Abstract
Phytoremediation is an eco-friendly biotechnology with low costs. The removal of copper (Cu) from polluted water by the two floating plant species Azolla filiculoides and Lemna minor was observed and recorded. Plants were exposed to different Cu (II) concentration (0.25–1.00 mg/L) and sampling time (Days 0, 1, 2, 5 and 7). Both plants can remove Cu at 1.00 mg Cu/L water, with the highest removal rates of 100% for A. filiculoides and 74% for L. minor on the fifth day of exposure. At the end of the exposure period (Day 7), the growth of A. filiculoides exposed to 1.00 mg Cu/L was inhibited by Cu, but the structure of the inner cells of A. filiculoides was well organized as compared to the initial treatment period. Regarding L. minor, Cu at 1.00 mg/L negatively impacted both the growth and morphology (shrinking of its inner structure) of this plant. This is due to the higher accumulation of Cu in L. minor (2.86 mg/g) than in A. filiculoides (1.49 mg/g). Additionally, the rate of Cu removal per dry mass of plant fitted a pseudo-second order model for both plants, whereas the adsorption equilibrium data fitted the Freundlich isotherm, indicating that Cu adsorption occurs in multiple layers. Based on the results, both species can be applied in the phytoremediation of Cu-polluted water.
Keywords: Heavy metals, Phytoremediation, Floating plant, Bioconcentration factor, Batch system
Heavy metals; Phytoremediation; Floating plant; Bioconcentration factor; Batch system.
1. Introduction
Copper (Cu) is one of the most toxic heavy metals and classified as a trace element (Bhat et al., 2022; Ghosh, 2010). In wastewater, Cu is generally derived from instruments, electroplating, glass, metal, ceramics, pipe infrastructure and plumbing activities (Ali et al., 2016). The discharge of industrial effluents, especially those containing toxic heavy metals, has a detrimental impact on aquatic environments and the survival of organisms, negatively impacting the food chain and human health (Titah et al., 2019). Excess exposure towards Cu can result in brain and kidney injury, liver cirrhosis, as well as stomach and intestinal inflammation (Wuana and Okieimen, 2011). In this sense, methods for the efficient treatment of wastewater effluents containing heavy metals are crucial.
Many treatment methods, such as physical, chemical and biological approaches, have been applied to remove heavy metals from industrial effluents (Rego et al., 2022). Of these, biosorption is a biological method for the removal and recovery of heavy metals from wastewater and characterised by a high-cost efficiency (Rahman and Hasegawa, 2011). This approach can be accomplished via microorganisms (Titah et al., 2018a; Huang et al., 2018; Hasan et al., 2016; Halmi et al., 2017; Subari et al., 2018; Kamaruzzaman et al., 2019) and plants (Ismail et al., 2017; Titah et al., 2018b; Tangahu et al., 2013; Selamat et al., 2018). Compared to physical and chemical treatment techniques such as coagulation-flocculation (Haan et al., 2018), membrane filtration, adsorption (Sahsiny et al., 2021), oxidation, chemical precipitation, ion-exchange and electrochemical methods, biosorption is more environmentally friendly (Panfili et al., 2017; Tangahu et al., 2013).
As a green biotechnology, biosorption with available floating plants has many advantages including its low cost, aesthetic values and simple operation when applied in constructed wetlands (Mutar et al., 2022; Arán et al., 2017). Several floating plants can accumulate heavy metals (Pb, Fe, Cu, Cd, Cr, Zn, Ni and As), such as Salvinia auriculata (Panfili et al., 2017; Espinoza-Qui˜nones et al., 2009), Lemna minor (Panfili et al., 2017), Lemna minuta and Azolla filiculoides (Bianchi et al., 2020), Pistia stratiotes (water lettuce), Spirodela polyrhiza (duckweed) and Eichhornia crassipes (water hyacinth) (Rai, 2019). However, the potential of A. filiculoides as a phytoremediation plant to remove Cu has not been investigated. Adsorption kinetics describes the rate at which a solute is adsorbed and the resident time of the adsorbates on the solid-liquid interface, whereas adsorption isotherms play an important role in determining the interaction between adsorbate and adsorbent and the optimum adsorption capacity of the adsorbent (Musah et al., 2022). In this study, A. filiculoides and L. minor were exposed to Cu-contaminated water to evaluate their phytoremediation potential along with the adsorption kinetics and isotherms.
2. Material and methods
2.1. Experimental set up for Cu exposure
Two perennial floating plants, A. filiculoides and L. minor, were studied regarding their ability to accumulate Cu within 7 days. A. filiculoides belongs to the Salviniaceace family (Dohaei et al., 2020) while L. minor on the other side belongs to Lemnaceae family (Ifayefunmi et al., 2021). Both plants have the ability to grow fast (Sathish et al., 2022) with high growth rate of 0.5 day−1 and doubling biomass time of 2 days (Chakrabarti et al., 2018; Dohaei et al., 2020).
The experiment was performed in the laboratory at a temperature range from 21–27 °C at Universiti Kebangsaan Malaysia. Both A. filiculoides and L. minor were propagated in a greenhouse in Hoagland nutrient medium (Costa et al., 2009; Khvatkov et al., 2019) for 7 days before being exposed to Cu-contaminated water (Figure 1S(a)). Subsequently, young specimens of A. filiculoides and L. minor were harvested and washed with fresh water (Figure 1S(b)) and allowed to achieve the log phase (rapid growth). According to Paul et al. (2021) and Kittiwongwattana and Vuttipongchaikij (2013), during this period, these plants achieved the log phase. According to Norhan et al. (2021), in the stationary phase, the plants grow slower than in the log phase, with a lower phytoremediation efficiency.
Each fresh plant was weighed (3 g wet weight) and transferred into a 100-mL glass container (6 cm in diameter) filled with 50 mL of water contaminated with different Cu concentrations (0, 0.25, 0.50, 0.75 and 1.00 mg/L). This weight represents 75% of the water surface of each container, with a density of 1.06 kg/m2, avoiding congestion. According to Van Hove (1989), a density of more than 2 kg of wet weight per m2 can result in congestion. Additional containers with plants and tap water were used as control (0 mg Cu/L), whereas the containers with the different Cu solutions were used as contaminant treatments. Each Cu concentration was tested in triplicate. Figure 2S illustrates the overall setup of this study for one plant species. The Cu solution was prepared by diluting 0.86 g of analytical-grade CuSO4.7H2O salt (Systerm, Malaysia) in 1 L of water to prepare a 1,000-mg/L of Cu (II) stock solution. This concentration was selected based on a preliminary study determining the Cu range in which plants can survive (Table 1S).
2.2. Water and plant analysis
Wastewater and plant samples were collected every 0, 1, 2, 5 and 7 days for analysis of the Cu concentration. At each sampling day, three replicate samples of water and each plant were sampled. Water analysis was performed in accordance to the APHA (2017). The water quality parameters pH and oxidization reduction potential (ORP, mV) were observed at each sampling day using a multi-probe IQ 150 (I.Q Scientific Instruments, U.K.). Plant samples were dried in a drying oven (MMM Laboratory Oven, Venti cell 707 Comfort) at 70 °C for 24 h.
Copper was extracted according to Ismail et al. (2019). First, the dried plant was mixed with 10 mL 69% HNO3 (R&M Chemicals) in a digestion tube covered with a glass slip and left to stand overnight. Subsequently, the sample was heated in a block digester (AIM 600 Digestion System) to 95 °C for 1.5 h and cooled down to 80 °C, followed by the addition of 8 mL 30% H2O2 (R&M Chemicals). Subsequently, the mixture was heated again to 95 °C for 2 h, and 2.5 mL of aqua regia (HNO3: HCl = 1:3) was added, followed by the addition of deionized water to reach a total volume of 50 mL. Finally, the sample was filtrated through a 0.45 μm cellulose acetate membrane filter (Whatman, England) to obtain the extract. The Cu contents extracted from water and plant tissue of A. filiculoides and L. minor were analysed using an Optima 7300DV ICP-OES instrument (PerkinElmer) at a wavelength of 324.8; Cu accumulation was calculated using Eq. (1) (Ismail et al., 2019):
| (1) |
where CCu = concentration of Cu in water analysed by ICP-OES (mg/L), Vs = volume of water sample extraction (0.05 L), DW = dry plant weight (g).
2.3. Bioconcentration factor (BCF) of Cu in plants
The ability of A. filiculoides and L. minor to accumulate Cu was assessed by calculating the concentration of Cu in the plant tissue relative to the contaminated growth medium. This value was determined using the bioconcentration factor (BCF) (Eq. ((2)) as described by Zhuang et al. (2007). The BCF of plants can be classified into four categories, namely no phytoaccumulation (BCF<0.01), low phytoaccumulation (0.01–0.1), moderate phytoaccumulation (0.1–1) and high phytoaccumulation (1–10) (Panfili et al., 2017; Sekabira et al., 2011). The equation is as follows:
| (2) |
where Cp = Cu concentration in plants (mg/L), Vse = volume of water sample extracted (L), Cm = Cu concentration in the water (mg/L), Vwm = total water volume (L).
2.4. Plant dry weight and response to Cu
The tolerance of the two plants to Cu was evaluated by dry weight throughout the 7-day Cu exposure. On each sampling day (0, 1, 2, 5 and 7 days), the plants were separated from the water using a sieve and washed with distilled water, followed by oven-drying at 60 °C for 24 h to determine the dry weight. Additionally, plant responses towards Cu contaminants were evaluated based on the relative growth rate (RGR) (Ismail et al., 2020), using Eq. (3):
| (3) |
where DW2 is the final dry weight (g) of floating plants, and DW1 represents the initial dry weight (g) of floating plants.
2.5. Microanalysis of plant leaves
Scanning electron microscopy (SEM) was used to obtain information about the morphological, physiological and biochemical characteristics of plants via high-resolution images of samples (Golinejad and Mirjalili, 2020). This was done either at the surface or the cross-section area. Prior to SEM analysis, the plant samples were prepared to obtain images with maximum maintenance of form and cell structure and with minimum cellular damage (Golinejad and Mirjalili, 2020). Preparation was performed at the Electron Microscopy Unit, Faculty of Science and Technology, Universiti Kebangsaan Malaysia (UKM). Briefly, samples were subjected to chemical fixation using 2% glutaraldehyde for 12–24 h at 4 °C. Subsequently, the samples were dehydrated using an ethanol series (30, 50, 70, 80, 90 and 100% for 10 min each); each series was applied three times. Critical point drying (CPD) was applied using a critical point dryer (Leica EM CPD300, Germany) for 1 h and 30 min. During CPD, the water in the samples is replaced with liquid carbon dioxide (CO2). The samples were then cut and placed on the stub, using carbon double-sided tape to stick the sample on the stub. The arranged samples were then coated with gold using a sputter coater (Quorum Q150R, Germany) and viewed under a Field-Emission Scanning Electron Microscope (FE-SEM) (Ziess Model Supra VP, Germany) at the i-CRIM Laboratory, UKM Research Complex, Centre of Research and Instrumentation Management (CRIM), UKM. Energy dispersive X-ray spectroscopy (EDX) and mapping analyses of the plants were also performed using FE-SEM. The analysis was conducted on the last day of the exposure period (Day 7) for control plants and plants exposed to 1.00 mg Cu/L.
2.6. Kinetic modelling of Cu removal and uptake
The adsorption ability and percentage removal of Cu (II) ion by floating plants were calculated using the following equations (Eqs. (4) and (5)) (Paz et al., 2022):
| (4) |
| (5) |
where C0, Ce and Ct (mg/L) are the liquid phase concentrations of Cu initially, at equilibrium and at a specific time t, respectively, V (L) is the synthetic wastewater volume, DW (g) is the dry weight of adsorbent used (floating plants).
To understand the biosorption of Cu by floating plants from synthetic wastewater contaminated with different Cu concentrations with respect to the equilibration time, kinetic models were studied (Table 2S). Three kinetic models, namely the pseudo-first-order model, the pseudo-second-order model and the intra-particle diffusion model, were used to consider the biosorption mechanism of Cu (II) by floating plants. The constant of the pseudo-first-order kinetic (k1) can be defined by plotting log (qe−qt) against t. The values of k1 and qe can be obtained from the slope and the intercept of the plot, respectively. For the pseudo-second-order model, the constant of the pseudo-second-order kinetic (k2) can be defined by plotting t/qt against t. The values of k2 and qe can be obtained from the intercept and the slope of the plot, respectively. Regarding of the intra-particle diffusion, the values of Kid and I were determined from the slopes and intercepts of the plots of qt vs. t1/2, respectively (Ghasemi et al., 2018). Additionally, the sorption isotherm was investigated by the three equilibrium models of Langmuir, Freundlich and Temkin, as shown in Table 3S.
2.7. Statistical analysis
Statistical analysis of dependent factors (Cu concentrations in water during each day of the treatment period, accumulation of Cu in plants, dry weights of plants and relative growth rates of plants) in accordance with the initial Cu concentrations in water, conditions (with and without plants) and treatment period were performed using IBM SPSS Statistics Version 23 (Norhan et al., 2021). A significance level of p < 0.05 was adopted (Othman et al., 2022; AL Sbani et al., 2021).
3. Results and discussion
3.1. Cu removal from water by floating plants
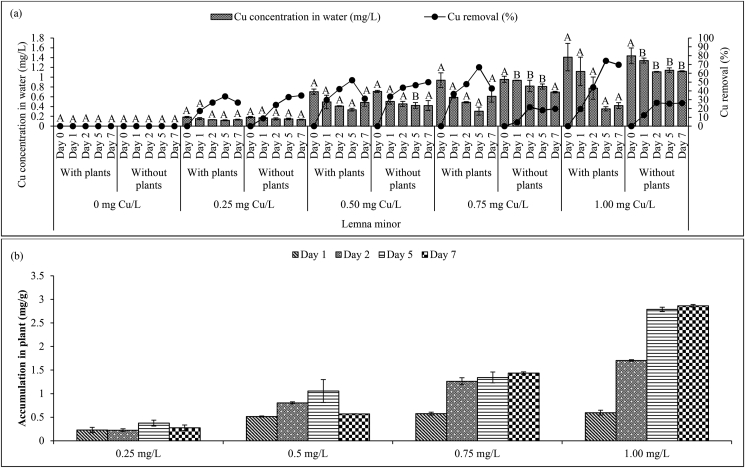
The Cu removal efficiencies for A. filiculoides and L. minor were determined. Figure 1Figure 1(a) and 2(a)2(a) illustrate the Cu removal efficiencies for the different Cu concentrations (0.25, 0.5, 0.75 and 1.00 mg/L). In general, the concentrations of Cu in both conditions (with and without plants) for both plant species decreased over time.
Figure 1.
(a) Concentration and removal of Cu by Azolla filiculoides in aqueous media and (b) accumulation of Cu per unit g of dry weight of plant. Mean ± SD (n = 3). Different letters (A–B) between treatments with and without plants within each initial Cu concentration for the same exposure period indicate significant differences (p < 0.05) between Cu concentration values in water and vice versa. The letter “c” indicates a significant difference at p < 0.05 in the Cu accumulation in plants (mg/g) between Days 1 and 7 within each initial Cu concentration.
Figure 2.
(a) Concentration and removal of Cu by Lemna minor in aqueous media and (b) accumulation of Cu per unit g of dry weight of plant. Mean ± SD (n = 3). Different letters (A–B) between treatments with and without plants within each initial Cu concentration for the same exposure period indicate significant differences (p < 0.05) between Cu concentration values in water and vice versa. The letter “c” indicates a significant difference at p < 0.05 in the Cu accumulation in plants (mg/g) between Days 1 and 7 within each initial Cu concentration.
The removal efficiency for A. filiculoides at low Cu concentrations of 0.25 and 0.5 mg/L was 100% after 1 day of exposure, whereas for 0.75 and 1.00 mg/L Cu, 100% removal efficiency was obtained after 2 days. In contrast, the maximum removal of Cu in the container without A. filiculoides reached 11, 40, 44 and 34% for Cu concentrations of 0.25, 0.5, 0.75 and 1.00 mg/L, respectively, on the last day of the treatment period. The presence of A. filiculoides in the glass container significantly increased the removal of Cu (p < 0.05) (represented by letters “A–B” in Figure 1(a)). The accumulation of Cu in A. filiculoides increased with increasing exposure period and initial Cu concentration (Figure 1(b)). At the end of the exposure period, 1 g of A. filiculoides had accumulated 0.32, 0.73, 0.13 and 1.49 mg Cu for initial Cu concentrations of 0.25,0.5, 0.75 and 1.00 mg/L respectively. The plants in the containers with and without Cu were able to survive, showing green leaves throughout the exposure period (Table 4S). Exposure to the highest Cu concentration (1.00 mg/L) showed no toxic effects on the plants.
Regarding L. minor, the maximum removal efficiencies for plants exposed to 0.25 and 0.5 mg/L were 34% and 52% after 5 days, whereas 74% was achieved for 1.00 mg/L Cu solution after 5 days of exposure. Additionally, the Cu removal efficiency gradually increased, for all Cu concentrations, until Day 5, followed by a decrease until Day 7. This was only observed for L. minor. According to Feigl et al. (2015), excess Cu can affect not only the growth of the leaves of Brassica juncea (L.) Czern and Brassica napus (L.) but also the physical appearance of the plants; i.e., the leaves turned yellow. Similar colour changes were observed for L. minor on Day 5, as shown in Table 4S. Additionally, the removal of Cu by L. minor was only significant at Cu concentrations of 0.75 and 1.00 mg/L (p < 0.05; represented by letters “A–B” in Figure 2(a)). The accumulation of Cu by 1 g of L. minor was 0.28, 0.57, 1.44 and 2.86 mg for initial Cu concentrations of 0.25, 0.5, 0.75 and 1.00 mg/L, respectively.
Overall, A. filiculoides showed a higher tolerance level towards Cu than L. minor, suggesting that A. filiculoides can remove more Cu from aqueous solution than L. minor. This might be explained by the lower accumulation of Cu in A. filiculoides (Figure 1(b)), which will not cause toxicity to this plant compared to L. minor (Figure 2(b)). This can be seen for the initial Cu concentration of 1.00 mg/L at Day 7, where Cu accumulation in 1 g of A. filiculoides was 1.49 mg, with a Cu removal of 100%, whereas 2.86 mg of Cu accumulated in 1 g of L. minor with a Cu removal of 74%. At the end of the exposure period, the leaves of A. filiculoides remained green, whereas those of L. minor had changed to a yellow colour. These findings highlight the potential of appropriate floating plant species to be employed during phytoremediation since different plant species resulted in distinct removal efficiency in removing Cu from contaminated water. This present study has demonstrated that Cu was efficiently removed by A. filiculoides with 26% removal higher than L. minor. In addition, there was significant relationship at p < 0.05 for each factor and among factors (Tables 1 and 2) for both plant species. It can be concluded that direct filtration of Cu via rhizosphere, followed with translocation within tissues and finally accumulation of Cu in leaves took place. Thus, rhizofiltration and phytoextraction are anticipated phytoremediation mechanisms for Cu as illustrated in Figure 3.
Table 1.
Two-way ANOVA results for the concentrations of Cu in water for each factor and between factors for both plant species.
| A. filliculoides |
L. minor |
|||
|---|---|---|---|---|
| F | Sig. | F | Sig. | |
| Cu concentration in water | 2,770 | <0.05 | 366 | <0.05 |
| Condition (with and without plants) | 3,670 | <0.05 | 41.4 | <0.05 |
| Treatment period | 1,230 | <0.05 | 38.3 | <0.05 |
| Cu concentration in water ∗ Condition | 811 | <0.05 | 38.2 | <0.05 |
| Cu concentration in water ∗ Treatment period | 207 | <0.05 | 8.17 | <0.05 |
| Condition ∗ Treatment period | 278 | <0.05 | 7.40 | <0.05 |
| Cu concentration in water ∗ Condition ∗ Treatment period | 170 | <0.05 | 6.31 | <0.05 |
| R2 | 0.997 | 0.954 | ||
| Adjusted R2 | 0.995 | 0.931 | ||
Table 2.
Two-way ANOVA results for the concentrations of Cu in plants at different factors and among factors.
|
A. filiculoides |
L. minor |
|||
|---|---|---|---|---|
| F | Sig. | F | Sig. | |
| Cu concentration in water | 370.360 | <0.05 | 96.916 | <0.05 |
| Treatment period | 159.362 | <0.05 | 49.463 | <0.05 |
| Cu concentration in water ∗ Treatment period | 39.676 | <0.05 | 21.732 | <0.05 |
| R2 | 0.982 | 0.949 | ||
| Adjusted R2 | 0.974 | 0.925 | ||
Figure 3.
Adsorption mechanism involved in the removal of Cu ions.
In a different study conducted by Rai (2019), the potential of the floating plants Pistia stratiotes, Spirodela polyrhiza and Eichhornia crassipes to remove mixtures of six heavy metals (Fe, Cu, Cd, Cr, Zn, Ni) over 15 days was investigated, obtaining removal efficiencies of more than 79%. Khellaf and Zerdaoui (2010) examined Lemna gibba growth at Cu concentrations from 0.003-0.3 mg/L. After 4 days of exposure, the Cu removal efficiencies for initial Cu levels of 0.3 and 0.1 mg/L were 60% and 80%, respectively.
3.2. Phytoremediation prospective for Cu by A. filiculoides and L. minor
Table 3 lists the BCF data for the two floating plants A. filiculoides and L. minor. The values were higher for A. filiculoides than for L. minor, ranging from 0.903-1.600 and 0.191–0.432, respectively. The bioconcentration factor (BCF) is an index of the ability of a plant to accumulate metals from polluted water (Rezania et al., 2016; Mimmo et al., 2015); in this study, the BCF values indicate that A. filiculoides is a greater Cu accumulator than L. minor.
Table 3.
Bioconcentration factor (BCF) values after 7 days of treatment for Azolla filiculoides and Lemna minor.
| Plant species | Azolla filiculoides | Lemna minor |
|---|---|---|
| Cu concentration (mg/L) | BCF | |
| 0.25 | 1.600 | 0.360 |
| 0.5 | 0.903 | 0.191 |
| 0.75 | 1.038 | 0.335 |
| 1.00 | 0.954 | 0.432 |
3.3. Growth evaluation of A. filiculoides and L. minor
Figure 4 depicts the dry weights of A. filiculoides (Figure 4(a)) and L. minor (Figure 4(b)) from Days 0–7. The dry weight for both plants increased over, with a slower increase in plants exposed to Cu. The dry weight of both plants in the control (0 mg Cu/L) significantly increased from Days 0–7 (p < 0.05). Regarding plants exposed to Cu, no significant increase in dry weight (p > 0.05) was observed from Days 0–7 for A. filiculoides in all Cu concentrations, but for L. minor, insignificant growth (p > 0.05) was only observed for Cu concentrations of 0.75 and 1 mg/L. Even though the growth of A. filiculoides exposed to Cu was lower than that of the control plants, this species still grew well, with green leaves throughout the 7-day exposure period and for all Cu concentrations. Unlike A. filiculoides, the growth of L. minor exposed to 0.75 and 1.00 mg/L was inhibited, and the plants showed yellow leaves at the end of the experiment. This phenomenon is known as chlorosis and was the result of excess Cu (Kumar et al., 2021). Different plant species can tolerate different Cu concentrations that they can tolerate. In this study, L. minor was able to tolerate 0.25 and 0.5 mg/L Babu et al. (2003) also showed L. gibba growth at a Cu concentration of 0.25 mg/L.
Figure 4.
Dry weights of the two floating plant species (a) A. filiculoides and (b) L. minor. Mean ± SD (n = 3). A-a indicate significant statistical differences (p < 0.05) for dry weight between initial and final exposure (Days 0 and 7, respectively) within each initial Cu concentration. Letters B-b indicate significant statistical differences (p < 0.05) for dry weight among the different initial Cu concentrations when compared with 0 mg/L at the same treatment period.
For the RGR, both plants showed similar trends of decreasing values as the concentrations of Cu increased, with values for A. filiculoides and L. minor in the control (0.0482 and 0.0376 g g−1 day−1) and treatment with 1 mg Cu/L (0.0098 and 0.0083 g g−1 day−1), respectively. The decrease in RGR indicates an adverse impact of Cu on the growth of A. filiculoides and L. minor. Plants exposed to the highest Cu concentration (1.00 mg/L) showed the lowest RGR values. Since A. filiculoides had a higher dry weight compared to L. minor (Figure 4), although the initial wet weights for both plants were the same, A. filiculoides is more tolerant on Cu than L. minor.
3.4. Microanalysis of plant leaves
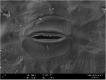
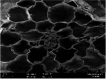
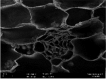
Photos of both plants on Day 0 and Day 7 when exposed to 1.00 mg Cu/L are shown in Table 4(a). The respective SEM images from Table 4(b) represent the stomata of A. filiculoides and L. minor. The stomata were open at the end of the exposure period in A. filiculoides, whereas in L. minor, the stomata were smaller than at the beginning of the exposure period. Table 4(c) and d represents the cross-section areas of both plants at 500X and 1000X magnification, respectively. The structure of the inner cells of A. filiculoides exposed to 1.00 mg Cu/L at the end of the exposure period was well organized as compared to the initial period. The Cu content of the water did not inhibit the growth of this plant (Table 4(a)). Unlike A. filiculoides, L. minor exhibited shrinking of its inner structure at the end of the exposure period. Regarding L. minor, Cu at 1.00 mg Cu/L inhibited the growth and the morphology of this plant.
Table 4.
(a) Physical appearance and SEM images of (b) stomata, (c) cross sections at 500× and (d) cross sections at 1,000× of Azolla filiculoides and Lemna minor at the beginning (Day 0) and end (Day 7) of exposure to 1.00 mg Cu/L.
|
Azolla filiculoides |
Lemna minor |
|||
|---|---|---|---|---|
| Day 0 | Day 7 | Day 0 | Day 7 | |
| (a) |  |
 |
 |
 |
| (b) |  |
 |
 |
 |
| (c) |  |
 |
 |
 |
| (d) |  |
 |
 |
 |
3.5. Kinetics and isotherms of Cu biosorption
Remaining Cu and plant dry weight data were used to generate the Cu removal kinetics. The sorption rate of Cu by floating plants was investigated using pseudo-first-order, pseudo-second-order and intra-particle diffusion models (Figure 3S-5S). The model with the highest R2 values was considered the best fitted model.
The adsorption of Cu (II) onto the two floating plants followed the pseudo-second-order model, with high R2 values in the range of 0.8–1.0 (Table 5). Moreover, the calculated qe values for the four Cu(II) concentrations by the two floating plants, A. filiculoids and L. minor adsorbent, obtained from the pseudo-second-order model, were 0.2297, 0.8190, 1.1733 and 1.6969 mg g−1 as well as 0.2879, 1.0385, 1.5201, and 4.6577 mg g−1, respectively, closer to the experimental data (0.230, 0.891, 1.168 and 1.682 mg g−1 as well as 0.249, 1.402, 2.432 and 4.047 mg g−1), indicating that the adsorption process is mainly controlled by chemisorption through sharing or exchanging electrons and the formation of complexes between floating plants and Cu (II) ions. The high correlation indicates the involvement of electrostatic interactions between plants and Cu (Wakkel et al., 2019). Chua et al. (2019), who used bamboo species for Cu uptake, obtained the best fit for Michaelis-Menten. In another study, Chrysopogon zizanioides L. (Vertiver grass) fitted the first-order removal model (Sun et al., 2016). Differences in kinetic models might be due to their responses towards the adsorbate.
Table 5.
Adsorption kinetics for adsorption of Cu (II) onto the two floating plant species.
| Model | Adsorbent | Sorbent |
Parameter |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu(II) Concentration (mg/L) | qe,exp (mg g−1) | qe,cal (mg g−1) | k1 (day−1) | R2 | |||||
| (a) | Pseudo-first-order | Azolla filiculoides | 0.25 | 0.2297 | 0.5049 | −0.1299 | 0.3309 | ||
| 0.5 | 0.8912 | 0.9480 | −0.0101 | 0.3309 | |||||
| 0.75 | 1.1676 | 0.4305 | −0.1292 | 0.1161 | |||||
| 1 | 1.6819 | 0.2202 | −0.1654 | 0.0675 | |||||
| Lemna minor | 0.25 | 0.2494 | 0.2121 | −0.2639 | 0.4903 | ||||
| 0.5 | 1.4020 | 0.6001 | 0.0226 | 0.0073 | |||||
| 0.75 | 2.4328 | 1.2900 | 0.0523 | 0.1014 | |||||
| 1 | 4.0473 | 3.8353 | 0.4470 | 0.7581 | |||||
| (b) | Model | Adsorbent | Sorbent | Parameter | |||||
| Cu(II) Concentration (mg/L) | qe,exp (mg g−1) | qe,cal (mg g−1) | k2 (g mg−1 day) | R2 | |||||
| Pseudo-second-order | Azolla filiculoides | 0.25 | 0.230 | 0.2297 | 3.7894 × 1015 | 1 | |||
| 0.5 | 0.891 | 0.819 | - | 1 | |||||
| 0.75 | 1.168 | 1.1733 | 27.6204 | 0.9999 | |||||
| 1 | 1.682 | 1.6969 | 9.9506 | 0.9996 | |||||
| Lemna minor | 0.25 | 0.249 | 0.2879 | 14.0513 | 0.9648 | ||||
| 0.5 | 1.402 | 1.0385 | −5.3286 | 0.9209 | |||||
| 0.75 | 2.432 | 1.5201 | −2.5817 | 0.8822 | |||||
| 1 | 4.047 | 4.6577 | 0.1259 | 0.8191 | |||||
| (c) | Model | Adsorbent | Sorbent | Parameter | |||||
| Cu(II) Concentration (mg/L) | ki (mg g−1 min−1) | I (mg g−1) | R2 | ||||||
| Intra-particle diffusion | Azolla filiculoides | 0.25 | 0.077 | 0.0714 | 0.6114 | ||||
| 0.5 | 0.2987 | 0.2771 | 0.6114 | ||||||
| 0.75 | 0.4006 | 0.3319 | 0.6619 | ||||||
| 1 | 0.5856 | 0.4462 | 0.696 | ||||||
| Lemna minor | 0.25 | 0.0843 | 0.0314 | 0.8137 | |||||
| 0.5 | 0.3823 | 0.2732 | 0.5774 | ||||||
| 0.75 | 0.6189 | 0.4506 | 0.5302 | ||||||
| 1 | 1.6135 | −0.0762 | 0.9455 | ||||||
The three isotherm models of Langmuir, Freundlich and Temkin were employed to study the equilibrium data of Cu(II) adsorption by A. filiculoides and L. minor. The models depicted the correlation between the amounts of Cu sorbed on plants (mg g−1) versus the Cu concentration in the solution. Figure 6S shows the linearized forms of the Langmuir, Freundlich and Temkin models. The values of the adsorption isotherm parameters are summarised in Table 6. The species A. filiculoids did not match all three models, whereas L. minor fitted Freundlich better, with a correlation coefficient R2 of 0.975. It can be inferred that the adsorption occurred in multiple layers.
Table 6.
Parameters of the isotherm models (a) Langmuir, (b) Freundlich and (c) Temkin for the two species tested.
| Model | Parameter | Adsorbent |
||
|---|---|---|---|---|
| Azolla filiculoides | Lemna minor | |||
| (a) | Langmuir | KL (L/mg) | 0 | 2.2973 |
| qm (mg/g) | 0 | 0.6980 | ||
| R2 | NA | 0.660 | ||
| RL | 1 | 0.7018 | ||
| (b) | Model | Parameter | Adsorbent | |
| Azolla filiculoides | Lemna minor | |||
| Freundlich | Kf (mg/g) | 0 | 26.1400 | |
| 1/n | 0 | 2.1722 | ||
| R2 | NA | 0.975 | ||
| (c) | Model | Parameter | Adsorbent | |
| Azolla filiculoides | Lemna minor | |||
| Temkin | B | 0 | 2.3176 | |
| KT (L/g) | 0 | 9.0460 | ||
| R2 | NA | 0.959 | ||
3.6. Chemical characteristics of the aquatic media
In this study, four concentrations of Cu (0.25, 0.50, 0.75 and 1.00 mg/L) were used to evaluate the phytoaccumulation abilities of the two floating plant species A. filiculoides and L. minor to treat water contaminated with Cu. The pH and ORP were observed and recorded as shown in Figure 5. The pH was slightly above neutral for both plants (Figure 5(a) and Figure 5(c)), suggesting that the possible mechanisms of heavy metal removal were immobilisation in the rhizosphere and absorption (Rana and Maiti, 2018). According to Kasim et al. (2017), the amounts and concentrations of heavy metals in groundwater are controlled by the pH and the redox potential. A higher pH contributes to a lower solubility of heavy metal ions, which may limit heavy metal uptake and translocation into plants (Sekabira et al., 2011). The ORP oscillated between -100 and +25 mV for A. filiculoides (Figure 5(b)) and L. minor (Figure 5(d)), respectively, indicating that conditions of heavy metal removal with floating plants fluctuated between anoxic and aerobic conditions (Al-Baldawi et al., 2021; AL Sbani et al., 2020), most likely because of oxygen leakage from the roots (Nivala et al., 2019).
Figure 5.
Variation in the chemical properties of media containing A. filiculoides for (a) pH and (b) ORP and L. minor for (c) pH and (d) ORP. Mean ± SD (n = 3).
4. Conclusions
We investigated the potential of two floating plant species (Azolla filiculoides and Lemna minor) to remove Cu from aqueous media. A. filiculoides showed a higher removal efficiency (100%) than L. minor (74%) with less toxicity observed physically and under the microscope during the last day of exposure. The accumulation loading of Cu in A. filiculoides is lesser (1.49 mg/g) than L. minor (2.86 mg/g). Higher accumulation of Cu per 1 g of plant biomass caused the toxicity on plant leaves leading to less removal efficiency of Cu. Phytoaccumulation by floating plants is an alternative and environmentally friendly approach to remediate Cu-polluted water. Choosing the right plant species before any treatment is crucial as this small act of decision making can affect the end results. Further studies are needed to investigate the potential of generating value-added products for a circular economy, related to the Sustainable Development Goals (SDGs) for environmental sustainability.
Declarations
Author contribution statement
Israa Abdulwahab Al-Baldawi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Safaa Rasheed Yasin; Salwa Shamran Jasim; Asia Fadhile Almansoory: Analyzed and interpreted the data; Wrote the paper.
Siti Rozaimah Sheikh Abdullah: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Nur ‘Izzati Ismail: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Salwa Shamran Jasim: Analyzed and interpreted the data.
Funding statement
This work was supported by Universiti Kebangsaan Malaysia under DIP-2021-002 research grant and Tasik Chini Research Centre.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are gratefully acknowledge the Electron Microscopy Unit, Faculty of Science and Technology, UKM, and i-CRIM Laboratory, Centre of Research and Instrumentation Management (CRIM), UKM, for support regarding the microscopic images (FESEM). The first author would like to acknowledge the Iraqi Ministry of Higher Education and Scientific Research for support. The authors would like to thank Universiti Kebangsaan Malaysia for sponsoring this study under DIP-2021-002 research grant and Tasik Chini Research Centre for supporting this research project.
Contributor Information
Siti Rozaimah Sheikh Abdullah, Email: rozaimah@ukm.edu.my.
Nur 'Izzati Ismail, Email: nurezatyismail@ukm.edu.my.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- AL Sbani N.H., Abdullah S.R.S., Idris M., Hasan H.A., Al-Baldawi I.A., Jehawi O.H., Ismail N.I. Remediation of PAHs-contaminated water and sand by tropical plant (Eleocharis ochrostachys) through sub-surface flow system. Environ. Technol. Innovat. 2020;20 [Google Scholar]
- AL Sbani N.H., Abdullah S.R.S., Idris M., Hasan H.A., Halmi M.I.E., Jehawi O.H., Ismail N.I. PAH-degrading rhizobacteria of Lepironia articulata for phytoremediation enhancement. J. Water Proc. Eng. 2021;39 [Google Scholar]
- Al-Baldawi I.A., Abdullah S.R.S., Ismail N.I., Almanso A.F., Jasim S.S. Phytotoxicity of Salvinia molesta in diesel exposure. Al-Khwarizmi Eng. J. 2021;17(3):13–21. [Google Scholar]
- Ali Z., Waheed H., Kazi A.G., Hayat A., Ahmad M. In: Plant Metal Interaction-Emerging Remediation Techniques. Ahmad P., editor. Elsevier; Amsterdam: 2016. Duckweed: chapter 16: an efficient hyperaccumulator of heavy metals in water bodies; pp. 411–429. [Google Scholar]
- APHA . American Public Health Association; Washington, DC: 2017. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- Arán D.S., Harguinteguy C.A., Fernandez-Cirelli A., Pignata M.L. Phytoextraction of Pb, Cr, Ni, and Zn using the aquatic plant Limnobium laevigatum and its potential use in the treatment of wastewater. Environ. Sci. Pollut. Control Ser. 2017;24:18295–18308. doi: 10.1007/s11356-017-9464-9. [DOI] [PubMed] [Google Scholar]
- Babu T., Akhtar T., Lampi M., Tripuranthakam S., Dixon D., Greenberg B. Similar stress responses are elicited by copper and ultraviolet radiation in the aquatic plant Lemna gibba: implication of reactive oxygen species as common signals. Plant Cell Physiol. 2003;44:1320–1329. doi: 10.1093/pcp/pcg160. [DOI] [PubMed] [Google Scholar]
- Bianchi E., Biancalani A., Berardi C., Antal A., Fibbi D., Coppi A., Lastrucci L., Bussotti N., Colzi I., Renai L., Scordo C., Bubba M.D., Gonnelli C. Improving the efficiency of wastewater treatment plants: bio-removal of heavy-metals and pharmaceuticals by Azolla filiculoides and Lemna minuta. Sci. Total Environ. 2020;746 doi: 10.1016/j.scitotenv.2020.141219. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R., Clark W.D., Sharma J.G., Goswami R.K., Shrivasta A.K., Tocher D.R. Mass production of Lemna minor and its amino acid and fatty acid profiles. Front. Chem. 2018;6:479. doi: 10.3389/fchem.2018.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua J., Banua J.M., Arcilla I., Orbecido A., de Castro M.E., Ledesma N., Deocaris C., Madrazo C., Belo L. Phytoremediation potential and copper uptake kinetics of Philippine bamboo species in copper contaminated substrate. Heliyon. 2019;5(9) doi: 10.1016/j.heliyon.2019.e02440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M.L., Santos M.C.R., Carrapiço F., Pereira A.L. Azolla-Anabaena’s behaviour in urban wastewater and artificial media - influence of combined nitrogen. Water Res. 2009;43 doi: 10.1016/j.watres.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Dohaei M., Karimi K., Rahimmalek M., Satari B. Integrated biorefinery of aquatic fern Azolla filiculoides for enhanced extraction of phenolics, protein, and lipid and methane production from the residues. J. Clean. Prod. 2020;276 [Google Scholar]
- Espinoza-Quinones F.R., Modenes A.N., Thome L.P., Palacio S.M., Trigueros D.E.G., Oliveira A.P., Szymanski N. Study of the bioaccumulation kinetic of lead by living aquatic macrophyte Salvinia auriculata. Chem. Eng. J. 2009;150:316–322. [Google Scholar]
- Feigl G., Kumar D., Lehotai N., Peto A., Molnnar A., Racz E., Ordog A., Erdei L., Kolbert Z., Laskay G. Comparing the effects of excess copper in the leaves of Brassica juncea (L.) Czern and Brassica napus (L.) seedlings: growth inhibition, oxidative stress and photosynthetic damage. Acta Biol. Hung. 2015;66(2):205–221. doi: 10.1556/018.66.2015.2.7. [DOI] [PubMed] [Google Scholar]
- Ghasemi N., Ghasemi M., Moazeni S., Parisa Ghasemi P., Alharbi N.S., Gupta V.K., Agarwal S., Burakova I.V., Tkachev A.G. Zn (II) removal by amino-functionalized magnetic nanoparticles: kinetics, isotherm, and thermodynamic aspects of adsorption. J. Ind. Eng. Chem. 2018;62:302–310. [Google Scholar]
- Golinejad S., Mirjalili M.H. Fast and cost-effective preparation of plant cells for scanning electron microscopy (SEM) analysis. Anal. Biochem. 2020;609 doi: 10.1016/j.ab.2020.113920. [DOI] [PubMed] [Google Scholar]
- Haan T.Y., Fen C.S., Radz M.F., Ganasen U. Comparative study for lake water remediation: chemical coagulation and electrocoagulation. Jurnal Kejuruteraan SI. 2018;1(6):81–87. [Google Scholar]
- Halmi M.I.E., Abdullah S.R.S., Shukor M.S. Characterization of chromate reducing Pseudomonas aeruginosa strain MIE3 isolated from Juru River sludge and its potential on azo dye decolorization. J. Chem. Pharmaceut. Sci. 2017;10:522–526. [Google Scholar]
- Hasan H.A., Abdullah S.R.S., Kofli N.T., Yeoh S.J. Interaction of environmental factors on simultaneous biosorption of lead and manganese ions by locally isolated Bacillus cereus. J. Ind. Eng. Chem. 2016;37:295–305. [Google Scholar]
- Huang H., Zhao Y., Xu Z., Ding Y., Zhang W., Wu L. Biosorption characteristics of a highly Mn (II) resistant Ralstonia pickettii strain isolated from Mn ore. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifayefunmi O.S., Mirseabasov O.A., Synzynys B.I. Indirect assessment of internal irradiation from tritium decay on Lemna minor duckweed. Nucl. Eng. Technol. 2021;53:1991–1999. [Google Scholar]
- Ismail N.I., Abdullah S.R.S., Idris M., Hasan H.A., Halmi M.I.E., AL Sbani N.H., Jehawi O.H., Sanusi S.N.A., Hashim M.H. Accumulation of Fe-Al by Scirpus grossus grown in synthetic bauxite mining wastewater and identification of resistant rhizobacteria. Environ. Eng. Sci. 2017;34:367–375. [Google Scholar]
- Ismail N.I., Abdullah S.R.S., Idris M., Hasan H.A., Halmi M.I.E., AL Sbani N.H., Jehawi O.H. Simultaneous bioaccumulation and translocation of iron and aluminium from mining wastewater by Scirpus grossus. Desalination Water Treat. 2019;163:133–142. [Google Scholar]
- Kamaruzzaman M.A., Abdullah S.R.S., Hasan H.A., Hassan M., Idris M., Ismail N.I. Potential of hexavalent chromium-resistant rhizosphere bacteria in promoting plant growth and hexavalent chromium reduction. J. Environ. Biol. 2019;40:427–433. [Google Scholar]
- Kasim N., Mahmoudi E., Mohammad A.W., Abdullah S.R.S. Influence of feed concentration and pH on iron and manganese rejection via nanohybrid polysulfone/Ag-GO ultrafiltration membrane. Desalination Water Treat. 2017;61:29–41. [Google Scholar]
- Khellaf N., Zerdaoui M. Growth response of the duckweed Lemna gibba L. to copper and nickel phytoaccumulation. Ecotoxicology. 2010;19:1363–1368. doi: 10.1007/s10646-010-0522-z. [DOI] [PubMed] [Google Scholar]
- Khvatkov P., Chernobrovkina M., Okuneva A., Dolgov S. Creation of culture media for efficient duckweeds micropropagation (Wolffia arrhiza and Lemna minor) using artificial mathematical optimization models. Plant Cell Tissue Organ Cult. 2019;136:85–100. [Google Scholar]
- Kittiwongwattana C., Vuttipongchaikij S. Effects of nutrient media on vegetative growth of Lemna minor and Landoltia punctata during in vitro and ex vitro cultivation. Maejo Int. J. Sci. Technol. 2013;7(1):60–69. [Google Scholar]
- Kumar V., Pandita S., Sidhu G.P.S., Sharma A., Khanna K., Kaur P., Bali A.S., Setia R. Copper bioavailability, uptake, toxicity and tolerance in plants: a comprehensive review. Chemosphere. 2021;262 doi: 10.1016/j.chemosphere.2020.127810. [DOI] [PubMed] [Google Scholar]
- Mimmo T., Bartucca M.L., Buono D., Cesco S. Italian ryegrass for the phytoremediation of solutions polluted with terbuthylazine. Chemosphere. 2015;119:31–36. doi: 10.1016/j.chemosphere.2014.04.114. [DOI] [PubMed] [Google Scholar]
- Musah M., Azeh Y., Mathew J.T., Umar M.T., Abdulhamid Z., Muhammad A.I. Adsorption kinetics and isotherm models: a review. Caliphate J. Sci. Technol. 2022;1:20–26. [Google Scholar]
- Mutar Z.H., Mohammed A.A., Al-Baldawi I.A., Abdullah S.R.S., Ismail N.I. Assessment of ornamental plants tolerance for acute exposure of acetaminophen and methylparaben in constructed wetlands- A preliminary study. Al-Khwarizmi Eng. J. 2022;18(3):26–36. [Google Scholar]
- Nivala J., Boog J., Headley T., Aubron T., Wallace S., Brix H., Mothes S., van Afferden M., Müller R.A. Side-by-side comparison of 15 pilot-scale conventional and intensified subsurface flow wetlands for treatment of domestic wastewater. Sci. Total Environ. 2019;658:1500–1513. doi: 10.1016/j.scitotenv.2018.12.165. [DOI] [PubMed] [Google Scholar]
- Norhan M.A., Abdullah S.R.S., Hasan H.A., Ismail N.I. A constructed wetland system for bio-polishing palm oil mill effluent and its future research opportunities. J. Water Proc. Eng. 2021;41 [Google Scholar]
- Othman A.R., Ismail N.S., Abdullah S.R.S., Hasan H.A., Kurniawan S.B., Sharuddin S.S.N., Ismail N.I. Potential of indigenous biosurfactant-producing fungi from real crude oil sludge in total petroleum hydrocarbon degradation and its future research prospects. J. Environ. Chem. Eng. 2022;10 [Google Scholar]
- Panfili I., Bartucca M.L., Ballerini E., Buono D.D. Combination of aquatic species and safeners improves the remediation of copper polluted water. Sci. Total Environ. 2017;601–602:1263–1270. doi: 10.1016/j.scitotenv.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Paul B., Sarkar A., Roy S. Appraising the stress responses in Azolla filiculoides elicited by short-term exposure of phenol. Plant Stress. 2021;2 [Google Scholar]
- Paz R., Viltres H., Gupta N.K., Rajput K., Roy D.R., Romero-Galarza A., Biesinger M.C., Carolina Leyva C. Zirconium-organic framework as a novel adsorbent for arsenate remediation from aqueous solutions. J. Mol. Liq. 2022;356 [Google Scholar]
- Rahman M.A., Hasegawa H. Review: aquatic arsenic: phytoremediation using floating macrophytes. Chemosphere. 2011;83:633–646. doi: 10.1016/j.chemosphere.2011.02.045. [DOI] [PubMed] [Google Scholar]
- Rai P.K. Heavy metals/metalloids remediation from wastewater using free floating macrophytes of a natural wetland. Environ. Technol. Innovat. 2019;15 [Google Scholar]
- Rana V., Maiti S.K. Municipal wastewater treatment potential and metal accumulation strategies of Colocasia esculenta (L.) Schott and Typha latifolia L. in a constructed wetland. Environ. Monit. Assess. 2018;190:328–333. doi: 10.1007/s10661-018-6705-4. [DOI] [PubMed] [Google Scholar]
- Rego R.M., Kurkuri M.D., Kigga M. A comprehensive review on water remediation using UiO-66 MOFs and their derivatives. Chemosphere. 2022;302 doi: 10.1016/j.chemosphere.2022.134845. [DOI] [PubMed] [Google Scholar]
- Rezania S., Taib S.M., Md Din M.F., Dahalan F.A., Kamyab H. Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. J. Hazard Mater. 2016;318:587–599. doi: 10.1016/j.jhazmat.2016.07.053. [DOI] [PubMed] [Google Scholar]
- Sahsiny J., Haizal M.W.B., Razali N.A.N.M., Ragunathan T., Abdullah S.R.S., Nordin D., Ali J.M. Perbandingan kecekapan penjerapan karbon teraktif daripada pelbagai herba. J. Kejuruteraan. 2021;33(3):593–621. [Google Scholar]
- Sathish S., Supriya S., Andal P., Prabu D., Aravind Kumar J., Rajasimman M., Ansar S., Rezania S. Effective utilization of Azolla filiculoides for biodiesel generation using graphene oxide nano catalyst derived from agro-waste. Fuel. 2022;329 [Google Scholar]
- Sekabira K., Oryem–Origa H., Mutumba G., Kakudidi E., Basamba T.A. Heavy metal phytoremediation by Commelina benghalensis (L) and Cynodon dactylon (L) growing in Urban stream sediments. Int. J. Plant Physiol. Biochem. 2011;3:133–142. [Google Scholar]
- Selamat S.N., Halmi M.I.E., Abdullah S.R.S., Idris M., Hassan H.A., Anuar N. Optimization of lead (Pb) bioaccumulation in Melastoma malabathricum L. by response surface methodology (RSM) Rend. Fis. Acc. Lincei. 2018;29:43–51. [Google Scholar]
- Subari F., Kamaruzzaman M.A., Abdullah S.R.S., Hasan H.A., Othman A.R. Simultaneous removal of ammonium and manganese in slow sand biofilter (SSB) by naturally grown bacteria from lake water and its diverse microbial community. J. Environ. Chem. Eng. 2018;6:6351–6358. [Google Scholar]
- Sun S.X., Li Y.M., Zheng Y., Hua Y., Datta R., Dan Y.M., Lv P., Sarkar D. Uptake of 2,4-bis(Isopropylamino)-6-methylthio-s-triazine by vetiver grass (Chrysopogon zizanioides L.) from hydroponic media. Bull. Environ. Contam. Toxicol. 2016;96:550–555. doi: 10.1007/s00128-016-1737-3. [DOI] [PubMed] [Google Scholar]
- Tangahu B.V., Abdullah S.R.S., Basri H., Idris M., Anuar N., Mukhlisin M. Phytoremediation of wastewater containing lead (Pb) in pilot reed bed using Scirpus grossus. Int. J. Phytoremediation. 2013;15:663–676. doi: 10.1080/15226514.2012.723069. [DOI] [PubMed] [Google Scholar]
- Titah H.S., Halmi M.I.E., Abdullah S.R.S., Hasan H.A., Idris M., Anuar N. Statistical optimization of the phytoremediation of arsenic by Ludwigia octovalvis in a pilot reed bed using response surface methodology (RSM) versus an artificial neural network (ANN) Int. J. Phytoremediation. 2018;20:721–729. doi: 10.1080/15226514.2017.1413337. [DOI] [PubMed] [Google Scholar]
- Titah H.S., Abdullah S.R.S., Idris M., Anuar N., Basri H., Mukhlisin M., Tangahu B.V., Purwanti I.F., Kurniawan S.B. Arsenic resistance and biosorption by isolated rhizobacteria from the roots of Ludwigia octovalvis. Internet J. Microbiol. 2018:1–10. doi: 10.1155/2018/3101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titah H.S., Purwanti I.F., Tangahu B.V., Kurniawan S.B., Imron M.F., Abdullah S.R.S., Ismail N.I. Kinetics of aluminium removal by locally isolated Brochothrix thermosphacta and Vibrio alginolyticus. J. Environ. Manag. 2019;238:194–200. doi: 10.1016/j.jenvman.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Van Hove C. food and agriculture organization; Rome, Italy: 1989. Azolla: and its Multiple Uses with Emphasis on Africa. [Google Scholar]
- Wakkel M., Khiari B., Zagrouba F. Textile wastewater treatment by agro-industrial waste: equilibrium modelling, thermodynamics and mass transfer mechanisms of cationic dyes adsorption onto low-cost lignocellulosic adsorbent. J. Taiwan Inst. Chem. Eng. 2019;96:439–452. [Google Scholar]
- Wuana R.A., Okieimen F.E. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Notices. 2011 [Google Scholar]
- Zhuang P., Yang Q.W., Wang H.B., Shu W.S. Phytoextraction of heavy metals by eight plant species in the field. Water. Air Soil Pollut. 2007;184:235–242. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.