Abstract
Although the concern for gastric cancer prevention has increased, gastric cancer has remained a heavy burden worldwide and is not just a local issue in East Asian countries. However, as several screening programs (listed below) have shown some success, it is important to determine whether the situation is changing in some other countries and whether similar methods should be recommended. Endoscopic screening has been performed as a national program in South Korea and Japan, and the results have shown a reduction in gastric cancer mortality. Although the efficacy of Helicobacter pylori eradication has been established, the efficacy of the screen-and-treat strategy is presently being evaluated in randomized controlled trials. The serum pepsinogen test and endoscopic examination can divide high-risk subjects with severe gastric atrophy from average-risk subjects. Risk stratification is anticipated to contribute to an efficient method of prediction of gastric cancer development when combined with endoscopic screening. Countries with a high incidence rate should realize the immediate need to reduce gastric cancer death directly by endoscopic screening and should recognize screen-and-treat as a second option to reduce future risk. However, all forms of gastric cancer prevention programs have some harms and potential to increase unnecessary examinations. A balance of the benefits and harms should be always considered. Although further study is needed to obtain sufficient evidence for gastric cancer prevention, the best available method should be examined in the context of each country.
Keywords: Stomach neoplasms, Mass screening, Helicobacter pylori antibodies, Serum pepsinogens, Endoscopes
INTRODUCTION
The burden of gastric cancer cannot be ignored worldwide as it is the fourth leading cause of cancer deaths worldwide at 768,793 in 2020.1 Gastric cancer has remained a heavy burden in East Asian countries. Although the age-standardized rates by the world population are 11.1 per 100,000 for incidence and 7.7 per 100,000 for mortality, the rates in East Asian countries are 22.4 per 100,000 and 14.6 per 100,000, respectively (Table 1).1 In addition to East Asian countries, a high incidence has been observed in Eastern European and South American countries. Although the incidence of gastric cancer is not high among non-Hispanic Whites in the United States, a high incidence has also been reported in Asian immigrants, particularly in South Korean and Japanese Americans.2,3 Regardless of the heavy burden being experienced in several countries and specific races, a coherent prevention program for gastric cancer has not yet been established. Recent advances have suggested new strategies for gastric cancer prevention and the potential to apply these strategies in countries outside of East Asia.4 These strategies include endoscopic screening, screen-and-treat, and risk stratification for gastric cancer development. Risk stratification can identify high-risk groups and provide intensive screening and priority of diagnostic examinations. It has already been adopted for human papillomavirus testing results in cervical cancer screening and hemoglobin concentration of fecal occult blood testing (FOBT) in colorectal cancer screening.5,6 In this review article the lines of evidence of the above-mentioned strategies are carefully assessed, particularly the use of risk stratification in combination with endoscopic screening as a forthcoming step for gastric cancer prevention.
Table 1.
Age-Standardized Incidence and Mortality by World Population
| Area | Incidence, per 100,000 | Incidence/mortality, per 100,000 | |||
|---|---|---|---|---|---|
| Men | Women | Incidence | Mortality | ||
| Eastern Asia | 32.5 | 13.2 | 22.4 | 14.6 | |
| China | 29.5 | 12.3 | 20.6 | 15.9 | |
| South Korea | 39.7 | 17.6 | 27.9 | 6.1 | |
| Japan | 48.1 | 17.3 | 31.6 | 8.2 | |
| Central and Eastern Europe | 17.4 | 7.1 | 11.3 | 8.3 | |
| World | 15.8 | 7.0 | 11.1 | 7.7 | |
| South America | 12.1 | 6.1 | 8.7 | 6.8 | |
| Western Asia | 11.4 | 6.1 | 8.5 | 7.1 | |
| Polynesia | 11.1 | 6.7 | 8.6 | 6.8 | |
| Southern Europe | 10.2 | 5.0 | 7.4 | 4.8 | |
| Melanesia | 9.9 | 6.2 | 7.9 | 6.3 | |
| Caribbean | 9.0 | 5.0 | 6.9 | 5.4 | |
| Central America | 8.7 | 6.1 | 7.3 | 5.6 | |
| Western Europe | 8.2 | 3.8 | 5.9 | 3.3 | |
| Micronesia | 7.7 | 3.9 | 5.8 | 4.1 | |
| South-Central Asia | 7.4 | 3.7 | 5.5 | 4.8 | |
| South-Eastern Asia | 7.3 | 4.0 | 5.5 | 4.5 | |
| Australia and New Zealand | 6.4 | 2.8 | 4.5 | 2.1 | |
| Northern Europe | 6.2 | 3.1 | 4.6 | 2.9 | |
| Northern Africa | 5.4 | 3.5 | 4.4 | 3.6 | |
| Northern America | 5.4 | 3.1 | 4.2 | 1.8 | |
| Eastern Africa | 4.9 | 4.2 | 4.5 | 4.0 | |
| Western Africa | 4.8 | 3.5 | 4.1 | 3.7 | |
| Southern Africa | 4.7 | 2.4 | 3.3 | 2.9 | |
| Middle Africa | 4.5 | 3.8 | 4.2 | 3.7 | |
Data from International Agency for Research on Cancer, GLOBOCAN 2020; Stomach.1
ENDOSCOPIC SCREENING
Endoscopic screening for gastric cancer has been provided as national programs in South Korea and Japan.7,8 In Japan, upper gastrointestinal series radiography has also been implemented for gastric cancer screening.9 Based on the success of South Korea and Japan in reducing gastric cancer mortality,10,11 attention to the use of endoscopic screening has increased in other countries.12 It is anticipated that endoscopic examination will become a common strategy for gastric cancer screening in other countries besides South Korea and Japan. In some Asian countries, opportunistic screening and research-based screening have also been performed.13-17
Two randomized controlled trials (RCTs) have been continuously conducted, and preliminary results have been published from China and Japan.18,19 However, the efficacy of endoscopic screening could not be comprehensively evaluated because of the small number of subjects examined. The effectiveness of endoscopic screening has been mainly evaluated in cohort and case-control studies published in South Korea, China, and Japan (Tables 2 and 3).20-29 Notably, the incidences of gastric cancer are higher in these countries than in others found in East Asia (Table 1). The results of observational studies were concordant and suggested reductions in mortality from gastric cancer. A recent systematic review and meta-analysis has included the above-mentioned six cohort studies and four case-control studies published in East Asian countries.30 The meta-analysis included 342,013 subjects and showed that endoscopic screening was associated with a reduction in mortality from gastric cancer by 40% (relative risk, 0.60, 95% confidence interval [CI], 0.49 to 0.73).
Table 2.
Cohort Study for the Evaluation of Endoscopic Screening
| Author (year) | Location | No. of subjects | Recruitment period | Age at entry, yr |
Follow-up, yr | Screening frequency | Screening interval, yr |
Comparator | Adjustment | GC incidence risk estimate (95% CI) | GC mortality risk estimate (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Riecken et al. (2002)20 |
China | GE: 4,394 | 1989–1994 | 35–64 | 11.5 (until July 2000) | 4 | Irregular (first cohort: 2/4 second cohort: 5) |
General population (Chinese Cancer Mortality Survey) | Age and sex | - | SMR All: 1.01 (0.72–1.37) Male: 1.13 (0.77–1.57) Female: 0.65 (0.26–1.32) |
| Matsumoto et al. (2007)23 |
Japan | UGI: 4,261 | UGI (1991–1995) |
≥40 | 6 (1990-1996) | ≥1 | 1/2 | General population (local town) | Age | - | UGI: SMR Male: 1.04 (0.50–1.58) Female: 1.54 (0.71–2.38) |
| GE: 7,178 | GE (1996–2003) |
10 (1997–2006) | GE: SMR Male 0.71 (0.33–1.10) Female: 0.62 (0.19–1.05) |
||||||||
| Hosokawa et al. (2008)24 |
Japan | GE: 2,192 No UGI/GE (hospital patients): 9,571 |
1990–1992 | 40–75 | 10 | 1 | - | No UGI/GE (hospital patients) | No | - | GE: RR All: 0.3465 (0.1396–0.8605) Male: 0.2174 (0.0676–0.6992) Female: 0.6835 (0.1595–2.9286) |
| Hamashima et al. (2015)25 |
Japan | GE: 9,950 UGI: 4,324 |
2007–2008 | 40–79 | 6 (until December 31, 2013) | ≥1 GE: 2.3 UGI: 2.2 |
1 | UGI | Age, sex and residence | HR: 0.988 (0.679–1.438) | HR: 0.327 (0.118–0.908) |
| Hamashima et al. (2015)26 | Japan | GE: 16,373 | 2005 | 40–79 | 5 | ≥1 | 1 | General population (local city) | Age and sex | - | GE: SMR All: 0.43 (0.30–0.57) Male: 0.49 (0.32–0.66) Female: 0.31 (0.12–0.54) |
| Clinic based UGI: 18,221 Mass survey UGI: 15,927 |
UGI: SMR All: 0.68 (0.55–0.79) Male: 0.72 (0.56–0.85) Female: 0.62 (0.39–0.80) |
||||||||||
| Kim et al. (2018)21 | South Korea | GE Screened: 4,356 Unscreened: 6,533 |
1993–2004 | GE (mean) Screened: 58.0±10.3 Unscreened: 57.3±11.7 |
10 (until December 31, 2013) | ≥1 | 2 | Never screened | Age and sex | GE HR: 1.21 (0.94–1.54) |
GE HR: 0.58 (0.36–0.94) |
| UGI Screened: 2,015 Unscreened: 2,758 |
UGI (mean) Screened: 58.9±10.2 Unscreened: 57.5±12.7 |
UGI HR: 0.83 (0.52–1.33) |
UGI HR: 0.91 (0.36–2.33) |
||||||||
| Suh et al. (2020)22 | South Korea | UGI: 34,122 GE: 82,653 No screening: 74,927 |
2004–2013 | ≥40 | 5 | ≥1 | 2 | Never screened | Age, sex, period, and treatment | - | UGI HR: 0.80 (0.78–0.82) |
| GE HR: 0.47 (0.46–0.48) |
GC, gastric cancer; CI, confidence interval; GE, gastrointestinal endoscopy; UGI, upper gastrointestinal series; SMR, standardized mortality ratio; RR, relative risk; HR, hazard ratio.
Table 3.
Case-Control Study for the Evaluation of Endoscopic Screening
| Author (year) | Location | No. of subjects | Age at GC diagnosis of case subjects, yr | Screening frequency |
Comparator | Odds ratio for GC mortality reduction (95% CI) |
|---|---|---|---|---|---|---|
| Hamashima et al. (2013)27 | Japan | Case: 410 | 40–79 | ≥1 | Never screened | Screened within 36 mo |
| Control: 2,292 | 0.695 (0.480–0.695) | |||||
| Matsumoto et al. (2013)28 | Japan | Case: 13 | 54–91 | ≥1 | Never screened | 0.206 (0.044–0.965) |
| Control: 130 | ||||||
| Chen et al. (2016)17 | China | Case: 313 | 40–69 | ≥1 | Never screened | 0.72 (0.54–0.97) |
| Control: 1,876 | ||||||
| Jun et al. (2017)29 | South Korea | Case: 54,418 | ≥40 | ≥1 | Never screened | GE: 0.53 (0.51–0.56) |
| UGI: 0.98 (0.95–1.01) | ||||||
| Control: 217,672 |
GC, gastric cancer; CI, confidence interval; GE, gastrointestinal endoscopy; UGI, upper gastrointestinal series.
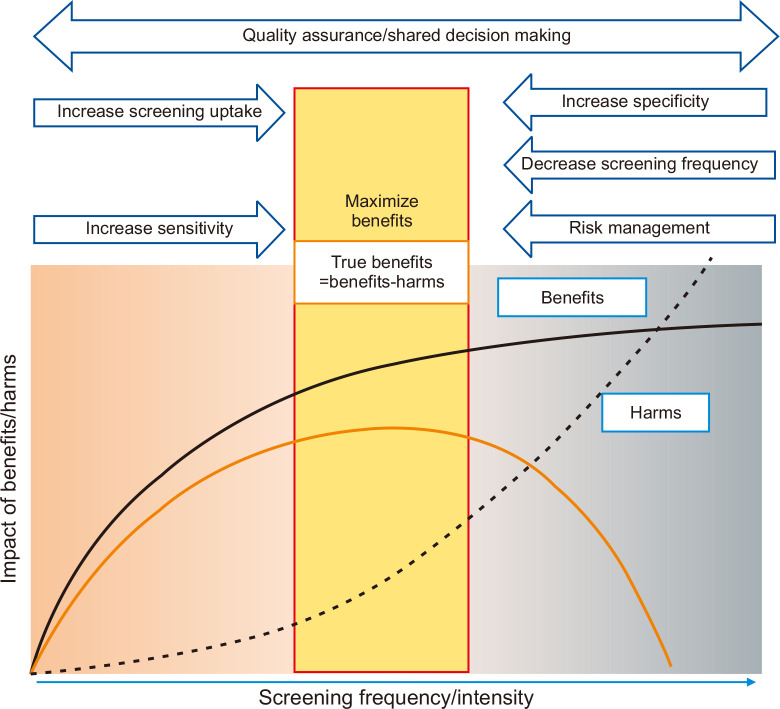
Endoscopic screening has also been reported to have several harms. These include infection, complications, false-positive results, and overdiagnosis.31 Both infection and complications can be managed by the establishment of a risk management system.32 On the other hand, false-positive results and overdiagnosis are inherent to the nature of cancer screening and these can easily increase according to the frequency of endoscopic examination. Although the specificity of endoscopic screening was over 85% in prevalence and incidence screening, this resulted from single one-off screenings.33 In endoscopic screening, false-positive cases included additional biopsy or repeated examinations. False-positive rates are below 15%, and the number of subjects is not so significant. However, individuals are required to participate in regular screening, and lifetime screening numbers are accumulated from multiple rounds. Hubbard et al.34 reported that annual colorectal cancer screening participants’ cumulative false-positive rate reached 23% and received at least one FOBT during a 10-year follow-up. There is a considerable possibility of overdiagnosis in endoscopic screening because endoscopy can diagnose cancers earlier than radiographic screening. About twice as many cases of gastric cancer were diagnosed in endoscopic screening compared with expected numbers.35 This suggests they included 46% of overdiagnosis cases at maximum from the total diagnostic cases of gastric cancer. Both overdiagnosis and false-positive cases increase according to increased screening frequency. One of the solutions to avoid these harms is to define the appropriate screening frequency (Fig. 1). Although a feasible approach is to define the starting and stopping age, there is no upper age limit for the national programs in South Korea and Japan.7,8 Expanding the screening interval is also helpful in decreasing the screening frequency, but there is a possibility of increasing interval cancers. Thus, continuous participation in regular screening is required to maintain the effectiveness of cancer screening if the screening interval is expanded. The benefits of screening become small beyond the optimal level of intensity, and harms continue to grow.36 The balance of benefits and harms should always be considered to maximize the benefits, but the best available threshold cannot be easily defined.
Fig. 1.
Maximizing real benefits of cancer screening. The benefit of cancer screening increases according to the increase of screening frequencies/intensities but becomes flattened beyond the optimal level.36 On the other hand, the harms directly increase according to the screening frequency. The real benefits lead to the difference between benefits and harms. To maximize the real benefits of screening, quality assurance and shared decision-making should be always considered. Increasing sensitivity and screening uptake lead to increased benefits until the optimal level is reached. Beyond this level, risk management, decrease in screening frequency and increase specificity could be helpful to reduce harms.
On the other hand, insufficient resources can be a barrier to endoscopic screening. Although endoscopic examination has become a common strategy worldwide, it requires a specific technique to ensure safety and accurate diagnosis. In Japan, endoscopic screening has been adopted as a national program, but most municipal governments have hesitated to implement it because of insufficient resources, particularly in rural areas.37 A similar problem has been discussed for colorectal cancer screening as the necessity for total colonoscopy is increased for work-up examinations after FOBT with positive results and surveillance.38-42 When endoscopic screening is introduced as population-based screening, its implementability context which includes the medical resources available should be considered.43
SCREEN-AND-TREAT STRATEGY
The screen-and-treat strategy consists of Helicobacter pylori screening and eradication for individuals with H. pylori infection. Regarding progression in the evaluation of the efficacy of H. pylori eradication, the concern of screen-and-treat strategy has increased. The International Agency for Research on Cancer (IARC) has recommended exploring screen-and-treat strategy in countries with high gastric cancer incidence based on expert opinion.44 In the screen-and-treat strategy, H. pylori screening is undertaken first to detect subjects with H. pylori infection, not gastric cancer, if H. pylori is detected it is subsequently targeted and eradicated. The efficacy of H. pylori eradication has been initially evaluated, and the efficacy of the screen-and-treat strategy is being continuously evaluated in some countries.
1. H. pylori screening
For H. pylori screening, the 13C-urea breath, H. pylori stool antigen, and serological tests are available.45 Although the 13C-urea breath test is more accurate, the serological test is commonly used for screening. The standard cutoff value is defined as 10.0 U/mL for the serological test, and it is occasionally used with the serum pepsinogen (PG) test. When the serological test is used as a screening technique, two functions are required. First, H. pylori screening discriminates subjects with H. pylori infection, which become the targets for H. pylori eradication. Second, H. pylori screening is expected as a predictor of gastric cancer. The first role consists of the screen-and-treat strategy, but the second role is expected to work as another form of risk stratification. Although H. pylori infection is the main cause of gastric cancer,46 it is not equal to an appropriate indicator for the prediction of gastric cancer development. As all subjects with a positive result will not have gastric cancer in the future, all subjects infected with H. pylori do not need treatment. However, H. pylori screening cannot be predicted correctly because of high sensitivity and low specificity.47 The estimates of H. pylori testing of prediction for gastric cancer development showed a sensitivity of 0.87 (95% CI, 0.76 to 0.94) and a specificity of 0.30 (95% CI, 0.23 to 0.49).48 As a result, H. pylori screening may result in unnecessary treatment after screening. H. pylori eradication has also a potential for producing harms such as the development of antibiotic resistance, obesity, and increased risk of gastroesophageal reflux diseases.45,49-52 If H. pylori infection is identified and eradicated as population program, experiences of harms remain in most people without gastric cancer.
2. Efficacy of H. pylori eradication
Several RCTs on H. pylori eradication have been carried out in clinical practice and communities.53-59 Although a decrease in gastric cancer incidence was not observed by H. pylori eradication in the Columbian and Chinese study,54,58 most studies confirmed a reduction of gastric cancer. Systematic reviews and meta-analyses reported results which were consistent with these studies.60-63 A recent meta-analysis has reported the efficacy of H. pylori eradication based on five population-based and two outpatient-based RCTs.63 Of 7,303 adults included in seven RCTs, 138 cases of gastric cancers were observed. H. pylori eradication prevented 35% of the gastric cancer incidence (hazard ratio [HR], 0.65; 95% CI, 0.41 to 1.0). Gastric cancer mortality was reduced by 41% through H. pylori eradication, although the result was not significant (HR, 0.59; 95% CI, 0.25 to 1.20). The all-cause mortality was also identified and showed a decreasing trend in five community-based RCTs (HR, 0.97; 95% CI, 0.69 to 1.28). Chen et al.62 focused on the background of the subjects. Although the incidence of gastric cancer did not decrease in subjects with intestinal metaplasia by H. pylori eradication, a decreased incidence of gastric cancer was confirmed in subjects with normal, non-atrophic, and atrophic gastritis (relative risk, 0.25; 95% CI, 0.08 to 0.81). Although sufficient evidence was not obtained because of the small sample size in the sub-analysis, it suggested critical results since the latter subjects consist of the screening population with mixed background risks.
3. Evaluation of screen-and-treat strategy
In the local islands of Taiwan with a high incidence of gastric cancer, a successful reduction in the gastric cancer incidence by screen-and-treat was reported.64 After performing the 13C-urea breath test, H. pylori eradication was conducted in patients with H. pylori infection. After six rounds of screen-and-treat, a coverage rate of 85.5% (6,512/7,616) for the targeted inhabitants in these local islands was achieved. With 15 years of follow-up, the gastric cancer incidence and mortality rates were reduced by 53% (95% CI, 30% to 69%) and 25% (95% CI, –14% to 51%), respectively, compared with a historical cohort without intervention.
To further confirm the efficacy of the screen-and-treat strategy, three community-based RCTs have been conducted in Taiwan, Latvia, and the United Kingdom (Table 4).65-67 The gastric cancer incidences were lower in these study areas than in East Asian countries which have high incidences. Although some variabilities in the H. pylori testing regimens were present among the studies from Taiwan, Latvia, and the United Kingdom, the regimens basically followed the standard treatment. The comparators were subjects who were offered the standard care in all the studies as they have no screening programs and surveillance in those areas. Gastric cancer incidence was the primary outcome in three RCTs, and gastric cancer mortality was also included as a secondary outcome. In the study conducted in Latvia, 30,000 individuals aged 40 to 64 years were recruited and then randomly allocated to the intervention and control groups.65 The control group received the usual care, whereas the intervention group received the H. pylori infection and serum PG tests. H. pylori eradication is usually offered to subjects who test positive for H. pylori infection, with a follow-up period of 15 years. In Taiwan, an RCT has been conducted based on colorectal cancer screening with fecal immunological testing (FIT).66 A total of 63,508 individuals were randomly allocated to the H. pylori stool antigen with FIT arm, and 88,995 were allocated to the FIT arm. At baseline, the incidences of colorectal and gastric cancers showed no significant differences between the two arms. Follow-up to assess gastric cancer mortality and incidence will be continued for 10 years. At present, an H. pylori screening study is being performed in the United Kingdom, and recruitment has been completed.67 If these results confirm the efficacy of screen-and-treat, it could be adopted to be one of the strategies for cancer control based on their evidence.
Table 4.
Randomized, Controlled Trials for the Screen-and-Treat Strategy
| Study | GISTAR Study | Taiwan | Helicobacter pylori Screening Study |
|---|---|---|---|
| Trial No. | NCT02047994 | NCT01741363 | ISRCTN71557037 |
| Publication | Protocol (Leja et al., 2017)65 | Baseline results (Lee et al., 2021)66 | Protocol67 |
| Country | Latvia/Russia/Belarus/Ukraine | Taiwan | UK |
| Age standardized incidence, per 100,000 | Lavita Men: 18.6 Women: 7.8 |
Men: 10.6 Women: 6.6 |
Men: 5.4 Women: 2.7 |
| Subjects | Healthy population | Healthy population | Healthy population |
| Intervention | |||
| Serological testing | H. pylori antibody + PG | H. pylori stool antigen + FIT | H. pylori testing (method unclear) |
| Treatment regime (first line) | Esomeprazole 40 mg Clarithromycin 500 mg Amoxicillin 1,000 mg (twice/day for 10 days) |
Days 1–5 Esomeprazole 40 mg once/day Clarithromycin 500 mg twice/day Days 6–10 Esomeprazole 40 mg once/day Clarithromycin 500 mg twice/day Metronidazole 500 mg twice/day |
Oral metronidazole, clarithromycin and lansoprazole (dose and days were unclear) |
| Comparator | Usual care (no screening) | Usual care (no screening + FIT) | Usual care (no screening) |
| Target age, yr | 40–64 | 50–69 | Men: 35–69 Women: 45–69 |
| Number invited | 30,000 | Intervention arm 63,508 Control arm 88,995 |
56,000 |
| Recruitments | On-going | Completed | Completed |
| Follow-up, yr | 15 | 10 | 15 |
| Primary outcome | Gastric cancer mortality | Gastric cancer incidence | Gastric cancer incidence/gastric cancer mortality |
PG, pepsinogen; FIT, fecal immunological testing.
RISK STRATIFICATION
The basic concept of risk stratification was considered based on the natural history of gastric cancer. The serum PG test and endoscopic examination have been identified as capable of discriminating the risk of gastric cancer. Although targeting high-risk groups is an attractive method, an application has not been established.
1. Serum PG test
The serum PG test has been used for both primary screening and risk stratification, and there has been an overlap of their abilities without a clear discrimination. As primary screening, the sensitivity of the serum PG test varied from 60% to 85%, but the specificity was reported as 70% to 82%.68,69 Because of low specificity, the recall rate exceeded 20% and does not match the basic requirement of primary screening. When it is combined with the H. pylori antibody, the sensitivity increases but specificity decreases. As such the failure of a primary screening using the serum PG test has been anticipated as a possible method of risk stratification.48,70-72 As chronic atrophic gastritis has been correlated with a stepwise regression in the serum PG I and PG I/II levels,72-74 the serum PG test can be used to diagnose chronic atrophic gastritis, which has the potential to progress to gastric cancer.46 Looking at a previous systematic review, eight studies were selected to examine the risk for gastric cancer.48 These studies were published from Japan, South Korea, and China, and reported gastric cancer incidence rates that varied from 21 to 260 (/100,000 person-years). Although the serum PG test was used to predict gastric cancer, the testing protocol and the cutoff values were different among these studies. The standard criteria (PG I ≤70 and PG I/II ≤3.0, which is defined as a positive result if it is matched) for the prediction of gastric cancer was mostly used. Cumulative risk was calculated and compared with subjects having negative results at an index testing based on 14,343 subjects in the eight studies. The odds ratio of developing gastric cancer for the subjects with positive results was 3.5 (95% CI, 2.7 to 4.7). In four studies involving 14,343 subjects, the meta-analysis showed that the subjects with positive results had a higher risk of developing gastric cancer than the subjects with negative results (HR, 3.54; 95% CI, 2.68 to 4.68). Even when the cutoff value was limited to the standard value, a similar result was obtained (HR, 3.13; 95% CI, 2.27 to 4.32). Ohata et al.71 compared the hazard risks for the intestinal type and diffuse type of gastric cancers. A stepwise increase was observed in the intestinal type according to the PG I/II level, whereas no clear change was observed in the diffuse type.
Yanaoka et al.72 reported the progression of gastric cancer based on a 10-year follow-up of 5,209 middle aged men in Japan. The sensitivity and specificity for gastric cancer prediction were calculated based on the index test results according to the different criteria of gastric atrophy. When the standard cutoff value was used, the sensitivity and specificity regarding the prediction of gastric cancer development was 58.7% (95% CI, 45.6% to 70.8%) and 73.4% (95% CI, 72.1% to 74.6%), respectively. A stepwise increase in specificity with a decrease in sensitivity was observed according to the strict criteria. Terasawa et al.48 reported the pooled sensitivity and specificity was 0.57 (95% CI, 0.49 to 0.65) and 0.76 (95% CI, 0.69 to 0.81), respectively based on the above-mentioned eight studies including 32,766 subjects. Although a combination method of serum PG test and H. pylori antibody (so called ABC classification) is the expected approach of the high sensitivity for the discrimination the risk of gastric cancer means, the decrease of specificity could not be avoided. In the case-control dataset from a large cohort study, prediction sensitivity and specificity of serum PG testing were 81.9% and 42.1%, respectively.47 The sensitivity and specificity of the combination method with the standard cutoff values were 97.2% and 21.1%, respectively.47 The area under the curve of the combination method was always lower than that of PG I/II, even when the cutoff values were changed. However, the results of the serum PG test are easily affected by proton inhibitors and non-steroidal anti-inflammatory drugs.75-77
After eradication, most results of the serum PG test became negative but the results of some PG-positive cases remained.78 The PG I and PG II levels were changed after treatment, but the PG I/II level was relatively stable and remained lower.79 Yanaoka et al.78 reported the use of the serum PG test after H. pylori eradication based on a 6-year follow-up. Although the results of the serum PG testing after H. pylori eradication became negative in most subjects, extensive chronic gastritis remained in subjects with PG-positive results, suggesting a risk of gastric cancer. When the cutoff value was changed, the high-risk group could be divided by PG I/II and the accuracy of the serum PG test was moderate (sensitivity, 65.9%; specificity, 79.3%).80 On the other hand, the Taiwan study reported that the level of PG I/II increased and moderately predicted gastric cancer development after the H. pylori eradication.81 Although there are some limitations in the use of the serum PG test, it might be a useful indicator of the risk for gastric cancer after H. pylori eradication.
2. Endoscopic screening
Chronic atrophic gastritis is a significant risk factor of gastric cancer,82-86 and it can be diagnosed by endoscopic examination. The Kimura and Takemoto classification of gastritis has commonly been used in clinical practice in Japan.87 The diagnosis of H. pylori infection and the category of gastric atrophy have been disseminated since the publication of the Kyoto global consensus report.88 Risk stratification of gastric atrophy could be easily applied in endoscopic screening. On the other hand, according to the dissemination of H. pylori eradication, subjects can be included after the treatment in endoscopic screening. Gastric cancer risk remained in chronic atrophic gastritis and intestinal metaplasia after completing eradication.78,89-91 In addition, there is another potential to develop gastric cancers in the non-atrophic area even if eradication is successful.81 Endoscopic screening will involve subjects with various background risks for gastric cancer, including individuals after eradication.
The Japanese study reported the results of risk stratification based on the endoscopic screening.92 The subjects were divided into the three types of gastric mucosa based on the Kimura and Takemoto classification; absence and slight atrophy (C1), medium atrophy (C2 and C3), and severe atrophy (O-1, O-2, and O-3). The distribution of the results was 44.4% for slight atrophy, 28.6% for medium atrophy, and 27.1% for severe atrophy. Annual progression rates depended on the severity, which was 0.10%, 0.16%, and 0.31%, respectively. The results suggested the possibility of adopting risk stratification using graded atrophy by endoscopic examination in population-based screening. A recent study examining the expansion of the screening interval has been started in Japan, which has taken into consideration the background risk.93 At the index screening, individual risks are divided into high-risk and low-risk groups based on the results of endoscopic diagnosis. The screening intervals were arranged by their risks and can be expanded for low-risk group. Risk stratification will be helpful in decreasing individual lifetime frequencies and harms of endoscopic screening for gastric cancer. It is also useful for promoting the efficient use of limited resources at the population level.
DISCUSSION
Although gastric cancer is still a heavy burden worldwide, the recent advance of technology has provided several options, including endoscopic screening and screen-and-treat strategy. When we consider the introduction of gastric cancer prevention, present and future burdens should be divided for priority setting in limited resources. Although the IARC recommended the screen-and-treat strategy in high-incidence countries,44 there is a time lag in reducing mortality after decreased gastric cancer incidence. The prevalence of H. pylori infection has reduced mainly in developed countries, and it is expected to reduce incidence of gastric cancer in the future.94 In countries with high incidence of gastric cancer, reducing the present burden of gastric cancer death is a matter of prior, and endoscopic screening can be a good solution. On the other hand, the prevalence of H. pylori infection has been high, investing in the future is required to reduce gastric cancer incidence. Although the screen-and-treat strategy is expected to reduce the future incidence of gastric cancer, it is not helpful in reducing present mortality from gastric cancer. A marked reduction in the substantial burden from gastric cancer is usually expected in high-incidence countries when cancer screening is introduced. The Japanese setting is a good example to consider priority setting of gastric cancer prevention. The prevalence of H. pylori infection in Japan is predicted to fall below 10% in subjects born in 2000.95 However, the incidence of gastric cancer is still high, and the number of deaths from gastric cancer reached 42,931 in 2019.96 Mortality reduction from gastric cancer is still needed, and gastric cancer screening still retains a significant role in cancer control. In Japan, H. pylori eradication is covered by national health insurance, individuals with H. pylori infection can be treated in clinical practice. Population program for screen-and-treat is the second option and depends on limited resources. Since the final goals are different between screen-and-treat strategy and endoscopic screening, a population program should be required to perform a shared role. Compatible population programs with endoscopic screening for the older people and screen-and-treat for the younger people might be available if there are enough resources. Based on the prediction of H. pylori infection and incidence of gastric cancer, assessment of the appropriate target population should also be considered from a balance of benefits, harms, and resources in each country.
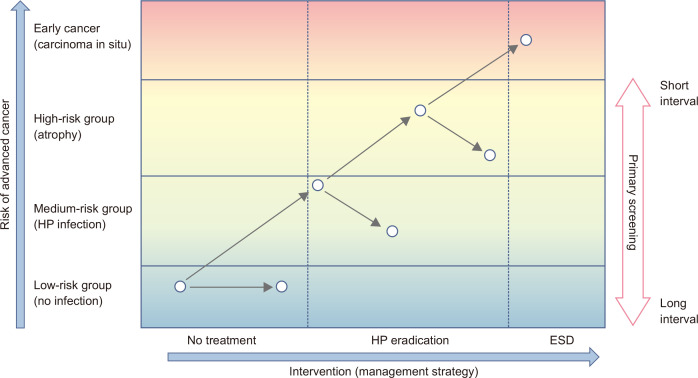
Although endoscopic screening is established based on the traditional concept of cancer screening, combining it with risk stratification might be an efficient way. There is a possibility to include various individuals with different levels of gastric cancer risk (Fig. 2). Individuals with H. pylori infection have gastric cancer risk, and the level of risk increases according to the severity of gastric atrophy.78,81 Even if they receive H. pylori eradication, their risk remains.78,89-91 They are often involved in screening programs if surveillance after H. pylori eradication has not been established. Although the actual target of gastric cancer screening is the average risk population, there are no universal thresholds targeting individuals for gastric cancer screening. The threshold depends on many factors, including the burden of gastric cancer, the healthcare system, and resources. The serum PG test and endoscopic examination have made it possible to divide high-risk subjects with severe gastric atrophy from average-risk subjects. Both methods can be used for the risk stratification of gastric cancer regardless of H. pylori eradication.78,81,90,91 Although serum PG test has been expected to be used as a primary screening method the low specificity is a barrier. However, it can be adopted as a triage for background risk for individuals for endoscopic screening if endoscopic diagnosis is difficult to define the severity of gastric atrophy. There is some potential for risk stratification using the serum PG test and endoscopic diagnosis for predicting gastric cancer development. If the background risk can be confirmed by endoscopic examinations, screening intervals could be arranged based on their risk (Fig. 2). Intensive screening with short screening intervals is beneficial for the high-risk group to decrease interval cancers, and the low-risk group can avoid harm by expanding screening intervals. It can decrease the frequency of lifetime screening and improve accessibility in limited resources. Beyond the individual usage of serological testing for risk stratification, combining the basic concept with established screening methods will also be useful.
Fig. 2.
Risk-stratified management for gastric cancer. The basic concept of risk-stratified management for gastric cancer is shown. The risk of gastric cancer is shown on the y-axis with intervention (management strategy) on the x-axis. Clinically relevant strata are shown from average risk to early cancer. On the population level, the risk of gastric cancer is basically low, and they are the main target population for cancer screening. However, gastric cancer screening is performed for subjects with various backgrounds. If the background risk is classified, the screening interval can be changed according to the background risk. Ideally, subjects with a high risk are screened at short intervals and subjects with low risk are screened at long intervals. However, the risk threshold of individuals for screening may vary between the healthcare systems and disease burden among countries.
HP, Helicobacter pylori; ESD, endoscopic mucosal resection.
The contexts of gastric cancer vary among countries. Mainly, the burden of gastric cancer and the availability of resources usually affect the introduction of a new prevention program. In the countries with established cancer screening programs, cancer screening in combination with risk stratification might prove to be a novel and efficient strategy for gastric cancer prevention. When cancer screening is introduced, we should always consider the balance of benefits and harms, and several strategies have been adopted to maximize the real benefits (Fig. 1). Risk stratification might be helpful to avoid harm decreasing the screening frequency considering gastric cancer risk. Since there is no one-size-fits-all solution for gastric cancer prevention, the IARC has suggested the need to consider the context of each country.44
CONCLUSIONS
Gastric cancer has remained a heavy burden worldwide. Unfortunately, gastric cancer prevention strategies have also remained limited. Fortunately, recent advances have offered new strategies for reducing the burden of gastric cancer not only in East Asian countries but also in other countries. Endoscopic screening has been adopted and performed as a national program in South Korea and Japan, with promising results of gastric cancer mortality reduction. The screen-and-treat strategy has also been expected to reduce the incidence of gastric cancer. As the natural history of gastric cancer is elucidated, risk stratification is highly anticipated to be a novel approach that can be used in combination with screening. Risk stratification has the potential to be a good management tool to decrease the harms of cancer screening. The countries with a high incidence have realized the immediate need to reduce gastric cancer directly and have recognized screen-and-treat as a second option to reduce future risk. Further study is needed to obtain sufficient evidence regarding this novel strategy, emphasizing the best available method should be examined in the context of each country.
ACKNOWLEDGEMENTS
This study was financially supported by a grant from the Japan Agency of Medical Research and Development Tokyo, Japan (grant number: 20ck0106527h).
The author is grateful to Dr. Edward F. Barroga (https://orcid.org/0000-0002-8920-2607), Medical and Nursing Science Editor and Professor of Academic Writing at St. Luke’s International University for reviewing and editing the manuscript. The author also appreciates Mr. Joshua Keane for proofreading the final manuscript.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.International Agency for Research on Cancer (IARC), author Cancer Today, 2020 [Internet] IARC; Lyon: c2020. [cited 2020 Oct 3]. Available from: https://gco.iarc.fr/today/home . [Google Scholar]
- 2.Shah SC, McKinley M, Gupta S, Peek RM, Jr, Martinez ME, Gomez SL. Population-based analysis of differences in gastric cancer incidence among races and ethnicities in individuals age 50 years and older. Gastroenterology. 2020;159:1705–1714. doi: 10.1053/j.gastro.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang RJ, Sharp N, Talamoa R, et al. Disaggregated mortality from gastrointestinal cancers in Asian Americans: analysis of United States death records. Int J Cancer. 2021;148:2954–2963. doi: 10.1002/ijc.33490. [DOI] [PubMed] [Google Scholar]
- 4.Liou JM, Malfertheiner P, Lee YC, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69:2093–2112. doi: 10.1136/gutjnl-2020-322368. [DOI] [PubMed] [Google Scholar]
- 5.Cuschieri K, Ronco G, Lorincz A, et al. Eurogin roadmap 2017: triage strategies for the management of HPV-positive women in cervical screening programs. Int J Cancer. 2018;143:735–745. doi: 10.1002/ijc.31261. [DOI] [PubMed] [Google Scholar]
- 6.Chen LS, Yen AM, Chiu SY, Liao CS, Chen HH. Baseline faecal occult blood concentration as a predictor of incident colorectal neoplasia: longitudinal follow-up of a Taiwanese population-based colorectal cancer screening cohort. Lancet Oncol. 2011;12:551–558. doi: 10.1016/S1470-2045(11)70101-2. [DOI] [PubMed] [Google Scholar]
- 7.Park HA, Nam SY, Lee SK, et al. The Korean guideline for gastric cancer screening. J Korean Med Assoc. 2015;58:373–384. doi: 10.5124/jkma.2015.58.5.373. [DOI] [Google Scholar]
- 8.Hamashima C Systematic Review Group and Guideline Development Group for Gastric Cancer Screening Guidelines, author. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J Clin Oncol. 2018;48:673–683. doi: 10.1093/jjco/hyy077. [DOI] [PubMed] [Google Scholar]
- 9.Hamashima C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn J Clin Oncol. 2018;48:278–286. doi: 10.1093/jjco/hyx190. [DOI] [PubMed] [Google Scholar]
- 10.Song M, Kang D, Yang JJ, et al. Age and sex interactions in gastric cancer incidence and mortality trends in Korea. Gastric Cancer. 2015;18:580–589. doi: 10.1007/s10120-014-0411-x. [DOI] [PubMed] [Google Scholar]
- 11.Katanoda K, Hori M, Saito E, et al. Updated trends in cancer in Japan: incidence in 1985-2015 and mortality in 1958-2018-a sign of decrease in cancer incidence. J Epidemiol. 2021;31:426–450. doi: 10.2188/jea.JE20200416.53bf69528beb4e509161df9683d55c4c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang RJ, Koh H, Hwang JH Summit Leaders, author. A summary of the 2020 Gastric Cancer Summit at Stanford University. Gastroenterology. 2020;159:1221–1226. doi: 10.1053/j.gastro.2020.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liou JM, Lin JT, Wang HP, et al. The optimal age threshold for screening upper endoscopy for uninvestigated dyspepsia in Taiwan, an area with a higher prevalence of gastric cancer in young adults. Gastrointest Endosc. 2005;61:819–825. doi: 10.1016/S0016-5107(05)00366-4. [DOI] [PubMed] [Google Scholar]
- 14.Leung WK, Wu MS, Kakugawa Y, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the National Cancer Screening Programme and the cancer screening status in Korea. Asian Pac J Cancer Prev. 2011;12:725–730. [PubMed] [Google Scholar]
- 16.Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut. 2013;62:676–682. doi: 10.1136/gutjnl-2012-302240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Q, Yu L, Hao CQ, et al. Effectiveness of endoscopic gastric cancer screening in a rural area of Linzhou, China: results from a case-control study. Cancer Med. 2016;5:2615–2622. doi: 10.1002/cam4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotoda T, Ishikawa H, Ohnishi H, et al. Randomized controlled trial comparing gastric cancer screening by gastrointestinal X-ray with serology for Helicobacter pylori and pepsinogens followed by gastrointestinal endoscopy. Gastric Cancer. 2015;18:605–611. doi: 10.1007/s10120-014-0408-5. [DOI] [PubMed] [Google Scholar]
- 19.Xiao HF, Yan SP, Chen YF, et al. Community-based upper gastrointestinal cancer screening in a randomized controlled trial: baseline results in a non-high-incidence area. Cancer Prev Res (Phila) 2020;13:317–328. doi: 10.1158/1940-6207.CAPR-19-0422. [DOI] [PubMed] [Google Scholar]
- 20.Riecken B, Pfeiffer R, Ma JL, et al. No impact of repeated endoscopic screens on gastric cancer mortality in a prospectively followed Chinese population at high risk. Prev Med. 2002;34:22–28. doi: 10.1006/pmed.2001.0925. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Hwang Y, Sung H, et al. Effectiveness of gastric cancer screening on gastric cancer incidence and mortality in a community-based prospective cohort. Cancer Res Treat. 2018;50:582–589. doi: 10.4143/crt.2017.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suh YS, Lee J, Woo H, et al. National cancer screening program for gastric cancer in Korea: nationwide treatment benefit and cost. Cancer. 2020;126:1929–1939. doi: 10.1002/cncr.32753. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto S, Yamasaki K, Tsuji K, Shirahama S. Results of mass endoscopic examination for gastric cancer in Kamigoto Hospital, Nagasaki Prefecture. World J Gastroenterol. 2007;13:4316–4320. doi: 10.3748/wjg.v13.i32.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosokawa O, Miyanaga T, Kaizaki Y, et al. Decreased death from gastric cancer by endoscopic screening: association with a population-based cancer registry. Scand J Gastroenterol. 2008;43:1112–1115. doi: 10.1080/00365520802085395. [DOI] [PubMed] [Google Scholar]
- 25.Hamashima C, Shabana M, Okada K, Okamoto M, Osaki Y. Mortality reduction from gastric cancer by endoscopic and radiographic screening. Cancer Sci. 2015;106:1744–1749. doi: 10.1111/cas.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamashima C, Ogoshi K, Narisawa R, et al. Impact of endoscopic screening on mortality reduction from gastric cancer. World J Gastroenterol. 2015;21:2460–2466. doi: 10.3748/wjg.v21.i8.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamashima C, Ogoshi K, Okamoto M, Shabana M, Kishimoto T, Fukao A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One. 2013;8:e79088. doi: 10.1371/journal.pone.0079088.4275dcf6748e4e63805561b9533700ec [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto S, Yoshida Y. Efficacy of endoscopic screening in an isolated island: a case-control study. Indian J Gastroenterol. 2014;33:46–49. doi: 10.1007/s12664-013-0378-2. [DOI] [PubMed] [Google Scholar]
- 29.Jun JK, Choi KS, Lee HY, et al. Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology. 2017;152:1319–1328. doi: 10.1053/j.gastro.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Li M, Chen S, et al. Endoscopic screening in Asian countries is associated with reduced gastric cancer mortality: a meta-analysis and systematic review. Gastroenterology. 2018;155:347–354. doi: 10.1053/j.gastro.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Hamashima C. Benefits and harms of endoscopic screening for gastric cancer. World J Gastroenterol. 2016;22:6385–6392. doi: 10.3748/wjg.v22.i28.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamashima C, Fukao A. Quality assurance manual of endoscopic screening for gastric cancer in Japanese communities. Jpn J Clin Oncol. 2016;46:1053–1061. doi: 10.1093/jjco/hyw106. [DOI] [PubMed] [Google Scholar]
- 33.Hamashima C, Okamoto M, Shabana M, Osaki Y, Kishimoto T. Sensitivity of endoscopic screening for gastric cancer by the incidence method. Int J Cancer. 2013;133:653–659. doi: 10.1002/ijc.28065. [DOI] [PubMed] [Google Scholar]
- 34.Hubbard RA, Johnson E, Hsia R, Rutter CM. The cumulative risk of false-positive fecal occult blood test after 10 years of colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2013;22:1612–1619. doi: 10.1158/1055-9965.EPI-13-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamashima C, Sobue T, Muramatsu Y, Saito H, Moriyama N, Kakizoe T. Comparison of observed and expected numbers of detected cancers in the research center for cancer prevention and screening program. Jpn J Clin Oncol. 2006;36:301–308. doi: 10.1093/jjco/hyl022. [DOI] [PubMed] [Google Scholar]
- 36.Harris RP, Wilt TJ, Qaseem A High Value Care Task Force of the American College of Physicians, author. A value framework for cancer screening: advice for high-value care from the American College of Physicians. Ann Intern Med. 2015;162:712–717. doi: 10.7326/M14-2327. [DOI] [PubMed] [Google Scholar]
- 37.Hamashima C, Goto R. Potential capacity of endoscopic screening for gastric cancer in Japan. Cancer Sci. 2017;108:101–107. doi: 10.1111/cas.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC's survey of endoscopic capacity. Gastroenterology. 2004;127:1670–1677. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 39.Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004;127:1661–1669. doi: 10.1053/j.gastro.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 40.van Turenhout ST, Terhaar sive Droste JS, Meijer GA, Masclée AA, Mulder CJ. Anticipating implementation of colorectal cancer screening in the Netherlands: a nation wide survey on endoscopic supply and demand. BMC Cancer. 2012;12:46. doi: 10.1186/1471-2407-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joseph DA, Meester RG, Zauber AG, et al. Colorectal cancer screening: estimated future colonoscopy need and current volume and capacity. Cancer. 2016;122:2479–2486. doi: 10.1002/cncr.30070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Comas M, Mendivil J, Andreu M, Hernández C, Castells X. Long-term prediction of the demand of colonoscopies generated by a population-based colorectal cancer screening program. PLoS One. 2016;11:e0164666. doi: 10.1371/journal.pone.0164666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andermann A, Blancquaert I, Beauchamp S, Déry V. Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Bull World Health Organ. 2008;86:317–319. doi: 10.2471/BLT.07.050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.IARC Helicobacter pylori Working Group, author. Helicobacter pylori eradication as a strategy for preventing gastric cancer: IARC Working Group Report Volume 8. International Agency for Research on Cancer; Lyon: 2014. [DOI] [Google Scholar]
- 45.O'Connor A, O'Morain CA, Ford AC. Population screening and treatment of Helicobacter pylori infection. Nat Rev Gastroenterol Hepatol. 2017;14:230–240. doi: 10.1038/nrgastro.2016.195. [DOI] [PubMed] [Google Scholar]
- 46.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process: First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 47.Hamashima C, Sasazuki S, Inoue M, Tsugane S JPHC Study Group, author. Receiver operating characteristic analysis of prediction for gastric cancer development using serum pepsinogen and Helicobacter pylori antibody tests. BMC Cancer. 2017;17:183. doi: 10.1186/s12885-017-3173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terasawa T, Nishida H, Kato K, et al. Prediction of gastric cancer development by serum pepsinogen test and Helicobacter pylori seropositivity in Eastern Asians: a systematic review and meta-analysis. PLoS One. 2014;9:e109783. doi: 10.1371/journal.pone.0109783.ea99ad424f994b58a4aec134a3cc60f1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuo YT, Liou JM, El-Omar EM, et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:707–715. doi: 10.1016/S2468-1253(17)30219-4. [DOI] [PubMed] [Google Scholar]
- 50.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 2018;155:1372–1382. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lane JA, Murray LJ, Harvey IM, Donovan JL, Nair P, Harvey RF. Randomised clinical trial: Helicobacter pylori eradication is associated with a significantly increased body mass index in a placebo-controlled study. Aliment Pharmacol Ther. 2011;33:922–929. doi: 10.1111/j.1365-2036.2011.04610.x. [DOI] [PubMed] [Google Scholar]
- 52.Wu JC, Chan FK, Ching JY, et al. Effect of Helicobacter pylori eradication on treatment of gastro-oesophageal reflux disease: a double blind, placebo controlled, randomised trial. Gut. 2004;53:174–179. doi: 10.1136/gut.2003.012641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104:488–492. doi: 10.1093/jnci/djs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 55.Wong BC, Zhang L, Ma JL, et al. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut. 2012;61:812–818. doi: 10.1136/gutjnl-2011-300154. [DOI] [PubMed] [Google Scholar]
- 56.Zhou L, Lin S, Ding S, et al. Relationship of Helicobacter pylori eradication with gastric cancer and gastric mucosal histological changes: a 10-year follow-up study. Chin Med J (Engl) 2014;127:1454–1458. [PubMed] [Google Scholar]
- 57.Saito D, Boku N, Fujioka T, et al. Impact of H-pylori eradication on gastric atrophy: current status of the Japanese intervention trial (JITHP study). Paper presented at: 6th International Gastric Cancer Congress; 2005 May 4-7; Yokohama, Japan. pp. 131–136. [Google Scholar]
- 58.Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–1888. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- 59.Tang J, Chen Y, Loke Y, et al. The effects of Helicobacter pylori eradication on histological changes of gastric mucosa and gastric cancer incidence: a three-year follow-up study. Mod Dig Intervention. 2010;15:47–49. [Google Scholar]
- 60.Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. doi: 10.1136/bmj.g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016;150:1113–1124. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 62.Chen HN, Wang Z, Li X, Zhou ZG. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric Cancer. 2016;19:166–175. doi: 10.1007/s10120-015-0462-7. [DOI] [PubMed] [Google Scholar]
- 63.Terasawa T, Hamashima C, Kato K, et al. Helicobacter pylori eradication treatment for gastric carcinoma prevention in asymptomatic or dyspeptic adults: systematic review and Bayesian meta-analysis of randomised controlled trials. BMJ Open. 2019;9:e026002. doi: 10.1136/bmjopen-2018-026002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiang TH, Chang WJ, Chen SL, et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut. 2021;70:243–250. doi: 10.1136/gutjnl-2020-322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leja M, Park JY, Murillo R, et al. Multicentric randomised study of Helicobacter pylori eradication and pepsinogen testing for prevention of gastric cancer mortality: the GISTAR study. BMJ Open. 2017;7:e016999. doi: 10.1136/bmjopen-2017-016999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee YC, Chiang TH, Chiu HM, et al. Community-based gastric cancer screening coupled with a National Colorectal Cancer Screening Program: baseline results. Gastroenterology. 2021;160:2159–2161. doi: 10.1053/j.gastro.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 67.BioMed Central, author. ISRCTN registry. ISRCTN71557037: Helicobacter pylori screening study [Internet] BioMed Central; London: c2019. [cited 2020 Oct 3]. Available from: https://doi.org/10.1186/ISRCTN71557037 . [DOI] [Google Scholar]
- 68.Hattori Y, Tashiro H, Kawamoto T, Kodama Y. Sensitivity and specificity of mass screening for gastric cancer using the measurment of serum pepsinogens. Jpn J Cancer Res. 1995;86:1210–1215. doi: 10.1111/j.1349-7006.1995.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kitahara F, Kobayashi K, Sato T, Kojima Y, Araki T, Fujino MA. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut. 1999;44:693–697. doi: 10.1136/gut.44.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song M, Camargo MC, Weinstein SJ, et al. Serum pepsinogen 1 and anti-Helicobacter pylori IgG antibodies as predictors of gastric cancer risk in Finnish males. Aliment Pharmacol Ther. 2018;47:494–503. doi: 10.1111/apt.14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138–143. doi: 10.1002/ijc.11680. [DOI] [PubMed] [Google Scholar]
- 72.Yanaoka K, Oka M, Mukoubayashi C, et al. Cancer high-risk subjects identified by serum pepsinogen tests: outcomes after 10-year follow-up in asymptomatic middle-aged males. Cancer Epidemiol Biomarkers Prev. 2008;17:838–845. doi: 10.1158/1055-9965.EPI-07-2762. [DOI] [PubMed] [Google Scholar]
- 73.Baak LC, Jansen JB, Biemond I, Lamers CB. Weekend treatment with 20 and 40 mg omeprazole: effect on intragastric pH, fasting and postprandial serum gastrin, and serum pepsinogens. Gut. 1991;32:977–982. doi: 10.1136/gut.32.9.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baak LC, Biemond I, Jansen JB, Lamers CB. Repeated intravenous bolus injections of omeprazole: effects on 24-hour intragastric pH, serum gastrin, and serum pepsinogen A and C. Scand J Gastroenterol. 1991;26:737–746. doi: 10.3109/00365529108998593. [DOI] [PubMed] [Google Scholar]
- 75.Shan J, Lei H, Shi W, Sun X, Tang Y, Ren C. High serum pepsinogen I and beta Helicobacter pylori infection are risk factors for aspirin-induced gastroduodenal injury. Dig Dis. 2018;36:66–71. doi: 10.1159/000477203. [DOI] [PubMed] [Google Scholar]
- 76.Samloff IM, Secrist DM, Passaro E., Jr A study of the relationship between serum group I pepsinogen levels and gastric acid secretion. Gastroenterology. 1975;69:1196–1200. doi: 10.1016/S0016-5085(19)32321-2. [DOI] [PubMed] [Google Scholar]
- 77.Iijima K, Koike T, Ara N, et al. Identification of a high-risk group for low-dose aspirin-induced gastropathy by measuring serum pepsinogen in H. pylori-infected subjects. J Gastroenterol. 2015;50:305–312. doi: 10.1007/s00535-014-0976-5. [DOI] [PubMed] [Google Scholar]
- 78.Yanaoka K, Oka M, Ohata H, et al. Eradication of Helicobacter pylori prevents cancer development in subjects with mild gastric atrophy identified by serum pepsinogen levels. Int J Cancer. 2009;125:2697–2703. doi: 10.1002/ijc.24591. [DOI] [PubMed] [Google Scholar]
- 79.Ohkusa T, Miwa H, Nomura T, et al. Improvement in serum pepsinogens and gastrin in long-term monitoring after eradication of Helicobacter pylori: comparison with H. pylori-negative patients. Aliment Pharmacol Ther. 2004;20 Suppl 1:25–32. doi: 10.1111/j.1365-2036.2004.01970.x. [DOI] [PubMed] [Google Scholar]
- 80.Haneda M, Kato M, Ishigaki S, et al. Identification of a high risk gastric cancer group using serum pepsinogen after successful eradication of Helicobacter pylori. J Gastroenterol Hepatol. 2013;28:78–83. doi: 10.1111/j.1440-1746.2012.07285.x. [DOI] [PubMed] [Google Scholar]
- 81.Chiang TH, Maeda M, Yamada H, et al. Risk stratification for gastric cancer after Helicobacter pylori eradication: a population-based study on Matsu Islands. J Gastroenterol Hepatol. 2021;36:671–679. doi: 10.1111/jgh.15187. [DOI] [PubMed] [Google Scholar]
- 82.Siurala M, Varis K, Wiljasalo M. Studies of patients with atrophic gastritis: a 10-15-year follow-up. Scand J Gastroenterol. 1966;1:40–48. doi: 10.1080/00365521.1966.11800612. [DOI] [PubMed] [Google Scholar]
- 83.Meister H, Holubarsch C, Haferkamp O, Schlag P, Herfarth C. Gastritis, intestinal metaplasia and dysplasia versus benign ulcer in stomach and duodenum and gastric carcinoma: a histotopographical study. Pathol Res Pract. 1979;164:259–269. doi: 10.1016/S0344-0338(79)80048-5. [DOI] [PubMed] [Google Scholar]
- 84.Sipponen P, Kekki M, Haapakoski J, Ihamäki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: statistical calculations of cross-sectional data. Int J Cancer. 1985;35:173–177. doi: 10.1002/ijc.2910350206. [DOI] [PubMed] [Google Scholar]
- 85.Testoni PA, Masci E, Marchi R, Guslandi M, Ronchi G, Tittobello A. Gastric cancer in chronic atrophic gastritis: associated gastric ulcer adds no further risk. J Clin Gastroenterol. 1987;9:298–302. doi: 10.1097/00004836-198706000-00011. [DOI] [PubMed] [Google Scholar]
- 86.Tatsuta M, Iishi H, Nakaizumi A, et al. Fundal atrophic gastritis as a risk factor for gastric cancer. Int J Cancer. 1993;53:70–74. doi: 10.1002/ijc.2910530114. [DOI] [PubMed] [Google Scholar]
- 87.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87–97. doi: 10.1055/s-0028-1098086. [DOI] [Google Scholar]
- 88.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mera R, Fontham ET, Bravo LE, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536–1540. doi: 10.1136/gut.2005.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kamada T, Hata J, Sugiu K, et al. Clinical features of gastric cancer discovered after successful eradication of Helicobacter pylori: results from a 9-year prospective follow-up study in Japan. Aliment Pharmacol Ther. 2005;21:1121–1126. doi: 10.1111/j.1365-2036.2005.02459.x. [DOI] [PubMed] [Google Scholar]
- 91.Kodama M, Murakami K, Okimoto T, et al. Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol. 2012;47:394–403. doi: 10.1007/s00535-011-0504-9. [DOI] [PubMed] [Google Scholar]
- 92.Kaji K, Hashiba A, Uotani C, et al. Grading of atrophic gastritis is useful for risk stratification in endoscopic screening for gastric cancer. Am J Gastroenterol. 2019;114:71–79. doi: 10.1038/s41395-018-0259-5. [DOI] [PubMed] [Google Scholar]
- 93.Hamashima C, Yoshimura K, Fukao A. A study protocol for expanding the screening interval of endoscopic screening for gastric cancer based on individual risks: prospective cohort study of gastric cancer screening. Ann Transl Med. 2020;8:1604. doi: 10.21037/atm-20-5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sonnenberg A. Review article: historic changes of Helicobacter pylori-associated diseases. Aliment Pharmacol Ther. 2013;38:329–342. doi: 10.1111/apt.12380. [DOI] [PubMed] [Google Scholar]
- 95.Wang C, Nishiyama T, Kikuchi S, et al. Changing trends in the prevalence of H. pylori infection in Japan (1908-2003): a systematic review and meta-regression analysis of 170,752 individuals. Sci Rep. 2017;7:15491. doi: 10.1038/s41598-017-15490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.National Cancer Center, author. Center for Cancer Control and Information Services [Internet] National Cancer Center; Tokyo: c2019. [cited 2021 Oct 3]. Available from: https://ganjoho.jp/public/index.html . [Google Scholar]