA sequencing, in vivo imaging and in vitro cell modeling are vastly improving our knowledge of the choroid and its role in normal health and in macular degeneration (AMD). Histologically loss of endothelial cells (ECs) of the choriocapillaris occurs early in AMD concomitant with elevated formation of the membrane attack complex of complement. Advanced imaging has allowed us to visualize early choroidal blood flow changes in AMD in living patients, supporting histological findings of loss of choroidal ECs. Single-cell RNA sequencing is being used to characterize choroidal cell types transcriptionally and discover their altered patterns of gene expression in aging and disease. Advances in iPSC protocols and 3D cultures will allow us to closely mimic the in vivo microenvironment of the choroid in vitro to gain a better understanding of the mechanism leading to choriocapillaris loss in AMD.
Overview of the retina-choroid interface
Vertebrate photoreceptor cells are uniquely adapted to detect light and transmit visual information to retinal bipolar cells which ultimately reaches the visual cortex. As discussed elsewhere in this volume, the process of phototransduction involves a conformational change in 11-cis retinal to all-trans retinal, followed by the recycling and regeneration of this retinoid through a series of trafficking and enzymatic processes. In addition to recycling, a considerable fraction of retinoids are delivered to the retina daily from the circulation. Thus, alterations in the quality or quantity of the outer retina’s vascular supply could profoundly affect dark adaptation (Owsley et al., 2016), discussed elsewhere in this volume.
Because this process is highly finessed, photoreceptor cells require an extracellular milieu that is tightly controlled, and even temporary perturbations in this microenvironment can lead to degeneration of photoreceptor cells, particularly rods. This microenvironment is maintained by the retinal pigment epithelium (RPE), where one RPE cell maintains 30 or more photoreceptor cells; the retinal Muller cells (which help provide retinoids to cones, as discussed elsewhere in this volume), and the interphotoreceptor matrix, which appears to be largely synthesized by the photoreceptor cells themselves and serves as the conduit for retinoids between the photoreceptor cells and RPE. Changes in the composition or volume of the fluid bathing photoreceptor cells can result in a sudden loss of vision.
At the same time, because of the retina’s exceptional energy consumption (the highest in the body (Ames, 1992)) photoreceptor cells require a constant and large supply of oxygen and generate a high volume of CO2, which must be converted to bicarbonate for removal through the action of carbonic anhydrases.
In summary, photoreceptor cells require a clear path of light through the retina with minimal distortion by vascular elements, a highly regulated microenvironment, and abundant metabolites. Collectively, these features all contribute to the need for a dense vascular supply located beneath (or external to) the photoreceptor cells. In humans, this need is met by the choroid (Figure 1).
Figure 1.
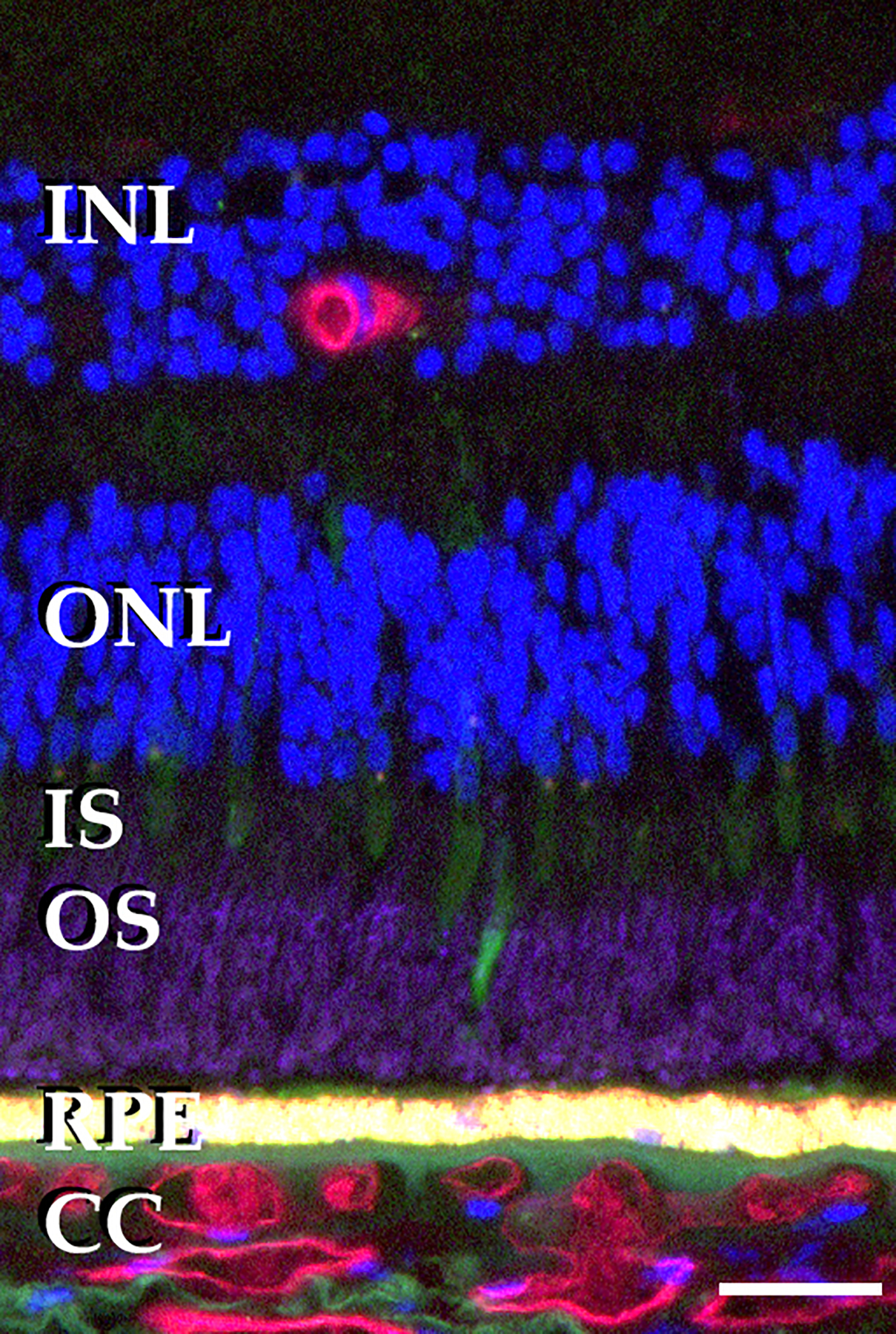
Relationship of photoreceptor cells, RPE and choriocapillaris. Section of human retina labeled with antibodies directed against rhodopsin (magenta), pooled anti-cone opsins (green), UEA-I, which binds vascular endothelial cells of the retina and choroid (red) and DAPI, which labels DNA (blue). Note the relatively sparse vasculature in the inner retina, absence of vascular elements in the outer retina, and dense, large diameter vessels in the choriocapillaris. Scalebar = 25μm. INL, inner nuclear layer; ONL, outer nuclear layer; IS, inner segments; OS, outer segments; RPE, retinal pigment epithelium; CC, choriocapillaris.
Choroid: structure
The choroid is a component of the peripheral vasculature outside of the blood retinal barrier that occupies the anatomical compartment between the retinal pigment epithelium and the sclera. It may be divided between the vascular elements and the surrounding connective tissue, the choroidal stroma. From outer to inner submacular choroid, the vascular elements progressively narrow as they branch, with layers of large, intermediate, and small vessels in Haller’s layer, Sattler’s layer, and the choriocapillaris, respectively (Figure 2). Notably, the smallest choroidal capillaries are still very large compared to those of the retinal vasculature, with biophysical implications for both velocity and diffusion.
Figure 2.
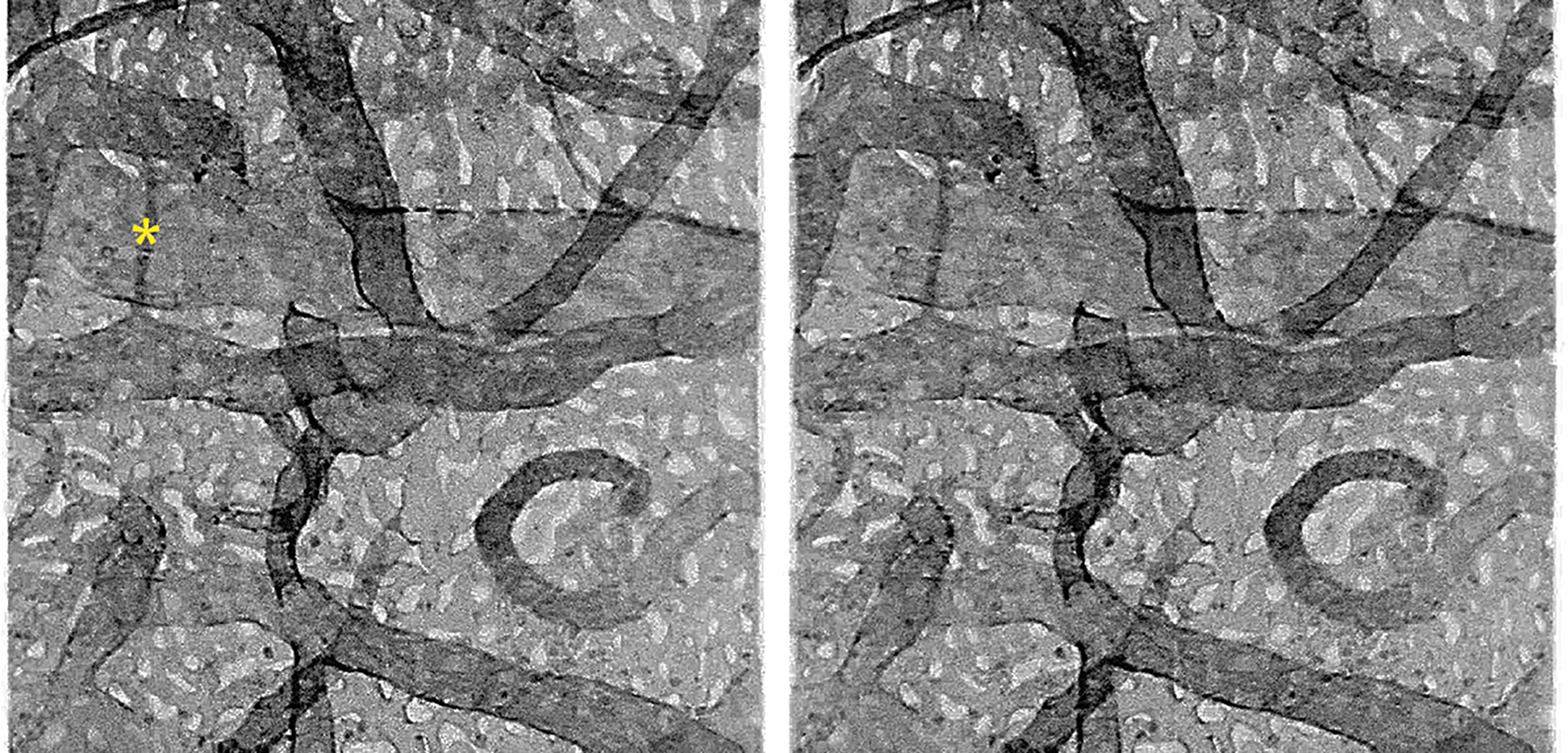
Confocal projection stereo pair of the human choroidal vasculature labeled with UEA-I lectin. Note the very dense honeycomb appearance of the choriocapillaris (superficial) and the complexity of the intermediate and large vessels. A potential A-V shunt is indicated (asterisk).
The choroid is supplied by the short posterior ciliary arteries (Figure 3) that enter the sclera at the posterior pole and drained by the vortex vein system at the eccentricity of the equator, where venules from the posterior pole converge and exit the eye through one of the ophthalmic vein branches. Each vortex vein typically drains around 20–25% of the choroid.
Figure 3.
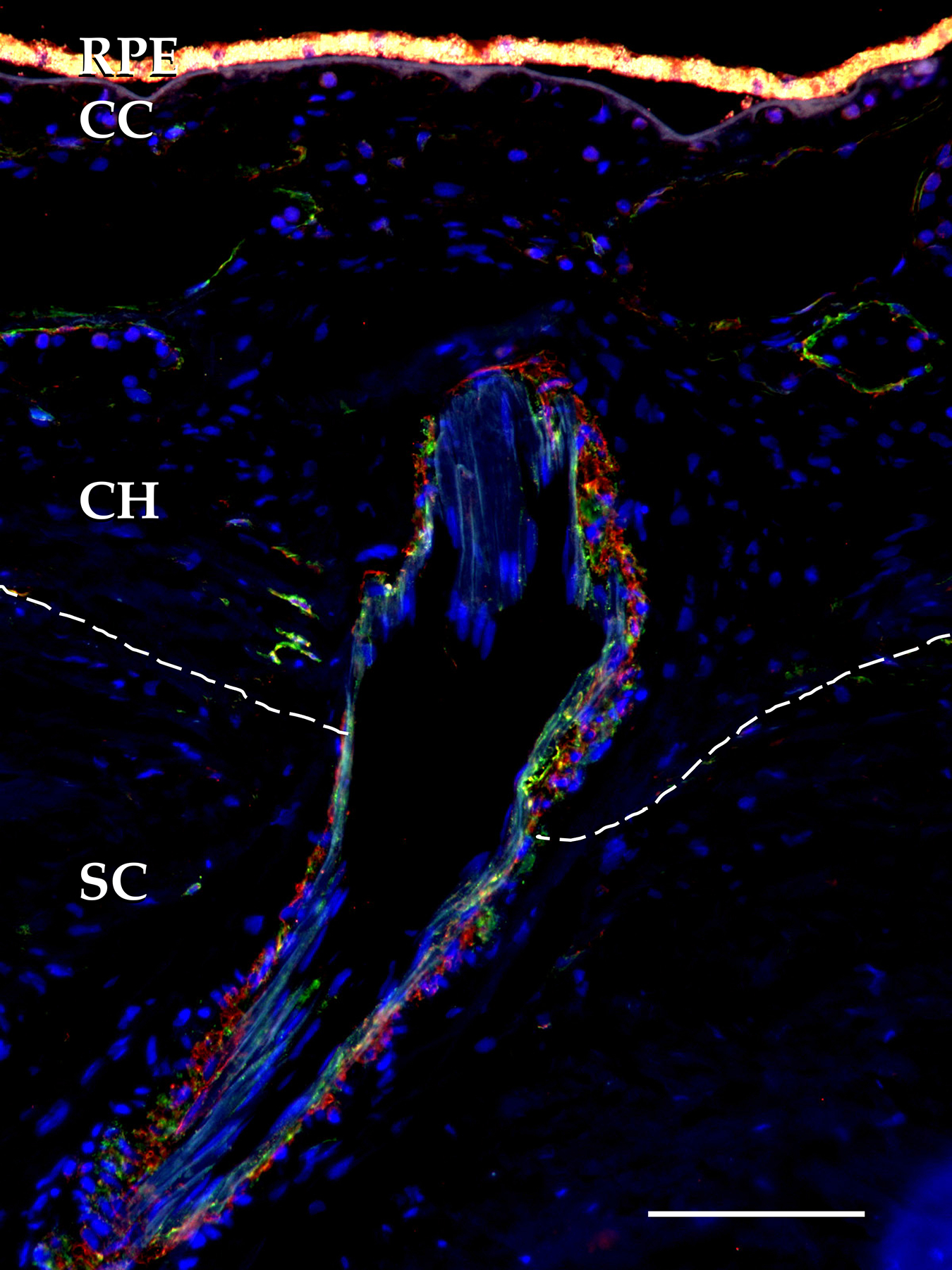
Short posterior ciliary artery penetrating the sclera (SC, margin indicated by dotted line) to supply the choroidal arterioles and ultimately choriocapillaris. Red, CC, choriocapillaris, CH, Saller’s and Hatler’s layers of the choroid, RPE, retinal pigment epithelium. Red, anti-smooth muscle actin antibody; green, anti-vascular adhesion protein antibody, scalebar = 100μm.
As in other vascular beds, the endothelial cells (ECs) of the choriocapillaris are supported by pericytes, which are normally distributed along the lateral and outer aspect of the endothelium. In contrast to retinal capillaries, the pericytes of the choroid do not circumscribe the endothelium and are not normally present between the endothelial lumen and the tissue it nourishes.
Choroid function:
The most obvious function of the choriocapillaris is supplying nutrients and oxygen and removing waste from the photoreceptor cells and RPE. The high level of photoreceptor metabolism results in CO2, water, and heat as byproducts of oxidative phosphorylation or aerobic glycolysis (Hurley et al., 2015). Carbonic anhydrases in the RPE and in the choriocapillaris (including CA4, which is restricted to choriocapillaris ECs) interconvert CO2 gas and bicarbonate ions. The removal of excess heat by the choriocapillaris (a consequence of both photoreceptor metabolism, highest in the dark, and absorption of photons by the RPE in the light) has been studied indirectly. Experimental elevation of intraocular pressure above the perfusion pressure of the choroid is associated with increased ocular temperature in both monkeys and humans, indicating that the normal choroidal circulation serves as a heat sink (Parver, 1991). The potential consequences of loss of choroidal flow in AMD (discussed below) and its impact on temperature at the photoreceptor/RPE/choroid interface has not been fully explored, however it is notable that RPE cells grown at elevated temperature show evidence of altered synthesis of extracellular matrix molecules (Sekiyama et al., 2012), which is a key feature of AMD (Chong et al., 2005; Grossniklaus et al., 1992; Sura et al., 2020).
The choroid also plays a critical role in accommodation in nonmammalian species by rapid shape changes (Nickla and Wallman, 2010) in contrast to the subtle and age-related thickness changes in humans, discussed below.
Cells of the choroid and their gene expression
While the vasculature of the choroid is very dense, especially at the level of the choriocapillaris where vessel lumens occupy more than 50% of cross-sectional area of the layer, ECs comprise a minority of choroidal nuclei (Voigt et al., 2019). Anatomically, in addition to the vascular cells lining blood vessel lumens, one can readily distinguish smooth muscle and pigmented melanocytes. Moreover, numerous nucleated cells, which include fibroblasts, mast cells, macrophages, lymphocytes, and nerve processes with supporting Schwann cells, are present in the choroid (Figure 4).
Figure 4.
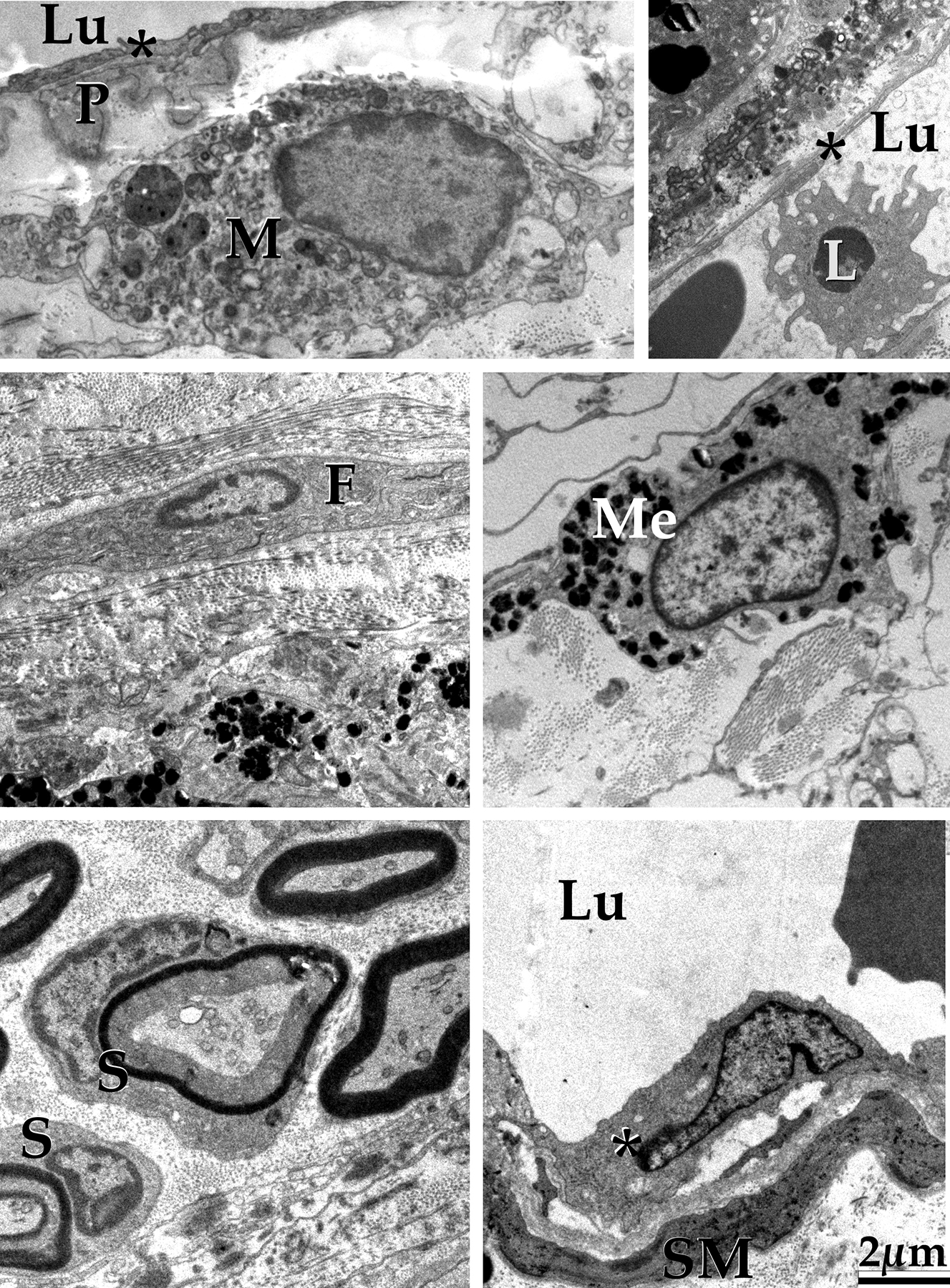
In addition to endothelial cells (asterisks), cells of the human choroid include immune cells, both within the stroma (M, macrophage) and circulating (L); fibroblasts (F); melanocytes (Me); Schwann cells (S), smooth muscle cells (SM) and pericytes (P). Lu, vascular lumen. Scalebar = 2μm.
In addition to identifying choroidal cells morphologically, gene expression patterns have been characterized for individual populations of choroidal cells with single-cell RNA sequencing (scRNA-seq). Transcriptomic signatures have been identified for all major choroidal cell populations in post-mortem human donors and mice, including distinct populations of fibroblasts, melanocytes, pericytes, ECs, smooth muscle cells, myelinating and non-myelinating Schwann cells, and multiple classes of resident and infiltrating leukocytes (Gautam et al., 2021; Lehmann et al., 2020; Voigt et al., 2019). Additionally, subpopulations of arteries, veins, and choriocapillaris ECs have been described by enriching for PECAM1-expressing cells prior to scRNA-seq, providing a high-resolution view of gene expression along the choroidal vascular tree (Figure 5, Table 1) (Rohlenova et al., 2020; Voigt et al., 2019; Voigt et al., 2020c).
Figure 5:
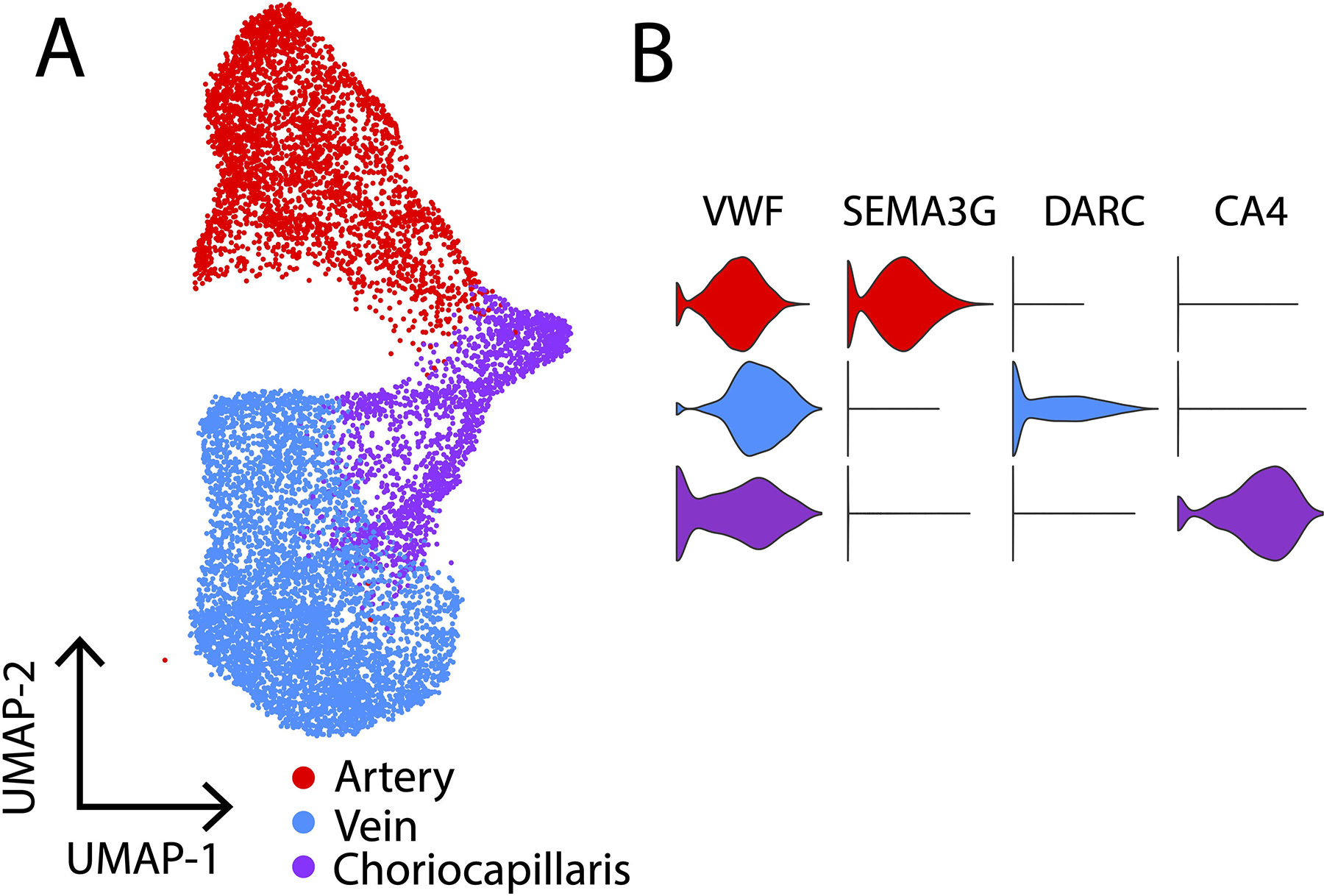
Choroidal arteries, veins, and choriocapillaris endothelial cells identified with single-cell RNA sequencing. A. UMAP plot of 10,576 choroidal endothelial cells isolated from 6 human donors (Voigt et al., 2020c). Each point represents the multidimensional transcriptome of one cell. Clusters of cells (red, blue, purple) are classified into different endothelial cell types (arteries, veins, choriocapillaris). B. Violin plots demonstrate that all choroidal endothelial cells highly express the pan-endothelial cell gene VWF. Choroidal arteries specifically express SEMA3G, choroidal veins specifically express DARC, and the choriocapillaris specifically expressed CA4.
Table 1: Genes enriched in different choroidal endothelial cell populations.
The top-ten genes enriched in choroidal arteries, veins, and choriocapillaris (CC) endothelial cells were identified by differential expression (Voigt et al., 2020c).
| Rank | Artery genes | Vein genes | CC genes |
|---|---|---|---|
| 1 | IGFBP3 | DARC | CA4 |
| 2 | ADAMTS1 | CLU | RGCC |
| 3 | CLDN5 | TIMP1 | PLVAP |
| 4 | HEY1 | TGFBR3 | VWA1 |
| 5 | GJA4 | GPR126 | STC1 |
| 6 | SEMA3G | NR2F2 | ADM |
| 7 | GJA5 | ACTN1 | BTNL9 |
| 8 | FAM107A | PKHD1L1 | EDNRB |
| 9 | BGN | SELE | IGFBP6 |
| 10 | CP | CPE | ITIH5 |
Moroever, these detailed expression atlases have allowed vision researchers to pinpoint which cell populations express genes previously implicated in AMD pathogenesis. For example, complement factor H (CFH) is a complement inhibitor with single-nucleotide variants strongly associated with AMD (Fritsche et al., 2016). However, CFH is a secreted protein, and the expression pattern of this important complement inhibitor has been a subject of debate. ScRNA-seq has clarified that CFH is most highly expressed by ECs (including the choriocapillaris), their surrounding pericytes, and fibroblasts in the choroid (Voigt et al., 2020a). Such gene expression information has been made rapidly available to the vision research community with web-based resources such as Spectacle (https://singlecell-eye.org) (Voigt et al., 2020b) and EndoDB (https://endotheliomics.shinyapps.io/murine_ectax/) (Khan et al., 2019).
In addition to generating detailed expression databases, scRNA-seq has been used to investigate how important biological variables central to AMD pathogenesis influence gene expression in the choroid. For example, macular versus peripheral transcriptomes have been compared in postmortem human donor choroid, and cell types such as pericytes have been shown to demonstrate modest regional expression differences (Voigt et al., 2019). Likewise, scRNA-seq was utilized to compare choroidal EC gene expression between young (< 2 years old) and aging (> 60 years old) human donors (Voigt et al., 2020c). Choriocapillaris ECs from aging donors demonstrated increased expression of pro-inflammatory genes such as ICAM-1, while the young choriocapillaris strongly expressed anti-inflammatory molecules such as CD34. Collectively, these gene expression patterns suggest that the choroidal vasculature develops a pro-inflammatory phenotype with advancing age.
Choroidal changes in aging
Both anatomical and molecular changes occur in the aging choroid. Anatomically, choroidal thickness (i.e., the distance between Bruch’s membrane and the inner surface of the sclera where the lamina fusca resides) decreases as a function of age, and this morphological feature of living humans was first noted by the application of enhanced depth imaging OCT (Margolis and Spaide, 2009; Spaide et al., 2008). Choroidal thicknesses have been measured predominantly subfoveally and show a normal distribution within a given population (Wei et al., 2013). The aging choroid exhibits a loss of approximately 3–4μm per year (Wakatsuki et al., 2015; Wei et al., 2013). Temporary increases in choroidal thickness can be experimentally achieved by increasing the blood volume (Arora et al., 2012), but these changes are relatively small compared to the age-related loss. In rodents, changes in choroidal thickness can be observed following experimental lesioning of central or peripheral nervous control of choroidal blood flow (Li et al., 2021; Steinle and Smith, 2002) with corresponding structural and functional deficits in the retina. In addition, choroidal thinning was also recently found to be related to levels of circulating C-reactive protein (CRP) (Chen et al., 2021b), an acute phase protein also elevated in patients with AMD (Seddon et al., 2004; Seddon et al., 2005). The mechanistic relationship between CRP and choroidal thinning is not well understood, however it is notable that CRP can activate the complement system, discussed below.
Loss of choroidal thickness is accompanied structurally by increased abundance and density of fibrillar collagen with corresponding decrease in ground substance in the choroidal stroma (Sohn et al., 2014). Both histological and swept source OCT studies suggest that the thickness of the choroid is unrelated to the density of the choriocapillaris and intermediate choroidal vessels, respectively (Sohn et al., 2014; Zhou et al., 2020).
Choriocapillaris degeneration:
In addition to choroidal thinning, there is a loss of choriocapillaris ECs in aging (Ramrattan et al., 1994). It is challenging to observe morphologic changes in AMD choriocapillaris cells with hematoxylin and eosin stained paraffin sections, and different investigators reached different conclusions about whether the choriocapillaris is lost in early AMD (Ramrattan et al., 1994; Spraul et al., 1996). We believe that this is due to the acellular choroidal capillary ghosts that present as clear lumens on H&E sections but can be readily discriminated using a label for intact ECs (Figure 6) (Lutty et al., 2020; Mullins et al., 2011).
Figure 6:
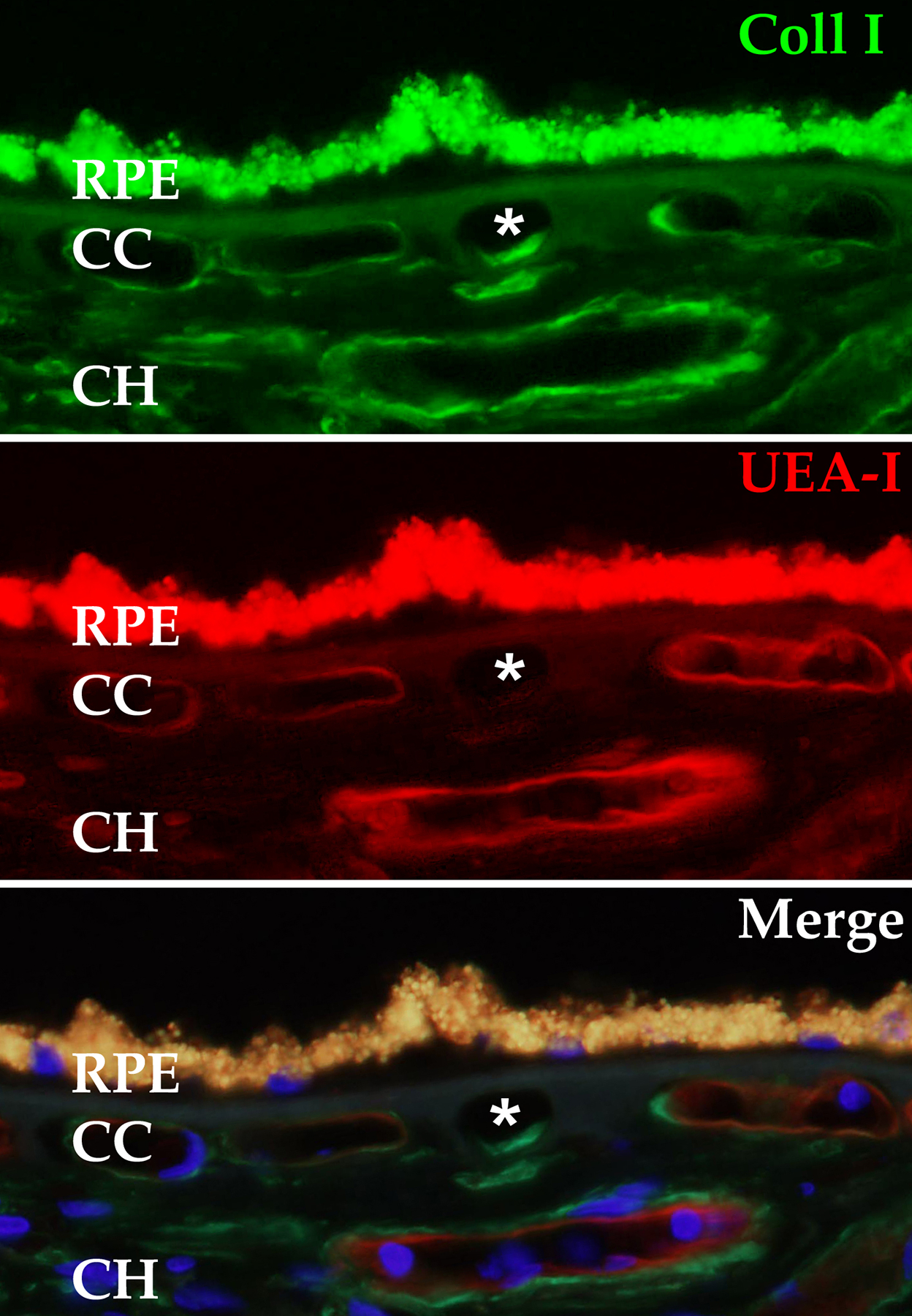
Ghost choriocapillaris vessel (asterisk) beneath intact RPE monolayer. This degenerated capillary segment appears normal when the choroid is labeled with antibodies directed against type I collagen (green, top panel), but is unreactive with the endothelial cell binding lectin UEA-I (red, middle panel). Lower panel depicts merged channels with DAPI nuclear counterstain. RPE, retinal pigment epithelium; CC. choriocapillaris, CH outer choroid.
Using the fucose-binding lectin Ulex europaeus agglutinin-I to label human choroidal whole mounts, Lutty and colleagues observed a loss of choriocapillaris area in early AMD from nearly 80% in controls to around 60% in early AMD eyes, a loss that was confined to the submacular choroid (Lutty et al., 2020). The degeneration of choriocapillaris and replacement of healthy capillaries with ghost vessels is further related to drusen density, in addition to drusen being spatially related with ghost choriocapillaris vessels (Mullins et al., 2011). The observed histological loss of choriocapillaris ECs is much more striking in eyes with geographic atrophy and this deterioration is observed under the still intact RPE (Sohn et al., 2019), consistent with recent OCT-A findings that widespread choriocapillaris flow voids (Kim et al., 2013; Zhang et al., 2018) precede atrophy in GA (Moreira-Neto et al., 2018; Moult et al., 2016). Based on available evidence, we propose that the most likely source of EC injury in aging eyes is the membrane attack complex (MAC) of complement, discussed below.
This loss, contributing to the impaired replenishment of bleached 11-cis retinal, likely contributes to the delayed dark adaptation observed in early AMD.
Biochemical changes in the aging choroid
A major feature of AMD is the accumulation of lipids and glycoproteins between the choriocapillaris and the cells it nourishes (reviewed in (Curcio et al., 2011)), and this accumulation can be in the form of discrete mounds (drusen) or in a linear confluent layer, which can further detach and form the appearance of soft drusen (Bressler et al., 1994; Chen et al., 2021a; Chen et al., 2020b). Other described changes include loss of the anti-angiogenic matricellular glycoprotein thrombospondin (Uno et al., 2006), increased advanced glycation end-products (Handa et al., 1999), decreased abundance of complement factor H (Bhutto et al., 2011), and accumulation of monomeric CRP (Bhutto et al., 2011; Chirco et al., 2018), which can both suppress and initiate complement activation in different contexts (Mihlan et al., 2011).
The activation of the complement system at the level of the choriocapillaris is of interest in the pathogenesis of AMD, especially considering the extensive evidence for genetic dysregulation of the complement system in numerous reports (recently reviewed (Armento et al., 2021)). The C5b-9 MAC of complement accumulates in aging choroid, with traces appearing in children and a steady increase through adulthood (Mullins et al., 2014). Biochemical quantification in human choroid using ELISA shows that the abundance of the MAC approximately doubles during normal aging, comparing young control (21–48 years) to aged control eyes (aged 71–96 years), and doubles again comparing aged control eyes to those with early AMD (77–99 years of age) (Mullins et al., 2014). We propose the joint findings that (a) the MAC accumulates at the level of the choriocapillaris, (b) choriocapillaris degeneration is a key early event in AMD pathogenesis and progression, (c) MAC levels are further related to the high risk Y402H variant in CFH (Chirco et al., 2018; Mullins et al., 2011), and (d) MAC can lyse primary choroidal ECs in vitro (Zeng et al., 2018), implicate the MAC as a major factor in AMD. Recent drug trials aimed at suppressing complement activation in atrophy have had variable results, but several of these are encouraging ((Wykoff et al., 2021), see below).
In Vivo Choroidal Imaging
Until a few years ago, in vivo imaging of the choroid was restricted to angiography that requires intravenous cannulation and injection of fluorescein and/or indocyanine green dye. In fluorescein angiography (FA), serial photographs are taken after dye injection using an excitation filter that allows blue light wavelengths to stimulate fluorescence and a barrier filter that allows yellow-green wavelengths from the fluorescent molecule to be imaged (Gass et al., 1967). In indocyanine green angiography (ICGA), different excitation and barrier filters are used (Alander et al., 2012). FA and ICGA can be performed simultaneously by coincident injection of both dyes and serial photography that alternates between two sets of filters.
Twenty percent of fluorescein in the circulation remains unbound to albumin and readily diffuses across the fenestrations of the choriocapillaris (Wolfe, 1986). This causes early diffuse hyperfluorescence (the “choroidal flush”) that obscures details of the choroidal circulation (Figure 7). Occasionally FA is useful in imaging the choroid, such as when there is delayed/absent choroidal perfusion in ocular ischemic syndrome, or in a hyperfluorescent “classic” choroidal neovascular membrane (1991). Unlike fluorescein, the ICG molecule remains protein-bound and normally remains in the circulation (Yoneya et al., 1998). The absence of ICG diffusion across the choriocapillaris enables visualization of the choroidal circulation (Figure 8) and of choroidal lesions such as choroidal hemangiomas. Two major strengths of dye-based angiography are the ability to visualize the temporal dynamics of dye transit and leakage, and to capture images despite poor fixation and significant media opacity. Weaknesses include the need for intravenous access, allergic contraindications and potential systemic adverse effects (Yannuzzi et al., 1986), slow image acquisition, and the lack of layer-specific angiography data.
Figure 7.
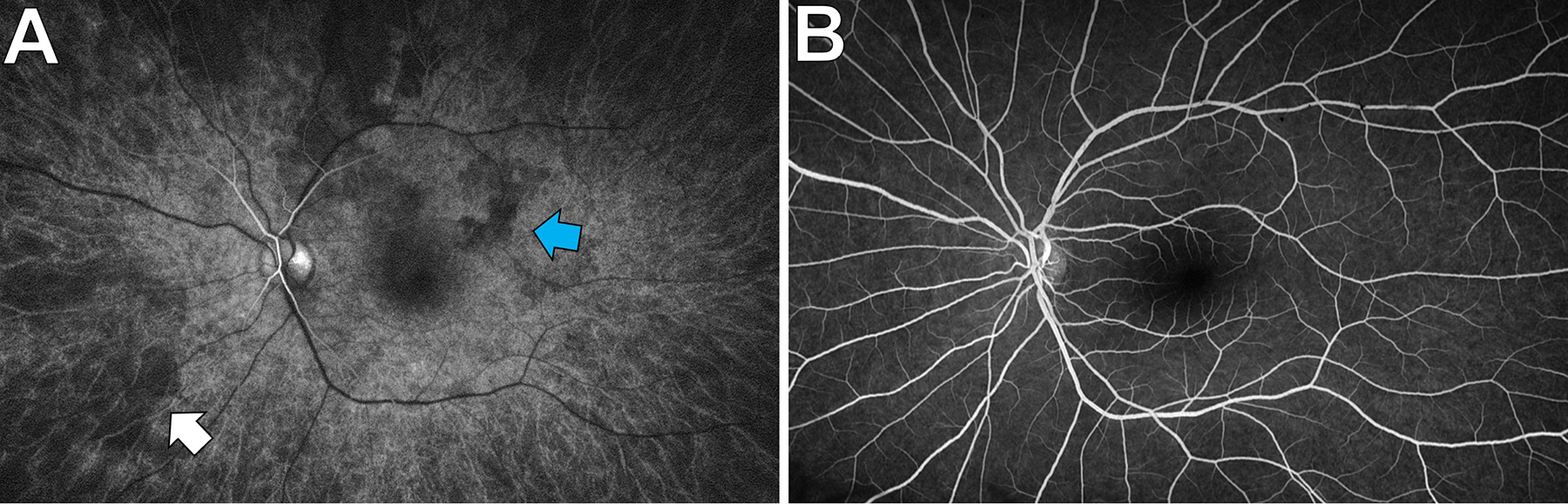
Choroidal visualization on a normal fluorescein angiogram. A: Seven seconds after dye injection, the retinal arteries have begun to fill. The large choroidal vessels are visible (white arrow) and there is patchy choroidal flush (blue arrow) in the posterior pole. B: Thirty seconds later, the retinal circulation has filled, the choroidal flush has become homogenous, and the large choroidal vessels are no longer visible.
Figure 8.
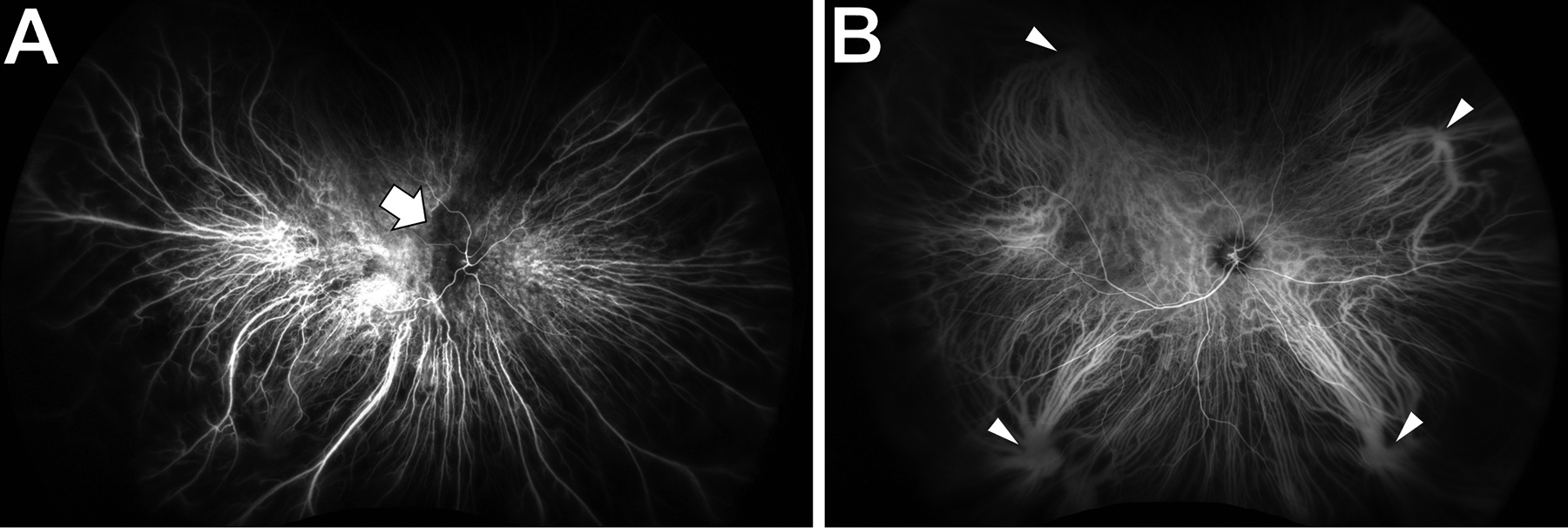
Choroidal visualization on a normal indocyanine green angiogram.
A: Sixteen seconds after dye injection, the retinal and choroidal circulations have begun to fill. There is patchy choroidal filling by the nerve (arrow).
B: Thirty seconds later, the entire retinal and choroidal circulations have filled. There is no choroidal flush to obscure the choroidal vessels. Vortex veins are indicated by arrowheads.
Optical coherence tomography (OCT) is a newer technology that measures the reflection of light waves to reconstruct ocular anatomy (Huang et al., 1991). Early OCT systems could not image the choroid well, but enhanced-depth spectral domain OCT enabled high-resolution imaging of the entire choroidal depth (Spaide et al., 2008), and swept source (SS) OCT can image the entire choroid even under retinal/sub-RPE lesions (Choi et al., 2013). Current versions of SS-OCT can image the entire posterior pole in a single, rapid scan (Russell et al., 2019). On OCT scans, the choroid consists of hyperreflective stroma and round to oval hyporeflective choroidal vessel lumens (Figure 9C, D). Many studies have catalogued choroidal thickness and choroidal vascularity (based on the ratio of lumen to stroma) in various conditions (Singh et al., 2019)(Figure 10). However, these studies are mostly limited to single OCT line scans of the subfoveal choroid, so there are few OCT studies of the choroid throughout the rest of the fundus. We recently compared normative values to human diabetic patients to show in vivo that the diabetic choroid is abnormal throughout the posterior pole (Russell et al., in revision), which had previously been demonstrated only with histopathology (Cao et al., 1998). A similar imaging strategy using SS-OCT can be applied to investigate choroidal dysfunction in many other retinal diseases such as AMD.
Figure 9.
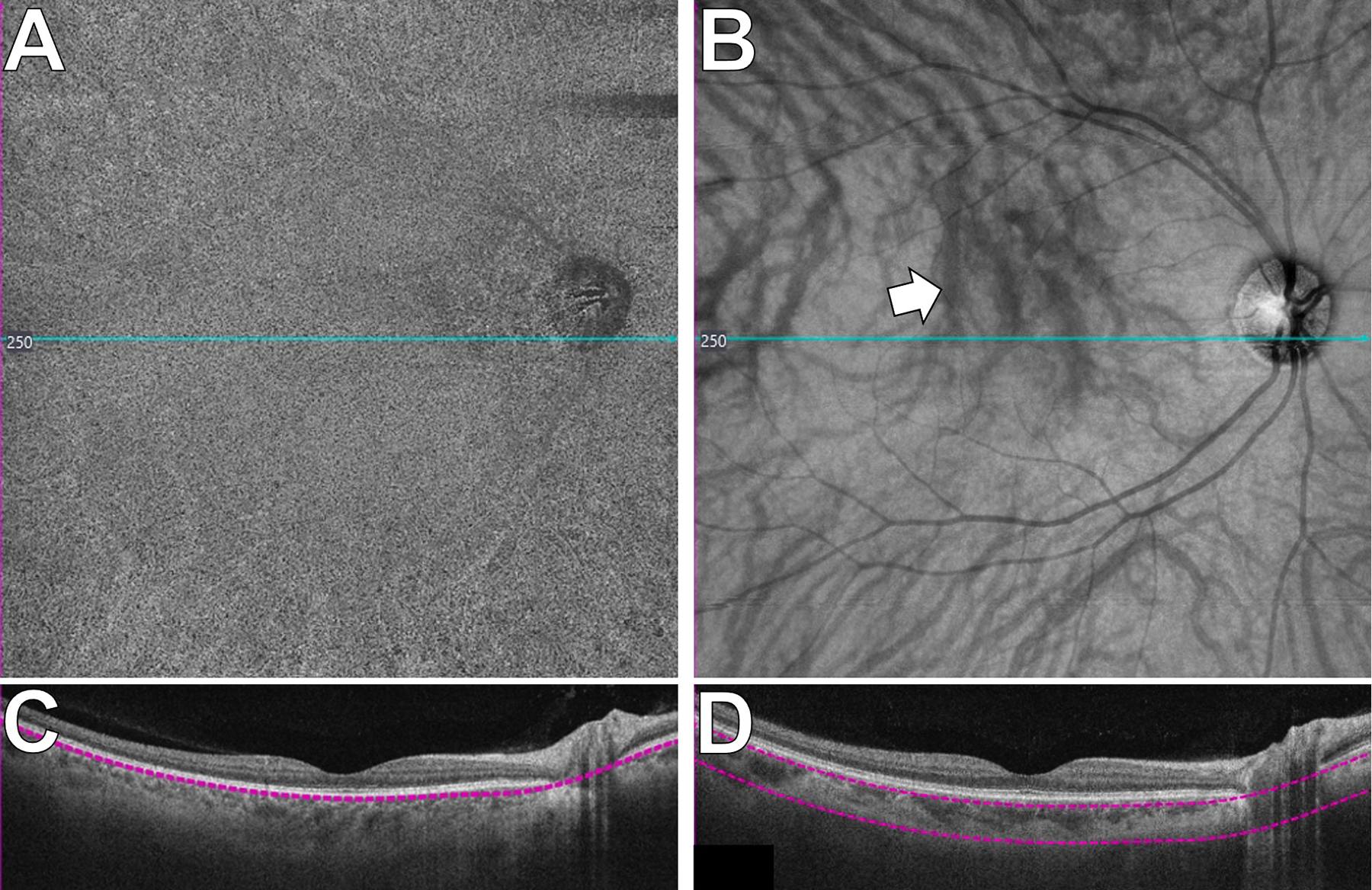
Choroidal visualization via swept source optical coherence tomography (SS-OCT) in the central 12×12mm of a normal retina.
A: SS-OCT angiogram of the choriocapillaris. The speckled pattern is normal.
B: En face SS-OCT of the large choroidal vessels. The choroidal vessels appear dark (arrow; see text). The overlying retinal vessels cause shadowing artefact.
The blue lines in A and B indicate the position of the OCT line scans in C and D, respectively. The purple segmentation lines in C and D define the depth of tissue depicted in the en face images (A and B).
Figure 10.
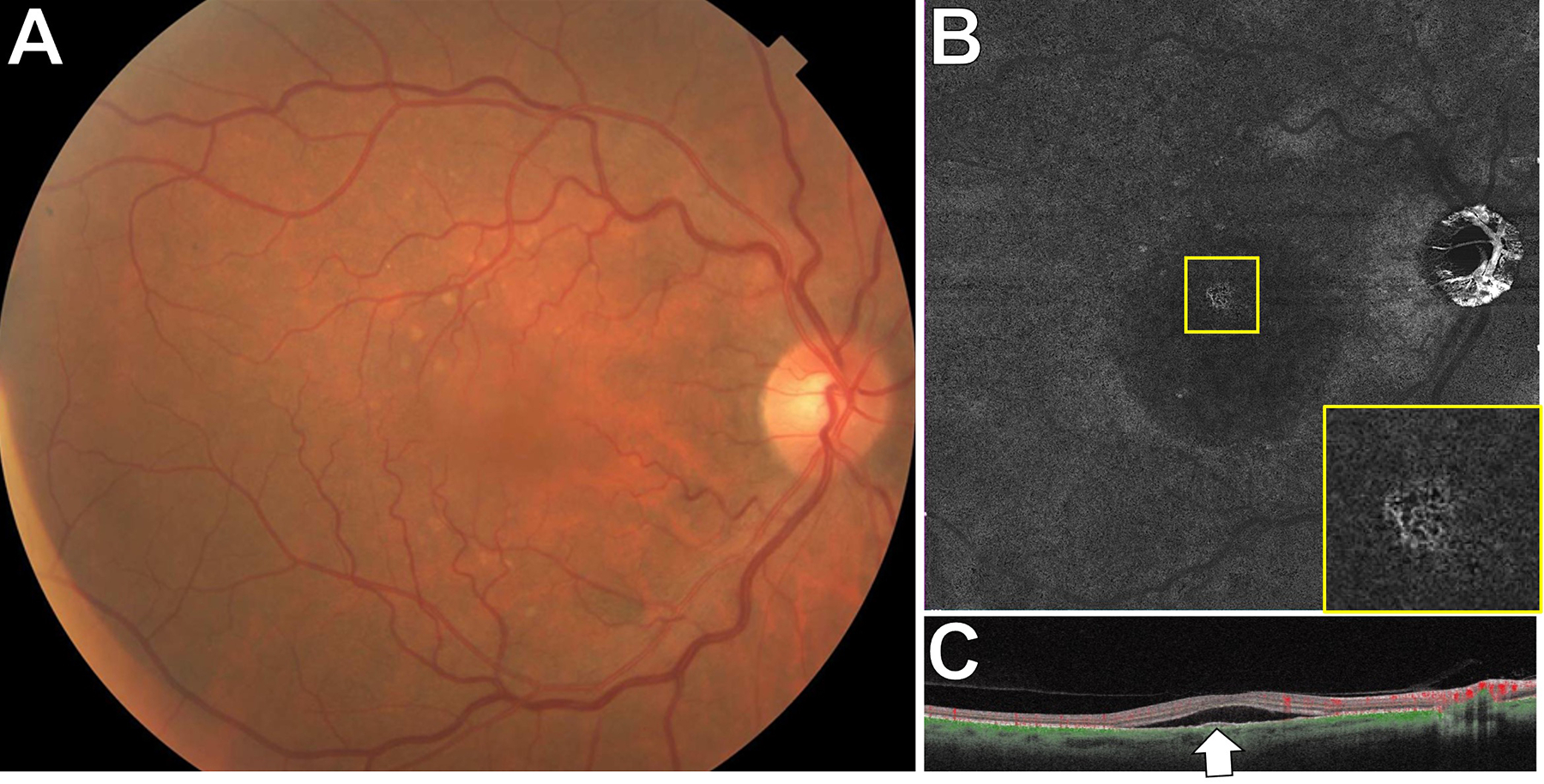
SS-OCTA of choroidal neovascularization in age-related macular degeneration. A: This 64 year-old patient was referred with a diagnosis of subretinal fluid from central serous chorioretinopathy. There were multiple large, soft drusen bilaterally.B: A 12×12mm SS-OCTA segmented to include detectable flow at the level of the RPE and a few microns below it showed a CNV (magnified view, inset). C: The corresponding SS-OCT line scan had an RPE elevation corresponding to the CNV.
OCT angiography (OCTA) utilizes repeated OCT scans to identify areas where reflectivity varies from scan to scan (Spaide et al., 2018). Variable reflectivity is inferred to result from vascular flow, so algorithms impute the presence of blood vessels in these areas and reconstruct two-dimensional angiograms of detectable flow within individual tissue layers (Spaide et al., 2018). With SS-OCTA, the individual vessels of the choriocapillaris can be visualized (Choi et al., 2013). However, flow through larger choroidal vessels cannot be measured with modern OCTA systems because when light passes through the choroid, most of the photons scatter through the blood and the residual reflected light is scattered by the RPE, so there is no detectable signal (Zhou et al., 2020). Hence, large choroidal vessels appear dark on OCT images. With the rapid scanning speed of SS-OCT/OCTA, it is possible to capture the choroidal vasculature throughout the posterior pole in a single scan (Figure 9A, B). In comparison to dye-based angiography of the choroid, SS-OCT/OCTA are less effective with media opacity and poor fixation, but they are faster, safer, non-invasive, repeatable, and provide high-resolution, layer-specific angiography data.
New insights into choroidal biology
Improvements in imaging living patients, new knowledge of choroidal pathology in AMD, applications of cutting-edge molecular biology, and cellular studies for both disease modeling and transplantation have brought the choroid into renewed focus.
New Insights: Imaging:
The advent of SS-OCT/OCTA for imaging the choroid has begun to revolutionize clinical practice and our understanding of choroidal dysfunction. The most immediate clinical application of SS-OCTA is for diagnosis and longitudinal evaluation of choroidal neovascularization (CNV). CNVs can be difficult or impossible to definitively identify with dye-based angiography (1991), but they are usually easily visualized with a quick SS-OCTA scan (Figure 11) (Miller et al., 2017). SS-OCTA has shown that some eyes with age-related macular degeneration (AMD) have no subretinal or intraretinal fluid but do have CNVs (De Oliveira et al., 2018; Querques et al., 2013). These lesions, also detected by histology (Chen et al., 2020a; Sarks, 1973), have been termed non-exudative CNVs (De Oliveira et al., 2018). They can persist for years without exudation (Yang et al., 2019), and it is not clear what triggers them to begin exudating. It has long been known that exudative AMD often evolves into geographic atrophy (GA) (Gemenetzi and Patel, 2017), but halting exudation with anti-VEGF injections may hasten GA (Gemenetzi and Patel, 2017). Conversely, slowing progression of GA via complement inhibition seems to increase the onset of exudative CNV (Wykoff et al., 2021). SS-OCTA in normal subjects has shown that choriocapillaris perfusion decreases with age in the macula, and this decrease is most pronounced in the fovea (Zheng et al., 2019). Longitudinal, in vivo SS-OCTA studies have demonstrated strong correlations between choriocapillaris ischemia and GA progression (Shi et al., 2021; Thulliez et al., 2019), and choriocapillaris flow correlates with retinal sensitivity in eyes with GA (Rinella et al., 2021).
Figure 11.
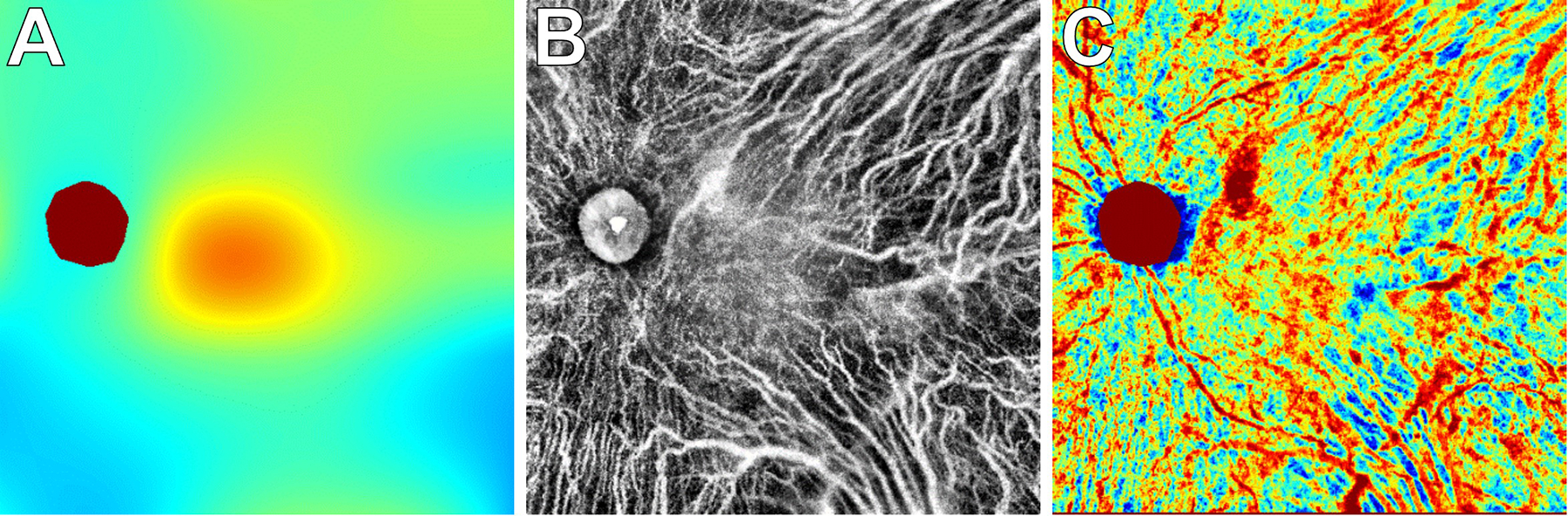
En face SS-OCT imaging to create choroidal thickness and vascularity maps.
This patient with proliferative diabetic retinopathy was imaged with 12×12mm SS-OCT to generate a choroidal thickness map (A), choroidal vascularity map (B), and choroidal vascularity index map (C). Areas with a thicker choroid or larger CVI measurements are depicted with warmer colors in the respective topographic heat maps (A, C). See Russell et al. (manuscript in revision) for more details.
We hypothesize that age, smoking, genetics, and metabolic factors cause choriocapillaris ischemia, which leads to dysfunction and atrophy of the RPE and outer retina in AMD (Chirco et al., 2017; Sohn et al., 2019; Whitmore et al., 2015). CNVs may then develop as an adaptive response to perfuse an ischemic RPE/outer retina to replace lost choriocapillaris vessels. If the flow through a non-exudative CNV exceeds the metabolic needs of the overlying tissue, exudation results. In contrast, if a non-exudative CNV fails to develop or cannot compensate for the metabolic needs of the overlying tissue, GA ensues. Therefore, one strategy for treating AMD is to promote non-exudative CNVs that safely and continuously nourish the RPE/outer retina. This will require a careful balance of molecular factors impacting angiogenesis, complement activation, and other pathways. SS-OCTA will be essential to identify eyes with choriocapillaris ischemia and non-exudative CNVs and to follow them longitudinally while titrating molecular therapy.
New Insights: The molecular biology of the choroid
ScRNA-seq has recently been applied to understanding AMD pathogenesis and this area is expected to greatly expand. In a recent report, gene expression was compared at the single-cell level between healthy mice and mice subjected to laser-induced choroidal neovascularization (CNV) (Rohlenova et al., 2020). In this study, choroids were isolated seven-days after laser-injury and enriched for PECAM1-expressing ECs. After clustering the cells, a distinct CNV endothelial population was identified that did not have any contributions from control choroids. This CNV-specific population demonstrated a gene expression pattern consistent with endothelial damage and was enriched in several metabolic pathways implicated in pathological angiogenesis (Rohlenova et al., 2020). In addition to this biological insight, this study provided further evidence that scRNA-seq is a valuable tool for interrogating small and rare populations of choroidal cells central to AMD pathogenesis.
Future studies will leverage scRNA-seq to further advance our understanding of AMD pathophysiology. While a total of two AMD-choroids have been examined with scRNA-seq to date (Voigt et al., 2019), larger cohorts of AMD and control donors may identify expression changes common to early or neovascular AMD. Likewise, scRNA-seq may help untangle important genotype-phenotype associations in AMD. For example, single-nucleotide variants near the genes HTRA1 and ARMS2 confer substantial AMD risk (Fritsche et al., 2016), and scRNA-seq may highlight specific cell populations that harbor large gene expression changes in response such variants. Lastly, advances in spatial transcriptomics have the potential to map gene expression changes to the spatial location of disease. This technology may be incredibly useful in studying AMD by characterizing how gene expression is perturbed around drusen or within neovascular membranes.
New Insights: Disease modeling in the choroid
The blood vessels of the choroid, like other vascular beds, are lined with ECs. Immortalized EC lines (e.g., primate chorioretinal endothelial cells RF/6A), human umbilical vein endothelial cells (HUVECS) and iPSC-derived endothelial cells can all be used to study EC function in vitro. Although HUVECs are widely used it is important to note they have a different gene expression profile compared to native choroidal ECs (Browning et al., 2012), bringing to light the importance of tissue specific ECs. ECs have specialized functions and expression patterns depending on their anatomical location (Nolan et al., 2013). For example, ECs in the choroid uniquely express CA4 (Baba et al., 2009; Hageman et al., 1991). Given the importance of tissue specific ECs, our lab generated an immortalized choroidal EC line from human donor choroidal ECs (Voigt et al., 2019) and we have developed a stepwise protocol to differentiate CA4-positive choroidal ECs from induced pluripotent stem cells (iPSCs) (Songstad et al., 2015; Songstad et al., 2017; (Mulfaul et al., 2020).
The first step in modeling the choroidal vasculature in vitro is to generate tissue-specific ECs in monolayer. Single-cell suspensions of iPSC derived ECs form tube-like structures in Matrigel (Mulfaul et al., 2020) however these vascular structures are viable for only about 24 hours. In vivo, choroidal ECs require trophic signals from the RPE for both development and homeostasis (Saint-Geniez et al., 2009). To model the choroid in vitro, other cell types need to be co-cultured with choroidal ECs to mimic the in vivo tissue. Co-culture models of RPE and ECs on transwell inserts (Fan et al., 2002; Geisen et al., 2006; Spencer et al., 2017), collagen gel (Sakamoto et al., 1995) or amniotic membranes (Hamilton et al., 2007) have been used to study the interaction between RPE and choroidal ECs. Most co-culture systems use synthetic material that do not mimic Bruch’s membrane.
Djigo and colleagues generated a tissue-engineered choroidal stroma by culturing choroidal stromal fibroblasts with ascorbic acid for six weeks to obtain ECM sheets which closely resembled the native human choroid. The tissue-engineered choroidal stroma supported growth of an RPE monolayer, a 3D vascular network of HUVECs and pigmented choroidal melanocytes (Djigo et al., 2019). Thus, this elegant system provides a non-synthetic alternative for RPE/choroid co-culture which supports vascular tube formation.
In addition to co-culture with RPE it is important to begin to culture choroid ECs in the presence of other supporting choroidal cell types to closely model the unique microenvironment of the choroid. A 3D RPE-choriocapillaris model using polyethylene glycol (PEG) hydrogels crosslinked with matrix metalloproteinase (MMP) degradable peptides highlights the importance of both RPE and mesenchyme in the development of fenestrated choroidal EC-like vasculature. Manian et al. encapsulated iPSC derived ECs in a PEG-MMP hydogel in the presence of mesenchymal stem cells followed by plating RPE cells on the apical surface of the hydrogel. ECs formed fenestrated vascular networks in the hydrogel consistent with in vivo development of the choroidal endothelium and co-cultured RPE-EC were stable for up to 60 days in culture (Manian et al., 2021).
As more is learned about the cellular and extracellular milieu of the human choroid, 3D models have the potential to incorporate all cell types present in the choroidal vasculature and stroma in vivo. Bioprinting allows for a combination of cell types to be incorporated into a hydrogel support. Bharti and colleagues have used this approach to bioprint iPSC derived fibroblasts, ECs and pericytes on the basal side of a biodegradable scaffold and seeded RPE on the apical side. As the 3D cultures matured the scaffold was replaced with extracellular matrix components mimicking Bruch’s membrane (Bharti et al., preprint). RPE developed apical processes, tight junctions, and basal infoldings and ECs formed fenestrated vascular capillary tubes. In the presence of human serum, the 3D cultures accumulated drusen-like deposits between the RPE and choroid and simulated CNV development using a Hif-1alpha activator, demonstrating the application of this model in the study of both dry and wet AMD.
In summary, the combination of innovations in in vivo imaging, gene expression analysis of single choroidal cells, quantitative biochemistry, and in vitro modeling of choroidal cells and their interactions are improving our understanding of this critical tissue. Future studies directed toward better understanding the environmental challenges faced by choroidal ECs, especially complement injury, and toward the rebuilding of the damaged choroid in AMD will offer improved opportunities for understanding and treating the choroidal EC loss observed in AMD.
Summary points.
The choroid is the vascular supply for the photoreceptor cells of the mammalian eye and is thus essential for maintaining vision.
Injury of the choriocapillaris layer of the choroid is an early initiating event in the pathogenesis of age-related macular degeneration and can precede the loss of the RPE in geographic atrophy yet this tissue has been understudied.
The application of novel approaches—including quantitative histology, in vivo functional imaging, molecular analysis at the single cell level of resolution, and in vitro induced pluripotent stem cell (iPSC) modeling techniques--is providing new insights into this fascinating tissue.
Future issues.
In light of the role of complement in damage to the choriocapillaris, inhibiting complement is being explored as a method to protect against the growth of geographic atrophy.
Imaging of the choriocapillaris in living patients will continue to improve and resolve some of the standing questions about flow voids and the relationships between the RPE, drusen and choriocapillaris health.
Studying complex intercellular interactions using stem cell derived RPE, choriocapillaris endothelial cells and structural cells such as pericytes to recreate a choroid like microenvironment.
Determining how genetic risk factors for AMD affect the physiology of the choroid prior to the onset of disease will provide insight into the mechanisms by which different variants contribute to macular disease.
In eyes that have lost vision due to AMD, restoring the choriocapillaris with patient derived endothelial cells will be a necessary component of restoring vision.
Acknowledgments
Supported in part by the Elmer and Sylvia Sramek Charitable Trust; NIH grants EY024605 and EY025580; Research to Prevent Blindness.
The authors gratefully acknowledge the eye donors and their families whose selfless acts of service made this research possible.
LITERATURE CITED
- Macular Photocoagulation Study Group 1991. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Archives of ophthalmology (Chicago, Ill. : 1960) 109, 1242–1257. [PubMed] [Google Scholar]
- Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, Venermo M, Välisuo P, 2012. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012, 940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A 3rd, 1992. Energy requirements of CNS cells as related to their function and to their vulnerability to ischemia: a commentary based on studies on retina. Can J Physiol Pharmacol 70 Suppl, S158–164. [DOI] [PubMed] [Google Scholar]
- Armento A, Ueffing M, Clark SJ, 2021. The complement system in age-related macular degeneration. Cell Mol Life Sci 78, 4487–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora KS, Jefferys JL, Maul EA, Quigley HA, 2012. Choroidal thickness change after water drinking is greater in angle closure than in open angle eyes. Investigative ophthalmology & visual science 53, 6393–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Grebe R, Hasegawa T, Bhutto I, Merges C, McLeod DS, Lutty GA, 2009. Maturation of the fetal human choriocapillaris. Invest Ophthalmol Vis Sci 50, 3503–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutto IA, Baba T, Merges C, Juriasinghani V, McLeod DS, Lutty GA, 2011. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. The British journal of ophthalmology 95, 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler NM, Silva JC, Bressler SB, Fine SL, Green WR, 1994. Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina (Philadelphia, Pa.) 14, 130–142. [PubMed] [Google Scholar]
- Browning AC, Halligan EP, Stewart EA, Swan DC, Dove R, Samaranayake GJ, Amoaku WM, 2012. Comparative gene expression profiling of human umbilical vein endothelial cells and ocular vascular endothelial cells. Br J Ophthalmol 96, 128–132. [DOI] [PubMed] [Google Scholar]
- Cao J, McLeod S, Merges CA, Lutty GA, 1998. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Archives of ophthalmology (Chicago, Ill. : 1960) 116, 589–597. [DOI] [PubMed] [Google Scholar]
- Chen L, Messinger JD, Kar D, Duncan JL, Curcio CA, 2021a. Biometrics, impact, and significance of basal linear deposit and subretinal drusenoid deposit in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 62, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Messinger JD, Sloan KR, Swain TA, Sugiura Y, Yannuzzi LA, Curcio CA, Freund KB, 2020a. Non-exudative neovascularization supporting outer retina in age-related macular degeneration, a clinicopathologic correlation. Ophthalmology 127, 931–947. [DOI] [PubMed] [Google Scholar]
- Chen L, Messinger JD, Sloan KR, Wong J, Roorda A, Duncan JL, Curcio CA, 2020b. Abundance and multimodal visibility of soft drusen in early age-related macular degeneration: a clinicopathologic correlation. Retina (Philadelphia, Pa.) 40, 1644–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RC, Palestine AG, Lynch AM, Patnaik JL, Wagner BD, Mathias MT, Mandava N, 2021b. Increased Systemic C-Reactive Protein Is Associated With Choroidal Thinning in Intermediate Age-Related Macular Degeneration. Transl Vis Sci Technol 10, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirco KR, Flamme-Wiese MJ, Wiley JS, Potempa LA, Stone EM, Tucker BA, Mullins RF, 2018. Evaluation of serum and ocular levels of membrane attack complex and C-reactive protein in CFH-genotyped human donors. Eye (London, England) 32, 1740–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirco KR, Sohn EH, Stone EM, Tucker BA, Mullins RF, 2017. Structural and molecular changes in the aging choroid: implications for age-related macular degeneration. Eye (London, England) 31, 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W, Mohler KJ, Potsaid B, Lu CD, Liu JJ, Jayaraman V, Cable AE, Duker JS, Huber R, Fujimoto JG, 2013. Choriocapillaris and choroidal microvasculature imaging with ultrahigh speed OCT angiography. PloS one 8, e81499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong NH, Keonin J, Luthert PJ, Frennesson CI, Weingeist DM, Wolf RL, Mullins RF, Hageman GS, 2005. Decreased thickness and integrity of the macular elastic layer of Bruch’s membrane correspond to the distribution of lesions associated with age-related macular degeneration. The American journal of pathology 166, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Johnson M, Rudolf M, Huang JD, 2011. The oil spill in ageing Bruch membrane. The British journal of ophthalmology 95, 1638–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira GS Jr., Errea M, Bialek J, Kendall MC, McCarthy RJ, 2018. The impact of health literacy on shared decision making before elective surgery: a propensity matched case control analysis. BMC Health Serv Res 18, 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djigo AD, Bérubé J, Landreville S, Proulx S, 2019. Characterization of a tissue-engineered choroid. Acta Biomater 84, 305–316. [DOI] [PubMed] [Google Scholar]
- Fan W, Zheng JJ, McLaughlin BJ, 2002. An in vitro model of the back of the eye for studying retinal pigment epithelial-choroidal endothelial interactions. In Vitro Cell Dev Biol Anim 38, 228–234. [DOI] [PubMed] [Google Scholar]
- Fritsche LG, Igl W, Bailey JN, Grassmann F, Sengupta S, Bragg-Gresham JL, Burdon KP, Hebbring SJ, Wen C, Gorski M, Kim IK, Cho D, Zack D, Souied E, Scholl HP, Bala E, Lee KE, Hunter DJ, Sardell RJ, Mitchell P, Merriam JE, Cipriani V, Hoffman JD, Schick T, Lechanteur YT, Guymer RH, Johnson MP, Jiang Y, Stanton CM, Buitendijk GH, Zhan X, Kwong AM, Boleda A, Brooks M, Gieser L, Ratnapriya R, Branham KE, Foerster JR, Heckenlively JR, Othman MI, Vote BJ, Liang HH, Souzeau E, McAllister IL, Isaacs T, Hall J, Lake S, Mackey DA, Constable IJ, Craig JE, Kitchner TE, Yang Z, Su Z, Luo H, Chen D, Ouyang H, Flagg K, Lin D, Mao G, Ferreyra H, Stark K, von Strachwitz CN, Wolf A, Brandl C, Rudolph G, Olden M, Morrison MA, Morgan DJ, Schu M, Ahn J, Silvestri G, Tsironi EE, Park KH, Farrer LA, Orlin A, Brucker A, Li M, Curcio CA, Mohand-Saïd S, Sahel JA, Audo I, Benchaboune M, Cree AJ, Rennie CA, Goverdhan SV, Grunin M, Hagbi-Levi S, Campochiaro P, Katsanis N, Holz FG, Blond F, Blanché H, Deleuze JF, Igo RP Jr., Truitt B, Peachey NS, Meuer SM, Myers CE, Moore EL, Klein R, Hauser MA, Postel EA, Courtenay MD, Schwartz SG, Kovach JL, Scott WK, Liew G, Tan AG, Gopinath B, Merriam JC, Smith RT, Khan JC, Shahid H, Moore AT, McGrath JA, Laux R, Brantley MA Jr., Agarwal A, Ersoy L, Caramoy A, Langmann T, Saksens NT, de Jong EK, Hoyng CB, Cain MS, Richardson AJ, Martin TM, Blangero J, Weeks DE, Dhillon B, van Duijn CM, Doheny KF, Romm J, Klaver CC, Hayward C, Gorin MB, Klein ML, Baird PN, den Hollander AI, Fauser S, Yates JR, Allikmets R, Wang JJ, Schaumberg DA, Klein BE, Hagstrom SA, Chowers I, Lotery AJ, Léveillard T, Zhang K, Brilliant MH, Hewitt AW, Swaroop A, Chew EY, Pericak-Vance MA, DeAngelis M, Stambolian D, Haines JL, Iyengar SK, Weber BH, Abecasis GR, Heid IM, 2016. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nature genetics 48, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JD, Sever RJ, Sparks D, Goren J, 1967. A combined technique of fluorescein funduscopy and angiography of the eye. Archives of ophthalmology (Chicago, Ill. : 1960) 78, 455–461. [DOI] [PubMed] [Google Scholar]
- Gautam P, Hamashima K, Chen Y, Zeng Y, Makovoz B, Parikh BH, Lee HY, Lau KA, Su X, Wong RCB, Chan WK, Li H, Blenkinsop TA, Loh YH, 2021. Multi-species single-cell transcriptomic analysis of ocular compartment regulons. Nat Commun 12, 5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisen P, McColm JR, Hartnett ME, 2006. Choroidal endothelial cells transmigrate across the retinal pigment epithelium but do not proliferate in response to soluble vascular endothelial growth factor. Exp Eye Res 82, 608–619. [DOI] [PubMed] [Google Scholar]
- Gemenetzi M, Patel PJ, 2017. A Systematic Review of the Treat and Extend Treatment Regimen with Anti-VEGF Agents for Neovascular Age-Related Macular Degeneration. Ophthalmol Ther 6, 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus HE, Martinez JA, Brown VB, Lambert HM, Sternberg P Jr., Capone A Jr., Aaberg TM, Lopez PF, 1992. Immunohistochemical and histochemical properties of surgically excised subretinal neovascular membranes in age-related macular degeneration. American journal of ophthalmology 114, 464–472. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Zhu XL, Waheed A, Sly WS, 1991. Localization of carbonic anhydrase IV in a specific capillary bed of the human eye. Proc Natl Acad Sci U S A 88, 2716–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RD, Foss AJ, Leach L, 2007. Establishment of a human in vitro model of the outer blood-retinal barrier. J Anat 211, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa JT, Verzijl N, Matsunaga H, Aotaki-Keen A, Lutty GA, te Koppele JM, Miyata T, Hjelmeland LM, 1999. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Investigative ophthalmology & visual science 40, 775–779. [PubMed] [Google Scholar]
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. , 1991. Optical coherence tomography. Science 254, 1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JB, Lindsay KJ, Du J, 2015. Glucose, lactate, and shuttling of metabolites in vertebrate retinas. Journal of neuroscience research 93, 1079–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Taverna F, Rohlenova K, Treps L, Geldhof V, de Rooij L, Sokol L, Pircher A, Conradi LC, Kalucka J, Schoonjans L, Eelen G, Dewerchin M, Karakach T, Li X, Goveia J, Carmeliet P, 2019. EndoDB: a database of endothelial cell transcriptomics data. Nucleic Acids Res 47, D736–d744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Fingler J, Zawadzki RJ, Park SS, Morse LS, Schwartz DM, Fraser SE, Werner JS, 2013. Optical imaging of the chorioretinal vasculature in the living human eye. Proceedings of the National Academy of Sciences of the United States of America 110, 14354–14359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann GL, Hanke-Gogokhia C, Hu Y, Bareja R, Salfati Z, Ginsberg M, Nolan DJ, Mendez-Huergo SP, Dalotto-Moreno T, Wojcinski A, Ochoa F, Zeng S, Cerliani JP, Panagis L, Zager PJ, Mullins RF, Ogura S, Lutty GA, Bang J, Zippin JH, Romano C, Rabinovich GA, Elemento O, Joyner AL, Rafii S, Rodriguez-Boulan E, Benedicto I, 2020. Single-cell profiling reveals an endothelium-mediated immunomodulatory pathway in the eye choroid. J Exp Med 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Fitzgerald MEC, Del Mar N, Wang H, Haughey C, Honig MG, Reiner A, 2021. Role of the superior salivatory nucleus in parasympathetic control of choroidal blood flow and in maintenance of retinal health. Experimental eye research 206, 108541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutty GA, McLeod DS, Bhutto IA, Edwards MM, Seddon JM, 2020. Choriocapillaris dropout in early age-related macular degeneration. Experimental eye research 192, 107939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manian KV, Galloway CA, Dalvi S, Emanuel AA, Mereness JA, Black W, Winschel L, Soto C, Li Y, Song Y, DeMaria W, Kumar A, Slukvin I, Schwartz MP, Murphy WL, Anand-Apte B, Chung M, Benoit DSW, Singh R, 2021. 3D iPSC modeling of the retinal pigment epithelium-choriocapillaris complex identifies factors involved in the pathology of macular degeneration. Cell Stem Cell 28, 846–862.e848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R, Spaide RF, 2009. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. American journal of ophthalmology 147, 811–815. [DOI] [PubMed] [Google Scholar]
- Mihlan M, Blom AM, Kupreishvili K, Lauer N, Stelzner K, Bergström F, Niessen HW, Zipfel PF, 2011. Monomeric C-reactive protein modulates classic complement activation on necrotic cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 25, 4198–4210. [DOI] [PubMed] [Google Scholar]
- Miller AR, Roisman L, Zhang Q, Zheng F, Rafael de Oliveira Dias J, Yehoshua Z, Schaal KB, Feuer W, Gregori G, Chu Z, Chen CL, Kubach S, An L, Stetson PF, Durbin MK, Wang RK, Rosenfeld PJ, 2017. Comparison Between Spectral-Domain and Swept-Source Optical Coherence Tomography Angiographic Imaging of Choroidal Neovascularization. Investigative ophthalmology & visual science 58, 1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira-Neto CA, Moult EM, Fujimoto JG, Waheed NK, Ferrara D, 2018. Choriocapillaris Loss in Advanced Age-Related Macular Degeneration. J Ophthalmol 2018, 8125267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moult EM, Waheed NK, Novais EA, Choi W, Lee B, Ploner SB, Cole ED, Louzada RN, Lu CD, Rosenfeld PJ, Duker JS, Fujimoto JG, 2016. SWEPT-SOURCE OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY REVEALS CHORIOCAPILLARIS ALTERATIONS IN EYES WITH NASCENT GEOGRAPHIC ATROPHY AND DRUSEN-ASSOCIATED GEOGRAPHIC ATROPHY. Retina (Philadelphia, Pa.) 36 Suppl 1, S2–s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulfaul K, Giacalone JC, Voigt AP, Riker MJ, Ochoa D, Han IC, Stone EM, Mullins RF, Tucker BA, 2020. Stepwise differentiation and functional characterization of human induced pluripotent stem cell-derived choroidal endothelial cells. Stem Cell Res Ther 11, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J, 2011. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Investigative ophthalmology & visual science 52, 1606–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Schoo DP, Sohn EH, Flamme-Wiese MJ, Workamelahu G, Johnston RM, Wang K, Tucker BA, Stone EM, 2014. The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. The American journal of pathology 184, 3142–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Wallman J, 2010. The multifunctional choroid. Progress in retinal and eye research 29, 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S, 2013. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 26, 204–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsley C, McGwin G Jr., Clark ME, Jackson GR, Callahan MA, Kline LB, Witherspoon CD, Curcio CA, 2016. Delayed Rod-Mediated Dark Adaptation Is a Functional Biomarker for Incident Early Age-Related Macular Degeneration. Ophthalmology 123, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parver LM, 1991. Temperature modulating action of choroidal blood flow. Eye (London, England) 5 (Pt 2), 181–185. [DOI] [PubMed] [Google Scholar]
- Querques G, Srour M, Massamba N, Georges A, Ben Moussa N, Rafaeli O, Souied EH, 2013. Functional characterization and multimodal imaging of treatment-naive “quiescent” choroidal neovascularization. Investigative ophthalmology & visual science 54, 6886–6892. [DOI] [PubMed] [Google Scholar]
- Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT, 1994. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Investigative ophthalmology & visual science 35, 2857–2864. [PubMed] [Google Scholar]
- Rinella NT, Zhou H, Wong J, Zhang Q, Nattagh K, Porco TC, Wang RK, Schwartz DM, Duncan JL, 2021. Correlation Between Localized Choriocapillaris Perfusion and Macular Function in Eyes with Geographic Atrophy: Choriocapillaris Flow Relates to Macular Function in AMD with GA. American journal of ophthalmology. [DOI] [PubMed] [Google Scholar]
- Rohlenova K, Goveia J, García-Caballero M, Subramanian A, Kalucka J, Treps L, Falkenberg KD, de Rooij L, Zheng Y, Lin L, Sokol L, Teuwen LA, Geldhof V, Taverna F, Pircher A, Conradi LC, Khan S, Stegen S, Panovska D, De Smet F, Staal FJT, McLaughlin RJ, Vinckier S, Van Bergen T, Ectors N, De Haes P, Wang J, Bolund L, Schoonjans L, Karakach TK, Yang H, Carmeliet G, Liu Y, Thienpont B, Dewerchin M, Eelen G, Li X, Luo Y, Carmeliet P, 2020. Single-Cell RNA Sequencing Maps Endothelial Metabolic Plasticity in Pathological Angiogenesis. Cell Metab 31, 862–877.e814. [DOI] [PubMed] [Google Scholar]
- Russell JF, Shi Y, Hinkle JW, Scott NL, Fan KC, Lyu C, Gregori G, Rosenfeld PJ, 2019. Longitudinal Wide-Field Swept-Source OCT Angiography of Neovascularization in Proliferative Diabetic Retinopathy after Panretinal Photocoagulation. Ophthalmol Retina 3, 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA, 2009. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proceedings of the National Academy of Sciences of the United States of America 106, 18751–18756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Sakamoto H, Murphy TL, Spee C, Soriano D, Ishibashi T, Hinton DR, Ryan SJ, 1995. Vessel formation by choroidal endothelial cells in vitro is modulated by retinal pigment epithelial cells. Arch Ophthalmol 113, 512–520. [DOI] [PubMed] [Google Scholar]
- Sarks SH, 1973. New vessel formation beneath the retinal pigment epithelium in senile eyes. British Journal of Ophthalmology 57, 951–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N, 2004. Association between C-reactive protein and age-related macular degeneration. Jama 291, 704–710. [DOI] [PubMed] [Google Scholar]
- Seddon JM, George S, Rosner B, Rifai N, 2005. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Archives of ophthalmology (Chicago, Ill. : 1960) 123, 774–782. [DOI] [PubMed] [Google Scholar]
- Sekiyama E, Saint-Geniez M, Yoneda K, Hisatomi T, Nakao S, Walshe TE, Maruyama K, Hafezi-Moghadam A, Miller JW, Kinoshita S, D’Amore PA, 2012. Heat treatment of retinal pigment epithelium induces production of elastic lamina components and antiangiogenic activity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 26, 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Zhang Q, Zhou H, Wang L, Chu Z, Jiang X, Shen M, Thulliez M, Lyu C, Feuer W, de Sisternes L, Durbin MK, Gregori G, Wang RK, Rosenfeld PJ, 2021. Correlations Between Choriocapillaris and Choroidal Measurements and the Growth of Geographic Atrophy Using Swept Source OCT Imaging. American journal of ophthalmology 224, 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SR, Vupparaboina KK, Goud A, Dansingani KK, Chhablani J, 2019. Choroidal imaging biomarkers. Survey of ophthalmology 64, 312–333. [DOI] [PubMed] [Google Scholar]
- Sohn EH, Flamme-Wiese MJ, Whitmore SS, Workalemahu G, Marneros AG, Boese EA, Kwon YH, Wang K, Abramoff MD, Tucker BA, Stone EM, Mullins RF, 2019. Choriocapillaris Degeneration in Geographic Atrophy. The American journal of pathology 189, 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn EH, Khanna A, Tucker BA, Abràmoff MD, Stone EM, Mullins RF, 2014. Structural and biochemical analyses of choroidal thickness in human donor eyes. Investigative ophthalmology & visual science 55, 1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G, 2018. Optical coherence tomography angiography. Progress in retinal and eye research 64, 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaide RF, Koizumi H, Pozzoni MC, 2008. Enhanced depth imaging spectral-domain optical coherence tomography. American journal of ophthalmology 146, 496–500. [DOI] [PubMed] [Google Scholar]
- Spencer C, Abend S, McHugh KJ, Saint-Geniez M, 2017. Identification of a synergistic interaction between endothelial cells and retinal pigment epithelium. J Cell Mol Med 21, 2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraul CW, Lang GE, Grossniklaus HE, 1996. Morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Investigative ophthalmology & visual science 37, 2724–2735. [PubMed] [Google Scholar]
- Steinle JJ, Smith PG, 2002. Role of adrenergic receptors in vascular remodelling of the rat choroid. Br J Pharmacol 136, 730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sura AA, Chen L, Messinger JD, Swain TA, McGwin G Jr, Freund KB, Curcio CA, 2020. Measuring the contributions of basal laminar deposit and Bruch’s membrane in age-related macular degeneration. Investigative ophthalmology & visual science 61, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulliez M, Zhang Q, Shi Y, Zhou H, Chu Z, de Sisternes L, Durbin MK, Feuer W, Gregori G, Wang RK, Rosenfeld PJ, 2019. Correlations between Choriocapillaris Flow Deficits around Geographic Atrophy and Enlargement Rates Based on Swept-Source OCT Imaging. Ophthalmol Retina 3, 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno K, Bhutto IA, McLeod DS, Merges C, Lutty GA, 2006. Impaired expression of thrombospondin-1 in eyes with age related macular degeneration. The British journal of ophthalmology 90, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt AP, Mulfaul K, Mullin NK, Flamme-Wiese MJ, Giacalone JC, Stone EM, Tucker BA, Scheetz TE, Mullins RF, 2019. Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration. Proc Natl Acad Sci U S A 116, 24100–24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt AP, Mullin NK, Stone EM, Tucker BA, Scheetz TE, Mullins RF, 2020a. Single-cell RNA sequencing in vision research: Insights into human retinal health and disease. Progress in retinal and eye research, 100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt AP, Whitmore SS, Lessing ND, DeLuca AP, Tucker BA, Stone EM, Mullins RF, Scheetz TE, 2020b. Spectacle: An interactive resource for ocular single-cell RNA sequencing data analysis. Experimental eye research 200, 108204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt AP, Whitmore SS, Mulfaul K, Chirco KR, Giacalone JC, Flamme-Wiese MJ, Stockman A, Stone EM, Tucker BA, Scheetz TE, Mullins RF, 2020c. Bulk and single-cell gene expression analyses reveal aging human choriocapillaris has pro-inflammatory phenotype. Microvasc Res, 104031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki Y, Shinojima A, Kawamura A, Yuzawa M, 2015. Correlation of Aging and Segmental Choroidal Thickness Measurement using Swept Source Optical Coherence Tomography in Healthy Eyes. PloS one 10, e0144156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei WB, Xu L, Jonas JB, Shao L, Du KF, Wang S, Chen CX, Xu J, Wang YX, Zhou JQ, You QS, 2013. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology 120, 175–180. [DOI] [PubMed] [Google Scholar]
- Whitmore SS, Sohn EH, Chirco KR, Drack AV, Stone EM, Tucker BA, Mullins RF, 2015. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Progress in retinal and eye research 45, 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe DR, 1986. Fluorescein angiography basic science and engineering. Ophthalmology 93, 1617–1620. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Rosenfeld PJ, Waheed NK, Singh RP, Ronca N, Slakter JS, Staurenghi G, Monés J, Baumal CR, Saroj N, Metlapally R, Ribeiro R, 2021. Characterizing New-Onset Exudation in the Randomized Phase 2 FILLY Trial of Complement Inhibitor Pegcetacoplan for Geographic Atrophy. Ophthalmology 128, 1325–1336. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang Q, Motulsky EH, Thulliez M, Shi Y, Lyu C, de Sisternes L, Durbin MK, Feuer W, Wang RK, Gregori G, Rosenfeld PJ, 2019. Two-Year Risk of Exudation in Eyes with Nonexudative Age-Related Macular Degeneration and Subclinical Neovascularization Detected with Swept Source Optical Coherence Tomography Angiography. American journal of ophthalmology 208, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannuzzi LA, Rohrer KT, Tindel LJ, Sobel RS, Costanza MA, Shields W, Zang E, 1986. Fluorescein angiography complication survey. Ophthalmology 93, 611–617. [DOI] [PubMed] [Google Scholar]
- Yoneya S, Saito T, Komatsu Y, Koyama I, Takahashi K, Duvoll-Young J, 1998. Binding properties of indocyanine green in human blood. Investigative ophthalmology & visual science 39, 1286–1290. [PubMed] [Google Scholar]
- Zeng S, Wen KK, Workalemahu G, Sohn EH, Wu M, Chirco KR, Flamme-Wiese MJ, Liu X, Stone EM, Tucker BA, Mullins RF, 2018. Imidazole Compounds for Protecting Choroidal Endothelial Cells from Complement Injury. Sci Rep 8, 13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Shi Y, Zhou H, Gregori G, Chu Z, Zheng F, Motulsky EH, de Sisternes L, Durbin M, Rosenfeld PJ, Wang RK, 2018. Accurate estimation of choriocapillaris flow deficits beyond normal intercapillary spacing with swept source OCT angiography. Quant Imaging Med Surg 8, 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Zhang Q, Shi Y, Russell JF, Motulsky EH, Banta JT, Chu Z, Zhou H, Patel NA, de Sisternes L, Durbin MK, Feuer W, Gregori G, Wang R, Rosenfeld PJ, 2019. Age-dependent Changes in the Macular Choriocapillaris of Normal Eyes Imaged With Swept-Source Optical Coherence Tomography Angiography. American journal of ophthalmology 200, 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Dai Y, Shi Y, Russell JF, Lyu C, Noorikolouri J, Feuer WJ, Chu Z, Zhang Q, de Sisternes L, Durbin MK, Gregori G, Rosenfeld PJ, Wang RK, 2020. Age-Related Changes in Choroidal Thickness and the Volume of Vessels and Stroma Using Swept-Source OCT and Fully Automated Algorithms. Ophthalmol Retina 4, 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]


