Abstract
Triplex-forming oligonucleotides (TFOs) are good candidates to be used as site-specific DNA-binding agents. Two obstacles encountered with TFOs are susceptibility to nuclease activity and a requirement for magnesium for triplex formation. Morpholino oligonucleotides were shown in one study to form triplexes in the absence of magnesium. In the current study, we have compared phosphodiester and morpholino oligonucleotides targeting a homopurine–homopyrimidine region in the human HER2/neu promoter. Using gel mobility shift analysis, our data demonstrate that triplex formation by phosphodiester oligonucleotides at the HER-2/neu promoter target is possible with pyrimidine-parallel, purine-antiparallel and mixed sequence (GT)-antiparallel motifs. Only the pyrimidine-parallel motif morpholino TFO was capable of efficient triple helix formation, which required low pH. Triplex formation with the morpholino TFO was efficient in low or no magnesium. The pyrimidine motif TFOs with either a phosphodiester or morpholino backbone were able to form triple helices in the presence of potassium ions, but required low pH. We have rationalized the experimental observations with detailed molecular modeling studies. These data demonstrate the potential for the development of TFOs based on the morpholino backbone modification and demonstrate that the pyrimidine motif is the preferred motif for triple helix formation by morpholino oligonucleotides.
INTRODUCTION
Oligonucleotides are being investigated for their use as potential therapeutic agents. They have been studied in antisense applications, where they are designed to target mRNAs, antigene applications, where they control gene expression via triple helix formation, and in applications that target proteins, where they are used as aptamers (1–4). As a result, oligonucleotides have been developed with modifications that could increase binding affinity and nuclease resistance.
In the triple helix or antigene strategy, the oligonucleotide binds in the major groove of double-stranded DNA via Hoogsteen hydrogen bonding to form a triple helix (5,6). Triplex-forming oligonucleotides (TFOs) bind homopurine–homopyrimidine sequences in double-stranded DNA. There are four structural motifs for triplex formation that have been described based on the third strand composition and its orientation relative to the purine-rich strand of the duplex. Purine motif TFOs (those that are comprised of G and A) form G*G:C and A*A:T triplets and bind in antiparallel orientation with regard to the purine strand of the duplex. Pyrimidine motif TFOs (C/T) form triplexes in parallel orientation and generally only at low pH due to the necessity of the cytosine bases being protonated in order to form Hoogsteen bonds; they form C+*G:C and T*A:T triplets. Finally, mixed purine and pyrimidine TFOs bind in either parallel or antiparallel orientation and form G*G:C and T*A:T triplets. The orientation in which the mixed motif TFOs bind is dependent upon the number of GpA and ApG steps in the homopurine tract (7). The antiparallel orientation is favored by a greater number of steps, while a low number of steps favors the parallel orientation (8).
Once the best motif for binding a particular target sequence is established, problems with natural phosphodiester oligonucleotides limit the success of the antigene approach and the therapeutic applications of oligonucleotides in general. Oligonucleotides with the natural phosphodiester backbone are susceptible to endo- and exonucleases. The predominant activity that degrades oligonucleotides is 3′-exonuclease activity, but endonuclease activity has also been observed in some settings (9,10). Thus, for application as therapeutics in vivo TFOs must be able to resist both exonuclease and endonuclease activity in order to reach their target. A backbone modification that confers nuclease resistance but allows binding to double-stranded DNA with high affinity is required for the in vivo application of TFOs.
Phosphorodiamidate morpholino oligomers are modified backbone oligonucleotides that have previously been investigated as antisense agents (11,12). Morpholino oligonucleotides have an uncharged backbone in which the deoxyribose sugar of DNA is replaced by a six membered ring and the phosphodiester linkage is replaced by a phosphorodiamidate linkage (13). Morpholino oligonucleotides are resistant to enzymatic degradation (14) and appear to function as antisense agents by arresting translation or interfering with pre-mRNA splicing rather than by activating RNase H (15,16). Morpholino oligonucleotides have been successfully delivered to tissue culture cells by methods that physically disrupt the cell membrane, and one study comparing several of these methods found that scrape loading was the most efficient method of delivery; however, because the morpholino backbone is uncharged, cationic lipids are not effective mediators of morpholino oligonucleotide uptake in cells (17). A recent report demonstrated triplex formation by a morpholino oligonucleotide and, because of the non-ionic backbone, these studies showed that the morpholino oligonucleotide was capable of triplex formation in the absence of magnesium (18).
Cations have been shown to play an important role in triple helix formation. When phosphodiester oligonucleotides are used as TFOs, magnesium is generally required for triplex formation with purine and mixed motif TFOs; it also speeds the reaction and stabilizes the triplex formed with pyrimidine motif TFOs (19–21). Other divalent cations have been shown to function in the same capacity as magnesium with regard to triplex formation (22). Magnesium occurs at a concentration of ∼0.8 mM in the cell and ∼1.5 mM in the blood (23), but most in vitro triplex reactions are performed in 5–10 mM MgCl2. Potassium occurs in the cell at a concentration of ∼140 mM, and at 4 mM in the blood. High concentrations of potassium can inhibit triplex formation with guanine-rich oligonucleotides designed as TFOs by favoring other secondary DNA structures, such as dimers and quadruplexes (8,24–28). It will be necessary to overcome the limited ability of phosphodiester TFOs to form a triplex in low magnesium and high potassium for them to be effective under physiological conditions. In a recent study of morpholino TFOs, triplex formation was demonstrated in the absence of magnesium and in the presence of potassium. These properties make morpholino TFOs good candidates for further study as antigene therapeutics.
In the current investigation, the target sequence is a 28 base, nearly perfect homopurine–homopyrimidine tract at –218 to –245 in the HER-2/neu promoter (Fig. 1). This target sequence has been previously described to form a triple helix with TFOs in purine and mixed motifs (29–31). Phosphodiester and morpholino backbone oligonucleotides in all possible triple helix motifs were investigated for their abilities to form triplexes with the HER-2/neu promoter by electrophoretic mobility shift analysis (EMSA). The structural motif required for triplex formation with morpholino TFOs in this target sequence was found to be the pyrimidine motif, and the cation and pH requirements for pyrimidine motif phosphodiester and morpholino TFOs were compared. Detailed molecular modeling analysis was used to rationalize the experimental data, indicating that the pyrimidine motif was the preferred motif for triplex formation by morpholino TFOs.
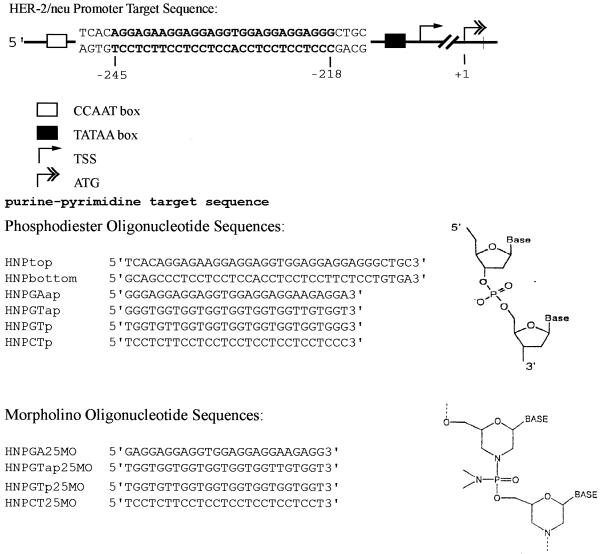
Figure 1.
Schematic diagram of the triplex target sequence in the HER-2/neu promoter and the sequences of the TFOs used in these studies. The map numbers for the HER-2/neu promoter are relative to the ATG translation start site at +1. TSS, transcription start site. The phosphodiester and morpholino backbone structures are illustrated.
MATERIALS AND METHODS
Oligodeoxyribonucleotide synthesis and purification
Unmodified phosphodiester oligonucleotides were synthesized by Sigma Genesys. Oligonucleotides were PAGE purified by standard methods. Morpholino oligonucleotides were synthesized and purified on Sepharose columns by GeneTools (13). The sequences of the oligonucleotides used in these studies and the target sequence in the HER-2/neu promoter are illustrated in Figure 1. The target sequence used for triplex formation consists of the 28 bp homopurine:homopyrimidine tract plus 4 bp of flanking sequence on each end.
Triplex reactions and native gel mobility shift analysis
The pyrimidine strand (HNPbottom) of the duplex target was end-labeled by T4 polynucleotide kinase with [γ-32P]ATP and annealed to a slight excess of the purine-rich strand (HNPtop). Labeled target duplex at a final concentration of 10 nM was incubated with serial 10-fold dilutions of each TFO over the range 0.01–10 µM in either TA, TAM or TBM (TA, 40 mM Tris titrated to pH 4–7.4 with acetic acid as indicated; TAM, 40 mM Tris titrated to pH 4–7.4 with acetic acid and 1–10 mM MgCl2 as indicated; TBM, 90 mM Tris, 90 mM borate and 10 mM MgCl2, pH 7.4) at 37°C for at least 24 h. The samples were loaded onto 16% native polyacrylamide gels made with the same buffer as the incubation buffer except that gels made with 1× TBM were made at pH 8.0 to prevent salt precipitation. The gels were run at 74 V at room temperature for 16 h. The pH of the buffer was checked before and after electrophoresis in representative experiments to ensure that a significant pH gradient did not develop during prolonged electrophoresis. Bands were visualized by autoradiography.
Calculation of dissociation constants
Autoradiographs were analyzed on a Stratagene Eagle Eye and the band intensity measured by densitometry. The dissociation constant (Kd) was calculated by first determining the fraction of triplex (y) at each concentration of TFO according to the formula: y = T/(D + T), where T and D represent the densitometry signal from the bands representing the triple helix and duplex, respectively. Kd was determined by applying a least squares curve fitting algorithm to the curve resulting from the equation y = [TFO]/([TFO] + Kd), where [TFO] represents the concentration of the TFO (8,32). This calculation is only accurate when the concentration of TFO is much greater than the concentration of target duplex. In some cases, a Kd was not strictly calculable by this approach because 10 nM target duplex was completely saturated by even a 10-fold excess of TFO (i.e. when [TFO] = 100 nM). In these cases, the Kd was approximated by plotting y versus [TFO] over the concentration range 10–100 nM and interpolating the mid point of the resulting curve using a least squares fitting algorithm, so that Kd is equal to [TFO] where y = 0.5 (8). In cases where the duplex was completely saturated at the lowest concentration of TFO used (10 nM) the Kd is given as <0.01 µM and when the TFO failed to saturate the target duplex at the highest concentration of TFO used (10 µM) the Kd is given as >10 µM.
Molecular modeling
The triplex system was based on 14 bases of the target duplex. The duplex used was d(GGAGAAGGAGGAGG)·d(CCTCCTCCTTCTCC) with either a parallel third strand d(CCTCTTCCTCCTCC) (with all third strand cytosines protonated) or an antiparallel third strand d(GGAGGAGGAAGAGG). Four simulations of triplexes were run, parallel DNA TFO, antiparallel phosphodiester TFO, parallel morpholino TFO and antiparallel morpholino TFO. The partial atomic charges of the morpholino residues (T, A, G and protonated C) were generated by ab initio calculations with the 6-31G** basis set using GAMESS (33). The necessary bond, angle and torsion parameters were empirically fitted from the ab initio calculations and AMBER parameters of analogous atoms.
The triplex starting structures were generated using fiber diffraction data (34,35) with initial in vacuo optimization to remove the imposed symmetry. The morpholino nucleotides were superimposed on the TFO strand bases to generate the morpholino starting structures, which were subsequently minimized in vacuo.
Models were hydrated in a 10 Å box of TIP3P waters using standard rules; Na+ cations were added and then Cl– counterions were placed randomly for charge neutrality. RDPARM was used to adjust each system to include 3877 waters for each model. Simulations were performed in the isothermal isobaric ensemble (300 K, 1 atm) with the AMBER 5.0 program (36) and AMBER-98 force field, using periodic boundary conditions and the Particle-Mesh-Ewald algorithm. Molecular dynamics (MD) simulations used the Sander routine (1.5 fs time step), with SHAKE to freeze all bonds involving hydrogen. All calculations were performed on a Silicon Graphics Origin 2000 server. Each system was slowly heated and equilibrated for 125 ps, with gradual removal of positional restraints for the DNA. The stepwise modeling protocol used was: (i) energy minimize waters only; (ii) 25 ps MD at 100 K, holding the triplex with a positional restraint of 100 kcal (mol·Å)–1; (iii) re-minimize waters; (iv) minimize whole system; (v) 25 ps MD at 100 K, restraining the triplex [100 kcal (mol·Å)–1]; and (vi) four sequential 25 ps MD steps at 300 K, with a gradual loosening of restraints for the triplex [i.e. 100, 50, 10, 1 kcal (mol·Å)–1]. After equilibration, production runs of 1 ns were used to obtain an average structure for each complex (taken from 100 snapshots accumulated in the last 150 ps), which was then fully minimized to give the final structures.
RESULTS
Analysis of phosphodiester TFOs in all possible motifs
The HER-2/neu promoter triplex target sequence and TFOs are illustrated in Figure 1. Phosphodiester TFOs in the pyrimidine-parallel, purine-antiparallel, mixed sequence-antiparallel and mixed sequence-parallel motifs were evaluated for their ability to undergo triple helix formation in the HER-2/neu promoter. EMSA showed the formation of triple helices in the purine, pyrimidine and mixed sequence motifs (Fig. 2A and B). Interestingly, these data demonstrate that the target sequence in the HER-2/neu promoter is capable of triplex formation with the pyrimidine-parallel, purine-antiparallel and mixed sequence-antiparallel motifs with Kd values of <50 nM. These experiments showed that the pyrimidine motif TFO had a high affinity for the duplex target, but demonstrated a sharp dependence on low pH for triplex formation. At physiological pH, the antiparallel mixed sequence motif TFO also showed a high affinity for the duplex target, with a Kd of <10 nM. The dissociation constants of the phosphodiester TFOs and the influence of pH on the pyrimidine-parallel motif TFO are given in Table 1.
Figure 2.
EMSA of triplex formation by phosphodiester TFOs in all possible structural motifs. The sequences of the TFOs are given in Figure 1. ss, single-stranded DNA; ds, double-stranded DNA; ts, triple-stranded DNA. (A) Triplex reactions and the non-denaturing gel were buffered in TBM, pH 7.4. (B) Triplex reactions and the non-denaturing gel were buffered in TAM, pH 5.0.
Table 1. Kd analysis of phosphodiester TFOs in all possible structural motifs.
| Motif | Orientation | pH | Kd (µM) |
|---|---|---|---|
| GA | antiparallel | 7.4 | 0.021 |
| GT | antiparallel | 7.4 | <0.01 |
| GT | parallel | 7.4 | 0.133 |
| CT | parallel | 7.4 | >10 |
| CT | parallel | 5.5 | 4.18 |
| CT | parallel | 5.0 | 0.03 |
| CT | parallel | 4.0 | 0.009 |
The mixed sequence motif TFOs designed to bind in both the parallel and antiparallel orientation demonstrated binding to the target duplex. However, it is unlikely that the same TFO sequence can bind in both orientations. The target sequence contains sufficient internal symmetry that the parallel mixed motif TFO (HNPGTp) may actually bind the target sequence in the antiparallel orientation. The resulting mismatches would explain the lower binding affinity and higher dissociation constant observed for the mixed sequence-parallel TFO compared to the mixed sequence-antiparallel TFO. Affinity cleavage studies would be required to prove the actual binding orientation of the TFOs.
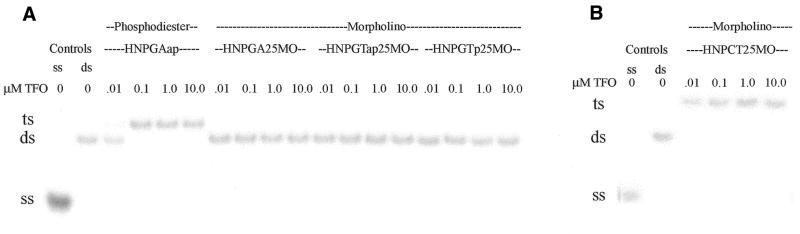
Analysis of morpholino TFOs in all possible motifs
With the knowledge that the target sequence in the HER-2/neu promoter supports triplex formation by phosphodiester TFOs in the pyrimidine, purine and mixed sequence motifs, morpholino TFOs in all four possible sequence motifs were studied for their ability to bind the target sequence. These studies showed that triplex formation in the HER-2/neu promoter with morpholino TFOs requires the pyrimidine motif; the purine and mixed motif TFOs failed to form triplex even at a TFO concentration of 10 µM (Fig. 3). The pyrimidine motif morpholino TFO forms a triplex with a Kd of <10 nM at pH 5.0 in 1× TAM (Table 2). The ability of the pyrimidine motif morpholino TFO to undergo triplex formation was highly pH dependent. Triplex formation was optimal at pH 5.0 and less and declined markedly as the pH approached 7.0.
Figure 3.
EMSA of triplex formation by morpholino TFOs with all possible structural motifs. The sequences of the TFOs are given in Figure 1. (A) Triplex reactions and the gel were buffered in TBM, pH 7.4. A phosphodiester TFO was included as a positive control. (B) Triplex reactions and the gel were buffered in TAM, pH 5.0. ss, single-stranded DNA; ds, double-stranded DNA; ts, triple-stranded DNA.
Table 2. Kd analysis of morpholino TFOs in the presence of 10 mM MgCl2.
| Motif | Backbone | Orientation | pH | Kd (µM) |
|---|---|---|---|---|
| GA | MO | antiparallel | 7.4 | >10 |
| GT | MO | antiparallel | 7.4 | >10 |
| GT | MO | parallel | 7.4 | >10 |
| CT | MO | parallel | 7.4 | >10 |
| CT | MO | parallel | 5.0 | <0.01 |
Formation of a triple helix in the absence of magnesium
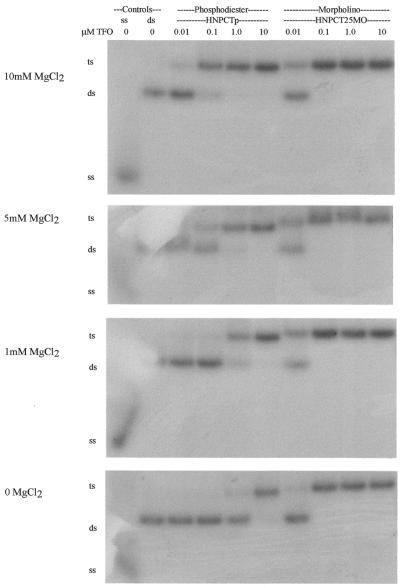
Many studies show that magnesium is necessary for fast and stable intermolecular triplex formation. In our studies we performed triplex reactions with both phosphodiester and morpholino pyrimidine motif TFOs in varying concentrations of magnesium (0, 1, 5 and 10 mM). All reactions were incubated in 1× TA (± Mg), pH 5.0, at 37°C for at least 24 h with the indicated concentrations of magnesium (Fig. 4). The pyrimidine-parallel phosphodiester TFOs showed triplex formation with a Kd of <150 nM with 5–10 mM MgCl2, whereas the morpholino TFO formed a triplex with high affinity (Kd < 50 nM) at every magnesium concentration. In the absence of magnesium, triplex formation with the phosphodiester oligonucleotide required very high concentrations of the TFO, indicating a Kd of 1.8 µM. The ability of the morpholino TFO to undergo triplex formation was nearly independent of magnesium concentration, with a Kd of 32 nM in the absence of magnesium (Table 3).
Figure 4.
EMSA of the effect of magnesium concentration on triplex formation by phosphodiester pyrimidine motif TFOs versus morpholino pyrimidine motif TFOs. Triplex reactions and the gel were buffered in TA, pH 5, with the concentration of magnesium indicated. ss, single-stranded DNA; ds, double-stranded DNA; ts, triple-stranded DNA.
Table 3. Kd comparison of morpholino and phosphodiester CT parallel TFOs under various buffering conditions.
| pH | [MgCl2] (mM) | [KCl] (mM) | Morpholino Kd (µM) | Phosphodiester Kd (µM) |
|---|---|---|---|---|
| 7.4 | 0 | 0 | >10 | >10 |
| 6.7 | 0 | 0 | >10 | >10 |
| 6.6 | 0 | 0 | >10 | >10 |
| 6.4 | 0 | 0 | 4.36 | >10 |
| 6.0 | 0 | 0 | 0.060 | >10 |
| 5.5 | 0 | 0 | 0.032 | >10 |
| 5.0 | 0 | 0 | 0.032 | 1.8 |
| 5.0 | 1 | 0 | <0.01 | 0.32 |
| 5.0 | 5 | 0 | <0.01 | 0.112 |
| 5.0 | 10 | 0 | <0.01 | 0.025 |
| 5.0 | 0 | 140 | 0.012 | 0.027 |
| 5.0 | 10 | 140 | 0.013 | 0.03 |
Triple helix formation in the presence of potassium
The effect of potassium on triplex formation with phosphodiester and morpholino oligonucleotides was also investigated. Morpholino or phosphodiester TFOs were incubated with the target duplex in the presence or absence of magnesium and potassium (1× TA, pH 5.0, alone or with 140 mM KCl or 140 mM KCl and 10 mM MgCl2 or 10 mM MgCl2) for at least 24 h at 37°C. Potassium ions did not adversely affect triplex formation by morpholino TFOs (Table 3).
Molecular models of triple helices
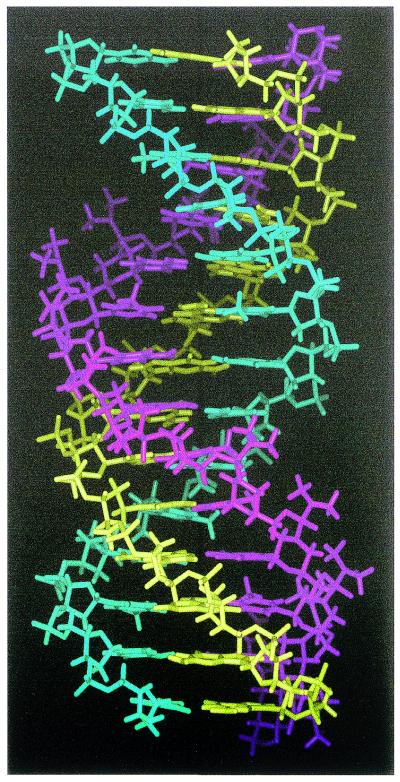
The triple helices were stable under MD simulation conditions for the parallel phosphodiester TFO, antiparallel phosphodiester TFO and parallel morpholino TFO (Fig. 5). To the authors’ knowledge this is the first report of detailed fully solvated MD simulations of a triplex comprising a DNA duplex and a morpholino TFO. The antiparallel morpholino TFO did not converge to a stable triplex and did not maintain the initial triplex structure. The reverse Hoogsteen hydrogen bonds were broken combined with severe loss of planarity of the base triplets.
Figure 5.
Molecular model of the parallel morpholino TFO–DNA triplex. The morpholino strand is in purple, the pyrimidine duplex strand is in cyan and the purine duplex strand is in yellow.
DISCUSSION
Our objective in this study was to find a nuclease-resistant TFO with the ability to bind and form a triplex with the duplex target in the promoter of the human HER-2/neu gene at a low concentration and under near physiological conditions. Considerations in the evaluation of antigene oligonucleotides include nuclease sensitivity, pH, interaction with cations and the possibility of non-sequence-specific effects described for some backbone modifications such as the phosphorothioates (37,38). The results of our studies show that the target in the promoter of the HER-2/neu gene is very favorable for triplex formation with the pyrimidine-parallel, purine-antiparallel and mixed sequence-antiparallel triplex motifs with phosphodiester TFOs. TFOs in each of these motifs formed triplexes with high affinity and the purine motif TFO and the mixed sequence motif TFOs formed triplexes at physiological pH. Because it is necessary for cytosines to be protonated in order to form Hoogsteen hydrogen bonds, the pyrimidine motif TFO formed a triplex with great affinity, but only at low pH.
Even though the phosphodiester TFOs formed triplexes with high affinity in vitro, the natural phosphodiester backbone TFO would be susceptible to nuclease activity in the cell. An alternative to phosphodiester TFOs is a modified oligonucleotide that has been investigated for its use in the antisense field, the morpholino oligonucleotide. Morpholino oligonucleotides offer nuclease resistance (14) and have been shown not to introduce any undesired activity or protein binding (13). Since information regarding the use of morpholino oligonucleotides as TFOs is so limited, we chose to study all structural motifs in order to find the best TFO for our target.
We found only pyrimidine-parallel morpholino oligonucleotides to be able to form a triplex with our duplex target. As expected, this motif required a low pH for triplex formation, as did the pyrimidine-parallel motif phosphodiester TFO. It may be possible to overcome this pH dependence with such substitutions as 5-methylcytosine for the cytosines in the TFO (39), however, this modification is currently unavailable commercially with the morpholino backbone. The stability of the parallel and antiparallel morpholino TFO triplexes in MD simulations is identical to and rationalizes the experimental results only for the pyrimidine-parallel morpholino oligonucleotide forming a triplex. The experimental and modeling data suggest that the morpholino oligonucleotides adopt a B-form helical structure capable of forming only forward Hoogsteen hydrogen bonds. RNA and RNA-mimetic backbone oligonucleotides such as the N3–P5 phosphoramidate backbone share this structure and also form triplexes only with forward Hoogsteen hydrogen bonds (40–42). In certain sequences with a limited number of GpA and ApG steps, the RNA-mimetic backbone of the N3–P5 phosphoramidate oligonucleotides can support triplex formation with mixed sequence-parallel motif TFOs with an even higher affinity for the target duplex than pyrimidine motif TFOs (43). The HER-2/neu promoter triplex target sequence is characterized by multiple GGA repeats and therefore contains many (14) ApG and GpA steps that are not favorable for triplex formation with mixed sequence-parallel TFOs (8). It is therefore not surprising that the pyrmidine motif (and low pH) is required for triplex formation by the morpholino TFOs in this particular target sequence, but it may be possible for mixed sequence-parallel motif TFOs to form a triple helix at neutral pH in other homopurine–homopyrimidine sequences with fewer GpA and ApG steps.
Even though the morpholino TFOs required low pH to form triplexes, they were able to undergo triplex formation in the presence of high concentrations of potassium and in the absence of magnesium, properties advantageous to the development of antigene oligonucleotides. Magnesium ions (or other divalent cations) can stabilize and speed triplex formation with phosphodiester TFOs by decreasing the charge repulsion of the anionic phosphodiester TFO backbone for the phosphodiester backbones of the duplex DNA target (19–22). Because the morpholino backbone is not charged, magnesium or another divalent cation is not required for triplex formation. We also found that triplex formation by the pyrimidine motif morpholino TFO was not inhibited by the presence of potassium ions, which may simply reflect the base composition of the TFO, since potassium likely inhibits triplex formation by favoring competing structures due to self-association of guanine-rich oligonucleotides (8,24–28).
In summary, morpholino oligonucleotides are nuclease-resistant oligonucleotides that can support triplex formation in the absence of magnesium and the presence of potassium, but they require a pyrimidine motif for triplex formation, at least in the HER-2/neu promoter sequence. Morpholino oligonucleotides are potentially viable candidates as antigene oligonucleotides, but will require that the limitation of dependence on non-physiological pH conditions be overcome.
REFERENCES
- 1.Helene C. and Toulme,J.J. (1990) Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim. Biophys. Acta, 1049, 99–125. [DOI] [PubMed] [Google Scholar]
- 2.Helene C. (1994) Control of oncogene expression by antisense nucleic acids. Eur. J. Cancer, 30A, 1721–1726. [DOI] [PubMed] [Google Scholar]
- 3.Stein C.A. and Cheng,Y.C. (1993) Antisense oligonucleotides as therapeutic agents—is the bullet really magical? Science, 261, 1004–1012. [DOI] [PubMed] [Google Scholar]
- 4.Wagner R.W. (1994) Gene inhibition using antisense oligodeoxynucleotides. Nature, 372, 333–335. [DOI] [PubMed] [Google Scholar]
- 5. Le Doan T., Perrouault,L., Praseuth,D., Habhoub,N., Decout,J.L., Thuong,N.T., Lhomme,J. and Helene,C. (1987) Sequence-specific recognition, photocrosslinking and cleavage of the DNA double helix by an oligo-α-thymidylate covalently linked to an azidoproflavine derivative. Nucleic Acids Res., 15, 7749–7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moser H.E. and Dervan,P.B. (1987) Sequence-specific cleavage of double helical DNA by triple helix formation. Science, 238, 645–650. [DOI] [PubMed] [Google Scholar]
- 7.Sun J.S., De Bizemont,T., Duval-Valentin,G., Montenay-Garestier,T. and Helene,C. (1991) Extension of the range of recognition sequences for triple helix formation by oligonucleotides containing guanines and thymines. C. R. Acad. Sci. III, 313, 585–590. [PubMed] [Google Scholar]
- 8.Gamper H.B.J., Kutyavin,I.V., Rhinehart,R.L., Lokhov,S.G., Reed,M.W. and Meyer,R.B. (1997) Modulation of Cm/T, G/A and G/T triplex stability by conjugate groups in the presence and absence of KCl. Biochemistry, 36, 14816–14826. [DOI] [PubMed] [Google Scholar]
- 9.Eder P.S., DeVine,R.J., Dagle,J.M. and Walder,J.A. (1991) Substrate specificity and kinetics of degradation of antisense oligonucleotides by a 3′ exonuclease in plasma. Antisense Res. Dev., 1, 141–151. [DOI] [PubMed] [Google Scholar]
- 10.Fisher T.L., Terhorst,T., Cao,X. and Wagner,R.W. (1993) Intracellular disposition and metabolism of fluorescently-labeled unmodified and modified oligonucleotides microinjected into mammalian cells. Nucleic Acids Res., 21, 3857–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein D., Foster,E., Huang,S.B., Weller,D. and Summerton,J. (1997) A specificity comparison of four antisense types: morpholino, 2′-O-methyl RNA, DNA and phosphorothioate DNA. Antisense Nucleic Acid Drug Dev., 7, 151–157. [DOI] [PubMed] [Google Scholar]
- 12.Summerton J., Stein,D., Huang,S.B., Matthews,P., Weller,D. and Partridge,M. (1997) Morpholino and phosphorothioate antisense oligomers compared in cell-free and in-cell systems. Antisense Nucleic Acid Drug Dev., 7, 63–70. [DOI] [PubMed] [Google Scholar]
- 13.Summerton J. and Weller,D. (1997) Morpholino antisense oligomers: design, preparation and properties. Antisense Nucleic Acid Drug Dev., 7, 187–195. [DOI] [PubMed] [Google Scholar]
- 14.Hudziak R.M., Barofsky,E., Barofsky,D.F., Weller,D.L., Huang,S.B. and Weller,D.D. (1996) Resistance of morpholino phosphorodiamidate oligomers to enzymatic degradation. Antisense Nucleic Acid Drug Dev., 6, 267–272. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh C., Stein,D., Weller,D. and Iversen,P. (2000) Evaluation of antisense mechanisms of action. Methods Enzymol., 313, 135–143. [DOI] [PubMed] [Google Scholar]
- 16.Giles R.V., Spiller,D.G., Clark,R.E. and Tidd,D.M. (1999) Antisense morpholino oligonucleotide analog induces missplicing of C-myc mRNA. Antisense Nucleic Acid Drug Dev., 9, 213–220. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh C. and Iversen,P.L. (2000) Intracellular delivery strategies for antisense phosphorodiamidate morpholino oligomers. Antisense Nucleic Acid Drug Dev., 10, 263–274. [DOI] [PubMed] [Google Scholar]
- 18.Lacroix L., Arimondo,P.B., Takasugi,M., Helene,C. and Mergny,J.L. (2000) Pyrimidine morpholino oligonucleotides form a stable triple helix in the absence of magnesium ions. Biochem. Biophys. Res. Commun., 270, 363–369. [DOI] [PubMed] [Google Scholar]
- 19.Rougee M., Faucon,B., Mergny,J.L., Barcelo,F., Giovannangeli,C., Garestier,T. and Helene,C. (1992) Kinetics and thermodynamics of triple-helix formation: effects of ionic strength and mismatches. Biochemistry, 31, 9269–9278. [DOI] [PubMed] [Google Scholar]
- 20.Maher L.J., Dervan,P.B. and Wold,B.J. (1990) Kinetic analysis of oligodeoxyribonucleotide-directed triple-helix formation on DNA. Biochemistry, 29, 8820–8826. [DOI] [PubMed] [Google Scholar]
- 21.Singleton S.F. and Dervan,P.B. (1993) Equilibrium association constants for oligonucleotide-directed triple helix formation at single DNA sites: linkage to cation valence and concentration. Biochemistry, 32, 13171–13179. [DOI] [PubMed] [Google Scholar]
- 22.Blume S.W., Lebowitz,J., Zacharias,W., Guarcello,V., Mayfield,C.A., Ebbinghaus,S.W., Bates,P., Jones,D.E.,Jr, Trent,J., Vigneswaran,N. et al. (1999) The integral divalent cation within the intermolecular purine*purine.pyrimidine structure: a variable determinant of the potential for and characteristics of the triple helical association. Nucleic Acids Res., 27, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. (1990) Transport across cell membranes. In Darnell,J., Lodish,H. and Baltimore,D. (eds), Molecular Cell Biology. Scientific American Books, New York, NY, pp. 531–578.
- 24.Suda T., Mishima,Y., Asakura,H. and Kominami,R. (1995) Formation of a parallel-stranded DNA homoduplex by d(GGA) repeat oligonucleotides. Nucleic Acids Res., 23, 3771–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivas W.M. and Maher,L.J. (1995) Overcoming potassium-mediated triplex inhibition. Nucleic Acids Res., 23, 1936–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svinarchuk F., Cherny,D., Debin,A., Delain,E. and Malvy,C. (1996) A new approach to overcome potassium-mediated inhibition of triplex formation. Nucleic Acids Res., 24, 3858–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faruqi A.F., Krawczyk,S.H., Matteucci,M.D. and Glazer,P.M. (1997) Potassium-resistant triple helix formation and improved intracellular gene targeting by oligodeoxyribonucleotides containing 7-deazaxanthine. Nucleic Acids Res., 25, 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blume S.W., Guarcello,V., Zacharias,W. and Miller,D.M. (1997) Divalent transition metal cations counteract potassium-induced quadruplex assembly of oligo(dG) sequences. Nucleic Acids Res., 25, 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebbinghaus S.W., Gee,J.E., Rodu,B., Mayfield,C.A., Sanders,G. and Miller,D.M. (1993) Triplex formation inhibits HER-2/neu transcription in vitro. J. Clin. Invest., 92, 2433–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noonberg S.B., Scott,G.K., Hunt,C.A., Hogan,M.E. and Benz,C.C. (1994) Inhibition of transcription factor binding to the HER2 promoter by triplex-forming oligodeoxyribonucleotides. Gene, 149, 123–126. [DOI] [PubMed] [Google Scholar]
- 31.Milligan J.F., Krawczyk,S.H., Wadwani,S. and Matteucci,M.D. (1993) An anti-parallel triple helix motif with oligodeoxynucleotides containing 2′-deoxyguanosine and 7-deaza-2′-deoxyxanthosine. Nucleic Acids Res., 21, 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arimondo P.B., Barcelo,F., Sun,J.S., Maurizot,J.C., Garestier,T. and Helene,C. (1998) Triple helix formation by (G,A)-containing oligonucleotides: asymmetric sequence effect. Biochemistry, 37, 16627–16635. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt M.W., Baldridge,K.K., Boatz,J.A., Elbert,S.T., Gordon,M.S., Jensen,J.H., Koseki,S., Matsunaga,N., Nguyen,K.A., Su,S.J. et al. (1993) General atomic and molecular-electronic structure system. J. Am. Chem. Soc., 14, 1347–1363. [Google Scholar]
- 34.Raghunathan G., Miles,H.T. and Sasisekharan,V. (1995) Symmetry and structure of RNA and DNA triple helices. Biopolymers, 36, 333–343. [DOI] [PubMed] [Google Scholar]
- 35.Raghunathan G., Miles,H.T. and Sasisekharan,V. (1993) Symmetry and molecular structure of a DNA triple helix: d(T)n.d(A)n.d(T)n. Biochemistry, 32, 455–462. [DOI] [PubMed] [Google Scholar]
- 36.Cornell W.D., Cieplak,P., Bayly,C.I., Gould,I.R., Merz,K.M., Ferguson,D.M., Spellmeyer,D.C., Fox,F., Caldwell,J.W. and Kollman,P.A. (1995) A second generation force field for the simulation of proteins, nucleic acids and organic molecules. J. Am. Chem. Soc., 117, 5197. [Google Scholar]
- 37.Torigoe H., Shimizume,R., Sarai,A. and Shindo,H. (1999) Triplex formation of chemically modified homopyrimidine oligonucleotides: thermodynamic and kinetic studies. Biochemistry, 38, 14653–14659. [DOI] [PubMed] [Google Scholar]
- 38.Meunier L., Monsigny,M. and Roche,A.C. (2001) Propynylated phosphodiester oligonucleotides inhibit ICAM-1 expression in A549 cells on electroporation. Antisense Nucleic Acid Drug Dev., 11, 117–123. [DOI] [PubMed] [Google Scholar]
- 39.Lin S.B., Kao,C.F., Lee,S.C. and Kan,L.S. (1994) DNA triplex formed by d-A-(G-A)7-G and d-mC-(T-mC)7-T in aqueous solution at neutral pH. Anticancer Drug Des., 9, 1–8. [PubMed] [Google Scholar]
- 40.Holland J.A. and Hoffman,D.W. (1996) Structural features and stability of an RNA triple helix in solution. Nucleic Acids Res., 24, 2841–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S. and Kool,E.T. (1995) Relative stabilities of triple helices composed of combinations of DNA, RNA and 2′-O-methyl-RNA backbones: chimeric circular oligonucleotides as probes. Nucleic Acids Res., 23, 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morvan F., Imbach,J.L. and Rayner,B. (1997) Comparative stability of eight different triple helices formed by differently modified DNA or RNA pyrimidine strands and a DNA hairpin. Antisense Nucleic Acid Drug Dev., 7, 327–334. [DOI] [PubMed] [Google Scholar]
- 43.Gryaznov S.M. (1999) Oligonucleotide N3′→P5′ phosphoramidates as potential therapeutic agents. Biochim. Biophys. Acta, 1489, 131–140. [DOI] [PubMed] [Google Scholar]