Abstract
Escherichia coli strains expressing Dr fimbriae are able to enter epithelial cells by interacting with a complement-regulatory protein, decay-accelerating factor. This model of bacterial internalization, with a well-characterized bacterial ligand and host receptor, provides a unique opportunity to investigate the early stages of invasion. We used immunofluorescence staining techniques to examine the distribution of receptor and cytoskeletal proteins in HeLa cells infected with E. coli recombinant strains that expressed Dr family of adhesins: Dr, Dr-II, F1845, AFA-I, and AFA-III. A major rearrangement of decay-accelerating factor was found at the adherence sites of recombinant strains expressing Dr, Dr-II, and F1845 adhesins. The changes in the distribution of receptor were significantly smaller on HeLa cells infected with E. coli bearing AFA-I or AFA-III afimbrial adhesins. Receptor aggregation was associated with the redistribution of cytoskeleton-associated proteins such as actin, α-actinin, ezrin, and occasionally tropomyosin. Purified Dr fimbriae coated on polystyrene beads were capable of triggering clustering of receptor and accumulating actin at the adhesion sites of beads to HeLa cells. Using scanning and transmission electron microscopic techniques, we have shown that beads coated with Dr fimbriae, as opposed to beads coated with bovine serum albumin, were enwrapped by cellular microvilli and ultimately internalized into HeLa cells. This indicates that interaction of Dr fimbriae with decay-accelerating factor is associated with redistribution of receptor and is sufficient to promote bacterial internalization.
Escherichia coli bearing Dr fimbriae or the related adhesins afimbrial adhesin I (AFA-I), afimbrial adhesin III (AFA-III), or F1845 adhesin is associated with urinary tract infections and diarrhea (5, 18). Despite similar genetic organization, the phenotypic expression among the Dr family of adhesins is associated with either fimbrial or afimbrial morphology. These morphological differences are attributed to the amino acid sequence of the major structural subunit. All members of the family display a similar pattern of binding to the natural receptor, decay-accelerating factor (DAF; CD55) (17). DAF, a complement regulatory protein expressed on most mammalian cells, consists of four 60-amino-acid short consensus repeat (SCR) domains and a serine/threonine-rich region followed by a carboxyl-terminal domain that allows the association with glycophosphatidylinositol anchor (GPI) (14). DAF protects cells from autologous complement-mediated damage by preventing the formation of C3 convertases. Recently, DAF has been recognized as a cell attachment receptor for coxsackievirus A21 and a number of hemagglutinating enteroviruses (1, 11, 21). One characteristic feature of GPI-anchored proteins is their lateral membrane mobility, which facilitates coupling to signaling molecules (20). Binding of the antibody to GPI-anchored protein can lead to cell activation. This effect is enhanced by anti-immunoglobulin antibodies, which promote clustering of cell surface immune complexes. Studies in which DAF is used as a model GPI-anchored protein demonstrated that DAF redistributes to a pole of T lymphocytes after incubation with anti-DAF monoclonal antibodies and anti-mouse immunoglobulin G (10). The redistribution (capping) of DAF was associated with cytoskeleton reorganization. GPI-anchored molecules associate with protein tyrosine kinases, which are important regulators of signal transduction (23). A DAF complex with the two src family protein tyrosine kinases p56lck and p59fyn was found in the murine thymoma EL-4 cell line transfected with DAF (22). The association between DAF and protein tyrosine kinases has also been found in the HeLa epithelial cell line (22). These studies pointed out a possible signaling pathway after DAF was cross-linked.
We have recently shown that the expression of E. coli Dr fimbrial operon allows bacteria to invade the epithelial cells (7). The Dr-positive clinical strain E. coli IH11128 and recombinant strain BN406 were able to enter cultured HeLa cells; the DraE-negative mutant was not. Binding of Dr-positive E. coli is a result of the interaction between bacterial adhesin and the short consensus repeat 3 (SCR3) domain of DAF. The monoclonal anti-SCR3 antibody is a potent inhibitor of receptor binding and subsequent invasion mediated by Dr fimbriae (17). Immunoelectron microscopy studies revealed morphologic interactions between DAF expressed on HeLa cells and bacterial ligand at the initial stages of internalization (7). The cellular cytoskeleton was involved in the process of internalization, since bacterial entry was prevented entirely by the microtubule inhibitor nocodazole and less efficiently by microfilament inhibitor cytochalasin D. In contrast to classic invasive pathogens like Salmonella or Shigella, with unknown receptor specificity, the advantage of our model of bacterial internalization relies on the fact that both components of the entry process, host receptor and bacterial ligand, are well characterized. This provides a unique opportunity to investigate how early bacterium-host cell interaction affects the distribution of receptor, cytoskeleton proteins, and intracellular signaling.
We hypothesized that binding of bacterial ligand might cause cross-linking of DAF. Following the clustering of DAF, cytoskeleton proteins rearrange to provide motor forces for bacterial entry. In this study, we used immunofluorescence (IF) techniques to compare the receptor and cytoskeletal component distribution in the HeLa epithelial cell line after interaction with Dr fimbriae and other members of Dr adhesin family. Electron microscopy was used to provide evidence that interaction of epithelial cells with purified Dr fimbriae promotes bacterial internalization.
MATERIALS AND METHODS
Recombinant strains.
The comparative studies of the interaction of E. coli recombinant strains bearing adhesins of the Dr family with epithelial cells may hypothetically be affected by the expression of adhesin operons in different vectors and in various host strains. The HindIII-HindIII 11.4-kb DNA fragment containing the Dr operon was originally expressed in the pACYC184 vector and carried in laboratory strain EC901 (16). The BamHI-HindIII 7.5-kb DNA fragment encoding the F1845 adhesin was expressed in the pUC18 vector and carried in E. coli LE392 (2). The 8.7-kb XhoI-HindIII fragment coding for Dr-II adhesin was expressed in pBluescript SK-II in E. coli DH5α (19). The EcoRI 6.7- and 8-kb fragments encoding AFA-I and AFA-III adhesins were originally expressed in pBR322 in two different E. coli host strains, HB101 and MC1061, respectively (12, 13). The DNA fragments containing operons of the Dr family of adhesins were mobilized from original vectors, ligated in the medium- to low-copy-number vector pBR322, and transformed to laboratory host strain E. coli DH5α. All procedures were performed by standard molecular biology methods (15).
We grew E. coli laboratory strain DH5α transformed with vector pBR322 and new constructs expressing Dr, Dr-II, F1845, AFA-I, and AFA-III adhesins on Luria (L) agar plates containing ampicillin (100 μg/ml). We also included the invasive strain Shigella flexneri SA100, which does not exploit DAF, as a receptor to evaluate DAF distribution in infected HeLa cells. We used a suspension of each strain made in phosphate-buffered saline (PBS) from overnight cultures and adjusted to an optical density at 600 nm (OD600) of 1.9 at to infect HeLa cell monolayers.
MAC assay.
The minimal agglutinating concentration (MAC) assay was performed with and without inhibitor substances, methyl-α-d-mannoside (1.1 mM) and chloramphenicol (5 mM) (Sigma Chemical Co., St. Louis, Mo.), to assess the amount of adhesin expressed by recombinant strains. The solution of inhibitors was prepared in PBS. The bacterial suspensions in PBS (OD600, 1.9) were prepared from overnight cultures grown on L agar with ampicillin (100 μg/ml). Twofold dilutions were made in PBS in the range from 1:2 to 1:4,096. To determine MAC, 20 μl of bacterial suspension was mixed on an agglutination plate with 30 μl of 3% suspension of human erythrocytes in PBS. The mixture was incubated for 5 min in a wet chamber with gentle rotation. The plate was examined for the highest dilutions showing hemagglutination.
Cell line.
The HeLa human cervical cell line (ATCC CCL2) was maintained in minimum essential medium Cellgro Eagle (Mediatech, Inc., Herndon, Va.) containing Earle’s salts and l-glutamine and supplemented with 10% heat-inactivated fetal calf serum.
Antibodies.
Bodipy FL Phallacidin was purchased from Molecular Probes, Inc. (Eugene, Oreg.), and used as recommended by the supplier. The mouse monoclonal anti-α-tubulin (clone DM 1A), anti-β-tubulin (clone TUB 2.1), anti-α-actinin (clone BM75.2), antitalin (clone 8d4), antivinculin (clone hVIN-1), and antitropomyosin (clone TM 311) antibodies were purchased from Sigma Chemical Co., and monoclonal antiezrin antibody (clone 18) was obtained from Transduction Laboratories (Lexington, Ky.). The monoclonal antibodies to the SCR-1 domain of DAF (clone 2D2-8) were kindly provided by John Moulds (Gamma Biologicals Inc., Houston, Tex.). Secondary goat anti-mouse antibodies and goat anti-rabbit antibodies conjugated with Oregon Green or Texas Red were purchased from Molecular Probes, Inc., and used at a dilution of 1:100 (20 μg/ml).
Purification of Dr fimbriae.
The recombinant strain bearing Dr fimbriae was grown overnight on L-agar plates with ampicillin (100 μg/ml). The bacteria were suspended in PBS. The suspension was vortexed for 5 min and centrifuged for 10 min at 10,000 × g in SS-34 centrifuge tubes. The supernatants were filtered through a 0.22-μm-pore-size membrane. Fimbrial protein was purified from the filtrate by ammonium sulfate precipitation and size exclusion chromatography. The column was connected to the Econo low-pressure liquid chromatography system (Bio-Rad, Hercules, Calif.). The eluted fimbriae were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (15% acrylamide).
Coating of polystyrene beads with purified Dr fimbriae.
Plain beads (Polybead polystyrene beads composed of 2.5% solid polystyrene; diameter, 1.072 μm; Polyscience, Inc., Warrington, Pa.) were diluted 1:10, washed three times with centrifugation, and resuspended in 0.1 M borate buffer (pH 8.5). A thawed fimbrial preparation containing 75 μg of purified fimbrial protein per ml was diluted 1:10 in the suspension of polystyrene beads. The control beads were mixed with a solution of albumin (bovine serum albumin [BSA], fraction V; Sigma Chemical Co.) to the same final concentration of protein as in the fimbrial sample. The suspensions were mixed end to end overnight at room temperature. Next, the suspensions were centrifuged for 5 min, resuspended in PBS containing 1% BSA and 0.1% glycerol (storage buffer), and kept at 4°C until assayed. The biological activity of beads coated with purified Dr fimbrial protein was confirmed by mannose-resistant, chloramphenicol-inhibitable agglutination of human erythrocytes. The Dr fimbria-coated beads also agglutinated with rabbit polyclonal anti-Dr serum.
Incubation of bacterial or polystyrene bead suspensions with HeLa cells monolayer.
Bacterial suspensions in PBS (OD600, 1.9) were diluted 1:100 in prewarmed minimal essential medium, added to HeLa cells monolayers grown on coverslips (15 mm in diameter) in 12-well tissue culture plates, and incubated for 3 h at 37°C under 5% CO2. The coverslips were washed three times with PBS, fixed for 10 min with 3% paraformaldehyde in PBS, washed with PBS, and permeabilized for 2 min with 0.1% Triton X-100. Permeabilization was omitted with monolayers subsequently stained for DAF. Similarly, the polystyrene beads coated with purified Dr fimbrial protein or BSA-coated control beads were diluted 1:10 in culture medium and incubated with the monolayer for the desired time. After being washed, the monolayers were processed in the same way as those incubated with bacterial suspensions.
IF staining.
The staining for polymerized actin was performed with Bodipy FL Phallacidin as recommended by the manufacturer. To visualize α-actinin, ezrin, talin, tropomyosin, tubulin, and vinculin, the paraformaldehyde-fixed monolayers were incubated for 20 min with 10% normal goat serum in PBS (blocking solution). They were subsequently incubated for 30 min with appropriate primary monoclonal antibodies diluted 1:50 in a solution of 1% BSA and 2% normal goat serum in PBS. In control staining, the primary antibody was replaced with preimmune mouse serum. After two washings with PBS, the cells were incubated for 30 min with goat anti-mouse secondary antibodies conjugated with Texas Red. Finally, the coverslips were stained with SYTO 9 (Molecular Probes, Inc.) to visualize cellular structures and individual bacterial cells. In some experiments, the double IF staining was performed to colocalize Dr fimbrial antigen. Slides were examined under an Eclipse 600 microscope (Nikon Inc., Melville, N.Y.) equipped with an epifluorescence attachment, Texas Red filter, double-pass Texas Red-fluorescein isothiocyanate filter, and U-III camera system.
Electron microscopy. (i) SEM.
Monolayers on coverslips for scanning electron microscopy (SEM) were fixed with a mixture of 1.25% formaldehyde, 2.5% glutaraldehyde, 0.03% trinitrophenol, and 0.03% CaCl2 in 0.05 M cacodylate buffer (pH 7.3) for at least 1 h at room temperature, postfixed in 1% OsO4 in 0.1 M cacodylate buffer, dehydrated in ethanol, treated with hexamethyldisalazane, and air dried (8). Next, the coverslips were mounted onto the stubs and sputter coated with palladium-gold alloy in a Bal-Tec SCD-004 Sputter Coater (Technotrade International, Manchester, N.H.) at 15 mA for 2 min. Samples were examined in a 525M scanning electron microscope (Philips Electron Optics, Eindhoven, The Netherlands) at 15 kV and at magnifications from ×3,000 to ×20,000.
(ii) TEM.
Monolayers for transmission electron microscopy (TEM) were fixed as for SEM, but between the fixation steps they were washed and scraped off the coverslips or plastic support and further processed as a pellet. They were stained en bloc with 1% uranyl acetate in 0.1 M maleate buffer (pH 5.2), dehydrated in ethanol, and embedded into Poly/Bed 812 resin (Polysciences, Inc). Ultrathin sections were cut on a Sorvall MT-6000 ultramicrotome (RMC, Tucson, Ariz.), stained with aqueous uranyl acetate and lead citrate, and examined in a Philips 201 transmission electron microscope at 60 kV.
RESULTS
The binding of recombinant E. coli expressing Dr adhesins triggers different patterns of distribution of DAF at the site of bacterial attachment.
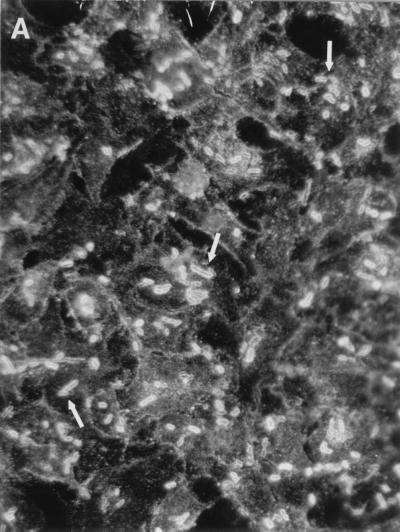
The interaction of an invasive bacterial pathogen like Salmonella typhimurium with mammalian cells may lead to significant clustering of the host cell surface proteins (6). We have used an IF double-staining technique to evaluate the changes in the DAF staining pattern at different time points after infection with E. coli recombinant strains bearing Dr fimbriae and other related adhesins. The double staining allowed colocalizing of the targeted cellular proteins and bacterial cells interacting with the monolayer. Significant differences were observed between the members of the Dr family. The noninfected monolayers of HeLa cells displayed an even distribution of granule-like staining with monoclonal antibodies raised to different domains of DAF (results not shown). The infection of HeLa cells with recombinant strains expressing Dr, Dr-II, and F1845 adhesins was associated with a rapid (15-min infection) and extensive redistribution of the receptor. The receptor formed tight profiles outlining the sites of bacterial interaction with the monolayer (Fig. 1A). HeLa cells infected with recombinant E. coli strains bearing AFA-I or AFA-III adhesins displayed shadow-like staining patterns corresponding to bacteria outlined by minute amounts of accumulated DAF (Fig. 1B, Table 1). The differences between the constructs in the agglutination titer of human erythrocytes did not affect the patterns of redistribution of DAF. For example, E. coli bearing F1845 fimbriae with the highest MAC (1:2,048) and E. coli expressing Dr hemagglutinin with a low MAC (1:64) do not differ in the pattern and intensity of DAF clustering. The DAF staining pattern on a monolayer infected with control invasive strain S. flexneri SA 100 (Fig. 1C) or with E. coli laboratory strain DH5α carrying the pBR322 vector remained unchanged and did not differ from that found in noninfected cells.
FIG. 1.
(A) Intense accumulation of DAF (arrows) outlining individual bacterial cells, characteristic of HeLa cells infected with recombinant E. coli strains expressing Dr, Dr-II, or F1845 adhesins. (B) Lack of appreciable redistribution of DAF and shadow-like staining pattern (arrows) on HeLa cells infected with recombinant strains expressing AFA-I or AFA-III adhesins. (C) HeLa cells infected with S. flexneri SA 100 do not exhibit any changes in the staining pattern of DAF.
TABLE 1.
Aggregation of DAF and rearrangements of cytoskeletal components associated with infection of HeLa cells with recombinant E. coli strains expressing Dr family adhesins
| Plasmid | HA titer | Adhesin phenotype | Redistribution of:
|
|||||
|---|---|---|---|---|---|---|---|---|
| DAF | Actin | α-Actinin | Ezrin | Tropomyosin | Vinculin, talin, and β-tubulinb | |||
| pSR406a | 1:64 | Dr | +++ | + | + | + | + | − |
| pSR411a | 1:256 | Dr-II | +++ | + | + | + | + | − |
| pSR1845a | 1:2,048 | F1845 | +++ | + | + | + | + | − |
| pIL22 | 1:128 | AFA-I | ± | + | + | + | + | − |
| pILL115 | 1:64 | AFA-III | ± | − | − | − | − | − |
| pBR322 | 0 | None | − | − | − | − | − | − |
Denotes new constructs; DNA fragments containing the operons of Dr family adhesins are expressed in plasmid pBR322 and E. coli laboratory strain DH5α.
Stained separately.
E. coli bearing adhesins of Dr family trigger different patterns of accumulation of cytoskeleton proteins in infected HeLa cells.
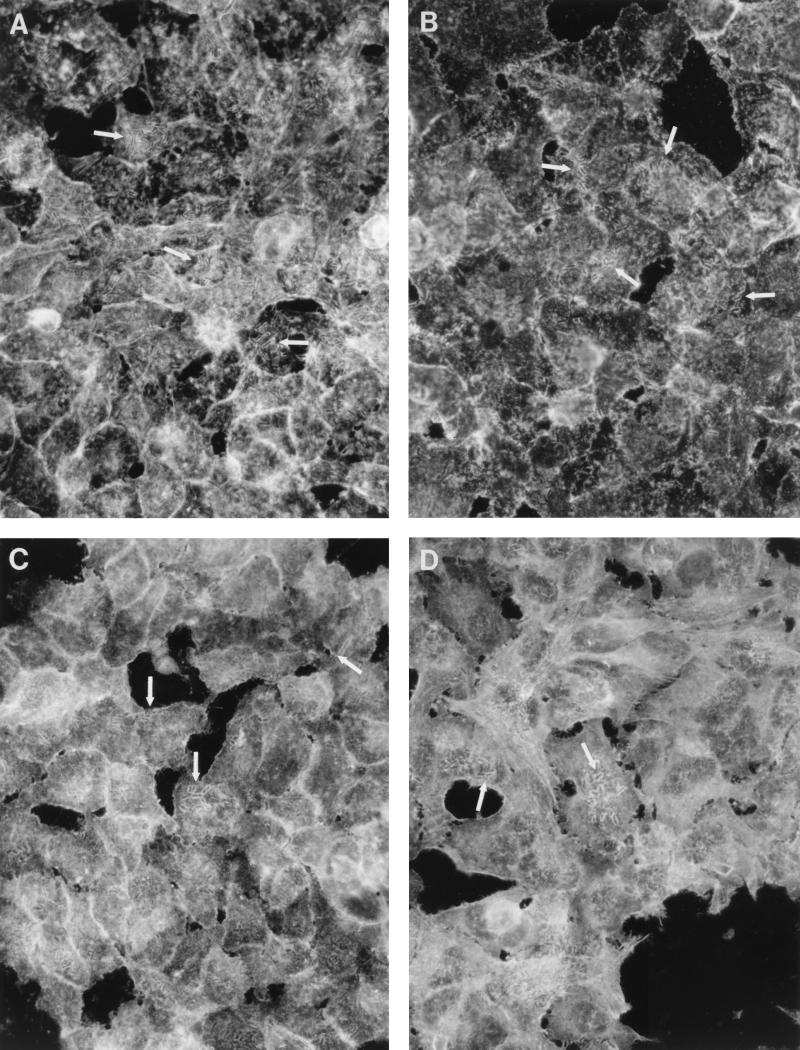
We have tested whether the infection of epithelial cells with recombinant E. coli strains carrying the Dr family of adhesins is associated with the redistribution of actin and actin-associated molecules. Infection of HeLa cells with recombinants carrying Dr, Dr-II, F1845, and AFA-I resulted in an accumulation of polymerized actin that completely or partially outlined individual adherent bacterial cells (Fig. 2A, Table 1). The first signs of actin redistribution were visible 10 to 15 min after actin was added to the bacteria. There was no evidence of actin redistribution in HeLa cells infected with E. coli expressing AFA-III adhesin (Table 1). A similar staining pattern was found with antibodies to α-actinin (Fig. 2B) and ezrin (Fig. 2C). Both molecules accumulated at the sites of bacterial binding and formed tight profiles outlining individual bacterial cells. Tropomyosin accumulated at the sites of bacterial attachment on cells infected with E. coli bearing Dr fimbriae and occasionally on HeLa cells incubated with Dr-II, AFA-I, and F1845 adhesins (Fig. 2D, Table 1). Cytoskeleton-associated proteins did not accumulate in monolayers infected with E. coli carrying the pBR322 vector. In addition, the redistribution was not seen on slides where primary antibodies were replaced with preimmune mouse serum (results not shown). Focal contact proteins, such as talin and vinculin, were not affected by adherence of recombinant E. coli strains bearing Dr family of adhesins (Table 1).
FIG. 2.
Rearrangement of the cytoskeleton component actin (A), ezrin (B), α-actinin (C), and tropomyosin (D) outlining individual bacterial cells (arrows) in HeLa cells infected with recombinant E. coli BN406 expressing Dr fimbriae.
The microtubules are involved in the internalization of Dr-positive E. coli but do not undergo redistribution.
We have found that internalization of Dr-positive E. coli is almost completely prevented by the microtubule inhibitor nocodazole (7). To estimate whether interaction of E. coli expressing Dr adhesins is associated with the rearrangements of microtubules, HeLa cells were incubated with bacterial suspensions for 3 h. Infection was interrupted at different time points, and immunostaining was performed to visualize microtubules or microtubules plus Dr antigen. Staining with monoclonal antibodies to either α- or β-tubulin did not reveal any reorganization of the microtubules during infection with E. coli expressing Dr and related adhesins (results not shown).
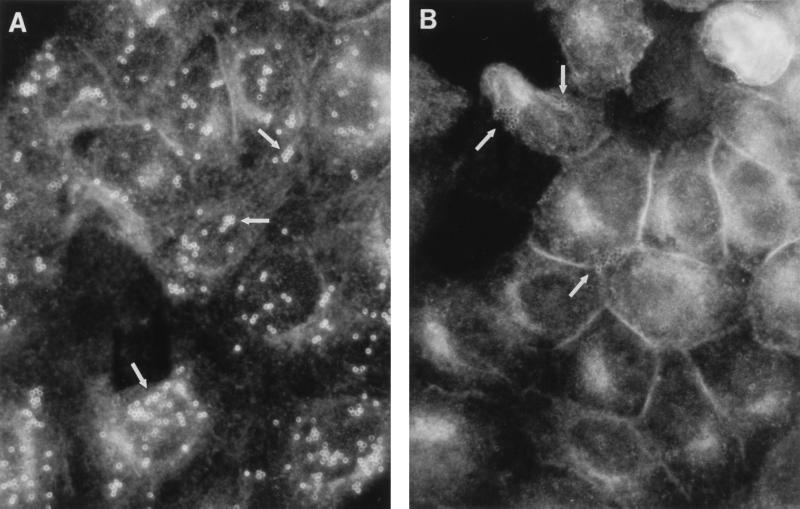
DAF and actin aggregate on HeLa cells interacting with polystyrene beads coated with purified Dr fimbriae.
The plain polystyrene beads coated with purified Dr fimbriae displayed intense binding to HeLa cells. The control beads coated with the equivalent amount of BSA exhibited insignificant attachment. Intense circle-like staining of DAF (Fig. 3A) and less intense staining of actin (results not shown) outlining the shape of individual beads were observed on HeLa cells incubated with Dr fimbria-coated particles. The DAF clusters and aggregates of actin were not seen on HeLa cells incubated with BSA-coated beads (Figure 3B).
FIG. 3.
Ring-like accumulation of DAF staining at the sites of attachment of polystyrene beads coated with purified Dr fimbriae (arrows) (A) and lack of DAF redistribution on HeLa cells interacting with BSA-coated beads (arrows) (B).
Polystyrene beads coated with purified Dr fimbrial protein internalize into HeLa cells.
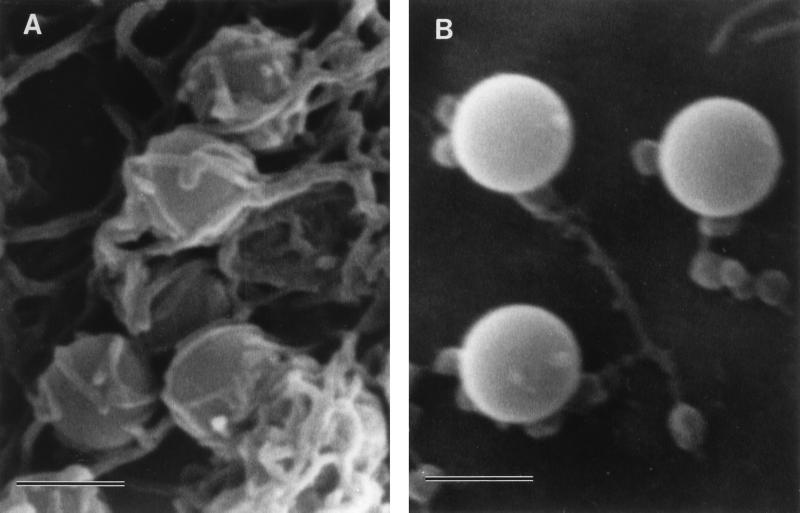
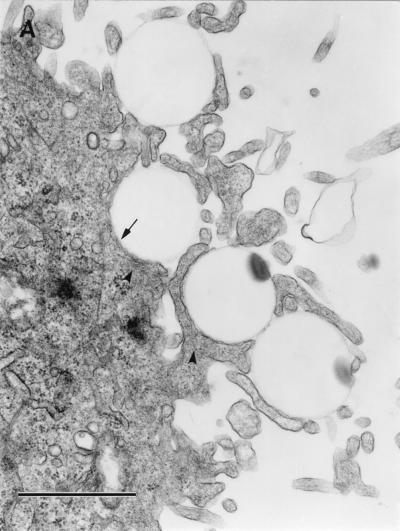
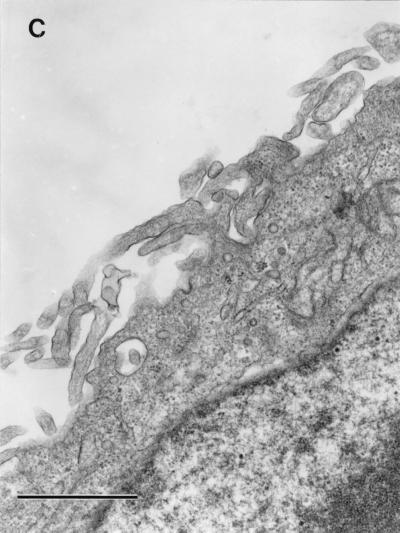
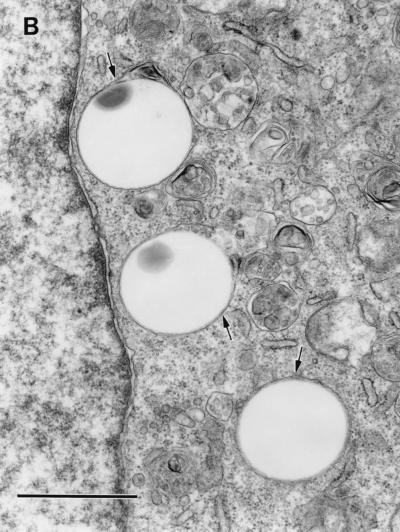
We hypothesized that interaction of purified Dr fimbriae protein with DAF initiates the reorganization of the cellular cytoskeleton and provides motor forces for bacterial entry. To estimate whether purified Dr fimbriae are able to promote the internalization into epithelial cells, SEM and TEM were performed on HeLa cells incubated with polystyrene beads coated with purified fimbriae and control, BSA-coated beads. After 1 h of incubation, beads coated with Dr fimbrial protein exhibited significant binding and were totally wrapped by cellular microvilli, as seen by SEM (Fig. 4A). Few adherent beads coated with BSA were found on the surface of HeLa cells after the same time of incubation (Fig. 4B). TEM confirmed the internalization of the beads with involvement of microvilli (Fig. 5A) and the intracellular location of Dr fimbria-coated beads within tight membrane-bound vacuoles (Fig. 5B). Concentrations of microfilaments, representing actin filaments, could be seen at the sites of internalization of the beads (Fig. 5A).
FIG. 4.
SEM. (A) Polystyrene beads coated with purified Dr fimbriae are tightly wrapped by HeLa cell microvilli after 1 h of incubation. (B) Few beads treated only with BSA are located at the surface of a HeLa cell, and they do not interact with its microvilli. Bars, 1 μm.
FIG. 5.
TEM. (A) Initial steps of internalization. Four beads coated with purified Dr fimbriae are wrapped with HeLa cell microvilli (arrow). Microfilament accumulation can be seen at the sites of internalization (arrowheads). (B) Three internalized Dr fimbria-coated beads are located in the cytoplasm within tight membrane-bound vacuoles (arrows). (C) Control, BSA-coated polystyrene were not found in ultrathin sections of HeLa cells. The microvilli on most of the cells were less strongly expressed and located closer to the cell surface. Bars, 1 μm.
Despite extensive examination by TEM, there was no evidence of adherence or internalization of control BSA-coated beads (Fig. 5C). Microvilli on HeLa cells incubated with control beads seemed to be less strongly expressed and were located closer to the cell surface (Fig. 4B and 5C) compared to those in cells interacting with adherent Dr-coated beads (Fig. 4A and 5A).
DISCUSSION
We have compared the distribution of receptors and the accumulation of cytoskeletal components that occur when recombinant E. coli strains bearing the Dr family of adhesins bind to and enter HeLa epithelial cells. Binding of recombinant E. coli bearing Dr, Dr-II, or F1845 adhesin was associated with intense accumulation of DAF, which outlined individual adherent bacterial cells. Weak or occasional redistribution of receptor occurred on monolayers infected with E. coli bearing AFA-I or AFA-III adhesins. Our earlier experiments revealed that DAF is a ligand for the Dr family of adhesins (17). The SCR3 domain of DAF appears to be crucial since deletion of the SCR3 domain abolished the binding of all Dr adhesins. Deletion of SCR2 resulted in lack of binding of all adhesins except AFA-I, which retained full binding capacity to DAFΔSCR2. The deletion in SCR4 was associated with a 50% reduction of binding of AFA-I and AFA-III. It is conceivable that the different patterns of DAF clustering may be attributed to the various DAF epitopes that the recombinant strains exploit for binding. Alternatively, the distinct morphology of the Dr family of adhesins may affect the pattern and intensity of DAF clustering. This possibility is supported by the fact that intense DAF clustering was associated with fimbrial or fimbria-like structures. Weak DAF accumulation occurred in HeLa cells infected with recombinant E. coli expressing afimbrial AFA-I and AFA-III adhesins.
The aggregation of DAF molecules in response to contact with Dr fimbriae resembles the capping of DAF on T lymphocytes after cross-linking with anti-DAF monoclonal antibodies and anti-murine IgG (10). The redistribution of DAF to a pole of the lymphocytes was associated with reorganization of cytoskeletal elements. We found that both polymerized actin and α-actinin colocalized at the sites of attachment of E. coli bearing Dr, Dr-II, F1845, and AFA-I adhesins and formed tight profiles outlining individual bacterial cells. The aggregates were weakly visible or not visible on the monolayers infected with E. coli expressing AFA-III adhesin. The actin and α-actinin redistribution appears to be different from that associated with the invasion of enteropathogenic E. coli (EPEC) or S. typhimurium into HeLa cells (3, 4). HeLa cells infected with EPEC display few aggregates located at the center of the groups of adherent bacteria within the first 2 h of infection. The rearrangement of actin caused by infection with recombinant E. coli expressing Dr or F1845 adhesins occurred during the first 10 to 15 min after addition of bacteria. The number of aggregates that outlined individual bacterial cells was proportional to the number of bacterial cells that encountered the surface of epithelial cells and increased with time of infection. With S. typhimurium, the redistribution of actin occurs in a manner similar to that for E. coli expressing Dr and F1845 adhesins soon after the addition of bacteria; however, actin aggregates are not detectable 1 h after the addition of bacteria (4).
Ezrin, a molecule involved in linking actin filaments to transmembrane receptors, was also found to aggregate at the sites of attachment of recombinants bearing Dr, Dr-II, F1845, and AFA-I but not AFA-III (25). We found a similar but occasional and less intense pattern of staining with antibodies to tropomyosin, a molecule suggested to be involved in actin-myosin-mediated movement (24). The adherence of Dr-positive E. coli and recombinant strains expressing related adhesins does not appear to affect the distribution of focal contact proteins, such as vinculin and talin.
The internalization of Dr-positive E. coli is significantly inhibited in the presence of the microtubule inhibitor nocodazole or colchicine. Therefore, we expected that infection of HeLa cells would result in rearranged microtubule organization. It was interesting that the staining with monoclonal antibodies to either α-tubulin or β-tubulin did not reveal a redistribution of microtubules at the areas of adherent bacteria. This finding is similar to that observed in EPEC strains (3). Internalization of EPEC strains is blocked by colchicine; however, internalization is not associated with visible changes in microtubule structure. In contrast, the infection of epithelial cells with S. typhimurium is unaffected by nocodazole and is known to be associated with the rearrangement of microtubules (3).
The results of EM studies on invasion of E. coli bearing Dr fimbriae led us to hypothesize that binding of bacterial ligand to the surface receptor is sufficient to trigger bacterial entry into epithelial cells (7). To provide experimental evidence supporting this hypothesis, polystyrene beads coated with purified Dr fimbrial protein were incubated with HeLa cells monolayers and examined by EM techniques. The purification of the Dr major structural subunit always resulted in a product that migrated as a single band on sodium dodecyl sulfate-polyacrylamide gels with a molecular mass of 15.6 kDa. We found that fimbrial protein-coated beads bound significantly to the microvilli and were entrapped by the microvilli of the epithelial cells; this process ultimately led to their internalization. The accumulation of DAF and actin was visible at the sites of attachment of polystyrene beads coated with Dr fimbriae. Therefore, this interaction appears to closely mimic Dr-positive E. coli-mediated internalization with the associated clustering of receptors. Studies on the invasion of E. coli carrying AFA-III adhesin showed that the bacterium-cell interaction involved two steps in which AfaE-III was required for adhesion and AfaD contributed to internalization (9). No evidence of AFA-III-mediated perturbation of actin polymerization was found (9). In conclusion, the differences in the recognition of DAF epitopes by Dr and related adhesins and/or differences in adhesin morphology may stimulate distinct patterns of cellular response. Further studies to understand how the Dr family of adhesins exploits the domains of DAF to promote their internalization into epithelial cells are in progress.
ACKNOWLEDGMENTS
We are grateful to Bogdan Nowicki and Stella Nowicki for helpful discussions and critical review of the manuscript. We thank John Moulds for sending us monoclonal antibodies to DAF and David W. Niesel for providing the S. flexneri strain. We are grateful to Robert G. McConnell and his staff of Publications, Grant, and Media Support for the Department of Obstetrics and Gynecology for their assistance in the preparation of the manuscript.
This work was supported in part by grant 2RO1DK42029 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
REFERENCES
- 1.Bergelson J M, Chan M, Solomon K R, St. John N F, Lin H, Finberg R W. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc Natl Acad Sci USA. 1994;91:6245–6248. doi: 10.1073/pnas.91.13.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilge S S, Clausen C R, Lau W, Moseley S L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989;171:4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finlay B B, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 5.Garcia M-I, Le Bouguénec C. Role of adhesion in pathogenicity of human uropathogenic and diarrhoeogenic Escherichia coli. Bull Inst Pasteur. 1996;94:201–236. [Google Scholar]
- 6.Garcia-del Portillo F, Pucciarelli M G, Jefferies W A, Finlay B B. Salmonella typhimurium induces selective aggregation and internalization of host cell surface proteins during invasion of epithelial cells. J Cell Sci. 1994;107:2005–2020. doi: 10.1242/jcs.107.7.2005. [DOI] [PubMed] [Google Scholar]
- 7.Goluszko P, Popov V, Selvarangan R, Nowicki S, Pham T, Nowicki B J. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the epithelial cell line. J Infect Dis. 1997;176:158–167. doi: 10.1086/514018. [DOI] [PubMed] [Google Scholar]
- 8.Ito S, Rikihisa T. Techniques for electron microscopy of rickettsiae. In: Burgdorfer W, Anacker R L, editors. Rickettsiae and rickettsial diseases. New York, N.Y: Academic Press, Inc.; 1981. pp. 213–227. [Google Scholar]
- 9.Jouve M, Garcia M I, Courcoux P, Labigne A, Gounon P, Le Bouguénec C. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the afa-3 gene cluster are mediated by the AfaE and AfaD proteins, respectively. Infect Immun. 1997;65:4082–4089. doi: 10.1128/iai.65.10.4082-4089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kammer G M, Walter E I, Medof M E. Association of cytoskeletal reorganization with capping of the complement decay-accelerating factor on T lymphocytes. J Immunol. 1988;141:2924–2928. [PubMed] [Google Scholar]
- 11.Karnauchow T M, Tolson D L, Harrison B A, Altman E, Lublin D M, Dimock K. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55) J Virol. 1996;70:5143–5152. doi: 10.1128/jvi.70.8.5143-5152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labigne-Roussel A, Schmidt M A, Walz W, Falkow S. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol. 1985;162:1285–1292. doi: 10.1128/jb.162.3.1285-1292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Bouguénec C, Garcia M I, Quin V, Desperier J-M, Gounon P, Labigne A. Characterization of plasmid-borne afa-3 gene clusters encoding afimbrial adhesins expressed by Escherichia coli strains associated with intestinal or urinary tract infections. Infect Immun. 1993;61:5106–5114. doi: 10.1128/iai.61.12.5106-5114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lublin D M, Atkinson J P. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- 15.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 16.Nowicki B, Svanborg-Edén C, Hull R, Hull S. Molecular cloning of the Escherichia coli O75X adhesin. Infect Immun. 1987;55:3168–3173. doi: 10.1128/iai.55.12.3168-3173.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowicki B, Hart A, Coyne K E, Lublin D M, Nowicki S. Short Consensus Repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli Dr adhesin in a model of cell-cell interaction. J Exp Med. 1993;178:2115–2121. doi: 10.1084/jem.178.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowicki B, Labigne A, Moseley S, Hull R, Hull S, Moulds J. The Dr hemagglutinin, afimbrial adhesins AFA-I and AFA-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with Dr receptor recognition. Infect Immun. 1990;58:279–281. doi: 10.1128/iai.58.1.279-281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pham T Q, Goluszko P, Popov V, Nowicki S, Nowicki B J. Molecular cloning and characterization of Dr-II, a nonfimbrial adhesin-I-like adhesin isolated from gestational pyelonephritis-associated Escherichia coli that binds to decay-accelerating factor. Infect Immun. 1997;65:4309–4318. doi: 10.1128/iai.65.10.4309-4318.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson P J. Signal transduction by GPI-anchored membrane proteins. Cell Biol Int Rep. 1991;15:761–767. doi: 10.1016/0309-1651(91)90031-d. [DOI] [PubMed] [Google Scholar]
- 21.Shafren D R, Dorahy D J, Ingham R A, Burns G F, Barry R D. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J Virol. 1997;71:4736–4743. doi: 10.1128/jvi.71.6.4736-4743.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shenoy-Scaria A M, Kwong J, Fujita T, Olszowy M W, Shaw A S, Lublin D M. Signal transduction through decay-accelerating factor. J Immunol. 1992;149:3535–3541. [PubMed] [Google Scholar]
- 23.Štefanová I, Hoejší V, Ansotegui I J, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- 24.Stossel T P. Contractile proteins in cell structure and function. Annu Rev Med. 1978;29:427–475. doi: 10.1146/annurev.me.29.020178.002235. [DOI] [PubMed] [Google Scholar]
- 25.Tsukita S, Yonemura S, Tsukita S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol. 1997;9:70–75. doi: 10.1016/s0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]