Abstract
Background/Aims
Screening for colorectal cancer (CRC) is important in reducing the morbidity and mortality of CRC. Thus, this study aimed to describe the trends of CRC screening in both organized and opportunistic settings in Korea from 2005 to 2020 according to sociodemographic characteristics.
Methods
This study analyzed the data of adults aged 50 to 74 years from the Korean National Cancer Screening Survey. Trends for CRC screening rates (fecal immunochemical test [FIT] within the last year, double-contrast barium enema within the last 5 years, or colonoscopy within the last 10 years for 2005–2018 and FIT within the last year or colonoscopy within the last 10 years for 2019–2020) were analyzed using Joinpoint regression. The trends were also analyzed according to sociodemographic characteristics, including age, sex, monthly household income, education level, and residential area.
Results
A total of 29,040 participants were included in the analysis. The CRC screening rate significantly increased from 25.0% to 60.1%, with an annual percent change (APC) of 9.2% between 2005 and 2014, followed by a nonsignificant increase to 64.4% between 2014 and 2020 (APC,1.7%). When the participants were stratified according to sociodemographic factors, the participants with higher household income and education levels generally had higher screening rates.
Conclusions
There has been substantial improvement in CRC screening rates in the general Korean population. However, it is necessary to determine why the screening rate has stabilized since 2014 and identify barriers that cause disparities in CRC screening rates among populations with lower socioeconomic status.
Keywords: Colorectal neoplasms, Early detection of cancer, Healthcare disparities, Social class
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related deaths worldwide.1 CRC is also a major public health problem in Korea as the fourth most incident, the third most prevalent cancer, and the third most deadly cancer in Korea in 2018.2 Most colorectal premalignant or malignant lesions are asymptomatic for years, with symptoms developing insidiously, and the prognosis for CRC is strongly related to the stage at diagnosis.3 CRC screening enables the detection of precancerous lesions or CRC in its early stages, and has been shown to reduce late-stage cancer.4-6 and consequently decrease the incidence and mortality associated with CRC by at least 60%.7,8
There are various commonly used and available CRC screening methods, including stool-based tests, such as fecal occult blood test and fecal immunochemical test (FIT), and optical approaches to direct examination of the colon and rectum, such as sigmoidoscopy and colonoscopy.9 The Korean Guideline for CRC Screening, initially developed in 200210 and revised in 2015,11 recommends annual or biennial FIT in asymptomatic adults aged 45 to 80 years (recommendation B; high certainty of moderate net benefit or moderate certainty of moderate to substantial net benefit) and selective colonoscopy based on professional judgment and individual preference (recommendation C; at least moderate certainty of small net befit). In Korea, CRC screening can be offered either through a population-based organized screening program or opportunistic cancer screening. A population-based organized screening program offers a standardized system of care based on a comprehensive guideline including eligible subjects, screening interval, screening modality, quality assurance, and public financing. On the other hand, attendance and services of opportunistic screening depends on individual or a health care professional. The Korean government established the National Cancer Screening Program (NCSP), a population-based organized screening program, and for CRC screening, the NCSP has provided annual FIT for adults aged 50 years or older, and colonoscopy or a double-contrast barium enema test to those with positive results from the FIT since 2004.12 Table 1 summarizes the Korean Guideline for CRC screening and the NCSP protocol for CRC. Opportunistic cancer screening is also available with various screening methods available and all fees paid entirely by the examinees.
Table 1.
The Korean Guideline and the NCSP Protocol for CRC Screening
| Korean Guideline for CRC Screening | Protocol of NCSP for CRC | ||||
|---|---|---|---|---|---|
| 200210 | 201511 | 2004 | 2016 | ||
| Target population | Adults aged ≥50 years | Adults aged 45–80 years | Adults aged ≥50 years | Adults aged ≥50 years | |
| Test | Colonoscopy | FIT | FIT† | FIT† | |
| Sigmoidoscopy & DCBE (not available for colonoscopy only) |
Colonoscopy* | ||||
| Interval | Colonoscopy: 5–10 years | FIT: 1–2 years | 2 Years | 1 Year‡ | |
| Sigmoidoscopy & DCBE: 5 years |
Colonoscopy* | ||||
| Additional study for confirmation |
DCBE or colonoscopy & biopsy |
DCBE or colonoscopy & biopsy |
|||
NCSP, National Cancer Screening Program; CRC, colorectal cancer; FIT, fecal immunochemical test; DCBE, double-contrast barium enema.
*Selectively offered or provided to individual screeners based on professional judgment and screening preferences (recommendation C); †Conducted quantitatively or qualitatively; ‡Performed from biennially rather than annually since 2012.
The main objective of this study was to describe the trends in CRC screening rates in Korea from 2005 to 2020 and investigate whether the trends vary according to demographic and socioeconomic status. We analyzed comprehensive data encompassing information on the screening rates from both opportunistic and population-based organized screening to understand the overall CRC screening rates in Korea. In addition, we considered various demographic and socioeconomic factors that might be associated with the screening rates.
MATERIALS AND METHODS
1. Data source and study population
This study analyzed data from the Korean National Cancer Screening Survey (KNCSS) conducted between 2005 and 2020. Although the KNCSS has been conducted since 2004, the results of the 2004 survey, which were conducted through telephone rather than face-to-face interviews, were excluded in this analysis. KNCSS is a cross-sectional nationwide, population-based survey of cancer-free men aged 40 to 74 years and women aged 20 to 74 years, conducted by the National Cancer Center. The KNCSS uses a stratified, multistage cluster sampling method based on geographical area, sex, and age, and excluded people who have already been diagnosed with cancer at the time of recruitment. The details of the sampling methods are described in a previous study.13 Of the participants in the KNCSS, the present study included men and women aged 50 to 74 years who were eligible for CRC screening according to the NCSP.
2. Variable definition
The experience of CRC screening was determined based on self-reports on whether the participants had been screened for CRC, and when and how the participants were screened. CRC screening rate was defined as the proportion of individuals who had FIT within the last 1 year, double-contrast barium enema within the last 5 years, or colonoscopy within the last 10 years for the survey year 2005–2018 and FIT within the last 1 year or colonoscopy within the last 10 years for the survey year 2019–2020.
We considered the following sociodemographic factors: sex, age, monthly household income, educational level, and residential area. Age was grouped into 50–59 years, 60–69 years, and 70–74 years. Monthly household income was divided into three groups based on tertiles for each year: lower, medium, and higher. Educational level was categorized into three groups depending on the number of years of education: lower (11 years or less), medium (between 12 and 15 years), and higher (16 years or more). Residential areas were categorized as rural and metropolitan.
3. Statistical analysis
A descriptive analysis was performed to evaluate the distribution of the participants’ sociodemographic characteristics. All estimates were weighted to account for the complex sampling design, so that the results could represent the entire Korean population.
Joinpoint regression analysis using raw values of the screening rates was used to fit a series of joined log-linear segments to the trends in the CRC screening rates from 2005 to 2020 and identify the points in which there is a statistically significant change in trend (p-value <0.05) using the best-fit data series.14,15 Joinpoint analysis tested whether a multi-segmented line that allows a maximum of two Joinpoints is a significantly better fit than a straight line. Then, the annual percentage changes (APCs) of each segment were calculated. Joinpoint regression analysis was also conducted according to sex, age group, monthly household income, education level, and residential area to identify differences in patterns of trends between subgroups. The trend was characterized according to the methodology of the National Cancer Institute.16 The trend was characterized as stable if the trend changed by ≤0.5% per year and the APC was not statistically significant. In contrast, the trend was characterized as nonsignificant change if the trend changed by >0.5% per year, and the APC was not statistically significant. The trend was characterized as rising or falling when the trend was statistically significant. Descriptive analyses were performed using the SAS software version 9.4 (SAS Institute, Cary, NC, USA), and Joinpoint regression analysis was performed using the Joinpoint Regression Program, version 4.7.0.0 (Statistical Research and Applications Branch; National Cancer Institute, Rockville, MD, USA).
4. Ethical statement
This study was approved by the Institutional Review Board of the National Cancer Center in Korea (IRB number: NCC2019-0233). All participants provided written informed consent.
RESULTS
The sample consisted of 29,040 participants aged 50 to 74 years who participated in the KNCSS from 2005 to 2020. In 2005 and 2020, 968 and 2,467 adults aged 50 to 74 years participated in the KNCSS, respectively, and the number of participants generally increased from 2005 to 2020. Supplementary Table 1 presents the number of participants and the sociodemographic characteristics of the participants for each survey year.
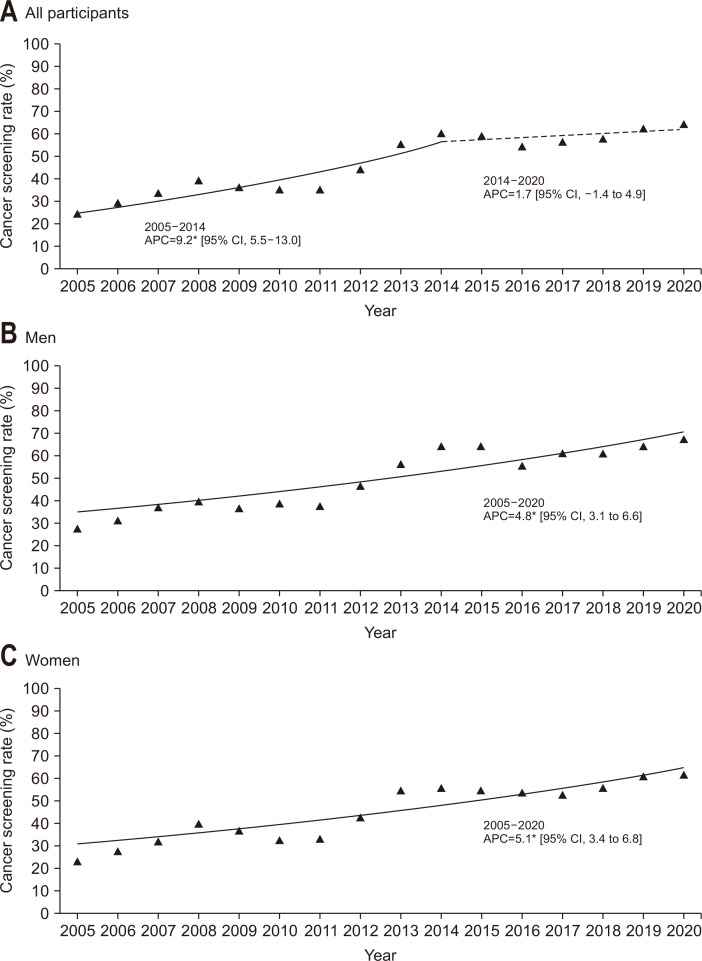
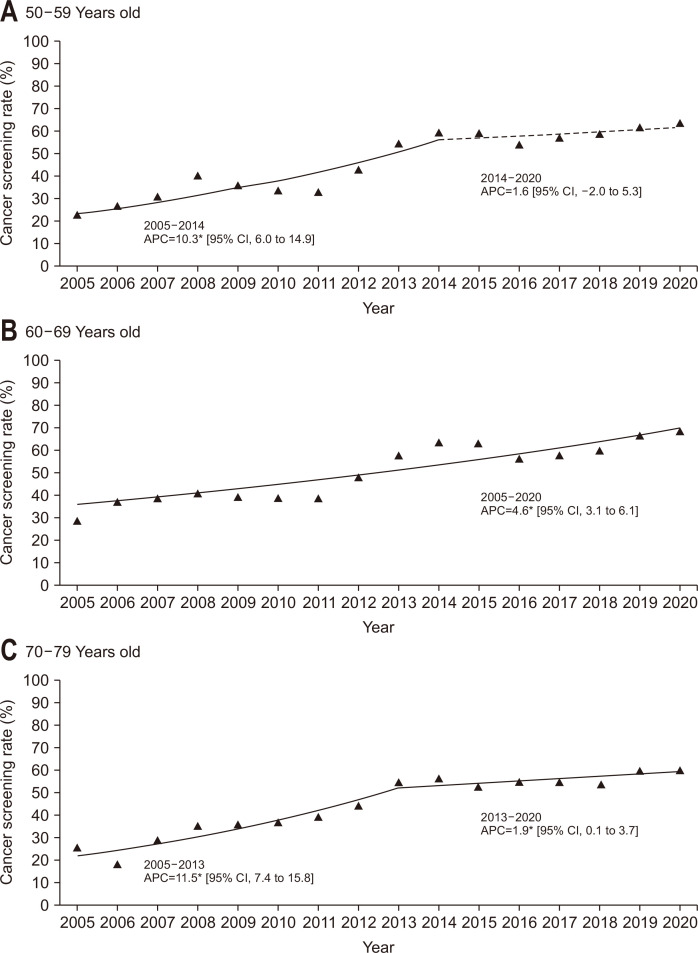
The screening rates are summarized in Table 2. Generally, the screening rate of all participants has increased from 25.0% in 2005 to 64.4% in 2020. The Joinpoint regression analysis showed that there was a significant increasing trend between 2005 and 2014 (APC, 9.2%), followed by a nonsignificant increase between 2014 and 2020 (APC, 1.7%) (Fig. 1A). Both men and women demonstrated a significant increase in the screening rate from 2005 to 2020, but the values of screening rates were generally higher in men (Fig. 1B and C). When the participants were grouped based on their age, the screening rate for the age group of 60 to 69 years was generally the highest for each survey year (Fig. 2).
Table 2.
Colorectal Cancer Screening Rates of Sociodemographic Characteristics of the Study Participants in the Korean National Cancer Screening Survey, 2005–2020
| Characteristics | Colorectal cancer screening rate of survey year, % | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | AAPC | |
| Total | 25.0 | 29.4 | 34.1 | 39.8 | 36.7 | 35.5 | 35.3 | 44.7 | 55.6 | 60.1 | 59.5 | 54.6 | 56.8 | 58.4 | 62.6 | 64.4 | 6.2* |
| Sex | |||||||||||||||||
| Male | 27.2 | 31.2 | 36.7 | 39.4 | 36.5 | 38.6 | 37.4 | 46.7 | 56.3 | 64.1 | 64.2 | 55.3 | 60.9 | 60.8 | 63.8 | 67.3 | 4.8* |
| Female | 23.1 | 27.9 | 31.9 | 40.1 | 36.8 | 32.5 | 33.3 | 42.8 | 54.9 | 56.2 | 55.0 | 53.9 | 52.7 | 56.1 | 61.5 | 61.6 | 5.1* |
| Age | |||||||||||||||||
| 50–59 yr | 22.9 | 26.2 | 30.7 | 40.2 | 35.5 | 33.3 | 32.7 | 42.9 | 54.7 | 59.0 | 59.0 | 53.8 | 56.9 | 58.6 | 61.2 | 63.4 | 6.8* |
| 60–69 yr | 28.7 | 37.1 | 38.3 | 40.7 | 38.8 | 38.4 | 38.3 | 48.0 | 57.4 | 63.3 | 62.9 | 55.7 | 57.2 | 59.6 | 66.2 | 68.0 | 4.6* |
| 70–74 yr | 25.9 | 18.6 | 29.0 | 35.3 | 35.6 | 36.9 | 39.6 | 44.4 | 55.0 | 56.4 | 52.6 | 55.0 | 54.6 | 53.8 | 59.8 | 60.3 | 6.9* |
| Monthly household income | |||||||||||||||||
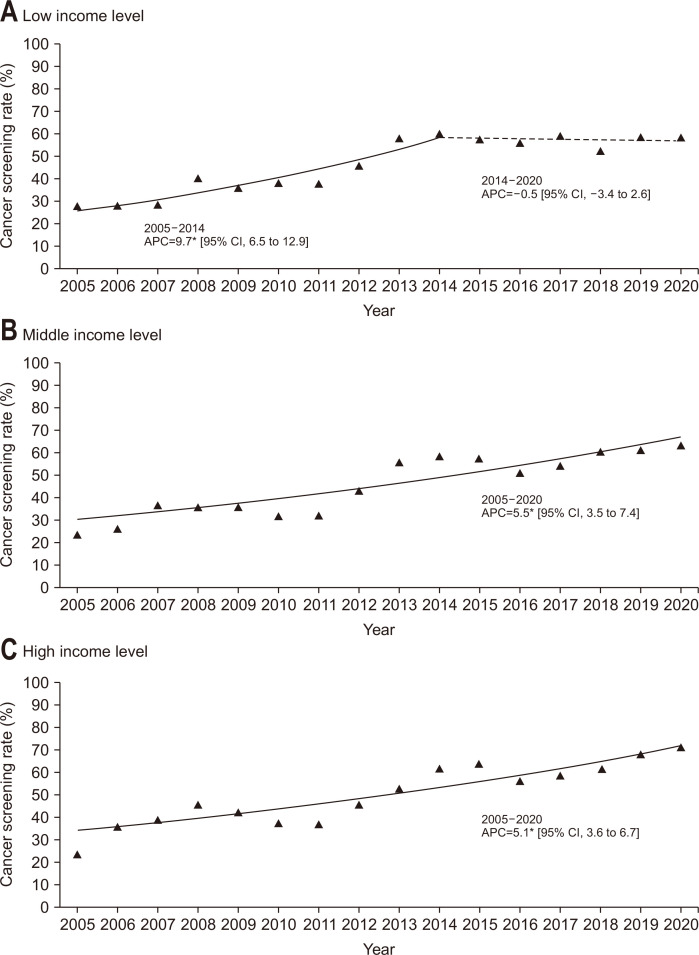
| Lower | 27.5 | 27.6 | 28.3 | 40.2 | 35.3 | 37.9 | 37.9 | 45.8 | 57.6 | 60.0 | 57.3 | 55.7 | 58.7 | 51.7 | 58.3 | 58.4 | 5.5* |
| Medium | 23.3 | 26.1 | 36.6 | 35.4 | 35.5 | 31.5 | 31.9 | 42.9 | 55.6 | 58.7 | 57.6 | 51.0 | 54.0 | 60.7 | 61.3 | 63.1 | 5.5* |
| Higher | 23.6 | 35.8 | 38.7 | 45.6 | 41.9 | 37.5 | 36.8 | 46.1 | 53.0 | 61.7 | 63.8 | 56.5 | 58.7 | 62.0 | 67.9 | 71.1 | 5.1* |
| Education | |||||||||||||||||
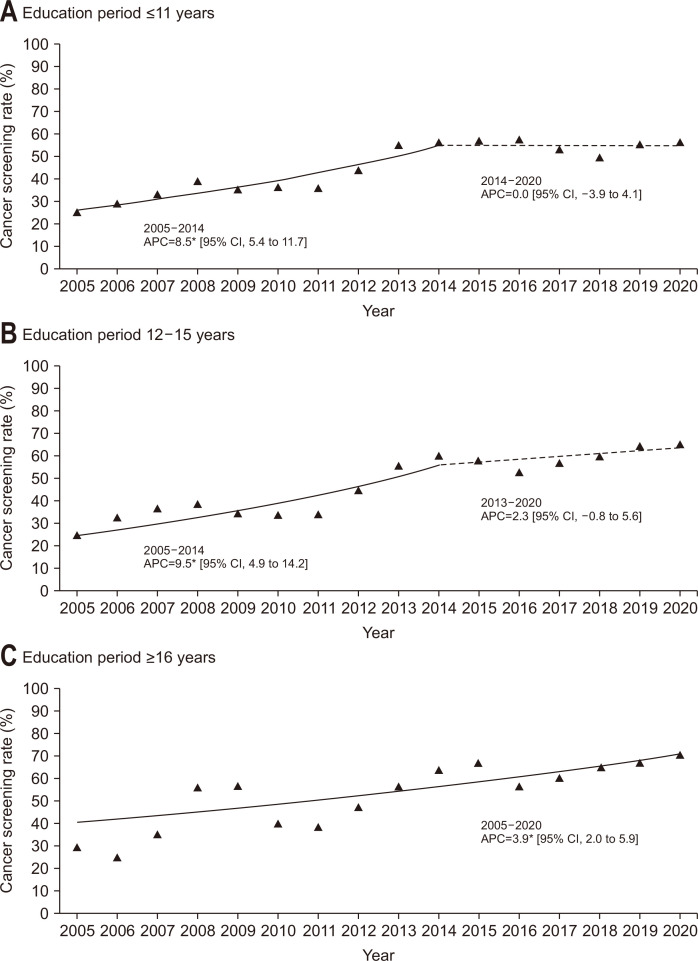
| Lower | 24.9 | 28.8 | 33.1 | 38.9 | 35.0 | 36.1 | 35.6 | 43.8 | 54.9 | 56.1 | 56.6 | 57.5 | 53.3 | 49.3 | 55.1 | 56.3 | 5.0* |
| Medium | 24.5 | 32.5 | 36.5 | 38.3 | 34.3 | 33.7 | 34.4 | 44.7 | 55.5 | 59.8 | 58.0 | 52.9 | 57.1 | 59.3 | 64.3 | 65.3 | 6.6* |
| Higher | 29.2 | 24.7 | 34.8 | 56.0 | 56.5 | 39.9 | 37.9 | 47.0 | 56.5 | 63.9 | 66.8 | 56.3 | 60.2 | 64.7 | 67.0 | 70.5 | 3.9* |
| Residential area | |||||||||||||||||
| Rural | 29.1 | 28.6 | 29.6 | 45.2 | 42.4 | 37.2 | 36.6 | 48.6 | 53.3 | 63.1 | 59.9 | 57.6 | 60.4 | 57.0 | 59.9 | 53.4 | 5.2* |
| Urban | 24.2 | 29.6 | 34.9 | 39.1 | 35.9 | 35.1 | 35.1 | 42.9 | 56.0 | 59.8 | 59.5 | 54.1 | 56.2 | 58.6 | 63.0 | 65.8 | 6.3* |
*Average annual percent change (AAPC) is significantly different from 0 at α=0.05.
Fig. 1.
Trends in colorectal cancer screening rates by sex, 2005–2020. (A-C) The solid line denotes a significant increasing trend, while the dashed line denotes a nonsignificant change.
CI, confidence interval. *p-value for the trend of annual percent change (APC) <0.05.
Fig. 2.
Trends of colorectal cancer screening rates by age group, 2005–2020. (A-C) The solid line denotes a significant increasing trend, while the dashed line denotes a nonsignificant change.
CI, confidence interval. *p-value for the trend of annual percent change (APC) <0.05.
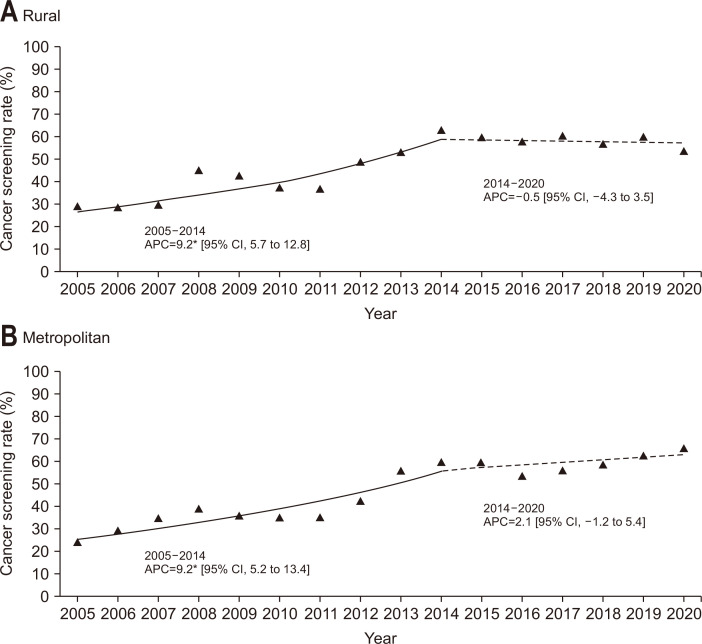
When the participants were grouped according to their monthly household income level (Fig. 3), the screening rates of participants with lower income exhibited a significant increase between 2005 and 2014 (APC, 9.7%), followed by a stable trend from 2014 to 2020. On the other hand, the slope of the screening rates significantly increased from 2005 to 2020 in the middle- and high-income groups (APC, 5.5% and 5.1%, respectively). Generally, the screening rate in the high-income group was higher than that in the middle- or lower-income groups over the study period. Fig. 4 demonstrates the results of the Joinpoint regression analysis when the participants were stratified according to education level. In the lower and middle education level groups, the screening rates were significantly increased between 2005 and 2014, and the slope afterwards were stable. On the other hand, the screening rate showed an increasing trend for the recent time period in the higher education level groups. The higher education level group generally showed the highest screening rate compared to the other groups. When the participants were stratified according to residential area (Fig. 5), the screening rates of participants living in both rural and metropolitan areas exhibited a significant increase between 2005 and 2014, followed by a stable trend from 2014 to 2020.
Fig. 3.
Trends of colorectal cancer screening rates by household income level, 2005–2020. Monthly household income status is classified by tertile. (A-C) The solid line denotes a significant increasing trend, while the dashed line denotes a non-significant change.
CI, confidence interval. *p-value for the trend of annual percent change (APC) <0.05.
Fig. 4.
Trends of colorectal cancer screening rates by education level, 2005–2020. The solid line denotes a significant increasing trend, while the dashed line denotes a non-significant change.
CI, confidence interval. *p-value for the trend of annual percent change (APC) <0.05.
Fig. 5.
Trends of colorectal cancer screening rates by residential area, 2005–2020. The solid line denotes a significant increasing trend, while the dashed line denotes a non-significant change.
CI, confidence interval. *p-value for the trend of annual percent change (APC) <0.05.
DISCUSSION
The present study demonstrated that the CRC screening rate increased significantly from 2005 to 2014, with an APC of 9.2%. When we analyzed the CRC screening rates according to participants’ socioeconomic status, the participants with higher household income and education level generally showed higher screening rates.
The screening rate in 2020 (64.4%) significantly improved compared to that in 2005 (25.0%). This could be because the number of medical institutions that provide CRC screening has increased continuously from 1,767 in 2007 to 4,367 in 2020, and thus the accessibility to CRC screening has improved. In addition, as of 2020, all but four out of 229 administrative districts had CRC screening institutions. In addition, mobile screening program is provided in remote areas to increase access to cancer screening. Therefore, it is unlikely that there is an issue with access to the CRC screening depending on the residential areas.
The CRC screening rate in Korea has improved to a relatively higher level compared to that in other countries. Although there are wide variations in terms of the types of screening programs, target age ranges, screening methods, and screening intervals among countries, participation rates are generally between 15% and 70% globally. In the United Kingdom, where the nationwide coverage for CRC screening started earlier than in Korea,17 a population-based organized screening program provides guaiac-based fecal occult blood test and FIT once every 2 years or flexible sigmoidoscopy once in a lifetime, with a screening rate of approximately 55.4%.18 In the United States, the U.S. Preventive Services Task Force recommended annual screening with FIT, screening every 10 years with sigmoidoscopy and annual screening with FIT, screening every 10 years with colonoscopy, or screening every 5 years with computed tomography colonography; it has been reported that approximately 60% of adults in the USA had been screened by endoscopy in 2015 under the opportunistic screening program.19
Although the CRC screening rate has improved substantially in Korea, there has been no significant increase in the screening rate from 2014 to 2020. The reason why the screening rate did not show a significant increase after 2014 is not fully understood. Because improving CRC screening rate is important to decrease morbidity and mortality from CRC,20-24 understanding the reasons for non-attendance for CRC screening and developing strategies to improve screening rates are necessary. Han et al.25 demonstrated the primary reasons for non-attendance for CRC screening through a survey and the reasons included “Without any symptoms (56.5%),” “Lack of time (14.4%),” and “Fear of exam procedure (11.0%).” In addition, according to the results of the 2019 KNCSS, 75.8% of people who have had a CRC screening according to the NCSP protocol reported to have a plan to receive a CRC screening again in the future, while 96.9% of those who have not had a screening reported that they have no plan of receiving it. This suggests that people who have not been screened have a fixed negative attitude toward screening; thus, a targeted strategy to improve their attitude toward screening seems to be needed.
In an effort to increase the CRC screening rate, the interval for the FIT provided by the NCSP has been changed from 2 years to 1 year since 2012. In addition, studies have been conducted to identify and lower the barriers that prevent an increase in CRC screening rates. Hong et al.26 found that CRC screening rates in rural areas in Korea increased when the FIT kit for stool sampling was postally distributed and collected to and from each subject. Shin et al.27 also found that the participants’ satisfaction and intention to undergo subsequent screening became higher when the conventional container for stool sampling was changed to a more convenient sampling bottle. These results suggest that an improvement in the inconvenience of FIT can increase CRC screening rates.
Many previous studies conducted in other countries have shown that low socioeconomic status is associated with decreased screening rate,28-33 although it is less associated with the existence of an organized screening program.34 The present study also demonstrated that the degree or pattern of increase in screening rates differed according to the sociodemographic characteristics of the subjects. In fact, the annual increase in the screening rate from 2005 to 2014 was larger in those who were less educated and had a lower income, probably because many national efforts have been made in Korea to expand coverage for screenings by lowering the economic burden of examinees for participating in the NCSP. Individuals eligible for free cancer screening through the NCSP have been gradually expanded so that Medical Aid recipients and National Health Insurance beneficiaries in the lower 50% income bracket have been provided with free CRC screening since 2005.12 Furthermore, for those who are not eligible for free cancer screening through the NCSP, patient cost sharing, which was initially 50%, gradually decreased to 20% in 2006, 10% in 2010, and has become fully covered by the NHIS since 2018.
However, the screening rate in participants with lower income and less education remained stable without significant change from 2014 onwards, while the screening rate increased consistently until recently in those who had a higher income and were more educated. This is consistent with a previous study that found the existence of screening inequality depending on the socioeconomic status in Korea,35,36 suggesting that there may still be remaining barriers to CRC screening participation with low socioeconomic status despite financial support through the NCSP. For example, because it is not required for employers to provide employees with mandated paid time off for cancer screening in Korea, taking paid time off to undertake a cancer screening might be more difficult for those from a lower socioeconomic status.
In addition, the increase in screening rates until recent years only in those with higher socioeconomic status could be because people with higher income and education choose to receive colonoscopy as the primary method of CRC screening through an opportunistic screening program. NCSP in Korea provides FIT as the primary CRC screening method, and colonoscopy is provided free of charge only to those with positive FIT results. On the other hand, under the opportunistic screening program, people can receive colonoscopy as the primary method of CRC screening, although the cost should be paid entirely by the examinee with no subsidy from the government. Because colonoscopy is approximately 20 times more expensive than FIT, which is even provided free of charge through the NCSP, the costs of the test can operate as a barrier to CRC screening, particularly for those with low socioeconomic status. However, a recent survey of 396 respondents who participated in the NCSP found that 68.7% of respondents preferred colonoscopy as a primary CRC screening test in the NCSP, and colonoscopy was preferred because it is accurate and can be used as a therapeutic option.37 In response to this, the Korean Ministry of Health and Welfare and National Cancer Center has been conducting a Korean colonoscopy screening pilot study since 2019 to evaluate the effectiveness, safety, feasibility, and acceptability of colonoscopy as a primary method for CRC screening.38
This study has some limitations. Since KNCSS only includes a non-institutionalized population and the response rate was between 34.5% and 55.5%, the results may have been influenced by selection bias. Moreover, the history of screening was self-reported by the participants, which could introduce either misclassification bias or recall bias. In addition, since the survey-participants were notdirectly enquired about the type of CRC screening they had undergone, we were unable to correctly identify whether the participants were screened through the NCSP or opportunistic screening. Instead, when inference was drawn indirectly using the responses to the questionnaire on who paid for the screening, the screening rates of those presumed to have been screened through the NCSP showed a similar trend to the screening rate of the entire participants. On the other hand, the screening rates of participants presumed to have undergone opportunistic screening exhibited a slight decrease from 2005 to 2020 (Supplementary Fig. 1). Despite these limitations, this study was able to investigate the changes in cancer screening rates over 10 years using data from a nationwide, population-based survey. Trends studies could provide an opportunity to better identify the determinants that may influence the trends.
In conclusion, this study demonstrated that CRC screening rates increased with a significant upward trend between 2005 and 2014, followed by a nonsignificant increase from 2014 to 2020. In addition, socioeconomic disparities in CRC screening occurred throughout the study period. Although there has been a lot of improvement in CRC screening rates, the results of our study indicate that there remains a need for more research to understand why the screening rates have been stagnant since 2014 and to identify multi-level remaining barriers to CRC screening participation specific to vulnerable populations.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl210419.
ACKNOWLEDGEMENTS
This study was supported by a Grant-in-Aid for Cancer Research and Control from the National Cancer Center of Korea (Grant No. 2210771).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Data analysis and interpretation, drafting of the manuscript: B.P. Data acquisition: M.S., K.S.C. Administrative, technical, and material support: S.Y.S. Statistical analysis: Y.Y.L., H.Y.S. Study concept and design, critical revision of the manuscript for important intellectual content, obtaining funding, and study supervision: J.K.J. All authors read and approved the final manuscript.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Hong S, Won YJ, Lee JJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021;53:301–315. doi: 10.4143/crt.2021.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maringe C, Walters S, Rachet B, et al. Stage at diagnosis and colorectal cancer survival in six high-income countries: a population-based study of patients diagnosed during 2000-2007. Acta Oncol. 2013;52:919–932. doi: 10.3109/0284186X.2013.764008. [DOI] [PubMed] [Google Scholar]
- 4.Lindebjerg J, Osler M, Bisgaard C. Colorectal cancers detected through screening are associated with lower stages and improved survival. Dan Med J. 2014;61:A4758. [PubMed] [Google Scholar]
- 5.Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer. 2014;120:2893–2901. doi: 10.1002/cncr.28794. [DOI] [PubMed] [Google Scholar]
- 6.Kubisch CH, Crispin A, Mansmann U, Goke B, Kolligs FT. Screening for colorectal cancer is associated with lower disease stage: a population-based study. Clin Gastroenterol Hepatol. 2016;14:1612–1618. doi: 10.1016/j.cgh.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284:1954–1961. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 8.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 9.Lauby-Secretan B, Vilahur N, Bianchini F, Guha N Straif K; International Agency for Research on Cancer Handbook Working Group, author. The IARC perspective on colorectal cancer screening. N Engl J Med. 2018;378:1734–1740. doi: 10.1056/NEJMsr1714643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee BH, Jeong SY. Korean national recommendation guidelines on screening and surveillance for early detection of colorectal cancers. J Korean Med Assoc. 2002;45:981–991. doi: 10.5124/jkma.2002.45.8.981. [DOI] [Google Scholar]
- 11.Sohn DK, Kim MJ, Park Y, et al. The Korean guideline for colorectal cancer screening. J Korean Med Assoc. 2015;58:420–432. doi: 10.5124/jkma.2015.58.5.420. [DOI] [Google Scholar]
- 12.Suh M, Song S, Cho HN, et al. Trends in participation rates for the national cancer screening program in Korea, 2002-2012. Cancer Res Treat. 2017;49:798–806. doi: 10.4143/crt.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HY, Park EC, Jun JK, et al. Trends in socioeconomic disparities in organized and opportunistic gastric cancer screening in Korea (2005-2009) Cancer Epidemiol Biomarkers Prev. 2010;19:1919–1926. doi: 10.1158/1055-9965.EPI-09-1308. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 15.Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28:3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute (NCI), author Cancer Trends Progress Report: Methodology for Characterizing Trends [Internet] NCI; Rockville: c2020. [cited 2021 Feb 21]. Available from: https://progressreport.cancer.gov/methodology . [Google Scholar]
- 17.Public Health England, author. NHS bowel cancer screening (BCSP) programme [Internet] Public Health England; Sheffield: c2010. [cited 2019 Mar 20]. Available from: http://www.cancerscreening.nhs.uk/bowel/index.html . [Google Scholar]
- 18.Ponti A, Anttila A, Ronco G, et al. Cancer Screening in the European Union (2017): report on the implementation of the council recommendation on cancer screening [Internet] European Commission; Brussels: c2017. [cited 2019 Mar 20]. Available from: https://ec.europa.eu/health/sites/health/files/major_chronic_diseases/docs/2017_cancerscreening_2ndreportimplementation_en.pdf . [Google Scholar]
- 19.American Cancer Society, author. Colorectal Cancer Facts & Figures 2017-2019 [Internet] American Cancer Society; Atlanta: c2017. [cited 2019 Apr 1]. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-factsand-statistics/colorectal-cancer-facts-and-figures/colorectalcancer-facts-and-figures-2017-2019.pdf . [Google Scholar]
- 20.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 22.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood: Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 23.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 24.Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–657. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 25.Han MA, Choi KS, Jun JK, Kim Y, Park EC, Lee HY. Factors associated with the intention to have colorectal cancer screening in Korean adults. Eur J Cancer Care (Engl) 2011;20:475–482. doi: 10.1111/j.1365-2354.2010.01209.x. [DOI] [PubMed] [Google Scholar]
- 26.Hong S, Shin HY, Lee B, Hwang NR, Hwang SH, Jun JK. Increase in the colorectal cancer screening rate by a round-mailed fecal immunochemical testing kit and associated factors in underserved regions of Korea: a community-based intervention study. Gut Liver. 2020;14:323–330. doi: 10.5009/gnl19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin HY, Suh M, Choi KS, et al. Higher satisfaction with an alternative collection device for stool sampling in colorectal cancer screening with fecal immunochemical test: a cross-sectional study. BMC Cancer. 2018;18:365. doi: 10.1186/s12885-018-4290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Cancer Institute (NCI), author Screening rates for several cancers miss their targets [Internet] NCI; Rockville: c2015. [cited 2019 Apr 1]. Available from: http://www.cancer.gov/ [Google Scholar]
- 29.Trivers KF, Shaw KM, Sabatino SA, Shapiro JA, Coates RJ. Trends in colorectal cancer screening disparities in people aged 50-64 years, 2000-2005. Am J Prev Med. 2008;35:185–193. doi: 10.1016/j.amepre.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Swan J, Breen N, Graubard BI, et al. Data and trends in cancer screening in the United States: results from the 2005 National Health Interview Survey. Cancer. 2010;116:4872–4881. doi: 10.1002/cncr.25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao Y, Jemal A, Zhang X, Ward EM. Trends in colorectal cancer incidence rates by age, race/ethnicity, and indices of access to medical care, 1995-2004 (United States) Cancer Causes Control. 2009;20:1855–1863. doi: 10.1007/s10552-009-9379-y. [DOI] [PubMed] [Google Scholar]
- 32.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:1611–1621. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole AM, Jackson JE, Doescher M. Urban-rural disparities in colorectal cancer screening: cross-sectional analysis of 1998-2005 data from the Centers for Disease Control's Behavioral Risk Factor Surveillance Study. Cancer Med. 2012;1:350–356. doi: 10.1002/cam4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh B, Silles M, O'Neill C. The importance of socio-economic variables in cancer screening participation: a comparison between population-based and opportunistic screening in the EU-15. Health Policy. 2011;101:269–276. doi: 10.1016/j.healthpol.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Mai TT, Lee YY, Suh M, et al. Socioeconomic inequalities in colorectal cancer screening in Korea, 2005-2015: after the introduction of the national cancer screening program. Yonsei Med J. 2018;59:1034–1040. doi: 10.3349/ymj.2018.59.9.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suh M, Choi KS, Lee HY, et al. Socioeconomic disparities in colorectal cancer screening in Korea: a nationwide cross-sectional study. Medicine (Baltimore) 2015;94:e1368. doi: 10.1097/MD.0000000000001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho YH, Kim DH, Cha JM, et al. Patients' preferences for primary colorectal cancer screening: a survey of the national colorectal cancer screening program in Korea. Gut Liver. 2017;11:821–827. doi: 10.5009/gnl17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park B, Jun JK, Kim BC, et al. Korean colonoscopy screening pilot study (K-cospi) for screening colorectal cancer: study protocol for the multicenter, community-based clinical trial. BMC Gastroenterol. 2021;21:36. doi: 10.1186/s12876-021-01610-1.1df23e345da040909d6d215fcf16f45a [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.